Hexazinone: Difference between revisions
No edit summary |
hazards |
||
| (35 intermediate revisions by 23 users not shown) | |||
| Line 5: | Line 5: | ||
| ImageFile = Hexazinone.png |
| ImageFile = Hexazinone.png |
||
| ImageSize = |
| ImageSize = |
||
| |
| PIN = 3-Cyclohexyl-6-(dimethylamino)-1-methyl-1,3,5-triazine-2,4(1''H'',3''H'')-dione |
||
| OtherNames = Velpar<br>Hexazinone |
| OtherNames = Velpar<br>Hexazinone |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| Line 11: | Line 11: | ||
| CASNo_Ref = {{cascite|correct|??}} |
| CASNo_Ref = {{cascite|correct|??}} |
||
| CASNo = 51235-04-2 |
| CASNo = 51235-04-2 |
||
| |
| ChEBI = 5705 |
||
| ChEMBL = 2252598 |
|||
| SMILES = O=C(N1C2CCCCC2)N=C(N(C)C)N(C)C1=O |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| PubChem = 39965 |
|||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
|||
| ChemSpiderID = 36542 |
| ChemSpiderID = 36542 |
||
| EC_number = 257-074-4 |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
| KEGG = C10926 |
|||
| PubChem = 39965 |
|||
| SMILES2 = O=C1/N=C(\N(C(=O)N1C2CCCCC2)C)N(C)C |
| SMILES2 = O=C1/N=C(\N(C(=O)N1C2CCCCC2)C)N(C)C |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = Y51727MR1Y |
|||
| SMILES = O=C(N1C2CCCCC2)N=C(N(C)C)N(C)C1=O |
|||
| InChI = 1/C12H20N4O2/c1-14(2)10-13-11(17)16(12(18)15(10)3)9-7-5-4-6-8-9/h9H,4-8H2,1-3H3 |
| InChI = 1/C12H20N4O2/c1-14(2)10-13-11(17)16(12(18)15(10)3)9-7-5-4-6-8-9/h9H,4-8H2,1-3H3 |
||
| InChIKey = CAWXEEYDBZRFPE-UHFFFAOYAU |
| InChIKey = CAWXEEYDBZRFPE-UHFFFAOYAU |
||
| Line 23: | Line 29: | ||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
||
| StdInChIKey = CAWXEEYDBZRFPE-UHFFFAOYSA-N |
| StdInChIKey = CAWXEEYDBZRFPE-UHFFFAOYSA-N |
||
| RTECS = |
|||
| MeSHName = |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| ChEBI = |
|||
| KEGG_Ref = {{keggcite|changed|kegg}} |
|||
| KEGG = C10926 |
|||
}} |
}} |
||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| Line 46: | Line 46: | ||
}} |
}} |
||
|Section7={{Chembox Hazards |
|Section7={{Chembox Hazards |
||
| EUClass = |
|||
| MainHazards = |
| MainHazards = |
||
| NFPA-H = |
| NFPA-H = |
||
| Line 52: | Line 51: | ||
| NFPA-R = |
| NFPA-R = |
||
| NFPA-S = |
| NFPA-S = |
||
| RPhrases = |
|||
| SPhrases = |
|||
| RSPhrases = |
|||
| FlashPt = |
| FlashPt = |
||
| AutoignitionPt = |
| AutoignitionPt = |
||
| ExploLimits = |
| ExploLimits = |
||
| PEL = |
| PEL = |
||
| GHS_ref=[https://pubchem.ncbi.nlm.nih.gov/compound/39965#section=Safety-and-Hazards] |
|||
| GHSPictograms = {{GHS07}}{{GHS09}} |
|||
| GHSSignalWord = Warning |
|||
| HPhrases = {{H-phrases|302|319|332|410}} |
|||
| PPhrases = {{P-phrases|261|264|264+265|270|271|273|280|301+317|304+340|305+351+338|317|330|337+317|391|501}} |
|||
}} |
}} |
||
}} |
}} |
||
'''Hexazinone''' is an [[organic compound]] that is used as a broad spectrum [[herbicide]]. It is a colorless solid. It exhibits some solubility in water but is highly soluble in most organic solvents except alkanes. A member |
'''Hexazinone''' is an [[organic compound]] that is used as a broad spectrum [[herbicide]]. It is a colorless solid. It exhibits some solubility in water but is highly soluble in most organic solvents except alkanes. A member of the [[triazine]] class herbicides, it is manufactured by [[DuPont]] and sold under the trade name '''Velpar'''.<ref>Arnold P. Appleby, Franz Müller, Serge Carpy "Weed Control" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. {{doi|10.1002/14356007.a28_165}}</ref> |
||
It functions by inhibiting photosynthesis and thus is a nonselective herbicide. It is used to control grasses, broadleaf, and woody plants. |
It functions by inhibiting photosynthesis and thus is a nonselective herbicide. It is used to control grasses, broadleaf, and woody plants. In the United States approximately 33% is used on alfalfa, 31% in forestry, 29% in industrial areas, 4% on rangeland and pastures, and < 2% on sugarcane.<ref>[http://pmep.cce.cornell.edu/profiles/herb-growthreg/fatty-alcohol-monuron/hexazinone/herb-prof-hexazinone.html Hexazinone], Herbicide Profile, Pesticide Management Education Program, [[Cornell University]]</ref> |
||
