Ruthenium: Difference between revisions
Johnjbarton (talk | contribs) Undid revision 1224955338 by 2603:8080:D03:89D4:7407:136:2E86:68AA (talk) |
Citation bot (talk | contribs) Added doi. | Use this bot. Report bugs. | Suggested by Headbomb | Linked from Wikipedia:WikiProject_Academic_Journals/Journals_cited_by_Wikipedia/Sandbox | #UCB_webform_linked 461/565 |
||
| (33 intermediate revisions by 11 users not shown) | |||
| Line 3: | Line 3: | ||
{{infobox ruthenium|phase=solid}} |
{{infobox ruthenium|phase=solid}} |
||
'''Ruthenium''' is a [[chemical element]]; it has [[Symbol (chemistry)|symbol]] '''Ru''' and [[atomic number]] 44. It is a rare [[transition metal]] belonging to the [[platinum group]] of the [[periodic table]]. Like the other metals of the platinum group, ruthenium is unreactive to most |
'''Ruthenium''' is a [[chemical element]]; it has [[Symbol (chemistry)|symbol]] '''Ru''' and [[atomic number]] 44. It is a rare [[transition metal]] belonging to the [[platinum group]] of the [[periodic table]]. Like the other metals of the platinum group, ruthenium is unreactive to most chemicals. [[Karl Ernst Claus]], a Russian scientist of Baltic-German ancestry, discovered the element in 1844 at [[Kazan State University]] and named it in honor of [[Russian Empire|Russia]], using the Latin name ''[[Ruthenia]]''. Ruthenium is usually found as a minor component of [[platinum]] ores; the annual production has risen from about 19 [[tonne]]s in 2009<ref name="JMM">[http://www.platinum.matthey.com/services/market-research/market-review-archive/platinum-2009 Summary. Ruthenium]. platinum.matthey.com, p. 9 (2009)</ref> to some 35.5 tonnes in 2017.<ref>[http://www.platinum.matthey.com/services/market-research/pgm-market-reports PGM Market Report.] platinum.matthey.com, p. 30 (May 2018)</ref> Most ruthenium produced is used in wear-resistant electrical contacts and thick-film resistors. A minor application for ruthenium is in platinum [[alloy]]s and as a chemistry [[Catalysis|catalyst]]. A new application of ruthenium is as the capping layer for extreme ultraviolet [[photomask]]s. Ruthenium is generally found in ores with the other platinum group metals in the [[Ural Mountains]] and in [[North America|North]] and [[South America]]. Small but commercially important quantities are also found in [[pentlandite]] extracted from [[Sudbury, Ontario]], and in [[pyroxenite]] deposits in [[South Africa]].{{sfnp|Haynes|2016|p=4.31}} |
||
==Characteristics== |
==Characteristics== |
||
| Line 12: | Line 12: | ||
{| class="wikitable" |
{| class="wikitable" |
||
|- |
|||
![[Atomic number|Z]] !! [[Chemical element|Element]] !! [[Electron shell|No. of electrons/shell]] |
![[Atomic number|Z]] !! [[Chemical element|Element]] !! [[Electron shell|No. of electrons/shell]] |
||
|- |
|- |
||
| Line 24: | Line 23: | ||
|} |
|} |
||
Whereas all other group 8 elements have two electrons in the outermost shell, in ruthenium |
Whereas all other group 8 elements have two electrons in the outermost shell, in ruthenium the outermost shell has only one electron (the final electron is in a lower shell). This anomaly is also observed in the neighboring metals [[niobium]] (41), [[molybdenum]] (42), and [[rhodium]] (45). |
||
===Chemical properties=== |
===Chemical properties=== |
||
| Line 31: | Line 30: | ||
Ruthenium is the first in a downward trend in the melting and boiling points and atomization enthalpy in the 4d transition metals after the maximum seen at [[molybdenum]], because the 4d subshell is more than half full and the electrons are contributing less to metallic bonding. ([[Technetium]], the previous element, has an exceptionally low value that is off the trend due to its half-filled [Kr]4d<sup>5</sup>5s<sup>2</sup> configuration, though it is not as far off the trend in the 4d series as [[manganese]] in the 3d transition series.){{sfnp|Greenwood|Earnshaw|1997|p=1075}} Unlike the lighter congener iron, ruthenium is [[paramagnetic]] at room temperature, as iron also is above its [[Curie point]].{{sfnp|Greenwood|Earnshaw|1997|p=1074}} |
Ruthenium is the first in a downward trend in the melting and boiling points and atomization enthalpy in the 4d transition metals after the maximum seen at [[molybdenum]], because the 4d subshell is more than half full and the electrons are contributing less to metallic bonding. ([[Technetium]], the previous element, has an exceptionally low value that is off the trend due to its half-filled [Kr]4d<sup>5</sup>5s<sup>2</sup> configuration, though it is not as far off the trend in the 4d series as [[manganese]] in the 3d transition series.){{sfnp|Greenwood|Earnshaw|1997|p=1075}} Unlike the lighter congener iron, ruthenium is [[paramagnetic]] at room temperature, as iron also is above its [[Curie point]].{{sfnp|Greenwood|Earnshaw|1997|p=1074}} |
||
The reduction potentials in acidic aqueous solution for some common ruthenium |
The reduction potentials in acidic aqueous solution for some common ruthenium species are shown below:{{sfnp|Greenwood|Earnshaw|1997|p=1077}} |
||
{|class=wikitable |
|||
{| |
|||
!Potential!!colspan=2|Reaction |
|||
|- |
|- |
||
| 0.455 V ||Ru<sup>2+</sup> + 2e<sup>−</sup>|| ↔ Ru |
| 0.455 V ||Ru<sup>2+</sup> + 2e<sup>−</sup>|| ↔ Ru |
||
|- |
|- |
||
| 0.249 V ||Ru<sup>3+</sup> + e<sup>−</sup>|| ↔ Ru< |
| 0.249 V ||Ru<sup>3+</sup> + e<sup>−</sup>|| ↔ Ru<sup>2+</sup> |
||
|- |
|- |
||
| 1.120 V ||RuO<sub>2</sub> + 4H<sup>+</sup> + 2e<sup>−</sup>|| ↔ Ru<sup>2+</sup> + 2H<sub>2</sub>O |
| 1.120 V ||RuO<sub>2</sub> + 4H<sup>+</sup> + 2e<sup>−</sup>|| ↔ Ru<sup>2+</sup> + 2H<sub>2</sub>O |
||
| Line 47: | Line 47: | ||
|} |
|} |
||
===Isotopes=== |
=== Isotopes === |
||
{{Main|Isotopes of ruthenium}} |
{{Main|Isotopes of ruthenium}} |
||
Naturally occurring ruthenium is composed of seven stable [[isotope]]s. Additionally, 34 [[radioactive isotopes]] have been discovered. Of these [[radioisotope]]s, the most stable are <sup>106</sup>Ru with a [[half-life]] of 373.59 days, <sup>103</sup>Ru with a half-life of 39.26 days and <sup>97</sup>Ru with a half-life of 2.9 days.<ref name="n1" /><ref name="n2" /> |
Naturally occurring ruthenium is composed of seven stable [[isotope]]s. Additionally, 34 [[radioactive isotopes]] have been discovered. Of these [[radioisotope]]s, the most stable are <sup>106</sup>Ru with a [[half-life]] of 373.59 days, <sup>103</sup>Ru with a half-life of 39.26 days and <sup>97</sup>Ru with a half-life of 2.9 days.<ref name="n1" /><ref name="n2" /> |
||
Fifteen other radioisotopes have been characterized with [[atomic weight]]s ranging from 89.93 |
Fifteen other radioisotopes have been characterized with [[atomic weight]]s ranging from {{val|89.93|ul=Da}} (<sup>90</sup>Ru) to 114.928 Da (<sup>115</sup>Ru). Most of these have half-lives that are less than five minutes; the exceptions are <sup>95</sup>Ru (half-life: 1.643 hours) and <sup>105</sup>Ru (half-life: 4.44 hours).<ref name="n1" /><ref name="n2" /> |
||
The primary [[decay mode]] before the most abundant isotope, <sup>102</sup>Ru, is [[electron capture]] while the primary mode after is [[beta emission]]. The primary [[decay product]] before <sup>102</sup>Ru is [[technetium]] and the primary decay product after is [[rhodium]].<ref name="n1">{{RubberBible86th}} Section 11, Table of the Isotopes</ref><ref name="n2">{{NUBASE 2003}}</ref> |
The primary [[decay mode]] before the most abundant isotope, <sup>102</sup>Ru, is [[electron capture]] while the primary mode after is [[beta emission]]. The primary [[decay product]] before <sup>102</sup>Ru is [[technetium]] and the primary decay product after is [[rhodium]].<ref name="n1">{{RubberBible86th}} Section 11, Table of the Isotopes</ref><ref name="n2">{{NUBASE 2003}}</ref> |
||
| Line 58: | Line 58: | ||
<sup>106</sup>Ru is a product of fission of a nucleus of [[uranium]] or [[plutonium]]. High concentrations of detected atmospheric <sup>106</sup>Ru were associated with an alleged [[Airborne radioactivity increase in Europe in autumn 2017|undeclared nuclear accident in Russia]] in 2017.<ref name="pnas2019">{{cite journal | title = Airborne concentrations and chemical considerations of radioactive ruthenium from an undeclared major nuclear release in 2017| journal = PNAS | date = 2019 | volume = 116 | issue = 34 | pages = 16750–16759 | doi = 10.1073/pnas.1907571116 | pmid = 31350352 | pmc = 6708381 | bibcode = 2019PNAS..11616750M | last1 = Masson | first1 = O. | last2 = Steinhauser | first2 = G. | last3 = Zok | first3 = D. | last4 = Saunier | first4 = O. | last5 = Angelov | first5 = H. | last6 = Babić | first6 = D. | last7 = Bečková | first7 = V. | last8 = Bieringer | first8 = J. | last9 = Bruggeman | first9 = M. | last10 = Burbidge | first10 = C. I. | last11 = Conil | first11 = S. | last12 = Dalheimer | first12 = A. | last13 = De Geer | first13 = L.-E. | last14 = De Vismes Ott | first14 = A. | last15 = Eleftheriadis | first15 = K. | last16 = Estier | first16 = S. | last17 = Fischer | first17 = H. | last18 = Garavaglia | first18 = M. G. | last19 = Gasco Leonarte | first19 = C. | last20 = Gorzkiewicz | first20 = K. | last21 = Hainz | first21 = D. | last22 = Hoffman | first22 = I. | last23 = Hýža | first23 = M. | last24 = Isajenko | first24 = K. | last25 = Karhunen | first25 = T. | last26 = Kastlander | first26 = J. | last27 = Katzlberger | first27 = C. | last28 = Kierepko | first28 = R. | last29 = Knetsch | first29 = G.-J. | last30 = Kövendiné Kónyi | first30 = J. | display-authors = 29 | doi-access = free }}</ref> |
<sup>106</sup>Ru is a product of fission of a nucleus of [[uranium]] or [[plutonium]]. High concentrations of detected atmospheric <sup>106</sup>Ru were associated with an alleged [[Airborne radioactivity increase in Europe in autumn 2017|undeclared nuclear accident in Russia]] in 2017.<ref name="pnas2019">{{cite journal | title = Airborne concentrations and chemical considerations of radioactive ruthenium from an undeclared major nuclear release in 2017| journal = PNAS | date = 2019 | volume = 116 | issue = 34 | pages = 16750–16759 | doi = 10.1073/pnas.1907571116 | pmid = 31350352 | pmc = 6708381 | bibcode = 2019PNAS..11616750M | last1 = Masson | first1 = O. | last2 = Steinhauser | first2 = G. | last3 = Zok | first3 = D. | last4 = Saunier | first4 = O. | last5 = Angelov | first5 = H. | last6 = Babić | first6 = D. | last7 = Bečková | first7 = V. | last8 = Bieringer | first8 = J. | last9 = Bruggeman | first9 = M. | last10 = Burbidge | first10 = C. I. | last11 = Conil | first11 = S. | last12 = Dalheimer | first12 = A. | last13 = De Geer | first13 = L.-E. | last14 = De Vismes Ott | first14 = A. | last15 = Eleftheriadis | first15 = K. | last16 = Estier | first16 = S. | last17 = Fischer | first17 = H. | last18 = Garavaglia | first18 = M. G. | last19 = Gasco Leonarte | first19 = C. | last20 = Gorzkiewicz | first20 = K. | last21 = Hainz | first21 = D. | last22 = Hoffman | first22 = I. | last23 = Hýža | first23 = M. | last24 = Isajenko | first24 = K. | last25 = Karhunen | first25 = T. | last26 = Kastlander | first26 = J. | last27 = Katzlberger | first27 = C. | last28 = Kierepko | first28 = R. | last29 = Knetsch | first29 = G.-J. | last30 = Kövendiné Kónyi | first30 = J. | display-authors = 29 | doi-access = free }}</ref> |
||
===Occurrence=== |
=== Occurrence === |
||
{{See also|Category:Ruthenium minerals}} |
{{See also|Category:Ruthenium minerals}} |
||
Ruthenium is found in about 100 [[parts per trillion]] in the crust, making it the 78th most abundant element.{{sfnp|Greenwood|Earnshaw|1997|p=1071}} It is generally found in ores with the other platinum group metals in the [[Ural Mountains]] and in North and South America. Small but commercially important quantities are also found in [[pentlandite]] extracted from [[Greater Sudbury|Sudbury]], [[Ontario]], Canada, and in [[pyroxenite]] deposits in [[South Africa]]. The native form of ruthenium is a very rare mineral (Ir replaces part of Ru in its structure).<ref name="USGS-YB-2006">{{cite web|url = http://minerals.usgs.gov/minerals/pubs/commodity/platinum/myb1-2006-plati.pdf |publisher = United States Geological Survey USGS|access-date = 2008-09-16|title = 2006 Minerals Yearbook: Platinum-Group Metals| first = Micheal W.|last = George}}</ref><ref name="USGS-CS-2008">{{cite web|url = http://minerals.usgs.gov/minerals/pubs/commodity/platinum/mcs-2008-plati.pdf |publisher = United States Geological Survey USGS|access-date = 2008-09-16|title = Commodity Report: Platinum-Group Metals}}</ref> Ruthenium has a relatively high [[fission product yield]] in nuclear fission and given that its most long |
Ruthenium is found in about 100 [[parts per trillion]] in the Earth's crust, making it the [[Abundance of the chemical elements|78th most abundant element]].{{sfnp|Greenwood|Earnshaw|1997|p=1071}} It is generally found in ores with the other platinum group metals in the [[Ural Mountains]] and in North and South America. Small but commercially important quantities are also found in [[pentlandite]] extracted from [[Greater Sudbury|Sudbury]], [[Ontario]], Canada, and in [[pyroxenite]] deposits in [[South Africa]]. The native form of ruthenium is a very rare mineral (Ir replaces part of Ru in its structure).<ref name="USGS-YB-2006">{{cite web|url = http://minerals.usgs.gov/minerals/pubs/commodity/platinum/myb1-2006-plati.pdf |publisher = United States Geological Survey USGS|access-date = 2008-09-16|title = 2006 Minerals Yearbook: Platinum-Group Metals| first = Micheal W.|last = George}}</ref><ref name="USGS-CS-2008">{{cite web|url = http://minerals.usgs.gov/minerals/pubs/commodity/platinum/mcs-2008-plati.pdf |publisher = United States Geological Survey USGS|access-date = 2008-09-16|title = Commodity Report: Platinum-Group Metals}}</ref> Ruthenium has a relatively high [[fission product yield]] in nuclear fission; and given that its most long-lived radioisotope has a half life of "only" around a year, there are often proposals to recover ruthenium in a new kind of [[nuclear reprocessing]] from [[spent fuel]]. An unusual ruthenium deposit can also be found at the [[natural nuclear fission reactor]] that was active in [[Oklo]], Gabon, some two billion years ago. Indeed, the isotope ratio of ruthenium found there was one of several ways used to confirm that a nuclear fission chain reaction had indeed occurred at that site in the geological past. Uranium is no longer mined at Oklo, and there have never been serious attempts to recover any of the platinum group metals present there. |
||
==Production== |
== Production == |
||
