Guanosine triphosphate: Difference between revisions
No edit summary |
Fixes image on dark mode |
||
| (20 intermediate revisions by 16 users not shown) | |||
| Line 1: | Line 1: | ||
{{short description|Chemical compound}} |
|||
{{distinguish|Adenosine triphosphate}} |
|||
{{chembox |
{{chembox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Watchedfields = changed |
| Watchedfields = changed |
||
| verifiedrevid = 477003555 |
| verifiedrevid = 477003555 |
||
| ImageFile |
| ImageFile = GTP.png |
||
| ImageSize = |
| ImageSize = 280 |
||
| ImageClass = skin-invert |
|||
| ImageAlt = Skeletal formula of guanosine triphosphate |
|||
| |
| ImageAlt = Skeletal formula of guanosine triphosphate |
||
| ImageFile1 = Guanosine-triphosphate-anion-3D-spacefill.png |
|||
| ImageSize1 = 220 |
|||
| ImageAlt1 = Space-filling model of the guanosine triphosphate anion |
| ImageSize1 = 220 |
||
| ImageAlt1 = Space-filling model of the guanosine triphosphate anion |
|||
| IUPACName = |
| IUPACName = Guanosine 5′-(tetrahydrogen triphosphate) |
||
| SystematicName = ''O''<sup>1</sup>-<nowiki/>{[(2''R'',3''S'',4''R'',5''R'')-5-(2-Amino-6-oxo-1,6-dihydro-9''H''-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl} tetrahydrogen triphosphate |
|||
| OtherNames = guanosine triphosphate, 9-β-<small>D</small>-ribofuranosylguanine-5'-triphosphate, 9-β-<small>D</small>-ribofuranosyl-2-amino-6-oxo-purine-5'-triphosphate |
| OtherNames = guanosine triphosphate, 9-β-<small>D</small>-ribofuranosylguanine-5'-triphosphate, 9-β-<small>D</small>-ribofuranosyl-2-amino-6-oxo-purine-5'-triphosphate |
||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| ChemSpiderID = 6569 |
| ChemSpiderID = 6569 |
||
| Line 22: | Line 26: | ||
| CASNo_Ref = {{cascite|correct|CAS}} |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
| CASNo = 86-01-1 |
| CASNo = 86-01-1 |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 01WV7J708X |
|||
| KEGG_Ref = {{keggcite|changed|kegg}} |
| KEGG_Ref = {{keggcite|changed|kegg}} |
||
| KEGG = C00044 |
| KEGG = C00044 |
||
| Line 31: | Line 37: | ||
| MeSHName = Guanosine+triphosphate |
| MeSHName = Guanosine+triphosphate |
||
}} |
}} |
||
| Section2 = {{Chembox Properties |
| Section2 = {{Chembox Properties |
||
| C=10|H=16|N=5|O=14|P=3 |
| C=10|H=16|N=5|O=14|P=3 |
||
| Appearance = |
| Appearance = |
||
| Line 38: | Line 44: | ||
| BoilingPt = |
| BoilingPt = |
||
}} |
}} |
||
| Section3 = {{Chembox Hazards |
| Section3 = {{Chembox Hazards |
||
| MainHazards = |
| MainHazards = |
||
| FlashPt = |
| FlashPt = |
||
| Line 45: | Line 51: | ||
}} |
}} |
||
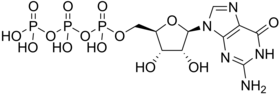
'''Guanosine-5'-triphosphate''' ('''GTP''') is a [[purine]] [[nucleoside triphosphate]]. It is one of the building blocks needed for the synthesis of [[RNA]] during the [[transcription (genetics)|transcription]] process. Its structure is similar to that of the [[ |
'''Guanosine-5'-triphosphate''' ('''GTP''') is a [[purine]] [[nucleoside triphosphate]]. It is one of the building blocks needed for the synthesis of [[RNA]] during the [[transcription (genetics)|transcription]] process. Its structure is similar to that of the [[guanosine]] [[nucleoside]], the only difference being that [[nucleotide]]s like GTP have [[phosphate]]s on their [[ribose]] sugar. GTP has the [[guanine]] [[nucleobase]] attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon. |
||
It also has the role of a source of energy or an activator of substrates in metabolic reactions, like that of [[adenosine triphosphate|ATP]], but more specific. It is used as a source of energy for [[protein biosynthesis|protein synthesis]] and [[gluconeogenesis]]. |
It also has the role of a source of energy or an activator of substrates in metabolic reactions, like that of [[adenosine triphosphate|ATP]], but more specific. It is used as a source of energy for [[protein biosynthesis|protein synthesis]] and [[gluconeogenesis]]. |
||
| Line 60: | Line 66: | ||
===Microtubule dynamic instability=== |
===Microtubule dynamic instability=== |
||
During [[microtubule]] polymerization, each heterodimer formed by an alpha and a beta tubulin molecule carries two GTP molecules, and the GTP is hydrolyzed to GDP when the tubulin dimers are added to the plus end of the growing microtubule. Such GTP hydrolysis is not mandatory for microtubule formation, but it appears that only GDP-bound tubulin molecules are able to depolymerize. Thus, a GTP-bound tubulin serves as a cap at the tip of microtubule to protect from depolymerization; and, once the GTP is hydrolyzed, the microtubule begins to depolymerize and shrink rapidly.<ref>{{cite web | url = http://www.cytochemistry.net/Cell-biology/microtubule_structure.htm | title = Microtubule structure | author = Gwen V. Childs | publisher = cytochemistry.net | url-status = dead | |
