Nanomaterials: Difference between revisions
m Dating maintenance tags: {{Update needed}} |
Citation bot (talk | contribs) Add: authors 1-1. Removed URL that duplicated identifier. Removed parameters. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | #UCB_CommandLine |
||
| (31 intermediate revisions by 23 users not shown) | |||
| Line 4: | Line 4: | ||
{{Nanotechnology}} |
{{Nanotechnology}} |
||
'''Nanomaterials''' describe, in principle, [[material]]s of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of [[nanoscopic scale|nanoscale]]<ref>{{cite journal |author1=Buzea, Cristina |author2=Pacheco, Ivan |author3=Robbie, Kevin |title=Nanomaterials and Nanoparticles: Sources and Toxicity |journal=Biointerphases |volume=2 |date=2007 |pages=MR17–MR71 |doi=10.1116/1.2815690 |pmid=20419892 |issue=4 |arxiv=0801.3280 |s2cid=35457219}}</ref>). |
'''Nanomaterials''' describe, in principle, chemical substances or [[material]]s of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of [[nanoscopic scale|nanoscale]]<ref>{{cite journal |author1=Buzea, Cristina |author2=Pacheco, Ivan |author3=Robbie, Kevin |title=Nanomaterials and Nanoparticles: Sources and Toxicity |journal=Biointerphases |volume=2 |date=2007 |pages=MR17–MR71 |doi=10.1116/1.2815690 |pmid=20419892 |issue=4 |arxiv=0801.3280 |s2cid=35457219}}</ref>). |
||
Nanomaterials research takes a [[materials science]]-based approach to [[nanotechnology]], leveraging advances in materials [[metrology]] and synthesis which have been developed in support of [[microfabrication]] research. Materials with structure at the nanoscale often have unique optical, electronic, thermo-physical or mechanical properties.<ref>{{cite journal |last1=Sadri |first1=Rad |title=A facile, bio-based, novel approach for synthesis of covalently functionalized graphene nanoplatelet nano-coolants toward improved thermo-physical and heat transfer properties |journal=Journal of Colloid and Interface Science |date=1 January 2018 |volume=509 |pages=140–152 |doi=10.1016/j.jcis.2017.07.052 |pmid=28898734 |bibcode=2018JCIS..509..140S |url=https://www.sciencedirect.com/science/article/abs/pii/S0021979717308202}}</ref><ref>{{cite journal |last1=Hubler |first1=A. |last2=Osuagwu |first2=O. |title=Digital quantum batteries: Energy and information storage in nanovacuum tube arrays |journal=Complexity |pages= |
Nanomaterials research takes a [[materials science]]-based approach to [[nanotechnology]], leveraging advances in materials [[metrology]] and synthesis which have been developed in support of [[microfabrication]] research. Materials with structure at the nanoscale often have unique optical, electronic, thermo-physical or mechanical properties.<ref>{{cite journal |last1=Sadri |first1=Rad |title=A facile, bio-based, novel approach for synthesis of covalently functionalized graphene nanoplatelet nano-coolants toward improved thermo-physical and heat transfer properties |journal=Journal of Colloid and Interface Science |date=1 January 2018 |volume=509 |pages=140–152 |doi=10.1016/j.jcis.2017.07.052 |pmid=28898734 |bibcode=2018JCIS..509..140S |url=https://www.sciencedirect.com/science/article/abs/pii/S0021979717308202}}</ref><ref>{{cite journal |last1=Hubler |first1=A. |last2=Osuagwu |first2=O. |title=Digital quantum batteries: Energy and information storage in nanovacuum tube arrays |journal=Complexity |pages=48–55 |date=2010 |volume=15 |issue=5 |doi=10.1002/cplx.20306|doi-access=free }}</ref><ref name="PortelaVidyasagar2020">{{cite journal |last1=Portela |first1=Carlos M. |last2=Vidyasagar |first2=A. |last3=Krödel |first3=Sebastian |last4=Weissenbach |first4=Tamara |last5=Yee |first5=Daryl W. |last6=Greer |first6=Julia R. |last7=Kochmann |first7=Dennis M. |title=Extreme mechanical resilience of self-assembled nanolabyrinthine materials |journal=Proceedings of the National Academy of Sciences |year=2020 |volume=117 |issue=11 |pages=5686–5693 |issn=0027-8424 |doi=10.1073/pnas.1916817117 |pmid=32132212 |pmc=7084143 |bibcode=2020PNAS..117.5686P |doi-access=free}}</ref> |
||
Nanomaterials are slowly becoming commercialized<ref name="nanotech-now">{{cite news |url=http://www.nanotech-now.com/columns/?article=835 |title=Achieving industry integration with nanomaterials through financial markets |date=8 January 2014 |publisher=Nanotechnology_Now |author=Eldridge, T.}}</ref> and beginning to emerge as commodities.<ref>{{cite journal |date=2010 |title=Commoditization of nanomaterials |journal=Nanotechnol. Perceptions |volume=6 |issue=3 |pages=155–178 |doi=10.4024/N15GO10A.ntp.06.03 |author=McGovern, C. |doi-access=free}}</ref> |
Nanomaterials are slowly becoming commercialized<ref name="nanotech-now">{{cite news |url=http://www.nanotech-now.com/columns/?article=835 |title=Achieving industry integration with nanomaterials through financial markets |date=8 January 2014 |publisher=Nanotechnology_Now |author=Eldridge, T.}}</ref> and beginning to emerge as commodities.<ref>{{cite journal |date=2010 |title=Commoditization of nanomaterials |journal=Nanotechnol. Perceptions |volume=6 |issue=3 |pages=155–178 |doi=10.4024/N15GO10A.ntp.06.03 |author=McGovern, C. |doi-access=free}}</ref> |
||
| Line 12: | Line 12: | ||
==Definition== |
==Definition== |
||
In [[ISO/TS 80004]], ''nanomaterial'' is defined as the "material with any external dimension in the nanoscale or having internal structure or surface structure in the nanoscale", with ''nanoscale'' defined as the "length range approximately from 1 |
In [[ISO/TS 80004]], ''nanomaterial'' is defined as the "material with any external dimension in the nanoscale or having internal structure or surface structure in the nanoscale", with ''nanoscale'' defined as the "length range approximately from 1 nm to 100 nm". This includes both ''nano-objects'', which are discrete pieces of material, and ''nanostructured materials'', which have internal or surface structure on the nanoscale; a nanomaterial may be a member of both these categories.<ref>{{cite web |url=https://www.iso.org/obp/ui/#iso:std:iso:ts:80004:-1:ed-2:v1:en |title=ISO/TS 80004-1:2015 - Nanotechnologies – Vocabulary – Part 1: Core terms |date=2015 |website=[[International Organization for Standardization]] |access-date=2018-01-08}}</ref> |
||
On 18 October 2011, the [[European Commission]] adopted the following definition of a nanomaterial: |
On 18 October 2011, the [[European Commission]] adopted the following definition of a nanomaterial:<ref>[http://ec.europa.eu/environment/chemicals/nanotech/ Nanomaterials]. European Commission. Last updated 18 October 2011</ref><blockquote>A natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1 nm – 100 nm. In specific cases and where warranted by concerns for the environment, health, safety or competitiveness the number size distribution threshold of 50% may be replaced by a threshold between 1% to 50%.</blockquote> |
||
==Sources== |
==Sources== |
||
===Engineered=== |
===Engineered=== |
||
Engineered nanomaterials have been deliberately engineered and manufactured by humans to have certain required properties.<ref name="PortelaVidyasagar2020"/><ref name="Current strategies for engineering">{{cite report |url=https://www.cdc.gov/niosh/docs/2014-102/default.html |title=Current Strategies for Engineering Controls in Nanomaterial Production and Downstream Handling Processes |date=November 2013 |website=U.S. [[National Institute for Occupational Safety and Health]] |pages=1–3, 7, 9–10, 17–20 |language=en-us |access-date=2017-03-05 |doi=10.26616/NIOSHPUB2014102 |doi-access=free}}</ref> |
[[Engineered nanomaterials]] have been deliberately engineered and manufactured by humans to have certain required properties.<ref name="PortelaVidyasagar2020"/><ref name="Current strategies for engineering">{{cite report |url=https://www.cdc.gov/niosh/docs/2014-102/default.html |title=Current Strategies for Engineering Controls in Nanomaterial Production and Downstream Handling Processes |date=November 2013 |website=U.S. [[National Institute for Occupational Safety and Health]] |pages=1–3, 7, 9–10, 17–20 |language=en-us |access-date=2017-03-05 |doi=10.26616/NIOSHPUB2014102 |doi-access=free}}</ref> |
||
Legacy nanomaterials are those that were in commercial production prior to the development of nanotechnology as incremental advancements over other [[colloid]]al or particulate materials.<ref>{{cite web |url=http://www.sun-fp7.eu/wp-content/uploads/2017/01/SUN-booklet-updates-for-web.pdf |title=A New Integrated Approach for Risk Assessment and Management of Nanotechnologies |date=2017 |website=EU Sustainable Nanotechnologies Project |pages=109–112 |access-date=2017-09-06}}</ref><ref>{{cite web |url=https://www.nanosafetycluster.eu/home/european-nanosafety-cluster-compendium.html |title=Compendium of Projects in the European NanoSafety Cluster |date=2017-06-26 |website=EU NanoSafety Cluster |page=10 |language=en |archive-url=https://web.archive.org/web/20120324111826/http://www.nanosafetycluster.eu/home/european-nanosafety-cluster-compendium.html |archive-date=2012-03-24 |url-status=dead |access-date=2017-09-07}}</ref><ref>{{cite web |url=http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2016)58&doclanguage=en |title=Future challenges related to the safety of manufactured nanomaterials |date=2016-11-04 |website=[[Organisation for Economic Co-operation and Development]] |page=11 |access-date=2017-09-06}}</ref> They include [[carbon black]] and [[titanium dioxide nanoparticle]]s.<ref>{{cite report |date=2016-08-18 |title=Taking Stock of the OSH Challenges of Nanotechnology: 2000 – 2015 |url=https://www.slideshare.net/windsgroup/taking-stock-of-the-osh-challenges-of-nanotechnology-2000-2015/115 |publisher=The Windsdor Consulting Group, Inc. |via=SlideShare}}</ref> |
Legacy nanomaterials are those that were in commercial production prior to the development of nanotechnology as incremental advancements over other [[colloid]]al or particulate materials.<ref>{{cite web |url=http://www.sun-fp7.eu/wp-content/uploads/2017/01/SUN-booklet-updates-for-web.pdf |title=A New Integrated Approach for Risk Assessment and Management of Nanotechnologies |date=2017 |website=EU Sustainable Nanotechnologies Project |pages=109–112 |access-date=2017-09-06}}</ref><ref>{{cite web |url=https://www.nanosafetycluster.eu/home/european-nanosafety-cluster-compendium.html |title=Compendium of Projects in the European NanoSafety Cluster |date=2017-06-26 |website=EU NanoSafety Cluster |page=10 |language=en |archive-url=https://web.archive.org/web/20120324111826/http://www.nanosafetycluster.eu/home/european-nanosafety-cluster-compendium.html |archive-date=2012-03-24 |url-status=dead |access-date=2017-09-07}}</ref><ref>{{cite web |url=http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2016)58&doclanguage=en |title=Future challenges related to the safety of manufactured nanomaterials |date=2016-11-04 |website=[[Organisation for Economic Co-operation and Development]] |page=11 |access-date=2017-09-06}}</ref> They include [[carbon black]] and [[titanium dioxide nanoparticle]]s.<ref>{{cite report |date=2016-08-18 |title=Taking Stock of the OSH Challenges of Nanotechnology: 2000 – 2015 |url=https://www.slideshare.net/windsgroup/taking-stock-of-the-osh-challenges-of-nanotechnology-2000-2015/115 |publisher=The Windsdor Consulting Group, Inc. |via=SlideShare}}</ref> |
||
| Line 26: | Line 26: | ||
===Natural=== |
===Natural=== |
||
Biological systems often feature natural, functional nanomaterials. The structure of [[foraminifera]] (mainly chalk) and viruses (protein, [[capsid]]), the wax crystals covering a [[Nelumbo nucifera|lotus]] or [[Tropaeolum|nasturtium]] leaf, spider and spider-mite silk,<ref>[http://phys.org/news/2013-05-natural-nanomaterial-spider-mite-genome-sequencing.html Novel natural nanomaterial spins off from spider-mite genome sequencing]. Phys.Org (23 May 2013)</ref> the blue hue of tarantulas,<ref>{{cite web |url=http://www.iflscience.com/plants-and-animals/why-are-tarantulas-blue |title=Why Are Tarantulas Blue? |publisher=iflscience}}</ref> the "spatulae" on the bottom of [[gecko]] feet, some [[Morpho rhetenor|butterfly]] wing scales, natural colloids ([[milk]], [[blood]]), horny materials ([[skin]], [[claw]]s, [[beak]]s, [[feather]]s, [[horn (anatomy)|horns]], [[hair]]), [[paper]], [[cotton]], [[nacre]], [[coral]]s, and even our own [[bone]] matrix are all natural ''organic'' nanomaterials. |
Biological systems often feature natural, functional nanomaterials. The structure of [[foraminifera]] (mainly chalk) and viruses (protein, [[capsid]]), the wax crystals covering a [[Nelumbo nucifera|lotus]] or [[Tropaeolum|nasturtium]] leaf, spider and spider-mite silk,<ref>[http://phys.org/news/2013-05-natural-nanomaterial-spider-mite-genome-sequencing.html Novel natural nanomaterial spins off from spider-mite genome sequencing]. Phys.Org (23 May 2013)</ref> the blue hue of tarantulas,<ref>{{cite web |url=http://www.iflscience.com/plants-and-animals/why-are-tarantulas-blue |title=Why Are Tarantulas Blue? |date=28 November 2015 |publisher=iflscience}}</ref> the "spatulae" on the bottom of [[gecko]] feet, some [[Morpho rhetenor|butterfly]] wing scales, natural colloids ([[milk]], [[blood]]), horny materials ([[skin]], [[claw]]s, [[beak]]s, [[feather]]s, [[horn (anatomy)|horns]], [[hair]]), [[paper]], [[cotton]], [[nacre]], [[coral]]s, and even our own [[bone]] matrix are all natural ''organic'' nanomaterials. |
||
Natural ''inorganic'' nanomaterials occur through crystal growth in the diverse chemical conditions of the [[Earth's crust]]. For example, [[clay]]s display complex nanostructures due to anisotropy of their underlying crystal structure, and volcanic activity can give rise to [[opal]]s, which are an instance of a naturally occurring [[photonic crystal]]s due to their nanoscale structure. Fires represent particularly complex reactions and can produce [[pigments]], [[cement]], [[fumed silica]] etc. |
Natural ''inorganic'' nanomaterials occur through crystal growth in the diverse chemical conditions of the [[Earth's crust]]. For example, [[clay]]s display complex nanostructures due to anisotropy of their underlying crystal structure, and volcanic activity can give rise to [[opal]]s, which are an instance of a naturally occurring [[photonic crystal]]s due to their nanoscale structure. Fires represent particularly complex reactions and can produce [[pigments]], [[cement]], [[fumed silica]] etc. |
||
| Line 36: | Line 36: | ||
File:Kapsid Schema-01.png|Viral [[capsid]] |
File:Kapsid Schema-01.png|Viral [[capsid]] |
||
File:Lotoseffekt.jpg|"[[Lotus effect]]", hydrophobic effect with self-cleaning ability |
File:Lotoseffekt.jpg|"[[Lotus effect]]", hydrophobic effect with self-cleaning ability |
||
File:Gecko foot on glass.JPG|Close-up of the underside of a gecko's foot as it walks on a glass wall (spatula: 200 × |
File:Gecko foot on glass.JPG|Close-up of the underside of a gecko's foot as it walks on a glass wall (spatula: 200 × 10–15 nm) |
||
File:SEM image of a Peacock wing, slant view 4.JPG|SEM micrograph of a butterfly wing scale (× 5000) |
File:SEM image of a Peacock wing, slant view 4.JPG|SEM micrograph of a butterfly wing scale (× 5000) |
||
File:Trevarno, pavo cristatus06.jpg|Peacock feather (detail) |
File:Trevarno, pavo cristatus06.jpg|Peacock feather (detail) |
||
File:62cts Brazilian Crystal Opal.JPG|Brazilian Crystal Opal. The play of color is caused by the interference and diffraction of light between silica spheres ( |
File:62cts Brazilian Crystal Opal.JPG|Brazilian Crystal Opal. The play of color is caused by the interference and diffraction of light between silica spheres (150–300 nm in diameter). |
||
File:Lasiodora parahybana, claws.JPG|Blue hue of a species of [[tarantula]] (450 nm ± 20 nm) |
File:Lasiodora parahybana, claws.JPG|Blue hue of a species of [[tarantula]] (450 nm ± 20 nm) |
||
</gallery> |
</gallery> |
||
==Types== |
==Types== |
||
Nano- |
Nano-materials are often categorized as to how many of their dimensions fall in the nanoscale. A ''[[nanoparticle]]'' is defined a nano-object with all three external dimensions in the nanoscale, whose longest and the shortest axes do not differ significantly. A ''[[nanofiber]]'' has two external dimensions in the nanoscale, with ''[[nanotube]]s'' being hollow nanofibers and ''[[nanorod]]s'' being solid nanofibers. A ''nanoplate/nanosheet'' has one external dimension in the nanoscale,<ref>{{cite journal |doi=10.1016/j.vacuum.2020.109700 |title=Structural, functional and magnetic ordering modifications in graphene oxide and graphite by 100 MeV gold ion irradiation |year=2020 |last1=Rawat |first1=Pankaj Singh |last2=Srivastava |first2=R.C. |last3=Dixit |first3=Gagan |last4=Asokan |first4=K. |journal=Vacuum |volume=182 |page=109700 |bibcode=2020Vacuu.182j9700R |s2cid=225410221}}</ref> and if the two larger dimensions are significantly different it is called a ''[[nanoribbon]]''. For nanofibers and nanoplates, the other dimensions may or may not be in the nanoscale, but must be significantly larger. In all of these cases, a significant difference is noted to typically be at least a factor of 3.<ref>{{cite web |url=https://www.iso.org/obp/ui/#iso:std:iso:ts:80004:-2:ed-1:v1:en |title=ISO/TS 80004-2:2015 - Nanotechnologies – Vocabulary – Part 2: Nano-objects |date=2015 |website=International Organization for Standardization |access-date=2018-01-08}}</ref> |
||
Nanostructured materials are often categorized by what [[phase (matter)|phases of matter]] they contain. A ''[[nanocomposite]]'' is a solid containing at least one physically or chemically distinct region or collection of regions, having at least one dimension in the nanoscale. A ''[[nanofoam]]'' has a liquid or solid matrix, filled with a gaseous phase, where one of the two phases has dimensions on the nanoscale. A [[nanoporous materials|''nanoporous material'']] is a solid material containing [[nanopore]]s, voids in the form of open or closed pores of sub-micron lengthscales. A ''[[nanocrystalline material]]'' has a significant fraction of crystal grains in the nanoscale.<ref>{{cite web |url=https://www.iso.org/obp/ui/#iso:std:iso:ts:80004:-4:ed-1:v1:en |title=ISO/TS 80004-4:2011 - Nanotechnologies – Vocabulary – Part 4: Nanostructured materials |date=2011 |website=International Organization for Standardization |access-date=2018-01-08}}</ref> |
Nanostructured materials are often categorized by what [[phase (matter)|phases of matter]] they contain. A ''[[nanocomposite]]'' is a solid containing at least one physically or chemically distinct region or collection of regions, having at least one dimension in the nanoscale. A ''[[nanofoam]]'' has a liquid or solid matrix, filled with a gaseous phase, where one of the two phases has dimensions on the nanoscale. A [[nanoporous materials|''nanoporous material'']] is a solid material containing [[nanopore]]s, voids in the form of open or closed pores of sub-micron lengthscales. A ''[[nanocrystalline material]]'' has a significant fraction of crystal grains in the nanoscale.<ref>{{cite web |url=https://www.iso.org/obp/ui/#iso:std:iso:ts:80004:-4:ed-1:v1:en |title=ISO/TS 80004-4:2011 - Nanotechnologies – Vocabulary – Part 4: Nanostructured materials |date=2011 |website=International Organization for Standardization |access-date=2018-01-08}}</ref> |
||
| Line 50: | Line 50: | ||
===Nanoporous materials=== |
===Nanoporous materials=== |
||
{{Main|Nanoporous materials}} |
{{Main|Nanoporous materials}} |
||
The term ''nanoporous materials'' contain subsets of microporous and mesoporous materials. Microporous materials are porous materials with a mean pore size smaller than 2 nm, while mesoporous materials are those with pores sizes in the region |
The term ''nanoporous materials'' contain subsets of microporous and mesoporous materials. Microporous materials are porous materials with a mean pore size smaller than 2 nm, while mesoporous materials are those with pores sizes in the region 2–50 nm.<ref name=porous>{{cite journal |vauthors=Doustkhah E et al. |title=Bispropylurea bridged polysilsesquioxane: A microporous MOF-likematerial for molecular recognition |journal=Chemosphere |year=2021 |volume=276 |pages=130181 |doi=10.1016/j.chemosphere.2021.130181 |pmid=33735650 |arxiv=2104.06715 |bibcode=2021Chmsp.27630181D |s2cid=232304875}}</ref> Microporous materials exhibit pore sizes with comparable length-scale to small molecules. For this reason such materials may serve valuable applications including separation membranes. Mesoporous materials are interesting towards applications that require high specific surface areas, while enabling penetration for molecules that may be too large to enter the pores of a microporous material. In some sources, nanoporous materials and nanofoam are sometimes considered nanostructures but not nanomaterials because only the voids and not the materials themselves are nanoscale.<ref name=":1"/> Although the ISO definition only considers round nano-objects to be [[nanoparticle]]s, other sources use the term nanoparticle for all shapes.<ref>{{cite book |title=Nanotechnology Standards |last1=Klaessig |first1=Fred |last2=Marrapese |first2=Martha |last3=Abe |first3=Shuji |date=2011 |publisher=Springer, New York, NY |isbn=9781441978523 |series=Nanostructure Science and Technology |pages=21–52 |language=en |doi=10.1007/978-1-4419-7853-0_2}}</ref> |
||
===Nanoparticles=== |
===Nanoparticles=== |
||
{{Main|Nanoparticle}} |
{{Main|Nanoparticle}} |
||
Nanoparticles have all three dimensions on the nanoscale. Nanoparticles can also be embedded in a bulk solid to form a nanocomposite.<ref name=":1">{{cite web |url=http://nanoparticles.org/pdf/nanometrology.pdf |title=Eighth Nanoforum Report: Nanometrology |date=July 2006 |website=Nanoforum |pages=13–14}}</ref> |
Nanoparticles have all three dimensions on the nanoscale. Nanoparticles can also be embedded in a bulk solid to form a nanocomposite.<ref name=":1">{{cite web |url=http://nanoparticles.org/pdf/nanometrology.pdf |title=Eighth Nanoforum Report: Nanometrology |date=July 2006 |website=Nanoforum |pages=13–14 |access-date=28 August 2017 |archive-date=20 October 2007 |archive-url=https://web.archive.org/web/20071020124419/http://nanoparticles.org/pdf/nanometrology.pdf |url-status=dead }}</ref> |
||
====Fullerenes==== |
====Fullerenes==== |
||
| Line 75: | Line 75: | ||
}}</ref> More recently, fullerenes have been detected in outer space.<ref>{{cite journal |doi=10.1126/science.1192035 |title=Detection of C<sub>60</sub> and C<sub>70</sub> in a Young Planetary Nebula |last1=Cami |first1=J |last2=Bernard-Salas |first2=J. |last3=Peeters |first3=E. |last4=Malek |first4=S. E. |date=2 September 2010 |journal=[[Science (journal)|Science]] |volume=329 |pmid=20651118 |bibcode=2010Sci...329.1180C |issue=5996 |pages=1180–2 |s2cid=33588270 |url=http://pdfs.semanticscholar.org/c14b/a4455e17f5045539de4a2d78bcf19825ce38.pdf |archive-url=https://web.archive.org/web/20190303024856/http://pdfs.semanticscholar.org/c14b/a4455e17f5045539de4a2d78bcf19825ce38.pdf |url-status=dead |archive-date=3 March 2019}}</ref> |
}}</ref> More recently, fullerenes have been detected in outer space.<ref>{{cite journal |doi=10.1126/science.1192035 |title=Detection of C<sub>60</sub> and C<sub>70</sub> in a Young Planetary Nebula |last1=Cami |first1=J |last2=Bernard-Salas |first2=J. |last3=Peeters |first3=E. |last4=Malek |first4=S. E. |date=2 September 2010 |journal=[[Science (journal)|Science]] |volume=329 |pmid=20651118 |bibcode=2010Sci...329.1180C |issue=5996 |pages=1180–2 |s2cid=33588270 |url=http://pdfs.semanticscholar.org/c14b/a4455e17f5045539de4a2d78bcf19825ce38.pdf |archive-url=https://web.archive.org/web/20190303024856/http://pdfs.semanticscholar.org/c14b/a4455e17f5045539de4a2d78bcf19825ce38.pdf |url-status=dead |archive-date=3 March 2019}}</ref> |
