Hydrogen fluoride: Difference between revisions
m added toxicity concern Tag: Reverted |
|||
| (57 intermediate revisions by 37 users not shown) | |||
| Line 1: | Line 1: | ||
{{distinguish|text = the element [[hafnium]], symbol Hf}} |
|||
{{chembox |
{{chembox |
||
| |
|verifiedrevid = 476992809 |
||
| |
|Name = Hydrogen fluoride |
||
| |
|ImageFileL1 = Hydrogen-fluoride-2D-dimensions.svg |
||
| |
|ImageFile = Hydrogen fluoride.svg |
||
| |
|ImageFileR1 = Hydrogen-fluoride-3D-vdW.svg |
||
| |
|OtherNames = Fluorane |
||
|Section1 = {{Chembox Identifiers |
|||
| IUPACName = |
|||
|UNII_Ref = {{fdacite|correct|FDA}} |
|||
| SystematicName = |
|||
|UNII = RGL5YE86CZ |
|||
| Section1 = {{Chembox Identifiers |
|||
| |
|KEGG_Ref = {{keggcite|correct|kegg}} |
||
| |
|KEGG = C16487 |
||
|InChI = 1/FH/h1H |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
|ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| KEGG = C16487 |
|||
| |
|ChEBI = 29228 |
||
|SMILES = F |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
|InChIKey = KRHYYFGTRYWZRS-UHFFFAOYAC |
|||
| ChEBI = 29228 |
|||
|StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| SMILES = F |
|||
|StdInChI = 1S/FH/h1H |
|||
| InChIKey = KRHYYFGTRYWZRS-UHFFFAOYAC |
|||
| |
|StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
||
|StdInChIKey = KRHYYFGTRYWZRS-UHFFFAOYSA-N |
|||
| StdInChI = 1S/FH/h1H |
|||
|CASNo = 7664-39-3 |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
|CASNo_Ref = {{cascite|correct|CAS}} |
|||
| StdInChIKey = KRHYYFGTRYWZRS-UHFFFAOYSA-N |
|||
|ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| CASNo = 7664-39-3 |
|||
|ChemSpiderID = 14214 |
|||
| CASNo_Ref = {{cascite|correct|CAS}} |
|||
|UNNumber = 1052 |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
|PubChem = 16211014 |
|||
| ChemSpiderID = 14214 |
|||
|RTECS = MW7875000 |
|||
| UNNumber = 1052 |
|||
}} |
|||
| PubChem = 16211014 |
|||
|Section2 = {{Chembox Properties |
|||
| RTECS = MW7875000 |
|||
| |
|H=1 | F=1 |
||
|Formula = HF |
|||
}} |
|||
|Appearance = colourless gas or colourless liquid (below 19.5 °C) |
|||
| Section2 = {{Chembox Properties |
|||
|Odor = unpleasant |
|||
| H=1 | F=1 |
|||
|Density = 1.15 g/L, gas (25 °C)<br />0.99 g/mL, liquid (19.5 °C)<br />1.663 g/mL, solid (–125 °C) |
|||
| Formula = HF |
|||
|Solubility = miscible (liquid) |
|||
| Appearance = colourless gas or colourless liquid (below 19.5 °C) |
|||
|MeltingPtC = -83.6 |
|||
| Odor = unpleasant |
|||
|BoilingPtC = 19.5 |
|||
| Density = 1.15 g/L, gas (25 °C)<br />0.99 g/mL, liquid (19.5 °C)<br />1.663 g/mL, solid (–125 °C) |
|||
|RefractIndex = 1.00001 |
|||
| Solubility = completely miscible (liquid) |
|||
|pKa = 3.17 (in water), |
|||
| MeltingPtC = -83.6 |
|||
| BoilingPtC = 19.5 |
|||
| RefractIndex = 1.00001 |
|||
| pKa = 3.17 (in water), |
|||
15 (in DMSO) <ref>{{Cite web|last=Evans|first=D. A.|title=pKa's of Inorganic and Oxo-Acids|url=http://ccc.chem.pitt.edu/wipf/MechOMs/evans_pKa_table.pdf|access-date=June 19, 2020}}</ref> |
15 (in DMSO) <ref>{{Cite web|last=Evans|first=D. A.|title=pKa's of Inorganic and Oxo-Acids|url=http://ccc.chem.pitt.edu/wipf/MechOMs/evans_pKa_table.pdf|access-date=June 19, 2020}}</ref> |
||
| |
|ConjugateAcid = [[Fluoronium]] |
||
| |
|ConjugateBase = [[Fluoride]] |
||
| |
|VaporPressure = 783 mmHg (20 °C)<ref name="PGCH" /> |
||
}} |
|||
| |
|Section3 = {{Chembox Structure |
||
| |
|MolShape = [[Linear molecular geometry|Linear]] |
