Bixin: Difference between revisions
Appearance
Content deleted Content added
added chembl id |
Mucketymuck (talk | contribs) m Non-restrictive clause. |
||
| (30 intermediate revisions by 24 users not shown) | |||
| Line 3: | Line 3: | ||
| verifiedrevid = 459980646 |
| verifiedrevid = 459980646 |
||
| Reference = <ref name="Merck">''[[Merck Index]]'', 11th Edition, '''1320'''</ref> |
| Reference = <ref name="Merck">''[[Merck Index]]'', 11th Edition, '''1320'''</ref> |
||
| ImageFile = |
| ImageFile = Bixina.svg |
||
| ImageSize = 300 |
| ImageSize = 300 |
||
| ImageName = Skeletal formula |
| ImageName = Skeletal formula |
||
| ⚫ | |||
| ImageFile1 = Cis-bixin-3D-balls-(rotated).png |
|||
| ImageSize1 = 310 |
|||
| ImageName1 = Ball-and-stick model |
|||
| ⚫ | |||
| OtherNames = ''cis''-Bixin; α-Bixin; 9-''cis''-6,6'-Diapo-ψ,ψ-carotenedioic acid, 6-methyl ester |
| OtherNames = ''cis''-Bixin; α-Bixin; 9-''cis''-6,6'-Diapo-ψ,ψ-carotenedioic acid, 6-methyl ester |
||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
||
| ChemSpiderID = 4444638 |
| ChemSpiderID = 4444638 |
||
| ChEBI = 3136 |
|||
| ⚫ | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} |
|||
| ⚫ | |||
| InChI = 1/C25H30O4/c1-20(12-8-14-22(3)16-18-24(26)27)10-6-7-11-21(2)13-9-15-23(4)17-19-25(28)29-5/h6-19H,1-5H3,(H,26,27)/b7-6+,12-8+,13-9+,18-16+,19-17+,20-10+,21-11+,22-14+,23-15- |
| InChI = 1/C25H30O4/c1-20(12-8-14-22(3)16-18-24(26)27)10-6-7-11-21(2)13-9-15-23(4)17-19-25(28)29-5/h6-19H,1-5H3,(H,26,27)/b7-6+,12-8+,13-9+,18-16+,19-17+,20-10+,21-11+,22-14+,23-15- |
||
| InChIKey = RAFGELQLHMBRHD-SLEZCNMEBU |
| InChIKey = RAFGELQLHMBRHD-SLEZCNMEBU |
||
| CASNo_Ref = {{cascite| |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
| CASNo = 6983-79-5 |
| CASNo = 6983-79-5 |
||
| CASNo2_Ref = {{cascite|correct|CAS}} |
|||
| CASOther = <br>39937-23-0 (''trans''-Bixin) |
|||
| CASNo2 = 39937-23-0 |
|||
| CASNo2_Comment = (''trans'') |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChI = 1S/C25H30O4/c1-20(12-8-14-22(3)16-18-24(26)27)10-6-7-11-21(2)13-9-15-23(4)17-19-25(28)29-5/h6-19H,1-5H3,(H,26,27)/b7-6+,12-8+,13-9+,18-16+,19-17+,20-10+,21-11+,22-14+,23-15+ |
| StdInChI = 1S/C25H30O4/c1-20(12-8-14-22(3)16-18-24(26)27)10-6-7-11-21(2)13-9-15-23(4)17-19-25(28)29-5/h6-19H,1-5H3,(H,26,27)/b7-6+,12-8+,13-9+,18-16+,19-17+,20-10+,21-11+,22-14+,23-15+ |
||
| Line 27: | Line 28: | ||
| UNII_Ref = {{fdacite|correct|FDA}} |
| UNII_Ref = {{fdacite|correct|FDA}} |
||
| UNII = 9L7T4VB66G |
| UNII = 9L7T4VB66G |
||
| UNII1_Ref = {{fdacite|correct|FDA}} |
|||
| UNII1 = 6JH6LEZ7HY |
|||
| UNII1_Comment = (''trans'') |
|||
| SMILES = O=C(O)\C=C\C(=C\C=C\C(=C\C=C\C=C(\C=C\C=C(/C=C/C(=O)OC)C)C)C)C |
| SMILES = O=C(O)\C=C\C(=C\C=C\C(=C\C=C\C=C(\C=C\C=C(/C=C/C(=O)OC)C)C)C)C |
||
}} |
}} |
||
| Line 33: | Line 37: | ||
| Appearance = Orange crystals |
| Appearance = Orange crystals |
||
| Density = |
| Density = |
||
| MeltingPt = |
| MeltingPt = 198 °C (cis-isomer) <br /> 217 °C (trans-isomer) |
||
| BoilingPt = |
| BoilingPt = |
||
| Solubility = Insoluble}} |
| Solubility = Insoluble}} |
||
| Section3 = {{Chembox Hazards |
| Section3 = {{Chembox Hazards |
||
| NFPA-H = 1 |
|||
| NFPA-F = 1 |
|||
| NFPA-R = 0 |
|||
| MainHazards = |
| MainHazards = |
||
| FlashPt = |
| FlashPt = |
||
| |
| AutoignitionPt = }} |
||
}} |
}} |
||
'''Bixin''' is an [[apocarotenoid]] found in |
