Formic acid: Difference between revisions
m convert special characters found by Wikipedia:Typo Team/moss (via WP:JWB) |
citation added |
||
| (33 intermediate revisions by 17 users not shown) | |||
| Line 15: | Line 15: | ||
| PIN = Formic acid<ref name=iupac2013>{{cite book | title = Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book) | publisher = [[Royal Society of Chemistry|The Royal Society of Chemistry]] | date = 2014 | location = Cambridge | page = 745 | doi = 10.1039/9781849733069 | isbn = 978-0-85404-182-4| last1 = Favre | first1 = Henri A. | last2 = Powell | first2 = Warren H. }}</ref> |
| PIN = Formic acid<ref name=iupac2013>{{cite book | title = Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book) | publisher = [[Royal Society of Chemistry|The Royal Society of Chemistry]] | date = 2014 | location = Cambridge | page = 745 | doi = 10.1039/9781849733069 | isbn = 978-0-85404-182-4| last1 = Favre | first1 = Henri A. | last2 = Powell | first2 = Warren H. }}</ref> |
||
| SystematicName = Methanoic acid<ref name=iupac2013 /> |
| SystematicName = Methanoic acid<ref name=iupac2013 /> |
||
| OtherNames = |
| OtherNames = {{Unbulleted list|Formylic acid|Methylic acid|Hydrogencarboxylic acid|Hydroxy(oxo)methane|Metacarbonoic acid|Oxocarbinic acid|Oxomethanol|Hydroxymethylene oxide}} |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| CASNo = 64-18-6 |
| CASNo = 64-18-6 |
||
| Line 50: | Line 50: | ||
| Density = 1.220{{nbsp}}g/mL |
| Density = 1.220{{nbsp}}g/mL |
||
| Solubility = Miscible |
| Solubility = Miscible |
||
| SolubleOther = Miscible with [[diethyl ether|ether]], [[acetone]], [[ethyl acetate]], [[glycerol]], [[methanol]], [[ethanol]] <br/> Partially soluble in [[benzene]], [[toluene]], [[xylene]]s |
| SolubleOther = Miscible with [[diethyl ether|ether]], [[acetone]], [[ethyl acetate]], [[glycerol]], [[methanol]], [[ethanol]] <br /> Partially soluble in [[benzene]], [[toluene]], [[xylene]]s |
||
| MeltingPtC = 8.4 |
| MeltingPtC = 8.4 |
||
| BoilingPtC = 100.8 |
| BoilingPtC = 100.8 |
||
| Line 62: | Line 62: | ||
}} |
}} |
||
|Section3={{Chembox Structure |
|Section3={{Chembox Structure |
||
| MolShape = [[ |
| MolShape = [[wikt:planar|Planar]] |
||
| Dipole = 1.41{{nbsp}}[[Debye|D]] (gas)}} |
| Dipole = 1.41{{nbsp}}[[Debye|D]] (gas)}} |
||
|Section5={{Chembox Thermochemistry |
|Section5={{Chembox Thermochemistry |
||
| Line 82: | Line 82: | ||
| FlashPtC = 69 |
| FlashPtC = 69 |
||
| AutoignitionPtC = 601 |
| AutoignitionPtC = 601 |
||
| ExploLimits = 14{{ndash}}34%{{citation needed|date=March 2015}}<br/> 18{{ndash}}57% (90% solution)<ref name=PGCH/> |
| ExploLimits = 14{{ndash}}34%{{citation needed|date=March 2015}}<br /> 18{{ndash}}57% (90% solution)<ref name=PGCH/> |
||
| GHSPictograms = {{GHS05}} |
| GHSPictograms = {{GHS02}} {{GHS05}} |
||
| GHSSignalWord = Danger |
| GHSSignalWord = Danger |
||
| HPhrases = {{H-phrases|314}} |
| HPhrases = {{H-phrases|314}} |
||
| Line 91: | Line 91: | ||
| IDLH = 30{{nbsp}}ppm<ref name=PGCH/> |
| IDLH = 30{{nbsp}}ppm<ref name=PGCH/> |
||
| REL = TWA 5{{nbsp}}ppm (9{{nbsp}}mg/m<sup>3</sup>)<ref name=PGCH/> |
| REL = TWA 5{{nbsp}}ppm (9{{nbsp}}mg/m<sup>3</sup>)<ref name=PGCH/> |
||
| LC50 = 7853{{nbsp}}ppm (rat, 15{{nbsp}}min)<br/>3246{{nbsp}}ppm (mouse, 15{{nbsp}}min)<ref name=IDLH/> |
| LC50 = 7853{{nbsp}}ppm (rat, 15{{nbsp}}min)<br />3246{{nbsp}}ppm (mouse, 15{{nbsp}}min)<ref name=IDLH/> |
||
}} |
}} |
||
|Section8={{Chembox Related |
|Section8={{Chembox Related |
||
| OtherFunction_label = [[carboxylic acid]]s |
| OtherFunction_label = [[carboxylic acid]]s |
||
| OtherFunction = [[Acetic acid]]<br/>[[Propionic acid]] |
| OtherFunction = [[Acetic acid]]<br />[[Propionic acid]] |
||
| OtherCompounds = [[Formaldehyde]]<br/>[[Methanol]]}} |
| OtherCompounds = [[Formaldehyde]]<br />[[Methanol]]}} |
||
}} |
}} |
||
| Line 103: | Line 103: | ||
==Natural occurrence== |
==Natural occurrence== |
||
{{See also|Insect defenses}} |
{{See also|Insect defenses}} |
||
Formic acid is found naturally in insects, weeds, fruits and vegetables, and forest emissions. It appears in most [[ants]] and in [[stingless bee]]s of the genus ''[[Oxytrigona]]''.<ref>{{cite journal |doi=10.1097/ACI.0b013e328339f325 |pmid=20445444 |title=Ant venoms |journal=Current Opinion in Allergy and Clinical Immunology |volume=10 |issue=4 |pages=342–6 |year=2010 |last1=Hoffman |first1=Donald R |s2cid=4999650 }}</ref><ref>{{cite journal| pmid=24302133 | doi=10.1007/BF01020539 | volume=13 | issue=5 | title=Formic acid in caustic cephalic secretions of stingless bee,Oxytrigona (Hymenoptera: Apidae) | year=1987 | journal=J Chem Ecol | pages=1079–86 | last1 = Roubik | first1 = DW | last2 = Smith | first2 = BH | last3 = Carlson | first3 = RG| s2cid=30511107 }}</ref> [[Formica rufa species group|Wood ants]] from the genus ''[[Formica]]'' can spray formic acid on their prey or to defend the nest. The [[Cerura vinula|puss moth caterpillar]] (''Cerura vinula'') will spray it as well when threatened by predators. It is also found in the [[trichome]]s of [[stinging nettle]] (''Urtica dioica''). Apart from that, this acid is incorporated in many fruits such as pineapple (0.21 mg per 100 g), apple (2 mg per 100 g) and kiwi (1 mg per 100 g), as well as in many vegetables, namely onion (45 mg per 100 g), eggplant (1.34 mg per 100 g) and, in extremely low concentrations, cucumber (0.11 mg per 100 g).<ref>{{cite journal| pmc=3349212 | pmid=22593694 | doi=10.1100/2012/564367 | volume=2012 | title=Phenolic compounds analysis of root, stalk, and leaves of nettle | year=2012 | journal=ScientificWorldJournal | page=564367 | last1 = Otles | first1 = S | last2 = Yalcin | first2 = B | doi-access=free }}</ref> Formic acid is a naturally occurring component of the [[Atmosphere of Earth|atmosphere]] primarily due to forest emissions.<ref>{{cite journal |doi=10.1029/91GL01565 |title=Emission of formic and acetic acids from tropical Savanna soils |journal=Geophysical Research Letters |volume=18 |issue=9 |pages=1707–10 |year=1991 |last1=Sanhueza |first1=Eugenio |last2=Andreae |first2=Meinrat O |bibcode=1991GeoRL..18.1707S }}</ref> |
|||
==History== |
==History== |
||
As early as the 15th century, some [[alchemy|alchemists]] and [[natural history|naturalists]] were aware that ant hills give off an acidic vapor. The first person to describe the isolation of this substance (by the distillation of large numbers of ants) was the English naturalist [[John Ray]], in 1671.<ref>{{cite journal |doi=10.1098/rstl.1670.0052 |title=Extract of a Letter, Written by Mr John Wray to the Publisher January 13. 1670. Concerning Some Un-Common Observations and Experiments Made with an Acid Juyce to be Found in Ants |journal=Philosophical Transactions of the Royal Society of London |volume=5 |issue=57–68 |pages=2063–2066 |year=1670 |last1=Wray |first1=J |bibcode=1670RSPT....5.2063W |doi-access=free }}</ref><ref>{{cite book | url = https://books.google.com/books?id=i1eS9LAe3PsC&pg=PA51 | title = History of the process and present state of animal chemistry | last1 = Johnson | first1 = W. B. | year = 1803}}</ref> Ants secrete the formic acid for attack and defense purposes. Formic acid was first synthesized from [[hydrocyanic acid]] by the French chemist [[Joseph Gay-Lussac]]. In 1855, another French chemist, [[Marcellin Berthelot]], developed a synthesis from [[carbon monoxide]] similar to the process used today. |
As early as the 15th century, some [[alchemy|alchemists]] and [[natural history|naturalists]] were aware that ant hills give off an acidic vapor. The first person to describe the isolation of this substance (by the distillation of large numbers of ants) was the English naturalist [[John Ray]], in 1671.<ref>{{cite journal |doi=10.1098/rstl.1670.0052 |title=Extract of a Letter, Written by Mr John Wray to the Publisher January 13. 1670. Concerning Some Un-Common Observations and Experiments Made with an Acid Juyce to be Found in Ants |journal=Philosophical Transactions of the Royal Society of London |volume=5 |issue=57–68 |pages=2063–2066 |year=1670 |last1=Wray |first1=J |bibcode=1670RSPT....5.2063W |doi-access=free }}</ref><ref>{{cite book | url = https://books.google.com/books?id=i1eS9LAe3PsC&pg=PA51 | title = History of the process and present state of animal chemistry | last1 = Johnson | first1 = W. B. | year = 1803}}</ref> Ants secrete the formic acid for attack and defense purposes. Formic acid was first synthesized from [[hydrocyanic acid]] by the French chemist [[Joseph Gay-Lussac]]. In 1855, another French chemist, [[Marcellin Berthelot]], developed a synthesis from [[carbon monoxide]] similar to the process used today.{{cn|date=June 2024}} |
||
