Piroxicam: Difference between revisions
m clean up, rmv redlink portal |
Tags: Mobile edit Mobile web edit |
||
| (33 intermediate revisions by 21 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound}} |
|||
{{Drugbox |
{{Drugbox |
||
| Watchedfields = changed |
| Watchedfields = changed |
||
| Line 16: | Line 17: | ||
| MedlinePlus = a684045 |
| MedlinePlus = a684045 |
||
| pregnancy_AU = C |
| pregnancy_AU = C |
||
| pregnancy_US = C |
|||
| pregnancy_category = D (US), if used during the 3rd trimester, as it may cause [[ductus arteriosus]]. |
|||
| legal_AU = S4 |
| legal_AU = S4 |
||
| legal_CA = Rx-only |
| legal_CA = Rx-only |
||
| legal_UK = POM |
| legal_UK = POM |
||
| legal_US = Rx-only |
| legal_US = Rx-only |
||
| legal_EU = Rx-only |
|||
| ⚫ | |||
| legal_EU_comment = <ref>{{cite web | title = Active substance: piroxicam | work = List of nationally authorised medicinal products | publisher = European Medicines Agency | date = 10 December 2020 | url = https://www.ema.europa.eu/documents/psusa/piroxicam-list-nationally-authorised-medicinal-products-psusa/00002438/202004_en.pdf}}</ref> |
|||
| ⚫ | |||
<!--Pharmacokinetic data--> |
<!--Pharmacokinetic data--> |
||
| bioavailability = |
| bioavailability = |
||
| Line 51: | Line 52: | ||
<!--Chemical data--> |
<!--Chemical data--> |
||
| C=15 | H=13 | N=3 | O=4 | S=1 |
| C=15 | H=13 | N=3 | O=4 | S=1 |
||
| |
| SMILES = OC=2c1ccccc1S(=O)(=O)N(C)C=2C(=O)Nc3ccccn3 |
||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChI = 1S/C15H13N3O4S/c1-18-13(15(20)17-12-8-4-5-9-16-12)14(19)10-6-2-3-7-11(10)23(18,21)22/h2-9,19H,1H3,(H,16,17,20) |
| StdInChI = 1S/C15H13N3O4S/c1-18-13(15(20)17-12-8-4-5-9-16-12)14(19)10-6-2-3-7-11(10)23(18,21)22/h2-9,19H,1H3,(H,16,17,20) |
||
| Line 58: | Line 59: | ||
}} |
}} |
||
<!-- Definition and medical uses --> |
<!-- Definition and medical uses --> |
||
'''Piroxicam''' is a [[nonsteroidal anti-inflammatory drug]] (NSAID) of the [[oxicam]] class used to relieve the symptoms of painful inflammatory conditions like [[arthritis]].<ref name = MD/><ref>{{cite |
'''Piroxicam''' is a [[nonsteroidal anti-inflammatory drug]] (NSAID) of the [[oxicam]] class used to relieve the symptoms of painful inflammatory conditions like [[arthritis]].<ref name = MD/><ref>{{cite web | title=TGA Approved Terminology for Medicines, Section 1 – Chemical Substances | date=July 1999 | work = Therapeutic Goods Administration, Department of Health and Ageing | publisher = Australian Government | page=97 | url=http://www.tga.gov.au/pdf/medicines-approved-terminology-chemical.pdf }}</ref> Piroxicam works by preventing the production of endogenous [[prostaglandins]] which are involved in the mediation of pain, stiffness, tenderness and swelling.<ref name = MD/> The medicine is available as [[capsule (pharmacy)|capsules]], [[tablet (pharmacy)|tablets]] and, in some countries, as a prescription-free [[gel]] 0.5%.<ref name = BNF/> It is also available in a betadex formulation, which allows a more rapid absorption of piroxicam from the digestive tract.<ref name = MD>{{cite web|title=Piroxicam|work=Martindale: The Complete Drug Reference|publisher=Pharmaceutical Press|date=14 January 2014|access-date=24 June 2014|url=http://www.medicinescomplete.com/mc/martindale/current/ms-2692-v.htm|veditors=Brayfield A|location=London, UK|archive-date=28 August 2021|archive-url=https://web.archive.org/web/20210828225223/https://about.medicinescomplete.com/wp-content/plugins/revslider/public/assets/js/jquery.themepunch.revolution.min.js?ver=5.4.5.2|url-status=dead}}</ref> Piroxicam is one of the few NSAIDs that can be given parenteral routes.{{cn|date=September 2022}} |
||
<!-- Society and culture --> |
<!-- Society and culture --> |
||
It was patented in 1968 by [[Pfizer]] and approved for medical use in 1979.<ref name=Fis2006>{{cite book | |
