Von Willebrand factor: Difference between revisions
better url |
Citation bot (talk | contribs) Alter: issue, pages. Formatted dashes. | Use this bot. Report bugs. | Suggested by Whoop whoop pull up | Category:Coagulation system | #UCB_Category 41/55 |
||
| (45 intermediate revisions by 27 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Mammalian protein involved in blood clotting}} |
|||
{{Lowercase title}} |
|||
{{Infobox_gene}} |
{{Infobox_gene}} |
||
| ⚫ | '''Von Willebrand factor''' ('''VWF''') ({{IPA|de|fɔn ˈvɪləbʁant|lang}}) is a [[blood]] [[glycoprotein]] that promotes [[hemostasis]], specifically, [[platelet]] [[Platelet#Adhesion|adhesion]]. It is deficient and/or defective in [[von Willebrand disease]] and is involved in many other diseases, including [[thrombotic thrombocytopenic purpura]], [[Heyde's syndrome]], and possibly [[hemolytic–uremic syndrome]].<ref name=Sadler>{{cite journal | vauthors = Sadler JE | title = Biochemistry and genetics of von Willebrand factor | journal = Annual Review of Biochemistry | volume = 67 | pages = 395–424 | year = 1998 | pmid = 9759493 | doi = 10.1146/annurev.biochem.67.1.395 | doi-access = free | author-link1 = J. Evan Sadler }}</ref> Increased plasma levels in many cardiovascular, neoplastic, metabolic (e.g. diabetes), and connective tissue diseases are presumed to arise from adverse changes to the [[endothelium]], and may predict an increased risk of [[thrombosis]].<ref>{{cite book | vauthors = Shahidi M | title = Thrombosis and Embolism: From Research to Clinical Practice | chapter = Thrombosis and von Willebrand Factor | series = Advances in Experimental Medicine and Biology | volume = 906 | pages = 285–306 | date = 2017 | pmid = 27628010 | doi = 10.1007/5584_2016_122 | isbn = 978-3-319-22107-6 }}</ref> |
||
| ⚫ | ''' |
||
== Biochemistry == |
== Biochemistry == |
||
| Line 15: | Line 16: | ||
** [[heparin]] |
** [[heparin]] |
||
** possibly [[collagen]] |
** possibly [[collagen]] |
||
* the A2 domain, which must partially unfold to expose the buried cleavage site for the specific [[ADAMTS13]] protease that inactivates VWF by making much smaller multimers. The partial unfolding is affected by shear flow in the blood, by calcium binding, and by the lump of a sequence-adjacent "vicinal disulfide" at the A2-domain C-terminus.<ref name=Jakobi>Jakobi AJ, Mashaghi A, Tans SJ, Huizinga EG |
* the A2 domain, which must partially unfold to expose the buried cleavage site for the specific [[ADAMTS13]] protease that inactivates VWF by making much smaller multimers. The partial unfolding is affected by shear flow in the blood, by calcium binding, and by the lump of a sequence-adjacent "vicinal disulfide" at the A2-domain C-terminus.<ref name=Jakobi>{{cite journal | vauthors = Jakobi AJ, Mashaghi A, Tans SJ, Huizinga EG | title = Calcium modulates force sensing by the von Willebrand factor A2 domain | journal = Nature Communications | volume = 2 | issue = | pages = 385 | date = July 2011 | pmid = 21750539 | pmc = 3144584 | doi = 10.1038/ncomms1385 | bibcode = 2011NatCo...2..385J }}</ref><ref>{{cite journal | vauthors = Luken BM, Winn LY, Emsley J, Lane DA, Crawley JT | title = The importance of vicinal cysteines, C1669 and C1670, for von Willebrand factor A2 domain function | journal = Blood | volume = 115 | issue = 23 | pages = 4910–4913 | date = June 2010 | pmid = 20354169 | pmc = 2890177 | doi = 10.1182/blood-2009-12-257949 }}</ref> |
||
* the A3 domain, which binds to collagen ([[von Willebrand factor type A domain]]) |
* the A3 domain, which binds to collagen ([[von Willebrand factor type A domain]]) |
||
* the C4 domain, in which the [[RGD motif]] binds to platelet [[Integrin αIIbβ3|integrin α<sub>IIb</sub>β<sub>3</sub>]] when this is activated ([[von Willebrand factor type C domain]]) |
* the C4 domain, in which the [[RGD motif]] binds to platelet [[Integrin αIIbβ3|integrin α<sub>IIb</sub>β<sub>3</sub>]] when this is activated ([[von Willebrand factor type C domain]]) |
||
* the other C domains, which may interact in ER dimers: the larger protein show six beads of (C and C-like) domains under [[cryo-EM]].<ref name="pmid22490677">{{cite journal |vauthors=Zhou YF, Eng ET, Zhu J, Lu C, Walz T, Springer TA|title=Sequence and structure relationships within von Willebrand factor |
* the other C domains, which may interact in ER dimers: the larger protein show six beads of (C and C-like) domains under [[cryo-EM]].<ref name="pmid22490677">{{cite journal | vauthors = Zhou YF, Eng ET, Zhu J, Lu C, Walz T, Springer TA | title = Sequence and structure relationships within von Willebrand factor | journal = Blood | volume = 120 | issue = 2 | pages = 449–458 | date = July 2012 | pmid = 22490677 | pmc = 3398765 | doi = 10.1182/blood-2012-01-405134 }}</ref> |
||
