Bicarbonate: Difference between revisions
m Reverted edits by 69.80.12.21 (talk) (HG) (3.4.10) |
→Other uses: clarified which types of products use ammonium bicarbonate and added citation |
||
| (24 intermediate revisions by 15 users not shown) | |||
| Line 1: | Line 1: | ||
{{short description|Polyatomic anion}} |
{{short description|Polyatomic anion}} |
||
{{use dmy dates |
{{use dmy dates|date=April 2021}} |
||
{{About||baking soda|sodium bicarbonate|the programming principle|Tim Toady Bicarbonate}}{{Distinguish|Dicarbonate}}{{Redirect|Hydrogen carbonate|the oxoacid|carbonic acid}}{{redirect|Hydrocarbonate|the gas|water gas}}{{Chembox |
{{About||baking soda|sodium bicarbonate|the programming principle|Tim Toady Bicarbonate}}{{Distinguish|Dicarbonate}}{{Redirect|Hydrogen carbonate|the oxoacid|carbonic acid}}{{redirect|Hydrocarbonate|the gas|water gas}}{{Chembox |
||
| ImageFile1 = Bicarbonate-resonance.png |
| ImageFile1 = Bicarbonate-resonance.png |
||
| ImageFile1_Ref = {{chemboximage|correct|??}} |
| ImageFile1_Ref = {{chemboximage|correct|??}} |
||
| ImageSize1 = 121 |
| ImageSize1 = 121 |
||
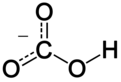
| ImageName1 = Skeletal formula of bicarbonate with the explicit hydrogen added |
| ImageName1 = Skeletal formula of bicarbonate with the explicit hydrogen added |
||
| ImageFile2 = Bicarbonate-ion-3D-balls.png |
| ImageFile2 = Bicarbonate-ion-3D-balls.png |
||
| ImageFile2_Ref = {{chemboximage|correct|??}} |
| ImageFile2_Ref = {{chemboximage|correct|??}} |
||
| ImageSize2 = 121 |
| ImageSize2 = 121 |
||
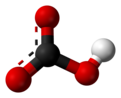
| ImageName2 = Ball and stick model of bicarbonate |
| ImageName2 = Ball and stick model of bicarbonate |
||
|IUPACName=Hydrogencarbonate |
|||
| SystematicName = Hydroxidodioxidocarbonate(1−)<ref name="hydrogencarbonate (CHEBI:17544)">{{Cite web|url = https://www.ebi.ac.uk/chebi/searchId.do?chebiId=17544|title = hydrogencarbonate (CHEBI:17544)|work = Chemical Entities of Biological Interest (ChEBI)|location = UK|publisher = European Institute of Bioinformatics|at = IUPAC Names|url-status = live|archive-url = https://web.archive.org/web/20150607062137/http://www.ebi.ac.uk/chebi/searchId.do?chebiId=17544|archive-date = 2015-06-07}}</ref> |
| SystematicName = Hydroxidodioxidocarbonate(1−)<ref name="hydrogencarbonate (CHEBI:17544)">{{Cite web|url = https://www.ebi.ac.uk/chebi/searchId.do?chebiId=17544|title = hydrogencarbonate (CHEBI:17544)|work = Chemical Entities of Biological Interest (ChEBI)|location = UK|publisher = European Institute of Bioinformatics|at = IUPAC Names|url-status = live|archive-url = https://web.archive.org/web/20150607062137/http://www.ebi.ac.uk/chebi/searchId.do?chebiId=17544|archive-date = 2015-06-07}}</ref> |
||
| OtherNames = |
| OtherNames = {{Unbulleted list|Hydrogen carbonate<ref name = "hydrogencarbonate (CHEBI:17544)"/>|Hydrocarbonate}} |
||
|Section1={{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| |
|CASNo = 71-52-3 |
||
| |
|CASNo_Ref = {{cascite|correct|CAS}} |
||
| |
|UNII_Ref = {{fdacite|correct|FDA}} |
||
| |
|UNII = HN1ZRA3Q20 |
||
| |
|PubChem = 769 |
||
| |
|ChemSpiderID = 749 |
||
| |
|ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| |
|KEGG = C00288 |
||
| |
|KEGG_Ref = {{keggcite|correct|kegg}} |
||
| |
|ChEBI = 17544 |
||
| |
|ChEBI_Ref = {{ebicite|correct|EBI}} |
||
| |
|ChEMBL = 363707 |
||
| |
|ChEMBL_Ref = {{ebicite|correct|EBI}} |
||
| |
|Beilstein = 3903504 |
||
| |
|Gmelin = 49249 |
||
| |
|3DMet = B00080 |
||
| |
|SMILES = OC([O-])=O |
||
| |
|StdInChI = 1S/CH2O3/c2-1(3)4/h(H2,2,3,4)/p-1 |
||
| |
|StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| |
