Phenibut: Difference between revisions
Gettinglit (talk | contribs) |
Rescuing 2 sources and tagging 0 as dead.) #IABot (v2.0.9.5 |
||
| (43 intermediate revisions by 17 users not shown) | |||
| Line 2: | Line 2: | ||
{{Use dmy dates|date=October 2020}} |
{{Use dmy dates|date=October 2020}} |
||
{{Infobox drug |
{{Infobox drug |
||
| Verifiedfields |
| Verifiedfields = verified |
||
| Watchedfields |
| Watchedfields = verified |
||
| verifiedrevid |
| verifiedrevid = 464373947 |
||
| IUPAC_name |
| IUPAC_name = 4-Amino-3-phenylbutanoic acid |
||
| image |
| image = Phenibut skeletal formula.svg |
||
| width |
| width = 200px |
||
| image2 |
| image2 = Phenibut ball-and-stick model.png |
||
| width2 |
| width2 = 200px |
||
<!--Clinical data-->| tradename |
<!--Clinical data-->| tradename = Anvifen, Fenibut, Noofen, others<ref name="DrobizhevFedotova2016">{{cite journal | vauthors = Drobizhev MY, Fedotova AV, Kikta SV, Antohin EY | year = 2016 | title = Феномен аминофенилмасляной кислоты | trans-title = Phenomenon of aminophenylbutyric acid | url = https://www.rmj.ru/articles/nevrologiya/Fenomen_aminofenilmaslyanoy_kisloty/ | language = Russian | journal = Russian Medical Journal | volume = 2017 | issue = 24 | pages = 1657–1663 | issn = 1382-4368 | access-date = 16 September 2017 | archive-date = 16 September 2017 | archive-url = https://web.archive.org/web/20170916053610/https://www.rmj.ru/articles/nevrologiya/Fenomen_aminofenilmaslyanoy_kisloty/ | url-status = live }}</ref> |
||
| pregnancy_AU |
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
||
| pregnancy_US |
| pregnancy_US = <!-- A / B / C / D / X --> |
||
| dependency_liability = |
| dependency_liability = |
||
| addiction_liability = |
| addiction_liability = |
||
| legal_AU |
| legal_AU = S4 |
||
| legal_CA |
| legal_CA = Unscheduled <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> |
||
| legal_BR |
| legal_BR = B1 |
||
| legal_DE |
| legal_DE = NPSG |
||
| legal_US |
| legal_US = Unapproved drug; use in [[dietary supplement]]s, [[food]], or [[medicine]] is unlawful.<ref>{{Cite journal |last=Nutrition |first=Center for Food Safety and Applied |date=2023-03-06 |title=Phenibut in Dietary Supplements |url=https://www.fda.gov/food/dietary-supplement-ingredient-directory/phenibut-dietary-supplements |journal=FDA |language=en |access-date=23 May 2023 |archive-date=23 May 2023 |archive-url=https://web.archive.org/web/20230523031247/https://www.fda.gov/food/dietary-supplement-ingredient-directory/phenibut-dietary-supplements |url-status=live }}</ref> |
||
| ⚫ | |||
Illegal in [[Alabama]]<ref name=APARX>{{cite web | title = HB2, Holmes, Tianeptine and Phenibut added to Schdule I Conrolled Substances | publisher = Alabama Pharmacy Association | url = https://www.aparx.org/page/24|archive-url=https://web.archive.org/web/20210601122529/https://www.aparx.org/page/24 | archive-date=2021-06-01 | url-status=dead}}</ref> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<!--Pharmacokinetic data-->| bioavailability = |
<!--Pharmacokinetic data-->| bioavailability = ≥63% (250 mg)<ref name="pmid11830761" /> |
||
| protein_bound = |
|||
| metabolism = [[Liver]] (minimal)<ref name="Phenibut-Label">{{citation | author = Ozon Pharm | title = Fenibut | access-date = 15 September 2017 | url = http://www.ozonpharm.ru/upload/iblock/608/nmntxzabdzjhlu%20-%20fbdoqpbtdj.ofzsxp%20tkbgeygfzj.pdf | archive-url = https://web.archive.org/web/20170916094855/http://www.ozonpharm.ru/upload/iblock/608/nmntxzabdzjhlu%20-%20fbdoqpbtdj.ofzsxp%20tkbgeygfzj.pdf | archive-date = 16 September 2017 | url-status = dead }}</ref><ref name="pmid11830761" /> |
|||
| ⚫ | |||
| protein_bound = |
|||
| metabolism = [[Liver]] (minimal)<ref name="Phenibut-Label" /><ref name="pmid11830761" /> |
|||
| ⚫ | |||
| elimination_half-life = 5.3 hours (250 mg)<ref name="pmid11830761" /> |
| elimination_half-life = 5.3 hours (250 mg)<ref name="pmid11830761" /> |
||
| onset |
| onset = [[Oral administration|Oral]]: 2–4 hours<ref name="pmid26693960" /><br />[[Rectal administration|Rectal]]: 20–30 minutes<ref name="pmid26693960" /> |
||
| duration_of_action = 15–24 hours (1–3 g)<ref name="pmid26693960" /> |
| duration_of_action = 15–24 hours (1–3 g)<ref name="pmid26693960" /> |
||
| excretion |
| excretion = [[Urine]]: 63% (unchanged)<ref name="pmid11830761" /> |
||
<!--Identifiers-->| CAS_number_Ref |
<!--Identifiers-->| CAS_number_Ref = {{cascite|correct|CAS}} |
||
| CAS_number |
| CAS_number = 1078-21-3 |
||
| CAS_supplemental |
| CAS_supplemental = <br />3060-41-1 ([[hydrochloride]]) |
||
| UNII_Ref |
| UNII_Ref = {{fdacite|changed|FDA}} |
||
| UNII |
| UNII = T2M58D6LA8 |
||
| ATC_prefix |
| ATC_prefix = N06 |
||
| ATC_suffix |
| ATC_suffix = BX22 |
||
| PubChem |
| PubChem = 14113 |
||
| DrugBank_Ref |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
||
| ChemSpiderID_Ref |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| ChemSpiderID |
| ChemSpiderID = 13491 |
||
| ChEMBL_Ref |
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
||
| ChEMBL |
| ChEMBL = 315818 |
||
| KEGG_Ref |
| KEGG_Ref = {{keggcite|correct|kegg}} |
||
| KEGG |
| KEGG = D10509 |
||
| synonyms |
| synonyms = Aminophenylbutyric acid; Fenibut; Fenigam; Phenigam; Phenybut; Phenygam; Phenylgamma; Phenigama; PHG; PhGABA; β-Phenyl-γ-aminobutyric acid; β-Phenyl-GABA<ref name="Elks2014">{{cite book| vauthors = Elks J |title=The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies|url=https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA69|date=14 November 2014|publisher=Springer|isbn=978-1-4757-2085-3|pages=69–}}</ref> |
||
<!--Chemical data-->| C |
<!--Chemical data-->| C = 10 |
||
| H |
| H = 13 |
||
| N |
| N = 1 |
||
| O |
| O = 2 |
||
| SMILES |
| SMILES = O=C(O)CC(c1ccccc1)CN |
||
| StdInChI_Ref |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChI |
| StdInChI = 1S/C10H13NO2/c11-7-9(6-10(12)13)8-4-2-1-3-5-8/h1-5,9H,6-7,11H2,(H,12,13) |
||
| StdInChIKey_Ref |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChIKey |
| StdInChIKey = DAFOCGYVTAOKAJ-UHFFFAOYSA-N |
||
<!--Physical data-->| melting_point |
<!--Physical data-->| melting_point = 253 |
||
}} |
}} |
||
<!-- Definition and medical uses --> |
<!-- Definition and medical uses --> |
||
'''Phenibut''', sold under the brand |
'''Phenibut''', sold under the brand name '''Anvifen''' among others,<ref name="DrobizhevFedotova2016" /> is a [[central nervous system]] (CNS) [[depressant]] with [[anxiolytic]] effects, and is used to treat [[anxiety]], [[insomnia]], and for a variety of other indications.<ref name="pmid11830761">{{cite journal | vauthors = Lapin I | title = Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug | journal = CNS Drug Reviews | volume = 7 | issue = 4 | pages = 471–81 | year = 2001 | pmid = 11830761 | pmc = 6494145 | doi = 10.1111/j.1527-3458.2001.tb00211.x }}</ref> It is usually taken [[oral administration|orally]] (swallowed by mouth), but may be given [[intravenously]].<ref name="Phenibut-Label" /><ref name="pmid11830761" /> |
||
<!-- Side effects and mechanism --> |
<!-- Side effects and mechanism --> |
||
Side effects of phenibut can include [[sedation]], [[sleepiness]], [[nausea]], [[irritability]], [[psychomotor agitation|agitation]], [[dizziness]], [[euphoria]] and sometimes [[headache]], among others.<ref name="Phenibut-Label" /><ref name="RLS-Phenibut" /> [[Overdose]] of phenibut can produce marked central nervous system depression including [[unconsciousness]].<ref name="Phenibut-Label" /><ref name="RLS-Phenibut" /> The medication is structurally related to the [[neurotransmitter]] [[γ-aminobutyric acid]] (GABA), and hence is a [[GABA analogue]].<ref name="pmid11830761" /> Phenibut is thought to act as a [[GABAB receptor|GABA<sub>B</sub> receptor]] [[agonist]], similarly to [[baclofen]] and [[γ-hydroxybutyrate]] (GHB).<ref name="pmid11830761" /> However, at low concentrations, phenibut mildly increases the concentration of [[dopamine]] in the brain, providing [[Stimulant|stimulatory]] effects in addition to the [[Anxiolytic|anxiolysis]].<ref>{{cite journal | vauthors = Lapin I | title = Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug | journal = CNS Drug Reviews | volume = 7 | issue = 4 | pages = 471–81 | date = 2006-06-07 | pmid = 11830761 | pmc = 6494145 | doi = 10.1111/j.1527-3458.2001.tb00211.x }}</ref> |