Roughly 30 tonnes of ruthenium are mined each year<ref name="usgs" /> |
Roughly 30 tonnes of ruthenium are mined each year,<ref name="usgs" /> and world reserves are estimated at 5,000 tonnes.<ref name="Emsley">{{cite book |last=Emsley |first=J. |title=Nature's Building Blocks: An A-Z Guide to the Elements |date=2003 |publisher=Oxford University Press |isbn=978-0-19-850340-8 |location=Oxford, England, UK |pages=[https://archive.org/details/naturesbuildingb0000emsl/page/368 368–370] |chapter=Ruthenium |chapter-url=https://archive.org/details/naturesbuildingb0000emsl/page/368}}</ref> The composition of the mined [[platinum group metal]] (PGM) mixtures varies widely, depending on the geochemical formation. For example, the PGMs mined in South Africa contain on average 11% ruthenium while the PGMs mined in the former USSR contain only 2% (1992).<ref>{{cite book|url = https://books.google.com/books?id=Wm6QMRaX9C4C&pg=PA69|page =69|isbn = 978-0-87335-100-3|editor = Hartman, H. L.|editor2 = Britton, S. G.|date = 1992|publisher = Society for Mining, Metallurgy, and Exploration|location = Littleton, Colo.|title = SME mining engineering handbook}}</ref><ref>{{cite journal |last1=Harris |first1=Donald C. |last2=Cabri |first2=Louis J. |title=The nomenclature of the natural alloys of osmium, iridium and ruthenium based on new compositional data of alloys from world-wide occurrences |journal=The Canadian Mineralogist |date=1 August 1973 |volume=12 |issue=2 |pages=104–112 |id={{NAID|20000798606}} |url=https://pubs.geoscienceworld.org/canmin/article-abstract/12/2/104/10913/The-nomenclature-of-the-natural-alloys-of-osmium }}</ref> Ruthenium, osmium, and iridium are considered the minor platinum group metals.{{sfnp|Greenwood|Earnshaw|1997|p=1074}} |
||
Ruthenium, like the other platinum group metals, is obtained commercially as a by-product from [[nickel]], |
Ruthenium, like the other platinum group metals, is obtained commercially as a by-product from processing of [[nickel]], [[copper]], and platinum metal ore. During [[Copper extraction techniques#Electrorefining|electrorefining of copper]] and nickel, noble metals such as silver, gold, and the platinum group metals precipitate as ''anode mud'', the [[feedstock]] for the extraction.<ref name="USGS-YB-2006" /><ref name="USGS-CS-2008" /> The metals are converted to ionized solutes by any of several methods, depending on the composition of the feedstock. One representative method is fusion with [[sodium peroxide]] followed by dissolution in [[aqua regia]], and solution in a mixture of [[chlorine]] with [[hydrochloric acid]].<ref name="ullmann-pt">{{cite book |doi=10.1002/14356007.a21_075 |chapter=Platinum Group Metals and Compounds |title=Ullmann's Encyclopedia of Industrial Chemistry |year=2001 |last1=Renner |first1=Hermann |last2=Schlamp |first2=Günther |last3=Kleinwächter |first3=Ingo |last4=Drost |first4=Ernst |last5=Lüschow |first5=Hans Martin |last6=Tews |first6=Peter |last7=Panster |first7=Peter |last8=Diehl |first8=Manfred |last9=Lang |first9=Jutta |last10=Kreuzer |first10=Thomas |last11=Knödler |first11=Alfons |last12=Starz |first12=Karl Anton |last13=Dermann |first13=Klaus |last14=Rothaut |first14=Josef |last15=Drieselmann |first15=Ralf |last16=Peter |first16=Catrin |last17=Schiele |first17=Rainer |isbn=978-3-527-30673-2 }}</ref><ref name="kirk-pt">{{cite book |title=Kirk Othmer Encyclopedia of Chemical Technology |first = R. J.|last = Seymour|author2=O'Farrelly, J. I. |chapter=Platinum-group metals |doi=10.1002/0471238961.1612012019052513.a01.pub2 |date=2001 |publisher=Wiley|isbn = 978-0471238966}}</ref> [[Osmium]], ruthenium, [[rhodium]], and [[iridium]] are insoluble in aqua regia and readily precipitate, leaving the other metals in solution. Rhodium is separated from the residue by treatment with molten sodium bisulfate. The insoluble residue, containing Ru, Os, and Ir is treated with sodium oxide, in which Ir is insoluble, producing dissolved Ru and Os salts. After oxidation to the volatile oxides, {{chem|RuO|4}} is separated from {{chem|OsO|4}} by precipitation of (NH<sub>4</sub>)<sub>3</sub>RuCl<sub>6</sub> with ammonium chloride or by distillation or extraction with organic solvents of the volatile osmium tetroxide.<ref>{{cite journal|title = The Platinum Metals|first = Raleigh|last = Gilchrist|journal = Chemical Reviews|date = 1943|volume = 32|issue = 3|pages = 277–372|doi = 10.1021/cr60103a002| s2cid=96640406 }}</ref> [[Hydrogen]] is used to reduce [[ammonium]] ruthenium chloride, yielding a powder.{{sfnp|Haynes|2016|p=4.31}}<ref name="cotton">{{cite book|last = Cotton|first = Simon|title = Chemistry of Precious Metals| pages = 1–20|publisher = Springer-Verlag New York, LLC|date = 1997|isbn = 978-0-7514-0413-5|url = https://books.google.com/books?id=6VKAs6iLmwcC&pg=PA2}}</ref> The product is reduced using hydrogen, yielding the metal as a powder or [[sponge metal]] that can be treated with [[powder metallurgy]] techniques or [[argon]]-[[arc welding]].{{sfnp|Haynes|2016|p=4.31}}<ref name="Hunt 1969 126–138">{{cite journal |first1=L. B. |last1=Hunt |last2=Lever |first2=F. M. |journal=Platinum Metals Review |volume=13 |issue=4 |date=1969 |pages=126–138 |title=Platinum Metals: A Survey of Productive Resources to industrial Uses |doi=10.1595/003214069X134126138 |s2cid=267561907 |url=http://www.platinummetalsreview.com/pdf/pmr-v13-i4-126-138.pdf |access-date=2 June 2009 |archive-date=29 October 2008 |archive-url=https://web.archive.org/web/20081029205825/http://www.platinummetalsreview.com/pdf/pmr-v13-i4-126-138.pdf |url-status=dead }}</ref> |
||
Ruthenium is contained in [[spent nuclear fuel]] both as a direct [[fission product]] and as a product of [[neutron absorption]] by [[long-lived fission product]] {{Chem|99|Tc| link= Technetium-99}}. After allowing the unstable [[isotopes of ruthenium]] to decay, chemical extraction could yield ruthenium for use |
Ruthenium is contained in [[spent nuclear fuel]], both as a direct [[fission product]] and as a product of [[neutron absorption]] by [[long-lived fission product]] {{Chem|99|Tc| link= Technetium-99}}. After allowing the unstable [[isotopes of ruthenium]] to decay, chemical extraction could yield ruthenium for use in all applications of ruthenium.<ref>{{cite journal |last1=Swain |first1=Pravati |last2=Mallika |first2=C. |last3=Srinivasan |first3=R. |last4=Mudali |first4=U. Kamachi |last5=Natarajan |first5=R. |title=Separation and recovery of ruthenium: a review |journal=Journal of Radioanalytical and Nuclear Chemistry |date=November 2013 |volume=298 |issue=2 |pages=781–796 |doi=10.1007/s10967-013-2536-5 |bibcode=2013JRNC..298..781S |s2cid=95804621 }}</ref><ref>{{cite journal |last1=Johal |first1=Sukhraaj Kaur |last2=Boxall |first2=Colin |last3=Gregson |first3=Colin |last4=Steele |first4=Carl |title=Ruthenium Volatilisation from Reprocessed Spent Nuclear Fuel – Studying the Baseline Thermodynamics of Ru(III) |journal=ECS Transactions |date=24 July 2015 |volume=66 |issue=21 |pages=31–42 |doi=10.1149/06621.0031ecst |bibcode=2015ECSTr..66u..31J |url=https://eprints.lancs.ac.uk/id/eprint/78523/2/SJohal_CBoxall_ECSTrans_Paper_16_July_2015_Final_v2.pdf }}</ref> |
||
Ruthenium can also be produced by deliberate [[nuclear transmutation]] from {{chem|99|Tc}}. Given |
Ruthenium can also be produced by deliberate [[nuclear transmutation]] from {{chem|99|Tc}}. Given its relatively long half life, high [[fission product yield]] and high chemical mobility in the environment, {{chem|99|Tc}} is among the most often proposed non-[[actinide]]s for commercial scale nuclear transmutation. {{Chem|99|Tc}} has a relatively large [[neutron cross section]], and because technetium has no stable isotopes, there would not be a problem of [[neutron activation]] of stable isotopes. Significant amounts of {{chem|99|Tc}} are produced in nuclear fission. They are also produced as a byproduct of the use of {{chem|99m|Tc| link= Technetium-99m}} in [[nuclear medicine]], because this isomer decays to {{chem|99|Tc}}. Exposing the {{chem|99|Tc}} target to strong enough [[neutron radiation]] will eventually yield appreciable quantities of ruthenium, which can be chemically separated while consuming {{chem|99|Tc}}.<ref>{{cite journal |last1=Konings |first1=R. J. M. |last2=Conrad |first2=R. |title=Transmutation of technetium – results of the EFTTRA-T2 experiment |journal=Journal of Nuclear Materials |date=1 September 1999 |volume=274 |issue=3 |pages=336–340 |doi=10.1016/S0022-3115(99)00107-5 |bibcode=1999JNuM..274..336K }}</ref><ref>{{cite journal |last1=Peretroukhine |first1=Vladimir |last2=Radchenko |first2=Viacheslav |last3=Kozar' |first3=Andrei |last4=Tarasov |first4=Valeriy |last5=Toporov |first5=Iury |last6=Rotmanov |first6=Konstantin |last7=Lebedeva |first7=Lidia |last8=Rovny |first8=Sergey |last9=Ershov |first9=Victor |title=Technetium transmutation and production of artificial stable ruthenium |journal=Comptes Rendus Chimie |date=December 2004 |volume=7 |issue=12 |pages=1215–1218 |doi=10.1016/j.crci.2004.05.002 |url=https://comptes-rendus.academie-sciences.fr/chimie/articles/10.1016/j.crci.2004.05.002/ }}</ref> |
||
==Chemical compounds== |
== Chemical compounds == |
||
{{See also|Category:Ruthenium compounds}} |
{{See also|Category:Ruthenium compounds}} |
||
The [[oxidation state]]s of ruthenium range from 0 to +8, and −2. The properties of ruthenium and osmium [[Chemical compound|compounds]] are often similar. The +2, +3, and +4 states are the most common. The most prevalent precursor is [[ruthenium trichloride]], a red solid that is poorly defined chemically but versatile synthetically.<ref name="cotton" /> |
The [[oxidation state]]s of ruthenium range from 0 to +8, and −2. The properties of ruthenium and osmium [[Chemical compound|compounds]] are often similar. The +2, +3, and +4 states are the most common. The most prevalent precursor is [[ruthenium trichloride]], a red solid that is poorly defined chemically but versatile synthetically.<ref name="cotton" /> |
||
===Oxides and chalcogenides=== |
=== Oxides and chalcogenides === |
||
Ruthenium can be [[oxidation|oxidized]] to [[ruthenium(IV) oxide]] (RuO<sub>2</sub>, oxidation state +4), which can, in turn, be oxidized by [[Sodium periodate|sodium metaperiodate]] to the volatile yellow tetrahedral [[ruthenium tetroxide]], RuO<sub>4</sub>, an aggressive, strong oxidizing agent with structure and properties analogous to [[osmium tetroxide]]. RuO<sub>4</sub> is mostly used as an intermediate in the purification of ruthenium from ores and radiowastes.<ref>{{cite journal|author1=Swain, P. |author2=Mallika, C. |author3=Srinivasan, R. |author4=Mudali, U. K. |author5=Natarajan, R. |s2cid=95804621|title=Separation and recovery of ruthenium: a review|journal=J. Radioanal. Nucl. Chem. |year=2013|volume=298|issue=2|pages=781–796|doi=10.1007/s10967-013-2536-5}}</ref> |
Ruthenium can be [[oxidation|oxidized]] to [[ruthenium(IV) oxide]] (RuO<sub>2</sub>, oxidation state +4), which can, in turn, be oxidized by [[Sodium periodate|sodium metaperiodate]] to the volatile yellow tetrahedral [[ruthenium tetroxide]], RuO<sub>4</sub>, an aggressive, strong oxidizing agent with structure and properties analogous to [[osmium tetroxide]]. RuO<sub>4</sub> is mostly used as an intermediate in the purification of ruthenium from ores and radiowastes.<ref>{{cite journal|author1=Swain, P. |author2=Mallika, C. |author3=Srinivasan, R. |author4=Mudali, U. K. |author5=Natarajan, R. |s2cid=95804621|title=Separation and recovery of ruthenium: a review|journal=J. Radioanal. Nucl. Chem. |year=2013|volume=298|issue=2|pages=781–796|doi=10.1007/s10967-013-2536-5|bibcode=2013JRNC..298..781S }}</ref> |
||
Dipotassium ruthenate (K<sub>2</sub>RuO<sub>4</sub>, +6) and potassium perruthenate (KRuO<sub>4</sub>, +7) are also known.{{sfnp|Greenwood|Earnshaw|1997|p={{pn|date=October 2023}}}} Unlike osmium tetroxide, ruthenium tetroxide is less stable, is strong enough as an oxidising agent to oxidise dilute [[hydrochloric acid]] and organic solvents like [[ethanol]] at room temperature, and is easily reduced to ruthenate ({{chem|RuO|4|2-}}) in aqueous alkaline solutions; it decomposes to form the dioxide above 100 °C. Unlike iron but like osmium, ruthenium does not form oxides in its lower +2 and +3 oxidation states.{{sfnp|Greenwood|Earnshaw|1997|pp=1080–1081}} Ruthenium forms di[[chalcogenide]]s, which are diamagnetic semiconductors crystallizing in the [[pyrite]] structure.{{sfnp|Greenwood|Earnshaw|1997|pp=1080–1081}} Ruthenium sulfide (RuS<sub>2</sub>) occurs naturally as the mineral [[laurite]]. |
Dipotassium ruthenate (K<sub>2</sub>RuO<sub>4</sub>, +6) and potassium perruthenate (KRuO<sub>4</sub>, +7) are also known.{{sfnp|Greenwood|Earnshaw|1997|p={{pn|date=October 2023}}}} Unlike osmium tetroxide, ruthenium tetroxide is less stable, is strong enough as an oxidising agent to oxidise dilute [[hydrochloric acid]] and organic solvents like [[ethanol]] at room temperature, and is easily reduced to ruthenate ({{chem|RuO|4|2-}}) in aqueous alkaline solutions; it decomposes to form the dioxide above 100 °C. Unlike iron but like osmium, ruthenium does not form oxides in its lower +2 and +3 oxidation states.{{sfnp|Greenwood|Earnshaw|1997|pp=1080–1081}} Ruthenium forms di[[chalcogenide]]s, which are diamagnetic semiconductors crystallizing in the [[pyrite]] structure.{{sfnp|Greenwood|Earnshaw|1997|pp=1080–1081}} Ruthenium sulfide (RuS<sub>2</sub>) occurs naturally as the mineral [[laurite]]. |
||
| Line 84: | Line 84: | ||
Some mixed oxides are also known, such as M<sup>II</sup>Ru<sup>IV</sup>O<sub>3</sub>, Na<sub>3</sub>Ru<sup>V</sup>O<sub>4</sub>, Na{{su|b=2}}Ru{{su|p=V|b=2}}O{{su|b=7}}, and M{{su|p=II|b=2}}Ln{{su|p=III}}Ru{{su|p=V}}O{{su|b=6}}.{{sfnp|Greenwood|Earnshaw|1997|p=1082}} |
Some mixed oxides are also known, such as M<sup>II</sup>Ru<sup>IV</sup>O<sub>3</sub>, Na<sub>3</sub>Ru<sup>V</sup>O<sub>4</sub>, Na{{su|b=2}}Ru{{su|p=V|b=2}}O{{su|b=7}}, and M{{su|p=II|b=2}}Ln{{su|p=III}}Ru{{su|p=V}}O{{su|b=6}}.{{sfnp|Greenwood|Earnshaw|1997|p=1082}} |
||
===Halides and oxyhalides=== |
=== Halides and oxyhalides === |
||
The highest known ruthenium halide is the [[ruthenium hexafluoride|hexafluoride]], a dark brown solid that melts at 54 °C. It hydrolyzes violently upon contact with water and easily disproportionates to form a mixture of lower ruthenium fluorides, releasing fluorine gas. [[Ruthenium pentafluoride]] is a tetrameric dark green solid that is also readily hydrolyzed, melting at 86.5 °C. The yellow [[ruthenium tetrafluoride]] is probably also polymeric and can be formed by reducing the pentafluoride with [[iodine]]. Among the binary compounds of ruthenium, these high oxidation states are known only in the oxides and fluorides.{{sfnp|Greenwood|Earnshaw|1997|p=1083}} |