It was patented in 1968 by [[Pfizer]] and approved for medical use in 1979.<ref name=Fis2006>{{cite book | vauthors = Fischer J, Ganellin CR |title=Analogue-based Drug Discovery |date=2006 |publisher=John Wiley & Sons |isbn=9783527607495 |page=519 |url=https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA519 |language=en}}</ref> It became generic in 1992,<ref name=NRDD/> and is marketed worldwide under many brandnames.<ref name=drugsInternat/> |
||
==Medical uses== |
==Medical uses== |
||
It is used in the treatment of [[rheumatoid arthritis|rheumatoid]] and [[osteoarthritis]], primary [[dysmenorrhoea]], postoperative pain; |
It is used in the treatment of certain inflammatory conditions like [[rheumatoid arthritis|rheumatoid]] and [[osteoarthritis]], primary [[dysmenorrhoea]], and postoperative pain; it acts as an [[analgesic]], especially where there is an [[inflammation|inflammatory]] component.<ref name = MD/> The [[European Medicines Agency]] issued a review of its use in 2007 and recommended that its use be limited to the treatment of chronic inflammatory conditions, as it is only in these circumstances that its risk-benefit ratio proves to be favourable.<ref name = BNF/><ref name = EMA>{{cite web|title=Committee for medicinal products for human use (CHMP) opinion following an Article 31(2) referral for Piroxicam containing medicinal products|work=European Medicines Agency|date=20 September 2007|location=London, UK|access-date=24 June 2014|url=http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Piroxicam_31/WC500011770.pdf}}</ref> |
||
==Adverse effects== |
==Adverse effects== |
||
{{See also|Nonsteroidal anti-inflammatory drug}} |
{{See also|Nonsteroidal anti-inflammatory drug}} |
||
As with other NSAIDs the principal side effects include: digestive complaints like [[nausea]], discomfort, [[diarrhoea]] and bleeds or ulceration of the [[stomach]], as well as [[headache]], dizziness, nervousness, [[depression (mood)|depression]], drowsiness, [[insomnia]], vertigo, hearing disturbances (such as [[tinnitus]]), [[hypertension|high blood pressure]], [[oedema]], light sensitivity, skin reactions (including, albeit rarely, [[Stevens–Johnson syndrome]] and [[toxic epidermal necrolysis]]) and rarely, [[kidney failure]], [[pancreatitis]], [[liver]] damage, visual disturbances, pulmonary [[eosinophilia]] and [[alveolitis]].<ref name="BNF">{{cite book | isbn = 978-0-85711-084-8 | title = British National Formulary (BNF) | last1 = Joint Formulary Committee | year = 2013 | publisher = Pharmaceutical Press | location = London, UK | edition = 65 | pages = [https://archive.org/details/bnf65britishnati0000unse/page/665 665, 673–674] | url = https://archive.org/details/bnf65britishnati0000unse/page/665 }}</ref> Compared to other NSAIDs it is more prone to causing gastrointestinal disturbances and serious skin reactions.<ref name = BNF/> |
As with other NSAIDs the principal side effects include: digestive complaints like [[nausea]], discomfort, [[diarrhoea]] and bleeds or ulceration of the [[stomach]], as well as [[headache]], dizziness, nervousness, [[depression (mood)|depression]], drowsiness, [[insomnia]], vertigo, hearing disturbances (such as [[tinnitus]]), [[hypertension|high blood pressure]], [[oedema]], light sensitivity, skin reactions (including, albeit rarely, [[Stevens–Johnson syndrome]] and [[toxic epidermal necrolysis]]) and rarely, [[kidney failure]], [[pancreatitis]], [[liver]] damage, visual disturbances, pulmonary [[eosinophilia]] and [[fibrosing alveolitis]].<ref name="BNF">{{cite book | isbn = 978-0-85711-084-8 | title = British National Formulary (BNF) | last1 = Joint Formulary Committee | year = 2013 | publisher = Pharmaceutical Press | location = London, UK | edition = 65 | pages = [https://archive.org/details/bnf65britishnati0000unse/page/665 665, 673–674] | url = https://archive.org/details/bnf65britishnati0000unse/page/665 }}</ref> Compared to other NSAIDs it is more prone to causing gastrointestinal disturbances and serious skin reactions.<ref name = BNF/> |
||
