Triarylmethane dye: Difference between revisions
→Xanthene dyes: main class |
Innerstream (talk | contribs) No edit summary |
||
| (3 intermediate revisions by 3 users not shown) | |||
| Line 8: | Line 8: | ||
Methyl violet dyes have dimethylamino groups at the ''p''-positions of two aryl groups. |
Methyl violet dyes have dimethylamino groups at the ''p''-positions of two aryl groups. |
||
<gallery caption="Methyl violet dyes" widths="180px" heights="120px" perrow="3"> |
<gallery caption="Methyl violet dyes" widths="180px" heights="120px" perrow="3"> |
||
Image:Methyl Violet 2B. |
Image:Methyl Violet 2B.svg|[[Methyl violet 2B]] |
||
Image:Methyl Violet 6B. |
Image:Methyl Violet 6B.svg|[[Methyl violet 6B]] |
||
Image: |
Image:Kristallviolett.svg|[[Crystal violet|Methyl violet 10B]] |
||
</gallery> |
</gallery> |
||
| Line 29: | Line 29: | ||
File:Phenol-red-zwitterionic-form-2D-skeletal.png|[[Phenol red]] |
File:Phenol-red-zwitterionic-form-2D-skeletal.png|[[Phenol red]] |
||
File:Chlorophenol red.png|[[Chlorophenol red]] |
File:Chlorophenol red.png|[[Chlorophenol red]] |
||
File: |
File:Cresol Red.svg|[[Cresol red]] |
||
File: |
File:Bromocresol purple.svg|[[Bromocresol purple]] |
||
File:Bromocresol green.svg|[[Bromocresol green]] |
File:Bromocresol green.svg|[[Bromocresol green]] |
||
</gallery> |
</gallery> |
||
| Line 54: | Line 54: | ||
===Xanthene dyes=== |
===Xanthene dyes=== |
||
{{see also| |
{{see also|fluoran}} |
||
Xanthene dyes feature a [[xanthene]] core. They are not widely used as textiles, but for other applications. |
Xanthene dyes feature a [[xanthene]] core. They are not widely used as textiles, but for other applications. |
||
<gallery caption="Xanthene dyes" widths="180px" heights="120px" perrow="3"> |
<gallery caption="Xanthene dyes" widths="180px" heights="120px" perrow="3"> |
||
| Line 65: | Line 65: | ||
==Bridged arenes== |
==Bridged arenes== |
||
Where two of the aryl groups are bridged by a [[heteroatom]], these triarylmethane compounds may be further categorized into [[acridine]]s (nitrogen-bridged), [[xanthene]]s (oxygen-bridged), and [[thioxanthene]]s (sulfur-bridged). |
Where two of the aryl groups are bridged by a [[heteroatom]], these triarylmethane compounds may be further categorized into [[acridine]]s (nitrogen-bridged), [[xanthene]]s (oxygen-bridged), and [[thioxanthene]]s (sulfur-bridged). |
||
==Synthesis== |
==Synthesis== |
||
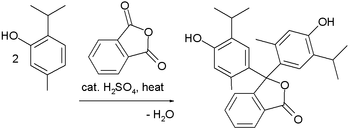
The amine-containing dyes are often prepared from [[Michler's ketone]] or its diethylamino analogue. In this way, the third aryl group is readily differentiated. The [[Friedel–Crafts alkylation]] reaction is a popular method to prepare many of the phenolic derivatives: |
The amine-containing dyes are often prepared from [[Michler's ketone]] or its diethylamino analogue. In this way, the third aryl group is readily differentiated. The [[Friedel–Crafts alkylation]] reaction is a popular method to prepare many of the phenolic derivatives: |
||
:[[File:ThymolphthaleinSynthesis.png|350px|Friedel–Crafts synthesis of thymolphthalein]] |
:[[File:ThymolphthaleinSynthesis.png|350px|Friedel–Crafts synthesis of thymolphthalein]] |
||
| Line 84: | Line 74: | ||
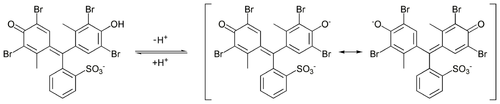
In addition to their dominant use as dyes, many of these dyes react reversibly with acid and base, and thus serve as [[pH indicator]]s.<ref name=Ull/> |
In addition to their dominant use as dyes, many of these dyes react reversibly with acid and base, and thus serve as [[pH indicator]]s.<ref name=Ull/> |
||
:[[File:Bromocresol green ionic equilibrium.png|500px|Bromocresol green reacts with acids and bases to give differently colored compounds]] |
:[[File:Bromocresol green ionic equilibrium.png|500px|Bromocresol green reacts with acids and bases to give differently colored compounds]] |
||
==See also== |
==See also== |
||
Latest revision as of 16:57, 7 December 2024
Triarylmethane dyes are synthetic organic compounds containing triphenylmethane backbones. As dyes, these compounds are intensely colored. They are produced industrially as dyes.[1]
Families
[edit]Triarylmethane dyes can be grouped into families according to the nature of the substituents on the aryl groups. In some cases, the anions associated with the cationic dyes (say crystal violet) vary even though the name of the dye does not. Often it is shown as chloride.
