Tetracycline: Difference between revisions
m v2.05b - Bot T20 CW#61 - Fix errors for CW project (Reference before punctuation) |
Citation bot (talk | contribs) Added bibcode. | Use this bot. Report bugs. | Suggested by Dominic3203 | Category:Biomarkers | #UCB_Category 57/82 |
||
| (14 intermediate revisions by 11 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Antibiotic used to treat a number of infections}} |
{{Short description|Antibiotic used to treat a number of infections}} |
||
{{About|the specific antibiotic|the family of antibiotics|Tetracycline antibiotics}} |
{{About|the specific antibiotic|the family of antibiotics|Tetracycline antibiotics}} |
||
{{cs1 config|name-list-style=vanc|display-authors=6}} |
{{Use dmy dates|date=June 2024}} |
||
{{cs1 config |name-list-style=vanc |display-authors=6}} |
|||
{{Drugbox |
{{Drugbox |
||
| Watchedfields = changed |
| Watchedfields = changed |
||
| Line 7: | Line 8: | ||
| drug_name = |
| drug_name = |
||
| image = [[File:Tetracycline skeletal.svg|frameless|tetracycline 2D skeletal]] |
| image = [[File:Tetracycline skeletal.svg|frameless|tetracycline 2D skeletal]] |
||
| ⚫ | |||
| image2 = [[File:Tetracycline.png|frameless|tetracycline 3D BS]] |
| image2 = [[File:Tetracycline.png|frameless|tetracycline 3D BS]] |
||
| ⚫ | |||
| ⚫ | |||
<!--Clinical data--> |
<!--Clinical data--> |
||
| pronounce = {{IPAc-en|ˌ|t|ɛ|t|r|ə|ˈ|s|aɪ|k|l|iː|n}} |
| pronounce = {{IPAc-en|ˌ|t|ɛ|t|r|ə|ˈ|s|aɪ|k|l|iː|n}} |
||
| tradename = |
| tradename = Tetracyn |
||
| Drugs.com = {{drugs.com|monograph|tetracycline}} |
| Drugs.com = {{drugs.com|monograph|tetracycline}} |
||
| MedlinePlus = a682098 |
| MedlinePlus = a682098 |
||
| |
| DailyMedID = Tetracycline |
||
| pregnancy_AU = D |
| pregnancy_AU = D |
||
| pregnancy_US = D |
|||
| ⚫ | |||
| routes_of_administration = [[Oral administration|By mouth]] |
| routes_of_administration = [[Oral administration|By mouth]] |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<!--Pharmacokinetic data--> |
<!--Pharmacokinetic data--> |
||
| Line 31: | Line 36: | ||
| CAS_number_Ref = {{cascite|correct|??}} |
| CAS_number_Ref = {{cascite|correct|??}} |
||
| CAS_number = 60-54-8 |
| CAS_number = 60-54-8 |
||
| CAS_number2 = 64-75-5 |
|||
| CAS_supplemental = <br>{{CAS|64-75-5}} (hydrochloride) <!-- Also CAS verified --> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| PubChem = 54675776 |
| PubChem = 54675776 |
||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
||
| Line 46: | Line 46: | ||
| KEGG_Ref = {{keggcite|correct|kegg}} |
| KEGG_Ref = {{keggcite|correct|kegg}} |
||
| KEGG = D00201 |
| KEGG = D00201 |
||
| ⚫ | |||
| ChEBI = 27902 |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
||
| ChEMBL = 1440 |
| ChEMBL = 1440 |
||
| PDB_ligand = TAC |
| PDB_ligand = TAC |
||
| ⚫ | |||
<!--Chemical data-->| C = 22 |
<!--Chemical data--> |
||
| C = 22 |
|||
| H = 24 |
| H = 24 |
||
| N = 2 |
| N = 2 |
||
| Line 62: | Line 66: | ||
<!-- Definition and medical uses --> |
<!-- Definition and medical uses --> |
||
'''Tetracycline''', sold under various brand names, is an |
'''Tetracycline''', sold under various brand names, is an [[antibiotic]] in the [[tetracyclines]] family of medications, used to treat a number of [[infections]],<ref name=AHFS2016/> including [[Acne vulgaris|acne]], [[cholera]], [[brucellosis]], [[plague (disease)|plague]], [[malaria]], and [[syphilis]].<ref name=AHFS2016/> It is available in oral and topical formulations.<ref>{{Cite web |title=Tetracycline Topical Dosage Guide + Max Dose, Adjustments |url=https://www.drugs.com/dosage/tetracycline-topical.html |access-date=2024-11-27 |website=Drugs.com |language=en}}</ref><ref>{{Cite web |title=Tetracycline Dosage Guide + Max Dose, Adjustments |url=https://www.drugs.com/dosage/tetracycline.html |access-date=2024-11-27 |website=Drugs.com |language=en}}</ref> |
||
<!-- Side effects and mechanism --> |
<!-- Side effects and mechanism --> |
||
| Line 68: | Line 72: | ||
<!-- Society and culture --> |
<!-- Society and culture --> |
||
Tetracycline was patented in 1953<ref>{{US patent|2699054A}}</ref> and was approved for prescription use in 1954.<ref name=History/><ref>{{cite book| vauthors = Fischer J, Ganellin CR |title=Analogue-based Drug Discovery|date=2006|publisher=John Wiley & Sons|isbn=9783527607495|page=489|url=https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA489|language=en|url-status=live|archive-url=https://web.archive.org/web/20161220084414/https://books.google.ca/books?id=FjKfqkaKkAAC&pg=PA489|archive-date= |
Tetracycline was patented in 1953<ref>{{US patent|2699054A}}</ref> and was approved for prescription use in 1954.<ref name=History/><ref>{{cite book| vauthors = Fischer J, Ganellin CR |title=Analogue-based Drug Discovery|date=2006|publisher=John Wiley & Sons|isbn=9783527607495|page=489|url=https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA489|language=en|url-status=live|archive-url=https://web.archive.org/web/20161220084414/https://books.google.ca/books?id=FjKfqkaKkAAC&pg=PA489|archive-date=20 December 2016}}</ref> It is on the [[World Health Organization's List of Essential Medicines]].<ref name="WHO21st">{{cite book | vauthors = ((World Health Organization)) | title = World Health Organization model list of essential medicines: 21st list 2019 | year = 2019 | hdl = 10665/325771 | author-link = World Health Organization | publisher = World Health Organization | location = Geneva | id = WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO }}</ref> Tetracycline is available as a [[generic medication]].<ref name=AHFS2016/> Tetracycline was originally made from bacteria of the genus ''[[Streptomyces]]''.<ref name=AHFS2016/> |
||
==Medical uses== |
==Medical uses== |
||
| Line 86: | Line 90: | ||
===Use as a biomarker=== |
===Use as a biomarker=== |
||
[[File:Tetracycline-HCl substance photo.jpg|thumb|Tetracycline hydrochloride is available as yellow crystalline powder.]] |
[[File:Tetracycline-HCl substance photo.jpg|thumb|Tetracycline hydrochloride is available as yellow crystalline powder.]] |
||
