HU-210: Difference between revisions
Bon courage (talk | contribs) →Effects and research: more primary |
consistent citation formatting; templated bare URL |
||
| (26 intermediate revisions by 10 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description| |
{{Short description|Synthetic cannabinoid}} |
||
{{cs1 config|name-list-style=vanc|display-authors=6}} |
|||
{{Drugbox |
{{Drugbox |
||
| Verifiedfields |
| Verifiedfields = changed |
||
| Watchedfields |
| Watchedfields = changed |
||
| verifiedrevid |
| verifiedrevid = 400102655 |
||
| IUPAC_name |
| IUPAC_name = (6a''R'',10a''R'')-9-(hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6''H'',6a''H'',7''H'',10''H'',10a''H''-benzo[''c'']isochromen-1-ol |
||
| image |
| image = HU-210 structure.svg |
||
| image2 |
| image2 = Hu210_bns.png |
||
<!--Clinical data-->| tradename |
<!--Clinical data-->| tradename = |
||
| legal_CA |
| legal_CA = Schedule II |
||
| legal_US |
| legal_US = Schedule I<ref name="USDOJ"/> |
||
| legal_UK |
| legal_UK = Class B |
||
| routes_of_administration = <!--Pharmacokinetic data--> |
| routes_of_administration = <!--Pharmacokinetic data--> |
||
| metabolism |
| metabolism = |
||
| elimination_half-life = |
| elimination_half-life = |
||
| excretion |
| excretion = <!--Identifiers--> |
||
| CAS_number_Ref |
| CAS_number_Ref = {{cascite|correct|??}} |
||
| CAS_number |
| CAS_number = 112830-95-2 |
||
| PubChem |
| PubChem = 9821569 |
||
| IUPHAR_ligand |
| IUPHAR_ligand = 731 |
||
| DrugBank_Ref |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
||
| ChemSpiderID_Ref |
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
||
| ChemSpiderID |
| ChemSpiderID = 7997318 |
||
| ChEMBL_Ref |
| ChEMBL_Ref = {{ebicite|changed|EBI}} |
||
| ChEMBL |
| ChEMBL = 307696 |
||
| UNII_Ref |
| UNII_Ref = {{fdacite|changed|FDA}} |
||
| UNII |
| UNII = 191042422P |
||
<!--Chemical data-->| C |
<!--Chemical data-->| C = 25 |
||
| H |
| H = 38 |
||
| O |
| O = 3 |
||
| smiles |
| smiles = CCCCCCC(C)(C)C1=CC2=C([C@@H]3CC(=CC[C@H]3C(O2)(C)C)CO)C(=C1)O |
||
| StdInChI_Ref |
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
||
| StdInChI |
| StdInChI = 1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19-,20-/m1/s1 |
||
| StdInChIKey_Ref |
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
||
| StdInChIKey |
| StdInChIKey = SSQJFGMEZBFMNV-WOJBJXKFSA-N |
||
| PDB_ligand = A1H66 |
|||
| synonyms |
| synonyms = 1,1-Dimethylheptyl- 11-hydroxy- tetrahydrocannabinol |
||
}} |
}} |
||
'''HU-210''' is a [[synthetic cannabinoid]] that was first [[chemical synthesis|synthesized]] in 1988 from (1''R'',5''S'')-[[myrtenol]]<ref name="Mechoulam et al 1990">{{cite journal | vauthors = Mechoulam R, Lander N, Zahalka J |title=Synthesis of the individual, pharmacologically distinct, enantiomers of a tetrahydrocannabinol derivative |journal=Tetrahedron: Asymmetry |date=January 1990 |volume=1 |issue=5 |pages=315–318 |doi=10.1016/S0957-4166(00)86322-3 }}</ref> by a group led by [[Raphael Mechoulam]] at the [[Hebrew University]].<ref>{{cite journal | vauthors = Mechoulam R, Feigenbaum JJ, Lander N, Segal M, Järbe TU, Hiltunen AJ, Consroe P | title = Enantiomeric cannabinoids: stereospecificity of psychotropic activity | journal = Experientia | volume = 44 | issue = 9 | pages = 762–4 | date = September 1988 | pmid = 3416993 | doi = 10.1007/BF01959156 | s2cid = 19589995 }}</ref><ref>{{cite journal | vauthors = Little PJ, Compton DR, Mechoulam R, Martin BR | title = Stereochemical effects of 11-OH-delta 8-THC-dimethylheptyl in mice and dogs | journal = Pharmacology, Biochemistry, and Behavior | volume = 32 | issue = 3 | pages = 661–6 | date = March 1989 | pmid = 2544901 | doi = 10.1016/0091-3057(89)90014-2 | s2cid = 140209484 }}</ref><ref>{{cite journal | vauthors = Järbe TU, Hiltunen AJ, Mechoulam R | title = Stereospecificity of the discriminative stimulus functions of the dimethylheptyl homologs of 11-hydroxy-delta 8-tetrahydrocannabinol in rats and pigeons | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 250 | issue = 3 | pages = 1000–5 | date = September 1989 | pmid = 2550611 }}</ref> HU-210 is 100 to 800 times more potent than natural [[Tetrahydrocannabinol|THC]] from [[cannabis (drug)|cannabis]] and has an extended duration of action.