During [[microtubule]] polymerization, each heterodimer formed by an alpha and a beta tubulin molecule carries two GTP molecules, and the GTP is hydrolyzed to GDP when the tubulin dimers are added to the plus end of the growing microtubule. Such GTP hydrolysis is not mandatory for microtubule formation, but it appears that only GDP-bound tubulin molecules are able to depolymerize. Thus, a GTP-bound tubulin serves as a cap at the tip of microtubule to protect from depolymerization; and, once the GTP is hydrolyzed, the microtubule begins to depolymerize and shrink rapidly.<ref>{{cite web | url = http://www.cytochemistry.net/Cell-biology/microtubule_structure.htm | title = Microtubule structure | author = Gwen V. Childs | publisher = cytochemistry.net | url-status = dead | archive-url = https://web.archive.org/web/20100215173527/http://www.cytochemistry.net/Cell-biology/microtubule_structure.htm | archive-date = 2010-02-15 }}</ref> |
||
===Mitochondrial function=== |
===Mitochondrial function=== |
||
The translocation of proteins into the mitochondrial matrix involves the interactions of both GTP and ATP. The importing of these proteins plays an important role in several pathways regulated within the mitochondria organelle,<ref>{{cite journal|last=Sepuri|first=Naresh Babu V. |author2=Norbert Schülke |author3=Debkumar Pain|title=GTP Hydrolysis Is Essential for Protein Import into the Mitochondrial Matrix|journal=Journal of Biological Chemistry|date=16 January 1998| |
The translocation of proteins into the mitochondrial matrix involves the interactions of both GTP and ATP. The importing of these proteins plays an important role in several pathways regulated within the mitochondria organelle,<ref>{{cite journal|last=Sepuri|first=Naresh Babu V. |author2=Norbert Schülke |author3=Debkumar Pain|title=GTP Hydrolysis Is Essential for Protein Import into the Mitochondrial Matrix|journal=Journal of Biological Chemistry|date=16 January 1998|volume=273 |issue=3|pages=1420–1424|doi=10.1074/jbc.273.3.1420|pmid=9430677 |doi-access=free}}</ref> such as converting [[oxaloacetate]] to [[phosphoenolpyruvate]] (PEP) in gluconeogenesis.{{fact|date=September 2017}} |
||
==Precursor for synthesis of riboflavin== |
|||
GTP, in combination with [[ribulose 5-phosphate]], are the precursor compounds for the synthesis of [[riboflavin]] (vitamin B<sub>2</sub>).<ref name=PKIN2020B2>{{cite book |vauthors=Merrill AH, McCormick DB |title = Present Knowledge in Nutrition, Eleventh Edition |chapter = Riboflavin |editor=BP Marriott |editor2=DF Birt |editor3=VA Stallings|editor4=AA Yates |publisher = Academic Press (Elsevier) |year=2020 |location = London, United Kingdom |pages = 189–208 |isbn=978-0-323-66162-1}}</ref> |
|||
==Biosynthesis== |
==Biosynthesis== |
||
| Line 70: | Line 79: | ||
* as a byproduct of the [[Succinyl-CoA]] to [[Succinate]] conversion catalysed by the [[Succinyl-CoA synthetase]] enzyme as part of the [[Krebs cycle]];<ref name=[[Stryer]] /> |
* as a byproduct of the [[Succinyl-CoA]] to [[Succinate]] conversion catalysed by the [[Succinyl-CoA synthetase]] enzyme as part of the [[Krebs cycle]];<ref name=[[Stryer]] /> |
||
* through exchanges of phosphate groups from ATP molecules by the [[Nucleoside-diphosphate kinase]], an enzyme tasked with maintaining an equilibrium between the concentrations of different nucleoside triphosphates.<ref name=[[Stryer]] /> |
* through exchanges of phosphate groups from ATP molecules by the [[Nucleoside-diphosphate kinase]], an enzyme tasked with maintaining an equilibrium between the concentrations of different nucleoside triphosphates.<ref name=[[Stryer]] /> |
||
==cGTP<!-- I was trying to find this cGTP information anywhere (NCBI, cited as [5] book); could not find such a thing like cGTP. Wasn't it intended to be trimeric G protein? Olfactory sensing is based on G protein => cAMP pathway, according to book [5]. -->== |
|||
'''Cyclic guanosine triphosphate''' (cGTP) helps [[cyclic adenosine monophosphate]] (cAMP) activate [[cyclic nucleotide-gated ion channel]]s in the [[olfactory system]].<ref name=boron>{{cite book | title = Medical Physiology | author = Boron & Boulpaep | isbn = 1-4160-2328-3 | publisher = Elsevier Saunders | year = 2005 | edition = Updated | page = 90}}</ref> |
|||
==See also== |
==See also== |
||
Latest revision as of 22:54, 16 December 2024
| |
| |
| Names | |
|---|---|
| IUPAC name
Guanosine 5′-(tetrahydrogen triphosphate)
| |
| Systematic IUPAC name
O1-{[(2R,3S,4R,5R)-5-(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl} tetrahydrogen triphosphate | |
| Other names
guanosine triphosphate, 9-β-D-ribofuranosylguanine-5'-triphosphate, 9-β-D-ribofuranosyl-2-amino-6-oxo-purine-5'-triphosphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.498 |
| KEGG | |
| MeSH | Guanosine+triphosphate |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H16N5O14P3 | |
| Molar mass | 523.180 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon.