||
| |
|Dipole = 1.86 [[Debye|D]] |
||
}} |
|||
| Coordination = |
|||
|Section4 = {{Chembox Thermochemistry |
|||
| CrystalStruct = |
|||
|DeltaHf = −13.66 kJ/g (gas) <br /> −14.99 kJ/g (liquid) |
|||
}} |
|||
|Entropy = 8.687 J/g K (gas) |
|||
| Section4 = {{Chembox Thermochemistry |
|||
}} |
|||
| DeltaHf = −13.66 kJ/g (gas) <br /> −14.99 kJ/g (liquid) |
|||
|Section5 = {{Chembox Hazards |
|||
| DeltaHc = |
|||
|MainHazards = Highly toxic, corrosive, irritant |
|||
| Entropy = 8.687 J/g K (gas) |
|||
|GHSPictograms = {{GHS corrosion}} {{GHS skull and crossbones}}{{GHS07}} |
|||
| HeatCapacity = |
|||
|GHSSignalWord = Danger |
|||
}} |
|||
|HPhrases = {{H-phrases|300+310+330|314}} |
|||
| Section5 = |
|||
|PPhrases = {{P-phrases|260|262|264|270|271|280|284|301+310|301+330+331|302+350|303+361+353|304+340|305+351+338|310|320|321|322|330|361|363|403+233|405|501}} |
|||
| Section6 = |
|||
|NFPA-H = 4 |
|||
| Section7 = {{Chembox Hazards |
|||
|NFPA-F = 0 |
|||
| MainHazards = Very toxic, corrosive. Irritant. |
|||
|NFPA-R = 1 |
|||
| ExternalSDS = |
|||
|NFPA-S = POI |
|||
| GHSPictograms = {{GHS corrosion}} {{GHS skull and crossbones}} |
|||
|FlashPt = none |
|||
| GHSSignalWord = Danger |
|||
|IDLH = 30 ppm<ref name="PGCH">{{PGCH|0334}}</ref> |
|||
| HPhrases = {{H-phrases|300+310+330|314}} |
|||
|REL = TWA 3 ppm (2.5 mg/m<sup>3</sup>) C 6 ppm (5 mg/m<sup>3</sup>) [15-minute]<ref name="PGCH" /> |
|||
| PPhrases = {{P-phrases|260|262|264|270|271|280|284|301+310|301+330+331|302+350|303+361+353|304+340|305+351+338|310|320|321|322|330|361|363|403+233|405|501}} |
|||
|PEL = TWA 3 ppm<ref name="PGCH" /> |
|||
| NFPA-H = 4 |
|||
|LD50 = 17 ppm (rat, oral) |
|||
| NFPA-F = 0 |
|||
|LC50 = 1276 ppm (rat, 1 hr)<br/>1774 ppm (monkey, 1 hr)<br/>4327 ppm (guinea pig, 15 min)<ref name="IDLH">{{IDLH|7664393|Hydrogen fluoride}}</ref> |
|||
| NFPA-R = 1 |
|||
|LCLo = 313 ppm (rabbit, 7 hr)<ref |
|||
| NFPA-S = POI |
|||
name="IDLH"/> |
|||
| FlashPt = none |
|||
}} |
|||
| IDLH = 30 ppm<ref name="PGCH">{{PGCH|0334}}</ref> |
|||
|Section6 = {{Chembox Related |
|||
| REL = TWA 3 ppm (2.5 mg/m<sup>3</sup>) C 6 ppm (5 mg/m<sup>3</sup>) [15-minute]<ref name="PGCH" /> |
|||
|OtherAnions = [[Hydrogen chloride]]<br />[[Hydrogen bromide]]<br />[[Hydrogen iodide]]<br />[[Hydrogen astatide]] |
|||
| PEL = TWA 3 ppm<ref name="PGCH" /> |
|||
|OtherCations = [[Sodium fluoride]]<br />[[Potassium fluoride]]<br />[[Rubidium fluoride]]<br />[[Caesium fluoride]] |
|||
| LC50 = 1276 ppm (rat, 1 hr)<br/>1774 ppm (monkey, 1 hr)<br/>4327 ppm (guinea pig, 15 min)<ref name="IDLH">{{IDLH|7664393|Hydrogen fluoride}}</ref> |
|||
|OtherCompounds = [[Properties of water|Water]]<br /> [[Ammonia]] |
|||
| LCLo = 313 ppm (rabbit, 7 hr)<ref name="IDLH" /> |
|||
}} |
|||
| Section8 = {{Chembox Related |
|||
| OtherAnions = [[Hydrogen chloride]]<br />[[Hydrogen bromide]]<br />[[Hydrogen iodide]]<br />[[Hydrogen astatide]] |
|||
| OtherCations = [[Sodium fluoride]]<br />[[Potassium fluoride]]<br />[[Rubidium fluoride]]<br />[[Caesium fluoride]] |
|||
| OtherCompounds = [[Properties of water|Water]]<br /> [[Ammonia]] |
|||
}} |
|||
}} |
}} |
||
'''Hydrogen fluoride''' (fluorane) is an [[Inorganic chemistry|inorganic compound]] with |