<ref name="pmid1317925">{{cite journal | vauthors = Devane WA, Breuer A, Sheskin T, Järbe TU, Eisen MS, Mechoulam R | title = A novel probe for the cannabinoid receptor | journal = Journal of Medicinal Chemistry | volume = 35 | issue = 11 | pages = 2065–9 | date = May 1992 | pmid = 1317925 | doi = 10.1021/jm00089a018 }} |
'''HU-210''' is a [[synthetic cannabinoid]] that was first [[chemical synthesis|synthesized]] in 1988 from (1''R'',5''S'')-[[myrtenol]]<ref name="Mechoulam et al 1990">{{cite journal | vauthors = Mechoulam R, Lander N, Zahalka J |title=Synthesis of the individual, pharmacologically distinct, enantiomers of a tetrahydrocannabinol derivative |journal=Tetrahedron: Asymmetry |date=January 1990 |volume=1 |issue=5 |pages=315–318 |doi=10.1016/S0957-4166(00)86322-3 }}</ref> by a group led by [[Raphael Mechoulam]] at the [[Hebrew University]].<ref>{{cite journal | vauthors = Mechoulam R, Feigenbaum JJ, Lander N, Segal M, Järbe TU, Hiltunen AJ, Consroe P | title = Enantiomeric cannabinoids: stereospecificity of psychotropic activity | journal = Experientia | volume = 44 | issue = 9 | pages = 762–4 | date = September 1988 | pmid = 3416993 | doi = 10.1007/BF01959156 | s2cid = 19589995 }}</ref><ref>{{cite journal | vauthors = Little PJ, Compton DR, Mechoulam R, Martin BR | title = Stereochemical effects of 11-OH-delta 8-THC-dimethylheptyl in mice and dogs | journal = Pharmacology, Biochemistry, and Behavior | volume = 32 | issue = 3 | pages = 661–6 | date = March 1989 | pmid = 2544901 | doi = 10.1016/0091-3057(89)90014-2 | s2cid = 140209484 }}</ref><ref>{{cite journal | vauthors = Järbe TU, Hiltunen AJ, Mechoulam R | title = Stereospecificity of the discriminative stimulus functions of the dimethylheptyl homologs of 11-hydroxy-delta 8-tetrahydrocannabinol in rats and pigeons | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 250 | issue = 3 | pages = 1000–5 | date = September 1989 | pmid = 2550611 }}</ref> HU-210 is 100 to 800 times more potent than natural [[Tetrahydrocannabinol|THC]] from [[cannabis (drug)|cannabis]] and has an extended duration of action.<ref name="pmid1317925">{{cite journal | vauthors = Devane WA, Breuer A, Sheskin T, Järbe TU, Eisen MS, Mechoulam R | title = A novel probe for the cannabinoid receptor | journal = Journal of Medicinal Chemistry | volume = 35 | issue = 11 | pages = 2065–9 | date = May 1992 | pmid = 1317925 | doi = 10.1021/jm00089a018 }}</ref> HU-210 has a binding affinity of 0.061 nM at [[Cannabinoid receptor 1|CB<sub>1</sub> receptors]]<ref>{{cite journal | vauthors = Stern E, Lambert DM | title = Medicinal chemistry endeavors around the phytocannabinoids | journal = Chemistry & Biodiversity | volume = 4 | issue = 8 | pages = 1707–1728 | date = August 2007 | pmid = 17712816 | doi = 10.1002/cbdv.200790149 | s2cid = 24920412 }}</ref> compared to 40.7 nM for Δ<sup>9</sup>-THC.<ref>{{cite journal | vauthors = Bow EW, Rimoldi JM | title = The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation | journal = Perspectives in Medicinal Chemistry | volume = 8 | pages = 17–39 | year = 2016 | pmid = 27398024 | pmc = 4927043 | doi = 10.4137/PMC.S32171 }}</ref> The binding pose of HU-210 to the CB<sub>1</sub> receptor is similar to other synthetic cannabinoids.<ref>{{cite journal | vauthors = Gloriam D, Thorsen T, Kulkarni Y, Sykes D, Bøggild A, Drace T, Hompluem P, Iliopoulos-Tsoutsouvas C, Nikas S, Daver H, Makriyannis A, Nissen P, Gajhede M, Veprintsev D, Boesen T, Kastrup J | title = Structural basis of Δ<sup>9</sup>-THC analog activity at the Cannabinoid 1 receptor | journal = Research Square | date = May 2024 | pmid = 38826401 | pmc = 11142349 | doi = 10.21203/rs.3.rs-4277209/v1 }}</ref> |