|StdInChIKey = BVKZGUZCCUSVTD-UHFFFAOYSA-M |
||
| |
|StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
||
}} |
}} |
||
|Section2={{Chembox Properties |
| Section2 = {{Chembox Properties |
||
| |
|Formula = {{Chem|HCO|3|-}} |
||
| |
|MolarMass = 61.0168 g mol<sup>−1</sup> |
||
| |
|LogP = −0.82 |
||
| |
|pKa = 10.3 |
||
| |
|pKb = 7.7 |
||
| |
|ConjugateAcid = [[Carbonic acid]] |
||
| |
|ConjugateBase = [[Carbonate]] |
||
}} |
}} |
||
}} |
}} |
||
In [[inorganic chemistry]], '''bicarbonate''' ([[International Union of Pure and Applied Chemistry|IUPAC]]-recommended nomenclature: '''hydrogencarbonate'''<ref>{{Citation |
In [[inorganic chemistry]], '''bicarbonate''' ([[International Union of Pure and Applied Chemistry|IUPAC]]-recommended nomenclature: '''hydrogencarbonate'''<ref>{{Citation|url = https://iupac.org/wp-content/uploads/2016/07/Red_Book_2005.pdf|title = Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005|publisher = IUPAC|page = 137}}</ref>) is an intermediate form in the [[deprotonation]] of [[carbonic acid]]. It is a [[Polyatomic ion|polyatomic]] [[anion]] with the chemical formula {{chem|[[Hydrogen|H]]|[[Carbon|C]]|[[Oxygen|O]]|3|-}}. |
||
|url = https://iupac.org/wp-content/uploads/2016/07/Red_Book_2005.pdf |
|||
|title = Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 |
|||
|publisher = IUPAC |
|||
|page = 137 |
|||
|url-status = live |
|||
}}</ref>) is an intermediate form in the [[deprotonation]] of [[carbonic acid]]. It is a [[Polyatomic ion|polyatomic]] [[anion]] with the chemical formula {{chem|[[Hydrogen|H]]|[[Carbon|C]]|[[Oxygen|O]]|3|-}}. |
|||
Bicarbonate serves a crucial biochemical role in the physiological [[pH]] [[buffering solution|buffering]] system.<ref name="veq">{{cite web |
Bicarbonate serves a crucial biochemical role in the physiological [[pH]] [[buffering solution|buffering]] system.<ref name="veq">{{cite web|url = http://www.biology.arizona.edu/biochemistry/problem_sets/medph/intro.html|publisher = Biology.arizona.edu|date = October 2006|title = Clinical correlates of pH levels: bicarbonate as a buffer|url-status = live|archive-url = https://web.archive.org/web/20150531000344/http://www.biology.arizona.edu/biochemistry/problem_sets/medph/intro.html|archive-date = 2015-05-31}}</ref> |
||
The term "bicarbonate" was [[Factitious airs#bicarbonate|coined]] in 1814 by the English chemist [[William Hyde Wollaston]].<ref>William Hyde Wollaston (1814) "A synoptic scale of chemical equivalents |
The term "bicarbonate" was [[Factitious airs#bicarbonate|coined]] in 1814 by the English chemist [[William Hyde Wollaston]].<ref>William Hyde Wollaston (1814) "A synoptic scale of chemical equivalents", ''Philosophical Transactions of the Royal Society'', '''104''': 1-22. [https://books.google.com/books?id=uYdJAAAAYAAJ&pg=PA11 On page 11], Wollaston coins the term "bicarbonate": "The next question that occurs relates to the composition of this crystallized carbonate of potash, which I am induced to call bi-carbonate of potash, for the purpose of marking more decidedly the distinction between this salt and that which is commonly called a subcarbonate, and in order to refer at once to the double dose of carbonic acid contained in it."</ref><ref>{{cite web|url=http://www.newton.dep.anl.gov/askasci/chem99/chem99492.htm|title=Baking Soda|publisher=[[Argonne National Laboratory]]|website=Newton – Ask a Scientist|access-date=2 May 2018|url-status=dead|archive-url=https://web.archive.org/web/20150226223438/http://newton.dep.anl.gov/askasci/chem99/chem99492.htm|archive-date=26 February 2015}}</ref> The name lives on as a [[trivial name]]. |
||
==Chemical properties== |