'''Bixin''' is an [[apocarotenoid]] found in the seeds of the [[Bixa orellana|achiote tree]] (''Bixa orellana'')<ref>{{Cite journal|last1=Bouvier|first1=Florence|last2=Dogbo|first2=Odette|last3=Camara|first3=Bilal|date=2003|title=Biosynthesis of the Food and Cosmetic Plant Pigment Bixin (Annatto)|url=https://www.jstor.org/stable/3834418|journal=Science|volume=300|issue=5628|pages=2089–2091|doi=10.1126/science.1085162 |jstor=3834418 |pmid=12829782 |bibcode=2003Sci...300.2089B |s2cid=560600 |issn=0036-8075}}</ref> from which it derives its name. It is commonly extracted from the seeds to form [[annatto]], a natural [[food coloring]], containing about 5% pigments, of which 70–80% are bixin.<ref>[https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/bixin_508.pdf Executive Summary Bixin] {{webarchive |url=https://web.archive.org/web/20110721055506/http://ntp-server.niehs.nih.gov/?objectid=F59ACAC5-F1F6-975E-7C563568F5F7351B#selection |date=July 21, 2011 }}, National Toxicology Program</ref> |
||
==Applications== |
|||
| ⚫ | |||
:[[image:Bixa orellana fruit open.jpg|thumb|center|Red seeds of the achiote tree]] |
|||
:[[file:CheetosCrop.jpg|thumb|Bixin is one of the colorants used in the snack Cheetos.]] |
|||
Several thousand tons are harvested annually.<ref>{{cite book |doi=10.1016/B978-0-12-803138-4.00006-X|chapter=Annatto/Urucum— Bixa orellana |title=Exotic Fruits |year=2018 |last1=Stringheta |first1=Paulo C. |last2=Silva |first2=Pollyanna I. |last3=Costa |first3=André G.V. |pages=23–30 |isbn=9780128031384 }}</ref> |
|||
==Chemical properties== |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
:[[File: |
:[[File:norbixin structure.png|thumb|center|300px|Chemical structure of norbixin]]{{clear-left}} |
||
:[[File:norbixin structure.png|thumb|left|300px|Chemical structure of norbixin]]{{clear-left}} |
|||
==References== |
==References== |
||
| Line 58: | Line 69: | ||
[[Category:Apocarotenoids]] |
[[Category:Apocarotenoids]] |
||
[[Category:E-number additives]] |
|||
[[ca:Bixina]] |
|||
[[de:Bixin]] |
|||
[[es:Bixina]] |
|||
[[fa:بیکسین]] |
|||
[[it:Bixina]] |
|||
[[lt:Biksinas]] |
|||
[[nl:Bixine]] |
|||
[[ja:ビキシン]] |
|||
[[pl:Biksyna]] |
|||
[[pt:Bixina]] |
|||
Latest revision as of 21:36, 6 December 2024
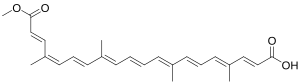
| |
| Names | |
|---|---|
| IUPAC name
(2E,4E,6E,8E,10E,12E,14E,16Z,18E)-20-Methoxy-4,8,13,17-tetramethyl-20-oxoicosa-2,4,6,8,10,12,14,16,18-nonaenoic acid
| |
| Other names
cis-Bixin; α-Bixin; 9-cis-6,6'-Diapo-ψ,ψ-carotenedioic acid, 6-methyl ester
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.499 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C25H30O4 | |
| Molar mass | 394.511 g·mol−1 |
| Appearance | Orange crystals |
| Melting point | 198 °C (cis-isomer) 217 °C (trans-isomer) |
| Insoluble | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bixin is an apocarotenoid found in the seeds of the achiote tree (Bixa orellana)[2] from which it derives its name. It is commonly extracted from the seeds to form annatto, a natural food coloring, containing about 5% pigments, of which 70–80% are bixin.[3]
Applications
[edit]Several thousand tons are harvested annually.[4]
Chemical properties
[edit]Bixin is unstable. It isomerizes into trans-bixin (β-bixin), the double-bond isomer.[1]
Bixin is soluble in fats and alcohols but insoluble in water. Upon exposure to alkali, the methyl ester is hydrolyzed to produce the dicarboxylic acid norbixin, a water-soluble derivative.
References
[edit]- ^ a b Merck Index, 11th Edition, 1320
- ^ Bouvier, Florence; Dogbo, Odette; Camara, Bilal (2003). "Biosynthesis of the Food and Cosmetic Plant Pigment Bixin (Annatto)". Science. 300 (5628): 2089–2091. Bibcode:2003Sci...300.2089B. doi:10.1126/science.1085162. ISSN 0036-8075. JSTOR 3834418. PMID 12829782. S2CID 560600.
- ^ Executive Summary Bixin Archived July 21, 2011, at the Wayback Machine, National Toxicology Program
- ^ Stringheta, Paulo C.; Silva, Pollyanna I.; Costa, André G.V. (2018). "Annatto/Urucum— Bixa orellana". Exotic Fruits. pp. 23–30. doi:10.1016/B978-0-12-803138-4.00006-X. ISBN 9780128031384.