Since tetracycline is absorbed into bone, it is used as a marker of bone growth for [[biopsies]] in humans. Tetracycline labeling is used to determine the amount of bone growth within a certain period of time, usually a period around 21 days. Tetracycline is incorporated into mineralizing bone and can be detected by its [[fluorescence]].<ref name=mayton>{{cite web | vauthors = Mayton CA | url = http://www.histosearch.com/histonet/Dec02/TetracyclinelabelingofbonA.html | title = Tetracycline labeling of bone | archive-url = https://web.archive.org/web/20070312193518/http://www.histosearch.com/histonet/Dec02/TetracyclinelabelingofbonA.html | archive-date= |
Since tetracycline is absorbed into bone, it is used as a marker of bone growth for [[biopsies]] in humans. Tetracycline labeling is used to determine the amount of bone growth within a certain period of time, usually a period around 21 days. Tetracycline is incorporated into mineralizing bone and can be detected by its [[fluorescence]].<ref name=mayton>{{cite web | vauthors = Mayton CA | url = http://www.histosearch.com/histonet/Dec02/TetracyclinelabelingofbonA.html | title = Tetracycline labeling of bone | archive-url = https://web.archive.org/web/20070312193518/http://www.histosearch.com/histonet/Dec02/TetracyclinelabelingofbonA.html | archive-date=12 March 2007 }}</ref> In "double tetracycline labeling", a second dose is given 11–14 days after the first dose, and the amount of bone formed during that interval can be calculated by measuring the distance between the two fluorescent labels.<ref>{{cite web | url = http://pathology2.jhu.edu/bonelab/4cycline.htm | work = The Johns Hopkins Medical Institutions. | title = Tetracycline Labeling | archive-url = https://archive.today/20121215013608/http://pathology2.jhu.edu/bonelab/4cycline.htm | archive-date=15 December 2012 | date = 8 January 2001 }}</ref> |
||
Tetracycline is also used as a biomarker in [[wildlife]] to detect consumption of medicine- or [[vaccine]]-containing baits.<ref>{{cite journal | vauthors = Olson CA, Mitchell KD, Werner PA | title = Bait ingestion by free-ranging raccoons and nontarget species in an oral rabies vaccine field trial in Florida | journal = Journal of Wildlife Diseases | volume = 36 | issue = 4 | pages = 734–743 | date = October 2000 | pmid = 11085436 | doi = 10.7589/0090-3558-36.4.734 | url = http://www.jwildlifedis.org/cgi/reprint/36/4/734 | url-status = dead | s2cid = 35102508 | archive-url = https://archive.today/20130415041932/http://www.jwildlifedis.org/cgi/reprint/36/4/734 | archive-date = |
Tetracycline is also used as a biomarker in [[wildlife]] to detect consumption of medicine- or [[vaccine]]-containing baits.<ref>{{cite journal | vauthors = Olson CA, Mitchell KD, Werner PA | title = Bait ingestion by free-ranging raccoons and nontarget species in an oral rabies vaccine field trial in Florida | journal = Journal of Wildlife Diseases | volume = 36 | issue = 4 | pages = 734–743 | date = October 2000 | pmid = 11085436 | doi = 10.7589/0090-3558-36.4.734 | url = http://www.jwildlifedis.org/cgi/reprint/36/4/734 | url-status = dead | s2cid = 35102508 | archive-url = https://archive.today/20130415041932/http://www.jwildlifedis.org/cgi/reprint/36/4/734 | archive-date = 15 April 2013 }}</ref> |
||
== Side effects == |
== Side effects == |
||
{{See also|Tooth bleaching}} |
{{See also|Tooth bleaching}} |
||
{{more med cn|section|date=November 2022}} |
{{more med cn|section|date=November 2022}} |
||
Use of [[tetracycline antibiotics]] can:<ref>{{Cite web|url=https://medlineplus.gov/druginfo/meds/a682098.html|title=Tetracycline: MedlinePlus Drug Information|website=medlineplus.gov|language=en|access-date=2017 |
Use of [[tetracycline antibiotics]] can:<ref>{{Cite web|url=https://medlineplus.gov/druginfo/meds/a682098.html|title=Tetracycline: MedlinePlus Drug Information|website=medlineplus.gov|language=en|access-date=19 May 2017|url-status=live|archive-url=https://web.archive.org/web/20170510164238/https://medlineplus.gov/druginfo/meds/a682098.html|archive-date=10 May 2017}}</ref> |
||
* Discolor permanent teeth (yellow-gray-brown), from prenatal period through childhood and adulthood.<ref>{{cite journal | vauthors = Sánchez AR, Rogers RS, Sheridan PJ | title = Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity | journal = International Journal of Dermatology | volume = 43 | issue = 10 | pages = 709–715 | date = October 2004 | pmid = 15485524 | doi = 10.1111/j.1365-4632.2004.02108.x }}</ref> Children receiving long- or short-term therapy with a tetracycline or glycylcycline may develop permanent brown discoloration of the teeth. |
* Discolor permanent teeth (yellow-gray-brown), from prenatal period through childhood and adulthood.<ref>{{cite journal | vauthors = Sánchez AR, Rogers RS, Sheridan PJ | title = Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity | journal = International Journal of Dermatology | volume = 43 | issue = 10 | pages = 709–715 | date = October 2004 | pmid = 15485524 | doi = 10.1111/j.1365-4632.2004.02108.x }}</ref><ref name=":0" /> Children receiving long- or short-term therapy with a tetracycline or glycylcycline may develop permanent brown discoloration of the teeth. |
||
* Be inactivated by calcium [[ion]]s, so are not to be taken with [[milk]], [[yogurt]], and other [[dairy product|dairy]] products |
* Be inactivated by calcium [[ion]]s, so are not to be taken with [[milk]], [[yogurt]], and other [[dairy product|dairy]] products |
||
* Be inactivated by [[aluminium]], [[iron]], and [[zinc]] ions, not to be taken at the same time as [[indigestion]] remedies (some common antacids and over-the-counter heartburn medicines) |
* Be inactivated by [[aluminium]], [[iron]], and [[zinc]] ions, not to be taken at the same time as [[indigestion]] remedies (some common antacids and over-the-counter heartburn medicines) |
||
* Cause [[skin]] [[photosensitivity]],<ref>{{cite book | vauthors = Shutter MC, Akhondi H | chapter = Tetracycline |date=2024 | title = StatPearls | chapter-url=http://www.ncbi.nlm.nih.gov/books/NBK549905/ |access-date= |
