Bacitracin: Difference between revisions
Artoria2e5 (talk | contribs) |
Ira Leviton (talk | contribs) m Fixed a reference. Please see Category:CS1 errors: dates. |
||
| (37 intermediate revisions by 15 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Polypeptide |
{{Short description|Polypeptide antibiotic}} |
||
{{Use dmy dates|date=January 2024}} |
|||
{{cs1 config |name-list-style=vanc |display-authors=6}} |
|||
{{Drugbox |
{{Drugbox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| verifiedrevid = 457285800 |
| verifiedrevid = 457285800 |
||
| ⚫ | | IUPAC_name = (4''R'')-4-[(2''S'')-2-({2-[(1''S'')-1-amino-2-methylbutyl]- 4,5-dihydro-1,3-thiazol-5-yl}formamido)-4-methylpentanamido]-4-<nowiki/>{[(1''S'')- 1-<nowiki/>{[(3''S'',6''R'',9''S'',12''R'',15''S'',18''R'',21''S'')- 18-(3-aminopropyl)-12-benzyl-15-(butan-2-yl)-3-(carbamoylmethyl)- 6-(carboxymethyl)-9-(1''H''-imidazol-5-ylmethyl)-2,5,8,11,14,17,20- heptaoxo-1,4,7,10,13,16,19-heptaazacyclopentacosan-21-yl]carbamoyl}- 2-methylbutyl]carbamoyl}butanoic acid |
||
| image = Bacitracin A.svg |
| image = Bacitracin A.svg |
||
| image2 = Bacitracin ball-and-stick.png |
| image2 = Bacitracin ball-and-stick.png |
||
<!--Clinical data--> |
<!-- Clinical data --> |
||
| tradename = Baciim |
| tradename = Baciguent, Baciim, others |
||
| Drugs.com = {{drugs.com|monograph|bacitracin}} |
| Drugs.com = {{drugs.com|monograph|bacitracin}} |
||
| pregnancy_AU = D |
| pregnancy_AU = D |
||
| ⚫ | |||
| pregnancy_US = C |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| legal_AU = S4 |
| legal_AU = S4 |
||
| legal_US = OTC |
| legal_US = OTC |
||
| legal_US_comment = |
| legal_US_comment = / Rx-only |
||
| ⚫ | |||
<!--Pharmacokinetic data--> |
<!--Pharmacokinetic data--> |
||
| bioavailability = |
| bioavailability = |
||
| Line 20: | Line 26: | ||
| metabolism = |
| metabolism = |
||
| elimination_half-life = |
| elimination_half-life = |
||
<!--Identifiers--> |
<!--Identifiers--> |
||
| CAS_number_Ref = {{cascite|correct|??}} |
| CAS_number_Ref = {{cascite|correct|??}} |
||
| CAS_number = 1405-87-4 |
| CAS_number = 1405-87-4 |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| PubChem = 439542 |
| PubChem = 439542 |
||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
||
| Line 38: | Line 42: | ||
| ChEMBL_Ref = {{ebicite|changed|EBI}} |
| ChEMBL_Ref = {{ebicite|changed|EBI}} |
||
| ChEMBL = 1200558 |
| ChEMBL = 1200558 |
||
<!--Chemical data--> |
<!--Chemical data--> |
||
| ⚫ | | IUPAC_name = (4''R'')-4-[(2''S'')-2-({2-[(1''S'')-1-amino-2-methylbutyl]- 4,5-dihydro-1,3-thiazol-5-yl}formamido)-4-methylpentanamido]-4-<nowiki/>{[(1''S'')- 1-<nowiki/>{[(3''S'',6''R'',9''S'',12''R'',15''S'',18''R'',21''S'')- 18-(3-aminopropyl)-12-benzyl-15-(butan-2-yl)-3-(carbamoylmethyl)- 6-(carboxymethyl)-9-(1''H''-imidazol-5-ylmethyl)-2,5,8,11,14,17,20- heptaoxo-1,4,7,10,13,16,19-heptaazacyclopentacosan-21-yl]carbamoyl}- 2-methylbutyl]carbamoyl}butanoic acid |
||
| C=66 | H=103 |
| C=66 | H=103 |
||
| N=17 | O=16 |
| N=17 | O=16 |
||
| Line 49: | Line 55: | ||
}} |
}} |
||
'''Bacitracin'''<ref>{{cite book| |
'''Bacitracin'''<ref>{{cite book| vauthors = Elks J, Ganellin CR |title=The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies|date=1990|publisher=Springer|isbn=978-1-4757-2085-3|pages=119–}}</ref> is a [[polypeptide antibiotic]]. It is a mixture of related [[cyclic peptides]] produced by ''[[Bacillus licheniformis]]'' bacteria, that was first isolated from the variety "Tracy I" ([[ATCC (company)|ATCC]] 10716) in 1945.<ref>Originally grouped under ''B. subtilis'', but nomenclature has since changed. See {{cite web | vauthors = Podstawka A |title=Bacillus licheniformis Tracy I {{!}} DSM 603, ATCC 10716, CCM 2181, IFO 12199, NBRC 12199, NCIB 8874, FDA BT1 {{!}} BacDiveID:686 |url=https://bacdive.dsmz.de/strain/686 |website=bacdive.dsmz.de |language=en |access-date=7 February 2022 |archive-date=7 February 2022 |archive-url=https://web.archive.org/web/20220207144504/https://bacdive.dsmz.de/strain/686 |url-status=live }}</ref> These peptides disrupt [[Gram-positive bacteria]] by interfering with [[cell wall]] and [[Peptidoglycan#Biosynthesis|peptidoglycan synthesis]]. |
