Norbaeocystin: Difference between revisions
Updating {{drugbox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'DrugBank_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Wikipedia talk:WikiProject |
m Open access bot: doi updated in citation with #oabot. |
||
| (36 intermediate revisions by 26 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound}} |
|||
{{Drugbox |
{{Drugbox |
||
| verifiedrevid = 447124257 |
| verifiedrevid = 447124257 |
||
| IUPAC_name = 3-(2-ammonioethyl)-1''H''-indol-4-yl hydrogen phosphate |
| IUPAC_name = 3-(2-ammonioethyl)-1''H''-indol-4-yl hydrogen phosphate |
||
| image = Norbaeocystin. |
| image = Norbaeocystin.svg |
||
| alt = Skeletal formula of norbaeocystin |
|||
| width = |
| width = 170 |
||
| image2 = Norbaeocystin zwitterion 3D spacefill.png |
|||
| width2 = 190 |
|||
| alt2 = Space-filling model of the norbaeocystin molecule as a zwitterion |
|||
<!--Clinical data--> |
<!--Clinical data--> |
||
| Line 13: | Line 18: | ||
| legal_CA = |
| legal_CA = |
||
| legal_UK = |
| legal_UK = |
||
| legal_US = Schedule I |
| legal_US = Schedule I |
||
| legal_US_comment = by analogue |
|||
| legal_status = |
| legal_status = |
||
| routes_of_administration = Oral |
| routes_of_administration = Oral |
||
| class = [[Serotonin receptor agonist]]; Non-[[hallucinogen]]ic [[serotonin]] [[5-HT2A receptor|5-HT<sub>2A</sub> receptor]] [[agonist]] |
|||
<!--Pharmacokinetic data--> |
<!--Pharmacokinetic data--> |
||
| Line 25: | Line 32: | ||
<!--Identifiers--> |
<!--Identifiers--> |
||
| CAS_number = |
| CAS_number = 21420-59-7 |
||
| ATC_prefix = none |
| ATC_prefix = none |
||
| ATC_suffix = |
| ATC_suffix = |
||
| PubChem = |
| PubChem = 9795063 |
||
| ⚫ | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| ⚫ | |||
| UNII = C8CA0W9FJ9 |
|||
| KEGG = C21778 |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| ChEBI = 139479 |
|||
| synonyms = 4-Phosphoryloxytryptamine; 4-PO-T; 4-Hydroxytryptamine 4-phosphate; 4-Hydroxytryptamine phosphate |
|||
<!--Chemical data--> |
<!--Chemical data--> |
||
| C=10 | H=13 | N=2 | O=4 | P=1 |
| C=10 | H=13 | N=2 | O=4 | P=1 |
||
| SMILES = NCCc1c[nH]c2cccc(OP(=O)(O)O)c12 |
|||
| molecular_weight = 256.19 g/mol |
|||
| ⚫ | |||
| smiles = [O-]P(O)(=O)Oc2cccc1nc(cc12)CC[NH3+] |
|||
| ⚫ | |||
| InChI = 1/C10H13N2O4P/c11-5-4-7-6-8-9(12-7)2-1-3-10(8)16-17(13,14)15/h1-3,6,12H,4-5,11H2,(H2,13,14,15) |
|||
| InChIKey = CUBFNIQZGIWBFC-UHFFFAOYAR |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| ⚫ | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| ⚫ | |||
| melting_point = |
|||
| melting_high = |
|||
}} |
}} |
||
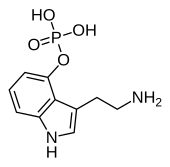
'''Norbaeocystin''', also known as '''4-phosphoryloxytryptamine''' ('''4-PO-T'''), is a [[psilocybin mushroom]] [[alkaloid]] and [[analog (chemistry)|analog]] of [[psilocybin]]. It is found as a minor compound in most [[psilocybin mushroom]]s together with [[psilocin]], [[psilocybin]], [[aeruginascin]], and [[baeocystin]], from which it is a derivative.<ref name="Leung_1968">{{cite journal | vauthors = Leung AY, Paul AG | title = Baeocystin and norbaeocystin: new analogs of psilocybin from Psilocybe baeocystis | journal = Journal of Pharmaceutical Sciences | volume = 57 | issue = 10 | pages = 1667–1671 | date = October 1968 | pmid = 5684732 | doi = 10.1002/jps.2600571007 }}</ref><ref>{{cite journal | vauthors = Gotvaldová K, Borovička J, Hájková K, Cihlářová P, Rockefeller A, Kuchař M | title = Extensive Collection of Psychotropic Mushrooms with Determination of Their Tryptamine Alkaloids | journal = International Journal of Molecular Sciences | volume = 23 | issue = 22 | pages = 14068 | date = November 2022 | pmid = 36430546 | doi = 10.3390/ijms232214068 | pmc = 9693126 | doi-access = free }}</ref> |