Formic acid was long considered a [[chemical compound]] of only minor interest in the chemical industry. In the late 1960s, significant quantities became available as a byproduct of [[acetic acid]] production. It now finds increasing use as a preservative and antibacterial in [[livestock]] feed. |
Formic acid was long considered a [[chemical compound]] of only minor interest in the chemical industry. In the late 1960s, significant quantities became available as a byproduct of [[acetic acid]] production. It now finds increasing use as a preservative and antibacterial in [[livestock]] feed.{{cn|date=June 2024}} |
||
==Properties== |
==Properties== |
||
[[File:Formic Acid Hydrogenbridge V.1.svg|thumb|left|Cyclic dimer of formic acid; dashed <span style="color:green;">'''green'''</span> lines represent hydrogen bonds]] |
[[File:Formic Acid Hydrogenbridge V.1.svg|thumb|left|Cyclic dimer of formic acid; dashed <span style="color:green;">'''green'''</span> lines represent hydrogen bonds]] |
||
Formic acid is a colorless liquid having a pungent, penetrating odor<ref>{{cite web|url=https://www.osha.gov/chemicaldata/chemResult.html?recNo=468|title=OSHA Occupational Chemical Database – Occupational Safety and Health Administration|website=osha.gov|access-date=17 April 2015|archive-date=29 April 2021|archive-url=https://web.archive.org/web/20210429045534/https://www.osha.gov/chemicaldata/chemResult.html?recNo=468|url-status=dead}}</ref> at room temperature, comparable to the related [[acetic acid]]. Formic acid is about ten times stronger than [[acetic acid]]. |
Formic acid is a colorless liquid having a pungent, penetrating odor<ref>{{cite web|url=https://www.osha.gov/chemicaldata/chemResult.html?recNo=468|title=OSHA Occupational Chemical Database – Occupational Safety and Health Administration|website=osha.gov|access-date=17 April 2015|archive-date=29 April 2021|archive-url=https://web.archive.org/web/20210429045534/https://www.osha.gov/chemicaldata/chemResult.html?recNo=468|url-status=dead}}</ref> at room temperature, comparable to the related [[acetic acid]]. Formic acid is about ten times stronger than [[acetic acid]].{{cn|date=June 2024}} |
||
It is [[Miscibility|miscible]] with water and most polar [[organic chemistry|organic]] [[solvent]]s, and is somewhat soluble in [[hydrocarbon]]s. In hydrocarbons and in the vapor phase, it consists of [[Hydrogen bond|hydrogen-bond]]ed [[dimer (chemistry)|dimers]] rather than individual molecules.<ref name=Ullmann_2009>{{cite book |doi=10.1002/14356007.a12_013 |chapter=Formic Acid |title=Ullmann's Encyclopedia of Industrial Chemistry |year=2000 |last1=Reutemann |first1=Werner |last2=Kieczka |first2=Heinz |isbn=978-3-527-30673-2 }}</ref><ref name=Balabin_2009>{{cite journal |doi=10.1021/jp9002643 |pmid=19344174 |title=Polar (Acyclic) Isomer of Formic Acid Dimer: Gas-Phase Raman Spectroscopy Study and Thermodynamic Parameters |journal=The Journal of Physical Chemistry A |volume=113 |issue=17 |pages=4910–8 |year=2009 |author= Roman M. Balabin |bibcode=2009JPCA..113.4910B }}</ref> Owing to its tendency to hydrogen-bond, gaseous formic acid does not obey the [[ideal gas law]].<ref name=Balabin_2009/> Solid formic acid, which can exist in either of two [[polymorphism (materials science)|polymorphs]], consists of an effectively endless network of hydrogen-bonded formic acid molecules. Formic acid forms a high-boiling [[azeotrope]] with water (107.3 °C; 77.5% formic acid). Liquid formic acid tends to [[supercooling|supercool]]. |
It is [[Miscibility|miscible]] with water and most polar [[organic chemistry|organic]] [[solvent]]s, and is somewhat soluble in [[hydrocarbon]]s. In hydrocarbons and in the vapor phase, it consists of [[Hydrogen bond|hydrogen-bond]]ed [[dimer (chemistry)|dimers]] rather than individual molecules.<ref name=Ullmann_2009>{{cite book |doi=10.1002/14356007.a12_013 |chapter=Formic Acid |title=Ullmann's Encyclopedia of Industrial Chemistry |year=2000 |last1=Reutemann |first1=Werner |last2=Kieczka |first2=Heinz |isbn=978-3-527-30673-2 }}</ref><ref name=Balabin_2009>{{cite journal |doi=10.1021/jp9002643 |pmid=19344174 |title=Polar (Acyclic) Isomer of Formic Acid Dimer: Gas-Phase Raman Spectroscopy Study and Thermodynamic Parameters |journal=The Journal of Physical Chemistry A |volume=113 |issue=17 |pages=4910–8 |year=2009 |author= Roman M. Balabin |bibcode=2009JPCA..113.4910B }}</ref> Owing to its tendency to hydrogen-bond, gaseous formic acid does not obey the [[ideal gas law]].<ref name=Balabin_2009/> Solid formic acid, which can exist in either of two [[polymorphism (materials science)|polymorphs]], consists of an effectively endless network of hydrogen-bonded formic acid molecules. Formic acid forms a high-boiling [[azeotrope]] with water (107.3 °C; 77.5% formic acid). Liquid formic acid tends to [[supercooling|supercool]]. |
||
| Line 123: | Line 124: | ||
:HCO<sub>2</sub>H → H<sub>2</sub>O + CO |
:HCO<sub>2</sub>H → H<sub>2</sub>O + CO |
||
Treatment of formic acid with |
Treatment of formic acid with sulfuric acid is a convenient laboratory source of CO.<ref name="koch">{{cite journal |doi=10.15227/orgsyn.044.0001 |title=1-Adamantanecarboxylic Acid |journal=Organic Syntheses |date=1964 |volume=44 |page=1|first1=H.|last1=Koch|first2=W.|last2=Haaf }}</ref> <ref name="coleman">{{OrgSynth|title=''p''-Tolualdehyde|author=Coleman, G. H.|author2=Craig, David|volume=12|pages=80|year=1932|doi=10.15227/orgsyn.012.0080}}</ref> |
||
In the presence of platinum, it decomposes with a release of [[hydrogen]] and [[carbon dioxide]]. |
In the presence of [[platinum]], it decomposes with a release of [[hydrogen]] and [[carbon dioxide]]. |
||
:HCO<sub>2</sub>H → H<sub>2</sub> + CO<sub>2</sub> |
:HCO<sub>2</sub>H → H<sub>2</sub> + CO<sub>2</sub> |
||
Soluble ruthenium catalysts are also effective.<ref name="Fellay2008">{{cite journal |doi=10.1002/anie.200800320 |pmid=18393267 |title=A Viable Hydrogen-Storage System Based on Selective Formic Acid Decomposition with a Ruthenium Catalyst |journal=Angewandte Chemie International Edition |volume=47 |issue=21 |year=2008 |last1=Fellay |first1=Céline |last2=Dyson |first2=Paul J. |last3=Laurenczy |first3=Gábor |pages=3966–8}}</ref |
Soluble [[ruthenium]] catalysts are also effective for producing carbon monoxide-free hydrogen.<ref name="Fellay2008">{{cite journal |doi=10.1002/anie.200800320 |pmid=18393267 |title=A Viable Hydrogen-Storage System Based on Selective Formic Acid Decomposition with a Ruthenium Catalyst |journal=Angewandte Chemie International Edition |volume=47 |issue=21 |year=2008 |last1=Fellay |first1=Céline |last2=Dyson |first2=Paul J. |last3=Laurenczy |first3=Gábor |pages=3966–8}}</ref> |
||
===Reactant=== |
===Reactant=== |
||
| Line 134: | Line 135: | ||
Formic acid is a source for a [[formyl]] group for example in the [[formylation]] of [[N-Methylaniline|''N''-methylaniline]] to ''N''-methylformanilide in [[toluene]].<ref>{{OrgSynth | title = ''N''-Methylformanilide | collvol = 3 | collvolpages = 590 | year = 1955 | prep = cv3p0590 | authorlink=Louis Fieser |author=L. F. Fieser |author2=J. E. Jones}}</ref> |
Formic acid is a source for a [[formyl]] group for example in the [[formylation]] of [[N-Methylaniline|''N''-methylaniline]] to ''N''-methylformanilide in [[toluene]].<ref>{{OrgSynth | title = ''N''-Methylformanilide | collvol = 3 | collvolpages = 590 | year = 1955 | prep = cv3p0590 | authorlink=Louis Fieser |author=L. F. Fieser |author2=J. E. Jones}}</ref> |
||
In [[organic synthesis|synthetic organic chemistry]], formic acid is often used as a source of [[hydride]] ion, as in the [[ |
In [[organic synthesis|synthetic organic chemistry]], formic acid is often used as a source of [[hydride]] ion, as in the [[Eschweiler–Clarke reaction]]: |
||
[[Image:Eschweiler-Clarke Reaction.svg|center|300px|The Eschweiler–Clark reaction]] |
[[Image:Eschweiler-Clarke Reaction.svg|center|300px|The Eschweiler–Clark reaction]] |
||
| Line 163: | Line 164: | ||
===Niche and obsolete chemical routes=== |
===Niche and obsolete chemical routes=== |
||
====By-product of acetic acid production==== |
====By-product of acetic acid production==== |
||
A significant amount of formic acid is produced as a byproduct in the manufacture of other chemicals. At one time, [[acetic acid]] was produced on a large scale by oxidation of [[alkane]]s, by a process that cogenerates significant formic acid.<ref name=Ullmann_2009/> This oxidative route to acetic acid has declined in importance so that the aforementioned dedicated routes to formic acid have become more important. |
A significant amount of formic acid is produced as a byproduct in the manufacture of other chemicals. At one time, [[acetic acid]] was produced on a large scale by oxidation of [[alkane]]s, by a process that cogenerates significant formic acid.<ref name=Ullmann_2009/> This oxidative route to acetic acid has declined in importance so that the aforementioned dedicated routes to formic acid have become more important.{{cn|date=June 2024}} |