||
| ⚫ | Bacitracin is primarily used as a topical preparation, as it can cause kidney damage when used internally.<ref name="Zintel">{{cite journal | vauthors = Zintel HA, Ma RA | title = The absorption, distribution, excretion and toxicity of bacitracin in man | journal = The American Journal of the Medical Sciences | volume = 218 | issue = 4 | pages = 439–445 | date = October 1949 | pmid = 18140540 | doi = 10.1097/00000441-194910000-00012 | s2cid = 2371497 }}</ref> It is generally safe when used topically, but in rare cases may cause [[hypersensitivity]], [[allergy|allergic]] or [[anaphylaxis|anaphylactic]] reactions, especially in people allergic to [[neomycin]].<ref>{{cite journal | vauthors = Spann CT, Taylor SC, Weinberg JM | title = Topical antimicrobial agents in dermatology | journal = Disease-a-Month | volume = 50 | issue = 7 | pages = 407–421 | date = July 2004 | pmid = 15280871 | doi = 10.1016/j.disamonth.2004.05.011 }}</ref><ref>{{cite journal | vauthors = Trookman NS, Rizer RL, Weber T | title = Treatment of minor wounds from dermatologic procedures: a comparison of three topical wound care ointments using a laser wound model | journal = Journal of the American Academy of Dermatology | volume = 64 | issue = 3 Suppl | pages = S8-15 | date = March 2011 | pmid = 21247665 | doi = 10.1016/j.jaad.2010.11.011 | doi-access = free }}</ref> |
||
Bacitracin is primarily used as a topical preparation, as it can cause kidney damage when used internally.<ref name="Zintel">{{cite journal |last1=Zintel |first1=H. A. |last2=Ma |first2=R. A. |last3=Nichols |first3=Anna C. |last4=Ellis |first4=Helen |title=The Absorption, Distribution, Excretion and Toxicity of Bacitracin in Man. |journal=American Journal of the Medical Sciences |date=1949 |volume=218 |issue=4 |pages=439–445}}</ref> |
|||
In 2022, it was the 323rd most commonly prescribed medication in the United States, with more than 100,000 prescriptions.<ref>{{cite web | title = Bacitracin Drug Usage Statistics, United States, 2013 - 2022 | website = ClinCalc | url = https://clincalc.com/DrugStats/Drugs/Bacitracin | access-date = 30 August 2024 }}</ref> |
|||
| ⚫ | |||
== Medical uses == |
== Medical uses == |
||
[[Image:Bacitracin ointment.jpg|thumb|left|A tube of bacitracin ointment for eyes]] Bacitracin is used in human medicine as a [[polypeptide antibiotic]] and is "approved by the [[ |
[[Image:Bacitracin ointment.jpg|thumb|left|A tube of bacitracin ointment for eyes]] Bacitracin is used in human medicine as a [[polypeptide antibiotic]] and is "approved by the US [[Food and Drug Administration]] (FDA) for use in chickens and turkeys," though use in animals contributes to [[antibiotic resistance]].<ref name="disc">{{cite web | vauthors = Pearl MC | date = 12 September 2007 | url = http://discovermagazine.com/2007/sep/better-planet | title = Antibiotic use on the farm hurts people—and doesn't help the bottom line. | work = [[Discover Magazine]] | archive-url = https://web.archive.org/web/20070925063617/http://discovermagazine.com/2007/sep/better-planet | archive-date = 25 September 2007 }}</ref> |
||
As bacitracin zinc salt, in combination with other topical antibiotics (usually [[polymyxin B]] and [[neomycin]]) as an [[ointment]] ("triple antibiotic ointment," with the brand name [[Neosporin]]), it is used for topical treatment of a variety of localized skin and eye infections, as well as for the prevention of wound [[infection]]s. A non-ointment form of ophthalmic solution is also available for eye infections.<ref>{{cite web|url=http://www.healthgrades.com/drug-ratings/drug/information/1246/Triple%20Antibiotic|title=Healthgrades > Find a Doctor > Doctor Reviews > Hospital Ratings|url-status=dead|archive-url=https://web.archive.org/web/20110523141000/http://www.healthgrades.com/drug-ratings/drug/information/1246/Triple%20Antibiotic|archive-date= |
