Sulfurous acid: Difference between revisions
No edit summary |
Moving from Category:Hydrogen compounds to Category:Oxoacids using Cat-a-lot |
||
| (41 intermediate revisions by 23 users not shown) | |||
| Line 2: | Line 2: | ||
{{distinguish|Sulfuric acid}} |
{{distinguish|Sulfuric acid}} |
||
{{chembox |
{{chembox |
||
| verifiedrevid = 470482579 |
| verifiedrevid = 470482579 |
||
| Name = Sulfurous acid |
| Name = Sulfurous acid |
||
| ImageFile_Ref = {{chemboximage|correct|??}} |
| ImageFile_Ref = {{chemboximage|correct|??}} |
||
| ImageFile = Sulfurous-acid-2D-pyramidal.png |
| ImageFile = Sulfurous-acid-2D-pyramidal.png |
||
| ImageSize = 150px |
| ImageSize = 150px |
||
| ImageName = Sulfuric(IV) acid |
| ImageName = Sulfuric(IV) acid |
||
| ImageFile1 = Sulfurous-acid-3D-balls.png |
| ImageFile1 = Sulfurous-acid-3D-balls.png |
||
| ImageName1 = Ball-and-stick model of sulfurous acid |
| ImageName1 = Ball-and-stick model of sulfurous acid |
||
| OtherNames = Sulfuric(IV) acid<br/>Thionic acid<br/>Sulfinic acid |
|||
| IUPACName = Sulfurous acid |
| IUPACName = Sulfurous acid |
||
| ⚫ | |||
| SystematicName = |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ChEBI = 48854 |
|||
| ⚫ | |||
| ⚫ | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| ChemSpiderID = 1069 |
| ChemSpiderID = 1069 |
||
| EC_number = 231-973-1 |
|||
| Gmelin = 1458 |
|||
| PubChem = 1100 |
| PubChem = 1100 |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
| UNII_Ref = {{fdacite|correct|FDA}} |
||
| Line 21: | Line 31: | ||
| InChI = 1/H2O3S/c1-4(2)3/h(H2,1,2,3) |
| InChI = 1/H2O3S/c1-4(2)3/h(H2,1,2,3) |
||
| InChIKey = LSNNMFCWUKXFEE-UHFFFAOYAJ |
| InChIKey = LSNNMFCWUKXFEE-UHFFFAOYAJ |
||
| ⚫ | |||
| ⚫ | |||
| |
| SMILES1 = O[S+](O)[O-] |
||
| ⚫ | |||
| SMILES1 = [O-][SH+2](O)[O-] |
|||
| SMILES1_Comment = Tautomer |
| SMILES1_Comment = Tautomer |
||
| ⚫ | |||
| ⚫ | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChI = 1S/H2O3S/c1-4(2)3/h(H2,1,2,3) |
| StdInChI = 1S/H2O3S/c1-4(2)3/h(H2,1,2,3) |
||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChIKey = LSNNMFCWUKXFEE-UHFFFAOYSA-N |
| StdInChIKey = LSNNMFCWUKXFEE-UHFFFAOYSA-N |
||
| ⚫ | |||
| ⚫ | |||
}} |
}} |
||
|Section2={{Chembox Properties |
| Section2 = {{Chembox Properties |
||
| Formula = |
| Formula = {{chem2|H2SO3}} |
||
| MolarMass = 82.07 g/mol |
| MolarMass = 82.07 g/mol |
||
| ConjugateBase = [[Bisulfite]] |
| ConjugateBase = [[Bisulfite]] |
||
| pKa = 1.857, 7.172<ref name=P82db>{{cite book|title=Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution|editor-first=D. D.|editor-last=Perrin|edition=2nd|series=[[IUPAC]] Chemical Data|issue=29|publisher=Pergamon|location=Oxford|year=1982|publication-date=1984|orig-date=1969|lccn=82-16524|isbn=0-08-029214-3|at=Entry 217}}</ref> |
|||
| pKa = 1.857, 7.172 |
|||
}} |
}} |
||
| Section3 = |
|||
| ⚫ | |||
| Section4 = |
|||
| Section5 = |
|||
| Section6 = |
|||
| ⚫ | |||
| ExternalSDS = [http://www.inchem.org/documents/icsc/icsc/eics0074.htm ICSC 0074] |
| ExternalSDS = [http://www.inchem.org/documents/icsc/icsc/eics0074.htm ICSC 0074] |
||
| GHSPictograms = {{GHS05}}{{GHS07}} |
|||
| EUClass = {{Hazchem C}} ('''C''') |
|||
| GHSSignalWord = Danger |
|||
| RPhrases = {{R20}}, {{R34}} |
|||
| HPhrases = {{H-phrases|314|332}} |
|||
| SPhrases = {{S1/2}}, {{S9}}, {{S26}}, {{S36/37/39}}, {{S45}} |
|||
| PPhrases = {{P-phrases|260|261|264|271|280|301+330+331|303+361+353|304+312|304+340|305+351+338|310|312|321|363|405|501}} |
|||
| FlashPt = Non-flammable |
| FlashPt = Non-flammable |
||
}} |
}} |
||
