Comins' reagent: Difference between revisions
Appearance
Content deleted Content added
Citation bot (talk | contribs) Add: publisher, authors 1-1. Removed parameters. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | Suggested by Dominic3203 | Category:Reagents for organic chemistry | #UCB_Category 10/228 |
Fixed incorrect IUPAC name |
||
| Line 4: | Line 4: | ||
| ImageName = Skeletal formula of Comin's Reagent |
| ImageName = Skeletal formula of Comin's Reagent |
||
| ImageFile2 = Comin's reagent-3D-balls.png |
| ImageFile2 = Comin's reagent-3D-balls.png |
||
| PIN = |
| PIN = N-(5-chloropyridin-2-yl)-1,1,1-trifluoro-N-((trifluoromethyl)sulfonyl)methanesulfonamide |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| CASNo = 145100-51-2 |
| CASNo = 145100-51-2 |
||
Latest revision as of 20:19, 2 December 2024
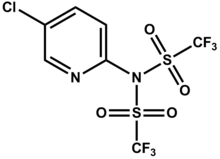
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
N-(5-chloropyridin-2-yl)-1,1,1-trifluoro-N-((trifluoromethyl)sulfonyl)methanesulfonamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.157.321 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H3ClF6N2O4S2 | |
| Molar mass | 392.67 g·mol−1 |
| Appearance | White solid |
| Melting point | 45 °C (113 °F; 318 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The Comins' reagent is a triflyl-donating reagent that is used to synthesize vinyl triflates from the corresponding ketone enolates or dienolates.[1]

It was first reported in 1992 by Daniel Comins.[2] The vinyl triflates prepared are useful as substrates in the Suzuki reaction.[3]
See also
[edit]References
[edit]- ^ Mundy, Bradford P.; Ellerd, Michael G.; Favaloro, Frank G. Jr. (2005). Name Reactions and Reagents in Organic Synthesis (2nd ed.). John Wiley & Sons. ISBN 978-0471228547.
- ^ Comins, Daniel L.; Dehghani, Ali (1992). "Pyridine-Derived Triflating Reagents: An Improved Preparation of Vinyl Triflates from Metallo Enolates". Tetrahedron Letters. 33 (42): 6299–6302. doi:10.1016/S0040-4039(00)60957-7.
- ^ Miyaura, Norio; Suzuki, Akira (1995). "Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds". Chemical Reviews. 95 (7): 2457–2483. CiteSeerX 10.1.1.735.7660. doi:10.1021/cr00039a007.