'''Hydrogen fluoride''' (fluorane) is an [[Inorganic chemistry|inorganic compound]] with [[chemical formula]] {{Chem2|HF|auto=yes}}. It is a very poisonous, colorless gas or liquid that dissolves in water to yield [[hydrofluoric acid]]. It is the principal industrial source of [[fluorine]], often in the form of hydrofluoric acid, and is an important [[feedstock]] in the preparation of many important compounds including pharmaceuticals and [[polymer]]s such as [[polytetrafluoroethylene]] (PTFE). HF is also widely used in the [[petrochemical industry]] as a component of [[superacid]]s. Due to strong and extensive [[hydrogen bond]]ing, it boils near room temperature, a much higher temperature than other [[hydrogen halide]]s. |
||
Hydrogen fluoride is an extremely dangerous gas, forming [[corrosive]] and penetrating [[hydrofluoric acid]] upon contact with [[moisture]]. The gas can also cause [[blindness]] by rapid destruction of the [[cornea]]s |
Hydrogen fluoride is an extremely dangerous gas, forming [[corrosive]] and penetrating [[hydrofluoric acid]] upon contact with [[moisture]]. The gas can also cause [[blindness]] by rapid destruction of the [[cornea]]s. |
||
==History== |
==History== |
||
In 1771 [[Carl Wilhelm Scheele]] prepared the aqueous solution, [[hydrofluoric acid]] in large quantities, although hydrofluoric acid had been known in the [[glass industry]] before then. |
In 1771 [[Carl Wilhelm Scheele]] prepared the aqueous solution, [[hydrofluoric acid]] in large quantities, although hydrofluoric acid had been known in the [[glass industry]] before then. |
||
French chemist [[Edmond Frémy]] (1814–1894) is credited with discovering |
French chemist [[Edmond Frémy]] (1814–1894) is credited with discovering hydrogen fluoride (HF) while trying to isolate [[fluorine]]. |
||
==Structure and reactions== |
==Structure and reactions== |
||
[[File:Hydrogen-fluoride-solid-2D-dimensions.png|left|295px|The structure of chains of HF in crystalline hydrogen fluoride.]]{{clear|left}} |
[[File:Hydrogen-fluoride-solid-2D-dimensions.png|thumb|left|295px|The structure of chains of HF in crystalline hydrogen fluoride.]]{{clear|left}} |
||
HF is diatomic in the gas-phase. As a liquid, HF forms relatively strong [[hydrogen bond]]s, hence its relatively high boiling point. Solid HF consists of zig-zag chains of HF molecules. The HF molecules, with a short covalent H–F bond of 95 pm length, are linked to neighboring molecules by intermolecular H–F distances of 155 pm.<ref>{{cite journal |author1=Johnson, M. W. |author2=Sándor, E. |author3=Arzi, E. | title = The Crystal Structure of Deuterium Fluoride | journal = [[Acta Crystallographica]] | year = 1975 | volume = B31 | pages = 1998–2003 | doi = 10.1107/S0567740875006711 | issue = 8 }}</ref> Liquid HF also consists of chains of HF molecules, but the chains are shorter, consisting on average of only five or six molecules.<ref>{{cite journal | title = On the Structure of Liquid Hydrogen Fluoride | journal = [[Angewandte Chemie International Edition]] | year = 2004 | volume = 43 | pages = 1952–55 | doi = 10.1002/anie.200353289 | author = McLain, Sylvia E. | pmid = 15065271 | last2 = Benmore | first2 = C. J. | last3 = Siewenie | first3 = J. E. | last4 = Urquidi | first4 = J. | last5 = Turner | first5 = J. F. | issue = 15 }}</ref> |
|||
===Comparison with other hydrogen halides=== |
===Comparison with other hydrogen halides=== |
||
