Ketonic decarboxylation: Difference between revisions
Tags: Mobile edit Mobile web edit |
→Intramolecular decarboxylations: rm some ambiguity |
||
| (37 intermediate revisions by 14 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|1=Chemical reaction which converts two –COOH groups to >C=O}} |
|||
| ⚫ | |||
'''Ketonic decarboxylation''' (also known as '''decarboxylative ketonization''') is a type of [[organic reaction]] and a [[decarboxylation]] converting two equivalents of a [[carboxylic acid]] ({{chem2|R\sC(\dO)OH}}) to a symmetric [[ketone]] ({{chem2|R2C\dO}}) by the application of heat. It can be thought of as a decarboxylative [[Claisen condensation]] of two identical molecules. [[Water]] and [[carbon dioxide]] are byproducts:<ref name=Pham>{{cite journal |doi=10.1021/cs400501h |title=Ketonization of Carboxylic Acids: Mechanisms, Catalysts, and Implications for Biomass Conversion |date=2013 |last1=Pham |first1=Tu N. |last2=Sooknoi |first2=Tawan |last3=Crossley |first3=Steven P. |last4=Resasco |first4=Daniel E. |journal=ACS Catalysis |volume=3 |issue=11 |pages=2456–2473 }}</ref> |
|||
:{{chem2|2 RCO2H -> R2CO + CO2 + H2O}} |
|||
| ⚫ | [[base (chemistry)|Bases]] promote this reaction. The [[reaction mechanism]] is proposed to involve [[nucleophilic attack]] of the [[alpha-carbon]] of one acid group on the other carboxylic acid group, possibly as a [[concerted reaction]] with the decarboxylation.<ref name=Pham/> The initial formation of an intermediate [[carbanion]] via decarboxylation of one of the acid groups prior to the nucleophilic attack is unlikely since the byproduct resulting from the carbanion's [[protonation]] by the acid has not been reported.<ref>{{cite journal | last1 = Renz | first1 = M | year = 2005 | title = Ketonization of Carboxylic Acids by Decarboxylation: Mechanism and Scope | journal = Eur. J. Org. Chem. | volume = 2005 | issue = 6| pages = 979–988 | doi = 10.1002/ejoc.200400546 | url = https://www.thevespiary.org/rhodium/Rhodium/Vespiary/talk/files/835-Thermal.Ketonization.Mechanism.and.Scopecc88.pdf | via = The Vespiary}}</ref> This reaction is different from [[oxidative decarboxylation]], which proceeds through a [[Radical (chemistry)|radical]] mechanism and is characterised by a different product distribution in [[isotopic labeling]] experiments with two different carboxylic acids. With two different carboxylic acids, the reaction behaves poorly because of poor selectivity except when one of the acids (for example, a small, volatile one) is used in large excess. |
||
==Examples== |
==Examples== |
||
The dry distillation of [[calcium acetate]] to [[acetone]] was reported by [[Charles Friedel]] in 1858 |
The dry distillation of [[calcium acetate]] to give [[acetone]] was reported by [[Charles Friedel]] in 1858<ref>{{cite journal|doi=10.1002/jlac.18581080124|title=Ueber s. G. gemischte Acetone|journal=Annalen der Chemie und Pharmacie|volume=108|pages=122–125|year=1858|last1=Friedel|first1=C.|url=https://zenodo.org/record/1427117}} |
||
</ref> and until [[World War I]] ketonization was the premier commercial method for its production.<ref>{{cite journal|doi=10.1021/ja02158a004|title=Improvement in the Manufacture of Acetone.1|journal=Journal of the American Chemical Society|volume=17|issue=3|pages=187–201|year=1895|last1=Squibb|first1=E. R.|url=https://zenodo.org/record/1428975}}</ref> |
|||
Ketonic decarboxylation of [[propanoic acid]] over a [[manganese(II) oxide]] catalyst in a [[tube furnace]]<ref>{{cite book|author1=Furniss, Brian |author2=Hannaford, Antony |author3=Smith, Peter |author4=Tatchell, Austin |title=Vogel's Textbook of Practical Organic Chemistry 5th Ed.|year=1996|publisher=Longman Science & Technical|location=London|isbn=9780582462366|page=613|url=https://archive.org/details/TextbookOfPracticalOrganicChemistry5thEd}}</ref> |
