Maleimide: Difference between revisions
m →top: Copy edit ▸ Presentation ▸ Outside article reference visually augmented via crossreferencing template. Tags: Mobile edit Mobile app edit Android app edit App full source |
|||
| (21 intermediate revisions by 12 users not shown) | |||
| Line 1: | Line 1: | ||
{{Use dmy dates|date=May 2022}} |
|||
{{Chembox |
{{Chembox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Line 71: | Line 72: | ||
}} |
}} |
||
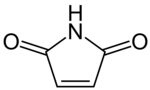
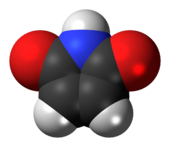
'''Maleimide''' is a [[chemical compound]] with the [[chemical formula|formula]] H<sub>2</sub>C<sub>2</sub>(CO)<sub>2</sub>NH (see diagram). This unsaturated [[imide]] is an important building block in [[organic synthesis]]. The name is a contraction of [[maleic acid]] and [[imide]], |
'''Maleimide''' is a [[chemical compound]] with the [[chemical formula|formula]] H<sub>2</sub>C<sub>2</sub>(CO)<sub>2</sub>NH (see diagram). This unsaturated [[imide]] is an important building block in [[organic synthesis]]. The name is a contraction of [[maleic acid]] and [[imide]], the -C(O)NHC(O)- [[functional group]]. Maleimides also describes a ''class'' of derivatives of the parent maleimide where the N''H'' group is replaced with [[alkyl]] or [[aryl]] groups such as a [[methyl]] or [[phenyl]], respectively. The substituent can also be a small molecule (such as [[biotin]], a fluorescent dye, an [[oligosaccharide]], or a [[nucleic acid]]), a reactive group, or a [[synthetic polymer]] such as [[polyethylene glycol]].<ref name="Hermanson2013">{{cite book | vauthors = Hermanson G | chapter = Chapter 6: Heterobifunctional Crosslinkers |title=Bioconjugate Techniques | doi = 10.1016/B978-0-12-382239-0.00006-6 |publisher= Elsevier |pages=299–339 |isbn=978-0-12-382239-0 |year=2013}}</ref> Human [[hemoglobin]] chemically modified with maleimide-polyethylene glycol is a [[blood substitute]] called MP4. |
||
==Organic chemistry== |
==Organic chemistry== |
||
Maleimide and its derivatives are prepared from [[maleic anhydride]] by treatment with [[amine]]s followed by dehydration.<ref>{{OrgSynth | |
Maleimide and its derivatives are prepared from [[maleic anhydride]] by treatment with [[amine]]s followed by dehydration.<ref>{{OrgSynth | vauthors = Cava MP, Deana AA, Muth K, Mitchell MJ | title = N-Phenylmaleimide | collvol = 5 | collvolpages = 944 | year = 1973 | prep = cv5p0944}}</ref> A special feature of the reactivity of maleimides is their susceptibility to additions across the double bond either by [[Michael addition]]s or via [[Diels-Alder]] reactions. '''Bismaleimides''' are a class of compounds with two maleimide groups connected by the nitrogen atoms via a linker, and are used as [[crosslinking reagent]]s in [[thermosetting polymer|thermoset polymer]] chemistry. Compounds containing a maleimide group linked with another reactive group, such as an activated [[N-hydroxysuccinimide]] ester, are called '''maleimide heterobifunctional reagents''' {{xref|(see [[Succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate|SMCC reagent]] for such an example)}}.<ref name="Hermanson2013" /> |
||
==Natural maleimides== |
==Natural maleimides== |
||
