Comins' reagent: Difference between revisions
Appearance
Content deleted Content added
Added general reference |
Fixed incorrect IUPAC name |
||
| (30 intermediate revisions by 22 users not shown) | |||
| Line 1: | Line 1: | ||
{{ |
{{Chembox |
||
| |
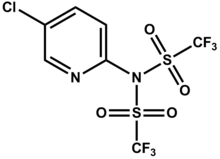
| Name = Comins' Reagent |
||
| |
| ImageFile = CominsReagent.png |
||
| ImageName = Skeletal formula of Comin's Reagent |
|||
| ImageSize = 200px |
|||
| |
| ImageFile2 = Comin's reagent-3D-balls.png |
||
| PIN = N-(5-chloropyridin-2-yl)-1,1,1-trifluoro-N-((trifluoromethyl)sulfonyl)methanesulfonamide |
|||
| IUPACName = |
|||
| |
|Section1={{Chembox Identifiers |
||
| |
| CASNo = 145100-51-2 |
||
| CASNo_Ref = {{Cascite|changed|CAS}} |
|||
| SMILES = |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| ⚫ | |||
| UNII = WS933U9U66 |
|||
| ⚫ | |||
| EINECS = 629-110-2 |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| |
| ChemSpiderID = 344376 |
||
| SMILES = O=S(=O)(N(c1ccc(Cl)cn1)S(=O)(=O)C(F)(F)F)C(F)(F)F |
|||
| ⚫ | |||
| InChI = 1/C7H3ClF6N2O4S2/c8-4-1-2-5(15-3-4)16(21(17,18)6(9,10)11)22(19,20)7(12,13)14/h1-3H |
|||
| ⚫ | |||
| InChIKey = TUFGVZMNGTYAQD-UHFFFAOYAK |
|||
| ⚫ | |||
| StdInChI = 1S/C7H3ClF6N2O4S2/c8-4-1-2-5(15-3-4)16(21(17,18)6(9,10)11)22(19,20)7(12,13)14/h1-3H |
|||
| ⚫ | |||
| StdInChIKey = TUFGVZMNGTYAQD-UHFFFAOYSA-N |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| Appearance = White solid |
|||
| MeltingPtC = 45 |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
|Section7={{Chembox Hazards |
|||
| ⚫ | |||
}} |
|||
}} |
}} |
||
Comins' |
The '''Comins' reagent''' is a [[Trifluoromethylsulfonyl|triflyl]]-donating reagent that is used to synthesize vinyl [[Trifluoromethanesulfonate|triflates]] from the corresponding ketone enolates or dienolates.<ref>{{cite book | last1 = Mundy | first1 = Bradford P. | last2 = Ellerd | first2 = Michael G. | last3 = Favaloro | first3 = Frank G. Jr. | title = Name Reactions and Reagents in Organic Synthesis | isbn = 978-0471228547 | edition = 2nd | year = 2005| publisher = John Wiley & Sons }}</ref> |
||
[[File:SampleReactionWithCominsReagent.png|center|500px|Sample Reaction With Comin's Reagent]] |
[[File:SampleReactionWithCominsReagent.png|center|500px|Sample Reaction With Comin's Reagent]] |
||
It was first reported in 1992 by Daniel Comins |
It was first reported in 1992 by Daniel Comins.<ref>{{cite journal | last1 = Comins | first1 = Daniel L. | last2 = Dehghani | first2 = Ali | title = Pyridine-Derived Triflating Reagents: An Improved Preparation of Vinyl Triflates from Metallo Enolates | journal = Tetrahedron Letters | year = 1992 | volume = 33 | issue = 42 | pages = 6299–6302 | doi = 10.1016/S0040-4039(00)60957-7}}</ref> The vinyl triflates prepared are useful as substrates in the [[Suzuki reaction]].<ref>{{cite journal|author1-link=Norio Miyaura|author2-link=Akira Suzuki (chemist) | last1 = Miyaura | first1 = Norio | last2 = Suzuki | first2 = Akira | title = Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds | journal = Chemical Reviews | year = 1995 | volume = 95 | issue = 7 | pages = 2457–2483 | doi = 10.1021/cr00039a007 | citeseerx = 10.1.1.735.7660 }}</ref> |
||
==See also== |
|||
* [[Bis(trifluoromethanesulfonyl)aniline]] |
|||
==References== |
|||
{{Reflist}} |
{{Reflist}} |
||
[[Category:Reagents for organic chemistry]] |
|||
[[Category:Chloropyridines]] |
|||
[[Category:Sulfonamides]] |
|||
[[Category:Trifluoromethyl compounds]] |
|||
[[Category:Substances discovered in the 1990s]] |
|||
{{organic-compound-stub}} |
|||
Latest revision as of 20:19, 2 December 2024
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
N-(5-chloropyridin-2-yl)-1,1,1-trifluoro-N-((trifluoromethyl)sulfonyl)methanesulfonamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.157.321 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H3ClF6N2O4S2 | |
| Molar mass | 392.67 g·mol−1 |
| Appearance | White solid |
| Melting point | 45 °C (113 °F; 318 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The Comins' reagent is a triflyl-donating reagent that is used to synthesize vinyl triflates from the corresponding ketone enolates or dienolates.[1]

It was first reported in 1992 by Daniel Comins.[2] The vinyl triflates prepared are useful as substrates in the Suzuki reaction.[3]
See also
[edit]References
[edit]- ^ Mundy, Bradford P.; Ellerd, Michael G.; Favaloro, Frank G. Jr. (2005). Name Reactions and Reagents in Organic Synthesis (2nd ed.). John Wiley & Sons. ISBN 978-0471228547.
- ^ Comins, Daniel L.; Dehghani, Ali (1992). "Pyridine-Derived Triflating Reagents: An Improved Preparation of Vinyl Triflates from Metallo Enolates". Tetrahedron Letters. 33 (42): 6299–6302. doi:10.1016/S0040-4039(00)60957-7.
- ^ Miyaura, Norio; Suzuki, Akira (1995). "Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds". Chemical Reviews. 95 (7): 2457–2483. CiteSeerX 10.1.1.735.7660. doi:10.1021/cr00039a007.