Hexazinone is a pervasive groundwater contaminant. Use of hexazinone causes groundwater to be at high risk of contamination due to the high leaching potential it exhibits.<ref>{{Cite journal |last1=da Silva |first1=Cydianne Cavalcante |last2=Souza |first2=Matheus de Freitas |last3=Passos |first3=Ana Beatriz Rocha de Jesus |last4=Silva |first4=Tatiane Severo |last5=Borges |first5=Maiara Pinheiro da Silva |last6=dos Santos |first6=Matheus Silva |last7=Silva |first7=Daniel Valadão |date=March 2022 |title=Risk of environmental contamination due to the hexazinone application in agricultural soils in northeastern Brazil |journal=Geoderma Regional |language=en |volume=28 |pages=e00481 |doi=10.1016/j.geodrs.2022.e00481|bibcode=2022GeodR..2800481D |s2cid=246338359 |doi-access= }}</ref> |
|||
Hexazinone is a pervasive groundwater contaminant, due to its high water solubility |
|||
== History == |
== History == |
||
Hexazinone is widely used as a herbicide. It is a non-selective herbicide from the triazine family. |
Hexazinone is widely used as a herbicide. It is a non-selective herbicide from the triazine family. It is used among a broad range of places. It is used to control weeds within all sort of applications. From sugarcane plantations, forestry field nurseries, pineapple plantations to high- and railway grasses and industrial plant sites.<ref>{{Cite journal|last1=Wang|first1=Huili|last2=Xu|first2=Shuxia|last3=Tan|first3=Chengxia|last4=Wang|first4=Xuedong|date=2009-05-30|title=Anaerobic biodegradation of hexazinone in four sediments|journal=Journal of Hazardous Materials|volume=164|issue=2–3|pages=806–811|doi=10.1016/j.jhazmat.2008.08.073|pmid=18824297|bibcode=2009JHzM..164..806W }}</ref> |
||
Hexazinone was first registered in 1975 for the overall control of weeds and later for uses in crops |
Hexazinone was first registered in 1975 for the overall control of weeds and later for uses in crops.<ref name=":0">{{Cite web|url=https://archive.epa.gov/pesticides/reregistration/web/pdf/0266fact.pdf|title=Hexazinone: Reregistration Eligibility Decision (RED) Fact Sheet}}</ref> |
||
In 1982 and 1988 registration standards were issued by the EPA. These product summaries required further research for chemistry, toxicology, ecological effects and environmental fate data. In 1988 the office for drinking water from the EPA issued a drinking water Health Advisory (HA) for hexazinone.<ref name=":0" /> |
|||
In 1989, hexazinone was deliberately used in an act of vandalism to poison [[Treaty Oak (Austin, Texas)|the Treaty Oak]] in Austin, Texas. |
|||
== Structure and reactivity == |
== Structure and reactivity == |
||
Triazines like hexazinone can bind to the D-1 quinone protein of the electron transport chain in photosystem II to inhibit the photosynthesis. These diverted electrons can thereby damage membranes and destroy cells.<ref>{{Cite web|url=http://agron-www.agron.iastate.edu/Courses/Agron317/Photosynthesis_Inhibitors.htm|title=Agronomy 317 - Iowa State University|website=agron-www.agron.iastate.edu|access-date=2017-03-15}}</ref> |
Triazines like hexazinone can bind to the D-1 quinone protein of the electron transport chain in photosystem II to inhibit the photosynthesis. These diverted electrons can thereby damage membranes and destroy cells.<ref>{{Cite web|url=http://agron-www.agron.iastate.edu/Courses/Agron317/Photosynthesis_Inhibitors.htm|title=Agronomy 317 - Iowa State University|website=agron-www.agron.iastate.edu|access-date=2017-03-15|archive-url=https://web.archive.org/web/20161123180144/http://agron-www.agron.iastate.edu/Courses/Agron317/Photosynthesis_Inhibitors.htm|archive-date=2016-11-23|url-status=dead}}</ref> |
||
== Synthesis == |
== Synthesis == |
||
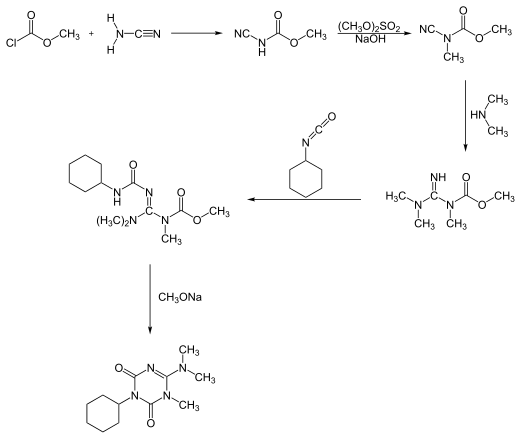
Hexazinone can be synthesized in two different reaction processes. One process starts with a reaction of [[methyl chloroformate]] with [[cyanamide]], forming hexazinone after a five-step pathway:<ref name=":1">{{Cite book |
Hexazinone can be synthesized in two different reaction processes. One process starts with a reaction of [[methyl chloroformate]] with [[cyanamide]], forming hexazinone after a five-step pathway:<ref name=":1">{{Cite book|title=Ullmann's agrochemicals.|date=2007-01-01|publisher=Wiley-VCH|isbn=9783527316045|oclc=470787466}}</ref> |
||
[[File:Hexazinon1.svg|border|frameless| |
[[File:Hexazinon1.svg|border|frameless|520x520px]] |
||
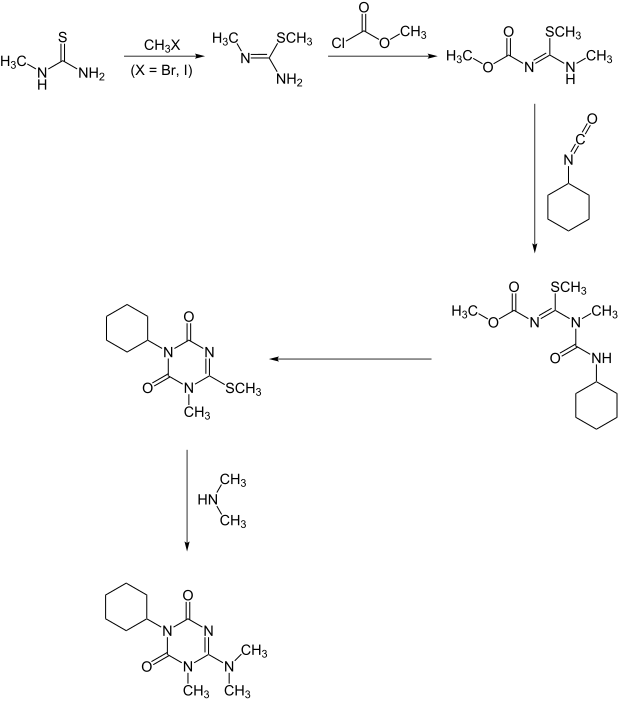
A second synthesis starts with methyl[[thiourea]] |
A second synthesis starts with methyl[[thiourea]].:<ref name=":1" /> |
||
[[File:Hexazinon2.svg|border|frameless| |
[[File:Hexazinon2.svg|border|frameless|618x818px]] |
||
== Degradation |
== Degradation == |
