Crohn's disease: Difference between revisions
Undid revision 1265409051 by InternetPresence (talk) MOS promo |
|||
| (299 intermediate revisions by 28 users not shown) | |||
| Line 4: | Line 4: | ||
{{Infobox medical condition (new) |
{{Infobox medical condition (new) |
||
| name = Crohn's disease |
| name = Crohn's disease |
||
| image = |
| image = CD colitis 2.jpg |
||
| caption = Endoscopic image of severe Crohn's colitis showing diffuse loss of mucosal architecture, friability of mucosa in sigmoid colon and exudate on wall. |
|||
| caption = The three most common sites of intestinal involvement in Crohn's disease (left) compared to the areas affected by ''ulcerative colitis'' (''[[colitis ulcerosa]]'', right) |
|||
| field = [[Gastroenterology]] |
| field = [[Gastroenterology]] |
||
| synonyms = Crohn disease, Crohn syndrome, granulomatous enteritis, regional enteritis, Leśniowski-Crohn disease |
| synonyms = Crohn disease, Crohn syndrome, granulomatous enteritis, regional enteritis, Leśniowski-Crohn disease |
||
| symptoms = [[Diarrhea]], [[abdominal pain]], [[fatigue]], [[weight loss]], [[fever]]<ref name="pmid32242028"/> |
|||
| symptoms = [[Abdominal pain]], [[diarrhea]] (may be bloody), [[fever]], [[weight loss]],<ref name="Baumgart2012" /> [[fatigue]], mouth sores, reduced appetite<ref name="AR">{{cite web |title=Crohn's disease |url=https://www.autoimmuneregistry.org/crohns-disease |access-date=June 15, 2022 |website=Autoimmune Registry Inc. |archive-date=June 15, 2022 |archive-url=https://web.archive.org/web/20220615184526/https://www.autoimmuneregistry.org/crohns-disease |url-status=live}}</ref> |
|||
| complications = [[Anemia]], [[ |
| complications = [[Anemia]], [[bowel cancer]], [[bowel obstruction]], [[strictures]], [[fistulas]], [[abscesses]], [[anal fissure]]<ref name="pmid32242028"/> |
||
| onset = |
| onset = 20–30 years<ref name="pmid28601423"/> |
||
| duration = |
| duration = Lifelong<ref name="pmid32242028"/> |
||
| causes = Uncertain |
| causes = Uncertain<ref name="pmid32242028"/> |
||
| risks = [[Genetic predisposition]], living in a [[developed country]], [[smoking]], [[diet (nutrition)|diet]],<ref name="pmid32242028"/> [[antibiotics]], [[oral contraceptives]], [[aspirin]], [[NSAIDS]]<ref name="pmid30611442"/> |
|||
| risks = [[Genetic predisposition]], living in a [[developed country]],<ref name="Baumgart2007">{{cite journal |vauthors=Baumgart DC, Carding SR |title=Inflammatory bowel disease: cause and immunobiology |journal=Lancet |volume=369 |issue=9573 |pages=1627–40 |date=May 2007 |pmid=17499605 |doi=10.1016/S0140-6736(07)60750-8 }}</ref><br />[[Psychological stress|stress]],<ref>{{cite journal |vauthors=Mawdsley JE, Rampton DS |title=Psychological stress in IBD: new insights into pathogenic and therapeutic implications |journal=Gut |volume=54 |issue=10 |pages=1481–91 |date=October 2005 |pmid=16162953 |pmc=1774724 |doi=10.1136/gut.2005.064261 }}</ref> [[tobacco smoking]],<ref name="Cosnes2004" /><br/>having undergone an [[appendectomy]]<ref>{{cite journal|archive-date=2019-06-13|archive-url=https://web.archive.org/web/20190613235036/https://rd.springer.com/article/10.1007/BF02237133|date=February 1999|first=I. E.|journal=Diseases of the Colon & Rectum|last=Koutroubakis|title=Appendectomy, tonsillectomy, and risk of inflammatory bowel disease|url=https://rd.springer.com/article/10.1007/BF02237133|url-status=live|year=1999|volume=42 |issue=2 |pages=225–230 |doi=10.1007/BF02237133 |pmid=10211500 |s2cid=31528819 }}<!-- auto-translated from Dutch by Module:CS1 translator --></ref><ref>{{cite journal |vauthors=Frisch M, Gridley G |title=Appendectomy in adulthood and the risk of inflammatory bowel diseases |journal=Scand J Gastroenterol |volume=37 |issue=10 |pages=1175–7 |date=October 2002 |pmid=12408522 |doi=10.1080/003655202760373380 }}</ref> or [[tonsillectomy]]<ref>{{cite journal|author=Weili Sun, Xiao Han, Siyuan Wu, Chuanhua Yang|date=2016-06-01|doi=10.1111/jgh.13273|issn=1440-1746|issue=6|journal=Journal of Gastroenterology and Hepatology|language=en|pages=1085–94|title=Tonsillectomy and the risk of inflammatory bowel disease: A systematic review and meta-analysis|url=http://onlinelibrary.wiley.com/doi/10.1111/jgh.13273/abstract|volume=31|pmid=26678358|s2cid=2625962|access-date=February 9, 2024|archive-date=August 16, 2017|archive-url=https://web.archive.org/web/20170816192410/http://onlinelibrary.wiley.com/doi/10.1111/jgh.13273/abstract|url-status=live}}<!-- auto-translated from Dutch by Module:CS1 translator --></ref> |
|||
| diagnosis = [[Colonoscopy]], [[capsule endoscopy]], [[medical imaging]], [[histopathology]]<ref name="pmid32242028"/>| differential = [[Ulcerative colitis]], [[Behçet's disease]], [[intestinal lymphoma]], [[intestinal tuberculosis]], [[ischaemic colitis]], [[irritable bowel syndrome]]<ref name="pmid32242028"/> |
|||
| diagnosis = [[Biopsy]], [[medical imaging]]<ref name="Baumgart2012" /> |
|||
| differential = [[Irritable bowel syndrome]], [[celiac disease]], [[Behçet's disease]], [[nonsteroidal anti-inflammatory drug]] enteropathy, intestinal [[tuberculosis]]<ref name="Baumgart2012" /><ref name="WGO-IBD" /> |
|||
| prevention = |
| prevention = |
||
| treatment = |
| treatment = |
||
| medication = [[ |
| medication = [[Biologics]] (especially [[TNF inhibitor|TNF blockers]]), [[immunosuppressants]] ([[thiopurine]]s and [[methotrexate]]), [[corticosteroid]]s,<ref name="pmid32242028"/> |
||
| prognosis = Slightly |
| prognosis = Slightly reduced life expectancy<ref name="pmid33168761"/> |
||
| frequency = |
| frequency = ~300 in 100,000 ([[North America]] and [[Western Europe]])<ref name="pmid32242028"/> |
||
| deaths = |
| deaths = |
||
| alt = |
| alt = |
||
| named after = {{ubl |
| named after = {{ubl|[[Burrill Bernard Crohn]]}} |
||
}} |
}} |
||
'''Crohn's disease''' is a chronic [[inflammatory bowel disease]] characterized by recurrent episodes of intestinal inflammation, primarily manifesting as [[diarrhea]] and [[abdominal pain]]. Unlike [[ulcerative colitis]], inflammation can occur anywhere in the gastrointestinal tract, though it most frequently affects the [[ileum]] and [[large intestine|colon]], involving all layers of the intestinal wall. Symptoms may be non-specific and progress gradually, often delaying diagnosis. About one-third of patients have colonic disease, another third have ileocolic disease, and the remaining third have isolated ileal disease. Systemic symptoms such as chronic [[fatigue]], [[unintentional weight loss|weight loss]], and low-grade [[fever]]s are common. Organs such as the skin and joints can also be affected. Complications can include [[bowel obstruction]]s, [[fistula]]s, nutrition problems, and an increased risk of [[intestinal cancer]]s.<ref name="pmid32242028"/> |
|||
'''Crohn's disease''' is a type of [[inflammatory bowel disease]] (IBD) that may affect any segment of the [[gastrointestinal tract]].<ref name="NIDDK2017" /> Symptoms often include [[abdominal pain]], [[diarrhea]], [[fever]], [[abdominal distension]], and [[weight loss]].<ref name="Baumgart2012">{{cite journal |vauthors=Baumgart DC, Sandborn WJ |title=Crohn's disease |journal=Lancet |volume=380 |issue=9853 |pages=1590–1605 |date=August 2012 |pmid=22914295 |doi=10.1016/S0140-6736(12)60026-9 |doi-access=free}}</ref><ref name="NIDDK2017">{{cite web |title=Crohn's Disease |website=[[National Institute of Diabetes and Digestive and Kidney Diseases]] (NIDDK) |url=https://www.niddk.nih.gov/health-information/digestive-diseases/crohns-disease/all-content |archive-url=https://web.archive.org/web/20191208230827/https://www.niddk.nih.gov/health-information/digestive-diseases/crohns-disease/all-content |archive-date=December 8, 2019 |url-status=live |access-date=December 8, 2019}}</ref> Complications outside of the gastrointestinal tract may include [[anemia]], [[skin rashes]], [[arthritis]], [[uveitis|inflammation of the eye]], and [[fatigue (medical)|fatigue]].<ref name="Baumgart2012" /> The skin rashes may be due to infections as well as [[pyoderma gangrenosum]] or [[erythema nodosum]].<ref name="Baumgart2012" /> [[Bowel obstruction]] may occur as a complication of chronic inflammation, and those with the disease are at greater risk of [[colon cancer]] and [[small bowel cancer]].<ref name="Baumgart2012" /> |
|||
Crohn's disease is influenced by genetic, environmental, and immunological factors. [[Smoking]] is a major modifiable risk factor, especially in Western countries, where it doubles the likelihood of developing the disease. Dietary shifts from high-fiber to processed foods may reduce microbiota diversity and increase risk, while high-fiber diets can offer some protection. Genetic predisposition plays a significant role, with first-degree relatives facing a five-fold increased risk, particularly due to mutations in genes like [[NOD2]] that affect immune response. The condition results from a dysregulated immune response to gut bacteria and increased intestinal permeability, alongside changes in the [[gut microbiome]].<ref name="pmid32242028"/> |
|||
Although the precise causes of Crohn's disease (CD) are unknown, it is believed to be caused by a combination of environmental, [[Immunity (medical)|immune]], and bacterial factors in genetically susceptible individuals.<ref name="NIDDK2017" /><ref>{{cite journal |vauthors=Cho JH, Brant SR |title=Recent insights into the genetics of inflammatory bowel disease |journal=Gastroenterology |volume=140 |issue=6 |pages=1704–12 |date=May 2011 |pmid=21530736 |pmc=4947143 |doi=10.1053/j.gastro.2011.02.046}}</ref><ref name="Bact08" /><ref>{{cite journal |vauthors=Stefanelli T, Malesci A, Repici A, Vetrano S, Danese S |title=New insights into inflammatory bowel disease pathophysiology: paving the way for novel therapeutic targets |journal=Current Drug Targets |volume=9 |issue=5 |pages=413–8 |date=May 2008 |pmid=18473770 |doi=10.2174/138945008784221170}}</ref> It results in a [[Immune-mediated inflammatory diseases|chronic inflammatory disorder]], in which the body's [[immune system]] defends the gastrointestinal tract, possibly targeting [[microbial]] [[antigens]].<ref name="Bact08">{{cite journal |vauthors=Dessein R, Chamaillard M, Danese S |title=Innate immunity in Crohn's disease: the reverse side of the medal |journal=Journal of Clinical Gastroenterology |volume=42 |issue=Suppl 3 Pt 1 |pages=S144–7 |date=September 2008 |pmid=18806708 |doi=10.1097/MCG.0b013e3181662c90}}</ref><ref name="pmid19437144">{{cite journal |vauthors=Marks DJ, Rahman FZ, Sewell GW, Segal AW |title=Crohn's disease: an immune deficiency state |journal=Clinical Reviews in Allergy & Immunology |volume=38 |issue=1 |pages=20–31 |date=February 2010 |pmid=19437144 |pmc=4568313 |doi=10.1007/s12016-009-8133-2}}</ref> While Crohn's is an immune-related disease, it does not seem to be an [[autoimmune disease]] (the immune system is not triggered by the body itself).<ref>{{cite journal |vauthors=Casanova JL, Abel L |title=Revisiting Crohn's disease as a primary immunodeficiency of macrophages |journal=The Journal of Experimental Medicine |volume=206 |issue=9 |pages=1839–43 |date=August 2009 |pmid=19687225 |pmc=2737171 |doi=10.1084/jem.20091683}}</ref> The exact underlying immune problem is not clear; however, it may be an [[immunodeficiency]] state.<ref name="pmid19437144" /><ref>{{cite journal |vauthors=Lalande JD, Behr MA |title=Mycobacteria in Crohn's disease: how innate immune deficiency may result in chronic inflammation |journal=Expert Review of Clinical Immunology |volume=6 |issue=4 |pages=633–641 |date=July 2010 |pmid=20594136 |doi=10.1586/eci.10.29 |s2cid=25402952}}</ref><ref>{{cite journal |vauthors=Yamamoto-Furusho JK, Korzenik JR |title=Crohn's disease: innate immunodeficiency? |journal=World Journal of Gastroenterology |volume=12 |issue=42 |pages=6751–5 |date=November 2006 |pmid=17106921 |pmc=4087427 |doi=10.3748/wjg.v12.i42.6751 |doi-access=free}}</ref> |
|||
Diagnosing Crohn's disease can be complex due to symptom overlap with other gastrointestinal disorders. It typically involves a combination of clinical history, physical examination, and various diagnostic tests. Key methods include [[colonoscopy|ileocolonoscopy]], which identifies the disease in about 90% of cases, and imaging techniques like [[Computed tomography enterography|CT]] and [[Magnetic resonance enterography|MRI enterography]], which help assess the extent of the disease and its complications. [[Histopathology|Histological examination]] of biopsy samples is the most reliable method for confirming diagnosis.<ref name="pmid32242028"/> |
|||
About half of the overall risk is related to genetics, with more than 70 [[gene]]s involved.<ref name="Baumgart2012" /><ref name="genetic_link">{{cite journal |vauthors=Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ |title=Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease |journal=Nature Genetics |volume=40 |issue=8 |pages=955–962 |date=August 2008 |pmid=18587394 |pmc=2574810 |doi=10.1038/ng.175}}</ref> Tobacco smokers are three times as likely to develop Crohn's disease as non-smokers.<ref name="Cosnes2004">{{cite journal |vauthors=Cosnes J |title=Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice |journal=Best Practice & Research. Clinical Gastroenterology |volume=18 |issue=3 |pages=481–496 |date=June 2004 |pmid=15157822 |doi=10.1016/j.bpg.2003.12.003}}</ref> It often begins after [[gastroenteritis]].<ref name="Baumgart2012" /> Other conditions with similar symptoms include [[irritable bowel syndrome]] and [[Behçet's disease]].<ref name="Baumgart2012" /> |
|||
Management of Crohn's disease is individualized, focusing on disease severity and location to achieve mucosal healing and improve long-term outcomes. Treatment may include [[corticosteroids]] for quick symptom relief, [[immunosuppressants]] for maintaining remission, and [[biologics]] like [[TNF inhibitors|anti-TNF therapies]], which are effective for both induction and maintenance. [[Bowel resection|Surgery]] may be necessary for complications such as blockages. Despite ongoing treatment, Crohn's disease is a chronic condition with no cure, often leading to a higher risk of related health issues and reduced life expectancy.<ref name="pmid32242028"/> |
|||
There is no known cure for Crohn's disease.<ref name="Baumgart2012" /><ref name="NIDDK2017" /> [[Treatment of Crohn's disease|Treatment options]] are intended to help with symptoms, maintain [[remission (medicine)|remission]], and prevent [[relapse]].<ref name="Baumgart2012" /> In those newly diagnosed, a [[corticosteroid]] may be used for a brief period of time to improve symptoms rapidly, alongside another medication such as either [[methotrexate]] or a [[thiopurine]] used to prevent recurrence.<ref name="Baumgart2012" /> Cessation of smoking is recommended for people with Crohn's disease.<ref name="Baumgart2012" /> One in five people with the disease is admitted to the hospital each year, and half of those with the disease will require surgery at some time during a ten-year period.<ref name="Baumgart2012" /> While surgery should be used as little as possible, it is necessary to address some [[abscess]]es, certain bowel obstructions, and cancers.<ref name="Baumgart2012" /> Checking for bowel cancer via [[colonoscopy]] is recommended every few years, starting eight years after the disease has begun.<ref name="Baumgart2012" /> |
|||
The disease is most prevalent in [[North America]] and [[Western Europe]], particularly among [[Ashkenazi Jews]], with prevalence rates of 322 per 100,000 in [[Germany]], 319 in [[Canada]],<ref name="pmid32242028"/> and 300 in the [[United States]].<ref name="pmid37223580"/> There is also a rising prevalence in newly industrialized countries, such as 18.6 per 100,000 in [[Hong Kong]] and 3.9 in [[Taiwan]]. The typical age of onset is between 20 and 30 years, with an increasing number of cases among children.<ref name="pmid32242028"/> |
|||
Crohn's disease affects about 3.2 per 1,000 people in Europe and North America;<ref name="Mol2012">{{cite journal |vauthors=Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG |s2cid=206223870 |title=Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review |journal=Gastroenterology |volume=142 |issue=1 |pages=46–54.e42; quiz e30 |date=January 2012 |pmid=22001864 |doi=10.1053/j.gastro.2011.10.001 |url=http://www.gastrojournal.org/article/S0016508511013783/pdf |access-date=October 7, 2022 |archive-date=October 7, 2022 |archive-url=https://web.archive.org/web/20221007193457/https://www.gastrojournal.org/article/S0016-5085(11)01378-3/pdf |url-status=live}}</ref> it is less common in Asia and Africa.<ref>{{cite journal |vauthors=Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC |title=Inflammatory bowel disease in Asia: a systematic review |journal=Journal of Gastroenterology and Hepatology |volume=27 |issue=8 |pages=1266–80 |date=August 2012 |pmid=22497584 |doi=10.1111/j.1440-1746.2012.07150.x |s2cid=205468282|doi-access=free}}</ref><ref name="Hov2012" /> It has historically been more common in the [[developed world]].<ref name="Bur2013" /> Rates have, however, been increasing, particularly in the developing world, since the 1970s.<ref name="Hov2012">{{cite journal |vauthors=Hovde Ø, Moum BA |title=Epidemiology and clinical course of Crohn's disease: results from observational studies |journal=World Journal of Gastroenterology |volume=18 |issue=15 |pages=1723–31 |date=April 2012 |pmid=22553396 |pmc=3332285 |doi=10.3748/wjg.v18.i15.1723 |doi-access=free}}</ref><ref name="Bur2013">{{cite journal |vauthors=Burisch J, Munkholm P |title=Inflammatory bowel disease epidemiology |journal=Current Opinion in Gastroenterology |volume=29 |issue=4 |pages=357–62 |date=July 2013 |pmid=23695429 |doi=10.1097/MOG.0b013e32836229fb |s2cid=9538639}}</ref> Inflammatory bowel disease resulted in 47,400 deaths in 2015,<ref>{{cite journal |title=Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015 |journal=The Lancet |volume=388 |issue=10053 |pages=1459–1544 |date=October 2016 |pmid=27733281 |pmc=5388903 |doi=10.1016/S0140-6736(16)31012-1 |author1=(( GBD 2015 Mortality Causes of Death Collaborators))}}</ref> and those with Crohn's disease have a slightly reduced [[life expectancy]].<ref name="Baumgart2012" /> It tends to start in adolescence and young adulthood, though it can occur at any age.<ref>{{Cite journal |last1=Shih |first1=I-Lun |last2=Lee |first2=Tsung-Chun |last3=Tu |first3=Chia-Hung |last4=Chang |first4=Chin-Chen |last5=Wang |first5=Yu-Fen |last6=Tseng |first6=Yao-Hui |last7=Chiu |first7=Han-Mo |last8=Wu |first8=Ming-Shiang |last9=Wang |first9=Hsiu-Po |last10=Shih |first10=Tiffany Ting-Fang |last11=Liu |first11=Kao-Lang |date=2016-12-01 |title=Intraobserver and interobserver agreement for identifying extraluminal manifestations of Crohn's disease with magnetic resonance enterography |journal=Advances in Digestive Medicine |language=en |volume=3 |issue=4 |pages=174–180 |doi=10.1016/j.aidm.2015.05.004 |s2cid=70796090 |doi-access=free}}</ref><ref name="Baumgart2012" /><ref name="NIDDK2017" /><ref name="eMedicineHealth" /> Males and females are equally affected.<ref name="NIDDK2017" /> |
