Autoinducer-2: Difference between revisions
Physchim62 (talk | contribs) +CSID |
abbreviation in box |
||
| (53 intermediate revisions by 32 users not shown) | |||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
| Verifiedfields = changed |
|||
| ⚫ | |||
| Watchedfields = changed |
|||
| ⚫ | |||
| verifiedrevid = 458783070 |
|||
| ⚫ | |||
| |
| Name = Autoinducer-2 |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| OtherNames = Dihydroxy[(2S,3R,4S)-2-methyldihydro- |
|||
| ⚫ | |||
| CASNo = |
|||
| Abbreviations = AI-2 |
|||
| ChEMBL = 1230903 |
|||
| KEGG = C16421 |
|||
| PubChem = 446576 |
|||
| ⚫ | |||
| ⚫ | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} |
|||
| ChEBI = 40646 |
|||
| SMILES = O[C@@H]1[C@]2(O[B-](O[C@]2(OC1)C)(O)O)O |
|||
| InChI = 1/C5H10BO7/c1-4-5(8,3(7)2-11-4)13-6(9,10)12-4/h3,7-10H,2H2,1H3/q-1/t3-,4+,5+/m0/s1 |
|||
| InChIKey = ACKRRKSNOOISSG-VPENINKCBL |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/C5H10BO7/c1-4-5(8,3(7)2-11-4)13-6(9,10)12-4/h3,7-10H,2H2,1H3/q-1/t3-,4+,5+/m0/s1 |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = ACKRRKSNOOISSG-VPENINKCSA-N |
|||
}} |
|||
|Section2={{Chembox Properties |
|||
| Formula = C<sub>5</sub>H<sub>10</sub>BO<sub>7</sub> |
|||
| MolarMass = 192.940 |
|||
| Appearance = |
|||
| Density = |
|||
| MeltingPt = |
|||
| BoilingPt = |
|||
| Solubility = |
|||
}} |
|||
|Section3={{Chembox Hazards |
|||
| MainHazards = |
|||
| FlashPt = |
|||
| AutoignitionPt = |
|||
}} |
}} |
||
}} |
}} |
||
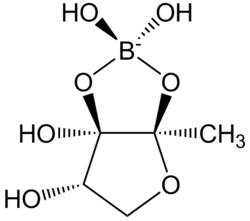
'''Autoinducer-2''' (AI-2) |
'''Autoinducer-2''' ('''AI-2''') is a furanosyl [[borate ester|borate diester]] or tetrahydroxy furan (species dependent) that—as the name suggests—is an [[autoinducer]], a member of a family of [[signaling molecule]]s used in [[quorum sensing]].<ref>{{Cite journal| last1= Cao | first1= Jie-Gang | last2= Meighen | first2= Edward A. | year= 1989 | title= Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi | journal= [[Journal of Biological Chemistry]] | volume= 264 | issue= 36 | pages= 21670–21676 | doi= 10.1016/S0021-9258(20)88238-6 | pmid= 2600086 | doi-access= free }}</ref> AI-2 is one of only a few known [[biomolecules]] incorporating [[boron]]. First identified in the [[marine bacterium]] ''[[Vibrio harveyi]]'', AI-2 is produced and recognized by many [[Gram-negative]] and [[Gram-positive bacteria]].<ref>{{Cite journal| doi= 10.1016/j.molcel.2004.07.020| last1= Miller | first1= Stephen T. | last2= Xavier | first2= Karina B. | last3= Campagna | first3= Shawn R. | last4= Taga | first4= Michiko E. | last5= Semmelhack | first5= Martin F. | last6= Bassler | first6= Bonnie L. | last7= Hughson | first7= Frederick M. | year= 2004 | title= Salmonella typhimurium Recognizes a Chemically Distinct Form of the Bacterial Quorum-Sensing Signal AI-2 | journal= [[Molecular Cell]] | volume= 15 | issue= 5 | pages= 677–687 | pmid= 15350213 | doi-access= free }}</ref><ref>{{Cite journal| doi= 10.1146/annurev.micro.55.1.165| last1= Miller | first1= M. B. | last2= Bassler | first2= B. L. | year= 2001 | title= Quorum sensing in bacteria | journal= [[Annual Review of Microbiology]] | volume= 55 | pages= 165–199 | pmid= 11544353 }}</ref> AI-2 arises by the reaction of [[4,5-Dihydroxy-2,3-pentanedione|4,5-dihydroxy-2,3-pentanedione]], which is produced enzymatically, with [[boric acid]]<ref>{{Cite web|url=http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/misc/AI2.html|title = Chemistry - Queen Mary University of London}}</ref> and is recognized by the two-component sensor kinase LuxPQ in [[Vibrionaceae]]. |