||
</ref> HU-210 has a binding affinity of 0.061nM at CB1 and 0.52nM at CB2 in cloned human cannabinoid receptors<ref>{{cite journal | vauthors = Stern E, Lambert DM | title = Medicinal chemistry endeavors around the phytocannabinoids | journal = Chemistry & Biodiversity | volume = 4 | issue = 8 | pages = 1707–1728 | date = August 2007 | pmid = 17712816 | doi = 10.1002/cbdv.200790149 | s2cid = 24920412 }}</ref> compared to Delta-9-THC of 40.7nM at CB1. <ref>{{cite journal | vauthors = Bow EW, Rimoldi JM | title = The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation | journal = Perspectives in Medicinal Chemistry | volume = 8 | pages = 17–39 | year = 2016 | pmid = 27398024 | pmc = 4927043 | doi = 10.4137/PMC.S32171 }}</ref> HU-210 is the (–)-1,1-dimethylheptyl analog of 11-hydroxy- Δ<sup>8</sup>- tetrahydrocannabinol; in some references it is called 1,1-dimethylheptyl- 11-hydroxytetrahydrocannabinol. The abbreviation "HU" stands for Hebrew University. |
|||
==Effects and research== |
==Effects and research== |
||
HU-210, the (–) [[enantiomer]] |
HU-210, the (–) [[enantiomer]], is an ultrapotent [[cannabinoid]], while its (+) enantiomer [[HU-211]] is not a cannabinoid, but an [[NMDA antagonist]] with [[neuroprotective]] effects.<ref>{{cite journal | vauthors = Howlett AC, Champion TM, Wilken GH, Mechoulam R | title = Stereochemical effects of 11-OH-delta 8-tetrahydrocannabinol-dimethylheptyl to inhibit adenylate cyclase and bind to the cannabinoid receptor | journal = Neuropharmacology | volume = 29 | issue = 2 | pages = 161–5 | date = February 1990 | pmid = 2158635 | doi = 10.1016/0028-3908(90)90056-w | s2cid = 28602221 }}</ref><ref name="pmid14534855">{{cite journal | vauthors = Darlington CL | title = Dexanabinol: a novel cannabinoid with neuroprotective properties | journal = IDrugs | volume = 6 | issue = 10 | pages = 976–9 | date = October 2003 | pmid = 14534855 | oclc = 112453448 }}</ref> |
||
HU-210 has an oral |
HU-210 has an oral [[Median lethal dose|LD<sub>50</sub>]] of 5,000 mg/kg in rats and 14,200 mg/kg in rabbits,<ref name="caymanchem 90083m">{{cite web | title = HU-210 | work = Material Safety Data Sheet | publisher = Cayman Chemical | url = https://www.caymanchem.com/msdss/90083m.pdf}}</ref> and an [[Lethal dose|LD<sub>LO</sub>]] (lowest lethal dose) of 143 mg/kg in humans.<ref name="caymanchem 90083m"/> This is more toxic than Δ<sup>8</sup>-THC; in monkeys and dogs, 9,000 mg/kg of Δ<sup>8</sup>-THC was nonlethal.<ref>{{cite journal | vauthors = Thompson GR, Rosenkrantz H, Schaeppi UH, Braude MC | title = Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys | journal = Toxicology and Applied Pharmacology | volume = 25 | issue = 3 | pages = 363–72 | date = July 1973 | pmid = 4199474 | doi = 10.1016/0041-008X(73)90310-4 | bibcode = 1973ToxAP..25..363T }}</ref><ref>{{Cite web |url= https://chem.nlm.nih.gov/chemidplus/rn/5957-75-5 |work = ChemIDplus | publisher = U.S. National Library of Medicine | title = delta-8-Tetrahydrocannabinol }}</ref> |
||
==Chemistry== |
==Chemistry== |
||
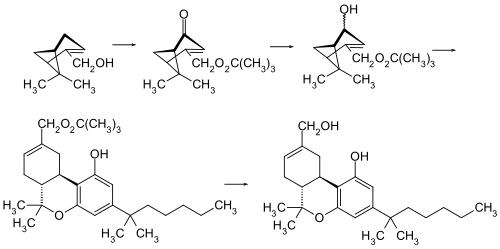
HU-210 is the [[enantiomer]] of [[HU-211]] ([[ |
HU-210 is the [[enantiomer]] of [[HU-211]] ([[dexanabinol]]). The original synthesis of HU-210 is based on an acid-catalyzed condensation of (–)-[[Myrtenol]] and [[1,1-Dimethylheptylresorcinol]] (3,5-Dihydroxy-1-(1,1-dimethylheptyl)benzol).<ref name="Mechoulam et al 1990"/> |
||
