Nirmatrelvir: Difference between revisions
step |
m →top: Fixed paragraph break Tags: Mobile edit Mobile app edit Android app edit App full source |
||
| (67 intermediate revisions by 32 users not shown) | |||
| Line 2: | Line 2: | ||
{{Use dmy dates|date=January 2022}} |
{{Use dmy dates|date=January 2022}} |
||
{{Use American English|date=January 2022}} |
{{Use American English|date=January 2022}} |
||
{{cs1 config|name-list-style=vanc|display-authors=6}} |
|||
{{Infobox drug |
{{Infobox drug |
||
| drug_name = |
| drug_name = |
||
| INN = |
| INN = |
||
| type = <!-- empty --> |
| type = <!-- empty --> |
||
| image = PF-07321332.svg |
| image = PF-07321332.svg |
||
| width = |
| width = |
||
| alt = |
| alt = |
||
| caption = |
| caption = |
||
<!-- Clinical data --> |
<!-- Clinical data --> |
||
| pronounce = {{IPAc-en |
| pronounce = {{IPAc-en|n|ɜːr|ˈ|m|æ|t|r|əl|v|ɪər}}<br />{{respell|nur|MAT|rəl|veer}} or {{IPAc-en|ˌ|n|ɜːr|m|ə|ˈ|t|r|ɛ|l|v|ɪər}}<br />{{respell|NUR|mə|TREL|veer}} |
||
| tradename = |
| tradename = |
||
| Drugs.com = |
| Drugs.com = |
||
| MedlinePlus = |
| MedlinePlus = |
||
| licence_EU = <!-- EMA uses INN (or special INN_EMA) --> |
| licence_EU = <!-- EMA uses INN (or special INN_EMA) --> |
||
| DailyMedID = Nirmatrelvir |
| DailyMedID = Nirmatrelvir |
||
| licence_US = <!-- FDA may use generic or brand name (generic name preferred) --> |
| licence_US = <!-- FDA may use generic or brand name (generic name preferred) --> |
||
| pregnancy_AU = B3 |
| pregnancy_AU = B3 |
||
| pregnancy_AU_comment = <ref>{{cite web | title=Updates to the Prescribing Medicines in Pregnancy database | website=Therapeutic Goods Administration (TGA) | date=12 May 2022 | url=https://www.tga.gov.au/updates-prescribing-medicines-pregnancy-database | access-date=13 May 2022}}</ref> |
| pregnancy_AU_comment = <ref>{{cite web | title=Updates to the Prescribing Medicines in Pregnancy database | website=Therapeutic Goods Administration (TGA) | date=12 May 2022 | url=https://www.tga.gov.au/resources/resource/guidance/updates-prescribing-medicines-pregnancy-database | access-date=13 May 2022 | archive-date=3 April 2022 | archive-url=https://web.archive.org/web/20220403064059/https://www.tga.gov.au/updates-prescribing-medicines-pregnancy-database | url-status=live }}</ref> |
||
| pregnancy_category = |
| pregnancy_category = |
||
| routes_of_administration = [[Oral administration|By mouth]] |
| routes_of_administration = [[Oral administration|By mouth]] |
||
| class = |
| class = |
||
| ATCvet = |
| ATCvet = |
||
| ATC_prefix = None |
| ATC_prefix = None |
||
| ATC_suffix = |
| ATC_suffix = |
||
| ATC_supplemental = |
| ATC_supplemental = |
||
<!-- Legal status --> |
<!-- Legal status --> |
||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled --> |
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled --> |
||
| legal_AU_comment = |
| legal_AU_comment = |
||
| legal_BR = <!-- OTC, A1, A2, A3, B1, B2, C1, C2, C3, C4, C5, D1, D2, E, F --> |
| legal_BR = <!-- OTC, A1, A2, A3, B1, B2, C1, C2, C3, C4, C5, D1, D2, E, F --> |
||
| legal_BR_comment = |
| legal_BR_comment = |
||
| legal_CA = Rx-only |
| legal_CA = Rx-only |
||
| legal_CA_comment = <ref>{{cite web | title=Notice: Nirmatrelvir (COVID-19) added to Prescription Drug List (PDL) | website=Health Canada | date=17 January 2022 | url=https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/prescription-drug-list/notices-changes/amendment-nirmatrelvir.html | access-date=29 May 2022}}</ref> |
| legal_CA_comment = <ref>{{cite web | title=Notice: Nirmatrelvir (COVID-19) added to Prescription Drug List (PDL) | website=Health Canada | date=17 January 2022 | url=https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/prescription-drug-list/notices-changes/amendment-nirmatrelvir.html | access-date=29 May 2022 | archive-date=29 May 2022 | archive-url=https://web.archive.org/web/20220529181817/https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/prescription-drug-list/notices-changes/amendment-nirmatrelvir.html | url-status=live }}</ref> |
||
| legal_DE = <!-- Anlage I, II, III or Unscheduled --> |
| legal_DE = <!-- Anlage I, II, III or Unscheduled --> |
||
| legal_DE_comment = |
| legal_DE_comment = |
||
| legal_NZ = <!-- Class A, B, C --> |
| legal_NZ = <!-- Class A, B, C --> |
||
| legal_NZ_comment = |
| legal_NZ_comment = |
||
| legal_UK = <!-- GSL, P, POM, CD, CD Lic, CD POM, CD No Reg POM, CD (Benz) POM, CD (Anab) POM or CD Inv POM / Class A, B, C --> |
| legal_UK = <!-- GSL, P, POM, CD, CD Lic, CD POM, CD No Reg POM, CD (Benz) POM, CD (Anab) POM or CD Inv POM / Class A, B, C --> |
||
| legal_UK_comment = |
| legal_UK_comment = |
||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
||
| legal_US_comment = |
| legal_US_comment = |
||
| legal_EU = |
| legal_EU = |
||
| legal_EU_comment = |
| legal_EU_comment = |
||
| legal_UN = <!-- N I, II, III, IV / P I, II, III, IV --> |
| legal_UN = <!-- N I, II, III, IV / P I, II, III, IV --> |
||
| legal_UN_comment = |
| legal_UN_comment = |
||
| legal_status = <!-- For countries not listed above --> |
| legal_status = <!-- For countries not listed above --> |
||
<!-- Pharmacokinetic data --> |
<!-- Pharmacokinetic data --> |
||
| bioavailability = |
| bioavailability = |
||
| protein_bound = |
| protein_bound = |
||
| metabolism = |
| metabolism = |
||
| metabolites = |
| metabolites = |
||
| onset = |
| onset = |
||
| elimination_half-life = |
| elimination_half-life = |
||
| duration_of_action = |
| duration_of_action = |
||
| excretion = |
| excretion = |
||
<!-- Identifiers --> |
<!-- Identifiers --> |
||
| CAS_number = 2628280-40-8 |
| CAS_number = 2628280-40-8 |
||
| CAS_supplemental = |
| CAS_supplemental = |
||
| PubChem = 155903259 |
| PubChem = 155903259 |
||
| IUPHAR_ligand = |
| IUPHAR_ligand = |
||
| DrugBank = DB16691 |
| DrugBank = DB16691 |
||
| ChemSpiderID = 114826566 |
| ChemSpiderID = 114826566 |
||
| Line 70: | Line 71: | ||
