Cyanohydrin reaction: Difference between revisions
Script-assisted style fixes: mainly date formats |
changing cyan to purple because cyan is completely illegible on my screen on a white background (presumably also a problem for others). Please feel free to choose a different colour |
||
| (17 intermediate revisions by 14 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Addition reaction of carbonyl compounds and cyanide}} |
|||
{{Use dmy dates|date= |
{{Use dmy dates|date=January 2024}} |
||
| ⚫ | |||
{{Reactionbox |
|||
| Name = Cyanohydrin reaction |
|||
| Type = Addition reaction |
|||
| NamedAfter = Friedrich Urech |
|||
}} |
|||
In [[organic chemistry]], a '''cyanohydrin reaction''' is an [[organic reaction]] in which an [[aldehyde]] ({{chem2|\sCH\dO}}) or [[ketone]] ({{chem2|>C\dO}}) reacts with a [[cyanide]] [[anion]] ({{chem2|N\tC-}}) or a [[nitrile]] ({{chem2|\sC\tN}}) to form a [[cyanohydrin]] ({{chem2|>C(OH)C\tN}}). For example: |
|||
<math chem display=block> |
|||
\ce{R}{\color{red} \ce{CH=O}} + \ce{R}{\color{purple} \ce{C#N}} \longrightarrow \ce{R2}{\color{red} \ce{C(OH)}}{\color{purple} \ce{C#N}} |
|||
</math> |
|||
| ⚫ | This [[nucleophilic addition]] is a [[reversible reaction]] but with [[aliphatic]] [[carbonyl]] compounds equilibrium is in favor of the reaction products. The cyanide source can be [[potassium cyanide]] (KCN), [[sodium cyanide]] (NaCN) or [[trimethylsilyl cyanide]] ({{chem2|(CH3)3SiCN}}). With aromatic aldehydes such as [[benzaldehyde]], the [[benzoin condensation]] is a competing reaction. The reaction is used in [[carbohydrate chemistry]] as a chain extension method for example that of D-[[xylose]]. |
||
== Examples == |
== Examples == |
||
| Line 7: | Line 20: | ||
[[Image:Benzoquinone cyanohydrin reaction.svg|thumb|600px|center|Reaction of [[benzoquinone]] with trimethylsilylcyanide, catalyst KCN is introduced as a 1:1 complex with the [[Crown ether]] 18-crown-6]] |
[[Image:Benzoquinone cyanohydrin reaction.svg|thumb|600px|center|Reaction of [[benzoquinone]] with trimethylsilylcyanide, catalyst KCN is introduced as a 1:1 complex with the [[Crown ether]] 18-crown-6]] |
||
[[Image:Xylose cyanohydrin reaction.svg|thumb|800px|center|chain extension of D-[[xylose]] in equilibrium with its [[hemiacetal]] with KCN to the cyclic [[ester]]]] |
|||
== Reaction mechanism == |
== Reaction mechanism == |
||
[[Image:Cyanohydrin-mechanism-2D.png|400px|center|Mechanism of the cyanohydrin reaction]] |
|||
== Asymmetric synthesis == |
== Asymmetric synthesis == |
||
The [[asymmetric synthesis|asymmetric]] cyanohydrin reaction of [[benzaldehyde]] with trimethylsilylcyanide is made possible by employment of (R)-[[Binol]]<ref> |
The [[asymmetric synthesis|asymmetric]] cyanohydrin reaction of [[benzaldehyde]] with trimethylsilylcyanide is made possible by employment of (R)-[[Binol]]<ref>{{cite journal |title=Chiral Lithium Binaphtholate Aqua Complex as a Highly Effective Asymmetric Catalyst for Cyanohydrin Synthesis |first1=Manabu |last1=Hatano |first2=Takumi |last2=Ikeno |first3=Takashi |last3=Miyamoto |first4=Kazuaki |last4=Ishihara |journal=[[J. Am. Chem. Soc.]] |year=2005 |volume=127 |issue=31 |pages=10776–77 |doi=10.1021/ja051125c|pmid=16076152 }}</ref> at 1–10% [[catalyst]] loading. This [[ligand]] firsts reacts with a lithium alkoxy compound to form a lithium binaphtholate Complex. |
||
[[Image: |
[[Image:Asym cyanohydrin reaction.svg|center|Asymmetric reaction of benzaldehyde with (R)–[[Binol]]–lithium(i-propyloxy) gives (S)-acetonitrile with 98% [[Enantiomer|ee]]]] |
||
{{center|<small>Asymmetric reaction of benzaldehyde with (R)–[[Binol]]–lithium(i-propyloxy) gives (S)-acetonitrile with 98% [[Enantiomer|ee]] </small>}} |