||
| ⚫ | AI-2 is actively transported by the Lsr ABC-type transporter into the cell in [[Enterobacteriaceae]] and few other bacterial taxa such as ''[[Pasteurella]]'', ''[[Photorhabdus]]'', ''[[Haemophilus]]'', and ''[[Bacillus]]'',<ref>{{cite journal|last1=Rezzonico|first1=F.|last2=Smits|first2=T.H.M.|last3=Duffy|first3=B.|title=Detection of AI-2 receptors in genomes of Enterobacteriaceae suggests a role of type-2 quorum sensing in closed ecosystems|journal=Sensors|date=2012|volume=12|issue=5|pages=6645–6665|doi=10.3390/s120506645|pmc=3386761|pmid=22778662|bibcode=2012Senso..12.6645R|doi-access=free}}</ref> where it is phosphorylated by [[LsrK]]. Then, Phospho-AI-2 binds the transcriptional repressor protein, LsrR, which subsequently is released from the promoter/operator region of the lsr operon – and transcription of the ''lsr'' genes is initiated. AI-2 signalling is also regulated by glucose and cAMP/CRP via the ''lsr'' operon. In the presence of glucose, low levels of cAMP/CRP result in almost no ''lsr'' operon (''lsrABCDFG'') expression. Without glucose, cAMP-CRP is needed to stimulate the ''lsr'' expression, while LsrR represses its expression in the absence of the inducer, phospho-AI-2. As AI-2 accumulates, more AI-2 is taken in via LsrABCD, phosphorylated via LsrK, and the ''lsr'' transcription is de-repressed, enabling even more AI-2 uptake.<ref>{{Cite journal| doi= 10.1128/JB.187.6.2066-2076.2005| last1= Wang | first1= Liang | last2= Hashimoto | first2= Yoshifumi | last3= Tsao | first3= Chen-Yu | last4= Valdes | first4= James J. | last5= Bentley | first5= William E. | year= 2005 | title= Cyclic AMP (cAMP) and cAMP Receptor Protein Influence both Synthesis and Uptake of Extracellular Autoinducer 2 in Escherichia coli | journal= [[Journal of Bacteriology]] | volume= 187 | issue= 6 | pages= 2066–2076 | pmid= 15743955| pmc= 1064054 }}</ref> |
||
Doubts have been expressed regarding AI-2's status as a universal signal. Although the ''luxS'' gene, which encodes the protein responsible for AI-2 production is widespread, the latter has mainly a primary metabolic role in the recycling of [[S-Adenosyl methionine|''S''-adenosyl-<small>L</small>-methionine]], with AI-2 being a by-product of that process.<ref>{{Cite journal | last1 = Diggle | first1 = S. P. | last2 = Gardner | first2 = A. | last3 = West | first3 = S. A. | last4 = Griffin | first4 = A. S. | title = Evolutionary theory of bacterial quorum sensing: when is a signal not a signal? | journal = Philosophical Transactions of the Royal Society B: Biological Sciences | volume = 362 | issue = 1483 | pages = 1241–1249 | year = 2007 | doi = 10.1098/rstb.2007.2049 | pmid=17360270 | pmc=2435587}}</ref> An unequivocally AI-2 related behavior was found to be restricted primarily to organisms bearing known AI-2 receptor genes.<ref>{{cite journal|last1=Rezzonico|first1=F.|last2=Duffy|first2=B.|title=Lack of genomic evidence of AI-2 receptors suggests a non-quorum sensing role for luxS in most bacteria|journal=BMC Microbiology|date=2008|volume=8|page=154|pmid=18803868|doi=10.1186/1471-2180-8-154|pmc=2561040 |doi-access=free }}</ref> Thus, while it is certainly true that some bacteria respond to AI-2, it is doubtful that it is always being produced for purposes of signaling. |
