Beta-peptide: Difference between revisions
Artoria2e5 (talk | contribs) No edit summary |
WP:CHEMCAPS updates |
||
| (7 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Class of peptides derived from β-amino acids}} |
{{Short description|Class of peptides derived from β-amino acids}} |
||
[[Image:Beta-alanineVSalpha-alanine.png|thumb|right|β-alanine, an example of a β |
[[Image:Beta-alanineVSalpha-alanine.png|thumb|right|β-alanine, an example of a β-amino acid. The amino group attaches not to the α carbon but to the β-carbon, which in this case is a [[methylene group]].]] |
||
'''Beta-peptides''' ('''β-peptides''') are peptides derived from '''β-amino acids''', in which the [[amino]] group is attached to the [[Alpha and beta carbon|β-carbon]] (i.e. the carbon two atoms away from the [[carboxylate]] group). The parent β-amino acid is [[Β-Alanine|β-alanine]] (H<sub>2</sub>NCH<sub>2</sub>CH<sub>2</sub>CO<sub>2</sub>H), a common natural substance, but most examples feature substituents in place of one or more C-H bonds. β-peptides usually do not occur in nature. β- |
'''Beta-peptides''' ('''β-peptides''') are peptides derived from '''β-amino acids''', in which the [[amino]] group is attached to the [[Alpha and beta carbon|β-carbon]] (i.e. the carbon two atoms away from the [[carboxylate]] group). The parent β-amino acid is [[Β-Alanine|β-alanine]] (H<sub>2</sub>NCH<sub>2</sub>CH<sub>2</sub>CO<sub>2</sub>H), a common natural substance, but most examples feature substituents in place of one or more C-H bonds. β-peptides usually do not occur in nature. β-Peptide-based antibiotics are being explored as ways of evading [[Antimicrobial resistance|antibiotic resistance]].<ref name="Seebach1996">{{cite journal |vauthors=Seebach D, Overhand M, ((Kühnle FNM)), Martinoni B, Oberer L, Hommel U, Widmer H |date=June 1996 |title=β-Peptides: Synthesis by Arndt-Eistert homologation with concomitant peptide coupling. Structure determination by NMR and CD spectroscopy and by X-ray crystallography. Helical secondary structure of a -hexapeptide in solution and its stability towards pepsin |journal=[[Helvetica Chimica Acta]] |volume=79 |issue=4 |pages=913–941 |doi=10.1002/hlca.19960790402}}</ref> Early studies in this field were published in 1996 by the group of [[Dieter Seebach]]<ref name="Seebach1996"/> and that of Samuel Gellman.<ref>{{cite journal|vauthors=Appella DH, Christianson LA, Karle IL, Powell DR, Gellman SH|year=1996|title=β-Peptide Foldamers: Robust Helix Formation in a New Family of -Amino Acid Oligomers|journal=[[J. Am. Chem. Soc.]]|volume=118|issue=51|pages=13071–2|doi=10.1021/ja963290l}}</ref> |
||
== Structure == |
== Structure == |
||
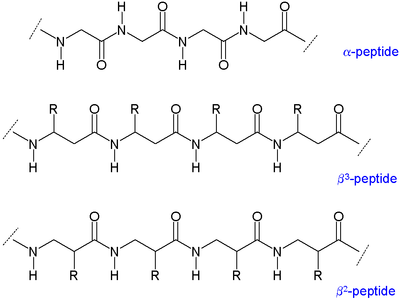
As there are two carbons available for substitution, β-amino acids have four sites (chirality included; as opposed to two in [[α-amino acid]]s) for attaching the organic residue group.<ref name=SeMa/> Accordingly, two main types β-amino acids exist differing by which carbon the residue is attached to: ones with the organic residue (R) next to the amine are called β<sup>3</sup> and those with position next to the carbonyl group are called β<sup>2</sup>. A β-peptide can consist of only one kind of these amino acids (β<sup>2</sup>-peptides and β<sup>3</sup>-peptides), or have a combination of the two. Furthermore, a β-amino acid can form a ring using both of its sites and also be incorporated into a peptide.<ref name=SeMa>{{cite journal |vauthors=Seebach D, Matthews JL |title=β-Peptides: a surprise at every turn |journal=[[Chem. Commun.]] |issue=21 |pages=2015–22 |year=1997 |url=https://chab.ethz.ch/content/dam/ethz/special-interest/chab/chab-dept/department/images/Emeriti/Seebach/PDFs/596_CC_1997.pdf |doi=10.1039/a704933a }}</ref> |
|||
[[Image:Beta-peptides.png|400px|center]] |
[[Image:Beta-peptides.png|400px|center]] |
||
| Line 17: | Line 17: | ||
==Clinical potential== |
==Clinical potential== |
||
β- |
