Chlorobis(dppe)iron hydride: Difference between revisions
rxns |
pubchem |
||
| (27 intermediate revisions by 10 users not shown) | |||
| Line 8: | Line 8: | ||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| CASNo = 32490-70-3 |
| CASNo = 32490-70-3 |
||
| PubChem = |
| PubChem = 165365653 |
||
| SMILES = Cl[FeH-4]56([P+](c1ccccc1)(c2ccccc2)CC[P+]5(c3ccccc3)c4ccccc4)[P+](c1ccccc1)(c2ccccc2)CC[P+]6(c3ccccc3)c4ccccc4 |
|||
| SMILES = }} |
|||
| StdInChI=1S/2C26H24P2.ClH.Fe.H/c2*1-5-13-23(14-6-1)27(24-15-7-2-8-16-24)21-22-28(25-17-9-3-10-18-25)26-19-11-4-12-20-26;;;/h2*1-20H,21-22H2;1H;;/q;;;+1;/p-1 |
|||
| StdInChIKey=NSXBDVRBULAONI-UHFFFAOYSA-M |
|||
}} |
|||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| C=52|H=49|P=4|Cl=1|Fe=1 |
| C=52|H=49|P=4|Cl=1|Fe=1 |
||
| Line 25: | Line 28: | ||
}} |
}} |
||
}} |
}} |
||
''' |
'''Chlorobis(dppe)iron hydride''' is a [[coordination complex]] with the formula HFeCl(dppe)<sub>2</sub>, where [[dppe]] is the bidentate ligand 1,2-bis(diphenylphosphino)ethane. It is a red-violet solid. The compound has attracted much attention as a precursor to [[dihydrogen complex]]es.<ref>{{cite journal |vauthors=Morris RH |title=Dihydrogen, Dihydride and in Between: Nmr and Structural Properties of Iron Group Complexes |journal=Coord. Chem. Rev. |year=2008 |volume=2252 |issue=21–22 |pages=2381–2394 |doi=10.1016/j.ccr.2008.01.010}}</ref> |
||
==Structure== |
==Structure== |
||
The |
The complex exhibits [[octahedral molecular geometry]]. The chloride and hydride ligands are mutually trans.<ref>Lee, J.; Jung, G.; Lee, S. W. Structure of trans-chlorohydridobis(diphenylphosphinoethane)iron(II). Bull. Korean. Chem. 1998, 19, 267. {{ISSN|0253-2964}}</ref> The bond distances between the iron metal atom and the coordinating ligands are given by the following: |
||
{|class="toccolours" border="1" style="margin: 1em; border-collapse: collapse;" |
{|class="toccolours" border="1" style="margin: 1em; border-collapse: collapse;" |
||
|- |
|- |
||
| Bond || Bond |
| Bond || Bond distance |
||
|- |
|- |
||
| Fe-P<sub>1</sub> || 2.238 |
| Fe-P<sub>1</sub> || 2.238 |
||
| Line 47: | Line 50: | ||
|} |
|} |
||
==Synthesis and reactions== |
==Synthesis and reactions== |
||
The compound is synthesized according to the following idealized reaction:<ref>{{cite book|author1=Giannoccaro, P. |author2=Sacco, A. |title=Inorganic Syntheses |chapter=Bis[Ethylenebis(Diphenylphosphine)]-Hydridoiron Complexes |journal=Inorg. Synth.|year=1977|volume=17|pages=69–71|doi=10.1002/9780470132487.ch19|isbn=9780470132487 }}</ref> |
|||
The compound is synthesized according to this idealized reaction: |
|||
:FeCl<sub>2</sub> + 2 dppe + Na[BH<sub>4</sub>] → NaCl + ½ B<sub>2</sub>H<sub>6</sub> + HFeCl |
:FeCl<sub>2</sub> + 2 dppe + Na[BH<sub>4</sub>] → NaCl + ½ B<sub>2</sub>H<sub>6</sub> + HFeCl(dppe)<sub>2</sub> |
||
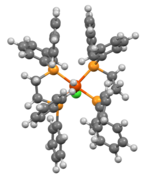
[[File:WEQGOOtopdown.png|thumb|left|144 px| |
[[File:WEQGOOtopdown.png|thumb|left|144 px|"Top-view" of the structure of HFeCl(dppe)<sub>2</sub>]] |
||
In the course of this conversion, high-spin complex FeCl<sub>2</sub>(dppe)<sub>2</sub> converts to low-spin HFeCl(dppe)<sub>2</sub>. |
|||
Reaction of the complex with [[sodium borohydride]] gives the dihydride complex: |
The complex HFeCl[(dppe)<sub>2</sub> exhibits a number of reactions associated with the remaining Fe-Cl bond. Reaction of the complex with [[sodium borohydride]] gives the dihydride complex: |
||
:HFeCl(dppe |
:HFeCl(dppe)<sub>2</sub> + NaBH<sub>4</sub> → H<sub>2</sub>Fe(dppe)<sub>2</sub> + NaCl + "BH<sub>3</sub>" |
||