* Cause [[skin]] [[photosensitivity]],<ref name=":0">{{cite book | vauthors = Shutter MC, Akhondi H | chapter = Tetracycline |date=2024 | title = StatPearls | chapter-url=http://www.ncbi.nlm.nih.gov/books/NBK549905/ |access-date=19 March 2024 |place=Treasure Island (FL) |publisher=StatPearls Publishing |pmid=31751095 }}</ref> so exposure to the [[sun]] or intense [[light]] is not recommended |
||
* Cause drug-induced [[Lupus erythematosus|lupus]], and [[hepatitis]] |
* Cause drug-induced [[Lupus erythematosus|lupus]], and [[hepatitis]] |
||
* Cause microvesicular [[fatty liver]]<ref>{{Citation |title=Demeclocycline |date=2012 |work=LiverTox: Clinical and Research Information on Drug-Induced Liver Injury |url=http://www.ncbi.nlm.nih.gov/books/NBK548848/ |access-date= |
* Cause microvesicular [[fatty liver]]<ref>{{Citation |title=Demeclocycline |date=2012 |work=LiverTox: Clinical and Research Information on Drug-Induced Liver Injury |url=http://www.ncbi.nlm.nih.gov/books/NBK548848/ |access-date=20 March 2024 |place=Bethesda (MD) |publisher=National Institute of Diabetes and Digestive and Kidney Diseases |pmid=31644155}}</ref> |
||
* Cause [[tinnitus]]<ref>{{Cite book|title=Antibiotics Manual : A Guide to Commonly Used Antimicrobials| vauthors = Schlossberg DL, Samuel R |publisher=John Wiley & Sons, Inc.|year=2017|pages=367|via=ProQuest Ebook Central}}</ref> |
* Cause [[tinnitus]]<ref>{{Cite web |date=15 August 2022 |title=Tinnitus: Ringing in the ears and what to do about it |url=https://www.health.harvard.edu/newsletter_article/tinnitus-ringing-in-the-ears-and-what-to-do-about-it |website=Harvard Health Publishing Harvard Medical School}}</ref><ref>{{Cite book|title=Antibiotics Manual : A Guide to Commonly Used Antimicrobials| vauthors = Schlossberg DL, Samuel R |publisher=John Wiley & Sons, Inc.|year=2017|pages=367|via=ProQuest Ebook Central}}</ref> |
||
* Cause [[epigastric pain]]<ref name=":0" /> |
|||
* Interfere with [[methotrexate]] by displacing it from the various protein-binding sites |
* Interfere with [[methotrexate]] by displacing it from the various protein-binding sites |
||
* Cause breathing complications, as well as [[anaphylactic shock]], in some individuals |
* Cause breathing complications, as well as [[anaphylactic shock]], in some individuals |
||
| Line 106: | Line 111: | ||
* [[Fanconi syndrome]] may result from ingesting expired tetracyclines. |
* [[Fanconi syndrome]] may result from ingesting expired tetracyclines. |
||
Caution should be exercised in long-term use when breastfeeding. Short-term use is safe; [[bioavailability]] in milk is low to nil.<ref>{{cite book | veditors = Riordan J, Wambach K | title = Breastfeeding and Human Lactation | publisher= Jones & Bartlett Learning | date = November 2010 | page = 179 }}</ref> According to the U.S. [[Food and Drug Administration]] (FDA), cases of [[Stevens–Johnson syndrome]], [[toxic epidermal necrolysis]], and [[erythema multiforme]] associated with [[doxycycline]] use have been reported, but a causative role has not been established.<ref name=AERSlist>{{cite web | url = https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm223734.htm | title = FDA Adverse Events Reporting System | website = [[Food and Drug Administration]] | date = 27 August 2010 | archive-url = https://web.archive.org/web/20110117121827/https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm223734.htm | archive-date= |
Caution should be exercised in long-term use when breastfeeding. Short-term use is safe; [[bioavailability]] in milk is low to nil.<ref>{{cite book | veditors = Riordan J, Wambach K | title = Breastfeeding and Human Lactation | publisher= Jones & Bartlett Learning | date = November 2010 | page = 179 }}</ref> According to the U.S. [[Food and Drug Administration]] (FDA), cases of [[Stevens–Johnson syndrome]], [[toxic epidermal necrolysis]], and [[erythema multiforme]] associated with [[doxycycline]] use have been reported, but a causative role has not been established.<ref name=AERSlist>{{cite web | url = https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm223734.htm | title = FDA Adverse Events Reporting System | website = [[Food and Drug Administration]] | date = 27 August 2010 | archive-url = https://web.archive.org/web/20110117121827/https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm223734.htm | archive-date=17 January 2011 | access-date = 14 January 2011 }}</ref> |
||
==Pharmacology== |
==Pharmacology== |
||
===Mechanism of action=== |
===Mechanism of action=== |
||
Tetracycline inhibits protein synthesis by blocking the attachment of charged [[aminoacyl-tRNA|tRNA]] at the [[Ribosome|P site]] peptide chain. Tetracycline blocks the A-site so that a hydrogen bond is not formed between the amino acids. Tetracycline binds to the 30S and 50S subunit of microbial ribosomes.<ref name=AHFS2016/> Thus, it prevents the formation of a peptide chain.<ref>{{cite web | vauthors = Mehta A |url=http://pharmaxchange.info/press/2011/05/mechanism-of-action-of-tetracyclines/ |title=Mechanism of Action of Tetracyclines |publisher=Pharmaxchange.info |date= |
Tetracycline inhibits protein synthesis by blocking the attachment of charged [[aminoacyl-tRNA|tRNA]] at the [[Ribosome|P site]] peptide chain. Tetracycline blocks the A-site so that a hydrogen bond is not formed between the amino acids. Tetracycline binds to the 30S and 50S subunit of microbial ribosomes.<ref name=AHFS2016/> Thus, it prevents the formation of a peptide chain.<ref>{{cite web | vauthors = Mehta A |url=http://pharmaxchange.info/press/2011/05/mechanism-of-action-of-tetracyclines/ |title=Mechanism of Action of Tetracyclines |publisher=Pharmaxchange.info |date=27 May 2011 |access-date=7 June 2012 |url-status=live |archive-url=https://web.archive.org/web/20120605235017/http://pharmaxchange.info/press/2011/05/mechanism-of-action-of-tetracyclines/ |archive-date=5 June 2012 }}</ref> The action is usually not inhibitory and irreversible even with the withdrawal of the drug. Mammalian cells are not vulnerable to the effect of Tetracycline as these cells contain no 30S ribosomal subunits so do not accumulate the drug.<ref>{{Cite web |title=Tetracycline, USP |url=https://toku-e.com/tetracycline-usp/ |access-date=2024-06-28 |website=TOKU-E |language=en}}</ref> This accounts for the relatively small off-site effect of tetracycline on human cells.<ref>{{cite book | vauthors = Todar K | chapter = Antimicrobial Agents in the Treatment of Infectious Disease. | title = Todars Online Text Book of Bacteriology | date = 2012 | chapter-url = http://textbookofbacteriology.net/antimicrobial_4.html |access-date=27 August 2013 |url-status=live |archive-url=https://web.archive.org/web/20131008103423/http://textbookofbacteriology.net/antimicrobial_4.html |archive-date=8 October 2013 }}</ref> |