|Section8={{Chembox Related |
| Section8 = {{Chembox Related |
||
| OtherCompounds = [[Sulfur dioxide]]<br/>[[Sulfuric acid]] |
| OtherCompounds = [[Sulfur dioxide]]<br/>[[Sulfuric acid]]<br/>[[Selenous acid]] |
||
}} |
}} |
||
}} |
}} |
||
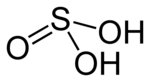
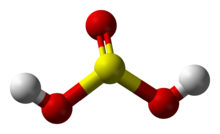
'''Sulfuric(IV) acid''' ([[United Kingdom]] spelling: '''sulphuric(IV) acid'''), also known as '''sulfurous''' (UK: '''sulphurous''') '''acid''' and '''thionic acid''',{{citation needed|date=July 2023}} is the [[chemical compound]] with the [[chemical formula|formula]] {{chem2|H2SO3}}. |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | [[Raman spectroscopy|Raman spectra]] of solutions of [[sulfur dioxide]] in water show only signals due to the |
||
| ⚫ | |||
<sup>17</sup>O [[NMR spectroscopy]] provided evidence that solutions of sulfurous acid and protonated sulfites contain a mixture of isomers, which is in equilibrium:<ref name="InorgChem">{{cite book |
<sup>17</sup>O [[NMR spectroscopy]] provided evidence that solutions of sulfurous acid and protonated sulfites contain a mixture of isomers, which is in equilibrium:<ref name="InorgChem">{{cite book |
||
| title = Inorganic Chemistry, 3rd Edition |
| title = Inorganic Chemistry, 3rd Edition |
||
| Line 67: | Line 78: | ||
| page = 520 |
| page = 520 |
||
}}</ref> |
}}</ref> |
||
: [H–OSO<sub>2</sub>]<sup>−</sup> ⇌ [H–SO<sub>3</sub>]<sup>−</sup> |
|||
{{block indent|{{chem2|[H\sOSO2]− ⇌ [H\sSO3]−}}}} |
|||
When trying to concentrate the solution by evaporation to produce waterless sulfurous acid it will decompose (reversing the forming reaction). In cooling down a [[clathrate]] SO<sub>2</sub>·{{frac|5|3|4}}H<sub>2</sub>O will crystallise which decomposes again at 7 °C. Thus sulfurous acid H<sub>2</sub>SO<sub>3</sub> cannot be isolated. |
|||
Attempts to concentrate the solutions of sulfurous acid simply reverses the equilibrium, producing sulfur dioxide and water vapor. A [[clathrate]] with the formula {{chem2|4SO2*23H2O}} has been crystallised. It decomposes above 7 °C. |
|||
==History and production== |
|||
| ⚫ | Sulfurous acid is commonly known to not exist in its free state, and due to this, it is stated in textbooks that it cannot be isolated in the water-free form.<ref>{{Greenwood&Earnshaw2nd|page=719}}</ref> However, the molecule has been detected in the gas phase in 1988 by the dissociative ionization of [[diethyl sulfite]].<ref>{{cite journal |author1=D. Sülzle |author2=M. Verhoeven |author3=J. K. Terlouw |author4=H. Schwarz | title = Generation and Characterization of Sulfurous Acid (H<sub>2</sub>SO<sub>3</sub>) and of Its Radical Cation as Stable Species in the Gas Phase | journal = [[Angew. Chem. Int. Ed. Engl.]] | volume = 27 | pages = 1533–4 | year = 1988 | doi = 10.1002/anie.198815331 | issue = 11}}</ref> The conjugate bases of this elusive acid are, however, common anions, [[bisulfite]] (or hydrogen sulfite) and [[sulfite]]. Sulfurous acid is an intermediate species in the formation of [[acid rain]] from sulfur dioxide.<ref>{{cite book|last1=McQuarrie |last2= Rock |date=1987 |title=General Chemistry |edition=2nd |publisher=W.H. Freeman and Company |location=New York |page=243 |ISBN=0-7167-1806-5}}</ref> |
||
==Uses== |
==Uses== |
||
| Line 82: | Line 98: | ||
==References== |
==References== |
||
{{reflist}} |
|||
<references/> |
|||
{{Hydrogen compounds}} |
{{Hydrogen compounds}} |
||
{{Sulfites}} |
|||
{{sulfur compounds}} |
|||
{{Authority control}} |
{{Authority control}} |
||
[[Category: |
[[Category:Oxoacids]] |
||
