Pancreas: Difference between revisions
→Structure: . |
m Substing templates: {{Format ISBN}}. See User:AnomieBOT/docs/TemplateSubster for info. |
||
| (25 intermediate revisions by 12 users not shown) | |||
| Line 7: | Line 7: | ||
| Greek = πάγκρεας (pánkreas) |
| Greek = πάγκρεας (pánkreas) |
||
| Image = Blausen_0699_PancreasAnatomy2.png |
| Image = Blausen_0699_PancreasAnatomy2.png |
||
| Caption = Anatomy of the pancreas |
| Caption = Anatomy of the human pancreas |
||
| Width = |
| Width = |
||
| Precursor = [[Pancreatic bud]]s |
| Precursor = [[Pancreatic bud]]s |
||
| Line 18: | Line 18: | ||
}} |
}} |
||
The '''pancreas''' is an [[Organ (anatomy)|organ]] of the [[Digestion|digestive system]] and [[endocrine system]] of [[vertebrate]]s. In humans, it is located in the [[abdominal cavity|abdomen]] behind the [[stomach]] and functions as a [[gland]]. The pancreas is a mixed or [[heterocrine gland]], i.e., it has both an [[endocrine]] and a digestive [[exocrine]] function.<ref>{{Cite journal|last1=Gyr|first1=K.|last2=Beglinger|first2=C.|last3=Stalder|first3=G. A.|date=1985-09-21|title=[Interaction of the endo- and exocrine pancreas] |
The '''pancreas''' is an [[Organ (anatomy)|organ]] of the [[Digestion|digestive system]] and [[endocrine system]] of [[vertebrate]]s. In humans, it is located in the [[abdominal cavity|abdomen]] behind the [[stomach]] and functions as a [[gland]]. The pancreas is a mixed or [[heterocrine gland]], i.e., it has both an [[endocrine]] and a digestive [[exocrine]] function.<ref>{{Cite journal|last1=Gyr|first1=K.|last2=Beglinger|first2=C.|last3=Stalder|first3=G. A.|date=1985-09-21|title=[Interaction of the endo- and exocrine pancreas]|journal=Schweizerische Medizinische Wochenschrift|volume=115|issue=38|pages=1299–1306|issn=0036-7672|pmid=2865807}}</ref> 99% of the pancreas is exocrine and 1% is endocrine.<ref>{{Cite web|title=Pancreas Gland - Endocrine System|url=https://www.innerbody.com/image/endo03.html|access-date=2021-06-12|website=Innerbody|language=en}}</ref>{{sfn|Beger's|2018|pp=124}}<ref>{{Cite web|title=Endocrine Pancreas - an overview ScienceDirect Topics|url=https://www.sciencedirect.com/topics/medicine-and-dentistry/endocrine-pancreas|access-date=2021-06-12|website=www.sciencedirect.com}}</ref><ref>{{Cite journal|last1=Henderson|first1=J. R.|last2=Daniel|first2=P. M.|last3=Fraser|first3=P. A.|date=February 1981|title=The pancreas as a single organ: the influence of the endocrine upon the exocrine part of the gland|journal=Gut|volume=22|issue=2|pages=158–167|doi=10.1136/gut.22.2.158|issn=0017-5749|pmc=1419227|pmid=6111521}}</ref> As an [[endocrine gland]], it functions mostly to regulate [[blood sugar level]]s, secreting the [[hormone]]s [[insulin]], [[glucagon]], [[somatostatin]] and [[pancreatic polypeptide]]. As a part of the digestive system, it functions as an [[exocrine gland]] secreting [[pancreatic juice]] into the [[duodenum]] through the [[pancreatic duct]]. This juice contains [[bicarbonate]], which neutralizes [[acid]] entering the duodenum from the stomach; and [[digestive enzyme]]s, which break down [[carbohydrates]], [[protein]]s and [[lipids|fats]] in food entering the duodenum from the stomach. |
||
Inflammation of the pancreas is known as [[pancreatitis]], with common causes including chronic [[Alcohol (drug)|alcohol]] use and [[gallstone]]s. Because of its role in the regulation of blood sugar, the pancreas is also a key organ in [[diabetes mellitus]]. [[Pancreatic cancer]] can arise following [[chronic pancreatitis]] or due to other reasons, and carries a very poor prognosis, as it is often only identified after it has spread to other areas of the body. |
Inflammation of the pancreas is known as [[pancreatitis]], with common causes including chronic [[Alcohol (drug)|alcohol]] use and [[gallstone]]s. Because of its role in the regulation of blood sugar, the pancreas is also a key organ in [[diabetes mellitus]]. [[Pancreatic cancer]] can arise following [[chronic pancreatitis]] or due to other reasons, and carries a very poor prognosis, as it is often only identified after it has spread to other areas of the body. |
||
| Line 27: | Line 27: | ||
[[File:Pancreas and nearby organs.jpg|thumb|The pancreas (shown here in pink) sits behind the [[stomach]], with the body near the curvature of the [[duodenum]], and the tail stretching to touch the [[spleen]].]] |
[[File:Pancreas and nearby organs.jpg|thumb|The pancreas (shown here in pink) sits behind the [[stomach]], with the body near the curvature of the [[duodenum]], and the tail stretching to touch the [[spleen]].]] |
||
The pancreas is an organ that in humans lies in the [[abdomen]], stretching from behind the [[stomach]] to the left upper abdomen near the [[spleen]]. In adults, it is about {{convert|12|-|15|cm}} long, {{wt|en|lobulated}}, and salmon-coloured in appearance.{{sfn|Gray's|2016|pp=1179–1189}} |
The pancreas is an organ that in humans lies in the [[abdomen]], stretching from behind the [[stomach]] to the left upper abdomen near the [[spleen]]. In adults, it is about {{convert|12|-|15|cm}} long, {{wt|en|lobulated}}, and salmon-coloured in appearance.{{sfn|Gray's|2016|pp=1179–1189}} |
||
Anatomically, the pancreas is divided into a ''head'', ''neck'', ''body'', and ''tail''. The pancreas stretches from the inner curvature of the [[duodenum]], where the head surrounds two [[blood vessel]]s: the [[superior mesenteric artery]] and [[superior mesenteric vein|vein]]. The longest part of the pancreas, the body, stretches across behind the stomach, and the tail of the pancreas ends adjacent to the [[spleen]].{{sfn|Gray's|2016|pp=1179–1189}} |
Anatomically, the pancreas is divided into a ''head'', ''neck'', ''body'', and ''tail''. The pancreas stretches from the inner curvature of the [[duodenum]], where the head surrounds two [[blood vessel]]s: the [[superior mesenteric artery]] and [[superior mesenteric vein|vein]]. The longest part of the pancreas, the body, stretches across behind the stomach, and the tail of the pancreas ends adjacent to the [[spleen]].{{sfn|Gray's|2016|pp=1179–1189}} |
||
| Line 54: | Line 54: | ||
The pancreas contains tissue with an [[endocrine]] and [[exocrine]] role, and this division is also visible when the pancreas is viewed under a microscope.{{sfn|Wheater's Histology|2013|pp=287-291}} |
The pancreas contains tissue with an [[endocrine]] and [[exocrine]] role, and this division is also visible when the pancreas is viewed under a microscope.{{sfn|Wheater's Histology|2013|pp=287-291}} |
||
The majority of pancreatic tissue has a digestive role. The cells with this role form clusters ({{ |
The majority of pancreatic tissue has a digestive role. The cells with this role form clusters ({{langx|la|acini||[[kernel]]s}}) around small [[duct (anatomy)|ducts]], and are arranged in lobes that have thin [[fibrous tissue|fibrous]] walls. The cells of each acinus secrete inactive digestive enzymes called [[zymogen]]s into the small ''intercalated'' ducts which they surround. In each acinus, the cells are pyramid-shaped and situated around the intercalated ducts, with the [[Cell nucleus|nuclei]] resting on the [[basement membrane]], a large [[endoplasmic reticulum]], and a number of zymogen granules visible within the [[cytoplasm]]. The intercalated ducts drain into larger ''intralobular'' ducts within the lobule, and finally ''interlobular'' ducts. The ducts are lined by a single layer of [[columnar epithelium|column-shaped cells]]. There is more than one layer of cells as the diameter of the ducts increases.{{sfn|Wheater's Histology|2013|pp=287-291}} |
||
The tissues with an [[endocrine]] role within the pancreas exist as clusters of cells called [[pancreatic islets]] (also called islets of [[Paul Langerhans|Langerhans]]) that are distributed throughout the pancreas.{{sfn|Wheater's Histology|2013|pp=332-333}} Pancreatic islets contain [[alpha cell]]s, [[beta cell]]s, and [[delta cell]]s, each of which releases a different hormone. These cells have characteristic positions, with alpha cells (secreting [[glucagon]]) tending to be situated around the periphery of the islet, and beta cells (secreting [[insulin]]) more numerous and found throughout the islet.{{sfn|Wheater's Histology|2013|pp=332-333}} [[Enterochromaffin cell]]s are also scattered throughout the islets.{{sfn|Wheater's Histology|2013|pp=332-333}} Islets are composed of up to 3,000 secretory cells, and contain several small arterioles to receive blood, and venules that allow the hormones secreted by the cells to enter the [[systemic circulation]].{{sfn|Wheater's Histology|2013|pp=332-333}} |
The tissues with an [[endocrine]] role within the pancreas exist as clusters of cells called [[pancreatic islets]] (also called islets of [[Paul Langerhans|Langerhans]]) that are distributed throughout the pancreas.{{sfn|Wheater's Histology|2013|pp=332-333}} Pancreatic islets contain [[alpha cell]]s, [[beta cell]]s, and [[delta cell]]s, each of which releases a different hormone. These cells have characteristic positions, with alpha cells (secreting [[glucagon]]) tending to be situated around the periphery of the islet, and beta cells (secreting [[insulin]]) more numerous and found throughout the islet.{{sfn|Wheater's Histology|2013|pp=332-333}} [[Enterochromaffin cell]]s are also scattered throughout the islets.{{sfn|Wheater's Histology|2013|pp=332-333}} Islets are composed of up to 3,000 secretory cells, and contain several small arterioles to receive blood, and venules that allow the hormones secreted by the cells to enter the [[systemic circulation]].{{sfn|Wheater's Histology|2013|pp=332-333}} |
||
| Line 63: | Line 63: | ||
=== Gene and protein expression === |
=== Gene and protein expression === |
||
{{Further |Bioinformatics#Gene and protein expression}} |
{{Further |Bioinformatics#Gene and protein expression}} |
||
10,000 protein coding genes (~50% of all human genes) are expressed in the normal human pancreas.<ref>{{Cite web|url=https://www.proteinatlas.org/humanproteome/pancreas|title=The human proteome in pancreas – The Human Protein Atlas|website=www.proteinatlas.org|access-date=2017-09-25}}</ref><ref>{{cite journal | vauthors = Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F | display-authors = 6 | title = Proteomics. Tissue-based map of the human proteome | journal = Science | volume = 347 | issue = 6220 | |