|||
'''Norbaeocystin''' is a [[mushroom]] [[alkaloid]] and [[analog (chemistry)|analog]] of the [[psychedelic]] [[Psychedelics, dissociatives and deliriants|hallucinogenic drug]] [[psilocybin]]. It is found as a minor compound in most psilocybin mushrooms together with [[psilocin]], [[psilocybin]] and [[baeocystin]]. |
|||
Norbaeocystin is an ''N''-demethylated derivative of baeocystin (itself an ''N''-demethylated derivative of psilocybin), and a phosphorylated derivative of [[4-hydroxytryptamine]] (4-HT). The latter is notable as a positional isomer of [[serotonin]], which is 5-hydroxytryptamine. |
|||
Norbaeocystin is thought to be a [[prodrug]] of 4-HT, analogously to how psilocybin is a prodrug of psilocin and baeocystin is thought to be a prodrug of [[norpsilocin]].<ref name="RakoczyRungeSen2024">{{cite journal | vauthors = Rakoczy RJ, Runge GN, Sen AK, Sandoval O, Wells HG, Nguyen Q, Roberts BR, Sciortino JH, Gibbons WJ, Friedberg LM, Jones JA, McMurray MS | title = Pharmacological and behavioural effects of tryptamines present in psilocybin-containing mushrooms | journal = Br J Pharmacol | volume = 181 | issue = 19 | pages = 3627–3641 | date = October 2024 | pmid = 38825326 | doi = 10.1111/bph.16466 | url = | doi-access = free }}</ref><ref name="SherwoodHalberstadtKlein2020">{{cite journal | vauthors = Sherwood AM, Halberstadt AL, Klein AK, McCorvy JD, Kaylo KW, Kargbo RB, Meisenheimer P | title = Synthesis and Biological Evaluation of Tryptamines Found in Hallucinogenic Mushrooms: Norbaeocystin, Baeocystin, Norpsilocin, and Aeruginascin | journal = J Nat Prod | volume = 83 | issue = 2 | pages = 461–467 | date = February 2020 | pmid = 32077284 | doi = 10.1021/acs.jnatprod.9b01061 | url = }}</ref> 4-HT is a [[potency (pharmacology)|potent]] and [[central nervous system|centrally penetrant]] serotonin [[5-HT2A receptor|5-HT<sub>2A</sub> receptor]] [[agonist]] and also interacts with other [[serotonin receptor]]s.<ref name="RakoczyRungeSen2024" /> In spite of this however, 4-HT and norbaeocystin do not produce the [[head-twitch response]], a behavioral proxy of [[serotonergic psychedelic|psychedelic]] effects, in animals, and hence are putatively non-[[hallucinogen]]ic.<ref name="RakoczyRungeSen2024" /><ref name="SherwoodHalberstadtKlein2020" /> The reasons for this are unknown, but may be due to [[β-arrestin2]]-preferring [[biased agonist|biased agonism]] of the serotonin 5-HT<sub>2A</sub> receptor.<ref name="RakoczyRungeSen2024" /> |
|||
== See also == |
== See also == |
||
* [[Aeruginascin]] |
|||
* [[Baeocystin]] |
* [[Baeocystin]] |
||
* [[ |
* [[Norpsilocin]] |
||
* [[Psilocybin]] |
* [[Psilocybin]] |
||
== References == |
== References == |
||
{{Reflist |
{{Reflist}} |
||
{{Hallucinogens}} |
|||
{{Serotonin receptor modulators}} |
|||
{{Tryptamines}} |
{{Tryptamines}} |
||
[[Category: |