Hydrogen fluoride does not boil until 20 °C in contrast to the heavier hydrogen halides, which boil between −85 °C (−120 °F) and −35 °C (−30 °F).<ref name="Pauling HF hydrogen bonds">{{cite book|last=Pauling|first=Linus A.|title=The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry|year=1960|publisher=Cornell University Press|isbn=978-0-8014-0333-0|pages=[https://archive.org/details/natureofchemical00paul/page/454 454]–464|url=https://archive.org/details/natureofchemical00paul|url-access=registration}}</ref><ref name="Atkins HF">{{cite book|last=Atkins|first=Peter|title=Chemical principles: The quest for insight|year=2008|publisher=W. H. Freeman & Co|isbn=978- |
Hydrogen fluoride does not boil until 20 °C in contrast to the heavier hydrogen halides, which boil between −85 °C (−120 °F) and −35 °C (−30 °F).<ref name="Pauling HF hydrogen bonds">{{cite book|last=Pauling|first=Linus A.|title=The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry|year=1960|publisher=Cornell University Press|isbn=978-0-8014-0333-0|pages=[https://archive.org/details/natureofchemical00paul/page/454 454]–464|url=https://archive.org/details/natureofchemical00paul|url-access=registration}}</ref><ref name="Atkins HF">{{cite book|last=Atkins|first=Peter|title=Chemical principles: The quest for insight|year=2008|publisher=W. H. Freeman & Co|isbn=978-1097774678|pages=184–185|url=https://books.google.com/books?id=4R6hb1OIMRUC&pg=PA184|author2=Jones, Loretta}}</ref><ref name="New Scientist HF">{{cite journal|last=Emsley|first=John|title=The hidden strength of hydrogen|journal=New Scientist|year=1981|volume=91|issue=1264|pages=291–292|url=https://books.google.com/books?id=ZbthaZCUXy4C&pg=PA292|access-date=25 December 2012|archive-date=22 July 2023|archive-url=https://web.archive.org/web/20230722094703/https://books.google.com/books?id=ZbthaZCUXy4C&pg=PA292|url-status=dead}}</ref> This hydrogen bonding between HF molecules gives rise to high [[viscosity]] in the liquid phase and lower than expected pressure in the gas phase. |
||
===Aqueous solutions=== |
===Aqueous solutions=== |
||
{{main|hydrofluoric acid}} |
{{main|hydrofluoric acid}} |
||
HF is [[miscible]] with water (dissolves in any proportion). In contrast, the other hydrogen halides exhibit limiting solubilities in water. Hydrogen fluoride forms a monohydrate HF<sup>.</sup>H<sub>2</sub>O with |
HF is [[miscible]] with water (dissolves in any proportion). In contrast, the other hydrogen halides exhibit limiting solubilities in water. Hydrogen fluoride forms a monohydrate HF<sup>.</sup>H<sub>2</sub>O with melting point −40 °C (−40 °F), which is 44 °C (79 °F) above the melting point of pure HF.<ref>{{cite book|last1=Greenwood|first1=N. N.|last2=Earnshaw|first2=A.|title=Chemistry of the Elements|date=1998|edition=2nd|publisher=Butterworth Heinemann|isbn=0-7506-3365-4|location=Oxford|pages=812–816}}</ref> |
||
{| style="margin:1em auto 1em 1em; text-align:left; width:505px; float:center;" cellpadding="0" cellspacing="0" |
{| style="margin:1em auto 1em 1em; text-align:left; width:505px; float:center;" cellpadding="0" cellspacing="0" |
||
| Line 126: | Line 119: | ||
==Production== |
==Production== |
||
Hydrogen fluoride is typically produced by the |
Hydrogen fluoride is typically produced by the reaction between [[sulfuric acid]] and pure grades of the mineral [[fluorite]]:<ref name="AigueperseMollard2000" /> |
||
:{{chem2|CaF2 + H2SO4 -> 2 HF + CaSO4}} |
:{{chem2|CaF2 + H2SO4 -> 2 HF + CaSO4}} |
||
| Line 152: | Line 145: | ||
===Precursor to metal fluorides and fluorine=== |
===Precursor to metal fluorides and fluorine=== |
||