In October 2020, the U.S. [[Food and Drug Administration]] (FDA) required the [[Drug labelling|drug label]] to be updated for all nonsteroidal anti-inflammatory medications to describe the risk of kidney problems in unborn babies that result in low amniotic fluid.<ref name="FDA PR 20201015" /><ref name="FDA safety 20201015" /> They recommend avoiding NSAIDs in pregnant women at 20 weeks or later in pregnancy.<ref name="FDA PR 20201015">{{cite press release | title=FDA Warns that Using a Type of Pain and Fever Medication in Second Half of Pregnancy Could Lead to Complications | website=U.S. [[Food and Drug Administration]] (FDA) | date=15 October 2020 | url=https://www.fda.gov/news-events/press-announcements/fda-warns-using-type-pain-and-fever-medication-second-half-pregnancy-could-lead-complications | access-date=15 October 2020}} {{PD-notice}}</ref><ref name="FDA safety 20201015">{{cite web | title=NSAIDs may cause rare kidney problems in fetuses| website=U.S. Food and Drug Administration | date=21 July 2017 | url=https://www.fda.gov/drugs/drug-safety-and-availability/fda-recommends-avoiding-use-nsaids-pregnancy-20-weeks-or-later-because-they-can-result-low-amniotic | access-date=15 October 2020}} {{PD-notice}}</ref> |
|||
==Mechanism of action== |
==Mechanism of action== |
||
{{See also|Nonsteroidal anti-inflammatory drug}} |
{{See also|Nonsteroidal anti-inflammatory drug}} |
||
Piroxicam is an NSAID and, as such, is a non-selective [[cyclooxygenase|COX]] inhibitor possessing both analgesic and [[antipyretic]] properties.<ref name = BNF/> |
Piroxicam is an NSAID and, as such, is a non-selective [[cyclooxygenase|COX]] [[COX-2 inhibitor|inhibitor]] possessing both analgesic and [[antipyretic]] properties.<ref name = BNF/> |
||
==Chemical properties== |
==Chemical properties== |
||
Piroxicam exists as [[Enol|alkenol]] [[tautomer]] in organic solvents and as [[zwitterion]]ic form in water.<ref>{{cite journal |vauthors=Ivanova D, Deneva V, Nedeltcheva D, Kamounah FS, Gergov G, Hansen PE, Kawauchi S, Antonov L | title = Tautomeric transformations of piroxicam in solution: a combined experimental and theoretical study | journal = RSC Advances | volume = 5 | issue = 40 | pages = 31852–31860 | year = 2015 | doi=10.1039/c5ra03653d| doi-access = free }}</ref> |
Piroxicam exists as [[Enol|alkenol]] [[tautomer]] in organic solvents and as [[zwitterion]]ic form in water.<ref>{{cite journal |vauthors=Ivanova D, Deneva V, Nedeltcheva D, Kamounah FS, Gergov G, Hansen PE, Kawauchi S, Antonov L | title = Tautomeric transformations of piroxicam in solution: a combined experimental and theoretical study | journal = RSC Advances | volume = 5 | issue = 40 | pages = 31852–31860 | year = 2015 | doi=10.1039/c5ra03653d| bibcode = 2015RSCAd...531852I | doi-access = free }}</ref> |
||
==History== |
==History== |
||
The project that produced piroxicam began in 1962 at [[Pfizer]]; the first clinical trial results were reported in 1977, and the product launched in 1980 under the brand name "Feldene".<ref name=NRDD>{{cite journal | |
The project that produced piroxicam began in 1962 at [[Pfizer]]; the first clinical trial results were reported in 1977, and the product launched in 1980 under the brand name "Feldene".<ref name=NRDD>{{cite journal | vauthors = Lombardino JG, Lowe JA | title = The role of the medicinal chemist in drug discovery--then and now | journal = Nature Reviews. Drug Discovery | volume = 3 | issue = 10 | pages = 853–862 | date = October 2004 | pmid = 15459676 | doi = 10.1038/nrd1523 | s2cid = 11225541 | doi-access = free }}. See: [http://www.nature.com/nrd/journal/v3/n10/box/nrd1523_BX1.html] Box 1: Discovery of piroxicam (1962–1980)</ref><ref>{{cite journal | vauthors = Weintraub M, Jacox RF, Angevine CD, Atwater EC | title = Piroxicam (CP 16171) in rheumatoid arthritis: a controlled clinical trial with novel assessment techniques | journal = The Journal of Rheumatology | volume = 4 | issue = 4 | pages = 393–404 | year = 1977 | pmid = 342691 }}</ref> Major patents expired in 1992<ref name=NRDD/> and the drug is marketed worldwide under many brandnames.<ref name=drugsInternat>{{cite web | work = Drugs.com | url = https://www.drugs.com/international/piroxicam.html | title = International listings for piroxicam | access-date = 3 July 2015 }}</ref> |
