Acyl-CoA: Difference between revisions
m Task 18 (cosmetic): eval 6 templates: hyphenate params (2×); |
|||
| (32 intermediate revisions by 18 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Group of coenzymes that metabolize fatty acids}} |
|||
[[File:Acyl-CoA2.svg|thumb|right|General chemical structure of an acyl-CoA, where R is a |
[[File:Acyl-CoA2.svg|thumb|right|General chemical structure of an acyl-CoA, where R is a carboxylic acid side chain]] |
||
'''Acyl-CoA''' is a group of [[coenzyme]]s that metabolize [[ |
'''Acyl-CoA''' is a group of [[coenzyme A|CoA]]-based [[coenzyme]]s that metabolize [[carboxylic acid]]s. [[Fatty acyl-CoA]]'s are susceptible to [[beta oxidation]], forming, ultimately, [[acetyl-CoA]]. The acetyl-CoA enters the [[citric acid cycle]], eventually forming several equivalents of [[Adenosine triphosphate|ATP]]. In this way, fats are converted to ATP, the common biochemical energy carrier. |
||
==Functions== |
==Functions== |
||
===Fatty acid activation=== |
===Fatty acid activation=== |
||
Fats are broken down by conversion to acyl-CoA. This conversion is one response to high energy demands such as exercise.<ref name=":0">{{Citation|last1=Talley|first1=Jacob T.|title=Biochemistry, Fatty Acid Oxidation|date=2020|url=http://www.ncbi.nlm.nih.gov/books/NBK556002/|work=StatPearls|place=Treasure Island (FL)|publisher=StatPearls Publishing|pmid=32310462|access-date=2021-02-23|last2=Mohiuddin|first2=Shamim S.}}</ref> |
|||
The oxidative degradation of fatty acids is a two-step process, catalyzed by [[Long-chain-fatty-acid—CoA ligase|acyl-CoA synthetase]]. First, the fatty acid reacts with ATP to form an acyl phosphate. This intermediate reacts subsequently to give acyl-CoA: |
|||
The oxidative degradation of fatty acids is a two-step process, catalyzed by [[Long-chain-fatty-acid—CoA ligase|acyl-CoA synthetase]].<ref>{{Cite journal |last1=Grevengoed |first1=Trisha J. |last2=Klett |first2=Eric L. |last3=Coleman |first3=Rosalind A. |date=2014-07-17 |title=Acyl-CoA Metabolism and Partitioning |journal=Annual Review of Nutrition |language=en |volume=34 |issue=1 |pages=1–30 |doi=10.1146/annurev-nutr-071813-105541 |issn=0199-9885 |pmc=5881898 |pmid=24819326}}</ref> Fatty acids are converted to their acyl phosphate, the precursor to acyl-CoA. The latter conversion is mediated by acyl-CoA synthase" |
|||
: |
:acyl-P + HS-CoA → acyl-S-CoA + P<sub>i</sub> + H<sup>+</sup> |
||
| ⚫ | Three types of acyl-CoA synthases are employed, depending on the chain length of the fatty acid.<ref>{{cite book |doi=10.1016/B978-0-12-803550-4.00015-X |chapter=Lipid Metabolism |title=Medical Biochemistry |pages=325–365 |year=2017 |last1=Blanco |first1=Antonio |last2=Blanco |first2=Gustavo |isbn=978-0-12-803550-4 }}</ref> For example, the substrates for medium chain acyl-CoA synthase are 4-11 carbon fatty acids.<ref name="Bhagavan et al 2015">{{cite book |doi=10.1016/B978-0-12-416687-5.00016-6 |chapter=Lipids I: Fatty Acids and Eicosanoids |title=Essentials of Medical Biochemistry |pages=269–297 |year=2015 |last1=Bhagavan |first1=N.V. |last2=Ha |first2=Chung-Eun |isbn=978-0-12-416687-5 }}</ref> The enzyme acyl-CoA thioesterase takes of the acyl-CoA to form a free fatty acid and coenzyme A.<ref name="Bhagavan et al 2015" /> |
||
Fatty acids are activated in the cytosol, but oxidation occurs in the mitochondria. Because there is no transport protein for CoA adducts, acyl groups must enter the mitochondria via a shuttle system involving the small molecule [[carnitine]].<ref>Pratt C.W., Cornely, K. ''Essential Biochemistry''. John Wiley & Sons, Inc. (2004){{page needed|date=May 2019}}</ref> |