| KEGG = D12244 |
| KEGG = D12244 |
||
| ChEBI = 170007 |
| ChEBI = 170007 |
||
| ChEMBL = |
| ChEMBL = |
||
| NIAID_ChemDB = |
| NIAID_ChemDB = |
||
| PDB_ligand = |
| PDB_ligand = |
||
| synonyms = PF-07321332 |
| synonyms = PF-07321332 |
||
<!-- Chemical and physical data --> |
<!-- Chemical and physical data --> |
||
| Line 84: | Line 85: | ||
| SMILES = CC1([C@@H]2[C@H]1[C@H](N(C2)C(=O)[C@H](C(C)(C)C)NC(=O)C(F)(F)F)C(=O)N[C@@H](C[C@@H]3CCNC3=O)C#N)C |
| SMILES = CC1([C@@H]2[C@H]1[C@H](N(C2)C(=O)[C@H](C(C)(C)C)NC(=O)C(F)(F)F)C(=O)N[C@@H](C[C@@H]3CCNC3=O)C#N)C |
||
| StdInChI = 1S/C23H32F3N5O4/c1-21(2,3)16(30-20(35)23(24,25)26)19(34)31-10-13-14(22(13,4)5)15(31)18(33)29-12(9-27)8-11-6-7-28-17(11)32/h11-16H,6-8,10H2,1-5H3,(H,28,32)(H,29,33)(H,30,35)/t11-,12-,13-,14-,15-,16+/m0/s1 |
| StdInChI = 1S/C23H32F3N5O4/c1-21(2,3)16(30-20(35)23(24,25)26)19(34)31-10-13-14(22(13,4)5)15(31)18(33)29-12(9-27)8-11-6-7-28-17(11)32/h11-16H,6-8,10H2,1-5H3,(H,28,32)(H,29,33)(H,30,35)/t11-,12-,13-,14-,15-,16+/m0/s1 |
||
| StdInChI_comment = |
| StdInChI_comment = |
||
| StdInChIKey = LIENCHBZNNMNKG-OJFNHCPVSA-N |
| StdInChIKey = LIENCHBZNNMNKG-OJFNHCPVSA-N |
||
| density = |
| density = |
||
| density_notes = |
| density_notes = |
||
| melting_point = 192.9 |
| melting_point = 192.9 |
||
| melting_high = |
| melting_high = |
||
| melting_notes = <ref name=Owen /> |
| melting_notes = <ref name=Owen /> |
||
| boiling_point = |
| boiling_point = |
||
| boiling_notes = |
| boiling_notes = |
||
| solubility = |
| solubility = |
||
| sol_units = |
| sol_units = |
||
| specific_rotation = |
| specific_rotation = |
||
}} |
}} |
||
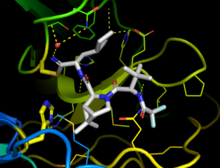
[[File:Xray crystal structure PDB-7si9.png|alt=Xray crystal structure PDB:7si9|thumb|X-ray crystal structure ([https://www.rcsb.org/structure/7SI9 PDB:7SI9] and [https://www.rcsb.org/structure/7VH8 7VH8]) of the SARS-CoV-2 protease inhibitor nirmatrelvir bound to the viral [[3CLpro]] protease enzyme. [[Ribbon diagram]] of the protein with the drug shown as sticks. The catalytic residues (His41, Cys145) are shown as yellow sticks.]] |
[[File:Xray crystal structure PDB-7si9.png|alt=Xray crystal structure PDB:7si9|thumb|X-ray crystal structure ([https://www.rcsb.org/structure/7SI9 PDB:7SI9] and [https://www.rcsb.org/structure/7VH8 7VH8]) of the SARS-CoV-2 protease inhibitor nirmatrelvir bound to the viral [[3CLpro]] protease enzyme. [[Ribbon diagram]] of the protein with the drug shown as sticks. The catalytic residues (His41, Cys145) are shown as yellow sticks.]] |
||
'''Nirmatrelvir''' is an [[antiviral medication]] developed by [[Pfizer]] which acts as an orally active [[3C-like protease|3C-like]] [[Protease inhibitor (pharmacology)|protease inhibitor]].<ref name=Owen /><ref>{{cite journal|vauthors=Şimşek-Yavuz S, Komsuoğlu Çelikyurt FI|date=August 2021|title=Antiviral treatment of COVID-19: An update|journal=Turkish Journal of Medical Sciences|volume=51|issue=SI-1|pages=3372–3390|doi=10.3906/sag-2106-250|pmid=34391321| pmc=8771049 |s2cid=237054672}}</ref><ref>{{cite journal|vauthors=Ahmad B, Batool M, Ain QU, Kim MS, Choi S|date=August 2021|title=Exploring the Binding Mechanism of PF-07321332 SARS-CoV-2 Protease Inhibitor through Molecular Dynamics and Binding Free Energy Simulations|journal=International Journal of Molecular Sciences|volume=22|issue=17|page=9124|doi=10.3390/ijms22179124|pmc=8430524|pmid=34502033|doi-access=free}}</ref><ref name="pfizer2">{{Cite press release |date=14 December 2021 |title=Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death | |
'''Nirmatrelvir''' is an [[antiviral medication]] developed by [[Pfizer]] which acts as an orally active [[3C-like protease|3C-like]] [[Protease inhibitor (pharmacology)|protease inhibitor]].<ref name=Owen /><ref>{{cite journal|vauthors=Şimşek-Yavuz S, Komsuoğlu Çelikyurt FI|date=August 2021|title=Antiviral treatment of COVID-19: An update|journal=Turkish Journal of Medical Sciences|volume=51|issue=SI-1|pages=3372–3390|doi=10.3906/sag-2106-250|pmid=34391321| pmc=8771049 |s2cid=237054672}}</ref><ref>{{cite journal|vauthors=Ahmad B, Batool M, Ain QU, Kim MS, Choi S|date=August 2021|title=Exploring the Binding Mechanism of PF-07321332 SARS-CoV-2 Protease Inhibitor through Molecular Dynamics and Binding Free Energy Simulations|journal=International Journal of Molecular Sciences|volume=22|issue=17|page=9124|doi=10.3390/ijms22179124|pmc=8430524|pmid=34502033|doi-access=free}}</ref><ref name="pfizer2">{{Cite press release |date=14 December 2021 |title=Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death |url=https://www.businesswire.com/news/home/20211214005548/en/Pfizer-Announces-Additional-Phase-23-Study-Results-Confirming-Robust-Efficacy-of-Novel-COVID-19-Oral-Antiviral-Treatment-Candidate-in-Reducing-Risk-of-Hospitalization-or-Death |publisher=[[Pfizer]] |via=Business Wire |access-date=25 December 2021 |archive-date=26 December 2021 |archive-url=https://web.archive.org/web/20211226044106/https://www.businesswire.com/news/home/20211214005548/en/Pfizer-Announces-Additional-Phase-23-Study-Results-Confirming-Robust-Efficacy-of-Novel-COVID-19-Oral-Antiviral-Treatment-Candidate-in-Reducing-Risk-of-Hospitalization-or-Death |url-status=live }}</ref><ref name=":0">{{cite journal|vauthors=Vandyck K, Deval J|date=August 2021|title=Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection|journal=Current Opinion in Virology|volume=49|pages=36–40|doi=10.1016/j.coviro.2021.04.006|pmc=8075814|pmid=34029993}}</ref> It is part of a [[nirmatrelvir/ritonavir]] combination used to treat [[COVID-19]] and sold under the brand name Paxlovid.<ref name="Paxlovid FDA label">{{cite web | title=Paxlovid- nirmatrelvir and ritonavir kit | website=DailyMed | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7bdddfba-bd31-44cb-ba9e-23a4e17a4691 | access-date=30 December 2021 | archive-date=31 December 2021 | archive-url=https://web.archive.org/web/20211231050453/https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7bdddfba-bd31-44cb-ba9e-23a4e17a4691 | url-status=live }}</ref> |