|||
| ⚫ | AI-2 is |
||
== Mimicry by host cells == |
|||
Doubts have been expressed regarding AI-2's status as a universal signal. The gene responsible for its production is the widespread ''luxS''; this gene has an important role in the recycling of S-adenosyl-L-methionine, with AI-2 being a metabolic by-product of that process.<ref>{{cite doi|10.1098/rstb.2007.2049}}</ref> While it is certainly true that some bacteria respond to AI-2, it is not yet clear that it is always being produced for purposes of signalling. |
|||
It has been found that in response to bacteria or to disruption of the [[tight junction]], [[mammal|mammalian]] [[epithelium|epithelial]] cells synthesize an AI-2 mimic that triggers quorum sensing and may play an important role in enlisting gut microbes that are known to assist in epithelial healing. In reporting these findings, the researchers write that their results “suggest that [[kingdom (biology)|crosskingdom]] communication occurs between [[eukaryotic]] cells and bacteria via the AI-2 bacterial quorum-sensing system.”<ref> {{cite journal |last1=Ismail |first1=Anisa S. |last2=Valastyan |first2=Julie S. |last3=Bassler |first3=Bonnie L. |date=March 17, 2016 |title= A Host-Produced Autoinducer-2 Mimic Activates Bacterial Quorum Sensing |journal=Cell Host & Microbe |volume=19 |issue=4 |pages=470–480 |doi=10.1016/j.chom.2016.02.020 |pmid=26996306 |pmc=4869860 }}</ref> |
|||
==References== |
== References == |
||
{{Reflist}} |
{{Reflist}} |
||
| Line 24: | Line 59: | ||
[[Category:Cell communication]] |
[[Category:Cell communication]] |
||
[[Category:Cell signaling]] |
[[Category:Cell signaling]] |
||
[[Category: |
[[Category:Tetrahydroxyborate esters]] |
||
[[Category:Boron heterocycles]] |
|||
[[Category:Furans]] |
|||
[[Category:Heterocyclic compounds with 2 rings]] |
|||
Latest revision as of 21:53, 4 November 2024
| |
| Names | |
|---|---|
| Preferred IUPAC name
(3aS,6S,6aR)-2,2,6,6a-Tetrahydroxy-3a-methyltetrahydro-2H-furo[2,3-d][1,3,2]dioxaborol-2-uide | |
| Other names
Dihydroxy[(2S,3R,4S)-2-methyldihydro-
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | AI-2 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H10BO7 | |
| Molar mass | 192.940 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Autoinducer-2 (AI-2) is a furanosyl borate diester or tetrahydroxy furan (species dependent) that—as the name suggests—is an autoinducer, a member of a family of signaling molecules used in quorum sensing.[1] AI-2 is one of only a few known biomolecules incorporating boron. First identified in the marine bacterium Vibrio harveyi, AI-2 is produced and recognized by many Gram-negative and Gram-positive bacteria.[2][3] AI-2 arises by the reaction of 4,5-dihydroxy-2,3-pentanedione, which is produced enzymatically, with boric acid[4] and is recognized by the two-component sensor kinase LuxPQ in Vibrionaceae.