The highest known ruthenium halide is the [[ruthenium hexafluoride|hexafluoride]], a dark brown solid that melts at 54 °C. It hydrolyzes violently upon contact with water and easily disproportionates to form a mixture of lower ruthenium fluorides, releasing fluorine gas. [[Ruthenium pentafluoride]] is a tetrameric dark green solid that is also readily hydrolyzed, melting at 86.5 °C. The yellow [[ruthenium tetrafluoride]] is probably also polymeric and can be formed by reducing the pentafluoride with [[iodine]]. Among the binary compounds of ruthenium, these high oxidation states are known only in the oxides and fluorides.{{sfnp|Greenwood|Earnshaw|1997|p=1083}} |
||
[[Ruthenium trichloride]] is a well-known compound, existing in a black α-form and a dark brown β-form: the trihydrate is red.{{sfnp|Greenwood|Earnshaw|1997|p=1084}} Of the known trihalides, trifluoride is dark brown and decomposes above 650 °C, tribromide is dark-brown and decomposes above 400 °C, and triiodide is black.{{sfnp|Greenwood|Earnshaw|1997|p=1083}} Of the dihalides, difluoride is not known, dichloride is brown, dibromide is black, and diiodide is blue.{{sfnp|Greenwood|Earnshaw|1997|p=1083}} The only known oxyhalide is the pale green ruthenium(VI) oxyfluoride, RuOF<sub>4</sub>.{{sfnp|Greenwood|Earnshaw|1997|p=1084}} |
[[Ruthenium trichloride]] is a well-known compound, existing in a black α-form and a dark brown β-form: the trihydrate is red.{{sfnp|Greenwood|Earnshaw|1997|p=1084}} Of the known trihalides, trifluoride is dark brown and decomposes above 650 °C, tribromide is dark-brown and decomposes above 400 °C, and triiodide is black.{{sfnp|Greenwood|Earnshaw|1997|p=1083}} Of the dihalides, difluoride is not known, dichloride is brown, dibromide is black, and diiodide is blue.{{sfnp|Greenwood|Earnshaw|1997|p=1083}} The only known oxyhalide is the pale green ruthenium(VI) oxyfluoride, RuOF<sub>4</sub>.{{sfnp|Greenwood|Earnshaw|1997|p=1084}} |
||
===Coordination and organometallic complexes=== |
=== Coordination and organometallic complexes === |
||
{{Main|Organoruthenium chemistry}} |
{{Main|Organoruthenium chemistry}} |
||
[[File:Tris(bipyridine)ruthenium(II)-chloride-powder.jpg|thumb|Tris(bipyridine)ruthenium(II) chloride]] |
[[File:Tris(bipyridine)ruthenium(II)-chloride-powder.jpg|thumb|Tris(bipyridine)ruthenium(II) chloride]] |
||
| Line 97: | Line 97: | ||
Ruthenium forms a wide range compounds with carbon–ruthenium bonds. [[Grubbs' catalyst]] is used for alkene metathesis.<ref>Hartwig, J. F. (2010) ''Organotransition Metal Chemistry, from Bonding to Catalysis'', University Science Books: New York. {{ISBN|1-891389-53-X}}</ref> [[Ruthenocene]] is analogous to [[ferrocene]] structurally, but exhibits distinctive redox properties. The colorless liquid [[ruthenium pentacarbonyl]] converts in the absence of CO pressure to the dark red solid [[triruthenium dodecacarbonyl]]. [[Ruthenium(III) chloride|Ruthenium trichloride]] reacts with carbon monoxide to give many derivatives including RuHCl(CO)(PPh<sub>3</sub>)<sub>3</sub> and Ru(CO)<sub>2</sub>(PPh<sub>3</sub>)<sub>3</sub> ([[Roper's complex]]). Heating solutions of ruthenium trichloride in alcohols with [[triphenylphosphine]] gives [[tris(triphenylphosphine)ruthenium dichloride]] (RuCl<sub>2</sub>(PPh<sub>3</sub>)<sub>3</sub>), which converts to the hydride complex chlorohydridotris(triphenylphosphine)ruthenium(II) (RuHCl(PPh<sub>3</sub>)<sub>3</sub>).<ref name="cotton" /> |
Ruthenium forms a wide range compounds with carbon–ruthenium bonds. [[Grubbs' catalyst]] is used for alkene metathesis.<ref>Hartwig, J. F. (2010) ''Organotransition Metal Chemistry, from Bonding to Catalysis'', University Science Books: New York. {{ISBN|1-891389-53-X}}</ref> [[Ruthenocene]] is analogous to [[ferrocene]] structurally, but exhibits distinctive redox properties. The colorless liquid [[ruthenium pentacarbonyl]] converts in the absence of CO pressure to the dark red solid [[triruthenium dodecacarbonyl]]. [[Ruthenium(III) chloride|Ruthenium trichloride]] reacts with carbon monoxide to give many derivatives including RuHCl(CO)(PPh<sub>3</sub>)<sub>3</sub> and Ru(CO)<sub>2</sub>(PPh<sub>3</sub>)<sub>3</sub> ([[Roper's complex]]). Heating solutions of ruthenium trichloride in alcohols with [[triphenylphosphine]] gives [[tris(triphenylphosphine)ruthenium dichloride]] (RuCl<sub>2</sub>(PPh<sub>3</sub>)<sub>3</sub>), which converts to the hydride complex chlorohydridotris(triphenylphosphine)ruthenium(II) (RuHCl(PPh<sub>3</sub>)<sub>3</sub>).<ref name="cotton" /> |
||
==History== |
== History == |
||
Though naturally occurring platinum alloys containing all six [[platinum-group metal]]s were used for a long time by [[pre-Columbian]] Americans and known as a material to European chemists from the mid-16th century, not until the mid-18th century was platinum identified as a pure element. That natural platinum contained palladium, rhodium, osmium and iridium was discovered in the first decade of the 19th century.<ref name="Weeks8">{{cite journal|doi = 10.1021/ed009p1017|title = The discovery of the elements. VIII. The platinum metals|date = 1932|last1 = Weeks|first1 = Mary Elvira|author-link1=Mary Elvira Weeks|journal = Journal of Chemical Education|volume = 9|page = 1017|bibcode = 1932JChEd...9.1017W|issue = 6}}</ref> Platinum in [[alluvium|alluvial sands]] of Russian rivers gave access to raw material for use in plates and medals and for the minting of [[ruble]] [[coins]], starting in 1828.<ref name="Roubles">{{cite journal |journal=Platinum Metals Review |volume=48 |issue=2 |date=2004 |pages=66–69 |title=The Minting of Platinum Roubles. Part I: History and Current Investigations |first=Christoph J. |last=Raub |doi=10.1595/003214004X4826669 |doi-access=free }}</ref> Residues from platinum production for coinage were available in the Russian Empire, and therefore most of the research on them was done in Eastern Europe. |
Though naturally occurring platinum alloys containing all six [[platinum-group metal]]s were used for a long time by [[pre-Columbian]] Americans and known as a material to European chemists from the mid-16th century, not until the mid-18th century was platinum identified as a pure element. That natural platinum contained palladium, rhodium, osmium and iridium was discovered in the first decade of the 19th century.<ref name="Weeks8">{{cite journal|doi = 10.1021/ed009p1017|title = The discovery of the elements. VIII. The platinum metals|date = 1932|last1 = Weeks|first1 = Mary Elvira|author-link1=Mary Elvira Weeks|journal = Journal of Chemical Education|volume = 9|page = 1017|bibcode = 1932JChEd...9.1017W|issue = 6}}</ref> Platinum in [[alluvium|alluvial sands]] of Russian rivers gave access to raw material for use in plates and medals and for the minting of [[ruble]] [[coins]], starting in 1828.<ref name="Roubles">{{cite journal |journal=Platinum Metals Review |volume=48 |issue=2 |date=2004 |pages=66–69 |title=The Minting of Platinum Roubles. Part I: History and Current Investigations |first=Christoph J. |last=Raub |doi=10.1595/003214004X4826669 |doi-access=free }}</ref> Residues from platinum production for coinage were available in the Russian Empire, and therefore most of the research on them was done in Eastern Europe. |
||
It is possible that the [[Poland|Polish]] chemist [[Jędrzej Śniadecki]] isolated element 44 (which he called "vestium" after the asteroid [[4 Vesta|Vesta]] discovered shortly before) from South American platinum ores in 1807. He published an announcement of his discovery in 1808.<ref>{{cite book |last1=Śniadecki |first1=Jędrzej |title=Rosprawa o nowym metallu w surowey platynie odkrytym |trans-title=A case about a new metal in raw platinum discovered |language=pl |date=1808 |publisher=Nakładém i Drukiém Józefa Zawadzkiego |url=https://www.dbc.wroc.pl/publication/5247 |oclc=739088520 }}</ref> His work was never confirmed, however, and he later withdrew his claim of discovery.<ref name="Emsley" /> |
It is possible that the [[Poland|Polish]] chemist [[Jędrzej Śniadecki]] isolated element 44 (which he called "vestium" after the asteroid [[4 Vesta|Vesta]] discovered shortly before) from South American platinum ores in 1807. He published an announcement of his discovery in 1808.<ref>{{cite book |last1=Śniadecki |first1=Jędrzej |title=Rosprawa o nowym metallu w surowey platynie odkrytym |trans-title=A case about a new metal in raw platinum discovered |language=pl |date=1808 |publisher=Nakładém i Drukiém Józefa Zawadzkiego |url=https://www.dbc.wroc.pl/publication/5247 |oclc=739088520 }}</ref> His work was never confirmed, however, and he later withdrew his claim of discovery.<ref name="Emsley" /> |
||
[[Jöns Berzelius]] and [[Gottfried Osann]] nearly discovered ruthenium in 1827.<ref>{{cite journal |title=New metals in the Uralian platina |journal=The Philosophical Magazine |date=1 November 1827 |volume=2 |issue=11 |pages=391–392 |doi=10.1080/14786442708674516 |url=https://books.google.com/books?id=x57C3yhRPUAC&pg=PA391 }}</ref> They examined residues that were left after dissolving crude platinum from the [[Ural Mountains]] in [[aqua regia]]. Berzelius did not find any unusual metals, but Osann thought he found three new metals, which he called pluranium, ruthenium, and polinium.{{sfnp|Haynes|2016|p=4.31}} This discrepancy led to a long-standing controversy between Berzelius and Osann about the composition of the residues.<ref name="DiscoRu" /> As Osann was not able to repeat his isolation of ruthenium, he eventually relinquished his claims.<ref name="DiscoRu" /><ref name="Osann2">{{cite journal | author = Osann, Gottfried | title = Berichtigung, meine Untersuchung des uralschen Platins betreffend | journal = [[Annalen der Physik|Poggendorffs Annalen der Physik und Chemie]] | volume = 15 | year = 1829 | page = 158 | url = http://gallica.bnf.fr/ark:/12148/bpt6k15100n.image.f168.langDE| doi = 10.1002/andp.18290910119 }}</ref> The name "ruthenium" was chosen by Osann because the analysed samples stemmed from the Ural Mountains in Russia.<ref name="Osann">{{cite journal |last1=Osann |first1=G. |title=Fortsetzung der Untersuchung des Platins vom Ural |trans-title=Continuation of the study of platinum from the Urals |language=de |journal=Annalen der Physik |date=1828 |volume=89 |issue=6 |pages=283–297 |doi=10.1002/andp.18280890609 |bibcode=1828AnP....89..283O |url=https://zenodo.org/record/1423520 }}</ref> |
[[Jöns Berzelius]] and [[Gottfried Osann]] nearly discovered ruthenium in 1827.<ref>{{cite journal |title=New metals in the Uralian platina |journal=The Philosophical Magazine |date=1 November 1827 |volume=2 |issue=11 |pages=391–392 |doi=10.1080/14786442708674516 |url=https://books.google.com/books?id=x57C3yhRPUAC&pg=PA391 }}</ref> They examined residues that were left after dissolving crude platinum from the [[Ural Mountains]] in [[aqua regia]]. Berzelius did not find any unusual metals, but Osann thought he found three new metals, which he called pluranium, ruthenium, and polinium.{{sfnp|Haynes|2016|p=4.31}} This discrepancy led to a long-standing controversy between Berzelius and Osann about the composition of the residues.<ref name="DiscoRu">{{cite journal | title = The Discovery of Ruthenium| first = V. N. | last = Pitchkov | journal = Platinum Metals Review | volume = 40 | issue = 4 | year = 1996 | pages =181–188 | doi = 10.1595/003214096X404181188 | url = http://www.platinummetalsreview.com/dynamic/article/view/pmr-v40-i4-181-188}}</ref><ref>{{cite journal |author = Claus, Karl |title=О способе добывания чистой платины из руд |trans-title=On the method of extracting pure platinum from ores |journal=Горный журнал (Mining Journal) |year=1845 | volume = 7 | issue = 3 | pages = 157–163 |language=ru}}</ref> As Osann was not able to repeat his isolation of ruthenium, he eventually relinquished his claims.<ref name="DiscoRu" /><ref name="Osann2">{{cite journal | author = Osann, Gottfried | title = Berichtigung, meine Untersuchung des uralschen Platins betreffend | journal = [[Annalen der Physik|Poggendorffs Annalen der Physik und Chemie]] | volume = 15 | year = 1829 | page = 158 | url = http://gallica.bnf.fr/ark:/12148/bpt6k15100n.image.f168.langDE| doi = 10.1002/andp.18290910119 }}</ref> The name "ruthenium" was chosen by Osann because the analysed samples stemmed from the Ural Mountains in Russia.<ref name="Osann">{{cite journal |last1=Osann |first1=G. |title=Fortsetzung der Untersuchung des Platins vom Ural |trans-title=Continuation of the study of platinum from the Urals |language=de |journal=Annalen der Physik |date=1828 |volume=89 |issue=6 |pages=283–297 |doi=10.1002/andp.18280890609 |bibcode=1828AnP....89..283O |url=https://zenodo.org/record/1423520 }}</ref> |
||
In 1844, [[Karl Ernst Claus]], a Russian scientist of [[Baltic German]] descent, showed that the compounds prepared by Gottfried Osann contained small amounts of ruthenium, which Claus had [[discovery of the chemical elements|discovered]] the same year.{{sfnp|Haynes|2016|p=4.31}}<ref name="Weeks8" /> Claus isolated ruthenium from the platinum residues of rouble production while he was working in [[Kazan University]], [[Kazan]],<ref name="DiscoRu" /> the same way its heavier congener osmium had been discovered four decades earlier.{{sfnp|Greenwood|Earnshaw|1997|p=1071}} Claus showed that ruthenium oxide contained a new metal and obtained 6 grams of ruthenium from the part of crude platinum that is insoluble in [[aqua regia]].<ref name="DiscoRu" /> Choosing the name for the new element, Claus stated: "I named the new body, in honour of my Motherland, ruthenium. I had every right to call it by this name because Mr. Osann relinquished his ruthenium and the word does not yet exist in chemistry."<ref name="DiscoRu" /><ref>{{cite journal |author = Claus, Karl |title=О способе добывания чистой платины из руд |trans-title=On the method of extracting pure platinum from ores |journal=Горный журнал (Mining Journal) |year=1845 | volume = 7 | issue = 3 | pages = 157–163 |language=ru}}</ref> In doing so, Claus started a trend that continues to this day – naming an element after a country.<ref name="Meija">{{cite journal |last1=Meija |first1=Juris |title=Politics at the periodic table |journal=Nature Chemistry |date=September 2021 |volume=13 |issue=9 |pages=814–816 |doi=10.1038/s41557-021-00780-5 |pmid=34480093 |bibcode=2021NatCh..13..814M |s2cid=237405162 }}</ref> |