==Chemical properties== |
||
| ⚫ | The bicarbonate ion (hydrogencarbonate ion) is an [[anion]] with the [[empirical formula]] {{Chem|HCO|3|-}} and a molecular mass of 61.01 [[atomic mass unit|daltons]]; it consists of one central carbon [[atom]] surrounded by three oxygen atoms in a [[trigonal planar]] arrangement, with a hydrogen atom attached to one of the oxygens. It is [[isoelectronic]] with [[nitric acid]] {{chem|HNO|3}}. The bicarbonate ion carries a negative one [[formal charge]] and is an [[Amphoterism#Amphiprotic molecules|amphiprotic]] species which has both acidic and basic properties. It is both the [[conjugate acid|conjugate base]] of [[carbonic acid]] {{chem|H|2|CO|3}}; and the [[conjugate acid]] of {{chem|CO|3|2−}}, the [[carbonate]] ion, as shown by these [[chemical equilibrium|equilibrium]] reactions: |
||
| ⚫ | |||
| ⚫ | The bicarbonate ion (hydrogencarbonate ion) is an [[anion]] with the [[empirical formula]] {{Chem|HCO|3|-}} and a molecular mass of 61.01 |
||
| ⚫ | |||
:H<sub>2</sub>CO<sub>3</sub> + 2 H<sub>2</sub>O {{eqm}} {{Chem|HCO|3|-}} + H<sub>3</sub>O<sup>+</sup> + H<sub>2</sub>O {{eqm}} {{chem|CO|3|2−}} + 2 H<sub>3</sub>O<sup>+</sup>. |
:H<sub>2</sub>CO<sub>3</sub> + 2 H<sub>2</sub>O {{eqm}} {{Chem|HCO|3|-}} + H<sub>3</sub>O<sup>+</sup> + H<sub>2</sub>O {{eqm}} {{chem|CO|3|2−}} + 2 H<sub>3</sub>O<sup>+</sup>. |
||
A bicarbonate salt forms when a [[cation|positively charged ion]] attaches to the negatively charged oxygen atoms of the ion, forming an [[ionic compound]]. Many bicarbonates are [[solubility|soluble]] in [[aqueous solution|water]] at [[standard temperature and pressure]]; in particular, sodium bicarbonate contributes to [[total dissolved solids]], a common parameter for assessing [[water quality]].<ref>{{cite book |
A bicarbonate salt forms when a [[cation|positively charged ion]] attaches to the negatively charged oxygen atoms of the ion, forming an [[ionic compound]]. Many bicarbonates are [[solubility|soluble]] in [[aqueous solution|water]] at [[standard temperature and pressure]]; in particular, sodium bicarbonate contributes to [[total dissolved solids]], a common parameter for assessing [[water quality]].<ref>{{cite book|last1=Geor|first1=Raymond J.|last2=Coenen|first2=Manfred|last3=Harris|first3=Pat|title=Equine Applied and Clinical Nutrition: Health, Welfare and Performance|date=31 January 2013|publisher=Elsevier Health Sciences|isbn=978-0-7020-5418-1|page=90|language=en|quote=The most common indicator of water quality is the concentration of total dissolved solids (TDS)}}</ref> |
||
==Physiological role== |
==Physiological role== |
||
[[File:Riassorbimento bicarbonati e respirazione cellulare.svg|450px|thumbnail|right|CO<sub>2</sub> produced as a waste product of the oxidation of sugars in the mitochondria reacts with water in a reaction catalyzed by [[carbonic anhydrase]] to form H<sub>2</sub>CO<sub>3</sub>, which is in equilibrium with the cation H<sup>+</sup> and anion HCO<sub>3</sub><sup>−</sup>. It is then carried to the lung, where the reverse reaction occurs and CO<sub>2</sub> gas is released. In the kidney (left), cells (green) lining the proximal tubule conserve bicarbonate by transporting it from the glomerular filtrate in the lumen (yellow) of the nephron back into the blood (red). The exact stoichiometry in the kidney is omitted for simplicity.]]{{clear left}} |
