Cannizzaro reaction: Difference between revisions
one of several possible meanings of "potash", and KOH is inconsistent with the next sentence Undid revision 863032338 by 117.211.38.144 (talk) |
m Reverted edit by 103.122.62.181 (talk) to last version by Troposphere hermit |
||
| (32 intermediate revisions by 22 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Organic chemical reaction}} |
|||
{{Reactionbox |
{{Reactionbox |
||
| Name = Cannizzaro reaction |
| Name = Cannizzaro reaction |
||
| Line 8: | Line 9: | ||
}} |
}} |
||
}} |
}} |
||
The '''Cannizzaro reaction''', named after its discoverer [[Stanislao Cannizzaro]], is a [[chemical reaction]] |
The '''Cannizzaro reaction''', named after its discoverer [[Stanislao Cannizzaro]], is a [[chemical reaction]] which involves the [[base (chemistry)|base]]-induced [[disproportionation]] of two molecules of a non-[[enol]]izable [[aldehyde]] to give a [[primary alcohol]] and a [[carboxylic acid]].<ref>{{cite journal |
||
| author = Cannizzaro, S. |
| author = Cannizzaro, S. |
||
| title = Ueber den der Benzoësäure entsprechenden Alkohol |
| title = Ueber den der Benzoësäure entsprechenden Alkohol |
||
| Line 29: | Line 31: | ||
| doi = 10.1002/jlac.18540900211 |
| doi = 10.1002/jlac.18540900211 |
||
}}</ref> |
}}</ref> |
||
:[[Image:Cannizzaro_reaction-benzaldehyde.svg|center]] |
|||
:[[Image:Cannizzarro Übersichtsreaktion-v8.svg|500px|center]] |
|||
| ⚫ | Cannizzaro first accomplished this transformation in 1853, when he obtained [[benzyl alcohol]] and [[potassium benzoate]] from the treatment of benzaldehyde with [[potash]] (potassium carbonate). More typically, the reaction would be conducted with [[sodium hydroxide |
||
| ⚫ | Cannizzaro first accomplished this transformation in 1853, when he obtained [[benzyl alcohol]] and [[potassium benzoate]] from the treatment of benzaldehyde with [[potash]] (potassium carbonate). More typically, the reaction would be conducted with [[sodium hydroxide]] or [[potassium hydroxide]], giving the sodium or potassium [[carboxylate]] salt of the carboxylic-acid product: |
||
:2 C<sub>6</sub>H<sub>5</sub>CHO + KOH → C<sub>6</sub>H<sub>5</sub>CH<sub>2</sub>OH + C<sub>6</sub>H<sub>5</sub>COOK |
:2 C<sub>6</sub>H<sub>5</sub>CHO + KOH → C<sub>6</sub>H<sub>5</sub>CH<sub>2</sub>OH + C<sub>6</sub>H<sub>5</sub>COOK |
||
The [[ |
The process is a [[redox]] reaction involving transfer of a [[hydride]] from one substrate molecule to the other: one aldehyde is oxidized to form the acid, the other is reduced to form the alcohol.<ref>Geissman, T. A. "The Cannizzaro Reaction" ''Org. React.'' '''1944''', ''2'', 94. {{doi|10.1002/0471264180.or002.03}}(Review)</ref> |
||
==Mechanism== |
==Mechanism== |
||
[[File:C-R startAnimGif.gif|thumb|left|Animated reaction mechanism]] |
|||
The reaction involves a [[nucleophilic acyl substitution]] on an aldehyde, with the [[leaving group]] concurrently attacking another aldehyde in the second step. First, hydroxide attacks a carbonyl. The resulting [[tetrahedral intermediate]] then collapses, re-forming the carbonyl and transferring [[hydride]] to attack another carbonyl.<ref>{{March6th}}</ref> In the final step of the reaction, the acid and alkoxide ions formed exchange a proton. |
The reaction involves a [[nucleophilic acyl substitution]] on an aldehyde, with the [[leaving group]] concurrently attacking another aldehyde in the second step. First, hydroxide attacks a carbonyl. The resulting [[tetrahedral intermediate]] then collapses, re-forming the carbonyl and transferring [[hydride]] to attack another carbonyl.<ref>{{March6th}}</ref> In the final step of the reaction, the acid and alkoxide ions formed exchange a proton. |