||
===Mechanisms of resistance=== |
===Mechanisms of resistance=== |
||
| Line 119: | Line 124: | ||
=== Discovery === |
=== Discovery === |
||
The tetracyclines, a large family of antibiotics, were discovered by [[Benjamin Minge Duggar]] in 1948 as natural products, and first prescribed in 1948.<ref>{{cite web | vauthors = Klajn R | url = http://www.chm.bris.ac.uk/motm/tetracycline/tetracycline.htm | title = Chemistry and chemical biology of tetracyclines | archive-url = https://web.archive.org/web/20070617003719/http://www.chm.bris.ac.uk/motm/tetracycline/tetracycline.htm | archive-date= |
The tetracyclines, a large family of antibiotics, were discovered by [[Benjamin Minge Duggar]] in 1948 as natural products, and first prescribed in 1948.<ref>{{cite web | vauthors = Klajn R | url = http://www.chm.bris.ac.uk/motm/tetracycline/tetracycline.htm | title = Chemistry and chemical biology of tetracyclines | archive-url = https://web.archive.org/web/20070617003719/http://www.chm.bris.ac.uk/motm/tetracycline/tetracycline.htm | archive-date=17 June 2007 | access-date = 20 June 2007}} {{better source needed|date=March 2017}}</ref> Benjamin Duggar, working under [[Yellapragada Subbarow]] at [[American Cyanamid|Lederle Laboratories]], discovered the first tetracycline antibiotic, [[chlortetracycline]] (Aureomycin), in 1945.<ref>{{cite journal | vauthors = Jukes TH | title = Some historical notes on chlortetracycline | journal = Reviews of Infectious Diseases | volume = 7 | issue = 5 | pages = 702–707 | date = 1985 | pmid = 3903946 | doi = 10.1093/clinids/7.5.702 | jstor = 4453725 }}</ref> The structure of Aureomycin was elucidated in 1952 and published in 1954 by the Pfizer-Woodward group.<ref>{{Cite journal | vauthors = Stephens CR, Conover LH, Pasternack R, Hochstein FA, Moreland WT, Regna PP, Pilgrim FJ, Brunings KJ, Woodward RB |date=July 1954 |title=The Structure of Aureomycin 1 |url=https://pubs.acs.org/doi/abs/10.1021/ja01642a064 |journal=Journal of the American Chemical Society |language=en |volume=76 |issue=13 |pages=3568–3575 |doi=10.1021/ja01642a064 |bibcode=1954JAChS..76.3568S |issn=0002-7863}}</ref> After the discovery of the structure, researchers at [[Pfizer]] began chemically modifying aureomycin by treating it with hydrogen in the presence of a [[Palladium on carbon|palladized carbon catalyst]]. This [[chemical]] reaction replaced a chlorine moiety with a hydrogen, creating a compound named tetracycline via [[hydrogenolysis]].<ref>{{Cite journal | vauthors = Conover LH, Moreland WT, English AR, Stephens CR, Pilgrim FJ |title=Terramycin. Xi. Tetracycline |date=September 1953 |url=https://pubs.acs.org/doi/abs/10.1021/ja01114a537 |journal=Journal of the American Chemical Society |language=en |volume=75 |issue=18 |pages=4622–4623 |doi=10.1021/ja01114a537 |bibcode=1953JAChS..75.4622C |issn=0002-7863}}</ref> Tetracycline displayed higher potency, better solubility, and more favorable pharmacology than the other antibiotics in its class, leading to its FDA approval in 1954. The new compound was one of the first commercially successful semi-synthetic antibiotics that was used, and laid the foundation for the development of Sancycline, [[Minocycline]], and later the [[Glycylcycline]]s.<ref name= History>{{cite journal | vauthors = Nelson ML, Levy SB | title = The history of the tetracyclines | journal = Annals of the New York Academy of Sciences | volume = 1241 | issue = 1 | pages = 17–32 | date = December 2011 | pmid = 22191524 | doi = 10.1111/j.1749-6632.2011.06354.x | bibcode = 2011NYASA1241...17N | s2cid = 34647314 }}</ref> |
||
===Evidence in antiquity=== |
===Evidence in antiquity=== |
||
| Line 130: | Line 135: | ||
==Society and culture== |
==Society and culture== |
||
=== |
===Economics=== |
||
According to data from EvaluatePharma and published in the ''[[Boston Globe]]'', in the USA the price of tetracycline rose from $0.06 per 250-[[Milligram|mg]] pill in 2013 to $4.06 a pill in 2015.<ref name="bostonglobe_2015_11">{{cite web | url=https://www.bostonglobe.com/business/2015/11/06/generic-drug-price-increases-alarm-insurers-providers-and-consumers/H3iA9CSxAUylnCdGjLNKVN/story.html?event=event25 | title=As competition wanes, prices for generics skyrocket | work=Boston Globe | date=6 November 2015 | access-date=18 November 2015 | vauthors = McCluskey PD | url-status=live | archive-url=https://web.archive.org/web/20151119073901/http://www.bostonglobe.com/business/2015/11/06/generic-drug-price-increases-alarm-insurers-providers-and-consumers/H3iA9CSxAUylnCdGjLNKVN/story.html?event=event25 | archive-date=19 November 2015 }}</ref> The ''Globe'' described the "big price hikes of some generic drugs" as a "relatively new phenomenon" which has left most pharmacists "grappling" with large upswings" in the "costs of generics, with 'overnight' price changes sometimes exceeding 1,000%."<ref name="bostonglobe_2015_11" /> |
According to data from EvaluatePharma and published in the ''[[Boston Globe]]'', in the USA the price of tetracycline rose from $0.06 per 250-[[Milligram|mg]] pill in 2013 to $4.06 a pill in 2015.<ref name="bostonglobe_2015_11">{{cite web | url=https://www.bostonglobe.com/business/2015/11/06/generic-drug-price-increases-alarm-insurers-providers-and-consumers/H3iA9CSxAUylnCdGjLNKVN/story.html?event=event25 | title=As competition wanes, prices for generics skyrocket | work=Boston Globe | date=6 November 2015 | access-date=18 November 2015 | vauthors = McCluskey PD | url-status=live | archive-url=https://web.archive.org/web/20151119073901/http://www.bostonglobe.com/business/2015/11/06/generic-drug-price-increases-alarm-insurers-providers-and-consumers/H3iA9CSxAUylnCdGjLNKVN/story.html?event=event25 | archive-date=19 November 2015 }}</ref> The ''Globe'' described the "big price hikes of some generic drugs" as a "relatively new phenomenon" which has left most pharmacists "grappling" with large upswings" in the "costs of generics, with 'overnight' price changes sometimes exceeding 1,000%."<ref name="bostonglobe_2015_11" /> |