[[File:Riassorbimento bicarbonati e respirazione cellulare.svg|450px|thumbnail|right|CO<sub>2</sub> produced as a waste product of the oxidation of sugars in the mitochondria reacts with water in a reaction catalyzed by [[carbonic anhydrase]] to form H<sub>2</sub>CO<sub>3</sub>, which is in equilibrium with the cation H<sup>+</sup> and anion HCO<sub>3</sub><sup>−</sup>. It is then carried to the lung, where the reverse reaction occurs and CO<sub>2</sub> gas is released. In the kidney (left), cells (green) lining the proximal tubule conserve bicarbonate by transporting it from the glomerular filtrate in the lumen (yellow) of the nephron back into the blood (red). The exact stoichiometry in the kidney is omitted for simplicity.]]{{clear left}} |
||
Bicarbonate ({{Chem|HCO|3|-}}) is a vital component of the [[pH]] [[Buffer solution|buffering system]]<ref name="veq" |
Bicarbonate ({{Chem|HCO|3|-}}) is a vital component of the [[pH]] [[Buffer solution|buffering system]]<ref name="veq"/> of the human body (maintaining [[acid–base homeostasis]]). 70%–75% of CO<sub>2</sub> in the body is converted into [[carbonic acid]] (H<sub>2</sub>CO<sub>3</sub>), which is the [[conjugate acid]] of {{Chem|HCO|3|-}} and can quickly turn into it.{{cn|date=February 2024}} |
||
| ⚫ | With carbonic acid as the [[Reaction intermediate|central intermediate]] [[Chemical species|species]], bicarbonate – in conjunction with water, [[hydronium|hydrogen ions]], and [[carbon dioxide]] – forms this buffering system, which is maintained at the volatile equilibrium<ref name="veq"/> required to provide prompt resistance to pH changes in both the acidic and [[Base (chemistry)|basic]] directions. This is especially important for protecting [[Tissue (biology)|tissues]] of the [[central nervous system]], where pH changes too far outside of the normal range in either direction could prove disastrous (see [[acidosis]] or [[alkalosis]]). Recently it has been also demonstrated that cellular bicarbonate metabolism can be regulated by mTORC1 signaling.<ref>{{cite journal|vauthors = Ali E, Liponska A, O'Hara B, Amici D, Torno M, Gao P, Asara J, Yap M-N F, Mendillo M, Ben-Sahra I|title = The mTORC1-SLC4A7 axis stimulates bicarbonate import to enhance de novo nucleotide synthesis|journal = Molecular Cell|volume = 82|issue = 1|pages = 3284–3298.e7|date = June 2022|doi = 10.1016/j.molcel.2022.06.008|pmid = 35772404|pmc = 9444906}}</ref> |
||
| ⚫ | With carbonic acid as the [[Reaction intermediate|central intermediate]] [[Chemical species|species]], bicarbonate – in conjunction with water, [[hydronium|hydrogen ions]], and [[carbon dioxide]] – forms this buffering system, which is maintained at the volatile equilibrium<ref name="veq" |
||
Additionally, bicarbonate plays a key role in the digestive system. It raises the internal pH of the stomach, after highly acidic digestive juices have finished in their digestion of food. Bicarbonate also acts to regulate pH in the small intestine. It is released from the [[pancreas]] in response to the hormone [[secretin]] to neutralize the acidic [[chyme]] entering the [[duodenum]] from the stomach.<ref>Berne & Levy, ''Principles of Physiology''</ref> |
Additionally, bicarbonate plays a key role in the digestive system. It raises the internal pH of the stomach, after highly acidic digestive juices have finished in their digestion of food. Bicarbonate also acts to regulate pH in the small intestine. It is released from the [[pancreas]] in response to the hormone [[secretin]] to neutralize the acidic [[chyme]] entering the [[duodenum]] from the stomach.<ref>Berne & Levy, ''Principles of Physiology''</ref> |
||
| Line 79: | Line 73: | ||