||
====Hydrogenation of carbon dioxide==== |
====Hydrogenation of carbon dioxide==== |
||
The catalytic [[hydrogenation]] of [[Carbon dioxide|CO<sub>2</sub>]] to formic acid has long been studied. This reaction can be conducted homogeneously.<ref>{{Cite book | author = P. G. Jessop | title = Handbook of Homogeneous Hydrogenation | editor = J. G. de Vries, C. J. Elsevier | publisher = Wiley-VCH | location = Weinheim, Germany | date = 2007 | pages = 489–511}}</ref><ref>{{cite journal |doi=10.1016/j.ccr.2004.05.019 |title=Recent advances in the homogeneous hydrogenation of carbon dioxide |journal=Coordination Chemistry Reviews |volume=248 |issue=21–24 |pages=2425 |year=2004 |last1=Jessop |first1=Philip G |last2=Joó |first2=Ferenc |last3=Tai |first3=Chih-Cheng }}</ref> |
The catalytic [[hydrogenation]] of [[Carbon dioxide|CO<sub>2</sub>]] to formic acid has long been studied. This reaction can be conducted homogeneously.<ref>{{Cite book | author = P. G. Jessop | title = Handbook of Homogeneous Hydrogenation | editor = J. G. de Vries, C. J. Elsevier | publisher = Wiley-VCH | location = Weinheim, Germany | date = 2007 | pages = 489–511}}</ref><ref>{{cite journal |doi=10.1016/j.ccr.2004.05.019 |title=Recent advances in the homogeneous hydrogenation of carbon dioxide |journal=Coordination Chemistry Reviews |volume=248 |issue=21–24 |pages=2425 |year=2004 |last1=Jessop |first1=Philip G |last2=Joó |first2=Ferenc |last3=Tai |first3=Chih-Cheng }}</ref><ref>{{Cite web |last=Sampson |first=Joanna |date=2 August 2020 |title=Wireless device makes clean fuel from sunlight, CO2 and water |url=https://www.gasworld.com/wireless-device-makes-clean-fuel-from-sunlight-co2-and-water-/2019694.article |access-date=2020-08-26 |website=Gasworld |language=en}}</ref> |
||
====Oxidation of biomass==== |
====Oxidation of biomass==== |
||
| Line 172: | Line 174: | ||
====Laboratory methods==== |
====Laboratory methods==== |
||
In the laboratory, formic acid can be obtained by heating [[oxalic acid]] in [[glycerol]] |
In the laboratory, formic acid can be obtained by heating [[oxalic acid]] in [[glycerol]] followed by steam distillation.<ref name="chattaway">{{cite journal |doi=10.1039/CT9140500151 |title=XX.—Interaction of glycerol and oxalic acid |journal=[[Journal of the Chemical Society, Transactions]] |volume=105 |pages=151–6 |year=1914 |last1=Chattaway |first1=Frederick Daniel |hdl=2027/mdp.39015067135775 |url=https://zenodo.org/record/2046509 }}</ref> Glycerol acts as a catalyst, as the reaction proceeds through a glyceryl oxalate intermediate. If the reaction mixture is heated to higher temperatures, [[allyl alcohol]] results. The net reaction is thus: |
||
:C<sub>2</sub>O<sub>4</sub>H<sub>2</sub> → HCO<sub>2</sub>H + CO<sub>2</sub><!--esoteric and not useful to anyone: Another preparation is the acid [[hydrolysis]] of ethyl isonitrile (C<sub>2</sub>H<sub>5</sub>NC) using [[hydrochloric acid|HCl]] solution.<ref name="cohen">{{Cite book | author = Cohen, Julius B. | title = Practical Organic Chemistry | publisher = MacMillan | date = 1930}}</ref> |
:C<sub>2</sub>O<sub>4</sub>H<sub>2</sub> → HCO<sub>2</sub>H + CO<sub>2</sub><!--esoteric and not useful to anyone: Another preparation is the acid [[hydrolysis]] of ethyl isonitrile (C<sub>2</sub>H<sub>5</sub>NC) using [[hydrochloric acid|HCl]] solution.<ref name="cohen">{{Cite book | author = Cohen, Julius B. | title = Practical Organic Chemistry | publisher = MacMillan | date = 1930}}</ref> |
||
:C<sub>2</sub>H<sub>5</sub>NC + 2 H<sub>2</sub>O → C<sub>2</sub>H<sub>5</sub>NH<sub>2</sub> + HCO<sub>2</sub>H |
:C<sub>2</sub>H<sub>5</sub>NC + 2 H<sub>2</sub>O → C<sub>2</sub>H<sub>5</sub>NH<sub>2</sub> + HCO<sub>2</sub>H |
||
| Line 180: | Line 182: | ||
====Electrochemical production==== |
====Electrochemical production==== |
||
Formate is formed by the [[electrochemical reduction]] of CO<sub>2</sub> (in the form of [[bicarbonate]]) at a [[lead]] [[cathode]] at pH 8.6:<ref>{{cite journal |display-authors=etal|last1=B. Innocent |title=Electro-reduction of carbon dioxide to formate on lead electrode in aqueous medium |journal=Journal of Applied Electrochemistry |date=Feb 2009 |doi=10.1007/s10800-008-9658-4 |volume=39 |issue=2 |pages=227–232|s2cid=98437382 }}</ref> |
|||
:{{chem|HCO|3|-}} + {{chem|H|2|O}} + 2e<sup>−</sup> → {{chem|HCO|2|-}} + 2{{Chem|OH|-}} |
:{{chem|HCO|3|-}} + {{chem|H|2|O}} + 2e<sup>−</sup> → {{chem|HCO|2|-}} + 2{{Chem|OH|-}} |
||
or |
or |
||
| Line 186: | Line 188: | ||
If the feed is {{chem|CO|2}} and oxygen is evolved at the anode, the total reaction is: |
If the feed is {{chem|CO|2}} and oxygen is evolved at the anode, the total reaction is: |
||
:{{CO2}} + {{chem|OH|-}} → {{chem|HCO|2|-}} + 1/2 {{O2}} |
:{{CO2}} + {{chem|OH|-}} → {{chem|HCO|2|-}} + 1/2 {{O2}} |
||
=== Artificial photosynthesis === |
|||
In August 2020, researchers at [[University of Cambridge|Cambridge University]] announced a stand-alone advanced 'photosheet' technology that converts sunlight, carbon dioxide and water into oxygen and formic acid with no other inputs.<ref>{{Cite web |last=Sampson |first=Joanna |date=2 August 2020 |title=Wireless device makes clean fuel from sunlight, CO2 and water |url=https://www.gasworld.com/wireless-device-makes-clean-fuel-from-sunlight-co2-and-water-/2019694.article |access-date=2020-08-26 |website=Gasworld |language=en}}</ref> |
|||
===Biosynthesis=== |
===Biosynthesis=== |
||
Formic acid is named after ants which have high concentrations of the compound in their venom |
Formic acid is named after ants which have high concentrations of the compound in their venom, derived from [[serine]] through a [[5,10-methenyltetrahydrofolate]] intermediate.<ref>{{cite journal|last1=Hefetz|first1=Abraham|last2=Blum|first2=Murray|title=Biosynthesis of formic acid by the poison glands of formicine ants|journal=Biochimica et Biophysica Acta (BBA) - General Subjects|date=1 November 1978|volume=543|issue=4|pages=484–496|doi=10.1016/0304-4165(78)90303-3|pmid=718985}}</ref> The conjugate base of formic acid, formate, also occurs widely in nature. An [[assay]] for formic acid in body fluids, designed for determination of formate after methanol poisoning, is based on the reaction of formate with bacterial [[formate dehydrogenase]].<ref>{{cite journal |doi=10.1016/0006-2944(75)90147-7 |pmid=1 |title=Formate assay in body fluids: Application in methanol poisoning |journal=Biochemical Medicine |volume=13 |issue=2 |pages=117–26 |year=1975 |last1=Makar |first1=A.B |last2=McMartin |first2=K.E |last3=Palese |first3=M |last4=Tephly |first4=T.R }}</ref> |
||
== Uses == |
== Uses == |
||
===Agriculture=== |
===Agriculture=== |
||
A major use of formic acid is as [[preservative]] and [[bacteria|antibacterial]] agent in livestock feed. In Europe, it is applied on [[silage]], including fresh hay, to promote the fermentation of [[lactic acid]] and to suppress the formation of [[butyric acid]]; it also allows fermentation to occur quickly, and at a lower temperature, reducing the loss of nutritional value.<ref name = Ullmann_2009/> |
A major use of formic acid is as a [[preservative]] and [[bacteria|antibacterial]] agent in livestock feed. It arrests certain decay processes and causes the feed to retain its nutritive value longer, |
||
In Europe, it is applied on [[silage]], including fresh hay, to promote the fermentation of [[lactic acid]] and to suppress the formation of [[butyric acid]]; it also allows fermentation to occur quickly, and at a lower temperature, reducing the loss of nutritional value.<ref name = Ullmann_2009/> It is widely used to preserve winter feed for [[cattle]],<ref>[https://books.google.com/books?id=7IrGwQTt1aMC&dq=formic+acid++winter+feed+for+cattle&pg=PA31 Organic Acids and Food Preservation], Maria M. Theron, J. F. Rykers Lues</ref> and is sometimes added to [[poultry]] feed to kill ''[[Escherichia coli|E. coli]]'' bacteria.<ref>{{cite journal |doi=10.1093/japr/14.4.750 |title=Alternatives to Antibiotics for Organic Poultry Production |journal=The Journal of Applied Poultry Research |volume=14 |issue=4 |pages=750 |year=2005 |last1=Griggs |first1=J. P |last2=Jacob |first2=J. P |doi-access=free }}</ref><ref>{{cite journal |doi=10.3382/japr.2006-00116 |title=Effect of Formic Acid and Plant Extracts on Growth, Nutrient Digestibility, Intestine Mucosa Morphology, and Meat Yield of Broilers |journal=The Journal of Applied Poultry Research |volume=16 |issue=4 |pages=555 |year=2007 |last1=Garcia |first1=V |last2=Catala-Gregori |first2=P |last3=Hernandez |first3=F |last4=Megias |first4=M. D |last5=Madrid |first5=J |doi-access=free }}</ref> Use as a preservative for silage and other animal feed constituted 30% of the global consumption in 2009.<ref name=CEH/> |