Ketonic decarboxylation of [[propanoic acid]] over a [[manganese(II) oxide]] catalyst in a [[tube furnace]] affords [[3-pentanone]].<ref>{{cite book|author1=Furniss, Brian |author2=Hannaford, Antony |author3=Smith, Peter |author4=Tatchell, Austin |title=Vogel's Textbook of Practical Organic Chemistry 5th Ed.|year=1996|publisher=Longman Science & Technical|location=London|isbn=9780582462366|page=[https://archive.org/details/TextbookOfPracticalOrganicChemistry5thEd/page/n637 613]|url=https://archive.org/details/TextbookOfPracticalOrganicChemistry5thEd}}</ref> |
||
[[5-Nonanone]], which is potentially of interest as a diesel fuel, can be produced from [[valeric acid]].<ref>{{cite journal|doi=10.1002/cssc.201501405|pmid=26847212|title=Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass|journal=ChemSusChem|volume=9|issue=6|pages=562–582|year=2016|last1=Pileidis|first1=Filoklis D.|last2=Titirici|first2=Maria-Magdalena|bibcode=2016ChSCh...9..562P |url=http://qmro.qmul.ac.uk/xmlui/handle/123456789/16048}}</ref> Stearone is prepared by heating [[magnesium stearate]].<ref>{{cite journal |author=A. G. Dobson and H. H. Hatt|doi=10.15227/orgsyn.033.0084|title=Stearone |journal=Organic Syntheses |year=1953 |volume=33 |page=84}}</ref> |
|||
| ⚫ | |||
===Metal oxide catalysts=== |
|||
:[[File:Barium adipate pyrolysis.png|frameless|400px]] |
|||
Dozens or more metal oxides have been investigated as catalysts for the decarboxylation. Early work focused on the oxides of calcium and thorium.<ref>{{cite journal |doi=10.1016/j.cattod.2017.09.044 |title=Ketonization of oxygenated hydrocarbons on metal oxide based catalysts |date=2018 |last1=Kumar |first1=Rawesh |last2=Enjamuri |first2=Nagasuresh |last3=Shah |first3=Sneha |last4=Al-Fatesh |first4=Ahmed Sadeq |last5=Bravo-Suárez |first5=Juan J. |last6=Chowdhury |first6=Biswajit |journal=Catalysis Today |volume=302 |pages=16–49 }}</ref> Of commercial interest are related ketonizations using [[cerium(IV) oxide]] and [[manganese dioxide]] on [[alumina]] as the [[catalyst]]s. The synthesis of [[4-heptanone]] illustrates the production of the metal carboxylate in situ. Iron powder and butyric acid are converted to iron butyrate. Pyrolysis of that salt gives the ketone. |
|||
<ref>{{cite journal |first1=Robert|last1=Davis|first2=Charles|last2=Granito|first3=Harry P.|last3= Schultz|doi=10.15227/orgsyn.047.0075|title=4-Heptanone|journal=Organic Syntheses|year=1967|volume=47|page=75}}</ref> |
|||
===Intramolecular decarboxylations=== |
|||
The intramolecular version of ketonic decarboxylation is often called the '''Ružička large-ring synthesis''' (or '''Ružička cyclization'''), [[named reaction|named]] for [[Lavoslav Ružička]] who developed the technique from prior methods that could synthesize small cyclic compounds from calcium salts of [[Dicarboxylic acid|dicarboxylic acids]].<ref>{{cite journal |author1=L. Ruzicka |author2=M. Stoll |author3=H. Schinz |year=1926 |title=Zur Kenntnis des Kohlenstoffringes II. Synthese der carbocyclischen Ketone vom Zehner- bis zum Achtzehnerring |journal=[[Helvetica Chimica Acta]] |volume=9 |issue=1 |pages=249–264 |doi=10.1002/hlca.19260090130}}</ref> It was the first synthesis to directly produce cyclic compounds with more than 8 members and was used by Ružička to produce [[Macrocycle|macrocyclic]] molecules with up to 34 carbon atoms. One target for such reactions are the naturally occurring fragrances [[civetone]] and [[muscone]]. The method involved dry distillation of dibasic salts of a dicarboxylic acid, such as thorium, cerium, and yttrium salts, mixed with copper powder to improve heat transfer. This method was low-yielding for large ring sizes and was eventually supplanted by various methods using the [[high dilution principle]].<ref>{{Cite book |last=Agrawal |first=O. P. |url=https://books.google.com/books?id=dyxob45rYkkC&pg=PA237 |title=Organic Chemistry – Reactions and Reagents (46th ed.). |publisher=Krishna Prakashan Media |isbn=978-81-87224-65-5 |editor-last=Agrawal |editor-first=Shipra |edition=46 |location=India |publication-date=2009 |pages=237–246 |language=en}}</ref> |
|||
