Aza-Diels–Alder reaction: Difference between revisions
Replacing Aza_DA_mechanism.gif with File:Aza_DA_mechanism.png (by CommonsDelinker because: file renamed on Commons). |
m Clean up spacing around commas and other punctuation fixes, replaced: ,b → , b |
||
| (16 intermediate revisions by 5 users not shown) | |||
| Line 7: | Line 7: | ||
}} |
}} |
||
}} |
}} |
||
The ''' |
The '''Aza-Diels–Alder reaction''' is a modification of the [[Diels–Alder reaction]] wherein a nitrogen replaces sp<sup>2</sup> carbon.<ref>{{cite book|title = Cycloaddition Reactions in Organic Synthesis|year = 2002|editor1-first = S.|editor1-last = Kobayashi|editor2-first = K. A.|editor2-last = Jørgensen|chapter = Catalytic Enantioselective Aza Diels-Alder Reactions|first = S.|last = Kobayashi|pages = 187–210|isbn = 9783527301591|publisher = [[John Wiley & Sons]]}}</ref> The nitrogen atom can be part of the [[diene]] or the [[dienophile]]. |
||
[[Image:Aza |
[[Image:Aza-Diels-Alder-reactie algemeen.png|200px|center|The Aza Diels–Alder reaction, general scope]] |
||
==Mechanism== |
|||
| ⚫ | The imine is often generated [[in situ]] from an [[amine]] and [[formaldehyde]]. An example is the reaction of [[cyclopentadiene]] with [[benzylamine]] to an aza [[norbornene]].<ref>''N-benzyl-2-azanorbornene |
||
The aza Diels-Alder (IDA) reaction may occur either by a concerted or stepwise process. The lowest-energy [[transition state]] for the concerted process places the imine lone pair (or coordinated Lewis acid) in an ''exo'' |
|||
position. Thus, (''E'') imines, in which the lone pair and larger imine carbon substituent are ''cis'', tend to give ''exo'' products.<ref>{{cite journal|author1=Whiting, A. |author2=Windsor, C. M. |journal=Tetrahedron|year=1998|volume=54|pages= 6035|doi=10.1016/S0040-4020(98)00284-1|title=What makes a neutral imino dieneophile undergo a thermal, non-catalysed, Diels-Alder reaction?|issue=22}}</ref> |
|||
{{center|[[File:iDAMech1.png]]}} |
|||
When the imine nitrogen is protonated or coordinated to a strong Lewis acid, the mechanism shifts to a stepwise, Mannich-Michael pathway.<ref>{{cite journal|author1=Hermitage, S. |author2=Jay, D. A. |author3=Whiting, A. |journal=Tetrahedron Lett.|year=2002|volume=43|pages= 9633|doi=10.1016/S0040-4039(02)02392-4|title=Evidence for the non-concerted \4+2]-cycloaddition of N-aryl imines when acting as both dienophiles and dienes under Lewis acid-catalysed conditions|issue=52}}</ref> |
|||
{{center|[[File:iDAMech2.png]]}} |
|||
Attaching an electron-withdrawing group to the imine nitrogen increases the rate. The ''exo'' isomer usually predominates (particularly when cyclic dienes are used), although selectivities vary.<ref>{{cite journal|author1=Corey, E. J. |author2=Yuen, P.-W. |journal=Tetrahedron Lett.|year=1989|volume=30|pages= 5825|doi=10.1016/S0040-4039(01)93481-1|title=A short, stereospecific route to chiral ''trans''-2,6-disubstituted quinuclidines|issue=43}}</ref> |
|||
| ⚫ | |||
{{center|[[File:iDAScope1.png]]}} |
|||
==Scope and limitations== |
|||
| ⚫ | In the [[enantioselective]] Diels–Alder |
||
===Stereoselective variants=== |
|||
In many cases, cyclic dienes give higher diastereoselectivities than acyclic dienes. Use of amino-acid-based chiral auxiliaries, for instance, leads to good diastereoselectivities in reactions of cyclopentadiene, but not in reactions of acyclic dienes.<ref>{{cite journal|author=Waldmann, H. |journal=Liebigs Ann. Chem.|year=1989|pages= 231–238|doi=10.1002/jlac.198919890145|title=Asymmetrische Hetero-Diels-Alder-Reaktionen in wäßriger Lösung unter Verwendung von Aminosäureestern als chiralen Auxiliaren|volume=1989|issue=3}}</ref> |
|||
{{center|[[File:iDAStereo2.png]]}} |
|||
===Asymmetric variants=== |
|||