|||
{{TOC limit}} |
|||
== Name controversy == |
|||
The disease was named after [[gastroenterology|gastroenterologist]] [[Burrill Bernard Crohn]], who in 1932, together with [[Leon Ginzburg]] (1898–1988) and [[Gordon D. Oppenheimer]] (1900–1974) at [[Mount Sinai Hospital, New York|Mount Sinai Hospital in New York]], described a series of patients with inflammation of the [[terminal ileum]] of the [[small intestine]], the area most commonly affected by the illness.<ref name="CrohnBB">{{cite journal |vauthors=Crohn BB, Ginzburg L, Oppenheimer GD |title=Regional ileitis: a pathologic and clinical entity. 1932 |journal=The Mount Sinai Journal of Medicine, New York |volume=67 |issue=3 |pages=263–8 |date=May 2000 |pmid=10828911}}</ref> Why the disease was named after Crohn has controversy around it.<ref>{{Cite journal |last1=Van Hootegem |first1=Philippe |last2=Travis |first2=Simon |date=2020-07-09 |title=Is Crohn's Disease a Rightly Used Eponym? |url=https://academic.oup.com/ecco-jcc/article/14/6/867/5614546 |journal=Journal of Crohn's and Colitis |language=en |volume=14 |issue=6 |pages=867–871 |doi=10.1093/ecco-jcc/jjz183 |pmid=31701137 |issn=1873-9946 |doi-access=free |access-date=July 8, 2023 |archive-date=July 8, 2023 |archive-url=https://web.archive.org/web/20230708104800/https://academic.oup.com/ecco-jcc/article/14/6/867/5614546 |url-status=live }}</ref><ref>{{Cite journal |last1=Mulder |first1=Daniel J. |last2=Noble |first2=Angela J. |last3=Justinich |first3=Christopher J. |last4=Duffin |first4=Jacalyn M. |date=May 2014 |title=A tale of two diseases: The history of inflammatory bowel disease |journal=Journal of Crohn's and Colitis |language=en |volume=8 |issue=5 |pages=341–8 |doi=10.1016/j.crohns.2013.09.009|pmid=24094598 |s2cid=13714394 |doi-access=free}}</ref> While Crohn, in his memoir, describes his original investigation of the disease, Ginzburg provided strong evidence of how he and Oppenheimer were the first to study the disease.<ref>{{Cite journal |last=Ginzburg |first=Leon |date=May 1986 |title=Regional enteritis: Historical perspective |journal=Gastroenterology |language=en |volume=90 |issue=5 |pages=1310–1 |doi=10.1016/0016-5085(86)90419-1|pmid=3514360 |doi-access=free}}</ref> |
|||
== Signs and symptoms == |
== Signs and symptoms == |
||
[[Image:Patterns of CD.jpg|right|thumb|alt=Diagram of the three most common sites of intestinal involvement in Crohn's disease.|The three most common sites of intestinal involvement in Crohn's disease are ileal, ileocolic and colonic.<ref name="pmid32242028"/>]] |
|||
{{Symptoms in CD vs. UC}} |
|||
Crohn's disease is characterized by recurring flares of intestinal inflammation, with diarrhea and abdominal pain as the primary symptoms. Symptoms may be non-specific and progress gradually, and many people have symptoms for years before diagnosis. Unlike [[ulcerative colitis]], inflammation can occur anywhere in the gastrointestinal tract, most often in the [[ileum]] and [[Large intestine|colon]], and can involve all layers of the intestine. Disease location tends to be stable, with a third of patients having colonic disease, a third having ileocolic disease, and a third having ileal disease. The disease may also involve perianal, upper gastrointestinal, and extraintestinal organs.<ref name="pmid32242028"/> |
|||
=== Gastrointestinal === |
=== Gastrointestinal === |
||
* [[Diarrhea]] affects 82% of people at the onset of Crohn's disease, with severity ranging from mild to severe enough to require substitution of water and electrolytes. With Crohn's disease, diarrhea is frequent and urgent rather than voluminous.<ref name="pmid22917170">{{cite journal | vauthors = Wenzl HH | title = Diarrhea in chronic inflammatory bowel diseases | journal = Gastroenterology Clinics of North America | volume = 41 | issue = 3 | pages = 651–675 | pmid = 22917170 | date = September 2012 | doi = 10.1016/j.gtc.2012.06.006 | doi-access = free }}</ref> |
|||
[[File:Aphtha2.jpg|thumb|An [[aphthous ulcer]] on the mucous membrane of the [[human mouth|mouth]] in Crohn's disease]] |
|||
* [[Abdominal pain]] affects at least 70% of people during the course of Crohn's disease. It can result directly from intestinal inflammation, or from complications such as strictures and fistulas.<ref name="pmid37867930">{{cite journal | vauthors = Coates MD, Clarke K, Williams E, Jeganathan N, Yadav S, Giampetro D, Gordin V, Smith S, Vrana K, Bobb A, Gazzio TT, Tressler H, Dalessio S | title = Abdominal Pain in Inflammatory Bowel Disease: An Evidence-Based, Multidisciplinary Review | journal = Crohns Colitis 360 | volume = 5 | issue = 4 | pmid = 37867930 | date = September 2023 | pages = otad055 | doi = 10.1093/crocol/otad055 | doi-access = free | pmc = 10588456 }}</ref> Pain most commonly occurs in the lower right abdomen.<ref name="ustekinumab">{{cite web |title=What I need to know about Crohn's Disease |url=http://www.niddk.nih.gov/health-information/health-topics/digestive-diseases/crohns-disease/Pages/ez.aspx#symptoms |website=www.niddk.nih.gov|access-date=December 11, 2015 |url-status=dead |archive-url = https://web.archive.org/web/20151121213407/http://www.niddk.nih.gov/health-information/health-topics/digestive-diseases/crohns-disease/Pages/ez.aspx#symptoms|archive-date=November 21, 2015}}</ref> |
|||
Many people with Crohn's disease have symptoms for years before the diagnosis.<ref name="Pimentel">{{cite journal |vauthors=Pimentel M, Chang M, Chow EJ, Tabibzadeh S, Kirit-Kiriak V, Targan SR, Lin HC |title=Identification of a prodromal period in Crohn's disease but not ulcerative colitis |journal=The American Journal of Gastroenterology |volume=95 |issue=12 |pages=3458–62 |date=December 2000 |doi=10.1111/j.1572-0241.2000.03361.x |pmid=11151877 |s2cid=2764694}}</ref> The usual onset is in the teens and twenties, but can occur at any age.<ref name="eMedicineHealth">{{cite web |url=http://www.emedicinehealth.com/crohn_disease/article_em.htm |title=Crohn's Disease: Get Facts on Symptoms and Diet |website=eMedicineHealth|url-status=live|archive-url=https://web.archive.org/web/20071020184248/http://www.emedicinehealth.com/crohn_disease/article_em.htm|archive-date=October 20, 2007}}</ref><ref name="Baumgart2012" /> Because of the 'patchy' nature of the [[gastrointestinal]] disease and the depth of tissue involvement, initial symptoms can be more subtle than those of [[ulcerative colitis]].{{Citation needed|date=May 2024}} People with Crohn's disease experience chronic recurring periods of flare-ups and [[remission (medicine)|remission]].<ref>{{cite book |vauthors=((National Research Council)) |title=Diagnosis and Control of Johne's Disease |year=2003 |chapter=Johne's Disease and Crohn's Disease |chapter-url=https://www.ncbi.nlm.nih.gov/books/NBK207651/ |publisher=National Academies Press |location=Washington, DC |doi=10.17226/10625 |pmid=25032299 |isbn=978-0-309-08611-0 |id=NBK207651 |access-date=August 30, 2017 |archive-date=September 6, 2017 |archive-url=https://web.archive.org/web/20170906121542/https://www.ncbi.nlm.nih.gov/books/NBK207651/ |url-status=live }}</ref> The symptoms experienced can change over time as inflammation increases and spreads. Symptoms can also be different depending on which organs are involved. It is generally thought that the presentation of Crohn's disease is different for each patient due to the high variability of symptoms, organ involvement, and initial presentation. |
|||
* [[Rectal bleeding]] is less common than in ulcerative colitis, and is more likely to occur with inflammation in the colon or rectum. Bleeding in the colon or rectum is bright red, whereas bleeding in higher segments causes dark or black stools.<ref name="CrohnsColitisCA">{{cite web | url = https://crohnsandcolitis.ca/About-Crohn-s-Colitis/IBD-Journey/Symptom-Management/Bleeding-and-Blood-in-the-Stool | title = Bleeding and Blood in the Stool | website = crohn's and colitis | access-date = 16 October 2024}}</ref> |
|||
* [[Bloating]], [[flatus]], and other symptoms of [[irritable bowel syndrome]] occur in 41% of people in remission.<ref name="pmid31496621">{{cite journal | vauthors = Barros LL, Farias AQ, Rezaie A | title = Gastrointestinal motility and absorptive disorders in patients with inflammatory bowel diseases: Prevalence, diagnosis and treatment | journal = World Journal of Gastroenterology | volume = 25 | issue = 31 | pages = 4414–4426 | pmid = 31496621 | pmc = 6710178 | date = August 2019 | doi = 10.3748/wjg.v25.i31.4414 | doi-access = free }}</ref> |
|||
=== Perianal === |
|||
* Perianal involvement occurs in 18–43% of cases, more frequently if the colon and rectum are inflamed, and can cause [[anal fistula|fistulas]], [[skin tags]], [[hemorrhoids]], [[anal fissure|fissures]], [[ulcers]], and [[anal stricture|strictures]].<ref name="pmid31507348">{{cite journal | vauthors = Pogacnik JS, Salgado G | title = Perianal Crohn's Disease | journal = Clinics in Colon and Rectal Surgery | volume = 32 | issue = 5 | pages = 377–385 | pmid = 31507348 | date = September 2019 | doi = 10.1055/s-0039-1687834 | doi-access = free | pmc = 6731113 }}</ref> |
|||
[[Perianal]] discomfort may also be prominent in Crohn's disease. Itchiness or pain around the [[anus]] may be suggestive of [[inflammation]] of the anus, or perianal complications such as [[anal fissure]]s, [[fistula]]e, or [[abscess]]es around the [[Anus|anal]] area.<ref name="Baumgart2012" /> Perianal [[acrochordon|skin tags]] are also common in Crohn's disease, and may appear with or without the presence of [[colorectal polyp]]s.<ref>{{cite journal |vauthors=Taylor BA, Williams GT, Hughes LE, Rhodes J |date=August 1989 |title=The histology of anal skin tags in Crohn's disease: an aid to confirmation of the diagnosis |journal=International Journal of Colorectal Disease |volume=4 |issue=3 |pages=197–9 |doi=10.1007/BF01649703 |pmid=2769004 |s2cid=7831833}}</ref> [[Fecal incontinence]] may accompany perianal Crohn's disease. |
|||
* Upper gastrointestinal involvement is rare, occurring in 0.5-16% of cases, and may cause symptoms such as [[odynophagia|pain while swallowing]], [[dysphagia|difficulty swallowing]], [[vomiting]], and [[nausea]].<ref name="pmid28708248">{{Cite journal |last1=Laube |first1=Robyn |last2=Liu |first2=Ken |last3=Schifter |first3=Mark |last4=Yang |first4=Jessica L |last5=Suen |first5=Michael K |last6=Leong |first6=Rupert W |date=February 2018 |title=Oral and upper gastrointestinal Crohn's disease |url=https://onlinelibrary.wiley.com/doi/10.1111/jgh.13866 |journal=Journal of Gastroenterology and Hepatology |language=en |volume=33 |issue=2 |pages=355–364 |doi=10.1111/jgh.13866 |pmid=28708248 |issn=0815-9319}}</ref> |
|||
=== Intestines === |
|||
The intestines, especially the colon and terminal ileum, are the areas of the body affected most commonly. [[Abdominal pain]] is a common initial symptom of Crohn's disease,<ref name="NIDDK2017" /> especially in the lower right abdomen.<ref name="ustekinumab">{{cite web |title=What I need to know about Crohn's Disease |url=http://www.niddk.nih.gov/health-information/health-topics/digestive-diseases/crohns-disease/Pages/ez.aspx#symptoms |website=www.niddk.nih.gov|access-date=December 11, 2015 |url-status=dead |archive-url = https://web.archive.org/web/20151121213407/http://www.niddk.nih.gov/health-information/health-topics/digestive-diseases/crohns-disease/Pages/ez.aspx#symptoms|archive-date=November 21, 2015}}</ref> Flatulence, bloating, and abdominal distension are additional symptoms and may also add to the intestinal discomfort. Pain is often accompanied by [[diarrhea]], which may or may not be bloody. Inflammation in different areas of the [[intestinal tract]] can affect the quality of the [[feces]]. [[Ileitis]] typically results in large-volume, watery feces, while [[colitis]] may result in a smaller volume of feces of greater frequency. Fecal consistency may range from solid to watery. In severe cases, an individual may have more than 20 [[bowel movements]] per day, and may need to awaken at night to defecate.<ref name="Baumgart2012" /><ref name="emed">{{EMedicine|article|172940|Crohn Disease}}</ref><ref name="Podolsky" /><ref>{{cite journal |vauthors=Mueller MH, Kreis ME, Gross ML, Becker HD, Zittel TT, Jehle EC |title=Anorectal functional disorders in the absence of anorectal inflammation in patients with Crohn's disease |journal=The British Journal of Surgery |volume=89 |issue=8 |pages=1027–31 |date=August 2002 |pmid=12153630 |doi=10.1046/j.1365-2168.2002.02173.x |s2cid=42383375|doi-access=free}}</ref> Visible bleeding in the feces is less common in Crohn's disease than in ulcerative colitis, but is not unusual.<ref name="Baumgart2012" /> Bloody bowel movements are usually intermittent, and may be bright red, dark maroon, or even black in color. The color of bloody stool depends on the location of the bleed. In severe Crohn's colitis, bleeding may be copious.<ref name="emed" /> |
|||
=== Stomach and esophagus=== |
|||
The stomach is rarely the sole or predominant site of CD. To date there are only a few documented case reports of adults with isolated gastric CD and no reports in the pediatric population. Isolated stomach involvement is very unusual presentation accounting for less than 0.07% of all gastrointestinal CD.<ref>{{Cite journal |last1=Ingle |first1=Sachin B |last2=Hinge |first2=Chitra R |last3=Dakhure |first3=Sarita |last4=Bhosale |first4=Smita S |date=May 16, 2013 |title=Isolated gastric Crohn's disease |journal=World Journal of Clinical Cases |volume=1 |issue=2 |pages=71–73 |doi=10.12998/wjcc.v1.i2.71 |issn=2307-8960 |pmc=3845940 |pmid=24303469 |doi-access=free}}</ref> However, the [[esophagus]] and [[stomach]] are increasingly understood to be affected in patients with intestinal CD. Recent studies suggest upper GI involvement occurs in 13-16% of cases, typically presenting after distal symptoms.<ref>{{Cite journal |last1=Greuter |first1=Thomas |last2=Piller |first2=Alberto |last3=Fournier |first3=Nicolas |last4=Safroneeva |first4=Ekaterina |last5=Straumann |first5=Alex |last6=Biedermann |first6=Luc |last7=Godat |first7=Sébastien |last8=Nydegger |first8=Andreas |last9=Scharl |first9=Michael |last10=Rogler |first10=Gerhard |last11=Vavricka |first11=Stephan R |last12=Schoepfer |first12=Alain M |date=2018-08-25 |title=Upper Gastrointestinal Tract Involvement in Crohn's Disease: Frequency, Risk Factors, and Disease Course |url=https://academic.oup.com/ecco-jcc/article/12/12/1399/5079482 |journal=Journal of Crohn's and Colitis |volume=12 |issue=12 |pages=1399–1409 |doi=10.1093/ecco-jcc/jjy121 |pmid=30165603 |issn=1873-9946}}</ref><ref>{{Cite journal |last1=Laube |first1=Robyn |last2=Liu |first2=Ken |last3=Schifter |first3=Mark |last4=Yang |first4=Jessica L |last5=Suen |first5=Michael K |last6=Leong |first6=Rupert W |date=February 2018 |title=Oral and upper gastrointestinal Crohn's disease |url=https://onlinelibrary.wiley.com/doi/10.1111/jgh.13866 |journal=Journal of Gastroenterology and Hepatology |language=en |volume=33 |issue=2 |pages=355–364 |doi=10.1111/jgh.13866 |pmid=28708248 |issn=0815-9319}}</ref><ref>{{Cite journal |last1=Pimentel |first1=Andréa Maia |last2=Rocha |first2=Raquel |last3=Santana |first3=Genoile Oliveira |date=2019-03-07 |title=Crohn's disease of esophagus, stomach and duodenum |journal=World Journal of Gastrointestinal Pharmacology and Therapeutics |volume=10 |issue=2 |pages=35–49 |doi=10.4292/wjgpt.v10.i2.35 |doi-access=free |issn=2150-5349 |pmc=6422852 |pmid=30891327}}</ref> Upper gastrointestinal symptoms may include difficulty swallowing ([[dysphagia]]), upper abdominal pain, and vomiting.<ref>{{cite journal |vauthors=Fix OK, Soto JA, Andrews CW, Farraye FA |date=December 2004 |title=Gastroduodenal Crohn's disease |journal=Gastrointestinal Endoscopy |volume=60 |issue=6 |pages=985 |doi=10.1016/S0016-5107(04)02200-X |pmid=15605018}}</ref> |
|||
===Oropharynx (mouth)=== |
|||
The mouth may be affected by recurrent sores ([[aphthous ulcer]]s). Recurrent aphthous ulcers are common; however, it is not clear whether this is due to Crohn's disease or simply that they are common in the general population. Other findings may include diffuse or nodular swelling of the mouth, a cobblestone appearance inside the mouth, granulomatous ulcers, or [[Pyostomatitis vegetans|pyostomatitis vegetans]]. Medications that are commonly prescribed to treat CD, such as anti-inflammatory and sulfa-containing drugs, may cause lichenoid drug reactions in the mouth. Fungal infection such as candidiasis is also common due to the immunosuppression required in the treatment of the disease. Signs of anemia such as pallor and angular cheilitis or glossitis are also common due to nutritional malabsorption.<ref name="pmid33477990">{{cite journal |vauthors=Antonelli E, Bassotti G, Tramontana M, Hansel K, Stingeni L, Ardizzone S, Genovese G, Marzano AV, Maconi G |title=Dermatological Manifestations in Inflammatory Bowel Diseases |journal=J Clin Med |volume=10 |issue=2 |date=January 2021 |page=364 |pmid=33477990 |pmc=7835974 |doi=10.3390/jcm10020364 | doi-access = free}}</ref> |
|||
People with Crohn's disease are also susceptible to [[angular stomatitis]], an inflammation of the corners of the mouth, and [[pyostomatitis vegetans]].<ref name="Cutaneous">{{Cite journal |last1=Aberumand |first1=Babak |last2=Howard |first2=Jessica |last3=Howard |first3=John |date=January 3, 2017 |title=Metastatic Crohn's Disease: An Approach to an Uncommon but Important Cutaneous Disorder |journal=BioMed Research International |volume=2017 |pages=e8192150 |doi=10.1155/2017/8192150 |pmid=28127561 |pmc=5239966 |issn=2314-6133|doi-access=free}}</ref> |
|||
=== Systemic === |
=== Systemic === |
||
Crohn's disease often presents with systemic symptoms, including: |
|||
Like many other chronic, inflammatory diseases, Crohn's disease can cause a variety of [[B symptoms|systemic symptoms]].<ref name="Baumgart2012" /> Among children, [[growth failure]] is common. Many children are first diagnosed with Crohn's disease based on [[failure to thrive|inability to maintain growth]].<ref name="Beattie" /> As it may manifest at the time of the growth spurt in [[puberty]], as many as 30% of children with Crohn's disease may have retardation of growth.<ref>{{cite journal |vauthors=Büller HA |title=Problems in diagnosis of IBD in children |journal=The Netherlands Journal of Medicine |volume=50 |issue=2 |pages=S8–11 |date=February 1997 |pmid=9050326 |doi=10.1016/S0300-2977(96)00064-2 |url=http://dare.uva.nl/personal/pure/en/publications/problems-in-diagnosis-of-ibd-in-children(59612226-ecf5-49df-8848-d79d03f0c5c8).html |type=Submitted manuscript |access-date=September 4, 2018 |archive-date=August 28, 2021 |archive-url=https://web.archive.org/web/20210828051037/https://dare.uva.nl/search?identifier=59612226-ecf5-49df-8848-d79d03f0c5c8 |url-status=live}}</ref> Fever may also be present, though fevers greater than 38.5 °C (101.3 °F) are uncommon unless there is a complication such as an abscess.<ref name="Baumgart2012" /> Among older individuals, Crohn's disease may manifest as weight loss, usually related to decreased food intake, since individuals with intestinal symptoms from Crohn's disease often feel better when they do not eat and might [[Anorexia (symptom)|lose their appetite]].<ref name="Beattie">{{cite journal |vauthors=Beattie RM, Croft NM, Fell JM, Afzal NA, Heuschkel RB |title=Inflammatory bowel disease |journal=Archives of Disease in Childhood |volume=91 |issue=5 |pages=426–432 |date=May 2006 |pmid=16632672 |pmc=2082730 |doi=10.1136/adc.2005.080481}}</ref> People with extensive [[small intestine]] disease may also have [[malabsorption]] of [[carbohydrate]]s or [[lipid]]s, which can further exacerbate weight loss.<ref name="pmid8898436">{{cite journal |vauthors=O'Keefe SJ |title=Nutrition and gastrointestinal disease |journal=Scandinavian Journal of Gastroenterology. Supplement |volume=220 |pages=52–9 |year=1996 |pmid=8898436 |doi=10.3109/00365529609094750}}</ref> |