As bacitracin zinc salt, in combination with other topical antibiotics (usually [[polymyxin B]] and [[neomycin]]) as an [[ointment]] ("triple antibiotic ointment," with the brand name [[Neosporin]]), it is used for topical treatment of a variety of localized skin and eye infections, as well as for the prevention of wound [[infection]]s. A non-ointment form of ophthalmic solution is also available for eye infections.<ref>{{cite web|url=http://www.healthgrades.com/drug-ratings/drug/information/1246/Triple%20Antibiotic|title=Healthgrades > Find a Doctor > Doctor Reviews > Hospital Ratings|url-status=dead|archive-url=https://web.archive.org/web/20110523141000/http://www.healthgrades.com/drug-ratings/drug/information/1246/Triple%20Antibiotic|archive-date=23 May 2011}}</ref> |
||
| ⚫ | |||
Although allergic cross-reaction with sulfa drugs has been occasionally reported, bacitracin-containing topical preparations remain a possible alternative to [[silver sulfadiazine]] (Silvadene) for burn patients with a sulfa allergy. |
|||
| ⚫ | |||
<br /> |
|||
Bacitracin can also be bought in pure form for those with allergies to the usual polymyxin B and neomycin components of the combination product. |
|||
Bacitracin is also commonly used as an aftercare antibiotic on tattoos and [[circumcision]]. It is preferred over combination products such as Neosporin because of its fewer ingredients, which lowers chances of an allergic reaction.<ref>{{cite web|url=http://tattoo.about.com/cs/tatfaq/a/aftrcr_cntrdctn.htm|title=The Right Way to Take Care of a New Tattoo|url-status=live|archive-url=https://web.archive.org/web/20070813200744/http://tattoo.about.com/cs/tatfaq/a/aftrcr_cntrdctn.htm|archive-date=2007-08-13}}</ref> |
|||
In 2005–06, it was the sixth-most-prevalent [[allergen]] in [[patch test]]s (9.2%).<ref>Zug KA, Warshaw EM, Fowler JF Jr, Maibach HI, Belsito DL, Pratt MD, Sasseville D, Storrs FJ, Taylor JS, Mathias CG, Deleo VA, Rietschel RL, Marks J. Patch-test results of the North American Contact Dermatitis Group 2005–2006. Dermatitis. 2009 May–Jun;20(3):149-60.</ref> |
|||
It was voted [[Allergen of the Year]] in 2003 by the American Contact Dermatitis Society.<ref>{{cite web | url = http://www.contactderm.org/i4a/pages/index.cfm?pageid=3467 | title = History of Allergen of the Year | archive-url = https://web.archive.org/web/20140425021024/http://www.contactderm.org/i4a/pages/index.cfm?pageid=3467 | archive-date = 2014-04-25 | work = [[American Contact Dermatitis Society]] }}</ref> |
|||
In infants, bacitracin is rarely administered [[intramuscular]]ly for the treatment of [[staphylococcal]] [[pneumonia]] and [[empyema]] when due to organisms shown susceptible to bacitracin. This use is extremely limited, since bacitracin is [[nephrotoxic]] and its concentration in the blood must be followed closely.<ref>{{cite web|url=http://www.sagentpharma.com/Products/Bacitracin/Catalog/Bacitracin_PI.pdf|title=FDA-approved IM injection package insert for bacitracin.|website=sagentpharma.com|url-status=dead|archive-url=https://web.archive.org/web/20110516001228/http://www.sagentpharma.com/Products/Bacitracin/Catalog/Bacitracin_PI.pdf|archive-date=2011-05-16}}</ref> |
|||
Bacitracin can be used to distinguish ''[[Streptococcus pyogenes]]'' from other [[streptococcus|streptococci]],<ref name="urlStreptococci">{{cite web |url=http://emedicine.medscape.com/article/228936-diagnosis |title=Streptococcus Group A Infections: Differential Diagnoses & Workup |access-date=Sep 23, 2009 |url-status=live |archive-url=https://web.archive.org/web/20090828230314/http://emedicine.medscape.com/article/228936-diagnosis |archive-date=2009-08-28 }}</ref> with ''[[S. pyogenes]]'' being sensitive to bacitracin and others resistant. In this case bacitracin is used to distinguish ''[[S. pyogenes]]'' from other ''[[streptococcus|β-hemolytic streptococci]]''. |