Bicarbonate is the dominant form of [[Total inorganic carbon|dissolved inorganic carbon]] in sea water,<ref>{{cite web|title=The chemistry of ocean acidification : OCB-OA|url=http://www.whoi.edu/OCB-OA/page.do?pid=112136|website=www.whoi.edu|publisher=Woods Hole Oceanographic Institution|access-date=17 May 2017|language=en-NZ|date=24 September 2012|url-status=live|archive-url=https://web.archive.org/web/20170519193604/http://www.whoi.edu/OCB-OA/page.do?pid=112136|archive-date=19 May 2017}}</ref> and in most fresh waters. As such it is an important sink in the [[carbon cycle]]. |
Bicarbonate is the dominant form of [[Total inorganic carbon|dissolved inorganic carbon]] in sea water,<ref>{{cite web|title=The chemistry of ocean acidification : OCB-OA|url=http://www.whoi.edu/OCB-OA/page.do?pid=112136|website=www.whoi.edu|publisher=Woods Hole Oceanographic Institution|access-date=17 May 2017|language=en-NZ|date=24 September 2012|url-status=live|archive-url=https://web.archive.org/web/20170519193604/http://www.whoi.edu/OCB-OA/page.do?pid=112136|archive-date=19 May 2017}}</ref> and in most fresh waters. As such it is an important sink in the [[carbon cycle]]. |
||
Some plants like ''[[Chara (alga)|Chara]]'' utilize carbonate and produce calcium carbonate (CaCO<sub>3</sub>) as result of biological metabolism.<ref>{{Cite journal|last1=Pełechaty|first1=Mariusz|last2=Pukacz|first2=Andrzej|last3=Apolinarska|first3=Karina|last4=Pełechata|first4=Aleksandra|last5=Siepak|first5=Marcin|date=June 2013|editor-last=Porta|editor-first=Giovanna Della|title=The significance of Chara vegetation in the precipitation of lacustrine calcium carbonate|url=https://onlinelibrary.wiley.com/doi/10.1111/sed.12020|journal=Sedimentology|language=en|volume=60|issue=4|pages=1017–1035|doi=10.1111/sed.12020|bibcode=2013Sedim..60.1017P |s2cid=128758128 }}</ref> |
|||
| ⚫ | In freshwater ecology, strong [[photosynthesis|photosynthetic]] activity by freshwater plants in daylight releases gaseous [[oxygen]] into the water and at the same time produces bicarbonate ions. These shift the pH upward until in certain circumstances the degree of alkalinity can become toxic to some organisms or can make other chemical constituents such as [[ammonia]] toxic. In darkness, when no photosynthesis occurs, respiration processes release carbon dioxide, and no new bicarbonate ions are produced, resulting in a rapid fall in pH. |
||
| ⚫ | In freshwater ecology, strong [[photosynthesis|photosynthetic]] activity by freshwater plants in daylight releases gaseous [[oxygen]] into the water and at the same time produces bicarbonate ions. These shift the pH upward until in certain circumstances the degree of alkalinity can become toxic to some organisms or can make other chemical constituents such as [[ammonia]] toxic. In darkness, when no photosynthesis occurs, respiration processes release carbon dioxide, and no new bicarbonate ions are produced, resulting in a rapid fall in pH.{{cn|date=July 2024}} |
||
The flow of bicarbonate ions from rocks weathered by the carbonic acid in rainwater is an important part of the [[carbon cycle]]. |
The flow of bicarbonate ions from rocks weathered by the carbonic acid in rainwater is an important part of the [[carbon cycle]]. |
||
==Other uses== |
==Other uses== |
||
The most common salt of the bicarbonate ion is [[sodium bicarbonate]], NaHCO<sub>3</sub>, which is commonly known as [[baking soda]]. When heated or exposed to an [[acid]] such as [[acetic acid]] ([[vinegar]]), sodium bicarbonate releases [[carbon dioxide]]. This is used as a [[leavening agent]] in [[baking]]. |
The most common salt of the bicarbonate ion is [[sodium bicarbonate]], NaHCO<sub>3</sub>, which is commonly known as [[baking soda]]. When heated or exposed to an [[acid]] such as [[acetic acid]] ([[vinegar]]), sodium bicarbonate releases [[carbon dioxide]]. This is used as a [[leavening agent]] in [[baking]].<ref>{{Cite web |title=How Baking Soda Works |url=https://www.seriouseats.com/cookie-science-how-baking-soda-works |access-date=2024-11-23 |website=Serious Eats |language=en}}</ref> |