It also has the role of a source of energy or an activator of substrates in metabolic reactions, like that of ATP, but more specific. It is used as a source of energy for protein synthesis and gluconeogenesis.
GTP is essential to signal transduction, in particular with G-proteins, in second-messenger mechanisms where it is converted to guanosine diphosphate (GDP) through the action of GTPases.
Uses
[edit]Energy transfer
[edit]GTP is involved in energy transfer within the cell. For instance, a GTP molecule is generated by one of the enzymes in the citric acid cycle. This is tantamount to the generation of one molecule of ATP, since GTP is readily converted to ATP with nucleoside-diphosphate kinase (NDK).[1]
Genetic translation
[edit]During the elongation stage of translation, GTP is used as an energy source for the binding of a new amino-bound tRNA to the A site of the ribosome. GTP is also used as an energy source for the translocation of the ribosome towards the 3' end of the mRNA.[2]
Microtubule dynamic instability
[edit]During microtubule polymerization, each heterodimer formed by an alpha and a beta tubulin molecule carries two GTP molecules, and the GTP is hydrolyzed to GDP when the tubulin dimers are added to the plus end of the growing microtubule. Such GTP hydrolysis is not mandatory for microtubule formation, but it appears that only GDP-bound tubulin molecules are able to depolymerize. Thus, a GTP-bound tubulin serves as a cap at the tip of microtubule to protect from depolymerization; and, once the GTP is hydrolyzed, the microtubule begins to depolymerize and shrink rapidly.[3]
Mitochondrial function
[edit]The translocation of proteins into the mitochondrial matrix involves the interactions of both GTP and ATP. The importing of these proteins plays an important role in several pathways regulated within the mitochondria organelle,[4] such as converting oxaloacetate to phosphoenolpyruvate (PEP) in gluconeogenesis.[citation needed]
Precursor for synthesis of riboflavin
[edit]GTP, in combination with ribulose 5-phosphate, are the precursor compounds for the synthesis of riboflavin (vitamin B2).[5]
Biosynthesis
[edit]In the cell, GTP is synthesised through many processes including:
- as a byproduct of the Succinyl-CoA to Succinate conversion catalysed by the Succinyl-CoA synthetase enzyme as part of the Krebs cycle;[1]
- through exchanges of phosphate groups from ATP molecules by the Nucleoside-diphosphate kinase, an enzyme tasked with maintaining an equilibrium between the concentrations of different nucleoside triphosphates.[1]
See also
[edit]References
[edit]- ^ a b c Berg, JM; JL Tymoczko; L Stryer (2002). Biochemistry (5th ed.). WH Freeman and Company. pp. 476. ISBN 0-7167-4684-0.
- ^ Solomon, EP; LR Berg; DW Martin (2005). Biology (7th ed.). pp. 244–245.
- ^ Gwen V. Childs. "Microtubule structure". cytochemistry.net. Archived from the original on 2010-02-15.
- ^ Sepuri, Naresh Babu V.; Norbert Schülke; Debkumar Pain (16 January 1998). "GTP Hydrolysis Is Essential for Protein Import into the Mitochondrial Matrix". Journal of Biological Chemistry. 273 (3): 1420–1424. doi:10.1074/jbc.273.3.1420. PMID 9430677.
- ^ Merrill AH, McCormick DB (2020). "Riboflavin". In BP Marriott, DF Birt, VA Stallings, AA Yates (eds.). Present Knowledge in Nutrition, Eleventh Edition. London, United Kingdom: Academic Press (Elsevier). pp. 189–208. ISBN 978-0-323-66162-1.
External links
[edit]- GTP bound to proteins in the PDB