10,000 protein coding genes (~50% of all human genes) are expressed in the normal human pancreas.<ref>{{Cite web|url=https://www.proteinatlas.org/humanproteome/pancreas|title=The human proteome in pancreas – The Human Protein Atlas|website=www.proteinatlas.org|access-date=2017-09-25}}</ref><ref>{{cite journal | vauthors = Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F | display-authors = 6 | title = Proteomics. Tissue-based map of the human proteome | journal = Science | volume = 347 | issue = 6220 | page = 1260419 | date = January 2015 | pmid = 25613900 | doi = 10.1126/science.1260419 | s2cid = 802377 }}</ref> Less than 100 of these genes are specifically expressed in the pancreas. Similar to the [[salivary gland]]s, most pancreas-specific genes encode for secreted proteins. Corresponding pancreas-specific proteins are either expressed in the exocrine cellular compartment and have functions related to digestion or food uptake such as digestive [[chymotrypsinogen]] enzymes and pancreatic lipase [[Pancreatic lipase family|PNLIP]], or are expressed in the various cells of the endocrine [[pancreatic islets]] and have functions related to secreted hormones such as [[insulin]], [[glucagon]], [[somatostatin]] and [[pancreatic polypeptide]].<ref>{{cite journal | vauthors = Danielsson A, Pontén F, Fagerberg L, Hallström BM, Schwenk JM, Uhlén M, Korsgren O, Lindskog C | display-authors = 6 | title = The human pancreas proteome defined by transcriptomics and antibody-based profiling | journal = PLOS ONE | volume = 9 | issue = 12 | pages = e115421 | date = 2014-12-29 | pmid = 25546435 | pmc = 4278897 | doi = 10.1371/journal.pone.0115421 | bibcode = 2014PLoSO...9k5421D | doi-access = free }}</ref> |
||
==Development== |
==Development== |
||
[[File:Suckale08FBS fig1 pancreas development.jpeg|thumbnail|300px|The pancreas originates from the [[foregut]], a precursor tube to part of the [[digestive tract]], as a [[Anatomical terms of location#Dorsal and ventral|dorsal and ventral]] bud. As it develops, the ventral bud rotates to the other side and the two buds fuse together.]] |
[[File:Suckale08FBS fig1 pancreas development.jpeg|thumbnail|300px|The pancreas originates from the [[foregut]], a precursor tube to part of the [[digestive tract]], as a [[Anatomical terms of location#Dorsal and ventral|dorsal and ventral]] bud. As it develops, the ventral bud rotates to the other side and the two buds fuse together.]] |
||
The pancreas forms during [[Prenatal development|development]] from two buds that arise from the [[duodenum|duodenal]] part of the [[foregut]], an embryonic tube that is a precursor to the [[gastrointestinal tract]].<ref name=Langmans2019>{{cite book |last1=Sadley |first1=TW |title=Langman's medical embryology |date=2019|location=Philadelphia|publisher=Wolters Kluwer |isbn= |
The pancreas forms during [[Prenatal development|development]] from two buds that arise from the [[duodenum|duodenal]] part of the [[foregut]], an embryonic tube that is a precursor to the [[gastrointestinal tract]].<ref name=Langmans2019>{{cite book |last1=Sadley |first1=TW |title=Langman's medical embryology |date=2019|location=Philadelphia|publisher=Wolters Kluwer |isbn=978-1-4963-8390-7 |edition=14th|pages=244–245}}</ref> It is of [[endoderm]]al origin.<ref name=Langmans2019 /> Pancreatic development begins with the formation of a [[Anatomical terms of location#Dorsal and ventral|dorsal and ventral]] [[pancreatic bud]]. Each joins with the foregut through a duct. The dorsal pancreatic bud forms the neck, body, and tail of the developed pancreas, and the ventral pancreatic bud forms the head and uncinate process.<ref name=Langmans2019 /> |
||
The definitive pancreas results from rotation of the ventral bud and the fusion of the two buds.<ref name=Langmans2019 /> During development, the duodenum rotates to the right, and the ventral bud rotates with it, moving to a position that becomes more dorsal. Upon reaching its final destination, the ventral pancreatic bud is below the larger dorsal bud, and eventually fuses with it. At this point of fusion, the main ducts of the ventral and dorsal pancreatic buds fuse, forming the main pancreatic duct. Usually, the duct of the dorsal bud regresses, leaving the [[main pancreatic duct]].<ref name=Langmans2019 /> |
The definitive pancreas results from rotation of the ventral bud and the fusion of the two buds.<ref name=Langmans2019 /> During development, the duodenum rotates to the right, and the ventral bud rotates with it, moving to a position that becomes more dorsal. Upon reaching its final destination, the ventral pancreatic bud is below the larger dorsal bud, and eventually fuses with it. At this point of fusion, the main ducts of the ventral and dorsal pancreatic buds fuse, forming the main pancreatic duct. Usually, the duct of the dorsal bud regresses, leaving the [[main pancreatic duct]].<ref name=Langmans2019 /> |
||
| Line 75: | Line 75: | ||
[[Pancreatic Progenitor Cell|Pancreatic progenitor cells]] are precursor cells that differentiate into the functional pancreatic cells, including exocrine acinar cells, endocrine islet cells, and ductal cells.<ref name="carlson"/> These progenitor cells are characterised by the co-expression of the [[transcription factor]]s [[PDX1]] and [[NKX6-1]].<ref name="carlson"/> |
[[Pancreatic Progenitor Cell|Pancreatic progenitor cells]] are precursor cells that differentiate into the functional pancreatic cells, including exocrine acinar cells, endocrine islet cells, and ductal cells.<ref name="carlson"/> These progenitor cells are characterised by the co-expression of the [[transcription factor]]s [[PDX1]] and [[NKX6-1]].<ref name="carlson"/> |
||
<!--Exocrine-->The cells of the exocrine pancreas differentiate through molecules that induce differentiation including [[follistatin]], [[fibroblast growth factor]]s, and activation of the [[Notch receptor]] system.<ref name="carlson">{{cite book | last = Carlson | first = Bruce M. | name-list-style = vanc |title=Human Embryology and Developmental Biology |publisher=Elsevier |location=St. Louis |year=2019 |pages=318–57 |isbn=978- |
<!--Exocrine-->The cells of the exocrine pancreas differentiate through molecules that induce differentiation including [[follistatin]], [[fibroblast growth factor]]s, and activation of the [[Notch receptor]] system.<ref name="carlson">{{cite book | last = Carlson | first = Bruce M. | name-list-style = vanc |title=Human Embryology and Developmental Biology |publisher=Elsevier |location=St. Louis |year=2019 |pages=318–57 |isbn=978-0-323-52375-2 }}</ref> Development of the exocrine acini progresses through three successive stages. These are the predifferentiated, protodifferentiated, and differentiated stages, which correspond to undetectable, low, and high levels of digestive enzyme activity, respectively.<ref name="carlson"/> |
||
<!--Endocrine-->Pancreatic progenitor cells differentiate into endocrine islet cells under the influence of [[neurogenins|neurogenin-3]] and [[ISL1]], but only in the absence of [[notch signaling pathway|notch receptor signaling]]. Under the direction of a [[Pax gene]], the endocrine precursor cells differentiate to form alpha and gamma cells. Under the direction of [[Pax-6]], the endocrine precursor cells differentiate to form beta and delta cells.<ref name="carlson"/> The pancreatic islets form as the endocrine cells migrate from the duct system to form small clusters around [[capillary|capillaries]].{{sfn|Wheater's Histology|2013|pp=332-333}} This occurs around the third month of development,<ref name=Langmans2019 /> and insulin and glucagon can be detected in the human [[fetal circulation]] by the fourth or fifth month of development.<ref name="carlson"/> |
<!--Endocrine-->Pancreatic progenitor cells differentiate into endocrine islet cells under the influence of [[neurogenins|neurogenin-3]] and [[ISL1]], but only in the absence of [[notch signaling pathway|notch receptor signaling]]. Under the direction of a [[Pax gene]], the endocrine precursor cells differentiate to form alpha and gamma cells. Under the direction of [[Pax-6]], the endocrine precursor cells differentiate to form beta and delta cells.<ref name="carlson"/> The pancreatic islets form as the endocrine cells migrate from the duct system to form small clusters around [[capillary|capillaries]].{{sfn|Wheater's Histology|2013|pp=332-333}} This occurs around the third month of development,<ref name=Langmans2019 /> and insulin and glucagon can be detected in the human [[fetal circulation]] by the fourth or fifth month of development.<ref name="carlson"/> |
||
| Line 90: | Line 90: | ||
Glucagon acts to increase glucose levels by promoting the [[gluconeogenesis|creation of glucose]] and the [[glycogenolysis|breakdown of glycogen]] to glucose in the liver. It also decreases the uptake of glucose in fat and muscle. Glucagon release is stimulated by low blood glucose or insulin levels, and during exercise.{{sfn|Harrison's|2015|pp=2853-4}} Insulin acts to decrease blood glucose levels by facilitating uptake by cells (particularly [[skeletal muscle]]), and promoting its use in the creation of proteins, fats and carbohydrates. Insulin is initially created as a precursor form called [[preproinsulin]]. This is converted to [[proinsulin]] and cleaved by [[C-peptide]] to [[insulin]] which is then stored in granules in beta cells. Glucose is taken into the beta cells and degraded. The end effect of this is to cause [[depolarisation]] of the cell membrane which stimulates the release of the insulin.{{sfn|Harrison's|2015|pp=2853-4}} |
Glucagon acts to increase glucose levels by promoting the [[gluconeogenesis|creation of glucose]] and the [[glycogenolysis|breakdown of glycogen]] to glucose in the liver. It also decreases the uptake of glucose in fat and muscle. Glucagon release is stimulated by low blood glucose or insulin levels, and during exercise.{{sfn|Harrison's|2015|pp=2853-4}} Insulin acts to decrease blood glucose levels by facilitating uptake by cells (particularly [[skeletal muscle]]), and promoting its use in the creation of proteins, fats and carbohydrates. Insulin is initially created as a precursor form called [[preproinsulin]]. This is converted to [[proinsulin]] and cleaved by [[C-peptide]] to [[insulin]] which is then stored in granules in beta cells. Glucose is taken into the beta cells and degraded. The end effect of this is to cause [[depolarisation]] of the cell membrane which stimulates the release of the insulin.{{sfn|Harrison's|2015|pp=2853-4}} |
||
<!--Influences-->The main factor influencing the secretion of insulin and glucagon are the levels of glucose in blood plasma.<ref name=Ganongs2019Islets>{{Cite book|title=Ganong's review of medical physiology|author1=Barrett, Kim E.|others=Barman, Susan M.,, Brooks, Heddwen L., Yuan, Jason X.-J.|isbn= |
<!--Influences-->The main factor influencing the secretion of insulin and glucagon are the levels of glucose in blood plasma.<ref name=Ganongs2019Islets>{{Cite book|title=Ganong's review of medical physiology|author1=Barrett, Kim E.|others=Barman, Susan M.,, Brooks, Heddwen L., Yuan, Jason X.-J.|isbn=978-1-260-12240-4|edition=26th|location=New York|oclc=1076268769|year=2019|pages=433–437|section=Regulation of Insulin Secretion; Glucagon}}</ref> Low blood sugar stimulates glucagon release, and high blood sugar stimulates insulin release. Other factors also influence the secretion of these hormones. Some [[amino acid]]s, that are byproducts of the digestion of [[protein]], stimulate insulin and glucagon release. Somatostatin acts as an inhibitor of both insulin and glucagon. The [[autonomic nervous system]] also plays a role. Activation of [[Beta-2 adrenergic receptor|Beta-2 receptors]] of the [[sympathetic nervous system]] by [[catecholamine]]s secreted from sympathetic nerves stimulates secretion of insulin and glucagon,<ref name=Ganongs2019Islets /><ref name=GuytonHall2016>{{cite book |last1=Hall |first1=John E |title=Guyton and Hall textbook of medical physiology |date=2016 |publisher=Elsevier |location=Philadelphia |isbn=978-1-4557-7016-8 |edition=13th|pages=990–994}}</ref> whereas activation of [[Alpha-1 adrenergic receptors|Alpha-1 receptors]] inhibits secretion.<ref name=Ganongs2019Islets /> [[Muscarinic acetylcholine receptor M3|M3 receptors]] of the [[parasympathetic nervous system]] act when stimulated by the right [[vagus nerve]] to stimulate release of insulin from beta cells.<ref name=Ganongs2019Islets /> |