|||
=== Beta oxidation of acyl-CoA === |
|||
| ⚫ | |||
The second step of fatty acid degradation is beta oxidation. Beta oxidation occurs in mitochondria.<ref name=":1">{{Cite web|title=Fatty acid beta oxidation {{!}} Abcam|url=https://www.abcam.com/pathways/fatty-acid-oxidation|access-date=2021-02-23|website=www.abcam.com}}</ref> After formation in the cytosol, acyl-CoA is transported into the mitochondria, the location of beta oxidation. Transport of acyl-CoA into the mitochondria requires [[Carnitine palmitoyltransferase I|carnitine palmitoyltransferase 1]] (CPT1), which converts acyl-CoA into acylcarnitine, which gets transported into the mitochondrial matrix.<ref name=":0" /> Once in the matrix, acylcarnitine is converted back to acyl-CoA by CPT2.<ref name=":1" /> Beta oxidation may begin now that Acyl-CoA is in the mitochondria. |
|||
Beta oxidation of acyl-CoA occurs in four steps. |
|||
| ⚫ | |||
| ⚫ | Heart muscle primarily metabolizes fat for energy and Acyl-CoA metabolism has been identified<ref>{{cite journal |last1=Goldenberg |first1=Joseph R. |last2=Carley |first2=Andrew N. |last3=Ji |first3=Ruiping |last4=Zhang |first4=Xiaokan |last5=Fasano |first5=Matt |last6=Schulze |first6=P. Christian |last7=Lewandowski |first7=E. Douglas |title=Preservation of Acyl-CoA Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking |journal=Circulation |volume=139 |issue=24 |pages=2765–2777 |date=26 March 2019 |doi=10.1161/CIRCULATIONAHA.119.039610 |pmid=30909726 |pmc=6557671 |
||
1. [[Acyl-CoA dehydrogenase]] catalyzes dehydrogenation of the acyl-CoA, creating a double bond between the alpha and beta carbons.<ref name=":2">{{Cite web|date=2016-02-26|title=6.11: Fatty Acid Oxidation|url=https://bio.libretexts.org/Bookshelves/Biochemistry/Book%3A_Biochemistry_Free_and_Easy_(Ahern_and_Rajagopal)/06%3A_Metabolism_I_-_Oxidative_Reductive_Processes/6.11%3A_Fatty_Acid_Oxidation|access-date=2021-02-23|website=Biology LibreTexts|language=en}}</ref> [[Flavin adenine dinucleotide|FAD]] is the hydrogen acceptor, yielding FADH2.<ref name=":3">{{Cite web|date=2017-06-03|title=Beta Oxidation|url=https://biologydictionary.net/beta-oxidation/|access-date=2021-02-23|website=Biology Dictionary|language=en-US}}</ref> |
|||
| ⚫ | Cellular acyl-CoA content correlates with insulin resistance, suggesting that it can mediate lipotoxicity in non-adipose tissues.<ref>{{cite journal |last1=Li |first1=Lei O. |last2=Klett |first2=Eric L. |last3=Coleman |first3=Rosalind A. |title=Acyl-CoA synthesis, lipid metabolism and lipotoxicity |journal=Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids |date=March 2010 |volume=1801 |issue=3 |pages=246–251 |doi=10.1016/j.bbalip.2009.09.024 |pmid=19818872 |pmc=2824076 }}</ref> Acyl-CoA: diacylglycerol acyltransferase (DGAT) plays an important role in energy metabolism on account of key enzyme in triglyceride biosynthesis. The synthetic role of DGAT in adipose tissue such as the liver and the intestine, sites where endogenous levels of its activity and triglyceride synthesis are high and comparatively clear. Also, any changes in the activity levels might cause changes in systemic insulin sensitivity and energy homeostasis.<ref>{{cite journal |last1=Yu |first1= |
||
2. [[Enoyl-CoA hydrase]] catalyzes the addition of water across the newly formed double bond to make an alcohol.<ref name=":1" /><ref name=":2" /> |
|||