||
== Development == |
== Development == |
||
===Pharmaceutical=== |
===Pharmaceutical=== |
||
Coronaviral [[protease]]s cleave multiple sites in the viral [[polyprotein]], usually after there are [[glutamine]] residues. Early work on related human [[rhinovirus]]es showed that the flexible |
Coronaviral [[protease]]s cleave multiple sites in the viral [[polyprotein]], usually after there are [[glutamine]] residues. Early work on related human [[rhinovirus]]es showed that the flexible glutamine side chain in inhibitors could be replaced by a rigid [[2-pyrrolidone|pyrrolidone]].<ref>{{cite journal | vauthors = Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R | title = Coronavirus Main Proteinase (3CLpro) Structure: Basis for Design of Anti-SARS Drugs | journal = Science | volume = 300 | issue = 5626 | pages = 1763–1767 | date = June 2003 | pmid = 12746549 | doi = 10.1126/science.1085658 | bibcode = 2003Sci...300.1763A | s2cid = 13031405 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Dragovich PS, Prins TJ, Zhou R, Webber SE, Marakovits JT, Fuhrman SA, Patick AK, Matthews DA, Lee CA, Ford CE, Burke BJ, Rejto PA, Hendrickson TF, Tuntland T, Brown EL, Meador JW, Ferre RA, Harr JE, Kosa MB, Worland ST | title = Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as L-glutamine replacements | journal = Journal of Medicinal Chemistry | volume = 42 | issue = 7 | pages = 1213–1224 | date = April 1999 | pmid = 10197965 | doi = 10.1021/jm9805384 }}</ref> These drugs had been further developed prior to the [[COVID-19 pandemic]] for other diseases including [[Severe acute respiratory syndrome|SARS]].<ref>{{cite journal | vauthors = Pillaiyar T, Manickam M, Namasivayam V, Hayashi Y, Jung SH | title = An Overview of Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) 3CL Protease Inhibitors: Peptidomimetics and Small Molecule Chemotherapy | journal = Journal of Medicinal Chemistry | volume = 59 | issue = 14 | pages = 6595–6628 | date = July 2016 | pmid = 26878082 | pmc = 7075650 | doi = 10.1021/acs.jmedchem.5b01461 }}</ref> The utility of targeting the 3CL protease in a real world setting was first demonstrated in 2018 when [[GC376]] (a [[prodrug]] of GC373) was used to treat the previously 100% lethal cat coronavirus disease, [[feline infectious peritonitis]], caused by [[feline coronavirus]].<ref>{{cite journal | vauthors = Pedersen NC, Kim Y, Liu H, Galasiti Kankanamalage AC, Eckstrand C, Groutas WC, Bannasch M, Meadows JM, Chang KO | title = Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis | journal = Journal of Feline Medicine and Surgery | volume = 20 | issue = 4 | pages = 378–392 | date = April 2018 | pmid = 28901812 | pmc = 5871025 | doi = 10.1177/1098612X17729626 | doi-access=free | title-link=doi }}</ref> Nirmatrelvir and GC373 are both [[peptidomimetic]]s, share the aforementioned [[2-pyrrolidone|pyrrolidone]] in P1 position and are competitive inhibitors. They use a [[nitrile]] and an [[aldehyde]] respectively to bind the catalytic [[cysteine]].<ref>{{cite journal | vauthors = Halford B | date = 7 April 2021 | title = Pfizer unveils its oral SARS-CoV-2 inhibitor | journal = Chemical & Engineering News | volume = 99 | issue = 13 | pages = 7 | doi = 10.47287/cen-09913-scicon3 | s2cid = 234887434 | doi-access= | title-link=doi }}</ref><ref>{{cite journal | vauthors = Vuong W, Khan MB, Fischer C, Arutyunova E, Lamer T, Shields J, Saffran HA, McKay RT, van Belkum MJ, Joyce MA, Young HS, Tyrrell DL, Vederas JC, Lemieux MJ | title = Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication | journal = Nature Communications | volume = 11 | issue = 1 | pages = 4282 | date = August 2020 | pmid = 32855413 | pmc = 7453019 | doi = 10.1038/s41467-020-18096-2 | doi-access = free }}</ref> Pfizer investigated two series of compounds, with nitrile and benzothiazol-2-yl ketone as the reactive group, respectively, and in the end settled on using nitrile.<ref name="Halford">{{cite magazine |vauthors=Halford B |title=How Pfizer scientists transformed an old drug lead into a COVID-19 antiviral: Behind the scenes of the medicinal chemistry campaign that led to the pill Paxlovid |magazine=Chemical & Engineering News |date=14 January 2022 |volume=100 |issue=3 |url=https://cen.acs.org/pharmaceuticals/drug-discovery/How-Pfizer-scientists-transformed-an-old-drug-lead-into-a-COVID-19-antiviral/100/i3 |access-date=14 January 2022 |archive-date=14 January 2022 |archive-url=https://web.archive.org/web/20220114181829/https://cen.acs.org/pharmaceuticals/drug-discovery/How-Pfizer-scientists-transformed-an-old-drug-lead-into-a-COVID-19-antiviral/100/i3 |url-status=live }}</ref> |
||
Nirmatrelvir was developed by modification of the earlier clinical candidate [[lufotrelvir]],<ref>{{ClinicalTrialsGov|NCT04535167|First-In-Human Study To Evaluate Safety, Tolerability, And Pharmacokinetics Following Single Ascending And Multiple Ascending Doses of PF-07304814 In Hospitalized Participants With COVID-19 }}</ref>{{Full citation needed|date=May 2022}}<ref>{{cite journal |doi=10.1038/s41467-021-26239-2 |title=Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19 |year=2021 | vauthors =Boras B, Jones RM, Anson BJ, Arenson D, Aschenbrenner L, Bakowski MA, Beutler N, Binder J, Chen E, Eng H, Hammond H, Hammond J, Haupt RE, Hoffman R, Kadar EP, Kania R, Kimoto E, Kirkpatrick MG, Lanyon L, Lendy EK, Lillis JR, Logue J, Luthra SA, Ma CL, Mason SW, McGrath ME, Noell S, Obach RS, O'Brien MN, O'Connor R |journal=Nature Communications |volume=12 |issue=1 |page=6055 |pmid=34663813 |pmc=8523698 |bibcode=2021NatCo..12.6055B | |