[[Image:Ružička-Cyclisierung.svg|center|400px|The Ruzicka large-ring synthesis]] |
|||
| ⚫ | A more conventional example of [[Intramolecular reaction|intramolecular]] ketonization is the conversion of [[adipic acid]] to [[cyclopentanone]] with [[barium hydroxide]].<ref>{{cite journal|journal=Org. Synth.|first1=J. F.|last1=Thorpe|first2=G. A. R.|last2=Kon|year=1925|title= Cyclopentanone|volume=5|pages=37|doi=10.15227/orgsyn.005.0037}}</ref> |
||
<!--== Further reading == |
|||
* {{cite journal |doi=10.1002/hlca.19260090130 |title=Zur Kenntnis des Kohlenstoffringes II. Synthese der carbocyclischen Ketone vom Zehner- bis zum Achtzehnerring |trans-title=Studies on Carbon Rings II. Syntheses of Carbocyclic Ketones from Ten-to Eighteen Rings|date=1926 |last1=Ruzicka |first1=L. |last2=Stoll |first2=M. |last3=Schinz |first3=H. |journal=Helvetica Chimica Acta |volume=9 |pages=249–264 }} |
|||
* {{cite journal |doi=10.1002/hlca.19260090140 |title=Zur Kenntnis des Kohlenstoffringes III. Über die Gewinnung des Cyclo-octanons aus Azelainsäure |trans-title=Studies on Carbon Rings III. About the production of cyclo-octanone from azelaic acid|date=1926 |last1=Ruzicka |first1=L. |last2=Brugger |first2=W. |journal=Helvetica Chimica Acta |volume=9 |pages=339–354 }} |
|||
* {{cite journal |doi=10.1002/hlca.19260090148 |title=Zur Kenntnis des Kohlenstoffringes IV. Über die Gewinnung des Cyclo-nonanons aus Sebacinsäure |trans-title=Studies on Carbon Rings IV. On the production of cyclononanone from sebacic acid|date=1926 |last1=Ruzicka |first1=L. |last2=Brugger |first2=W. |journal=Helvetica Chimica Acta |volume=9 |pages=389–398 }} |
|||
* {{cite journal |doi=10.1002/hlca.19260090164 |title=Zur Kenntnis des Kohlenstoffringes VI. Über die relative Bildungsleichtigkeit, die relative Beständigkeit und den räumlichen Bau der gesättigten Kohlenstoffringe |trans-title=Studies on Carbon Rings VI. About the relative ease of formation, the relative stability and the spatial structure of the saturated carbon rings|date=1926 |last1=Ruzicka |first1=L. |last2=Brugger |first2=W. |last3=Pfeiffer |first3=M. |last4=Schinz |first4=H. |last5=Stoll |first5=M. |journal=Helvetica Chimica Acta |volume=9 |pages=499–520 }}--> |
|||
==Ketonization (other meaning)== |
|||
Ketonization can also refer to the conversion of some [[enol]]s to the ketone. Such a conversion is the reverse of [[enolization]].<ref>{{March6th|page=794}}</ref> |
|||
==References== |
==References== |
||
| Line 14: | Line 37: | ||
[[Category:Organic reactions]] |
[[Category:Organic reactions]] |
||
[[Category:Substitution reactions]] |
|||
[[Category:Name reactions]] |
|||
{{Organic-chemistry-stub}} |
|||
Latest revision as of 13:27, 4 December 2024
Ketonic decarboxylation (also known as decarboxylative ketonization) is a type of organic reaction and a decarboxylation converting two equivalents of a carboxylic acid (R−C(=O)OH) to a symmetric ketone (R2C=O) by the application of heat. It can be thought of as a decarboxylative Claisen condensation of two identical molecules. Water and carbon dioxide are byproducts:[1]
- 2 RCO2H → R2CO + CO2 + H2O
Bases promote this reaction. The reaction mechanism is proposed to involve nucleophilic attack of the alpha-carbon of one acid group on the other carboxylic acid group, possibly as a concerted reaction with the decarboxylation.[1] The initial formation of an intermediate carbanion via decarboxylation of one of the acid groups prior to the nucleophilic attack is unlikely since the byproduct resulting from the carbanion's protonation by the acid has not been reported.[2] This reaction is different from oxidative decarboxylation, which proceeds through a radical mechanism and is characterised by a different product distribution in isotopic labeling experiments with two different carboxylic acids. With two different carboxylic acids, the reaction behaves poorly because of poor selectivity except when one of the acids (for example, a small, volatile one) is used in large excess.