The electrowinning of [[aluminium]] relies on the electrolysis of aluminium fluoride in molten cryolite. Several kilograms of HF are consumed per ton of Al produced. Other metal fluorides are produced using HF, including [[uranium |
The [[electrowinning]] of [[aluminium]] relies on the electrolysis of aluminium fluoride in molten cryolite. Several kilograms of HF are consumed per ton of Al produced. Other metal fluorides are produced using HF, including [[uranium tetrafluoride]].<ref name="AigueperseMollard2000" /> |
||
HF is the precursor to elemental [[fluorine]], F<sub>2</sub>, by [[electrolysis]] of a solution of HF and [[potassium bifluoride]]. The potassium bifluoride is needed because anhydrous HF does not conduct electricity. Several thousand tons of F<sub>2</sub> are produced annually.<ref>{{Ullmann|author=M. Jaccaud, R. Faron, D. Devilliers, R. Romano|title=Fluorine|year=2005|doi=10.1002/14356007.a11_293}}.</ref> |
HF is the precursor to elemental [[fluorine]], F<sub>2</sub>, by [[electrolysis]] of a solution of HF and [[potassium bifluoride]]. The potassium bifluoride is needed because anhydrous HF does not conduct electricity. Several thousand tons of F<sub>2</sub> are produced annually.<ref>{{Ullmann|author=M. Jaccaud, R. Faron, D. Devilliers, R. Romano|title=Fluorine|year=2005|doi=10.1002/14356007.a11_293}}.</ref> |
||
| Line 163: | Line 156: | ||
==Health effects== |
==Health effects== |
||
{{Main|Hydrofluoric acid|Hydrofluoric acid burn}} |
|||
[[File:HF burned hands.jpg|thumb|250px|alt=left and right hands, two views, burned index fingers|HF burns, not evident until a day after]] |
[[File:HF burned hands.jpg|thumb|250px|alt=left and right hands, two views, burned index fingers|HF burns, not evident until a day after]] |
||
{{Main|Hydrofluoric acid|Hydrofluoric acid burn}} |
|||
Hydrogen fluoride is highly corrosive and a powerful contact poison. Exposure requires immediate medical attention.<ref name="emergency.cdc.gov">[http://emergency.cdc.gov/agent/hydrofluoricacid/basics/facts.asp Facts About Hydrogen Fluoride (Hydrofluoric Acid)]</ref> It can cause blindness by rapid destruction of the [[cornea]]s. Breathing in hydrogen fluoride at high levels or in combination with skin contact can cause death from an [[Cardiac dysrhythmia|irregular heartbeat]] or from [[pulmonary edema]] (fluid buildup in the lungs).<ref name="emergency.cdc.gov" /> |
Hydrogen fluoride is highly corrosive and a powerful contact poison. Exposure requires immediate medical attention.<ref name="emergency.cdc.gov">[http://emergency.cdc.gov/agent/hydrofluoricacid/basics/facts.asp Facts About Hydrogen Fluoride (Hydrofluoric Acid)]</ref> It can cause blindness by rapid destruction of the [[cornea]]s. Breathing in hydrogen fluoride at high levels or in combination with skin contact can cause death from an [[Cardiac dysrhythmia|irregular heartbeat]] or from [[pulmonary edema]] (fluid buildup in the lungs).<ref name="emergency.cdc.gov" /> |
||
| Line 171: | Line 164: | ||
== References == |
== References == |
||
{{Reflist |
{{Reflist|30em}} |
||
==External links== |
==External links== |
||
| Line 177: | Line 170: | ||
*[https://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=38 Fluorides, Hydrogen Fluoride, and Fluorine] at [[ATSDR]]. Retrieved September 30, 2019 |
*[https://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=38 Fluorides, Hydrogen Fluoride, and Fluorine] at [[ATSDR]]. Retrieved September 30, 2019 |