One natural maleimide is the [[Cytotoxicity|cytotoxic]] [[showdomycin]] from ''[[Streptomyces showdoensis]]'',<ref name="PencolideRef">{{cite journal | vauthors = Birkinshaw JH, Kalyanpur MG, Stickings CE | title = Studies in the biochemistry of micro-organisms. 113. Pencolide, a nitrogen-containing metabolite of Penicillium multicolor Grigorieva-Manilova and Poradielova | journal = The Biochemical Journal | volume = 86 | issue = 2 | pages = 237–243 | date = February 1963 | pmid = 13971137 | pmc = 1201741 | doi = 10.1042/bj0860237 | name-list-style = amp }}</ref> and [[pencolide]] from ''Pe. multicolor''<ref name="PencolideRef" /> – have been reported. [[Farinomalein]] was first isolated in 2009 from the [[entomopathogenic fungus]] ''Isaria farinosa'' (''Paecilomyces farinosus'') – source H599 (Japan).<ref name="DiscoveryTeam">{{cite journal | vauthors = Putri SP, Kinoshita H, Ihara F, Igarashi Y, Nihira T | title = Farinomalein, a maleimide-bearing compound from the entomopathogenic fungus Paecilomyces farinosus | journal = Journal of Natural Products | volume = 72 | issue = 8 | pages = 1544–6 | date = August 2009 | pmid = 19670877 | doi = 10.1021/np9002806 }}</ref> |
|||
==Biotechnology and pharmaceutical applications== |
==Biotechnology and pharmaceutical applications== |
||
Maleimide-mediated methodologies are among the most used in [[bioconjugation]].<ref>{{cite journal | |
Maleimide-mediated methodologies are among the most used in [[bioconjugation]].<ref>{{cite journal | vauthors = Koniev O, Wagner A | title = Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation | journal = Chemical Society Reviews | volume = 44 | issue = 15 | pages = 5495–5551 | date = August 2015 | pmid = 26000775 | doi = 10.1039/C5CS00048C | doi-access = free }}</ref><ref>{{cite journal | vauthors = Francis MB, Carrico IS | title = New frontiers in protein bioconjugation | journal = Current Opinion in Chemical Biology | volume = 14 | issue = 6 | pages = 771–773 | date = December 2010 | pmid = 21112236 | doi = 10.1016/j.cbpa.2010.11.006 }}</ref> Due to fast reactions and high selectivity towards [[cysteine]] residues in [[proteins]], a large variety of maleimide heterobifunctional reagents are used for the preparation of targeted therapeutics, assemblies for studying proteins in their biological context, protein-based microarrays, or proteins immobilisation.<ref>{{cite book | vauthors = Hermanson G | chapter = Chapter 1 - Introduction to Bioconjugation |title=Bioconjugate Techniques | doi = 10.1016/B978-0-12-382239-0.00001-7 |publisher= Elsevier |pages=1–125 |isbn=978-0-12-382239-0|year=2013}}</ref> |
||
For instance |
For instance, [[antibody-drug conjugates]], are constituted of three main components: a [[monoclonal antibody]], a cytotoxic drug, and a linker molecule often containing a maleimide group, which conjugates the drug through thiols or dienes to the antibody.<ref>{{cite journal | vauthors = Beck A, Goetsch L, Dumontet C, Corvaïa N | title = Strategies and challenges for the next generation of antibody-drug conjugates | journal = Nature Reviews. Drug Discovery | volume = 16 | issue = 5 | pages = 315–337 | date = May 2017 | pmid = 28303026 | doi = 10.1038/nrd.2016.268 | s2cid = 22045270 }}</ref><ref name=Lahn/> |
||
Maleimides linked to [[polyethylene glycol]] chains are often used as flexible linking molecules to attach proteins to surfaces. The double bond readily |
Maleimides linked to [[polyethylene glycol]] chains are often used as flexible linking molecules to attach proteins to surfaces. The double bond readily undergoes a retro-Michael reaction with the [[thiol]] group found on [[cysteine]] to form a stable carbon-sulfur bond. Cysteines are often used for site-selective modifications for therapeutic purposes because of the rapid rate of complete bioconjugation with sulfhydryl groups, allowing for higher levels of cytotoxic drug incorporations.<ref name=":1">{{Cite journal |last1=Ravasco |first1=João M. J. M. |last2=Faustino |first2=Hélio |last3=Trindade |first3=Alexandre |last4=Gois |first4=Pedro M. P. |date=2018-11-19 |title=Bioconjugation with Maleimides: A Useful Tool for Chemical Biology |url=http://dx.doi.org/10.1002/chem.201803174 |journal=Chemistry – A European Journal |volume=25 |issue=1 |pages=43–59 |doi=10.1002/chem.201803174 |pmid=30095185 |issn=0947-6539}}</ref> Attaching the other end of the polyethylene chain to a bead or solid support allows for easy separation of protein from other molecules in solution, provided these molecules do not also possess thiol groups. |