[[File:HU-210 synthesis.svg|HU-210 synthesis|center|500px]] |
[[File:HU-210 synthesis.svg|HU-210 synthesis|center|500px]] |
||
| Line 60: | Line 61: | ||
===United States=== |
===United States=== |
||
HU-210 is not explicitly listed in the [[List of Schedule I drugs (US)|list of scheduled controlled substances]] in the USA.<ref name="PART 1308 — SCHEDULES OF CONTROLLED SUBSTANCES - 1308.11 Schedule I">{{cite web|url=http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm|title=PART 1308 - Section 1308.11 Schedule I | work = Office of Diversion Control | publisher = Drug Enforcement Administration, U.S. Department of Justice |access-date=3 May 2018|url-status=live|archive-url= https://web.archive.org/web/20090827043725/http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm |archive-date=27 August 2009}}</ref> A brief profile of HU-210 written and published by the [[Drug Enforcement Administration]] (DEA) in 2009, but removed in later years, stated that HU-210 is a Schedule I controlled substance under the [[Controlled Substances Act]] due to being similar to [[THC]].<ref>{{cite web |url= http://www.deadiversion.usdoj.gov/drugs_concern/spice/spice_hu210.htm |title=Spice Cannabinoid - HU-210|url-status=dead |archive-url=https://web.archive.org/web/20120117131045/http://www.deadiversion.usdoj.gov/drugs_concern/spice/spice_hu210.htm|archive-date=2012-01-17 | work = Office of Diversion Control | publisher = Drug Enforcement Administration, U.S. Department of Justice }}</ref> A version of the document (updated in 2013), now in PDF form, exists on the DEA Office of Diversion Control's website.<ref>{{cite web | url = http://www.deadiversion.usdoj.gov/drug_chem_info/spice/spice_hu210.pdf | title = HU-210 | quote = 6aR,10aR)-9-(hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c] chromen-1-ol)] [Purported Ingredient of “Spice” | archive-url = https://web.archive.org/web/20161228044049/https://www.deadiversion.usdoj.gov/drug_chem_info/spice/spice_hu210.pdf | archive-date = 2016-12-28 | work = Office of Diversion Control | publisher = Drug Enforcement Administration, U.S. Department of Justice | date = January 2013 }}</ref> In that PDF, DEA reasserts that HU-210 is a Schedule I substance. DEA currently considers HU-210 a Schedule I controlled substance under the umbrella of ‘tetrahydrocannabinols’ |
HU-210 is not explicitly listed in the [[List of Schedule I drugs (US)|list of scheduled controlled substances]] in the USA.<ref name="PART 1308 — SCHEDULES OF CONTROLLED SUBSTANCES - 1308.11 Schedule I">{{cite web|url=http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm|title=PART 1308 - Section 1308.11 Schedule I | work = Office of Diversion Control | publisher = Drug Enforcement Administration, U.S. Department of Justice |access-date=3 May 2018|url-status=live|archive-url= https://web.archive.org/web/20090827043725/http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm |archive-date=27 August 2009}}</ref> A brief profile of HU-210 written and published by the [[Drug Enforcement Administration]] (DEA) in 2009, but removed in later years, stated that HU-210 is a Schedule I controlled substance under the [[Controlled Substances Act]] due to being similar to [[THC]].<ref>{{cite web |url= http://www.deadiversion.usdoj.gov/drugs_concern/spice/spice_hu210.htm |title=Spice Cannabinoid - HU-210|url-status=dead |archive-url=https://web.archive.org/web/20120117131045/http://www.deadiversion.usdoj.gov/drugs_concern/spice/spice_hu210.htm|archive-date=2012-01-17 | work = Office of Diversion Control | publisher = Drug Enforcement Administration, U.S. Department of Justice }}</ref> A version of the document (updated in 2013), now in PDF form, exists on the DEA Office of Diversion Control's website.