In 1844, [[Karl Ernst Claus]], a Russian scientist of [[Baltic German]] descent, showed that the compounds prepared by Gottfried Osann contained small amounts of ruthenium, which Claus had [[discovery of the chemical elements|discovered]] the same year.{{sfnp|Haynes|2016|p=4.31}}<ref name="Weeks8" /> Claus isolated ruthenium from the platinum residues of rouble production while he was working in [[Kazan University]], [[Kazan]],<ref name="DiscoRu" /> the same way its heavier congener osmium had been discovered four decades earlier.{{sfnp|Greenwood|Earnshaw|1997|p=1071}} Claus showed that ruthenium oxide contained a new metal and obtained 6 grams of ruthenium from the part of crude platinum that is insoluble in [[aqua regia]].<ref name="DiscoRu" /> Choosing the name for the new element, Claus stated: "I named the new body, in honour of my Motherland, ruthenium. I had every right to call it by this name because Mr. Osann relinquished his ruthenium and the word does not yet exist in chemistry."<ref name="DiscoRu" /><ref>{{cite journal |author = Claus, Karl |title=О способе добывания чистой платины из руд |trans-title=On the method of extracting pure platinum from ores |journal=Горный журнал (Mining Journal) |year=1845 | volume = 7 | issue = 3 | pages = 157–163 |language=ru}}</ref> The name itself derives from the Latin word ''[[Ruthenia]]''.<ref name=Lewis2019/><!-- ref def in Template:infobox ruthenium--><ref name=Pitchkov1996/><!-- ref def in Template:infobox ruthenium--> |
||
In doing so, Claus started a trend that continues to this day – naming an element after a country.<ref name="Meija">{{cite journal |last1=Meija |first1=Juris |title=Politics at the periodic table |journal=Nature Chemistry |date=September 2021 |volume=13 |issue=9 |pages=814–816 |doi=10.1038/s41557-021-00780-5 |pmid=34480093 |bibcode=2021NatCh..13..814M |s2cid=237405162 }}</ref> |
|||
==Applications== |
== Applications == |
||
Approximately 30.9 tonnes of ruthenium were consumed in 2016, 13.8 of them in electrical applications, 7.7 in catalysis, and 4.6 in electrochemistry.<ref name="usgs">Loferski, Patricia J.; Ghalayini, Zachary T. and Singerling, Sheryl A. (2018) [https://d9-wret.s3-us-west-2.amazonaws.com/assets/palladium/production/atoms/files/myb1-2016-plati.pdf Platinum-group metals]. ''2016 Minerals Yearbook''. USGS. p. 57.3.</ref> |
Approximately 30.9 tonnes of ruthenium were consumed in 2016, 13.8 of them in electrical applications, 7.7 in catalysis, and 4.6 in electrochemistry.<ref name="usgs">Loferski, Patricia J.; Ghalayini, Zachary T. and Singerling, Sheryl A. (2018) [https://d9-wret.s3-us-west-2.amazonaws.com/assets/palladium/production/atoms/files/myb1-2016-plati.pdf Platinum-group metals]. ''2016 Minerals Yearbook''. USGS. p. 57.3.</ref> |
||
Because it hardens platinum and palladium alloys, ruthenium is used in [[Switch#Contacts|electrical contacts]], where a thin film is sufficient to achieve the desired durability. With its similar properties to and lower cost than rhodium,<ref name="Hunt 1969 126–138" /> electric contacts are a major use of ruthenium.<ref name="USGS-YB-2006"/><ref>{{cite journal |doi=10.1016/j.ccr.2004.08.015 |title=Chemical and electrochemical depositions of platinum group metals and their applications |date=2005 |author=Rao, C. |journal=Coordination Chemistry Reviews |volume=249 |page=613 |last2=Trivedi |first2=D. |issue=5–6}}</ref> The ruthenium plate is applied to the electrical contact and electrode base metal by electroplating<ref>{{cite journal |doi=10.1016/S0026-0576(00)83089-5 |title=Ruthenium plating |date=1999 |author=Weisberg, A. |journal=Metal Finishing |volume=97 |page=297}}</ref> or [[sputtering]].<ref>{{cite book |isbn=978-0-87170-285-2| url=https://books.google.com/books?id=EkStW7v8VPkC&pg=RA3-PA550 |page=184 |collaboration=ASM International Handbook Committee |author=Merrill L. Minges |date=1989 |publisher=ASM International |location=Materials Park, OH |title=Electronic materials handbook}}</ref> |
Because it hardens platinum and palladium alloys, ruthenium is used in [[Switch#Contacts|electrical contacts]], where a thin film is sufficient to achieve the desired durability. With its similar properties to and lower cost than rhodium,<ref name="Hunt 1969 126–138" /> electric contacts are a major use of ruthenium.<ref name="USGS-YB-2006"/><ref>{{cite journal |doi=10.1016/j.ccr.2004.08.015 |title=Chemical and electrochemical depositions of platinum group metals and their applications |date=2005 |author=Rao, C. |journal=Coordination Chemistry Reviews |volume=249 |page=613 |last2=Trivedi |first2=D. |issue=5–6}}</ref> The ruthenium plate is applied to the electrical contact and electrode base metal by electroplating<ref>{{cite journal |doi=10.1016/S0026-0576(00)83089-5 |title=Ruthenium plating |date=1999 |author=Weisberg, A. |journal=Metal Finishing |volume=97 |page=297}}</ref> or [[sputtering]].<ref>{{cite book |isbn=978-0-87170-285-2| url=https://books.google.com/books?id=EkStW7v8VPkC&pg=RA3-PA550 |page=184 |collaboration=ASM International Handbook Committee |author=Merrill L. Minges |date=1989 |publisher=ASM International |location=Materials Park, OH |title=Electronic materials handbook}}</ref> |
||
Ruthenium dioxide with [[lead]] and [[bismuth]] ruthenates are used in thick-film chip resistors.<ref>{{cite journal|doi =10.1007/s10854-006-0036-x|title =Microstructure development and electrical properties of RuO<sub>2</sub>-based lead-free thick film resistors|date =2006|author =Busana, M. G.|journal =Journal of Materials Science: Materials in Electronics|volume =17|page =951|last2 =Prudenziati|first2 =M.|last3 =Hormadaly|first3 =J.|s2cid =135485712|issue =11|hdl =11380/303403}}</ref><ref>{{cite journal|doi = 10.1016/j.matlet.2006.05.015|title = Environment friendly perovskite ruthenate based thick film resistors|date = 2007|author = Rane, Sunit|journal = Materials Letters|volume = 61|page = 595|last2 = Prudenziati|first2 = Maria|last3 = Morten|first3 = Bruno|issue = 2|hdl = 11380/307664}}</ref><ref>{{cite book|isbn = 978-0-8247-1934-0| url = https://books.google.com/books?id=c2YxCCaM9RIC&pg=PA184|pages = 184, 345|editor = Slade, Paul G.|date = 1999|publisher = Dekker|location = New York, NY|title = Electrical contacts : principles and applications}}</ref><!--http://md1.csa.com/partners/viewrecord.php?requester=gs&collection=TRD&recid=N8113268AH--> These two electronic applications account for 50% of the ruthenium consumption.<ref name="Emsley" /> |
Ruthenium dioxide with [[lead]] and [[bismuth]] ruthenates are used in thick-film chip resistors.<ref>{{cite journal|doi =10.1007/s10854-006-0036-x|title =Microstructure development and electrical properties of RuO<sub>2</sub>-based lead-free thick film resistors|date =2006|author =Busana, M. G.|journal =Journal of Materials Science: Materials in Electronics|volume =17|page =951|last2 =Prudenziati|first2 =M.|last3 =Hormadaly|first3 =J.|s2cid =135485712|issue =11|hdl =11380/303403}}</ref><ref>{{cite journal|doi = 10.1016/j.matlet.2006.05.015|title = Environment friendly perovskite ruthenate based thick film resistors|date = 2007|author = Rane, Sunit|journal = Materials Letters|volume = 61|page = 595|last2 = Prudenziati|first2 = Maria|last3 = Morten|first3 = Bruno|issue = 2| bibcode=2007MatL...61..595R |hdl = 11380/307664}}</ref><ref>{{cite book|isbn = 978-0-8247-1934-0| url = https://books.google.com/books?id=c2YxCCaM9RIC&pg=PA184|pages = 184, 345|editor = Slade, Paul G.|date = 1999|publisher = Dekker|location = New York, NY|title = Electrical contacts : principles and applications}}</ref><!--http://md1.csa.com/partners/viewrecord.php?requester=gs&collection=TRD&recid=N8113268AH--> These two electronic applications account for 50% of the ruthenium consumption.<ref name="Emsley" /> |
||
Ruthenium is seldom alloyed with metals outside the platinum group, where small quantities improve some properties. The added corrosion resistance in [[titanium]] alloys led to the development of a special alloy with 0.1% ruthenium.<ref>{{cite journal |last1=Schutz |first1=R. W. |title=Ruthenium Enhanced Titanium Alloys |journal=Platinum Metals Review |date=April 1996 |volume=40 |issue=2 |pages=54–61 |doi=10.1595/003214096X4025461 |citeseerx=10.1.1.630.7411 |s2cid=267551174 }}</ref> Ruthenium is also used in some advanced high-temperature single-crystal [[superalloy]]s, with applications that include the turbines in [[jet engines]]. Several nickel based superalloy compositions are described, such as EPM-102 (with 3% Ru), TMS-162 (with 6% Ru), TMS-138,<ref>{{cite news| title=Fourth generation nickel base single crystal superalloy. TMS-138 / 138A |url=http://sakimori.nims.go.jp/catalog/TMS-138-A.pdf |date=July 2006|work=High Temperature Materials Center, National Institute for Materials Science, Japan|archive-url=https://web.archive.org/web/20130418105851/http://sakimori.nims.go.jp/catalog/TMS-138-A.pdf|archive-date=18 April 2013}}</ref> and TMS-174,<ref>{{cite journal|author=Koizumi, Yutaka |display-authors=etal |title=Development of a Next-Generation Ni-base Single Crystal Superalloy |url=http://nippon.zaidan.info/seikabutsu/2003/00916/pdf/igtc2003tokyo_ts119.pdf |journal=Proceedings of the International Gas Turbine Congress, Tokyo 2–7 November 2003 |archive-date=10 January 2014 |archive-url=https://web.archive.org/web/20140110170053/http://nippon.zaidan.info/seikabutsu/2003/00916/pdf/igtc2003tokyo_ts119.pdf}}</ref><ref>{{cite news| title=Joint Development of a Fourth Generation Single Crystal Superalloy |author=Walston, S. |author2=Cetel, A. |author3=MacKay, R. |author4=O'Hara, K. |author5=Duhl, D. |author6=Dreshfield, R. |date=December 2004 |work=NASA |url=https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20050019231_2005000097.pdf}}</ref> the latter two containing 6% [[rhenium]].<ref>{{cite journal|doi = 10.1007/s11041-006-0099-6|title = Effect of high-gradient directed crystallization on the structure and properties of rhenium-bearing single-crystal alloy|date = 2006|last1 = Bondarenko |first1 = Yu. A.|journal = Metal Science and Heat Treatment|volume = 48|page = 360|last2 = Kablov|first2 = E. N.|last3 = Surova|first3 = V. A.|last4 = Echin|first4 = A. B.|s2cid = 136907279|issue = 7–8|bibcode = 2006MSHT...48..360B}}</ref> [[Fountain pen]] nibs are frequently tipped with ruthenium alloy. From 1944 onward, the [[Parker 51]] fountain pen was fitted with the "RU" nib, a 14K gold nib tipped with 96.2% ruthenium and 3.8% [[iridium]].<ref>{{cite journal |url=http://www.nibs.com/article4.html |journal=The PENnant |volume=XIII |issue=2 |date=1999 |title=Notes from the Nib Works—Where's the Iridium? |author=Mottishaw, J. |archive-url=https://web.archive.org/web/20020604135505/http://www.nibs.com/article4.html|archive-date=4 June 2002}}</ref> |
Ruthenium is seldom alloyed with metals outside the platinum group, where small quantities improve some properties. The added corrosion resistance in [[titanium]] alloys led to the development of a special alloy with 0.1% ruthenium.<ref>{{cite journal |last1=Schutz |first1=R. W. |title=Ruthenium Enhanced Titanium Alloys |journal=Platinum Metals Review |date=April 1996 |volume=40 |issue=2 |pages=54–61 |doi=10.1595/003214096X4025461 |citeseerx=10.1.1.630.7411 |s2cid=267551174 }}</ref> Ruthenium is also used in some advanced high-temperature single-crystal [[superalloy]]s, with applications that include the turbines in [[jet engines]]. Several nickel based superalloy compositions are described, such as EPM-102 (with 3% Ru), TMS-162 (with 6% Ru), TMS-138,<ref>{{cite news| title=Fourth generation nickel base single crystal superalloy. TMS-138 / 138A |url=http://sakimori.nims.go.jp/catalog/TMS-138-A.pdf |date=July 2006|work=High Temperature Materials Center, National Institute for Materials Science, Japan|archive-url=https://web.archive.org/web/20130418105851/http://sakimori.nims.go.jp/catalog/TMS-138-A.pdf|archive-date=18 April 2013}}</ref> and TMS-174,<ref>{{cite journal|author=Koizumi, Yutaka |display-authors=etal |title=Development of a Next-Generation Ni-base Single Crystal Superalloy |url=http://nippon.zaidan.info/seikabutsu/2003/00916/pdf/igtc2003tokyo_ts119.pdf |journal=Proceedings of the International Gas Turbine Congress, Tokyo 2–7 November 2003 |archive-date=10 January 2014 |archive-url=https://web.archive.org/web/20140110170053/http://nippon.zaidan.info/seikabutsu/2003/00916/pdf/igtc2003tokyo_ts119.pdf}}</ref><ref>{{cite news| title=Joint Development of a Fourth Generation Single Crystal Superalloy |author=Walston, S. |author2=Cetel, A. |author3=MacKay, R. |author4=O'Hara, K. |author5=Duhl, D. |author6=Dreshfield, R. |date=December 2004 |work=NASA |url=https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20050019231_2005000097.pdf}}</ref> the latter two containing 6% [[rhenium]].<ref>{{cite journal|doi = 10.1007/s11041-006-0099-6|title = Effect of high-gradient directed crystallization on the structure and properties of rhenium-bearing single-crystal alloy|date = 2006|last1 = Bondarenko |first1 = Yu. A.|journal = Metal Science and Heat Treatment|volume = 48|page = 360|last2 = Kablov|first2 = E. N.|last3 = Surova|first3 = V. A.|last4 = Echin|first4 = A. B.|s2cid = 136907279|issue = 7–8|bibcode = 2006MSHT...48..360B}}</ref> [[Fountain pen]] nibs are frequently tipped with ruthenium alloy. From 1944 onward, the [[Parker 51]] fountain pen was fitted with the "RU" nib, a 14K gold nib tipped with 96.2% ruthenium and 3.8% [[iridium]].<ref>{{cite journal |url=http://www.nibs.com/article4.html |journal=The PENnant |volume=XIII |issue=2 |date=1999 |title=Notes from the Nib Works—Where's the Iridium? |author=Mottishaw, J. |archive-url=https://web.archive.org/web/20020604135505/http://www.nibs.com/article4.html|archive-date=4 June 2002}}</ref> |
||
| Line 117: | Line 118: | ||