Side effects of phenibut can include [[sedation]], [[sleepiness]], [[nausea]], [[irritability]], [[psychomotor agitation|agitation]], [[dizziness]], [[euphoria]], and sometimes [[headache]], among others.<ref name="Phenibut-Label" /><ref name="RLS-Phenibut" /> [[Overdose]] of phenibut can produce marked central nervous system depression including [[unconsciousness]].<ref name="Phenibut-Label" /><ref name="RLS-Phenibut" /> The medication is structurally related to the [[neurotransmitter]] [[γ-aminobutyric acid]] (GABA), and hence is a [[GABA analogue]].<ref name="pmid11830761" /> Phenibut is thought to act as a [[GABAB receptor|GABA<sub>B</sub> receptor]] [[agonist]], similarly to [[baclofen]] and [[γ-hydroxybutyrate]] (GHB).<ref name="pmid11830761" /> However, at low concentrations, phenibut mildly increases the concentration of [[dopamine]] in the brain, providing [[Stimulant|stimulatory]] effects in addition to the [[Anxiolytic|anxiolysis]].<ref>{{cite journal | vauthors = Lapin I | title = Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug | journal = CNS Drug Reviews | volume = 7 | issue = 4 | pages = 471–81 | date = 2006-06-07 | pmid = 11830761 | pmc = 6494145 | doi = 10.1111/j.1527-3458.2001.tb00211.x }}</ref> |
||
<!-- History, society, and culture --> |
<!-- History, society, and culture --> |
||
Phenibut was developed in the [[Soviet Union]] and was introduced for medical use in the 1960s.<ref name="pmid11830761" /> Today, it is marketed for medical use in Russia, Ukraine, Belarus, Kazakhstan, and Latvia.<ref name="pmid11830761" /> The medication is not approved for clinical use in the United States and most of Europe, but it is sold on the Internet as a [[dietary supplement|supplement]] and purported [[nootropic]].<ref name="pmid26693960" /><ref name="ClinicalTox">{{cite journal | vauthors = Cohen PA, Ellison RR, Travis JC, Gaufberg SV, Gerona R | title = Quantity of phenibut in dietary supplements before and after FDA warnings | journal = Clinical Toxicology | volume = 60 | issue = 4 | pages = 486–488 | date = April 2022 | pmid = 34550038 | doi = 10.1080/15563650.2021.1973020 | s2cid = 237594860 }}</ref> Phenibut has been used [[recreational drug|recreationally]] and can produce [[euphoria]] as well as [[drug addiction|addiction]], [[drug dependence|dependence]], and [[drug withdrawal|withdrawal]].<ref name="pmid26693960" /> It is a [[controlled substance]] in Australia, and it has been suggested that its legal status should be reconsidered in Europe as well.<ref name="pmid26693960" /> In Germany, phenibut is not approved as a drug and, as a food supplement, is controlled under the German New Psychoactive Substances Act.<ref>{{Cite web |title=Anlage NpSG - Einzelnorm |url=https://www.gesetze-im-internet.de/npsg/anlage.html |access-date=2024-06-07 |website=www.gesetze-im-internet.de}}</ref> |
Phenibut was developed in the [[Soviet Union]] and was introduced for medical use in the 1960s.<ref name="pmid11830761" /> Today, it is marketed for medical use in Russia, Ukraine, Belarus, Kazakhstan, and Latvia.<ref name="pmid11830761" /> The medication is not approved for clinical use in the United States and most of Europe, but it is sold on the Internet as a [[dietary supplement|supplement]] and purported [[nootropic]].<ref name="pmid26693960" /><ref name="ClinicalTox">{{cite journal | vauthors = Cohen PA, Ellison RR, Travis JC, Gaufberg SV, Gerona R | title = Quantity of phenibut in dietary supplements before and after FDA warnings | journal = Clinical Toxicology | volume = 60 | issue = 4 | pages = 486–488 | date = April 2022 | pmid = 34550038 | doi = 10.1080/15563650.2021.1973020 | s2cid = 237594860 }}</ref> Phenibut has been used [[recreational drug|recreationally]] and can produce [[euphoria]] as well as [[drug addiction|addiction]], [[drug dependence|dependence]], and [[drug withdrawal|withdrawal]].<ref name="pmid26693960" /> It is a [[controlled substance]] in Australia, and it has been suggested that its legal status should be reconsidered in Europe as well.<ref name="pmid26693960" /> In Germany, phenibut is not approved as a drug and, as a food supplement, is controlled under the German New Psychoactive Substances Act.<ref name="Anlage NpSG - Einzelnorm">{{Cite web |title=Anlage NpSG - Einzelnorm |url=https://www.gesetze-im-internet.de/npsg/anlage.html |access-date=2024-06-07 |website=www.gesetze-im-internet.de}}</ref> |
||
In a 2023 assessment, the U.S. [[Food and Drug Administration]] (FDA) determined that phenibut does not meet the definition of a dietary ingredient, thereby making phenibut supplement products misbranded and illegal for marketing.<ref name="fda-23">{{cite web |title=Phenibut in Dietary Supplements |url=https://www.fda.gov/food/information-select-dietary-supplement-ingredients-and-other-substances/phenibut-dietary-supplements |publisher=US Food and Drug Administration |access-date=14 September 2024 |date=12 July 2023}}</ref> [[FDA warning letter]]s had been issued to supplement manufacturers marketing phenibut products as [[Adulterant|adulterated]].<ref name="fda-19">{{cite web |title=FDA Acts on Dietary Supplements Containing DMHA and Phenibut |url=https://www.fda.gov/food/cfsan-constituent-updates/fda-acts-dietary-supplements-containing-dmha-and-phenibut |publisher=US Food and Drug Administration |access-date=14 September 2024 |date=29 April 2019 |archive-date=14 June 2022 |archive-url=https://web.archive.org/web/20220614224615/https://www.fda.gov/food/cfsan-constituent-updates/fda-acts-dietary-supplements-containing-dmha-and-phenibut |url-status=live }}</ref> |
|||
==Medical uses== |
==Medical uses== |
||
| Line 92: | Line 93: | ||
==Side effects== |
==Side effects== |
||
Phenibut is generally well-[[tolerability|tolerated]].<ref name="pmid11830761" /><ref name="RLS-Phenibut" /> Possible [[side effect]]s may include [[sedation]], [[somnolence]], [[nausea]], [[irritability]], [[psychomotor agitation|agitation]], [[anxiety]], [[dizziness]], [[headache]], and [[allergic reaction]]s such as [[skin rash]] and [[itching]].<ref name="Phenibut-Label" /><ref name="RLS-Phenibut" /> At high doses, [[motor incoordination]], [[loss of balance]], and [[hangover]]s may occur.<ref name="pmid26693960" /> Due to its |
Phenibut is generally well-[[tolerability|tolerated]].<ref name="pmid11830761" /><ref name="RLS-Phenibut" /> Possible [[side effect]]s may include [[sedation]], [[somnolence]], [[nausea]], [[irritability]], [[psychomotor agitation|agitation]], [[anxiety]], [[dizziness]], [[headache]], and [[allergic reaction]]s such as [[skin rash]] and [[itching]].<ref name="Phenibut-Label" /><ref name="RLS-Phenibut" /> At high doses, [[motor incoordination]], [[loss of balance]], and [[hangover]]s may occur.<ref name="pmid26693960" /> Due to its CNS depressant effects, people taking phenibut should refrain from potentially dangerous activities such as operating heavy machinery.<ref name="Phenibut-Label" /><ref name="RLS-Phenibut" /> With prolonged use of phenibut, particularly at high doses, the [[liver]] and [[blood]] should be [[medical monitoring|monitored]], due to risk of [[fatty liver disease]] and [[eosinophilia]].<ref name="Phenibut-Label" /><ref name="RLS-Phenibut" /> |
||
==Overdose== |
==Overdose== |
