Fulvenes: Difference between revisions
added Category:Vinylidene compounds using HotCat Tag: Reverted |
M97uzivatel (talk | contribs) →Preparation: full name when mentioned for the first time |
||
| (9 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:Fulvene_with_hydrogens.svg|thumb|right|upright|Chemical structure of [[fulvene]]]] |
[[File:Fulvene_with_hydrogens.svg|thumb|right|upright|Chemical structure of [[fulvene]]]] |
||
'''Fulvenes''' are the class of [[hydrocarbon]] obtained by formally [[cross-conjugation|cross-conjugating]] one [[Cycloalkane|ring]] and [[methylidene]] through a common [[exocyclic]] [[alkene|double bond]].<ref>{{citation | last = Agranat | first = Israel | title = Ground-State Versus Excited-State Polarity of Triafulvenes: A Study of Solvent Effects on Molecular Electronic Spectra | journal = The Jerusalem Symposia on Quantum Chemistry and Biochemistry | year = 2012 | volume = 8 | pages = 573–583 | doi = 10.1007/978-94-010-1837-1_36 }}</ref><ref>{{citation | last = Neuenschwander | first = Markus | title = Synthetic and NMR spectroscopic investigations of fulvenes and fulvalenes | journal = Pure Appl. Chem. | year = 1986 | volume = 58 | issue = 1 | pages = 55–66 | doi = 10.1351/pac198658010055|url = http://pac.iupac.org/publications/pac/pdf/1986/pdf/5801x0055.pdf |
'''Fulvenes''' are the class of [[hydrocarbon]] obtained by formally [[cross-conjugation|cross-conjugating]] one [[Cycloalkane|ring]] and [[methylidene]] through a common [[exocyclic]] [[alkene|double bond]].<ref>{{citation | last = Agranat | first = Israel | title = Ground-State Versus Excited-State Polarity of Triafulvenes: A Study of Solvent Effects on Molecular Electronic Spectra | journal = The Jerusalem Symposia on Quantum Chemistry and Biochemistry | year = 2012 | volume = 8 | pages = 573–583 | doi = 10.1007/978-94-010-1837-1_36 | isbn = 978-94-010-1839-5}}</ref><ref>{{citation | last = Neuenschwander | first = Markus | title = Synthetic and NMR spectroscopic investigations of fulvenes and fulvalenes | journal = Pure Appl. Chem. | year = 1986 | volume = 58 | issue = 1 | pages = 55–66 | doi = 10.1351/pac198658010055|url = http://pac.iupac.org/publications/pac/pdf/1986/pdf/5801x0055.pdf}}</ref> |
||
The name is derived from [[fulvene]], which has one pentagonal ring. Other examples include [[methylenecyclopropene]] (triafulvene) and heptafulvene. |
The name is derived from [[fulvene]], which has one pentagonal ring. Other examples include [[methylenecyclopropene]] (triafulvene) and heptafulvene. |
||
Fulvenes are generally named based on the number of ring atoms. Thus [[methylenecyclopropene]] is "triafulvene", methylenecyclopentadiene is "[[pentafulvene]]", etc.<ref>{{GoldBookRef|file=F02550|title=Fulvenes}}</ref> |
|||
==Subclasses== |
|||
Several types of fulvenes are defined.<ref>{{GoldBookRef|file=F02550|title=Fulvenes}}</ref> They are: |
|||
*pentafulvene |
|||
*[[Methylenecyclopropene|triafulvene]] |
|||
*heptafulvene |
|||
*nonafulvene |
|||
==Preparation== |
==Preparation== |
||
Fulvenes are readily prepared by the condensation of [[cyclopentadiene]] and [[aldehyde]]s and [[ketone]]s: |
Fulvenes are readily prepared by the condensation of [[cyclopentadiene]] and [[aldehyde]]s and [[ketone]]s: |
||
:C<sub>5</sub>H<sub>6</sub> + R<sub>2</sub>C=O → C<sub>4</sub>H<sub>4</sub>C=CR<sub>2</sub> + H<sub>2</sub>O |
