Aminolysis: Difference between revisions
m image size |
Reconrabbit (talk | contribs) |
||
| (26 intermediate revisions by 15 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Breakdown of a molecule via reaction with ammonia or an amine}} |
|||
| ⚫ | ''' |
||
| ⚫ | In [[chemistry]], '''aminolysis''' (/am·i·nol·y·sis/) is any [[chemical reaction]] in which a molecule is [[Chemical decomposition|lysed]] (split into two parts) by reacting with [[ammonia]] ({{chem2|NH3}}) or an [[amine]].<ref>{{cite web |url=https://medical-dictionary.thefreedictionary.com/aminolysis |title=Aminolysis|publisher=Dorland's Medical Dictionary for Health Consumers |date=2007 |website= The Free Dictionary |access-date=May 21, 2019}}</ref> The case where the reaction involves ammonia may be more specifically referred to as [[ammonolysis]]. |
||
==Reactions== |
|||
==Reaction== |
|||
===Alkyl group=== |
===Alkyl group=== |
||
An example of an aminolysis reaction is the replacement of a [[halogen]] in an [[alkyl]] group (R |
An example of an aminolysis reaction is the replacement of a [[halogen]] in an [[alkyl]] group ({{chem2|R\sX}}) by an [[amine]] ({{chem2|R'\sNH2}}) and the elimination of [[hydrogen halide]] (HX). |
||
:R-X + R'- |
:<chem>R-X + R'-NH2 -> R-NH-R' + HX</chem> |
||
===Synthesis of peptides=== |
===Synthesis of peptides=== |
||
Another common example is the reaction of a primary amine or secondary amine with a [[carboxylic acid]] or with a carboxylic acid derivative to form an [[amide]]. This reaction is widely used, especially in the synthesis of [[peptide]]s. On the simple addition of an amine to a carboxylic acid, a salt of the organic acid and base is obtained. To overcome this, the carboxylic acid first needs to be "activated". This is usually done by converting the acid into a more reactive derivative (i.e. [[anhydride]], [[acid halide]]) or by using a [[coupling agent]]. In some cases, high temperatures (>200 °C) can overcome salt formation by driving off water, without the need for "activation" of the carboxyl group. The downside to this simple reaction is that the compounds may decompose at these elevated temperatures. |
Another common example is the reaction of a primary amine or secondary amine with a [[carboxylic acid]] or with a carboxylic acid derivative to form an [[amide]]. This reaction is widely used, especially in the synthesis of [[peptide]]s. On the simple addition of an amine to a carboxylic acid, a salt of the organic acid and base is obtained. To overcome this, the carboxylic acid first needs to be "activated". This is usually done by converting the acid into a more reactive derivative (i.e. [[anhydride]], [[acid halide]]) or by using a [[coupling agent]]. In some cases, high temperatures (>200 °C) can overcome salt formation by driving off water, without the need for "activation" of the carboxyl group. The downside to this simple reaction is that the compounds may decompose at these elevated temperatures. |
||
| Line 16: | Line 17: | ||
===Synthesis of amides from carboxylic acids=== |
===Synthesis of amides from carboxylic acids=== |
||
Making an amide is one of the processes which require ammonia as a reactant. There are other processes of preparing an amide such as from acid anhydrides and acyl chloride.<ref>{{cite web |url=https://www.chemguide.co.uk/organicprops/amides/preparation.html |title=Making Amide|date=n.d. |website= Chemguide |access-date=May 21, 2019}}</ref> |
Making an amide is one of the processes which require ammonia as a reactant. There are other processes of preparing an amide such as from acid anhydrides and acyl chloride.<ref>{{cite web |url=https://www.chemguide.co.uk/organicprops/amides/preparation.html |title=Making Amide|date=n.d. |website= Chemguide |access-date=May 21, 2019}}</ref> |
||
Carboxylic acids react with [[ammonium carbonate]], to convert the carboxylic acids to ammonium salts. For example, [[acetic acid]] reacts with ammonium carbonate to produce [[ammonium acetate]]<ref>{{cite web |url=https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Carboxylic_Acids/Reactivity_of_Carboxylic_Acids/Simple_Reactions_of_Carboxylic_Acids_as_Acids |title=Simple Reactions of Carboxylic Acids as Acids|date=June 6, 2019 |website= Chemistry Libretexts |access-date=June 21, 2019}}</ref> |