||
| Line 102: | Line 103: | ||
== Interactions == |
== Interactions == |
||
Phenibut may mutually potentiate and extend the duration of the effects of other |
Phenibut may mutually potentiate and extend the duration of the effects of other CNS depressants, including [[anxiolytic]]s, [[antipsychotic]]s, [[sedative]]s, [[opioid]]s, [[anticonvulsant]]s, and [[alcohol (drug)|alcohol]].<ref name="Phenibut-Label" /><ref name="RLS-Phenibut" /> |
||
==Pharmacology== |
==Pharmacology== |
||
| Line 133: | Line 134: | ||
|} |
|} |
||
Phenibut acts as a [[full agonist]] of the [[GABAB receptor|GABA<sub>B</sub> receptor]], similarly to [[baclofen]].<ref name="AcademicPress2010">{{cite book|title=GABAb Receptor Pharmacology: A Tribute to Norman Bowery: A Tribute to Norman Bowery|url=https://books.google.com/books?id=_iMDQOA2UIsC&pg=PA25|date=21 September 2010|publisher=Academic Press|isbn=978-0-12-378648-7|pages=25–}}</ref><ref name="pmid18275958">{{cite journal | vauthors = Dambrova M, Zvejniece L, Liepinsh E, Cirule H, Zharkova O, Veinberg G, Kalvinsh I | title = Comparative pharmacological activity of optical isomers of phenibut | journal = European Journal of Pharmacology | volume = 583 | issue = 1 | pages = 128–134 | date = March 2008 | pmid = 18275958 | doi = 10.1016/j.ejphar.2008.01.015 }}</ref> It has between 30- and 68-fold lower [[affinity (pharmacology)|affinity]] for the GABA<sub>B</sub> receptor than baclofen, and, in accordance, is used at far higher doses in comparison.<ref name="AcademicPress2010" /> (''R'')-Phenibut has more than 100-fold higher affinity for the GABA<sub>B</sub> receptor than does (''S'')-phenibut; hence, (''R'')-phenibut is the active enantiomer at the GABA<sub>B</sub> receptor.<ref name="AllanBates1990">{{cite journal | vauthors = Allan RD, Bates MC, Drew CA, Duke RK, Hambley TW, Johnston GA, Mewett KN, Spence I | display-authors= 6 |title=A new synthesis resolution and in vitro activities of (R)- and (S)-β-Phenyl-Gaba|journal=Tetrahedron|volume=46|issue=7|year=1990|pages=2511–2524|issn=0040-4020|doi=10.1016/S0040-4020(01)82032-9}}</ref> |
Phenibut acts as a [[full agonist]] of the [[GABAB receptor|GABA<sub>B</sub> receptor]], similarly to [[baclofen]].<ref name="AcademicPress2010">{{cite book|title=GABAb Receptor Pharmacology: A Tribute to Norman Bowery: A Tribute to Norman Bowery|url=https://books.google.com/books?id=_iMDQOA2UIsC&pg=PA25|date=21 September 2010|publisher=Academic Press|isbn=978-0-12-378648-7|pages=25–}}</ref><ref name="pmid18275958">{{cite journal | vauthors = Dambrova M, Zvejniece L, Liepinsh E, Cirule H, Zharkova O, Veinberg G, Kalvinsh I | title = Comparative pharmacological activity of optical isomers of phenibut | journal = European Journal of Pharmacology | volume = 583 | issue = 1 | pages = 128–134 | date = March 2008 | pmid = 18275958 | doi = 10.1016/j.ejphar.2008.01.015 }}</ref> It has between 30- and 68-fold lower [[affinity (pharmacology)|affinity]] for the GABA<sub>B</sub> receptor than baclofen, and, in accordance, is used at far higher doses in comparison.<ref name="AcademicPress2010" /> (''R'')-Phenibut has more than 100-fold higher affinity for the GABA<sub>B</sub> receptor than does (''S'')-phenibut; hence, (''R'')-phenibut is the active enantiomer at the GABA<sub>B</sub> receptor.<ref name="AllanBates1990">{{cite journal | vauthors = Allan RD, Bates MC, Drew CA, Duke RK, Hambley TW, Johnston GA, Mewett KN, Spence I | display-authors= 6 |title=A new synthesis resolution and in vitro activities of (R)- and (S)-β-Phenyl-Gaba|journal=Tetrahedron|volume=46|issue=7|year=1990|pages=2511–2524|issn=0040-4020|doi=10.1016/S0040-4020(01)82032-9}}</ref> |
||
{| class="wikitable floatright" |
{| class="wikitable floatright" |
||
| Line 156: | Line 157: | ||
Phenibut also binds to and blocks [[Voltage-dependent calcium channel#α2δ Subunit|α<sub>2</sub>δ subunit]]-containing VDCCs, similarly to [[gabapentin]] and [[pregabalin]], and hence is a [[gabapentinoid]].<ref name="pmid26234470" /><ref name="pmid26621244">{{cite journal | vauthors = Vavers E, Zvejniece L, Svalbe B, Volska K, Makarova E, Liepinsh E, Rizhanova K, Liepins V, Dambrova M | display-authors = 6 | title = The neuroprotective effects of R-phenibut after focal cerebral ischemia | journal = Pharmacological Research | volume = 113 | issue = Pt B | pages = 796–801 | date = November 2016 | pmid = 26621244 | doi = 10.1016/j.phrs.2015.11.013 }}</ref> Both (''R'')-phenibut and (''S'')-phenibut display this action with similar [[affinity (pharmacology)|affinity]] (K<sub>i</sub> = 23 and 39 μM, respectively).<ref name="pmid26234470" /> |
Phenibut also binds to and blocks [[Voltage-dependent calcium channel#α2δ Subunit|α<sub>2</sub>δ subunit]]-containing VDCCs, similarly to [[gabapentin]] and [[pregabalin]], and hence is a [[gabapentinoid]].<ref name="pmid26234470" /><ref name="pmid26621244">{{cite journal | vauthors = Vavers E, Zvejniece L, Svalbe B, Volska K, Makarova E, Liepinsh E, Rizhanova K, Liepins V, Dambrova M | display-authors = 6 | title = The neuroprotective effects of R-phenibut after focal cerebral ischemia | journal = Pharmacological Research | volume = 113 | issue = Pt B | pages = 796–801 | date = November 2016 | pmid = 26621244 | doi = 10.1016/j.phrs.2015.11.013 }}</ref> Both (''R'')-phenibut and (''S'')-phenibut display this action with similar [[affinity (pharmacology)|affinity]] (K<sub>i</sub> = 23 and 39 μM, respectively).<ref name="pmid26234470" /> |
||
It is often claimed on websites about [[nootropic]]s and elsewhere on the internet that phenibut increases dopamine. Three papers published in Russian by Soviet scientists in 1979, 1986, and 1990 report that phenibut increases dopamine in the striatum of rats and in the mouse brain.<ref name="dopaminw">{{cite journal |last1=Lapin |first1=Izyaslav |title=Phenibut ( |
It is often claimed on websites about [[nootropic]]s and elsewhere on the internet that phenibut increases dopamine. Three papers published in Russian by Soviet scientists in 1979, 1986, and 1990 report that phenibut increases dopamine in the striatum of rats and in the mouse brain.<ref name="dopaminw">{{cite journal |last1=Lapin |first1=Izyaslav |title=Phenibut (β-Phenyl-GABA): A Tranquilizer and Nootropic Drug |journal=CNS Drug Reviews |date=December 2001 |volume=7 |issue=4 |pages=471–481 |doi=10.1111/j.1527-3458.2001.tb00211.x|pmid=11830761 |pmc=6494145 }}</ref> The mechanism underlying this putative effect is unclear.<ref name="dopaminw"/> Structurally, phenibut can also be considered a derivative of [[phenethylamine]], and some research suggests that phenibut antagonizes the action of phenethylamine.<ref name="dopaminw"/> |
||
===Pharmacokinetics=== |
===Pharmacokinetics=== |
||
| Line 169: | Line 170: | ||
[[File:Phenibut and analogues.png|thumb|right|350px|Chemical structures of phenibut and analogues.]] |
[[File:Phenibut and analogues.png|thumb|right|350px|Chemical structures of phenibut and analogues.]] |
||
Phenibut is a [[chemical derivative|derivative]] of the [[inhibitory postsynaptic potential|inhibitory]] neurotransmitter GABA.<ref name="pmid11830761" /> Hence, it is a [[GABA analogue]].<ref name="pmid11830761" /> Phenibut is specifically the [[structural analog|analogue]] of GABA with a [[phenyl ring]] substituted in at the β-position.<ref name="pmid11830761" /> As such, its chemical name is β-phenyl-γ-aminobutyric acid, which can be abbreviated as β-phenyl-GABA.<ref name="pmid11830761" /> The presence of the phenyl ring allows phenibut to cross the [[blood–brain barrier]] significantly, unlike |