Chiral auxiliaries have been employed on either the imino nitrogen<ref>{{cite journal|author1=Hedberg, C. |author2=Pinho, P. |author3=Roth, P. |author4=Andersson, P. G. |journal=J. Org. Chem.|year=2000|volume=65|pages= 2810–2|doi=10.1021/jo9916683|pmid=10808461|title=Diels-Alder reaction of heterocyclic imine dienophiles|issue=9}}</ref> or imino carbon<ref>{{cite journal|author1=Ishimaru, K. |author2=Watanabe, K. |author3=Yamamoto, Y. |author4=Akiba, K.-Y. |journal=Synlett|year=1994|pages= 495|doi=10.1055/s-1994-22902|title=Stereocontrol in [4+2]Type Cycloaddition of an Aldimine Derived from (''S'')-Ethyl Lactate with 2-Siloxy-1,3-butadienes|volume=1994|issue=7}}</ref> to effect diastereoselection. |
|||
{{center|[[File:iDAStereo1.png]]}} |
|||
| ⚫ | In the [[enantioselective]] Diels–Alder reaction of an [[aniline]], [[formaldehyde]] and a [[cyclohexenone]] [[catalysis|catalyzed]] by (''S'')-[[proline]] even the diene is masked.<ref>{{cite journal |title= Direct Catalytic Enantioselective Aza-Diels-Alder Reactions |first1= Henrik |last1= Sundén |first2= Ismail |last2= Ibrahem |first3= Lars |last3= Eriksson |first4= Armando |last4= Córdova |journal= [[Angewandte Chemie International Edition]] |pages= 4877–4880 |year= 2005 |doi= 10.1002/anie.200500811 |volume= 44 |issue= 31}}</ref> |
||
[[Image:Aza Diels Alder proline.gif|center|S-proline enantioselective Aza Diels–Alder reaction]] |
[[Image:Aza Diels Alder proline.gif|center|S-proline enantioselective Aza Diels–Alder reaction]] |
||
===In situ generated imines=== |
|||
| ⚫ | The imine is often generated [[in situ]] from an [[amine]] and [[formaldehyde]]. An example is the reaction of [[cyclopentadiene]] with [[benzylamine]] to an aza [[norbornene]].<ref>{{cite journal|title = ''N''-benzyl-2-azanorbornene|first1 = P. A.|last1 = Grieco|first2 = S. D.|last2 = Larsen|journal = [[Organic Syntheses]]|year = 1990|volume = 68|page = 206|url = http://www.orgsyn.org/orgsyn/prep.asp?prep=cv8p0031|doi = 10.15227/orgsyn.068.0206}}</ref> |
||
| ⚫ | |||
The [[catalytic cycle]] starts with the reactions of the aromatic amine with [[formaldehyde]] to the [[imine]] and the reaction of the [[ketone]] with [[proline]] to the diene. The second step, an [[endo trig cyclisation]], is driven to one of the two possible [[enantiomer]]s (99% [[enantiomeric excess|ee]]) because the imine nitrogen atom forms a [[hydrogen bond]] with the [[carboxylic acid]] group of proline on the [[Si face]]. Hydrolysis of the final complex releases the product and regenerates the catalyst. |
The [[catalytic cycle]] starts with the reactions of the aromatic amine with [[formaldehyde]] to the [[imine]] and the reaction of the [[ketone]] with [[proline]] to the diene. The second step, an [[endo trig cyclisation]], is driven to one of the two possible [[enantiomer]]s (99% [[enantiomeric excess|ee]]) because the imine nitrogen atom forms a [[hydrogen bond]] with the [[carboxylic acid]] group of proline on the [[Si face]]. Hydrolysis of the final complex releases the product and regenerates the catalyst. |
||
[[Image:Aza DA mechanism.png|center|catalytic cycle for S-proline enantioselective Aza Diels–Alder reaction]] |
[[Image:Aza DA mechanism.png|center|catalytic cycle for S-proline enantioselective Aza Diels–Alder reaction]] |
||
Tosylimines may be generated ''in situ'' from tosylisocyanate and aldehydes. Cycloadditions of |
|||
these intermediates with dienes give single constitutional isomers, but proceed with moderate stereoselectivity.<ref>{{cite journal|author1=Schrader, T. |author2=Steglich, W. |journal=Synthesis|year=1990|pages= 1153|doi=10.1055/s-1990-27122|title=Phosphoranaloge von Aminosäuren IV.1Synthesen ungewöhnlicher 1-Aminophosphonsäuren über Diels-Alder-Reaktionen von (N-Acyliminomethyl)phosphonsäurediethylestern|volume=1990|issue=12}}</ref> |
|||
{{center|[[File:iDAScope2.png]]}} |
|||