||
The degradation of hexazinone has long been studied.<ref>{{Cite journal|last1=Helling|first1=C. S.|last2=Kearney|first2=P. C.|last3=Alexander|first3=M.|year=1971|title=Behavior of pesticides in soil|journal=Adv. Agron.|volume=23|pages=147–240|doi=10.1016/S0065-2113(08)60153-4|series=Advances in Agronomy|isbn=9780120007233}}</ref> It degrades approximately 10% in five weeks, when exposed to artificial sunlight in distilled water. However, degradation in natural waters can be three to seven times greater. Surprisingly, the [[pH]] and the temperature of the water do not affect the [[photodegradation]] significantly.<ref name=":2">{{Cite journal|last=Rhodes|first=R. C.|year=1980b|title=Studies with 14C-labeled hexazinone in water and bluegill sunfish|journal=J. Agric. Food Chem.|volume=28|issue=2|pages=306–310|doi=10.1021/jf60228a002|pmid=7391368}}</ref> It is mainly degraded by aerobic microorganisms in soils.<ref>{{Cite journal|last=Rhodes|first=R. C.|year=1980a|title=Soil Studies with 14C-labeled hexazinone|journal=J. Agric. Food Chem.|volume=28|issue=2|pages=311–315|doi=10.1021/jf60228a012}}</ref> |
|||
'''Volatilization''' |
|||
Although hexazinone does not volatilize rapidly in the field, the potential for volatilization increases with temperature, increasing soil moisture and decreasing clay and organic content.<ref>{{Cite journal|last=Helling|first=C. S.|last2=Kearney|first2=P. C.|last3=Alexander|first3=M.|year=1971|title=Behavior of pesticides in soil|url=|journal=Adv. Agron.|volume=23|pages=147–240|via=}}</ref> |
|||
'''Photodegradation''' |
|||
Hexazinone degrades approximately 10% in five weeks, when exposed to artificial sunlight in distilled water. However, degradation in natural waters can be three to seven times greater. Surprisingly, the pH and the temperature of the water do not affect the photodegradation significantly.<ref name=":2">{{Cite journal|last=Rhodes|first=R. C.|year=1980b|title=Studies with 14C-labeled hexazinone in water and bluegill sunfish|url=|journal=J. Agric. Food Chem.|volume=28|pages=306–310|via=}}</ref> |
|||
'''Microbial degradation''' |
|||
Hexazinone is mainly degraded by aerobic microorganisms in the soils. In soils which were kept in anaerobic conditions, no degradation occurred. However, in aerobic soils 45-75% of the applied hexazinone was released as CO2 as a result of microbial degradation.<ref>{{Cite journal|last=Rhodes|first=R. C.|year=1980a|title=Soil Studies with 14C-labeled hexazinone|url=|journal=J. Agric. Food Chem.|volume=28|pages=311–315|via=}}</ref> |
|||
'''Adsorption''' |
|||
Adsorption of hexazinone to soil particles is low, but increases with increasing organic content, pH, and clay exchange capacity. The soil temperature however does not affect the adsorption significantly.<ref>{{Cite journal|last=Koskiene|first=W.C.|last2=Stone|first2=D.M.|last3=Harris|first3=A.R.|year=1996|title=Sorption of hexazinone, sulfometuron methyl, and tebuthiuron on acid, low base saturated sands|url=|journal=Chemosphere|volume=32(9)|pages=1681-1689|via=}}</ref> |
|||
'''Chemical decomposition''' |
|||
Hexazinone degrades into eight or more different metabolites: A through H. Only metabolite B is toxic to plants, however it still has only 1% of the toxicity of hexazinone.<ref name=":2" /> The ratio between the different metabolites depends on the environmental conditions.<ref>{{Cite journal|last=Roy|first=D.N.|last2=Konar|first2=S.K.|last3=Charles|first3=D.A.|last4=Feng|first4=J.C.|last5=Prasad|first5=R.|last6=Campbell|first6=R.A.|year=1989|title=Determination of persistence, movement, and degradation of hexazinone in selected Canadian boreal forest soils|url=|journal=J. Agric. Food Chem.|volume=37|pages=443-447|via=}}</ref> |
|||
== Mechanism of action == |
== Mechanism of action == |
||
Hexazinone is a broad-spectrum residual and contact [[herbicide]], rapidly absorbed by the leaves and roots. It is tolerated by conifers, and therefore it is a very effective [[herbicide]] for the control for [[Annual plant|annual]] and [[Perennial plant|perennial]] [[Broad-leaved tree|broadleaf]] weeds, some grasses, and some woody species. Hexazinone works as rain or snowmelt makes it possible for the [[herbicide]] to move downward into the soil. There the hexazinone is absorbed from the soil by the roots.<ref |