||
[[Ammonium bicarbonate]] is used in the manufacturing of some cookies, crackers, and biscuits.<ref>{{Cite web |title=Ammonium Bicarbonate – Bicarbonate Applications |url=https://www.ahperformance.com/media-library/ammonium-bicarbonate-bicarbonate-applications/ |access-date=2024-11-23 |website=www.ahperformance.com |language=en-US}}</ref> |
|||
[[Ammonium bicarbonate]] is used in [[digestive biscuit]] manufacture. |
|||
==Diagnostics== |
==Diagnostics== |
||
In [[diagnostic medicine]], the [[blood value]] of bicarbonate is one of several indicators of the state of [[acid–base physiology]] in the body. It is measured, along with |
In [[diagnostic medicine]], the [[blood value]] of bicarbonate is one of several indicators of the state of [[acid–base physiology]] in the body. It is measured, along with [[chloride]], [[potassium]], and [[sodium]], to assess [[electrolyte]] levels in an [[electrolyte panel]] test (which has [[Current Procedural Terminology]], CPT, code 80051).{{cn|date=July 2024}} |
||
The parameter ''standard bicarbonate concentration'' (SBC<sub>e</sub>) is the bicarbonate concentration in the blood at a [[PaCO2|P<sub>a</sub>CO<sub>2</sub>]] of {{convert|40|mmHg|kPa|2|abbr=on}}, full oxygen saturation and 36 |
The parameter ''standard bicarbonate concentration'' (SBC<sub>e</sub>) is the bicarbonate concentration in the blood at a [[PaCO2|P<sub>a</sub>CO<sub>2</sub>]] of {{convert|40|mmHg|kPa|2|abbr=on}}, full oxygen saturation and 36 °C.<ref>[http://www.nda.ox.ac.uk/wfsa/html/u13/u1312_03.htm Acid Base Balance (page 3)] {{webarchive|url=https://web.archive.org/web/20020613020114/http://www.nda.ox.ac.uk/wfsa/html/u13/u1312_03.htm|date=2002-06-13}}</ref> |
||
[[File:Reference ranges for blood tests - by molarity.png|thumb|550px|center|[[Reference ranges for blood tests]], comparing blood content of bicarbonate (shown in blue at right) with other constituents.]] |
[[File:Reference ranges for blood tests - by molarity.png|thumb|550px|center|[[Reference ranges for blood tests]], comparing blood content of bicarbonate (shown in blue at right) with other constituents.]] |
||
== |
==Bicarbonate compounds== |
||
* [[Sodium bicarbonate]] |
* [[Sodium bicarbonate]] |
||
* [[Potassium bicarbonate]] |
* [[Potassium bicarbonate]] |
||
| Line 106: | Line 101: | ||
==See also== |
==See also== |
||
* [[Carbon dioxide]] |
* [[Carbon dioxide]] |
||
* [[Carbonate]] |
* [[Carbonate]] |
||
| Line 112: | Line 106: | ||
* [[Hard water]] |
* [[Hard water]] |
||
* [[Arterial blood gas test]] |
* [[Arterial blood gas test]] |
||
* [[Henderson-Hasselbach equation]] |
|||
==References== |
==References== |
||
| Line 126: | Line 121: | ||
[[Category:Amphoteric compounds]] |
[[Category:Amphoteric compounds]] |
||
[[Category:Anions]] |
[[Category:Anions]] |
||
[[Category:Bicarbonates| |
[[Category:Bicarbonates|Bicarbonates]] |
||
Latest revision as of 17:01, 23 November 2024
| |
| |
| Names | |
|---|---|
| IUPAC name
Hydrogencarbonate
| |
| Systematic IUPAC name
Hydroxidodioxidocarbonate(1−)[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 3903504 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| 49249 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HCO− 3 | |
| Molar mass | 61.0168 g mol−1 |
| log P | −0.82 |
| Acidity (pKa) | 10.3 |
| Basicity (pKb) | 7.7 |
| Conjugate acid | Carbonic acid |
| Conjugate base | Carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate[2]) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula HCO−
3.