|||
The chemist Urech in 1872 was the first to synthesize cyanohydrins from ketones with alkali cyanides and acetic acid<ref>Urech |
The chemist Urech in 1872 was the first to synthesize cyanohydrins from ketones with alkali cyanides and acetic acid<ref>{{ cite journal| last1=Urech |first1=Friedrich |title=Ueber einige Cyanderivate des Acetons |journal=[[Liebigs Ann.]] |volume=164 |page=255 |year=1872 |issue=2 |doi=10.1002/jlac.18721640207|url=https://zenodo.org/record/1427311 }}</ref> and therefore this reaction also goes by the name of '''Urech cyanohydrin method'''. |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
== References == |
== References == |
||
{{reflist}} |
{{reflist}} |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
{{Organic reactions}} |
|||
| ⚫ | |||
[[Category:Carbohydrate chemistry]] |
[[Category:Carbohydrate chemistry]] |
||
[[Category:Cyanohydrins]] |
[[Category:Cyanohydrins]] |
||
Latest revision as of 10:00, 24 October 2024
| Cyanohydrin reaction | |
|---|---|
| Named after | Friedrich Urech |
| Reaction type | Addition reaction |
In organic chemistry, a cyanohydrin reaction is an organic reaction in which an aldehyde (−CH=O) or ketone (>C=O) reacts with a cyanide anion (N≡C−) or a nitrile (−C≡N) to form a cyanohydrin (>C(OH)C≡N). For example:
This nucleophilic addition is a reversible reaction but with aliphatic carbonyl compounds equilibrium is in favor of the reaction products. The cyanide source can be potassium cyanide (KCN), sodium cyanide (NaCN) or trimethylsilyl cyanide ((CH3)3SiCN). With aromatic aldehydes such as benzaldehyde, the benzoin condensation is a competing reaction. The reaction is used in carbohydrate chemistry as a chain extension method for example that of D-xylose.
Examples
[edit]

Reaction mechanism
[edit]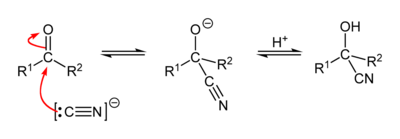
Asymmetric synthesis
[edit]The asymmetric cyanohydrin reaction of benzaldehyde with trimethylsilylcyanide is made possible by employment of (R)-Binol[1] at 1–10% catalyst loading. This ligand firsts reacts with a lithium alkoxy compound to form a lithium binaphtholate Complex.
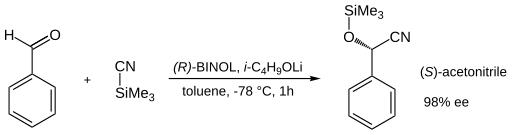
The chemist Urech in 1872 was the first to synthesize cyanohydrins from ketones with alkali cyanides and acetic acid[2] and therefore this reaction also goes by the name of Urech cyanohydrin method.
References
[edit]- ^ Hatano, Manabu; Ikeno, Takumi; Miyamoto, Takashi; Ishihara, Kazuaki (2005). "Chiral Lithium Binaphtholate Aqua Complex as a Highly Effective Asymmetric Catalyst for Cyanohydrin Synthesis". J. Am. Chem. Soc. 127 (31): 10776–77. doi:10.1021/ja051125c. PMID 16076152.
- ^ Urech, Friedrich (1872). "Ueber einige Cyanderivate des Acetons". Liebigs Ann. 164 (2): 255. doi:10.1002/jlac.18721640207.
External links
[edit]- Cyanohydrin reaction of formaldehyde to hydroxyacetonitrile or glycolonitrile with sodium cyanide in Organic Syntheses Coll. Vol. 2, p. 387; Vol. 13, p. 56 Article
- Cyanohydrin reaction of formaldehyde with potassium cyanide Organic Syntheses Coll. Vol. 3, p. 436; Vol. 27, p. 41 Article
- Cyanohydrin reaction of acetophenone with potassium cyanide Organic Syntheses Coll. Vol. 4, p. 58; Vol. 33, p. 7 Article
- Cyanohydrin reaction of D-xylose with potassium cyanide Organic Syntheses Coll. Vol. 4, p. 506; Vol. 36, p. 38 Article
- Cyanohydrin reaction of acetone with potassium cyanide Organic Syntheses Coll. Vol. 2, p. 7; Vol. 15, p. 1 Article
- Cyanohydrin reaction of benzoquinone with trimethylsilylcyanide Organic Syntheses Coll. Vol. 7, p. 517; Vol. 60, p. 126 Article