Nirmatrelvir was developed by modification of the earlier clinical candidate [[lufotrelvir]],<ref>{{ClinicalTrialsGov|NCT04535167|First-In-Human Study To Evaluate Safety, Tolerability, And Pharmacokinetics Following Single Ascending And Multiple Ascending Doses of PF-07304814 In Hospitalized Participants With COVID-19 }}</ref>{{Full citation needed|date=May 2022}}<ref>{{cite journal |doi=10.1038/s41467-021-26239-2 |title=Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19 |year=2021 | vauthors =Boras B, Jones RM, Anson BJ, Arenson D, Aschenbrenner L, Bakowski MA, Beutler N, Binder J, Chen E, Eng H, Hammond H, Hammond J, Haupt RE, Hoffman R, Kadar EP, Kania R, Kimoto E, Kirkpatrick MG, Lanyon L, Lendy EK, Lillis JR, Logue J, Luthra SA, Ma CL, Mason SW, McGrath ME, Noell S, Obach RS, O'Brien MN, O'Connor R |journal=Nature Communications |volume=12 |issue=1 |page=6055 |pmid=34663813 |pmc=8523698 |bibcode=2021NatCo..12.6055B }}</ref><ref>{{cite journal | vauthors = Galli M, Migliano F, Fasano V, Silvani A, Passarella D, Citarella A | title = Nirmatrelvir: From Discovery to Modern and Alternative Synthetic Approaches. | journal = Processes | date = 2024 | volume = 12 | issue = 6 | pages = 1242 | doi = 10.3390/pr12061242 | doi-access = free }}</ref> which is also a covalent protease inhibitor but its active element is a phosphate [[prodrug]] of a [[hydroxyketone]]. Lufotrelvir needs to be administered [[Intravenous therapy|intravenously]] limiting its use to a hospital setting. Stepwise modification of the [[tripeptide]] [[peptidomimetic]] led to nirmatrelvir, which is suitable for [[oral administration]].<ref name=Owen>{{cite journal | vauthors = Owen DR, Allerton CM, Anderson AS, Aschenbrenner L, Avery M, Berritt S, Boras B, Cardin RD, Carlo A, Coffman KJ, Dantonio A, Di L, Eng H, Ferre R, Gajiwala KS, Gibson SA, Greasley SE, Hurst BL, Kadar EP, Kalgutkar AS, Lee JC, Lee J, Liu W, Mason SW, Noell S, Novak JJ, Obach RS, Ogilvie K, Patel NC, Pettersson M, Rai DK, Reese MR, Sammons MF, Sathish JG, Singh RS, Steppan CM, Stewart AE, Tuttle JB, Updyke L, Verhoest PR, Wei L, Yang Q, Zhu Y | title = An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19 | journal = Science | pages = 1586–1593 | date = November 2021 | pmid = 34726479 | doi = 10.1126/science.abl4784 | doi-access=free | s2cid = 240422219 | title-link=doi | volume = 374 | issue = 6575 | bibcode = 2021Sci...374.1586O }}</ref> Key changes include a reduction in the number of [[hydrogen bond]] donors, and the [[Lipinski's rule of five|number of rotatable bonds]] by introducing a rigid bicyclic [[noncanonical amino acid incorporation|non-canonical amino acid]] (specifically, a "fused cyclopropyl ring with two methyl groups"<ref name="Halford" />), which mimics the [[leucine]] residue found in earlier inhibitors. This residue had previously been used in the synthesis of [[boceprevir]].<ref name="pmid18193821">{{cite journal | vauthors = Njoroge FG, Chen KX, Shih NY, Piwinski JJ | title = Challenges in modern drug discovery: a case study of boceprevir, an HCV protease inhibitor for the treatment of hepatitis C virus infection | journal = Accounts of Chemical Research | volume = 41 | issue = 1 | pages = 50–59 | date = January 2008 | pmid = 18193821 | doi = 10.1021/ar700109k | s2cid = 2629035 }}</ref> |
||
The leucine-like residue resulted in loss of a nearby contact with a [[glutamine]] on the 3C-like protease.<ref name="Halford" /> To compensate Pfizer tried adding methane [[sulfonamide]], [[acetamide]] |
Tert-leucine (abbreviation: Tle) used in the P3 position of nirmatrelvir was identified first as optimal non-canonical amino acid in potential drug targeting SARS-CoV-2 3C-like protease using [[combinatorial chemistry]] (hybrid combinatorial substrate library technology).<ref>{{cite journal |vauthors=Poreba M, Salvesen GS, Drag M |title=Synthesis of a HyCoSuL peptide substrate library to dissect protease substrate specificity |journal=Nature Protocols |pages=2189–2214 |date=October 2017 |volume=12 |issue=10 |doi=10.1038/nprot.2017.091 |pmid=28933778 |s2cid=23895951 |url=https://zenodo.org/record/3629507 |access-date=25 December 2021 |archive-date=27 December 2021 |archive-url=https://web.archive.org/web/20211227183040/https://zenodo.org/record/3629507 |url-status=live }}</ref><ref>{{cite journal |vauthors=Rut W, Groborz K, Zhang L, Sun X, Zmudzinski M, Pawlik B, Wang X, Jochmans D, Neyts J, Młynarski W, Hilgenfeld R, Drag M |title=SARS-CoV-2 M pro inhibitors and activity-based probes for patient-sample imaging |journal=Nature Chemical Biology |date=October 2020 |volume=17 |issue=2 |pages=222–228 |doi=10.1038/s41589-020-00689-z |pmid=33093684 |s2cid=224827220 | doi-access=free | title-link=doi }}</ref> The leucine-like residue resulted in loss of a nearby contact with a [[glutamine]] on the 3C-like protease.<ref name="Halford" /> To compensate, Pfizer tried adding methane [[sulfonamide]], [[acetamide]] and [[trifluoroacetamide]], discovering that of the three, trifluoroacetamide resulted in superior oral bioavailability.<ref name="Halford" /> |
||
==Chemistry and pharmacology== |
==Chemistry and pharmacology== |
||
Full details of the synthesis of nirmatrelvir were first published by scientists from Pfizer. |
Full details of the synthesis of nirmatrelvir were first published by scientists from Pfizer. |
||
:[[File:PF-07321332 synthesis.svg|650px]] |
:[[File:PF-07321332 synthesis.svg|650px]] |
||
In the penultimate step a synthetic [[homochiral]] [[amino acid]] is coupled with a homochiral amino [[amide]] using the water-soluble [[carbodiimide]] [[EDCI]] as a [[coupling reaction|coupling]] agent. The resulting intermediate is then treated with [[Burgess reagent]], which [[Dehydration reaction|dehydrates]] the amide group to the [[nitrile]] of the product.<ref name=Owen /> |
In the penultimate step a synthetic [[homochiral]] [[amino acid]] is coupled with a homochiral amino [[amide]] using the water-soluble [[carbodiimide]] [[EDCI]] as a [[coupling reaction|coupling]] agent. The resulting intermediate is then treated with [[Burgess reagent]], which [[Dehydration reaction|dehydrates]] the amide group to the [[nitrile]] of the product.<ref name=Owen /> |