|||
* [[Fatigue#Chronic|Chronic fatigue]], which lasts for at least 6 months and cannot be cured by rest, occurs in 80% of people with Crohn's disease, including 30% of people who are in remission.<ref name="pmid37629549">{{cite journal | vauthors = Włodarczyk M, Makaro A, Prusisz M, Włodarczyk J, Nowocień M, Maryńczak K, Fichna J, Dziki Ł | title = The Role of Chronic Fatigue in Patients with Crohn's Disease | journal = Life (Basel) | volume = 13 | issue = 8 | pages = 1692 | pmid = 37629549 | date = August 2023 | doi = 10.3390/life13081692 | doi-access = free | pmc = 10455565 | bibcode = 2023Life...13.1692W }}</ref> |
|||
* [[Fevers]], typically low-grade, are often reported as initial symptoms of Crohn's disease. High-grade fevers are often a result of abscesses.<ref name="StatPearls">{{cite web |title=Crohn Disease |date=2024 |url=https://www.ncbi.nlm.nih.gov/books/NBK436021/| access-date=18 October 2024 | publisher=StatPearls Publishing |pmid=28613792 | vauthors = Ranasinghe IR, Tian C, Hsu R }}</ref> |
|||
* [[Unintentional weight loss|Weight loss]] often occurs due to diarrhea and reduced appetite.<ref name="StatPearls"/> |
|||
=== Extraintestinal === |
=== Extraintestinal === |
||
Extraintestinal manifestations occur in 21–47% of cases, and include symptoms such as:<ref name="pmid32242028"/> |
|||
Crohn's disease can affect many [[organ system]]s beyond the [[gastrointestinal tract]].<ref name="Harbord">{{cite journal|vauthors=Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, Burisch J, De Vos M, De Vries AM, Dick AD, Juillerat P, Karlsen TH, Koutroubakis I, Lakatos PL, Orchard T, Papay P, Raine T, Reinshagen M, Thaci D, Tilg H, Carbonnel F |date=March 2016 |title=The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease |journal=Journal of Crohn's & Colitis |volume=10 |issue=3 |pages=239–254 |doi=10.1093/ecco-jcc/jjv213 |pmc=4957476 |pmid=26614685}}</ref> |
|||
* Mouth ulcers, such as [[canker sores]].<ref name="pmid33477990">{{cite journal |vauthors=Antonelli E, Bassotti G, Tramontana M, Hansel K, Stingeni L, Ardizzone S, Genovese G, Marzano AV, Maconi G |title=Dermatological Manifestations in Inflammatory Bowel Diseases |journal=J Clin Med |volume=10 |issue=2 |date=January 2021 |page=364 |pmid=33477990 |pmc=7835974 |doi=10.3390/jcm10020364 | doi-access = free}}</ref> |
|||
{{Complications of CD vs. UC}} |
|||
* Eye inflammation, such as [[uveitis]], [[scleritis]], and [[episcleritis]].<ref name="pmid32242028"/> |
|||
* Skin inflammation, such as [[erythema nodosum]] and [[pyoderma gangrenosum]].<ref name="pmid32242028"/> |
|||
* Blood conditions such as [[portal hypertension]], [[thromboembolism]], [[thrombosis]], [[pulmonary embolism]].<ref name="pmid32242028"/> |
|||
* Joint inflammation such as [[arthritis]], [[ankylosing spondylitis]], [[sacroiliitis]].<ref name="pmid32242028"/> |
|||
* Respiratory conditions such as [[obstructive sleep apnea]] and [[chest infections]].<ref name="pmid32242028"/> |
|||
* Liver, bile duct, and gallbladder conditions such as [[primary sclerosing cholangitis]] and [[cirrhosis]].<ref name="pmid32242028"/> |
|||
* Mental disorders such as [[Major depressive disorder|depression]] and [[Anxiety disorder|anxiety]].<ref name="pmid35732730">{{cite journal | vauthors = Bisgaard TH, Allin KH, Keefer L, Ananthakrishnan AN, Jess T | title = Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment | journal = Nature Reviews Gastroenterology & Hepatology | volume = 19 | issue = 11 | pages = 717–726 | pmid = 35732730 | date = March 2023 | doi = 10.1038/s41575-022-00634-6 | doi-access = free }}</ref> |
|||
* [[Metabolic bone disease]].<ref name="pmid32242028"/> |
|||
=== |
=== Complications === |
||
[[Image:Colorectal cancer endo 2.jpg|right|thumb| alt=Image of colon cancer identified during a colonoscopy in Crohn's disease.|Image of colon cancer identified in the sigmoid colon of a person with Crohn's disease during a colonoscopy.]] |
|||
Bowel damage due to inflammation occurs in half of cases within 10 years of diagnosis, and can lead to stricturing or penetrating disease forms. This can cause complications such as: |
|||
Inflammation of the interior portion of the eye, known as [[uveitis]], can cause blurred vision and eye pain, especially when exposed to light ([[photophobia]]).<ref name="Trikudanathan2012" /> Uveitis can lead to loss of vision if untreated.<ref name="Harbord" /> |
|||
* [[Bowel obstruction]]s may occur due to strictures, particularly in the small intestine, and may require surgical removal of the affected segment ([[bowel resection]]) or surgical dilation ([[stricturoplasty]]).<ref name="pmid28601423"/> |
|||
* [[Fistulas]], openings in the gut, result from penetrating disease and can cause diarrhea, [[urinary tract infections]], and stool leakage to the vagina or skin. It is treated by bowel resection or [[fistulotomy]]<ref name="pmid28601423"/> |
|||
* [[Abscesses]], infected pockets, can also result from penetrating disease, causing abdominal pain, fever, and chills. It may be treated by [[Incision and drainage|surgical drainage]].<ref name="pmid28601423">{{cite journal | vauthors = Feuerstein JD, Cheifetz AS | title = Crohn Disease: Epidemiology, Diagnosis, and Management | journal = Mayo Clinic Proceedings | volume = 92 | issue = 7 | pages = 1088–1103 | pmid = 28601423 | date = June 2017 | doi = 10.1016/j.mayocp.2017.04.010 | doi-access = free }}</ref> |
|||
Malnutrition occurs in 38.9% of people in remission and 82.8% of people with active disease due to malabsorption in the small intestine, reduced appetite, and drug interactions.<ref name="pmid37111210"/> This can cause complications such as: |
|||
Inflammation may also involve the white part of the eye ([[sclera]]) or the overlying connective tissue ([[episclera]]), which causes conditions called [[scleritis]] and [[episcleritis]], respectively.<ref name="Trikudanathan2012" /> |
|||
* [[Anemia]] occurs in 6–74% of cases as a result of [[iron deficiency]] and blood loss. It is treated by [[oral iron]] or [[intravenous iron]] depending on disease activity.<ref name="pmid37111210"/> |
|||
* [[Vitamin D deficiency]] is prevalent and can cause [[osteoporosis]], and is treated by oral supplementation.<ref name="pmid37111210"/> |
|||
* [[Folate deficiency|Folic acid]], [[Vitamin B12 deficiency|vitamin B12]], [[Zinc deficiency|zinc]], [[Magnesium deficiency|magnesium]], and [[Selenium deficiency|selenium]] deficiencies may also occur, and are treated through oral supplementation.<ref name="pmid37111210">{{cite journal | vauthors = Jabłońska B, Mrowiec S | title = Nutritional Status and Its Detection in Patients with Inflammatory Bowel Diseases | journal = Nutrients | volume = 15 | issue = 8 | pages = 1991 | pmid = 37111210 | pmc = 10143611 | date = April 2023 | doi = 10.3390/nu15081991 | doi-access = free }}</ref> |
|||
* [[Stunted growth|Impaired growth]] and nutritional deficiency occur in 65–85% of children with Crohn's disease.<ref name="pmid25309059">{{cite journal | vauthors = Gasparetto M, Guariso G | title = Crohn's disease and growth deficiency in children and adolescents | journal = World Journal of Gastroenterology | volume = 20 | issue = 37 | pages = 13219–13233 | pmid = 25309059 | pmc = 4188880 | date = October 2014 | doi = 10.3748/wjg.v20.i37.13219 | doi-access = free }}</ref> |
|||
Intestinal cancers may develop as a result of prolonged or severe inflammation.<ref name="pmid37627182"/> This includes: |
|||
Other very rare ophthalmological manifestations include: [[conjunctivitis]], [[glaucoma]], and retinal vascular disease.<ref name="Manifestations">{{Cite journal |last1=Jose |first1=Folashade A. |last2=Heyman |first2=Melvin B. |date=February 2008 |title=Extraintestinal Manifestations of Inflammatory Bowel Disease |journal=Journal of Pediatric Gastroenterology and Nutrition |volume=46 |issue=2 |pages=124–133 |doi=10.1097/MPG.0b013e318093f4b0 |issn=0277-2116 |pmc=3245880 |pmid=18223370}}</ref> |
|||
* [[Colorectal cancer]] has a prevalence of 7% at 30 years after diagnosis and accounts for 15% of deaths in people with Crohn's. Risk is higher if the disease occurs in most of the colon. Endoscopic surveillance is performed to detect and remove polyps, while surgery is required for dysplasia beyond the mucosal surface.<ref name="pmid37627182">{{cite journal | vauthors = Sato Y, Tsujinaka S, Miura T, Kitamura Y, Suzuki H, Shibata C | title = Inflammatory Bowel Disease and Colorectal Cancer: Epidemiology, Etiology, Surveillance, and Management | journal = Cancers (Basel) | volume = 15 | issue = 16 | pages = 4154 | pmid = 37627182 | pmc = 10452690 | date = August 2023 | doi = 10.3390/cancers15164154 | doi-access = free }}</ref> |
|||
* [[Small bowel cancer]] has a prevalence of 1.6%, at least 12-times greater in people with Crohn's disease. Unlike colorectal cancer, endoscopic surveillance is ineffective and not recommended for small bowel cancer.<ref name="pmid38188070">{{cite journal | vauthors = Bhatt H, Mathis KL | title = Small Bowel Carcinoma in the Setting of Inflammatory Bowel Disease | journal = Clinics in Colon and Rectal Surgery | volume = 37 | issue = 1 | pages = 46–52 | pmid = 38188070 | pmc = 10769580 | date = March 2023 | doi = 10.1055/s-0043-1762929 | doi-access = free }}</ref> |
|||
== Causes == |
|||
====Gallbladder and liver==== |
|||
=== Risk factors === |
|||
Smoking is a major modifiable risk factor for Crohn's disease, particularly in Western countries, where it doubles the risk. This risk is higher in females and varies with age. Smoking is also linked to earlier disease onset, increased need for immunosuppression, more surgeries, and higher recurrence rates. Ethnic differences have been noted, with studies in Japan linking passive smoking to the disease.<ref name="pmid32242028"/> Proposed mechanisms for smoking's effects include impaired [[autophagy]], direct toxicity to immune cells, and changes in the [[Gut microbiota|microbiome]].<ref name="pmid30611442"/> |
|||
Diet may influence the development of Crohn's disease by affecting the gut microbiome. The shift from high-fiber, low-fat foods to processed foods reduces microbiota diversity, increasing the risk of Crohn's disease.<ref name="pmid32242028"/> Conversely, high-fiber diets may reduce risk by up to 40%, likely due to the production of anti-inflammatory [[short-chain fatty acids]] from fiber metabolism by gut bacteria.<ref name="pmid30611442"/> The [[Mediterranean diet]] is also linked to a lower risk of later-onset Crohn's disease. Since diet's effect on the microbiome is temporary, its role in gut dysbiosis is controversial.<ref name="pmid32242028"/> |
|||
Crohn's disease that affects the [[ileum]] may result in an increased risk of [[gallstone]]s. This is due to a decrease in [[Enterohepatic circulation|bile acid resorption in the ileum]], and the [[bile]] gets excreted in the stool. As a result, the [[cholesterol]]/bile ratio increases in the [[gallbladder]], resulting in an increased risk for gallstones.<ref name="Trikudanathan2012" /> Although the association is greater in the context of [[ulcerative colitis]], Crohn's disease may also be associated with [[primary sclerosing cholangitis]], a type of inflammation of the [[bile duct]]s.<ref>{{cite book |title=Robbins and Cotran: Pathologic Basis of Disease |vauthors=Kumar V, Abbas AK, Fausto N |date=July 30, 2004 |publisher=Elsevier Saunders |isbn=978-0-7216-0187-8 |edition=7th |location=Philadelphia, Pennsylvania |page=847 |chapter=The Gastrointestinal Tract}}</ref> |
|||
Childhood antibiotic exposure is linked to a higher risk of Crohn's disease due to changes in the intestinal microbiome, which shapes the immune system in early life. Other medications, like [[oral contraceptives]], [[aspirin]], and [[NSAIDs]], may also increase risk by up to two-fold. Conversely, [[breastfeeding]] and [[statin]] use may reduce risk, though breastfeeding's effects are inconsistent. Early life factors such as mode of delivery, pet exposure, and infections—related to the [[hygiene hypothesis]]—also significantly influence risk, likely due to influences on the microbiome.<ref name="pmid30611442"/> |
|||
Liver involvement of Crohn's disease can include [[cirrhosis]] and [[steatosis]]. [[Non-alcoholic fatty liver disease|Nonalcoholic fatty liver disease]] (nonalcoholic steatohepatitis, NAFLD) are relatively common and can slowly progress to end-stage liver disease. NAFLD sensitizes the liver to injury and increases the risk of developing acute or chronic liver failure following another liver injury.<ref name="Manifestations" /> |
|||
=== Genetics === |
|||
Other rare hepatobiliary manifestations of Crohn's disease include: [[cholangiocarcinoma]], granulomatous hepatitis, cholelithiasis, [[autoimmune hepatitis]], [[Liver abscess|hepatic abscess]], and pericholangitis.<ref name="Manifestations" /> |
|||
Genetics significantly influences the risk of Crohn's disease. First-degree relatives of affected individuals have a five-fold increased risk, while identical twins have a 38–50% risk if one twin is affected. [[Genome-wide association studies]] have identified around 200 loci linked to Crohn's, most found in non-coding regions that regulate gene expression and overlap with other immune-related conditions, such as [[ankylosing spondylitis]] and [[psoriasis]].<ref name="pmid30611442"/> While genetics can predict disease location, it does not determine complications like stricturing. A substantial portion of inherited risk is attributed to a few key polymorphisms.<ref name="pmid32242028"/> |
|||
* [[NOD2]] mutations are the primary genetic risk factor for ileal Crohn's disease, impairing the function of immune cells, particularly [[Paneth cells]]. These mutations are found in 10–27% of individuals with Crohn's disease, predominantly in Caucasian populations. Heterozygotes (one mutated copy) have a three-fold risk, while homozygotes (two copies) have a 20–40 fold risk.<ref name="pmid38582044">{{cite journal | vauthors = Kayali S, Fantasia S, Gaiani F, Cavallaro LG, de'Angelis GL, Laghi L | title = NOD2 and Crohn's Disease Clinical Practice: From Epidemiology to Diagnosis and Therapy, Rewired | journal = Inflammatory Bowel Diseases | pmid = 38582044 | date = April 2024 | doi = 10.1093/ibd/izae075 | doi-access = free }}</ref> |
|||
====Renal and urological==== |
|||
* [[ATG16L1]] mutations impair autophagy and immune defense, and are more common in Caucasians.<ref name="pmid32242028"/> |
|||
* [[IL23R]] mutations increase inflammatory signaling of the [[interleukin-23]] pathway, and are more common in Caucasians.<ref name="pmid32242028"/> |
|||
* [[TNFSF15]] mutations are the primary genetic risk factor in Asian populations.<ref name="pmid32242028"/> |
|||
* [[IL10RA]] mutations impair the anti-inflammatory signaling of [[interleukin-10]], causing early-onset Crohn's disease with high [[penetrance]]. |
|||
== Mechanism == |
|||
[[Nephrolithiasis]], [[obstructive uropathy]], and [[Fistula|fistulization]] of the urinary tract directly result from the underlying disease process. Nephrolithiasis is due to calcium oxalate or uric acid stones. Calcium oxalate is due to hyperoxaluria typically associated with either distal ileal CD or ileal resection. Oxalate absorption increases in the presence of unabsorbed fatty acids in the colon. The fatty acids compete with oxalate to bind calcium, displacing the oxalate, which can then be absorbed as unbound sodium oxalate across colonocytes and excreted into the urine. Because sodium oxalate only is absorbed in the colon, calcium-oxalate stones form only in patients with an intact colon. Patients with an [[ileostomy]] are prone to formation of uric-acid stones because of frequent dehydration. The sudden onset of severe abdominal, back, or flank pain in patients with IBD, particularly if different from the usual discomfort, should lead to inclusion of a renal stone in the differential diagnosis.<ref name="Manifestations" /> |
|||
[[File:Crohn's Disease Mechanism.png|thumb|upright=2|alt=diagram of mechanism of Crohn's disease|The intestinal barrier and immune system in health and during Crohn's disease. In health, immune cells secrete TGFβ and retinoic acid to promote the differentiation of Tregs, which regulate the inflammatory behavior of effector T cells.<ref name="pmid27590281">{{cite journal | vauthors = Plitas G, Rudensky AY | title = Regulatory T Cells: Differentiation and Function | journal = Cancer Immunology Research | volume = 4 | issue = 9 | pages = 721–725 | pmid = 27590281 | date = September 2016 | doi = 10.1158/2326-6066.CIR-16-0193 | doi-access = free | pmc = 5026325 }}</ref> During Crohn's disease, microbiome alterations, intestinal barrier permeability, and deficient innate immunity enable pathogens to enter the gut tissue. This causes antigen-presenting cells to upregulate IL-12, IL-18, and IL-23, increasing the differentiation of Th1 and Th17 cells. These cells secrete inflammatory cytokines such as IL-17, IFNγ, and TNF to perpetuate inflammation.<ref name="pmid30611442"/>]] |
|||
Crohn's disease is believed to be caused by a dysregulated immune response to gut bacteria, though the exact mechanism is unknown. This is evidenced by the disease's links to genes involved in bacteria defense and its occurrence in the [[ileum]] and [[Large intestine|colon]], the most bacteria-dense segments of the intestine.<ref name="pmid25234148">{{cite journal | vauthors = Mowat AM, Agace WW | title = Regional specialization within the intestinal immune system | journal = Nature Reviews Immunology | volume = 14 | issue = 10 | pages = 667–85 | pmid = 25234148 | date = October 2014 | doi = 10.1038/nri3738 | doi-access = free }}</ref> In Crohn's disease, a permeable intestinal barrier and a deficient [[innate immune response]] enable bacteria to enter intestinal tissue, causing an excessive inflammatory response from [[T helper 1]] (Th1) and [[T helper 17]] (Th17) cells. An altered [[microbiome]] may also be causatory and serve as the link to environmental factors.<ref name="pmid30611442">{{cite journal | vauthors = Ramos GP, Papadakis KA | title = Mechanisms of Disease: Inflammatory Bowel Diseases | journal = Mayo Clinic Proceedings | volume = 94 | issue = 1 | pages = 155–165 | pmid = 30611442 | pmc = 6386158 | date = January 2019 | doi = 10.1016/j.mayocp.2018.09.013 | doi-access = free }}</ref> |