Carboxylic acids react with [[ammonium carbonate]], to convert the carboxylic acids to ammonium salts. For example, [[acetic acid]] reacts with ammonium carbonate to produce [[ammonium acetate]].<ref>{{cite web |url=https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Carboxylic_Acids/Reactivity_of_Carboxylic_Acids/Simple_Reactions_of_Carboxylic_Acids_as_Acids |title=Simple Reactions of Carboxylic Acids as Acids|date=June 6, 2019 |website= Chemistry Libretexts |access-date=June 21, 2019}}</ref> |
||
[[File:Carboxylic acid react with ammonium carbonate.png|frameless|500px|Carboxylic acid reacts with ammonium carbonate]] |
[[File:Carboxylic acid react with ammonium carbonate.png|frameless|500px|Carboxylic acid reacts with ammonium carbonate]] |
||
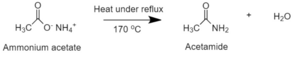
After the reaction is completed, ammonium acetate is heated under reflux (170 |
After the reaction is completed, ammonium acetate is heated under reflux (170 °C) to dehydrate the salt and eliminate excess acetic acid and water producing [[acetamide]]:<ref>{{cite web |url=http://www.orgsyn.org/demo.aspx?prep=CV1P0003 |title=ACETAMIDE |last1=Coleman |first1=G. H. |last2=Alvarado |first2=A. M. |date=30 April 2005 |website=Organic synthesis |access-date=21 June 2019}}</ref> |
||
[[File:Dehydrate ammonium acetate.png|frameless|300px|Dehydrate ammonium acetate]] |
[[File:Dehydrate ammonium acetate.png|frameless|300px|Dehydrate ammonium acetate]] |
||
| Line 29: | Line 29: | ||
==Usage== |
==Usage== |
||
===PET degradation=== |
===PET degradation=== |
||
PET (Polyethylene terephthalate) belongs to the polyester family, it can be used for many purposes such as plastic bottles and filter cloth as it is thermoplastic polymer.<ref>{{cite web |url=http://omnexus.specialchem.com/selection-guide/polyethylene-terephthalate-pet-plastic |title=Polyethylene Terephthalate (PET): A Comprehensive Review |
PET (Polyethylene terephthalate) belongs to the polyester family, it can be used for many purposes such as plastic bottles and filter cloth as it is thermoplastic polymer.<ref>{{cite web |url=http://omnexus.specialchem.com/selection-guide/polyethylene-terephthalate-pet-plastic |title=Polyethylene Terephthalate (PET): A Comprehensive Review |date=n.d. |website=Omnexus |access-date=29 June 2019}}</ref> PET can be degraded by using aminolysis which works similarly to solvolytic reaction and aminoglycolysis. For aminolysis, PET reacts with DETA (diethylenetriamine) or TETA (triethylenetetramine) which is [[polyamine]]. The reaction involves 200 - 210 Celsius. From this reaction, the products are symmetrical primary amides, asymmetrical primary/ secondary diamides, and symmetrical secondary diamides. The remaining waste material products can be used for hardening of epoxy resins. Similarly, in solvolytic reaction, the polyester reacts with water, acid, amine or alcohol, and in aminoglycolysis reaction, the polyester reacts with TEA (triethanolamine).<ref>{{cite book |url=https://books.google.com/books?id=ktJKDwAAQBAJ&q=aminolysis+usage&pg=PA114|title=Recycling of Polyethylene Terephthalate Bottles |last1=Thomas |first1= Sabu |last2= Rane |first2=Ajay |last3=Kanny |first3=Krishnan |publisher=Matthew Deans |date=2019 |access-date=29 June 2019|isbn=9780323509671 }}</ref><ref>{{cite journal |url=https://link.springer.com/article/10.1007/s10163-000-0036-5|title=Aminolysis and aminoglycolysis of waste poly(ethylene terephthalate) |last1= Spychaj |first1= Tadeusz |last2= Fabrycy |first2=Ewa |last3=Spychaj |first3= Stanislawa |publisher=Springer-Verlag |date=21 September 2000 |website=Springer Link |access-date=29 June 2019|doi=10.1007/s10163-000-0036-5 |s2cid=94364571 }}</ref> |
||
This is PET degradation with polyamines through aminolysis route. |
|||
[[File:PET degradation with polyamines through aminolysis route.png|frameless|950px|PET degradation with polyamines through aminolysis route.png]] |
|||
==Notes== |
|||
{{ref|ammonolysis|α}} For additional details, see [[Solvolysis#Ammonolysis]]. |
|||
== |
== See also == |
||
<references/> |
|||
* [[Solvolysis]] |
|||
== References == |
|||
{{Reflist}} |
|||
[[Category:Substitution reactions]] |
[[Category:Substitution reactions]] |
||
Latest revision as of 14:56, 28 October 2024
In chemistry, aminolysis (/am·i·nol·y·sis/) is any chemical reaction in which a molecule is lysed (split into two parts) by reacting with ammonia (NH3) or an amine.[1] The case where the reaction involves ammonia may be more specifically referred to as ammonolysis.