||
===Digestion=== |
===Digestion=== |
||
[[File:1820 The Pancreas.jpg|thumb|The pancreas has a role in digestion, highlighted here. Ducts in the pancreas (green) conduct digestive enzymes into the duodenum. This image also shows a [[pancreatic islet]], part of the endocrine pancreas, which contains cells responsible for secretion of [[insulin]] and [[glucagon]].]] |
[[File:1820 The Pancreas.jpg|thumb|The pancreas has a role in digestion, highlighted here. Ducts in the pancreas (green) conduct digestive enzymes into the duodenum. This image also shows a [[pancreatic islet]], part of the endocrine pancreas, which contains cells responsible for secretion of [[insulin]] and [[glucagon]].]] |
||
The pancreas plays a vital role in the [[digestive system]]. It does this by secreting a fluid that contains digestive enzymes into the [[duodenum]], the first part of the [[small intestine]] that receives food from the [[stomach]]. These enzymes help to break down carbohydrates, proteins and lipids (fats). This role is called the "exocrine" role of the pancreas. The cells that do this are arranged in clusters called ''[[acini]]''. Secretions into the middle of the acinus accumulate in [[intralobular ducts]], which drain to the main [[pancreatic duct]], which drains directly into the [[duodenum]]. About 1. |
The pancreas plays a vital role in the [[digestive system]]. It does this by secreting a fluid that contains digestive enzymes into the [[duodenum]], the first part of the [[small intestine]] that receives food from the [[stomach]]. These enzymes help to break down carbohydrates, proteins and lipids (fats). This role is called the "exocrine" role of the pancreas. The cells that do this are arranged in clusters called ''[[acini]]''. Secretions into the middle of the acinus accumulate in [[intralobular ducts]], which drain to the main [[pancreatic duct]], which drains directly into the [[duodenum]]. About 1.5–3 liters of fluid are secreted in this manner every day.{{sfn|Gray's|2008|pp=1183-1190}}{{sfn|Harrison's|2015|pp=2086-2102}} |
||
The cells in each acinus are filled with granules containing the digestive enzymes. These are secreted in an inactive form termed [[zymogen]]s or proenzymes. When released into the duodenum, they are activated by the enzyme [[enterokinase]] present in the lining of the duodenum. The proenzymes are cleaved, creating a cascade of activating enzymes.{{sfn|Harrison's|2015|pp=2086-2102}} |
The cells in each acinus are filled with granules containing the digestive enzymes. These are secreted in an inactive form termed [[zymogen]]s or proenzymes. When released into the duodenum, they are activated by the enzyme [[enterokinase]] present in the lining of the duodenum. The proenzymes are cleaved, creating a cascade of activating enzymes.{{sfn|Harrison's|2015|pp=2086-2102}} |
||
| Line 106: | Line 106: | ||
===Additional functions=== |
===Additional functions=== |
||
The pancreas also secretes [[vasoactive intestinal peptide]] and [[pancreatic polypeptide]]. [[Enterochromaffin cell]]s of the pancreas secrete the hormones [[motilin]], [[serotonin]], and [[substance P]].{{sfn|Wheater's Histology|2013|pp=332-333}} |
The pancreas also secretes [[vasoactive intestinal peptide]] and [[pancreatic polypeptide]]. [[Enterochromaffin cell]]s of the pancreas secrete the hormones [[motilin]], [[serotonin]], and [[substance P]].{{sfn|Wheater's Histology|2013|pp=332-333}} It has been demonstrated that pancreatic tissue is a strong accumulator and secretor in the intestine of radioactive cesium ([[Cs-137]]).<ref>{{Cite journal |last=Bandazhevsky Y.I. |year=2003 |title=Chronic Cs-137 incorporation in children's organs. |journal=Swiss Med. Wkly. |volume=133 |issue=35–36 |pages=488–90 |doi=10.4414/smw.2003.10226 |pmid=14652805 |s2cid=28184979 |doi-access=free}}</ref><ref>{{cite journal |last1=Venturi |first1=Sebastiano |title=Cesium in Biology, Pancreatic Cancer, and Controversy in High and Low Radiation Exposure Damage—Scientific, Environmental, Geopolitical, and Economic Aspects |journal=International Journal of Environmental Research and Public Health |date=January 2021 |volume=18 |issue=17 |page=8934 |doi=10.3390/ijerph18178934 |pmid=34501532 |language=en|pmc=8431133 |doi-access=free }}</ref> |
||
==Clinical significance== |
==Clinical significance== |
||
| Line 122: | Line 122: | ||
{{Main|Pancreatic cancer}} |
{{Main|Pancreatic cancer}} |
||
[[File:Pancreatic Cancer.jpg|thumb|Pancreatic cancer, shown here, most commonly occurs as an [[adenocarcinoma]] in the head of the pancreas. Because symptoms (such as skin yellowing, pain, or itch) do not occur until later in the disease, it often presents at a later [[Cancer staging|stage]] and has limited treatment options.]] |
[[File:Pancreatic Cancer.jpg|thumb|Pancreatic cancer, shown here, most commonly occurs as an [[adenocarcinoma]] in the head of the pancreas. Because symptoms (such as skin yellowing, pain, or itch) do not occur until later in the disease, it often presents at a later [[Cancer staging|stage]] and has limited treatment options.]] |
||
[[File:Relative incidence of pancreatic neoplasms.png|thumb|350px|Relative incidences of various pancreatic [[neoplasm]]s, with pancreatic cancers in red/pink color.<ref>{{cite journal |last1=Wang |first1=Y |last2=Miller |first2=FH |last3=Chen |first3=ZE |last4=Merrick |first4=L |last5=Mortele |first5=KJ |last6=Hoff |first6=FL | display-authors=etal| title=Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. | journal=Radiographics | year= 2011 | volume= 31 | issue= 3 | pages= E47-64 | pmid=21721197 | doi=10.1148/rg.313105174 | pmc= |
[[File:Relative incidence of pancreatic neoplasms.png|thumb|350px|Relative incidences of various pancreatic [[neoplasm]]s, with pancreatic cancers in red/pink color.<ref>{{cite journal |last1=Wang |first1=Y |last2=Miller |first2=FH |last3=Chen |first3=ZE |last4=Merrick |first4=L |last5=Mortele |first5=KJ |last6=Hoff |first6=FL | display-authors=etal| title=Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. | journal=Radiographics | year= 2011 | volume= 31 | issue= 3 | pages= E47-64 | pmid=21721197 | doi=10.1148/rg.313105174 | pmc= }}<br />Diagram by Mikael Häggström, M.D.</ref>]] |
||
[[Pancreatic cancer]]s, particularly the most common type, pancreatic [[adenocarcinoma]], remain very difficult to treat, and are mostly diagnosed only at a stage that is too late for surgery, which is the only curative treatment. Pancreatic cancer is rare in people younger than 40 and the [[median]] age of [[diagnosis]] is 71.<ref name=NEJM14>{{cite journal | vauthors = Ryan DP, Hong TS, Bardeesy N | title = Pancreatic adenocarcinoma | journal = The New England Journal of Medicine | volume = 371 | issue = 11 | pages = 1039–49 | date = September 2014 | pmid = 25207767 | doi = 10.1056/NEJMra1404198 }}</ref> Risk factors include chronic pancreatitis, older age, smoking, [[obesity]], [[diabetes mellitus|diabetes]], and certain rare genetic conditions including [[multiple endocrine neoplasia type 1]], [[hereditary nonpolyposis colon cancer]] and [[dysplastic nevus syndrome]] among others.{{sfn|Davidson's|2018|p=837-844}}<ref name=PDQ2014>{{cite web|title=Pancreatic Cancer Treatment (PDQ®) Patient Version|url=http://www.cancer.gov/cancertopics/pdq/treatment/pancreatic/Patient/page1/AllPages|website=National Cancer Institute|access-date=8 June 2014|date=2014-04-17}}</ref> About 25% of cases are attributable to [[tobacco smoking]],<ref>{{cite journal | vauthors = Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH | title = Recent progress in pancreatic cancer | journal = CA: A Cancer Journal for Clinicians | volume = 63 | issue = 5 | pages = 318–48 | date = September 2013 | pmid = 23856911 | pmc = 3769458 | doi = 10.3322/caac.21190 }}</ref> while 5–10% of cases are linked to [[heredity|inherited genes]].<ref name="NEJM14" /> |
[[Pancreatic cancer]]s, particularly the most common type, pancreatic [[adenocarcinoma]], remain very difficult to treat, and are mostly diagnosed only at a stage that is too late for surgery, which is the only curative treatment. Pancreatic cancer is rare in people younger than 40 and the [[median]] age of [[diagnosis]] is 71.<ref name=NEJM14>{{cite journal | vauthors = Ryan DP, Hong TS, Bardeesy N | title = Pancreatic adenocarcinoma | journal = The New England Journal of Medicine | volume = 371 | issue = 11 | pages = 1039–49 | date = September 2014 | pmid = 25207767 | doi = 10.1056/NEJMra1404198 }}</ref> Risk factors include chronic pancreatitis, older age, smoking, [[obesity]], [[diabetes mellitus|diabetes]], and certain rare genetic conditions including [[multiple endocrine neoplasia type 1]], [[hereditary nonpolyposis colon cancer]] and [[dysplastic nevus syndrome]] among others.{{sfn|Davidson's|2018|p=837-844}}<ref name=PDQ2014>{{cite web|title=Pancreatic Cancer Treatment (PDQ®) Patient Version|url=http://www.cancer.gov/cancertopics/pdq/treatment/pancreatic/Patient/page1/AllPages|website=National Cancer Institute|access-date=8 June 2014|date=2014-04-17}}</ref> About 25% of cases are attributable to [[tobacco smoking]],<ref>{{cite journal | vauthors = Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH | title = Recent progress in pancreatic cancer | journal = CA: A Cancer Journal for Clinicians | volume = 63 | issue = 5 | pages = 318–48 | date = September 2013 | pmid = 23856911 | pmc = 3769458 | doi = 10.3322/caac.21190 }}</ref> while 5–10% of cases are linked to [[heredity|inherited genes]].<ref name="NEJM14" /> |
||
Pancreatic adenocarcinoma is the most common form of pancreatic cancer, and is cancer arising from the exocrine digestive part of the pancreas. Most occur in the head of the pancreas.{{sfn|Davidson's|2018|p=837-844}} Symptoms tend to arise late in the course of the cancer, when it causes abdominal pain, weight loss, or yellowing of the skin ([[jaundice]]). Jaundice occurs when the outflow of [[bile]] is blocked by the cancer. Other less common symptoms include nausea, vomiting, pancreatitis, diabetes or recurrent [[venous thrombosis]].{{sfn|Davidson's|2018|p=837-844}} Pancreatic cancer is usually diagnosed by [[medical imaging]] in the form of an [[ultrasound]] or [[CT scan]] with contrast enhancement. An [[endoscopic ultrasound]] may be used if a tumour is being considered for surgical removal, and biopsy guided by [[ERCP]] or ultrasound can be used to confirm an uncertain diagnosis.{{sfn|Davidson's|2018|p=837-844}} |