:C<sub>5</sub>H<sub>6</sub> + R<sub>2</sub>C=O → C<sub>4</sub>H<sub>4</sub>C=CR<sub>2</sub> + H<sub>2</sub>O |
||
[[Johannes Thiele (chemist)|Thiele]] is credited with discovering this reaction.<ref>{{cite journal |
[[Johannes Thiele (chemist)|Johannes Thiele]] is credited with discovering this reaction.<ref>{{cite journal |
||
| author = Thiele, J. |
| author = Thiele, J. |
||
| title = <!--no umlaut-->Ueber Ketonreactionen bei dem Cyclopentadiën |
| title = <!--no umlaut-->Ueber Ketonreactionen bei dem Cyclopentadiën |
||
| Line 25: | Line 20: | ||
Modern synthesis of fulvenes employ buffer systems.<ref>{{Cite journal|last1=Coşkun|first1=Necdet|last2=Erden|first2=Ihsan|date=2011-11-11|title=An efficient catalytic method for fulvene synthesis|journal=Tetrahedron|volume=67|issue=45|pages=8607–8614|doi=10.1016/j.tet.2011.09.036|issn=0040-4020|pmc=3196336|pmid=22021940}}</ref><ref>{{Cite journal|last1=Sieverding|first1=Paul|last2=Osterbrink|first2=Johanna|last3=Besson|first3=Claire|last4=Kögerler|first4=Paul|date=2019-01-18|title=Kinetics and mechanism of pyrrolidine buffer-catalyzed fulvene formation|journal=J. Org. Chem.|volume=84|issue=2|pages=486–494|doi=10.1021/acs.joc.8b01660|pmid=30540466|issn=0022-3263}}</ref> |
Modern synthesis of fulvenes employ buffer systems.<ref>{{Cite journal|last1=Coşkun|first1=Necdet|last2=Erden|first2=Ihsan|date=2011-11-11|title=An efficient catalytic method for fulvene synthesis|journal=Tetrahedron|volume=67|issue=45|pages=8607–8614|doi=10.1016/j.tet.2011.09.036|issn=0040-4020|pmc=3196336|pmid=22021940}}</ref><ref>{{Cite journal|last1=Sieverding|first1=Paul|last2=Osterbrink|first2=Johanna|last3=Besson|first3=Claire|last4=Kögerler|first4=Paul|date=2019-01-18|title=Kinetics and mechanism of pyrrolidine buffer-catalyzed fulvene formation|journal=J. Org. Chem.|volume=84|issue=2|pages=486–494|doi=10.1021/acs.joc.8b01660|pmid=30540466|issn=0022-3263}}</ref> |
||
==Properties== |
|||
The cross-conjugation generally destabilizes the exocyclic double bond, as (per Hückel's rules) polarization of the π electrons would lead to an aromatic ring ion. Consequently, fulvenes add nucleo- and electrophiles easily. They also have a small [[HOMO-LUMO gap]], typically leading to the eponymous [[visible spectrum|visible coloration]] ("[[wikt:fulvus|fulvus]]" is Latin for "yellow").<ref>{{cite book|doi=10.1002/9780470772256.ch4|title=The Chemistry of Double-Bonded Functional Groups|volume=Supplement A, Part 2|editor-first=Saul|editor-last=Patai|publisher=Wiley|series=The Chemistry of Functional Groups|year=1989|chapter=Fulvenes|first=M.|last=Neuenschwander|pages=1132–1136|isbn=978-0-470-77225-6}}</ref> |
|||
==Ligand in organometallic chemistry== |
==Ligand in organometallic chemistry== |
||
| Line 42: | Line 40: | ||
{{Authority control}} |
{{Authority control}} |
||
[[Category:Hydrocarbons]] |
|||
[[Category:Cycloalkenes]] |
[[Category:Cycloalkenes]] |
||
[[Category:Vinylidene compounds]] |
|||
Latest revision as of 15:50, 29 December 2024

Fulvenes are the class of hydrocarbon obtained by formally cross-conjugating one ring and methylidene through a common exocyclic double bond.[1][2]
The name is derived from fulvene, which has one pentagonal ring. Other examples include methylenecyclopropene (triafulvene) and heptafulvene.