||
Maleimide-functionalised polymers and liposomes exhibit enhanced ability to adhere to mucosal surfaces ([[mucoadhesion]]) due to the reactions with thiol-containing mucins.<ref>{{ |
Maleimide-functionalised polymers and liposomes exhibit enhanced ability to adhere to mucosal surfaces ([[mucoadhesion]]) due to the reactions with thiol-containing mucins.<ref>{{cite journal | vauthors = Tonglairoum P, Brannigan RP, Opanasopit P, Khutoryanskiy VV | title = Maleimide-bearing nanogels as novel mucoadhesive materials for drug delivery | journal = Journal of Materials Chemistry B | volume = 4 | issue = 40 | pages = 6581–6587 | date = October 2016 | pmid = 32263701 | doi = 10.1039/C6TB02124G | doi-access = free }}</ref><ref>{{cite journal | vauthors = Kaldybekov DB, Tonglairoum P, Opanasopit P, Khutoryanskiy VV | title = Mucoadhesive maleimide-functionalised liposomes for drug delivery to urinary bladder | journal = European Journal of Pharmaceutical Sciences | volume = 111 | pages = 83–90 | date = January 2018 | pmid = 28958893 | doi = 10.1016/j.ejps.2017.09.039 | s2cid = 35605027 | url = https://centaur.reading.ac.uk/72941/5/Manuscript_Maleimide_liposomes_VKaccepted.pdf }}</ref><ref>{{cite journal | vauthors = Moiseev RV, Kaldybekov DB, Filippov SK, Radulescu A, Khutoryanskiy VV | title = Maleimide-Decorated PEGylated Mucoadhesive Liposomes for Ocular Drug Delivery | journal = Langmuir | volume = 38 | issue = 45 | pages = 13870–13879 | date = November 2022 | pmid = 36327096 | pmc = 9671038 | doi = 10.1021/acs.langmuir.2c02086 }}</ref> This could be applicable in the design of dosage forms for transmucosal drug delivery. |
||
The retro-Michael reactions resulting in maleimide-thiol adducts require precise control. The targeting ability of drugs containing the adducts can be easily hindered or lost due to their instability in vivo.<ref name=":2">{{Cite journal |last1=Huang |first1=Wenmao |last2=Wu |first2=Xin |last3=Gao |first3=Xiang |last4=Yu |first4=Yifei |last5=Lei |first5=Hai |last6=Zhu |first6=Zhenshu |last7=Shi |first7=Yi |last8=Chen |first8=Yulan |last9=Qin |first9=Meng |last10=Wang |first10=Wei |last11=Cao |first11=Yi |date=2019-02-04 |title=Maleimide–thiol adducts stabilized through stretching |url=http://dx.doi.org/10.1038/s41557-018-0209-2 |journal=Nature Chemistry |volume=11 |issue=4 |pages=310–319 |doi=10.1038/s41557-018-0209-2 |pmid=30718898 |issn=1755-4330}}</ref> The instability is mainly attributed to the formation of the thiosuccinimide which might be involved in thiol exchange reaction with glutathione. B-elimination reaction follows, resulting in off-target activity and a loss of efficacy of the drugs.<ref name=Lahn>{{Cite journal |last1=Lahnsteiner |first1=Marianne |last2=Kastner |first2=Alexander |last3=Mayr |first3=Josef |last4=Roller |first4=Alexander |last5=Keppler |first5=Bernhard K. |last6=Kowol |first6=Christian R. |date=2020-10-27 |title=Improving the Stability of Maleimide–Thiol Conjugation for Drug Targeting |url=http://dx.doi.org/10.1002/chem.202003951 |journal=Chemistry – A European Journal |volume=26 |issue=68 |pages=15867–15870 |doi=10.1002/chem.202003951 |pmid=32871016 |issn=0947-6539|pmc=7756610 }}</ref> |
|||