|||
It is also commonly used to distinguish ''[[Haemophilus influenzae]]'' colonies amongst respiratory flora; since ''[[Haemophilus influenzae|H. influenzae]]'' is intrinsically resistant to bacitracin, colonies form within the [[Agar diffusion test|zone of inhibition]]. |
|||
=== Spectrum of activity and susceptibility data === |
=== Spectrum of activity and susceptibility data === |
||
Bacitracin is a narrow-spectrum antibiotic. It targets Gram-positive |
Bacitracin is a narrow-spectrum antibiotic. It targets Gram-positive bacteria, especially those that cause skin infections. The following represents susceptibility data for a few medically significant microorganisms.<ref>{{cite web | title = Bacitracin Susceptibility and Minimum Inhibitory Concentration (MIC) Data | url = http://www.toku-e.com/Assets/MIC/Bacitracin.pdf | work = TOKU-E | access-date = 12 August 2013 | archive-date = 22 December 2018 | archive-url = https://web.archive.org/web/20181222141944/http://www.toku-e.com/Assets/MIC/Bacitracin.pdf | url-status = live }}</ref> |
||
* ''[[Staphylococcus aureus]]'' – ≤0.03 μg/mL – 700 μg/mL |
* ''[[Staphylococcus aureus]]'' – ≤0.03 μg/mL – 700 μg/mL |
||
| Line 86: | Line 77: | ||
== Mechanism of action == |
== Mechanism of action == |
||
{{main|Bactoprenol phosphate}} |
{{main|Bactoprenol phosphate}} |
||
Bacitracin interferes with the dephosphorylation of [[C55-isoprenyl pyrophosphate|C<sub>55</sub>-isoprenyl pyrophosphate]], and a related molecule known as [[bactoprenol]] pyrophosphate; both of these lipids function as membrane carrier molecules that transport the building-blocks of the [[peptidoglycan]] bacterial [[cell wall]] outside of the inner membrane.<ref name="pmid4332017">{{cite journal | vauthors = Stone KJ, Strominger JL | title = Mechanism of action of bacitracin: complexation with metal ion and C 55 -isoprenyl pyrophosphate | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 68 | issue = 12 | pages = 3223–7 | date = December 1971 | pmid = 4332017 | pmc = 389626 | doi = 10.1073/pnas.68.12.3223 | bibcode = 1971PNAS...68.3223S | doi-access = free }}</ref> |
Bacitracin interferes with the dephosphorylation of [[C55-isoprenyl pyrophosphate|C<sub>55</sub>-isoprenyl pyrophosphate]], and a related molecule known as [[bactoprenol]] pyrophosphate; both of these lipids function as membrane carrier molecules that transport the building-blocks of the [[peptidoglycan]] bacterial [[cell wall]] outside of the inner membrane.<ref name="pmid4332017">{{cite journal | vauthors = Stone KJ, Strominger JL | title = Mechanism of action of bacitracin: complexation with metal ion and C 55 -isoprenyl pyrophosphate | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 68 | issue = 12 | pages = 3223–7 | date = December 1971 | pmid = 4332017 | pmc = 389626 | doi = 10.1073/pnas.68.12.3223 | bibcode = 1971PNAS...68.3223S | doi-access = free | title-link = doi }}</ref> |
||
| ⚫ | |||
== History == |
== History == |
||
Bacitracin was isolated by [[Balbina Johnson]], a [[bacteriologist]] at the [[Columbia University College of Physicians and Surgeons]].<ref name="pmid17770204"/> Its name derives from the fact that a compound produced by a microbe in young Margaret |
Bacitracin was isolated by [[Balbina Johnson]], a [[bacteriologist]] at the [[Columbia University College of Physicians and Surgeons]].<ref name="pmid17770204"/> Its name derives from the fact that a compound produced by a microbe in young Margaret Tracy's (1936–1994)<ref>{{cite web |url=https://files.nyu.edu/jmm257/public/other/bacitracin.html |title=Margaret Tracy & Balbina Johnson: The Women Behind Bacitracin |access-date=30 September 2014 |url-status=dead |archive-url= https://web.archive.org/web/20140428190211/https://files.nyu.edu/jmm257/public/other/bacitracin.html |archive-date=28 April 2014 }}</ref> leg injury showed antibacterial activity.<ref>{{Cite web|url=https://healthmatters.nyp.org/bacitracin-discovery/|title=NewYork-Presbyterian | the Discovery of Bacitracin|date=7 February 2017|access-date=9 April 2020|archive-date=27 February 2021|archive-url=https://web.archive.org/web/20210227164020/https://healthmatters.nyp.org/bacitracin-discovery/|url-status=live}}</ref> |