||
==See also== |
== See also == |
||
*[[Meloxicam]] |
* [[Meloxicam]] |
||
*[[Isoxicam]] |
* [[Isoxicam]] |
||
*[[Lornoxicam]] |
* [[Lornoxicam]] |
||
==References== |
== References == |
||
{{ |
{{Reflist}} |
||
==Further reading== |
== Further reading == |
||
{{refbegin}} |
|||
* {{cite book | title=Medical Genetics Summaries | chapter=Piroxicam Therapy and CYP2C9 Genotype | chapter-url=https://www.ncbi.nlm.nih.gov/books/NBK537367/ | veditors=Pratt VM, McLeod HL, Rubinstein WS, Scott SA, Dean LC, Kattman BL, Malheiro AJ | display-editors=3 | publisher=[[National Center for Biotechnology Information]] (NCBI) | year=2019 | pmid=30742401 | id=Bookshelf ID: NBK537367 | vauthors=Dean L | url=https://www.ncbi.nlm.nih.gov/books/NBK61999/ }} |
* {{cite book | title=Medical Genetics Summaries | chapter=Piroxicam Therapy and CYP2C9 Genotype | chapter-url=https://www.ncbi.nlm.nih.gov/books/NBK537367/ | veditors=Pratt VM, McLeod HL, Rubinstein WS, Scott SA, Dean LC, Kattman BL, Malheiro AJ | display-editors=3 | publisher=[[National Center for Biotechnology Information]] (NCBI) | year=2019 | pmid=30742401 | id=Bookshelf ID: NBK537367 | vauthors=Dean L | url=https://www.ncbi.nlm.nih.gov/books/NBK61999/ }} |
||
{{refend}} |
|||
{{Anti-inflammatory and antirheumatic products}} |
{{Anti-inflammatory and antirheumatic products}} |
||
{{Topical products for joint and muscular pain}} |
{{Topical products for joint and muscular pain}} |
||
{{Prostanoid signaling modulators}} |
|||
{{Prostanoidergics}} |
|||
{{Portal bar | Medicine}} |
{{Portal bar | Medicine}} |
||
[[Category:Dermatoxins]] |
|||
[[Category:Nonsteroidal anti-inflammatory drugs]] |
[[Category:Nonsteroidal anti-inflammatory drugs]] |
||
[[Category: |
[[Category:2-Pyridyl compounds]] |
||
[[Category:Carboxamides]] |
[[Category:Carboxamides]] |
||
[[Category:Benzothiazines]] |
[[Category:Benzothiazines]] |
||
[[Category:Hepatotoxins]] |
[[Category:Hepatotoxins]] |
||
[[Category: |
[[Category:Drugs developed by Pfizer]] |
||
[[Category:Sultams]] |
|||
Latest revision as of 18:31, 26 October 2024
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /paɪˈrɒksɪˌkæm/ |
| Trade names | Feldene, others[1] |
| Other names | Piroksikam, piroxikam |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684045 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99%[4] |
| Metabolism | Liver-mediated hydroxylation and glucuronidation[4] |
| Elimination half-life | 50 hours[4] |
| Excretion | Urine, faeces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.144 |
| Chemical and physical data | |
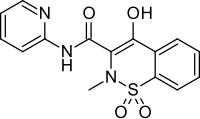
| Formula | C15H13N3O4S |
| Molar mass | 331.35 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Piroxicam is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class used to relieve the symptoms of painful inflammatory conditions like arthritis.[4][5] Piroxicam works by preventing the production of endogenous prostaglandins which are involved in the mediation of pain, stiffness, tenderness and swelling.[4] The medicine is available as capsules, tablets and, in some countries, as a prescription-free gel 0.5%.[6] It is also available in a betadex formulation, which allows a more rapid absorption of piroxicam from the digestive tract.[4] Piroxicam is one of the few NSAIDs that can be given parenteral routes.[citation needed]
It was patented in 1968 by Pfizer and approved for medical use in 1979.[7] It became generic in 1992,[8] and is marketed worldwide under many brandnames.[1]
Medical uses
[edit]It is used in the treatment of certain inflammatory conditions like rheumatoid and osteoarthritis, primary dysmenorrhoea, and postoperative pain; it acts as an analgesic, especially where there is an inflammatory component.[4] The European Medicines Agency issued a review of its use in 2007 and recommended that its use be limited to the treatment of chronic inflammatory conditions, as it is only in these circumstances that its risk-benefit ratio proves to be favourable.[6][9]