Phenibut is a [[chemical derivative|derivative]] of the [[inhibitory postsynaptic potential|inhibitory]] neurotransmitter [[GABA]].<ref name="pmid11830761" /> Hence, it is a [[GABA analogue]].<ref name="pmid11830761" /> Phenibut is specifically the [[structural analog|analogue]] of GABA with a [[phenyl ring]] substituted in at the β-position.<ref name="pmid11830761" /> As such, its chemical name is β-phenyl-γ-aminobutyric acid, which can be abbreviated as β-phenyl-GABA.<ref name="pmid11830761" /> The presence of the phenyl ring allows phenibut to cross the [[blood–brain barrier]] significantly, unlike GABA.<ref name="pmid11830761" /> Phenibut also contains the [[trace amine]] [[β-phenethylamine]] in its [[chemical structure|structure]].<ref name="pmid11830761" /> |
||
Phenibut is closely related to a variety of other GABA analogues including [[baclofen]] (β-(4-chlorophenyl)-GABA), [[4-fluorophenibut]] (β-(4-fluorophenyl)-GABA), [[tolibut]] (β-(4-methylphenyl)-GABA), [[pregabalin]] ((''S'')-β-isobutyl-GABA), [[gabapentin]] (1-(aminomethyl)cyclohexane acetic acid), and [[GABOB]] (β-hydroxy-GABA).<ref name="pmid11830761" /><ref name="pmid26234470" /> It has almost the same chemical structure as baclofen, differing from it only in having a [[hydrogen]] atom instead of a [[chlorine]] atom at the ''para'' position of the phenyl ring.<ref name="pmid11830761" /> Phenibut is also close in structure to pregabalin, which has an [[isobutane|isobutyl]] group at the β position instead of phenibut's phenyl ring.<ref name="pmid26234470" /> |
Phenibut is closely related to a variety of other GABA analogues including [[baclofen]] (β-(4-chlorophenyl)-GABA), [[4-fluorophenibut]] (β-(4-fluorophenyl)-GABA), [[tolibut]] (β-(4-methylphenyl)-GABA), [[pregabalin]] ((''S'')-β-isobutyl-GABA), [[gabapentin]] (1-(aminomethyl)cyclohexane acetic acid), and [[GABOB]] (β-hydroxy-GABA).<ref name="pmid11830761" /><ref name="pmid26234470" /> It has almost the same chemical structure as baclofen, differing from it only in having a [[hydrogen]] atom instead of a [[chlorine]] atom at the ''para'' position of the phenyl ring.<ref name="pmid11830761" /> Phenibut is also close in structure to pregabalin, which has an [[isobutane|isobutyl]] group at the β position instead of phenibut's phenyl ring.<ref name="pmid26234470" /> |
||
| Line 188: | Line 189: | ||
===Brand names=== |
===Brand names=== |
||
Phenibut is marketed in [[Russia]], [[Ukraine]], [[Belarus]] and [[Latvia]] under the brand names Anvifen, Fenibut, Bifren and Noofen ([[Russian language|Russian]]: Анвифен, Фенибут, Бифрен and Ноофен, respectively).<ref name="DrobizhevFedotova2016" /> |
Phenibut is marketed in [[Russia]], [[Ukraine]], [[Belarus]], and [[Latvia]] under the brand names Anvifen, Fenibut, Bifren, and Noofen ([[Russian language|Russian]]: Анвифен, Фенибут, Бифрен and Ноофен, respectively).<ref name="DrobizhevFedotova2016" /> |
||
===Availability=== |
===Availability=== |
||
Phenibut is approved in [[Russia]], [[Ukraine]], [[Belarus]] and [[Latvia]] for medical use.<ref name="pmid26693960" /> It is not approved or available as a [[pharmaceutical drug|medication]] in other countries in the [[European Union]], the [[United States]], or [[Australia]].<ref name="pmid26693960" /> In countries where phenibut is not a licensed pharmaceutical drug, it is sold online [[over-the-counter|without a prescription]] as a "[[nutritional supplement]]".<ref name="pmid26693960" /><ref name="ClinicalTox" /> It is often used as a form of [[self-medication]] for [[social anxiety]].<ref name="pmid26693960" /> |
Phenibut is approved in [[Russia]], [[Ukraine]], [[Belarus]], and [[Latvia]] for medical use.<ref name="pmid26693960" /> It is not approved or available as a [[pharmaceutical drug|medication]] in other countries in the [[European Union]], the [[United States]], or [[Australia]].<ref name="pmid26693960" /> In countries where phenibut is not a licensed pharmaceutical drug, it is sold online [[over-the-counter|without a prescription]] as a "[[nutritional supplement]]".<ref name="pmid26693960" /><ref name="ClinicalTox" /> It is often used as a form of [[self-medication]] for [[social anxiety]].<ref name="pmid26693960" /> |
||
===Recreational use=== |
===Recreational use=== |
||
Phenibut is used [[recreational drug use|recreationally]] due to its ability to produce [[euphoria]], [[anxiolysis]], and increased [[sociability]],<ref name="pmid26693960" /> as well as remaining undetected in routine urinalysis. Because of its delayed onset of effects, first-time users often mistakenly take an additional dose of phenibut in the belief that the initial dose did not work.<ref name="pmid26693960" /> Recreational users usually take the drug orally; there are a few case reports of rectal administration and one report of [[Insufflation (medicine)|insufflation]], which was described as "very painful" and causing swollen [[nostril]]s.<ref name="pmid26693960" /> |
Phenibut is used [[recreational drug use|recreationally]] due to its ability to produce [[euphoria]], [[anxiolysis]], and increased [[sociability]],<ref name="pmid26693960" /> as well as remaining undetected in routine urinalysis. Because of its delayed onset of effects, first-time users often mistakenly take an additional dose of phenibut in the belief that the initial dose did not work.<ref name="pmid26693960" /> Recreational users usually take the drug [[Oral administration|orally]]; there are a few case reports of rectal administration and one report of [[Insufflation (medicine)|insufflation]], which was described as "very painful" and causing swollen [[nostril]]s.<ref name="pmid26693960" /> |
||
===Legal status=== |
===Legal status=== |
||
As of 2021, phenibut is a [[controlled substance]] in Australia,<ref name="pmid26693960"/> France,<ref>Par [https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000032106035/] {{Webarchive|url=https://web.archive.org/web/20210508163545/https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000032106035/|date=8 May 2021}} La liste des substances psychotropes</ref> Hungary,<ref>{{Cite web|url=http://kozlonyok.hu/nkonline/MKPDF/hiteles/MK18170.pdf|title=39/2018. (XI. 8.) EMMI rendelet Az új pszichoaktív anyaggá minősített anyagokról vagy vegyületcsoportokról szóló 55/2014. (XII. 30.) EMMI rendelet módosításáról|access-date=14 November 2018|archive-date=14 November 2018|archive-url=https://web.archive.org/web/20181114141557/http://kozlonyok.hu/nkonline/MKPDF/hiteles/MK18170.pdf|url-status=live}}</ref> Italy,<ref name="Gazzetta Ufficiale 11/08/20">{{cite web | url= https://www.gazzettaufficiale.it/eli/id/2020/08/21/20A04540/SG | title= Gazzetta Ufficiale 11/08/20 | publisher= Lorenzo Arbolino | date= 11 August 2020 | access-date= 27 August 2020 | archive-date= 2 May 2024 | archive-url= https://web.archive.org/web/20240502165522/https://www.gazzettaufficiale.it/eli/id/2020/08/21/20A04540/SG | url-status= live }}</ref>Lithuania |