||
In the presence of a very high concentration of base, the aldehyde first forms a doubly charged anion from which a hydride ion is transferred to the second molecule of aldehyde to form carboxylate and alkoxide ions. Subsequently, the alkoxide ion acquires a proton from the solvent. |
In the presence of a very high concentration of base, the aldehyde first forms a doubly charged anion from which a hydride ion is transferred to the second molecule of aldehyde to form carboxylate and alkoxide ions. Subsequently, the alkoxide ion acquires a proton from the solvent. |
||
{{clear-left}} |
|||
[[File:Cannizzaro reaction mechanism.svg|center]] |
|||
Overall, the reaction follows third-order kinetics. It is second order in aldehyde and first order in base: |
Overall, the reaction follows third-order kinetics. It is second order in aldehyde and first order in base: |
||
: rate = k[RCHO]<sup>2</sup>[OH<sup>−</sup>] |
|||
At very high base a second path (k') becomes important that is second order in base: |
At very high base a second path (k') becomes important that is second order in base: |
||
: rate = k[RCHO]<sup>2</sup>[OH<sup>−</sup>] + k'[RCHO]<sup>2</sup>[OH<sup>−</sup>]<sup>2</sup> |
|||
The k' pathway implicates a reaction between the doubly charged anion (RCHO<sub>2</sub><sup>2−</sup>) and the aldehyde. The direct transfer of hydride ion is evident from the observation that the recovered alcohol does not contain any deuterium attached to the α-carbon when the reaction is performed in the presence of D<sub>2</sub>O. |
The k' pathway implicates a reaction between the doubly charged anion (RCHO<sub>2</sub><sup>2−</sup>) and the aldehyde. The direct transfer of hydride ion is evident from the observation that the recovered alcohol does not contain any deuterium attached to the α-carbon when the reaction is performed in the presence of D<sub>2</sub>O. |
||
==Scope== |
==Scope== |
||
Due to the strongly [[alkaline]] reaction conditions, aldehydes that have alpha hydrogen atom(s) instead undergo deprotonation there, leading to [[enolate]]s and possible [[aldol reaction]]s. Under ideal conditions the reaction produces |
Due to the strongly [[alkaline]] reaction conditions, aldehydes that have alpha hydrogen atom(s) instead undergo deprotonation there, leading to [[enolate]]s and possible [[aldol reaction]]s. Under ideal conditions the reaction produces 50% of both the alcohol and the carboxylic acid (it takes two aldehydes to produce one acid and one alcohol).<ref>{{OrgSynth|author=W. C. Wilson|year=1941|title=2-Furancarboxylic Acid and 2-Furylcarbinol|collvol=1| collvolpages=276|prep=CV1P0276}}</ref> This can be economically viable if the products can be separated and both have a value; the commercial conversion of [[furfural]] into [[furfuryl alcohol]] and [[2-furoic acid]] is an example of this.<ref name="MariscalMaireles-Torres2016">{{cite journal|last1=Mariscal|first1=R.|last2=Maireles-Torres|first2=P.|last3=Ojeda|first3=M.|last4=Sádaba|first4=I.|last5=López Granados|first5=M.|title=Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels|journal=Energy Environ. Sci.|volume=9|issue=4|year=2016|pages=1144–1189|issn=1754-5692|doi=10.1039/C5EE02666K|url=https://digital.csic.es/bitstream/10261/184700/1/Mariscal_Furfural-A%20renewable_versatile_2016_postprint.pdf|hdl=10261/184700|s2cid=101343477 |hdl-access=free}}</ref> Alternatively, higher yields of one product (usually the alcohol) can be achieved in the '''crossed Cannizzaro reaction''', in which a sacrificial aldehyde is used in combination with a more valuable chemical. In this variation, the reductant is [[formaldehyde]], which is oxidized to [[formic acid|sodium formate]] and the other aldehyde chemical is reduced to the alcohol. Thus, the yield of the valuable chemical is high, although the [[atom economy]] can be low. The final stage in the synthesis of [[pentaerythritol]] is an example. |