AI-2 is actively transported by the Lsr ABC-type transporter into the cell in Enterobacteriaceae and few other bacterial taxa such as Pasteurella, Photorhabdus, Haemophilus, and Bacillus,[5] where it is phosphorylated by LsrK. Then, Phospho-AI-2 binds the transcriptional repressor protein, LsrR, which subsequently is released from the promoter/operator region of the lsr operon – and transcription of the lsr genes is initiated. AI-2 signalling is also regulated by glucose and cAMP/CRP via the lsr operon. In the presence of glucose, low levels of cAMP/CRP result in almost no lsr operon (lsrABCDFG) expression. Without glucose, cAMP-CRP is needed to stimulate the lsr expression, while LsrR represses its expression in the absence of the inducer, phospho-AI-2. As AI-2 accumulates, more AI-2 is taken in via LsrABCD, phosphorylated via LsrK, and the lsr transcription is de-repressed, enabling even more AI-2 uptake.[6]
Doubts have been expressed regarding AI-2's status as a universal signal. Although the luxS gene, which encodes the protein responsible for AI-2 production is widespread, the latter has mainly a primary metabolic role in the recycling of S-adenosyl-L-methionine, with AI-2 being a by-product of that process.[7] An unequivocally AI-2 related behavior was found to be restricted primarily to organisms bearing known AI-2 receptor genes.[8] Thus, while it is certainly true that some bacteria respond to AI-2, it is doubtful that it is always being produced for purposes of signaling.
Mimicry by host cells
[edit]It has been found that in response to bacteria or to disruption of the tight junction, mammalian epithelial cells synthesize an AI-2 mimic that triggers quorum sensing and may play an important role in enlisting gut microbes that are known to assist in epithelial healing. In reporting these findings, the researchers write that their results “suggest that crosskingdom communication occurs between eukaryotic cells and bacteria via the AI-2 bacterial quorum-sensing system.”[9]
References
[edit]- ^ Cao, Jie-Gang; Meighen, Edward A. (1989). "Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi". Journal of Biological Chemistry. 264 (36): 21670–21676. doi:10.1016/S0021-9258(20)88238-6. PMID 2600086.
- ^ Miller, Stephen T.; Xavier, Karina B.; Campagna, Shawn R.; Taga, Michiko E.; Semmelhack, Martin F.; Bassler, Bonnie L.; Hughson, Frederick M. (2004). "Salmonella typhimurium Recognizes a Chemically Distinct Form of the Bacterial Quorum-Sensing Signal AI-2". Molecular Cell. 15 (5): 677–687. doi:10.1016/j.molcel.2004.07.020. PMID 15350213.
- ^ Miller, M. B.; Bassler, B. L. (2001). "Quorum sensing in bacteria". Annual Review of Microbiology. 55: 165–199. doi:10.1146/annurev.micro.55.1.165. PMID 11544353.
- ^ "Chemistry - Queen Mary University of London".
- ^ Rezzonico, F.; Smits, T.H.M.; Duffy, B. (2012). "Detection of AI-2 receptors in genomes of Enterobacteriaceae suggests a role of type-2 quorum sensing in closed ecosystems". Sensors. 12 (5): 6645–6665. Bibcode:2012Senso..12.6645R. doi:10.3390/s120506645. PMC 3386761. PMID 22778662.
- ^ Wang, Liang; Hashimoto, Yoshifumi; Tsao, Chen-Yu; Valdes, James J.; Bentley, William E. (2005). "Cyclic AMP (cAMP) and cAMP Receptor Protein Influence both Synthesis and Uptake of Extracellular Autoinducer 2 in Escherichia coli". Journal of Bacteriology. 187 (6): 2066–2076. doi:10.1128/JB.187.6.2066-2076.2005. PMC 1064054. PMID 15743955.
- ^ Diggle, S. P.; Gardner, A.; West, S. A.; Griffin, A. S. (2007). "Evolutionary theory of bacterial quorum sensing: when is a signal not a signal?". Philosophical Transactions of the Royal Society B: Biological Sciences. 362 (1483): 1241–1249. doi:10.1098/rstb.2007.2049. PMC 2435587. PMID 17360270.
- ^ Rezzonico, F.; Duffy, B. (2008). "Lack of genomic evidence of AI-2 receptors suggests a non-quorum sensing role for luxS in most bacteria". BMC Microbiology. 8: 154. doi:10.1186/1471-2180-8-154. PMC 2561040. PMID 18803868.
- ^ Ismail, Anisa S.; Valastyan, Julie S.; Bassler, Bonnie L. (March 17, 2016). "A Host-Produced Autoinducer-2 Mimic Activates Bacterial Quorum Sensing". Cell Host & Microbe. 19 (4): 470–480. doi:10.1016/j.chom.2016.02.020. PMC 4869860. PMID 26996306.