|||
[[Urology|Urological]] manifestations in patients with IBD may include ureteral calculi, enterovesical [[fistula]], perivesical infection, perinephric abscess, and obstructive uropathy with [[hydronephrosis]]. Ureteral compression is associated with retroperitoneal extension of the phlegmonous inflammatory process involving the [[Ileum|terminal ileum]] and [[cecum]], and may result in [[hydronephrosis]] severe enough to cause [[hypertension]].<ref name="Manifestations" /> |
|||
=== Intestinal barrier === |
|||
Immune complex [[glomerulonephritis]] presenting with [[proteinuria]] and [[hematuria]] has been described in children and adults with CD or UC. Diagnosis is by renal biopsy, and treatment parallels the underlying IBD.<ref name="Manifestations" /> |
|||
The epithelial barrier is a single layer of [[epithelial cells]] covered in antimicrobial mucus that protects the intestine from gut bacteria.<ref name="pmid25234148"/> Epithelial cells are joined by [[tight junction proteins]], which are reduced by Crohn's-linked polymorphisms. In particular, [[claudin-5]] and [[CLDN8|claudin-8]] are reduced, while pore-forming [[claudin-2]] is increased, causing intestinal permeability. Epithelial cells under stress emit inflammatory signals such as the [[unfolded protein response]] to stimulate the immune system, and Crohn's-linked polymorphisms to the [[ATG16L1]] gene lower the threshold at which this response is triggered.<ref name="pmid32242028">{{cite journal | vauthors = Roda G, Chien Ng S, Kotze PG, Argollo M, Panaccione R, Spinelli A, Kaser A, Peyrin-Biroulet L, Danese S | title = Crohn's Disease | journal = Nature Reviews Disease Primers | volume = 6 | issue = 1 | pages = 22 | pmid = 32242028 | date = April 2020 | doi = 10.1038/s41572-020-0156-2 | doi-access = free }}</ref> |
|||
In a functional state, the intestinal epithelium and IgA dimers work together to manage and keep the luminal microflora distinct from the mucosal immune system.<ref>{{cite journal | url=https://doi.org/10.1016/S0140-6736(16)31711-1 | doi=10.1016/S0140-6736(16)31711-1 | title=Crohn's disease | date=2017 | journal=The Lancet | volume=389 | issue=10080 | pages=1741–1755 | pmid=27914655 | vauthors = Torres J, Mehandru S, Colombel J, Peyrin-Biroulet L }}</ref> [[Paneth cells]] exist in the epithelial barrier of the small intestine and secrete [[Alpha defensin|α-defensins]] to prevent bacteria from entering gut tissue.<ref name="pmid25234148"/> Genetic polymorphisms associated with Crohn's disease can impair this ability and lead to Crohn's disease in the ileum. [[NOD2]] is a receptor produced by Paneth cells to sense bacteria, and mutations to NOD2 can inhibit the antimicrobial activity of Paneth cells. ATG16L1, [[IRGM]], and [[LRRK2]] are proteins involved in selective [[autophagy]], the mechanism by which Paneth cells secrete α-defensins, and mutations to these genes also impair the antimicrobial activity of Paneth cells.<ref name="pmid32242028"/> |
|||
[[Amyloidosis]] (see endocrinological involvement) secondary to Crohn's disease has been described and is known to affect the kidneys.<ref name="Manifestations" /> |
|||
[[Intraepithelial lymphocytes]] (IELs) are immune cells that exist in the epithelial barrier, consisting mostly of activated [[T cells]]. They interact with gut bacteria directly and emit signals to regulate the intestinal immune system. IELs in Crohn's disease produce increased levels of inflammatory cytokines [[Interleukin 17|IL-17]], [[IFNγ]], and [[TNF]].<ref name="pmid32242028"/> It is hypothesized that inflammatory signals from the immune system and alterations to the gut microbiome influence IELs to produce inflammatory signals, contributing to Crohn's disease.<ref name="pmid29242771">{{cite journal | vauthors = Hu MD, Edelblum KL | title = Sentinels at the frontline: the role of intraepithelial lymphocytes in inflammatory bowel disease | journal = Current Pharmacology Reports | volume = 3 | issue = 6 | pages = 321–334 | pmid = 29242771 | date = August 2017 | doi = 10.1007/s40495-017-0105-2 | doi-access = free | pmc = 5724577 }}</ref> |
|||
====Pancreatic==== |
|||
=== Immune system === |
|||
[[Pancreatitis]] may be associated with both UC and CD. The most common cause is [[Iatrogenesis|iatrogenic]] and involves sensitivity to medications used to treat IBD (3% of patients), including [[sulfasalazine]], [[Mesalazine|mesalamine]], [[Mercaptopurine|6-mercaptopurine]], and [[azathioprine]]. Pancreatitis may present as symptomatic (in 2%) or more commonly asymptomatic (8–21%) disease in adults with IBD.<ref name="Manifestations" /> |
|||
Normally, intestinal [[macrophages]] have reduced inflammatory behavior while retaining their ability to consume and destroy pathogens. In Crohn's disease, the number and activity of macrophages is reduced, enabling the entrance of pathogens into intestinal tissue.<ref name="pmid30611442"/> Macrophages degrade internal pathogens through autophagy, which is impaired by Crohn's-linked polymorphisms in genes such as NOD2 and ATG16L1.<ref name="pmid32242028"/> Additionally, people with Crohn's tend to have a separate abnormal population of macrophages that secrete proinflammatory cytokines such as TNF and [[Interleukin 6|IL-6]].<ref name="pmid30611442"/> |
|||
[[Neutrophils]] are recruited from the bloodstream in response to inflammatory signals, and defend tissue by secreting antimicrobial substances and consuming pathogens.<ref name="pmid25234148"/> In Crohn's disease, neutrophil recruitment is delayed and autophagy is impaired, allowing bacteria to survive in intestinal tissue.<ref name="pmid30611442"/> Dysfunction in neutrophil secretion of [[reactive oxygen species]], which are toxic to bacteria, is associated with very early onset Crohn's disease. Although neutrophils are important in bacterial defense, their subsequent accumulation in Crohn's disease damages the epithelial barrier and perpetuates inflammation.<ref name="pmid32242028"/> |
|||
====Cardiovascular and circulatory==== |
|||
[[Innate lymphoid cell]]s (ILCs) consist of subtypes including ILC1s, ILC2s, and ILC3s. ILC3s are particularly important for regenerating the epithelial barrier through secretion of IL-17 by NCR- ILC3s and [[Interleukin 22|IL-22]] by NCR+ ILC3s. During Crohn's disease, inflammatory signals from antigen-presenting cells, such as IL-23, cause excessive IL-17 and IL-22 secretion. Although these cytokines protect the intestinal barrier, excessive production damages the barrier through increased inflammation and neutrophil recruitment. Additionally, [[Interleukin 12|IL-12]] from activated dendritic cells influence NCR+ ILC3s to transform into inflammatory [[IFNγ]]-producing ILC1s.<ref name="pmid30962426">{{cite journal | vauthors = Zeng B, Shi S, Ashworth G, Dong C, Liu J, Xing F | title = ILC3 function as a double-edged sword in inflammatory bowel diseases | journal = Cell Death & Disease | volume = 10 | issue = 4 | pages = 315 | pmid = 30962426 | pmc = 6453898 | date = April 2019 | doi = 10.1038/s41419-019-1540-2 | doi-access = free }}</ref> |
|||
Children and adults with IBD have been rarely (<1%) reported developing [[Pericarditis|pleuropericarditis]] either at initial presentation or during active or quiescent disease. The pathogenesis of pleuropericarditis is unknown, although certain medications (e.g., [[sulfasalazine]] and [[Mesalazine|mesalamine]] derivatives) have been implicated in some cases. The clinical presentation may include chest pain, [[Shortness of breath|dyspnea]], or in severe cases [[Cardiac tamponade|pericardial tamponade]] requiring rapid drainage. [[Nonsteroidal anti-inflammatory drug]]s have been used as therapy, although this should be weighed against the hypothetical risk of exacerbating the underlying IBD.<ref name="Manifestations" /> |
|||
Naive T cells are activated primarily by dendritic cells, which then differentiate into anti-inflammatory [[Regulatory T cell|T regulatory cells]] (Tregs) or inflammatory T helper cells to maintain balance. In Crohn's disease, macrophages and antigen-presenting cells secrete IL-12, [[Interleukin 18|IL-18]], and IL-23 in response to pathogens, increasing Th1 and T17 differentiation and promoting inflammation via [[Interleukin 17|IL-17]], IFNγ and TNF. IL-23 is particularly important, and IL-23 receptor polymorphisms that increase activity are linked with Crohn's disease. Tregs suppress inflammation via [[Interleukin 10|IL-10]], and mutations to IL-10 and its receptor cause very early onset Crohn's disease.<ref name="pmid32242028"/> |
|||
In rare cases, [[cardiomyopathy]], [[endocarditis]], and [[myocarditis]] have been described.<ref name="Manifestations" /> |
|||
=== Microbiome === |
|||
Crohn's disease also increases the risk of [[blood clot]]s;<ref name="Trikudanathan2012" /> painful swelling of the lower legs can be a sign of [[deep venous thrombosis]], while difficulty breathing may be a result of [[pulmonary embolism]]. |
|||
People with Crohn's disease tend to have altered microbiomes, although no disease-specific microorganisms have been identified. An altered microbiome may link environmental factors with Crohn's, though causality is uncertain. [[Firmicutes]] tend to be reduced, particularly [[Faecalibacterium prausnitzii]], which produces [[short-chain fatty acids]] that reduce inflammation. [[Bacteroidetes]] and [[proteobacteria]] tend to be increased, particularly adherent-invasive [[E. coli]], which attaches to intestinal epithelial cells. Additionally, mucolytic and sulfate-reducing bacteria are elevated, contributing to damage to the intestinal barrier.<ref name="pmid30611442"/> |
|||
Alterations in gut viral and fungal communities may contribute to Crohn's disease. [[Caudovirales]] bacteriophage sequences found in children with Crohn's suggest a potential biomarker for early-onset disease. A meta-analysis showed lower viral diversity in Crohn's patients compared to healthy individuals, with increased [[Synechococcus phage S CBS1]] and [[Retroviridae]] viruses. Additionally, a Japanese study found that the fungal microbiota in Crohn's patients differs significantly from that of healthy individuals, particularly with an abundance of [[Candida albicans|Candida]].<ref name="pmid32242028"/> |
|||
====Respiratory==== |
|||
== Diagnosis == |
|||
[[Larynx|Laryngeal]] involvement in inflammatory bowel disease is extremely rare. Only 12 cases of laryngeal involvement in Crohn's disease have been reported {{as of|2019|lc=y}}. Moreover, only one case of laryngeal manifestations in ulcerative colitis has been reported as of the same date.<ref>{{Cite journal |last1=Loos |first1=Elke |last2=Lemkens |first2=Peter |last3=Poorten |first3=Vincent Vander |last4=Humblet |first4=Evelien |last5=Laureyns |first5=Griet |date=January 2019 |title=Laryngeal Manifestations of Inflammatory Bowel Disease |journal=Journal of Voice |volume=33 |issue=1 |pages=1–6 |doi=10.1016/j.jvoice.2017.09.021 |pmid=29605161 |s2cid=4565046 }}</ref> Nine patients complained of difficulty in breathing due to [[edema]] and [[ulcer]]ation from the [[larynx]] to the [[hypopharynx]].<ref>{{Cite journal |last1=Hasegawa |first1=Naoko |last2=Ishimoto |first2=Shin-Ichi |last3=Takazoe |first3=Masakazu |last4=Tsunoda |first4=Koichi |last5=Fujimaki |first5=Youko |last6=Shiraishi |first6=Aiko |last7=Kinoshita |first7=Makoto |last8=Okada |first8=Kazunari |date=July 2009 |title=Recurrent hoarseness due to inflammatory vocal fold lesions in a patient with Crohn's disease |journal=The Annals of Otology, Rhinology, and Laryngology |volume=118 |issue=7 |pages=532–5 |doi=10.1177/000348940911800713 |pmid=19708494 |s2cid=8472904 }}</ref> Hoarseness, sore throat, and [[odynophagia]] are other symptoms of laryngeal involvement of Crohn's disease.<ref>{{Cite journal |last1=Li |first1=Cong J. |last2=Aronowitz |first2=Paul |date=March 2013 |title=Sore throat, odynophagia, hoarseness, and a muffled, high-pitched voice |journal=Cleveland Clinic Journal of Medicine |volume=80 |issue=3 |pages=144–145 |doi=10.3949/ccjm.80a.12056 |pmid=23456463 |s2cid=31002546 |issn=0891-1150 |doi-access=free}}</ref> |
|||
Diagnosis of Crohn's disease may be challenging since its symptoms overlap with other gastrointestinal diseases. An accurate diagnosis requires a combined assessment of clinical history, physical examination, and diagnostic tests. |
|||
=== Endoscopy === |
|||
Considering extraintestinal manifestations of CD, those involving the [[lung]] are relatively rare. However, there is a wide array of lung manifestations, ranging from subclinical alterations, airway diseases and lung [[parenchyma]]l diseases to [[Pleural cavity|pleural]] diseases and drug-related diseases. The most frequent manifestation is bronchial inflammation and [[Pus|suppuration]] with or without bronchiectasis. There are a number of mechanisms by which the lungs may become involved in CD. These include the same embryological origin of the lung and gastrointestinal tract by ancestral intestine, similar immune systems in the pulmonary and intestinal mucosa, the presence of circulating immune complexes and auto-antibodies, and the adverse pulmonary effects of some drugs.<ref>{{Cite journal |last1=Lu |first1=De-Gan |last2=Ji |first2=Xiao-Qing |last3=Liu |first3=Xun |last4=Li |first4=Hong-Jia |last5=Zhang |first5=Cai-Qing |date=January 7, 2014 |title=Pulmonary manifestations of Crohn's disease |journal=World Journal of Gastroenterology |volume=20 |issue=1 |pages=133–141 |doi=10.3748/wjg.v20.i1.133 |issn=1007-9327 |pmc=3886002 |pmid=24415866 |doi-access=free}}</ref> A complete list of known pulmonary manifestations include: fibrosing alveolitis, pulmonary [[vasculitis]], apical [[fibrosis]], [[bronchiectasis]], [[bronchitis]], [[bronchiolitis]], [[Laryngotracheal stenosis|tracheal stenosis]], [[granuloma]]tous lung disease, and abnormal pulmonary function.<ref name="Manifestations" /> |
|||
[[Image:CD colitis.jpg|thumb|right|alt=Image of deep ulcers in the colon of a person with Crohn's colitis.|Image of a colon showing deep ulceration due to Crohn's disease.]] |
|||
[[Image:CD serpiginous ulcer.jpg|thumb|right|alt=Image of a serpiginous ulcer due to Crohn's disease found during a colonoscopy.|Image of a serpiginous ulcer in the colon, a classic finding in Crohn's disease]] |
|||
====Musculoskeletal==== |
|||
[[Colonoscopy|Ileocolonoscopy]] is the primary procedure for diagnosing Crohn's disease in the ileum and colon, accurately identifying it in about 90% of cases.<ref name="pmid29972402">{{Cite journal |last1=Passos |first1=Márcio Alexandre Terra |last2=Chaves |first2=Fernanda Correa |last3=Chaves-Junior |first3=Nilson |date=2018 |title=The Importance of Colonoscopy in Inflammatory Bowel Diseases |journal=Arquivos Brasileiros de Cirurgia Digestiva |volume=31 |issue=2 |pages=e1374 |doi=10.1590/0102-672020180001e1374 |pmc=6044200 |pmid=29972402 |doi-access=free}}</ref> During this exam, doctors closely examine the intestinal lining and take small tissue samples for further testing. Signs of Crohn's disease include uneven inflammation and 'skip lesions', which are patches of inflammation separated by healthy tissue. The ulcers can be small (less than 5 mm) or larger (over 5 mm), often appearing cobblestone-like. Their depth helps determine disease severity. Unlike [[ulcerative colitis]], Crohn's disease usually does not affect the rectum or cause continuous inflammation around the bowel.<ref name="pmid32242028"/> |
|||
Crohn's disease is associated with a type of [[Rheumatology|rheumatologic disease]] known as [[spondyloarthropathy|seronegative spondyloarthropathy]].<ref name="Trikudanathan2012" /> This group of diseases is characterized by inflammation of one or more [[joint]]s ([[arthritis]]) or muscle insertions ([[enthesitis]]).<ref name="Trikudanathan2012" /> The arthritis in Crohn's disease can be divided into two types. The first type affects larger weight-bearing joints such as the knee (most common), hips, shoulders, wrists, or elbows.<ref name="Trikudanathan2012" /> The second type symmetrically involves five or more of the small joints of the hands and feet.<ref name="Trikudanathan2012" /> The arthritis may also involve the [[Vertebral column|spine]], leading to [[ankylosing spondylitis]] if the entire spine is involved, or simply [[sacroiliitis]] if only the [[sacroiliac joint]] is involved.<ref name="Trikudanathan2012" /> |
|||
In certain cases, such as disease in the upper small bowel, standard colonoscopy may be ineffective. Physicians may then opt for [[Enteroscopy|device-assisted enteroscopy]] or [[capsule endoscopy]]. While capsule endoscopy is effective in detecting abnormalities, it may not reliably diagnose Crohn's disease and carries a risk of retention, which is about 1.6% when Crohn's disease is suspected and increases to 13% if already diagnosed. To reduce this risk, physicians typically perform small-bowel imaging and use a [[patency capsule]] that disintegrates within 48 to 72 hours. Once the patency capsule has passed through the intestine, capsule endoscopy may be performed.<ref name="pmid28601423"/> |
|||
Crohn's disease increases the risk of [[osteoporosis]] or thinning of the bones.<ref name="Trikudanathan2012" /> Individuals with osteoporosis are at increased risk of [[bone fracture]]s.<ref name="Bernstein">{{cite journal |vauthors=Bernstein M, Irwin S, Greenberg GR |date=September 2005 |title=Maintenance infliximab treatment is associated with improved bone mineral density in Crohn's disease |journal=The American Journal of Gastroenterology |volume=100 |issue=9 |pages=2031–5 |doi=10.1111/j.1572-0241.2005.50219.x |pmid=16128948 |s2cid=28982700}}</ref> |
|||
[[Enteroscopy|Device-assisted enteroscopy]] is not typically the first choice for diagnosing small-bowel Crohn's disease due to its invasiveness and higher costs.<ref name="pmid32242028"/> The procedure closely examines the small intestine using specialized tools, such as longer endoscopes or balloon-assisted devices, making it easier for doctors to visualize and treat issues.<ref name="pmid31367155">{{Cite journal |vauthors=Schneider M, Höllerich J, Beyna T |date=July 2019 |title=Device-assisted enteroscopy: A review of available techniques and upcoming new technologies |journal=World Journal of Gastroenterology |volume=25 |issue=27 |pages=3538–3545 |doi=10.3748/wjg.v25.i27.3538 |pmc=6658397 |pmid=31367155 |doi-access=free}}</ref> It often requires sedation and is generally reserved for patients needing a tissue sample or immediate treatment.<ref name="pmid32242028"/> |
|||
====Dermatological==== |
|||
[[File:A single EN.JPG|thumb|A single lesion of erythema nodosum]] |
|||
=== Cross-sectional Imaging === |
|||