||
[[File:Pentaerythritol Synthesis.svg|600px|center]] |
|||
| ⚫ | A solvent-free reaction has been reported involving grinding liquid 2-chlorobenzaldehyde with [[potassium hydroxide]] in a [[mortar and pestle]]:<ref>''A Facile Solvent-Free Cannizzaro Reaction'' Phonchaiya, Sonthi; Panijpan, Bhinyo Rajviroongit, Shuleewan; Wright, Tony; Blanchfield, Joanne T. "A Facile Solvent-Free Cannizzaro Reaction" [[J. Chem. Educ.]] '''2009''', volume 86, page 85. {{ |
||
| ⚫ | A solvent-free reaction has been reported involving grinding liquid 2-chlorobenzaldehyde with [[potassium hydroxide]] in a [[mortar and pestle]]:<ref>''A Facile Solvent-Free Cannizzaro Reaction'' Phonchaiya, Sonthi; Panijpan, Bhinyo Rajviroongit, Shuleewan; Wright, Tony; Blanchfield, Joanne T. "A Facile Solvent-Free Cannizzaro Reaction" [[J. Chem. Educ.]] '''2009''', volume 86, page 85. {{doi|10.1021/ed086p85}}</ref> |
||
[[Image:Cannizzaro reaction Phonchaiya 2009.svg|Solvent-free Cannizzaro reaction|center]] |
[[Image:Cannizzaro reaction Phonchaiya 2009.svg|Solvent-free Cannizzaro reaction|center]] |
||
| Line 56: | Line 62: | ||
==Variations== |
==Variations== |
||
In the [[Tishchenko reaction]], the base used is an [[alkoxide]] rather than hydroxide, and the product is an [[ester]] rather than the separate alcohol and carboxylate groups. After the nucleophilic base attacks an aldehyde, the resulting new oxygen anion attacks another aldehyde to give a hemiacetal linkage between two of the formerly aldehyde-containing reactants rather than undergoing tetrahedral collapse. Eventually tetrahedral collapse does occur, giving the stable ester product. |
In the [[Tishchenko reaction]], the base used is an [[alkoxide]] rather than hydroxide, and the product is an [[ester]] rather than the separate alcohol and carboxylate groups. After the nucleophilic base attacks an aldehyde, the resulting new oxygen anion attacks another aldehyde to give a hemiacetal linkage between two of the formerly aldehyde-containing reactants rather than undergoing tetrahedral collapse. Eventually tetrahedral collapse does occur, giving the stable ester product. |
||
:[[File:Tishchenko reaction.svg| |
:[[File:Tishchenko reaction.svg|400px]] |
||
Certain [[ketone]]s can undergo a Cannizzaro-type reaction, transferring one of their two carbon groups rather than the hydride that would be present on an aldehyde.<ref>{{cite journal |title= Efficient synthesis of tetradecafluoro-4-phenylheptan-4-ol by a Cannizzaro-type reaction and application of the alcohol as a bulky Martin ligand variant for a new anti-apicophilic phosphorane |first1= Xin-Dong |last1= Jiang |first2= Shiro |last2= Matsukawa |first3= Ken-ichiro |last3= Kakuda |first4= Yuta |last4= Fukuzaki |first5= Wei-Li |last5= Zhao |first6= Lin-Song |last6= Li |first7= Huai-Bin |last7= Shen |first8= Satoshi |last8= Kojim |first9= Yohsuke |last9= Yamamoto |journal= Dalton Trans. |year= 2010 |volume= 39 |pages= 9823–9829 |doi= 10.1039/C0DT00539H }}</ref> |