Hexazinone is a broad-spectrum residual and contact [[herbicide]], rapidly absorbed by the leaves and roots. It is tolerated by many conifers, and therefore it is a very effective [[herbicide]] for the control for [[Annual plant|annual]] and [[Perennial plant|perennial]] [[Broad-leaved tree|broadleaf]] weeds, some grasses, and some woody species. Hexazinone works as rain or snowmelt makes it possible for the [[herbicide]] to move downward into the soil. There the hexazinone is absorbed from the soil by the roots.<ref>{{Cite book|title=Environmental fates and impacts of major forest use pesticides|last=Ghassemi|first=M.|display-authors=etal|year=1981|location=Washington D.C.|pages=169–194}}</ref> It moves through the conductive tissues to the leaves, where it blocks the [[photosynthesis]] of the plant within the [[chloroplast]]s. Hexazinone binds to a protein of the [[photosystem II]] complex, which blocks the electron transport. The result are multiple following reactions. First triplet-state [[chlorophyll]] reacts with [[oxygen]] to form [[singlet oxygen]]. Both [[chlorophyll]] and [[singlet oxygen]] then remove [[hydrogen ion]]s from the unsaturated [[lipid]]s present in de cells and the organelle membranes, forming lipid radicals. These radicals will oxidize other lipids and proteins, eventually resulting in loss of the membrane integrity of the cells and organelles. This will result in a loss of [[chlorophyll]], leakage of cellular contents, cell death, and eventually death of the plant.<ref>{{Cite web|url=http://wssa.net/|title=Weed Science Society of America|website=wssa.net|language=en-US|access-date=2017-03-15}}</ref> Woody plants first show yellowing of the leaves before they start to defoliate, eventually they will die.<ref>{{Cite journal|last1=Sidhu|first1=S. S.|last2=Feng.|first2=J. C.|year=1993|title=Hexazinone and its metabolites in boreal forest vegetation.|journal=Weed Sci.|volume=41|issue=2|pages=281–287|doi=10.1017/S0043174500076177|s2cid=83421922 }}</ref> Sometimes plants are able to refoliate and defoliate again during the growing season. |
||
== Metabolism == |
|||
Hexazinone has seven naturally occurring metabolites A, B, C, D, E, G, and H. These metabolites occur through the degradation of hexazinone, plant metabolism being the leading factor since it is a herbicide. The major pathways of hexazinone degradation are photodegradation and biological decomposition.<ref>{{cite book|last1=Ghassemi|first1=M|last2=et al|title=Environmental fates and impacts of major forest use pesticides.|date=1981|location=Washington D.C.|pages=169-194}}</ref> In aqueous solution a slow degradation under light occurs into metabolite A by hydroxylation, and into metabolites B and H by demethylation.<ref>{{cite book|last1=Rhodes|first1=R.C.|title=Decomposition of “Velpar” weed killer in soil.|date=1987}}</ref> Degradation into metabolites A, B, C, and D happens under the influence of microbes this process occurs more eagerly than photodegradation.<ref>{{cite book|last1=Neary|first1=D.G.|last2=Bush|first2=P.B.|last3=Michael|first3=J.L.|title=Fate, dissipation and environmental effects of pesticides in southern forests: A review of a decade of research progress.|date=1993|publisher=J. of Environ. Toxicol. and Chem.|page=12:411-428}}</ref> It is found that this degradation is primarily by hydroxylation and demethylation of the cyclohexyl ring.<ref>{{cite book|last1=Rhodes|first1=R.C.|title=Soil studies with 14C-labeled hexazinone.|date=1980|publisher=J. of Agric. and Food Chem. Vol. 28, No 2.|page=311-315}}</ref> Hexazinone can also be metabolised within multiple plant species, this may vary from study to study; nontheles degradation into metabolites A, B, C, D, and E have been observed in one or more of these studies.<ref>{{cite book|last1=Baron|first1=J.L.|last2=Monaco|first2=T.J.|title=Uptake, translocation, and metabolism of hexazinone in blueberry (Vaccinium spl.) and Hollow Goldenrod (Solidago fistulosa).|date=1986|publisher=Weed Science.|pages=34:824- 829.}}</ref><ref>{{cite book|last1=Jensen|first1=K.I.N.|last2=Kimball|first2=E.R.|title=Uptake and metabolism of hexazinone in Rubus hispidus L. and Pyrus melanocarpa (Michx.) Willd.|date=1990|publisher=Weed Res.|pages=30: 35-41}}</ref><ref>{{cite book|last1=Sidhu|first1=S.S.|last2=Feng|first2=J.C.|title=Hexazinone and its metabolites in boreal forest vegetation.|date=1993|publisher=Weed Sci.|pages=41:281-287}}</ref> |
|||
== Indications == |
|||