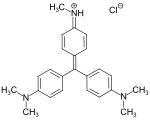
Methyl violet dyes
[edit]Methyl violet dyes have dimethylamino groups at the p-positions of two aryl groups.
- Methyl violet dyes
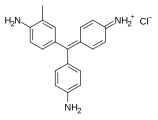
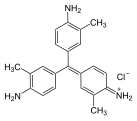
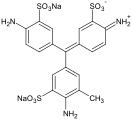
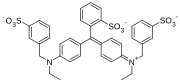
Fuchsine dyes
[edit]Fuchsine dyes have primary or secondary amines (NH2 or NHMe) functional groups at the p-positions of each aryl group.
- Fuchsine dyes
-
Fuchsine (hydrochloride salt)
-
New fuchsine (As chloride)
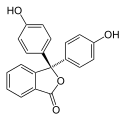
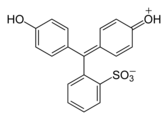
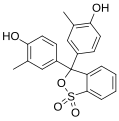
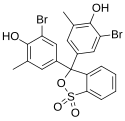
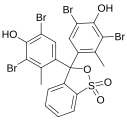
Phenol dyes
[edit]Phenol dyes have hydroxyl groups at the p positions of at least two aryl groups.
- Phenol dyes
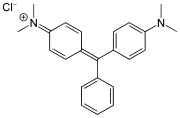
Malachite green dyes
[edit]Malachite green dyes are related to the methyl violet dyes, except that they contain one phenyl (C6H5) group.
- Malachite green dyes
-
Brilliant blue FCF, a common food colorant
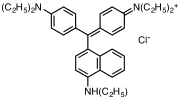
Victoria blue dyes
[edit]Victoria blue dyes are related to the methyl violet dyes, except they contain one naphthylamino group. Variation is found is dimethylamine vs diethylamino substituents on the phenyl rings and variations of the secondary amine on the naphthyl group.
- Victoria blue dyes
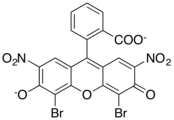
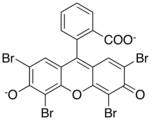
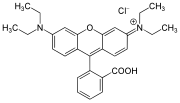
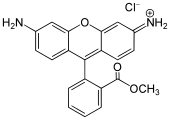
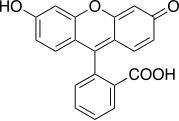
Xanthene dyes
[edit]Xanthene dyes feature a xanthene core. They are not widely used as textiles, but for other applications.
- Xanthene dyes
Bridged arenes
[edit]Where two of the aryl groups are bridged by a heteroatom, these triarylmethane compounds may be further categorized into acridines (nitrogen-bridged), xanthenes (oxygen-bridged), and thioxanthenes (sulfur-bridged).
Synthesis
[edit]The amine-containing dyes are often prepared from Michler's ketone or its diethylamino analogue. In this way, the third aryl group is readily differentiated. The Friedel–Crafts alkylation reaction is a popular method to prepare many of the phenolic derivatives:
Applications
[edit]In addition to their dominant use as dyes, many of these dyes react reversibly with acid and base, and thus serve as pH indicators.[1]
See also
[edit]References
[edit]- ^ a b Gessner, Thomas; Mayer, Udo (2000). "Triarylmethane and Diarylmethane Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_179. ISBN 978-3527306732.