| ⚫ | A rare disease called multiple acyl-CoA dehydrogenase deficiency (MADD)<ref name="Rashmi et al 2017">{{cite journal |last1=Rashmi |first1=S. |last2=Gayathri |first2=N. |last3=Kumar |first3=M. Veerendra |last4=Sumanth |first4=S. |last5=Subasree |first5=R. |last6=Pooja |first6=M. |title=Multiple Acyl CoA dehydrogenase deficiency: Uncommon yet treatable disorder |journal=Neurology India |date=1 January 2017 |volume=65 |issue=1 |pages=177–8 |doi=10.4103/0028-3886.198186 |pmid=28084266 |doi- |
||
3. [[3-hydroxyacyl-CoA dehydrogenase]] oxidizes the alcohol group to a ketone.<ref name=":1" /> NADH is produced from [[Nicotinamide adenine dinucleotide|NAD+]].<ref name=":2" /> |
|||
4. [[HADHB|Thiolase]] cleaves between the [[alpha carbon]] and ketone to release one molecule of [[Acetyl-CoA]] and the Acyl-CoA which is now 2 carbons shorter.<ref name=":2" /> |
|||
This four step process repeats until acyl-CoA has removed all carbons from the chain, leaving only Acetyl-CoA. During one cycle of beta oxidation, Acyl-CoA creates one molecule of Acetyl-CoA, FADH2, and NADH.<ref name=":3" /> Acetyl-CoA is then used in the [[citric acid cycle]] while FADH2 and NADH are sent to the [[electron transport chain]].<ref name=":4">{{Cite web|title=6.32 Fatty Acid Oxidation (Beta-oxidation) {{!}} Nutrition Flexbook|url=https://courses.lumenlearning.com/suny-nutrition/chapter/6-32-fatty-acid-oxidation-beta-oxidation/|access-date=2021-02-23|website=courses.lumenlearning.com}}</ref> These intermediates all end up providing energy for the body as they are ultimately converted to [[Adenosine triphosphate|ATP]].<ref name=":4" /> |
|||
[[File:Beta Oxidation Process.png|alt=The process of Beta Oxidation of an activated Acyl-CoA molecule.|thumb|Example of Beta Oxidation using Stearic Acid]] |
|||
Beta oxidation, as well as alpha-oxidation, also occurs in the [[peroxisome]].<ref name=":0" /> The peroxisome handles beta oxidation of fatty acids that have more than 20 carbons in their chain because the peroxisome contains [[Very long-chain acyl-CoA synthetase|very-long-chain Acyl-CoA synthetases]].<ref name=":5">{{Cite journal|last1=Reddy|first1=Janardan K|last2=Hashimoto|first2=Takashi|date=2001-07-01|title=PEROXISOMAL β-OXIDATION AND PEROXISOME PROLIFERATOR–ACTIVATED RECEPTOR α: An Adaptive Metabolic System|url=https://www.annualreviews.org/doi/10.1146/annurev.nutr.21.1.193|journal=Annual Review of Nutrition|volume=21|issue=1|pages=193–230|doi=10.1146/annurev.nutr.21.1.193|pmid=11375435|issn=0199-9885}}</ref> These enzymes are better equipped to oxidize Acyl-CoA with long chains that the mitochondria cannot handle. |
|||
==== Example using stearic acid ==== |
|||
Beta oxidation removes 2 carbons at a time, so in the oxidation of an 18 carbon fatty acid such as [[Stearic acid|Stearic Acid]] 8 cycles will need to occur to completely break down Acyl-CoA.<ref name=":5" /> This will produce 9 Acetyl-CoA that have 2 carbons each, 8 FADH2, and 8 NADH. |
|||
| ⚫ | |||
| ⚫ | Heart muscle primarily metabolizes fat for energy and Acyl-CoA metabolism has been identified<ref>{{cite journal |last1=Goldenberg |first1=Joseph R. |last2=Carley |first2=Andrew N. |last3=Ji |first3=Ruiping |last4=Zhang |first4=Xiaokan |last5=Fasano |first5=Matt |last6=Schulze |first6=P. Christian |last7=Lewandowski |first7=E. Douglas |title=Preservation of Acyl-CoA Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking |journal=Circulation |volume=139 |issue=24 |pages=2765–2777 |date=26 March 2019 |doi=10.1161/CIRCULATIONAHA.119.039610 |pmid=30909726 |pmc=6557671}} |
||
*{{lay source |template=cite news |author=Marti Leitch |title=Scientists find metabolic target to prevent, treat heart failure at earliest stage |url=https://news.osu.edu/scientists-find-metabolic-target-to-prevent-treat-heart-failure-at-earliest-stage |date=March 26, 2019 |work=Ohio State News}}</ref> as a critical molecule in early stage heart muscle pump failure. |