|||
[[Beekeeper]]s use formic acid as a [[miticide]] against the tracheal mite (''[[Acarapis woodi]]'') and the [[Varroa destructor|''Varroa destructor'' mite]] and [[Varroa jacobsoni|''Varroa jacobsoni'' mite]].<ref>{{cite journal |author= Hoppe, H. |author2=Ritter, W. |author3=Stephen, E. W. C.|year= 1989 |title= The control of parasitic bee mites: Varroa jacobsoni, Acarapis woodi and Tropilaelaps clareae with formic acid |journal= American Bee Journal}}</ref> |
[[Beekeeper]]s use formic acid as a [[miticide]] against the tracheal mite (''[[Acarapis woodi]]'') and the [[Varroa destructor|''Varroa destructor'' mite]] and [[Varroa jacobsoni|''Varroa jacobsoni'' mite]].<ref>{{cite journal |author= Hoppe, H. |author2=Ritter, W. |author3=Stephen, E. W. C.|year= 1989 |title= The control of parasitic bee mites: Varroa jacobsoni, Acarapis woodi and Tropilaelaps clareae with formic acid |journal= American Bee Journal}}</ref> |
||
===Energy=== |
===Energy=== |
||
Formic acid |
Formic acid can be used directly in [[formic acid fuel cell]]s or indirectly in hydrogen [[fuel cell]]s.<ref>{{cite journal |doi=10.1016/j.jpowsour.2004.12.031 |title=Performance characterization of Pd/C nanocatalyst for direct formic acid fuel cells |journal=Journal of Power Sources |volume=144 |issue=1 |pages=28–34 |year=2005 |last1=Ha |first1=S |last2=Larsen |first2=R |last3=Masel |first3=R.I |bibcode=2005JPS...144...28H }}</ref><ref>{{cite news |title=Ant power: Take a ride on a bus that runs on formic acid |author=Jorn Madslien |url=https://www.bbc.com/news/business-40403351 |publisher=[[BBC News]] |date=27 June 2017 |access-date=11 July 2017}}</ref> |
||
Electrolytic conversion of electrical energy to chemical fuel has been proposed as a large-scale source of formate by various groups.<ref>{{Cite journal|last1=Yishai|first1=Oren|last2=Lindner|first2=Steffen N|last3=Gonzalez de la Cruz|first3=Jorge|last4=Tenenboim|first4=Hezi|last5=Bar-Even|first5=Arren|date=December 2016|title=The formate bio-economy|journal=Current Opinion in Chemical Biology|language=en|volume=35|pages=1–9|doi=10.1016/j.cbpa.2016.07.005|pmid=27459678}}</ref> The formate could be used as feed to modified ''[[Escherichia coli|E. coli]]'' bacteria for producing [[biomass]].<ref>{{cite journal |display-authors=etal|last1=Shmuel Gleizer |title=Conversion of ''Escherichia coli'' to Generate All Biomass Carbon from CO<sub>2</sub> |journal=Cell |date=Nov 2019 |doi=10.1016/j.cell.2019.11.009 |volume=179 |issue=6 |pages=1255–1263.e12|pmid=31778652 |doi-access=free |pmc=6904909 }}</ref><ref>{{Cite journal|last1=Kim|first1=Seohyoung|last2=Lindner|first2=Steffen N.|last3=Aslan|first3=Selçuk|last4=Yishai|first4=Oren|last5=Wenk|first5=Sebastian|last6=Schann|first6=Karin|last7=Bar-Even|first7=Arren|date=2020-02-10|title=Growth of E. coli on formate and methanol via the reductive glycine pathway|url=https://www.nature.com/articles/s41589-020-0473-5|journal=Nature Chemical Biology|language=en|pages=538–545|doi=10.1038/s41589-020-0473-5|issn=1552-4469|volume=16|issue=5|pmid=32042198|s2cid=211074951}}</ref> Natural microbes |
Electrolytic conversion of electrical energy to chemical fuel has been proposed as a large-scale source of formate by various groups.<ref>{{Cite journal|last1=Yishai|first1=Oren|last2=Lindner|first2=Steffen N|last3=Gonzalez de la Cruz|first3=Jorge|last4=Tenenboim|first4=Hezi|last5=Bar-Even|first5=Arren|date=December 2016|title=The formate bio-economy|journal=Current Opinion in Chemical Biology|language=en|volume=35|pages=1–9|doi=10.1016/j.cbpa.2016.07.005|pmid=27459678}}</ref> The formate could be used as feed to modified ''[[Escherichia coli|E. coli]]'' bacteria for producing [[biomass]].<ref>{{cite journal |display-authors=etal|last1=Shmuel Gleizer |title=Conversion of ''Escherichia coli'' to Generate All Biomass Carbon from CO<sub>2</sub> |journal=Cell |date=Nov 2019 |doi=10.1016/j.cell.2019.11.009 |volume=179 |issue=6 |pages=1255–1263.e12|pmid=31778652 |doi-access=free |pmc=6904909 }}</ref><ref>{{Cite journal|last1=Kim|first1=Seohyoung|last2=Lindner|first2=Steffen N.|last3=Aslan|first3=Selçuk|last4=Yishai|first4=Oren|last5=Wenk|first5=Sebastian|last6=Schann|first6=Karin|last7=Bar-Even|first7=Arren|date=2020-02-10|title=Growth of E. coli on formate and methanol via the reductive glycine pathway|url=https://www.nature.com/articles/s41589-020-0473-5|journal=Nature Chemical Biology|language=en|pages=538–545|doi=10.1038/s41589-020-0473-5|issn=1552-4469|volume=16|issue=5|pmid=32042198|s2cid=211074951}}</ref> Natural [[methylotroph]] microbes can feed on formic acid or formate. |
||
Formic acid has been considered as a means of [[hydrogen storage]].<ref>{{cite journal |doi=10.1002/cssc.200800133 |pmid=18781551 |title=Breakthroughs in Hydrogen Storage-Formic Acid as a Sustainable Storage Material for Hydrogen |journal=ChemSusChem |volume=1 |issue=10 |pages=805–8 |year=2008 |last1=Joó |first1=Ferenc }}</ref> The co-product of this decomposition, carbon dioxide, can be rehydrogenated back to formic acid in a second step. Formic acid contains 53 g/L hydrogen at room temperature and atmospheric pressure, which is three and a half times as much as compressed hydrogen gas can attain at 350 bar pressure (14.7 g/L). Pure formic acid is a liquid with a [[flash point]] of 69 °C, much higher than that of gasoline ( |
Formic acid has been considered as a means of [[hydrogen storage]].<ref>{{cite journal |doi=10.1002/cssc.200800133 |pmid=18781551 |title=Breakthroughs in Hydrogen Storage-Formic Acid as a Sustainable Storage Material for Hydrogen |journal=ChemSusChem |volume=1 |issue=10 |pages=805–8 |year=2008 |last1=Joó |first1=Ferenc }}</ref> The co-product of this decomposition, carbon dioxide, can be rehydrogenated back to formic acid in a second step. Formic acid contains 53 g/L hydrogen at room temperature and atmospheric pressure, which is three and a half times as much as compressed hydrogen gas can attain at 350 bar pressure (14.7 g/L). Pure formic acid is a liquid with a [[flash point]] of 69 °C, much higher than that of gasoline (−40 °C) or ethanol (13 °C).{{citation needed|date=November 2017}} |
||
It is possible to use formic acid as an intermediary to produce [[isobutanol]] from {{CO2}} using microbes.<ref>{{Cite web|url=https://newenergyandfuel.com/http:/newenergyandfuel/com/2012/03/30/ucla-researchers-use-electricity-and-co2-to-make-butanol/|title=UCLA Researchers Use Electricity and CO2 to Make Butanol|date=30 March 2012 }}</ref><ref>{{Cite journal|title=Integrated Electromicrobial Conversion of CO2 to Higher Alcohols|first1=James C.|last1=Liao|first2=Kwang Myung|last2=Cho|first3=Yi-Xin|last3=Huo|first4=Peter|last4=Malati|first5=Wendy|last5=Higashide|first6=Tung-Yun|last6=Wu|first7=Steve|last7=Rogers|first8=David G.|last8=Wernick|first9=Paul H.|last9=Opgenorth|first10=Han|last10=Li|date=30 March 2012|journal=Science|volume=335|issue=6076|pages=1596|doi=10.1126/science.1217643|pmid=22461604|bibcode=2012Sci...335.1596L|s2cid=24328552}}</ref> |
It is possible to use formic acid as an intermediary to produce [[isobutanol]] from {{CO2}} using microbes.<ref>{{Cite web|url=https://newenergyandfuel.com/http:/newenergyandfuel/com/2012/03/30/ucla-researchers-use-electricity-and-co2-to-make-butanol/|title=UCLA Researchers Use Electricity and CO2 to Make Butanol|date=30 March 2012 }}</ref><ref>{{Cite journal|title=Integrated Electromicrobial Conversion of CO2 to Higher Alcohols|first1=James C.|last1=Liao|first2=Kwang Myung|last2=Cho|first3=Yi-Xin|last3=Huo|first4=Peter|last4=Malati|first5=Wendy|last5=Higashide|first6=Tung-Yun|last6=Wu|first7=Steve|last7=Rogers|first8=David G.|last8=Wernick|first9=Paul H.|last9=Opgenorth|first10=Han|last10=Li|date=30 March 2012|journal=Science|volume=335|issue=6076|pages=1596|doi=10.1126/science.1217643|pmid=22461604|bibcode=2012Sci...335.1596L|s2cid=24328552}}</ref> |
||
===Soldering=== |
===Soldering=== |
||
Formic acid has a potential application in [[soldering]]. Due to its capacity to reduce oxide layers, formic acid gas can be blasted at an oxide surface to increase solder [[Soldering#Flux|wettability]]. |
Formic acid has a potential application in [[soldering]]. Due to its capacity to reduce oxide layers, formic acid gas can be blasted at an oxide surface to increase solder [[Soldering#Flux|wettability]].{{cn|date=June 2024}} |
||
===Chromatography=== |
===Chromatography=== |
||
| Line 226: | Line 227: | ||