Certain [[ketone]]s can undergo a Cannizzaro-type reaction, transferring one of their two carbon groups rather than the hydride that would be present on an aldehyde.<ref>{{cite journal |title= Efficient synthesis of tetradecafluoro-4-phenylheptan-4-ol by a Cannizzaro-type reaction and application of the alcohol as a bulky Martin ligand variant for a new anti-apicophilic phosphorane |first1= Xin-Dong |last1= Jiang |first2= Shiro |last2= Matsukawa |first3= Ken-ichiro |last3= Kakuda |first4= Yuta |last4= Fukuzaki |first5= Wei-Li |last5= Zhao |first6= Lin-Song |last6= Li |first7= Huai-Bin |last7= Shen |first8= Satoshi |last8= Kojim |first9= Yohsuke |last9= Yamamoto |journal= Dalton Trans. |year= 2010 |volume= 39 |issue= 41 |pages= 9823–9829 |doi= 10.1039/C0DT00539H |pmid= 20859600 }}</ref> |
||
==See also== |
==See also== |
||
*[[Formose reaction]] - slow self-reaction of formaldehyde in hydroxide to form aldose sugars |
|||
*[[Meerwein–Ponndorf–Verley reduction]] |
|||
*[[Benzoin condensation]] - self-reaction of aldehydes to give α-hydroxy ketones |
|||
*[[Oppenauer oxidation]] |
|||
*[[Meerwein–Ponndorf–Verley reduction]] and [[Oppenauer oxidation]] - related interconversions of ketones and secondary alcohols via disproportionations |
|||
==References== |
==References== |
||
{{Reflist}} |
{{Reflist}} |
||
{{Organic reactions}} |
|||
[[Category:Organic redox reactions]] |
[[Category:Organic redox reactions]] |
||
[[Category:Name reactions]] |
[[Category:Name reactions]] |
||
Latest revision as of 01:53, 14 November 2024
| Cannizzaro reaction | |
|---|---|
| Named after | Stanislao Cannizzaro |
| Reaction type | Organic redox reaction |
| Identifiers | |
| Organic Chemistry Portal | cannizzaro-reaction |
| RSC ontology ID | RXNO:0000218 |
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction which involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to give a primary alcohol and a carboxylic acid.[1][2]
Cannizzaro first accomplished this transformation in 1853, when he obtained benzyl alcohol and potassium benzoate from the treatment of benzaldehyde with potash (potassium carbonate). More typically, the reaction would be conducted with sodium hydroxide or potassium hydroxide, giving the sodium or potassium carboxylate salt of the carboxylic-acid product:
- 2 C6H5CHO + KOH → C6H5CH2OH + C6H5COOK
The process is a redox reaction involving transfer of a hydride from one substrate molecule to the other: one aldehyde is oxidized to form the acid, the other is reduced to form the alcohol.[3]
Mechanism
[edit]
The reaction involves a nucleophilic acyl substitution on an aldehyde, with the leaving group concurrently attacking another aldehyde in the second step. First, hydroxide attacks a carbonyl. The resulting tetrahedral intermediate then collapses, re-forming the carbonyl and transferring hydride to attack another carbonyl.[4] In the final step of the reaction, the acid and alkoxide ions formed exchange a proton. In the presence of a very high concentration of base, the aldehyde first forms a doubly charged anion from which a hydride ion is transferred to the second molecule of aldehyde to form carboxylate and alkoxide ions. Subsequently, the alkoxide ion acquires a proton from the solvent.

Overall, the reaction follows third-order kinetics. It is second order in aldehyde and first order in base:
- rate = k[RCHO]2[OH−]
At very high base a second path (k') becomes important that is second order in base:
- rate = k[RCHO]2[OH−] + k'[RCHO]2[OH−]2
The k' pathway implicates a reaction between the doubly charged anion (RCHO22−) and the aldehyde. The direct transfer of hydride ion is evident from the observation that the recovered alcohol does not contain any deuterium attached to the α-carbon when the reaction is performed in the presence of D2O.