<ref name=USDOJ>{{cite web | url = http://www.deadiversion.usdoj.gov/drug_chem_info/spice/spice_hu210.pdf | title = HU-210 | quote = 6aR,10aR)-9-(hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c] chromen-1-ol)] [Purported Ingredient of “Spice” | archive-url = https://web.archive.org/web/20161228044049/https://www.deadiversion.usdoj.gov/drug_chem_info/spice/spice_hu210.pdf | archive-date = 2016-12-28 | work = Office of Diversion Control | publisher = Drug Enforcement Administration, U.S. Department of Justice | date = January 2013 }}</ref> In that PDF, the DEA reasserts that HU-210 is a Schedule I substance. The DEA currently considers HU-210 a Schedule I controlled substance under the umbrella of ‘tetrahydrocannabinols’ under CSCN 7370.<ref>{{cite book | title = Lists of Scheduling Actions, Controlled Substances, and Regulated Chemicals | url = https://www.deadiversion.usdoj.gov/schedules/orangebook/orangebook.pdf | date = November 2024 | publisher = Drug and Chemical Evaluation Section, Diversion Control Division, Drug Enforcement Administration Division, U.S. Department of Justice }}</ref> |
||
=== Alabama === |
=== Alabama === |
||
| Line 76: | Line 77: | ||
====Vermont==== |
====Vermont==== |
||
Effective January 1, 2016, HU-210 is a regulated drug in Vermont designated as a "Hallucinogenic Drug."<ref>{{cite book |title = Code of Vermont Rules | chapter = Chapter 8 – Alcohol and Drug Abuse Subchapter 9: Regulated Drug Rule | publisher = Vermont Department of Health | date = 15 July 2017 |access-date=3 May 2018 | chapter-url = http://www.healthvermont.gov/sites/default/files/documents/2017/01/REG_regulated-drugs.pdf |url-status= live |archive-url= https://web.archive.org/web/20170127230917/http://healthvermont.gov/sites/default/files/documents/2017/01/REG_regulated-drugs.pdf | archive-date = 27 January 2017 }}</ref> |
Effective January 1, 2016, HU-210 is a regulated drug in Vermont designated as a "Hallucinogenic Drug."<ref>{{cite book |title = Code of Vermont Rules | chapter = Chapter 8 – Alcohol and Drug Abuse Subchapter 9: Regulated Drug Rule | publisher = Vermont Department of Health | date = 15 July 2017 |access-date=3 May 2018 | chapter-url = http://www.healthvermont.gov/sites/default/files/documents/2017/01/REG_regulated-drugs.pdf |url-status= live |archive-url= https://web.archive.org/web/20170127230917/http://healthvermont.gov/sites/default/files/documents/2017/01/REG_regulated-drugs.pdf | archive-date = 27 January 2017 }}</ref> |
||
{{quote|“Hallucinogenic Drug” means those specified in Section 7 of this rule including [[stramonium]], [[mescaline]] or [[peyote]], [[lysergic acid diethylamide]], and [[psilocybin]], and all synthetic equivalents of chemicals contained in resinous extractives of [[Cannabis sativa]], or any salts or derivatives or compounds of any preparations or mixtures thereof, and any other substance having a hallucinogenic effect in the regulations adopted by the Board of Health under 18 V.S.A.§ 4202. |
|||
... |
|||
• Cannabimimetic Agents means, collectively, any chemical that is a [[cannabinoid receptor type 1]] (CB1) or [[cannabinoid receptor type 2]] (CB2) agonist, or any salts, [[isomers]], derivatives, or analogs of these chemicals. Structural classes include but are not limited to: |
|||
(a) 2-(3-hydroxycyclohexyl)phenol with substitution at the 5-position of the phenolic ring by alkyl or alkenyl, whether or not substituted on the cyclohexyl ring to any extent. |
|||
(b) 3-(1-naphthoyl)indole or 3-(1-naphthyl)indole with substitution at the nitrogen atom of the indole ring, whether or not further substituted on the indole ring to any extent, whether or not substituted on the naphthoyl or naphthyl ring to any extent. |
|||
(c) 3-(1-naphthoyl)pyrrole with substitution at the nitrogen atom of the pyrrole ring, whether or not further substituted in the pyrrole ring to any extent, whether or not substituted on the naphthoyl ring to any extent. |