Removal of chloride using sodium tetrafluorborate under and atmostphere of hydrogen or nitrogen gives the [[dinitrogen complex|dinitrogen]] and [[dihydrogen complex]]es:<ref>{{cite journal|vauthors=Bautista MT, Bynum LD, Schauer CK |title=Synthesis of η<sup>2</sup>-Dihydrogen Complex, ''trans''-<nowiki>{</nowiki>Fe(η<sup>2</sup>-H<sub>2</sub>)(H)[1,2-bis(diphenylphosphino)ethane]<sub>2</sub><nowiki>}</nowiki>[BF<sub>4</sub>]: An Experiment for an Advanced Inorganic Chemistry Laboratory Involving Synthesis and NMR Properties of an η<sup>2</sup>-H<sub>2</sub> Complex |journal=Journal of Chemical Education |year=1996 |volume=73 |issue=10 |pages=988–991 |doi=10.1021/ed073p988}}</ref> |
|||
:HFeCl(dppe |
:HFeCl(dppe)<sub>2</sub> + NaBF<sub>4</sub> + N<sub>2</sub> → [HFe(N<sub>2</sub>)(dppe)<sub>2</sub>]BF<sub>4</sub> + NaCl |
||
:HFeCl(dppe)<sub>2</sub> + NaBF<sub>4</sub> + H<sub>2</sub> → [HFe(H<sub>2</sub>)(dppe)<sub>2</sub>]BF<sub>4</sub> + NaCl |
|||
==References== |
==References== |
||
<references /> |
<references /> |
||
[[Category: |
[[Category:Hydrido complexes]] |
||
[[Category:Iron compounds]] |
[[Category:Iron(II) compounds]] |
||
[[Category:Chloro complexes]] |
[[Category:Chloro complexes]] |
||
[[Category:Phosphine complexes]] |
[[Category:Phosphine complexes]] |
||
[[Category:Iron complexes]] |
|||
[[Category:1,2-Ethanediyl compounds]] |
|||
Latest revision as of 07:06, 30 November 2024

| |

| |
| Names | |
|---|---|
| IUPAC name
Chlorohydridobis(bis-1,2-(diphenylphosphino)ethane)iron(II)
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C52H49ClFeP4 | |
| Molar mass | 889.09 |
| Appearance | red-violet solid |
| Melting point | 195 °C (383 °F; 468 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chlorobis(dppe)iron hydride is a coordination complex with the formula HFeCl(dppe)2, where dppe is the bidentate ligand 1,2-bis(diphenylphosphino)ethane. It is a red-violet solid. The compound has attracted much attention as a precursor to dihydrogen complexes.[1]
Structure
[edit]The complex exhibits octahedral molecular geometry. The chloride and hydride ligands are mutually trans.[2] The bond distances between the iron metal atom and the coordinating ligands are given by the following:
| Bond | Bond distance |
| Fe-P1 | 2.238 |
| Fe-P2 | 2.256 |
| Fe-P3 | 2.236 |
| Fe-P4 | 2.255 |
| Fe-Cl | 2.404 |
| Fe-H | 1.313 |
Synthesis and reactions
[edit]The compound is synthesized according to the following idealized reaction:[3]
- FeCl2 + 2 dppe + Na[BH4] → NaCl + ½ B2H6 + HFeCl(dppe)2
In the course of this conversion, high-spin complex FeCl2(dppe)2 converts to low-spin HFeCl(dppe)2.
The complex HFeCl[(dppe)2 exhibits a number of reactions associated with the remaining Fe-Cl bond. Reaction of the complex with sodium borohydride gives the dihydride complex:
- HFeCl(dppe)2 + NaBH4 → H2Fe(dppe)2 + NaCl + "BH3"
Removal of chloride using sodium tetrafluorborate under and atmostphere of hydrogen or nitrogen gives the dinitrogen and dihydrogen complexes:[4]
- HFeCl(dppe)2 + NaBF4 + N2 → [HFe(N2)(dppe)2]BF4 + NaCl
- HFeCl(dppe)2 + NaBF4 + H2 → [HFe(H2)(dppe)2]BF4 + NaCl
References
[edit]- ^ Morris RH (2008). "Dihydrogen, Dihydride and in Between: Nmr and Structural Properties of Iron Group Complexes". Coord. Chem. Rev. 2252 (21–22): 2381–2394. doi:10.1016/j.ccr.2008.01.010.
- ^ Lee, J.; Jung, G.; Lee, S. W. Structure of trans-chlorohydridobis(diphenylphosphinoethane)iron(II). Bull. Korean. Chem. 1998, 19, 267. ISSN 0253-2964
- ^ Giannoccaro, P.; Sacco, A. (1977). "Bis[Ethylenebis(Diphenylphosphine)]-Hydridoiron Complexes". Inorganic Syntheses. Vol. 17. pp. 69–71. doi:10.1002/9780470132487.ch19. ISBN 9780470132487.
{{cite book}}:|journal=ignored (help) - ^ Bautista MT, Bynum LD, Schauer CK (1996). "Synthesis of η2-Dihydrogen Complex, trans-{Fe(η2-H2)(H)[1,2-bis(diphenylphosphino)ethane]2}[BF4]: An Experiment for an Advanced Inorganic Chemistry Laboratory Involving Synthesis and NMR Properties of an η2-H2 Complex". Journal of Chemical Education. 73 (10): 988–991. doi:10.1021/ed073p988.