||
Nirmatrelvir is a covalent inhibitor, binding directly to the catalytic [[cysteine]] (Cys145) [[amino acid residue|residue]] of the [[cysteine protease]] enzyme.<ref>{{cite journal |vauthors=Pavan M, Bolcato G, Bassani D, Sturlese M, Moro S |title=Supervised Molecular Dynamics (SuMD) Insights into the mechanism of action of SARS-CoV-2 main protease inhibitor PF-07321332 |journal=Journal of Enzyme Inhibition and Medicinal Chemistry |volume=36 |issue=1 |pages=1646–1650 |date=December 2021 |pmid=34289752 |pmc=8300928 |doi=10.1080/14756366.2021.1954919 }}</ref> |
Nirmatrelvir is a covalent inhibitor, binding directly to the catalytic [[cysteine]] (Cys145) [[amino acid residue|residue]] of the [[cysteine protease]] enzyme.<ref>{{cite journal |vauthors=Pavan M, Bolcato G, Bassani D, Sturlese M, Moro S |title=Supervised Molecular Dynamics (SuMD) Insights into the mechanism of action of SARS-CoV-2 main protease inhibitor PF-07321332 |journal=Journal of Enzyme Inhibition and Medicinal Chemistry |volume=36 |issue=1 |pages=1646–1650 |date=December 2021 |pmid=34289752 |pmc=8300928 |doi=10.1080/14756366.2021.1954919 }}</ref> |
||
In the co-packaged medication [[nirmatrelvir/ritonavir]], [[ritonavir]] serves to slow the metabolism of nirmatrelvir via [[Cytochrome P450|cytochrome enzyme]] inhibition, thereby increasing the circulating concentration of the main drug.<ref>{{cite |
In the co-packaged medication [[nirmatrelvir/ritonavir]], [[ritonavir]] serves to slow the metabolism of nirmatrelvir via [[Cytochrome P450|cytochrome enzyme]] inhibition, thereby increasing the circulating concentration of the main drug.<ref>{{cite news|vauthors=Woodley M|date=19 October 2021|title=What is Australia's potential new COVID treatment?|url=https://www1.racgp.org.au/newsgp/clinical/what-is-australia-s-potential-new-covid-treatment|access-date=6 November 2021|newspaper=Newsgp|archive-date=5 November 2021|archive-url=https://web.archive.org/web/20211105155311/https://www1.racgp.org.au/newsgp/clinical/what-is-australia-s-potential-new-covid-treatment|url-status=live}}</ref> This effect is also used in [[Management of HIV/AIDS|HIV therapy]], where ritonavir is used in combination with another [[Protease inhibitor (pharmacology)|protease inhibitor]] to similarly enhance their pharmacokinetics.<ref>{{cite journal | vauthors = Hull MW, Montaner JS | title = Ritonavir-boosted protease inhibitors in HIV therapy | journal = Annals of Medicine | volume = 43 | issue = 5 | pages = 375–388 | date = April 2011 | pmid = 21501034 | doi = 10.3109/07853890.2011.572905 | s2cid = 21400449 }}</ref> |
||
== Society and culture == |
== Society and culture == |
||
=== Licensing === |
=== Licensing === |
||
In November 2021, Pfizer signed a license agreement with the [[United Nations]]–backed [[Medicines Patent Pool]] to allow nirmatrelvir to be manufactured and sold in 95 countries.<ref>{{cite press release | title=Pfizer and The Medicines Patent Pool (MPP) Sign Licensing Agreement for COVID-19 Oral Antiviral Treatment Candidate to Expand Access in Low- and Middle-Income Countries | publisher=[[Pfizer]] | via=Business Wire | date=16 November 2021 | url=https://www.businesswire.com/news/home/20211116005353/en/ | access-date=17 November 2021}}</ref> Pfizer stated that the agreement will allow local medicine manufacturers to produce the pill "with the goal of facilitating greater access to the global population". The deal excludes several countries with major COVID-19 outbreaks including Brazil, China, Russia, Argentina, and Thailand.<ref>{{cite news |title=Covid-19: Pfizer to allow developing nations to make its treatment pill |url=https://www.bbc.com/news/world-us-canada-59310582 |access-date=17 November 2021 |work=[[BBC News]] |date=16 November 2021 |archive-url=https://web.archive.org/web/20211116203444/https://www.bbc.com/news/world-us-canada-59310582 |archive-date=16 November 2021|url-status=live}}</ref><ref>{{cite news | title=Pfizer Will Allow Its Covid Pill to Be Made and Sold Cheaply in Poor Countries | website=The New York Times | date=16 November 2021 | url=https://www.nytimes.com/2021/11/16/health/covid-pill-pfizer.html | access-date=17 November 2021}}</ref> |
In November 2021, Pfizer signed a license agreement with the [[United Nations]]–backed [[Medicines Patent Pool]] to allow nirmatrelvir to be manufactured and sold in 95 countries.<ref>{{cite press release | title=Pfizer and The Medicines Patent Pool (MPP) Sign Licensing Agreement for COVID-19 Oral Antiviral Treatment Candidate to Expand Access in Low- and Middle-Income Countries | publisher=[[Pfizer]] | via=Business Wire | date=16 November 2021 | url=https://www.businesswire.com/news/home/20211116005353/en/ | access-date=17 November 2021 | archive-date=17 November 2021 | archive-url=https://web.archive.org/web/20211117184448/https://www.businesswire.com/news/home/20211116005353/en/ | url-status=live }}</ref> Pfizer stated that the agreement will allow local medicine manufacturers to produce the pill "with the goal of facilitating greater access to the global population". The deal excludes several countries with major COVID-19 outbreaks including Brazil, China, Russia, Argentina, and Thailand.<ref>{{cite news |title=Covid-19: Pfizer to allow developing nations to make its treatment pill |url=https://www.bbc.com/news/world-us-canada-59310582 |access-date=17 November 2021 |work=[[BBC News]] |date=16 November 2021 |archive-url=https://web.archive.org/web/20211116203444/https://www.bbc.com/news/world-us-canada-59310582 |archive-date=16 November 2021|url-status=live}}</ref><ref>{{cite news | title=Pfizer Will Allow Its Covid Pill to Be Made and Sold Cheaply in Poor Countries | website=The New York Times | date=16 November 2021 | url=https://www.nytimes.com/2021/11/16/health/covid-pill-pfizer.html | access-date=17 November 2021 | archive-date=17 November 2021 | archive-url=https://web.archive.org/web/20211117000251/https://www.nytimes.com/2021/11/16/health/covid-pill-pfizer.html | url-status=live }}</ref> |
