Peroxymonosulfuric acid: Difference between revisions
removed Category:Oxidizing agents; added Category:Oxidizing acids using HotCat |
alkali metal salts discussion is better suited for our oxone article |
||
| (13 intermediate revisions by 10 users not shown) | |||
| Line 41: | Line 41: | ||
| Density = 2.239 g cm<sup>−3</sup> |
| Density = 2.239 g cm<sup>−3</sup> |
||
| ConjugateBase = [[Peroxomonosulfate]] |
| ConjugateBase = [[Peroxomonosulfate]] |
||
| pKa = 1, 9.3<ref name=P82db>{{cite book|title=Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution|editor-first=D. D.|editor-last=Perrin|edition=2nd|series=[[IUPAC]] Chemical Data|issue=29|publisher=Pergamon|location=Oxford|year=1982|publication-date=1984|orig-date=1969|lccn=82-16524|isbn=0-08-029214-3|at=Entry 176}}</ref> |
|||
| MeltingPt = 45 °C |
| MeltingPt = 45 °C |
||
}} |
}} |
||
| Line 50: | Line 51: | ||
}} |
}} |
||
}} |
}} |
||
'''Peroxymonosulfuric acid''', |
'''Peroxymonosulfuric acid''', also known as '''persulfuric acid''', '''peroxysulfuric acid''' is the [[inorganic compound]] with the formula {{chem2|H2SO5}}. It is a white solid. It is a component of '''Caro's acid''', which is a solution of peroxymonosulfuric acid in [[sulfuric acid]] containing small amounts of water.<ref name=Ullmann/> Peroxymonosulfuric acid is a very strong oxidant ([[Electrode potential|''E''<sup>0</sup>]] = +2.51 V). |
||
==Structure== |
|||
{{Chem|H|2|S|O|5}} is sometimes confused with {{Chem|H|2|S|2|O|8}}, known as [[peroxydisulfuric acid]]. The disulfuric acid, which appears to be more widely used as its alkali metal salts, has the structure HO–S(O)<sub>2</sub>–O–O–S(O)<sub>2</sub>–OH. |
|||
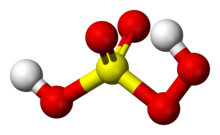
In peroxymonosulfuric acid, the S(VI) center adopts its characteristic tetrahedral geometry; the connectivity is indicated by the formula HO–O–S(O)<sub>2</sub>–OH. The S-O-''H'' proton is more acidic.<ref name=Ullmann>{{cite book |doi=10.1002/14356007.a19_177.pub2 |chapter=Peroxo Compounds, Inorganic |title=Ullmann's Encyclopedia of Industrial Chemistry |date=2007 |last1=Jakob |first1=Harald |last2=Leininger |first2=Stefan |last3=Lehmann |first3=Thomas |last4=Jacobi |first4=Sylvia |last5=Gutewort |first5=Sven |isbn=978-3-527-30673-2 }}</ref> |
|||
<!--what about (HOO)2SO2?, e.g. SO2Cl2 + 2 H2O2 to give the diperoxide?--> |
|||
==History== |
==History== |
||
The German chemist [[Heinrich Caro]] first reported investigations of mixtures of hydrogen peroxide and sulfuric acid.<ref>{{cite journal |first=H. |last=Caro |year=1898 |title=Zur Kenntniss der Oxydation aromatischer Amine |trans-title=[Contribution] to [our] knowledge of the oxidation of aromatic amines |journal=[[Angewandte Chemie|Zeitschrift für angewandte Chemie]] |volume=11 |issue=36 |pages=845–846 |url=http://babel.hathitrust.org/cgi/pt?id=mdp.39015073166319;view=1up;seq=855 |doi=10.1002/ange.18980113602 }}</ref> |
|||
==Synthesis and production== |
==Synthesis and production== |
||
One laboratory scale preparation of Caro's acid involves the combination of [[chlorosulfuric acid]] and [[hydrogen peroxide]]:<ref name="PrepChem">{{Cite web |url=http://www.prepchem.com/synthesis-caros-acid/ |title=Synthesis of Caro's acid |date=2017-02-13 |website=PrepChem.com |language=en-US |access-date=2018-10-12}}</ref> |
|||
:{{chem2 | H2O2 + ClSO2OH <-> H2SO5 + HCl }} |
|||
::{{Chem|H|2|O|2}} + {{Chem|Cl|S|O|2|OH}} ⇌ {{Chem|H|2|S|O|5}} + HCl <ref name="PrepChem">{{Cite web |url=http://www.prepchem.com/synthesis-caros-acid/ |title=Synthesis of Caro's acid |date=2017-02-13 |website=PrepChem.com |language=en-US |access-date=2018-10-12}}</ref> |
|||