* the "[[cystine knot]]" domain (at the C-terminal end of the protein), which VWF shares with [[platelet-derived growth factor]] (PDGF), [[transforming growth factor]]-β (TGFβ) and β-[[human chorionic gonadotropin]] (βHCG, of [[pregnancy test]] fame). ([[von Willebrand factor type C domain]]) |
* the "[[cystine knot]]" domain (at the C-terminal end of the protein), which VWF shares with [[platelet-derived growth factor]] (PDGF), [[transforming growth factor]]-β (TGFβ) and β-[[human chorionic gonadotropin]] (βHCG, of [[pregnancy test]] fame). ([[von Willebrand factor type C domain]]) |
||
Monomers are subsequently [[glycosylation|N-glycosylated]], arranged into dimers in the [[endoplasmic reticulum]] and into multimers in the [[Golgi apparatus]] by crosslinking of [[cysteine]] residues via [[disulfide bond]]s. With respect to the glycosylation, VWF is one of only a few proteins that carry [[ABO blood group system]] antigens.<ref name=Sadler/> VWFs coming out of the Golgi are packaged into storage organelles, [[Weibel–Palade body|Weibel-Palade bodies]] (WPBs) in endothelial cells and [[Platelet alpha-granule|α-granules]] in platelets.<ref name=pmid25712991>{{cite journal | vauthors = Lenting PJ, Christophe OD, Denis CV | title = von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends | journal = Blood | volume = 125 | issue = 13 | pages = |
Monomers are subsequently [[glycosylation|N-glycosylated]], arranged into dimers in the [[endoplasmic reticulum]] and into multimers in the [[Golgi apparatus]] by crosslinking of [[cysteine]] residues via [[disulfide bond]]s. With respect to the glycosylation, VWF is one of only a few proteins that carry [[ABO blood group system]] antigens.<ref name=Sadler/> VWFs coming out of the Golgi are packaged into storage organelles, [[Weibel–Palade body|Weibel-Palade bodies]] (WPBs) in endothelial cells and [[Platelet alpha-granule|α-granules]] in platelets.<ref name=pmid25712991>{{cite journal | vauthors = Lenting PJ, Christophe OD, Denis CV | title = von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends | journal = Blood | volume = 125 | issue = 13 | pages = 2019–2028 | date = March 2015 | pmid = 25712991 | doi = 10.1182/blood-2014-06-528406 | s2cid = 27785232 | doi-access = free }}</ref> |
||
Multimers of VWF can be extremely large, >20,000 [[kDa]], and consist of over 80 subunits of 250 kDa each. Only the large multimers are functional. Some cleavage products that result from VWF production are also secreted but probably serve no function.<ref name=Sadler/> |
Multimers of VWF can be extremely large, >20,000 [[kDa]], and consist of over 80 subunits of 250 kDa each. Only the large multimers are functional. Some cleavage products that result from VWF production are also secreted but probably serve no function.<ref name=Sadler/> |
||
| Line 27: | Line 28: | ||
=== Function === |
=== Function === |
||
[[Image:VWF-GP1ba.png|thumb|300px|right|The interaction of VWF and GP1b alpha. The GP1b receptor on the surface of platelets allows the platelet to bind to VWF, which is exposed upon damage to |
[[Image:VWF-GP1ba.png|thumb|300px|right|The interaction of VWF and GP1b alpha. The GP1b receptor on the surface of platelets allows the platelet to bind to VWF, which is exposed upon damage to vasculature. The VWF A1 domain (yellow) interacts with the extracellular domain of GP1ba (blue).]] |
||
Von Willebrand Factor's primary function is binding to other proteins, in particular [[factor VIII]], and it is important in [[Platelet#Adhesion and aggregation|platelet adhesion]] to wound sites.<ref name=Sadler/> It is not an [[enzyme]] and, thus, has no catalytic activity. |
Von Willebrand Factor's primary function is binding to other proteins, in particular [[factor VIII]], and it is important in [[Platelet#Adhesion and aggregation|platelet adhesion]] to wound sites.<ref name=Sadler/> It is not an [[enzyme]] and, thus, has no catalytic activity. |
||
VWF binds to a number of cells and molecules. The most important ones are:<ref name=Sadler/> |
VWF binds to a number of cells and molecules. The most important ones are:<ref name=Sadler/> |
||
* Factor VIII is bound to VWF while inactive in circulation; factor VIII degrades rapidly when not bound to VWF. Factor VIII is released from VWF by the action of [[thrombin]]. In the absence of VWF, factor VIII has a half-life of |
* Factor VIII is bound to VWF while inactive in circulation; factor VIII degrades rapidly when not bound to VWF. Factor VIII is released from VWF by the action of [[thrombin]]. In the absence of VWF, factor VIII has a half-life of 1–2 hours; when carried by intact VWF, factor VIII has a half-life of 8–12 hours. |
||
* VWF binds to collagen, e.g., when collagen is exposed beneath [[endothelium|endothelial cells]] due to damage occurring to the blood vessel. Endothelium also releases VWF which forms additional links between the platelets' glycoprotein Ib/IX/V and the collagen fibrils |