Formic acid is readily metabolized and eliminated by the body. Nonetheless, it has specific [[toxic]] effects; the formic acid and [[formaldehyde]] produced as metabolites of [[methanol]] are responsible for the [[optic nerve]] damage, causing blindness, seen in [[methanol poisoning]].<ref>{{cite journal |doi=10.1136/jnnp.72.4.423 |pmid=11909893 |pmc=1737836 |year=2002 |last1=Sadun |first1=A. A |title=Mitochondrial optic neuropathies |journal=Journal of Neurology, Neurosurgery, and Psychiatry |volume=72 |issue=4 |pages=423–5 }}</ref> Some chronic effects of formic acid exposure have been documented. Some experiments on bacterial species have demonstrated it to be a [[mutagen]].<ref name="osha.gov">{{cite web|url=http://www.osha.gov/SLTC/healthguidelines/formicacid/recognition.html|title=Occupational Safety and Health Guideline for Formic Acid|publisher=OSHA|access-date=28 May 2011|archive-date=20 September 2011|archive-url=https://web.archive.org/web/20110920200807/http://www.osha.gov/SLTC/healthguidelines/formicacid/recognition.html|url-status=dead}}</ref> Chronic exposure in humans may cause kidney damage.<ref name="osha.gov"/> Another possible effect of chronic exposure is development of a skin [[allergy]] that manifests upon re-exposure to the chemical. |
Formic acid is readily metabolized and eliminated by the body. Nonetheless, it has specific [[toxic]] effects; the formic acid and [[formaldehyde]] produced as metabolites of [[methanol]] are responsible for the [[optic nerve]] damage, causing blindness, seen in [[methanol poisoning]].<ref>{{cite journal |doi=10.1136/jnnp.72.4.423 |pmid=11909893 |pmc=1737836 |year=2002 |last1=Sadun |first1=A. A |title=Mitochondrial optic neuropathies |journal=Journal of Neurology, Neurosurgery, and Psychiatry |volume=72 |issue=4 |pages=423–5 }}</ref> Some chronic effects of formic acid exposure have been documented. Some experiments on bacterial species have demonstrated it to be a [[mutagen]].<ref name="osha.gov">{{cite web|url=http://www.osha.gov/SLTC/healthguidelines/formicacid/recognition.html|title=Occupational Safety and Health Guideline for Formic Acid|publisher=OSHA|access-date=28 May 2011|archive-date=20 September 2011|archive-url=https://web.archive.org/web/20110920200807/http://www.osha.gov/SLTC/healthguidelines/formicacid/recognition.html|url-status=dead}}</ref> Chronic exposure in humans may cause kidney damage.<ref name="osha.gov"/> Another possible effect of chronic exposure is development of a skin [[allergy]] that manifests upon re-exposure to the chemical. |
||
Concentrated formic acid slowly decomposes to carbon monoxide and water, leading to pressure buildup in the containing vessel. For this reason, 98% formic acid is shipped in plastic bottles with self-venting caps. |
Concentrated formic acid slowly decomposes to carbon monoxide and water, leading to pressure buildup in the containing vessel. For this reason, 98% formic acid is shipped in plastic bottles with self-venting caps.{{cn|date=June 2024}} |
||
The hazards of solutions of formic acid depend on the concentration. The following table lists the [[Globally Harmonized System of Classification and Labelling of Chemicals]] for formic acid solutions:{{citation needed|date=October 2021}} |
The hazards of solutions of formic acid depend on the concentration. The following table lists the [[Globally Harmonized System of Classification and Labelling of Chemicals]] for formic acid solutions:{{citation needed|date=October 2021}} |
||
| Line 249: | Line 250: | ||
|} |
|} |
||
Formic acid in 85% concentration is flammable, and diluted formic acid is on the U.S. Food and Drug Administration list of food additives.<ref>{{CodeFedReg|21|186|1316}}, {{CodeFedReg|21|172|515}}</ref> The principal danger from formic acid is from skin or eye contact with the concentrated liquid or vapors. The U.S. [[Occupational Safety and Health Administration|OSHA]] Permissible Exposure Level ([[Permissible exposure limit|PEL]]) of formic acid vapor in the work environment is 5 [[parts per million]] (ppm) of air. |
Formic acid in 85% concentration is flammable, and diluted formic acid is on the U.S. Food and Drug Administration list of food additives.<ref>{{CodeFedReg|21|186|1316}}, {{CodeFedReg|21|172|515}}</ref> The principal danger from formic acid is from skin or eye contact with the concentrated liquid or vapors. The U.S. [[Occupational Safety and Health Administration|OSHA]] Permissible Exposure Level ([[Permissible exposure limit|PEL]]) of formic acid vapor in the work environment is 5 [[parts per million]] (ppm) of air.<ref>{{Cite web |title=CDC - NIOSH Pocket Guide to Chemical Hazards - Formic acid |url=https://www.cdc.gov/niosh/npg/npgd0296.html |access-date=2024-11-01 |website=www.cdc.gov}}</ref> |
||
==See also== |
==See also== |
||
Latest revision as of 17:07, 1 November 2024
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Formic acid[1] | |||
| Systematic IUPAC name
Methanoic acid[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1209246 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.527 | ||
| EC Number |
| ||
| E number | E236 (preservatives) | ||
| 1008 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH2O2 | |||
| Molar mass | 46.025 g·mol−1 | ||
| Appearance | Colorless fuming liquid | ||
| Odor | Pungent, penetrating | ||
| Density | 1.220 g/mL | ||
| Melting point | 8.4 °C (47.1 °F; 281.5 K) | ||
| Boiling point | 100.8 °C (213.4 °F; 373.9 K) | ||
| Miscible | |||
| Solubility | Miscible with ether, acetone, ethyl acetate, glycerol, methanol, ethanol Partially soluble in benzene, toluene, xylenes | ||
| log P | −0.54 | ||
| Vapor pressure | 35 mmHg (20 °C)[2] | ||
| Acidity (pKa) | 3.745[3] | ||
| Conjugate base | Formate | ||
| −19.90×10−6 cm3/mol | |||
Refractive index (nD)
|
1.3714 (20 °C) | ||
| Viscosity | 1.57 cP at 268 °C | ||
| Structure | |||
| Planar | |||
| 1.41 D (gas) | |||
| Thermochemistry | |||
Std molar
entropy (S⦵298) |
131.8 J/mol K | ||
Std enthalpy of
formation (ΔfH⦵298) |
−425.0 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−254.6 kJ/mol | ||
| Pharmacology | |||
| QP53AG01 (WHO) | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Corrosive; irritant; sensitizer | ||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H314 | |||
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 69 °C (156 °F; 342 K) | ||
| 601 °C (1,114 °F; 874 K) | |||
| Explosive limits | 14–34%[citation needed] 18–57% (90% solution)[2] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
700 mg/kg (mouse, oral), 1100 mg/kg (rat, oral), 4000 mg/kg (dog, oral)[4] | ||
LC50 (median concentration)
|
7853 ppm (rat, 15 min) 3246 ppm (mouse, 15 min)[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 5 ppm (9 mg/m3)[2] | ||
REL (Recommended)
|
TWA 5 ppm (9 mg/m3)[2] | ||
IDLH (Immediate danger)
|
30 ppm[2] | ||
| Safety data sheet (SDS) | MSDS from JT Baker | ||
| Related compounds | |||
Related carboxylic acids
|
Acetic acid Propionic acid | ||
Related compounds
|
Formaldehyde Methanol | ||
| Supplementary data page | |||
| Formic acid (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
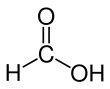
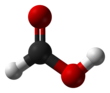
Formic acid (from Latin formica 'ant'), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure H−C(=O)−O−H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Esters, salts and the anion derived from formic acid are called formates. Industrially, formic acid is produced from methanol.[5]
Natural occurrence
[edit]Formic acid is found naturally in insects, weeds, fruits and vegetables, and forest emissions. It appears in most ants and in stingless bees of the genus Oxytrigona.[6][7] Wood ants from the genus Formica can spray formic acid on their prey or to defend the nest. The puss moth caterpillar (Cerura vinula) will spray it as well when threatened by predators. It is also found in the trichomes of stinging nettle (Urtica dioica). Apart from that, this acid is incorporated in many fruits such as pineapple (0.21 mg per 100 g), apple (2 mg per 100 g) and kiwi (1 mg per 100 g), as well as in many vegetables, namely onion (45 mg per 100 g), eggplant (1.34 mg per 100 g) and, in extremely low concentrations, cucumber (0.11 mg per 100 g).[8] Formic acid is a naturally occurring component of the atmosphere primarily due to forest emissions.[9]
History
[edit]As early as the 15th century, some alchemists and naturalists were aware that ant hills give off an acidic vapor. The first person to describe the isolation of this substance (by the distillation of large numbers of ants) was the English naturalist John Ray, in 1671.[10][11] Ants secrete the formic acid for attack and defense purposes. Formic acid was first synthesized from hydrocyanic acid by the French chemist Joseph Gay-Lussac. In 1855, another French chemist, Marcellin Berthelot, developed a synthesis from carbon monoxide similar to the process used today.[citation needed]
Formic acid was long considered a chemical compound of only minor interest in the chemical industry. In the late 1960s, significant quantities became available as a byproduct of acetic acid production. It now finds increasing use as a preservative and antibacterial in livestock feed.[citation needed]