||
=== Names === |
|||
Nirmatrelvir is the [[international nonproprietary name]]<ref>{{cite journal | vauthors = ((World Health Organization)) | year = 2022 | title = International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 88 | journal = WHO Drug Information | volume = 36 | issue = 3 | hdl = 10665/363551 | hdl-access = free | author-link = World Health Organization }}</ref> |
|||
== Research == |
== Research == |
||
The research that led to nirmatrelvir began |
The research that led to nirmatrelvir began in March 2020, when Pfizer formally launched a project at its [[Cambridge, Massachusetts]] site to develop antiviral drugs for treating COVID-19.<ref name="Halford" /> In July 2020, Pfizer chemists were able to synthesize nirmatrelvir for the first time.<ref name="Halford" /> In September 2020, Pfizer completed a [[Pharmacokinetics|pharmacokinetic]] study in rats which suggested that nirmatrelvir could be administered orally.<ref name="Halford" /> The actual synthesis of the drug for laboratory research and for clinical trials was carried out at Pfizer's [[Groton, Connecticut]] site.<ref name="Green">{{cite news |vauthors=Green R |title=Pfizer scientists in Groton played a critical role in development of new COVID-19 pill |url=https://www.courant.com/coronavirus/hc-news-coronavirus-pfizer-paxlovid-connecticut-groton-researchers-20211223-hsurttz4lrbt7o4nijvoq5qxui-story.html |work=The Hartford Courant |date=23 December 2021 |access-date=15 January 2022 |archive-date=15 January 2022 |archive-url=https://web.archive.org/web/20220115024158/https://www.courant.com/coronavirus/hc-news-coronavirus-pfizer-paxlovid-connecticut-groton-researchers-20211223-hsurttz4lrbt7o4nijvoq5qxui-story.html |url-status=live }}</ref> |
||
In February 2021, Pfizer launched the company's first [[Phases of clinical research#Phase I|phase I trial]] of PF-07321332 (nirmatrelvir)<ref>{{ |
In February 2021, Pfizer launched the company's first [[Phases of clinical research#Phase I|phase I trial]] of PF-07321332 (nirmatrelvir)<ref>{{clinicalTrialsGov|NCT04756531|Study Of PF-07321332 In Healthy Participants}}</ref> at its clinical research unit in [[New Haven, Connecticut]].<ref name="Green" /> |
||
A study published in March 2023 reported that treatment with nirmatrelvir within five days of initial infection reduced the risk of [[long COVID]] relative to patients who did not receive Paxlovid.<ref>{{cite journal | vauthors = Xie Y, Choi T, Al-Aly Z | title = Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition | journal = [[JAMA Internal Medicine]] | date = March 2023 | volume = 183 | issue = 6 | pages = 554–564 | pmid = 36951829 | doi = 10.1001/jamainternmed.2023.0743 | pmc = 10037200 }}</ref> |
|||
A 2024 study found that "the time to sustained alleviation of all signs and symptoms of Covid-19 did not differ significantly between participants who received nirmatrelvir–ritonavir and those who received placebo."<ref>{{cite journal | vauthors = Hammond J, Fountaine RJ, Yunis C, Fleishaker D, Almas M, Bao W, Wisemandle W, Baniecki ML, Hendrick VM, Kalfov V, Simón-Campos JA, Pypstra R, Rusnak JM | title = Nirmatrelvir for Vaccinated or Unvaccinated Adult Outpatients with Covid-19 | journal = The New England Journal of Medicine | volume = 390 | issue = 13 | pages = 1186–1195 | date = April 2024 | pmid = 38598573 | doi = 10.1056/NEJMoa2309003 | pmc = 11156287 }}</ref> |
|||
== References == |
== References == |
||
{{Reflist}} |
{{Reflist}} |
||
== External links == |
|||
* {{cite web | url = https://druginfo.nlm.nih.gov/drugportal/name/nirmatrelvir | publisher = U.S. National Library of Medicine | work = Drug Information Portal | title = Nirmatrelvir }} |
|||
{{RNA antivirals}} |
{{RNA antivirals}} |
||
| Line 141: | Line 148: | ||
[[Category:Amides]] |
[[Category:Amides]] |
||
[[Category:Combination antiviral drugs]] |
|||
[[Category:COVID-19 drug development]] |
[[Category:COVID-19 drug development]] |
||
[[Category:Cyclopropanes]] |
[[Category:Cyclopropanes]] |
||
[[Category:Nitriles]] |
[[Category:Nitriles]] |
||
[[Category: |
[[Category:Drugs developed by Pfizer]] |
||
[[Category:Pyrrolidones]] |
[[Category:Pyrrolidones]] |
||
[[Category:SARS-CoV-2 main protease inhibitors]] |
|||
[[Category:Trifluoromethyl compounds]] |
[[Category:Trifluoromethyl compounds]] |
||
Latest revision as of 15:17, 10 October 2024
 | |
| Clinical data | |
|---|---|
| Pronunciation | /nɜːrˈmætrəlvɪər/ nur-MAT-rəl-veer or /ˌnɜːrməˈtrɛlvɪər/ NUR-mə-TREL-veer |
| Other names | PF-07321332 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H32F3N5O4 |
| Molar mass | 499.535 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 192.9 °C (379.2 °F) [3] |
| |
| |
Nirmatrelvir is an antiviral medication developed by Pfizer which acts as an orally active 3C-like protease inhibitor.[3][4][5][6][7] It is part of a nirmatrelvir/ritonavir combination used to treat COVID-19 and sold under the brand name Paxlovid.[8]
Development
[edit]Pharmaceutical
[edit]Coronaviral proteases cleave multiple sites in the viral polyprotein, usually after there are glutamine residues. Early work on related human rhinoviruses showed that the flexible glutamine side chain in inhibitors could be replaced by a rigid pyrrolidone.[9][10] These drugs had been further developed prior to the COVID-19 pandemic for other diseases including SARS.[11] The utility of targeting the 3CL protease in a real world setting was first demonstrated in 2018 when GC376 (a prodrug of GC373) was used to treat the previously 100% lethal cat coronavirus disease, feline infectious peritonitis, caused by feline coronavirus.[12] Nirmatrelvir and GC373 are both peptidomimetics, share the aforementioned pyrrolidone in P1 position and are competitive inhibitors. They use a nitrile and an aldehyde respectively to bind the catalytic cysteine.[13][14] Pfizer investigated two series of compounds, with nitrile and benzothiazol-2-yl ketone as the reactive group, respectively, and in the end settled on using nitrile.[15]