No general method exist for stabilizing thioesters, such as thiosuccinimides, so that their off-target effects can be eliminated in drugs. Problems associated with thiol exchange can be mitigated by hydrolyzing the thiosuccinimide, which prevents elimination of the maleimide-thiol bond. The process of ring-opening hydrolysis requires special catalysts and bases, which may not be biocompatible and lead to harsh conditions. Alternatively, cysteines in the positively charged environment or an electron-withdrawing group enable the thiosuccinimide ring to undergo self-hydrolysis.<ref name=":2"/> |
|||
Another problem with hydrolysis arises if it is applied to ''N''-alkyl-substituted derivatives instead of the N-aryl-substituted derivatives because they hydrolyze at a rate that’s too slow to yield consistently stable adducts.<ref name=Lahn/> |
|||
==Technological applications== |
==Technological applications== |
||
Analogous to [[Styrene maleic anhydride]], [[copolymer]]s of maleimides and [[styrene]] have been commercialized.<ref>{{cite book |doi=10.1002/14356007.a21_615.pub2 |chapter=Polystyrene and Styrene Copolymers |title=Ullmann's Encyclopedia of Industrial Chemistry |date=2007 |last1=Maul |first1=Jürgen |last2=Frushour |first2=Bruce G. |last3=Kontoff |first3=Jeffrey R. |last4=Eichenauer |first4=Herbert |last5=Ott |first5=Karl-Heinz |last6=Schade |first6=Christian |isbn=978-3-527-30385-4 }}</ref> |
|||
| ⚫ | Mono- and bismaleimide-based polymers are used for high temperature applications up to {{cvt|250|C|F|sigfig=2}}.<ref>{{cite journal | journal = Polymer | |
||
| ⚫ | Mono- and bismaleimide-based polymers are used for high temperature applications up to {{cvt|250|C|F|sigfig=2}}.<ref>{{cite journal | journal = Polymer | vauthors = Lin KF, Lin JS, Cheng CH | title = High temperature resins based on allylamine/bismaleimides | volume = 37 | pages = 4729–4737 | year = 1996 | doi = 10.1016/S0032-3861(96)00311-4 | issue = 21|url=http://ntur.lib.ntu.edu.tw/bitstream/246246/93332/1/19.pdf }}</ref> Maleimides linked to rubber chains are often used as flexible linking molecules to reinforce rubber in [[tires]]. The double bond readily reacts with all [[Hydroxyl|hydroxy]], [[amine]] or [[thiol]] groups found on the matrix to form a stable carbon-oxygen, carbon-nitrogen, or carbon-sulfur bond, respectively. These polymers are used in aerospace for high temperature applications of composites. Lockheed Martin's [[Lockheed Martin F-22 Raptor|F-22]] extensively uses thermoset composites, with bismaleimide and toughened epoxy comprising up to 17.5% and 6.6% of the structure by weight respectively.<ref>{{cite journal | vauthors = Anderson WD, Mortara S |date=23-26 April 2007 |title=F-22 Aeroelastic Design and Test Validation |journal=American Institute of Aeronautics and Astronautics (AIAA) |page=4 |doi=10.2514/6.2007-1764 |isbn=978-1-62410-013-0 }}</ref> Lockheed Martin's F-35B (a STOVL version of this US fighter) is reportedly composed of bismaleimide materials, in addition to the use of advanced carbon fiber [[thermoset polymer matrix]] composites.<ref>{{cite web | url=http://www.isciencetimes.com/articles/5929/20130821/lockheed-martin-f35b-ufo-stealth-fighter-video.htm | archive-url = https://web.archive.org/web/20140221074332/http://www.isciencetimes.com/articles/5929/20130821/lockheed-martin-f35b-ufo-stealth-fighter-video.htm | archive-date = 21 February 2014 | title=Lockheed Martin F-35B Boasts UFO Technology, Fights For Team USA | date=21 August 2013 | publisher=International Science Times |access-date=28 January 2014}}</ref> |
||
==See also== |
== See also == |
||
* [[N-Methylmaleimide|''N''-Methylmaleimide]] |
* [[N-Methylmaleimide|''N''-Methylmaleimide]] |
||
* [[Succinimide]] |
* [[Succinimide]] |
||
==References== |
== References == |
||
{{Reflist}} |
{{Reflist}} |
||
==External links== |
== External links== |
||
* [http://www.chm.bris.ac.uk/motm/mpg/ The MP4 website], Molecule of the Month, December 2004 |
* [http://www.chm.bris.ac.uk/motm/mpg/ The MP4 website], Molecule of the Month, December 2004 |