[[Category:Sulfites]] |
[[Category:Sulfites]] |
||
[[Category:Sulfur oxoacids]] |
[[Category:Sulfur oxoacids]] |
||
[[Category:Hypothetical chemical compounds]] |
|||
Latest revision as of 13:28, 13 December 2024
| |
| |
| Names | |
|---|---|
| IUPAC name
Sulfurous acid
| |
| Other names
Sulfuric(IV) acid
Thionic acid Sulfinic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.029.066 |
| EC Number |
|
| 1458 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H2SO3 | |
| Molar mass | 82.07 g/mol |
| Acidity (pKa) | 1.857, 7.172[1] |
| Conjugate base | Bisulfite |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H314, H332 | |
| P260, P261, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P363, P405, P501 | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | ICSC 0074 |
| Related compounds | |
Related compounds
|
Sulfur dioxide Sulfuric acid Selenous acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sulfuric(IV) acid (United Kingdom spelling: sulphuric(IV) acid), also known as sulfurous (UK: sulphurous) acid and thionic acid,[citation needed] is the chemical compound with the formula H2SO3.
Raman spectra of solutions of sulfur dioxide in water show only signals due to the SO2 molecule and the bisulfite ion, HSO−3.[2] The intensities of the signals are consistent with the following equilibrium:
17O NMR spectroscopy provided evidence that solutions of sulfurous acid and protonated sulfites contain a mixture of isomers, which is in equilibrium:[3]
Attempts to concentrate the solutions of sulfurous acid simply reverses the equilibrium, producing sulfur dioxide and water vapor. A clathrate with the formula 4SO2·23H2O has been crystallised. It decomposes above 7 °C.
History and production
[edit]Sulfurous acid is commonly known to not exist in its free state, and due to this, it is stated in textbooks that it cannot be isolated in the water-free form.[4] However, the molecule has been detected in the gas phase in 1988 by the dissociative ionization of diethyl sulfite.[5] The conjugate bases of this elusive acid are, however, common anions, bisulfite (or hydrogen sulfite) and sulfite. Sulfurous acid is an intermediate species in the formation of acid rain from sulfur dioxide.[6]
Uses
[edit]Aqueous solutions of sulfur dioxide, which sometimes are referred to as sulfurous acid, are used as reducing agents and as disinfectants, as are solutions of bisulfite and sulfite salts. They are oxidised to sulfuric acid or sulfate by accepting another oxygen atom.[7]
See also
[edit]References
[edit]- ^ Perrin, D. D., ed. (1982) [1969]. Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution. IUPAC Chemical Data (2nd ed.). Oxford: Pergamon (published 1984). Entry 217. ISBN 0-08-029214-3. LCCN 82-16524.
- ^ Jolly, William L. (1991). Modern Inorganic Chemistry (2nd ed.). New York: McGraw-Hill. ISBN 0-07-032768-8.
- ^ Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 16: The group 16 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 520. ISBN 978-0-13-175553-6.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 719. ISBN 978-0-08-037941-8.
- ^ D. Sülzle; M. Verhoeven; J. K. Terlouw; H. Schwarz (1988). "Generation and Characterization of Sulfurous Acid (H2SO3) and of Its Radical Cation as Stable Species in the Gas Phase". Angew. Chem. Int. Ed. Engl. 27 (11): 1533–4. doi:10.1002/anie.198815331.
- ^ McQuarrie; Rock (1987). General Chemistry (2nd ed.). New York: W.H. Freeman and Company. p. 243. ISBN 0-7167-1806-5.
- ^ L. Kolditz, Anorganische Chemie, VEB Deutscher Verlag der Wissenschaften, Berlin 1983, S. 476.