Nirmatrelvir was developed by modification of the earlier clinical candidate lufotrelvir,[16][full citation needed][17][18] which is also a covalent protease inhibitor but its active element is a phosphate prodrug of a hydroxyketone. Lufotrelvir needs to be administered intravenously limiting its use to a hospital setting. Stepwise modification of the tripeptide peptidomimetic led to nirmatrelvir, which is suitable for oral administration.[3] Key changes include a reduction in the number of hydrogen bond donors, and the number of rotatable bonds by introducing a rigid bicyclic non-canonical amino acid (specifically, a "fused cyclopropyl ring with two methyl groups"[15]), which mimics the leucine residue found in earlier inhibitors. This residue had previously been used in the synthesis of boceprevir.[19]
Tert-leucine (abbreviation: Tle) used in the P3 position of nirmatrelvir was identified first as optimal non-canonical amino acid in potential drug targeting SARS-CoV-2 3C-like protease using combinatorial chemistry (hybrid combinatorial substrate library technology).[20][21] The leucine-like residue resulted in loss of a nearby contact with a glutamine on the 3C-like protease.[15] To compensate, Pfizer tried adding methane sulfonamide, acetamide and trifluoroacetamide, discovering that of the three, trifluoroacetamide resulted in superior oral bioavailability.[15]
Chemistry and pharmacology
[edit]Full details of the synthesis of nirmatrelvir were first published by scientists from Pfizer.
In the penultimate step a synthetic homochiral amino acid is coupled with a homochiral amino amide using the water-soluble carbodiimide EDCI as a coupling agent. The resulting intermediate is then treated with Burgess reagent, which dehydrates the amide group to the nitrile of the product.[3]
Nirmatrelvir is a covalent inhibitor, binding directly to the catalytic cysteine (Cys145) residue of the cysteine protease enzyme.[22]
In the co-packaged medication nirmatrelvir/ritonavir, ritonavir serves to slow the metabolism of nirmatrelvir via cytochrome enzyme inhibition, thereby increasing the circulating concentration of the main drug.[23] This effect is also used in HIV therapy, where ritonavir is used in combination with another protease inhibitor to similarly enhance their pharmacokinetics.[24]
Society and culture
[edit]Licensing
[edit]In November 2021, Pfizer signed a license agreement with the United Nations–backed Medicines Patent Pool to allow nirmatrelvir to be manufactured and sold in 95 countries.[25] Pfizer stated that the agreement will allow local medicine manufacturers to produce the pill "with the goal of facilitating greater access to the global population". The deal excludes several countries with major COVID-19 outbreaks including Brazil, China, Russia, Argentina, and Thailand.[26][27]
Names
[edit]Nirmatrelvir is the international nonproprietary name[28]
Research
[edit]The research that led to nirmatrelvir began in March 2020, when Pfizer formally launched a project at its Cambridge, Massachusetts site to develop antiviral drugs for treating COVID-19.[15] In July 2020, Pfizer chemists were able to synthesize nirmatrelvir for the first time.[15] In September 2020, Pfizer completed a pharmacokinetic study in rats which suggested that nirmatrelvir could be administered orally.[15] The actual synthesis of the drug for laboratory research and for clinical trials was carried out at Pfizer's Groton, Connecticut site.[29]
In February 2021, Pfizer launched the company's first phase I trial of PF-07321332 (nirmatrelvir)[30] at its clinical research unit in New Haven, Connecticut.[29]
A study published in March 2023 reported that treatment with nirmatrelvir within five days of initial infection reduced the risk of long COVID relative to patients who did not receive Paxlovid.[31]
A 2024 study found that "the time to sustained alleviation of all signs and symptoms of Covid-19 did not differ significantly between participants who received nirmatrelvir–ritonavir and those who received placebo."[32]
References
[edit]- ^ "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 12 May 2022. Archived from the original on 3 April 2022. Retrieved 13 May 2022.
- ^ "Notice: Nirmatrelvir (COVID-19) added to Prescription Drug List (PDL)". Health Canada. 17 January 2022. Archived from the original on 29 May 2022. Retrieved 29 May 2022.
- ^ a b c d Owen DR, Allerton CM, Anderson AS, Aschenbrenner L, Avery M, Berritt S, et al. (November 2021). "An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19". Science. 374 (6575): 1586–1593. Bibcode:2021Sci...374.1586O. doi:10.1126/science.abl4784. PMID 34726479. S2CID 240422219.
- ^ Şimşek-Yavuz S, Komsuoğlu Çelikyurt FI (August 2021). "Antiviral treatment of COVID-19: An update". Turkish Journal of Medical Sciences. 51 (SI-1): 3372–3390. doi:10.3906/sag-2106-250. PMC 8771049. PMID 34391321. S2CID 237054672.
- ^ Ahmad B, Batool M, Ain QU, Kim MS, Choi S (August 2021). "Exploring the Binding Mechanism of PF-07321332 SARS-CoV-2 Protease Inhibitor through Molecular Dynamics and Binding Free Energy Simulations". International Journal of Molecular Sciences. 22 (17): 9124. doi:10.3390/ijms22179124. PMC 8430524. PMID 34502033.
- ^ "Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death" (Press release). Pfizer. 14 December 2021. Archived from the original on 26 December 2021. Retrieved 25 December 2021 – via Business Wire.
- ^ Vandyck K, Deval J (August 2021). "Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection". Current Opinion in Virology. 49: 36–40. doi:10.1016/j.coviro.2021.04.006. PMC 8075814. PMID 34029993.
- ^ "Paxlovid- nirmatrelvir and ritonavir kit". DailyMed. Archived from the original on 31 December 2021. Retrieved 30 December 2021.