||
For the past decade, the chemical and physical properties of fullerenes have been a hot topic in the field of research and development, and are likely to continue to be for a long time. In April |
For the past decade, the chemical and physical properties of fullerenes have been a hot topic in the field of research and development, and are likely to continue to be for a long time. In April 2003, fullerenes were under study for [[nanomedicine|potential medicinal use]]: binding specific [[antibiotic]]s to the structure of resistant [[bacterium|bacteria]] and even target certain types of [[cancer]] cells such as [[melanoma]]. The October 2005 issue of Chemistry and Biology contains an article describing the use of fullerenes as light-activated [[antimicrobial]] agents. In the field of [[nanotechnology]], heat resistance and [[superconductivity]] are among the |
||
properties attracting intense research. |
properties attracting intense research. |
||
| Line 83: | Line 83: | ||
====Metal-based nanoparticles==== |
====Metal-based nanoparticles==== |
||
Inorganic nanomaterials, (e.g. [[quantum dot]]s, [[nanowire]]s, and [[nanorod]]s) because of their interesting [[optical]] and electrical properties, could be used in [[optoelectronics]].<ref>{{cite journal |last1=Zeng |first1=S. |last2=Baillargeat |first2=Dominique |last3=Ho |first3=Ho-Pui |last4=Yong |first4=Ken-Tye |date=2014 |title=Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications |journal=Chemical Society Reviews |volume=43 |issue=10 |pages=3426–3452 |doi=10.1039/C3CS60479A |pmid=24549396 |hdl=10356/102043 |hdl-access=free}}</ref> Furthermore, the optical and electronic properties of nanomaterials which depend on their size and shape can be tuned via synthetic techniques. There are the possibilities to use those materials in organic material based optoelectronic devices such as [[organic solar cells]], [[OLEDs]] etc. The operating principles of such devices are governed by photoinduced processes like [[photoinduced electron transfer|electron transfer]] and energy transfer. The performance of the devices depends on the efficiency of the photoinduced process responsible for their functioning. Therefore, better understanding of those photoinduced processes in organic/inorganic nanomaterial composite systems is necessary in order to use them in optoelectronic devices. |
Inorganic nanomaterials, (e.g. [[quantum dot]]s,<ref>{{Cite journal |last1=Shishodia |first1=Shubham |last2=Chouchene |first2=Bilel |last3=Gries |first3=Thomas |last4=Schneider |first4=Raphaël |date=2023-10-31 |title=Selected I-III-VI2 Semiconductors: Synthesis, Properties and Applications in Photovoltaic Cells |journal=Nanomaterials |language=en |volume=13 |issue=21 |pages=2889 |doi=10.3390/nano13212889 |issn=2079-4991 |doi-access=free |pmid=37947733 |pmc=10648425 }}</ref> [[nanowire]]s, and [[nanorod]]s) because of their interesting [[optical]] and electrical properties, could be used in [[optoelectronics]].<ref>{{cite journal |last1=Zeng |first1=S. |last2=Baillargeat |first2=Dominique |last3=Ho |first3=Ho-Pui |last4=Yong |first4=Ken-Tye |date=2014 |title=Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications |journal=Chemical Society Reviews |volume=43 |issue=10 |pages=3426–3452 |doi=10.1039/C3CS60479A |pmid=24549396 |hdl=10356/102043 |hdl-access=free}}</ref> Furthermore, the optical and electronic properties of nanomaterials which depend on their size and shape can be tuned via synthetic techniques. There are the possibilities to use those materials in organic material based optoelectronic devices such as [[organic solar cells]], [[OLEDs]] etc. The operating principles of such devices are governed by photoinduced processes like [[photoinduced electron transfer|electron transfer]] and energy transfer. The performance of the devices depends on the efficiency of the photoinduced process responsible for their functioning. Therefore, better understanding of those photoinduced processes in organic/inorganic nanomaterial composite systems is necessary in order to use them in optoelectronic devices. |
||
Nanoparticles or |
Nanoparticles or nanocrystals made of metals, semiconductors, or oxides are of particular interest for their mechanical, electrical, magnetic, optical, chemical and other properties.<ref>{{cite journal |last1=Stephenson |first1=C. |last2=Hubler |first2=A. |date=2015 |title=Stability and conductivity of self assembled wires in a transverse electric field |journal=Sci. Rep. |volume=5 |pages=15044 |doi=10.1038/srep15044 |pmid=26463476 |pmc=4604515 |bibcode=2015NatSR...515044S}}</ref><ref>{{cite journal |last1=Hubler |first1=A. |last2=Lyon |first2=D. |date=2013 |title=Gap size dependence of the dielectric strength in nano vacuum gaps |journal=[[IEEE Transactions on Dielectrics and Electrical Insulation]] |volume=20 |issue=4 |pages=1467–1471 |doi=10.1109/TDEI.2013.6571470 |s2cid=709782}}</ref> Nanoparticles have been used as [[quantum dot]]s and as chemical [[catalyst]]s such as [[nanomaterial-based catalyst]]s. Recently, a range of nanoparticles are extensively investigated for [[biomedical]] applications including [[tissue engineering]], [[drug delivery]], [[biosensor]].<ref>{{cite journal |vauthors=Valenti G, Rampazzo R, Bonacchi S, Petrizza L, Marcaccio M, Montalti M, Prodi L, Paolucci F |title=Variable Doping Induces Mechanism Swapping in Electrogenerated Chemiluminescence of Ru(bpy)32+ Core−Shell Silica Nanoparticles |journal=J. Am. Chem. Soc. |year=2016 |pages=15935–15942 |volume=138 |issue=49 |doi=10.1021/jacs.6b08239 |pmid=27960352 |hdl=11585/583548 |hdl-access=free}}</ref><ref>{{cite journal |last1=Kerativitayanan |first1=P |last2=Carrow |first2=JK |last3=Gaharwar |first3=AK |date=26 May 2015 |title=Nanomaterials for Engineering Stem Cell Responses |journal=Advanced Healthcare Materials |volume=4 |issue=11 |pages=1600–27 |doi=10.1002/adhm.201500272 |pmid=26010739 |s2cid=21582516}}</ref> |
||
Nanoparticles are of great scientific interest as they are effectively a bridge between bulk materials and [[atom]]ic or [[molecular]] structures. A bulk material should have constant physical properties regardless of its size, but at the nano-scale this is often not the case. Size-dependent properties are observed such as [[quantum confinement]] in [[semiconductor]] particles, [[surface plasmon resonance]] in some metal particles, and [[superparamagnetism]] in [[magnetic]] materials. |
Nanoparticles are of great scientific interest as they are effectively a bridge between bulk materials and [[atom]]ic or [[molecular]] structures. A bulk material should have constant physical properties regardless of its size, but at the nano-scale this is often not the case. Size-dependent properties are observed such as [[quantum confinement]] in [[semiconductor]] particles, [[surface plasmon resonance]] in some metal particles, and [[superparamagnetism]] in [[magnetic]] materials. |
||
| Line 108: | Line 108: | ||
{{Main|Applications of nanotechnology}} |
{{Main|Applications of nanotechnology}} |
||
Nano materials are used in a variety of, manufacturing processes, products and healthcare including paints, filters, insulation and lubricant additives. In healthcare [[artificial enzyme#Nanozymes|Nanozymes]] are nanomaterials with enzyme-like characteristics.<ref>{{cite journal |last1=Wei |first1=Hui |last2=Wang |first2=Erkang |date=2013-06-21 |title=Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes |journal=Chemical Society Reviews |volume=42 |issue=14 |doi=10.1039/C3CS35486E |pmid=23740388 |pages=6060–93}}</ref> They are an emerging type of [[artificial enzyme]], which have been used for wide applications in such as biosensing, bioimaging, tumor diagnosis,<ref>{{cite journal |title=Highly sensitive electrochemiluminescence detection of a prostate cancer biomarker |year=2017 |last1=Juzgado |first1=A. |last2=Solda |first2=A. |last3=Ostric |first3=A. |last4=Criado |first4=A. |last5=Valenti |first5=G. |last6=Rapino |first6=S. |last7=Conti |first7=G. |last8=Fracasso |first8=G. |last9=Paolucci |first9=F. |last10=Prato |first10=M. |journal=J. Mater. Chem. B |volume=5 |issue=32 |pages=6681–6687 |doi=10.1039/c7tb01557g |pmid=32264431}}</ref> antibiofouling and more. High quality filters may be produced using nanostructures, these filters are capable of removing particulate as small as a virus as seen in a water filter created by Seldon Technologies. Nanomaterials membrane bioreactor (NMs-MBR), the next generation of conventional [[membrane bioreactor|MBR]], are recently proposed for the advanced treatment of wastewater.<ref>{{cite journal |last1=Pervez |first1=Md Nahid |last2=Balakrishnan |first2=Malini |last3=Hasan |first3=Shadi Wajih |last4=Choo |first4=Kwang-Ho |last5=Zhao |first5=Yaping |last6=Cai |first6=Yingjie |last7=Zarra |first7=Tiziano |last8=Belgiorno |first8=Vincenzo |last9=Naddeo |first9=Vincenzo |date=2020-11-05 |title=A critical review on nanomaterials membrane bioreactor (NMs-MBR) for wastewater treatment |journal= |
Nano materials are used in a variety of, manufacturing processes, products and healthcare including [[paints]], [[filtration|filters]], insulation and [[lubricant]] additives. In healthcare [[artificial enzyme#Nanozymes|Nanozymes]] are nanomaterials with enzyme-like characteristics.<ref>{{cite journal |last1=Wei |first1=Hui |last2=Wang |first2=Erkang |date=2013-06-21 |title=Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes |journal=Chemical Society Reviews |volume=42 |issue=14 |doi=10.1039/C3CS35486E |pmid=23740388 |pages=6060–93}}</ref> They are an emerging type of [[artificial enzyme]], which have been used for wide applications in such as biosensing, bioimaging, tumor diagnosis,<ref>{{cite journal |title=Highly sensitive electrochemiluminescence detection of a prostate cancer biomarker |year=2017 |last1=Juzgado |first1=A. |last2=Solda |first2=A. |last3=Ostric |first3=A. |last4=Criado |first4=A. |last5=Valenti |first5=G. |last6=Rapino |first6=S. |last7=Conti |first7=G. |last8=Fracasso |first8=G. |last9=Paolucci |first9=F. |last10=Prato |first10=M. |journal=J. Mater. Chem. B |volume=5 |issue=32 |pages=6681–6687 |doi=10.1039/c7tb01557g |pmid=32264431}}</ref> antibiofouling and more. High quality filters may be produced using nanostructures, these filters are capable of removing particulate as small as a virus as seen in a [[water filter]] created by Seldon Technologies. Nanomaterials membrane bioreactor (NMs-MBR), the next generation of conventional [[membrane bioreactor|MBR]], are recently proposed for the advanced treatment of wastewater.<ref>{{cite journal |last1=Pervez |first1=Md Nahid |last2=Balakrishnan |first2=Malini |last3=Hasan |first3=Shadi Wajih |last4=Choo |first4=Kwang-Ho |last5=Zhao |first5=Yaping |last6=Cai |first6=Yingjie |last7=Zarra |first7=Tiziano |last8=Belgiorno |first8=Vincenzo |last9=Naddeo |first9=Vincenzo |date=2020-11-05 |title=A critical review on nanomaterials membrane bioreactor (NMs-MBR) for wastewater treatment |journal=npj Clean Water |language=en |volume=3 |issue=1 |page=43 |doi=10.1038/s41545-020-00090-2 |issn=2059-7037 |doi-access=free|bibcode=2020npjCW...3...43P }}</ref> In the air purification field, nano technology was used to combat the spread of [[Middle East respiratory syndrome|MERS]] in Saudi Arabian hospitals in 2012.<ref name="Nanovate">{{cite book |last1=Anis |first1=Mohab |last2=AlTaher |first2=Ghada |last3=Sarhan |first3=Wesam |last4=Elsemary |first4=Mona |title=Nanovate |date=2017 |publisher=Springer |isbn=9783319448619 |page=105}}</ref> Nanomaterials are being used in modern and human-safe insulation technologies; in the past they were found in [[Asbestos]]-based insulation.<ref>{{cite web |url=http://www.asbestosindustry.asn.au/health-effects |title=Health Effects |website=Asbestos Industry Association |access-date=2017-08-28 |archive-date=9 April 2013 |archive-url=https://web.archive.org/web/20130409063318/http://www.asbestosindustry.asn.au/health-effects |url-status=dead }}</ref>{{unreliable inline|date=March 2024}} As a lubricant additive, nano materials have the ability to reduce friction in moving parts. Worn and corroded parts can also be repaired with self-assembling anisotropic nanoparticles called TriboTEX.<ref name="Nanovate"/> Nanomaterials have also been applied in a range of industries and consumer products. Mineral nanoparticles such as [[titanium-oxide]] have been used to improve UV protection in [[sunscreen]]. Phosphorus, carbon and nitrogen doped titanium-oxide nanoparticles are used as additive to water based paint for self-cleaning properties.<ref>{{Cite journal |last1=Maqbool |first1=Qaisar |last2=Favoni |first2=Orlando |last3=Wicht |first3=Thomas |last4=Lasemi |first4=Niusha |last5=Sabbatini |first5=Simona |last6=Stöger-Pollach |first6=Michael |last7=Ruello |first7=Maria Letizia |last8=Tittarelli |first8=Francesca |last9=Rupprechter |first9=Günther |date=2024-04-05 |title=Highly Stable Self-Cleaning Paints Based on Waste-Valorized PNC-Doped TiO 2 Nanoparticles |journal=ACS Catalysis |language=en |volume=14 |issue=7 |pages=4820–4834 |doi=10.1021/acscatal.3c06203 |issn=2155-5435 |pmc=11003396 |pmid=38601782}}</ref> In the sports industry, lighter bats to have been produced with carbon nanotubes to improve performance. Another application is in the military, where mobile pigment nanoparticles have been used to create more effective camouflage. Nanomaterials can also be used in three-way-catalyst applications, which have the advantage of controlling the emission of nitrogen oxides (NO<sub>x</sub>), which are precursors to acid rain and smog.<ref>{{cite journal |last1=Pham |first1=Phuong |last2=Minh |first2=Thang |last3=Nguyen |first3=Tien |last4=Van Driessche |first4=Isabel |title=Ceo2 Based Catalysts for the Treatment of Propylene in Motorcycle's Exhaust Gases |journal=Materials |date=17 November 2014 |volume=7 |issue=11 |pages=7379–7397 |doi=10.3390/ma7117379 |pmid=28788253 |pmc=5512641 |bibcode=2014Mate....7.7379P |doi-access=free}}</ref> In core-shell structure, nanomaterials form shell as the catalyst support to protect the noble metals such as palladium and rhodium.<ref>{{cite journal |last1=Kašpar |first1=Jan |last2=Fornasiero |first2=Paolo |last3=Hickey |first3=Neal |title=Automotive catalytic converters: current status and some perspectives |journal=Catalysis Today |date=January 2003 |volume=77 |issue=4 |pages=419–449 |doi=10.1016/S0920-5861(02)00384-X}}</ref> The primary function is that the supports can be used for carrying catalysts active components, making them highly dispersed, reducing the use of noble metals, enhancing catalysts activity, and potentially improving the stability.<ref name="TankardPCCP2024">{{cite journal |last1=Tankard |first1=Rikke Egeberg |last2=Romeggio |first2=Filippo |last3=Akazawa |first3=Stefan Kei |last4=Krabbe |first4=Alexander |last5=Sloth |first5=Olivia Fjord |last6=Secher |first6=Niklas Mørch |last7=Colding-Fagerholt |first7=Sofie |last8=Helveg |first8=Stig |last9=Palmer |first9=Richard |last10=Damsgaard |first10=Christian Danvad |last11=Kibsgaard |first11=Jakob |last12=Chorkendorff |first12=Ib|title=Stable mass-selected AuTiOx nanoparticles for CO oxidation |journal=Physical Chemistry Chemical Physics |date=2024 |volume=26 |issue=12 |pages=9253–9263 |doi=10.1039/D4CP00211C |doi-access=free |pmid=38445363 |bibcode=2024PCCP...26.9253T }}</ref> |
||
==Synthesis== |
==Synthesis== |
||
| Line 118: | Line 118: | ||
Chaotic processes involve elevating the constituent atoms or molecules to a chaotic state and then suddenly changing the conditions so as to make that state unstable. Through the clever manipulation of any number of parameters, products form largely as a result of the insuring kinetics. The collapse from the chaotic state can be difficult or impossible to control and so ensemble statistics often govern the resulting size distribution and average size. Accordingly, nanoparticle formation is controlled through manipulation of the end state of the products. Examples of chaotic processes are laser ablation,<ref name="Elsevier">{{cite book |last1=Wang |first1=Shujun |last2=Gao |first2=Lihong |title=Industrial Applications of Nanomaterials |date=2019 |publisher=Elsevier |isbn=978-0-12-815749-7 |pages=181–203 |chapter=Laser-driven nanomaterials and laser-enabled nanofabrication for industrial applications |doi=10.1016/B978-0-12-815749-7.00007-4 |s2cid=202212003}}</ref> exploding wire, arc, flame pyrolysis, combustion,<ref>Rawat, Pankaj Singh, R. C. Srivastava, Gagan Dixit, G. C. Joshi, and K. Asokan. "Facile synthesis and temperature dependent dielectric properties of MnFe2O4 nanoparticles." In AIP Conference Proceedings, vol. 2115, no. 1, p. 030104. AIP Publishing LLC, 2019.https://doi.org/10.1063/1.5112943</ref> and precipitation synthesis techniques. |
Chaotic processes involve elevating the constituent atoms or molecules to a chaotic state and then suddenly changing the conditions so as to make that state unstable. Through the clever manipulation of any number of parameters, products form largely as a result of the insuring kinetics. The collapse from the chaotic state can be difficult or impossible to control and so ensemble statistics often govern the resulting size distribution and average size. Accordingly, nanoparticle formation is controlled through manipulation of the end state of the products. Examples of chaotic processes are laser ablation,<ref name="Elsevier">{{cite book |last1=Wang |first1=Shujun |last2=Gao |first2=Lihong |title=Industrial Applications of Nanomaterials |date=2019 |publisher=Elsevier |isbn=978-0-12-815749-7 |pages=181–203 |chapter=Laser-driven nanomaterials and laser-enabled nanofabrication for industrial applications |doi=10.1016/B978-0-12-815749-7.00007-4 |s2cid=202212003}}</ref> exploding wire, arc, flame pyrolysis, combustion,<ref>Rawat, Pankaj Singh, R. C. Srivastava, Gagan Dixit, G. C. Joshi, and K. Asokan. "Facile synthesis and temperature dependent dielectric properties of MnFe2O4 nanoparticles." In AIP Conference Proceedings, vol. 2115, no. 1, p. 030104. AIP Publishing LLC, 2019.https://doi.org/10.1063/1.5112943</ref> and precipitation synthesis techniques. |
||
Controlled processes involve the controlled delivery of the constituent atoms or molecules to the site(s) of nanoparticle formation such that the nanoparticle can grow to a prescribed sizes in a controlled manner. Generally the state of the constituent atoms or molecules are never far from that needed for nanoparticle formation. Accordingly, nanoparticle formation is controlled through the control of the state of the reactants. Examples of controlled processes are self-limiting growth solution, [[Atomic Layer Deposition|self-limited chemical vapor deposition]], shaped pulse femtosecond laser techniques, and [[molecular beam epitaxy]]. |
Controlled processes involve the controlled delivery of the constituent atoms or molecules to the site(s) of nanoparticle formation such that the nanoparticle can grow to a prescribed sizes in a controlled manner. Generally the state of the constituent atoms or molecules are never far from that needed for nanoparticle formation. Accordingly, nanoparticle formation is controlled through the control of the state of the reactants. Examples of controlled processes are self-limiting growth solution, [[Atomic Layer Deposition|self-limited chemical vapor deposition]], shaped pulse femtosecond laser techniques, plant and microbial approaches<ref>{{Cite journal |last1=Alsaiari |first1=Norah Salem |last2=Alzahrani |first2=Fatimah Mohammed |last3=Amari |first3=Abdelfattah |last4=Osman |first4=Haitham |last5=Harharah |first5=Hamed N. |last6=Elboughdiri |first6=Noureddine |last7=Tahoon |first7=Mohamed A. |date=January 2023 |title=Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives |journal=Molecules |language=en |volume=28 |issue=1 |pages=463 |doi=10.3390/molecules28010463 |pmid=36615655 |pmc=9823860 |issn=1420-3049 |doi-access=free }}</ref> and [[molecular beam epitaxy]]. |
||
===Top-down methods=== |
===Top-down methods=== |
||
Top-down methods adopt some 'force' (e. g. mechanical force, laser) to break bulk materials into nanoparticles. A popular method involves mechanical break apart bulk materials into nanomaterials is 'ball milling'. Besides, nanoparticles can also be made by laser ablation which apply short pulse lasers (e. g. femtosecond laser) to ablate a target (solid).<ref name="Elsevier"/> |
Top-down methods adopt some 'force' (e. g. mechanical force, laser) to break bulk materials into nanoparticles. A popular method involves mechanical break apart bulk materials into nanomaterials is 'ball milling'. Besides that, nanoparticles can also be made by laser ablation which apply short pulse lasers (e. g. femtosecond laser) to ablate a target (solid).<ref name="Elsevier"/> |
||
==Characterization== |
==Characterization== |
||
| Line 139: | Line 139: | ||
There is also a group of traditional techniques for characterizing [[surface charge]] or [[zeta potential]] of nano-particles in solutions. This information is required for proper system stabilization, preventing its aggregation or [[flocculation]]. These methods include [[microelectrophoresis]], [[electrophoretic light scattering]], and [[acoustical engineering|electroacoustics]]. The last one, for instance [[colloid vibration current]] method is suitable for characterizing concentrated systems. |
There is also a group of traditional techniques for characterizing [[surface charge]] or [[zeta potential]] of nano-particles in solutions. This information is required for proper system stabilization, preventing its aggregation or [[flocculation]]. These methods include [[microelectrophoresis]], [[electrophoretic light scattering]], and [[acoustical engineering|electroacoustics]]. The last one, for instance [[colloid vibration current]] method is suitable for characterizing concentrated systems. |
||
==Mechanical |
==Mechanical properties== |
||
The ongoing research has shown that mechanical properties can vary significantly in nanomaterials compared to bulk material. Nanomaterials have substantial mechanical properties due to the volume, surface, and quantum effects of nanoparticles. This is observed when the nanoparticles are added to common bulk material, the nanomaterial refines the grain and forms intergranular and intragranular structures which improve the grain boundaries and therefore the mechanical properties of the materials.{{ |
The ongoing research has shown that mechanical properties can vary significantly in nanomaterials compared to bulk material. Nanomaterials have substantial mechanical properties due to the volume, surface, and quantum effects of nanoparticles. This is observed when the nanoparticles are added to common bulk material, the nanomaterial refines the grain and forms intergranular and intragranular structures which improve the grain boundaries and therefore the mechanical properties of the materials.{{citation needed|date=April 2023}} Grain boundary refinements provide strengthening by increasing the stress required to cause intergranular or transgranular fractures. A common example where this can be observed is the addition of nano Silica to cement, which improves the tensile strength, compressive strength, and bending strength by the mechanisms just mentioned. The understanding of these properties will enhance the use of nanoparticles in novel applications in various fields such as surface engineering, tribology, nanomanufacturing, and nanofabrication. |
||