||
Latest revision as of 23:53, 2 November 2024
| |
| |
| Names | |
|---|---|
| IUPAC name
Maleimide
| |
| Preferred IUPAC name
1H-Pyrrole-2,5-dione | |
| Other names
2,5-Pyrroledione
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.990 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H3NO2 | |
| Molar mass | 97.07 g/mol |
| Melting point | 91 to 93 °C (196 to 199 °F; 364 to 366 K) |
| organic solvents | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H301, H314, H317 | |
| P260, P261, P264, P270, P272, P280, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P330, P333+P313, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Maleimide is a chemical compound with the formula H2C2(CO)2NH (see diagram). This unsaturated imide is an important building block in organic synthesis. The name is a contraction of maleic acid and imide, the -C(O)NHC(O)- functional group. Maleimides also describes a class of derivatives of the parent maleimide where the NH group is replaced with alkyl or aryl groups such as a methyl or phenyl, respectively. The substituent can also be a small molecule (such as biotin, a fluorescent dye, an oligosaccharide, or a nucleic acid), a reactive group, or a synthetic polymer such as polyethylene glycol.[1] Human hemoglobin chemically modified with maleimide-polyethylene glycol is a blood substitute called MP4.
Organic chemistry
[edit]Maleimide and its derivatives are prepared from maleic anhydride by treatment with amines followed by dehydration.[2] A special feature of the reactivity of maleimides is their susceptibility to additions across the double bond either by Michael additions or via Diels-Alder reactions. Bismaleimides are a class of compounds with two maleimide groups connected by the nitrogen atoms via a linker, and are used as crosslinking reagents in thermoset polymer chemistry. Compounds containing a maleimide group linked with another reactive group, such as an activated N-hydroxysuccinimide ester, are called maleimide heterobifunctional reagents .[1]
Natural maleimides
[edit]One natural maleimide is the cytotoxic showdomycin from Streptomyces showdoensis,[3] and pencolide from Pe. multicolor[3] – have been reported. Farinomalein was first isolated in 2009 from the entomopathogenic fungus Isaria farinosa (Paecilomyces farinosus) – source H599 (Japan).[4]
Biotechnology and pharmaceutical applications
[edit]Maleimide-mediated methodologies are among the most used in bioconjugation.[5][6] Due to fast reactions and high selectivity towards cysteine residues in proteins, a large variety of maleimide heterobifunctional reagents are used for the preparation of targeted therapeutics, assemblies for studying proteins in their biological context, protein-based microarrays, or proteins immobilisation.[7] For instance, antibody-drug conjugates, are constituted of three main components: a monoclonal antibody, a cytotoxic drug, and a linker molecule often containing a maleimide group, which conjugates the drug through thiols or dienes to the antibody.[8][9]
Maleimides linked to polyethylene glycol chains are often used as flexible linking molecules to attach proteins to surfaces. The double bond readily undergoes a retro-Michael reaction with the thiol group found on cysteine to form a stable carbon-sulfur bond. Cysteines are often used for site-selective modifications for therapeutic purposes because of the rapid rate of complete bioconjugation with sulfhydryl groups, allowing for higher levels of cytotoxic drug incorporations.[10] Attaching the other end of the polyethylene chain to a bead or solid support allows for easy separation of protein from other molecules in solution, provided these molecules do not also possess thiol groups.