* VWF binds to collagen, e.g., when collagen is exposed beneath [[endothelium|endothelial cells]] due to damage occurring to the blood vessel. Endothelium also releases VWF which forms additional links between the platelets' glycoprotein Ib/IX/V and the collagen fibrils |
||
* VWF binds to platelet [[ |
* VWF binds to platelet [[GpIb]] when it forms a complex with [[gpIX]] and [[factor V|gpV]]; this binding occurs under all circumstances, but is most efficient under high [[shear stress]] (i.e., rapid blood flow in narrow blood vessels, see below). |
||
* VWF binds to other platelet receptors when they are activated, e.g., by [[thrombin]] (i.e., when coagulation has been stimulated). |
* VWF binds to other platelet receptors when they are activated, e.g., by [[thrombin]] (i.e., when coagulation has been stimulated). |
||
VWF plays a major role in blood coagulation. Therefore, VWF deficiency or dysfunction (von Willebrand disease) leads to a bleeding tendency, which is most apparent in tissues having high blood flow [[Shear (fluid)|shear]] in narrow vessels. From studies it appears that VWF uncoils under these circumstances, decelerating passing platelets.<ref name=Sadler/> Recent research also suggests that von Willebrand Factor is involved in the [[angiogenesis|formation of blood vessels themselves]], which would explain why some people with von Willebrand disease develop vascular malformations (predominantly in the [[digestive tract]]) that can [[gastrointestinal bleeding|bleed excessively]].<ref>{{cite journal | vauthors = Randi AM, Laffan MA | title = Von Willebrand |
VWF plays a major role in blood coagulation. Therefore, VWF deficiency or dysfunction (von Willebrand disease) leads to a bleeding tendency, which is most apparent in tissues having high blood flow [[Shear (fluid)|shear]] in narrow vessels. From studies it appears that VWF uncoils under these circumstances, decelerating passing platelets.<ref name=Sadler/> Recent research also suggests that von Willebrand Factor is involved in the [[angiogenesis|formation of blood vessels themselves]], which would explain why some people with von Willebrand disease develop vascular malformations (predominantly in the [[digestive tract]]) that can [[gastrointestinal bleeding|bleed excessively]].<ref>{{cite journal | vauthors = Randi AM, Laffan MA | title = Von Willebrand factor and angiogenesis: basic and applied issues | journal = Journal of Thrombosis and Haemostasis | volume = 15 | issue = 1 | pages = 13–20 | date = January 2017 | pmid = 27778439 | doi = 10.1111/jth.13551 | hdl = 10044/1/42796 | s2cid = 3490036 | doi-access = free | hdl-access = free }}</ref> |
||
===Catabolism=== |
===Catabolism=== |
||
The biological breakdown ([[catabolism]]) of VWF is largely mediated by the enzyme [[ADAMTS13]] (acronym of "''a'' ''d''isintegrin-like ''a''nd ''m''etalloprotease with ''t''hrombo''s''pondin type 1 motif no. ''13''"). It is a [[metalloproteinase]] that [[proteolysis|cleaves]] VWF between [[tyrosine]] at position 842 and [[methionine]] at position 843 (or 1605–1606 of the gene) in the A2 domain. This breaks down the multimers into smaller units, which are degraded by other [[peptidase]]s.<ref>{{cite journal | vauthors = Levy GG, Motto DG, Ginsburg D | title = ADAMTS13 turns 3 | journal = Blood | volume = 106 | issue = 1 | pages = |
The biological breakdown ([[catabolism]]) of VWF is largely mediated by the enzyme [[ADAMTS13]] (acronym of "''a'' ''d''isintegrin-like ''a''nd ''m''etalloprotease with ''t''hrombo''s''pondin type 1 motif no. ''13''"). It is a [[metalloproteinase]] that [[proteolysis|cleaves]] VWF between [[tyrosine]] at position 842 and [[methionine]] at position 843 (or 1605–1606 of the gene) in the A2 domain. This breaks down the multimers into smaller units, which are degraded by other [[peptidase]]s.<ref>{{cite journal | vauthors = Levy GG, Motto DG, Ginsburg D | title = ADAMTS13 turns 3 | journal = Blood | volume = 106 | issue = 1 | pages = 11–17 | date = July 2005 | pmid = 15774620 | doi = 10.1182/blood-2004-10-4097 | s2cid = 25645477 | doi-access = free }}</ref> |
||
The half-life of vWF in human plasma is around 16 hours; glycosylation variation on vWF molecules from different individuals result in a larger range of 4.2 to 26 hours. Liver cells as well as [[macrophage]]s take up vWF for clearance via [[Asialoglycoprotein receptor|ASGPRs]] and [[LRP1]]. [[SIGLEC5]] and [[CLEC4M]] also recognize vWF.<ref name=pmid25712991/> |
The half-life of vWF in human plasma is around 16 hours; glycosylation variation on vWF molecules from different individuals result in a larger range of 4.2 to 26 hours. Liver cells as well as [[macrophage]]s take up vWF for clearance via [[Asialoglycoprotein receptor|ASGPRs]] and [[LRP1]]. [[SIGLEC5]] and [[CLEC4M]] also recognize vWF.<ref name=pmid25712991/> |