Adverse effects
[edit]As with other NSAIDs the principal side effects include: digestive complaints like nausea, discomfort, diarrhoea and bleeds or ulceration of the stomach, as well as headache, dizziness, nervousness, depression, drowsiness, insomnia, vertigo, hearing disturbances (such as tinnitus), high blood pressure, oedema, light sensitivity, skin reactions (including, albeit rarely, Stevens–Johnson syndrome and toxic epidermal necrolysis) and rarely, kidney failure, pancreatitis, liver damage, visual disturbances, pulmonary eosinophilia and fibrosing alveolitis.[6] Compared to other NSAIDs it is more prone to causing gastrointestinal disturbances and serious skin reactions.[6]
In October 2020, the U.S. Food and Drug Administration (FDA) required the drug label to be updated for all nonsteroidal anti-inflammatory medications to describe the risk of kidney problems in unborn babies that result in low amniotic fluid.[10][11] They recommend avoiding NSAIDs in pregnant women at 20 weeks or later in pregnancy.[10][11]
Mechanism of action
[edit]Piroxicam is an NSAID and, as such, is a non-selective COX inhibitor possessing both analgesic and antipyretic properties.[6]
Chemical properties
[edit]Piroxicam exists as alkenol tautomer in organic solvents and as zwitterionic form in water.[12]
History
[edit]The project that produced piroxicam began in 1962 at Pfizer; the first clinical trial results were reported in 1977, and the product launched in 1980 under the brand name "Feldene".[8][13] Major patents expired in 1992[8] and the drug is marketed worldwide under many brandnames.[1]
See also
[edit]References
[edit]- ^ a b c "International listings for piroxicam". Drugs.com. Retrieved 3 July 2015.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Active substance: piroxicam" (PDF). List of nationally authorised medicinal products. European Medicines Agency. 10 December 2020.
- ^ a b c d e f g Brayfield A, ed. (14 January 2014). "Piroxicam". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. Archived from the original on 28 August 2021. Retrieved 24 June 2014.
- ^ "TGA Approved Terminology for Medicines, Section 1 – Chemical Substances" (PDF). Therapeutic Goods Administration, Department of Health and Ageing. Australian Government. July 1999. p. 97.
- ^ a b c d e Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. pp. 665, 673–674. ISBN 978-0-85711-084-8.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 519. ISBN 9783527607495.
- ^ a b c Lombardino JG, Lowe JA (October 2004). "The role of the medicinal chemist in drug discovery--then and now". Nature Reviews. Drug Discovery. 3 (10): 853–862. doi:10.1038/nrd1523. PMID 15459676. S2CID 11225541.. See: [1] Box 1: Discovery of piroxicam (1962–1980)
- ^ "Committee for medicinal products for human use (CHMP) opinion following an Article 31(2) referral for Piroxicam containing medicinal products" (PDF). European Medicines Agency. London, UK. 20 September 2007. Retrieved 24 June 2014.
- ^ a b "FDA Warns that Using a Type of Pain and Fever Medication in Second Half of Pregnancy Could Lead to Complications". U.S. Food and Drug Administration (FDA) (Press release). 15 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "NSAIDs may cause rare kidney problems in fetuses". U.S. Food and Drug Administration. 21 July 2017. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Ivanova D, Deneva V, Nedeltcheva D, Kamounah FS, Gergov G, Hansen PE, Kawauchi S, Antonov L (2015). "Tautomeric transformations of piroxicam in solution: a combined experimental and theoretical study". RSC Advances. 5 (40): 31852–31860. Bibcode:2015RSCAd...531852I. doi:10.1039/c5ra03653d.
- ^ Weintraub M, Jacox RF, Angevine CD, Atwater EC (1977). "Piroxicam (CP 16171) in rheumatoid arthritis: a controlled clinical trial with novel assessment techniques". The Journal of Rheumatology. 4 (4): 393–404. PMID 342691.
Further reading
[edit]- Dean L (2019). "Piroxicam Therapy and CYP2C9 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 30742401. Bookshelf ID: NBK537367.