β-Peptides are stable against [[Proteolysis|proteolytic degradation]] [[in vitro]] and [[in vivo]], a potential advantage over natural peptides.<ref>{{cite journal |vauthors=Beke T, Somlai C, Perczel A |title=Toward a rational design of β-peptide structures |journal=J Comput Chem |volume=27 |issue=1 |pages=20–38 |date=January 2006 |pmid=16247761 |doi=10.1002/jcc.20299|s2cid=35579693 }}</ref> β-Peptides have been used to mimic natural peptide-based antibiotics such as [[magainin]]s, which are highly potent but difficult to use as drugs because they are degraded by proteolytic enzymes.<ref>{{cite journal |vauthors=Porter EA, Weisblum B, Gellman SH |title=Mimicry of host-defense peptides by unnatural oligomers: antimicrobial β-peptides |journal=[[J. Am. Chem. Soc.]] |volume=124 |issue=25 |pages=7324–30 |year=2002 |doi=10.1021/ja0260871 |pmid=12071741}}</ref> |
||
== |
==Examples== |
||
β-Amino acids with a wide variety of substituents exist. Named by analogy to the biological [[α-amino acid]]s, the following have been found naturally: β-alanine, [[β-leucine]], [[β-lysine]], β-arginine, β-glutamate, β-glutamine, β-phenylalanine and β-tyrosine.<ref name="Juaristi2005">{{cite book |last1=Juaristi |first1=E. |last2=Soloshonok |first2=Vadim A. |url=https://books.google.com/books?id=WpgRvHGa0zIC&dq=8%20beta%20forms&pg=PA23 |title=Enantioselective Synthesis of Beta-Amino Acids |date=6 May 2005 |publisher=Wiley Inc. |isbn=9780471698470 |location=Hoboken, New Jersey (NJ) |oclc=559972352 |access-date=7 May 2022 |archive-date=7 May 2022 |archive-url=https://web.archive.org/web/20220507184706/https://www.google.co.in/books/edition/Enantioselective_Synthesis_of_Beta_Amino/WpgRvHGa0zIC?hl=en&gbpv=1&dq=8+beta+forms&pg=PA23 |url-status=live }}</ref>{{rp|23}} Of these, β-alanine is found in mammals and incorporated in [[pantothenic acid]], an essential nutrient.<ref name="Juaristi2005"/>{{rp|2}} Two α-amino acids are also structurally β-amino acids: [[aspartic acid]] and [[asparagine]].<ref name="Juaristi2005"/>{{rp|218}} [[Microcystin]]s are a class of compounds containing a β-isoaspartyl (i.e. aspartic acid linked with its beta-carboxyl) residue.<ref name="Juaristi2005"/>{{rp|23}} |
|||
==See also== |
==See also== |
||
| Line 26: | Line 26: | ||
==References== |
==References== |
||
{{Reflist| |
{{Reflist|25em}} |
||
{{Non-proteinogenic amino acids}} |
{{Non-proteinogenic amino acids}} |
||
Latest revision as of 02:30, 19 November 2024
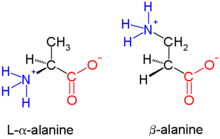
Beta-peptides (β-peptides) are peptides derived from β-amino acids, in which the amino group is attached to the β-carbon (i.e. the carbon two atoms away from the carboxylate group). The parent β-amino acid is β-alanine (H2NCH2CH2CO2H), a common natural substance, but most examples feature substituents in place of one or more C-H bonds. β-peptides usually do not occur in nature. β-Peptide-based antibiotics are being explored as ways of evading antibiotic resistance.[1] Early studies in this field were published in 1996 by the group of Dieter Seebach[1] and that of Samuel Gellman.[2]
Structure
[edit]As there are two carbons available for substitution, β-amino acids have four sites (chirality included; as opposed to two in α-amino acids) for attaching the organic residue group.[3] Accordingly, two main types β-amino acids exist differing by which carbon the residue is attached to: ones with the organic residue (R) next to the amine are called β3 and those with position next to the carbonyl group are called β2. A β-peptide can consist of only one kind of these amino acids (β2-peptides and β3-peptides), or have a combination of the two. Furthermore, a β-amino acid can form a ring using both of its sites and also be incorporated into a peptide.[3]
Synthesis
[edit]β-Amino acids have been prepared by many routes,[4][5] including some based on the Arndt-Eistert synthesis.
Secondary structure
[edit]Because their backbones are longer than those of normal peptides, β-peptides form disparate secondary structures. The alkyl substituents at both the α and β positions in a β-amino acid favor a gauche conformation about the bond between the α-carbon and β-carbon. This also affects the thermodynamic stability of the structure.