Crohn's disease may also involve the skin, blood, and [[endocrine system]]. [[Erythema nodosum]] is the most common type of skin problem, occurring in around 8% of people with Crohn's disease, producing raised, tender red [[Nodule (medicine)|nodule]]s usually appearing on the [[Tibia|shin]]s.<ref name="Trikudanathan2012">{{cite journal |vauthors=Trikudanathan G, Venkatesh PG, Navaneethan U |title=Diagnosis and therapeutic management of extra-intestinal manifestations of inflammatory bowel disease |journal=Drugs |volume=72 |issue=18 |pages=2333–49 |date=December 2012 |pmid=23181971 |doi=10.2165/11638120-000000000-00000 |s2cid=10078879}}</ref><ref name="Thrash2013">{{cite journal |vauthors=Thrash B, Patel M, Shah KR, Boland CR, Menter A |title=Cutaneous manifestations of gastrointestinal disease: part II |journal=Journal of the American Academy of Dermatology |volume=68 |issue=2 |pages=211.e1-33; quiz 244–6 |date=February 2013 |pmid=23317981 |doi=10.1016/j.jaad.2012.10.036|s2cid=1819416}}</ref><ref name="PMID30682031">{{cite journal |vauthors=Roth N, Biedermann L, Fournier N, Butter M, Vavricka SR, Navarini AA, Rogler G, Scharl M |title=Occurrence of skin manifestations in patients of the Swiss Inflammatory Bowel Disease Cohort Study |journal=PLOS ONE |volume=14 |issue=1 |pages=e0210436 |date=2019 |pmid=30682031 |pmc=6347222 |doi=10.1371/journal.pone.0210436 |s2cid=59275029 |bibcode=2019PLoSO..1410436R | doi-access = free}}</ref> Erythema nodosum is due to inflammation of the underlying [[subcutaneous tissue]], and is characterized by [[septal]] [[panniculitis]].<ref name="Thrash2013" /> |
|||
[[Image:CT scan gastric CD.jpg|thumb|right|alt=CT scan of Crohn's disease in the fundus of the stomach.|CT scan showing Crohn's disease in the stomach]] |
|||
Cross-sectional imaging techniques, like [[Abdominal ultrasonography|bowel ultrasonography]] (BUS), [[Computed tomography enterography|CT enterography]] (CTE), and [[Magnetic resonance enterography|MRI enterography]] (MRE), are essential for understanding how extensive Crohn's disease is and whether there are any complications, like blockages or abnormal connections between organs. All three methods are quite accurate for diagnosing Crohn's disease and spotting these complications.<ref name="pmid32242028"/> |
|||
[[Pyoderma gangrenosum]] is a less common skin problem, occurring in under 2%,<ref name="PMID30682031" /> and is typically a painful ulcerating nodule.<ref name="Thrash2013" /><ref name="Harbord" /> |
|||
* CTE involves [[radiation]] and requires the use of [[contrast agents]] (substances that help show details in images). Despite this, CTE is very effective, with over 80% accuracy in diagnosing the disease and its complications, including blockages and fistulas (abnormal connections).<ref name="pmid32242028"/> |
|||
[[Nail clubbing|Clubbing]], a deformity of the ends of the fingers, may also be a result of Crohn's disease.<ref>{{Cite journal |last1=Kitis |first1=G |last2=Thompson |first2=H |last3=Allan |first3=R N |date=1979-10-06 |title=Finger clubbing in inflammatory bowel disease: its prevalence and pathogenesis. |journal=British Medical Journal |volume=2 |issue=6194 |pages=825–828 |doi=10.1136/bmj.2.6194.825 |issn=0007-1447 |pmc=1596648 |pmid=509114}}</ref> |
|||
* MRE is particularly good for detecting intestinal narrowing, with 89% sensitivity and 94% specificity. It is the preferred option for examining fistulas and abscesses in the pelvic area.<ref name="pmid32242028"/> |
|||
* BUS is a non-invasive method that doesn't involve radiation and is effective for assessing the intestinal wall and related issues, such as fistulas and abscesses. Despite its limitations, it can accurately identify signs of Crohn's disease when the bowel wall thickens to more than 3 mm, achieving high accuracy rates (88–100%) when also considering factors like fistulas and abscesses.<ref name="pmid32242028"/> |
|||
=== Histology === |
|||
Other very rare dermatological manifestations include: [[pyostomatitis vegetans]], [[erythema multiforme]], [[Epidermolysis bullosa acquisita|epidermolysis bullosa acquista]] (described in a case report), and metastatic CD (the spread of Crohn's inflammation to the skin<ref name="Cutaneous" />).<ref name="Manifestations" /> It is unknown if [[Sweet's syndrome]] is connected to Crohn's disease.<ref name="Manifestations" /> |
|||
[[File:Histopathology of granuloma of colonic mucosa.jpg|thumb|alt=Tissue stain of a granuloma due to Crohn's disease.|Tissue stain of a granuloma in the colonic mucosa due to Crohn's disease, consisting of an aggregate of [[histiocytes]] in the center of the image.]] |
|||
[[Image:Crohn's transmural path.jpg|thumb|right|alt=Tissue stain of colon showing deep inflammation across all layers.|Tissue stain of colon showing deep inflammation across all layers.]] |
|||
====Neurological==== |
|||
The most reliable way to confirm a diagnosis of Crohn's disease is through a [[histopathology|histological examination]] of biopsy samples or tissue removed during surgery. This process helps distinguish Crohn's disease from ulcerative colitis and other types of colitis, particularly infections. While no features are unique to Crohn's disease, typical signs include patchy chronic inflammation, irregularities in the intestinal lining, [[granulomas]] (not related to tissue injury), and abnormal [[intestinal villus|villi]] structure in the terminal ileum. A pathologist specializing in inflammatory bowel disease is important for accurate Crohn's disease diagnoses. Even if biopsy results are unclear, doctors can still suggest a Crohn's disease diagnosis based on clinical symptoms, endoscopic findings, and imaging results.<ref name="pmid32242028"/> |
|||
Crohn's disease can also cause [[neurological]] complications (reportedly in up to 15%).<ref name="pro">[http://professionals.epilepsy.com/page/inflammatory_crohn.html Crohn's disease] {{webarchive|url=https://web.archive.org/web/20070805041654/http://professionals.epilepsy.com/page/inflammatory_crohn.html |date=August 5, 2007}}. professionals.epilepsy.com. Retrieved July 13, 2007.</ref> The most common of these are [[seizures]], [[stroke]], [[myopathy]], [[peripheral neuropathy]], [[headache]], and [[Depression (mood)|depression]].<ref name="pro" /> |
|||
=== Disease activity indexes === |
|||
Central and peripheral neurological disorders are described in patients with IBD and include peripheral [[Peripheral neuropathy|neuropathies]], [[Myopathy|myopathies]], focal [[central nervous system]] defects, [[convulsion]]s, confusional episodes, [[meningitis]], [[Syncope (medicine)|syncope]], [[optic neuritis]], and [[Sensorineural hearing loss|sensorineural]] loss. [[Autoimmunity|Autoimmune]] mechanisms are proposed for involvement with IBD. Nutritional deficiencies associated with neurological manifestations, such as [[vitamin B12 deficiency|vitamin B<sub>12</sub> deficiency]], should be investigated. Spinal [[abscess]] has been reported in both a child and an adult with initial complaints of severe back pain due to extension of a psoas abscess from the epidural space to the subarachnoid space.<ref name="Manifestations" /> |
|||
The Crohn's Disease Activity Index (CDAI) is a scoring system to assess the symptoms associated with Crohn's disease. It assigns a score based on eight clinical factors, including overall well-being, frequency of loose stools, abdominal pain, presence of abdominal masses, changes in weight, low [[hemoglobin]] levels, and use of opiates for diarrhea. The CDAI is primarily used in clinical trials to evaluate the effectiveness of treatments and to determine whether the disease is in remission. This is particularly significant, as approximately 50% of patients who report feeling well may still exhibit signs of active disease in the intestine, while some patients with symptoms may present with normal intestinal findings.<ref name="pmid32242028"/> |
|||
The Harvey–Bradshaw Index (HBI) provides a more streamlined approach by assessing only clinical factors, thus eliminating the need for laboratory tests. Neither the CDAI nor the HBI incorporates diagnostic procedures such as endoscopies or imaging studies; instead, they focus exclusively on symptom tracking. The HBI is generally considered easier to apply than the CDAI and may be more suitable for certain clinical trials and routine practice due to its simplicity in calculation and reduced reliance on patient recall of symptoms.<ref name="pmid32242028"/> |
|||
====Psychiatric and psychological==== |
|||
The Crohn's Disease Endoscopic Index of Severity (CDEIS) is a scoring system used during endoscopy to evaluate Crohn's disease severity. It assesses six factors: deep and shallow ulcers, nonulcerated and ulcerated stenosis, the area covered by ulcers, and the overall disease-affected area across five intestinal sections. Scores range from 0 to 44, with higher scores indicating more severe disease. While often seen as the standard for measuring severity, CDEIS can be complex to calculate and may underestimate severity if only one segment, particularly the ileum, is affected. There are also no clear score cutoffs for specific outcomes or treatment responses, limiting its effectiveness in determining remission.<ref name="pmid27184635">{{cite journal | vauthors = Koutroumpakis E, Katsanos KH | title = Implementation of the simple endoscopic activity score in crohn's disease | journal = Saudi Journal of Gastroenterology | volume = 22 | issue = 3 | pages = 183–191 | pmid = 31367155 | pmc = 4898086 | date = May 2016 | doi = 10.4103/1319-3767.182455 | doi-access = free }}</ref> |
|||
Crohn's disease is linked to many psychological disorders, including [[Depression (mood)|depression]] and [[anxiety]], denial of one's disease, the need for dependence or dependent behaviors, feeling overwhelmed, and having a poor self-image.<ref>{{Cite web |title=Mental and Emotional Well-Being |url=https://www.crohnscolitisfoundation.org/mental-health |access-date=September 6, 2021 |website=Crohn's & Colitis Foundation |archive-date=October 7, 2022 |archive-url=https://web.archive.org/web/20221007193457/https://www.crohnscolitisfoundation.org/mental-health |url-status=live}}</ref> |
|||
The Simple Endoscopic Score for Crohn's Disease (SES-CD) offers a more straightforward approach than the CDEIS scoring system, using four key factors to evaluate Crohn's disease during an endoscopy. These factors include the presence and size of ulcers, the area affected by ulcers, the overall extent of the disease, and any narrowing of the intestine (stenosis). The first three factors are scored from 0 to 3 in each of the five sections of the intestine, with a maximum score of 15 for each section. Stenosis is scored separately, ranging from 0 to 11. This results in a total SES-CD score that can range from 0 to 56, with higher scores indicating more severe disease.<ref name="pmid27184635"/> |
|||
Many studies have found that patients with IBD report a higher frequency of depressive and anxiety disorders than the general population; most studies confirm that women with IBD are more likely than men to develop affective disorders and show that up to 65% of them may have [[Major depressive disorder|depression]] and [[anxiety disorder]].<ref>{{cite journal |vauthors=Fracas E, Costantino A, Vecchi M, Buoli M |title=Depressive and Anxiety Disorders in Patients with Inflammatory Bowel Diseases: Are There Any Gender Differences? |journal=Int J Environ Res Public Health |volume=20 |issue=13 |date=June 2023 |page=6255 |pmid=37444101 |pmc=10340762 |doi=10.3390/ijerph20136255 |doi-access=free }}</ref><ref>{{cite journal |vauthors=Barberio B, Zamani M, Black CJ, Savarino EV, Ford AC |title=Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis |journal=Lancet Gastroenterol Hepatol |volume=6 |issue=5 |pages=359–370 |date=May 2021 |pmid=33721557 |doi=10.1016/S2468-1253(21)00014-5 |url=https://eprints.whiterose.ac.uk/173607/3/LGH_Manuscript_clean.pdf |access-date=May 26, 2024 |archive-date=December 3, 2023 |archive-url=https://web.archive.org/web/20231203051934/https://eprints.whiterose.ac.uk/173607/3/LGH_Manuscript_clean.pdf |url-status=live }}</ref> |
|||
=== Laboratory testing === |
|||
====Endocrinological or hematological==== |
|||
While no lab test can definitively confirm or rule out Crohn's disease, results from serum and stool tests can help support the diagnosis:<ref name="pmid28601423"/> |
|||
* The antimicrobial [[antibody]] [[Anti–Saccharomyces cerevisiae antibody|ASCA]] is a well-known blood test marker used in the diagnosis of Crohn's disease. Approximately 60–70% of individuals with Crohn's disease test positive for ASCA, while only 10–15% of those with ulcerative colitis and less than 5% of patients with other types of colitis have positive results.<ref name="pmid32242028"/> |
|||
*The [[autoantibody]] [[P-ANCA|pANCA]] is found in 10–15% of Crohn's disease cases, in 60–70% of ulcerative colitis cases, and in less than 5% of patients with other types of colitis that aren't inflammatory bowel disease. Additionally, patients with Crohn's disease who test positive for pANCA often show symptoms similar to those of ulcerative colitis.<ref name="pmid32242028"/> |
|||
*[[C-reactive protein]] (CRP) is a blood marker that indicates inflammation and can help monitor Crohn's disease activity. However, about one third of patients with active disease may have normal CRP levels, while one third with high levels of CRP have inactive disease. Moreover, CRP's ability to predict disease progression is not well established.<ref name="pmid32242028"/> |
|||
*[[Fecal calprotectin]] is a stool test used to differentiate inflammatory bowel disease, like Crohn's disease, from irritable bowel syndrome. While there are no official cut-off values, it is commonly used to assess disease activity in Crohn's.<ref name="pmid32242028"/> Elevated levels may also indicate other intestinal infections or inflammatory conditions, not just Crohn's disease.<ref name="pmid28601423"/> |
|||
=== Differential diagnosis === |
|||
[[Autoimmune hemolytic anemia]], a condition in which the [[immune system]] attacks the [[red blood cells]], is also more common in Crohn's disease and may cause [[fatigue]], a pale appearance, and other symptoms common in [[anemia]]. |
|||
Crohn's disease has similar endoscopic, radiographic and histological features with other inflammatory or infectious diseases. 10% of people with Crohn's disease are initially diagnosed with indeterminate colitis.<ref name="pmid32242028"/> |
|||
* [[Behçet’s disease]] can cause intestinal inflammation, primarily featuring single ulcers and symptoms outside the intestines, which differ from Crohn's disease. Recurrent sores in the mouth and genitals raise suspicion for Behçet's. A pathergy test, where the skin is lightly pricked to check for a red bump or ulcer, can help confirm the diagnosis. Eye inflammation (uveitis) and skin issues are also common in Behçet's disease.<ref name="pmid32242028"/> |
|||
Secondary [[amyloidosis]] (AA) is another rare but serious complication of [[inflammatory bowel disease]] (IBD), generally seen in Crohn's disease. At least 1% of patients with Crohn's disease develop amyloidosis. In the literature, the time lapse between the onset of Crohn's disease and the diagnosis of amyloidosis has been reported to range from 1 to 21 years. |
|||
* [[Intestinal lymphoma]] lacks symptoms that differentiate it from other conditions; diagnosis can only be confirmed through histological examination of tissue samples.<ref name="pmid32242028"/> |
|||
* [[Intestinal tuberculosis]] can cause symptoms such as fever, night sweats, and ulcers in the transverse colon, along with a swollen ileocecal valve. Key tissue changes include granulomas, which may be caseating, confluent, or large. A positive smear test for acid-fast bacillus and imaging that detects necrotic lymph nodes are also important indicators of the disease.<ref name="pmid32242028"/> |
|||
[[Leukocytosis]] and [[thrombocytopenia]] are usually due to [[immunosuppressant]] treatments or [[sulfasalazine]]. Plasma [[erythropoietin]] levels often are lower in patients with IBD than expected, in conjunction with severe [[anemia]].<ref name="Manifestations" /> |
|||
* [[Ischemic colitis]] is also a possible alternative diagnosis to Crohn's disease. It often shows swelling and redness of the inner lining of the colon, while the rectum usually remains unaffected.<ref name="pmid32242028"/> |
|||
[[Thrombocythemia|Thrombocytosis]] and [[thromboembolic]] events resulting from a [[Thrombophilia|hypercoagulable]] state in patients with IBD can lead to [[pulmonary embolism]] or [[thrombosis]] elsewhere in the body. Thrombosis has been reported in 1.8% of patients with UC and 3.1% of patients with CD. Thromboembolism and thrombosis are less frequently reported among pediatric patients, with three patients with UC and one with CD described in case reports.<ref name="Manifestations" /> |
|||
In rare cases, hypercoagulation disorders and [[portal vein thrombosis]] have been described.<ref name="Manifestations" /> |
|||
====Malnutrition symptoms==== |
|||
People with Crohn's disease may develop anemia due to [[vitamin B12|vitamin B<sub>12</sub>]], [[folate]], [[iron deficiency]], or due to [[anemia of chronic disease]].<ref name="Lomer2011">{{cite journal |vauthors=Lomer MC |date=August 2011 |title=Dietary and nutritional considerations for inflammatory bowel disease |journal=The Proceedings of the Nutrition Society |volume=70 |issue=3 |pages=329–35 |doi=10.1017/S0029665111000097 |pmid=21450124|doi-access=free}}</ref><ref name="Gerasimidis2011">{{cite journal |vauthors=Gerasimidis K, McGrogan P, Edwards CA |date=August 2011 |title=The aetiology and impact of malnutrition in paediatric inflammatory bowel disease |journal=Journal of Human Nutrition and Dietetics |type=Review |volume=24 |issue=4 |pages=313–26 |doi=10.1111/j.1365-277X.2011.01171.x |pmid=21564345|doi-access=free}}</ref> The most common is [[iron deficiency anemia]]<ref name="Lomer2011" /> from chronic [[blood loss]], reduced dietary intake, and persistent inflammation leading to increased [[hepcidin]] levels, restricting [[iron]] absorption in the duodenum.<ref name="Gerasimidis2011" /> As Crohn's disease most commonly affects the [[terminal ileum]] where the vitamin B<sub>12</sub>/[[intrinsic factor]] complex is absorbed, B<sub>12</sub> deficiency may be seen.<ref name="Gerasimidis2011" /> This is particularly common after surgery to remove the ileum.<ref name="Lomer2011" /> Involvement of the duodenum and [[jejunum]] can impair the absorption of many other nutrients including [[folate]]. People with Crohn's often also have issues with [[small bowel bacterial overgrowth syndrome]], which can produce micronutrient deficiencies.<ref>{{MedlinePlusEncyclopedia|000222|Small bowel bacterial overgrowth}}</ref><ref name="mimicking">{{Cite journal |last1=Klaus |first1=Jochen |last2=Spaniol |first2=Ulrike |last3=Adler |first3=Guido |last4=Mason |first4=Richard A. |last5=Reinshagen |first5=Max |last6=von Tirpitz C |first6=Christian |date=July 30, 2009 |title=Small intestinal bacterial overgrowth mimicking acute flare as a pitfall in patients with Crohn's Disease |journal=BMC Gastroenterology |volume=9 |issue=1 |pages=61 |doi=10.1186/1471-230X-9-61 |issn=1471-230X |pmc=2728727 |pmid=19643023 |doi-access=free}} [[File:CC BY-SA icon.svg|50px]] Text was copied from this source, which is available under a [https://creativecommons.org/licenses/by/2.0/ Creative Commons Attribution 2.0 Generic (CC BY 2.0)] {{Webarchive|url=https://web.archive.org/web/20110223001542/http://creativecommons.org/licenses/by/2.0/ |date=February 23, 2011}} license.</ref> |