In a case report where a 26 year old woman inhaled an unknown amount of hexazinone dust it was found that the only occurrence of indication was vomiting within 24 hours. No other symptoms were reported and no further treatment was administered.<ref>{{cite book|last1=Environmental Protection Agency|title=Health Advisories for 50 Pesticides.|date=1988|publisher=Simazine (USNTIS, PB 88-245931), Washington DC, EPA Office of Drinking Water|pages=765–788}}</ref> |
|||
== Efficacy and adverse effects == |
|||
=== Efficacy === |
|||
Hexazinone is being used as a herbicide. It is absorbed from the soil and inhibits photosynthesis in plants. In this way, the growth of unwanted plants in controlled.<ref>{{Cite web|url=https://hazmap.nlm.nih.gov/category-details?table=copytblagents&id=1766|title=Haz-Map Category Details|website=hazmap.nlm.nih.gov|language=en-US|access-date=2017-03-17}}</ref> Because of the high water-solubility, hexazinone is very mobile in the soil.<ref name=":5">{{Cite web|url=http://ace.orst.edu/info/extoxnet/|title=Pesticide Information Profiles.|last=EXTOXNET 1996|first=|date=|website=Extension Toxicology Network|archive-url=|archive-date=|dead-url=|access-date=}}</ref> It is also very persistent, and has a half-life between a couple of days<ref>{{Cite journal|last=Solomon|first=K.R.|last2=Bowhey|first2=C.S.|last3=Liber|first3=K.|last4=Stephenson|first4=G.R.|year=1988|title=Persistence of hexazinone (Velpar), triclopyr (Garlon), and 2,4-D in a Northern Ontario aquatic environment|url=|journal=J. Agric. Food Chem.|volume=36|pages=1314-1318|via=}}</ref> to more than nine months<ref>{{Cite journal|last=Thompson|first=D.G.|last2=MacDonald|first2=L.M.|last3=Staznik|first3=B.|year=1992|title=Persistence of hexazinone and metsulfuron-methyl in a mixed-wood/boreal forest lake|url=|journal=J. Agric. Food Chem.|volume=40|pages=1444-1449|via=}}</ref>. The effect of a single application of hexazinone can be observed for a long period. |
|||
The benefit of using hexazinone compared to mechanical weed control techniques is investigated. The application of hexazinone causes less changes in water quality than the mechanical techniques.<ref>{{Cite journal|last=Neary|first=D.G.|last2=Bush|first2=P.B.|last3=Grant|first3=A.|year=1986|title=Water quality of ephemeral forest streams after site preparation with the herbicide hexazinone|url=|journal=For. Ecol. Manage.|volume=14|pages=23-40|via=}}</ref> |
|||
=== Adverse effects === |
|||
Because it is non-selective, not only the weed, but also the crop can be affected. The water-solubility and the persistence in water are not only advantages for the efficacy, but can also be disadvantages. It can move through the soil and can affect plants 100 meters away from the location where the chemical is administered.<ref>{{Cite journal|last=Allender|first=W.J.|year=1991|title=Movement of bromacil and hexazinone in a municipal site.|url=|journal=Bull. Environ. Contam. Toxicol.|volume=46|pages=284-291|via=}}</ref> |
|||
By moving through ground water, the hexazinone also affects the organisms living around the agriculture area. Organisms take in the chemical by drinking water or eating contaminated plants. For small mammals and aquatic and terrestrial plants the levels of hexazinone even exceed the LOC (levels of concern). It is also highly toxic for algae. This affects all the aquatic wildlife, because the aquatic plants and algae are the food and oxygen source for fish.<ref name=":0" /> |
|||
Larch trees (Larix spp.) experience toxic effects when they take up hexazinone, so the herbicide cannot be applicated in region where these trees vegetate.<ref name=":6">{{Cite book|title=Pesticide profiles. Toxicity, Environmenal Impact, and Fate.|last=Kamrin|first=M.A.|publisher=Lewis Publishers|year=1997|isbn=|location=New York,United States of America|pages=351-354}}</ref> |
|||
== Toxicity == |
|||
Animal studies have shown that hexazinone causes acute, severe eye irritation. Therefore, it has been labelled as a Toxicity Category I compound for primary eye irritation, which is the highest classification for toxicity. There is a relatively low acute toxicity in case of ingestion (Toxicity Category III), inhalation or skin contact (Toxicity Category IV).<ref name=":0" /> |
|||
The EPA has classified hexazinone as a Group D carcinogen. If a chemical is listed as a Group D, it means there is no evidence for carcinogenicity, but there is also no evidence that it is not carcinogenic. Thus, the results from studies do not accept nor reject the carcinogenicity.<ref name=":0" /> |