Bicarbonate serves a crucial biochemical role in the physiological pH buffering system.[3]
The term "bicarbonate" was coined in 1814 by the English chemist William Hyde Wollaston.[4][5] The name lives on as a trivial name.
Chemical properties
[edit]The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula HCO−
3 and a molecular mass of 61.01 daltons; it consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. It is isoelectronic with nitric acid HNO
3. The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties. It is both the conjugate base of carbonic acid H
2CO
3; and the conjugate acid of CO2−
3, the carbonate ion, as shown by these equilibrium reactions:
- CO2−
3 + 2 H2O ⇌ HCO−
3 + H2O + OH− ⇌ H2CO3 + 2 OH−
- H2CO3 + 2 H2O ⇌ HCO−
3 + H3O+ + H2O ⇌ CO2−
3 + 2 H3O+.
A bicarbonate salt forms when a positively charged ion attaches to the negatively charged oxygen atoms of the ion, forming an ionic compound. Many bicarbonates are soluble in water at standard temperature and pressure; in particular, sodium bicarbonate contributes to total dissolved solids, a common parameter for assessing water quality.[6]
Physiological role
[edit]
Bicarbonate (HCO−
3) is a vital component of the pH buffering system[3] of the human body (maintaining acid–base homeostasis). 70%–75% of CO2 in the body is converted into carbonic acid (H2CO3), which is the conjugate acid of HCO−
3 and can quickly turn into it.[citation needed]
With carbonic acid as the central intermediate species, bicarbonate – in conjunction with water, hydrogen ions, and carbon dioxide – forms this buffering system, which is maintained at the volatile equilibrium[3] required to provide prompt resistance to pH changes in both the acidic and basic directions. This is especially important for protecting tissues of the central nervous system, where pH changes too far outside of the normal range in either direction could prove disastrous (see acidosis or alkalosis). Recently it has been also demonstrated that cellular bicarbonate metabolism can be regulated by mTORC1 signaling.[7]
Additionally, bicarbonate plays a key role in the digestive system. It raises the internal pH of the stomach, after highly acidic digestive juices have finished in their digestion of food. Bicarbonate also acts to regulate pH in the small intestine. It is released from the pancreas in response to the hormone secretin to neutralize the acidic chyme entering the duodenum from the stomach.[8]
Bicarbonate in the environment
[edit]Bicarbonate is the dominant form of dissolved inorganic carbon in sea water,[9] and in most fresh waters. As such it is an important sink in the carbon cycle.
Some plants like Chara utilize carbonate and produce calcium carbonate (CaCO3) as result of biological metabolism.[10]
In freshwater ecology, strong photosynthetic activity by freshwater plants in daylight releases gaseous oxygen into the water and at the same time produces bicarbonate ions. These shift the pH upward until in certain circumstances the degree of alkalinity can become toxic to some organisms or can make other chemical constituents such as ammonia toxic. In darkness, when no photosynthesis occurs, respiration processes release carbon dioxide, and no new bicarbonate ions are produced, resulting in a rapid fall in pH.[citation needed]
The flow of bicarbonate ions from rocks weathered by the carbonic acid in rainwater is an important part of the carbon cycle.