||
*[https://www.cdc.gov/niosh/npg/npgd0334.html CDC - NIOSH Pocket Guide to Chemical Hazards] |
*[https://www.cdc.gov/niosh/npg/npgd0334.html CDC - NIOSH Pocket Guide to Chemical Hazards] |
||
*[https://www.turi.org/TURI_Publications/TURI_Chemical_Fact_Sheets/Hydrogen_Fluoride_Fact_Sheet Hydrogen Fluoride Fact Sheet] at [[Toxics Use Reduction Institute]] |
*[https://www.turi.org/TURI_Publications/TURI_Chemical_Fact_Sheets/Hydrogen_Fluoride_Fact_Sheet Hydrogen Fluoride Fact Sheet]{{dead link|date=August 2024}} at [[Toxics Use Reduction Institute]] |
||
{{Hydrogen compounds}} |
{{Hydrogen compounds}} |
||
| Line 192: | Line 185: | ||
[[Category:Inorganic solvents]] |
[[Category:Inorganic solvents]] |
||
[[Category:Nonmetal halides]] |
[[Category:Nonmetal halides]] |
||
[[Category:Diatomic molecules]] |
|||
Latest revision as of 14:42, 5 January 2025

| |||
| |||
| Names | |||
|---|---|---|---|
| Other names
Fluorane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.759 | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1052 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| HF | |||
| Molar mass | 20.006 g·mol−1 | ||
| Appearance | colourless gas or colourless liquid (below 19.5 °C) | ||
| Odor | unpleasant | ||
| Density | 1.15 g/L, gas (25 °C) 0.99 g/mL, liquid (19.5 °C) 1.663 g/mL, solid (–125 °C) | ||
| Melting point | −83.6 °C (−118.5 °F; 189.6 K) | ||
| Boiling point | 19.5 °C (67.1 °F; 292.6 K) | ||
| miscible (liquid) | |||
| Vapor pressure | 783 mmHg (20 °C)[1] | ||
| Acidity (pKa) | 3.17 (in water),
15 (in DMSO) [2] | ||
| Conjugate acid | Fluoronium | ||
| Conjugate base | Fluoride | ||
Refractive index (nD)
|
1.00001 | ||
| Structure | |||
| Linear | |||
| 1.86 D | |||
| Thermochemistry | |||
Std molar
entropy (S⦵298) |
8.687 J/g K (gas) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−13.66 kJ/g (gas) −14.99 kJ/g (liquid) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Highly toxic, corrosive, irritant | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H300+H310+H330, H314 | |||
| P260, P262, P264, P270, P271, P280, P284, P301+P310, P301+P330+P331, P302+P350, P303+P361+P353, P304+P340, P305+P351+P338, P310, P320, P321, P322, P330, P361, P363, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | none | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
17 ppm (rat, oral) | ||
LC50 (median concentration)
|
1276 ppm (rat, 1 hr) 1774 ppm (monkey, 1 hr) 4327 ppm (guinea pig, 15 min)[3] | ||
LCLo (lowest published)
|
313 ppm (rabbit, 7 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 3 ppm[1] | ||
REL (Recommended)
|
TWA 3 ppm (2.5 mg/m3) C 6 ppm (5 mg/m3) [15-minute][1] | ||
IDLH (Immediate danger)
|
30 ppm[1] | ||
| Related compounds | |||
Other anions
|
Hydrogen chloride Hydrogen bromide Hydrogen iodide Hydrogen astatide | ||
Other cations
|
Sodium fluoride Potassium fluoride Rubidium fluoride Caesium fluoride | ||
Related compounds
|
Water Ammonia | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Hydrogen fluoride (fluorane) is an inorganic compound with chemical formula HF. It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides.
Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas.
History
[edit]In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid had been known in the glass industry before then. French chemist Edmond Frémy (1814–1894) is credited with discovering hydrogen fluoride (HF) while trying to isolate fluorine.