Properties
[edit]
Formic acid is a colorless liquid having a pungent, penetrating odor[12] at room temperature, comparable to the related acetic acid. Formic acid is about ten times stronger than acetic acid.[citation needed]
It is miscible with water and most polar organic solvents, and is somewhat soluble in hydrocarbons. In hydrocarbons and in the vapor phase, it consists of hydrogen-bonded dimers rather than individual molecules.[13][14] Owing to its tendency to hydrogen-bond, gaseous formic acid does not obey the ideal gas law.[14] Solid formic acid, which can exist in either of two polymorphs, consists of an effectively endless network of hydrogen-bonded formic acid molecules. Formic acid forms a high-boiling azeotrope with water (107.3 °C; 77.5% formic acid). Liquid formic acid tends to supercool.
Chemical reactions
[edit]Decomposition
[edit]Formic acid readily decomposes by dehydration in the presence of concentrated sulfuric acid to form carbon monoxide and water:
- HCO2H → H2O + CO
Treatment of formic acid with sulfuric acid is a convenient laboratory source of CO.[15] [16]
In the presence of platinum, it decomposes with a release of hydrogen and carbon dioxide.
- HCO2H → H2 + CO2
Soluble ruthenium catalysts are also effective for producing carbon monoxide-free hydrogen.[17]
Reactant
[edit]Formic acid shares most of the chemical properties of other carboxylic acids. Because of its high acidity, solutions in alcohols form esters spontaneously; in Fischer esterifications of formic acid, it self-catalyzes the reaction and no additional acid catalyst is needed.[18] Formic acid shares some of the reducing properties of aldehydes, reducing solutions of metal oxides to their respective metal.[19]
Formic acid is a source for a formyl group for example in the formylation of N-methylaniline to N-methylformanilide in toluene.[20]
In synthetic organic chemistry, formic acid is often used as a source of hydride ion, as in the Eschweiler–Clarke reaction:
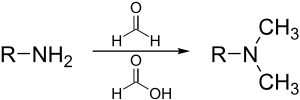
It is used as a source of hydrogen in transfer hydrogenation, as in the Leuckart reaction to make amines, and (in aqueous solution or in its azeotrope with triethylamine) for hydrogenation of ketones.[21]
Addition to alkenes
[edit]Formic acid is unique among the carboxylic acids in its ability to participate in addition reactions with alkenes. Formic acids and alkenes readily react to form formate esters. In the presence of certain acids, including sulfuric and hydrofluoric acids, however, a variant of the Koch reaction occurs instead, and formic acid adds to the alkene to produce a larger carboxylic acid.[22]
Formic acid anhydride
[edit]An unstable formic anhydride, H(C=O)−O−(C=O)H, can be obtained by dehydration of formic acid with N,N′-dicyclohexylcarbodiimide in ether at low temperature.[23]
Production
[edit]In 2009, the worldwide capacity for producing formic acid was 720 thousand tonnes (1.6 billion pounds) per year, roughly equally divided between Europe (350 thousand tonnes or 770 million pounds, mainly in Germany) and Asia (370 thousand tonnes or 820 million pounds, mainly in China) while production was below 1 thousand tonnes or 2.2 million pounds per year in all other continents.[24] It is commercially available in solutions of various concentrations between 85 and 99 w/w %.[13] As of 2009[update], the largest producers are BASF, Eastman Chemical Company, LC Industrial, and Feicheng Acid Chemicals, with the largest production facilities in Ludwigshafen (200 thousand tonnes or 440 million pounds per year, BASF, Germany), Oulu (105 thousand tonnes or 230 million pounds, Eastman, Finland), Nakhon Pathom (n/a, LC Industrial), and Feicheng (100 thousand tonnes or 220 million pounds, Feicheng, China). 2010 prices ranged from around €650/tonne (equivalent to around $800/tonne) in Western Europe to $1250/tonne in the United States.[24]
From methyl formate and formamide
[edit]When methanol and carbon monoxide are combined in the presence of a strong base, the result is methyl formate, according to the chemical equation:[13]
- CH3OH + CO → HCO2CH3
In industry, this reaction is performed in the liquid phase at elevated pressure. Typical reaction conditions are 80 °C and 40 atm. The most widely used base is sodium methoxide. Hydrolysis of the methyl formate produces formic acid:
- HCO2CH3 + H2O → HCOOH + CH3OH
Efficient hydrolysis of methyl formate requires a large excess of water. Some routes proceed indirectly by first treating the methyl formate with ammonia to give formamide, which is then hydrolyzed with sulfuric acid:
- HCO2CH3 + NH3 → HC(O)NH2 + CH3OH
- 2 HC(O)NH2 + 2H2O + H2SO4 → 2HCO2H + (NH4)2SO4
A disadvantage of this approach is the need to dispose of the ammonium sulfate byproduct. This problem has led some manufacturers to develop energy-efficient methods of separating formic acid from the excess water used in direct hydrolysis. In one of these processes, used by BASF, the formic acid is removed from the water by liquid-liquid extraction with an organic base.[citation needed]
Niche and obsolete chemical routes
[edit]By-product of acetic acid production
[edit]A significant amount of formic acid is produced as a byproduct in the manufacture of other chemicals. At one time, acetic acid was produced on a large scale by oxidation of alkanes, by a process that cogenerates significant formic acid.[13] This oxidative route to acetic acid has declined in importance so that the aforementioned dedicated routes to formic acid have become more important.[citation needed]
Hydrogenation of carbon dioxide
[edit]The catalytic hydrogenation of CO2 to formic acid has long been studied. This reaction can be conducted homogeneously.[25][26][27]
Oxidation of biomass
[edit]Formic acid can also be obtained by aqueous catalytic partial oxidation of wet biomass by the OxFA process.[28][29] A Keggin-type polyoxometalate (H5PV2Mo10O40) is used as the homogeneous catalyst to convert sugars, wood, waste paper, or cyanobacteria to formic acid and CO2 as the sole byproduct. Yields of up to 53% formic acid can be achieved.[citation needed]
Laboratory methods
[edit]In the laboratory, formic acid can be obtained by heating oxalic acid in glycerol followed by steam distillation.[30] Glycerol acts as a catalyst, as the reaction proceeds through a glyceryl oxalate intermediate. If the reaction mixture is heated to higher temperatures, allyl alcohol results. The net reaction is thus:
- C2O4H2 → HCO2H + CO2
Another illustrative method involves the reaction between lead formate and hydrogen sulfide, driven by the formation of lead sulfide.[31]
- Pb(HCOO)2 + H2S → 2HCOOH + PbS
Electrochemical production
[edit]Formate is formed by the electrochemical reduction of CO2 (in the form of bicarbonate) at a lead cathode at pH 8.6:[32]
- HCO−
3 + H
2O + 2e− → HCO−
2 + 2OH−
or
- CO
2 + H
2O + 2e− → HCO−
2 + OH−
If the feed is CO
2 and oxygen is evolved at the anode, the total reaction is:
- CO2 + OH−
→ HCO−
2 + 1/2 O2
Biosynthesis
[edit]Formic acid is named after ants which have high concentrations of the compound in their venom, derived from serine through a 5,10-methenyltetrahydrofolate intermediate.[33] The conjugate base of formic acid, formate, also occurs widely in nature. An assay for formic acid in body fluids, designed for determination of formate after methanol poisoning, is based on the reaction of formate with bacterial formate dehydrogenase.[34]
Uses
[edit]Agriculture
[edit]A major use of formic acid is as a preservative and antibacterial agent in livestock feed. It arrests certain decay processes and causes the feed to retain its nutritive value longer,
In Europe, it is applied on silage, including fresh hay, to promote the fermentation of lactic acid and to suppress the formation of butyric acid; it also allows fermentation to occur quickly, and at a lower temperature, reducing the loss of nutritional value.[13] It is widely used to preserve winter feed for cattle,[35] and is sometimes added to poultry feed to kill E. coli bacteria.[36][37] Use as a preservative for silage and other animal feed constituted 30% of the global consumption in 2009.[24]
Beekeepers use formic acid as a miticide against the tracheal mite (Acarapis woodi) and the Varroa destructor mite and Varroa jacobsoni mite.[38]
Energy
[edit]Formic acid can be used directly in formic acid fuel cells or indirectly in hydrogen fuel cells.[39][40]
Electrolytic conversion of electrical energy to chemical fuel has been proposed as a large-scale source of formate by various groups.[41] The formate could be used as feed to modified E. coli bacteria for producing biomass.[42][43] Natural methylotroph microbes can feed on formic acid or formate.