||
== Role in disease == |
== Role in disease == |
||
{{main article| |
{{main article|von Willebrand disease}} |
||
[[Genetic disorder|Hereditary]] or acquired defects of VWF lead to [[von Willebrand disease]] (vWD), a [[bleeding diathesis]] of the skin and mucous membranes, causing [[nosebleed]]s, [[menorrhagia]], and [[gastrointestinal bleed]]ing. The point at which the [[mutation]] occurs determines the severity of the bleeding diathesis. There are three types (I, II and III), and type II is further divided in several subtypes. Treatment depends on the nature of the abnormality and the severity of the symptoms.<ref>{{cite journal | vauthors = Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, Holmberg L, Ingerslev J, Lee CA, Lillicrap D, Mannucci PM, Mazurier C, Meyer D, Nichols WL, Nishino M, Peake IR, Rodeghiero F, Schneppenheim R, Ruggeri ZM, Srivastava A, Montgomery RR, Federici AB | title = Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor | journal = Journal of Thrombosis and Haemostasis | volume = 4 | issue = 10 | pages = |
[[Genetic disorder|Hereditary]] or acquired defects of VWF lead to [[von Willebrand disease]] (vWD), a [[bleeding diathesis]] of the skin and mucous membranes, causing [[nosebleed]]s, [[menorrhagia]], and [[gastrointestinal bleed]]ing. The point at which the [[mutation]] occurs determines the severity of the bleeding diathesis. There are three types (I, II and III), and type II is further divided in several subtypes. Treatment depends on the nature of the abnormality and the severity of the symptoms.<ref>{{cite journal | vauthors = Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, Holmberg L, Ingerslev J, Lee CA, Lillicrap D, Mannucci PM, Mazurier C, Meyer D, Nichols WL, Nishino M, Peake IR, Rodeghiero F, Schneppenheim R, Ruggeri ZM, Srivastava A, Montgomery RR, Federici AB | display-authors = 6 | title = Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor | journal = Journal of Thrombosis and Haemostasis | volume = 4 | issue = 10 | pages = 2103–2114 | date = October 2006 | pmid = 16889557 | doi = 10.1111/j.1538-7836.2006.02146.x | s2cid = 23875096 | doi-access = }}</ref> Most cases of vWD are hereditary, but abnormalities of VWF may be acquired; [[aortic valve stenosis]], for instance, has been linked to vWD type IIA, causing [[gastrointestinal bleeding]] - an association known as [[Heyde's syndrome]].<ref>{{cite journal | vauthors = Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, Bauters A, Decoene C, Goudemand J, Prat A, Jude B | display-authors = 6 | title = Acquired von Willebrand syndrome in aortic stenosis | journal = The New England Journal of Medicine | volume = 349 | issue = 4 | pages = 343–349 | date = July 2003 | pmid = 12878741 | doi = 10.1056/NEJMoa022831 | doi-access = free }}</ref> |
||
In [[thrombotic thrombocytopenic purpura]] (TTP) and [[hemolytic–uremic syndrome]] (HUS), ADAMTS13 either is deficient or has been inhibited by [[antibody|antibodies]] directed at the enzyme. This leads to decreased breakdown of the ultra-large multimers of VWF and [[microangiopathic hemolytic anemia]] with deposition of fibrin and platelets in small vessels, and capillary necrosis. In TTP, the organ most obviously affected is the brain; in HUS, the kidney.<ref>{{cite journal | vauthors = Moake JL | title = von Willebrand |
In [[thrombotic thrombocytopenic purpura]] (TTP) and [[hemolytic–uremic syndrome]] (HUS), [[ADAMTS13]] either is deficient or has been inhibited by [[antibody|antibodies]] directed at the enzyme. This leads to decreased breakdown of the ultra-large multimers of VWF and [[microangiopathic hemolytic anemia]] with deposition of fibrin and platelets in small vessels, and capillary necrosis. In TTP, the organ most obviously affected is the brain; in HUS, the kidney.<ref>{{cite journal | vauthors = Moake JL | title = von Willebrand factor, ADAMTS-13, and thrombotic thrombocytopenic purpura | journal = Seminars in Hematology | volume = 41 | issue = 1 | pages = 4–14 | date = January 2004 | pmid = 14727254 | doi = 10.1053/j.seminhematol.2003.10.003 }}</ref> |
||
Higher levels of VWF are more common among people that have had [[ischemic stroke]] (from blood-clotting) for the first time.<ref>{{cite journal| |