Examples
[edit]The dry distillation of calcium acetate to give acetone was reported by Charles Friedel in 1858[3] and until World War I ketonization was the premier commercial method for its production.[4]
Ketonic decarboxylation of propanoic acid over a manganese(II) oxide catalyst in a tube furnace affords 3-pentanone.[5]
5-Nonanone, which is potentially of interest as a diesel fuel, can be produced from valeric acid.[6] Stearone is prepared by heating magnesium stearate.[7]
Metal oxide catalysts
[edit]Dozens or more metal oxides have been investigated as catalysts for the decarboxylation. Early work focused on the oxides of calcium and thorium.[8] Of commercial interest are related ketonizations using cerium(IV) oxide and manganese dioxide on alumina as the catalysts. The synthesis of 4-heptanone illustrates the production of the metal carboxylate in situ. Iron powder and butyric acid are converted to iron butyrate. Pyrolysis of that salt gives the ketone. [9]
Intramolecular decarboxylations
[edit]The intramolecular version of ketonic decarboxylation is often called the Ružička large-ring synthesis (or Ružička cyclization), named for Lavoslav Ružička who developed the technique from prior methods that could synthesize small cyclic compounds from calcium salts of dicarboxylic acids.[10] It was the first synthesis to directly produce cyclic compounds with more than 8 members and was used by Ružička to produce macrocyclic molecules with up to 34 carbon atoms. One target for such reactions are the naturally occurring fragrances civetone and muscone. The method involved dry distillation of dibasic salts of a dicarboxylic acid, such as thorium, cerium, and yttrium salts, mixed with copper powder to improve heat transfer. This method was low-yielding for large ring sizes and was eventually supplanted by various methods using the high dilution principle.[11]
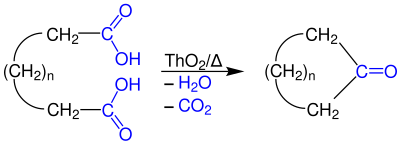
A more conventional example of intramolecular ketonization is the conversion of adipic acid to cyclopentanone with barium hydroxide.[12]
Ketonization (other meaning)
[edit]Ketonization can also refer to the conversion of some enols to the ketone. Such a conversion is the reverse of enolization.[13]
References
[edit]- ^ a b Pham, Tu N.; Sooknoi, Tawan; Crossley, Steven P.; Resasco, Daniel E. (2013). "Ketonization of Carboxylic Acids: Mechanisms, Catalysts, and Implications for Biomass Conversion". ACS Catalysis. 3 (11): 2456–2473. doi:10.1021/cs400501h.
- ^ Renz, M (2005). "Ketonization of Carboxylic Acids by Decarboxylation: Mechanism and Scope" (PDF). Eur. J. Org. Chem. 2005 (6): 979–988. doi:10.1002/ejoc.200400546 – via The Vespiary.
- ^ Friedel, C. (1858). "Ueber s. G. gemischte Acetone". Annalen der Chemie und Pharmacie. 108: 122–125. doi:10.1002/jlac.18581080124.
- ^ Squibb, E. R. (1895). "Improvement in the Manufacture of Acetone.1". Journal of the American Chemical Society. 17 (3): 187–201. doi:10.1021/ja02158a004.
- ^ Furniss, Brian; Hannaford, Antony; Smith, Peter; Tatchell, Austin (1996). Vogel's Textbook of Practical Organic Chemistry 5th Ed. London: Longman Science & Technical. p. 613. ISBN 9780582462366.
- ^ Pileidis, Filoklis D.; Titirici, Maria-Magdalena (2016). "Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass". ChemSusChem. 9 (6): 562–582. Bibcode:2016ChSCh...9..562P. doi:10.1002/cssc.201501405. PMID 26847212.
- ^ A. G. Dobson and H. H. Hatt (1953). "Stearone". Organic Syntheses. 33: 84. doi:10.15227/orgsyn.033.0084.
- ^ Kumar, Rawesh; Enjamuri, Nagasuresh; Shah, Sneha; Al-Fatesh, Ahmed Sadeq; Bravo-Suárez, Juan J.; Chowdhury, Biswajit (2018). "Ketonization of oxygenated hydrocarbons on metal oxide based catalysts". Catalysis Today. 302: 16–49. doi:10.1016/j.cattod.2017.09.044.
- ^ Davis, Robert; Granito, Charles; Schultz, Harry P. (1967). "4-Heptanone". Organic Syntheses. 47: 75. doi:10.15227/orgsyn.047.0075.
- ^ L. Ruzicka; M. Stoll; H. Schinz (1926). "Zur Kenntnis des Kohlenstoffringes II. Synthese der carbocyclischen Ketone vom Zehner- bis zum Achtzehnerring". Helvetica Chimica Acta. 9 (1): 249–264. doi:10.1002/hlca.19260090130.
- ^ Agrawal, O. P. (2009). Agrawal, Shipra (ed.). Organic Chemistry – Reactions and Reagents (46th ed.) (46 ed.). India: Krishna Prakashan Media. pp. 237–246. ISBN 978-81-87224-65-5.
- ^ Thorpe, J. F.; Kon, G. A. R. (1925). "Cyclopentanone". Org. Synth. 5: 37. doi:10.15227/orgsyn.005.0037.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 794, ISBN 978-0-471-72091-1