As of 2021, phenibut is a [[controlled substance]] in Australia,<ref name="pmid26693960"/> France,<ref>Par [https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000032106035/] {{Webarchive|url=https://web.archive.org/web/20210508163545/https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000032106035/|date=8 May 2021}} La liste des substances psychotropes</ref> Hungary,<ref>{{Cite web|url=http://kozlonyok.hu/nkonline/MKPDF/hiteles/MK18170.pdf|title=39/2018. (XI. 8.) EMMI rendelet Az új pszichoaktív anyaggá minősített anyagokról vagy vegyületcsoportokról szóló 55/2014. (XII. 30.) EMMI rendelet módosításáról|access-date=14 November 2018|archive-date=14 November 2018|archive-url=https://web.archive.org/web/20181114141557/http://kozlonyok.hu/nkonline/MKPDF/hiteles/MK18170.pdf|url-status=live}}</ref> Italy,<ref name="Gazzetta Ufficiale 11/08/20">{{cite web | url= https://www.gazzettaufficiale.it/eli/id/2020/08/21/20A04540/SG | title= Gazzetta Ufficiale 11/08/20 | publisher= Lorenzo Arbolino | date= 11 August 2020 | access-date= 27 August 2020 | archive-date= 2 May 2024 | archive-url= https://web.archive.org/web/20240502165522/https://www.gazzettaufficiale.it/eli/id/2020/08/21/20A04540/SG | url-status= live }}</ref> Lithuania,<ref>{{Cite web|url=http://ntakd.lrv.lt/lt/naujienos/rinkos-ribojimo-priemones-fenibutui|title=RINKOS RIBOJIMO PRIEMONĖS FENIBUTUI!|website=ntakd.lrv.lt|language=lt|access-date=2020-01-27|archive-date=6 October 2021|archive-url=https://web.archive.org/web/20211006065759/https://ntakd.lrv.lt/lt/naujienos/rinkos-ribojimo-priemones-fenibutui|url-status=dead}}</ref><ref name=":0">{{Cite web|url=https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/9aff1ef11ce911eaadfcfdb735b57421?jfwid=-bgd9aafbv|title=V-1431 Dėl Lietuvos Respublikos sveikatos apsaugos ministro 2000 m. sausio 6 d. įsakymo Nr. 5 "Dėl Narko...|website=e-seimas.lrs.lt|language=lt|access-date=2020-01-27|archive-date=27 January 2020|archive-url=https://web.archive.org/web/20200127002637/https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/9aff1ef11ce911eaadfcfdb735b57421%3Fjfwid%3D-bgd9aafbv|url-status=live}}</ref> and Germany<ref name="Anlage NpSG - Einzelnorm"/> where, nevertheless, it is readily obtained online.<ref>{{Cite journal |vauthors=Bonnet U, Scherbaum N, Schaper A, Soyka M|title=Phenibut — an Illegal Food Supplement With Psychotropic Effects and Health Risks|date=5 April 2024 |doi= 10.3238/arztebl.m2024.0003|volume=121|pages=222–7|url=https://www.aerzteblatt.de/int/archive/article?id=238384 |journal=Deutsches Ärzteblatt International|issue=7 |pmid=38377332 |language=en|pmc=11539871}}</ref> |
||
However, so far, this regulation has not prevented the easy online procurement of phenibut in Germany.<ref>{{Cite web |last=Ärzteblatt |first=Deutscher Ärzteverlag GmbH, Redaktion Deutsches |title=Phenibutan—an Illegal Food Supplement With Psychotropic Effects and Health Risks (05.04.2024) |url=https://www.aerzteblatt.de/int/archive/article?id=238384 |access-date=2024-06-07 |website=Deutsches Ärzteblatt |language=en}}</ref> |
|||
In 2015, it was suggested that the legal status of phenibut in Europe should be reconsidered due to its recreational potential.<ref name="pmid26693960" /> In February 2018, the Australian [[Therapeutic Goods Administration]] declared it a prohibited (schedule 9) substance, citing health concerns due to withdrawal and overdose.<ref>{{cite web|publisher=Australian Government Department of Health.|work=Administration Therapeutic Goods Administration|title=3.3 Phenibut|url=https://www.tga.gov.au/book-page/33-phenibut|access-date=6 November 2017|language=en|date=31 October 2017|archive-date=27 March 2020|archive-url=https://web.archive.org/web/20200327065227/https://www.tga.gov.au/book-page/33-phenibut|url-status=live}}</ref><ref>{{cite news | url= http://www.abc.net.au/news/2018-02-22/detectives-probe-if-drug-behind-school-overdose-bought-online/9471958 | title= Mass school overdose investigation focuses on banned Russian drug | newspaper= ABC News | publisher= Australian Broadcasting Corporation | date= 22 February 2018 | access-date= 22 February 2018 | archive-date= 22 February 2018 | archive-url= https://web.archive.org/web/20180222023735/http://www.abc.net.au/news/2018-02-22/detectives-probe-if-drug-behind-school-overdose-bought-online/9471958 | url-status= live }}</ref> |
In 2015, it was suggested that the legal status of phenibut in Europe should be reconsidered due to its recreational potential.<ref name="pmid26693960" /> In February 2018, the Australian [[Therapeutic Goods Administration]] declared it a prohibited (schedule 9) substance, citing health concerns due to withdrawal and overdose.<ref>{{cite web|publisher=Australian Government Department of Health.|work=Administration Therapeutic Goods Administration|title=3.3 Phenibut|url=https://www.tga.gov.au/book-page/33-phenibut|access-date=6 November 2017|language=en|date=31 October 2017|archive-date=27 March 2020|archive-url=https://web.archive.org/web/20200327065227/https://www.tga.gov.au/book-page/33-phenibut|url-status=live}}</ref><ref>{{cite news | url= http://www.abc.net.au/news/2018-02-22/detectives-probe-if-drug-behind-school-overdose-bought-online/9471958 | title= Mass school overdose investigation focuses on banned Russian drug | newspaper= ABC News | publisher= Australian Broadcasting Corporation | date= 22 February 2018 | access-date= 22 February 2018 | archive-date= 22 February 2018 | archive-url= https://web.archive.org/web/20180222023735/http://www.abc.net.au/news/2018-02-22/detectives-probe-if-drug-behind-school-overdose-bought-online/9471958 | url-status= live }}</ref> |
||
As of 14 November 2018, Hungary added phenibut and 10 other items to its New Psychoactive Substances ban list |
As of 14 November 2018, Hungary added phenibut and 10 other items to its New Psychoactive Substances ban list,<ref>{{cite web | url= https://net.jogtar.hu/jogszabaly?docid=A1400055.EMM | title= EMMI Decree substances or groups of compounds classified as new psychoactive substances | publisher= Wolters Kluwer | date= 1 January 2015 | access-date= 5 August 2020 | archive-date= 8 August 2020 | archive-url= https://web.archive.org/web/20200808213352/https://net.jogtar.hu/jogszabaly?docid=A1400055.EMM | url-status= dead }}</ref> and, as of 26 August 2020, Italy added phenibut to its New Psychoactive Substances ban list.<ref name="Gazzetta Ufficiale 11/08/20"/> As of 18 September 2020, France added phenibut to the controlled psychoactive substances list, prohibiting production, sale, storage, and use.<ref>{{Cite web |url=https://www.legeneraliste.fr/actu-medicale/sante-publique/le-phenibut-interdit-en-france |title=Le phénibut interdit en France {{!}} Le Généraliste |work=Le Généraliste |access-date=28 April 2021 |archive-date=22 January 2021 |archive-url=https://web.archive.org/web/20210122213622/https://www.legeneraliste.fr/actu-medicale/sante-publique/le-phenibut-interdit-en-france |url-status=live }}</ref> |
||
In the United States, phenibut is an unapproved drug, but is often misleadingly marketed as a dietary supplement. It is readily available without a prescription.<ref>{{cite journal |vauthors=Penzak SR, Bulloch M |title=Phenibut: Review and Pharmacologic Approaches to Treating Withdrawal |journal=J Clin Pharmacol |volume=64 |issue=6 |pages=652–671 |date=June 2024 |pmid=38339875 |doi=10.1002/jcph.2414 |type=Review}}</ref><ref>{{cite journal |vauthors=Jouney EA |title=Phenibut (β-Phenyl-γ-Aminobutyric Acid): an Easily Obtainable "Dietary Supplement" With Propensities for Physical Dependence and Addiction |journal=Curr Psychiatry Rep |volume=21 |issue=4 |pages=23 |date=March 2019 |pmid=30852710 |doi=10.1007/s11920-019-1009-0 |url=}}</ref> |
|||
As of 26 August 2020, Italy added phenibut to its New Psychoactive Substances ban list.<ref name="Gazzetta Ufficiale 11/08/20"/> |
|||
In [[Alabama]], phenibut was made a [[Controlled Substances Act#Schedule II controlled substances|Schedule II substance]] at the state level in November 2021.<ref>{{cite web|url=https://www.alabamapublichealth.gov/blog/assets/controlledsubstanceslist.pdf|title=Controlled Substances List; Schedule II, page 70|date=22 February 2024|publisher=Alabama State Board of Health|accessdate=13 September 2024|archive-date=8 August 2024|archive-url=https://web.archive.org/web/20240808233205/https://www.alabamapublichealth.gov/blog/assets/controlledsubstanceslist.pdf|url-status=live}}</ref> |
|||
==See also== |
|||
In the United States, phenibut is not a [[Controlled Substances Act|Controlled Substance.]] However, [[Dietary supplement]]s that contain phenibut are unlawful to introduce into interstate commerce, because phenibut is considered a "[[Federal Food, Drug, and Cosmetic Act|New Drug]]" and any food, supplement, cosmetic, or drug that contains phenibut is therefore misbranded. In Alabama, Phenibut was made a [Controlled Substances Act#Schedule II controlled substances|schedule II]] at the state level on November 14th, 2021.<ref>https://www.alabamapublichealth.gov/blog/assets/controlledsubstanceslist.pdf</ref> |