|||
(d) 1-(1-naphthylmethyl)indene with substitution of the 3-position of the indene ring, whether or not further substituted in the indene ring to any extent, whether or not substituted on the naphthyl ring to any extent. |
|||
(e) 3-phenylacetylindole or 3-benzoylindole with substitution at the nitrogen atom of the indole ring, whether or not further substituted in the indole ring to any extent, whether or not substituted on the phenyl ring to any extent. |
|||
(f) indole- (2,2,3,3-tetramethylcyclopropyl)methanone, with substitution at the nitrogen atom of the indole ring, whether or not further substituted in the indole ring to any extent, whether or not substituted on the phenyl ring to any extent. |
|||
(g) N- adamantyl-indole-3-carboxamide, with substitution at the nitrogen atom of the indole ring, whether or not further substituted in the indole ring to any extent, whether or not substituted on the phenyl ring to any extent. |
|||
(h) (1,3-thiazol-2- ylidine)-2,2,3,3- tetramethylcyclopropane-1-carboxamide, with substitution to any extent at any position of the thiazolylidine ring. |
|||
... |
|||
• HU-210; (6aR,10aR)-9-(hydroxymethyl)-6,6-dimethyl-3- (2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c] chromen-1-ol; OR [(6aR,10aR)-9-(hy droxymethyl)- 6,6-dimethyl-3-(2-methyl octan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol; OR 1,1-Dimethylheptyl-11-hydroxytetrahydrocannabinol}} |
|||
==Other HU Cannabinoids== |
|||
{{colbegin}} |
|||
| ⚫ | |||
* [[HU-239]] |
|||
* [[HU-243]] |
|||
* [[HU-308]] |
|||
* [[HU-320]] |
|||
* [[HU-331]] |
|||
* [[HU-336]] |
|||
* [[HU-345]] |
|||
{{colend}} |
|||
== See also == |
== See also == |
||
* [[11-Hydroxy-Delta-8-THC]] |
* [[11-Hydroxy-Delta-8-THC]] |
||
| ⚫ | |||
* [[CP 47,497]] |
* [[CP 47,497]] |
||
* [[JWH-018]] |
|||
== References == |
== References == |
||
| Line 137: | Line 101: | ||
[[Category:HU cannabinoids]] |
[[Category:HU cannabinoids]] |
||
[[Category:Designer drugs]] |
[[Category:Designer drugs]] |
||
[[Category: |
[[Category:Hydroxyarenes]] |
||
Latest revision as of 07:28, 14 December 2024
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 1,1-Dimethylheptyl- 11-hydroxy- tetrahydrocannabinol |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H38O3 |
| Molar mass | 386.576 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol[2] by a group led by Raphael Mechoulam at the Hebrew University.[3][4][5] HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended duration of action.[6] HU-210 has a binding affinity of 0.061 nM at CB1 receptors[7] compared to 40.7 nM for Δ9-THC.[8] The binding pose of HU-210 to the CB1 receptor is similar to other synthetic cannabinoids.[9]
Effects and research
[edit]HU-210, the (–) enantiomer, is an ultrapotent cannabinoid, while its (+) enantiomer HU-211 is not a cannabinoid, but an NMDA antagonist with neuroprotective effects.[10][11]
HU-210 has an oral LD50 of 5,000 mg/kg in rats and 14,200 mg/kg in rabbits,[12] and an LDLO (lowest lethal dose) of 143 mg/kg in humans.[12] This is more toxic than Δ8-THC; in monkeys and dogs, 9,000 mg/kg of Δ8-THC was nonlethal.[13][14]
Chemistry
[edit]HU-210 is the enantiomer of HU-211 (dexanabinol). The original synthesis of HU-210 is based on an acid-catalyzed condensation of (–)-Myrtenol and 1,1-Dimethylheptylresorcinol (3,5-Dihydroxy-1-(1,1-dimethylheptyl)benzol).[2]
Legal status
[edit]HU-210 is not listed in the schedules set out by the United Nations' Single Convention on Narcotic Drugs from 1961 nor their Convention on Psychotropic Substances from 1971,[15] so the signatory countries to these international drug control treaties are not required by said treaties to control HU-210.