Pancreatic adenocarcinoma is the most common form of pancreatic cancer, and is cancer arising from the exocrine digestive part of the pancreas. Most occur in the head of the pancreas.{{sfn|Davidson's|2018|p=837-844}} Symptoms tend to arise late in the course of the cancer, when it causes abdominal pain, weight loss, or yellowing of the skin ([[jaundice]]). Jaundice occurs when the outflow of [[bile]] is blocked by the cancer. Other less common symptoms include nausea, vomiting, pancreatitis, diabetes or recurrent [[venous thrombosis]].{{sfn|Davidson's|2018|p=837-844}} Pancreatic cancer is usually diagnosed by [[medical imaging]] in the form of an [[ultrasound]] or [[CT scan]] with contrast enhancement. An [[endoscopic ultrasound]] may be used if a tumour is being considered for surgical removal, and biopsy guided by [[ERCP]] or ultrasound can be used to confirm an uncertain diagnosis.{{sfn|Davidson's|2018|p=837-844}} |
||
Because of the late development of symptoms, most cancer presents at an advanced [[Cancer staging|stage]].{{sfn|Davidson's|2018|p=837-844}} Only 10 to 15% of tumours are suitable for surgical resection.{{sfn|Davidson's|2018|p=837-844}} {{As of|2018}}, when chemotherapy is given the [[FOLFIRINOX]] regimen containing [[fluorouracil]], [[irinotecan]], [[oxaliplatin]] and [[leucovorin]] has been shown to extend survival beyond traditional [[gemcitabine]] regimens.{{sfn|Davidson's|2018|p=837-844}} For the most part, treatment is [[palliative]], focus on the management of symptoms that develop. This may include management of [[itch]], a [[Bile duct#Drainage|choledochojejunostomy]] or the insertion of stents with [[ERCP]] to facilitate the drainage of bile, and medications to help control pain.{{sfn|Davidson's|2018|p=837-844}} In the United States pancreatic cancer is the fourth most common cause of deaths due to cancer.<ref name="PMC2504856">{{cite journal | vauthors = Hariharan D, Saied A, Kocher HM | title = Analysis of mortality rates for pancreatic cancer across the world | journal = HPB | volume = 10 | issue = 1 | pages = 58–62 | year = 2008 | pmid = 18695761 | pmc = 2504856 | doi = 10.1080/13651820701883148 }}</ref> The disease occurs more often in the developed world, which had 68% of new cases in 2012.<ref name=WCR2014>{{cite book|title=World Cancer Report 2014|date=2014|publisher=World Health Organization|isbn=978- |
Because of the late development of symptoms, most cancer presents at an advanced [[Cancer staging|stage]].{{sfn|Davidson's|2018|p=837-844}} Only 10 to 15% of tumours are suitable for surgical resection.{{sfn|Davidson's|2018|p=837-844}} {{As of|2018}}, when chemotherapy is given the [[FOLFIRINOX]] regimen containing [[fluorouracil]], [[irinotecan]], [[oxaliplatin]] and [[leucovorin]] has been shown to extend survival beyond traditional [[gemcitabine]] regimens.{{sfn|Davidson's|2018|p=837-844}} For the most part, treatment is [[palliative]], focus on the management of symptoms that develop. This may include management of [[itch]], a [[Bile duct#Drainage|choledochojejunostomy]] or the insertion of stents with [[ERCP]] to facilitate the drainage of bile, and medications to help control pain.{{sfn|Davidson's|2018|p=837-844}} In the United States pancreatic cancer is the fourth most common cause of deaths due to cancer.<ref name="PMC2504856">{{cite journal | vauthors = Hariharan D, Saied A, Kocher HM | title = Analysis of mortality rates for pancreatic cancer across the world | journal = HPB | volume = 10 | issue = 1 | pages = 58–62 | year = 2008 | pmid = 18695761 | pmc = 2504856 | doi = 10.1080/13651820701883148 }}</ref> The disease occurs more often in the developed world, which had 68% of new cases in 2012.<ref name=WCR2014>{{cite book|title=World Cancer Report 2014|date=2014|publisher=World Health Organization|isbn=978-92-832-0429-9|chapter=Chapter 5.7}}</ref> Pancreatic adenocarcinoma typically has poor outcomes with the average percentage alive for at least one and five years after [[diagnosis]] being 25% and 5% respectively.<ref name=WCR2014/><ref name="ACS-CFF-2010">{{cite web| title=American Cancer Society: Cancer Facts & Figures 2010: see page 4 for incidence estimates, and page 19 for survival percentages| url=http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf| archive-url=https://web.archive.org/web/20150114080100/http://www.cancer.org/acs/groups/content/%40nho/documents/document/acspc-024113.pdf| archive-date=2015-01-14}}</ref> In localized disease where the cancer is small (< 2 cm) the number alive at five years is approximately 20%.<ref name=PDQ2014P>{{cite web|title=Pancreatic Cancer Treatment (PDQ®) Health Professional Version|url=http://www.cancer.gov/cancertopics/pdq/treatment/pancreatic/HealthProfessional/page1/AllPages|website=NCI|access-date=8 June 2014|date=2014-02-21}}</ref> |
||
There are several types of pancreatic cancer, involving both the endocrine and exocrine tissue. The many types of [[pancreatic endocrine tumor]]s are all uncommon or rare, and have varied outlooks. However the [[Incidence (epidemiology)|incidence]] of these cancers has been rising sharply; it is not clear to what extent this reflects increased detection, especially through [[medical imaging]], of tumors that would be very slow to develop. [[Insulinoma]]s (largely benign) and [[gastrinoma]]s are the most common types.<ref>{{cite journal | vauthors = Burns WR, Edil BH | title = Neuroendocrine pancreatic tumors: guidelines for management and update | journal = Current Treatment Options in Oncology | volume = 13 | issue = 1 | pages = 24–34 | date = March 2012 | pmid = 22198808 | doi = 10.1007/s11864-011-0172-2 | s2cid = 7329783 }}</ref> For those with neuroendocrine cancers the number alive after five years is much better at 65%, varying considerably with type.<ref name=WCR2014/> |
There are several types of pancreatic cancer, involving both the endocrine and exocrine tissue. The many types of [[pancreatic endocrine tumor]]s are all uncommon or rare, and have varied outlooks. However the [[Incidence (epidemiology)|incidence]] of these cancers has been rising sharply; it is not clear to what extent this reflects increased detection, especially through [[medical imaging]], of tumors that would be very slow to develop. [[Insulinoma]]s (largely benign) and [[gastrinoma]]s are the most common types.<ref>{{cite journal | vauthors = Burns WR, Edil BH | title = Neuroendocrine pancreatic tumors: guidelines for management and update | journal = Current Treatment Options in Oncology | volume = 13 | issue = 1 | pages = 24–34 | date = March 2012 | pmid = 22198808 | doi = 10.1007/s11864-011-0172-2 | s2cid = 7329783 }}</ref> For those with neuroendocrine cancers the number alive after five years is much better at 65%, varying considerably with type.<ref name=WCR2014/> |
||
| Line 136: | Line 136: | ||
====Type 1 diabetes==== |
====Type 1 diabetes==== |
||
{{Main|Diabetes mellitus type 1}} |
{{Main|Diabetes mellitus type 1}} |
||
[[Diabetes mellitus type 1]] is a chronic [[autoimmune disease]] in which the [[immune system]] attacks the insulin-secreting beta cells of the pancreas.<ref name=Ganongs2019DM>{{Cite book|title=Ganong's review of medical physiology|publisher=McGraw-Hill Education |first1=Kim E. |last1=Barrett |first2=Susan M. |last2=Barman |first3=Heddwen L. |last3=Brooks |first4=Jason X.-J. |last4=Yuan |first5=William F. |last5=Ganong |isbn= |
[[Diabetes mellitus type 1]] is a chronic [[autoimmune disease]] in which the [[immune system]] attacks the insulin-secreting beta cells of the pancreas.<ref name=Ganongs2019DM>{{Cite book|title=Ganong's review of medical physiology|publisher=McGraw-Hill Education |first1=Kim E. |last1=Barrett |first2=Susan M. |last2=Barman |first3=Heddwen L. |last3=Brooks |first4=Jason X.-J. |last4=Yuan |first5=William F. |last5=Ganong |isbn=978-1-260-12240-4|edition=26th|location=New York|oclc=1076268769|year=2019|pages=440–441|section=Hypoglycaemia & Diabetes Mellitus in Humans}}</ref> Insulin is needed to keep [[blood sugar]] levels within optimal ranges, and its lack can lead to [[hyperglycemia|high blood sugar]]. As an untreated chronic condition, complications including accelerated [[vascular disease]], [[diabetic retinopathy]], [[diabetic nephropathy|kidney disease]] and [[diabetic neuropathy|neuropathy]] can result.<ref name=Ganongs2019DM /> In addition, if there is not enough insulin for glucose to be used within cells, the medical emergency [[diabetic ketoacidosis]], which is often the first symptom that a person with type 1 diabetes may have, can result.{{sfn|Davidson's|2018|p=735}} Type 1 diabetes can develop at any age but is most often diagnosed before age 40.<ref name=Ganongs2019DM /> For people living with type 1 diabetes, insulin injections are critical for survival.<ref name=Ganongs2019DM /> An experimental procedure to treat type 1 diabetes is [[pancreas transplantation]] or isolated [[islet cell transplantation|transplantation of islet cells]] to supply a person with functioning beta cells.<ref name=Ganongs2019DM /> |
||
====Type 2 diabetes==== |
====Type 2 diabetes==== |
||
| Line 147: | Line 147: | ||
==History== |
==History== |
||
The pancreas was first identified by [[Herophilus]] (335–280 BC), a [[Greeks|Greek]] [[anatomist]] and [[surgery|surgeon]].<ref>{{Cite book | url = https://books.google.com/books?id=rlLPBgAAQBAJ | title = History of the Pancreas: Mysteries of a Hidden Organ | last1 = Howard | first1 = John M. | last2 = Hess | first2 = Walter | name-list-style = vanc | date = 2012 | publisher = Springer Science & Business Media | isbn = 978- |
The pancreas was first identified by [[Herophilus]] (335–280 BC), a [[Greeks|Greek]] [[anatomist]] and [[surgery|surgeon]].<ref>{{Cite book | url = https://books.google.com/books?id=rlLPBgAAQBAJ | title = History of the Pancreas: Mysteries of a Hidden Organ | last1 = Howard | first1 = John M. | last2 = Hess | first2 = Walter | name-list-style = vanc | date = 2012 | publisher = Springer Science & Business Media | isbn = 978-1-4615-0555-6 | page = 24 | language = en }}</ref> A few hundred years later, [[Rufus of Ephesus]], another Greek anatomist, gave the pancreas its name. Etymologically, the term "pancreas", a modern [[Latin]] adaptation of [[Greek language|Greek]] πάγκρεας,<ref name="O'Brien">{{cite book| first = Terry | last = O'Brien | name-list-style = vanc |title=A2Z Book of word Origins|url=https://books.google.com/books?id=Rgzh83cChl0C&pg=PT86|publisher=Rupa Publications|isbn=978-81-291-1809-7|page=86|year=2015}}</ref> [πᾶν ("all", "whole"), and κρέας ("flesh")],<ref>{{cite web |last=Harper |first=Douglas |title=Pancreas |work=Online Etymology Dictionary |url=http://www.etymonline.com/index.php?term=pancreas |access-date=2007-04-04}}</ref> originally means [[sweetbread]],<ref name="Green2008">{{cite book| first = Tamara M. | last = Green | name-list-style = vanc |title=The Greek and Latin Roots of English|url=https://books.google.com/books?id=6gknyzq0uD8C&pg=PA176|year=2008|publisher=Rowman & Littlefield|isbn=978-0-7425-4780-3|page=176}}</ref> although literally meaning all-flesh, presumably because of its fleshy consistency. It was only in 1889 when [[Oskar Minkowski]] discovered that removing the pancreas from a dog caused it to become diabetic.<ref name="Karamanou2016">{{cite journal |last1=Karamanou |first1=M. |last2=Protogerou |first2=A. |last3=Tsoucalas |first3=G. |last4=Androutsos |first4=G. |last5=Poulakou-Rebelakou |first5=E. |title=Milestones in the history of diabetes mellitus: The main contributors. |journal=World Journal of Diabetes |date=10 January 2016 |volume=7 |issue=1 |pages=1–7 |doi=10.4239/wjd.v7.i1.1 |pmid=26788261|pmc=4707300 |doi-access=free }}</ref> Insulin was later isolated from pancreatic islets by [[Frederick Banting]] and [[Charles Best (medical scientist)|Charles Best]] in 1921.<ref name=Karamanou2016 /> |