Patents include more than one reaction for preparation of Caro's acid, usually as an intermediate for the production of [[Potassium peroxymonosulfate|potassium monopersulfate (PMPS)]], a bleaching and oxidizing agent. One route employs the following reaction:<ref name="Martin patent">{{Citation |title=A method and apparatus for producing a peroxyacid solution |url=https://patents.google.com/patent/WO2005016511A1/da |access-date=2018-10-12}}</ref> |
|||
:{{chem2 | H2O2 + H2SO4 <-> H2SO5 + H2O }} |
|||
::{{Chem|H|2|O|2}} + {{Chem|H|2|S|O|4}} ⇌ {{Chem|H|2|S|O|5}} + {{Chem|H|2|O}}<!-- probably also made electrochemically --> <ref name="Martin patent">{{Citation |title=A method and apparatus for producing a peroxyacid solution |url=https://patents.google.com/patent/WO2005016511A1/da |access-date=2018-10-12}}</ref> |
|||
| ⚫ | |||
| ⚫ | |||
==Uses in industry== |
==Uses in industry== |
||
{{Chem|H|2|S|O|5}} and Caro's acid have been used for a variety of disinfectant and cleaning applications, e.g., swimming pool treatment and denture cleaning. It is used in [[gold mining]] to destroy the [[cyanide]] in the waste stream ("[[Tailings]]"). |
|||
{{Chem|H|2|S|O|5}} has been used for a variety of disinfectant and cleaning applications, e.g., swimming pool treatment and denture cleaning. Alkali metal salts of {{Chem|H|2|S|O|5}} show promise for the [[lignin|delignification]] of wood.<ref>{{cite journal |title=Treatment of softwood kraft pulps with peroxymonosulfate before oxygen delignification |last=Springer |first=E. L. |last2=McSweeny |first2=J. D. |journal=TAPPI Journal |year=1993 |volume=76 |issue=8 |pages=194–199 |url=http://www.tappi.org/Bookstore/Technical-Papers/Journal-Articles/TAPPI-JOURNAL/Archives/1993/August/Treatment-of-softwood-kraft-pulps-with-peroxymonosulfate-before-oxygen-delignification-TAPPI-JOURNA.aspx |issn=0734-1415 |access-date=2011-05-14 |archive-url=https://web.archive.org/web/20110929012348/http://www.tappi.org/Bookstore/Technical-Papers/Journal-Articles/TAPPI-JOURNAL/Archives/1993/August/Treatment-of-softwood-kraft-pulps-with-peroxymonosulfate-before-oxygen-delignification-TAPPI-JOURNA.aspx |archive-date=2011-09-29 |url-status=dead }}</ref> It is also used in laboratories as a last resort in removing organic materials since {{Chem|H|2|S|O|5}} can fully [[oxidize]] any organic materials.{{citation needed|date=June 2020}} |
|||
[[Ammonium]], [[sodium]], and [[potassium]] salts of {{Chem|H|2|S|O|5}} are used in the [[plastics industry]] as [[radical initiator]]s for [[polymerization]]. They are also used as [[etching|etchants]], [[Desizing#Oxidative desizing|oxidative desizing]] agents for textile fabrics, and for decolorizing and deodorizing oils. |
|||
Alkali metal salts of {{Chem|H|2|S|O|5}}, especially [[oxone]], are widely investigated. |
|||
==Hazards== |
==Hazards== |
||
These peroxy acids can be explosive. Explosions have been reported at [[Brown University]]<ref>{{cite journal | last1 = Edwards | first1 = J.O. | journal = [[Chem. Eng. News]] | volume = 33 | pages = 3336 | year = 1955 | doi = 10.1021/cen-v033n032.p3336 | title = Safety | issue = 32}}</ref> and [[Sun Oil]]. As with all strong oxidizing agents, peroxysulfuric acid is incompatible with [[organic compound]]s. |
|||
==See also== |
==See also== |
||
* [[Peroxydisulfuric acid]] |
* [[Peroxydisulfuric acid]] {{Chem|H|2|S|2|O|8}}, |
||
* [[Peroxomonosulfate]] |
* [[Peroxomonosulfate]] |
||
| Line 87: | Line 85: | ||
{{Hydrogen compounds}} |
{{Hydrogen compounds}} |
||
{{Persulfates}} |
|||
{{sulfur compounds}} |
|||
{{DEFAULTSORT:Peroxymonosulfuric Acid}} |
{{DEFAULTSORT:Peroxymonosulfuric Acid}} |
||
| Line 95: | Line 93: | ||
[[Category:Persulfates]] |
[[Category:Persulfates]] |
||
[[Category:Peroxy acids]] |
[[Category:Peroxy acids]] |
||
[[Category:Oxidizing acids]] |
|||
[[Category:Explosive chemicals]] |
[[Category:Explosive chemicals]] |