'''Techniques used:''' |
'''Techniques used:''' |
||
| Line 152: | Line 152: | ||
ẟc0 : deflection ofset |
ẟc0 : deflection ofset |
||
AFM allows us to obtain a high-resolution image of multiple types of surfaces while the tip of the cantilever can be used to obtain information about mechanical properties. Computer simulations are also being progressively used to test theories and complement experimental studies. The most used computer method is molecular dynamics simulation,<ref>{{cite journal |last1=Luan |first1=Binquan |last2=Robbins |first2=Mark O. |date=June 2005 |title=The breakdown of continuum models for mechanical contacts |url=http://dx.doi.org/10.1038/nature03700 |journal=Nature |volume=435 |issue=7044 |pages=929–932 |doi=10.1038/nature03700 |pmid=15959512 |s2cid=4398925 |issn=0028-0836}}</ref> which uses newton's equations of motion for the atoms or molecules in the system. Other techniques such direct probe method are used to determine the adhesive properties of nanomaterials. Both the technique and simulation are coupled with transmission electron microscope (TEM) and AFM techniques to provide results. |
AFM allows us to obtain a high-resolution image of multiple types of surfaces while the tip of the cantilever can be used to obtain information about mechanical properties. Computer simulations are also being progressively used to test theories and complement experimental studies. The most used computer method is molecular dynamics simulation,<ref>{{cite journal |last1=Luan |first1=Binquan |last2=Robbins |first2=Mark O. |date=June 2005 |title=The breakdown of continuum models for mechanical contacts |url=http://dx.doi.org/10.1038/nature03700 |journal=Nature |volume=435 |issue=7044 |pages=929–932 |doi=10.1038/nature03700 |pmid=15959512 |bibcode=2005Natur.435..929L |s2cid=4398925 |issn=0028-0836}}</ref> which uses newton's equations of motion for the atoms or molecules in the system. Other techniques such direct probe method are used to determine the adhesive properties of nanomaterials. Both the technique and simulation are coupled with transmission electron microscope (TEM) and AFM techniques to provide results. |
||
'''Mechanical properties of common nanomaterials classes:''' |
'''Mechanical properties of common nanomaterials classes:''' |
||
'''Crystalline metal nanomaterials''': Dislocations are one of the major contributors toward elastic properties within nanomaterials similar to bulk crystalline materials. Despite the traditional view of there being no dislocations in nanomaterials. Ramos,<ref>{{cite journal |last1=Ramos |first1=Manuel |last2=Ortiz-Jordan |first2=Luis |last3=Hurtado-Macias |first3=Abel |last4=Flores |first4=Sergio |last5=Elizalde-Galindo |first5=José |last6=Rocha |first6=Carmen |last7=Torres |first7=Brenda |last8=Zarei-Chaleshtori |first8=Maryam |last9=Chianelli |first9=Russell |date=2013-01-14 |title=Hardness and Elastic Modulus on Six-Fold Symmetry Gold Nanoparticles |journal=Materials |volume=6 |issue=1 |pages=198–205 |doi=10.3390/ma6010198 |pmid=28809302 |pmc=5452105 |issn=1996-1944 |doi-access=free}}</ref> experimental work has shown that the hardness of gold nanoparticles is much higher than their bulk counterparts, as there are stacking faults and dislocations forming that activate multiple strengthening mechanisms in the material. Through these experiments, more research has shown that via nanoindentation techniques,<ref>{{cite journal |last1=Mordehai |first1=Dan |last2=Lee |first2=Seok-Woo |last3=Backes |first3=Björn |last4=Srolovitz |first4=David J. |last5=Nix |first5=William D. |last6=Rabkin |first6=Eugen |date=August 2011 |title=Size effect in compression of single-crystal gold microparticles |url=http://dx.doi.org/10.1016/j.actamat.2011.04.057 |journal=Acta Materialia |volume=59 |issue=13 |pages=5202–5215 |doi=10.1016/j.actamat.2011.04.057 |issn=1359-6454}}</ref> material strength; compressive stress, increases under compression with decreasing particle size, because of nucleating dislocations. These dislocations have been observed using TEM techniques, coupled with nanoindentation. Silicon nanoparticles strength and hardness are four times more than the value of the bulk material.<ref name="Carlton 1134–1139"/> The resistance to pressure applied can be attributed to the line defects inside the particles as well as a dislocation that provides strengthening of the mechanical properties of the nanomaterial. Furthermore, the addition of nanoparticles strengthens a matrix because the pinning of particles inhibits grain growth. This refines the grain, and hence improves the mechanical properties.<ref name="Borisenko 455–459"/> However, not all additions of nanomaterials lead to an increase in properties for example nano-Cu. But this is attributed to the inherent properties of the material being weaker than the matrix. |
'''Crystalline metal nanomaterials''': Dislocations are one of the major contributors toward elastic properties within nanomaterials similar to bulk crystalline materials. Despite the traditional view of there being no dislocations in nanomaterials. Ramos,<ref>{{cite journal |last1=Ramos |first1=Manuel |last2=Ortiz-Jordan |first2=Luis |last3=Hurtado-Macias |first3=Abel |last4=Flores |first4=Sergio |last5=Elizalde-Galindo |first5=José |last6=Rocha |first6=Carmen |last7=Torres |first7=Brenda |last8=Zarei-Chaleshtori |first8=Maryam |last9=Chianelli |first9=Russell |date=2013-01-14 |title=Hardness and Elastic Modulus on Six-Fold Symmetry Gold Nanoparticles |journal=Materials |volume=6 |issue=1 |pages=198–205 |doi=10.3390/ma6010198 |pmid=28809302 |pmc=5452105 |bibcode=2013Mate....6..198R |issn=1996-1944 |doi-access=free}}</ref> experimental work has shown that the hardness of gold nanoparticles is much higher than their bulk counterparts, as there are stacking faults and dislocations forming that activate multiple strengthening mechanisms in the material. Through these experiments, more research has shown that via nanoindentation techniques,<ref>{{cite journal |last1=Mordehai |first1=Dan |last2=Lee |first2=Seok-Woo |last3=Backes |first3=Björn |last4=Srolovitz |first4=David J. |last5=Nix |first5=William D. |last6=Rabkin |first6=Eugen |date=August 2011 |title=Size effect in compression of single-crystal gold microparticles |url=http://dx.doi.org/10.1016/j.actamat.2011.04.057 |journal=Acta Materialia |volume=59 |issue=13 |pages=5202–5215 |doi=10.1016/j.actamat.2011.04.057 |bibcode=2011AcMat..59.5202M |issn=1359-6454}}</ref> material strength; compressive stress, increases under compression with decreasing particle size, because of nucleating dislocations. These dislocations have been observed using TEM techniques, coupled with nanoindentation. Silicon nanoparticles strength and hardness are four times more than the value of the bulk material.<ref name="Carlton 1134–1139"/> The resistance to pressure applied can be attributed to the line defects inside the particles as well as a dislocation that provides strengthening of the mechanical properties of the nanomaterial. Furthermore, the addition of nanoparticles strengthens a matrix because the pinning of particles inhibits grain growth. This refines the grain, and hence improves the mechanical properties.<ref name="Borisenko 455–459"/> However, not all additions of nanomaterials lead to an increase in properties for example nano-Cu. But this is attributed to the inherent properties of the material being weaker than the matrix. |
||
'''Nonmetallic nanoparticles and nanomaterials:''' Size-dependent behavior of mechanical properties is still not clear in the case of polymer nanomaterials however, in one research by Lahouij they found that the compressive moduli of polystyrene nanoparticles were found to be less than that of the bulk counterparts. This can be associated with the functional groups being hydrated.<ref name="Tan 7015–7020">{{cite journal |last1=Tan |first1=Susheng |last2=Sherman |first2=Robert L. |last3=Ford |first3=Warren T. |date=2004-07-21 |title=Nanoscale Compression of Polymer Microspheres by Atomic Force Microscopy |url=http://dx.doi.org/10.1021/la049597c |journal=Langmuir |volume=20 |issue=17 |pages=7015–7020 |doi=10.1021/la049597c |pmid=15301482 |issn=0743-7463}}</ref> Furthermore, nonmetallic nanomaterials can lead to agglomerates forming inside the matrix they are being added to and hence decrease the mechanical properties by leading to fracture under even low mechanical loads, such as the addition of CNTs. The agglomerates will act as slip planes as well as planes in which cracks can easily propagate (9). However, most organic nanomaterials are flexible and these and the mechanical properties such as hardness etc. are not dominant.<ref name="Tan 7015–7020"/> |
'''Nonmetallic nanoparticles and nanomaterials:''' Size-dependent behavior of mechanical properties is still not clear in the case of polymer nanomaterials however, in one research by Lahouij they found that the compressive moduli of polystyrene nanoparticles were found to be less than that of the bulk counterparts. This can be associated with the functional groups being hydrated.<ref name="Tan 7015–7020">{{cite journal |last1=Tan |first1=Susheng |last2=Sherman |first2=Robert L. |last3=Ford |first3=Warren T. |date=2004-07-21 |title=Nanoscale Compression of Polymer Microspheres by Atomic Force Microscopy |url=http://dx.doi.org/10.1021/la049597c |journal=Langmuir |volume=20 |issue=17 |pages=7015–7020 |doi=10.1021/la049597c |pmid=15301482 |issn=0743-7463}}</ref> Furthermore, nonmetallic nanomaterials can lead to agglomerates forming inside the matrix they are being added to and hence decrease the mechanical properties by leading to fracture under even low mechanical loads, such as the addition of CNTs. The agglomerates will act as slip planes as well as planes in which cracks can easily propagate (9). However, most organic nanomaterials are flexible and these and the mechanical properties such as hardness etc. are not dominant.<ref name="Tan 7015–7020"/> |
||
'''Nanowires and nanotubes''': The elastic moduli of some nanowires namely lead and silver, decrease with increasing diameter. This has been associated with surface stress, oxidation layer, and surface roughness.<ref>{{cite journal |last1=Jing |first1=G. Y. |last2=Duan |first2=H. L. |last3=Sun |first3=X. M. |last4=Zhang |first4=Z. S. |last5=Xu |first5=J. |last6=Li |first6=Y. D. |last7=Wang |first7=J. X. |last8=Yu |first8=D. P. |date=2006-06-13 |title=Surface effects on elastic properties of silver nanowires: Contact atomic-force microscopy |url=http://dx.doi.org/10.1103/physrevb.73.235409 |journal=Physical Review B |volume=73 |issue=23 |doi=10.1103/physrevb.73.235409 |issn=1098-0121}}</ref> However, the elastic behavior of ZnO nanowires does not get affected by surface effects but their fracture properties do. So, it is generally dependent on material behavior and their bonding as well.<ref>{{cite journal |last1=Jing |first1=Guangyin |last2=Zhang |first2=Xinzheng |last3=Yu |first3=Dapeng |date=2010-05-18 |title=Effect of surface morphology on the mechanical properties of ZnO nanowires |url=http://dx.doi.org/10.1007/s00339-010-5736-7 |journal=Applied Physics A |volume=100 |issue=2 |pages=473–478 |doi=10.1007/s00339-010-5736-7 |s2cid=95077632 |issn=0947-8396}}</ref> |
'''Nanowires and nanotubes''': The elastic moduli of some nanowires namely lead and silver, decrease with increasing diameter. This has been associated with surface stress, oxidation layer, and surface roughness.<ref>{{cite journal |last1=Jing |first1=G. Y. |last2=Duan |first2=H. L. |last3=Sun |first3=X. M. |last4=Zhang |first4=Z. S. |last5=Xu |first5=J. |last6=Li |first6=Y. D. |last7=Wang |first7=J. X. |last8=Yu |first8=D. P. |date=2006-06-13 |title=Surface effects on elastic properties of silver nanowires: Contact atomic-force microscopy |url=http://dx.doi.org/10.1103/physrevb.73.235409 |journal=Physical Review B |volume=73 |issue=23 |page=235409 |doi=10.1103/physrevb.73.235409 |bibcode=2006PhRvB..73w5409J |issn=1098-0121}}</ref> However, the elastic behavior of ZnO nanowires does not get affected by surface effects but their fracture properties do. So, it is generally dependent on material behavior and their bonding as well.<ref>{{cite journal |last1=Jing |first1=Guangyin |last2=Zhang |first2=Xinzheng |last3=Yu |first3=Dapeng |date=2010-05-18 |title=Effect of surface morphology on the mechanical properties of ZnO nanowires |url=http://dx.doi.org/10.1007/s00339-010-5736-7 |journal=Applied Physics A |volume=100 |issue=2 |pages=473–478 |doi=10.1007/s00339-010-5736-7 |bibcode=2010ApPhA.100..473J |s2cid=95077632 |issn=0947-8396}}</ref> |
||
The reason why mechanical properties of nanomaterials are still a hot topic for research is that measuring the mechanical properties of individual nanoparticles is a complicated method, involving multiple control factors. Nonetheless, Atomic force microscopy has been widely used to measure the mechanical properties of nanomaterials. |
The reason why mechanical properties of nanomaterials are still a hot topic for research is that measuring the mechanical properties of individual nanoparticles is a complicated method, involving multiple control factors. Nonetheless, Atomic force microscopy has been widely used to measure the mechanical properties of nanomaterials. |
||
| Line 166: | Line 166: | ||
'''Adhesion and friction of nanoparticles''' |
'''Adhesion and friction of nanoparticles''' |
||
When talking about the application of a material adhesion and friction play a critical role in determining the outcome of the application. Therefore, it is critical to see how these properties also get affected by the size of a material. Again, AFM is a technique most used to measure these properties and to determine the adhesive strength of nanoparticles to any solid surface, along with the colloidal probe technique and other chemical properties.<ref>{{cite journal |last1=Mate |first1=C. Mathew |last2=McClelland |first2=Gary M. |last3=Erlandsson |first3=Ragnar |last4=Chiang |first4=Shirley |date=1987-10-26 |title=Atomic-scale friction of a tungsten tip on a graphite surface |url=http://dx.doi.org/10.1103/physrevlett.59.1942 |journal=Physical Review Letters |volume=59 |issue=17 |pages=1942–1945 |doi=10.1103/physrevlett.59.1942 |pmid=10035374 |issn=0031-9007}}</ref> Furthermore, the forces playing a role in providing these adhesive properties to nanomaterials are either the electrostatic forces, VdW, capillary forces, solvation forces, structure force, etc. It has been found that the addition of nanomaterials in bulk materials substantially increases their adhesive capabilities by increasing their strength through various bonding mechanisms.<ref>{{cite journal |last1=Lee |first1=Chang-Gun |last2=Hwang |first2=Yu-Jin |last3=Choi |first3=Young-Min |last4=Lee |first4=Jae-Keun |last5=Choi |first5=Cheol |last6=Oh |first6=Je-Myung |date=January 2009 |title=A study on the tribological characteristics of graphite nano lubricants |url=http://dx.doi.org/10.1007/s12541-009-0013-4 |journal=International Journal of Precision Engineering and Manufacturing |volume=10 |issue=1 |pages=85–90 |doi=10.1007/s12541-009-0013-4 |s2cid=135542937 |issn=1229-8557}}</ref> Nanomaterials dimension approaches zero, which means that the fraction of the particle's surface to overall atoms increases. |
When talking about the application of a material adhesion and friction play a critical role in determining the outcome of the application. Therefore, it is critical to see how these properties also get affected by the size of a material. Again, AFM is a technique most used to measure these properties and to determine the adhesive strength of nanoparticles to any solid surface, along with the colloidal probe technique and other chemical properties.<ref>{{cite journal |last1=Mate |first1=C. Mathew |last2=McClelland |first2=Gary M. |last3=Erlandsson |first3=Ragnar |last4=Chiang |first4=Shirley |date=1987-10-26 |title=Atomic-scale friction of a tungsten tip on a graphite surface |url=http://dx.doi.org/10.1103/physrevlett.59.1942 |journal=Physical Review Letters |volume=59 |issue=17 |pages=1942–1945 |doi=10.1103/physrevlett.59.1942 |pmid=10035374 |bibcode=1987PhRvL..59.1942M |issn=0031-9007}}</ref> Furthermore, the forces playing a role in providing these adhesive properties to nanomaterials are either the electrostatic forces, VdW, capillary forces, solvation forces, structure force, etc. It has been found that the addition of nanomaterials in bulk materials substantially increases their adhesive capabilities by increasing their strength through various bonding mechanisms.<ref>{{cite journal |last1=Lee |first1=Chang-Gun |last2=Hwang |first2=Yu-Jin |last3=Choi |first3=Young-Min |last4=Lee |first4=Jae-Keun |last5=Choi |first5=Cheol |last6=Oh |first6=Je-Myung |date=January 2009 |title=A study on the tribological characteristics of graphite nano lubricants |url=http://dx.doi.org/10.1007/s12541-009-0013-4 |journal=International Journal of Precision Engineering and Manufacturing |volume=10 |issue=1 |pages=85–90 |doi=10.1007/s12541-009-0013-4 |s2cid=135542937 |issn=1229-8557}}</ref> Nanomaterials dimension approaches zero, which means that the fraction of the particle's surface to overall atoms increases. |
||
Along with surface effects, the movement of nanoparticles also plays a role in dictating their mechanical properties such as shearing capabilities. The movement of particles can be observed under TEM. For example, the movement behavior of MoS2 <ref>{{cite journal |last1=Lahouij |first1=Imène |last2=Dassenoy |first2=Fabrice |last3=de Knoop |first3=Ludvig |last4=Martin |first4=Jean-Michel |last5=Vacher |first5=Béatrice |date=2011-02-04 |title=In Situ TEM Observation of the Behavior of an Individual Fullerene-Like MoS2 Nanoparticle in a Dynamic Contact |url=http://dx.doi.org/10.1007/s11249-011-9755-0 |journal=Tribology Letters |volume=42 |issue=2 |pages=133–140 |doi=10.1007/s11249-011-9755-0 |s2cid=138069848 |issn=1023-8883}}</ref> nanoparticles dynamic contact was directly observed in situ which led to the conclusion that fullerenes can shear via rolling or sliding. However, observing these properties is again a very complicated process due to multiple contributing factors. |
Along with surface effects, the movement of nanoparticles also plays a role in dictating their mechanical properties such as shearing capabilities. The movement of particles can be observed under TEM. For example, the movement behavior of MoS2 <ref>{{cite journal |last1=Lahouij |first1=Imène |last2=Dassenoy |first2=Fabrice |last3=de Knoop |first3=Ludvig |last4=Martin |first4=Jean-Michel |last5=Vacher |first5=Béatrice |date=2011-02-04 |title=In Situ TEM Observation of the Behavior of an Individual Fullerene-Like MoS2 Nanoparticle in a Dynamic Contact |url=http://dx.doi.org/10.1007/s11249-011-9755-0 |journal=Tribology Letters |volume=42 |issue=2 |pages=133–140 |doi=10.1007/s11249-011-9755-0 |s2cid=138069848 |issn=1023-8883}}</ref> nanoparticles dynamic contact was directly observed in situ which led to the conclusion that fullerenes can shear via rolling or sliding. However, observing these properties is again a very complicated process due to multiple contributing factors. |
||
Applications specific to Mechanical Properties:<ref>{{cite journal |last1=Guo |first1=Dan |last2=Xie |first2=Guoxin |last3=Luo |first3=Jianbin |date=2013-12-03 |title=Mechanical properties of nanoparticles: basics and applications |
Applications specific to Mechanical Properties:<ref>{{cite journal |last1=Guo |first1=Dan |last2=Xie |first2=Guoxin |last3=Luo |first3=Jianbin |date=2013-12-03 |title=Mechanical properties of nanoparticles: basics and applications |journal=Journal of Physics D: Applied Physics |volume=47 |issue=1 |pages=013001 |doi=10.1088/0022-3727/47/1/013001 |s2cid=4778703 |issn=0022-3727|doi-access=free }}</ref> |
||
* Lubrication |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
==Uniformity== |
==Uniformity== |
||
The chemical processing and synthesis of high performance technological components for the private, industrial and military sectors requires the use of high purity [[ceramic materials|ceramics]], [[polymer]]a, [[glass-ceramic]]s, and [[composite material]]s. In condensed bodies formed from fine powders, the irregular sizes and shapes of [[nanoparticles]] in a typical powder often lead to non-uniform packing morphologies that result in packing density variations in the powder compact. |
The chemical processing and synthesis of high performance technological components for the private, industrial and military sectors requires the use of high purity [[ceramic materials|ceramics]], [[polymer]]a, [[glass-ceramic]]s, and [[composite material]]s. In condensed bodies formed from fine powders, the irregular sizes and shapes of [[nanoparticles]] in a typical powder often lead to non-uniform packing morphologies that result in packing density variations in the powder compact. |
||
Uncontrolled [[flocculation|agglomeration]] of powders due to [[force|attractive]] [[van der Waals forces]] can also give rise to in microstructural inhomogeneities. Differential stresses that develop as a result of non-uniform drying shrinkage are directly related to the rate at which the [[solvent]] can be removed, and thus highly dependent upon the distribution of [[porosity]]. Such stresses have been associated with a plastic-to-brittle transition in consolidated bodies, and can yield to [[crack propagation]] in the unfired body if not relieved.<ref>{{cite book |editor=Onoda, G.Y. Jr. |editor2=Hench, L.L. |title=Ceramic Processing Before Firing |publisher=Wiley & Sons |place=New York |date=1979 |isbn=978-0-471-65410-0}}</ref><ref>{{cite journal |author=Aksay, I.A. |author2=Lange, F.F. |author3=Davis, B.I. |journal=J. Am. Ceram. Soc. |volume=66 |page=C–190 |date=1983 |doi=10.1111/j.1151-2916.1983.tb10550.x |title=Uniformity of Al<sub>2</sub>O<sub>3</sub>-ZrO<sub>2</sub> Composites by Colloidal Filtration |issue=10}}</ref> |
Uncontrolled [[flocculation|agglomeration]] of powders due to [[force|attractive]] [[van der Waals forces]] can also give rise to in microstructural inhomogeneities. Differential stresses that develop as a result of non-uniform drying shrinkage are directly related to the rate at which the [[solvent]] can be removed, and thus highly dependent upon the distribution of [[porosity]]. Such stresses have been associated with a plastic-to-brittle transition in consolidated bodies, and can yield to [[crack propagation]] in the unfired body if not relieved.<ref>{{cite book |editor=Onoda, G.Y. Jr. |editor2=Hench, L.L. |title=Ceramic Processing Before Firing |publisher=Wiley & Sons |place=New York |date=1979 |isbn=978-0-471-65410-0}}</ref><ref>{{cite journal |author=Aksay, I.A. |author2=Lange, F.F. |author3=Davis, B.I. |journal=J. Am. Ceram. Soc. |volume=66 |page=C–190 |date=1983 |doi=10.1111/j.1151-2916.1983.tb10550.x |title=Uniformity of Al<sub>2</sub>O<sub>3</sub>-ZrO<sub>2</sub> Composites by Colloidal Filtration |issue=10}}</ref><ref>{{cite journal |author=Franks, G.V. |author2=Lange, F.F. |name-list-style=amp |journal=J. Am. Ceram. Soc. |volume=79 |pages=3161–3168 |date=1996 |doi=10.1111/j.1151-2916.1996.tb08091.x |title=Plastic-to-Brittle Transition of Saturated, Alumina Powder Compacts |issue=12}}</ref> |
||
<ref>{{cite journal |author=Franks, G.V. |author2=Lange, F.F. |name-list-style=amp |journal=J. Am. Ceram. Soc. |volume=79 |pages=3161–3168 |date=1996 |doi=10.1111/j.1151-2916.1996.tb08091.x |title=Plastic-to-Brittle Transition of Saturated, Alumina Powder Compacts |issue=12}}</ref> |
|||