|||
| ⚫ | Cellular acyl-CoA content correlates with insulin resistance, suggesting that it can mediate lipotoxicity in non-adipose tissues.<ref>{{cite journal |last1=Li |first1=Lei O. |last2=Klett |first2=Eric L. |last3=Coleman |first3=Rosalind A. |title=Acyl-CoA synthesis, lipid metabolism and lipotoxicity |journal=Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids |date=March 2010 |volume=1801 |issue=3 |pages=246–251 |doi=10.1016/j.bbalip.2009.09.024 |pmid=19818872 |pmc=2824076 }}</ref> Acyl-CoA: diacylglycerol acyltransferase (DGAT) plays an important role in energy metabolism on account of key enzyme in triglyceride biosynthesis. The synthetic role of DGAT in adipose tissue such as the liver and the intestine, sites where endogenous levels of its activity and triglyceride synthesis are high and comparatively clear. Also, any changes in the activity levels might cause changes in systemic insulin sensitivity and energy homeostasis.<ref>{{cite journal |last1=Yu |first1=Yi-Hao |last2=Ginsberg |first2=Henry |title=The role of acyl-CoA:diacylglycerol acyltransferase (DGAT) in energy metabolism |journal=Annals of Medicine |date=8 July 2009 |volume=36 |issue=4 |pages=252–261 |doi=10.1080/07853890410028429 |pmid=15224651 |s2cid=9174481 |doi-access=free }}</ref> |
||
| ⚫ | A rare disease called multiple acyl-CoA dehydrogenase deficiency (MADD)<ref name="Rashmi et al 2017">{{cite journal |last1=Rashmi |first1=S. |last2=Gayathri |first2=N. |last3=Kumar |first3=M. Veerendra |last4=Sumanth |first4=S. |last5=Subasree |first5=R. |last6=Pooja |first6=M. |title=Multiple Acyl CoA dehydrogenase deficiency: Uncommon yet treatable disorder |journal=Neurology India |date=1 January 2017 |volume=65 |issue=1 |pages=177–8 |doi=10.4103/0028-3886.198186 |pmid=28084266 |doi-access=free | url=https://neurologyindia.com/article.asp?issn=0028-3886;year=2017;volume=65;issue=1;spage=177;epage=178;aulast=Pooja}}</ref> is a fatty acid metabolism disorder. Acyl-CoA is important because this enzyme helps make Acyl-CoA from free fatty acids, and this activates the fatty acid to be metabolized. This compromised fatty acid oxidation leads to many different symptoms, including severe symptoms such as cardiomyopathy and liver disease and mild symptoms such as episodic metabolic decomposition, muscle weakness and respiratory failure. MADD is a genetic disorder, caused by a mutation in the ETFA, ETFB, and ETFDH genes. MADD is known as an "autosomal recessive disorder"<ref name="Rashmi et al 2017"/> because for one to get this disorder, one must receive this recessive gene from both parents. |
||
==See also== |
==See also== |
||
| Line 25: | Line 46: | ||
* [[Acyl CoA dehydrogenase]] |
* [[Acyl CoA dehydrogenase]] |
||
* [[Fatty acid metabolism]] |
* [[Fatty acid metabolism]] |
||
* [[Fatty acyl-CoA esters]] |
|||
==References== |
==References== |
||
| Line 33: | Line 55: | ||
{{Fatty-acid metabolism intermediates}} |
{{Fatty-acid metabolism intermediates}} |
||
{{portal bar|Metabolism}} |
|||
[[Category:Metabolism]] |
[[Category:Metabolism]] |
||
Latest revision as of 22:29, 7 November 2024

Acyl-CoA is a group of CoA-based coenzymes that metabolize carboxylic acids. Fatty acyl-CoA's are susceptible to beta oxidation, forming, ultimately, acetyl-CoA. The acetyl-CoA enters the citric acid cycle, eventually forming several equivalents of ATP. In this way, fats are converted to ATP, the common biochemical energy carrier.