||
=== |
===Brand names=== |
||
It is marketed under the brand names Sumycin, Tetracyn, and Panmycin, among others. Actisite is a thread-like fiber formulation used in dental applications.{{ |
It is marketed under the brand names Sumycin, Tetracyn, and Panmycin, among others. Actisite is a thread-like fiber formulation used in dental applications.<ref>{{cite journal | vauthors = Litch JM, Encarnacion M, Chen S, Leonard J, Burkoth TL | title = Use of the polymeric matrix as internal standard for quantitation of in vivo delivery of tetracycline HCl from Actisite tetracycline fiber during periodontal treatment | journal = Journal of Periodontal Research | volume = 31 | issue = 8 | pages = 540–544 | date = November 1996 | pmid = 8971652 | doi = 10.1111/j.1600-0765.1996.tb00518.x }}</ref> |
||
It is also used to produce several semisynthetic derivatives, which together are known as the [[tetracycline antibiotics]]. The term "tetracycline" is also used to denote the four-ring system of this compound; "tetracyclines" are related substances that contain the same four-ring system.{{cn|date=March 2023}} |
It is also used to produce several semisynthetic derivatives, which together are known as the [[tetracycline antibiotics]]. The term "tetracycline" is also used to denote the four-ring system of this compound; "tetracyclines" are related substances that contain the same four-ring system.{{cn|date=March 2023}} |
||
| Line 147: | Line 152: | ||
In [[genetic engineering]], tetracycline is used in [[Tetracycline-controlled transcriptional activation|transcriptional activation]]. It has been used as an engineered "control switch" in [[chronic myelogenous leukemia]] models in mice. Engineers were able to develop a retrovirus that induced a particular type of leukemia in mice, and could then "switch" the cancer on and off through tetracycline administration. This could be used to grow the cancer in mice and then halt it at a particular stage to allow for further experimentation or study.<ref>{{cite journal | vauthors = Dugray A, Geay JF, Foudi A, Bonnet ML, Vainchenker W, Wendling F, Louache F, Turhan AG | title = Rapid generation of a tetracycline-inducible BCR-ABL defective retrovirus using a single autoregulatory retroviral cassette | journal = Leukemia | volume = 15 | issue = 10 | pages = 1658–1662 | date = October 2001 | pmid = 11587226 | doi = 10.1038/sj.leu.2402225 | s2cid = 40155100 | doi-access = }}</ref> |
In [[genetic engineering]], tetracycline is used in [[Tetracycline-controlled transcriptional activation|transcriptional activation]]. It has been used as an engineered "control switch" in [[chronic myelogenous leukemia]] models in mice. Engineers were able to develop a retrovirus that induced a particular type of leukemia in mice, and could then "switch" the cancer on and off through tetracycline administration. This could be used to grow the cancer in mice and then halt it at a particular stage to allow for further experimentation or study.<ref>{{cite journal | vauthors = Dugray A, Geay JF, Foudi A, Bonnet ML, Vainchenker W, Wendling F, Louache F, Turhan AG | title = Rapid generation of a tetracycline-inducible BCR-ABL defective retrovirus using a single autoregulatory retroviral cassette | journal = Leukemia | volume = 15 | issue = 10 | pages = 1658–1662 | date = October 2001 | pmid = 11587226 | doi = 10.1038/sj.leu.2402225 | s2cid = 40155100 | doi-access = }}</ref> |
||
A technique being developed for the control of the [[mosquito]] species ''[[Aedes aegypti]]'' (the infection [[Vector (epidemiology)|vector]] for [[yellow fever]], [[dengue fever]], [[Zika fever]], and several other diseases) uses a strain that is [[genetically modified]] to require tetracycline to develop beyond the larval stage. Modified males raised in a laboratory develop normally as they are supplied with this chemical and can be released into the wild. Their subsequent offspring inherit this trait, but find no tetracycline in their environments, so never develop into adults.<ref>{{cite news| vauthors = Urquhart C | title= Can GM mosquitoes rid the world of a major killer?| newspaper= The Observer| date= 15 July 2012| url= https://www.theguardian.com/environment/2012/jul/15/gm-mosquitoes-dengue-fever-feature| access-date= |
A technique being developed for the control of the [[mosquito]] species ''[[Aedes aegypti]]'' (the infection [[Vector (epidemiology)|vector]] for [[yellow fever]], [[dengue fever]], [[Zika fever]], and several other diseases) uses a strain that is [[genetically modified]] to require tetracycline to develop beyond the larval stage. Modified males raised in a laboratory develop normally as they are supplied with this chemical and can be released into the wild. Their subsequent offspring inherit this trait, but find no tetracycline in their environments, so never develop into adults.<ref>{{cite news| vauthors = Urquhart C | title= Can GM mosquitoes rid the world of a major killer?| newspaper= The Observer| date= 15 July 2012| url= https://www.theguardian.com/environment/2012/jul/15/gm-mosquitoes-dengue-fever-feature| access-date= 15 July 2012| url-status= live| archive-url= https://web.archive.org/web/20131205105805/http://www.theguardian.com/environment/2012/jul/15/gm-mosquitoes-dengue-fever-feature| archive-date= 5 December 2013}}</ref> |
||
== References == |
== References == |
||
{{Reflist}} |
{{Reflist}} |
||
== External links == |
|||
* {{cite web | url = https://druginfo.nlm.nih.gov/drugportal/name/tetracycline | publisher = U.S. National Library of Medicine | work = Drug Information Portal | title = Tetracycline }} |
|||
{{Stomatological preparations}} |
{{Stomatological preparations}} |
||
| Line 161: | Line 163: | ||
{{Otologicals}} |
{{Otologicals}} |
||