In addition, any fluctuations in packing density in the compact as it is prepared for the kiln are often amplified during the [[sintering]] process, yielding inhomogeneous densification. Some pores and other structural [[crystallographic defect|defect]]s associated with density variations have been shown to play a detrimental role in the sintering process by growing and thus limiting end-point densities. Differential stresses arising from inhomogeneous densification have also been shown to result in the propagation of internal cracks, thus becoming the strength-controlling flaws. |
In addition, any fluctuations in packing density in the compact as it is prepared for the kiln are often amplified during the [[sintering]] process, yielding inhomogeneous densification. Some pores and other structural [[crystallographic defect|defect]]s associated with density variations have been shown to play a detrimental role in the sintering process by growing and thus limiting end-point densities. Differential stresses arising from inhomogeneous densification have also been shown to result in the propagation of internal cracks, thus becoming the strength-controlling flaws.<ref>{{cite journal |author1=Evans, A.G. |author2=Davidge, R.W. |journal=Phil. Mag. |volume=20 |issue=164 |pages=373–388 |date=1969 |doi=10.1080/14786436908228708 |title=The strength and fracture of fully dense polycrystalline magnesium oxide |bibcode=1969PMag...20..373E}}</ref><ref>{{cite journal |author=Lange, F.F. |author2=Metcalf, M. |name-list-style=amp |journal=J. Am. Ceram. Soc. |volume=66 |pages=398–406 |date=1983 |doi=10.1111/j.1151-2916.1983.tb10069.x |title=Processing-Related Fracture Origins: II, Agglomerate Motion and Cracklike Internal Surfaces Caused by Differential Sintering |issue=6}}</ref> |
||
<ref>{{cite journal |author1=Evans, A.G. |author2=Davidge, R.W. |journal=Phil. Mag. |volume=20 |issue=164 |pages=373–388 |date=1969 |doi=10.1080/14786436908228708 |title=The strength and fracture of fully dense polycrystalline magnesium oxide |bibcode=1969PMag...20..373E}}</ref><ref>{{cite journal |author=Lange, F.F. |author2=Metcalf, M. |name-list-style=amp |journal=J. Am. Ceram. Soc. |volume=66 |pages=398–406 |date=1983 |doi=10.1111/j.1151-2916.1983.tb10069.x |title=Processing-Related Fracture Origins: II, Agglomerate Motion and Cracklike Internal Surfaces Caused by Differential Sintering |issue=6}}</ref> |
|||
It would therefore appear desirable to process a material in such a way that it is physically uniform with regard to the distribution of components and porosity, rather than using particle size distributions which will maximize the green density. The containment of a uniformly dispersed assembly of strongly interacting particles in suspension requires total control over particle-particle interactions. A number of dispersants such as ammonium citrate (aqueous) and imidazoline or [[oleyl alcohol]] (nonaqueous) are promising solutions as possible additives for enhanced dispersion and deagglomeration. [[Monodisperse]] nanoparticles and colloids provide this potential.<ref>{{cite journal |author=Evans, A.G. |journal=J. Am. Ceram. Soc. |volume=65 |pages=497–501 |date=1987 |doi=10.1111/j.1151-2916.1982.tb10340.x |title=Considerations of Inhomogeneity Effects in Sintering |issue=10}}</ref> |
It would therefore appear desirable to process a material in such a way that it is physically uniform with regard to the distribution of components and porosity, rather than using particle size distributions which will maximize the green density. The containment of a uniformly dispersed assembly of strongly interacting particles in suspension requires total control over particle-particle interactions. A number of dispersants such as ammonium citrate (aqueous) and imidazoline or [[oleyl alcohol]] (nonaqueous) are promising solutions as possible additives for enhanced dispersion and deagglomeration. [[Monodisperse]] nanoparticles and colloids provide this potential.<ref>{{cite journal |author=Evans, A.G. |journal=J. Am. Ceram. Soc. |volume=65 |pages=497–501 |date=1987 |doi=10.1111/j.1151-2916.1982.tb10340.x |title=Considerations of Inhomogeneity Effects in Sintering |issue=10}}</ref> |
||
Monodisperse powders of colloidal [[silica]], for example, may therefore be stabilized sufficiently to ensure a high degree of order in the [[colloidal crystal]] or [[polycrystalline]] colloidal solid which results from aggregation. The degree of order appears to be limited by the time and space allowed for longer-range correlations to be established. Such defective polycrystalline colloidal structures would appear to be the basic elements of sub-micrometer colloidal materials science, and, therefore, provide the first step in developing a more rigorous understanding of the mechanisms involved in microstructural evolution in high performance materials and components. |
Monodisperse powders of colloidal [[silica]], for example, may therefore be stabilized sufficiently to ensure a high degree of order in the [[colloidal crystal]] or [[polycrystalline]] colloidal solid which results from aggregation. The degree of order appears to be limited by the time and space allowed for longer-range correlations to be established. Such defective polycrystalline colloidal structures would appear to be the basic elements of sub-micrometer colloidal materials science, and, therefore, provide the first step in developing a more rigorous understanding of the mechanisms involved in microstructural evolution in high performance materials and components.<ref>{{cite journal |title=Molecular Self-Assembly and Nanochemistry: A Chemical Strategy for the Synthesis of Nanostructures |journal=Science |volume=254 |date=1991 |doi=10.1126/science.1962191 |pmid=1962191 |bibcode=1991Sci...254.1312W |issue=5036 |pages=1312–9 |last1=Whitesides |first1=George M. |display-authors=1 |last2=Mathias |first2=John P. |last3=Seto |first3=Christopher T. |url=https://apps.dtic.mil/sti/pdfs/ADA243530.pdf |archive-url=https://web.archive.org/web/20170821140138/http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA243530&Location=U2&doc=GetTRDoc.pdf |url-status=live |archive-date=21 August 2017}}</ref><ref>{{cite journal |author=Dubbs D. M |author2=Aksay I.A. |title=Self-Assembled Ceramics Produced by Complex-Fluid Templation |journal=Annu. Rev. Phys. Chem. |volume=51 |date=2000 |pmid=11031294 |doi=10.1146/annurev.physchem.51.1.601 |bibcode=2000ARPC...51..601D |pages=601–22 |s2cid=14113689 |url=http://pdfs.semanticscholar.org/a21d/fb4c37e66c0f1fb932dc42c6402d15ce5b38.pdf |archive-url=https://web.archive.org/web/20200925040042/http://pdfs.semanticscholar.org/a21d/fb4c37e66c0f1fb932dc42c6402d15ce5b38.pdf |url-status=dead |archive-date=2020-09-25}}</ref> |
||
<ref>{{cite journal |title=Molecular Self-Assembly and Nanochemistry: A Chemical Strategy for the Synthesis of Nanostructures |journal=Science |volume=254 |date=1991 |doi=10.1126/science.1962191 |pmid=1962191 |bibcode=1991Sci...254.1312W |issue=5036 |pages=1312–9 |last1=Whitesides |first1=George M. |display-authors=1 |last2=Mathias |first2=John P. |last3=Seto |first3=Christopher T. |url=https://apps.dtic.mil/sti/pdfs/ADA243530.pdf |archive-url=https://web.archive.org/web/20170821140138/http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA243530&Location=U2&doc=GetTRDoc.pdf |url-status=live |archive-date=21 August 2017}}</ref><ref>{{cite journal |author=Dubbs D. M |author2=Aksay I.A. |title=Self-Assembled Ceramics Produced by Complex-Fluid Templation |journal=Annu. Rev. Phys. Chem. |volume=51 |date=2000 |pmid=11031294 |doi=10.1146/annurev.physchem.51.1.601 |bibcode=2000ARPC...51..601D |pages=601–22 |s2cid=14113689 |url=http://pdfs.semanticscholar.org/a21d/fb4c37e66c0f1fb932dc42c6402d15ce5b38.pdf |archive-url=https://web.archive.org/web/20200925040042/http://pdfs.semanticscholar.org/a21d/fb4c37e66c0f1fb932dc42c6402d15ce5b38.pdf |url-status=dead |archive-date=2020-09-25}}</ref> |
|||
==Nanomaterials in articles, patents, and products== |
==Nanomaterials in articles, patents, and products== |
||
The quantitative analysis of nanomaterials showed that nanoparticles, nanotubes, nanocrystalline materials, nanocomposites, and graphene have been mentioned in |
The quantitative analysis of nanomaterials showed that nanoparticles, nanotubes, nanocrystalline materials, nanocomposites, and graphene have been mentioned in 400,000, 181,000, 144,000, 140,000, and 119,000 ISI-indexed articles, respectively, by September 2018. As far as patents are concerned, nanoparticles, nanotubes, nanocomposites, graphene, and nanowires have been played a role in 45,600, 32,100, 12,700, 12,500, and 11,800 patents, respectively. Monitoring approximately 7,000 commercial nano-based products available on global markets revealed that the properties of around 2,330 products have been enabled or enhanced aided by nanoparticles. Liposomes, nanofibers, nanocolloids, and aerogels were also of the most common nanomaterials in consumer products.<ref name=":2">{{cite web |url=http://statnano.com/nanomaterials |title=Statnano |access-date=2018-09-28}}</ref> |
||
The [ |
The [[European Union Observatory for Nanomaterials]] (EUON) has produced a database ([https://nanodata.echa.europa.eu/ NanoData]) that provides information on specific patents, products, and research publications on nanomaterials. |
||
==Health and safety== |
==Health and safety== |
||
{{Main|Health and safety hazards of nanomaterials}} |
{{Main|Health and safety hazards of nanomaterials}} |
||
{{Update section|reason= [https://pubmed.ncbi.nlm.nih.gov/38482025/] Quote: |
|||
“... nanotechnology products classified (2019–2023) according with industrial areas: electronics, medicine, construction, cosmetics,etc...”|date=March 2024}} |
|||
===World Health Organization guidelines=== |
===World Health Organization guidelines=== |
||
The World Health Organization (WHO) published a guideline on protecting workers from potential risk of manufactured nanomaterials at the end of 2017.<ref>{{cite web |url=https://www.who.int/occupational_health/publications/manufactured-nanomaterials/en/ |archive-url=https://web.archive.org/web/20171219115614/http://www.who.int/occupational_health/publications/manufactured-nanomaterials/en/ |url-status=dead |archive-date=19 December 2017 |title=WHO {{!}} WHO guidelines on protecting workers from potential risks of manufactured nanomaterials|website=WHO|access-date=2018-02-20}}</ref> WHO used a precautionary approach as one of its guiding principles. This means that exposure has to be reduced, despite uncertainty about the adverse health effects, when there are reasonable indications to do so. This is highlighted by recent scientific studies that demonstrate a capability of nanoparticles to cross [[cell |
The World Health Organization (WHO) published a guideline on protecting workers from potential risk of manufactured nanomaterials at the end of 2017.<ref>{{cite web |url=https://www.who.int/occupational_health/publications/manufactured-nanomaterials/en/ |archive-url=https://web.archive.org/web/20171219115614/http://www.who.int/occupational_health/publications/manufactured-nanomaterials/en/ |url-status=dead |archive-date=19 December 2017 |title=WHO {{!}} WHO guidelines on protecting workers from potential risks of manufactured nanomaterials|website=WHO|access-date=2018-02-20}}</ref> WHO used a precautionary approach as one of its guiding principles. This means that exposure has to be reduced, despite uncertainty about the adverse health effects, when there are reasonable indications to do so. This is highlighted by recent scientific studies that demonstrate a capability of nanoparticles to cross [[cell barrier]]s and interact with cellular structures.<ref>{{cite book |title=Comprehensive Nanoscience and Technology |publisher=Academic Press |year=2010 |isbn=9780123743961 |location=Cambridge, MA |pages=169}}</ref><ref>{{cite journal |last1=Verma |first1=Ayush |last2=Stellacci |first2=Francesco |date=2010 |title=Effect of Surface Properties on Nanoparticle-Cell Interactions |journal=Small |volume=6 |issue=1 |pages=12–21 |doi=10.1002/smll.200901158 |pmid=19844908}}</ref> In addition, the hierarchy of controls was an important guiding principle. This means that when there is a choice between control measures, those measures that are closer to the root of the problem should always be preferred over measures that put a greater burden on workers, such as the use of personal protective equipment (PPE). WHO commissioned systematic reviews for all important issues to assess the current state of the science and to inform the recommendations according to the process set out in the WHO Handbook for guideline development. The recommendations were rated as "strong" or "conditional" depending on the quality of the scientific evidence, values and preferences, and costs related to the recommendation. |
||
The WHO guidelines contain the following recommendations for safe handling of manufactured nanomaterials (MNMs) |
The WHO guidelines contain the following recommendations for safe handling of manufactured nanomaterials (MNMs) |
||
| Line 220: | Line 216: | ||
# WHO suggests preventing dermal exposure by occupational hygiene measures such as surface cleaning, and the use of appropriate gloves (conditional recommendation, low quality evidence). |
# WHO suggests preventing dermal exposure by occupational hygiene measures such as surface cleaning, and the use of appropriate gloves (conditional recommendation, low quality evidence). |
||
# When assessment and measurement by a workplace safety expert is not available, WHO suggests using control banding for nanomaterials to select exposure control measures in the workplace. Owing to a lack of studies, WHO cannot recommend one method of control banding over another (conditional recommendation, very low-quality evidence). |
# When assessment and measurement by a workplace safety expert is not available, WHO suggests using control banding for nanomaterials to select exposure control measures in the workplace. Owing to a lack of studies, WHO cannot recommend one method of control banding over another (conditional recommendation, very low-quality evidence). |
||
For health surveillance WHO could not make a recommendation for targeted MNM-specific health surveillance programmes over existing health surveillance programmes that are already in use owing to the lack of evidence. WHO considers training of workers and worker involvement in health and safety issues to be best practice but could not recommend one form of training of workers over another, or one form of worker involvement over another, owing to the lack of studies available. It is expected that there will be considerable progress in validated measurement methods and risk assessment and WHO expects to update these guidelines in five years' time, in 2022.{{update |
For health surveillance WHO could not make a recommendation for targeted MNM-specific health surveillance programmes over existing health surveillance programmes that are already in use owing to the lack of evidence. WHO considers training of workers and worker involvement in health and safety issues to be best practice but could not recommend one form of training of workers over another, or one form of worker involvement over another, owing to the lack of studies available. It is expected that there will be considerable progress in validated measurement methods and risk assessment and WHO expects to update these guidelines in five years' time, in 2022.{{update inline|date=April 2023}} |
||
===Other guidance=== |
===Other guidance=== |
||
Because nanotechnology is a recent development, the health and safety effects of exposures to nanomaterials, and what levels of exposure may be acceptable, are subjects of ongoing research.<ref name="Current strategies for engineering"/> Of the possible hazards, [[inhalation exposure]] appears to present the most concern. Animal studies indicate that [[carbon nanotube]]s and [[carbon nanofiber]]s can cause pulmonary effects including [[inflammation]], [[granuloma]]s, and [[pulmonary fibrosis]], which were of similar or greater potency when compared with other known [[fibrosis|fibrogenic]] materials such as [[silica gel|silica]], [[asbestos]], and ultrafine [[carbon black]]. Acute inhalation exposure of healthy animals to biodegradable inorganic nanomaterials have not demonstrated significant toxicity effects.<ref>{{cite journal |last1=Mapanao |first1=Ana Katrina |last2=Giannone |first2=Giulia |last3=Summa |first3=Maria |last4=Ermini |first4=Maria Laura |last5=Zamborlin |first5=Agata |last6=Santi |first6=Melissa |last7=Cassano |first7=Domenico |last8=Bertorelli |first8=Rosalia |last9=Voliani |first9=Valerio |date=2020 |title=Biokinetics and clearance of inhaled gold ultrasmall-in-nano architectures |journal=Nanoscale Advances |volume=2 |issue=9 |language=en |pages=3815–3820 |doi=10.1039/D0NA00521E |pmid=36132776 |pmc=9417912 |bibcode=2020NanoA...2.3815M |issn=2516-0230 |doi-access=free}}</ref> Although the extent to which animal data may predict clinically significant lung effects in workers is not known, the toxicity seen in the short-term animal studies indicate a need for protective action for workers exposed to these nanomaterials, although no reports of actual adverse health effects in workers using or producing these nanomaterials were known as of 2013.<ref name="Current intelligence bulletin 65 - Carbon nanotubes">{{cite journal |url=https://www.cdc.gov/niosh/docs/2013-145/ |title=Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers |date=April 2013 |website=U.S. National Institute for Occupational Safety and Health |pages=v–x, 33–35, 43, 63–64 |language=en-us |access-date=2017-04-26 |doi=10.26616/NIOSHPUB2013145 |doi-access=free}}</ref> Additional concerns include skin contact and ingestion exposure,<ref name="Current intelligence bulletin 65 - Carbon nanotubes"/><ref name="Approaches to safe nanotechnology2">{{cite journal |url=https://www.cdc.gov/niosh/docs/2009-125/ |title=Approaches to Safe Nanotechnology: Managing the Health and Safety Concerns Associated with Engineered Nanomaterials |date=March 2009 |website=U.S. National Institute for Occupational Safety and Health |page=12 |language=en-us |access-date=2017-04-26 |doi=10.26616/NIOSHPUB2009125 |doi-access=free}}</ref><ref>[https://emagazine.com/eating-nano/ Eating Nano]. By Brita Belli. ''E – The Environmental Magazine'', 3 November 2012.</ref> and [[dust explosion]] hazards.<ref name="Screening of allotropes">{{cite journal |last1=Turkevich |first1=Leonid A. |last2=Fernback |first2=Joseph |last3=Dastidar |first3=Ashok G. |last4=Osterberg |first4=Paul |date=2016-05-01 |title=Potential explosion hazard of carbonaceous nanoparticles: screening of allotropes |url=https://www.cdc.gov/niosh/nioshtic-2/20047783.html |journal=Combustion and Flame |volume=167 |pages=218–227 |doi=10.1016/j.combustflame.2016.02.010 |pmid=27468178 |pmc=4959120}}</ref><ref name="Fire and explosion properties">{{cite web |url=http://www.hse.gov.uk/research/rrhtm/rr782.htm |title=Fire and explosion properties of nanopowders |date=2010 |website=U.K. [[Health and Safety Executive]] |pages=2, 13–15, 61–62 |access-date=2017-04-28}}</ref> |
Because nanotechnology is a recent development, the health and safety effects of exposures to nanomaterials, and what levels of exposure may be acceptable, are subjects of ongoing research.<ref name="Current strategies for engineering"/> Of the possible hazards, [[inhalation exposure]] appears to present the most concern. Animal studies indicate that [[carbon nanotube]]s and [[carbon nanofiber]]s can cause pulmonary effects including [[inflammation]], [[granuloma]]s, and [[pulmonary fibrosis]], which were of similar or greater potency when compared with other known [[fibrosis|fibrogenic]] materials such as [[silica gel|silica]], [[asbestos]], and ultrafine [[carbon black]]. Acute inhalation exposure of healthy animals to biodegradable inorganic nanomaterials have not demonstrated significant toxicity effects.<ref>{{cite journal |last1=Mapanao |first1=Ana Katrina |last2=Giannone |first2=Giulia |last3=Summa |first3=Maria |last4=Ermini |first4=Maria Laura |last5=Zamborlin |first5=Agata |last6=Santi |first6=Melissa |last7=Cassano |first7=Domenico |last8=Bertorelli |first8=Rosalia |last9=Voliani |first9=Valerio |date=2020 |title=Biokinetics and clearance of inhaled gold ultrasmall-in-nano architectures |journal=Nanoscale Advances |volume=2 |issue=9 |language=en |pages=3815–3820 |doi=10.1039/D0NA00521E |pmid=36132776 |pmc=9417912 |bibcode=2020NanoA...2.3815M |issn=2516-0230 |doi-access=free}}</ref> Although the extent to which animal data may predict clinically significant lung effects in workers is not known, the toxicity seen in the short-term animal studies indicate a need for protective action for workers exposed to these nanomaterials, although no reports of actual adverse health effects in workers using or producing these nanomaterials were known as of 2013.<ref name="Current intelligence bulletin 65 - Carbon nanotubes">{{cite journal |url=https://www.cdc.gov/niosh/docs/2013-145/ |title=Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers |date=April 2013 |website=U.S. National Institute for Occupational Safety and Health |pages=v–x, 33–35, 43, 63–64 |language=en-us |access-date=2017-04-26 |doi=10.26616/NIOSHPUB2013145 |doi-access=free}}</ref> Additional concerns include skin contact and ingestion exposure,<ref name="Current intelligence bulletin 65 - Carbon nanotubes"/><ref name="Approaches to safe nanotechnology2">{{cite journal |url=https://www.cdc.gov/niosh/docs/2009-125/ |title=Approaches to Safe Nanotechnology: Managing the Health and Safety Concerns Associated with Engineered Nanomaterials |date=March 2009 |website=U.S. National Institute for Occupational Safety and Health |page=12 |language=en-us |access-date=2017-04-26 |doi=10.26616/NIOSHPUB2009125 |doi-access=free}}</ref><ref>[https://emagazine.com/eating-nano/ Eating Nano]. By Brita Belli. ''E – The Environmental Magazine'', 3 November 2012.</ref> and [[dust explosion]] hazards.<ref name="Screening of allotropes">{{cite journal |last1=Turkevich |first1=Leonid A. |last2=Fernback |first2=Joseph |last3=Dastidar |first3=Ashok G. |last4=Osterberg |first4=Paul |date=2016-05-01 |title=Potential explosion hazard of carbonaceous nanoparticles: screening of allotropes |url=https://www.cdc.gov/niosh/nioshtic-2/20047783.html |journal=Combustion and Flame |volume=167 |pages=218–227 |doi=10.1016/j.combustflame.2016.02.010 |pmid=27468178 |pmc=4959120|bibcode=2016CoFl..167..218T }}</ref><ref name="Fire and explosion properties">{{cite web |url=http://www.hse.gov.uk/research/rrhtm/rr782.htm |title=Fire and explosion properties of nanopowders |date=2010 |website=U.K. [[Health and Safety Executive]] |pages=2, 13–15, 61–62 |access-date=2017-04-28}}</ref> |
||