||
<blockquote>''One strain isolated from tissue debrided from a compound fracture of the tibia was particularly active. We named this growth-antagonistic strain for the patient, "Tracy I." When cell-free filtrates of broth cultures of this bacillus proved to possess strong antibiotic activity and to be non-toxic, further study seemed warranted. We have called this active principle "Bacitracin.''<ref name="pmid17770204">{{cite journal | vauthors = Johnson BA, Anker H, Meleney FL | title = Bacitracin: a new antibiotic produced by a member of the B. subtilis group | journal = Science | volume = 102 | issue = 2650 | pages = 376–7 | date = October 1945 | pmid = 17770204 | doi = 10.1126/science.102.2650.376 | bibcode = 1945Sci...102..376J | s2cid = 51066 }}</ref></blockquote> |
<blockquote>''One strain isolated from tissue debrided from a compound fracture of the tibia was particularly active. We named this growth-antagonistic strain for the patient, "Tracy I." When cell-free filtrates of broth cultures of this bacillus proved to possess strong antibiotic activity and to be non-toxic, further study seemed warranted. We have called this active principle "Bacitracin.''<ref name="pmid17770204">{{cite journal | vauthors = Johnson BA, Anker H, Meleney FL | title = Bacitracin: a new antibiotic produced by a member of the B. subtilis group | journal = Science | volume = 102 | issue = 2650 | pages = 376–7 | date = October 1945 | pmid = 17770204 | doi = 10.1126/science.102.2650.376 | bibcode = 1945Sci...102..376J | s2cid = 51066 }}</ref></blockquote> |
||
Bacitracin was approved by FDA in 1948.<ref>{{Cite web|title=Drugs@FDA: FDA-Approved Drugs|url=https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=060733|access-date= |
Bacitracin was approved by the US FDA in 1948.<ref>{{Cite web|title=Drugs@FDA: FDA-Approved Drugs|url=https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=060733|access-date=17 September 2021|website=U.S. [[Food and Drug Administration]] (FDA)|archive-date=27 July 2021|archive-url=https://web.archive.org/web/20210727171910/https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=060733|url-status=dead}}</ref> |
||
== Synthesis == |
== Synthesis == |
||
Bacitracin is synthesised via [[nonribosomal peptide synthetase]]s (NRPSs), which means that [[ribosome]]s are not directly involved in its [[nonribosomal peptide|synthesis]]. The three-enzyme [[operon]] is called BacABC, not to be confused with BacABCDE of [[bacilycin]] synthesis.<ref>{{cite journal | vauthors = Konz D, Klens A, Schörgendorfer K, Marahiel MA | title = The bacitracin biosynthesis operon of Bacillus licheniformis ATCC 10716: molecular characterization of three multi-modular peptide synthetases | journal = Chemistry & Biology | volume = 4 | issue = 12 | pages = 927–937 | date = December 1997 | pmid = 9427658 | doi = 10.1016/s1074-5521(97)90301-x | title-link = doi | doi-access = free }}</ref> |
|||
{{missing information|section|commercial strain improvement by mutagenesis; these strains themselves have names}} |
|||
Bacitracin is synthesised via [[nonribosomal peptide synthetase]]s (NRPSs), which means that [[ribosome]]s are not directly involved in its [[nonribosomal peptide|synthesis]]. |
|||
| ⚫ | |||