Other uses
[edit]The most common salt of the bicarbonate ion is sodium bicarbonate, NaHCO3, which is commonly known as baking soda. When heated or exposed to an acid such as acetic acid (vinegar), sodium bicarbonate releases carbon dioxide. This is used as a leavening agent in baking.[11]
Ammonium bicarbonate is used in the manufacturing of some cookies, crackers, and biscuits.[12]
Diagnostics
[edit]In diagnostic medicine, the blood value of bicarbonate is one of several indicators of the state of acid–base physiology in the body. It is measured, along with chloride, potassium, and sodium, to assess electrolyte levels in an electrolyte panel test (which has Current Procedural Terminology, CPT, code 80051).[citation needed]
The parameter standard bicarbonate concentration (SBCe) is the bicarbonate concentration in the blood at a PaCO2 of 40 mmHg (5.33 kPa), full oxygen saturation and 36 °C.[13]

Bicarbonate compounds
[edit]- Sodium bicarbonate
- Potassium bicarbonate
- Caesium bicarbonate
- Magnesium bicarbonate
- Calcium bicarbonate
- Ammonium bicarbonate
- Carbonic acid
See also
[edit]- Carbon dioxide
- Carbonate
- Carbonic anhydrase
- Hard water
- Arterial blood gas test
- Henderson-Hasselbach equation
References
[edit]- ^ a b "hydrogencarbonate (CHEBI:17544)". Chemical Entities of Biological Interest (ChEBI). UK: European Institute of Bioinformatics. IUPAC Names. Archived from the original on 7 June 2015.
- ^ Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 (PDF), IUPAC, p. 137
- ^ a b c "Clinical correlates of pH levels: bicarbonate as a buffer". Biology.arizona.edu. October 2006. Archived from the original on 31 May 2015.
- ^ William Hyde Wollaston (1814) "A synoptic scale of chemical equivalents", Philosophical Transactions of the Royal Society, 104: 1-22. On page 11, Wollaston coins the term "bicarbonate": "The next question that occurs relates to the composition of this crystallized carbonate of potash, which I am induced to call bi-carbonate of potash, for the purpose of marking more decidedly the distinction between this salt and that which is commonly called a subcarbonate, and in order to refer at once to the double dose of carbonic acid contained in it."
- ^ "Baking Soda". Newton – Ask a Scientist. Argonne National Laboratory. Archived from the original on 26 February 2015. Retrieved 2 May 2018.
- ^ Geor, Raymond J.; Coenen, Manfred; Harris, Pat (31 January 2013). Equine Applied and Clinical Nutrition: Health, Welfare and Performance. Elsevier Health Sciences. p. 90. ISBN 978-0-7020-5418-1.
The most common indicator of water quality is the concentration of total dissolved solids (TDS)
- ^ Ali E, Liponska A, O'Hara B, Amici D, Torno M, Gao P, Asara J, Yap M-N F, Mendillo M, Ben-Sahra I (June 2022). "The mTORC1-SLC4A7 axis stimulates bicarbonate import to enhance de novo nucleotide synthesis". Molecular Cell. 82 (1): 3284–3298.e7. doi:10.1016/j.molcel.2022.06.008. PMC 9444906. PMID 35772404.
- ^ Berne & Levy, Principles of Physiology
- ^ "The chemistry of ocean acidification : OCB-OA". www.whoi.edu. Woods Hole Oceanographic Institution. 24 September 2012. Archived from the original on 19 May 2017. Retrieved 17 May 2017.
- ^ Pełechaty, Mariusz; Pukacz, Andrzej; Apolinarska, Karina; Pełechata, Aleksandra; Siepak, Marcin (June 2013). Porta, Giovanna Della (ed.). "The significance of Chara vegetation in the precipitation of lacustrine calcium carbonate". Sedimentology. 60 (4): 1017–1035. Bibcode:2013Sedim..60.1017P. doi:10.1111/sed.12020. S2CID 128758128.
- ^ "How Baking Soda Works". Serious Eats. Retrieved 23 November 2024.
- ^ "Ammonium Bicarbonate – Bicarbonate Applications". www.ahperformance.com. Retrieved 23 November 2024.
- ^ Acid Base Balance (page 3) Archived 2002-06-13 at the Wayback Machine
External links
[edit]- Bicarbonates at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