[[Hazard elimination|Elimination]] and [[hazard substitution|substitution]] are the most desirable approaches to [[hierarchy of hazard controls|hazard control]]. While the nanomaterials themselves often cannot be eliminated or substituted with conventional materials,<ref name="Current strategies for engineering"/> it may be possible to choose properties of the nanoparticle such as [[particle size|size]], [[nanoparticle#Morphology and structure|shape]], [[surface functionalisation|functionalization]], [[surface charge]], [[solubility]], [[flocculation|agglomeration]], and [[particle aggregation|aggregation state]] to improve their toxicological properties while retaining the desired functionality.<ref name="Building a Safety Program">{{cite journal |url=https://www.cdc.gov/niosh/docs/2016-102/ |title=Building a Safety Program to Protect the Nanotechnology Workforce: A Guide for Small to Medium-Sized Enterprises |date=March 2016 |website=U.S. National Institute for Occupational Safety and Health |pages=8, 12–15 |language=en-us |access-date=2017-03-05 |doi=10.26616/NIOSHPUB2016102 |doi-access=free}}</ref> Handling procedures can also be improved, for example, using a nanomaterial [[slurry]] or [[colloid|suspension]] in a liquid solvent instead of a dry powder will reduce dust exposure.<ref name="Current strategies for engineering"/> [[Engineering controls]] are physical changes to the workplace that isolate workers from hazards, mainly ventilation systems such as [[fume hood]]s, [[glovebox]]es, [[biosafety cabinet]]s, and [[vented balance safety enclosure|vented balance enclosures]].<ref name="General safe practices for working4">{{cite journal |url=https://www.cdc.gov/niosh/docs/2012-147/ |title=General Safe Practices for Working with Engineered Nanomaterials in Research Laboratories |date=May 2012 |website=U.S. National Institute for Occupational Safety and Health |pages=15–28 |language=en-us |access-date=2017-03-05 |doi=10.26616/NIOSHPUB2012147 |doi-access=free}}</ref> [[Administrative controls]] are changes to workers' behavior to mitigate a hazard, including training on [[best practice]]s for safe handling, storage, and disposal of nanomaterials, proper awareness of hazards through labeling and warning signage, and encouraging a general [[safety culture]]. [[Personal protective equipment]] must be worn on the worker's body and is the least desirable option for controlling hazards.<ref name="Current strategies for engineering"/> Personal protective equipment normally used for typical chemicals are also appropriate for nanomaterials, including long pants, long-sleeve shirts, and closed-toed shoes, and the use of [[safety glove]]s, [[goggles]], and impervious [[laboratory coat]]s.<ref name="General safe practices for working4"/> In some circumstances [[respirator]]s may be used.<ref name="Building a Safety Program"/> |
[[Hazard elimination|Elimination]] and [[hazard substitution|substitution]] are the most desirable approaches to [[hierarchy of hazard controls|hazard control]]. While the nanomaterials themselves often cannot be eliminated or substituted with conventional materials,<ref name="Current strategies for engineering"/> it may be possible to choose properties of the nanoparticle such as [[particle size|size]], [[nanoparticle#Morphology and structure|shape]], [[surface functionalisation|functionalization]], [[surface charge]], [[solubility]], [[flocculation|agglomeration]], and [[particle aggregation|aggregation state]] to improve their toxicological properties while retaining the desired functionality.<ref name="Building a Safety Program">{{cite journal |url=https://www.cdc.gov/niosh/docs/2016-102/ |title=Building a Safety Program to Protect the Nanotechnology Workforce: A Guide for Small to Medium-Sized Enterprises |date=March 2016 |website=U.S. National Institute for Occupational Safety and Health |pages=8, 12–15 |language=en-us |access-date=2017-03-05 |doi=10.26616/NIOSHPUB2016102 |doi-access=free|hdl=10919/76615 |hdl-access=free }}</ref> Handling procedures can also be improved, for example, using a nanomaterial [[slurry]] or [[colloid|suspension]] in a liquid solvent instead of a dry powder will reduce dust exposure.<ref name="Current strategies for engineering"/> [[Engineering controls]] are physical changes to the workplace that isolate workers from hazards, mainly ventilation systems such as [[fume hood]]s, [[glovebox]]es, [[biosafety cabinet]]s, and [[vented balance safety enclosure|vented balance enclosures]].<ref name="General safe practices for working4">{{cite journal |url=https://www.cdc.gov/niosh/docs/2012-147/ |title=General Safe Practices for Working with Engineered Nanomaterials in Research Laboratories |date=May 2012 |website=U.S. National Institute for Occupational Safety and Health |pages=15–28 |language=en-us |access-date=2017-03-05 |doi=10.26616/NIOSHPUB2012147 |doi-access=free}}</ref> [[Administrative controls]] are changes to workers' behavior to mitigate a hazard, including training on [[best practice]]s for safe handling, storage, and disposal of nanomaterials, proper awareness of hazards through labeling and warning signage, and encouraging a general [[safety culture]]. [[Personal protective equipment]] must be worn on the worker's body and is the least desirable option for controlling hazards.<ref name="Current strategies for engineering"/> Personal protective equipment normally used for typical chemicals are also appropriate for nanomaterials, including long pants, long-sleeve shirts, and closed-toed shoes, and the use of [[safety glove]]s, [[goggles]], and impervious [[laboratory coat]]s.<ref name="General safe practices for working4"/> In some circumstances [[respirator]]s may be used.<ref name="Building a Safety Program"/> |
||
[[Exposure assessment]] is a set of methods used to monitor contaminant release and exposures to workers. These methods include personal sampling, where samplers are located in the personal breathing zone of the worker, often attached to a shirt collar to be as close to the nose and mouth as possible; and area/background sampling, where they are placed at static locations. The assessment should use both [[particle counter]]s, which monitor the real-time quantity of nanomaterials and other background particles; and filter-based samples, which can be used to identify the nanomaterial, usually using [[electron microscope|electron microscopy]] and [[elemental analysis]].<ref name="Building a Safety Program"/><ref name="Refinement (JOEH)">{{cite journal |last1=Eastlake |first1=Adrienne C. |last2=Beaucham |first2=Catherine |last3=Martinez |first3=Kenneth F. |last4=Dahm |first4=Matthew M. |last5=Sparks |first5=Christopher |last6=Hodson |first6=Laura L. |last7=Geraci |first7=Charles L. |date=2016-09-01 |title=Refinement of the Nanoparticle Emission Assessment Technique into the Nanomaterial Exposure Assessment Technique (NEAT 2.0) |journal=Journal of Occupational and Environmental Hygiene |volume=13 |issue=9 |pages=708–717 |doi=10.1080/15459624.2016.1167278 |pmc=4956539 |pmid=27027845}}</ref> As of 2016, quantitative [[occupational exposure limit]]s have not been determined for most nanomaterials. The U.S. [[National Institute for Occupational Safety and Health]] has determined non-regulatory [[recommended exposure limit]]s for [[carbon nanotube]]s, [[carbon nanofiber]]s,<ref name="Current intelligence bulletin 65 - Carbon nanotubes"/> and [[ultrafine particle|ultrafine]] [[titanium dioxide]].<ref name="Current intelligence bulletin 63 - TiO22">{{cite journal |url=https://www.cdc.gov/niosh/docs/2011-160/ |title=Current Intelligence Bulletin 63: Occupational Exposure to Titanium Dioxide |date=April 2011 |website=U.S. National Institute for Occupational Safety and Health |pages=vii, 77–78 |language=en-us |access-date=2017-04-27 |doi=10.26616/NIOSHPUB2011160 |doi-access=free}}</ref> Agencies and organizations from other countries, including the [[British Standards Institute]]<ref>{{cite web |url=http://shop.bsigroup.com/forms/Nano/PD-6699-2/Confirmation/ |title=Nanotechnologies – Part 2: Guide to safe handling and disposal of manufactured nanomaterials |date=December 2007 |website=[[British Standards Institute]] |archive-url=https://web.archive.org/web/20141102051352/http://shop.bsigroup.com/forms/Nano/PD-6699-2/Confirmation/ |archive-date=2014-11-02 |url-status=dead |access-date=2017-04-21}}</ref> and the [[Institute for Occupational Safety and Health]] in Germany,<ref>{{cite web |url=http://www.dguv.de/ifa;/fachinfos/nanopartikel-am-arbeitsplatz/beurteilung-von-schutzmassnahmen/index-2.jsp |title=Criteria for assessment of the effectiveness of protective measures |date=2009 |website=[[Institute for Occupational Safety and Health of the German Social Accident Insurance]] |language=en |access-date=2017-04-21}}</ref> have established OELs for some nanomaterials, and some companies have supplied OELs for their products.<ref name="Current strategies for engineering"/> |
[[Exposure assessment]] is a set of methods used to monitor contaminant release and exposures to workers. These methods include personal sampling, where samplers are located in the personal breathing zone of the worker, often attached to a shirt collar to be as close to the nose and mouth as possible; and area/background sampling, where they are placed at static locations. The assessment should use both [[particle counter]]s, which monitor the real-time quantity of nanomaterials and other background particles; and filter-based samples, which can be used to identify the nanomaterial, usually using [[electron microscope|electron microscopy]] and [[elemental analysis]].<ref name="Building a Safety Program"/><ref name="Refinement (JOEH)">{{cite journal |last1=Eastlake |first1=Adrienne C. |last2=Beaucham |first2=Catherine |last3=Martinez |first3=Kenneth F. |last4=Dahm |first4=Matthew M. |last5=Sparks |first5=Christopher |last6=Hodson |first6=Laura L. |last7=Geraci |first7=Charles L. |date=2016-09-01 |title=Refinement of the Nanoparticle Emission Assessment Technique into the Nanomaterial Exposure Assessment Technique (NEAT 2.0) |journal=Journal of Occupational and Environmental Hygiene |volume=13 |issue=9 |pages=708–717 |doi=10.1080/15459624.2016.1167278 |pmc=4956539 |pmid=27027845}}</ref> As of 2016, quantitative [[occupational exposure limit]]s have not been determined for most nanomaterials. The U.S. [[National Institute for Occupational Safety and Health]] has determined non-regulatory [[recommended exposure limit]]s for [[carbon nanotube]]s, [[carbon nanofiber]]s,<ref name="Current intelligence bulletin 65 - Carbon nanotubes"/> and [[ultrafine particle|ultrafine]] [[titanium dioxide]].<ref name="Current intelligence bulletin 63 - TiO22">{{cite journal |url=https://www.cdc.gov/niosh/docs/2011-160/ |title=Current Intelligence Bulletin 63: Occupational Exposure to Titanium Dioxide |date=April 2011 |website=U.S. National Institute for Occupational Safety and Health |pages=vii, 77–78 |language=en-us |access-date=2017-04-27 |doi=10.26616/NIOSHPUB2011160 |doi-access=free}}</ref> Agencies and organizations from other countries, including the [[British Standards Institute]]<ref>{{cite web |url=http://shop.bsigroup.com/forms/Nano/PD-6699-2/Confirmation/ |title=Nanotechnologies – Part 2: Guide to safe handling and disposal of manufactured nanomaterials |date=December 2007 |website=[[British Standards Institute]] |archive-url=https://web.archive.org/web/20141102051352/http://shop.bsigroup.com/forms/Nano/PD-6699-2/Confirmation/ |archive-date=2014-11-02 |url-status=dead |access-date=2017-04-21}}</ref> and the [[Institute for Occupational Safety and Health]] in Germany,<ref>{{cite web |url=http://www.dguv.de/ifa;/fachinfos/nanopartikel-am-arbeitsplatz/beurteilung-von-schutzmassnahmen/index-2.jsp |title=Criteria for assessment of the effectiveness of protective measures |date=2009 |website=[[Institute for Occupational Safety and Health of the German Social Accident Insurance]] |language=en |access-date=2017-04-21}}</ref> have established OELs for some nanomaterials, and some companies have supplied OELs for their products.<ref name="Current strategies for engineering"/> |
||
| Line 233: | Line 229: | ||
Nanotechnology has been making headlines in the medical field,<ref>{{cite journal |last=Prasad |first=Paras |date=Jan 22, 2016 |title=Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy |url=https://pubs.acs.org/doi/10.1021/acs.chemrev.5b00148 |journal=Chemical Reviews |volume=116 |issue=5 |pages=2827, 2841, 2850 |doi=10.1021/acs.chemrev.5b00148 |pmid=26799741}}</ref> being responsible for biomedical imaging. The unique optical, magnetic and chemical properties of materials on the Nano scale has allowed the development of imaging probes with multi-functionality such as better contrast enhancement, better spatial information, controlled bio distribution, and multi-modal imaging across various scanning devices. These developments have had advantages such as being able to detect the location of tumors and inflammations, accurate assessment of disease progression, and personalized medicine. |
Nanotechnology has been making headlines in the medical field,<ref>{{cite journal |last=Prasad |first=Paras |date=Jan 22, 2016 |title=Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy |url=https://pubs.acs.org/doi/10.1021/acs.chemrev.5b00148 |journal=Chemical Reviews |volume=116 |issue=5 |pages=2827, 2841, 2850 |doi=10.1021/acs.chemrev.5b00148 |pmid=26799741}}</ref> being responsible for biomedical imaging. The unique optical, magnetic and chemical properties of materials on the Nano scale has allowed the development of imaging probes with multi-functionality such as better contrast enhancement, better spatial information, controlled bio distribution, and multi-modal imaging across various scanning devices. These developments have had advantages such as being able to detect the location of tumors and inflammations, accurate assessment of disease progression, and personalized medicine. |
||
# '''Silica nanoparticles-''' Silica nanoparticles<ref>{{cite journal |last=Haynes |first=Christy |date=Jan 11, 2012 |title=Critical Considerations in the Biomedical Use of Mesoporous Silica Nanoparticles |url=https://academic.oup.com/ib/article-abstract/5/1/74/5208312?redirectedFrom=fulltext |journal=The Journal of Physical Chemistry Letters |volume=3 |issue=3 |pages=364–374 |doi=10.1021/jz2013837 |pmid=26285853}}</ref> can be classified into solid, non-porous, and mesoporous. They have large surface are, hydrophilic surface, and chemical and physical stabilities. Silica nanoparticles are made by the use of the Stöber process. Which is the hydrolysis of silyl ethers such as tetraethyl silicate into silanols (Si-OH) using ammonia in a mixture of water and alcohol followed by the condensation of silanols into |
# '''Silica nanoparticles-''' Silica nanoparticles<ref>{{cite journal |last=Haynes |first=Christy |date=Jan 11, 2012 |title=Critical Considerations in the Biomedical Use of Mesoporous Silica Nanoparticles |url=https://academic.oup.com/ib/article-abstract/5/1/74/5208312?redirectedFrom=fulltext |journal=The Journal of Physical Chemistry Letters |volume=3 |issue=3 |pages=364–374 |doi=10.1021/jz2013837 |pmid=26285853}}</ref> can be classified into solid, non-porous, and mesoporous. They have large surface are, hydrophilic surface, and chemical and physical stabilities. Silica nanoparticles are made by the use of the Stöber process. Which is the hydrolysis of silyl ethers such as tetraethyl silicate into silanols (Si-OH) using ammonia in a mixture of water and alcohol followed by the condensation of silanols into 50–2000 nm silica particles. The size of the particle can be controlled by varying the concentration of silyl ether and alcohol or the micro emulsion method. Mesoporous silica nanoparticles are synthesized by the sol-gel process. They have pores that range in diameter from 2 nm to 50 nm. They are synthesized in a water-based solution in the presence of a base catalyst and a pore forming agent known as a surfactant. Surfactants are molecules that present the particularity to have a hydrophobic tail (alkyl chain) and a hydrophilic head (charged group, such as a quaternary amine for example). As these surfactants are added to a water-based solution, they will coordinate to form micelles with increasing concentration in order to stabilize the hydrophobic tails. Varying the pH of the solution and composition of the solvents, and the addition of certain swelling agents can control the pore size. Their hydrophilic surface is what makes silica nanoparticles so important and allows them to carry out functions such as drug and gene delivery, bio imaging and therapy. In order for this application to be successful, assorted surface functional groups are necessary and can be added either by the co-condensation process during preparation or by post surface modification. The high surface area of silica nanoparticles allows them to carry much larger amounts of the desired drug than through conventional methods like polymers and liposomes. It allows for site specific targeting, especially in the treatment of cancer. Once the particles have reached their destination, they can act as a reporter, release a compound, or be remotely heated to damage biological structures in close proximity. Targeting is typically accomplished by modifying the surface of the nanoparticle with a chemical or biological compound. They accumulate at tumor sites through Enhanced Permeability Retention (EPR), where the tumor vessels accelerate the delivery of the nanoparticles directly into the tumor. The porous shell of the silica allows control over the rate at which the drug diffuses out of the nanoparticle. The shell can be modified to have an affinity for the drug, or even to be triggered by pH, heat, light, salts, or other signaling molecules. Silica nanoparticles are also used in bio imaging because they can accommodate fluorescent/MRI/PET/ SPECT contrast agents and drug/DNA molecules to their adaptable surface and pores. This is made possible by using the silica nanoparticle as a vector for the expression of fluorescent proteins. Several different types of fluorescent probes, like cyanine dyes, methyl violegen, or semiconductor quantum dots can be conjugated to silica nanoparticles and delivered into specific cells or injected in vivo. Carrier molecule RGD peptide has been very useful of targeted in vivo imaging. |
||
# '''Topically applied surface-enhanced resonance Raman ratiometric spectroscopy (TAS3RS)'''<ref>{{cite journal |last=Kircher |first=Moritz F. |date=Dec 19, 2016 |title=Folate-Targeted Surface-Enhanced Resonance Raman Scattering Nanoprobe Ratiometry for Detection of Microscopic Ovarian Cancer |journal=ACS Nano |volume=11 |issue=2 |pages=1488–1497 |doi=10.1021/acsnano.6b06796 |pmid=27992724 |pmc=5502101}}</ref>'''-''' TAS3RS is another technique that is starting to make advancement in the medical field. It is an imaging technique that uses Folate Receptors (FR) to detect tumor lesions as small as 370 micrometers. Folate Receptors are membrane bound surface proteins that bind folates and folate conjugates with high affinity. FR is frequently overexpressed in a number of human malignancies including cancer of the ovary, lung, kidney, breast, bladder, brain, and endometrium. Raman imaging is a type of spectroscopy that is used in chemistry to provide structural fingerprint by which molecules can be identified. It relies upon inelastic scattering of photons, which result in ultra high sensitivity. There was a study that was done where two different surface enhanced resonance Raman scattering were synthesized (SERRS). One of the SERRS was a |
# '''Topically applied surface-enhanced resonance Raman ratiometric spectroscopy (TAS3RS)'''<ref>{{cite journal |last=Kircher |first=Moritz F. |date=Dec 19, 2016 |title=Folate-Targeted Surface-Enhanced Resonance Raman Scattering Nanoprobe Ratiometry for Detection of Microscopic Ovarian Cancer |journal=ACS Nano |volume=11 |issue=2 |pages=1488–1497 |doi=10.1021/acsnano.6b06796 |pmid=27992724 |pmc=5502101}}</ref>'''-''' TAS3RS is another technique that is starting to make advancement in the medical field. It is an imaging technique that uses Folate Receptors (FR) to detect tumor lesions as small as 370 micrometers. Folate Receptors are membrane bound surface proteins that bind folates and folate conjugates with high affinity. FR is frequently overexpressed in a number of human malignancies including cancer of the ovary, lung, kidney, breast, bladder, brain, and endometrium. Raman imaging is a type of spectroscopy that is used in chemistry to provide structural fingerprint by which molecules can be identified. It relies upon inelastic scattering of photons, which result in ultra high sensitivity. There was a study that was done where two different surface enhanced resonance Raman scattering were synthesized (SERRS). One of the SERRS was a "targeted nanoprobe functionalized with an anti-folate-receptor antibody (αFR-Ab) via a PEG-maleimide-succinimide and using the infrared dye IR780 as the Raman reporter, henceforth referred to as αFR-NP, and a nontargeted probe (nt-NP) coated with PEG5000-maleimide and featuring the IR140 infrared dye as the Raman reporter." These two different mixtures were injected into tumor bearing mice and healthy controlled mice. The mice were imaged with Bioluminescence (BLI) signal that produces light energy within an organism's body. They were also scanned with the Raman microscope in order to be able to see the correlation between the TAS3RS and the BLI map. TAS3RS did not show anything in the healthy mice, but was able to locate the tumor lesions in the infected mice and also able to create a TAS3RS map that could be used as guidance during surgery. TAS3RS shows to be promising in being able to combat ovarian and peritoneal cancer as it allows early detection with high accuracy. This technique can be administered locally, which is an advantage as it does not have to enter the bloodstream and therefore bypassing the toxicity concerns circulating nanoprobes. This technique is also more photostable than fluorochromes because SERRS nanoparticles cannot form from biomolecules and therefore there would not be any false positives in TAS3RS as there is in fluorescence imaging. |
||
==See also== |
==See also== |
||
| Line 252: | Line 248: | ||
*[http://www.oecd.org/department/0,3355,en_2649_37015404_1_1_1_1_1,00.html Safety of Manufactured Nanomaterials: OECD Environment Directorate] |
*[http://www.oecd.org/department/0,3355,en_2649_37015404_1_1_1_1_1,00.html Safety of Manufactured Nanomaterials: OECD Environment Directorate] |
||
*[http://copublications.greenfacts.org/en/nanotechnologies/index.htm Assessing health risks of nanomaterials] summary by [[GreenFacts]] of the European Commission SCENIHR assessment |
*[http://copublications.greenfacts.org/en/nanotechnologies/index.htm Assessing health risks of nanomaterials] summary by [[GreenFacts]] of the European Commission SCENIHR assessment |
||
| ⚫ | |||
*[http://www.liposome.org International Liposome Society] (gone) |
|||
| ⚫ | |||
*[http://www.iop.org/EJ/article/0957-4484/14/3/201/t303R1.pdf?request-id=NENUvFK63BGH-Bna2wi7Kg IOP.org Article]{{Dead link|date=May 2020}} |
|||
*[https://books.google.com/books?id=_pbtbJwkj5YC&pg=PA5 Nano Structured Material] |
*[https://books.google.com/books?id=_pbtbJwkj5YC&pg=PA5 Nano Structured Material] |
||
*[https://web.archive.org/web/20150605092355/https://nanohub.org/resources/1914 Online course MSE 376-Nanomaterials by Mark C. Hersam (2006)] |
*[https://web.archive.org/web/20150605092355/https://nanohub.org/resources/1914 Online course MSE 376-Nanomaterials by Mark C. Hersam (2006)] |
||
| Line 268: | Line 262: | ||
[[Category:Nanomaterials| ]] |
[[Category:Nanomaterials| ]] |
||
[[Category:Emerging technologies]] |
|||
Latest revision as of 15:24, 7 December 2024
| Part of a series of articles on |
| Nanomaterials |
|---|
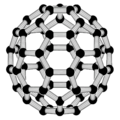 |
| Carbon nanotubes |
| Fullerenes |
| Other nanoparticles |
| Nanostructured materials |
| Part of a series of articles on |
| Nanotechnology |
|---|
| Impact and applications |
| Nanomaterials |
| Molecular self-assembly |
| Nanoelectronics |
| Nanometrology |
| Molecular nanotechnology |
Nanomaterials describe, in principle, chemical substances or materials of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of nanoscale[1]).