Ruthenium is a component of [[mixed-metal oxide]] (MMO) anodes used for cathodic protection of underground and submerged structures, and for electrolytic cells for such processes as [[Chlorine production|generating chlorine]] from salt water.<ref>{{cite book|title =Materials Handbook: A Concise Desktop Reference|chapter-url = https://books.google.com/books?id=ArsfQZig_9AC&pg=PT612|pages = 581–582| first1 = François|last1 = Cardarelli|chapter = Dimensionally Stable Anodes (DSA) for Chlorine Evolution|isbn = 978-1-84628-668-1|date =2008|publisher =Springer|location =London}}</ref> The [[fluorescence]] of some ruthenium complexes is quenched by oxygen, finding use in [[optode]] sensors for oxygen.<ref>{{cite book|title = Chemical sensors in oceanography|chapter = Oxygen Microoptode|page = 150|first1 = Mark S.|last1 = Varney|date = 2000|isbn = 978-90-5699-255-2|publisher = Gordon & Breach|location = Amsterdam}}</ref> [[Ruthenium red]], [(NH<sub>3</sub>)<sub>5</sub>Ru-O-Ru(NH<sub>3</sub>)<sub>4</sub>-O-Ru(NH<sub>3</sub>)<sub>5</sub>]<sup>6+</sup>, is a [[biological stain]] used to stain [[polyanion]]ic molecules such as [[pectin]] and [[nucleic acids]] for [[light microscopy]] and [[electron microscopy]].<ref>{{cite book|title = Stains and cytochemical methods|chapter = Ruthenium red|first1 = M. A.|last1 = Hayat|chapter-url = https://books.google.com/books?id=oGj7MLioFlQC&pg=PA305|pages = [https://archive.org/details/stainscytochemic0000haya/page/305 305–310]|isbn = 978-0-306-44294-0|date = 1993|publisher = Plenum Press|location = New York, NY|url = https://archive.org/details/stainscytochemic0000haya/page/305}}</ref> The beta-decaying isotope 106 of ruthenium is used in radiotherapy of eye tumors, mainly [[malignant melanoma]]s of the [[uvea]].<ref>{{cite book|url = https://books.google.com/books?id=Aa83RoXCNk0C&pg=PA97|title = Radiotherapy of ocular disease, Ausgabe 13020|first1 = T.|last1 = Wiegel|isbn = 978-3-8055-6392-5|date = 1997|publisher = Karger|location = Basel, Freiburg}}</ref> Ruthenium-centered complexes are being researched for possible anticancer properties.<ref>{{cite journal |last1=Richards |first1=Adair D. |last2=Rodger |first2=Alison |title=Synthetic metallomolecules as agents for the control of DNA structure |journal=Chem. Soc. Rev. |date=2007 |volume=36 |issue=3 |pages=471–483 |doi=10.1039/b609495c |pmid=17325786 |url=http://wrap.warwick.ac.uk/2189/1/WRAP_Richards_Revised_article1.pdf }}</ref> Compared with platinum complexes, those of ruthenium show greater resistance to hydrolysis and more selective action on tumors.{{citation needed|date = April 2012}} |
Ruthenium is a component of [[mixed-metal oxide]] (MMO) anodes used for cathodic protection of underground and submerged structures, and for electrolytic cells for such processes as [[Chlorine production|generating chlorine]] from salt water.<ref>{{cite book|title =Materials Handbook: A Concise Desktop Reference|chapter-url = https://books.google.com/books?id=ArsfQZig_9AC&pg=PT612|pages = 581–582| first1 = François|last1 = Cardarelli|chapter = Dimensionally Stable Anodes (DSA) for Chlorine Evolution|isbn = 978-1-84628-668-1|date =2008|publisher =Springer|location =London}}</ref> The [[fluorescence]] of some ruthenium complexes is quenched by oxygen, finding use in [[optode]] sensors for oxygen.<ref>{{cite book|title = Chemical sensors in oceanography|chapter = Oxygen Microoptode|page = 150|first1 = Mark S.|last1 = Varney|date = 2000|isbn = 978-90-5699-255-2|publisher = Gordon & Breach|location = Amsterdam}}</ref> [[Ruthenium red]], [(NH<sub>3</sub>)<sub>5</sub>Ru-O-Ru(NH<sub>3</sub>)<sub>4</sub>-O-Ru(NH<sub>3</sub>)<sub>5</sub>]<sup>6+</sup>, is a [[biological stain]] used to stain [[polyanion]]ic molecules such as [[pectin]] and [[nucleic acids]] for [[light microscopy]] and [[electron microscopy]].<ref>{{cite book|title = Stains and cytochemical methods|chapter = Ruthenium red|first1 = M. A.|last1 = Hayat|chapter-url = https://books.google.com/books?id=oGj7MLioFlQC&pg=PA305|pages = [https://archive.org/details/stainscytochemic0000haya/page/305 305–310]|isbn = 978-0-306-44294-0|date = 1993|publisher = Plenum Press|location = New York, NY|url = https://archive.org/details/stainscytochemic0000haya/page/305}}</ref> The beta-decaying isotope 106 of ruthenium is used in radiotherapy of eye tumors, mainly [[malignant melanoma]]s of the [[uvea]].<ref>{{cite book|url = https://books.google.com/books?id=Aa83RoXCNk0C&pg=PA97|title = Radiotherapy of ocular disease, Ausgabe 13020|first1 = T.|last1 = Wiegel|isbn = 978-3-8055-6392-5|date = 1997|publisher = Karger|location = Basel, Freiburg}}</ref> Ruthenium-centered complexes are being researched for possible anticancer properties.<ref>{{cite journal |last1=Richards |first1=Adair D. |last2=Rodger |first2=Alison |title=Synthetic metallomolecules as agents for the control of DNA structure |journal=Chem. Soc. Rev. |date=2007 |volume=36 |issue=3 |pages=471–483 |doi=10.1039/b609495c |pmid=17325786 |url=http://wrap.warwick.ac.uk/2189/1/WRAP_Richards_Revised_article1.pdf }}</ref> Compared with platinum complexes, those of ruthenium show greater resistance to hydrolysis and more selective action on tumors.{{citation needed|date = April 2012}} |
||
[[Ruthenium tetroxide]] exposes latent fingerprints by reacting on contact with fatty oils or fats with sebaceous contaminants and producing brown/black ruthenium dioxide pigment.<ref>[https://www.ncjrs.gov/App/publications/abstract.aspx?ID=172645 NCJRS Abstract – National Criminal Justice Reference Service]. Ncjrs.gov. Retrieved on 2017-02-28.</ref> |
[[Ruthenium tetroxide]] exposes latent fingerprints by reacting on contact with fatty oils or fats with sebaceous contaminants and producing brown/black ruthenium dioxide pigment.<ref>[https://www.ncjrs.gov/App/publications/abstract.aspx?ID=172645 NCJRS Abstract – National Criminal Justice Reference Service] {{Webarchive|url=https://web.archive.org/web/20220620131906/https://www.ncjrs.gov/App/publications/abstract.aspx?ID=172645 |date=20 June 2022 }}. Ncjrs.gov. Retrieved on 2017-02-28.</ref> |
||
| ⚫ | |||
[[File:Ru-intercalated halloysite nanotubes 3.jpg|thumb|[[Halloysite]] nanotubes intercalated with ruthenium catalytic [[nanoparticle]]s<ref name="stam">{{cite journal|doi=10.1080/14686996.2016.1278352|title=Formation of metal clusters in halloysite clay nanotubes|pmc=5402758|journal=Science and Technology of Advanced Materials|volume=18|issue=1|pages=147–151|year=2017|last1=Vinokurov|first1=Vladimir A.|last2=Stavitskaya|first2=Anna V.|last3=Chudakov|first3=Yaroslav A.|last4=Ivanov|first4=Evgenii V.|last5=Shrestha|first5=Lok Kumar|last6=Ariga|first6=Katsuhiko|last7=Darrat|first7=Yusuf A.|last8=Lvov|first8=Yuri M.|pmid=28458738|bibcode=2017STAdM..18..147V}}</ref>]] |
|||
| ⚫ | |||
Electronics is the largest use of ruthenium.<ref name="usgs"/> Ru metal is particularly nonvolatile, which is advantageous in [[microelectronic]] devices. Ru and its main oxide RuO<sub>2</sub> have comparable electrical resistivities.<ref>{{cite journal |last1=Kwon |first1=Oh-Kyum |last2=Kim |first2=Jae-Hoon |last3=Park |first3=Hyoung-Sang |last4=Kang |first4=Sang-Won |title=Atomic Layer Deposition of Ruthenium Thin Films for Copper Glue Layer |journal=Journal of the Electrochemical Society |date=2004 |volume=151 |issue=2 |pages=G109 |doi=10.1149/1.1640633 |bibcode=2004JElS..151G.109K }}</ref> Copper can be directly electroplated onto ruthenium,<ref>{{cite journal |last1=Moffat |first1=T. P. |last2=Walker |first2=M. |last3=Chen |first3=P. J. |last4=Bonevich |first4=J. E. |last5=Egelhoff |first5=W. F. |last6=Richter |first6=L. |last7=Witt |first7=C. |last8=Aaltonen |first8=T. |last9=Ritala |first9=M. |last10=Leskelä |first10=M. |last11=Josell |first11=D. |title=Electrodeposition of Cu on Ru Barrier Layers for Damascene Processing |journal=Journal of the Electrochemical Society |date=2006 |volume=153 |issue=1 |pages=C37 |doi=10.1149/1.2131826 |bibcode=2006JElS..153C..37M |url=https://zenodo.org/record/1236224 }}</ref> particular applications include [[barrier layer]]s, transistor gates, and interconnects.<ref>{{cite journal |doi=10.1149/2.0281901jes|title=Review—Ruthenium as Diffusion Barrier Layer in Electronic Interconnects: Current Literature with a Focus on Electrochemical Deposition Methods|year=2019|last1=Bernasconi|first1=R.|last2=Magagnin|first2=L.|journal=Journal of the Electrochemical Society|volume=166|issue=1|pages=D3219–D3225|bibcode=2019JElS..166D3219B|s2cid=104430143|doi-access=free}}</ref> Ru films can be deposited by [[chemical vapor deposition]] using volatile complexes such as [[ruthenium tetroxide]] and the [[organoruthenium compound]] ([[cyclohexadiene]])Ru(CO)<sub>3</sub>.<ref>{{cite journal |doi=10.1134/S106373971001004X|title=Low-temperature pulsed CVD of ruthenium thin films for micro- and nanoelectronic applications, Part 1: Equipment and methodology|year=2010|last1=Vasilyev|first1=V. Yu.|journal=Russian Microelectronics|volume=39|pages=26–33|s2cid=122854468}}</ref> |
Electronics is the largest use of ruthenium.<ref name="usgs"/> Ru metal is particularly nonvolatile, which is advantageous in [[microelectronic]] devices. Ru and its main oxide RuO<sub>2</sub> have comparable electrical resistivities.<ref>{{cite journal |last1=Kwon |first1=Oh-Kyum |last2=Kim |first2=Jae-Hoon |last3=Park |first3=Hyoung-Sang |last4=Kang |first4=Sang-Won |title=Atomic Layer Deposition of Ruthenium Thin Films for Copper Glue Layer |journal=Journal of the Electrochemical Society |date=2004 |volume=151 |issue=2 |pages=G109 |doi=10.1149/1.1640633 |bibcode=2004JElS..151G.109K }}</ref> Copper can be directly electroplated onto ruthenium,<ref>{{cite journal |last1=Moffat |first1=T. P. |last2=Walker |first2=M. |last3=Chen |first3=P. J. |last4=Bonevich |first4=J. E. |last5=Egelhoff |first5=W. F. |last6=Richter |first6=L. |last7=Witt |first7=C. |last8=Aaltonen |first8=T. |last9=Ritala |first9=M. |last10=Leskelä |first10=M. |last11=Josell |first11=D. |title=Electrodeposition of Cu on Ru Barrier Layers for Damascene Processing |journal=Journal of the Electrochemical Society |date=2006 |volume=153 |issue=1 |pages=C37 |doi=10.1149/1.2131826 |bibcode=2006JElS..153C..37M |url=https://zenodo.org/record/1236224 }}</ref> particular applications include [[barrier layer]]s, transistor gates, and interconnects.<ref>{{cite journal |doi=10.1149/2.0281901jes|title=Review—Ruthenium as Diffusion Barrier Layer in Electronic Interconnects: Current Literature with a Focus on Electrochemical Deposition Methods|year=2019|last1=Bernasconi|first1=R.|last2=Magagnin|first2=L.|journal=Journal of the Electrochemical Society|volume=166|issue=1|pages=D3219–D3225|bibcode=2019JElS..166D3219B|s2cid=104430143|doi-access=free}}</ref> Ru films can be deposited by [[chemical vapor deposition]] using volatile complexes such as [[ruthenium tetroxide]] and the [[organoruthenium compound]] ([[cyclohexadiene]])Ru(CO)<sub>3</sub>.<ref>{{cite journal |doi=10.1134/S106373971001004X|title=Low-temperature pulsed CVD of ruthenium thin films for micro- and nanoelectronic applications, Part 1: Equipment and methodology|year=2010|last1=Vasilyev|first1=V. Yu.|journal=Russian Microelectronics|volume=39|pages=26–33|s2cid=122854468}}</ref> |
||
===Catalysis=== |
=== Catalysis === |
||
| ⚫ | Many ruthenium-containing compounds exhibit useful catalytic properties. Solutions containing [[ruthenium trichloride]] are highly active for [[olefin metathesis]]. Such catalysts are used commercially for the production of polynorbornene for example.<ref name="KO">{{cite encyclopedia |encyclopedia=Kirk-Othmer Encyclopedia of Chemical Technology |author1=Delaude, Lionel |author2=Noels, Alfred F. |year=2005| doi=10.1002/0471238961.metanoel.a01 |place=Weinheim|publisher=Wiley-VCH |chapter=Metathesis|isbn=978-0471238966}}</ref> Well defined ruthenium [[carbene]] and [[alkylidene]] complexes show similar reactivity but are only used on small-scale.<ref>{{cite journal|doi = 10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G|title=Olefin Metathesis and Beyond|author=Fürstner, Alois|journal=Angewandte Chemie International Edition|volume=39 |issue=17|date=2000|pages=3012–3043 |pmid=11028025|bibcode=2000AngCh..39.3012F }}</ref> The [[Grubbs' catalyst]]s for example have been employed in the preparation of drugs and advanced materials. |
||
Many ruthenium-containing compounds exhibit useful catalytic properties. The catalysts are conveniently divided into those that are soluble in the reaction medium, [[homogeneous catalyst]]s, and those that are not, which are called [[heterogeneous catalyst]]s. |
|||
| ⚫ | |||
====Homogeneous catalysis==== |
|||
| ⚫ | Solutions containing [[ruthenium trichloride]] are highly active for [[olefin metathesis]]. Such catalysts are used commercially for the production of polynorbornene for example.<ref name="KO">{{cite encyclopedia |encyclopedia=Kirk-Othmer Encyclopedia of Chemical Technology |author1=Delaude, Lionel |author2=Noels, Alfred F. |year=2005| doi=10.1002/0471238961.metanoel.a01 |place=Weinheim|publisher=Wiley-VCH |chapter=Metathesis|isbn=978-0471238966}}</ref> Well defined ruthenium [[carbene]] and [[alkylidene]] complexes show similar reactivity but are only used on small-scale.<ref>{{cite journal|doi = 10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G|title=Olefin Metathesis and Beyond|author=Fürstner, Alois|journal=Angewandte Chemie International Edition|volume=39 |issue=17|date=2000|pages=3012–3043 |pmid=11028025}}</ref> The [[Grubbs' catalyst]]s for example have been employed in the preparation of drugs and advanced materials. |
||
| ⚫ | Some ruthenium complexes are highly active catalysts for [[transfer hydrogenation]]s (sometimes referred to as "borrowing hydrogen" reactions). [[Chiral]] ruthenium complexes, introduced by [[Ryoji Noyori]], are employed for the [[asymmetric hydrogenation|enantioselective hydrogenation]] of [[ketone]]s, [[aldehyde]]s, and [[imine]]s.<ref name="citation 21">{{citation |author1=Noyori, R. |author2=Ohkuma, T. |author3=Kitamura, M. |author4=Takaya, H. |author5=Sayo, N. |author6=Kumobayashi, H. |author7=Akutagawa, S. |journal=[[Journal of the American Chemical Society]]|title=Asymmetric Hydrogenation of β-Keto Carboxylic Esters. A Practical, Purely Chemical Access to β-Hydroxy Esters in High Enantiomeric Purity|year=1987|volume=109|issue=19 |pages=5856|doi=10.1021/ja00253a051}}</ref> A typical catalyst is (cymene)Ru(S,S-Ts[[DPEN]]):<ref>{{OrgSynth | author = Ikariya, Takao; Hashiguchi, Shohei; Murata, Kunihiko and [[Ryōji Noyori|Noyori, Ryōji]]| title = Preparation of Optically Active (R,R)-Hydrobenzoin from Benzoin or Benzil| vol = 82 | pages = 10 | year = 2005 | prep = v82p0010}}</ref><ref>{{Cite journal | title = Synthesis of Optically Active 1,2,3,4-Tetrahydroquinolines via Asymmetric Hydrogenation Using Iridium-Diamine Catalyst|journal=Org. Synth.|volume = 92 | pages = 213–226 | year = 2015 | doi = 10.15227/orgsyn.092.0213|last1=Chen|first1=Fei|doi-access = free}}</ref>A [[Nobel Prize in Chemistry]] was awarded in 2001 to [[Ryōji Noyori]] for contributions to the field of [[asymmetric hydrogenation]]. |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
A [[Nobel Prize in Chemistry]] was awarded in 2001 to [[Ryōji Noyori]] for contributions to the field of [[asymmetric hydrogenation]]. |