Higher levels of VWF are more common among people that have had [[ischemic stroke]] (from blood-clotting) for the first time.<ref>{{cite journal | vauthors = Denorme F, De Meyer SF | title = The VWF-GPIb axis in ischaemic stroke: lessons from animal models | journal = Thrombosis and Haemostasis | volume = 116 | issue = 4 | pages = 597–604 | date = September 2016 | pmid = 27029413 | doi = 10.1160/TH16-01-0036 | s2cid = 4964177 }}</ref> Occurrence is not affected by ADAMTS13, and the only significant genetic factor is the person's [[Blood type|blood]] group. High plasma VWF levels were found to be an independent predictor of major bleeding in anticoagulated [[atrial fibrillation]] patients.<ref>{{cite journal | vauthors = Roldán V, Marín F, Muiña B, Torregrosa JM, Hernández-Romero D, Valdés M, Vicente V, Lip GY | display-authors = 6 | title = Plasma von Willebrand factor levels are an independent risk factor for adverse events including mortality and major bleeding in anticoagulated atrial fibrillation patients | journal = Journal of the American College of Cardiology | volume = 57 | issue = 25 | pages = 2496–2504 | date = June 2011 | pmid = 21497043 | doi = 10.1016/j.jacc.2010.12.033 | doi-access = free }}</ref> VWF is a marker of [[endothelial dysfunction]], and is consistently elevated in atrial fibrillation, associated with adverse outcomes.<ref name="pmid31631989">{{cite journal | vauthors = Khan AA, Thomas GN, Lip G, Shantsila A | title=Endothelial function in patients with atrial fibrillation | journal= [[Annals of Medicine]] | volume=52 | issue=1–2 | pages=1–11 | year=2020 | doi= 10.1080/07853890.2019.1711158 | pmc=7877921 | pmid=31903788 }}</ref> |
||
== History == |
== History == |
||
{{See also|Erik Adolf von Willebrand#Von Willebrand disease}} |
{{See also|Erik Adolf von Willebrand#Von Willebrand disease}} |
||
VWF is named after [[Erik Adolf von Willebrand]], a Finnish physician who in 1926 first described a hereditary bleeding disorder in families from |
VWF is named after [[Erik Adolf von Willebrand]], a Finnish physician who in 1926 first described a hereditary bleeding disorder in families from [[Åland]]. Although von Willebrand did not identify the definite cause, he distinguished von Willebrand disease (vWD) from [[hemophilia]] and other forms of [[bleeding diathesis]].<ref>{{cite journal| vauthors = von Willebrand EA | title = Hereditär pseudohemofili | trans-title = Hereditary pseudo haemophilia | language = sv | journal = Fin Läkaresällsk Handl | year = 1926 | volume = 68 | pages = 87–112}} Reproduced in {{cite journal | vauthors = Von Willebrand EA | title = Hereditary pseudohaemophilia | journal = Haemophilia | volume = 5 | issue = 3 | pages = 223–31; discussion 222 | date = May 1999 | pmid = 10444294 | doi = 10.1046/j.1365-2516.1999.00302.x | s2cid = 221750622 }}</ref> |
||
In the 1950s, vWD was shown to be caused by a plasma factor deficiency (instead of being caused by platelet disorders), and, in the 1970s, the VWF protein was purified.<ref name=Sadler/> |
In the 1950s, vWD was shown to be caused by a plasma factor deficiency (instead of being caused by platelet disorders), and, in the 1970s, the VWF protein was purified.<ref name=Sadler/> |
||
[[Harvey J. Weiss]]<ref name="PMID-4127287">{{cite journal | |
[[Harvey J. Weiss]]<ref name="PMID-4127287">{{cite journal | vauthors = Weiss HJ, Hoyer IW | title = Von Willebrand factor: dissociation from antihemophilic factor procoagulant activity | journal = Science | volume = 182 | issue = 4117 | pages = 1149–1151 | date = December 1973 | pmid = 4127287 | doi = 10.1126/science.182.4117.1149 | s2cid = 41340436 | bibcode = 1973Sci...182.1149W }}</ref> and coworkers developed a quantitative assay for VWF function that remains a mainstay of laboratory |
||
evaluation for VWD to this day.<ref name="PMID-4201262">{{cite journal | |
evaluation for VWD to this day.<ref name="PMID-4201262">{{cite journal | vauthors = Weiss HJ, Rogers J, Brand H | title = Defective ristocetin-induced platelet aggregation in von Willebrand's disease and its correction by factor VIII | journal = The Journal of Clinical Investigation | volume = 52 | issue = 11 | pages = 2697–2707 | date = November 1973 | pmid = 4201262 | pmc = 302536 | doi = 10.1172/JCI107464 }}</ref> |
||
== Interactions == |
== Interactions == |
||
Von Willebrand Factor has been shown to [[Protein-protein interaction|interact]] with [[Collagen, type I, alpha 1]].<ref name="pmid3490481">{{cite journal | vauthors = Pareti FI, Fujimura Y, Dent JA, Holland LZ, Zimmerman TS, Ruggeri ZM | title = Isolation and characterization of a collagen binding domain in human von Willebrand factor | journal = The Journal of Biological Chemistry | volume = 261 | issue = 32 | pages = 15310–15315 | date = November 1986 | doi = 10.1016/S0021-9258(18)66869-3 | pmid = 3490481 | doi-access = free }}</ref> |
|||
Recently, It has been reported that the cooperation and interactions within the |
Recently, It has been reported that the cooperation and interactions within the von Willebrand Factors enhances the adsorption probability in the primary haemostasis. Such cooperation is proven by calculating the adsorption probability of flowing VWF once it crosses another adsorbed one. Such cooperation is held within a wide range of shear rates.<ref>{{cite journal | vauthors = Heidari M, Mehrbod M, Ejtehadi MR, Mofrad MR | title = Cooperation within von Willebrand factors enhances adsorption mechanism | journal = Journal of the Royal Society, Interface | volume = 12 | issue = 109 | pages = 20150334 | date = August 2015 | pmid = 26179989 | pmc = 4535404 | doi = 10.1098/rsif.2015.0334 }}</ref> |