|||
=== Complications === |
|||
====Intestinal damage==== |
|||
[[File:Histopathology of granuloma of colonic mucosa.jpg|thumb|Histopathology of a non-necrotizing granuloma of colonic mucosa in a patient with Crohn's disease, H&E stain. It is seen as an aggregate of histiocytes in the center of the image, having ample eosinophilic cytoplasm.]] |
|||
Crohn's disease can lead to several mechanical complications within the [[intestine]]s, including [[Bowel obstruction|obstruction]],<ref>{{cite web |url=http://www.merckmanuals.com/home/digestive-disorders/gastrointestinal-emergencies/intestinal-obstruction |title=Intestinal Obstruction |website=MERCK MANUAL Consumer Version|access-date=June 27, 2016|url-status=live|archive-url=https://web.archive.org/web/20160710102459/http://www.merckmanuals.com/home/digestive-disorders/gastrointestinal-emergencies/intestinal-obstruction|archive-date=July 10, 2016}}</ref> [[fistula]]e,<ref>{{cite web |url=http://www.merckmanuals.com/home/digestive-disorders/anal-and-rectal-disorders/anorectal-fistula |title=Anorectal Fistula |website=MERCK MANUAL Consumer Version|access-date=June 27, 2016|url-status=live|archive-url=https://web.archive.org/web/20160710213544/http://www.merckmanuals.com/home/digestive-disorders/anal-and-rectal-disorders/anorectal-fistula|archive-date=July 10, 2016}}</ref> and [[abscess]]es.<ref>{{cite web |url=http://www.merckmanuals.com/home/digestive-disorders/anal-and-rectal-disorders/anorectal-abscess |title=Anorectal Abscess |website=MERCK MANUAL Consumer Version|access-date=June 27, 2016|url-status=live|archive-url=https://web.archive.org/web/20160614095919/http://www.merckmanuals.com/home/digestive-disorders/anal-and-rectal-disorders/anorectal-abscess|archive-date=June 14, 2016}}</ref> Obstruction typically occurs from [[strictures]] or [[adhesions]] that narrow the [[Lumen (anatomy)|lumen]], blocking the passage of the intestinal contents. A fistula can develop between two loops of bowel, between the bowel and [[bladder]], between the bowel and [[vagina]], and between the bowel and skin. Abscesses are walled-off concentrations of [[infection]], which can occur in the [[abdomen]] or in the [[perianal]] area. Crohn's is responsible for 10% of vesicoenteric fistulae, and is the most common cause of [[Ileum|ileovesical]] [[fistula]]e.<ref>{{EMedicine|article|442000|Enterovesical Fistula}}</ref> |
|||
Symptoms caused by [[bowel obstruction|intestinal stenosis]], or the tightening and narrowing of the bowel, are also common in Crohn's disease. Abdominal pain is often most severe in areas of the bowel with [[stenosis]]. Persistent [[vomit]]ing and [[nausea]] may indicate stenosis from [[small bowel obstruction]] or disease involving the [[stomach]], [[pylorus]], or [[duodenum]].<ref name="emed" /> |
|||
Intestinal [[granuloma]]s are a walled-off portions of the intestine by macrophages in order to isolate infections. Granuloma formation is more often seen in younger patients, and mainly in the severe, active penetrating disease.<ref name="granuloma">{{Cite journal |last1=Molnár |first1=Tamás |last2=Tiszlavicz |first2=László |last3=Gyulai |first3=Csaba |last4=Nagy |first4=Ferenc |last5=Lonovics |first5=János |date=May 28, 2005 |title=Clinical significance of granuloma in Crohn's disease |journal=World Journal of Gastroenterology |volume=11 |issue=20 |pages=3118–3121 |doi=10.3748/wjg.v11.i20.3118 |issn=1007-9327 |pmc=4305850 |pmid=15918200 |doi-access=free}}</ref> Granuloma is considered the hallmark of microscopic diagnosis in Crohn's disease (CD), but granulomas can be detected in only 21–60% of CD patients.<ref name="granuloma" /> |
|||
====Cancer==== |
|||
Crohn's disease also increases the risk of [[cancer]] in the area of inflammation. For example, individuals with Crohn's disease involving the [[small bowel]] are at higher risk for [[small intestinal cancer]].<ref>{{cite journal |vauthors=Bye WA, Nguyen TM, Parker CE, Jairath V, East JE |title=Strategies for detecting colon cancer in patients with inflammatory bowel disease |journal=The Cochrane Database of Systematic Reviews |volume=2017 |pages=CD000279 |date=September 2017 |issue=9 |pmid=28922695 |pmc=6483622 |doi=10.1002/14651858.CD000279.pub4}}</ref> Similarly, people with [[Crohn's colitis]] have a [[relative risk]] of 5.6 for developing [[colon cancer]].<ref>{{cite journal |vauthors=Ekbom A, Helmick C, Zack M, Adami HO |title=Increased risk of large-bowel cancer in Crohn's disease with colonic involvement |journal=Lancet |volume=336 |issue=8711 |pages=357–9 |date=August 1990 |pmid=1975343 |doi=10.1016/0140-6736(90)91889-I |url=https://zenodo.org/record/1258309 |s2cid=2046255 |access-date=September 4, 2018 |archive-date=August 5, 2020 |archive-url=https://web.archive.org/web/20200805072047/https://zenodo.org/record/1258309 |url-status=live}}</ref> Screening for colon cancer with [[colonoscopy]] is recommended for anyone who has had Crohn's colitis for at least eight years.<ref>{{cite journal |vauthors=Itzkowitz SH, Present DH |title=Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease |journal=Inflammatory Bowel Diseases |volume=11 |issue=3 |pages=314–21 |date=March 2005 |pmid=15735438 |doi=10.1097/01.mib.0000160811.76729.d5 |collaboration=Crohn's and Colitis Foundation of America Colon Cancer in IBD Study Group}}</ref> |
|||
Some studies suggest there is a role for chemoprotection in the prevention of colorectal cancer in Crohn's involving the colon; two agents have been suggested, [[folate]] and [[mesalamine]] preparations.<ref>{{cite journal |vauthors=Zisman TL, Rubin DT |title=Colorectal cancer and dysplasia in inflammatory bowel disease |journal=World Journal of Gastroenterology |volume=14 |issue=17 |pages=2662–9 |date=May 2008 |pmid=18461651 |pmc=2709054 |doi=10.3748/wjg.14.2662 |doi-access=free}}</ref> Also, [[immunotherapy|immunomodulators]] and [[biological therapy for inflammatory bowel disease|biologic agents]] used to treat this disease may promote developing extra-intestinal cancers.<ref>{{cite journal |vauthors=Axelrad JE, Lichtiger S, Yajnik V |title=Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment |journal=World Journal of Gastroenterology |volume=22 |issue=20 |pages=4794–801 |date=May 2016 |pmid=27239106 |pmc=4873872 |doi=10.3748/wjg.v22.i20.4794 |type=Review |doi-access=free}}</ref> |
|||
Some cancers, such as [[Acute myeloid leukemia|acute myelocytic leukaemia]] have been described in cases of Crohn's disease.<ref name="Manifestations" /> [[Hepatosplenic T-cell lymphoma]] (HSTCL) is a rare, lethal disease generally seen in young male patients with inflammatory bowel disease. TNF-α Inhibitor treatments ([[infliximab]], [[adalimumab]], [[Certolizumab pegol|certolizumab]], [[natalizumab]], and [[etanercept]]) are thought to be the cause of this rare disease.<ref>{{Cite journal |last1=Parakkal |first1=Deepak |last2=Sifuentes |first2=Humberto |last3=Semer |first3=Rumi |last4=Ehrenpreis |first4=Eli Daniel |date=November 2011 |title=Hepatosplenic T-cell lymphoma in patients receiving TNF-α inhibitor therapy: expanding the groups at risk |journal=European Journal of Gastroenterology & Hepatology |volume=23 |issue=12 |pages=1150–6 |doi=10.1097/MEG.0b013e32834bb90a |issn=1473-5687 |pmid=21941193 |s2cid=27267004 }}</ref> |
|||
[[File:Colorectal cancer endo 2.jpg|thumb|[[Colonoscopy|Endoscopic]] image of colon cancer identified in the sigmoid colon on screening [[colonoscopy]] for Crohn's disease]] |
|||
====Major complications==== |
|||
Major complications of Crohn's disease include [[bowel obstruction]], abscesses, free [[Bowel perforation|perforation]], and [[hemorrhage]], which in rare cases may be fatal.<ref>{{cite news |date=September 1, 1985 |title=Man of Many Problems Comes to City for Help |page=B1 |work=Richmond Times-Dispatch |location=Richmond, Virginia, USA |url=http://infoweb.newsbank.com/iw-search/we/InfoWeb?p_product=AWNB&p_theme=aggregated5&p_action=doc&p_docid=0EB4F60CC90DCE22&p_docnum=2&p_queryname=4 |vauthors=Carrillo M}}</ref><ref>{{cite news |date=April 3, 2014 |title=Kay, Laura Lynn |work=Richmond Times-Dispatch |location=Richmond, Virginia, USA |url=http://infoweb.newsbank.com/iw-search/we/InfoWeb?p_product=AWNB&p_theme=aggregated5&p_action=doc&p_docid=14CF552B8BBF3B90&p_docnum=5&p_queryname=1}} <br /> {{cite news |date=March 2, 2014 |title=Doris L. Johnson, 82, of Westminster |work=Carroll County Times |location=Westminster, Maryland, USA |url=http://infoweb.newsbank.com/iw-search/we/InfoWeb?p_product=AWNB&p_theme=aggregated5&p_action=doc&p_docid=14C4D200CC6C63B0&p_docnum=13&p_queryname=1 |vauthors=Loebenberg P}} <br /> {{cite news |date=December 31, 2013 |title=In memoriam: Dan Hodges Jr. |work=The Roanoke Times |location=Roanoke, Virginia, USA |url=http://infoweb.newsbank.com/iw-search/we/InfoWeb?p_product=AWNB&p_theme=aggregated5&p_action=doc&p_docid=14B0CCC8A20C3B78&p_docnum=29&p_queryname=1 |vauthors=Berrier Jr R}} <br /> {{cite news |date=May 4, 2014 |title=Cynthia Meredith Routt |page=A11 |work=Daily Press |location=Newport News, Virginia, USA |url=http://infoweb.newsbank.com/iw-search/we/InfoWeb?p_product=AWNB&p_theme=aggregated5&p_action=doc&p_docid=14D99C943586A6E8&p_docnum=1&p_queryname=1}}</ref> |
|||
====Other complications==== |
|||
Individuals with Crohn's disease are at risk of [[malnutrition]] for many reasons, including decreased food intake and [[malabsorption]]. The risk increases following resection of the [[small bowel]]. Such individuals may require oral supplements to increase their [[caloric intake]], or in severe cases, [[total parenteral nutrition]] (TPN). Most people with moderate or severe Crohn's disease are referred to a [[dietitian]] for assistance in nutrition.<ref>{{cite journal |vauthors=Evans JP, Steinhart AH, Cohen Z, McLeod RS |title=Home total parenteral nutrition: an alternative to early surgery for complicated inflammatory bowel disease |journal=Journal of Gastrointestinal Surgery |volume=7 |issue=4 |pages=562–566 |year=2003 |pmid=12763417 |doi=10.1016/S1091-255X(02)00132-4 |s2cid=195305419}}</ref> |
|||
[[Small intestinal bacterial overgrowth]] (SIBO) is characterized by excessive proliferation of colonic bacterial species in the small bowel. Potential causes of SIBO include fistulae, strictures, or motility disturbances. Hence, patients with Crohn's disease are especially predisposed to develop SIBO. As result, CD patients may experience malabsorption and report symptoms such as weight loss, watery diarrhea, [[meteorism]], flatulence, and abdominal pain, mimicking acute flare in these patients.<ref name="mimicking" /> |
|||
====Pregnancy==== |
|||
Crohn's disease can be problematic during [[pregnancy]], and some medications can cause adverse outcomes for the [[fetus]] or mother. Consultation with an [[obstetrician]] and [[gastroenterologist]] about Crohn's disease and all medications facilitates preventive measures. In some cases, remission occurs during pregnancy. Certain medications can also lower [[sperm count]] or otherwise adversely affect a man's [[fertility]].<ref>{{cite web |url=http://www.ccfa.org/about/news/pregnancy |publisher=[[Crohn's and Colitis Foundation of America]] |date=October 21, 2005 |title=IBD and Pregnancy: What You Need to Know |vauthors=Kaplan C |access-date=November 7, 2009 |archive-url=https://web.archive.org/web/20120217151655/http://www.ccfa.org/about/news/pregnancy |archive-date=February 17, 2012}}</ref> |
|||
====Ostomy-related complications==== |
|||
Common complications of an [[ostomy]] (a common surgery in Crohn's disease) are: mucosal edema, peristomal dermatitis, retraction, ostomy prolapse, mucosal/skin detachment, hematoma, necrosis, parastomal hernia, and stenosis.<ref>{{Cite book |last1=Rodrigues |first1=Francielle Profeta |url=https://www.intechopen.com/chapters/67036 |title=Intestinal Ostomy Complications and Care |last2=Novaes |first2=Jane Andrea Vieira |last3=Pinheiro |first3=Marcela Monteiro |last4=Martins |first4=Paula |last5=Cunha-Melo |first5=José Renan |date=October 23, 2019 |publisher=IntechOpen |isbn=978-1-78984-186-2 |access-date=September 6, 2021 |archive-date=October 7, 2022 |archive-url=https://web.archive.org/web/20221007193456/https://www.intechopen.com/chapters/67036 |url-status=live}}</ref> |
|||
== Etiology == |
|||
The [[etiology]] of Crohn's disease is unknown. Many theories have been disputed, with four main theories hypothesized to be the primary mechanism of Crohn's disease. In [[autoimmune diseases]], [[Antibody|antibodies]] and [[T cell|T lymphocytes]] are the primary mode of inflammation. These cells and bodies are part of the [[adaptive immune system]], or the part of the immune system that learns to fight foreign bodies when first identified.<ref>{{Cite web|title=Definition of Autoimmunity & Autoimmune Disease - Autoimmune Disease {{!}} Johns Hopkins Pathology|url=https://pathology.jhu.edu/autoimmune/definitions|access-date=October 3, 2021|website=pathology.jhu.edu|archive-date=October 3, 2021|archive-url=https://web.archive.org/web/20211003015525/https://pathology.jhu.edu/autoimmune/definitions|url-status=live}}</ref> [[Autoinflammatory disease]]s are diseases where the [[innate immune system]], or the immune system we are genetically coded with, is designed to attack our own cells.<ref name="Ciccarelli 261–269">{{Cite journal |last1=Ciccarelli |first1=F. |last2=Martinis |first2=M. De |last3=Ginaldi |first3=L. |date=January 2013 |title=An Update on Autoinflammatory Diseases |journal=Current Medicinal Chemistry |volume=21 |issue=3 |pages=261–269 |doi=10.2174/09298673113206660303 |issn=0929-8673 |pmc=3905709 |pmid=24164192}}</ref> Crohn's disease likely has involvement of both the adaptive and innate immune systems.<ref name="Li 15–22">{{Cite journal |last1=Li |first1=Na |last2=Shi |first2=Rui-Hua |date=January 7, 2018 |title=Updated review on immune factors in pathogenesis of Crohn's disease |journal=World Journal of Gastroenterology |volume=24 |issue=1 |pages=15–22 |doi=10.3748/wjg.v24.i1.15 |issn=1007-9327 |pmc=5757119 |pmid=29358878 |doi-access=free}}</ref> |
|||
=== Autoinflammatory theory === |
|||
Crohn's disease can be described as a multifactorial autoinflammatory disease. The etiopathogenesis of Crohn's disease is still unknown. In any event, a loss of the regulatory capacity of the immune apparatus would be implicated in the onset of the disease. In this respect interestingly enough, as for [[Blau syndrome|Blau's disease]] (a [[Monogenic (genetics)|monogenic]] autoinflammatory disease), the [[NOD2]] gene mutations have been linked to Crohn's disease. However, in Crohn's disease, NOD2 mutations act as a risk factor, being more common among Crohn's disease patients than the background population, while in Blau's disease NOD2 mutations are linked directly to this syndrome, as it is an [[Autosomal dominant|autosomal-dominant]] disease. All this new knowledge in the [[pathogenesis]] of Crohn's disease allows us to put this multifactorial disease in the group of [[Autoinflammatory diseases|autoinflammatory]] syndromes.<ref name="Ciccarelli 261–269" /> |
|||
Some examples of how the innate immune system affects bowel inflammation have been described.<ref name="Li 15–22" /> A meta-analysis of CD [[genome]]-wide association studies revealed 71 distinct CD-susceptibility [[Locus (genetics)|loci]]. Interestingly, three very important CD-susceptibility genes (the intracellular pathogen-recognition receptor, NOD2; the autophagy-related 16-like 1, [[ATG16L1]] and the immunity-related GTPase M, [[IRGM]]) are involved in innate immune responses against gut microbiota, while one (the X-box binding protein 1) is involved in regulation of the [adaptive] immune pathway via [[MHC class II]],<ref>{{Citation |last1=Roggenbuck |first1=D. |title=Chapter Two - Autoimmunity in Crohn's Disease—A Putative Stratification Factor of the Clinical Phenotype |date=January 1, 2016 |url=https://www.sciencedirect.com/science/article/pii/S0065242316300361 |journal=Advances in Clinical Chemistry |volume=77 |pages=77–101|editor-last=Makowski|editor-first=Gregory S. |publisher=Elsevier |doi=10.1016/bs.acc.2016.06.002|access-date=November 4, 2021 |last2=Reinhold |first2=D. |last3=Baumgart |first3=D. C. |last4=Schierack |first4=P. |last5=Conrad |first5=K. |last6=Laass |first6=M. W. |pmid=27717419}}</ref> resulting in autoinflammatory inflammation. Studies have also found that increased [[Type 3 innate lymphoid cells|ILC3]] can [[overexpress]] [[Major histocompatibility complex|major histocompatibility complex (MHC) II]]. MHC class II can induce [[CD4+ T cell]] [[apoptosis]], thus avoiding the [[T cell]] response to normal bowel micro bacteria. Further studies of IBD patients compared with non-IBD patients found that the expression of MHC II by ILC3 was significantly reduced in IBD patients, thus causing an immune reaction against intestinal cells or normal bowel bacteria and damaging the intestines. This can also make the intestines more susceptible to environmental factors, such as food or bacteria.<ref name="Li 15–22" /> |
|||
The thinking is that because Crohn's disease has strong innate immune system involvement and has NOD2 mutations as a predisposition, Crohn's disease is more likely an autoinflammatory disease than an autoimmune disease.<ref name="Li 15–22" /> |
|||
=== Immunodeficiency theory === |
|||
{{Update section|date=May 2024|reason=All current sources are from 2010}} |
|||
A substantial body of data has emerged in recent years to suggest that the primary defect in Crohn's disease is actually one of relative [[immunodeficiency]].<ref name="Marks 20–31">{{Cite journal |last1=Marks |first1=Daniel J. B. |last2=Rahman |first2=Farooq Z. |last3=Sewell |first3=Gavin W. |last4=Segal |first4=Anthony W. |date=February 2010 |title=Crohn's Disease: an Immune Deficiency State |journal=Clinical Reviews in Allergy & Immunology |volume=38 |issue=1 |pages=20–31 |doi=10.1007/s12016-009-8133-2 |issn=1080-0549 |pmc=4568313 |pmid=19437144}}</ref> This view has been bolstered recently by novel immunological and clinical studies that have confirmed gross aberrations in this early response, consistent with subsequent genetic studies that highlighted molecules important for [[Innate immune system|innate immune function]]. The suggestion therefore is that Crohn's [[pathogenesis]] actually results from partial immunodeficiency, a theory that coincides with the frequent recognition of a virtually identical, non-infectious inflammatory bowel disease arising in patients with [[congenital]] [[Monogenic (genetics)|monogenic]] disorders impairing [[phagocyte]] function.<ref name="Marks 20–31" /> |
|||
== Risk factors == |
|||
{{Risk factors in CD vs. UC}} |
|||