|||
====Heterogeneous catalysis==== |
|||
Ruthenium-promoted cobalt catalysts are used in [[Fischer–Tropsch process|Fischer–Tropsch synthesis]].<ref>{{cite journal|doi=10.1016/S0926-860X(99)00160-X|title=Short history and present trends of Fischer–Tropsch synthesis|journal=Applied Catalysis A: General|volume=186|issue=1–2|pages=3–12|year=1999|last1=Schulz|first1=Hans}}</ref> |
Ruthenium-promoted cobalt catalysts are used in [[Fischer–Tropsch process|Fischer–Tropsch synthesis]].<ref>{{cite journal|doi=10.1016/S0926-860X(99)00160-X|title=Short history and present trends of Fischer–Tropsch synthesis|journal=Applied Catalysis A: General|volume=186|issue=1–2|pages=3–12|year=1999|last1=Schulz|first1=Hans}}</ref> |
||
=== Biology === |
|||
The inorganic dye ammoniated ruthenium oxychloride, also known as [[ruthenium red]], is used in [[histology]] to [[Staining|stain]] [[aldehyde]] [[Fixation (histology)|fixed]] [[mucopolysaccharides]]. |
|||
===Emerging applications=== |
===Emerging applications=== |
||
| ⚫ | Ruthenium-based compounds are components of [[dye-sensitized solar cell]]s, which are proposed as [[low-cost solar cell]] system.<ref>{{cite journal|doi =10.1021/ja058540p|title =High Molar Extinction Coefficient Heteroleptic Ruthenium Complexes for Thin Film Dye-Sensitized Solar Cells|date =2006|last1 =Kuang|first1 =Daibin|last2 =Ito|first2 =Seigo|last3 =Wenger|first3 =Bernard|last4 =Klein|first4 =Cedric|last5 =Moser|first5 =Jacques-E|last6 =Humphry-Baker|first6 =Robin|last7 =Zakeeruddin|first7 =Shaik M.|last8 =Grätzel|first8 =Michael|s2cid =39111991|journal =Journal of the American Chemical Society|volume =128|pages =4146–54|pmid =16551124|issue =12}}</ref> |
||
| ⚫ | |||
| ⚫ | |||
Many ruthenium-based oxides show very unusual properties, such as a [[quantum critical point]] behavior,<ref>{{cite journal|last1 = Perry|first1 = R.|last2 = Kitagawa|first2 = K.|last3 = Grigera|first3 = S.|last4 = Borzi|first4 = R.|last5 = MacKenzie|first5 = A.|last6 = Ishida|first6 = K.|last7 = Maeno|first7 = Y.|s2cid = 26241456|title = Multiple First-Order Metamagnetic Transitions and Quantum Oscillations in Ultrapure Sr.<sub>3</sub>Ru<sub>2</sub>O<sub>7</sub>|journal = Physical Review Letters|volume = 92|date = 2004|doi = 10.1103/PhysRevLett.92.166602|pmid = 15169251|bibcode=2004PhRvL..92p6602P|arxiv = cond-mat/0401371|issue = 16|pages = 166602}}</ref> exotic [[superconductivity]] (in its [[distrontium ruthenate|strontium ruthenate]] form),<ref>{{cite journal|last1 = Maeno|first1 = Yoshiteru|last2 = Rice|first2 = T. Maurice|last3 = Sigrist|first3 = Manfred|title = The Intriguing Superconductivity of Strontium Ruthenate|doi = 10.1063/1.1349611|date = 2001|page = 42|volume = 54|issue = 1|journal = Physics Today|url = http://repository.kulib.kyoto-u.ac.jp/dspace/bitstream/2433/49957/1/PTO000042.pdf|bibcode = 2001PhT....54a..42M| hdl=2433/49957 |doi-access = free}}</ref> and high-temperature [[ferromagnetism]].<ref>{{cite journal|last1 = Shlyk|first1 = Larysa|last2 = Kryukov|first2 = Sergiy|last3 = Schüpp-Niewa|first3 = Barbara|last4 = Niewa|first4 = Rainer|last5 = De Long|first5 = Lance E.|title = High-Temperature Ferromagnetism and Tunable Semiconductivity of (Ba, Sr)M<sub>2±x</sub>Ru<sub>4∓x</sub>O<sub>11</sub> (M = Fe, Co): A New Paradigm for Spintronics|journal = Advanced Materials|volume = 20|page = 1315|date = 2008|doi = 10.1002/adma.200701951|issue = 7| bibcode=2008AdM....20.1315S |s2cid = 136558050}}</ref> |
|||
| ⚫ | |||
Little is known about the health effects of ruthenium<ref name=":0">{{Cite web|title=Ruthenium|url=https://www.espimetals.com/index.php/msds/237-Ruthenium|access-date=2020-07-26|website=espimetals.com}}</ref> and it is relatively rare for people to encounter ruthenium compounds.<ref name=":1">{{Cite web|title=Ruthenium (Ru) - Chemical properties, Health and Environmental effects|url=https://www.lenntech.com/periodic/elements/ru.htm|access-date=2020-07-26|website=lenntech.com}}</ref> Metallic ruthenium is [[Chemically inert|inert]] (is not [[Reactivity (chemistry)|chemically reactive]]).<ref name=":0" /> Some compounds such as [[RuO4|ruthenium oxide (RuO<sub>4</sub>)]] are highly toxic and volatile.<ref name=":1" /> |
Little is known about the health effects of ruthenium<ref name=":0">{{Cite web|title=Ruthenium|url=https://www.espimetals.com/index.php/msds/237-Ruthenium|access-date=2020-07-26|website=espimetals.com}}</ref> and it is relatively rare for people to encounter ruthenium compounds.<ref name=":1">{{Cite web|title=Ruthenium (Ru) - Chemical properties, Health and Environmental effects|url=https://www.lenntech.com/periodic/elements/ru.htm|access-date=2020-07-26|website=lenntech.com}}</ref> Metallic ruthenium is [[Chemically inert|inert]] (is not [[Reactivity (chemistry)|chemically reactive]]).<ref name=":0" /> Some compounds such as [[RuO4|ruthenium oxide (RuO<sub>4</sub>)]] are highly toxic and volatile.<ref name=":1" /> |
||
==See also== |
== See also == |
||
* [[Airborne radioactivity increase in Europe in autumn 2017]] |
* [[Airborne radioactivity increase in Europe in autumn 2017]] |
||
==Notes== |
== Notes == |
||
{{notelist}} |
{{notelist}} |
||
==References== |
== References == |
||
{{ |
{{reflist|30em}} |
||
==Bibliography== |
== Bibliography == |
||
* {{Greenwood&Earnshaw2nd}} |
* {{Greenwood&Earnshaw2nd}} |
||
*{{cite book | editor-last= Haynes |editor-first=William M. | date = 2016| title = CRC Handbook of Chemistry and Physics | edition = 97th | publisher = [[CRC Press]] | isbn = 9781498754293| title-link = CRC Handbook of Chemistry and Physics}} |
* {{cite book | editor-last= Haynes |editor-first=William M. | date = 2016| title = CRC Handbook of Chemistry and Physics | edition = 97th | publisher = [[CRC Press]] | isbn = 9781498754293| title-link = CRC Handbook of Chemistry and Physics}} |
||
==External links== |
== External links == |
||
{{Commons|Ruthenium}} |
{{Commons|Ruthenium}} |
||
{{Wiktionary|ruthenium}} |
{{Wiktionary|ruthenium}} |
||
Latest revision as of 02:37, 16 November 2024
 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ruthenium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ruːˈθiːniəm/ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white metallic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Ru) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ruthenium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 44 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d7 5s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 15, 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2607 K (2334 °C, 4233 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 4423 K (4150 °C, 7502 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 12.364 g/cm3[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 10.65 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 38.59 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 619 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 24.06 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | common: +3, +4 −2,[4] +1,[4] +2,[4] +5,[4] +6,[4] +7,[4] +8[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 134 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 146±7 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) (hP2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lattice constants | a = 270.58 pm c = 428.16 pm (at 20 °C)[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 6.78×10−6/K (at 20 °C)[3][a] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 117
αa 5.77 αc 8.80 αavr 6.78 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 71 nΩ⋅m (at 0 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | +39×10−6 cm3/mol (298 K)[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 447 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 173 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 220 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 5970 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 6.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 2160 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-18-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | from Latin Ruthenia for Russia[6][7] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Karl Ernst Claus (1844) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of ruthenium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ruthenium is a chemical element; it has symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is unreactive to most chemicals. Karl Ernst Claus, a Russian scientist of Baltic-German ancestry, discovered the element in 1844 at Kazan State University and named it in honor of Russia, using the Latin name Ruthenia. Ruthenium is usually found as a minor component of platinum ores; the annual production has risen from about 19 tonnes in 2009[9] to some 35.5 tonnes in 2017.[10] Most ruthenium produced is used in wear-resistant electrical contacts and thick-film resistors. A minor application for ruthenium is in platinum alloys and as a chemistry catalyst. A new application of ruthenium is as the capping layer for extreme ultraviolet photomasks. Ruthenium is generally found in ores with the other platinum group metals in the Ural Mountains and in North and South America. Small but commercially important quantities are also found in pentlandite extracted from Sudbury, Ontario, and in pyroxenite deposits in South Africa.[11]
Characteristics
[edit]Physical properties
[edit]
Ruthenium, a polyvalent hard white metal, is a member of the platinum group and is in group 8 of the periodic table:
| Z | Element | No. of electrons/shell |
|---|---|---|
| 26 | iron | 2, 8, 14, 2 |
| 44 | ruthenium | 2, 8, 18, 15, 1 |
| 76 | osmium | 2, 8, 18, 32, 14, 2 |
| 108 | hassium | 2, 8, 18, 32, 32, 14, 2 |
Whereas all other group 8 elements have two electrons in the outermost shell, in ruthenium the outermost shell has only one electron (the final electron is in a lower shell). This anomaly is also observed in the neighboring metals niobium (41), molybdenum (42), and rhodium (45).
Chemical properties
[edit]Ruthenium has four crystal modifications and does not tarnish at ambient conditions; it oxidizes upon heating to 800 °C (1,070 K). Ruthenium dissolves in fused alkalis to give ruthenates (RuO2−
4). It is not attacked by acids (even aqua regia) but is attacked by sodium hypochlorite at room temperature, and halogens at high temperatures.[11] Ruthenium is most readily attacked by oxidizing agents.[12] Small amounts of ruthenium can increase the hardness of platinum and palladium. The corrosion resistance of titanium is increased markedly by the addition of a small amount of ruthenium.[11] The metal can be plated by electroplating and by thermal decomposition. A ruthenium–molybdenum alloy is known to be superconductive at temperatures below 10.6 K.[11] Ruthenium is the only 4d transition metal that can assume the group oxidation state +8, and even then it is less stable there than the heavier congener osmium: this is the first group from the left of the table where the second and third-row transition metals display notable differences in chemical behavior. Like iron but unlike osmium, ruthenium can form aqueous cations in its lower oxidation states of +2 and +3.[13]
Ruthenium is the first in a downward trend in the melting and boiling points and atomization enthalpy in the 4d transition metals after the maximum seen at molybdenum, because the 4d subshell is more than half full and the electrons are contributing less to metallic bonding. (Technetium, the previous element, has an exceptionally low value that is off the trend due to its half-filled [Kr]4d55s2 configuration, though it is not as far off the trend in the 4d series as manganese in the 3d transition series.)[14] Unlike the lighter congener iron, ruthenium is paramagnetic at room temperature, as iron also is above its Curie point.[15]
The reduction potentials in acidic aqueous solution for some common ruthenium species are shown below:[16]
| Potential | Reaction | |
|---|---|---|
| 0.455 V | Ru2+ + 2e− | ↔ Ru |
| 0.249 V | Ru3+ + e− | ↔ Ru2+ |
| 1.120 V | RuO2 + 4H+ + 2e− | ↔ Ru2+ + 2H2O |
| 1.563 V | RuO2− 4 + 8H+ + 4e− |
↔ Ru2+ + 4H2O |
| 1.368 V | RuO− 4 + 8H+ + 5e− |
↔ Ru2+ + 4H2O |
| 1.387 V | RuO4 + 4H+ + 4e− | ↔ RuO2 + 2H2O |
Isotopes
[edit]Naturally occurring ruthenium is composed of seven stable isotopes. Additionally, 34 radioactive isotopes have been discovered. Of these radioisotopes, the most stable are 106Ru with a half-life of 373.59 days, 103Ru with a half-life of 39.26 days and 97Ru with a half-life of 2.9 days.[17][18]
Fifteen other radioisotopes have been characterized with atomic weights ranging from 89.93 Da (90Ru) to 114.928 Da (115Ru). Most of these have half-lives that are less than five minutes; the exceptions are 95Ru (half-life: 1.643 hours) and 105Ru (half-life: 4.44 hours).[17][18]
The primary decay mode before the most abundant isotope, 102Ru, is electron capture while the primary mode after is beta emission. The primary decay product before 102Ru is technetium and the primary decay product after is rhodium.[17][18]
106Ru is a product of fission of a nucleus of uranium or plutonium. High concentrations of detected atmospheric 106Ru were associated with an alleged undeclared nuclear accident in Russia in 2017.[19]
Occurrence
[edit]Ruthenium is found in about 100 parts per trillion in the Earth's crust, making it the 78th most abundant element.[20] It is generally found in ores with the other platinum group metals in the Ural Mountains and in North and South America. Small but commercially important quantities are also found in pentlandite extracted from Sudbury, Ontario, Canada, and in pyroxenite deposits in South Africa. The native form of ruthenium is a very rare mineral (Ir replaces part of Ru in its structure).[21][22] Ruthenium has a relatively high fission product yield in nuclear fission; and given that its most long-lived radioisotope has a half life of "only" around a year, there are often proposals to recover ruthenium in a new kind of nuclear reprocessing from spent fuel. An unusual ruthenium deposit can also be found at the natural nuclear fission reactor that was active in Oklo, Gabon, some two billion years ago. Indeed, the isotope ratio of ruthenium found there was one of several ways used to confirm that a nuclear fission chain reaction had indeed occurred at that site in the geological past. Uranium is no longer mined at Oklo, and there have never been serious attempts to recover any of the platinum group metals present there.