||
== See also == |
== See also == |
||
*[[von Willebrand disease]] |
* [[von Willebrand disease]] |
||
*[[Bernard–Soulier syndrome]] |
* [[Bernard–Soulier syndrome]] |
||
== References == |
== References == |
||
{{ |
{{Reflist}} |
||
== External links == |
== External links == |
||
Latest revision as of 05:51, 4 December 2024
| VWF | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | VWF, F8VWD, von Willebrand factor | ||||||||||||||||||||||||||||||||||||||||||||||||||
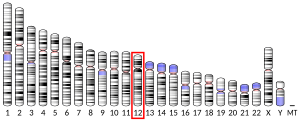
| External IDs | OMIM: 613160; MGI: 98941; HomoloGene: 466; GeneCards: VWF; OMA:VWF - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Von Willebrand factor (VWF) (German: [fɔn ˈvɪləbʁant]) is a blood glycoprotein that promotes hemostasis, specifically, platelet adhesion. It is deficient and/or defective in von Willebrand disease and is involved in many other diseases, including thrombotic thrombocytopenic purpura, Heyde's syndrome, and possibly hemolytic–uremic syndrome.[5] Increased plasma levels in many cardiovascular, neoplastic, metabolic (e.g. diabetes), and connective tissue diseases are presumed to arise from adverse changes to the endothelium, and may predict an increased risk of thrombosis.[6]
Biochemistry
[edit]Synthesis
[edit]VWF is a large multimeric glycoprotein present in blood plasma and produced constitutively as ultra-large VWF in endothelium (in the Weibel–Palade bodies), megakaryocytes (α-granules of platelets), and subendothelial connective tissue.[5]
Structure
[edit]The basic VWF monomer is a 2050-amino acid protein. Every monomer contains a number of specific domains with a specific function; elements of note are:[5]
- the D'/D3 domain, which binds to factor VIII (von Willebrand factor type D domain).[7]
- the A1 domain, which binds to:
- the A2 domain, which must partially unfold to expose the buried cleavage site for the specific ADAMTS13 protease that inactivates VWF by making much smaller multimers. The partial unfolding is affected by shear flow in the blood, by calcium binding, and by the lump of a sequence-adjacent "vicinal disulfide" at the A2-domain C-terminus.[8][9]
- the A3 domain, which binds to collagen (von Willebrand factor type A domain)
- the C4 domain, in which the RGD motif binds to platelet integrin αIIbβ3 when this is activated (von Willebrand factor type C domain)
- the other C domains, which may interact in ER dimers: the larger protein show six beads of (C and C-like) domains under cryo-EM.[7]
- the "cystine knot" domain (at the C-terminal end of the protein), which VWF shares with platelet-derived growth factor (PDGF), transforming growth factor-β (TGFβ) and β-human chorionic gonadotropin (βHCG, of pregnancy test fame). (von Willebrand factor type C domain)
Monomers are subsequently N-glycosylated, arranged into dimers in the endoplasmic reticulum and into multimers in the Golgi apparatus by crosslinking of cysteine residues via disulfide bonds. With respect to the glycosylation, VWF is one of only a few proteins that carry ABO blood group system antigens.[5] VWFs coming out of the Golgi are packaged into storage organelles, Weibel-Palade bodies (WPBs) in endothelial cells and α-granules in platelets.[10]
Multimers of VWF can be extremely large, >20,000 kDa, and consist of over 80 subunits of 250 kDa each. Only the large multimers are functional. Some cleavage products that result from VWF production are also secreted but probably serve no function.[5]

Function
[edit]
Von Willebrand Factor's primary function is binding to other proteins, in particular factor VIII, and it is important in platelet adhesion to wound sites.[5] It is not an enzyme and, thus, has no catalytic activity.
VWF binds to a number of cells and molecules. The most important ones are:[5]
- Factor VIII is bound to VWF while inactive in circulation; factor VIII degrades rapidly when not bound to VWF. Factor VIII is released from VWF by the action of thrombin. In the absence of VWF, factor VIII has a half-life of 1–2 hours; when carried by intact VWF, factor VIII has a half-life of 8–12 hours.
- VWF binds to collagen, e.g., when collagen is exposed beneath endothelial cells due to damage occurring to the blood vessel. Endothelium also releases VWF which forms additional links between the platelets' glycoprotein Ib/IX/V and the collagen fibrils
- VWF binds to platelet GpIb when it forms a complex with gpIX and gpV; this binding occurs under all circumstances, but is most efficient under high shear stress (i.e., rapid blood flow in narrow blood vessels, see below).