{{Xenobiotic-sensing receptor modulators}} |
{{Xenobiotic-sensing receptor modulators}} |
||
{{Portal bar|Medicine}} |
{{Portal bar | Medicine}} |
||
{{Authority control}} |
|||
[[Category:1948 introductions]] |
[[Category:1948 introductions]] |
||
[[Category:Anti-acne preparations]] |
[[Category:Anti-acne preparations]] |
||
Latest revision as of 06:16, 28 November 2024
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌtɛtrəˈsaɪkliːn/ |
| Trade names | Tetracyn |
| Other names | TE/TET/TC/TCY[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682098 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 80% |
| Metabolism | Not metabolized |
| Elimination half-life | 8–11 hours, 57–108 hours (kidney impairment) |
| Excretion | Urine (>60%), feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.438 |
| Chemical and physical data | |
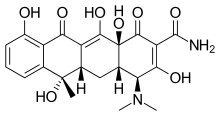
| Formula | C22H24N2O8 |
| Molar mass | 444.440 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Tetracycline, sold under various brand names, is an antibiotic in the tetracyclines family of medications, used to treat a number of infections,[3] including acne, cholera, brucellosis, plague, malaria, and syphilis.[3] It is available in oral and topical formulations.[4][5]
Common side effects include vomiting, diarrhea, rash, and loss of appetite.[3] Other side effects include poor tooth development if used by children less than eight years of age, kidney problems, and sunburning easily.[3] Use during pregnancy may harm the baby.[3] It works by inhibiting protein synthesis in bacteria.[3]
Tetracycline was patented in 1953[6] and was approved for prescription use in 1954.[7][8] It is on the World Health Organization's List of Essential Medicines.[9] Tetracycline is available as a generic medication.[3] Tetracycline was originally made from bacteria of the genus Streptomyces.[3]
Medical uses
[edit]Spectrum of activity
[edit]Tetracyclines have a broad spectrum of antibiotic action. Originally, they possessed some level of bacteriostatic activity against almost all medically relevant aerobic and anaerobic bacterial genera, both Gram-positive and Gram-negative, with a few exceptions, such as Pseudomonas aeruginosa and Proteus spp., which display intrinsic resistance. However, acquired (as opposed to inherent) resistance has proliferated in many pathogenic organisms and greatly eroded the formerly vast versatility of this group of antibiotics. Resistance amongst Staphylococcus spp., Streptococcus spp., Neisseria gonorrhoeae, anaerobes, members of the Enterobacteriaceae, and several other previously sensitive organisms is now quite common. Tetracyclines remain especially useful in the management of infections by certain obligately intracellular bacterial pathogens such as Chlamydia, Mycoplasma, and Rickettsia. They are also of value in spirochaetal infections, such as syphilis, and Lyme disease. Certain rare or exotic infections, including anthrax, plague, and brucellosis, are also susceptible to tetracyclines. Tetracycline tablets were used in the plague outbreak in India in 1994.[10] Tetracycline is first-line therapy for Rocky Mountain spotted fever (Rickettsia), Lyme disease (B. burgdorferi), Q fever (Coxiella), psittacosis, Mycoplasma pneumoniae, and nasal carriage of meningococci.[citation needed]
It is also one of a group of antibiotics which together may be used to treat peptic ulcers caused by bacterial infections. The mechanism of action for the antibacterial effect of tetracyclines relies on disrupting protein translation in bacteria, thereby damaging the ability of microbes to grow and repair; however, protein translation is also disrupted in eukaryotic mitochondria leading to effects that may confound experimental results.[11][12]
The following list presents MIC susceptibility data for some medically significant microorganisms:
- Escherichia coli: 1 μg/mL to >128 μg/mL
- Shigella spp.: 1 μg/mL to 128 μg/mL[13]
Anti-eukaryote use
[edit]The tetracyclines also have activity against certain eukaryotic parasites, including those responsible for diseases such as dysentery caused by an amoeba, malaria (a plasmodium), and balantidiasis (a ciliate).[citation needed]
Use as a biomarker
[edit]
Since tetracycline is absorbed into bone, it is used as a marker of bone growth for biopsies in humans. Tetracycline labeling is used to determine the amount of bone growth within a certain period of time, usually a period around 21 days. Tetracycline is incorporated into mineralizing bone and can be detected by its fluorescence.[14] In "double tetracycline labeling", a second dose is given 11–14 days after the first dose, and the amount of bone formed during that interval can be calculated by measuring the distance between the two fluorescent labels.[15]
Tetracycline is also used as a biomarker in wildlife to detect consumption of medicine- or vaccine-containing baits.[16]
Side effects
[edit]This section needs more reliable medical references for verification or relies too heavily on primary sources. (November 2022) |  |
Use of tetracycline antibiotics can:[17]
- Discolor permanent teeth (yellow-gray-brown), from prenatal period through childhood and adulthood.[18][19] Children receiving long- or short-term therapy with a tetracycline or glycylcycline may develop permanent brown discoloration of the teeth.
- Be inactivated by calcium ions, so are not to be taken with milk, yogurt, and other dairy products
- Be inactivated by aluminium, iron, and zinc ions, not to be taken at the same time as indigestion remedies (some common antacids and over-the-counter heartburn medicines)
- Cause skin photosensitivity,[19] so exposure to the sun or intense light is not recommended
- Cause drug-induced lupus, and hepatitis
- Cause microvesicular fatty liver[20]
- Cause tinnitus[21][22]
- Cause epigastric pain[19]
- Interfere with methotrexate by displacing it from the various protein-binding sites
- Cause breathing complications, as well as anaphylactic shock, in some individuals
- Affect bone growth of the fetus, so should be avoided during pregnancy
- Fanconi syndrome may result from ingesting expired tetracyclines.