|||
* [[Gabapentin]] |
|||
* [[Pregabalin]] |
|||
* [[List of Russian drugs]] |
|||
==References== |
==References== |
||
Latest revision as of 12:16, 27 December 2024
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Anvifen, Fenibut, Noofen, others[1] |
| Other names | Aminophenylbutyric acid; Fenibut; Fenigam; Phenigam; Phenybut; Phenygam; Phenylgamma; Phenigama; PHG; PhGABA; β-Phenyl-γ-aminobutyric acid; β-Phenyl-GABA[2] |
| Routes of administration | Common: Oral[3] Uncommon: Rectal[3] |
| Drug class | GABAB receptor agonist; Gabapentinoid |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ≥63% (250 mg)[5] |
| Metabolism | Liver (minimal)[6][5] |
| Metabolites | Inactive[6] |
| Onset of action | Oral: 2–4 hours[3] Rectal: 20–30 minutes[3] |
| Elimination half-life | 5.3 hours (250 mg)[5] |
| Duration of action | 15–24 hours (1–3 g)[3] |
| Excretion | Urine: 63% (unchanged)[5] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.800 |
| Chemical and physical data | |
| Formula | C10H13NO2 |
| Molar mass | 179.219 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 253 °C (487 °F) |
| |
| |
| (verify) | |
Phenibut, sold under the brand name Anvifen among others,[1] is a central nervous system (CNS) depressant with anxiolytic effects, and is used to treat anxiety, insomnia, and for a variety of other indications.[5] It is usually taken orally (swallowed by mouth), but may be given intravenously.[6][5]
Side effects of phenibut can include sedation, sleepiness, nausea, irritability, agitation, dizziness, euphoria, and sometimes headache, among others.[6][7] Overdose of phenibut can produce marked central nervous system depression including unconsciousness.[6][7] The medication is structurally related to the neurotransmitter γ-aminobutyric acid (GABA), and hence is a GABA analogue.[5] Phenibut is thought to act as a GABAB receptor agonist, similarly to baclofen and γ-hydroxybutyrate (GHB).[5] However, at low concentrations, phenibut mildly increases the concentration of dopamine in the brain, providing stimulatory effects in addition to the anxiolysis.[8]
Phenibut was developed in the Soviet Union and was introduced for medical use in the 1960s.[5] Today, it is marketed for medical use in Russia, Ukraine, Belarus, Kazakhstan, and Latvia.[5] The medication is not approved for clinical use in the United States and most of Europe, but it is sold on the Internet as a supplement and purported nootropic.[3][9] Phenibut has been used recreationally and can produce euphoria as well as addiction, dependence, and withdrawal.[3] It is a controlled substance in Australia, and it has been suggested that its legal status should be reconsidered in Europe as well.[3] In Germany, phenibut is not approved as a drug and, as a food supplement, is controlled under the German New Psychoactive Substances Act.[10]
In a 2023 assessment, the U.S. Food and Drug Administration (FDA) determined that phenibut does not meet the definition of a dietary ingredient, thereby making phenibut supplement products misbranded and illegal for marketing.[11] FDA warning letters had been issued to supplement manufacturers marketing phenibut products as adulterated.[12]
Medical uses
[edit]Phenibut is used in Russia, Ukraine, Belarus and Latvia as a pharmaceutical drug to treat anxiety and to improve sleep (e.g., in the treatment of insomnia).[5][6] It is also used for various other indications, including the treatment of asthenia, depression, alcoholism, alcohol withdrawal syndrome, post-traumatic stress disorder, stuttering, tics, vestibular disorders, Ménière's disease, dizziness, for the prevention of motion sickness, and for the prevention of anxiety before or after surgical procedures or painful diagnostic tests.[6][5]
Available forms
[edit]Phenibut is available as a medication in the form of 250 mg or 500 mg tablets for oral administration and as a solution at a concentration of 10 mg/mL for infusion.[6][7][13] In the US, dietary supplements labeled as containing phenibut have been found to contain zero to greater than 1,100 mg of phenibut per serving.[9]
Contraindications
[edit]Contraindications of phenibut include:[6][7]
- Intolerance to phenibut
- Pregnancy and breastfeeding
- Children who are younger than two years of age
- Liver insufficiency or failure
- Ulcerative lesions of the gastrointestinal tract
Phenibut should not be combined with alcohol.[7]
Side effects
[edit]Phenibut is generally well-tolerated.[5][7] Possible side effects may include sedation, somnolence, nausea, irritability, agitation, anxiety, dizziness, headache, and allergic reactions such as skin rash and itching.[6][7] At high doses, motor incoordination, loss of balance, and hangovers may occur.[3] Due to its CNS depressant effects, people taking phenibut should refrain from potentially dangerous activities such as operating heavy machinery.[6][7] With prolonged use of phenibut, particularly at high doses, the liver and blood should be monitored, due to risk of fatty liver disease and eosinophilia.[6][7]
Overdose
[edit]In overdose, phenibut can cause severe drowsiness, nausea, vomiting, eosinophilia, lowered blood pressure, renal impairment, and, above 7 grams, fatty liver degeneration.[6][7] There are no specific antidotes for phenibut overdose.[7] Lethargy, somnolence, agitation, delirium, tonic–clonic seizures, reduced consciousness or unconsciousness, and unresponsiveness have been reported in recreational users who have overdosed.[3] Management of phenibut overdose includes activated charcoal, gastric lavage, induction of vomiting, and symptom-based treatment.[6][7] There have been three associated deaths which found Phenibut in the users system but only one of these cases single-handedly included Phenibut.[14]
Dependency and withdrawal
[edit]Tolerance to phenibut easily develops with repeated use leading to dependency.[5][needs update] Withdrawal symptoms may occur upon discontinuation, and, in recreational users taking high doses, have been reported to include severe rebound anxiety, insomnia, anger, irritability, agitation, visual and auditory hallucinations, and acute psychosis.[3] Baclofen has successfully been used for treatment of phenibut dependence.[15]
Interactions
[edit]Phenibut may mutually potentiate and extend the duration of the effects of other CNS depressants, including anxiolytics, antipsychotics, sedatives, opioids, anticonvulsants, and alcohol.[6][7]
Pharmacology
[edit]Pharmacodynamics
[edit]| Compound | GABAB | GABAA |
|---|---|---|
| GABA | 0.08 | 0.12 |
| GHB | >100 | >100 |
| GABOB | 1.10 | 1.38 |
| Phenibut | 9.6 | >100 |
| 4-F-phenibut | 1.70 | >100 |
| Baclofen | 0.13 | >100 |
| (R)-Baclofen | 0.13 | >100 |
| (S)-Baclofen | 74.0 | >100 |
| Values are IC50 (μM) in rat brain. | ||
Phenibut acts as a full agonist of the GABAB receptor, similarly to baclofen.[17][18] It has between 30- and 68-fold lower affinity for the GABAB receptor than baclofen, and, in accordance, is used at far higher doses in comparison.[17] (R)-Phenibut has more than 100-fold higher affinity for the GABAB receptor than does (S)-phenibut; hence, (R)-phenibut is the active enantiomer at the GABAB receptor.[19]
| Compound | α2δ | GABAB |
|---|---|---|
| Phenibut | ND | 177 |
| (R)-Phenibut | 23 | 92 |
| (S)-Phenibut | 39 | >1,000 |
| Baclofen | 156 | 6 |
| Gabapentin | 0.05 | >1,000 |
| Values are Ki (μM) in rat brain. | ||
Phenibut also binds to and blocks α2δ subunit-containing VDCCs, similarly to gabapentin and pregabalin, and hence is a gabapentinoid.[20][21] Both (R)-phenibut and (S)-phenibut display this action with similar affinity (Ki = 23 and 39 μM, respectively).[20]