||
The way the tissue of the pancreas has been viewed has also changed. Previously, it was viewed using simple [[staining]] methods such as [[H&E stain]]s. Now, [[immunohistochemistry]] can be used to more easily differentiate cell types. This involves visible antibodies to the products of certain cell types, and helps identify with greater ease cell types such as alpha and beta cells.{{sfn|Wheater's Histology|2013|pp=332-333}} |
The way the tissue of the pancreas has been viewed has also changed. Previously, it was viewed using simple [[staining]] methods such as [[H&E stain]]s. Now, [[immunohistochemistry]] can be used to more easily differentiate cell types. This involves visible antibodies to the products of certain cell types, and helps identify with greater ease cell types such as alpha and beta cells.{{sfn|Wheater's Histology|2013|pp=332-333}} |
||
==Other animals== |
==Other animals== |
||
Pancreatic tissue is present in all [[vertebrate]]s, but its precise form and arrangement varies widely. There may be up to three separate pancreases, two of which arise from the [[pancreatic bud]], and the other [[dorsally]]. In most species (including humans), these "fuse" in the adult, but there are several exceptions. Even when a single pancreas is present, two or three pancreatic ducts may persist, each draining separately into the duodenum (or equivalent part of the foregut). [[Bird]]s, for example, typically have three such ducts.<ref name=VB>{{cite book | last1 = Romer | first1 = Alfred Sherwood| last2 = Parsons | first2 = Thomas S. | name-list-style = vanc |year=1977 |title=The Vertebrate Body |publisher=Holt-Saunders International |location= Philadelphia, PA|pages= 357–59|url=https://books.google.com/books?id=EKhqAAAAMAAJ |isbn= 978- |
Pancreatic tissue is present in all [[vertebrate]]s, but its precise form and arrangement varies widely. There may be up to three separate pancreases, two of which arise from the [[pancreatic bud]], and the other [[dorsally]]. In most species (including humans), these "fuse" in the adult, but there are several exceptions. Even when a single pancreas is present, two or three pancreatic ducts may persist, each draining separately into the duodenum (or equivalent part of the foregut). [[Bird]]s, for example, typically have three such ducts.<ref name=VB>{{cite book | last1 = Romer | first1 = Alfred Sherwood| last2 = Parsons | first2 = Thomas S. | name-list-style = vanc |year=1977 |title=The Vertebrate Body |publisher=Holt-Saunders International |location= Philadelphia, PA|pages= 357–59|url=https://books.google.com/books?id=EKhqAAAAMAAJ |isbn= 978-0-03-910284-5}}</ref> |
||
In [[teleost]] fish, and a few other species (such as [[rabbit]]s), there is no discrete pancreas at all, with pancreatic tissue being distributed diffusely across the [[mesentery]] and even within other nearby organs, such as the [[liver]] or [[spleen]]. In a few teleost species, the endocrine tissue has fused to form a distinct gland within the abdominal cavity, but otherwise it is distributed among the exocrine components. The most primitive arrangement, however, appears to be that of [[lamprey]]s and [[lungfish]], in which pancreatic tissue is found as a number of discrete nodules within the wall of the gut itself, with the exocrine portions being little different from other glandular structures of the intestine.<ref name=VB/> |
In [[teleost]] fish, and a few other species (such as [[rabbit]]s), there is no discrete pancreas at all, with pancreatic tissue being distributed diffusely across the [[mesentery]] and even within other nearby organs, such as the [[liver]] or [[spleen]]. In a few teleost species, the endocrine tissue has fused to form a distinct gland within the abdominal cavity, but otherwise it is distributed among the exocrine components. The most primitive arrangement, however, appears to be that of [[lamprey]]s and [[lungfish]], in which pancreatic tissue is found as a number of discrete nodules within the wall of the gut itself, with the exocrine portions being little different from other glandular structures of the intestine.<ref name=VB/> |
||
| Line 158: | Line 158: | ||
==Cuisine== |
==Cuisine== |
||
The pancreas of [[veal|calf]] (''ris de veau'') or [[Lamb and mutton|lamb]] (''ris d'agneau''), and, less commonly, of [[beef]] or [[pork]], are used as food under the [[culinary name]] of [[sweetbread]].<ref>''[[Oxford Companion to Food]]''; ''[[Oxford English Dictionary]]''</ref><ref name="Leiths">{{cite book |last1=Spaull |first1=Susan |last2=Bruce-Gardyne |first2=Lucinda | name-list-style = vanc |title=Leiths Techniques Bible |date=2003 |publisher=Bloomsbury |isbn= |
The pancreas of [[veal|calf]] (''ris de veau'') or [[Lamb and mutton|lamb]] (''ris d'agneau''), and, less commonly, of [[beef]] or [[pork]], are used as food under the [[culinary name]] of [[sweetbread]].<ref>''[[Oxford Companion to Food]]''; ''[[Oxford English Dictionary]]''</ref><ref name="Leiths">{{cite book |last1=Spaull |first1=Susan |last2=Bruce-Gardyne |first2=Lucinda | name-list-style = vanc |title=Leiths Techniques Bible |date=2003 |publisher=Bloomsbury |isbn=0-7475-6046-3 |page=451 |edition= 1 }}</ref> |
||
== Additional images == |
== Additional images == |
||
| Line 175: | Line 175: | ||
===Bibliography=== |
===Bibliography=== |
||
* {{Cite book|title=Ganong's review of medical physiology|author1=Barrett, Kim E.|others=Barman, Susan M.,, Brooks, Heddwen L., Yuan, Jason X.-J.|isbn= |
* {{Cite book|title=Ganong's review of medical physiology|author1=Barrett, Kim E.|others=Barman, Susan M.,, Brooks, Heddwen L., Yuan, Jason X.-J.|isbn=978-1-260-12240-4|edition=26th|location=New York|oclc=1076268769|year=2019|ref={{harvid|Ganong's Physiology|2019}}}} |
||
* {{Cite book |editor=Beger HG|title=The pancreas |
* {{Cite book |editor=Beger HG|title=The pancreas: an integrated textbook of basic science, medicine, and surgery|date=2018|isbn=978-1-119-18841-4|location=Hoboken, NJ|oclc=1065547789|edition=third|ref={{harvid|Beger's|2018}}}} |
||
* {{cite book |last1=Kasper |first1=Dennis |last2=Fauci |first2=Anthony |last3=Hauser |first3=Stephen |last4=Longo |first4=Dan |last5=Jameson |first5=J. |last6=Loscalzo |first6=Joseph | name-list-style = vanc |title=Harrison's Principles of Internal Medicine |date=2015 |publisher=McGraw-Hill Professional |isbn= |
* {{cite book |last1=Kasper |first1=Dennis |last2=Fauci |first2=Anthony |last3=Hauser |first3=Stephen |last4=Longo |first4=Dan |last5=Jameson |first5=J. |last6=Loscalzo |first6=Joseph | name-list-style = vanc |title=Harrison's Principles of Internal Medicine |date=2015 |publisher=McGraw-Hill Professional |isbn=978-0-07-180215-4 |edition= 19|ref={{harvid|Harrison's|2015}}}} |
||
* {{cite book |editor-last1=Ralston |editor-first1=Stuart H. |editor-last2=Penman |editor-first2=Ian D. |editor-last3=Strachan |editor-first3=Mark W. |editor-last4=Hobson |editor-first4=Richard P. | name-list-style = vanc |title=Davidson's principles and practice of medicine |date=2018 |publisher=Elsevier |isbn=978-0-7020-7028-0 |edition= 23rd|ref={{harvid|Davidson's|2018}}}} |
* {{cite book |editor-last1=Ralston |editor-first1=Stuart H. |editor-last2=Penman |editor-first2=Ian D. |editor-last3=Strachan |editor-first3=Mark W. |editor-last4=Hobson |editor-first4=Richard P. | name-list-style = vanc |title=Davidson's principles and practice of medicine |date=2018 |publisher=Elsevier |isbn=978-0-7020-7028-0 |edition= 23rd|ref={{harvid|Davidson's|2018}}}} |
||
* {{cite book |editor=Standring, Susan|editor2=Borley, Neil R.|title=Gray's anatomy |
* {{cite book |editor=Standring, Susan|editor2=Borley, Neil R.|title=Gray's anatomy: the anatomical basis of clinical practice|date=2008|publisher=Churchill Livingstone|location=London|isbn=978-0-8089-2371-8|edition= 40th|ref={{harvid|Gray's|2008}}|display-editors=etal}} |
||
* {{Cite book |editor=Standring, Susan|title=Gray's anatomy |
* {{Cite book |editor=Standring, Susan|title=Gray's anatomy: the anatomical basis of clinical practice|isbn=978-0-7020-5230-9|edition=41st|location=Philadelphia|oclc=920806541|year=2016|ref={{harvid|Gray's|2016}}}} |
||
* {{cite book |last1=Young |first1=Barbara |last2=O'Dowd |first2=Geraldine |last3=Woodford |first3=Phillip |title=Wheater's functional histology: a text and colour atlas.|publisher=Elsevier |location=Philadelphia |date=2013|isbn= |
* {{cite book |last1=Young |first1=Barbara |last2=O'Dowd |first2=Geraldine |last3=Woodford |first3=Phillip |title=Wheater's functional histology: a text and colour atlas.|publisher=Elsevier |location=Philadelphia |date=2013|isbn=978-0-7020-4747-3 |edition=6th|ref={{harvid|Wheater's Histology|2013}}}} |
||
== External links == |
== External links == |
||
Latest revision as of 14:55, 15 December 2024
| Pancreas | |
|---|---|
 Anatomy of the human pancreas | |
| Details | |
| Pronunciation | /ˈpæŋkriəs/ |
| Precursor | Pancreatic buds |
| System | Digestive system and endocrine system |
| Artery | Inferior pancreaticoduodenal artery, anterior superior pancreaticoduodenal artery, posterior superior pancreaticoduodenal artery, splenic artery |
| Vein | Pancreaticoduodenal veins, pancreatic veins |
| Nerve | Pancreatic plexus, celiac ganglia, vagus nerve[1] |
| Lymph | Splenic lymph nodes, celiac lymph nodes and superior mesenteric lymph nodes |
| Identifiers | |
| Latin | pancreas |
| Greek | πάγκρεας (pánkreas) |
| MeSH | D010179 |
| TA98 | A05.9.01.001 |
| TA2 | 3114 |
| FMA | 7198 |
| Anatomical terminology | |
The pancreas is an organ of the digestive system and endocrine system of vertebrates. In humans, it is located in the abdomen behind the stomach and functions as a gland. The pancreas is a mixed or heterocrine gland, i.e., it has both an endocrine and a digestive exocrine function.[2] 99% of the pancreas is exocrine and 1% is endocrine.[3][4][5][6] As an endocrine gland, it functions mostly to regulate blood sugar levels, secreting the hormones insulin, glucagon, somatostatin and pancreatic polypeptide. As a part of the digestive system, it functions as an exocrine gland secreting pancreatic juice into the duodenum through the pancreatic duct. This juice contains bicarbonate, which neutralizes acid entering the duodenum from the stomach; and digestive enzymes, which break down carbohydrates, proteins and fats in food entering the duodenum from the stomach.
Inflammation of the pancreas is known as pancreatitis, with common causes including chronic alcohol use and gallstones. Because of its role in the regulation of blood sugar, the pancreas is also a key organ in diabetes mellitus. Pancreatic cancer can arise following chronic pancreatitis or due to other reasons, and carries a very poor prognosis, as it is often only identified after it has spread to other areas of the body.