Maleimide-functionalised polymers and liposomes exhibit enhanced ability to adhere to mucosal surfaces (mucoadhesion) due to the reactions with thiol-containing mucins.[11][12][13] This could be applicable in the design of dosage forms for transmucosal drug delivery.
The retro-Michael reactions resulting in maleimide-thiol adducts require precise control. The targeting ability of drugs containing the adducts can be easily hindered or lost due to their instability in vivo.[14] The instability is mainly attributed to the formation of the thiosuccinimide which might be involved in thiol exchange reaction with glutathione. B-elimination reaction follows, resulting in off-target activity and a loss of efficacy of the drugs.[9]
No general method exist for stabilizing thioesters, such as thiosuccinimides, so that their off-target effects can be eliminated in drugs. Problems associated with thiol exchange can be mitigated by hydrolyzing the thiosuccinimide, which prevents elimination of the maleimide-thiol bond. The process of ring-opening hydrolysis requires special catalysts and bases, which may not be biocompatible and lead to harsh conditions. Alternatively, cysteines in the positively charged environment or an electron-withdrawing group enable the thiosuccinimide ring to undergo self-hydrolysis.[14]
Another problem with hydrolysis arises if it is applied to N-alkyl-substituted derivatives instead of the N-aryl-substituted derivatives because they hydrolyze at a rate that’s too slow to yield consistently stable adducts.[9]
Technological applications
[edit]Analogous to Styrene maleic anhydride, copolymers of maleimides and styrene have been commercialized.[15]
Mono- and bismaleimide-based polymers are used for high temperature applications up to 250 °C (480 °F).[16] Maleimides linked to rubber chains are often used as flexible linking molecules to reinforce rubber in tires. The double bond readily reacts with all hydroxy, amine or thiol groups found on the matrix to form a stable carbon-oxygen, carbon-nitrogen, or carbon-sulfur bond, respectively. These polymers are used in aerospace for high temperature applications of composites. Lockheed Martin's F-22 extensively uses thermoset composites, with bismaleimide and toughened epoxy comprising up to 17.5% and 6.6% of the structure by weight respectively.[17] Lockheed Martin's F-35B (a STOVL version of this US fighter) is reportedly composed of bismaleimide materials, in addition to the use of advanced carbon fiber thermoset polymer matrix composites.[18]
See also
[edit]References
[edit]- ^ a b Hermanson G (2013). "Chapter 6: Heterobifunctional Crosslinkers". Bioconjugate Techniques. Elsevier. pp. 299–339. doi:10.1016/B978-0-12-382239-0.00006-6. ISBN 978-0-12-382239-0.
- ^ Cava MP, Deana AA, Muth K, Mitchell MJ (1973). "N-Phenylmaleimide". Organic Syntheses; Collected Volumes, vol. 5, p. 944.
- ^ a b Birkinshaw JH, Kalyanpur MG, Stickings CE (February 1963). "Studies in the biochemistry of micro-organisms. 113. Pencolide, a nitrogen-containing metabolite of Penicillium multicolor Grigorieva-Manilova and Poradielova". The Biochemical Journal. 86 (2): 237–243. doi:10.1042/bj0860237. PMC 1201741. PMID 13971137.
- ^ Putri SP, Kinoshita H, Ihara F, Igarashi Y, Nihira T (August 2009). "Farinomalein, a maleimide-bearing compound from the entomopathogenic fungus Paecilomyces farinosus". Journal of Natural Products. 72 (8): 1544–6. doi:10.1021/np9002806. PMID 19670877.
- ^ Koniev O, Wagner A (August 2015). "Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation". Chemical Society Reviews. 44 (15): 5495–5551. doi:10.1039/C5CS00048C. PMID 26000775.
- ^ Francis MB, Carrico IS (December 2010). "New frontiers in protein bioconjugation". Current Opinion in Chemical Biology. 14 (6): 771–773. doi:10.1016/j.cbpa.2010.11.006. PMID 21112236.