It is often claimed on websites about nootropics and elsewhere on the internet that phenibut increases dopamine. Three papers published in Russian by Soviet scientists in 1979, 1986, and 1990 report that phenibut increases dopamine in the striatum of rats and in the mouse brain.[22] The mechanism underlying this putative effect is unclear.[22] Structurally, phenibut can also be considered a derivative of phenethylamine, and some research suggests that phenibut antagonizes the action of phenethylamine.[22]
Pharmacokinetics
[edit]Little information thus far has been published on the clinical pharmacokinetics of phenibut.[5] The drug is reported to be well-absorbed.[6] It distributes widely throughout the body and across the blood–brain barrier.[6] Approximately 0.1% of an administered dose of phenibut reportedly penetrates into the brain, with this said to occur to a much greater extent in young people and the elderly.[6] Following a single 250 mg dose in healthy volunteers, its elimination half-life was approximately 5.3 hours and the drug was largely (63%) excreted in the urine unchanged.[5]
Some limited information has been described on the pharmacokinetics of phenibut in recreational users taking much higher doses (e.g., 1–3 grams) than typical clinical doses.[3][23] In these individuals, the onset of action of phenibut has been reported to be 2 to 4 hours orally and 20 to 30 minutes rectally, the peak effects are described as occurring 4 to 6 hours following oral ingestion, and the total duration for the oral route has been reported to be 15 to 24 hours (or about 3 to 5 terminal half-lives).[3]
Chemistry
[edit]Phenibut is a synthetic aromatic amino acid. It is a chiral molecule and thus has two potential configurations, as (R)- and (S)-enantiomers.[18]
Structure and analogues
[edit]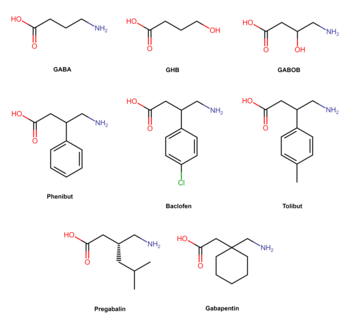
Phenibut is a derivative of the inhibitory neurotransmitter GABA.[5] Hence, it is a GABA analogue.[5] Phenibut is specifically the analogue of GABA with a phenyl ring substituted in at the β-position.[5] As such, its chemical name is β-phenyl-γ-aminobutyric acid, which can be abbreviated as β-phenyl-GABA.[5] The presence of the phenyl ring allows phenibut to cross the blood–brain barrier significantly, unlike GABA.[5] Phenibut also contains the trace amine β-phenethylamine in its structure.[5]
Phenibut is closely related to a variety of other GABA analogues including baclofen (β-(4-chlorophenyl)-GABA), 4-fluorophenibut (β-(4-fluorophenyl)-GABA), tolibut (β-(4-methylphenyl)-GABA), pregabalin ((S)-β-isobutyl-GABA), gabapentin (1-(aminomethyl)cyclohexane acetic acid), and GABOB (β-hydroxy-GABA).[5][20] It has almost the same chemical structure as baclofen, differing from it only in having a hydrogen atom instead of a chlorine atom at the para position of the phenyl ring.[5] Phenibut is also close in structure to pregabalin, which has an isobutyl group at the β position instead of phenibut's phenyl ring.[20]
A glutamate-derivative analogue of phenibut is glufimet (dimethyl 3-phenylglutamate hydrochloride).[24]
Synthesis
[edit]A chemical synthesis of phenibut has been published.[13]
History
[edit]Phenibut was synthesized at the A. I. Herzen Leningrad Pedagogical Institute (USSR) by Professor Vsevolod Perekalin's team and tested at the Institute of Experimental Medicine, USSR Academy of Medical Sciences.[5] It was introduced into clinical use in Russia in the 1960s.[5]
Society and culture
[edit]
Other names
[edit]Alternate spellings include fenibut and phenybut.[2] It is also sometimes referred to as aminophenylbutyric acid.[1] The word phenibut is a contraction of the chemical name of the drug, β-phenyl-γ-aminobutyric acid.[5] In early publications, phenibut was referred to as fenigam and phenigama.[5][25] The drug has not been assigned an INN.[2][6]
Brand names
[edit]Phenibut is marketed in Russia, Ukraine, Belarus, and Latvia under the brand names Anvifen, Fenibut, Bifren, and Noofen (Russian: Анвифен, Фенибут, Бифрен and Ноофен, respectively).[1]
Availability
[edit]Phenibut is approved in Russia, Ukraine, Belarus, and Latvia for medical use.[3] It is not approved or available as a medication in other countries in the European Union, the United States, or Australia.[3] In countries where phenibut is not a licensed pharmaceutical drug, it is sold online without a prescription as a "nutritional supplement".[3][9] It is often used as a form of self-medication for social anxiety.[3]
Recreational use
[edit]Phenibut is used recreationally due to its ability to produce euphoria, anxiolysis, and increased sociability,[3] as well as remaining undetected in routine urinalysis. Because of its delayed onset of effects, first-time users often mistakenly take an additional dose of phenibut in the belief that the initial dose did not work.[3] Recreational users usually take the drug orally; there are a few case reports of rectal administration and one report of insufflation, which was described as "very painful" and causing swollen nostrils.[3]
Legal status
[edit]As of 2021, phenibut is a controlled substance in Australia,[3] France,[26] Hungary,[27] Italy,[28] Lithuania,[29][30] and Germany[10] where, nevertheless, it is readily obtained online.[31]
In 2015, it was suggested that the legal status of phenibut in Europe should be reconsidered due to its recreational potential.[3] In February 2018, the Australian Therapeutic Goods Administration declared it a prohibited (schedule 9) substance, citing health concerns due to withdrawal and overdose.[32][33]
As of 14 November 2018, Hungary added phenibut and 10 other items to its New Psychoactive Substances ban list,[34] and, as of 26 August 2020, Italy added phenibut to its New Psychoactive Substances ban list.[28] As of 18 September 2020, France added phenibut to the controlled psychoactive substances list, prohibiting production, sale, storage, and use.[35]
In the United States, phenibut is an unapproved drug, but is often misleadingly marketed as a dietary supplement. It is readily available without a prescription.[36][37]
In Alabama, phenibut was made a Schedule II substance at the state level in November 2021.[38]
See also
[edit]References
[edit]- ^ a b c d Drobizhev MY, Fedotova AV, Kikta SV, Antohin EY (2016). "Феномен аминофенилмасляной кислоты" [Phenomenon of aminophenylbutyric acid]. Russian Medical Journal (in Russian). 2017 (24): 1657–1663. ISSN 1382-4368. Archived from the original on 16 September 2017. Retrieved 16 September 2017.
- ^ a b c Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 69–. ISBN 978-1-4757-2085-3.
- ^ a b c d e f g h i j k l m n o p q r s t u v Owen DR, Wood DM, Archer JR, Dargan PI (September 2016). "Phenibut (4-amino-3-phenyl-butyric acid): Availability, prevalence of use, desired effects and acute toxicity". Drug and Alcohol Review. 35 (5): 591–6. doi:10.1111/dar.12356. hdl:10044/1/30073. PMID 26693960.
- ^ Nutrition, Center for Food Safety and Applied (6 March 2023). "Phenibut in Dietary Supplements". FDA. Archived from the original on 23 May 2023. Retrieved 23 May 2023.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab Lapin I (2001). "Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug". CNS Drug Reviews. 7 (4): 471–81. doi:10.1111/j.1527-3458.2001.tb00211.x. PMC 6494145. PMID 11830761.
- ^ a b c d e f g h i j k l m n o p q r s Ozon Pharm, Fenibut (PDF), archived from the original (PDF) on 16 September 2017, retrieved 15 September 2017
- ^ a b c d e f g h i j k l m Регистр лекарственных средств России ([Russian Medicines Register]). "Фенибут (Phenybutum)" [Fenibut (Phenybutum)] (in Russian). Archived from the original on 3 March 2009. Retrieved 15 September 2017.