The word pancreas comes from the Greek πᾶν (pân, "all") & κρέας (kréas, "flesh"). The function of the pancreas in diabetes has been known since at least 1889, with its role in insulin production identified in 1921.
Structure
[edit]
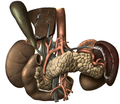
The pancreas is an organ that in humans lies in the abdomen, stretching from behind the stomach to the left upper abdomen near the spleen. In adults, it is about 12–15 centimetres (4.7–5.9 in) long, lobulated, and salmon-coloured in appearance.[7]
Anatomically, the pancreas is divided into a head, neck, body, and tail. The pancreas stretches from the inner curvature of the duodenum, where the head surrounds two blood vessels: the superior mesenteric artery and vein. The longest part of the pancreas, the body, stretches across behind the stomach, and the tail of the pancreas ends adjacent to the spleen.[7]
Two ducts, the main pancreatic duct and a smaller accessory pancreatic duct run through the body of the pancreas. The main pancreatic duct joins with the common bile duct forming a small ballooning called the ampulla of Vater (hepatopancreatic ampulla). This ampulla is surrounded by a muscle, the sphincter of Oddi. This ampulla opens into the descending part of the duodenum. The opening of the common bile duct into main pancreatic duct is controlled by sphincter of Boyden. The accessory pancreatic duct opens into duodenum with separate openings located above the opening of the main pancreatic duct.[7]
Parts
[edit]The head of the pancreas sits within the curvature of the duodenum, and wraps around the superior mesenteric artery and vein. To the right sits the descending part of the duodenum, and between these travel the superior and inferior pancreaticoduodenal arteries. Behind rests the inferior vena cava, and the common bile duct. In front sits the peritoneal membrane and the transverse colon.[7] A small uncinate process emerges from below the head, situated behind the superior mesenteric vein and sometimes artery.[7]
The neck of the pancreas separates the head of the pancreas, located in the curvature of the duodenum, from the body. The neck is about 2 cm (0.79 in) wide, and sits in front of where the portal vein is formed. The neck lies mostly behind the pylorus of the stomach, and is covered with peritoneum. The anterior superior pancreaticoduodenal artery travels in front of the neck of the pancreas.[7]
The body is the largest part of the pancreas, and mostly lies behind the stomach, tapering along its length. The peritoneum sits on top of the body of the pancreas, and the transverse colon in front of the peritoneum.[7] Behind the pancreas are several blood vessels, including the aorta, the splenic vein, and the left renal vein, as well as the beginning of the superior mesenteric artery.[7] Below the body of the pancreas sits some of the small intestine, specifically the last part of the duodenum and the jejunum to which it connects, as well as the suspensory ligament of the duodenum which falls between these two. In front of the pancreas sits the transverse colon.[8]
The pancreas narrows towards the tail, which sits near to the spleen.[7] It is usually between 1.3–3.5 cm (0.51–1.38 in) long, and sits between the layers of the ligament between the spleen and the left kidney. The splenic artery and vein, which also passes behind the body of the pancreas, pass behind the tail of the pancreas.[7]
Blood supply
[edit]The pancreas has a rich blood supply, with vessels originating as branches of both the coeliac artery and superior mesenteric artery.[7] The splenic artery, the largest branch of the celiac trunk, runs along the top of the pancreas, and supplies the left part of the body and the tail of the pancreas through its pancreatic branches, the largest of which is called the greater pancreatic artery.[7] The superior and inferior pancreaticoduodenal arteries run along the back and front surfaces of the head of the pancreas adjacent to the duodenum. These supply the head of the pancreas. These vessels join together (anastamose) in the middle.[7]
The body and neck of the pancreas drain into the splenic vein, which sits behind the pancreas.[7] The head drains into, and wraps around, the superior mesenteric and portal veins, via the pancreaticoduodenal veins.[7]
The pancreas drains into lymphatic vessels that travel alongside its arteries, and has a rich lymphatic supply.[7] The lymphatic vessels of the body and tail drain into splenic lymph nodes, and eventually into lymph nodes that lie in front of the aorta, between the coeliac and superior mesenteric arteries. The lymphatic vessels of the head and neck drain into intermediate lymphatic vessels around the pancreaticoduodenal, mesenteric and hepatic arteries, and from there into the lymph nodes that lie in front of the aorta.[7]
Microanatomy
[edit]

The pancreas contains tissue with an endocrine and exocrine role, and this division is also visible when the pancreas is viewed under a microscope.[10]
The majority of pancreatic tissue has a digestive role. The cells with this role form clusters (Latin: acini, lit. 'kernels') around small ducts, and are arranged in lobes that have thin fibrous walls. The cells of each acinus secrete inactive digestive enzymes called zymogens into the small intercalated ducts which they surround. In each acinus, the cells are pyramid-shaped and situated around the intercalated ducts, with the nuclei resting on the basement membrane, a large endoplasmic reticulum, and a number of zymogen granules visible within the cytoplasm. The intercalated ducts drain into larger intralobular ducts within the lobule, and finally interlobular ducts. The ducts are lined by a single layer of column-shaped cells. There is more than one layer of cells as the diameter of the ducts increases.[10]
The tissues with an endocrine role within the pancreas exist as clusters of cells called pancreatic islets (also called islets of Langerhans) that are distributed throughout the pancreas.[9] Pancreatic islets contain alpha cells, beta cells, and delta cells, each of which releases a different hormone. These cells have characteristic positions, with alpha cells (secreting glucagon) tending to be situated around the periphery of the islet, and beta cells (secreting insulin) more numerous and found throughout the islet.[9] Enterochromaffin cells are also scattered throughout the islets.[9] Islets are composed of up to 3,000 secretory cells, and contain several small arterioles to receive blood, and venules that allow the hormones secreted by the cells to enter the systemic circulation.[9]
Variation
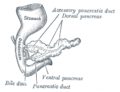
[edit]The size of the pancreas varies considerably.[7] Several anatomical variations exist, relating to the embryological development of the two pancreatic buds. The pancreas develops from these buds on either side of the duodenum. The ventral bud rotates to lie next to the dorsal bud, eventually fusing. In about 10% of adults, an accessory pancreatic duct may be present if the main duct of the dorsal bud of the pancreas does not regress; this duct opens into the minor duodenal papilla.[11] If the two buds themselves, each having a duct, do not fuse, a pancreas may exist with two separate ducts, a condition known as a pancreas divisum. This condition has no physiologic consequence.[12] If the ventral bud does not fully rotate, an annular pancreas may exist, where part or all of the duodenum is encircled by the pancreas. This may be associated with duodenal atresia.[13]
Gene and protein expression
[edit]10,000 protein coding genes (~50% of all human genes) are expressed in the normal human pancreas.[14][15] Less than 100 of these genes are specifically expressed in the pancreas. Similar to the salivary glands, most pancreas-specific genes encode for secreted proteins. Corresponding pancreas-specific proteins are either expressed in the exocrine cellular compartment and have functions related to digestion or food uptake such as digestive chymotrypsinogen enzymes and pancreatic lipase PNLIP, or are expressed in the various cells of the endocrine pancreatic islets and have functions related to secreted hormones such as insulin, glucagon, somatostatin and pancreatic polypeptide.[16]
Development
[edit]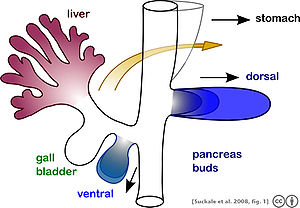
The pancreas forms during development from two buds that arise from the duodenal part of the foregut, an embryonic tube that is a precursor to the gastrointestinal tract.[11] It is of endodermal origin.[11] Pancreatic development begins with the formation of a dorsal and ventral pancreatic bud. Each joins with the foregut through a duct. The dorsal pancreatic bud forms the neck, body, and tail of the developed pancreas, and the ventral pancreatic bud forms the head and uncinate process.[11]
The definitive pancreas results from rotation of the ventral bud and the fusion of the two buds.[11] During development, the duodenum rotates to the right, and the ventral bud rotates with it, moving to a position that becomes more dorsal. Upon reaching its final destination, the ventral pancreatic bud is below the larger dorsal bud, and eventually fuses with it. At this point of fusion, the main ducts of the ventral and dorsal pancreatic buds fuse, forming the main pancreatic duct. Usually, the duct of the dorsal bud regresses, leaving the main pancreatic duct.[11]
Cellular development
[edit]Pancreatic progenitor cells are precursor cells that differentiate into the functional pancreatic cells, including exocrine acinar cells, endocrine islet cells, and ductal cells.[17] These progenitor cells are characterised by the co-expression of the transcription factors PDX1 and NKX6-1.[17]
The cells of the exocrine pancreas differentiate through molecules that induce differentiation including follistatin, fibroblast growth factors, and activation of the Notch receptor system.[17] Development of the exocrine acini progresses through three successive stages. These are the predifferentiated, protodifferentiated, and differentiated stages, which correspond to undetectable, low, and high levels of digestive enzyme activity, respectively.[17]
Pancreatic progenitor cells differentiate into endocrine islet cells under the influence of neurogenin-3 and ISL1, but only in the absence of notch receptor signaling. Under the direction of a Pax gene, the endocrine precursor cells differentiate to form alpha and gamma cells. Under the direction of Pax-6, the endocrine precursor cells differentiate to form beta and delta cells.[17] The pancreatic islets form as the endocrine cells migrate from the duct system to form small clusters around capillaries.[9] This occurs around the third month of development,[11] and insulin and glucagon can be detected in the human fetal circulation by the fourth or fifth month of development.[17]
Function
[edit]The pancreas is involved in blood sugar control and metabolism within the body, and also in the secretion of substances (collectively pancreatic juice) that help digestion. These are divided into an "endocrine" role, relating to the secretion of insulin and other substances within pancreatic islets that help control blood sugar levels and metabolism within the body, and an "exocrine" role, relating to the secretion of enzymes involved in digesting substances in the digestive tract.[10]
Blood glucose regulation
[edit]
Cells within the pancreas help to maintain blood glucose levels (homeostasis). The cells that do this are located within the pancreatic islets that are present throughout the pancreas. When blood glucose levels are low, alpha cells secrete glucagon, which increases blood glucose levels. When blood glucose levels are high beta cells secrete insulin to decrease glucose in blood. Delta cells in the islet also secrete somatostatin which decreases the release of insulin and glucagon.[9]
Glucagon acts to increase glucose levels by promoting the creation of glucose and the breakdown of glycogen to glucose in the liver. It also decreases the uptake of glucose in fat and muscle. Glucagon release is stimulated by low blood glucose or insulin levels, and during exercise.[18] Insulin acts to decrease blood glucose levels by facilitating uptake by cells (particularly skeletal muscle), and promoting its use in the creation of proteins, fats and carbohydrates. Insulin is initially created as a precursor form called preproinsulin. This is converted to proinsulin and cleaved by C-peptide to insulin which is then stored in granules in beta cells. Glucose is taken into the beta cells and degraded. The end effect of this is to cause depolarisation of the cell membrane which stimulates the release of the insulin.[18]
The main factor influencing the secretion of insulin and glucagon are the levels of glucose in blood plasma.[19] Low blood sugar stimulates glucagon release, and high blood sugar stimulates insulin release. Other factors also influence the secretion of these hormones. Some amino acids, that are byproducts of the digestion of protein, stimulate insulin and glucagon release. Somatostatin acts as an inhibitor of both insulin and glucagon. The autonomic nervous system also plays a role. Activation of Beta-2 receptors of the sympathetic nervous system by catecholamines secreted from sympathetic nerves stimulates secretion of insulin and glucagon,[19][20] whereas activation of Alpha-1 receptors inhibits secretion.[19] M3 receptors of the parasympathetic nervous system act when stimulated by the right vagus nerve to stimulate release of insulin from beta cells.[19]
Digestion
[edit]