Formic acid has been considered as a means of hydrogen storage.[44] The co-product of this decomposition, carbon dioxide, can be rehydrogenated back to formic acid in a second step. Formic acid contains 53 g/L hydrogen at room temperature and atmospheric pressure, which is three and a half times as much as compressed hydrogen gas can attain at 350 bar pressure (14.7 g/L). Pure formic acid is a liquid with a flash point of 69 °C, much higher than that of gasoline (−40 °C) or ethanol (13 °C).[citation needed]
It is possible to use formic acid as an intermediary to produce isobutanol from CO2 using microbes.[45][46]
Soldering
[edit]Formic acid has a potential application in soldering. Due to its capacity to reduce oxide layers, formic acid gas can be blasted at an oxide surface to increase solder wettability.[citation needed]
Chromatography
[edit]Formic acid is used as a volatile pH modifier in HPLC and capillary electrophoresis. Formic acid is often used as a component of mobile phase in reversed-phase high-performance liquid chromatography (RP-HPLC) analysis and separation techniques for the separation of hydrophobic macromolecules, such as peptides, proteins and more complex structures including intact viruses. Especially when paired with mass spectrometry detection, formic acid offers several advantages over the more traditionally used phosphoric acid.[47][48]
Other uses
[edit]Formic acid is also significantly used in the production of leather, including tanning (23% of the global consumption in 2009[24]), and in dyeing and finishing textiles (9% of the global consumption in 2009[24]) because of its acidic nature. Use as a coagulant in the production of rubber[13] consumed 6% of the global production in 2009.[24]
Formic acid is also used in place of mineral acids for various cleaning products,[13] such as limescale remover and toilet bowl cleaner. Some formate esters are artificial flavorings and perfumes.
Formic acid application has been reported to be an effective treatment for warts.[49]
Safety
[edit]Formic acid has low toxicity (hence its use as a food additive), with an LD50 of 1.8 g/kg (tested orally on mice). The concentrated acid is corrosive to the skin.[13]
Formic acid is readily metabolized and eliminated by the body. Nonetheless, it has specific toxic effects; the formic acid and formaldehyde produced as metabolites of methanol are responsible for the optic nerve damage, causing blindness, seen in methanol poisoning.[50] Some chronic effects of formic acid exposure have been documented. Some experiments on bacterial species have demonstrated it to be a mutagen.[51] Chronic exposure in humans may cause kidney damage.[51] Another possible effect of chronic exposure is development of a skin allergy that manifests upon re-exposure to the chemical.
Concentrated formic acid slowly decomposes to carbon monoxide and water, leading to pressure buildup in the containing vessel. For this reason, 98% formic acid is shipped in plastic bottles with self-venting caps.[citation needed]
The hazards of solutions of formic acid depend on the concentration. The following table lists the Globally Harmonized System of Classification and Labelling of Chemicals for formic acid solutions:[citation needed]
| Concentration (weight percent) | Pictogram | H-Phrases |
|---|---|---|
| 2–10% | 
|
H315 |
| 10–90% | 
|
H313 |
| >90% | 
|
H314 |
Formic acid in 85% concentration is flammable, and diluted formic acid is on the U.S. Food and Drug Administration list of food additives.[52] The principal danger from formic acid is from skin or eye contact with the concentrated liquid or vapors. The U.S. OSHA Permissible Exposure Level (PEL) of formic acid vapor in the work environment is 5 parts per million (ppm) of air.[53]
See also
[edit]References
[edit]- ^ a b Favre, Henri A.; Powell, Warren H. (2014). Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. p. 745. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0296". National Institute for Occupational Safety and Health (NIOSH).
- ^ Smith, Robert M.; Martell, Arthur E. (1989). Critical Stability Constants Volume 6: Second Supplement. New York: Plenum Press. p. 299. ISBN 0-306-43104-1.
- ^ a b "Formic acid". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health. 4 December 2014. Retrieved 26 March 2015.
- ^ "Formic acid". American Chemical Society. Retrieved 21 November 2023.
- ^ Hoffman, Donald R (2010). "Ant venoms". Current Opinion in Allergy and Clinical Immunology. 10 (4): 342–6. doi:10.1097/ACI.0b013e328339f325. PMID 20445444. S2CID 4999650.
- ^ Roubik, DW; Smith, BH; Carlson, RG (1987). "Formic acid in caustic cephalic secretions of stingless bee,Oxytrigona (Hymenoptera: Apidae)". J Chem Ecol. 13 (5): 1079–86. doi:10.1007/BF01020539. PMID 24302133. S2CID 30511107.
- ^ Otles, S; Yalcin, B (2012). "Phenolic compounds analysis of root, stalk, and leaves of nettle". ScientificWorldJournal. 2012: 564367. doi:10.1100/2012/564367. PMC 3349212. PMID 22593694.
- ^ Sanhueza, Eugenio; Andreae, Meinrat O (1991). "Emission of formic and acetic acids from tropical Savanna soils". Geophysical Research Letters. 18 (9): 1707–10. Bibcode:1991GeoRL..18.1707S. doi:10.1029/91GL01565.
- ^ Wray, J (1670). "Extract of a Letter, Written by Mr John Wray to the Publisher January 13. 1670. Concerning Some Un-Common Observations and Experiments Made with an Acid Juyce to be Found in Ants". Philosophical Transactions of the Royal Society of London. 5 (57–68): 2063–2066. Bibcode:1670RSPT....5.2063W. doi:10.1098/rstl.1670.0052.
- ^ Johnson, W. B. (1803). History of the process and present state of animal chemistry.
- ^ "OSHA Occupational Chemical Database – Occupational Safety and Health Administration". osha.gov. Archived from the original on 29 April 2021. Retrieved 17 April 2015.
- ^ a b c d e f g h Reutemann, Werner; Kieczka, Heinz (2000). "Formic Acid". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a12_013. ISBN 978-3-527-30673-2.
- ^ a b Roman M. Balabin (2009). "Polar (Acyclic) Isomer of Formic Acid Dimer: Gas-Phase Raman Spectroscopy Study and Thermodynamic Parameters". The Journal of Physical Chemistry A. 113 (17): 4910–8. Bibcode:2009JPCA..113.4910B. doi:10.1021/jp9002643. PMID 19344174.
- ^ Koch, H.; Haaf, W. (1964). "1-Adamantanecarboxylic Acid". Organic Syntheses. 44: 1. doi:10.15227/orgsyn.044.0001.
- ^ Coleman, G. H.; Craig, David (1932). "p-Tolualdehyde". Organic Syntheses. 12: 80. doi:10.15227/orgsyn.012.0080.
- ^ Fellay, Céline; Dyson, Paul J.; Laurenczy, Gábor (2008). "A Viable Hydrogen-Storage System Based on Selective Formic Acid Decomposition with a Ruthenium Catalyst". Angewandte Chemie International Edition. 47 (21): 3966–8. doi:10.1002/anie.200800320. PMID 18393267.