Production
[edit]Roughly 30 tonnes of ruthenium are mined each year,[23] and world reserves are estimated at 5,000 tonnes.[24] The composition of the mined platinum group metal (PGM) mixtures varies widely, depending on the geochemical formation. For example, the PGMs mined in South Africa contain on average 11% ruthenium while the PGMs mined in the former USSR contain only 2% (1992).[25][26] Ruthenium, osmium, and iridium are considered the minor platinum group metals.[15]
Ruthenium, like the other platinum group metals, is obtained commercially as a by-product from processing of nickel, copper, and platinum metal ore. During electrorefining of copper and nickel, noble metals such as silver, gold, and the platinum group metals precipitate as anode mud, the feedstock for the extraction.[21][22] The metals are converted to ionized solutes by any of several methods, depending on the composition of the feedstock. One representative method is fusion with sodium peroxide followed by dissolution in aqua regia, and solution in a mixture of chlorine with hydrochloric acid.[27][28] Osmium, ruthenium, rhodium, and iridium are insoluble in aqua regia and readily precipitate, leaving the other metals in solution. Rhodium is separated from the residue by treatment with molten sodium bisulfate. The insoluble residue, containing Ru, Os, and Ir is treated with sodium oxide, in which Ir is insoluble, producing dissolved Ru and Os salts. After oxidation to the volatile oxides, RuO
4 is separated from OsO
4 by precipitation of (NH4)3RuCl6 with ammonium chloride or by distillation or extraction with organic solvents of the volatile osmium tetroxide.[29] Hydrogen is used to reduce ammonium ruthenium chloride, yielding a powder.[11][30] The product is reduced using hydrogen, yielding the metal as a powder or sponge metal that can be treated with powder metallurgy techniques or argon-arc welding.[11][31]
Ruthenium is contained in spent nuclear fuel, both as a direct fission product and as a product of neutron absorption by long-lived fission product 99
Tc. After allowing the unstable isotopes of ruthenium to decay, chemical extraction could yield ruthenium for use in all applications of ruthenium.[32][33]
Ruthenium can also be produced by deliberate nuclear transmutation from 99
Tc. Given its relatively long half life, high fission product yield and high chemical mobility in the environment, 99
Tc is among the most often proposed non-actinides for commercial scale nuclear transmutation. 99
Tc has a relatively large neutron cross section, and because technetium has no stable isotopes, there would not be a problem of neutron activation of stable isotopes. Significant amounts of 99
Tc are produced in nuclear fission. They are also produced as a byproduct of the use of 99m
Tc in nuclear medicine, because this isomer decays to 99
Tc. Exposing the 99
Tc target to strong enough neutron radiation will eventually yield appreciable quantities of ruthenium, which can be chemically separated while consuming 99
Tc.[34][35]
Chemical compounds
[edit]The oxidation states of ruthenium range from 0 to +8, and −2. The properties of ruthenium and osmium compounds are often similar. The +2, +3, and +4 states are the most common. The most prevalent precursor is ruthenium trichloride, a red solid that is poorly defined chemically but versatile synthetically.[30]
Oxides and chalcogenides
[edit]Ruthenium can be oxidized to ruthenium(IV) oxide (RuO2, oxidation state +4), which can, in turn, be oxidized by sodium metaperiodate to the volatile yellow tetrahedral ruthenium tetroxide, RuO4, an aggressive, strong oxidizing agent with structure and properties analogous to osmium tetroxide. RuO4 is mostly used as an intermediate in the purification of ruthenium from ores and radiowastes.[36]
Dipotassium ruthenate (K2RuO4, +6) and potassium perruthenate (KRuO4, +7) are also known.[37] Unlike osmium tetroxide, ruthenium tetroxide is less stable, is strong enough as an oxidising agent to oxidise dilute hydrochloric acid and organic solvents like ethanol at room temperature, and is easily reduced to ruthenate (RuO2−
4) in aqueous alkaline solutions; it decomposes to form the dioxide above 100 °C. Unlike iron but like osmium, ruthenium does not form oxides in its lower +2 and +3 oxidation states.[38] Ruthenium forms dichalcogenides, which are diamagnetic semiconductors crystallizing in the pyrite structure.[38] Ruthenium sulfide (RuS2) occurs naturally as the mineral laurite.
Like iron, ruthenium does not readily form oxoanions and prefers to achieve high coordination numbers with hydroxide ions instead. Ruthenium tetroxide is reduced by cold dilute potassium hydroxide to form black potassium perruthenate, KRuO4, with ruthenium in the +7 oxidation state. Potassium perruthenate can also be produced by oxidising potassium ruthenate, K2RuO4, with chlorine gas. The perruthenate ion is unstable and is reduced by water to form the orange ruthenate. Potassium ruthenate may be synthesized by reacting ruthenium metal with molten potassium hydroxide and potassium nitrate.[39]
Some mixed oxides are also known, such as MIIRuIVO3, Na3RuVO4, Na
2RuV
2O
7, and MII
2LnIII
RuV
O
6.[39]
Halides and oxyhalides
[edit]The highest known ruthenium halide is the hexafluoride, a dark brown solid that melts at 54 °C. It hydrolyzes violently upon contact with water and easily disproportionates to form a mixture of lower ruthenium fluorides, releasing fluorine gas. Ruthenium pentafluoride is a tetrameric dark green solid that is also readily hydrolyzed, melting at 86.5 °C. The yellow ruthenium tetrafluoride is probably also polymeric and can be formed by reducing the pentafluoride with iodine. Among the binary compounds of ruthenium, these high oxidation states are known only in the oxides and fluorides.[40]
Ruthenium trichloride is a well-known compound, existing in a black α-form and a dark brown β-form: the trihydrate is red.[41] Of the known trihalides, trifluoride is dark brown and decomposes above 650 °C, tribromide is dark-brown and decomposes above 400 °C, and triiodide is black.[40] Of the dihalides, difluoride is not known, dichloride is brown, dibromide is black, and diiodide is blue.[40] The only known oxyhalide is the pale green ruthenium(VI) oxyfluoride, RuOF4.[41]
Coordination and organometallic complexes
[edit]
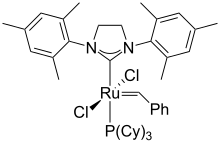
Ruthenium forms a variety of coordination complexes. Examples are the many pentaammine derivatives [Ru(NH3)5L]n+ that often exist for both Ru(II) and Ru(III). Derivatives of bipyridine and terpyridine are numerous, best known being the luminescent tris(bipyridine)ruthenium(II) chloride.
Ruthenium forms a wide range compounds with carbon–ruthenium bonds. Grubbs' catalyst is used for alkene metathesis.[42] Ruthenocene is analogous to ferrocene structurally, but exhibits distinctive redox properties. The colorless liquid ruthenium pentacarbonyl converts in the absence of CO pressure to the dark red solid triruthenium dodecacarbonyl. Ruthenium trichloride reacts with carbon monoxide to give many derivatives including RuHCl(CO)(PPh3)3 and Ru(CO)2(PPh3)3 (Roper's complex). Heating solutions of ruthenium trichloride in alcohols with triphenylphosphine gives tris(triphenylphosphine)ruthenium dichloride (RuCl2(PPh3)3), which converts to the hydride complex chlorohydridotris(triphenylphosphine)ruthenium(II) (RuHCl(PPh3)3).[30]
History
[edit]Though naturally occurring platinum alloys containing all six platinum-group metals were used for a long time by pre-Columbian Americans and known as a material to European chemists from the mid-16th century, not until the mid-18th century was platinum identified as a pure element. That natural platinum contained palladium, rhodium, osmium and iridium was discovered in the first decade of the 19th century.[43] Platinum in alluvial sands of Russian rivers gave access to raw material for use in plates and medals and for the minting of ruble coins, starting in 1828.[44] Residues from platinum production for coinage were available in the Russian Empire, and therefore most of the research on them was done in Eastern Europe.
It is possible that the Polish chemist Jędrzej Śniadecki isolated element 44 (which he called "vestium" after the asteroid Vesta discovered shortly before) from South American platinum ores in 1807. He published an announcement of his discovery in 1808.[45] His work was never confirmed, however, and he later withdrew his claim of discovery.[24]
Jöns Berzelius and Gottfried Osann nearly discovered ruthenium in 1827.[46] They examined residues that were left after dissolving crude platinum from the Ural Mountains in aqua regia. Berzelius did not find any unusual metals, but Osann thought he found three new metals, which he called pluranium, ruthenium, and polinium.[11] This discrepancy led to a long-standing controversy between Berzelius and Osann about the composition of the residues.[47][48] As Osann was not able to repeat his isolation of ruthenium, he eventually relinquished his claims.[47][49] The name "ruthenium" was chosen by Osann because the analysed samples stemmed from the Ural Mountains in Russia.[50]
In 1844, Karl Ernst Claus, a Russian scientist of Baltic German descent, showed that the compounds prepared by Gottfried Osann contained small amounts of ruthenium, which Claus had discovered the same year.[11][43] Claus isolated ruthenium from the platinum residues of rouble production while he was working in Kazan University, Kazan,[47] the same way its heavier congener osmium had been discovered four decades earlier.[20] Claus showed that ruthenium oxide contained a new metal and obtained 6 grams of ruthenium from the part of crude platinum that is insoluble in aqua regia.[47] Choosing the name for the new element, Claus stated: "I named the new body, in honour of my Motherland, ruthenium. I had every right to call it by this name because Mr. Osann relinquished his ruthenium and the word does not yet exist in chemistry."[47][51] The name itself derives from the Latin word Ruthenia.[6][7] In doing so, Claus started a trend that continues to this day – naming an element after a country.[52]
Applications
[edit]Approximately 30.9 tonnes of ruthenium were consumed in 2016, 13.8 of them in electrical applications, 7.7 in catalysis, and 4.6 in electrochemistry.[23]
Because it hardens platinum and palladium alloys, ruthenium is used in electrical contacts, where a thin film is sufficient to achieve the desired durability. With its similar properties to and lower cost than rhodium,[31] electric contacts are a major use of ruthenium.[21][53] The ruthenium plate is applied to the electrical contact and electrode base metal by electroplating[54] or sputtering.[55]
Ruthenium dioxide with lead and bismuth ruthenates are used in thick-film chip resistors.[56][57][58] These two electronic applications account for 50% of the ruthenium consumption.[24]
Ruthenium is seldom alloyed with metals outside the platinum group, where small quantities improve some properties. The added corrosion resistance in titanium alloys led to the development of a special alloy with 0.1% ruthenium.[59] Ruthenium is also used in some advanced high-temperature single-crystal superalloys, with applications that include the turbines in jet engines. Several nickel based superalloy compositions are described, such as EPM-102 (with 3% Ru), TMS-162 (with 6% Ru), TMS-138,[60] and TMS-174,[61][62] the latter two containing 6% rhenium.[63] Fountain pen nibs are frequently tipped with ruthenium alloy. From 1944 onward, the Parker 51 fountain pen was fitted with the "RU" nib, a 14K gold nib tipped with 96.2% ruthenium and 3.8% iridium.[64]
Ruthenium is a component of mixed-metal oxide (MMO) anodes used for cathodic protection of underground and submerged structures, and for electrolytic cells for such processes as generating chlorine from salt water.[65] The fluorescence of some ruthenium complexes is quenched by oxygen, finding use in optode sensors for oxygen.[66] Ruthenium red, [(NH3)5Ru-O-Ru(NH3)4-O-Ru(NH3)5]6+, is a biological stain used to stain polyanionic molecules such as pectin and nucleic acids for light microscopy and electron microscopy.[67] The beta-decaying isotope 106 of ruthenium is used in radiotherapy of eye tumors, mainly malignant melanomas of the uvea.[68] Ruthenium-centered complexes are being researched for possible anticancer properties.[69] Compared with platinum complexes, those of ruthenium show greater resistance to hydrolysis and more selective action on tumors.[citation needed]
Ruthenium tetroxide exposes latent fingerprints by reacting on contact with fatty oils or fats with sebaceous contaminants and producing brown/black ruthenium dioxide pigment.[70]
Electronics
[edit]Electronics is the largest use of ruthenium.[23] Ru metal is particularly nonvolatile, which is advantageous in microelectronic devices. Ru and its main oxide RuO2 have comparable electrical resistivities.[71] Copper can be directly electroplated onto ruthenium,[72] particular applications include barrier layers, transistor gates, and interconnects.[73] Ru films can be deposited by chemical vapor deposition using volatile complexes such as ruthenium tetroxide and the organoruthenium compound (cyclohexadiene)Ru(CO)3.[74]
Catalysis
[edit]Many ruthenium-containing compounds exhibit useful catalytic properties. Solutions containing ruthenium trichloride are highly active for olefin metathesis. Such catalysts are used commercially for the production of polynorbornene for example.[75] Well defined ruthenium carbene and alkylidene complexes show similar reactivity but are only used on small-scale.[76] The Grubbs' catalysts for example have been employed in the preparation of drugs and advanced materials.

RuCl3-catalyzed ring-opening metathesis polymerization reaction giving polynorbornene
Some ruthenium complexes are highly active catalysts for transfer hydrogenations (sometimes referred to as "borrowing hydrogen" reactions). Chiral ruthenium complexes, introduced by Ryoji Noyori, are employed for the enantioselective hydrogenation of ketones, aldehydes, and imines.[77] A typical catalyst is (cymene)Ru(S,S-TsDPEN):[78][79]A Nobel Prize in Chemistry was awarded in 2001 to Ryōji Noyori for contributions to the field of asymmetric hydrogenation.

[RuCl(S,S-TsDPEN)(cymene)]-catalysed (R,R)-hydrobenzoin synthesis (yield 100%, ee >99%)
Ruthenium-promoted cobalt catalysts are used in Fischer–Tropsch synthesis.[80]
Emerging applications
[edit]Ruthenium-based compounds are components of dye-sensitized solar cells, which are proposed as low-cost solar cell system.[81]
Health effects
[edit]Little is known about the health effects of ruthenium[82] and it is relatively rare for people to encounter ruthenium compounds.[83] Metallic ruthenium is inert (is not chemically reactive).[82] Some compounds such as ruthenium oxide (RuO4) are highly toxic and volatile.[83]
See also
[edit]Notes
[edit]- ^ The thermal expansion is anisotropic: the parameters (at 20 °C) for each crystal axis are αa = 5.77×10−6/K, αc = 8.80×10−6/K, and αaverage = αV = 6.78×10−6/K.[3]
References
[edit]- ^ "Standard Atomic Weights: Ruthenium". CIAAW. 1983.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (4 May 2022). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c d Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ a b c d e f g Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- ^ a b Haynes, p. 4.130
- ^ a b Lewis, David E. (2 September 2019). "The Minor Impurity in Spent Ores of the "Siberian Metal": Ruthenium Turns 175". Chemistry – A European Journal. 25 (49): 11394–11401. doi:10.1002/chem.201901922. ISSN 0947-6539.
- ^ a b Pitchkov, By V. N. (1 October 1996). "The Discovery of Ruthenium: "I Named The New Body, in Honour of my Motherland"". Platinum Metals Review. 40 (4): 181–188. doi:10.1595/003214096X404181188. ISSN 0032-1400.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ Summary. Ruthenium. platinum.matthey.com, p. 9 (2009)
- ^ PGM Market Report. platinum.matthey.com, p. 30 (May 2018)
- ^ a b c d e f g h Haynes (2016), p. 4.31.
- ^ Greenwood & Earnshaw (1997), p. 1076.
- ^ Greenwood & Earnshaw (1997), p. 1078.
- ^ Greenwood & Earnshaw (1997), p. 1075.
- ^ a b Greenwood & Earnshaw (1997), p. 1074.
- ^ Greenwood & Earnshaw (1997), p. 1077.
- ^ a b c Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5. Section 11, Table of the Isotopes
- ^ a b c Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ Masson, O.; Steinhauser, G.; Zok, D.; Saunier, O.; Angelov, H.; Babić, D.; Bečková, V.; Bieringer, J.; Bruggeman, M.; Burbidge, C. I.; Conil, S.; Dalheimer, A.; De Geer, L.-E.; De Vismes Ott, A.; Eleftheriadis, K.; Estier, S.; Fischer, H.; Garavaglia, M. G.; Gasco Leonarte, C.; Gorzkiewicz, K.; Hainz, D.; Hoffman, I.; Hýža, M.; Isajenko, K.; Karhunen, T.; Kastlander, J.; Katzlberger, C.; Kierepko, R.; Knetsch, G.-J.; et al. (2019). "Airborne concentrations and chemical considerations of radioactive ruthenium from an undeclared major nuclear release in 2017". PNAS. 116 (34): 16750–16759. Bibcode:2019PNAS..11616750M. doi:10.1073/pnas.1907571116. PMC 6708381. PMID 31350352.