- ^ Lapin I (7 June 2006). "Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug". CNS Drug Reviews. 7 (4): 471–81. doi:10.1111/j.1527-3458.2001.tb00211.x. PMC 6494145. PMID 11830761.
- ^ a b c Cohen PA, Ellison RR, Travis JC, Gaufberg SV, Gerona R (April 2022). "Quantity of phenibut in dietary supplements before and after FDA warnings". Clinical Toxicology. 60 (4): 486–488. doi:10.1080/15563650.2021.1973020. PMID 34550038. S2CID 237594860.
- ^ a b "Anlage NpSG - Einzelnorm". www.gesetze-im-internet.de. Retrieved 7 June 2024.
- ^ "Phenibut in Dietary Supplements". US Food and Drug Administration. 12 July 2023. Retrieved 14 September 2024.
- ^ "FDA Acts on Dietary Supplements Containing DMHA and Phenibut". US Food and Drug Administration. 29 April 2019. Archived from the original on 14 June 2022. Retrieved 14 September 2024.
- ^ a b Sivchik VV, Grygoryan HO, Survilo VL, Trukhachova TV (2012), Синтез γ-амино-β-фенилмасляной кислоты (фенибута) [Synthesis of β-phenyl-γ-aminobutyric acid (phenibut)] (PDF) (in Russian), archived (PDF) from the original on 16 September 2017, retrieved 16 September 2017
- ^ Graves JM, Dilley J, Kubsad S, Liebelt E (September 2020). "Notes from the Field: Phenibut Exposures Reported to Poison Centers - United States, 2009–2019". MMWR. Morbidity and Mortality Weekly Report. 69 (35): 1227–1228. doi:10.15585/mmwr.mm6935a5. PMC 7470459. PMID 32881852.
- ^ Samokhvalov AV, Paton-Gay CL, Balchand K, Rehm J (February 2013). "Phenibut dependence". BMJ Case Reports. 2013: bcr2012008381. doi:10.1136/bcr-2012-008381. PMC 3604470. PMID 23391959.
- ^ Bowery NG, Hill DR, Hudson AL (January 1983). "Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes". British Journal of Pharmacology. 78 (1): 191–206. doi:10.1111/j.1476-5381.1983.tb09380.x. PMC 2044790. PMID 6297646.
- ^ a b GABAb Receptor Pharmacology: A Tribute to Norman Bowery: A Tribute to Norman Bowery. Academic Press. 21 September 2010. pp. 25–. ISBN 978-0-12-378648-7.
- ^ a b Dambrova M, Zvejniece L, Liepinsh E, Cirule H, Zharkova O, Veinberg G, Kalvinsh I (March 2008). "Comparative pharmacological activity of optical isomers of phenibut". European Journal of Pharmacology. 583 (1): 128–134. doi:10.1016/j.ejphar.2008.01.015. PMID 18275958.
- ^ Allan RD, Bates MC, Drew CA, Duke RK, Hambley TW, Johnston GA, et al. (1990). "A new synthesis resolution and in vitro activities of (R)- and (S)-β-Phenyl-Gaba". Tetrahedron. 46 (7): 2511–2524. doi:10.1016/S0040-4020(01)82032-9. ISSN 0040-4020.
- ^ a b c d e Zvejniece L, Vavers E, Svalbe B, Veinberg G, Rizhanova K, Liepins V, et al. (October 2015). "R-phenibut binds to the α2-δ subunit of voltage-dependent calcium channels and exerts gabapentin-like anti-nociceptive effects". Pharmacology, Biochemistry, and Behavior. 137: 23–9. doi:10.1016/j.pbb.2015.07.014. PMID 26234470. S2CID 42606053.
- ^ Vavers E, Zvejniece L, Svalbe B, Volska K, Makarova E, Liepinsh E, et al. (November 2016). "The neuroprotective effects of R-phenibut after focal cerebral ischemia". Pharmacological Research. 113 (Pt B): 796–801. doi:10.1016/j.phrs.2015.11.013. PMID 26621244.
- ^ a b c Lapin, Izyaslav (December 2001). "Phenibut (β-Phenyl-GABA): A Tranquilizer and Nootropic Drug". CNS Drug Reviews. 7 (4): 471–481. doi:10.1111/j.1527-3458.2001.tb00211.x. PMC 6494145. PMID 11830761.
- ^ Schifano F, Orsolini L, Duccio Papanti G, Corkery JM (February 2015). "Novel psychoactive substances of interest for psychiatry". World Psychiatry. 14 (1): 15–26. doi:10.1002/wps.20174. PMC 4329884. PMID 25655145.
- ^ Perfilova VN, Popova TA, Prokofiev II, Mokrousov IS, Ostrovskii OV, Tyurenkov IN (June 2017). "Effect of Phenibut and Glufimet, a Novel Glutamic Acid Derivative, on Respiration of Heart and Brain Mitochondria from Animals Exposed to Stress against the Background of Inducible NO-Synthase Blockade". Bulletin of Experimental Biology and Medicine. 163 (2): 226–229. doi:10.1007/s10517-017-3772-4. PMID 28726197. S2CID 4907409.
- ^ Khaunina RA, Lapin IP (1976). "Fenibut, a new tranquilizer". Pharmaceutical Chemistry Journal. 10 (12): 1703–1705. doi:10.1007/BF00760021. ISSN 0091-150X. S2CID 29071385.
- ^ Par [1] Archived 8 May 2021 at the Wayback Machine La liste des substances psychotropes
- ^ "39/2018. (XI. 8.) EMMI rendelet Az új pszichoaktív anyaggá minősített anyagokról vagy vegyületcsoportokról szóló 55/2014. (XII. 30.) EMMI rendelet módosításáról" (PDF). Archived (PDF) from the original on 14 November 2018. Retrieved 14 November 2018.
- ^ a b "Gazzetta Ufficiale 11/08/20". Lorenzo Arbolino. 11 August 2020. Archived from the original on 2 May 2024. Retrieved 27 August 2020.
- ^ "RINKOS RIBOJIMO PRIEMONĖS FENIBUTUI!". ntakd.lrv.lt (in Lithuanian). Archived from the original on 6 October 2021. Retrieved 27 January 2020.
- ^ "V-1431 Dėl Lietuvos Respublikos sveikatos apsaugos ministro 2000 m. sausio 6 d. įsakymo Nr. 5 "Dėl Narko..." e-seimas.lrs.lt (in Lithuanian). Archived from the original on 27 January 2020. Retrieved 27 January 2020.
- ^ Bonnet U, Scherbaum N, Schaper A, Soyka M (5 April 2024). "Phenibut — an Illegal Food Supplement With Psychotropic Effects and Health Risks". Deutsches Ärzteblatt International. 121 (7): 222–7. doi:10.3238/arztebl.m2024.0003. PMC 11539871. PMID 38377332.
- ^ "3.3 Phenibut". Administration Therapeutic Goods Administration. Australian Government Department of Health. 31 October 2017. Archived from the original on 27 March 2020. Retrieved 6 November 2017.
- ^ "Mass school overdose investigation focuses on banned Russian drug". ABC News. Australian Broadcasting Corporation. 22 February 2018. Archived from the original on 22 February 2018. Retrieved 22 February 2018.
- ^ "EMMI Decree substances or groups of compounds classified as new psychoactive substances". Wolters Kluwer. 1 January 2015. Archived from the original on 8 August 2020. Retrieved 5 August 2020.
- ^ "Le phénibut interdit en France | Le Généraliste". Le Généraliste. Archived from the original on 22 January 2021. Retrieved 28 April 2021.
- ^ Penzak SR, Bulloch M (June 2024). "Phenibut: Review and Pharmacologic Approaches to Treating Withdrawal". J Clin Pharmacol (Review). 64 (6): 652–671. doi:10.1002/jcph.2414. PMID 38339875.
- ^ Jouney EA (March 2019). "Phenibut (β-Phenyl-γ-Aminobutyric Acid): an Easily Obtainable "Dietary Supplement" With Propensities for Physical Dependence and Addiction". Curr Psychiatry Rep. 21 (4): 23. doi:10.1007/s11920-019-1009-0. PMID 30852710.
- ^ "Controlled Substances List; Schedule II, page 70" (PDF). Alabama State Board of Health. 22 February 2024. Archived (PDF) from the original on 8 August 2024. Retrieved 13 September 2024.