- ^ Furniss, Brian S.; Hannaford, Antony, J.; Smith, Peter W. G.; Tatchell, Austin S. (1989). Vogel's Textbook of Practical Organic Chemistry (5th ed.). Longman Scientific & Technical. p. 696, 701. ISBN 978-0582462366.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Ozawa, Naoto; Okubo, Tatsuo; Matsuda, Jun; Sakai, Tatsuo (October 2016). "Observation and analysis of metal oxide reduction by formic acid for soldering". 2016 11th International Microsystems, Packaging, Assembly and Circuits Technology Conference (IMPACT). pp. 148–151. doi:10.1109/IMPACT.2016.7799990. ISBN 978-1-5090-4769-7. S2CID 32545113.
- ^ L. F. Fieser; J. E. Jones (1955). "N-Methylformanilide". Organic Syntheses; Collected Volumes, vol. 3, p. 590.
- ^ Zhou, Xiaowei; et al. (2012). "Varying the ratio of formic acid to triethylamine impacts on asymmetric transfer hydrogenation of ketones". Journal of Molecular Catalysis A: Chemical. 357: 133–140. doi:10.1016/j.molcata.2012.02.002. ISSN 1381-1169.
- ^ Haaf, Wolfgang (1966). "Die Synthese sekundärer Carbonsäuren nach der Ameisensäure-Methode". Chemische Berichte. 99 (4): 1149–52. doi:10.1002/cber.19660990410.
- ^ Wu, G; Shlykov, S; Van Alseny, F. S; Geise, H. J; Sluyts, E; Van Der Veken, B. J (1995). "Formic Anhydride in the Gas Phase, Studied by Electron Diffraction and Microwave and Infrared Spectroscopy, Supplemented with Ab-Initio Calculations of Geometries and Force Fields". The Journal of Physical Chemistry. 99 (21): 8589–98. doi:10.1021/j100021a022.
- ^ a b c d e f S. N. Bizzari; M. Blagoev (June 2010). "CEH Marketing Research Report: FORMIC ACID". Chemical Economics Handbook. SRI consulting. Archived from the original on 14 September 2011.
- ^ P. G. Jessop (2007). J. G. de Vries, C. J. Elsevier (ed.). Handbook of Homogeneous Hydrogenation. Weinheim, Germany: Wiley-VCH. pp. 489–511.
- ^ Jessop, Philip G; Joó, Ferenc; Tai, Chih-Cheng (2004). "Recent advances in the homogeneous hydrogenation of carbon dioxide". Coordination Chemistry Reviews. 248 (21–24): 2425. doi:10.1016/j.ccr.2004.05.019.
- ^ Sampson, Joanna (2 August 2020). "Wireless device makes clean fuel from sunlight, CO2 and water". Gasworld. Retrieved 26 August 2020.
- ^ Wölfel, Rene; Taccardi, Nicola; Bösmann, Andreas; Wasserscheid, Peter (2011). "Selective catalytic conversion of biobased carbohydrates to formic acid using molecular oxygen". Green Chemistry. 13 (10): 2759. doi:10.1039/C1GC15434F. S2CID 97572039.
- ^ Albert, Jakob; Wölfel, Rene; Bösmann, Andreas; Wasserscheid, Peter (2012). "Selective oxidation of complex, water-insoluble biomass to formic acid using additives as reaction accelerators". Energy & Environmental Science. 5 (7): 7956. doi:10.1039/C2EE21428H. S2CID 93224286.
- ^ Chattaway, Frederick Daniel (1914). "XX.—Interaction of glycerol and oxalic acid". Journal of the Chemical Society, Transactions. 105: 151–6. doi:10.1039/CT9140500151. hdl:2027/mdp.39015067135775.
- ^ Arthur Sutcliffe (1930). Practical Chemistry for Advanced Students (1949 ed.). London: John Murray.
- ^ B. Innocent; et al. (February 2009). "Electro-reduction of carbon dioxide to formate on lead electrode in aqueous medium". Journal of Applied Electrochemistry. 39 (2): 227–232. doi:10.1007/s10800-008-9658-4. S2CID 98437382.
- ^ Hefetz, Abraham; Blum, Murray (1 November 1978). "Biosynthesis of formic acid by the poison glands of formicine ants". Biochimica et Biophysica Acta (BBA) - General Subjects. 543 (4): 484–496. doi:10.1016/0304-4165(78)90303-3. PMID 718985.
- ^ Makar, A.B; McMartin, K.E; Palese, M; Tephly, T.R (1975). "Formate assay in body fluids: Application in methanol poisoning". Biochemical Medicine. 13 (2): 117–26. doi:10.1016/0006-2944(75)90147-7. PMID 1.
- ^ Organic Acids and Food Preservation, Maria M. Theron, J. F. Rykers Lues
- ^ Griggs, J. P; Jacob, J. P (2005). "Alternatives to Antibiotics for Organic Poultry Production". The Journal of Applied Poultry Research. 14 (4): 750. doi:10.1093/japr/14.4.750.
- ^ Garcia, V; Catala-Gregori, P; Hernandez, F; Megias, M. D; Madrid, J (2007). "Effect of Formic Acid and Plant Extracts on Growth, Nutrient Digestibility, Intestine Mucosa Morphology, and Meat Yield of Broilers". The Journal of Applied Poultry Research. 16 (4): 555. doi:10.3382/japr.2006-00116.
- ^ Hoppe, H.; Ritter, W.; Stephen, E. W. C. (1989). "The control of parasitic bee mites: Varroa jacobsoni, Acarapis woodi and Tropilaelaps clareae with formic acid". American Bee Journal.
- ^ Ha, S; Larsen, R; Masel, R.I (2005). "Performance characterization of Pd/C nanocatalyst for direct formic acid fuel cells". Journal of Power Sources. 144 (1): 28–34. Bibcode:2005JPS...144...28H. doi:10.1016/j.jpowsour.2004.12.031.
- ^ Jorn Madslien (27 June 2017). "Ant power: Take a ride on a bus that runs on formic acid". BBC News. Retrieved 11 July 2017.
- ^ Yishai, Oren; Lindner, Steffen N; Gonzalez de la Cruz, Jorge; Tenenboim, Hezi; Bar-Even, Arren (December 2016). "The formate bio-economy". Current Opinion in Chemical Biology. 35: 1–9. doi:10.1016/j.cbpa.2016.07.005. PMID 27459678.
- ^ Shmuel Gleizer; et al. (November 2019). "Conversion of Escherichia coli to Generate All Biomass Carbon from CO2". Cell. 179 (6): 1255–1263.e12. doi:10.1016/j.cell.2019.11.009. PMC 6904909. PMID 31778652.
- ^ Kim, Seohyoung; Lindner, Steffen N.; Aslan, Selçuk; Yishai, Oren; Wenk, Sebastian; Schann, Karin; Bar-Even, Arren (10 February 2020). "Growth of E. coli on formate and methanol via the reductive glycine pathway". Nature Chemical Biology. 16 (5): 538–545. doi:10.1038/s41589-020-0473-5. ISSN 1552-4469. PMID 32042198. S2CID 211074951.
- ^ Joó, Ferenc (2008). "Breakthroughs in Hydrogen Storage-Formic Acid as a Sustainable Storage Material for Hydrogen". ChemSusChem. 1 (10): 805–8. doi:10.1002/cssc.200800133. PMID 18781551.
- ^ "UCLA Researchers Use Electricity and CO2 to Make Butanol". 30 March 2012.
- ^ Liao, James C.; Cho, Kwang Myung; Huo, Yi-Xin; Malati, Peter; Higashide, Wendy; Wu, Tung-Yun; Rogers, Steve; Wernick, David G.; Opgenorth, Paul H.; Li, Han (30 March 2012). "Integrated Electromicrobial Conversion of CO2 to Higher Alcohols". Science. 335 (6076): 1596. Bibcode:2012Sci...335.1596L. doi:10.1126/science.1217643. PMID 22461604. S2CID 24328552.
- ^ "Archived copy". Archived from the original on 7 November 2017. Retrieved 7 November 2017.
{{cite web}}: CS1 maint: archived copy as title (link)[full citation needed] - ^ Heukeshoven, Jochen; Dernick, Rudolf (1982). "Reversed-phase high-performance liquid chromatography of virus proteins and other large hydrophobic proteins in formic acid containing solvents". Journal of Chromatography A. 252: 241–54. doi:10.1016/S0021-9673(01)88415-6. PMID 6304128.
- ^ Bhat, Ramesh M; Vidya, Krishna; Kamath, Ganesh (2001). "Topical formic acid puncture technique for the treatment of common warts". International Journal of Dermatology. 40 (6): 415–9. doi:10.1046/j.1365-4362.2001.01242.x. PMID 11589750. S2CID 42351889.
- ^ Sadun, A. A (2002). "Mitochondrial optic neuropathies". Journal of Neurology, Neurosurgery, and Psychiatry. 72 (4): 423–5. doi:10.1136/jnnp.72.4.423. PMC 1737836. PMID 11909893.
- ^ a b "Occupational Safety and Health Guideline for Formic Acid". OSHA. Archived from the original on 20 September 2011. Retrieved 28 May 2011.
- ^ 21 CFR 186.1316, 21 CFR 172.515
- ^ "CDC - NIOSH Pocket Guide to Chemical Hazards - Formic acid". www.cdc.gov. Retrieved 1 November 2024.