SNi: Difference between revisions
mNo edit summary |
m Reverted 1 edit by 49.43.249.108 (talk) to last revision by AnomieBOT |
||
| (20 intermediate revisions by 19 users not shown) | |||
| Line 1: | Line 1: | ||
{{DISPLAYTITLE:S |
{{DISPLAYTITLE:S{{sub|N}}i}} |
||
{{short description|Mechanism for nucleophilic substitution reactions}} |
|||
{{Other uses|SNI (disambiguation)}} |
{{Other uses|SNI (disambiguation)}} |
||
| ⚫ | '''S |
||
| ⚫ | In [[chemistry]], '''S{{sub|N}}i''' ('''substitution nucleophilic internal''') refers to a specific, regio-selective but not often encountered [[reaction mechanism]] for [[nucleophilic aliphatic substitution]]. The name was introduced by Cowdrey et al. in 1937 to label [[nucleophilic]] reactions which occur with retention of configuration,<ref>{{cite journal|last1=Hughes|first1=Edward D.|last2=Ingold|first2=Christopher K.|last3=Scott|first3=Alan D.|title=258. The mechanism of elimination reactions. Part I. Unimolecular olefin formation from alkyl halides in sulphur dioxide and formic acid|journal=Journal of the Chemical Society (Resumed)|date=1937|pages=1271|doi=10.1039/JR9370001271}}</ref> but later was employed to describe various reactions that proceed with a similar mechanism. |
||
| ⚫ | A typical representative [[organic reaction]] displaying this mechanism is the [[ |
||
| ⚫ | A typical representative [[organic reaction]] displaying this mechanism is the [[chlorination reaction|chlorination]] of [[Alcohol (chemistry)|alcohol]]s with [[thionyl chloride]], or the decomposition of [[Chloroformate|alkyl chloroformates]], the main feature is retention of [[stereochemical]] configuration. Some examples for this reaction were reported by Edward S. Lewis and Charles E. Boozer in 1952.<ref>{{cite journal|last1=Lewis|first1=Edward S.|last2=Boozer|first2=Charles E.|title=The Kinetics and Stereochemistry of the Decomposition of Secondary Alkyl Chlorosulfites|journal=Journal of the American Chemical Society|date=January 1952|volume=74|issue=2|pages=308–311|doi=10.1021/ja01122a005}}</ref> Mechanistic and kinetic studies were reported few years later by various researchers.<ref name="Cram">{{cite journal|last1=Cram|first1=Donald J.|title=Studies in Stereochemistry. XVI. Ionic Intermediates in the Decomposition of Certain Alkyl Chlorosulfites|journal=Journal of the American Chemical Society|date=January 1953|volume=75|issue=2|pages=332–338|doi=10.1021/ja01098a024}}</ref><ref>{{cite journal|last1=Lee|first1=C.C.|last2=Clayton|first2=J.W.|last3=Lee|first3=D.G.|last4=Finlayson|first4=A.J.|title=Rearrangement studies with 14C—XIII|journal=Tetrahedron|date=January 1962|volume=18|issue=12|pages=1395–1402|doi=10.1016/S0040-4020(01)99294-4}}</ref> |
||
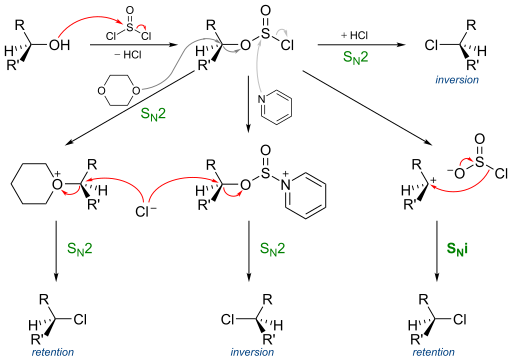
| ⚫ | Thionyl chloride first reacts with the alcohol to form an [[sulfite|alkyl chloro sulfite]], actually forming an [[intimate ion pair]]. The second step is the |
||
| ⚫ | Thionyl chloride first reacts with the alcohol to form an [[sulfite|alkyl chloro sulfite]], actually forming an [[intimate ion pair]]. The second step is the loss of a [[sulfur dioxide]] molecule and its replacement by the chloride, which was attached to the sulphite group. The difference between S{{sub|N}}1 and S{{sub|N}}i is actually that the <em>ion pair is not completely dissociated</em>, and therefore no real carbocation is formed, which else would lead to a racemisation.{{Citation needed|reason=No citation for lack of real carbocation |date=October 2024}} |
||
This reaction type is linked to many forms of [[neighbouring group participation]], for instance the reaction of the [[sulfur]] or [[nitrogen]] [[lone pair]] in [[sulfur mustard]] or [[nitrogen mustard]] to form the [[cation]]ic [[reactive intermediate|intermediate]]. |
This reaction type is linked to many forms of [[neighbouring group participation]], for instance the reaction of the [[sulfur]] or [[nitrogen]] [[lone pair]] in [[sulfur mustard]] or [[nitrogen mustard]] to form the [[cation]]ic [[reactive intermediate|intermediate]]. |
||
| Line 11: | Line 13: | ||
This reaction mechanism is supported by the observation that addition of [[pyridine]] to the reaction leads to [[Walden inversion|inversion]]. The reasoning behind this finding is that pyridine reacts with the intermediate sulfite replacing chlorine. The dislodged chlorine has to resort to nucleophilic attack from the rear as in a regular nucleophilic substitution.<ref name="Cram" /> |
This reaction mechanism is supported by the observation that addition of [[pyridine]] to the reaction leads to [[Walden inversion|inversion]]. The reasoning behind this finding is that pyridine reacts with the intermediate sulfite replacing chlorine. The dislodged chlorine has to resort to nucleophilic attack from the rear as in a regular nucleophilic substitution.<ref name="Cram" /> |