Nanomaterials research takes a materials science-based approach to nanotechnology, leveraging advances in materials metrology and synthesis which have been developed in support of microfabrication research. Materials with structure at the nanoscale often have unique optical, electronic, thermo-physical or mechanical properties.[2][3][4]
Nanomaterials are slowly becoming commercialized[5] and beginning to emerge as commodities.[6]
Definition
[edit]In ISO/TS 80004, nanomaterial is defined as the "material with any external dimension in the nanoscale or having internal structure or surface structure in the nanoscale", with nanoscale defined as the "length range approximately from 1 nm to 100 nm". This includes both nano-objects, which are discrete pieces of material, and nanostructured materials, which have internal or surface structure on the nanoscale; a nanomaterial may be a member of both these categories.[7]
On 18 October 2011, the European Commission adopted the following definition of a nanomaterial:[8]
A natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1 nm – 100 nm. In specific cases and where warranted by concerns for the environment, health, safety or competitiveness the number size distribution threshold of 50% may be replaced by a threshold between 1% to 50%.
Sources
[edit]Engineered
[edit]Engineered nanomaterials have been deliberately engineered and manufactured by humans to have certain required properties.[4][9]
Legacy nanomaterials are those that were in commercial production prior to the development of nanotechnology as incremental advancements over other colloidal or particulate materials.[10][11][12] They include carbon black and titanium dioxide nanoparticles.[13]
Incidental
[edit]Nanomaterials may be unintentionally produced as a byproduct of mechanical or industrial processes through combustion and vaporization. Sources of incidental nanoparticles include vehicle engine exhausts, smelting, welding fumes, combustion processes from domestic solid fuel heating and cooking. For instance, the class of nanomaterials called fullerenes are generated by burning gas, biomass, and candle.[14] It can also be a byproduct of wear and corrosion products.[15] Incidental atmospheric nanoparticles are often referred to as ultrafine particles, which are unintentionally produced during an intentional operation, and could contribute to air pollution.[16][17]
Natural
[edit]Biological systems often feature natural, functional nanomaterials. The structure of foraminifera (mainly chalk) and viruses (protein, capsid), the wax crystals covering a lotus or nasturtium leaf, spider and spider-mite silk,[18] the blue hue of tarantulas,[19] the "spatulae" on the bottom of gecko feet, some butterfly wing scales, natural colloids (milk, blood), horny materials (skin, claws, beaks, feathers, horns, hair), paper, cotton, nacre, corals, and even our own bone matrix are all natural organic nanomaterials.
Natural inorganic nanomaterials occur through crystal growth in the diverse chemical conditions of the Earth's crust. For example, clays display complex nanostructures due to anisotropy of their underlying crystal structure, and volcanic activity can give rise to opals, which are an instance of a naturally occurring photonic crystals due to their nanoscale structure. Fires represent particularly complex reactions and can produce pigments, cement, fumed silica etc.
Natural sources of nanoparticles include combustion products forest fires, volcanic ash, ocean spray, and the radioactive decay of radon gas. Natural nanomaterials can also be formed through weathering processes of metal- or anion-containing rocks, as well as at acid mine drainage sites.[16]
- Gallery of natural nanomaterials
-
Viral capsid
-
"Lotus effect", hydrophobic effect with self-cleaning ability
-
Close-up of the underside of a gecko's foot as it walks on a glass wall (spatula: 200 × 10–15 nm)
-
SEM micrograph of a butterfly wing scale (× 5000)
-
Peacock feather (detail)
-
Brazilian Crystal Opal. The play of color is caused by the interference and diffraction of light between silica spheres (150–300 nm in diameter).
-
Blue hue of a species of tarantula (450 nm ± 20 nm)
Types
[edit]Nano-materials are often categorized as to how many of their dimensions fall in the nanoscale. A nanoparticle is defined a nano-object with all three external dimensions in the nanoscale, whose longest and the shortest axes do not differ significantly. A nanofiber has two external dimensions in the nanoscale, with nanotubes being hollow nanofibers and nanorods being solid nanofibers. A nanoplate/nanosheet has one external dimension in the nanoscale,[20] and if the two larger dimensions are significantly different it is called a nanoribbon. For nanofibers and nanoplates, the other dimensions may or may not be in the nanoscale, but must be significantly larger. In all of these cases, a significant difference is noted to typically be at least a factor of 3.[21]
Nanostructured materials are often categorized by what phases of matter they contain. A nanocomposite is a solid containing at least one physically or chemically distinct region or collection of regions, having at least one dimension in the nanoscale. A nanofoam has a liquid or solid matrix, filled with a gaseous phase, where one of the two phases has dimensions on the nanoscale. A nanoporous material is a solid material containing nanopores, voids in the form of open or closed pores of sub-micron lengthscales. A nanocrystalline material has a significant fraction of crystal grains in the nanoscale.[22]
Nanoporous materials
[edit]The term nanoporous materials contain subsets of microporous and mesoporous materials. Microporous materials are porous materials with a mean pore size smaller than 2 nm, while mesoporous materials are those with pores sizes in the region 2–50 nm.[23] Microporous materials exhibit pore sizes with comparable length-scale to small molecules. For this reason such materials may serve valuable applications including separation membranes. Mesoporous materials are interesting towards applications that require high specific surface areas, while enabling penetration for molecules that may be too large to enter the pores of a microporous material. In some sources, nanoporous materials and nanofoam are sometimes considered nanostructures but not nanomaterials because only the voids and not the materials themselves are nanoscale.[24] Although the ISO definition only considers round nano-objects to be nanoparticles, other sources use the term nanoparticle for all shapes.[25]
Nanoparticles
[edit]Nanoparticles have all three dimensions on the nanoscale. Nanoparticles can also be embedded in a bulk solid to form a nanocomposite.[24]
Fullerenes
[edit]The fullerenes are a class of allotropes of carbon which conceptually are graphene sheets rolled into tubes or spheres. These include the carbon nanotubes (or silicon nanotubes) which are of interest both because of their mechanical strength and also because of their electrical properties.[26]

The first fullerene molecule to be discovered, and the family's namesake, buckminsterfullerene (C60), was prepared in 1985 by Richard Smalley, Robert Curl, James Heath, Sean O'Brien, and Harold Kroto at Rice University. The name was a homage to Buckminster Fuller, whose geodesic domes it resembles. Fullerenes have since been found to occur in nature.[27] More recently, fullerenes have been detected in outer space.[28]
For the past decade, the chemical and physical properties of fullerenes have been a hot topic in the field of research and development, and are likely to continue to be for a long time. In April 2003, fullerenes were under study for potential medicinal use: binding specific antibiotics to the structure of resistant bacteria and even target certain types of cancer cells such as melanoma. The October 2005 issue of Chemistry and Biology contains an article describing the use of fullerenes as light-activated antimicrobial agents. In the field of nanotechnology, heat resistance and superconductivity are among the properties attracting intense research.
A common method used to produce fullerenes is to send a large current between two nearby graphite electrodes in an inert atmosphere. The resulting carbon plasma arc between the electrodes cools into sooty residue from which many fullerenes can be isolated.
There are many calculations that have been done using ab-initio Quantum Methods applied to fullerenes. By DFT and TDDFT methods one can obtain IR, Raman, and UV spectra. Results of such calculations can be compared with experimental results.
Metal-based nanoparticles
[edit]Inorganic nanomaterials, (e.g. quantum dots,[29] nanowires, and nanorods) because of their interesting optical and electrical properties, could be used in optoelectronics.[30] Furthermore, the optical and electronic properties of nanomaterials which depend on their size and shape can be tuned via synthetic techniques. There are the possibilities to use those materials in organic material based optoelectronic devices such as organic solar cells, OLEDs etc. The operating principles of such devices are governed by photoinduced processes like electron transfer and energy transfer. The performance of the devices depends on the efficiency of the photoinduced process responsible for their functioning. Therefore, better understanding of those photoinduced processes in organic/inorganic nanomaterial composite systems is necessary in order to use them in optoelectronic devices.
Nanoparticles or nanocrystals made of metals, semiconductors, or oxides are of particular interest for their mechanical, electrical, magnetic, optical, chemical and other properties.[31][32] Nanoparticles have been used as quantum dots and as chemical catalysts such as nanomaterial-based catalysts. Recently, a range of nanoparticles are extensively investigated for biomedical applications including tissue engineering, drug delivery, biosensor.[33][34]
Nanoparticles are of great scientific interest as they are effectively a bridge between bulk materials and atomic or molecular structures. A bulk material should have constant physical properties regardless of its size, but at the nano-scale this is often not the case. Size-dependent properties are observed such as quantum confinement in semiconductor particles, surface plasmon resonance in some metal particles, and superparamagnetism in magnetic materials.
Nanoparticles exhibit a number of special properties relative to bulk material. For example, the bending of bulk copper (wire, ribbon, etc.) occurs with movement of copper atoms/clusters at about the 50 nm scale. Copper nanoparticles smaller than 50 nm are considered super hard materials that do not exhibit the same malleability and ductility as bulk copper. The change in properties is not always desirable. Ferroelectric materials smaller than 10 nm can switch their polarization direction using room temperature thermal energy, thus making them useless for memory storage. Suspensions of nanoparticles are possible because the interaction of the particle surface with the solvent is strong enough to overcome differences in density, which usually result in a material either sinking or floating in a liquid. Nanoparticles often have unexpected visual properties because they are small enough to confine their electrons and produce quantum effects. For example, gold nanoparticles appear deep red to black in solution.
The often very high surface area to volume ratio of nanoparticles provides a tremendous driving force for diffusion, especially at elevated temperatures. Sintering is possible at lower temperatures and over shorter durations than for larger particles. This theoretically does not affect the density of the final product, though flow difficulties and the tendency of nanoparticles to agglomerate do complicate matters. The surface effects of nanoparticles also reduces the incipient melting temperature.
One-dimensional nanostructures
[edit]The smallest possible crystalline wires with cross-section as small as a single atom can be engineered in cylindrical confinement.[35][36][37] Carbon nanotubes, a natural semi-1D nanostructure, can be used as a template for synthesis. Confinement provides mechanical stabilization and prevents linear atomic chains from disintegration; other structures of 1D nanowires are predicted to be mechanically stable even upon isolation from the templates.[36][37]
Two-dimensional nanostructures
[edit]2D materials are crystalline materials consisting of a two-dimensional single layer of atoms. The most important representative graphene was discovered in 2004. Thin films with nanoscale thicknesses are considered nanostructures, but are sometimes not considered nanomaterials because they do not exist separately from the substrate.[24][38]
Bulk nanostructured materials
[edit]Some bulk materials contain features on the nanoscale, including nanocomposites, nanocrystalline materials, nanostructured films, and nanotextured surfaces.[24]
Box-shaped graphene (BSG) nanostructure is an example of 3D nanomaterial.[39] BSG nanostructure has appeared after mechanical cleavage of pyrolytic graphite. This nanostructure is a multilayer system of parallel hollow nanochannels located along the surface and having quadrangular cross-section. The thickness of the channel walls is approximately equal to 1 nm. The typical width of channel facets makes about 25 nm.
Applications
[edit]Nano materials are used in a variety of, manufacturing processes, products and healthcare including paints, filters, insulation and lubricant additives. In healthcare Nanozymes are nanomaterials with enzyme-like characteristics.[40] They are an emerging type of artificial enzyme, which have been used for wide applications in such as biosensing, bioimaging, tumor diagnosis,[41] antibiofouling and more. High quality filters may be produced using nanostructures, these filters are capable of removing particulate as small as a virus as seen in a water filter created by Seldon Technologies. Nanomaterials membrane bioreactor (NMs-MBR), the next generation of conventional MBR, are recently proposed for the advanced treatment of wastewater.[42] In the air purification field, nano technology was used to combat the spread of MERS in Saudi Arabian hospitals in 2012.[43] Nanomaterials are being used in modern and human-safe insulation technologies; in the past they were found in Asbestos-based insulation.[44][unreliable source?] As a lubricant additive, nano materials have the ability to reduce friction in moving parts. Worn and corroded parts can also be repaired with self-assembling anisotropic nanoparticles called TriboTEX.[43] Nanomaterials have also been applied in a range of industries and consumer products. Mineral nanoparticles such as titanium-oxide have been used to improve UV protection in sunscreen. Phosphorus, carbon and nitrogen doped titanium-oxide nanoparticles are used as additive to water based paint for self-cleaning properties.[45] In the sports industry, lighter bats to have been produced with carbon nanotubes to improve performance. Another application is in the military, where mobile pigment nanoparticles have been used to create more effective camouflage. Nanomaterials can also be used in three-way-catalyst applications, which have the advantage of controlling the emission of nitrogen oxides (NOx), which are precursors to acid rain and smog.[46] In core-shell structure, nanomaterials form shell as the catalyst support to protect the noble metals such as palladium and rhodium.[47] The primary function is that the supports can be used for carrying catalysts active components, making them highly dispersed, reducing the use of noble metals, enhancing catalysts activity, and potentially improving the stability.[48]
Synthesis
[edit]The goal of any synthetic method for nanomaterials is to yield a material that exhibits properties that are a result of their characteristic length scale being in the nanometer range (1 – 100 nm). Accordingly, the synthetic method should exhibit control of size in this range so that one property or another can be attained. Often the methods are divided into two main types, "bottom up" and "top down".
Bottom-up methods
[edit]Bottom-up methods involve the assembly of atoms or molecules into nanostructured arrays. In these methods the raw material sources can be in the form of gases, liquids, or solids. The latter require some sort of disassembly prior to their incorporation onto a nanostructure. Bottom up methods generally fall into two categories: chaotic and controlled.
Chaotic processes involve elevating the constituent atoms or molecules to a chaotic state and then suddenly changing the conditions so as to make that state unstable. Through the clever manipulation of any number of parameters, products form largely as a result of the insuring kinetics. The collapse from the chaotic state can be difficult or impossible to control and so ensemble statistics often govern the resulting size distribution and average size. Accordingly, nanoparticle formation is controlled through manipulation of the end state of the products. Examples of chaotic processes are laser ablation,[49] exploding wire, arc, flame pyrolysis, combustion,[50] and precipitation synthesis techniques.
Controlled processes involve the controlled delivery of the constituent atoms or molecules to the site(s) of nanoparticle formation such that the nanoparticle can grow to a prescribed sizes in a controlled manner. Generally the state of the constituent atoms or molecules are never far from that needed for nanoparticle formation. Accordingly, nanoparticle formation is controlled through the control of the state of the reactants. Examples of controlled processes are self-limiting growth solution, self-limited chemical vapor deposition, shaped pulse femtosecond laser techniques, plant and microbial approaches[51] and molecular beam epitaxy.
Top-down methods
[edit]Top-down methods adopt some 'force' (e. g. mechanical force, laser) to break bulk materials into nanoparticles. A popular method involves mechanical break apart bulk materials into nanomaterials is 'ball milling'. Besides that, nanoparticles can also be made by laser ablation which apply short pulse lasers (e. g. femtosecond laser) to ablate a target (solid).[49]
Characterization
[edit]Novel effects can occur in materials when structures are formed with sizes comparable to any one of many possible length scales, such as the de Broglie wavelength of electrons, or the optical wavelengths of high energy photons. In these cases quantum mechanical effects can dominate material properties. One example is quantum confinement where the electronic properties of solids are altered with great reductions in particle size. The optical properties of nanoparticles, e.g. fluorescence, also become a function of the particle diameter. This effect does not come into play by going from macrosocopic to micrometer dimensions, but becomes pronounced when the nanometer scale is reached.
In addition to optical and electronic properties, the novel mechanical properties of many nanomaterials is the subject of nanomechanics research. When added to a bulk material, nanoparticles can strongly influence the mechanical properties of the material, such as the stiffness or elasticity. For example, traditional polymers can be reinforced by nanoparticles (such as carbon nanotubes) resulting in novel materials which can be used as lightweight replacements for metals. Such composite materials may enable a weight reduction accompanied by an increase in stability and improved functionality.[52]
Finally, nanostructured materials with small particle size, such as zeolites and asbestos, are used as catalysts in a wide range of critical industrial chemical reactions. The further development of such catalysts can form the basis of more efficient, environmentally friendly chemical processes.
The first observations and size measurements of nano-particles were made during the first decade of the 20th century. Zsigmondy made detailed studies of gold sols and other nanomaterials with sizes down to 10 nm and less. He published a book in 1914.[53] He used an ultramicroscope that employs a dark field method for seeing particles with sizes much less than light wavelength.
There are traditional techniques developed during the 20th century in interface and colloid science for characterizing nanomaterials. These are widely used for first generation passive nanomaterials specified in the next section.
These methods include several different techniques for characterizing particle size distribution. This characterization is imperative because many materials that are expected to be nano-sized are actually aggregated in solutions. Some of methods are based on light scattering. Others apply ultrasound, such as ultrasound attenuation spectroscopy for testing concentrated nano-dispersions and microemulsions.[54]
There is also a group of traditional techniques for characterizing surface charge or zeta potential of nano-particles in solutions. This information is required for proper system stabilization, preventing its aggregation or flocculation. These methods include microelectrophoresis, electrophoretic light scattering, and electroacoustics. The last one, for instance colloid vibration current method is suitable for characterizing concentrated systems.
Mechanical properties
[edit]The ongoing research has shown that mechanical properties can vary significantly in nanomaterials compared to bulk material. Nanomaterials have substantial mechanical properties due to the volume, surface, and quantum effects of nanoparticles. This is observed when the nanoparticles are added to common bulk material, the nanomaterial refines the grain and forms intergranular and intragranular structures which improve the grain boundaries and therefore the mechanical properties of the materials.[citation needed] Grain boundary refinements provide strengthening by increasing the stress required to cause intergranular or transgranular fractures. A common example where this can be observed is the addition of nano Silica to cement, which improves the tensile strength, compressive strength, and bending strength by the mechanisms just mentioned. The understanding of these properties will enhance the use of nanoparticles in novel applications in various fields such as surface engineering, tribology, nanomanufacturing, and nanofabrication.
Techniques used:
Steinitz in 1943 used the micro-indentation technique to test the hardness of microparticles, and now nanoindentation has been employed to measure elastic properties of particles at about 5-micron level.[55] These protocols are frequently used to calculate the mechanical characteristics of nanoparticles via atomic force microscopy (AFM) techniques. To measure the elastic modulus; indentation data is obtained via AFM force-displacement curves being converted to force-indentation curves. Hooke's law is used to determine the cantilever deformation and depth of the tip, and in conclusion, the pressure equation can be written as:[56]
P=k (ẟc - ẟc0) [57]
ẟc : cantilever deformation
ẟc0 : deflection ofset
AFM allows us to obtain a high-resolution image of multiple types of surfaces while the tip of the cantilever can be used to obtain information about mechanical properties. Computer simulations are also being progressively used to test theories and complement experimental studies. The most used computer method is molecular dynamics simulation,[58] which uses newton's equations of motion for the atoms or molecules in the system. Other techniques such direct probe method are used to determine the adhesive properties of nanomaterials. Both the technique and simulation are coupled with transmission electron microscope (TEM) and AFM techniques to provide results.
Mechanical properties of common nanomaterials classes:
Crystalline metal nanomaterials: Dislocations are one of the major contributors toward elastic properties within nanomaterials similar to bulk crystalline materials. Despite the traditional view of there being no dislocations in nanomaterials. Ramos,[59] experimental work has shown that the hardness of gold nanoparticles is much higher than their bulk counterparts, as there are stacking faults and dislocations forming that activate multiple strengthening mechanisms in the material. Through these experiments, more research has shown that via nanoindentation techniques,[60] material strength; compressive stress, increases under compression with decreasing particle size, because of nucleating dislocations. These dislocations have been observed using TEM techniques, coupled with nanoindentation. Silicon nanoparticles strength and hardness are four times more than the value of the bulk material.[57] The resistance to pressure applied can be attributed to the line defects inside the particles as well as a dislocation that provides strengthening of the mechanical properties of the nanomaterial. Furthermore, the addition of nanoparticles strengthens a matrix because the pinning of particles inhibits grain growth. This refines the grain, and hence improves the mechanical properties.[55] However, not all additions of nanomaterials lead to an increase in properties for example nano-Cu. But this is attributed to the inherent properties of the material being weaker than the matrix.