The pancreas plays a vital role in the digestive system. It does this by secreting a fluid that contains digestive enzymes into the duodenum, the first part of the small intestine that receives food from the stomach. These enzymes help to break down carbohydrates, proteins and lipids (fats). This role is called the "exocrine" role of the pancreas. The cells that do this are arranged in clusters called acini. Secretions into the middle of the acinus accumulate in intralobular ducts, which drain to the main pancreatic duct, which drains directly into the duodenum. About 1.5–3 liters of fluid are secreted in this manner every day.[8][21]
The cells in each acinus are filled with granules containing the digestive enzymes. These are secreted in an inactive form termed zymogens or proenzymes. When released into the duodenum, they are activated by the enzyme enterokinase present in the lining of the duodenum. The proenzymes are cleaved, creating a cascade of activating enzymes.[21]
- Enzymes that break down proteins begin with activation of trypsinogen to trypsin. The free trypsin then cleaves the rest of the trypsinogen, as well as chymotrypsinogen to its active form chymotrypsin.[21]
- Enzymes secreted involved in the digestion of fats include lipase, phospholipase A2, lysophospholipase, and cholesterol esterase.[21]
- Enzymes that break down starch and other carbohydrates include amylase.[21]
These enzymes are secreted in a fluid rich in bicarbonate. Bicarbonate helps maintain an alkaline pH for the fluid, a pH in which most of the enzymes act most efficiently, and also helps to neutralise the stomach acids that enter the duodenum.[21] Secretion is influenced by hormones including secretin, cholecystokinin, and VIP, as well as acetylcholine stimulation from the vagus nerve. Secretin is released from the S cells which form part of the lining of the duodenum in response to stimulation by gastric acid. Along with VIP, it increases the secretion of enzymes and bicarbonate. Cholecystokinin is released from Ito cells of the lining of the duodenum and jejunum mostly in response to long chain fatty acids, and increases the effects of secretin.[21] At a cellular level, bicarbonate is secreted from centroacinar and ductal cells through a sodium and bicarbonate cotransporter that acts because of membrane depolarisation caused by the cystic fibrosis transmembrane conductance regulator. Secretin and VIP act to increase the opening of the cystic fibrosis transmembrane conductance regulator, which leads to more membrane depolarisation and more secretion of bicarbonate.[22][23][24]
A variety of mechanisms act to ensure that the digestive action of the pancreas does not act to digest pancreatic tissue itself. These include the secretion of inactive enzymes (zymogens), the secretion of the protective enzyme trypsin inhibitor, which inactivates trypsin, the changes in pH that occur with bicarbonate secretion that stimulate digestion only when the pancreas is stimulated, and the fact that the low calcium within cells causes inactivation of trypsin.[21]
Additional functions
[edit]The pancreas also secretes vasoactive intestinal peptide and pancreatic polypeptide. Enterochromaffin cells of the pancreas secrete the hormones motilin, serotonin, and substance P.[9] It has been demonstrated that pancreatic tissue is a strong accumulator and secretor in the intestine of radioactive cesium (Cs-137).[25][26]
Clinical significance
[edit]Inflammation
[edit]Inflammation of the pancreas is known as pancreatitis. Pancreatitis is most often associated with recurrent gallstones or chronic alcohol use, with other common causes including traumatic damage, damage following an ERCP, some medications, infections such as mumps and very high blood triglyceride levels. Acute pancreatitis is likely to cause intense pain in the central abdomen, that often radiates to the back, and may be associated with nausea or vomiting. Severe pancreatitis may lead to bleeding or perforation of the pancreas resulting in shock or a systemic inflammatory response syndrome, bruising of the flanks or the region around the belly button. These severe complications are often managed in an intensive care unit.[27]
In pancreatitis, enzymes of the exocrine pancreas damage the structure and tissue of the pancreas. Detection of some of these enzymes, such as amylase and lipase in the blood, along with symptoms and findings on medical imaging such as ultrasound or a CT scan, are often used to indicate that a person has pancreatitis. Pancreatitis is often managed medically with pain relief, and monitoring to prevent or manage shock, and management of any identified underlying causes. This may include removal of gallstones, lowering of blood triglyceride or glucose levels, the use of corticosteroids for autoimmune pancreatitis, and the cessation of any medication triggers.[27]
Chronic pancreatitis refers to the development of pancreatitis over time. It shares many similar causes, with the most common being chronic alcohol use, with other causes including recurrent acute episodes and cystic fibrosis. Abdominal pain, characteristically relieved by sitting forward or drinking alcohol, is the most common symptom. When the digestive function of the pancreas is severely affected, this may lead to problems with fat digestion and the development of steatorrhoea; when the endocrine function is affected, this may lead to diabetes. Chronic pancreatitis is investigated in a similar way to acute pancreatitis. In addition to management of pain and nausea, and management of any identified causes (which may include alcohol cessation), because of the digestive role of the pancreas, enzyme replacement may be needed to prevent malabsorption.[27]
Cancer
[edit]

Pancreatic cancers, particularly the most common type, pancreatic adenocarcinoma, remain very difficult to treat, and are mostly diagnosed only at a stage that is too late for surgery, which is the only curative treatment. Pancreatic cancer is rare in people younger than 40 and the median age of diagnosis is 71.[29] Risk factors include chronic pancreatitis, older age, smoking, obesity, diabetes, and certain rare genetic conditions including multiple endocrine neoplasia type 1, hereditary nonpolyposis colon cancer and dysplastic nevus syndrome among others.[27][30] About 25% of cases are attributable to tobacco smoking,[31] while 5–10% of cases are linked to inherited genes.[29]
Pancreatic adenocarcinoma is the most common form of pancreatic cancer, and is cancer arising from the exocrine digestive part of the pancreas. Most occur in the head of the pancreas.[27] Symptoms tend to arise late in the course of the cancer, when it causes abdominal pain, weight loss, or yellowing of the skin (jaundice). Jaundice occurs when the outflow of bile is blocked by the cancer. Other less common symptoms include nausea, vomiting, pancreatitis, diabetes or recurrent venous thrombosis.[27] Pancreatic cancer is usually diagnosed by medical imaging in the form of an ultrasound or CT scan with contrast enhancement. An endoscopic ultrasound may be used if a tumour is being considered for surgical removal, and biopsy guided by ERCP or ultrasound can be used to confirm an uncertain diagnosis.[27]
Because of the late development of symptoms, most cancer presents at an advanced stage.[27] Only 10 to 15% of tumours are suitable for surgical resection.[27] As of 2018[update], when chemotherapy is given the FOLFIRINOX regimen containing fluorouracil, irinotecan, oxaliplatin and leucovorin has been shown to extend survival beyond traditional gemcitabine regimens.[27] For the most part, treatment is palliative, focus on the management of symptoms that develop. This may include management of itch, a choledochojejunostomy or the insertion of stents with ERCP to facilitate the drainage of bile, and medications to help control pain.[27] In the United States pancreatic cancer is the fourth most common cause of deaths due to cancer.[32] The disease occurs more often in the developed world, which had 68% of new cases in 2012.[33] Pancreatic adenocarcinoma typically has poor outcomes with the average percentage alive for at least one and five years after diagnosis being 25% and 5% respectively.[33][34] In localized disease where the cancer is small (< 2 cm) the number alive at five years is approximately 20%.[35]
There are several types of pancreatic cancer, involving both the endocrine and exocrine tissue. The many types of pancreatic endocrine tumors are all uncommon or rare, and have varied outlooks. However the incidence of these cancers has been rising sharply; it is not clear to what extent this reflects increased detection, especially through medical imaging, of tumors that would be very slow to develop. Insulinomas (largely benign) and gastrinomas are the most common types.[36] For those with neuroendocrine cancers the number alive after five years is much better at 65%, varying considerably with type.[33]
A solid pseudopapillary tumour is a low-grade malignant tumour of the pancreas of papillary architecture that typically afflicts young women.[37]
Diabetes mellitus
[edit]Type 1 diabetes
[edit]Diabetes mellitus type 1 is a chronic autoimmune disease in which the immune system attacks the insulin-secreting beta cells of the pancreas.[38] Insulin is needed to keep blood sugar levels within optimal ranges, and its lack can lead to high blood sugar. As an untreated chronic condition, complications including accelerated vascular disease, diabetic retinopathy, kidney disease and neuropathy can result.[38] In addition, if there is not enough insulin for glucose to be used within cells, the medical emergency diabetic ketoacidosis, which is often the first symptom that a person with type 1 diabetes may have, can result.[39] Type 1 diabetes can develop at any age but is most often diagnosed before age 40.[38] For people living with type 1 diabetes, insulin injections are critical for survival.[38] An experimental procedure to treat type 1 diabetes is pancreas transplantation or isolated transplantation of islet cells to supply a person with functioning beta cells.[38]
Type 2 diabetes
[edit]Diabetes mellitus type 2 is the most common form of diabetes.[38] The causes for high blood sugar in this form of diabetes usually are a combination of insulin resistance and impaired insulin secretion, with both genetic and environmental factors playing a role in the development of the disease.[40] Over time, pancreatic beta cells may become "exhausted" and less functional.[38] The management of type 2 diabetes involves a combination of lifestyle measures, medications if required and potentially insulin.[41] With relevance to the pancreas, several medications act to enhance the secretion of insulin from beta cells, particularly sulphonylureas, which act directly on beta cells; incretins which replicate the action of the hormones glucagon-like peptide 1, increasing the secretion of insulin from beta cells after meals, and are more resistant to breakdown; and DPP-4 inhibitors, which slow the breakdown of incretins.[41]
Removal
[edit]It is possible for a person to live without a pancreas, provided that the person takes insulin for proper regulation of blood glucose concentration and pancreatic enzyme supplements to aid digestion.[42]
History
[edit]The pancreas was first identified by Herophilus (335–280 BC), a Greek anatomist and surgeon.[43] A few hundred years later, Rufus of Ephesus, another Greek anatomist, gave the pancreas its name. Etymologically, the term "pancreas", a modern Latin adaptation of Greek πάγκρεας,[44] [πᾶν ("all", "whole"), and κρέας ("flesh")],[45] originally means sweetbread,[46] although literally meaning all-flesh, presumably because of its fleshy consistency. It was only in 1889 when Oskar Minkowski discovered that removing the pancreas from a dog caused it to become diabetic.[47] Insulin was later isolated from pancreatic islets by Frederick Banting and Charles Best in 1921.[47]
The way the tissue of the pancreas has been viewed has also changed. Previously, it was viewed using simple staining methods such as H&E stains. Now, immunohistochemistry can be used to more easily differentiate cell types. This involves visible antibodies to the products of certain cell types, and helps identify with greater ease cell types such as alpha and beta cells.[9]
Other animals
[edit]Pancreatic tissue is present in all vertebrates, but its precise form and arrangement varies widely. There may be up to three separate pancreases, two of which arise from the pancreatic bud, and the other dorsally. In most species (including humans), these "fuse" in the adult, but there are several exceptions. Even when a single pancreas is present, two or three pancreatic ducts may persist, each draining separately into the duodenum (or equivalent part of the foregut). Birds, for example, typically have three such ducts.[48]
In teleost fish, and a few other species (such as rabbits), there is no discrete pancreas at all, with pancreatic tissue being distributed diffusely across the mesentery and even within other nearby organs, such as the liver or spleen. In a few teleost species, the endocrine tissue has fused to form a distinct gland within the abdominal cavity, but otherwise it is distributed among the exocrine components. The most primitive arrangement, however, appears to be that of lampreys and lungfish, in which pancreatic tissue is found as a number of discrete nodules within the wall of the gut itself, with the exocrine portions being little different from other glandular structures of the intestine.[48]
Cuisine
[edit]The pancreas of calf (ris de veau) or lamb (ris d'agneau), and, less commonly, of beef or pork, are used as food under the culinary name of sweetbread.[49][50]
Additional images
[edit]-
A normal pancreas on ultrasound.