Lewis-acid catalyzed reactions of sulfonyl imines also exhibit moderate stereoselectivity.<ref>{{cite journal|author1=Krow, G. R. |author2=Pyun, C. |author3=Rodebaugh, R. |author4=Marakowski, J. |journal=Tetrahedron|year=1974|volume=30|pages= 2977|doi=10.1016/S0040-4020(01)97542-8|title=Heterodienophiles—V|issue=17}}</ref> |
|||
{{center|[[File:iDAScope3.png]]}} |
|||
Simple unactivated imines react with hydrocarbon dienes only with the help of a Lewis acid; however, both electron-rich |
|||
and electron-poor dienes react with unactivated imines when heated. Vinylketenes, for instance, afford dihydropyridones upon [4+2] cycloaddition with imines. Regio- and stereoselectivity are unusually high in reactions of this class of dienes.<ref>{{cite journal|author1=Bennett, D. M. |author2=Okamoto, I.|authorlink3=Rick L. Danheiser |author3=Danheiser, R. L. |journal=Org. Lett.|year=1999|volume=1|pages= 641–4|doi=10.1021/ol9907217|pmid=10823193|title=Hetero 4 + 2 cycloadditions of (trialkylsilyl)vinylketenes. Synthesis of alpha,beta-unsaturated delta-valerolactones and -lactams|issue=4}}</ref> |
|||
{{center|[[File:iDAScope4.png]]}} |
|||
Vinylallenes react similarly in the presence of a Lewis acid, often with high diastereoselectivity.<ref>{{cite journal|author1=Regas, D. |author2=Afonso, M. M. |author3=Rodriguez, M. L. |author4=Palenzuela, J. A. |journal=J. Org. Chem.|year=2003|volume=68|pages= 7845–52|doi=10.1021/jo034480z|pmid=14510565|title=Synthesis of octahydroquinolines through the Lewis acid catalyzed reaction of vinyl allenes and imines|issue=20}}</ref> |
|||
{{center|[[File:iDAScope5.png]]}} |
|||
===Acyliminium substrates=== |
|||
Acyliminium ions also participate in cycloadditions. These cations are generated by removal of chloride from chloromethylated amides:<ref>{{cite journal |doi=10.1021/cr00097a008 |title=N-acyl imines and related hetero dienes in [4+2]-cycloaddition reactions |date=1989 |last1=Weinreb |first1=Steven M. |last2=Scola |first2=Paul M. |journal=Chemical Reviews |volume=89 |issue=7 |pages=1525–1534 }}</ref> |
|||
:{{chem2| RCONRCH2Cl -> RCONR/dCH2+ + Cl-}} |
|||
The resulting acyl iminium cations serve as hetero[[diene]]s as well as [[dienophile]]. |
|||
===Use in natural products synthesis=== |
|||
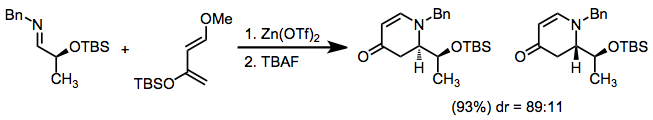
In 2014, Doyle and coworkers reported a Zn(OTf)<sub>2</sub>-catalyzed [4+2] cycloaddition reaction between two imines to form tetrahydropyrimidine products.<ref>Mandler, M. D.; Truong, P. M.; Zavalij, P. Y.; Doyle, M. P. Org. Lett. 2014, 16, 740-743.</ref> |
|||
The aza-Diels–Alder reaction has been applied to the synthesis of a number of alkaloid natural products. [[Danishefsky's diene]] is used to form a six-membered ring en route to phyllanthine.<ref>{{cite journal|author1=Han, G. |author2=LaPorte, M. G. |author3=Folmer, J. J. |author4=Werner, K. M. |author5=Weinreb, S. M. |journal=J. Org. Chem.|year=2000|volume=65|pages= 6293–306|doi=10.1021/jo000260z|pmid=11052071|title=Total syntheses of the Securinega alkaloids (+)-14,15-dihydronorsecurinine, (−)-norsecurinine, and phyllanthine|issue=20}}</ref> |
|||
{{center|[[File:iDASynth.png]]}} |
|||
==See also== |
==See also== |
||
* [[Oxo |
* [[Oxo-Diels–Alder reaction]] |
||
* [[Imine Diels–Alder reaction]] |
|||
== |
==References== |
||
{{Reflist|35em}} |
|||
<references/> |
|||
{{DEFAULTSORT:Aza-Diels-Alder reaction}} |
{{DEFAULTSORT:Aza-Diels-Alder reaction}} |
||
Latest revision as of 03:58, 27 November 2024
| Aza-Diels–Alder reaction | |
|---|---|
| Named after | Otto Diels Kurt Alder |
| Reaction type | Cycloaddition |
| Identifiers | |
| RSC ontology ID | RXNO:0000092 |
The Aza-Diels–Alder reaction is a modification of the Diels–Alder reaction wherein a nitrogen replaces sp2 carbon.[1] The nitrogen atom can be part of the diene or the dienophile.