Nonmetallic nanoparticles and nanomaterials: Size-dependent behavior of mechanical properties is still not clear in the case of polymer nanomaterials however, in one research by Lahouij they found that the compressive moduli of polystyrene nanoparticles were found to be less than that of the bulk counterparts. This can be associated with the functional groups being hydrated.[61] Furthermore, nonmetallic nanomaterials can lead to agglomerates forming inside the matrix they are being added to and hence decrease the mechanical properties by leading to fracture under even low mechanical loads, such as the addition of CNTs. The agglomerates will act as slip planes as well as planes in which cracks can easily propagate (9). However, most organic nanomaterials are flexible and these and the mechanical properties such as hardness etc. are not dominant.[61]
Nanowires and nanotubes: The elastic moduli of some nanowires namely lead and silver, decrease with increasing diameter. This has been associated with surface stress, oxidation layer, and surface roughness.[62] However, the elastic behavior of ZnO nanowires does not get affected by surface effects but their fracture properties do. So, it is generally dependent on material behavior and their bonding as well.[63]
The reason why mechanical properties of nanomaterials are still a hot topic for research is that measuring the mechanical properties of individual nanoparticles is a complicated method, involving multiple control factors. Nonetheless, Atomic force microscopy has been widely used to measure the mechanical properties of nanomaterials.
Adhesion and friction of nanoparticles
When talking about the application of a material adhesion and friction play a critical role in determining the outcome of the application. Therefore, it is critical to see how these properties also get affected by the size of a material. Again, AFM is a technique most used to measure these properties and to determine the adhesive strength of nanoparticles to any solid surface, along with the colloidal probe technique and other chemical properties.[64] Furthermore, the forces playing a role in providing these adhesive properties to nanomaterials are either the electrostatic forces, VdW, capillary forces, solvation forces, structure force, etc. It has been found that the addition of nanomaterials in bulk materials substantially increases their adhesive capabilities by increasing their strength through various bonding mechanisms.[65] Nanomaterials dimension approaches zero, which means that the fraction of the particle's surface to overall atoms increases.
Along with surface effects, the movement of nanoparticles also plays a role in dictating their mechanical properties such as shearing capabilities. The movement of particles can be observed under TEM. For example, the movement behavior of MoS2 [66] nanoparticles dynamic contact was directly observed in situ which led to the conclusion that fullerenes can shear via rolling or sliding. However, observing these properties is again a very complicated process due to multiple contributing factors.
Applications specific to Mechanical Properties:[67]
- Lubrication
- Nano-manufacturing
- Coatings
Uniformity
[edit]The chemical processing and synthesis of high performance technological components for the private, industrial and military sectors requires the use of high purity ceramics, polymera, glass-ceramics, and composite materials. In condensed bodies formed from fine powders, the irregular sizes and shapes of nanoparticles in a typical powder often lead to non-uniform packing morphologies that result in packing density variations in the powder compact.
Uncontrolled agglomeration of powders due to attractive van der Waals forces can also give rise to in microstructural inhomogeneities. Differential stresses that develop as a result of non-uniform drying shrinkage are directly related to the rate at which the solvent can be removed, and thus highly dependent upon the distribution of porosity. Such stresses have been associated with a plastic-to-brittle transition in consolidated bodies, and can yield to crack propagation in the unfired body if not relieved.[68][69][70]
In addition, any fluctuations in packing density in the compact as it is prepared for the kiln are often amplified during the sintering process, yielding inhomogeneous densification. Some pores and other structural defects associated with density variations have been shown to play a detrimental role in the sintering process by growing and thus limiting end-point densities. Differential stresses arising from inhomogeneous densification have also been shown to result in the propagation of internal cracks, thus becoming the strength-controlling flaws.[71][72]
It would therefore appear desirable to process a material in such a way that it is physically uniform with regard to the distribution of components and porosity, rather than using particle size distributions which will maximize the green density. The containment of a uniformly dispersed assembly of strongly interacting particles in suspension requires total control over particle-particle interactions. A number of dispersants such as ammonium citrate (aqueous) and imidazoline or oleyl alcohol (nonaqueous) are promising solutions as possible additives for enhanced dispersion and deagglomeration. Monodisperse nanoparticles and colloids provide this potential.[73]
Monodisperse powders of colloidal silica, for example, may therefore be stabilized sufficiently to ensure a high degree of order in the colloidal crystal or polycrystalline colloidal solid which results from aggregation. The degree of order appears to be limited by the time and space allowed for longer-range correlations to be established. Such defective polycrystalline colloidal structures would appear to be the basic elements of sub-micrometer colloidal materials science, and, therefore, provide the first step in developing a more rigorous understanding of the mechanisms involved in microstructural evolution in high performance materials and components.[74][75]
Nanomaterials in articles, patents, and products
[edit]The quantitative analysis of nanomaterials showed that nanoparticles, nanotubes, nanocrystalline materials, nanocomposites, and graphene have been mentioned in 400,000, 181,000, 144,000, 140,000, and 119,000 ISI-indexed articles, respectively, by September 2018. As far as patents are concerned, nanoparticles, nanotubes, nanocomposites, graphene, and nanowires have been played a role in 45,600, 32,100, 12,700, 12,500, and 11,800 patents, respectively. Monitoring approximately 7,000 commercial nano-based products available on global markets revealed that the properties of around 2,330 products have been enabled or enhanced aided by nanoparticles. Liposomes, nanofibers, nanocolloids, and aerogels were also of the most common nanomaterials in consumer products.[76]
The European Union Observatory for Nanomaterials (EUON) has produced a database (NanoData) that provides information on specific patents, products, and research publications on nanomaterials.
Health and safety
[edit]This section needs to be updated. The reason given is: [1] Quote:
“... nanotechnology products classified (2019–2023) according with industrial areas: electronics, medicine, construction, cosmetics,etc...”. (March 2024) |
World Health Organization guidelines
[edit]The World Health Organization (WHO) published a guideline on protecting workers from potential risk of manufactured nanomaterials at the end of 2017.[77] WHO used a precautionary approach as one of its guiding principles. This means that exposure has to be reduced, despite uncertainty about the adverse health effects, when there are reasonable indications to do so. This is highlighted by recent scientific studies that demonstrate a capability of nanoparticles to cross cell barriers and interact with cellular structures.[78][79] In addition, the hierarchy of controls was an important guiding principle. This means that when there is a choice between control measures, those measures that are closer to the root of the problem should always be preferred over measures that put a greater burden on workers, such as the use of personal protective equipment (PPE). WHO commissioned systematic reviews for all important issues to assess the current state of the science and to inform the recommendations according to the process set out in the WHO Handbook for guideline development. The recommendations were rated as "strong" or "conditional" depending on the quality of the scientific evidence, values and preferences, and costs related to the recommendation.
The WHO guidelines contain the following recommendations for safe handling of manufactured nanomaterials (MNMs)
A. Assess health hazards of MNMs
- WHO recommends assigning hazard classes to all MNMs according to the Globally Harmonized System (GHS) of Classification and Labelling of Chemicals for use in safety data sheets. For a limited number of MNMs this information is made available in the guidelines (strong recommendation, moderate-quality evidence).
- WHO recommends updating safety data sheets with MNM-specific hazard information or indicating which toxicological end-points did not have adequate testing available (strong recommendation, moderate-quality evidence).
- For the respirable fibres and granular biopersistent particles' groups, the GDG suggests using the available classification of MNMs for provisional classification of nanomaterials of the same group (conditional recommendation, low-quality evidence).
B. Assess exposure to MNMs
- WHO suggests assessing workers' exposure in workplaces with methods similar to those used for the proposed specific occupational exposure limit (OEL) value of the MNM (conditional recommendation, low-quality evidence).
- Because there are no specific regulatory OEL values for MNMs in workplaces, WHO suggests assessing whether workplace exposure exceeds a proposed OEL value for the MNM. A list of proposed OEL values is provided in an annex of the guidelines. The chosen OEL should be at least as protective as a legally mandated OEL for the bulk form of the material (conditional recommendation, low-quality evidence).
- If specific OELs for MNMs are not available in workplaces, WHO suggests a step-wise approach for inhalation exposure with, first an assessment of the potential for exposure; second, conducting basic exposure assessment and third, conducting a comprehensive exposure assessment such as those proposed by the Organisation for Economic Cooperation and Development (OECD) or Comité Européen de Normalisation (the European Committee for Standardization, CEN) (conditional recommendation, moderate quality evidence).
- For dermal exposure assessment, WHO found that there was insufficient evidence to recommend one method of dermal exposure assessment over another.
C. Control exposure to MNMs
- Based on a precautionary approach, WHO recommends focusing control of exposure on preventing inhalation exposure with the aim of reducing it as much as possible (strong recommendation, moderate-quality evidence).
- WHO recommends reduction of exposures to a range of MNMs that have been consistently measured in workplaces especially during cleaning and maintenance, collecting material from reaction vessels and feeding MNMs into the production process. In the absence of toxicological information, WHO recommends implementing the highest level of controls to prevent workers from any exposure. When more information is available, WHO recommends taking a more tailored approach (strong recommendation, moderate-quality evidence).
- WHO recommends taking control measures based on the principle of hierarchy of controls, meaning that the first control measure should be to eliminate the source of exposure before implementing control measures that are more dependent on worker involvement, with PPE being used only as a last resort. According to this principle, engineering controls should be used when there is a high level of inhalation exposure or when there is no, or very little, toxicological information available. In the absence of appropriate engineering controls PPE should be used, especially respiratory protection, as part of a respiratory protection programme that includes fit-testing (strong recommendation, moderate-quality evidence).
- WHO suggests preventing dermal exposure by occupational hygiene measures such as surface cleaning, and the use of appropriate gloves (conditional recommendation, low quality evidence).
- When assessment and measurement by a workplace safety expert is not available, WHO suggests using control banding for nanomaterials to select exposure control measures in the workplace. Owing to a lack of studies, WHO cannot recommend one method of control banding over another (conditional recommendation, very low-quality evidence).
For health surveillance WHO could not make a recommendation for targeted MNM-specific health surveillance programmes over existing health surveillance programmes that are already in use owing to the lack of evidence. WHO considers training of workers and worker involvement in health and safety issues to be best practice but could not recommend one form of training of workers over another, or one form of worker involvement over another, owing to the lack of studies available. It is expected that there will be considerable progress in validated measurement methods and risk assessment and WHO expects to update these guidelines in five years' time, in 2022.[needs update]
Other guidance
[edit]Because nanotechnology is a recent development, the health and safety effects of exposures to nanomaterials, and what levels of exposure may be acceptable, are subjects of ongoing research.[9] Of the possible hazards, inhalation exposure appears to present the most concern. Animal studies indicate that carbon nanotubes and carbon nanofibers can cause pulmonary effects including inflammation, granulomas, and pulmonary fibrosis, which were of similar or greater potency when compared with other known fibrogenic materials such as silica, asbestos, and ultrafine carbon black. Acute inhalation exposure of healthy animals to biodegradable inorganic nanomaterials have not demonstrated significant toxicity effects.[80] Although the extent to which animal data may predict clinically significant lung effects in workers is not known, the toxicity seen in the short-term animal studies indicate a need for protective action for workers exposed to these nanomaterials, although no reports of actual adverse health effects in workers using or producing these nanomaterials were known as of 2013.[81] Additional concerns include skin contact and ingestion exposure,[81][82][83] and dust explosion hazards.[84][85]
Elimination and substitution are the most desirable approaches to hazard control. While the nanomaterials themselves often cannot be eliminated or substituted with conventional materials,[9] it may be possible to choose properties of the nanoparticle such as size, shape, functionalization, surface charge, solubility, agglomeration, and aggregation state to improve their toxicological properties while retaining the desired functionality.[86] Handling procedures can also be improved, for example, using a nanomaterial slurry or suspension in a liquid solvent instead of a dry powder will reduce dust exposure.[9] Engineering controls are physical changes to the workplace that isolate workers from hazards, mainly ventilation systems such as fume hoods, gloveboxes, biosafety cabinets, and vented balance enclosures.[87] Administrative controls are changes to workers' behavior to mitigate a hazard, including training on best practices for safe handling, storage, and disposal of nanomaterials, proper awareness of hazards through labeling and warning signage, and encouraging a general safety culture. Personal protective equipment must be worn on the worker's body and is the least desirable option for controlling hazards.[9] Personal protective equipment normally used for typical chemicals are also appropriate for nanomaterials, including long pants, long-sleeve shirts, and closed-toed shoes, and the use of safety gloves, goggles, and impervious laboratory coats.[87] In some circumstances respirators may be used.[86]
Exposure assessment is a set of methods used to monitor contaminant release and exposures to workers. These methods include personal sampling, where samplers are located in the personal breathing zone of the worker, often attached to a shirt collar to be as close to the nose and mouth as possible; and area/background sampling, where they are placed at static locations. The assessment should use both particle counters, which monitor the real-time quantity of nanomaterials and other background particles; and filter-based samples, which can be used to identify the nanomaterial, usually using electron microscopy and elemental analysis.[86][88] As of 2016, quantitative occupational exposure limits have not been determined for most nanomaterials. The U.S. National Institute for Occupational Safety and Health has determined non-regulatory recommended exposure limits for carbon nanotubes, carbon nanofibers,[81] and ultrafine titanium dioxide.[89] Agencies and organizations from other countries, including the British Standards Institute[90] and the Institute for Occupational Safety and Health in Germany,[91] have established OELs for some nanomaterials, and some companies have supplied OELs for their products.[9]
Nanoscale diagnostics
Nanotechnology has been making headlines in the medical field,[92] being responsible for biomedical imaging. The unique optical, magnetic and chemical properties of materials on the Nano scale has allowed the development of imaging probes with multi-functionality such as better contrast enhancement, better spatial information, controlled bio distribution, and multi-modal imaging across various scanning devices. These developments have had advantages such as being able to detect the location of tumors and inflammations, accurate assessment of disease progression, and personalized medicine.
- Silica nanoparticles- Silica nanoparticles[93] can be classified into solid, non-porous, and mesoporous. They have large surface are, hydrophilic surface, and chemical and physical stabilities. Silica nanoparticles are made by the use of the Stöber process. Which is the hydrolysis of silyl ethers such as tetraethyl silicate into silanols (Si-OH) using ammonia in a mixture of water and alcohol followed by the condensation of silanols into 50–2000 nm silica particles. The size of the particle can be controlled by varying the concentration of silyl ether and alcohol or the micro emulsion method. Mesoporous silica nanoparticles are synthesized by the sol-gel process. They have pores that range in diameter from 2 nm to 50 nm. They are synthesized in a water-based solution in the presence of a base catalyst and a pore forming agent known as a surfactant. Surfactants are molecules that present the particularity to have a hydrophobic tail (alkyl chain) and a hydrophilic head (charged group, such as a quaternary amine for example). As these surfactants are added to a water-based solution, they will coordinate to form micelles with increasing concentration in order to stabilize the hydrophobic tails. Varying the pH of the solution and composition of the solvents, and the addition of certain swelling agents can control the pore size. Their hydrophilic surface is what makes silica nanoparticles so important and allows them to carry out functions such as drug and gene delivery, bio imaging and therapy. In order for this application to be successful, assorted surface functional groups are necessary and can be added either by the co-condensation process during preparation or by post surface modification. The high surface area of silica nanoparticles allows them to carry much larger amounts of the desired drug than through conventional methods like polymers and liposomes. It allows for site specific targeting, especially in the treatment of cancer. Once the particles have reached their destination, they can act as a reporter, release a compound, or be remotely heated to damage biological structures in close proximity. Targeting is typically accomplished by modifying the surface of the nanoparticle with a chemical or biological compound. They accumulate at tumor sites through Enhanced Permeability Retention (EPR), where the tumor vessels accelerate the delivery of the nanoparticles directly into the tumor. The porous shell of the silica allows control over the rate at which the drug diffuses out of the nanoparticle. The shell can be modified to have an affinity for the drug, or even to be triggered by pH, heat, light, salts, or other signaling molecules. Silica nanoparticles are also used in bio imaging because they can accommodate fluorescent/MRI/PET/ SPECT contrast agents and drug/DNA molecules to their adaptable surface and pores. This is made possible by using the silica nanoparticle as a vector for the expression of fluorescent proteins. Several different types of fluorescent probes, like cyanine dyes, methyl violegen, or semiconductor quantum dots can be conjugated to silica nanoparticles and delivered into specific cells or injected in vivo. Carrier molecule RGD peptide has been very useful of targeted in vivo imaging.
- Topically applied surface-enhanced resonance Raman ratiometric spectroscopy (TAS3RS)[94]- TAS3RS is another technique that is starting to make advancement in the medical field. It is an imaging technique that uses Folate Receptors (FR) to detect tumor lesions as small as 370 micrometers. Folate Receptors are membrane bound surface proteins that bind folates and folate conjugates with high affinity. FR is frequently overexpressed in a number of human malignancies including cancer of the ovary, lung, kidney, breast, bladder, brain, and endometrium. Raman imaging is a type of spectroscopy that is used in chemistry to provide structural fingerprint by which molecules can be identified. It relies upon inelastic scattering of photons, which result in ultra high sensitivity. There was a study that was done where two different surface enhanced resonance Raman scattering were synthesized (SERRS). One of the SERRS was a "targeted nanoprobe functionalized with an anti-folate-receptor antibody (αFR-Ab) via a PEG-maleimide-succinimide and using the infrared dye IR780 as the Raman reporter, henceforth referred to as αFR-NP, and a nontargeted probe (nt-NP) coated with PEG5000-maleimide and featuring the IR140 infrared dye as the Raman reporter." These two different mixtures were injected into tumor bearing mice and healthy controlled mice. The mice were imaged with Bioluminescence (BLI) signal that produces light energy within an organism's body. They were also scanned with the Raman microscope in order to be able to see the correlation between the TAS3RS and the BLI map. TAS3RS did not show anything in the healthy mice, but was able to locate the tumor lesions in the infected mice and also able to create a TAS3RS map that could be used as guidance during surgery. TAS3RS shows to be promising in being able to combat ovarian and peritoneal cancer as it allows early detection with high accuracy. This technique can be administered locally, which is an advantage as it does not have to enter the bloodstream and therefore bypassing the toxicity concerns circulating nanoprobes. This technique is also more photostable than fluorochromes because SERRS nanoparticles cannot form from biomolecules and therefore there would not be any false positives in TAS3RS as there is in fluorescence imaging.
See also
[edit]- Artificial enzyme
- Directional freezing
- List of software for nanostructures modeling
- Nanostructure
- Nanotopography
- Nanozymes
References
[edit]- ^ Buzea, Cristina; Pacheco, Ivan; Robbie, Kevin (2007). "Nanomaterials and Nanoparticles: Sources and Toxicity". Biointerphases. 2 (4): MR17–MR71. arXiv:0801.3280. doi:10.1116/1.2815690. PMID 20419892. S2CID 35457219.
- ^ Sadri, Rad (1 January 2018). "A facile, bio-based, novel approach for synthesis of covalently functionalized graphene nanoplatelet nano-coolants toward improved thermo-physical and heat transfer properties". Journal of Colloid and Interface Science. 509: 140–152. Bibcode:2018JCIS..509..140S. doi:10.1016/j.jcis.2017.07.052. PMID 28898734.
- ^ Hubler, A.; Osuagwu, O. (2010). "Digital quantum batteries: Energy and information storage in nanovacuum tube arrays". Complexity. 15 (5): 48–55. doi:10.1002/cplx.20306.
- ^ a b Portela, Carlos M.; Vidyasagar, A.; Krödel, Sebastian; Weissenbach, Tamara; Yee, Daryl W.; Greer, Julia R.; Kochmann, Dennis M. (2020). "Extreme mechanical resilience of self-assembled nanolabyrinthine materials". Proceedings of the National Academy of Sciences. 117 (11): 5686–5693. Bibcode:2020PNAS..117.5686P. doi:10.1073/pnas.1916817117. ISSN 0027-8424. PMC 7084143. PMID 32132212.
- ^ Eldridge, T. (8 January 2014). "Achieving industry integration with nanomaterials through financial markets". Nanotechnology_Now.
- ^ McGovern, C. (2010). "Commoditization of nanomaterials". Nanotechnol. Perceptions. 6 (3): 155–178. doi:10.4024/N15GO10A.ntp.06.03.
- ^ "ISO/TS 80004-1:2015 - Nanotechnologies – Vocabulary – Part 1: Core terms". International Organization for Standardization. 2015. Retrieved 8 January 2018.
- ^ Nanomaterials. European Commission. Last updated 18 October 2011
- ^ a b c d e f Current Strategies for Engineering Controls in Nanomaterial Production and Downstream Handling Processes. U.S. National Institute for Occupational Safety and Health (Report). November 2013. pp. 1–3, 7, 9–10, 17–20. doi:10.26616/NIOSHPUB2014102. Retrieved 5 March 2017.
- ^ "A New Integrated Approach for Risk Assessment and Management of Nanotechnologies" (PDF). EU Sustainable Nanotechnologies Project. 2017. pp. 109–112. Retrieved 6 September 2017.
- ^ "Compendium of Projects in the European NanoSafety Cluster". EU NanoSafety Cluster. 26 June 2017. p. 10. Archived from the original on 24 March 2012. Retrieved 7 September 2017.
- ^ "Future challenges related to the safety of manufactured nanomaterials". Organisation for Economic Co-operation and Development. 4 November 2016. p. 11. Retrieved 6 September 2017.
- ^ Taking Stock of the OSH Challenges of Nanotechnology: 2000 – 2015 (Report). The Windsdor Consulting Group, Inc. 18 August 2016 – via SlideShare.
- ^ Barcelo, Damia; Farre, Marinella (2012). Analysis and Risk of Nanomaterials in Environmental and Food Samples. Oxford: Elsevier. p. 291. ISBN 9780444563286.
- ^ Sahu, Saura; Casciano, Daniel (2009). Nanotoxicity: From in Vivo and in Vitro Models to Health Risks. Chichester, West Sussex: John Wiley & Sons. p. 227. ISBN 9780470741375.
- ^ a b "Radiation Safety Aspects of Nanotechnology". National Council on Radiation Protection and Measurements. 2 March 2017. pp. 11–15. Retrieved 7 July 2017.
- ^ Kim, Richard (2014). Asphalt Pavements, Vol. 1. Boca Raton, FL: CRC Press. p. 41. ISBN 9781138027121.
- ^ Novel natural nanomaterial spins off from spider-mite genome sequencing. Phys.Org (23 May 2013)
- ^ "Why Are Tarantulas Blue?". iflscience. 28 November 2015.
- ^ Rawat, Pankaj Singh; Srivastava, R.C.; Dixit, Gagan; Asokan, K. (2020). "Structural, functional and magnetic ordering modifications in graphene oxide and graphite by 100 MeV gold ion irradiation". Vacuum. 182: 109700. Bibcode:2020Vacuu.182j9700R. doi:10.1016/j.vacuum.2020.109700. S2CID 225410221.
- ^ "ISO/TS 80004-2:2015 - Nanotechnologies – Vocabulary – Part 2: Nano-objects". International Organization for Standardization. 2015. Retrieved 8 January 2018.
- ^ "ISO/TS 80004-4:2011 - Nanotechnologies – Vocabulary – Part 4: Nanostructured materials". International Organization for Standardization. 2011. Retrieved 8 January 2018.