bacABC is involved in synthesis.<ref>{{cite journal | vauthors = Murphy T, Roy I, Harrop A, Dixon K, Keshavarz T | title = Effect of oligosaccharide elicitors on bacitracin A production and evidence of transcriptional level control | journal = Journal of Biotechnology | volume = 131 | issue = 4 | pages = 397–403 | date = September 2007 | pmid = 17825450 | doi = 10.1016/j.jbiotec.2007.07.943 }}</ref> |
|||
| ⚫ | Bacitracin is composed of a mixture of related compounds with varying degrees of antibacterial activity. Notable fractions include bacitracin A, A1, B, B1, B2, C, D, E, F, G, and X.<ref>"Committee for Veterinary Medicinal Products Bacitracin." Ema.europa.eu. The European Agency for the Evaluation of Medicinal Products, June 1998. Web. 18 January 2013</ref> Bacitracin A has been found to have the most antibacterial activity. Bacitracin B1 and B2 have similar potencies and are approximately 90% as active as bacitracin A.<ref name="pmid1601975">{{cite journal | vauthors = Bell RG | title = Preparative high-performance liquid chromatographic separation and isolation of bacitracin components and their relationship to microbiological activity | journal = Journal of Chromatography | volume = 590 | issue = 1 | pages = 163–8 | date = January 1992 | pmid = 1601975 | doi = 10.1016/0021-9673(92)87018-4 | doi-access = free }}</ref> |
||
== Society and culture == |
|||
Bacitracin is commercially manufactured by growing the bacteria ''Bacillus subtilis var Tracy I'' in a container of liquid [[growth medium]]. Over time, the bacteria synthesizes the antibiotic and secretes the antibiotic into the medium. The antibiotic is then extracted from the medium using chemical processes. |
|||
=== Controversies === |
|||
| ⚫ | Claims that bacitracin is a [[protein disulfide isomerase]] inhibitor are disputed by ''in vitro'' studies.<ref>{{cite journal | vauthors = Karala AR, Ruddock LW | title = Bacitracin is not a specific inhibitor of protein disulfide isomerase | journal = The FEBS Journal | volume = 277 | issue = 11 | pages = 2454–62 | date = June 2010 | pmid = 20477872 | doi = 10.1111/j.1742-4658.2010.07660.x | s2cid = 37519169 }}</ref><ref>{{cite journal | vauthors = Weston BS, Wahab NA, Roberts T, Mason RM | title = Bacitracin inhibits fibronectin matrix assembly by mesangial cells in high glucose | journal = Kidney International | volume = 60 | issue = 5 | pages = 1756–64 | date = November 2001 | pmid = 11703593 | doi = 10.1046/j.1523-1755.2001.00991.x | doi-access = free | title-link = doi }}</ref> |
||
| ⚫ | |||
| ⚫ | Bacitracin is composed of a mixture of related compounds with varying degrees of antibacterial activity. Notable fractions include bacitracin A, A1, B, B1, B2, C, D, E, F, G, and X.<ref>"Committee for Veterinary Medicinal Products Bacitracin." Ema.europa.eu. The European Agency for the Evaluation of Medicinal Products, June 1998. Web. 18 |
||
== References == |
== References == |
||
{{Reflist |
{{Reflist}} |
||
{{Other antibacterials}} |
|||
{{Cell wall disruptive antibiotics}} |
|||
{{Antibiotics and chemotherapeutics for dermatological use}} |
{{Antibiotics and chemotherapeutics for dermatological use}} |
||
{{Throat preparations}} |
{{Throat preparations}} |
||
{{Portal bar | Medicine}} |
|||
{{Authority control}} |
|||
[[Category:Polypeptide antibiotics]] |
[[Category:Polypeptide antibiotics]] |
||
Latest revision as of 17:49, 2 December 2024
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Baciguent, Baciim, others |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Topical, intramuscular, Ophthalmic drug administration |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
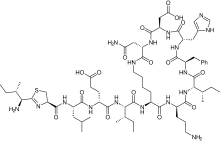
| Formula | C66H103N17O16S |
| Molar mass | 1422.71 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bacitracin[1] is a polypeptide antibiotic. It is a mixture of related cyclic peptides produced by Bacillus licheniformis bacteria, that was first isolated from the variety "Tracy I" (ATCC 10716) in 1945.[2] These peptides disrupt Gram-positive bacteria by interfering with cell wall and peptidoglycan synthesis.