New Zealand
[edit]HU-210 is banned in New Zealand as of 8 May 2014.[16]
United States
[edit]HU-210 is not explicitly listed in the list of scheduled controlled substances in the USA.[17] A brief profile of HU-210 written and published by the Drug Enforcement Administration (DEA) in 2009, but removed in later years, stated that HU-210 is a Schedule I controlled substance under the Controlled Substances Act due to being similar to THC.[18] A version of the document (updated in 2013), now in PDF form, exists on the DEA Office of Diversion Control's website.[1] In that PDF, the DEA reasserts that HU-210 is a Schedule I substance. The DEA currently considers HU-210 a Schedule I controlled substance under the umbrella of ‘tetrahydrocannabinols’ under CSCN 7370.[19]
Alabama
[edit]HU-210 is a Schedule I controlled substance in Alabama.[20]
(4)a. A synthetic controlled substance that is any material, mixture, or preparation that contains any quantity of the following chemical compounds, their salts, isomers and salts of isomers, unless specifically excepted, whenever the existence of these salts, isomers and salts of isomers is possible within the specific chemical designation or compound:
...
9. (6aR, 10aR)-9-(hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol, some trade or other names: HU-210.
Florida
[edit]HU-210 is a Schedule I controlled substance, categorized as a hallucinogen, making it illegal to buy, sell, or possess in the state of Florida without a license.[21]
(c) Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation that contains any quantity of the following hallucinogenic substances or that contains any of their salts, isomers, including optical, positional, or geometric isomers, homologues, nitrogen-heterocyclic analogs, esters, ethers, and salts of isomers, homologues, nitrogen-heterocyclic analogs, esters, or ethers, if the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation or class description: ... 47. HU-210 [(6aR,10aR)-9-(Hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol].
Vermont
[edit]Effective January 1, 2016, HU-210 is a regulated drug in Vermont designated as a "Hallucinogenic Drug."[22]
See also
[edit]References
[edit]- ^ a b "HU-210" (PDF). Office of Diversion Control. Drug Enforcement Administration, U.S. Department of Justice. January 2013. Archived from the original (PDF) on 2016-12-28.
6aR,10aR)-9-(hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c] chromen-1-ol)] [Purported Ingredient of "Spice"
- ^ a b Mechoulam R, Lander N, Zahalka J (January 1990). "Synthesis of the individual, pharmacologically distinct, enantiomers of a tetrahydrocannabinol derivative". Tetrahedron: Asymmetry. 1 (5): 315–318. doi:10.1016/S0957-4166(00)86322-3.
- ^ Mechoulam R, Feigenbaum JJ, Lander N, Segal M, Järbe TU, Hiltunen AJ, et al. (September 1988). "Enantiomeric cannabinoids: stereospecificity of psychotropic activity". Experientia. 44 (9): 762–4. doi:10.1007/BF01959156. PMID 3416993. S2CID 19589995.
- ^ Little PJ, Compton DR, Mechoulam R, Martin BR (March 1989). "Stereochemical effects of 11-OH-delta 8-THC-dimethylheptyl in mice and dogs". Pharmacology, Biochemistry, and Behavior. 32 (3): 661–6. doi:10.1016/0091-3057(89)90014-2. PMID 2544901. S2CID 140209484.
- ^ Järbe TU, Hiltunen AJ, Mechoulam R (September 1989). "Stereospecificity of the discriminative stimulus functions of the dimethylheptyl homologs of 11-hydroxy-delta 8-tetrahydrocannabinol in rats and pigeons". The Journal of Pharmacology and Experimental Therapeutics. 250 (3): 1000–5. PMID 2550611.