Functions
[edit]Fatty acid activation
[edit]Fats are broken down by conversion to acyl-CoA. This conversion is one response to high energy demands such as exercise.[1] The oxidative degradation of fatty acids is a two-step process, catalyzed by acyl-CoA synthetase.[2] Fatty acids are converted to their acyl phosphate, the precursor to acyl-CoA. The latter conversion is mediated by acyl-CoA synthase"
- acyl-P + HS-CoA → acyl-S-CoA + Pi + H+
Three types of acyl-CoA synthases are employed, depending on the chain length of the fatty acid.[3] For example, the substrates for medium chain acyl-CoA synthase are 4-11 carbon fatty acids.[4] The enzyme acyl-CoA thioesterase takes of the acyl-CoA to form a free fatty acid and coenzyme A.[4]
Beta oxidation of acyl-CoA
[edit]The second step of fatty acid degradation is beta oxidation. Beta oxidation occurs in mitochondria.[5] After formation in the cytosol, acyl-CoA is transported into the mitochondria, the location of beta oxidation. Transport of acyl-CoA into the mitochondria requires carnitine palmitoyltransferase 1 (CPT1), which converts acyl-CoA into acylcarnitine, which gets transported into the mitochondrial matrix.[1] Once in the matrix, acylcarnitine is converted back to acyl-CoA by CPT2.[5] Beta oxidation may begin now that Acyl-CoA is in the mitochondria.
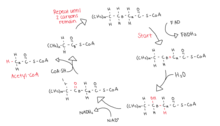
Beta oxidation of acyl-CoA occurs in four steps.
1. Acyl-CoA dehydrogenase catalyzes dehydrogenation of the acyl-CoA, creating a double bond between the alpha and beta carbons.[6] FAD is the hydrogen acceptor, yielding FADH2.[7]
2. Enoyl-CoA hydrase catalyzes the addition of water across the newly formed double bond to make an alcohol.[5][6]
3. 3-hydroxyacyl-CoA dehydrogenase oxidizes the alcohol group to a ketone.[5] NADH is produced from NAD+.[6]
4. Thiolase cleaves between the alpha carbon and ketone to release one molecule of Acetyl-CoA and the Acyl-CoA which is now 2 carbons shorter.[6]
This four step process repeats until acyl-CoA has removed all carbons from the chain, leaving only Acetyl-CoA. During one cycle of beta oxidation, Acyl-CoA creates one molecule of Acetyl-CoA, FADH2, and NADH.[7] Acetyl-CoA is then used in the citric acid cycle while FADH2 and NADH are sent to the electron transport chain.[8] These intermediates all end up providing energy for the body as they are ultimately converted to ATP.[8]
Beta oxidation, as well as alpha-oxidation, also occurs in the peroxisome.[1] The peroxisome handles beta oxidation of fatty acids that have more than 20 carbons in their chain because the peroxisome contains very-long-chain Acyl-CoA synthetases.[9] These enzymes are better equipped to oxidize Acyl-CoA with long chains that the mitochondria cannot handle.
Example using stearic acid
[edit]Beta oxidation removes 2 carbons at a time, so in the oxidation of an 18 carbon fatty acid such as Stearic Acid 8 cycles will need to occur to completely break down Acyl-CoA.[9] This will produce 9 Acetyl-CoA that have 2 carbons each, 8 FADH2, and 8 NADH.
Clinical significance
[edit]Heart muscle primarily metabolizes fat for energy and Acyl-CoA metabolism has been identified[10] as a critical molecule in early stage heart muscle pump failure.
Cellular acyl-CoA content correlates with insulin resistance, suggesting that it can mediate lipotoxicity in non-adipose tissues.[11] Acyl-CoA: diacylglycerol acyltransferase (DGAT) plays an important role in energy metabolism on account of key enzyme in triglyceride biosynthesis. The synthetic role of DGAT in adipose tissue such as the liver and the intestine, sites where endogenous levels of its activity and triglyceride synthesis are high and comparatively clear. Also, any changes in the activity levels might cause changes in systemic insulin sensitivity and energy homeostasis.[12]
A rare disease called multiple acyl-CoA dehydrogenase deficiency (MADD)[13] is a fatty acid metabolism disorder. Acyl-CoA is important because this enzyme helps make Acyl-CoA from free fatty acids, and this activates the fatty acid to be metabolized. This compromised fatty acid oxidation leads to many different symptoms, including severe symptoms such as cardiomyopathy and liver disease and mild symptoms such as episodic metabolic decomposition, muscle weakness and respiratory failure. MADD is a genetic disorder, caused by a mutation in the ETFA, ETFB, and ETFDH genes. MADD is known as an "autosomal recessive disorder"[13] because for one to get this disorder, one must receive this recessive gene from both parents.