Caution should be exercised in long-term use when breastfeeding. Short-term use is safe; bioavailability in milk is low to nil.[23] According to the U.S. Food and Drug Administration (FDA), cases of Stevens–Johnson syndrome, toxic epidermal necrolysis, and erythema multiforme associated with doxycycline use have been reported, but a causative role has not been established.[24]
Pharmacology
[edit]Mechanism of action
[edit]Tetracycline inhibits protein synthesis by blocking the attachment of charged tRNA at the P site peptide chain. Tetracycline blocks the A-site so that a hydrogen bond is not formed between the amino acids. Tetracycline binds to the 30S and 50S subunit of microbial ribosomes.[3] Thus, it prevents the formation of a peptide chain.[25] The action is usually not inhibitory and irreversible even with the withdrawal of the drug. Mammalian cells are not vulnerable to the effect of Tetracycline as these cells contain no 30S ribosomal subunits so do not accumulate the drug.[26] This accounts for the relatively small off-site effect of tetracycline on human cells.[27]
Mechanisms of resistance
[edit]Bacteria usually acquire resistance to tetracycline from horizontal transfer of a gene that either encodes an efflux pump or a ribosomal protection protein. Efflux pumps actively eject tetracycline from the cell, preventing the build up of an inhibitory concentration of tetracycline in the cytoplasm.[28] Ribosomal protection proteins interact with the ribosome and dislodge tetracycline from the ribosome, allowing for translation to continue.[29]
History
[edit]Discovery
[edit]The tetracyclines, a large family of antibiotics, were discovered by Benjamin Minge Duggar in 1948 as natural products, and first prescribed in 1948.[30] Benjamin Duggar, working under Yellapragada Subbarow at Lederle Laboratories, discovered the first tetracycline antibiotic, chlortetracycline (Aureomycin), in 1945.[31] The structure of Aureomycin was elucidated in 1952 and published in 1954 by the Pfizer-Woodward group.[32] After the discovery of the structure, researchers at Pfizer began chemically modifying aureomycin by treating it with hydrogen in the presence of a palladized carbon catalyst. This chemical reaction replaced a chlorine moiety with a hydrogen, creating a compound named tetracycline via hydrogenolysis.[33] Tetracycline displayed higher potency, better solubility, and more favorable pharmacology than the other antibiotics in its class, leading to its FDA approval in 1954. The new compound was one of the first commercially successful semi-synthetic antibiotics that was used, and laid the foundation for the development of Sancycline, Minocycline, and later the Glycylcyclines.[7]
Evidence in antiquity
[edit]Tetracycline has a high affinity for calcium and is incorporated into bones during the active mineralization of hydroxyapatite. When incorporated into bones, tetracycline can be identified using ultraviolet light.[34]
There is evidence that early inhabitants of Northeastern Africa consumed tetracycline antibiotics. Nubian mummies from between 350 and 550 A.D. were found to exhibit patterns of fluorescence identical with that of modern tetracycline labelled bone.[35]
It is conjectured that the beer brewed by the Nubians was the source of the tetracycline found in these bones.[36]
Society and culture
[edit]Economics
[edit]According to data from EvaluatePharma and published in the Boston Globe, in the USA the price of tetracycline rose from $0.06 per 250-mg pill in 2013 to $4.06 a pill in 2015.[37] The Globe described the "big price hikes of some generic drugs" as a "relatively new phenomenon" which has left most pharmacists "grappling" with large upswings" in the "costs of generics, with 'overnight' price changes sometimes exceeding 1,000%."[37]
Brand names
[edit]It is marketed under the brand names Sumycin, Tetracyn, and Panmycin, among others. Actisite is a thread-like fiber formulation used in dental applications.[38]
It is also used to produce several semisynthetic derivatives, which together are known as the tetracycline antibiotics. The term "tetracycline" is also used to denote the four-ring system of this compound; "tetracyclines" are related substances that contain the same four-ring system.[citation needed]
Media
[edit]Due to the drug's association with fighting infections, it serves as the main "commodity" in the science fiction series Aftermath, with the search for tetracycline becoming a major preoccupation in later episodes.[39]
Tetracycline is also represented in Bohemia Interactive's survival sandbox, DayZ. In the game, players may find the antibiotic to treat the common cold, influenza, cholera and infected wounds, but does not portray any side effects associated with tetracycline.
Research
[edit]Genetic engineering
[edit]In genetic engineering, tetracycline is used in transcriptional activation. It has been used as an engineered "control switch" in chronic myelogenous leukemia models in mice. Engineers were able to develop a retrovirus that induced a particular type of leukemia in mice, and could then "switch" the cancer on and off through tetracycline administration. This could be used to grow the cancer in mice and then halt it at a particular stage to allow for further experimentation or study.[40]
A technique being developed for the control of the mosquito species Aedes aegypti (the infection vector for yellow fever, dengue fever, Zika fever, and several other diseases) uses a strain that is genetically modified to require tetracycline to develop beyond the larval stage. Modified males raised in a laboratory develop normally as they are supplied with this chemical and can be released into the wild. Their subsequent offspring inherit this trait, but find no tetracycline in their environments, so never develop into adults.[41]
References
[edit]- ^ "Antibiotic abbreviations list". Retrieved 22 June 2023.
- ^ "Tetracycline". PubChem. National Center for Biotechnology Information.
- ^ a b c d e f g h i "Tetracycline". The American Society of Health-System Pharmacists. Archived from the original on 28 December 2016. Retrieved 8 December 2016.
- ^ "Tetracycline Topical Dosage Guide + Max Dose, Adjustments". Drugs.com. Retrieved 27 November 2024.
- ^ "Tetracycline Dosage Guide + Max Dose, Adjustments". Drugs.com. Retrieved 27 November 2024.
- ^ U.S. patent 2699054A
- ^ a b Nelson ML, Levy SB (December 2011). "The history of the tetracyclines". Annals of the New York Academy of Sciences. 1241 (1): 17–32. Bibcode:2011NYASA1241...17N. doi:10.1111/j.1749-6632.2011.06354.x. PMID 22191524. S2CID 34647314.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 489. ISBN 9783527607495. Archived from the original on 20 December 2016.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Lippincott's Illustrated Reviews: Pharmacology, 4th ed. Harvery RA, Champe, PC. Lippincott, Williams & Wilkins, 2009
- ^ Moullan N, Mouchiroud L, Wang X, Ryu D, Williams EG, Mottis A, et al. (March 2015). "Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research". Cell Reports. 10 (10): 1681–1691. doi:10.1016/j.celrep.2015.02.034. PMC 4565776. PMID 25772356.
- ^ Chatzispyrou IA, Held NM, Mouchiroud L, Auwerx J, Houtkooper RH (November 2015). "Tetracycline antibiotics impair mitochondrial function and its experimental use confounds research". Cancer Research. 75 (21): 4446–4449. doi:10.1158/0008-5472.CAN-15-1626. PMC 4631686. PMID 26475870.