|||
The ADI (acceptable daily intake) for hexazinone is 0.1 mg/kg/day and the NOAEL (No Observed Adverse Effect Level) is 10 mg/kg/day.<ref>{{Cite web|url=https://www.cropcare.com.au/assets/2210/1/BarrageSDS0867_0516.pdf|title=Barrage Herbicide, SDS0867|last=|first=|date=|website=Cropcare|archive-url=|archive-date=|dead-url=|access-date=17-03-2017}}</ref> |
|||
People working in the agriculture sector do not have to wear additional protection, but should be careful during the application of hexazinone due to the acute eye toxicity.<ref name=":0" /> |
|||
== Effects on animals == |
|||
To birds and mammals hexazinone has a low toxicity. The uptake from treated crops will not be enough to reach a toxic level in animals.<ref name=":4" /> Beside the excretion in animals occurs rapidly whereby there is no accumulation in the organism.<ref name=":6" /> The NOAEL in rats is 200 ppm and NOAEL in dogs is 1000 ppm.<ref>{{Cite journal|last=Gerald|first=L.|last2=Kennedy Jr.|first2=A.|year=1984|title=Chronic toxicity, reproductive, and teratogenic studies of hexazinone.|url=|journal=Fundamental and Applied Toxicology|volume=4|pages=980-971|via=}}</ref> To most fish and reptiles hexazinone is slightly toxic.<ref name=":5" /> The LC50 values for rainbow trout and bluegill are respectively 320 mg/L and 370 mg/L.<ref name=":6" /> |
|||
Because hexazinone can have a long half-life in water, the residues exceed the LOC. Consequently, a high toxicity arises for aquatic plants and algae. Therefore, the fish eating the algae and plants also undergo the toxic effects or die because of a lack of food. This can result in a collapse of the food chain, which influences the ecological environment.<ref>{{Cite journal|last=Peterson|first=H.G.|last2=Boutin|first2=C.|last3=Martin|first3=P.A.|last4=Freemark|first4=K.E.|last5=Ruecker|first5=N.J.|last6=Moody|first6=M.J.|year=1994|title=Aquatic phytotoxicity of 23 pesticides applied at expected environmental concentrations.|url=|journal=Aquat. Toxicol.|volume=28|pages=275-292|via=}}</ref> |
|||
== Risk assessment and regulations == |
|||
'''Usage'''<br />Herbicides containing hexazinone should not be used on lawns, driveways, walks, tennis courts, and similar areas.<ref>{{cite web|title=Hexazinone|url=https://pubchem.ncbi.nlm.nih.gov/compound/hexazinone#section=Experimental-Properties|website=PubChem|accessdate=16 March 2017}}</ref> Drift of dry herbicide powder must be prevented, it should only be applied to the desired plants. Contamination of any body of water must also be prevented. |
|||
'''Disposal'''<br />The safest way to dispose of excess herbicides containing hexazinone is to, in case of small amounts use it. If usage of the remaining herbicide is not possible, dispose of it in a household hazardous waste collection program or a similar program for the elimination of unwanted, residual herbicides. Verify with your local solid waste management authority, health department, or environmental agency to find out where this is locally possible. Information about any local regulations for the waste disposal of herbicides can also be found at these authorities. Empty herbicide container should be regarded as full because they are residually contaminated. These containers should not be reused and disposed of following the directions on the container. |
|||
'''Allowable Tolerances'''<br />Established tolerances for combined residues containing hexazinone herbicides and its metabolites on food are as follows: |
|||
{| class="wikitable" |
|||
|- |
|||
! Allowed residues !! Food products |
|||
|- |
|||
| 0.1 ppm || Cattle fat, meat, meat by-products; goat fat, meat, meat by-products; hog fat, meat, meat by-products; horse fat, meat, meat by-products; sheep fat, meat, meat by-products; milk |
|||
|- |
|||
| 0.2 ppm || Blueberry |
|||
|- |
|||
| 0.5 ppm || Pineapple (whole fruit) |
|||
|- |
|||
| 2.0 ppm || Alfalfa green forage |
|||
|- |
|||
| 8.0 ppm || Alfalfa hay |
|||
|- |
|||
| 10 ppm || Grass pasture, range |
|||
|} |
|||
'''Hazards'''<br />The following health and environmental hazards apply to hexazinone herbicides:<ref>{{cite web|title=hexazinone (Ref: DPX A3674)|url=http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/384.htm#none|website=PPDB: Pesticide Properties DataBase|accessdate=14 March 2017}}</ref><ref>{{cite web|title=Hexazinone|url=http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.detail&language=EN&selectedID=1456|website=EU Pesticides database|accessdate=14 March 2017}}</ref> |