Bacitracin is primarily used as a topical preparation, as it can cause kidney damage when used internally.[3] It is generally safe when used topically, but in rare cases may cause hypersensitivity, allergic or anaphylactic reactions, especially in people allergic to neomycin.[4][5]
In 2022, it was the 323rd most commonly prescribed medication in the United States, with more than 100,000 prescriptions.[6]
Medical uses
[edit]
Bacitracin is used in human medicine as a polypeptide antibiotic and is "approved by the US Food and Drug Administration (FDA) for use in chickens and turkeys," though use in animals contributes to antibiotic resistance.[7]
As bacitracin zinc salt, in combination with other topical antibiotics (usually polymyxin B and neomycin) as an ointment ("triple antibiotic ointment," with the brand name Neosporin), it is used for topical treatment of a variety of localized skin and eye infections, as well as for the prevention of wound infections. A non-ointment form of ophthalmic solution is also available for eye infections.[8]

Spectrum of activity and susceptibility data
[edit]Bacitracin is a narrow-spectrum antibiotic. It targets Gram-positive bacteria, especially those that cause skin infections. The following represents susceptibility data for a few medically significant microorganisms.[9]
- Staphylococcus aureus – ≤0.03 μg/mL – 700 μg/mL
- Staphylococcus epidermidis – 0.25 μg/mL – >16 μg/mL
- Streptococcus pyogenes – 0.5 μg/mL – >16 μg/mL
Mechanism of action
[edit]Bacitracin interferes with the dephosphorylation of C55-isoprenyl pyrophosphate, and a related molecule known as bactoprenol pyrophosphate; both of these lipids function as membrane carrier molecules that transport the building-blocks of the peptidoglycan bacterial cell wall outside of the inner membrane.[10]
History
[edit]Bacitracin was isolated by Balbina Johnson, a bacteriologist at the Columbia University College of Physicians and Surgeons.[11] Its name derives from the fact that a compound produced by a microbe in young Margaret Tracy's (1936–1994)[12] leg injury showed antibacterial activity.[13]
One strain isolated from tissue debrided from a compound fracture of the tibia was particularly active. We named this growth-antagonistic strain for the patient, "Tracy I." When cell-free filtrates of broth cultures of this bacillus proved to possess strong antibiotic activity and to be non-toxic, further study seemed warranted. We have called this active principle "Bacitracin.[11]
Bacitracin was approved by the US FDA in 1948.[14]
Synthesis
[edit]Bacitracin is synthesised via nonribosomal peptide synthetases (NRPSs), which means that ribosomes are not directly involved in its synthesis. The three-enzyme operon is called BacABC, not to be confused with BacABCDE of bacilycin synthesis.[15]
Composition
[edit]Bacitracin is composed of a mixture of related compounds with varying degrees of antibacterial activity. Notable fractions include bacitracin A, A1, B, B1, B2, C, D, E, F, G, and X.[16] Bacitracin A has been found to have the most antibacterial activity. Bacitracin B1 and B2 have similar potencies and are approximately 90% as active as bacitracin A.[17]
Society and culture
[edit]Controversies
[edit]Claims that bacitracin is a protein disulfide isomerase inhibitor are disputed by in vitro studies.[18][19]
References
[edit]- ^ Elks J, Ganellin CR (1990). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 119–. ISBN 978-1-4757-2085-3.