- ^ "Tetracycline hydrochloride" (PDF). Susceptibility and Minimum Inhibitory Concentration (MIC) Data. TOKU-E. 8 September 2015. Archived from the original (PDF) on 8 September 2015.
- ^ Mayton CA. "Tetracycline labeling of bone". Archived from the original on 12 March 2007.
- ^ "Tetracycline Labeling". The Johns Hopkins Medical Institutions. 8 January 2001. Archived from the original on 15 December 2012.
- ^ Olson CA, Mitchell KD, Werner PA (October 2000). "Bait ingestion by free-ranging raccoons and nontarget species in an oral rabies vaccine field trial in Florida". Journal of Wildlife Diseases. 36 (4): 734–743. doi:10.7589/0090-3558-36.4.734. PMID 11085436. S2CID 35102508. Archived from the original on 15 April 2013.
- ^ "Tetracycline: MedlinePlus Drug Information". medlineplus.gov. Archived from the original on 10 May 2017. Retrieved 19 May 2017.
- ^ Sánchez AR, Rogers RS, Sheridan PJ (October 2004). "Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity". International Journal of Dermatology. 43 (10): 709–715. doi:10.1111/j.1365-4632.2004.02108.x. PMID 15485524.
- ^ a b c Shutter MC, Akhondi H (2024). "Tetracycline". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 31751095. Retrieved 19 March 2024.
- ^ "Demeclocycline", LiverTox: Clinical and Research Information on Drug-Induced Liver Injury, Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, 2012, PMID 31644155, retrieved 20 March 2024
- ^ "Tinnitus: Ringing in the ears and what to do about it". Harvard Health Publishing Harvard Medical School. 15 August 2022.
- ^ Schlossberg DL, Samuel R (2017). Antibiotics Manual : A Guide to Commonly Used Antimicrobials. John Wiley & Sons, Inc. p. 367 – via ProQuest Ebook Central.
- ^ Riordan J, Wambach K, eds. (November 2010). Breastfeeding and Human Lactation. Jones & Bartlett Learning. p. 179.
- ^ "FDA Adverse Events Reporting System". Food and Drug Administration. 27 August 2010. Archived from the original on 17 January 2011. Retrieved 14 January 2011.
- ^ Mehta A (27 May 2011). "Mechanism of Action of Tetracyclines". Pharmaxchange.info. Archived from the original on 5 June 2012. Retrieved 7 June 2012.
- ^ "Tetracycline, USP". TOKU-E. Retrieved 28 June 2024.
- ^ Todar K (2012). "Antimicrobial Agents in the Treatment of Infectious Disease.". Todars Online Text Book of Bacteriology. Archived from the original on 8 October 2013. Retrieved 27 August 2013.
- ^ Chopra I, Roberts M (June 2001). "Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance". Microbiology and Molecular Biology Reviews. 65 (2): 232–60, second page, table of contents. doi:10.1128/MMBR.65.2.232-260.2001. PMC 99026. PMID 11381101.
- ^ Connell SR, Tracz DM, Nierhaus KH, Taylor DE (December 2003). "Ribosomal protection proteins and their mechanism of tetracycline resistance". Antimicrobial Agents and Chemotherapy. 47 (12): 3675–3681. doi:10.1128/AAC.47.12.3675-3681.2003. PMC 296194. PMID 14638464.
- ^ Klajn R. "Chemistry and chemical biology of tetracyclines". Archived from the original on 17 June 2007. Retrieved 20 June 2007. [better source needed]
- ^ Jukes TH (1985). "Some historical notes on chlortetracycline". Reviews of Infectious Diseases. 7 (5): 702–707. doi:10.1093/clinids/7.5.702. JSTOR 4453725. PMID 3903946.
- ^ Stephens CR, Conover LH, Pasternack R, Hochstein FA, Moreland WT, Regna PP, et al. (July 1954). "The Structure of Aureomycin 1". Journal of the American Chemical Society. 76 (13): 3568–3575. Bibcode:1954JAChS..76.3568S. doi:10.1021/ja01642a064. ISSN 0002-7863.
- ^ Conover LH, Moreland WT, English AR, Stephens CR, Pilgrim FJ (September 1953). "Terramycin. Xi. Tetracycline". Journal of the American Chemical Society. 75 (18): 4622–4623. Bibcode:1953JAChS..75.4622C. doi:10.1021/ja01114a537. ISSN 0002-7863.
- ^ Pautke C, Vogt S, Kreutzer K, Haczek C, Wexel G, Kolk A, et al. (July 2010). "Characterization of eight different tetracyclines: advances in fluorescence bone labeling". Journal of Anatomy. 217 (1): 76–82. doi:10.1111/j.1469-7580.2010.01237.x. PMC 2913014. PMID 20456523.
- ^ Bassett EJ, Keith MS, Armelagos GJ, Martin DL, Villanueva AR (September 1980). "Tetracycline-labeled human bone from ancient Sudanese Nubia (A.D. 350)". Science. 209 (4464): 1532–1534. Bibcode:1980Sci...209.1532B. doi:10.1126/science.7001623. PMID 7001623.
- ^ Armelagos G (2000). "Take Two Beers and Call Me in 1,600 Years: Use of Tetracycline by Nubians and Ancient Egyptians". Natural History. 109 (4): 50–53.
- ^ a b McCluskey PD (6 November 2015). "As competition wanes, prices for generics skyrocket". Boston Globe. Archived from the original on 19 November 2015. Retrieved 18 November 2015.
- ^ Litch JM, Encarnacion M, Chen S, Leonard J, Burkoth TL (November 1996). "Use of the polymeric matrix as internal standard for quantitation of in vivo delivery of tetracycline HCl from Actisite tetracycline fiber during periodontal treatment". Journal of Periodontal Research. 31 (8): 540–544. doi:10.1111/j.1600-0765.1996.tb00518.x. PMID 8971652.
- ^ "Aftermath Episode Recap". SyFy Channel. Archived from the original on 13 October 2017. Retrieved 15 April 2020.
- ^ Dugray A, Geay JF, Foudi A, Bonnet ML, Vainchenker W, Wendling F, et al. (October 2001). "Rapid generation of a tetracycline-inducible BCR-ABL defective retrovirus using a single autoregulatory retroviral cassette". Leukemia. 15 (10): 1658–1662. doi:10.1038/sj.leu.2402225. PMID 11587226. S2CID 40155100.
- ^ Urquhart C (15 July 2012). "Can GM mosquitoes rid the world of a major killer?". The Observer. Archived from the original on 5 December 2013. Retrieved 15 July 2012.