See also
[edit]- Acetyl-CoA
- Beta oxidation
- Coenzyme A
- Acyl CoA dehydrogenase
- Fatty acid metabolism
- Fatty acyl-CoA esters
References
[edit]- ^ a b c Talley, Jacob T.; Mohiuddin, Shamim S. (2020), "Biochemistry, Fatty Acid Oxidation", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 32310462, retrieved 2021-02-23
- ^ Grevengoed, Trisha J.; Klett, Eric L.; Coleman, Rosalind A. (2014-07-17). "Acyl-CoA Metabolism and Partitioning". Annual Review of Nutrition. 34 (1): 1–30. doi:10.1146/annurev-nutr-071813-105541. ISSN 0199-9885. PMC 5881898. PMID 24819326.
- ^ Blanco, Antonio; Blanco, Gustavo (2017). "Lipid Metabolism". Medical Biochemistry. pp. 325–365. doi:10.1016/B978-0-12-803550-4.00015-X. ISBN 978-0-12-803550-4.
- ^ a b Bhagavan, N.V.; Ha, Chung-Eun (2015). "Lipids I: Fatty Acids and Eicosanoids". Essentials of Medical Biochemistry. pp. 269–297. doi:10.1016/B978-0-12-416687-5.00016-6. ISBN 978-0-12-416687-5.
- ^ a b c d "Fatty acid beta oxidation | Abcam". www.abcam.com. Retrieved 2021-02-23.
- ^ a b c d "6.11: Fatty Acid Oxidation". Biology LibreTexts. 2016-02-26. Retrieved 2021-02-23.
- ^ a b "Beta Oxidation". Biology Dictionary. 2017-06-03. Retrieved 2021-02-23.
- ^ a b "6.32 Fatty Acid Oxidation (Beta-oxidation) | Nutrition Flexbook". courses.lumenlearning.com. Retrieved 2021-02-23.
- ^ a b Reddy, Janardan K; Hashimoto, Takashi (2001-07-01). "PEROXISOMAL β-OXIDATION AND PEROXISOME PROLIFERATOR–ACTIVATED RECEPTOR α: An Adaptive Metabolic System". Annual Review of Nutrition. 21 (1): 193–230. doi:10.1146/annurev.nutr.21.1.193. ISSN 0199-9885. PMID 11375435.
- ^ Goldenberg, Joseph R.; Carley, Andrew N.; Ji, Ruiping; Zhang, Xiaokan; Fasano, Matt; Schulze, P. Christian; Lewandowski, E. Douglas (26 March 2019). "Preservation of Acyl-CoA Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking". Circulation. 139 (24): 2765–2777. doi:10.1161/CIRCULATIONAHA.119.039610. PMC 6557671. PMID 30909726.
- Lay summary in: Marti Leitch (March 26, 2019). "Scientists find metabolic target to prevent, treat heart failure at earliest stage". Ohio State News.
- ^ Li, Lei O.; Klett, Eric L.; Coleman, Rosalind A. (March 2010). "Acyl-CoA synthesis, lipid metabolism and lipotoxicity". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1801 (3): 246–251. doi:10.1016/j.bbalip.2009.09.024. PMC 2824076. PMID 19818872.
- ^ Yu, Yi-Hao; Ginsberg, Henry (8 July 2009). "The role of acyl-CoA:diacylglycerol acyltransferase (DGAT) in energy metabolism". Annals of Medicine. 36 (4): 252–261. doi:10.1080/07853890410028429. PMID 15224651. S2CID 9174481.
- ^ a b Rashmi, S.; Gayathri, N.; Kumar, M. Veerendra; Sumanth, S.; Subasree, R.; Pooja, M. (1 January 2017). "Multiple Acyl CoA dehydrogenase deficiency: Uncommon yet treatable disorder". Neurology India. 65 (1): 177–8. doi:10.4103/0028-3886.198186. PMID 28084266.
External links
[edit]- Acyl+Coenzyme+A at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