- ^ Hermanson G (2013). "Chapter 1 - Introduction to Bioconjugation". Bioconjugate Techniques. Elsevier. pp. 1–125. doi:10.1016/B978-0-12-382239-0.00001-7. ISBN 978-0-12-382239-0.
- ^ Beck A, Goetsch L, Dumontet C, Corvaïa N (May 2017). "Strategies and challenges for the next generation of antibody-drug conjugates". Nature Reviews. Drug Discovery. 16 (5): 315–337. doi:10.1038/nrd.2016.268. PMID 28303026. S2CID 22045270.
- ^ a b c Lahnsteiner, Marianne; Kastner, Alexander; Mayr, Josef; Roller, Alexander; Keppler, Bernhard K.; Kowol, Christian R. (27 October 2020). "Improving the Stability of Maleimide–Thiol Conjugation for Drug Targeting". Chemistry – A European Journal. 26 (68): 15867–15870. doi:10.1002/chem.202003951. ISSN 0947-6539. PMC 7756610. PMID 32871016.
- ^ Ravasco, João M. J. M.; Faustino, Hélio; Trindade, Alexandre; Gois, Pedro M. P. (19 November 2018). "Bioconjugation with Maleimides: A Useful Tool for Chemical Biology". Chemistry – A European Journal. 25 (1): 43–59. doi:10.1002/chem.201803174. ISSN 0947-6539. PMID 30095185.
- ^ Tonglairoum P, Brannigan RP, Opanasopit P, Khutoryanskiy VV (October 2016). "Maleimide-bearing nanogels as novel mucoadhesive materials for drug delivery". Journal of Materials Chemistry B. 4 (40): 6581–6587. doi:10.1039/C6TB02124G. PMID 32263701.
- ^ Kaldybekov DB, Tonglairoum P, Opanasopit P, Khutoryanskiy VV (January 2018). "Mucoadhesive maleimide-functionalised liposomes for drug delivery to urinary bladder" (PDF). European Journal of Pharmaceutical Sciences. 111: 83–90. doi:10.1016/j.ejps.2017.09.039. PMID 28958893. S2CID 35605027.
- ^ Moiseev RV, Kaldybekov DB, Filippov SK, Radulescu A, Khutoryanskiy VV (November 2022). "Maleimide-Decorated PEGylated Mucoadhesive Liposomes for Ocular Drug Delivery". Langmuir. 38 (45): 13870–13879. doi:10.1021/acs.langmuir.2c02086. PMC 9671038. PMID 36327096.
- ^ a b Huang, Wenmao; Wu, Xin; Gao, Xiang; Yu, Yifei; Lei, Hai; Zhu, Zhenshu; Shi, Yi; Chen, Yulan; Qin, Meng; Wang, Wei; Cao, Yi (4 February 2019). "Maleimide–thiol adducts stabilized through stretching". Nature Chemistry. 11 (4): 310–319. doi:10.1038/s41557-018-0209-2. ISSN 1755-4330. PMID 30718898.
- ^ Maul, Jürgen; Frushour, Bruce G.; Kontoff, Jeffrey R.; Eichenauer, Herbert; Ott, Karl-Heinz; Schade, Christian (2007). "Polystyrene and Styrene Copolymers". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a21_615.pub2. ISBN 978-3-527-30385-4.
- ^ Lin KF, Lin JS, Cheng CH (1996). "High temperature resins based on allylamine/bismaleimides" (PDF). Polymer. 37 (21): 4729–4737. doi:10.1016/S0032-3861(96)00311-4.
- ^ Anderson WD, Mortara S (23–26 April 2007). "F-22 Aeroelastic Design and Test Validation". American Institute of Aeronautics and Astronautics (AIAA): 4. doi:10.2514/6.2007-1764. ISBN 978-1-62410-013-0.
- ^ "Lockheed Martin F-35B Boasts UFO Technology, Fights For Team USA". International Science Times. 21 August 2013. Archived from the original on 21 February 2014. Retrieved 28 January 2014.
External links
[edit]- The MP4 website, Molecule of the Month, December 2004