Scope
[edit]Due to the strongly alkaline reaction conditions, aldehydes that have alpha hydrogen atom(s) instead undergo deprotonation there, leading to enolates and possible aldol reactions. Under ideal conditions the reaction produces 50% of both the alcohol and the carboxylic acid (it takes two aldehydes to produce one acid and one alcohol).[5] This can be economically viable if the products can be separated and both have a value; the commercial conversion of furfural into furfuryl alcohol and 2-furoic acid is an example of this.[6] Alternatively, higher yields of one product (usually the alcohol) can be achieved in the crossed Cannizzaro reaction, in which a sacrificial aldehyde is used in combination with a more valuable chemical. In this variation, the reductant is formaldehyde, which is oxidized to sodium formate and the other aldehyde chemical is reduced to the alcohol. Thus, the yield of the valuable chemical is high, although the atom economy can be low. The final stage in the synthesis of pentaerythritol is an example.

A solvent-free reaction has been reported involving grinding liquid 2-chlorobenzaldehyde with potassium hydroxide in a mortar and pestle:[7]
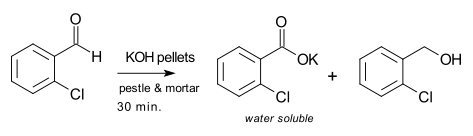
Variations
[edit]In the Tishchenko reaction, the base used is an alkoxide rather than hydroxide, and the product is an ester rather than the separate alcohol and carboxylate groups. After the nucleophilic base attacks an aldehyde, the resulting new oxygen anion attacks another aldehyde to give a hemiacetal linkage between two of the formerly aldehyde-containing reactants rather than undergoing tetrahedral collapse. Eventually tetrahedral collapse does occur, giving the stable ester product.
Certain ketones can undergo a Cannizzaro-type reaction, transferring one of their two carbon groups rather than the hydride that would be present on an aldehyde.[8]
See also
[edit]- Formose reaction - slow self-reaction of formaldehyde in hydroxide to form aldose sugars
- Benzoin condensation - self-reaction of aldehydes to give α-hydroxy ketones
- Meerwein–Ponndorf–Verley reduction and Oppenauer oxidation - related interconversions of ketones and secondary alcohols via disproportionations
References
[edit]- ^ Cannizzaro, S. (1853). "Ueber den der Benzoësäure entsprechenden Alkohol" [On the alcohol corresponding to benzoic acid]. Liebigs Annalen der Chemie und Pharmacie. 88: 129–130. doi:10.1002/jlac.18530880114.
- ^ List, K.; Limpricht, H. (1854). "Ueber das sogenannte Benzoëoxyd und einige andere gepaarte Verbindungen" [On so-called benzoic oxide and some other paired compounds]. Liebigs Annalen der Chemie und Pharmacie. 90 (2): 190–210. doi:10.1002/jlac.18540900211.
- ^ Geissman, T. A. "The Cannizzaro Reaction" Org. React. 1944, 2, 94. doi:10.1002/0471264180.or002.03(Review)
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ W. C. Wilson (1941). "2-Furancarboxylic Acid and 2-Furylcarbinol". Organic Syntheses; Collected Volumes, vol. 1, p. 276.
- ^ Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. (2016). "Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels" (PDF). Energy Environ. Sci. 9 (4): 1144–1189. doi:10.1039/C5EE02666K. hdl:10261/184700. ISSN 1754-5692. S2CID 101343477.
- ^ A Facile Solvent-Free Cannizzaro Reaction Phonchaiya, Sonthi; Panijpan, Bhinyo Rajviroongit, Shuleewan; Wright, Tony; Blanchfield, Joanne T. "A Facile Solvent-Free Cannizzaro Reaction" J. Chem. Educ. 2009, volume 86, page 85. doi:10.1021/ed086p85
- ^ Jiang, Xin-Dong; Matsukawa, Shiro; Kakuda, Ken-ichiro; Fukuzaki, Yuta; Zhao, Wei-Li; Li, Lin-Song; Shen, Huai-Bin; Kojim, Satoshi; Yamamoto, Yohsuke (2010). "Efficient synthesis of tetradecafluoro-4-phenylheptan-4-ol by a Cannizzaro-type reaction and application of the alcohol as a bulky Martin ligand variant for a new anti-apicophilic phosphorane". Dalton Trans. 39 (41): 9823–9829. doi:10.1039/C0DT00539H. PMID 20859600.