|||
{| class="wikitable" |
|||
|- |
|||
! Code !! Applies to !! Statement |
|||
|- |
|||
| H302 || Acute oral toxicity, category 4 || “Harmful if swallowed” |
|||
|- |
|||
| H319 || Severe eye damage/irritation, category 2A || “Causes serious eye irritation” |
|||
|- |
|||
| H400 || Acute danger for aquatic environment, category 1 || “Very toxic to aquatic life” |
|||
|- |
|||
| H410 || Chronic danger for aquatic environment, category 1 || “Very toxic to aquatic life with long-lasting effects” |
|||
|} |
|||
'''Precaution'''<br />The following prevention, response, and disposal precautionary statements apply to hexazinone herbicides:<ref>{{cite web|title=Hexazinone|url=https://pubchem.ncbi.nlm.nih.gov/compound/hexazinone#section=Experimental-Properties|website=PubChem|accessdate=16 March 2017}}</ref> |
|||
{| class="wikitable" |
|||
|- |
|||
! Code !! Statement |
|||
|- |
|||
| P261 || “Avoid breathing dust/fumes/gas/mist/vapours/spray” |
|||
|- |
|||
| P264 || “Wash … thoroughly after handling” |
|||
|- |
|||
| P270 || “Do not eat, drink, or smoke when using this product” |
|||
|- |
|||
| P273 || “Avoid release to the environment” |
|||
|- |
|||
| P280 || “Wear protective gloves/protective clothing/eye protection/face protection” |
|||
|- |
|||
| P301+P330+P312 || “IF SWALLOWED: Rinse mouth. Call a POISON CENTER/doctor if you feel unwell” |
|||
|- |
|||
| P304+P340 || “IF INHALED: Remove person to fresh air and keep comfortable for breathing” |
|||
|- |
|||
| P305+P351+P338 || “IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do. Continue rinsing” |
|||
|- |
|||
| P337+P313 || “If eye irritation persists: Get medical adviece/attention” |
|||
|- |
|||
| P391 || “Collect spillage” |
|||
|- |
|||
| P501 || “Dispose of contents/container to … [… in accordance with local/regional/national/international regulation (as specified under Disposal)]” |
|||
|} |
|||
==References== |
|||
<references/> |
<references/> |
||
| Line 224: | Line 106: | ||
[[Category:Triazines]] |
[[Category:Triazines]] |
||
[[Category:Lactams]] |
[[Category:Lactams]] |
||
[[Category:Cyclohexyl compounds]] |
|||
[[Category:Dimethylamino compounds]] |
|||
Latest revision as of 21:26, 10 December 2024

| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Cyclohexyl-6-(dimethylamino)-1-methyl-1,3,5-triazine-2,4(1H,3H)-dione | |
| Other names
Velpar
Hexazinone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.051.869 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H20N4O2 | |
| Molar mass | 252.31 |
| Appearance | White crystalline solid |
| Density | 1.25 g/cm3 |
| Melting point | 116 °C (241 °F; 389 K) |
| Soluble | |
| Hazards | |
| GHS labelling:[1] | |
 
| |
| Warning | |
| H302, H319, H332, H410 | |
| P261, P264, P264+P265, P270, P271, P273, P280, P301+P317, P304+P340, P305+P351+P338, P317, P330, P337+P317, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hexazinone is an organic compound that is used as a broad spectrum herbicide. It is a colorless solid. It exhibits some solubility in water but is highly soluble in most organic solvents except alkanes. A member of the triazine class herbicides, it is manufactured by DuPont and sold under the trade name Velpar.[1]
It functions by inhibiting photosynthesis and thus is a nonselective herbicide. It is used to control grasses, broadleaf, and woody plants. In the United States approximately 33% is used on alfalfa, 31% in forestry, 29% in industrial areas, 4% on rangeland and pastures, and < 2% on sugarcane.[2]
Hexazinone is a pervasive groundwater contaminant. Use of hexazinone causes groundwater to be at high risk of contamination due to the high leaching potential it exhibits.[3]
History
[edit]Hexazinone is widely used as a herbicide. It is a non-selective herbicide from the triazine family. It is used among a broad range of places. It is used to control weeds within all sort of applications. From sugarcane plantations, forestry field nurseries, pineapple plantations to high- and railway grasses and industrial plant sites.[4]
Hexazinone was first registered in 1975 for the overall control of weeds and later for uses in crops.[5]
Structure and reactivity
[edit]Triazines like hexazinone can bind to the D-1 quinone protein of the electron transport chain in photosystem II to inhibit the photosynthesis. These diverted electrons can thereby damage membranes and destroy cells.[6]