- VWF binds to other platelet receptors when they are activated, e.g., by thrombin (i.e., when coagulation has been stimulated).
VWF plays a major role in blood coagulation. Therefore, VWF deficiency or dysfunction (von Willebrand disease) leads to a bleeding tendency, which is most apparent in tissues having high blood flow shear in narrow vessels. From studies it appears that VWF uncoils under these circumstances, decelerating passing platelets.[5] Recent research also suggests that von Willebrand Factor is involved in the formation of blood vessels themselves, which would explain why some people with von Willebrand disease develop vascular malformations (predominantly in the digestive tract) that can bleed excessively.[11]
Catabolism
[edit]The biological breakdown (catabolism) of VWF is largely mediated by the enzyme ADAMTS13 (acronym of "a disintegrin-like and metalloprotease with thrombospondin type 1 motif no. 13"). It is a metalloproteinase that cleaves VWF between tyrosine at position 842 and methionine at position 843 (or 1605–1606 of the gene) in the A2 domain. This breaks down the multimers into smaller units, which are degraded by other peptidases.[12]
The half-life of vWF in human plasma is around 16 hours; glycosylation variation on vWF molecules from different individuals result in a larger range of 4.2 to 26 hours. Liver cells as well as macrophages take up vWF for clearance via ASGPRs and LRP1. SIGLEC5 and CLEC4M also recognize vWF.[10]
Role in disease
[edit]Hereditary or acquired defects of VWF lead to von Willebrand disease (vWD), a bleeding diathesis of the skin and mucous membranes, causing nosebleeds, menorrhagia, and gastrointestinal bleeding. The point at which the mutation occurs determines the severity of the bleeding diathesis. There are three types (I, II and III), and type II is further divided in several subtypes. Treatment depends on the nature of the abnormality and the severity of the symptoms.[13] Most cases of vWD are hereditary, but abnormalities of VWF may be acquired; aortic valve stenosis, for instance, has been linked to vWD type IIA, causing gastrointestinal bleeding - an association known as Heyde's syndrome.[14]
In thrombotic thrombocytopenic purpura (TTP) and hemolytic–uremic syndrome (HUS), ADAMTS13 either is deficient or has been inhibited by antibodies directed at the enzyme. This leads to decreased breakdown of the ultra-large multimers of VWF and microangiopathic hemolytic anemia with deposition of fibrin and platelets in small vessels, and capillary necrosis. In TTP, the organ most obviously affected is the brain; in HUS, the kidney.[15]
Higher levels of VWF are more common among people that have had ischemic stroke (from blood-clotting) for the first time.[16] Occurrence is not affected by ADAMTS13, and the only significant genetic factor is the person's blood group. High plasma VWF levels were found to be an independent predictor of major bleeding in anticoagulated atrial fibrillation patients.[17] VWF is a marker of endothelial dysfunction, and is consistently elevated in atrial fibrillation, associated with adverse outcomes.[18]
History
[edit]VWF is named after Erik Adolf von Willebrand, a Finnish physician who in 1926 first described a hereditary bleeding disorder in families from Åland. Although von Willebrand did not identify the definite cause, he distinguished von Willebrand disease (vWD) from hemophilia and other forms of bleeding diathesis.[19]
In the 1950s, vWD was shown to be caused by a plasma factor deficiency (instead of being caused by platelet disorders), and, in the 1970s, the VWF protein was purified.[5] Harvey J. Weiss[20] and coworkers developed a quantitative assay for VWF function that remains a mainstay of laboratory evaluation for VWD to this day.[21]
Interactions
[edit]Von Willebrand Factor has been shown to interact with Collagen, type I, alpha 1.[22]
Recently, It has been reported that the cooperation and interactions within the von Willebrand Factors enhances the adsorption probability in the primary haemostasis. Such cooperation is proven by calculating the adsorption probability of flowing VWF once it crosses another adsorbed one. Such cooperation is held within a wide range of shear rates.[23]
See also
[edit]References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000110799 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000001930 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c d e f g h i Sadler JE (1998). "Biochemistry and genetics of von Willebrand factor". Annual Review of Biochemistry. 67: 395–424. doi:10.1146/annurev.biochem.67.1.395. PMID 9759493.
- ^ Shahidi M (2017). "Thrombosis and von Willebrand Factor". Thrombosis and Embolism: From Research to Clinical Practice. Advances in Experimental Medicine and Biology. Vol. 906. pp. 285–306. doi:10.1007/5584_2016_122. ISBN 978-3-319-22107-6. PMID 27628010.
- ^ a b Zhou YF, Eng ET, Zhu J, Lu C, Walz T, Springer TA (July 2012). "Sequence and structure relationships within von Willebrand factor". Blood. 120 (2): 449–458. doi:10.1182/blood-2012-01-405134. PMC 3398765. PMID 22490677.
- ^ Jakobi AJ, Mashaghi A, Tans SJ, Huizinga EG (July 2011). "Calcium modulates force sensing by the von Willebrand factor A2 domain". Nature Communications. 2: 385. Bibcode:2011NatCo...2..385J. doi:10.1038/ncomms1385. PMC 3144584. PMID 21750539.