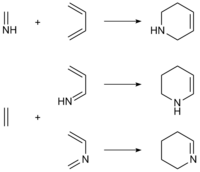
Mechanism
[edit]The aza Diels-Alder (IDA) reaction may occur either by a concerted or stepwise process. The lowest-energy transition state for the concerted process places the imine lone pair (or coordinated Lewis acid) in an exo position. Thus, (E) imines, in which the lone pair and larger imine carbon substituent are cis, tend to give exo products.[2]
When the imine nitrogen is protonated or coordinated to a strong Lewis acid, the mechanism shifts to a stepwise, Mannich-Michael pathway.[3]
Attaching an electron-withdrawing group to the imine nitrogen increases the rate. The exo isomer usually predominates (particularly when cyclic dienes are used), although selectivities vary.[4]
Scope and limitations
[edit]Stereoselective variants
[edit]In many cases, cyclic dienes give higher diastereoselectivities than acyclic dienes. Use of amino-acid-based chiral auxiliaries, for instance, leads to good diastereoselectivities in reactions of cyclopentadiene, but not in reactions of acyclic dienes.[5]
Asymmetric variants
[edit]Chiral auxiliaries have been employed on either the imino nitrogen[6] or imino carbon[7] to effect diastereoselection.
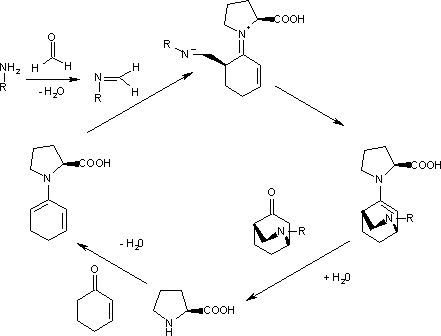
In the enantioselective Diels–Alder reaction of an aniline, formaldehyde and a cyclohexenone catalyzed by (S)-proline even the diene is masked.[8]

In situ generated imines
[edit]The imine is often generated in situ from an amine and formaldehyde. An example is the reaction of cyclopentadiene with benzylamine to an aza norbornene.[9]

The catalytic cycle starts with the reactions of the aromatic amine with formaldehyde to the imine and the reaction of the ketone with proline to the diene. The second step, an endo trig cyclisation, is driven to one of the two possible enantiomers (99% ee) because the imine nitrogen atom forms a hydrogen bond with the carboxylic acid group of proline on the Si face. Hydrolysis of the final complex releases the product and regenerates the catalyst.
Tosylimines may be generated in situ from tosylisocyanate and aldehydes. Cycloadditions of these intermediates with dienes give single constitutional isomers, but proceed with moderate stereoselectivity.[10]
Lewis-acid catalyzed reactions of sulfonyl imines also exhibit moderate stereoselectivity.[11]
Simple unactivated imines react with hydrocarbon dienes only with the help of a Lewis acid; however, both electron-rich and electron-poor dienes react with unactivated imines when heated. Vinylketenes, for instance, afford dihydropyridones upon [4+2] cycloaddition with imines. Regio- and stereoselectivity are unusually high in reactions of this class of dienes.[12]
Vinylallenes react similarly in the presence of a Lewis acid, often with high diastereoselectivity.[13]
Acyliminium substrates
[edit]Acyliminium ions also participate in cycloadditions. These cations are generated by removal of chloride from chloromethylated amides:[14]
- RCONRCH2Cl → RCONR/dCH+2 + Cl−
The resulting acyl iminium cations serve as heterodienes as well as dienophile.
Use in natural products synthesis
[edit]The aza-Diels–Alder reaction has been applied to the synthesis of a number of alkaloid natural products. Danishefsky's diene is used to form a six-membered ring en route to phyllanthine.[15]
See also
[edit]References
[edit]- ^ Kobayashi, S. (2002). "Catalytic Enantioselective Aza Diels-Alder Reactions". In Kobayashi, S.; Jørgensen, K. A. (eds.). Cycloaddition Reactions in Organic Synthesis. John Wiley & Sons. pp. 187–210. ISBN 9783527301591.