- ^ Doustkhah E, et al. (2021). "Bispropylurea bridged polysilsesquioxane: A microporous MOF-likematerial for molecular recognition". Chemosphere. 276: 130181. arXiv:2104.06715. Bibcode:2021Chmsp.27630181D. doi:10.1016/j.chemosphere.2021.130181. PMID 33735650. S2CID 232304875.
- ^ a b c d "Eighth Nanoforum Report: Nanometrology" (PDF). Nanoforum. July 2006. pp. 13–14. Archived from the original (PDF) on 20 October 2007. Retrieved 28 August 2017.
- ^ Klaessig, Fred; Marrapese, Martha; Abe, Shuji (2011). Nanotechnology Standards. Nanostructure Science and Technology. Springer, New York, NY. pp. 21–52. doi:10.1007/978-1-4419-7853-0_2. ISBN 9781441978523.
- ^ "Fullerenes". Encyclopædia Britannica.
- ^ Buseck, P.R.; Tsipursky, S.J.; Hettich, R. (1992). "Fullerenes from the Geological Environment". Science. 257 (5067): 215–7. Bibcode:1992Sci...257..215B. doi:10.1126/science.257.5067.215. PMID 17794751. S2CID 4956299.
- ^ Cami, J; Bernard-Salas, J.; Peeters, E.; Malek, S. E. (2 September 2010). "Detection of C60 and C70 in a Young Planetary Nebula" (PDF). Science. 329 (5996): 1180–2. Bibcode:2010Sci...329.1180C. doi:10.1126/science.1192035. PMID 20651118. S2CID 33588270. Archived from the original (PDF) on 3 March 2019.
- ^ Shishodia, Shubham; Chouchene, Bilel; Gries, Thomas; Schneider, Raphaël (31 October 2023). "Selected I-III-VI2 Semiconductors: Synthesis, Properties and Applications in Photovoltaic Cells". Nanomaterials. 13 (21): 2889. doi:10.3390/nano13212889. ISSN 2079-4991. PMC 10648425. PMID 37947733.
- ^ Zeng, S.; Baillargeat, Dominique; Ho, Ho-Pui; Yong, Ken-Tye (2014). "Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications". Chemical Society Reviews. 43 (10): 3426–3452. doi:10.1039/C3CS60479A. hdl:10356/102043. PMID 24549396.
- ^ Stephenson, C.; Hubler, A. (2015). "Stability and conductivity of self assembled wires in a transverse electric field". Sci. Rep. 5: 15044. Bibcode:2015NatSR...515044S. doi:10.1038/srep15044. PMC 4604515. PMID 26463476.
- ^ Hubler, A.; Lyon, D. (2013). "Gap size dependence of the dielectric strength in nano vacuum gaps". IEEE Transactions on Dielectrics and Electrical Insulation. 20 (4): 1467–1471. doi:10.1109/TDEI.2013.6571470. S2CID 709782.
- ^ Valenti G, Rampazzo R, Bonacchi S, Petrizza L, Marcaccio M, Montalti M, Prodi L, Paolucci F (2016). "Variable Doping Induces Mechanism Swapping in Electrogenerated Chemiluminescence of Ru(bpy)32+ Core−Shell Silica Nanoparticles". J. Am. Chem. Soc. 138 (49): 15935–15942. doi:10.1021/jacs.6b08239. hdl:11585/583548. PMID 27960352.
- ^ Kerativitayanan, P; Carrow, JK; Gaharwar, AK (26 May 2015). "Nanomaterials for Engineering Stem Cell Responses". Advanced Healthcare Materials. 4 (11): 1600–27. doi:10.1002/adhm.201500272. PMID 26010739. S2CID 21582516.
- ^ Suenaga R, Komsa H, Liu Z, Hirose-Takai K, Krasheninnikov A, Suenaga K (2014). "Atomic structure and dynamic behaviour of truly one-dimensional ionic chains inside carbon nanotubes". Nat. Mater. 13 (11): 1050–1054. Bibcode:2014NatMa..13.1050S. doi:10.1038/nmat4069. PMID 25218060.
- ^ a b Medeiros PV, Marks S, Wynn JM, Vasylenko A, Ramasse QM, Quigley D, Sloan J, Morris AJ (2017). "Single-Atom Scale Structural Selectivity in Te Nanowires Encapsulated inside Ultranarrow, Single-Walled Carbon Nanotubes". ACS Nano. 11 (6): 6178–6185. arXiv:1701.04774. doi:10.1021/acsnano.7b02225. PMID 28467832. S2CID 30388342.
- ^ a b Vasylenko A, Marks S, Wynn JM, Medeiros PV, Ramasse QM, Morris AJ, Sloan J, Quigley D (2018). "Electronic Structure Control of Sub-nanometer 1D SnTe via Nanostructuring within Single-Walled Carbon Nanotubes" (PDF). ACS Nano. 12 (6): 6023–6031. doi:10.1021/acsnano.8b02261. PMID 29782147.
- ^ "Structural, functional and magnetic ordering modifications in graphene oxide and graphite by 100 MeV gold ion irradiation". Vacuum. 182: 109700. 2020-12-01. doi:10.1016/j.vacuum.2020.109700
- ^ Lapshin, Rostislav V. (January 2016). "STM observation of a box-shaped graphene nanostructure appeared after mechanical cleavage of pyrolytic graphite". Applied Surface Science. 360: 451–460. arXiv:1611.04379. Bibcode:2016ApSS..360..451L. doi:10.1016/j.apsusc.2015.09.222. S2CID 119369379.
- ^ Wei, Hui; Wang, Erkang (21 June 2013). "Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes". Chemical Society Reviews. 42 (14): 6060–93. doi:10.1039/C3CS35486E. PMID 23740388.
- ^ Juzgado, A.; Solda, A.; Ostric, A.; Criado, A.; Valenti, G.; Rapino, S.; Conti, G.; Fracasso, G.; Paolucci, F.; Prato, M. (2017). "Highly sensitive electrochemiluminescence detection of a prostate cancer biomarker". J. Mater. Chem. B. 5 (32): 6681–6687. doi:10.1039/c7tb01557g. PMID 32264431.
- ^ Pervez, Md Nahid; Balakrishnan, Malini; Hasan, Shadi Wajih; Choo, Kwang-Ho; Zhao, Yaping; Cai, Yingjie; Zarra, Tiziano; Belgiorno, Vincenzo; Naddeo, Vincenzo (5 November 2020). "A critical review on nanomaterials membrane bioreactor (NMs-MBR) for wastewater treatment". npj Clean Water. 3 (1): 43. Bibcode:2020npjCW...3...43P. doi:10.1038/s41545-020-00090-2. ISSN 2059-7037.
- ^ a b Anis, Mohab; AlTaher, Ghada; Sarhan, Wesam; Elsemary, Mona (2017). Nanovate. Springer. p. 105. ISBN 9783319448619.
- ^ "Health Effects". Asbestos Industry Association. Archived from the original on 9 April 2013. Retrieved 28 August 2017.
- ^ Maqbool, Qaisar; Favoni, Orlando; Wicht, Thomas; Lasemi, Niusha; Sabbatini, Simona; Stöger-Pollach, Michael; Ruello, Maria Letizia; Tittarelli, Francesca; Rupprechter, Günther (5 April 2024). "Highly Stable Self-Cleaning Paints Based on Waste-Valorized PNC-Doped TiO 2 Nanoparticles". ACS Catalysis. 14 (7): 4820–4834. doi:10.1021/acscatal.3c06203. ISSN 2155-5435. PMC 11003396. PMID 38601782.
- ^ Pham, Phuong; Minh, Thang; Nguyen, Tien; Van Driessche, Isabel (17 November 2014). "Ceo2 Based Catalysts for the Treatment of Propylene in Motorcycle's Exhaust Gases". Materials. 7 (11): 7379–7397. Bibcode:2014Mate....7.7379P. doi:10.3390/ma7117379. PMC 5512641. PMID 28788253.
- ^ Kašpar, Jan; Fornasiero, Paolo; Hickey, Neal (January 2003). "Automotive catalytic converters: current status and some perspectives". Catalysis Today. 77 (4): 419–449. doi:10.1016/S0920-5861(02)00384-X.
- ^ Tankard, Rikke Egeberg; Romeggio, Filippo; Akazawa, Stefan Kei; Krabbe, Alexander; Sloth, Olivia Fjord; Secher, Niklas Mørch; Colding-Fagerholt, Sofie; Helveg, Stig; Palmer, Richard; Damsgaard, Christian Danvad; Kibsgaard, Jakob; Chorkendorff, Ib (2024). "Stable mass-selected AuTiOx nanoparticles for CO oxidation". Physical Chemistry Chemical Physics. 26 (12): 9253–9263. Bibcode:2024PCCP...26.9253T. doi:10.1039/D4CP00211C. PMID 38445363.
- ^ a b Wang, Shujun; Gao, Lihong (2019). "Laser-driven nanomaterials and laser-enabled nanofabrication for industrial applications". Industrial Applications of Nanomaterials. Elsevier. pp. 181–203. doi:10.1016/B978-0-12-815749-7.00007-4. ISBN 978-0-12-815749-7. S2CID 202212003.
- ^ Rawat, Pankaj Singh, R. C. Srivastava, Gagan Dixit, G. C. Joshi, and K. Asokan. "Facile synthesis and temperature dependent dielectric properties of MnFe2O4 nanoparticles." In AIP Conference Proceedings, vol. 2115, no. 1, p. 030104. AIP Publishing LLC, 2019.https://doi.org/10.1063/1.5112943
- ^ Alsaiari, Norah Salem; Alzahrani, Fatimah Mohammed; Amari, Abdelfattah; Osman, Haitham; Harharah, Hamed N.; Elboughdiri, Noureddine; Tahoon, Mohamed A. (January 2023). "Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives". Molecules. 28 (1): 463. doi:10.3390/molecules28010463. ISSN 1420-3049. PMC 9823860. PMID 36615655.
- ^ Ramsden, J.J. (2011) Nanotechnology: An Introduction, Elsevier, Amsterdam
- ^ Zsigmondy, R. (1914) "Colloids and the Ultramicroscope", J. Wiley and Sons, NY
- ^ Dukhin, A.S. & Goetz, P.J. (2002). Ultrasound for characterizing colloids. Elsevier.
- ^ a b Borisenko, V. A.; Alfintseva, R. A. (June 1978). "Temperature dependence of the hardness of dispersion-strengthened molybdenum alloys". Soviet Powder Metallurgy and Metal Ceramics. 17 (6): 455–459. doi:10.1007/bf00795801. ISSN 0038-5735. S2CID 137512360.
- ^ Paik, P.; Kar, K.K.; Deva, D.; Sharma, A. (2007). "Measurement of mechanical properties of polymer nanospheres by atomic force microscopy: effects of particle size". Micro & Nano Letters. 2 (3): 72. doi:10.1049/mnl:20070030. ISSN 1750-0443.
- ^ a b Carlton, C.E.; Ferreira, P.J. (November 2012). "In situ TEM nanoindentation of nanoparticles". Micron. 43 (11): 1134–1139. doi:10.1016/j.micron.2012.03.002. ISSN 0968-4328. PMID 22484052.
- ^ Luan, Binquan; Robbins, Mark O. (June 2005). "The breakdown of continuum models for mechanical contacts". Nature. 435 (7044): 929–932. Bibcode:2005Natur.435..929L. doi:10.1038/nature03700. ISSN 0028-0836. PMID 15959512. S2CID 4398925.
- ^ Ramos, Manuel; Ortiz-Jordan, Luis; Hurtado-Macias, Abel; Flores, Sergio; Elizalde-Galindo, José; Rocha, Carmen; Torres, Brenda; Zarei-Chaleshtori, Maryam; Chianelli, Russell (14 January 2013). "Hardness and Elastic Modulus on Six-Fold Symmetry Gold Nanoparticles". Materials. 6 (1): 198–205. Bibcode:2013Mate....6..198R. doi:10.3390/ma6010198. ISSN 1996-1944. PMC 5452105. PMID 28809302.
- ^ Mordehai, Dan; Lee, Seok-Woo; Backes, Björn; Srolovitz, David J.; Nix, William D.; Rabkin, Eugen (August 2011). "Size effect in compression of single-crystal gold microparticles". Acta Materialia. 59 (13): 5202–5215. Bibcode:2011AcMat..59.5202M. doi:10.1016/j.actamat.2011.04.057. ISSN 1359-6454.
- ^ a b Tan, Susheng; Sherman, Robert L.; Ford, Warren T. (21 July 2004). "Nanoscale Compression of Polymer Microspheres by Atomic Force Microscopy". Langmuir. 20 (17): 7015–7020. doi:10.1021/la049597c. ISSN 0743-7463. PMID 15301482.
- ^ Jing, G. Y.; Duan, H. L.; Sun, X. M.; Zhang, Z. S.; Xu, J.; Li, Y. D.; Wang, J. X.; Yu, D. P. (13 June 2006). "Surface effects on elastic properties of silver nanowires: Contact atomic-force microscopy". Physical Review B. 73 (23): 235409. Bibcode:2006PhRvB..73w5409J. doi:10.1103/physrevb.73.235409. ISSN 1098-0121.
- ^ Jing, Guangyin; Zhang, Xinzheng; Yu, Dapeng (18 May 2010). "Effect of surface morphology on the mechanical properties of ZnO nanowires". Applied Physics A. 100 (2): 473–478. Bibcode:2010ApPhA.100..473J. doi:10.1007/s00339-010-5736-7. ISSN 0947-8396. S2CID 95077632.
- ^ Mate, C. Mathew; McClelland, Gary M.; Erlandsson, Ragnar; Chiang, Shirley (26 October 1987). "Atomic-scale friction of a tungsten tip on a graphite surface". Physical Review Letters. 59 (17): 1942–1945. Bibcode:1987PhRvL..59.1942M. doi:10.1103/physrevlett.59.1942. ISSN 0031-9007. PMID 10035374.
- ^ Lee, Chang-Gun; Hwang, Yu-Jin; Choi, Young-Min; Lee, Jae-Keun; Choi, Cheol; Oh, Je-Myung (January 2009). "A study on the tribological characteristics of graphite nano lubricants". International Journal of Precision Engineering and Manufacturing. 10 (1): 85–90. doi:10.1007/s12541-009-0013-4. ISSN 1229-8557. S2CID 135542937.
- ^ Lahouij, Imène; Dassenoy, Fabrice; de Knoop, Ludvig; Martin, Jean-Michel; Vacher, Béatrice (4 February 2011). "In Situ TEM Observation of the Behavior of an Individual Fullerene-Like MoS2 Nanoparticle in a Dynamic Contact". Tribology Letters. 42 (2): 133–140. doi:10.1007/s11249-011-9755-0. ISSN 1023-8883. S2CID 138069848.
- ^ Guo, Dan; Xie, Guoxin; Luo, Jianbin (3 December 2013). "Mechanical properties of nanoparticles: basics and applications". Journal of Physics D: Applied Physics. 47 (1): 013001. doi:10.1088/0022-3727/47/1/013001. ISSN 0022-3727. S2CID 4778703.
- ^ Onoda, G.Y. Jr.; Hench, L.L., eds. (1979). Ceramic Processing Before Firing. New York: Wiley & Sons. ISBN 978-0-471-65410-0.
- ^ Aksay, I.A.; Lange, F.F.; Davis, B.I. (1983). "Uniformity of Al2O3-ZrO2 Composites by Colloidal Filtration". J. Am. Ceram. Soc. 66 (10): C–190. doi:10.1111/j.1151-2916.1983.tb10550.x.
- ^ Franks, G.V. & Lange, F.F. (1996). "Plastic-to-Brittle Transition of Saturated, Alumina Powder Compacts". J. Am. Ceram. Soc. 79 (12): 3161–3168. doi:10.1111/j.1151-2916.1996.tb08091.x.
- ^ Evans, A.G.; Davidge, R.W. (1969). "The strength and fracture of fully dense polycrystalline magnesium oxide". Phil. Mag. 20 (164): 373–388. Bibcode:1969PMag...20..373E. doi:10.1080/14786436908228708.
- ^ Lange, F.F. & Metcalf, M. (1983). "Processing-Related Fracture Origins: II, Agglomerate Motion and Cracklike Internal Surfaces Caused by Differential Sintering". J. Am. Ceram. Soc. 66 (6): 398–406. doi:10.1111/j.1151-2916.1983.tb10069.x.
- ^ Evans, A.G. (1987). "Considerations of Inhomogeneity Effects in Sintering". J. Am. Ceram. Soc. 65 (10): 497–501. doi:10.1111/j.1151-2916.1982.tb10340.x.
- ^ Whitesides, George M.; et al. (1991). "Molecular Self-Assembly and Nanochemistry: A Chemical Strategy for the Synthesis of Nanostructures" (PDF). Science. 254 (5036): 1312–9. Bibcode:1991Sci...254.1312W. doi:10.1126/science.1962191. PMID 1962191. Archived (PDF) from the original on 21 August 2017.
- ^ Dubbs D. M; Aksay I.A. (2000). "Self-Assembled Ceramics Produced by Complex-Fluid Templation" (PDF). Annu. Rev. Phys. Chem. 51: 601–22. Bibcode:2000ARPC...51..601D. doi:10.1146/annurev.physchem.51.1.601. PMID 11031294. S2CID 14113689. Archived from the original (PDF) on 25 September 2020.
- ^ "Statnano". Retrieved 28 September 2018.
- ^ "WHO | WHO guidelines on protecting workers from potential risks of manufactured nanomaterials". WHO. Archived from the original on 19 December 2017. Retrieved 20 February 2018.
- ^ Comprehensive Nanoscience and Technology. Cambridge, MA: Academic Press. 2010. p. 169. ISBN 9780123743961.
- ^ Verma, Ayush; Stellacci, Francesco (2010). "Effect of Surface Properties on Nanoparticle-Cell Interactions". Small. 6 (1): 12–21. doi:10.1002/smll.200901158. PMID 19844908.
- ^ Mapanao, Ana Katrina; Giannone, Giulia; Summa, Maria; Ermini, Maria Laura; Zamborlin, Agata; Santi, Melissa; Cassano, Domenico; Bertorelli, Rosalia; Voliani, Valerio (2020). "Biokinetics and clearance of inhaled gold ultrasmall-in-nano architectures". Nanoscale Advances. 2 (9): 3815–3820. Bibcode:2020NanoA...2.3815M. doi:10.1039/D0NA00521E. ISSN 2516-0230. PMC 9417912. PMID 36132776.
- ^ a b c "Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers". U.S. National Institute for Occupational Safety and Health: v–x, 33–35, 43, 63–64. April 2013. doi:10.26616/NIOSHPUB2013145. Retrieved 26 April 2017.
- ^ "Approaches to Safe Nanotechnology: Managing the Health and Safety Concerns Associated with Engineered Nanomaterials". U.S. National Institute for Occupational Safety and Health: 12. March 2009. doi:10.26616/NIOSHPUB2009125. Retrieved 26 April 2017.
- ^ Eating Nano. By Brita Belli. E – The Environmental Magazine, 3 November 2012.
- ^ Turkevich, Leonid A.; Fernback, Joseph; Dastidar, Ashok G.; Osterberg, Paul (1 May 2016). "Potential explosion hazard of carbonaceous nanoparticles: screening of allotropes". Combustion and Flame. 167: 218–227. Bibcode:2016CoFl..167..218T. doi:10.1016/j.combustflame.2016.02.010. PMC 4959120. PMID 27468178.
- ^ "Fire and explosion properties of nanopowders". U.K. Health and Safety Executive. 2010. pp. 2, 13–15, 61–62. Retrieved 28 April 2017.
- ^ a b c "Building a Safety Program to Protect the Nanotechnology Workforce: A Guide for Small to Medium-Sized Enterprises". U.S. National Institute for Occupational Safety and Health: 8, 12–15. March 2016. doi:10.26616/NIOSHPUB2016102. hdl:10919/76615. Retrieved 5 March 2017.
- ^ a b "General Safe Practices for Working with Engineered Nanomaterials in Research Laboratories". U.S. National Institute for Occupational Safety and Health: 15–28. May 2012. doi:10.26616/NIOSHPUB2012147. Retrieved 5 March 2017.
- ^ Eastlake, Adrienne C.; Beaucham, Catherine; Martinez, Kenneth F.; Dahm, Matthew M.; Sparks, Christopher; Hodson, Laura L.; Geraci, Charles L. (1 September 2016). "Refinement of the Nanoparticle Emission Assessment Technique into the Nanomaterial Exposure Assessment Technique (NEAT 2.0)". Journal of Occupational and Environmental Hygiene. 13 (9): 708–717. doi:10.1080/15459624.2016.1167278. PMC 4956539. PMID 27027845.
- ^ "Current Intelligence Bulletin 63: Occupational Exposure to Titanium Dioxide". U.S. National Institute for Occupational Safety and Health: vii, 77–78. April 2011. doi:10.26616/NIOSHPUB2011160. Retrieved 27 April 2017.
- ^ "Nanotechnologies – Part 2: Guide to safe handling and disposal of manufactured nanomaterials". British Standards Institute. December 2007. Archived from the original on 2 November 2014. Retrieved 21 April 2017.
- ^ "Criteria for assessment of the effectiveness of protective measures". Institute for Occupational Safety and Health of the German Social Accident Insurance. 2009. Retrieved 21 April 2017.
- ^ Prasad, Paras (22 January 2016). "Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy". Chemical Reviews. 116 (5): 2827, 2841, 2850. doi:10.1021/acs.chemrev.5b00148. PMID 26799741.
- ^ Haynes, Christy (11 January 2012). "Critical Considerations in the Biomedical Use of Mesoporous Silica Nanoparticles". The Journal of Physical Chemistry Letters. 3 (3): 364–374. doi:10.1021/jz2013837. PMID 26285853.
- ^ Kircher, Moritz F. (19 December 2016). "Folate-Targeted Surface-Enhanced Resonance Raman Scattering Nanoprobe Ratiometry for Detection of Microscopic Ovarian Cancer". ACS Nano. 11 (2): 1488–1497. doi:10.1021/acsnano.6b06796. PMC 5502101. PMID 27992724.
External links
[edit]- European Union Observatory for Nanomaterials (EUON)
- Acquisition, evaluation and public orientated presentation of societal relevant data and findings for nanomaterials (DaNa)
- Safety of Manufactured Nanomaterials: OECD Environment Directorate
- Assessing health risks of nanomaterials summary by GreenFacts of the European Commission SCENIHR assessment
- Textiles Nanotechnology Laboratory at Cornell University
- Nano Structured Material
- Online course MSE 376-Nanomaterials by Mark C. Hersam (2006)
- Nanomaterials: Quantum Dots, Nanowires and Nanotubes online presentation by Dr Sands
- Lecture Videos for the Second International Symposium on the Risk Assessment of Manufactured Nanomaterials, NEDO 2012
- Nader Engheta: Wave interaction with metamaterials, SPIE Newsroom 2016
- Managing nanomaterials in the Workplace by the European Agency for Safety and Health at Work.