- ^ Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R (June 2003). "Coronavirus Main Proteinase (3CLpro) Structure: Basis for Design of Anti-SARS Drugs". Science. 300 (5626): 1763–1767. Bibcode:2003Sci...300.1763A. doi:10.1126/science.1085658. PMID 12746549. S2CID 13031405.
- ^ Dragovich PS, Prins TJ, Zhou R, Webber SE, Marakovits JT, Fuhrman SA, et al. (April 1999). "Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as L-glutamine replacements". Journal of Medicinal Chemistry. 42 (7): 1213–1224. doi:10.1021/jm9805384. PMID 10197965.
- ^ Pillaiyar T, Manickam M, Namasivayam V, Hayashi Y, Jung SH (July 2016). "An Overview of Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) 3CL Protease Inhibitors: Peptidomimetics and Small Molecule Chemotherapy". Journal of Medicinal Chemistry. 59 (14): 6595–6628. doi:10.1021/acs.jmedchem.5b01461. PMC 7075650. PMID 26878082.
- ^ Pedersen NC, Kim Y, Liu H, Galasiti Kankanamalage AC, Eckstrand C, Groutas WC, et al. (April 2018). "Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis". Journal of Feline Medicine and Surgery. 20 (4): 378–392. doi:10.1177/1098612X17729626. PMC 5871025. PMID 28901812.
- ^ Halford B (7 April 2021). "Pfizer unveils its oral SARS-CoV-2 inhibitor". Chemical & Engineering News. 99 (13): 7. doi:10.47287/cen-09913-scicon3. S2CID 234887434.
- ^ Vuong W, Khan MB, Fischer C, Arutyunova E, Lamer T, Shields J, et al. (August 2020). "Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication". Nature Communications. 11 (1): 4282. doi:10.1038/s41467-020-18096-2. PMC 7453019. PMID 32855413.
- ^ a b c d e f g Halford B (14 January 2022). "How Pfizer scientists transformed an old drug lead into a COVID-19 antiviral: Behind the scenes of the medicinal chemistry campaign that led to the pill Paxlovid". Chemical & Engineering News. Vol. 100, no. 3. Archived from the original on 14 January 2022. Retrieved 14 January 2022.
- ^ Clinical trial number NCT04535167 for "First-In-Human Study To Evaluate Safety, Tolerability, And Pharmacokinetics Following Single Ascending And Multiple Ascending Doses of PF-07304814 In Hospitalized Participants With COVID-19 " at ClinicalTrials.gov
- ^ Boras B, Jones RM, Anson BJ, Arenson D, Aschenbrenner L, Bakowski MA, et al. (2021). "Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19". Nature Communications. 12 (1): 6055. Bibcode:2021NatCo..12.6055B. doi:10.1038/s41467-021-26239-2. PMC 8523698. PMID 34663813.
- ^ Galli M, Migliano F, Fasano V, Silvani A, Passarella D, Citarella A (2024). "Nirmatrelvir: From Discovery to Modern and Alternative Synthetic Approaches". Processes. 12 (6): 1242. doi:10.3390/pr12061242.
- ^ Njoroge FG, Chen KX, Shih NY, Piwinski JJ (January 2008). "Challenges in modern drug discovery: a case study of boceprevir, an HCV protease inhibitor for the treatment of hepatitis C virus infection". Accounts of Chemical Research. 41 (1): 50–59. doi:10.1021/ar700109k. PMID 18193821. S2CID 2629035.
- ^ Poreba M, Salvesen GS, Drag M (October 2017). "Synthesis of a HyCoSuL peptide substrate library to dissect protease substrate specificity". Nature Protocols. 12 (10): 2189–2214. doi:10.1038/nprot.2017.091. PMID 28933778. S2CID 23895951. Archived from the original on 27 December 2021. Retrieved 25 December 2021.
- ^ Rut W, Groborz K, Zhang L, Sun X, Zmudzinski M, Pawlik B, et al. (October 2020). "SARS-CoV-2 M pro inhibitors and activity-based probes for patient-sample imaging". Nature Chemical Biology. 17 (2): 222–228. doi:10.1038/s41589-020-00689-z. PMID 33093684. S2CID 224827220.
- ^ Pavan M, Bolcato G, Bassani D, Sturlese M, Moro S (December 2021). "Supervised Molecular Dynamics (SuMD) Insights into the mechanism of action of SARS-CoV-2 main protease inhibitor PF-07321332". Journal of Enzyme Inhibition and Medicinal Chemistry. 36 (1): 1646–1650. doi:10.1080/14756366.2021.1954919. PMC 8300928. PMID 34289752.
- ^ Woodley M (19 October 2021). "What is Australia's potential new COVID treatment?". Newsgp. Archived from the original on 5 November 2021. Retrieved 6 November 2021.
- ^ Hull MW, Montaner JS (April 2011). "Ritonavir-boosted protease inhibitors in HIV therapy". Annals of Medicine. 43 (5): 375–388. doi:10.3109/07853890.2011.572905. PMID 21501034. S2CID 21400449.
- ^ "Pfizer and The Medicines Patent Pool (MPP) Sign Licensing Agreement for COVID-19 Oral Antiviral Treatment Candidate to Expand Access in Low- and Middle-Income Countries" (Press release). Pfizer. 16 November 2021. Archived from the original on 17 November 2021. Retrieved 17 November 2021 – via Business Wire.
- ^ "Covid-19: Pfizer to allow developing nations to make its treatment pill". BBC News. 16 November 2021. Archived from the original on 16 November 2021. Retrieved 17 November 2021.
- ^ "Pfizer Will Allow Its Covid Pill to Be Made and Sold Cheaply in Poor Countries". The New York Times. 16 November 2021. Archived from the original on 17 November 2021. Retrieved 17 November 2021.
- ^ World Health Organization (2022). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 88". WHO Drug Information. 36 (3). hdl:10665/363551.
- ^ a b Green R (23 December 2021). "Pfizer scientists in Groton played a critical role in development of new COVID-19 pill". The Hartford Courant. Archived from the original on 15 January 2022. Retrieved 15 January 2022.
- ^ Clinical trial number NCT04756531 for "Study Of PF-07321332 In Healthy Participants" at ClinicalTrials.gov
- ^ Xie Y, Choi T, Al-Aly Z (March 2023). "Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition". JAMA Internal Medicine. 183 (6): 554–564. doi:10.1001/jamainternmed.2023.0743. PMC 10037200. PMID 36951829.
- ^ Hammond J, Fountaine RJ, Yunis C, Fleishaker D, Almas M, Bao W, et al. (April 2024). "Nirmatrelvir for Vaccinated or Unvaccinated Adult Outpatients with Covid-19". The New England Journal of Medicine. 390 (13): 1186–1195. doi:10.1056/NEJMoa2309003. PMC 11156287. PMID 38598573.