||
Latest revision as of 21:19, 4 November 2024

| |
| |
| Names | |
|---|---|
| IUPAC names
Peroxysulfuric acid
Sulfuroperoxoic acid[1] | |
| Systematic IUPAC name | |
| Other names
Peroxosulfuric acid[1]
Peroxomonosulfuric acid[citation needed] Persulfuric acid[citation needed] Caro's acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.879 |
| EC Number |
|
| 101039 | |
PubChem CID
|
|
| UNII | |
| UN number | 1483 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H 2SO 5 | |
| Molar mass | 114.078 g mol−1 |
| Appearance | White crystals |
| Density | 2.239 g cm−3 |
| Melting point | 45 °C |
| Acidity (pKa) | 1, 9.3[3] |
| Conjugate base | Peroxomonosulfate |
| Structure | |
| Tetrahedral at S | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
strong oxidizer |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Peroxymonosulfuric acid, also known as persulfuric acid, peroxysulfuric acid is the inorganic compound with the formula H2SO5. It is a white solid. It is a component of Caro's acid, which is a solution of peroxymonosulfuric acid in sulfuric acid containing small amounts of water.[4] Peroxymonosulfuric acid is a very strong oxidant (E0 = +2.51 V).
Structure
[edit]In peroxymonosulfuric acid, the S(VI) center adopts its characteristic tetrahedral geometry; the connectivity is indicated by the formula HO–O–S(O)2–OH. The S-O-H proton is more acidic.[4]
History
[edit]The German chemist Heinrich Caro first reported investigations of mixtures of hydrogen peroxide and sulfuric acid.[5]
Synthesis and production
[edit]One laboratory scale preparation of Caro's acid involves the combination of chlorosulfuric acid and hydrogen peroxide:[6]
- H2O2 + ClSO2OH ⇌ H2SO5 + HCl
Patents include more than one reaction for preparation of Caro's acid, usually as an intermediate for the production of potassium monopersulfate (PMPS), a bleaching and oxidizing agent. One route employs the following reaction:[7]
- H2O2 + H2SO4 ⇌ H2SO5 + H2O
This reaction is related to "piranha solution".
Uses in industry
[edit]H
2SO
5 and Caro's acid have been used for a variety of disinfectant and cleaning applications, e.g., swimming pool treatment and denture cleaning. It is used in gold mining to destroy the cyanide in the waste stream ("Tailings").
Alkali metal salts of H
2SO
5, especially oxone, are widely investigated.
Hazards
[edit]These peroxy acids can be explosive. Explosions have been reported at Brown University[8] and Sun Oil. As with all strong oxidizing agents, peroxysulfuric acid is incompatible with organic compounds.
See also
[edit]References
[edit]- ^ a b c International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 139. Electronic version.
- ^ "Peroxysulfuric acid (CHEBI:29286)". Chemical Entities of Biological Interest. UK: European Bioinformatics Institute. 20 November 2007. Retrieved 17 November 2011.
- ^ Perrin, D. D., ed. (1982) [1969]. Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution. IUPAC Chemical Data (2nd ed.). Oxford: Pergamon (published 1984). Entry 176. ISBN 0-08-029214-3. LCCN 82-16524.
- ^ a b Jakob, Harald; Leininger, Stefan; Lehmann, Thomas; Jacobi, Sylvia; Gutewort, Sven (2007). "Peroxo Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a19_177.pub2. ISBN 978-3-527-30673-2.
- ^ Caro, H. (1898). "Zur Kenntniss der Oxydation aromatischer Amine" [[Contribution] to [our] knowledge of the oxidation of aromatic amines]. Zeitschrift für angewandte Chemie. 11 (36): 845–846. doi:10.1002/ange.18980113602.
- ^ "Synthesis of Caro's acid". PrepChem.com. 2017-02-13. Retrieved 2018-10-12.
- ^ A method and apparatus for producing a peroxyacid solution, retrieved 2018-10-12
- ^ Edwards, J.O. (1955). "Safety". Chem. Eng. News. 33 (32): 3336. doi:10.1021/cen-v033n032.p3336.