While the exact cause or causes are unknown, Crohn's disease seems to be due to a combination of [[environmental factor]]s and [[genetic predisposition]].<ref>{{cite journal |vauthors=Braat H, Peppelenbosch MP, Hommes DW |title=Immunology of Crohn's disease |journal=Annals of the New York Academy of Sciences |volume=1072 |issue=1 |pages=135–54 |date=August 2006 |pmid=17057196 |doi=10.1196/annals.1326.039 |bibcode=2006NYASA1072..135B |s2cid=2627465}}</ref> Crohn's is the first genetically complex disease in which the relationship between genetic [[Risk factor (epidemiology)|risk factor]]s and the immune system is understood in considerable detail.<ref>{{cite journal |vauthors=Henckaerts L, Figueroa C, Vermeire S, Sans M |title=The role of genetics in inflammatory bowel disease |journal=Current Drug Targets |volume=9 |issue=5 |pages=361–8 |date=May 2008 |pmid=18473763 |doi=10.2174/138945008784221161}}</ref> Each individual risk [[mutation]] makes a small contribution to the overall risk of Crohn's (approximately 1:200). The genetic data, and direct assessment of immunity, indicates a malfunction in the [[innate immune system]].<ref name="Marks2006"> |
|||
{{cite journal |vauthors=Marks DJ, Harbord MW, MacAllister R, Rahman FZ, Young J, Al-Lazikani B, Lees W, Novelli M, Bloom S, Segal AW |title=Defective acute inflammation in Crohn's disease: a clinical investigation |journal=Lancet |volume=367 |issue=9511 |pages=668–78 |date=February 2006 |pmid=16503465 |doi=10.1016/S0140-6736(06)68265-2 |s2cid=13898663}}</ref> In this view, the chronic inflammation of Crohn's is caused when the [[adaptive immune system]] tries to compensate for a deficient innate immune system.<ref>{{cite journal |vauthors=Comalada M, Peppelenbosch MP |title=Impaired innate immunity in Crohn's disease |journal=Trends in Molecular Medicine |volume=12 |issue=9 |pages=397–9 |date=September 2006 |pmid=16890491 |doi=10.1016/j.molmed.2006.07.005}}</ref> |
|||
=== Genetics === |
|||
[[File:NOD2 structure model domain diagram.png|thumb|upright=1.4|[[NOD2]] protein model with schematic diagram. Two N-terminal [[CARD domain|CARD]] domains (red) connected via helical linker (blue) with central [[NOD-like receptor|NBD]] domain (green). At C-terminus [[Leucine-rich repeat|LRR]] domain (cyan) is located. Additionally, some mutations which are associated with certain disease patterns in Crohn's disease are marked in red wire representation.<ref>{{cite journal |vauthors=Nakagome S, Mano S, Kozlowski L, Bujnicki JM, Shibata H, Fukumaki Y, Kidd JR, Kidd KK, Kawamura S, Oota H |title=Crohn's disease risk alleles on the NOD2 locus have been maintained by natural selection on standing variation |journal=Molecular Biology and Evolution |volume=29 |issue=6 |pages=1569–85 |date=June 2012 |pmid=22319155 |pmc=3697811 |doi=10.1093/molbev/mss006}}</ref>]] |
|||
Crohn's has a genetic component.<ref>{{cite web |url=http://www.ccfa.org/reuters/geneticlink |title=Crohn's disease has strong genetic link: study |publisher=[[Crohn's and Colitis Foundation of America]] |date=April 16, 2007 |access-date=November 7, 2009 |url-status=dead |archive-url = https://web.archive.org/web/20070502210012/http://www.ccfa.org/reuters/geneticlink |archive-date=May 2, 2007}}</ref> Because of this, siblings of known people with Crohn's are 30 times more likely to develop Crohn's than the general population.<ref>{{cite journal |vauthors=Liu JZ, Anderson CA |title=Genetic studies of Crohn's disease: past, present and future |journal=Best Practice & Research. Clinical Gastroenterology |volume=28 |issue=3 |pages=373–86 |date=June 2014 |pmid=24913378 |pmc=4075408 |doi=10.1016/j.bpg.2014.04.009}}</ref> |
|||
The first mutation found to be associated with Crohn's was a [[frameshift mutation|frameshift]] in the [[NOD2]] gene (also known as the [[CARD15]] gene),<ref>{{cite journal |vauthors=Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G, Cho JH |title=A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease |journal=Nature |volume=411 |issue=6837 |pages=603–6 |date=May 2001 |pmid=11385577 |doi=10.1038/35079114 | hdl-access = free |s2cid=205017657 |bibcode=2001Natur.411..603O |hdl=2027.42/62856}}</ref> followed by the discovery of [[point mutation]]s.<ref>{{cite journal |vauthors=Cuthbert AP, Fisher SA, Mirza MM, King K, Hampe J, Croucher PJ, Mascheretti S, Sanderson J, Forbes A, Mansfield J, Schreiber S, Lewis CM, Mathew CG |title=The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease |journal=Gastroenterology |volume=122 |issue=4 |pages=867–74 |date=April 2002 |pmid=11910337 |doi=10.1053/gast.2002.32415}}</ref> Over 30 genes have been associated with Crohn's; a [[biological function]] is known for most of them. For example, one association is with mutations in the [[XBP1]] gene, which is involved in the [[unfolded protein response]] pathway of the [[endoplasmic reticulum]].<ref>{{cite journal |vauthors=Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS |title=XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease |journal=Cell |volume=134 |issue=5 |pages=743–56 |date=September 2008 |pmid=18775308 |pmc=2586148 |doi=10.1016/j.cell.2008.07.021}}</ref><ref>{{cite journal |vauthors=Clevers H |title=Inflammatory bowel disease, stress, and the endoplasmic reticulum |journal=The New England Journal of Medicine |volume=360 |issue=7 |pages=726–7 |date=February 2009 |pmid=19213688 |doi=10.1056/NEJMcibr0809591}}</ref> The gene variants of NOD2/CARD15 seem to be related with small-bowel involvement.<ref>{{cite journal |vauthors=Vermeire S |title=NOD2/CARD15: relevance in clinical practice |journal=Best Practice & Research. Clinical Gastroenterology |volume=18 |issue=3 |pages=569–75 |date=June 2004 |pmid=15157828 |doi=10.1016/j.bpg.2003.12.008 |type=Review}}</ref> Other well documented genes which increase the risk of developing Crohn's disease are [[ATG16L1]],<ref name="Prescott NJ, Fisher SA, Franke A, Hampe J, Onnie CM, Soars D, Bagnall R, Mirza MM, Sanderson J, Forbes A, Mansfield JC, Lewis CM, Schreiber S, Mathew CG 2007 1665–71">{{cite journal |vauthors=Prescott NJ, Fisher SA, Franke A, Hampe J, Onnie CM, Soars D, Bagnall R, Mirza MM, Sanderson J, Forbes A, Mansfield JC, Lewis CM, Schreiber S, Mathew CG |title=A nonsynonymous SNP in ATG16L1 predisposes to ileal Crohn's disease and is independent of CARD15 and IBD5 |journal=Gastroenterology |volume=132 |issue=5 |pages=1665–71 |date=May 2007 |pmid=17484864 |doi=10.1053/j.gastro.2007.03.034|doi-access=free}}</ref> [[IL23R]],<ref>{{cite journal |vauthors=Diegelmann J, Czamara D, Le Bras E, Zimmermann E, Olszak T, Bedynek A, Göke B, Franke A, Glas J, Brand S |title=Intestinal DMBT1 expression is modulated by Crohn's disease-associated IL23R variants and by a DMBT1 variant which influences binding of the transcription factors CREB1 and ATF-2 |journal=PLOS ONE |volume=8 |issue=11 |pages=e77773 |year=2013 |pmid=24223725 |pmc=3818382 |doi=10.1371/journal.pone.0077773 |bibcode=2013PLoSO...877773D | doi-access = free}}</ref> [[IRGM]],<ref>{{cite journal |vauthors=Prescott NJ, Dominy KM, Kubo M, Lewis CM, Fisher SA, Redon R, Huang N, Stranger BE, Blaszczyk K, Hudspith B, Parkes G, Hosono N, Yamazaki K, Onnie CM, Forbes A, Dermitzakis ET, Nakamura Y, Mansfield JC, Sanderson J, Hurles ME, Roberts RG, Mathew CG |title=Independent and population-specific association of risk variants at the IRGM locus with Crohn's disease |journal=Human Molecular Genetics |volume=19 |issue=9 |pages=1828–39 |date=May 2010 |pmid=20106866 |pmc=2850616 |doi=10.1093/hmg/ddq041}}</ref> and [[SLC11A1]].<ref>{{cite journal |vauthors=Chermesh I, Azriel A, Alter-Koltunoff M, Eliakim R, Karban A, Levi BZ |title=Crohn's disease and SLC11A1 promoter polymorphism |journal=Digestive Diseases and Sciences |volume=52 |issue=7 |pages=1632–5 |date=July 2007 |pmid=17385031 |doi=10.1007/s10620-006-9682-3 |s2cid=11429585}}</ref> |
|||
There is considerable overlap between susceptibility loci for IBD and [[mycobacterial]] infections.<ref name="pmid23128233">{{cite journal |vauthors=Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D'Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH |title=Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease |journal=Nature |volume=491 |issue=7422 |pages=119–24 |date=November 2012 |pmid=23128233 |pmc=3491803 |doi=10.1038/nature11582 |bibcode=2012Natur.491..119.}}</ref> [[Genome]]-wide association studies have shown that Crohn's disease is genetically linked to [[coeliac disease]].<ref>{{cite journal |vauthors=Walker MM, Murray JA |title=An update in the diagnosis of coeliac disease |journal=Histopathology |volume=59 |issue=2 |pages=166–79 |date=August 2011 |pmid=21054494 |doi=10.1111/j.1365-2559.2010.03680.x |s2cid=5196629 |type=Review |quote=Recent genome-wide association studies have shown that chronic inflammatory and autoimmune diseases are linked genetically to coeliac disease; for example, type 1 diabetes mellitus, Grave's disease and Crohn's disease.}}</ref> |
|||
Crohn's has been linked to the gene [[LRRK2]] with one variant potentially increasing the risk of developing the disease by 70%, while another lowers it by 25%. The gene is responsible for making a [[protein]], which collects and eliminates [[waste product]] in cells, and is also associated with [[Parkinson's disease]].<ref>{{cite news |vauthors=Coghlan A |title=A single gene can either raise or lower Crohn's disease risk |url=https://www.newscientist.com/article/single-gene-can-either-raise-lower-crohns-disease-risk/ |work=New Scientist |date=January 10, 2018 |access-date=November 5, 2020 |archive-date=November 9, 2020 |archive-url=https://web.archive.org/web/20201109041205/https://www.newscientist.com/article/single-gene-can-either-raise-lower-crohns-disease-risk/ |url-status=live}}</ref> |
|||
=== Immune system === |
|||
There was a prevailing view that Crohn's disease is a primary [[T cell]] autoimmune disorder; however, a newer theory hypothesizes that Crohn's results from an impaired innate immunity.<ref>{{cite journal |vauthors=Marks DJ, Segal AW |title=Innate immunity in inflammatory bowel disease: a disease hypothesis |journal=The Journal of Pathology |volume=214 |issue=2 |pages=260–6 |date=January 2008 |pmid=18161747 |pmc=2635948 |doi=10.1002/path.2291}}</ref> The later hypothesis describes impaired [[cytokine]] secretion by [[macrophage]]s, which contributes to impaired innate immunity and leads to a sustained microbial-induced inflammatory response in the colon, where the bacterial load is high.<ref name="Bact08" /><ref name="Marks2006" /> Another theory is that the inflammation of Crohn's was caused by an overactive [[Th2 cells|T<sub>h</sub>1]] and [[Th17|T<sub>h</sub>17]] [[cytokine]] response.<ref>{{cite journal |vauthors=Cobrin GM, Abreu MT |title=Defects in mucosal immunity leading to Crohn's disease |journal=Immunological Reviews |volume=206 |issue=1 |pages=277–95 |date=August 2005 |pmid=16048555 |doi=10.1111/j.0105-2896.2005.00293.x |s2cid=37353838}}</ref><ref>{{cite journal |vauthors=Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA |title=Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice |journal=Gastroenterology |volume=132 |issue=7 |pages=2359–70 |date=June 2007 |pmid=17570211 |doi=10.1053/j.gastro.2007.03.104}}</ref> |
|||
In 2007, the ATG16L1 gene was implicated in Crohn's disease, which may induce [[Autophagy (cellular)|autophagy]] and hinder the body's ability to attack invasive bacteria.<ref name="Prescott NJ, Fisher SA, Franke A, Hampe J, Onnie CM, Soars D, Bagnall R, Mirza MM, Sanderson J, Forbes A, Mansfield JC, Lewis CM, Schreiber S, Mathew CG 2007 1665–71" /> Another study theorized that the human immune system traditionally evolved with the presence of [[parasite]]s inside the body and that the lack thereof due to modern hygiene standards has weakened the immune system. Test subjects were reintroduced to harmless parasites, with positive responses.<ref>{{cite news |url=https://www.nytimes.com/2008/06/29/magazine/29wwln-essay-t.html |work=[[The New York Times]] |title=The Worm Turns |vauthors=Velasquez-Manoff M |date=June 29, 2008 |url-status=live |archive-url = https://web.archive.org/web/20170107144610/http://www.nytimes.com/2008/06/29/magazine/29wwln-essay-t.html |archive-date=January 7, 2017}}</ref> |
|||
=== Microbes === |
|||
It is hypothesized that maintenance of [[commensal]] [[microorganism]] growth in the [[GI tract]] is dysregulated, either as a result or cause of [[immune dysregulation]].<ref>{{cite journal |vauthors=Sartor RB |title=Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis |journal=Nature Clinical Practice. Gastroenterology & Hepatology |volume=3 |issue=7 |pages=390–407 |date=July 2006 |pmid=16819502 |doi=10.1038/ncpgasthep0528 |s2cid=3329677}}</ref><ref name="pmid22508665">{{cite journal |vauthors=Dogan B, Scherl E, Bosworth B, Yantiss R, Altier C, McDonough PL, Jiang ZD, Dupont HL, Garneau P, Harel J, Rishniw M, Simpson KW |title=Multidrug resistance is common in Escherichia coli associated with ileal Crohn's disease |journal=Inflammatory Bowel Diseases |volume=19 |issue=1 |pages=141–50 |date=January 2013 |pmid=22508665 |doi=10.1002/ibd.22971 |s2cid=25518704|doi-access=free}}</ref> |
|||
There is an apparent connection between Crohn's disease, ''[[Mycobacterium]]'', other pathogenic bacteria, and [[genetic marker]]s.<ref>{{cite journal |vauthors=Subramanian S, Roberts CL, Hart CA, Martin HM, Edwards SW, Rhodes JM, Campbell BJ |date=February 2008 |title=Replication of Colonic Crohn's Disease Mucosal Escherichia coli Isolates within Macrophages and Their Susceptibility to Antibiotics |journal=Antimicrobial Agents and Chemotherapy |volume=52 |issue=2 |pages=427–34 |doi=10.1128/AAC.00375-07 |pmc=2224732 |pmid=18070962}}</ref><ref>{{cite journal |vauthors=Mpofu CM, Campbell BJ, Subramanian S, Marshall-Clarke S, Hart CA, Cross A, Roberts CL, McGoldrick A, Edwards SW, Rhodes JM |date=November 2007 |title=Microbial mannan inhibits bacterial killing by macrophages: a possible pathogenic mechanism for Crohn's disease |journal=Gastroenterology |volume=133 |issue=5 |pages=1487–98 |doi=10.1053/j.gastro.2007.08.004 |pmid=17919633 |url=http://download.journals.elsevierhealth.com/pdfs/journals/0016-5085/PIIS0016508507014503.pdf}}</ref> A number of studies have suggested a causal role for [[Mycobacterium avium subspecies paratuberculosis|''Mycobacterium avium'' subspecies ''paratuberculosis'']] (MAP), which causes a similar disease, [[Johne's disease]], in cattle.<ref name="pmid16306778">{{cite journal |vauthors=Naser SA, Collins MT |date=December 2005 |title=Debate on the lack of evidence of Mycobacterium avium subsp. paratuberculosis in Crohn's disease |journal=Inflammatory Bowel Diseases |volume=11 |issue=12 |pages=1123 |doi=10.1097/01.MIB.0000191609.20713.ea |pmid=16306778|doi-access=free}}</ref><ref name="PMC4064085">{{cite journal |vauthors=Naser SA, Sagramsingh SR, Naser AS, Thanigachalam S |date=June 2014 |title=Mycobacterium avium subspecies paratuberculosis causes Crohn's disease in some inflammatory bowel disease patients |journal=World Journal of Gastroenterology |volume=20 |issue=23 |pages=7403–15 |doi=10.3748/wjg.v20.i23.7403 |pmc=4064085 |pmid=24966610 |doi-access=free}}</ref> In many individuals, genetic factors predispose individuals to ''[[Mycobacterium avium]]'' subsp.'' [[paratuberculosis]]'' infection. This bacterium may produce certain compounds containing [[mannose]], which may protect both itself and various other bacteria from [[phagocytosis]], thereby possibly causing a variety of [[secondary infection]]s.<ref>{{cite web |title=New insights into Crohn's Disease |url=http://www.liv.ac.uk/researchintelligence/issue33/crohns.htm|url-status=dead|archive-url=https://web.archive.org/web/20130923151342/http://www.liv.ac.uk/researchintelligence/issue33/crohns.htm|archive-date=September 23, 2013}}</ref> |
|||
NOD2 is a gene involved in Crohn's genetic susceptibility. It is associated with [[macrophage]]s' diminished ability to [[phagocytize]] MAP. This same gene may reduce innate and adaptive immunity in gastrointestinal tissue and impair the ability to resist infection by the MAP bacterium.<ref>{{cite journal |vauthors=Glubb DM, Gearry RB, Barclay ML, Roberts RL, Pearson J, Keenan JI, McKenzie J, Bentley RW |title=NOD2 and ATG16L1 polymorphisms affect monocyte responses in Crohn's disease |journal=World Journal of Gastroenterology |volume=17 |issue=23 |pages=2829–37 |date=June 2011 |pmid=21734790 |pmc=3120942 |doi=10.3748/wjg.v17.i23.2829 |doi-broken-date=September 12, 2024 |doi-access=free}}</ref> Macrophages that ingest the MAP bacterium are associated with high production of [[TNF-α]].<ref>{{cite journal |vauthors=Clancy R, Ren Z, Turton J, Pang G, Wettstein A |title=Molecular evidence for Mycobacterium avium subspecies paratuberculosis (MAP) in Crohn's disease correlates with enhanced TNF-alpha secretion |journal=Digestive and Liver Disease |volume=39 |issue=5 |pages=445–51 |date=May 2007 |pmid=17317344 |doi=10.1016/j.dld.2006.12.006}}</ref><ref>{{cite journal |vauthors=Nakase H, Tamaki H, Matsuura M, Chiba T, Okazaki K |title=Involvement of mycobacterium avium subspecies paratuberculosis in TNF-α production from macrophage: possible link between MAP and immune response in Crohn's disease |journal=Inflammatory Bowel Diseases |volume=17 |issue=11 |pages=E140–2 |date=November 2011 |pmid=21990211 |doi=10.1002/ibd.21750}}</ref> |
|||
Other studies have linked specific strains of [[entero]]adherent ''[[Escherichia coli|E. coli]]'' to the disease.<ref name="Baumgart_2007">{{cite journal |vauthors=Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, Orsi RH, Wiedmann M, McDonough P, Kim SG, Berg D, Schukken Y, Scherl E, Simpson KW |title=Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum |journal=The ISME Journal |volume=1 |issue=5 |pages=403–18 |date=September 2007 |pmid=18043660 |doi=10.1038/ismej.2007.52 | bibcode = 2007ISMEJ...1..403B | doi-access = free}}</ref> Adherent-invasive [[Escherichia coli]] (AIEC), more common in people with CD,<ref>{{cite journal |vauthors=Sasaki M, Sitaraman SV, Babbin BA, Gerner-Smidt P, Ribot EM, Garrett N, Alpern JA, Akyildiz A, Theiss AL, Nusrat A, Klapproth JM |title=Invasive Escherichia coli are a feature of Crohn's disease |journal=Laboratory Investigation; A Journal of Technical Methods and Pathology |volume=87 |issue=10 |pages=1042–54 |date=October 2007 |pmid=17660846 |doi=10.1038/labinvest.3700661 | doi-access = free}}</ref><ref>{{cite journal |vauthors=Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF |title=High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease |journal=Gastroenterology |volume=127 |issue=2 |pages=412–21 |date=August 2004 |pmid=15300573 |doi=10.1053/j.gastro.2004.04.061|doi-access=free}}</ref><ref name="Baumgart_2007" /> have the ability to make strong [[biofilm]]s compared to non-AIEC strains correlating with high adhesion and invasion indices<ref>{{cite journal |vauthors=Nickerson KP, McDonald C |title=Crohn's disease-associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin |journal=PLOS ONE |volume=7 |issue=12 |pages=e52132 |year=2012 |pmid=23251695 |pmc=3520894 |doi=10.1371/journal.pone.0052132 | doi-access = free |veditors=Mizoguchi E |bibcode=2012PLoSO...752132N}}</ref><ref>{{cite journal |vauthors=Martinez-Medina M, Naves P, Blanco J, Aldeguer X, Blanco JE, Blanco M, Ponte C, Soriano F, Darfeuille-Michaud A, Garcia-Gil LJ |title=Biofilm formation as a novel phenotypic feature of adherent-invasive Escherichia coli (AIEC) |journal=BMC Microbiology |volume=9 |issue=1 |pages=202 |date=September 2009 |pmid=19772580 |pmc=2759958 |doi=10.1186/1471-2180-9-202 |doi-access=free}}</ref> of [[neutrophil]]s and the ability to block [[autophagy]] at the autolysosomal step, which allows for intracellular survival of the bacteria and induction of inflammation.<ref name="pmid23272151">{{cite journal |vauthors=Chargui A, Cesaro A, Mimouna S, Fareh M, Brest P, Naquet P, Darfeuille-Michaud A, Hébuterne X, Mograbi B, Vouret-Craviari V, Hofman P |title=Subversion of autophagy in adherent invasive Escherichia coli-infected neutrophils induces inflammation and cell death |journal=PLOS ONE |volume=7 |issue=12 |pages=e51727 |year=2012 |pmid=23272151 |pmc=3522719 |doi=10.1371/journal.pone.0051727 |bibcode=2012PLoSO...751727C | doi-access = free}}</ref> Inflammation drives the proliferation of AIEC and [[dysbiosis]] in the ileum, irrespective of genotype.<ref name="pmid22848538">{{cite journal |vauthors=Craven M, Egan CE, Dowd SE, McDonough SP, Dogan B, Denkers EY, Bowman D, Scherl EJ, Simpson KW |title=Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn's disease |journal=PLOS ONE |volume=7 |issue=7 |pages=e41594 |year=2012 |pmid=22848538 |pmc=3404971 |doi=10.1371/journal.pone.0041594 |bibcode=2012PLoSO...741594C | doi-access = free}}</ref> AIEC strains replicate extensively inside macrophages inducing the secretion of very large amounts of TNF-α.<ref>{{cite journal |vauthors=Barnich N, Darfeuille-Michaud A |title=Adherent-invasive Escherichia coli and Crohn's disease |journal=Current Opinion in Gastroenterology |volume=23 |issue=1 |pages=16–20 |date=January 2007 |pmid=17133079 |doi=10.1097/MOG.0b013e3280105a38 |s2cid=23564986}}</ref> |