-
Identifying pancreas on abdominal ultrasonography when it is partly obscured by bowel gas.
-
Pancreas of a human embryo at end of sixth week
-
The pancreas and its surrounding structures
-
Duodenum and pancreas. Deep dissection.
References
[edit]- ^ Nosek, Thomas M. "Section 6/6ch2/s6ch2_30". Essentials of Human Physiology. Archived from the original on 2016-03-24.
- ^ Gyr, K.; Beglinger, C.; Stalder, G. A. (1985-09-21). "[Interaction of the endo- and exocrine pancreas]". Schweizerische Medizinische Wochenschrift. 115 (38): 1299–1306. ISSN 0036-7672. PMID 2865807.
- ^ "Pancreas Gland - Endocrine System". Innerbody. Retrieved 2021-06-12.
- ^ Beger's 2018, pp. 124.
- ^ "Endocrine Pancreas - an overview ScienceDirect Topics". www.sciencedirect.com. Retrieved 2021-06-12.
- ^ Henderson, J. R.; Daniel, P. M.; Fraser, P. A. (February 1981). "The pancreas as a single organ: the influence of the endocrine upon the exocrine part of the gland". Gut. 22 (2): 158–167. doi:10.1136/gut.22.2.158. ISSN 0017-5749. PMC 1419227. PMID 6111521.
- ^ a b c d e f g h i j k l m n o p q r Gray's 2016, pp. 1179–1189.
- ^ a b Gray's 2008, pp. 1183–1190.
- ^ a b c d e f g h i Wheater's Histology 2013, pp. 332–333.
- ^ a b c Wheater's Histology 2013, pp. 287–291.
- ^ a b c d e f g Sadley, TW (2019). Langman's medical embryology (14th ed.). Philadelphia: Wolters Kluwer. pp. 244–245. ISBN 978-1-4963-8390-7.
- ^ Kanth, Rajan; Roy, Praveen K; Al Samman, Mounzer; Patti, Marco G (2019-11-21). "Pancreatic Divisum: Pathophysiology". emedicine.medscape.com. Medscape.
- ^ Noh, TH; Lee, SE; Park, JM (February 2012). "Laparoscopic treatment of annular pancreas in adults: report of a case". Korean Journal of Hepato-biliary-pancreatic Surgery. 16 (1): 43–5. doi:10.14701/kjhbps.2012.16.1.43. PMC 4575014. PMID 26388905.
- ^ "The human proteome in pancreas – The Human Protein Atlas". www.proteinatlas.org. Retrieved 2017-09-25.
- ^ Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. (January 2015). "Proteomics. Tissue-based map of the human proteome". Science. 347 (6220): 1260419. doi:10.1126/science.1260419. PMID 25613900. S2CID 802377.
- ^ Danielsson A, Pontén F, Fagerberg L, Hallström BM, Schwenk JM, Uhlén M, et al. (2014-12-29). "The human pancreas proteome defined by transcriptomics and antibody-based profiling". PLOS ONE. 9 (12): e115421. Bibcode:2014PLoSO...9k5421D. doi:10.1371/journal.pone.0115421. PMC 4278897. PMID 25546435.
- ^ a b c d e f Carlson BM (2019). Human Embryology and Developmental Biology. St. Louis: Elsevier. pp. 318–57. ISBN 978-0-323-52375-2.
- ^ a b Harrison's 2015, pp. 2853–4.
- ^ a b c d Barrett, Kim E. (2019). "Regulation of Insulin Secretion; Glucagon". Ganong's review of medical physiology. Barman, Susan M.,, Brooks, Heddwen L., Yuan, Jason X.-J. (26th ed.). New York. pp. 433–437. ISBN 978-1-260-12240-4. OCLC 1076268769.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Hall, John E (2016). Guyton and Hall textbook of medical physiology (13th ed.). Philadelphia: Elsevier. pp. 990–994. ISBN 978-1-4557-7016-8.
- ^ a b c d e f g h Harrison's 2015, pp. 2086–2102.
- ^ Harrison's 2015, pp. 2090.
- ^ Beger's 2018, pp. 16, 57.
- ^ Pandol, Stephen (2011). The Exocrine Pancreas. Colloquium Series on Integrated Systems Physiology: From Molecule to Function. Vol. 3. San Rafael, CA: Morgan & Claypool Life Sciences. pp. 1–64. doi:10.4199/C00026ED1V01Y201102ISP014. ISBN 978-1-61504-138-1. ISSN 2154-560X. OCLC 708084265. PMID 21634067. Archived from the original on Nov 18, 2022.
- ^ Bandazhevsky Y.I. (2003). "Chronic Cs-137 incorporation in children's organs". Swiss Med. Wkly. 133 (35–36): 488–90. doi:10.4414/smw.2003.10226. PMID 14652805. S2CID 28184979.
- ^ Venturi, Sebastiano (January 2021). "Cesium in Biology, Pancreatic Cancer, and Controversy in High and Low Radiation Exposure Damage—Scientific, Environmental, Geopolitical, and Economic Aspects". International Journal of Environmental Research and Public Health. 18 (17): 8934. doi:10.3390/ijerph18178934. PMC 8431133. PMID 34501532.
- ^ a b c d e f g h i j k Davidson's 2018, p. 837-844.
- ^ Wang, Y; Miller, FH; Chen, ZE; Merrick, L; Mortele, KJ; Hoff, FL; et al. (2011). "Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas". Radiographics. 31 (3): E47-64. doi:10.1148/rg.313105174. PMID 21721197.
Diagram by Mikael Häggström, M.D. - ^ a b Ryan DP, Hong TS, Bardeesy N (September 2014). "Pancreatic adenocarcinoma". The New England Journal of Medicine. 371 (11): 1039–49. doi:10.1056/NEJMra1404198. PMID 25207767.
- ^ "Pancreatic Cancer Treatment (PDQ®) Patient Version". National Cancer Institute. 2014-04-17. Retrieved 8 June 2014.
- ^ Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH (September 2013). "Recent progress in pancreatic cancer". CA: A Cancer Journal for Clinicians. 63 (5): 318–48. doi:10.3322/caac.21190. PMC 3769458. PMID 23856911.
- ^ Hariharan D, Saied A, Kocher HM (2008). "Analysis of mortality rates for pancreatic cancer across the world". HPB. 10 (1): 58–62. doi:10.1080/13651820701883148. PMC 2504856. PMID 18695761.
- ^ a b c "Chapter 5.7". World Cancer Report 2014. World Health Organization. 2014. ISBN 978-92-832-0429-9.
- ^ "American Cancer Society: Cancer Facts & Figures 2010: see page 4 for incidence estimates, and page 19 for survival percentages" (PDF). Archived from the original (PDF) on 2015-01-14.
- ^ "Pancreatic Cancer Treatment (PDQ®) Health Professional Version". NCI. 2014-02-21. Retrieved 8 June 2014.
- ^ Burns WR, Edil BH (March 2012). "Neuroendocrine pancreatic tumors: guidelines for management and update". Current Treatment Options in Oncology. 13 (1): 24–34. doi:10.1007/s11864-011-0172-2. PMID 22198808. S2CID 7329783.
- ^ Patil TB, Shrikhande SV, Kanhere HA, Saoji RR, Ramadwar MR, Shukla PJ (2006). "Solid pseudopapillary neoplasm of the pancreas: a single institution experience of 14 cases". HPB. 8 (2): 148–50. doi:10.1080/13651820510035721. PMC 2131425. PMID 18333264.
- ^ a b c d e f g Barrett, Kim E.; Barman, Susan M.; Brooks, Heddwen L.; Yuan, Jason X.-J.; Ganong, William F. (2019). "Hypoglycaemia & Diabetes Mellitus in Humans". Ganong's review of medical physiology (26th ed.). New York: McGraw-Hill Education. pp. 440–441. ISBN 978-1-260-12240-4. OCLC 1076268769.
- ^ Davidson's 2018, p. 735.
- ^ Davidson's 2018, p. 730-732.
- ^ a b Davidson's 2018, p. 745-751.
- ^ Banks PA, Conwell DL, Toskes PP (February 2010). "The management of acute and chronic pancreatitis". Gastroenterology & Hepatology. 6 (2 Suppl 3): 1–16. PMC 2886461. PMID 20567557.
- ^ Howard JM, Hess W (2012). History of the Pancreas: Mysteries of a Hidden Organ. Springer Science & Business Media. p. 24. ISBN 978-1-4615-0555-6.
- ^ O'Brien T (2015). A2Z Book of word Origins. Rupa Publications. p. 86. ISBN 978-81-291-1809-7.
- ^ Harper, Douglas. "Pancreas". Online Etymology Dictionary. Retrieved 2007-04-04.
- ^ Green TM (2008). The Greek and Latin Roots of English. Rowman & Littlefield. p. 176. ISBN 978-0-7425-4780-3.
- ^ a b Karamanou, M.; Protogerou, A.; Tsoucalas, G.; Androutsos, G.; Poulakou-Rebelakou, E. (10 January 2016). "Milestones in the history of diabetes mellitus: The main contributors". World Journal of Diabetes. 7 (1): 1–7. doi:10.4239/wjd.v7.i1.1. PMC 4707300. PMID 26788261.
- ^ a b Romer AS, Parsons TS (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 357–59. ISBN 978-0-03-910284-5.
- ^ Oxford Companion to Food; Oxford English Dictionary
- ^ Spaull S, Bruce-Gardyne L (2003). Leiths Techniques Bible (1 ed.). Bloomsbury. p. 451. ISBN 0-7475-6046-3.
Bibliography
[edit]- Barrett, Kim E. (2019). Ganong's review of medical physiology. Barman, Susan M.,, Brooks, Heddwen L., Yuan, Jason X.-J. (26th ed.). New York. ISBN 978-1-260-12240-4. OCLC 1076268769.
{{cite book}}: CS1 maint: location missing publisher (link) - Beger HG, ed. (2018). The pancreas: an integrated textbook of basic science, medicine, and surgery (third ed.). Hoboken, NJ. ISBN 978-1-119-18841-4. OCLC 1065547789.
{{cite book}}: CS1 maint: location missing publisher (link) - Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J (2015). Harrison's Principles of Internal Medicine (19 ed.). McGraw-Hill Professional. ISBN 978-0-07-180215-4.
- Ralston SH, Penman ID, Strachan MW, Hobson RP, eds. (2018). Davidson's principles and practice of medicine (23rd ed.). Elsevier. ISBN 978-0-7020-7028-0.
- Standring, Susan; Borley, Neil R.; et al., eds. (2008). Gray's anatomy: the anatomical basis of clinical practice (40th ed.). London: Churchill Livingstone. ISBN 978-0-8089-2371-8.
- Standring, Susan, ed. (2016). Gray's anatomy: the anatomical basis of clinical practice (41st ed.). Philadelphia. ISBN 978-0-7020-5230-9. OCLC 920806541.
{{cite book}}: CS1 maint: location missing publisher (link) - Young, Barbara; O'Dowd, Geraldine; Woodford, Phillip (2013). Wheater's functional histology: a text and colour atlas (6th ed.). Philadelphia: Elsevier. ISBN 978-0-7020-4747-3.
External links
[edit] Media related to Pancreas at Wikimedia Commons
Media related to Pancreas at Wikimedia Commons- Pancreas at the Human Protein Atlas
- Pancreatic Diseases – English – The Gastro Specialist