Fulvenes are generally named based on the number of ring atoms. Thus methylenecyclopropene is "triafulvene", methylenecyclopentadiene is "pentafulvene", etc.[3]
Preparation
[edit]Fulvenes are readily prepared by the condensation of cyclopentadiene and aldehydes and ketones:
- C5H6 + R2C=O → C4H4C=CR2 + H2O
Johannes Thiele is credited with discovering this reaction.[4][5]
Modern synthesis of fulvenes employ buffer systems.[6][7]
Properties
[edit]The cross-conjugation generally destabilizes the exocyclic double bond, as (per Hückel's rules) polarization of the π electrons would lead to an aromatic ring ion. Consequently, fulvenes add nucleo- and electrophiles easily. They also have a small HOMO-LUMO gap, typically leading to the eponymous visible coloration ("fulvus" is Latin for "yellow").[8]
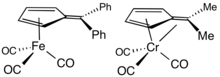
Ligand in organometallic chemistry
[edit]Fulvenes are common ligands and ligand precursors in organometallic chemistry.[9] 2,3,4,5-Tetramethylfulvene, abbreviated Me4Fv, results from the deprotonation of cationic pentamethylcyclopentadienyl complexes.[10] Some Me4Fv complexes are called tuck-in complexes.
References
[edit]- ^ Agranat, Israel (2012), "Ground-State Versus Excited-State Polarity of Triafulvenes: A Study of Solvent Effects on Molecular Electronic Spectra", The Jerusalem Symposia on Quantum Chemistry and Biochemistry, 8: 573–583, doi:10.1007/978-94-010-1837-1_36, ISBN 978-94-010-1839-5
- ^ Neuenschwander, Markus (1986), "Synthetic and NMR spectroscopic investigations of fulvenes and fulvalenes" (PDF), Pure Appl. Chem., 58 (1): 55–66, doi:10.1351/pac198658010055
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Fulvenes". doi:10.1351/goldbook.F02550
- ^ Thiele, J. (1900). "Ueber Ketonreactionen bei dem Cyclopentadiën". Chemische Berichte. 33: 666–673. doi:10.1002/cber.190003301113.
- ^ Hafner, K.; Vöpel, K. H.; Ploss, G.; König, C. (1967). "6-(Dimethylamino)Fulvene". Organic Syntheses. 47: 52. doi:10.15227/orgsyn.047.0052.
- ^ Coşkun, Necdet; Erden, Ihsan (2011-11-11). "An efficient catalytic method for fulvene synthesis". Tetrahedron. 67 (45): 8607–8614. doi:10.1016/j.tet.2011.09.036. ISSN 0040-4020. PMC 3196336. PMID 22021940.
- ^ Sieverding, Paul; Osterbrink, Johanna; Besson, Claire; Kögerler, Paul (2019-01-18). "Kinetics and mechanism of pyrrolidine buffer-catalyzed fulvene formation". J. Org. Chem. 84 (2): 486–494. doi:10.1021/acs.joc.8b01660. ISSN 0022-3263. PMID 30540466.
- ^ Neuenschwander, M. (1989). "Fulvenes". In Patai, Saul (ed.). The Chemistry of Double-Bonded Functional Groups. The Chemistry of Functional Groups. Vol. Supplement A, Part 2. Wiley. pp. 1132–1136. doi:10.1002/9780470772256.ch4. ISBN 978-0-470-77225-6.
- ^ Strohfeldt, Katja; Tacke, Matthias (2008). "Bioorganometallic fulvene-derived titanocene anti-cancer drugs". Chemical Society Reviews. 37 (6): 1174–87. doi:10.1039/B707310K. PMID 18497930.
- ^ Kreindlin, A. Z.; Rybinskaya, M. A. (2004). "Cationic and Neutral Transition Metal Complexes with a Tetramethylfulvene or Trimethylallyldiene Ligand". Russian Chemical Reviews. 73 (5): 417–432. Bibcode:2004RuCRv..73..417K. doi:10.1070/RC2004v073n05ABEH000842.