- ^ a b Greenwood & Earnshaw (1997), p. 1071.
- ^ a b c George, Micheal W. "2006 Minerals Yearbook: Platinum-Group Metals" (PDF). United States Geological Survey USGS. Retrieved 16 September 2008.
- ^ a b "Commodity Report: Platinum-Group Metals" (PDF). United States Geological Survey USGS. Retrieved 16 September 2008.
- ^ a b c Loferski, Patricia J.; Ghalayini, Zachary T. and Singerling, Sheryl A. (2018) Platinum-group metals. 2016 Minerals Yearbook. USGS. p. 57.3.
- ^ a b c Emsley, J. (2003). "Ruthenium". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. pp. 368–370. ISBN 978-0-19-850340-8.
- ^ Hartman, H. L.; Britton, S. G., eds. (1992). SME mining engineering handbook. Littleton, Colo.: Society for Mining, Metallurgy, and Exploration. p. 69. ISBN 978-0-87335-100-3.
- ^ Harris, Donald C.; Cabri, Louis J. (1 August 1973). "The nomenclature of the natural alloys of osmium, iridium and ruthenium based on new compositional data of alloys from world-wide occurrences". The Canadian Mineralogist. 12 (2): 104–112. NAID 20000798606.
- ^ Renner, Hermann; Schlamp, Günther; Kleinwächter, Ingo; Drost, Ernst; Lüschow, Hans Martin; Tews, Peter; Panster, Peter; Diehl, Manfred; Lang, Jutta; Kreuzer, Thomas; Knödler, Alfons; Starz, Karl Anton; Dermann, Klaus; Rothaut, Josef; Drieselmann, Ralf; Peter, Catrin; Schiele, Rainer (2001). "Platinum Group Metals and Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a21_075. ISBN 978-3-527-30673-2.
- ^ Seymour, R. J.; O'Farrelly, J. I. (2001). "Platinum-group metals". Kirk Othmer Encyclopedia of Chemical Technology. Wiley. doi:10.1002/0471238961.1612012019052513.a01.pub2. ISBN 978-0471238966.
- ^ Gilchrist, Raleigh (1943). "The Platinum Metals". Chemical Reviews. 32 (3): 277–372. doi:10.1021/cr60103a002. S2CID 96640406.
- ^ a b c Cotton, Simon (1997). Chemistry of Precious Metals. Springer-Verlag New York, LLC. pp. 1–20. ISBN 978-0-7514-0413-5.
- ^ a b Hunt, L. B.; Lever, F. M. (1969). "Platinum Metals: A Survey of Productive Resources to industrial Uses" (PDF). Platinum Metals Review. 13 (4): 126–138. doi:10.1595/003214069X134126138. S2CID 267561907. Archived from the original (PDF) on 29 October 2008. Retrieved 2 June 2009.
- ^ Swain, Pravati; Mallika, C.; Srinivasan, R.; Mudali, U. Kamachi; Natarajan, R. (November 2013). "Separation and recovery of ruthenium: a review". Journal of Radioanalytical and Nuclear Chemistry. 298 (2): 781–796. Bibcode:2013JRNC..298..781S. doi:10.1007/s10967-013-2536-5. S2CID 95804621.
- ^ Johal, Sukhraaj Kaur; Boxall, Colin; Gregson, Colin; Steele, Carl (24 July 2015). "Ruthenium Volatilisation from Reprocessed Spent Nuclear Fuel – Studying the Baseline Thermodynamics of Ru(III)" (PDF). ECS Transactions. 66 (21): 31–42. Bibcode:2015ECSTr..66u..31J. doi:10.1149/06621.0031ecst.
- ^ Konings, R. J. M.; Conrad, R. (1 September 1999). "Transmutation of technetium – results of the EFTTRA-T2 experiment". Journal of Nuclear Materials. 274 (3): 336–340. Bibcode:1999JNuM..274..336K. doi:10.1016/S0022-3115(99)00107-5.
- ^ Peretroukhine, Vladimir; Radchenko, Viacheslav; Kozar', Andrei; Tarasov, Valeriy; Toporov, Iury; Rotmanov, Konstantin; Lebedeva, Lidia; Rovny, Sergey; Ershov, Victor (December 2004). "Technetium transmutation and production of artificial stable ruthenium". Comptes Rendus Chimie. 7 (12): 1215–1218. doi:10.1016/j.crci.2004.05.002.
- ^ Swain, P.; Mallika, C.; Srinivasan, R.; Mudali, U. K.; Natarajan, R. (2013). "Separation and recovery of ruthenium: a review". J. Radioanal. Nucl. Chem. 298 (2): 781–796. Bibcode:2013JRNC..298..781S. doi:10.1007/s10967-013-2536-5. S2CID 95804621.
- ^ Greenwood & Earnshaw (1997), p. [page needed].
- ^ a b Greenwood & Earnshaw (1997), pp. 1080–1081.
- ^ a b Greenwood & Earnshaw (1997), p. 1082.
- ^ a b c Greenwood & Earnshaw (1997), p. 1083.
- ^ a b Greenwood & Earnshaw (1997), p. 1084.
- ^ Hartwig, J. F. (2010) Organotransition Metal Chemistry, from Bonding to Catalysis, University Science Books: New York. ISBN 1-891389-53-X
- ^ a b Weeks, Mary Elvira (1932). "The discovery of the elements. VIII. The platinum metals". Journal of Chemical Education. 9 (6): 1017. Bibcode:1932JChEd...9.1017W. doi:10.1021/ed009p1017.
- ^ Raub, Christoph J. (2004). "The Minting of Platinum Roubles. Part I: History and Current Investigations". Platinum Metals Review. 48 (2): 66–69. doi:10.1595/003214004X4826669.
- ^ Śniadecki, Jędrzej (1808). Rosprawa o nowym metallu w surowey platynie odkrytym [A case about a new metal in raw platinum discovered] (in Polish). Nakładém i Drukiém Józefa Zawadzkiego. OCLC 739088520.
- ^ "New metals in the Uralian platina". The Philosophical Magazine. 2 (11): 391–392. 1 November 1827. doi:10.1080/14786442708674516.
- ^ a b c d e Pitchkov, V. N. (1996). "The Discovery of Ruthenium". Platinum Metals Review. 40 (4): 181–188. doi:10.1595/003214096X404181188.
- ^ Claus, Karl (1845). "О способе добывания чистой платины из руд" [On the method of extracting pure platinum from ores]. Горный журнал (Mining Journal) (in Russian). 7 (3): 157–163.
- ^ Osann, Gottfried (1829). "Berichtigung, meine Untersuchung des uralschen Platins betreffend". Poggendorffs Annalen der Physik und Chemie. 15: 158. doi:10.1002/andp.18290910119.
- ^ Osann, G. (1828). "Fortsetzung der Untersuchung des Platins vom Ural" [Continuation of the study of platinum from the Urals]. Annalen der Physik (in German). 89 (6): 283–297. Bibcode:1828AnP....89..283O. doi:10.1002/andp.18280890609.
- ^ Claus, Karl (1845). "О способе добывания чистой платины из руд" [On the method of extracting pure platinum from ores]. Горный журнал (Mining Journal) (in Russian). 7 (3): 157–163.
- ^ Meija, Juris (September 2021). "Politics at the periodic table". Nature Chemistry. 13 (9): 814–816. Bibcode:2021NatCh..13..814M. doi:10.1038/s41557-021-00780-5. PMID 34480093. S2CID 237405162.
- ^ Rao, C.; Trivedi, D. (2005). "Chemical and electrochemical depositions of platinum group metals and their applications". Coordination Chemistry Reviews. 249 (5–6): 613. doi:10.1016/j.ccr.2004.08.015.
- ^ Weisberg, A. (1999). "Ruthenium plating". Metal Finishing. 97: 297. doi:10.1016/S0026-0576(00)83089-5.
- ^ Merrill L. Minges; et al. (ASM International Handbook Committee) (1989). Electronic materials handbook. Materials Park, OH: ASM International. p. 184. ISBN 978-0-87170-285-2.
- ^ Busana, M. G.; Prudenziati, M.; Hormadaly, J. (2006). "Microstructure development and electrical properties of RuO2-based lead-free thick film resistors". Journal of Materials Science: Materials in Electronics. 17 (11): 951. doi:10.1007/s10854-006-0036-x. hdl:11380/303403. S2CID 135485712.
- ^ Rane, Sunit; Prudenziati, Maria; Morten, Bruno (2007). "Environment friendly perovskite ruthenate based thick film resistors". Materials Letters. 61 (2): 595. Bibcode:2007MatL...61..595R. doi:10.1016/j.matlet.2006.05.015. hdl:11380/307664.
- ^ Slade, Paul G., ed. (1999). Electrical contacts : principles and applications. New York, NY: Dekker. pp. 184, 345. ISBN 978-0-8247-1934-0.
- ^ Schutz, R. W. (April 1996). "Ruthenium Enhanced Titanium Alloys". Platinum Metals Review. 40 (2): 54–61. CiteSeerX 10.1.1.630.7411. doi:10.1595/003214096X4025461. S2CID 267551174.
- ^ "Fourth generation nickel base single crystal superalloy. TMS-138 / 138A" (PDF). High Temperature Materials Center, National Institute for Materials Science, Japan. July 2006. Archived from the original (PDF) on 18 April 2013.
- ^ Koizumi, Yutaka; et al. "Development of a Next-Generation Ni-base Single Crystal Superalloy" (PDF). Proceedings of the International Gas Turbine Congress, Tokyo 2–7 November 2003. Archived from the original (PDF) on 10 January 2014.
- ^ Walston, S.; Cetel, A.; MacKay, R.; O'Hara, K.; Duhl, D.; Dreshfield, R. (December 2004). "Joint Development of a Fourth Generation Single Crystal Superalloy" (PDF). NASA.
- ^ Bondarenko, Yu. A.; Kablov, E. N.; Surova, V. A.; Echin, A. B. (2006). "Effect of high-gradient directed crystallization on the structure and properties of rhenium-bearing single-crystal alloy". Metal Science and Heat Treatment. 48 (7–8): 360. Bibcode:2006MSHT...48..360B. doi:10.1007/s11041-006-0099-6. S2CID 136907279.
- ^ Mottishaw, J. (1999). "Notes from the Nib Works—Where's the Iridium?". The PENnant. XIII (2). Archived from the original on 4 June 2002.
- ^ Cardarelli, François (2008). "Dimensionally Stable Anodes (DSA) for Chlorine Evolution". Materials Handbook: A Concise Desktop Reference. London: Springer. pp. 581–582. ISBN 978-1-84628-668-1.
- ^ Varney, Mark S. (2000). "Oxygen Microoptode". Chemical sensors in oceanography. Amsterdam: Gordon & Breach. p. 150. ISBN 978-90-5699-255-2.
- ^ Hayat, M. A. (1993). "Ruthenium red". Stains and cytochemical methods. New York, NY: Plenum Press. pp. 305–310. ISBN 978-0-306-44294-0.
- ^ Wiegel, T. (1997). Radiotherapy of ocular disease, Ausgabe 13020. Basel, Freiburg: Karger. ISBN 978-3-8055-6392-5.
- ^ Richards, Adair D.; Rodger, Alison (2007). "Synthetic metallomolecules as agents for the control of DNA structure" (PDF). Chem. Soc. Rev. 36 (3): 471–483. doi:10.1039/b609495c. PMID 17325786.
- ^ NCJRS Abstract – National Criminal Justice Reference Service Archived 20 June 2022 at the Wayback Machine. Ncjrs.gov. Retrieved on 2017-02-28.
- ^ Kwon, Oh-Kyum; Kim, Jae-Hoon; Park, Hyoung-Sang; Kang, Sang-Won (2004). "Atomic Layer Deposition of Ruthenium Thin Films for Copper Glue Layer". Journal of the Electrochemical Society. 151 (2): G109. Bibcode:2004JElS..151G.109K. doi:10.1149/1.1640633.
- ^ Moffat, T. P.; Walker, M.; Chen, P. J.; Bonevich, J. E.; Egelhoff, W. F.; Richter, L.; Witt, C.; Aaltonen, T.; Ritala, M.; Leskelä, M.; Josell, D. (2006). "Electrodeposition of Cu on Ru Barrier Layers for Damascene Processing". Journal of the Electrochemical Society. 153 (1): C37. Bibcode:2006JElS..153C..37M. doi:10.1149/1.2131826.
- ^ Bernasconi, R.; Magagnin, L. (2019). "Review—Ruthenium as Diffusion Barrier Layer in Electronic Interconnects: Current Literature with a Focus on Electrochemical Deposition Methods". Journal of the Electrochemical Society. 166 (1): D3219–D3225. Bibcode:2019JElS..166D3219B. doi:10.1149/2.0281901jes. S2CID 104430143.
- ^ Vasilyev, V. Yu. (2010). "Low-temperature pulsed CVD of ruthenium thin films for micro- and nanoelectronic applications, Part 1: Equipment and methodology". Russian Microelectronics. 39: 26–33. doi:10.1134/S106373971001004X. S2CID 122854468.
- ^ Delaude, Lionel; Noels, Alfred F. (2005). "Metathesis". Kirk-Othmer Encyclopedia of Chemical Technology. Weinheim: Wiley-VCH. doi:10.1002/0471238961.metanoel.a01. ISBN 978-0471238966.
- ^ Fürstner, Alois (2000). "Olefin Metathesis and Beyond". Angewandte Chemie International Edition. 39 (17): 3012–3043. Bibcode:2000AngCh..39.3012F. doi:10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G. PMID 11028025.
- ^ Noyori, R.; Ohkuma, T.; Kitamura, M.; Takaya, H.; Sayo, N.; Kumobayashi, H.; Akutagawa, S. (1987), "Asymmetric Hydrogenation of β-Keto Carboxylic Esters. A Practical, Purely Chemical Access to β-Hydroxy Esters in High Enantiomeric Purity", Journal of the American Chemical Society, 109 (19): 5856, doi:10.1021/ja00253a051
- ^ Ikariya, Takao; Hashiguchi, Shohei; Murata, Kunihiko and Noyori, Ryōji (2005). "Preparation of Optically Active (R,R)-Hydrobenzoin from Benzoin or Benzil". Organic Syntheses: 10
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Chen, Fei (2015). "Synthesis of Optically Active 1,2,3,4-Tetrahydroquinolines via Asymmetric Hydrogenation Using Iridium-Diamine Catalyst". Org. Synth. 92: 213–226. doi:10.15227/orgsyn.092.0213.
- ^ Schulz, Hans (1999). "Short history and present trends of Fischer–Tropsch synthesis". Applied Catalysis A: General. 186 (1–2): 3–12. doi:10.1016/S0926-860X(99)00160-X.
- ^ Kuang, Daibin; Ito, Seigo; Wenger, Bernard; Klein, Cedric; Moser, Jacques-E; Humphry-Baker, Robin; Zakeeruddin, Shaik M.; Grätzel, Michael (2006). "High Molar Extinction Coefficient Heteroleptic Ruthenium Complexes for Thin Film Dye-Sensitized Solar Cells". Journal of the American Chemical Society. 128 (12): 4146–54. doi:10.1021/ja058540p. PMID 16551124. S2CID 39111991.
- ^ a b "Ruthenium". espimetals.com. Retrieved 26 July 2020.
- ^ a b "Ruthenium (Ru) - Chemical properties, Health and Environmental effects". lenntech.com. Retrieved 26 July 2020.
Bibliography
[edit]- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
External links
[edit]- Ruthenium at The Periodic Table of Videos (University of Nottingham)
- Nano-layer of ruthenium stabilizes magnetic sensors Archived 5 April 2016 at the Wayback Machine