Structure and reactions
[edit]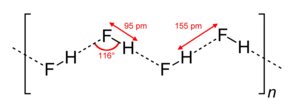
HF is diatomic in the gas-phase. As a liquid, HF forms relatively strong hydrogen bonds, hence its relatively high boiling point. Solid HF consists of zig-zag chains of HF molecules. The HF molecules, with a short covalent H–F bond of 95 pm length, are linked to neighboring molecules by intermolecular H–F distances of 155 pm.[4] Liquid HF also consists of chains of HF molecules, but the chains are shorter, consisting on average of only five or six molecules.[5]
Comparison with other hydrogen halides
[edit]Hydrogen fluoride does not boil until 20 °C in contrast to the heavier hydrogen halides, which boil between −85 °C (−120 °F) and −35 °C (−30 °F).[6][7][8] This hydrogen bonding between HF molecules gives rise to high viscosity in the liquid phase and lower than expected pressure in the gas phase.
Aqueous solutions
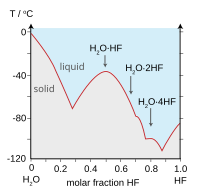
[edit]HF is miscible with water (dissolves in any proportion). In contrast, the other hydrogen halides exhibit limiting solubilities in water. Hydrogen fluoride forms a monohydrate HF.H2O with melting point −40 °C (−40 °F), which is 44 °C (79 °F) above the melting point of pure HF.[9]
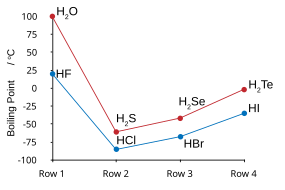
| HF and H2O similarities | |
|
|
| Boiling points of the hydrogen halides (blue) and hydrogen chalcogenides (red): HF and H2O break trends. | Freezing point of HF/ H2O mixtures: arrows indicate compounds in the solid state. |
Aqueous solutions of HF are called hydrofluoric acid. When dilute, hydrofluoric acid behaves like a weak acid, unlike the other hydrohalic acids, due to the formation of hydrogen-bonded ion pairs [H3O+·F−]. However concentrated solutions are strong acids, because bifluoride anions are predominant, instead of ion pairs. In liquid anhydrous HF, self-ionization occurs:[10][11]
- 3 HF ⇌ H2F+ + HF−2
which forms an extremely acidic liquid (H0 = −15.1).
Reactions with Lewis acids
[edit]Like water, HF can act as a weak base, reacting with Lewis acids to give superacids. A Hammett acidity function (H0) of −21 is obtained with antimony pentafluoride (SbF5), forming fluoroantimonic acid.[12][13]
Production
[edit]Hydrogen fluoride is typically produced by the reaction between sulfuric acid and pure grades of the mineral fluorite:[14]
- CaF2 + H2SO4 → 2 HF + CaSO4
About 20% of manufactured HF is a byproduct of fertilizer production, which generates hexafluorosilicic acid. This acid can be degraded to release HF thermally and by hydrolysis:
- H2SiF6 → 2 HF + SiF4
- SiF4 + 2 H2O → 4 HF + SiO2
Use
[edit]In general, anhydrous hydrogen fluoride is more common industrially than its aqueous solution, hydrofluoric acid. Its main uses, on a tonnage basis, are as a precursor to organofluorine compounds and a precursor to cryolite for the electrolysis of aluminium.[14]
Precursor to organofluorine compounds
[edit]HF reacts with chlorocarbons to give fluorocarbons. An important application of this reaction is the production of tetrafluoroethylene (TFE), precursor to Teflon. Chloroform is fluorinated by HF to produce chlorodifluoromethane (R-22):[14]
- CHCl3 + 2 HF → CHClF2 + 2 HCl
Pyrolysis of chlorodifluoromethane (at 550-750 °C) yields TFE.
HF is a reactive solvent in the electrochemical fluorination of organic compounds. In this approach, HF is oxidized in the presence of a hydrocarbon and the fluorine replaces C–H bonds with C–F bonds. Perfluorinated carboxylic acids and sulfonic acids are produced in this way.[15]
1,1-Difluoroethane is produced by adding HF to acetylene using mercury as a catalyst.[15]
- HC≡CH + 2 HF → CH3CHF2
The intermediate in this process is vinyl fluoride or fluoroethylene, the monomeric precursor to polyvinyl fluoride.