- ^ Luken BM, Winn LY, Emsley J, Lane DA, Crawley JT (June 2010). "The importance of vicinal cysteines, C1669 and C1670, for von Willebrand factor A2 domain function". Blood. 115 (23): 4910–4913. doi:10.1182/blood-2009-12-257949. PMC 2890177. PMID 20354169.
- ^ a b Lenting PJ, Christophe OD, Denis CV (March 2015). "von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends". Blood. 125 (13): 2019–2028. doi:10.1182/blood-2014-06-528406. PMID 25712991. S2CID 27785232.
- ^ Randi AM, Laffan MA (January 2017). "Von Willebrand factor and angiogenesis: basic and applied issues". Journal of Thrombosis and Haemostasis. 15 (1): 13–20. doi:10.1111/jth.13551. hdl:10044/1/42796. PMID 27778439. S2CID 3490036.
- ^ Levy GG, Motto DG, Ginsburg D (July 2005). "ADAMTS13 turns 3". Blood. 106 (1): 11–17. doi:10.1182/blood-2004-10-4097. PMID 15774620. S2CID 25645477.
- ^ Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, Holmberg L, et al. (October 2006). "Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor". Journal of Thrombosis and Haemostasis. 4 (10): 2103–2114. doi:10.1111/j.1538-7836.2006.02146.x. PMID 16889557. S2CID 23875096.
- ^ Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, et al. (July 2003). "Acquired von Willebrand syndrome in aortic stenosis". The New England Journal of Medicine. 349 (4): 343–349. doi:10.1056/NEJMoa022831. PMID 12878741.
- ^ Moake JL (January 2004). "von Willebrand factor, ADAMTS-13, and thrombotic thrombocytopenic purpura". Seminars in Hematology. 41 (1): 4–14. doi:10.1053/j.seminhematol.2003.10.003. PMID 14727254.
- ^ Denorme F, De Meyer SF (September 2016). "The VWF-GPIb axis in ischaemic stroke: lessons from animal models". Thrombosis and Haemostasis. 116 (4): 597–604. doi:10.1160/TH16-01-0036. PMID 27029413. S2CID 4964177.
- ^ Roldán V, Marín F, Muiña B, Torregrosa JM, Hernández-Romero D, Valdés M, et al. (June 2011). "Plasma von Willebrand factor levels are an independent risk factor for adverse events including mortality and major bleeding in anticoagulated atrial fibrillation patients". Journal of the American College of Cardiology. 57 (25): 2496–2504. doi:10.1016/j.jacc.2010.12.033. PMID 21497043.
- ^ Khan AA, Thomas GN, Lip G, Shantsila A (2020). "Endothelial function in patients with atrial fibrillation". Annals of Medicine. 52 (1–2): 1–11. doi:10.1080/07853890.2019.1711158. PMC 7877921. PMID 31903788.
- ^ von Willebrand EA (1926). "Hereditär pseudohemofili" [Hereditary pseudo haemophilia]. Fin Läkaresällsk Handl (in Swedish). 68: 87–112. Reproduced in Von Willebrand EA (May 1999). "Hereditary pseudohaemophilia". Haemophilia. 5 (3): 223–31, discussion 222. doi:10.1046/j.1365-2516.1999.00302.x. PMID 10444294. S2CID 221750622.
- ^ Weiss HJ, Hoyer IW (December 1973). "Von Willebrand factor: dissociation from antihemophilic factor procoagulant activity". Science. 182 (4117): 1149–1151. Bibcode:1973Sci...182.1149W. doi:10.1126/science.182.4117.1149. PMID 4127287. S2CID 41340436.
- ^ Weiss HJ, Rogers J, Brand H (November 1973). "Defective ristocetin-induced platelet aggregation in von Willebrand's disease and its correction by factor VIII". The Journal of Clinical Investigation. 52 (11): 2697–2707. doi:10.1172/JCI107464. PMC 302536. PMID 4201262.
- ^ Pareti FI, Fujimura Y, Dent JA, Holland LZ, Zimmerman TS, Ruggeri ZM (November 1986). "Isolation and characterization of a collagen binding domain in human von Willebrand factor". The Journal of Biological Chemistry. 261 (32): 15310–15315. doi:10.1016/S0021-9258(18)66869-3. PMID 3490481.
- ^ Heidari M, Mehrbod M, Ejtehadi MR, Mofrad MR (August 2015). "Cooperation within von Willebrand factors enhances adsorption mechanism". Journal of the Royal Society, Interface. 12 (109): 20150334. doi:10.1098/rsif.2015.0334. PMC 4535404. PMID 26179989.
External links
[edit]- GeneReviews/NCBI/NIH/UW entry on von Willebrand Factor Deficiency. Includes: Type 1 von Willebrand Disease, Type 2A von Willebrand Disease, Type 2B von Willebrand Disease, Type 2M von Willebrand Disease, Type 2N von Willebrand Disease, Type 3 von Willebrand Disease
- Overview of all the structural information available in the PDB for UniProt: P04275 (von Willebrand factor) at the PDBe-KB.