- ^ Originally grouped under B. subtilis, but nomenclature has since changed. See Podstawka A. "Bacillus licheniformis Tracy I | DSM 603, ATCC 10716, CCM 2181, IFO 12199, NBRC 12199, NCIB 8874, FDA BT1 | BacDiveID:686". bacdive.dsmz.de. Archived from the original on 7 February 2022. Retrieved 7 February 2022.
- ^ Zintel HA, Ma RA (October 1949). "The absorption, distribution, excretion and toxicity of bacitracin in man". The American Journal of the Medical Sciences. 218 (4): 439–445. doi:10.1097/00000441-194910000-00012. PMID 18140540. S2CID 2371497.
- ^ Spann CT, Taylor SC, Weinberg JM (July 2004). "Topical antimicrobial agents in dermatology". Disease-a-Month. 50 (7): 407–421. doi:10.1016/j.disamonth.2004.05.011. PMID 15280871.
- ^ Trookman NS, Rizer RL, Weber T (March 2011). "Treatment of minor wounds from dermatologic procedures: a comparison of three topical wound care ointments using a laser wound model". Journal of the American Academy of Dermatology. 64 (3 Suppl): S8-15. doi:10.1016/j.jaad.2010.11.011. PMID 21247665.
- ^ "Bacitracin Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ Pearl MC (12 September 2007). "Antibiotic use on the farm hurts people—and doesn't help the bottom line". Discover Magazine. Archived from the original on 25 September 2007.
- ^ "Healthgrades > Find a Doctor > Doctor Reviews > Hospital Ratings". Archived from the original on 23 May 2011.
- ^ "Bacitracin Susceptibility and Minimum Inhibitory Concentration (MIC) Data" (PDF). TOKU-E. Archived (PDF) from the original on 22 December 2018. Retrieved 12 August 2013.
- ^ Stone KJ, Strominger JL (December 1971). "Mechanism of action of bacitracin: complexation with metal ion and C 55 -isoprenyl pyrophosphate". Proceedings of the National Academy of Sciences of the United States of America. 68 (12): 3223–7. Bibcode:1971PNAS...68.3223S. doi:10.1073/pnas.68.12.3223. PMC 389626. PMID 4332017.
- ^ a b Johnson BA, Anker H, Meleney FL (October 1945). "Bacitracin: a new antibiotic produced by a member of the B. subtilis group". Science. 102 (2650): 376–7. Bibcode:1945Sci...102..376J. doi:10.1126/science.102.2650.376. PMID 17770204. S2CID 51066.
- ^ "Margaret Tracy & Balbina Johnson: The Women Behind Bacitracin". Archived from the original on 28 April 2014. Retrieved 30 September 2014.
- ^ "NewYork-Presbyterian | the Discovery of Bacitracin". 7 February 2017. Archived from the original on 27 February 2021. Retrieved 9 April 2020.
- ^ "Drugs@FDA: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 27 July 2021. Retrieved 17 September 2021.
- ^ Konz D, Klens A, Schörgendorfer K, Marahiel MA (December 1997). "The bacitracin biosynthesis operon of Bacillus licheniformis ATCC 10716: molecular characterization of three multi-modular peptide synthetases". Chemistry & Biology. 4 (12): 927–937. doi:10.1016/s1074-5521(97)90301-x. PMID 9427658.
- ^ "Committee for Veterinary Medicinal Products Bacitracin." Ema.europa.eu. The European Agency for the Evaluation of Medicinal Products, June 1998. Web. 18 January 2013
- ^ Bell RG (January 1992). "Preparative high-performance liquid chromatographic separation and isolation of bacitracin components and their relationship to microbiological activity". Journal of Chromatography. 590 (1): 163–8. doi:10.1016/0021-9673(92)87018-4. PMID 1601975.
- ^ Karala AR, Ruddock LW (June 2010). "Bacitracin is not a specific inhibitor of protein disulfide isomerase". The FEBS Journal. 277 (11): 2454–62. doi:10.1111/j.1742-4658.2010.07660.x. PMID 20477872. S2CID 37519169.
- ^ Weston BS, Wahab NA, Roberts T, Mason RM (November 2001). "Bacitracin inhibits fibronectin matrix assembly by mesangial cells in high glucose". Kidney International. 60 (5): 1756–64. doi:10.1046/j.1523-1755.2001.00991.x. PMID 11703593.