- ^ Devane WA, Breuer A, Sheskin T, Järbe TU, Eisen MS, Mechoulam R (May 1992). "A novel probe for the cannabinoid receptor". Journal of Medicinal Chemistry. 35 (11): 2065–9. doi:10.1021/jm00089a018. PMID 1317925.
- ^ Stern E, Lambert DM (August 2007). "Medicinal chemistry endeavors around the phytocannabinoids". Chemistry & Biodiversity. 4 (8): 1707–1728. doi:10.1002/cbdv.200790149. PMID 17712816. S2CID 24920412.
- ^ Bow EW, Rimoldi JM (2016). "The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation". Perspectives in Medicinal Chemistry. 8: 17–39. doi:10.4137/PMC.S32171. PMC 4927043. PMID 27398024.
- ^ Gloriam D, Thorsen T, Kulkarni Y, Sykes D, Bøggild A, Drace T, et al. (May 2024). "Structural basis of Δ9-THC analog activity at the Cannabinoid 1 receptor". Research Square. doi:10.21203/rs.3.rs-4277209/v1. PMC 11142349. PMID 38826401.
- ^ Howlett AC, Champion TM, Wilken GH, Mechoulam R (February 1990). "Stereochemical effects of 11-OH-delta 8-tetrahydrocannabinol-dimethylheptyl to inhibit adenylate cyclase and bind to the cannabinoid receptor". Neuropharmacology. 29 (2): 161–5. doi:10.1016/0028-3908(90)90056-w. PMID 2158635. S2CID 28602221.
- ^ Darlington CL (October 2003). "Dexanabinol: a novel cannabinoid with neuroprotective properties". IDrugs. 6 (10): 976–9. OCLC 112453448. PMID 14534855.
- ^ a b "HU-210" (PDF). Material Safety Data Sheet. Cayman Chemical.
- ^ Thompson GR, Rosenkrantz H, Schaeppi UH, Braude MC (July 1973). "Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys". Toxicology and Applied Pharmacology. 25 (3): 363–72. Bibcode:1973ToxAP..25..363T. doi:10.1016/0041-008X(73)90310-4. PMID 4199474.
- ^ "delta-8-Tetrahydrocannabinol". ChemIDplus. U.S. National Library of Medicine.
- ^ "International Drug Control Conventions". United Nations Office on Drugs and Crime. Archived from the original on 12 January 2018. Retrieved 3 May 2018.
- ^ "Synthetic cannabinoids: What they are". New Zealand Drug Foundation. Archived from the original on 2015-09-21. Retrieved 2015-07-18.
- ^ "PART 1308 - Section 1308.11 Schedule I". Office of Diversion Control. Drug Enforcement Administration, U.S. Department of Justice. Archived from the original on 27 August 2009. Retrieved 3 May 2018.
- ^ "Spice Cannabinoid - HU-210". Office of Diversion Control. Drug Enforcement Administration, U.S. Department of Justice. Archived from the original on 2012-01-17.
- ^ Lists of Scheduling Actions, Controlled Substances, and Regulated Chemicals (PDF). Drug and Chemical Evaluation Section, Diversion Control Division, Drug Enforcement Administration Division, U.S. Department of Justice. November 2024.
- ^ "Controlled substances, Schedule I, additional synthetic controlled substances and analogue substances included in, trafficking in controlled substance analogues, requisite weight increased, Secs. 13A-12-231, 20-2-23 am'd". Alabama Senate Bill 333. March 2014. Archived from the original on 4 March 2016. Retrieved 2 February 2017.
- ^ "Chapter 893: Drug Abuse Prevention and Control". The 2020 Florida Statutes. The Florida Legislature. Archived from the original on 14 March 2018. Retrieved 3 May 2018.
- ^ "Chapter 8 – Alcohol and Drug Abuse Subchapter 9: Regulated Drug Rule" (PDF). Code of Vermont Rules. Vermont Department of Health. 15 July 2017. Archived (PDF) from the original on 27 January 2017. Retrieved 3 May 2018.
Further reading
[edit]- Brumfiel G (13 October 2005). "Marijuana may make your brain grow". Nature. doi:10.1038/news051010-12.
- Bush DM, Woodwell DA (16 October 2014). "Update: Drug-Related Emergency Department Visits Involving Synthetic Cannabinoids" (PDF). The CBHSQ Report. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. PMID 27030867.