Synthesis
[edit]Hexazinone can be synthesized in two different reaction processes. One process starts with a reaction of methyl chloroformate with cyanamide, forming hexazinone after a five-step pathway:[7]
A second synthesis starts with methylthiourea.:[7]
Degradation
[edit]The degradation of hexazinone has long been studied.[8] It degrades approximately 10% in five weeks, when exposed to artificial sunlight in distilled water. However, degradation in natural waters can be three to seven times greater. Surprisingly, the pH and the temperature of the water do not affect the photodegradation significantly.[9] It is mainly degraded by aerobic microorganisms in soils.[10]
Mechanism of action
[edit]Hexazinone is a broad-spectrum residual and contact herbicide, rapidly absorbed by the leaves and roots. It is tolerated by many conifers, and therefore it is a very effective herbicide for the control for annual and perennial broadleaf weeds, some grasses, and some woody species. Hexazinone works as rain or snowmelt makes it possible for the herbicide to move downward into the soil. There the hexazinone is absorbed from the soil by the roots.[11] It moves through the conductive tissues to the leaves, where it blocks the photosynthesis of the plant within the chloroplasts. Hexazinone binds to a protein of the photosystem II complex, which blocks the electron transport. The result are multiple following reactions. First triplet-state chlorophyll reacts with oxygen to form singlet oxygen. Both chlorophyll and singlet oxygen then remove hydrogen ions from the unsaturated lipids present in de cells and the organelle membranes, forming lipid radicals. These radicals will oxidize other lipids and proteins, eventually resulting in loss of the membrane integrity of the cells and organelles. This will result in a loss of chlorophyll, leakage of cellular contents, cell death, and eventually death of the plant.[12] Woody plants first show yellowing of the leaves before they start to defoliate, eventually they will die.[13] Sometimes plants are able to refoliate and defoliate again during the growing season.
References
[edit]- ^ Arnold P. Appleby, Franz Müller, Serge Carpy "Weed Control" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a28_165
- ^ Hexazinone, Herbicide Profile, Pesticide Management Education Program, Cornell University
- ^ da Silva, Cydianne Cavalcante; Souza, Matheus de Freitas; Passos, Ana Beatriz Rocha de Jesus; Silva, Tatiane Severo; Borges, Maiara Pinheiro da Silva; dos Santos, Matheus Silva; Silva, Daniel Valadão (March 2022). "Risk of environmental contamination due to the hexazinone application in agricultural soils in northeastern Brazil". Geoderma Regional. 28: e00481. Bibcode:2022GeodR..2800481D. doi:10.1016/j.geodrs.2022.e00481. S2CID 246338359.
- ^ Wang, Huili; Xu, Shuxia; Tan, Chengxia; Wang, Xuedong (2009-05-30). "Anaerobic biodegradation of hexazinone in four sediments". Journal of Hazardous Materials. 164 (2–3): 806–811. Bibcode:2009JHzM..164..806W. doi:10.1016/j.jhazmat.2008.08.073. PMID 18824297.
- ^ "Hexazinone: Reregistration Eligibility Decision (RED) Fact Sheet" (PDF).
- ^ "Agronomy 317 - Iowa State University". agron-www.agron.iastate.edu. Archived from the original on 2016-11-23. Retrieved 2017-03-15.
- ^ a b Ullmann's agrochemicals. Wiley-VCH. 2007-01-01. ISBN 9783527316045. OCLC 470787466.
- ^ Helling, C. S.; Kearney, P. C.; Alexander, M. (1971). "Behavior of pesticides in soil". Adv. Agron. Advances in Agronomy. 23: 147–240. doi:10.1016/S0065-2113(08)60153-4. ISBN 9780120007233.
- ^ Rhodes, R. C. (1980b). "Studies with 14C-labeled hexazinone in water and bluegill sunfish". J. Agric. Food Chem. 28 (2): 306–310. doi:10.1021/jf60228a002. PMID 7391368.
- ^ Rhodes, R. C. (1980a). "Soil Studies with 14C-labeled hexazinone". J. Agric. Food Chem. 28 (2): 311–315. doi:10.1021/jf60228a012.
- ^ Ghassemi, M.; et al. (1981). Environmental fates and impacts of major forest use pesticides. Washington D.C. pp. 169–194.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ "Weed Science Society of America". wssa.net. Retrieved 2017-03-15.
- ^ Sidhu, S. S.; Feng., J. C. (1993). "Hexazinone and its metabolites in boreal forest vegetation". Weed Sci. 41 (2): 281–287. doi:10.1017/S0043174500076177. S2CID 83421922.
External links
[edit]- DuPont webpage on Velpar
- Hexazinone in the Pesticide Properties DataBase (PPDB)