Many types of helix structures consisting of β-peptides have been reported. These conformation types are distinguished by the number of atoms in the hydrogen-bonded ring that is formed in solution; 8-helix, 10-helix, 12-helix, 14-helix,[6] and 10/12-helix have been reported. Generally speaking, β-peptides form a more stable helix than α-peptides.[7]
Clinical potential
[edit]β-Peptides are stable against proteolytic degradation in vitro and in vivo, a potential advantage over natural peptides.[8] β-Peptides have been used to mimic natural peptide-based antibiotics such as magainins, which are highly potent but difficult to use as drugs because they are degraded by proteolytic enzymes.[9]
Examples
[edit]β-Amino acids with a wide variety of substituents exist. Named by analogy to the biological α-amino acids, the following have been found naturally: β-alanine, β-leucine, β-lysine, β-arginine, β-glutamate, β-glutamine, β-phenylalanine and β-tyrosine.[10]: 23 Of these, β-alanine is found in mammals and incorporated in pantothenic acid, an essential nutrient.[10]: 2 Two α-amino acids are also structurally β-amino acids: aspartic acid and asparagine.[10]: 218 Microcystins are a class of compounds containing a β-isoaspartyl (i.e. aspartic acid linked with its beta-carboxyl) residue.[10]: 23
See also
[edit]References
[edit]- ^ a b Seebach D, Overhand M, Kühnle FNM, Martinoni B, Oberer L, Hommel U, Widmer H (June 1996). "β-Peptides: Synthesis by Arndt-Eistert homologation with concomitant peptide coupling. Structure determination by NMR and CD spectroscopy and by X-ray crystallography. Helical secondary structure of a -hexapeptide in solution and its stability towards pepsin". Helvetica Chimica Acta. 79 (4): 913–941. doi:10.1002/hlca.19960790402.
- ^ Appella DH, Christianson LA, Karle IL, Powell DR, Gellman SH (1996). "β-Peptide Foldamers: Robust Helix Formation in a New Family of -Amino Acid Oligomers". J. Am. Chem. Soc. 118 (51): 13071–2. doi:10.1021/ja963290l.
- ^ a b Seebach D, Matthews JL (1997). "β-Peptides: a surprise at every turn" (PDF). Chem. Commun. (21): 2015–22. doi:10.1039/a704933a.
- ^ Basler B, Schuster O, Bach T (November 2005). "Conformationally constrained β-amino acid derivatives by intramolecular [2 + 2]-photocycloaddition of a tetronic acid amide and subsequent lactone ring opening". J. Org. Chem. 70 (24): 9798–808. doi:10.1021/jo0515226. PMID 16292808.
- ^ Murray JK, Farooqi B, Sadowsky JD, et al. (September 2005). "Efficient synthesis of a β-peptide combinatorial library with microwave irradiation". J. Am. Chem. Soc. 127 (38): 13271–80. doi:10.1021/ja052733v. PMID 16173757.
- ^ Vasantha, Basavalingappa; George, Gijo; Raghothama, Srinivasarao; Balaram, Padmanabhan (January 2017). "Homooligomeric β3 (R)-valine peptides: Transformation between C14 and C12 helical structures induced by a guest Aib residue". Biopolymers. 108 (1): e22935. doi:10.1002/bip.22935. ISSN 1097-0282. PMID 27539268. S2CID 205497333. Archived from the original on 2022-02-13. Retrieved 2022-05-07.
- ^ Gademann K, Hintermann T, Schreiber JV (October 1999). "Beta-peptides: twisting and turning". Curr. Med. Chem. 6 (10): 905–25. doi:10.2174/092986730610220401154606. PMID 10519905. S2CID 247917035.
- ^ Beke T, Somlai C, Perczel A (January 2006). "Toward a rational design of β-peptide structures". J Comput Chem. 27 (1): 20–38. doi:10.1002/jcc.20299. PMID 16247761. S2CID 35579693.
- ^ Porter EA, Weisblum B, Gellman SH (2002). "Mimicry of host-defense peptides by unnatural oligomers: antimicrobial β-peptides". J. Am. Chem. Soc. 124 (25): 7324–30. doi:10.1021/ja0260871. PMID 12071741.
- ^ a b c d Juaristi, E.; Soloshonok, Vadim A. (6 May 2005). Enantioselective Synthesis of Beta-Amino Acids. Hoboken, New Jersey (NJ): Wiley Inc. ISBN 9780471698470. OCLC 559972352. Archived from the original on 7 May 2022. Retrieved 7 May 2022.