[[Category:Experimental non-hallucinogens]] |
||
[[Category: |
[[Category:Non-hallucinogenic 5-HT2A receptor agonists]] |
||
[[Category:Organophosphates]] |
[[Category:Organophosphates]] |
||
[[Category:Prodrugs]] |
|||
[[Category:Psychedelic tryptamines]] |
|||
[[Category:Tryptamine alkaloids]] |
|||
{{ |
{{Psychoactive-stub}} |
||
[[de:Norbaeocystin]] |
|||
[[fr:Norbaeocystine]] |
|||
[[fi:Norbaeosystiini]] |
|||
[[sv:Norbaeocystin]] |
|||
Latest revision as of 17:10, 6 November 2024
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 4-Phosphoryloxytryptamine; 4-PO-T; 4-Hydroxytryptamine 4-phosphate; 4-Hydroxytryptamine phosphate |
| Routes of administration | Oral |
| Drug class | Serotonin receptor agonist; Non-hallucinogenic serotonin 5-HT2A receptor agonist |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C10H13N2O4P |
| Molar mass | 256.198 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Norbaeocystin, also known as 4-phosphoryloxytryptamine (4-PO-T), is a psilocybin mushroom alkaloid and analog of psilocybin. It is found as a minor compound in most psilocybin mushrooms together with psilocin, psilocybin, aeruginascin, and baeocystin, from which it is a derivative.[1][2]
Norbaeocystin is an N-demethylated derivative of baeocystin (itself an N-demethylated derivative of psilocybin), and a phosphorylated derivative of 4-hydroxytryptamine (4-HT). The latter is notable as a positional isomer of serotonin, which is 5-hydroxytryptamine.
Norbaeocystin is thought to be a prodrug of 4-HT, analogously to how psilocybin is a prodrug of psilocin and baeocystin is thought to be a prodrug of norpsilocin.[3][4] 4-HT is a potent and centrally penetrant serotonin 5-HT2A receptor agonist and also interacts with other serotonin receptors.[3] In spite of this however, 4-HT and norbaeocystin do not produce the head-twitch response, a behavioral proxy of psychedelic effects, in animals, and hence are putatively non-hallucinogenic.[3][4] The reasons for this are unknown, but may be due to β-arrestin2-preferring biased agonism of the serotonin 5-HT2A receptor.[3]
See also
[edit]References
[edit]- ^ Leung AY, Paul AG (October 1968). "Baeocystin and norbaeocystin: new analogs of psilocybin from Psilocybe baeocystis". Journal of Pharmaceutical Sciences. 57 (10): 1667–1671. doi:10.1002/jps.2600571007. PMID 5684732.
- ^ Gotvaldová K, Borovička J, Hájková K, Cihlářová P, Rockefeller A, Kuchař M (November 2022). "Extensive Collection of Psychotropic Mushrooms with Determination of Their Tryptamine Alkaloids". International Journal of Molecular Sciences. 23 (22): 14068. doi:10.3390/ijms232214068. PMC 9693126. PMID 36430546.
- ^ a b c d Rakoczy RJ, Runge GN, Sen AK, Sandoval O, Wells HG, Nguyen Q, Roberts BR, Sciortino JH, Gibbons WJ, Friedberg LM, Jones JA, McMurray MS (October 2024). "Pharmacological and behavioural effects of tryptamines present in psilocybin-containing mushrooms". Br J Pharmacol. 181 (19): 3627–3641. doi:10.1111/bph.16466. PMID 38825326.
- ^ a b Sherwood AM, Halberstadt AL, Klein AK, McCorvy JD, Kaylo KW, Kargbo RB, Meisenheimer P (February 2020). "Synthesis and Biological Evaluation of Tryptamines Found in Hallucinogenic Mushrooms: Norbaeocystin, Baeocystin, Norpsilocin, and Aeruginascin". J Nat Prod. 83 (2): 461–467. doi:10.1021/acs.jnatprod.9b01061. PMID 32077284.