- ^ Whiting, A.; Windsor, C. M. (1998). "What makes a neutral imino dieneophile undergo a thermal, non-catalysed, Diels-Alder reaction?". Tetrahedron. 54 (22): 6035. doi:10.1016/S0040-4020(98)00284-1.
- ^ Hermitage, S.; Jay, D. A.; Whiting, A. (2002). "Evidence for the non-concerted \4+2]-cycloaddition of N-aryl imines when acting as both dienophiles and dienes under Lewis acid-catalysed conditions". Tetrahedron Lett. 43 (52): 9633. doi:10.1016/S0040-4039(02)02392-4.
- ^ Corey, E. J.; Yuen, P.-W. (1989). "A short, stereospecific route to chiral trans-2,6-disubstituted quinuclidines". Tetrahedron Lett. 30 (43): 5825. doi:10.1016/S0040-4039(01)93481-1.
- ^ Waldmann, H. (1989). "Asymmetrische Hetero-Diels-Alder-Reaktionen in wäßriger Lösung unter Verwendung von Aminosäureestern als chiralen Auxiliaren". Liebigs Ann. Chem. 1989 (3): 231–238. doi:10.1002/jlac.198919890145.
- ^ Hedberg, C.; Pinho, P.; Roth, P.; Andersson, P. G. (2000). "Diels-Alder reaction of heterocyclic imine dienophiles". J. Org. Chem. 65 (9): 2810–2. doi:10.1021/jo9916683. PMID 10808461.
- ^ Ishimaru, K.; Watanabe, K.; Yamamoto, Y.; Akiba, K.-Y. (1994). "Stereocontrol in [4+2]Type Cycloaddition of an Aldimine Derived from (S)-Ethyl Lactate with 2-Siloxy-1,3-butadienes". Synlett. 1994 (7): 495. doi:10.1055/s-1994-22902.
- ^ Sundén, Henrik; Ibrahem, Ismail; Eriksson, Lars; Córdova, Armando (2005). "Direct Catalytic Enantioselective Aza-Diels-Alder Reactions". Angewandte Chemie International Edition. 44 (31): 4877–4880. doi:10.1002/anie.200500811.
- ^ Grieco, P. A.; Larsen, S. D. (1990). "N-benzyl-2-azanorbornene". Organic Syntheses. 68: 206. doi:10.15227/orgsyn.068.0206.
- ^ Schrader, T.; Steglich, W. (1990). "Phosphoranaloge von Aminosäuren IV.1Synthesen ungewöhnlicher 1-Aminophosphonsäuren über Diels-Alder-Reaktionen von (N-Acyliminomethyl)phosphonsäurediethylestern". Synthesis. 1990 (12): 1153. doi:10.1055/s-1990-27122.
- ^ Krow, G. R.; Pyun, C.; Rodebaugh, R.; Marakowski, J. (1974). "Heterodienophiles—V". Tetrahedron. 30 (17): 2977. doi:10.1016/S0040-4020(01)97542-8.
- ^ Bennett, D. M.; Okamoto, I.; Danheiser, R. L. (1999). "Hetero 4 + 2 cycloadditions of (trialkylsilyl)vinylketenes. Synthesis of alpha,beta-unsaturated delta-valerolactones and -lactams". Org. Lett. 1 (4): 641–4. doi:10.1021/ol9907217. PMID 10823193.
- ^ Regas, D.; Afonso, M. M.; Rodriguez, M. L.; Palenzuela, J. A. (2003). "Synthesis of octahydroquinolines through the Lewis acid catalyzed reaction of vinyl allenes and imines". J. Org. Chem. 68 (20): 7845–52. doi:10.1021/jo034480z. PMID 14510565.
- ^ Weinreb, Steven M.; Scola, Paul M. (1989). "N-acyl imines and related hetero dienes in [4+2]-cycloaddition reactions". Chemical Reviews. 89 (7): 1525–1534. doi:10.1021/cr00097a008.
- ^ Han, G.; LaPorte, M. G.; Folmer, J. J.; Werner, K. M.; Weinreb, S. M. (2000). "Total syntheses of the Securinega alkaloids (+)-14,15-dihydronorsecurinine, (−)-norsecurinine, and phyllanthine". J. Org. Chem. 65 (20): 6293–306. doi:10.1021/jo000260z. PMID 11052071.