Reaction
[edit]Alkyl group
[edit]An example of an aminolysis reaction is the replacement of a halogen in an alkyl group (R−X) by an amine (R'−NH2) and the elimination of hydrogen halide (HX).
Synthesis of peptides
[edit]Another common example is the reaction of a primary amine or secondary amine with a carboxylic acid or with a carboxylic acid derivative to form an amide. This reaction is widely used, especially in the synthesis of peptides. On the simple addition of an amine to a carboxylic acid, a salt of the organic acid and base is obtained. To overcome this, the carboxylic acid first needs to be "activated". This is usually done by converting the acid into a more reactive derivative (i.e. anhydride, acid halide) or by using a coupling agent. In some cases, high temperatures (>200 °C) can overcome salt formation by driving off water, without the need for "activation" of the carboxyl group. The downside to this simple reaction is that the compounds may decompose at these elevated temperatures.
The carboxylic acid derivatives can be esters, anhydrides, acid halides or any other activated species.
The choice of activated carboxyl group or coupling agent can be very important in peptide synthesis, as using the wrong one can lead to racemization.
Synthesis of amides from carboxylic acids
[edit]Making an amide is one of the processes which require ammonia as a reactant. There are other processes of preparing an amide such as from acid anhydrides and acyl chloride.[2]
Carboxylic acids react with ammonium carbonate, to convert the carboxylic acids to ammonium salts. For example, acetic acid reacts with ammonium carbonate to produce ammonium acetate.[3]
After the reaction is completed, ammonium acetate is heated under reflux (170 °C) to dehydrate the salt and eliminate excess acetic acid and water producing acetamide:[4]
Usage
[edit]PET degradation
[edit]PET (Polyethylene terephthalate) belongs to the polyester family, it can be used for many purposes such as plastic bottles and filter cloth as it is thermoplastic polymer.[5] PET can be degraded by using aminolysis which works similarly to solvolytic reaction and aminoglycolysis. For aminolysis, PET reacts with DETA (diethylenetriamine) or TETA (triethylenetetramine) which is polyamine. The reaction involves 200 - 210 Celsius. From this reaction, the products are symmetrical primary amides, asymmetrical primary/ secondary diamides, and symmetrical secondary diamides. The remaining waste material products can be used for hardening of epoxy resins. Similarly, in solvolytic reaction, the polyester reacts with water, acid, amine or alcohol, and in aminoglycolysis reaction, the polyester reacts with TEA (triethanolamine).[6][7]
This is PET degradation with polyamines through aminolysis route.
See also
[edit]References
[edit]- ^ "Aminolysis". The Free Dictionary. Dorland's Medical Dictionary for Health Consumers. 2007. Retrieved May 21, 2019.
- ^ "Making Amide". Chemguide. n.d. Retrieved May 21, 2019.
- ^ "Simple Reactions of Carboxylic Acids as Acids". Chemistry Libretexts. June 6, 2019. Retrieved June 21, 2019.
- ^ Coleman, G. H.; Alvarado, A. M. (30 April 2005). "ACETAMIDE". Organic synthesis. Retrieved 21 June 2019.
- ^ "Polyethylene Terephthalate (PET): A Comprehensive Review". Omnexus. n.d. Retrieved 29 June 2019.
- ^ Thomas, Sabu; Rane, Ajay; Kanny, Krishnan (2019). Recycling of Polyethylene Terephthalate Bottles. Matthew Deans. ISBN 9780323509671. Retrieved 29 June 2019.
- ^ Spychaj, Tadeusz; Fabrycy, Ewa; Spychaj, Stanislawa (21 September 2000). "Aminolysis and aminoglycolysis of waste poly(ethylene terephthalate)". Springer Link. Springer-Verlag. doi:10.1007/s10163-000-0036-5. S2CID 94364571. Retrieved 29 June 2019.