Precursor to metal fluorides and fluorine
[edit]The electrowinning of aluminium relies on the electrolysis of aluminium fluoride in molten cryolite. Several kilograms of HF are consumed per ton of Al produced. Other metal fluorides are produced using HF, including uranium tetrafluoride.[14]
HF is the precursor to elemental fluorine, F2, by electrolysis of a solution of HF and potassium bifluoride. The potassium bifluoride is needed because anhydrous HF does not conduct electricity. Several thousand tons of F2 are produced annually.[16]
Catalyst
[edit]HF serves as a catalyst in alkylation processes in refineries. It is used in the majority of the installed linear alkyl benzene production facilities in the world. The process involves dehydrogenation of n-paraffins to olefins, and subsequent reaction with benzene using HF as catalyst. For example, in oil refineries "alkylate", a component of high-octane petrol (gasoline), is generated in alkylation units, which combine C3 and C4 olefins and iso-butane.[14]
Solvent
[edit]Hydrogen fluoride is an excellent solvent. Reflecting the ability of HF to participate in hydrogen bonding, even proteins and carbohydrates dissolve in HF and can be recovered from it. In contrast, most non-fluoride inorganic chemicals react with HF rather than dissolving.[17]
Health effects
[edit]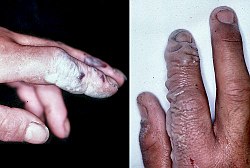
Hydrogen fluoride is highly corrosive and a powerful contact poison. Exposure requires immediate medical attention.[18] It can cause blindness by rapid destruction of the corneas. Breathing in hydrogen fluoride at high levels or in combination with skin contact can cause death from an irregular heartbeat or from pulmonary edema (fluid buildup in the lungs).[18]
References
[edit]- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0334". National Institute for Occupational Safety and Health (NIOSH).
- ^ Evans, D. A. "pKa's of Inorganic and Oxo-Acids" (PDF). Retrieved June 19, 2020.
- ^ a b "Hydrogen fluoride". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Johnson, M. W.; Sándor, E.; Arzi, E. (1975). "The Crystal Structure of Deuterium Fluoride". Acta Crystallographica. B31 (8): 1998–2003. doi:10.1107/S0567740875006711.
- ^ McLain, Sylvia E.; Benmore, C. J.; Siewenie, J. E.; Urquidi, J.; Turner, J. F. (2004). "On the Structure of Liquid Hydrogen Fluoride". Angewandte Chemie International Edition. 43 (15): 1952–55. doi:10.1002/anie.200353289. PMID 15065271.
- ^ Pauling, Linus A. (1960). The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry. Cornell University Press. pp. 454–464. ISBN 978-0-8014-0333-0.
- ^ Atkins, Peter; Jones, Loretta (2008). Chemical principles: The quest for insight. W. H. Freeman & Co. pp. 184–185. ISBN 978-1097774678.
- ^ Emsley, John (1981). "The hidden strength of hydrogen". New Scientist. 91 (1264): 291–292. Archived from the original on 22 July 2023. Retrieved 25 December 2012.
- ^ Greenwood, N. N.; Earnshaw, A. (1998). Chemistry of the Elements (2nd ed.). Oxford: Butterworth Heinemann. pp. 812–816. ISBN 0-7506-3365-4.
- ^ C. E. Housecroft and A. G. Sharpe Inorganic Chemistry, p. 221.
- ^ F. A. Cotton and G. Wilkinson Advanced Inorganic Chemistry, p. 111.
- ^ W. L. Jolly "Modern Inorganic Chemistry" (McGraw-Hill 1984), p. 203. ISBN 0-07-032768-8.
- ^ F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry (5th ed.) John Wiley and Sons: New York, 1988. ISBN 0-471-84997-9. p. 109.
- ^ a b c d e J. Aigueperse, P. Mollard, D. Devilliers, M. Chemla, R. Faron, R. Romano, J. P. Cuer (2000). "Fluorine Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_307. ISBN 3527306730.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - ^ a b G. Siegemund, W. Schwertfeger, A. Feiring, B. Smart, F. Behr, H. Vogel, B. McKusick (2005). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - ^ M. Jaccaud, R. Faron, D. Devilliers, R. Romano (2005). "Fluorine". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_293. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link). - ^ Greenwood and Earnshaw, "Chemistry of the Elements", pp. 816–819.
- ^ a b Facts About Hydrogen Fluoride (Hydrofluoric Acid)
External links
[edit]- Fluorides, Hydrogen Fluoride, and Fluorine at ATSDR. Retrieved September 30, 2019
- CDC - NIOSH Pocket Guide to Chemical Hazards
- Hydrogen Fluoride Fact Sheet[dead link] at Toxics Use Reduction Institute