||
In the complete picture for this reaction the sulfite reacts with a chlorine ion in a standard [[SN2 reaction|S |
In the complete picture for this reaction the sulfite reacts with a chlorine ion in a standard [[SN2 reaction|S{{sub|N}}2]] reaction with ''inversion'' of configuration. When the solvent is also a [[nucleophile]] such as [[dioxane]] two successive S{{sub|N}}2 reactions take place and the stereochemistry is again ''retention''. With standard S{{sub|N}}1 reaction conditions the reaction outcome is ''retention'' via a competing S{{sub|N}}i mechanism and not racemization and with pyridine added the result is again ''inversion''.<ref>{{cite book|last1=March|first1=Jerry|editor1-last=Knipe|editor1-first=A.C.|title=March's Advanced Organic Chemistry Reactions, Mechanisms, and Structure.|date=2007|publisher=John Wiley & Sons|location=Hoboken|isbn=9780470084946|pages=468-469|edition=6th}}</ref><ref name="Cram" /> |
||
| ⚫ | |||
Sn1 occurs in tertiary carbon while Sn2 occurs in primary carbon]] |
|||
| ⚫ | |||
== See also == |
== See also == |
||
| Line 23: | Line 27: | ||
{{Reaction mechanisms}} |
{{Reaction mechanisms}} |
||
[[Category: |
[[Category:Nucleophilic substitution reactions]] |
||
[[Category:Reaction mechanisms]] |
[[Category:Reaction mechanisms]] |
||
Latest revision as of 15:18, 27 December 2024
In chemistry, SNi (substitution nucleophilic internal) refers to a specific, regio-selective but not often encountered reaction mechanism for nucleophilic aliphatic substitution. The name was introduced by Cowdrey et al. in 1937 to label nucleophilic reactions which occur with retention of configuration,[1] but later was employed to describe various reactions that proceed with a similar mechanism.
A typical representative organic reaction displaying this mechanism is the chlorination of alcohols with thionyl chloride, or the decomposition of alkyl chloroformates, the main feature is retention of stereochemical configuration. Some examples for this reaction were reported by Edward S. Lewis and Charles E. Boozer in 1952.[2] Mechanistic and kinetic studies were reported few years later by various researchers.[3][4]
Thionyl chloride first reacts with the alcohol to form an alkyl chloro sulfite, actually forming an intimate ion pair. The second step is the loss of a sulfur dioxide molecule and its replacement by the chloride, which was attached to the sulphite group. The difference between SN1 and SNi is actually that the ion pair is not completely dissociated, and therefore no real carbocation is formed, which else would lead to a racemisation.[citation needed]
This reaction type is linked to many forms of neighbouring group participation, for instance the reaction of the sulfur or nitrogen lone pair in sulfur mustard or nitrogen mustard to form the cationic intermediate.
This reaction mechanism is supported by the observation that addition of pyridine to the reaction leads to inversion. The reasoning behind this finding is that pyridine reacts with the intermediate sulfite replacing chlorine. The dislodged chlorine has to resort to nucleophilic attack from the rear as in a regular nucleophilic substitution.[3]
In the complete picture for this reaction the sulfite reacts with a chlorine ion in a standard SN2 reaction with inversion of configuration. When the solvent is also a nucleophile such as dioxane two successive SN2 reactions take place and the stereochemistry is again retention. With standard SN1 reaction conditions the reaction outcome is retention via a competing SNi mechanism and not racemization and with pyridine added the result is again inversion.[5][3]
See also
[edit]References
[edit]- ^ Hughes, Edward D.; Ingold, Christopher K.; Scott, Alan D. (1937). "258. The mechanism of elimination reactions. Part I. Unimolecular olefin formation from alkyl halides in sulphur dioxide and formic acid". Journal of the Chemical Society (Resumed): 1271. doi:10.1039/JR9370001271.
- ^ Lewis, Edward S.; Boozer, Charles E. (January 1952). "The Kinetics and Stereochemistry of the Decomposition of Secondary Alkyl Chlorosulfites". Journal of the American Chemical Society. 74 (2): 308–311. doi:10.1021/ja01122a005.
- ^ a b c Cram, Donald J. (January 1953). "Studies in Stereochemistry. XVI. Ionic Intermediates in the Decomposition of Certain Alkyl Chlorosulfites". Journal of the American Chemical Society. 75 (2): 332–338. doi:10.1021/ja01098a024.
- ^ Lee, C.C.; Clayton, J.W.; Lee, D.G.; Finlayson, A.J. (January 1962). "Rearrangement studies with 14C—XIII". Tetrahedron. 18 (12): 1395–1402. doi:10.1016/S0040-4020(01)99294-4.
- ^ March, Jerry (2007). Knipe, A.C. (ed.). March's Advanced Organic Chemistry Reactions, Mechanisms, and Structure (6th ed.). Hoboken: John Wiley & Sons. pp. 468–469. ISBN 9780470084946.