|||
Mouse studies have suggested some symptoms of Crohn's disease, ulcerative colitis, and [[irritable bowel syndrome]] have the same underlying cause. [[Biopsy]] samples taken from the colons of all three patient groups were found to produce elevated levels of a [[serine protease]].<ref>{{cite journal |vauthors=Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N |title=Role for protease activity in visceral pain in irritable bowel syndrome |journal=The Journal of Clinical Investigation |volume=117 |issue=3 |pages=636–47 |date=March 2007 |pmid=17304351 |pmc=1794118 |doi=10.1172/JCI29255}}</ref> Experimental introduction of the serine [[protease]] into mice has been found to produce widespread pain associated with irritable bowel syndrome, as well as colitis, which is associated with all three diseases.<ref>{{cite journal |vauthors=Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R, Bueno L, Vergnolle N |title=Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2 |journal=The American Journal of Pathology |volume=161 |issue=5 |pages=1903–15 |date=November 2002 |pmid=12414536 |pmc=1850779 |doi=10.1016/S0002-9440(10)64466-5}}</ref> Regional and temporal variations in those illnesses follow those associated with infection with the protozoan ''[[Blastocystis]]''.<ref>{{cite journal |vauthors=Boorom KF, Smith H, Nimri L, Viscogliosi E, Spanakos G, Parkar U, Li LH, Zhou XN, Ok UZ, Leelayoova S, Jones MS |title=Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection |journal=Parasites & Vectors |volume=1 |issue=1 |pages=40 |date=October 2008 |pmid=18937874 |pmc=2627840 |doi=10.1186/1756-3305-1-40 |doi-access=free}}</ref> |
|||
The "cold-chain" hypothesis is that [[psychrotrophic bacteria]] such as ''[[Yersinia]]'' and ''[[Listeria]]'' species contribute to the disease. A statistical correlation was found between the advent of the use of refrigeration in the United States and various parts of Europe and the rise of the disease.<ref name="pmid14683664">{{cite journal |vauthors=Hugot JP, Alberti C, Berrebi D, Bingen E, Cézard JP |title=Crohn's disease: the cold chain hypothesis |journal=Lancet |volume=362 |issue=9400 |pages=2012–5 |date=December 2003 |pmid=14683664 |doi=10.1016/S0140-6736(03)15024-6 |s2cid=10254395}}</ref><ref>{{cite news |title=Fridges blamed for Crohn's disease rise |work=Medical News Today |date=December 12, 2003 |url=http://www.medicalnewstoday.com/articles/4849.php |url-status=live |archive-url = https://web.archive.org/web/20090103190132/http://www.medicalnewstoday.com/articles/4849.php |archive-date=January 3, 2009}}</ref><ref>{{cite journal |vauthors=Forbes A, Kalantzis T |title=Crohn's disease: the cold chain hypothesis |journal=International Journal of Colorectal Disease |volume=21 |issue=5 |pages=399–401 |date=July 2006 |pmid=16059694 |doi=10.1007/s00384-005-0003-7 |s2cid=13271176}}</ref> |
|||
There is also a tentative association between ''[[Candida (fungus)|Candida]]'' colonization and Crohn's disease.<ref>{{cite journal |vauthors=Kumamoto CA |title=Inflammation and gastrointestinal Candida colonization |journal=Current Opinion in Microbiology |volume=14 |issue=4 |pages=386–91 |date=August 2011 |pmid=21802979 |pmc=3163673 |doi=10.1016/j.mib.2011.07.015}}</ref> |
|||
Still, these relationships between specific pathogens and Crohn's disease remain unclear.<ref name="scah_out38">{{cite web |title=Possible links between Crohn's disease and Paratuberculosis |url=http://ec.europa.eu/food/fs/sc/scah/out38_en.pdf|url-status=dead|archive-url=https://web.archive.org/web/20081217094053/http://ec.europa.eu/food/fs/sc/scah/out38_en.pdf|archive-date=December 17, 2008|access-date=November 7, 2009 |publisher=European Commission Directorate-General Health & Consumer Protection}}</ref><ref>{{cite journal |vauthors=Gui GP, Thomas PR, Tizard ML, Lake J, Sanderson JD, Hermon-Taylor J |date=March 1997 |title=Two-year-outcomes analysis of Crohn's disease treated with rifabutin and macrolide antibiotics |journal=The Journal of Antimicrobial Chemotherapy |volume=39 |issue=3 |pages=393–400 |doi=10.1093/jac/39.3.393 |pmid=9096189|doi-access=free}}</ref> |
|||
=== Environmental factors === |
|||
The increased incidence of Crohn's in the [[industrialized]] world indicates an environmental component. Crohn's is associated with an increased intake of animal [[protein]], [[milk protein]], and an increased ratio of [[Omega-6 fatty acid|omega-6]] to [[Omega-3 fatty acid|omega-3]] [[Polyunsaturated fat|polyunsaturated fatty acids]].<ref name="Shoda"> |
|||
{{cite journal |vauthors=Shoda R, Matsueda K, Yamato S, Umeda N |title=Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan |journal=The American Journal of Clinical Nutrition |volume=63 |issue=5 |pages=741–5 |date=May 1996 |pmid=8615358 |doi=10.1093/ajcn/63.5.741 | doi-access = free}}</ref> |
|||
Those who consume vegetable proteins appear to have a lower incidence of Crohn's disease. Consumption of fish protein has no association.<ref name="Shoda" /> |
|||
[[Tobacco smoking|Smoking]] increases the risk of the return of active disease (flares).<ref name="Cosnes2004" /> The introduction of [[hormonal contraception]] in the United States in the 1960s is associated with a dramatic increase in incidence, and one hypothesis is that these drugs work on the digestive system in ways similar to smoking.<ref> |
|||
{{cite journal |vauthors=Lesko SM, Kaufman DW, Rosenberg L, Helmrich SP, Miller DR, Stolley PD, Shapiro S |title=Evidence for an increased risk of Crohn's disease in oral contraceptive users |journal=Gastroenterology |volume=89 |issue=5 |pages=1046–9 |date=November 1985 |pmid=4043662 |doi=10.1016/0016-5085(85)90207-0}}</ref> [[Isotretinoin]] is associated with Crohn's.<ref>{{cite journal |vauthors=Reddy D, Siegel CA, Sands BE, Kane S |title=Possible association between isotretinoin and inflammatory bowel disease |journal=The American Journal of Gastroenterology |volume=101 |issue=7 |pages=1569–73 |date=July 2006 |doi=10.1111/j.1572-0241.2006.00632.x |pmid=16863562 |s2cid=27663573}}</ref><ref>{{cite journal |vauthors=Borobio E, Arín A, Valcayo A, Iñarrairaegui M, Nantes O, Prieto C |title=[Isotretinoin and ulcerous colitis] |language=es |journal=Anales del Sistema Sanitario de Navarra |volume=27 |issue=2 |pages=241–3 |year=2004 |pmid=15381956 |doi=10.4321/S1137-66272004000300009 | doi-access = free}}</ref><ref>{{cite journal |vauthors=Reniers DE, Howard JM |title=Isotretinoin-induced inflammatory bowel disease in an adolescent |journal=The Annals of Pharmacotherapy |volume=35 |issue=10 |pages=1214–6 |date=October 2001 |pmid=11675849 |doi=10.1345/aph.10368 |s2cid=22216642 |url=http://www.theannals.com/cgi/pmidlookup?view=long&pmid=11675849 |archive-url = https://archive.today/20120629103405/http://www.theannals.com/cgi/pmidlookup?view=long&pmid=11675849 |url-status=dead |archive-date=June 29, 2012 |access-date=November 1, 2010}}</ref> |
|||
Although [[stress (physiology)|stress]] is sometimes claimed to exacerbate Crohn's disease, there is no concrete evidence to support such claim.<ref name="NIDDK2017" /> Still, it is well known that immune function is related to stress.<ref>{{Cite journal |last1=Segerstrom |first1=Suzanne C. |last2=Miller |first2=Gregory E. |date=July 2004 |title=Psychological Stress and the Human Immune System: A Meta-Analytic Study of 30 Years of Inquiry |journal=Psychological Bulletin |volume=130 |issue=4 |pages=601–630 |doi=10.1037/0033-2909.130.4.601 |issn=0033-2909 |pmc=1361287 |pmid=15250815}}</ref> Dietary [[microparticle]]s, such as those found in toothpaste, have been studied as they produce effects on immunity, but they were not consumed in greater amounts in patients with Crohn's.<ref>{{cite journal |vauthors=Lomer MC, Hutchinson C, Volkert S, Greenfield SM, Catterall A, Thompson RP, Powell JJ |title=Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn's disease |journal=The British Journal of Nutrition |volume=92 |issue=6 |pages=947–55 |date=December 2004 |pmid=15613257 |doi=10.1079/bjn20041276 | doi-access = free}}</ref><ref>{{cite journal |vauthors=Powell JJ, Thoree V, Pele LC |title=Dietary microparticles and their impact on tolerance and immune responsiveness of the gastrointestinal tract |journal=The British Journal of Nutrition |volume=98 |issue=Suppl 1 |pages=S59–63 |date=October 2007 |pmid=17922962 |pmc=2737314 |doi=10.1017/S0007114507832922}}</ref> The use of [[doxycycline]] has also been associated with increased risk of developing inflammatory bowel diseases.<ref>{{cite journal |vauthors=Lee TW, Russell L, Deng M, Gibson PR |title=Association of doxycycline use with the development of gastroenteritis, irritable bowel syndrome and inflammatory bowel disease in Australians deployed abroad |journal=Internal Medicine Journal |volume=43 |issue=8 |pages=919–26 |date=August 2013 |pmid=23656210 |doi=10.1111/imj.12179 |s2cid=9418654}}</ref><ref name="tetracycline">{{cite journal |vauthors=Margolis DJ, Fanelli M, Hoffstad O, Lewis JD |title=Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease |journal=The American Journal of Gastroenterology |volume=105 |issue=12 |pages=2610–6 |date=December 2010 |pmid=20700115 |doi=10.1038/ajg.2010.303 |s2cid=20085592}}</ref><ref>{{cite journal |vauthors=Garrett JP, Margolis DJ |title=Impact of Long-Term Antibiotic Use for Acne on Bacterial Ecology and Health Outcomes: A Review of Observational Studies |journal=Current Dermatology Reports |date=March 2012 |volume=1 |issue=1 |pages=23–28 |doi=10.1007/s13671-011-0001-7 |doi-access=free}}</ref> In one large retrospective study, patients who were prescribed [[doxycycline]] for their [[acne]] had a 2.25-fold greater risk of developing Crohn's disease.<ref name="tetracycline" /> |
|||
== Pathophysiology == |
|||
{{Pathophysiology in CD vs. UC}} |
|||
During a [[colonoscopy]], [[Biopsy|biopsies]] of the colon are often taken to confirm the diagnosis. Certain characteristic features of the [[pathology]] seen point toward Crohn's disease; it shows a transmural pattern of [[inflammation]], meaning the inflammation may span the entire depth of the [[Gastrointestinal wall|intestinal wall]].<ref name="Baumgart2012" /> |
|||
[[Granuloma]]s, aggregates of macrophage derivatives known as giant cells, are found in 50% of cases and are most specific for Crohn's disease. The granulomas of Crohn's disease do not show "caseation", a cheese-like appearance on microscopic examination characteristic of granulomas associated with infections, such as [[tuberculosis]]. Biopsies may also show chronic mucosal damage, as evidenced by blunting of the intestinal [[Intestinal villus|villi]], atypical branching of the crypts, and a change in the tissue type ([[metaplasia]]). One example of such metaplasia, ''Paneth cell metaplasia'', involves the development of Paneth cells (typically found in the small intestine and a key regulator of intestinal microbiota) in other parts of the gastrointestinal system.<ref>{{cite book |vauthors=Crawford JM |chapter=17. The Gastrointestinal tract |veditors=Cotran RS, Kumar V, Robbins SL |title=Robbins Pathologic Basis of Disease |publisher=W.B. Saunders |edition=5th |date=1994 |isbn=0-7216-5032-5 |oclc=29702821}}</ref><ref>{{cite journal |vauthors=Zhang T, DeSimone RA, Jiao X, Rohlf FJ, Zhu W, Gong QQ, Hunt SR, Dassopoulos T, Newberry RD, Sodergren E, Weinstock G, Robertson CE, Frank DN, Li E |title=Host genes related to paneth cells and xenobiotic metabolism are associated with shifts in human ileum-associated microbial composition |journal=PLOS ONE |volume=7 |issue=6 |pages=e30044 |date=June 13, 2012 |pmid=22719822 |pmc=3374611 |doi=10.1371/journal.pone.0030044 |bibcode=2012PLoSO...730044Z | doi-access = free}}</ref> |
|||
== Diagnosis == |
|||
The diagnosis of Crohn's disease can sometimes be challenging,<ref name="Pimentel" /> and many tests are often required to assist the physician in making the diagnosis.<ref name="emed" /> Even with a full battery of tests, it may not be possible to diagnose Crohn's with complete certainty; a colonoscopy is approximately 70% effective in diagnosing the disease, with further tests being less effective. Disease in the small bowel is particularly difficult to diagnose, as a traditional colonoscopy allows access to only the colon and lower portions of the small intestines; introduction of the [[capsule endoscopy]]<ref>{{Cite web|url=http://www.givenimaging.com/en-us/HealthcareProfessionals/Pages/pageHCP.aspx|archiveurl=https://web.archive.org/web/20080616005419/http://www.givenimaging.com/en-us/HealthCareProfessionals/Pages/pageHCP.aspx|url-status=dead|title=HCP: Pill Cam, Capsule Endoscopy, Esophageal Endoscopy|archivedate=June 16, 2008}}</ref> aids in endoscopic diagnosis. [[Giant cells|Giant (multinucleate) cells]], a common finding in the lesions of Crohn's disease, are less common in the lesions of [[lichen nitidus]].<ref>{{cite journal |vauthors=Scheinfeld NS, Teplitz E, McClain SA |title=Crohn's disease and lichen nitidus: a case report and comparison of common histopathologic features |journal=Inflammatory Bowel Diseases |volume=7 |issue=4 |pages=314–8 |date=November 2001 |pmid=11720321 |doi=10.1097/00054725-200111000-00006 |s2cid=35892792|doi-access=free}}</ref> |
|||
<gallery> |
|||
CD colitis.jpg|Endoscopic image of Crohn's colitis showing deep ulceration |
|||
CT scan gastric CD.jpg|[[CT scan]] showing Crohn's disease in the fundus of the stomach |
|||
Crohn's transmural path.jpg|Section of [[colectomy]] showing transmural inflammation |
|||
ResectedIleum.jpg|Resected ileum from a person with Crohn's disease |
|||
</gallery> |
|||
=== Classification === |
=== Classification === |
||
The [[Montreal classification system]] is a widely used framework for categorizing the phenotypes of Crohn's disease. It considers three primary factors: the age at diagnosis (divided into three groups: less than 16 years, 17 to 40 years, and over 40 years), the location of the disease (which can be ileal, colonic, ileocolonic, or isolated upper), and the behavior of the disease (including non-stricturing/non-penetrating, stricturing, penetrating, and perianal types).<ref name="pmid38065612">{{cite journal | vauthors = Cockburn E, Kamal S, Chan A, Rao V, Liu T, Huang JY, Segal JP | title = Crohn's disease: an update | journal = Clinical Medicine (London) | volume = 23 | issue = 6 | pages = 549–557 | pmid = 38065612 | pmc = 11298500 | date = November 2023 | doi = 10.7861/clinmed.2023-0493 | doi-access = free }}</ref> |
|||
[[File:Distribution of CD.svg|thumb|upright=1.4|Distribution of gastrointestinal Crohn's disease]] |
|||
Crohn's disease is one type of [[inflammatory bowel disease]] (IBD). It typically manifests in the gastrointestinal tract and can be categorized by the specific tract region affected. |
|||
== Management == |
|||
Gastroduodenal Crohn's disease causes inflammation in the stomach and the first part of the small intestine called the duodenum. Jejunoileitis causes spotty patches of inflammation in the top half of the small intestine, called the jejunum.<ref>{{cite journal |vauthors=Tan WC, Allan RN |title=Diffuse jejunoileitis of Crohn's disease |journal=Gut |volume=34 |issue=10 |pages=1374–8 |date=October 1993 |pmid=8244104 |pmc=1374544 |doi=10.1136/gut.34.10.1374}}</ref> The disease can attack any part of the digestive tract, from mouth to anus. However, individuals affected by the disease rarely fall outside these three classifications, with presentations in other areas.<ref name="Baumgart2012" /> |
|||
{{Main|Management of Crohn's disease}} |
|||
The management of Crohn's disease is customized based on the severity, location, and behavior of the disease. Providers also assess the risk of aggressive disease to determine the need for more intensive treatment. Risk factors include diagnosis before age 30, extensive disease involvement, perianal complications, deep ulcers, and history of surgery. A key goal of treatment is to achieve mucosal healing, which restores the intestinal lining. Mucosal healing is linked to better outcomes, such as fewer flare-ups, reduced hospitalizations, steroid-free remission, and a longer interval without surgery.<ref name="pmid32242028"/> |
|||
=== Corticosteroids === |
|||
Crohn's disease may also be categorized by the behavior of disease as it progresses. These categorizations formalized in the Vienna classification of the disease.<ref name="Vienna">{{cite journal |vauthors=Gasche C, Scholmerich J, Brynskov J, D'Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ, Sutherland LR |title=A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998 |journal=Inflammatory Bowel Diseases |volume=6 |issue=1 |pages=8–15 |date=February 2000 |pmid=10701144 |doi=10.1002/ibd.3780060103}}</ref> There are three categories of disease presentation in Crohn's disease: stricturing, penetrating, and inflammatory. Stricturing disease causes narrowing of the bowel that may lead to bowel obstruction or changes in the caliber of the [[feces]]. Penetrating disease creates abnormal passageways (fistulae) between the bowel and other structures, such as the skin. [[Inflammatory disease]] (or nonstricturing, nonpenetrating disease) causes inflammation without causing strictures or fistulae.<ref name="Vienna" /><ref>{{cite journal |vauthors=Dubinsky MC, Fleshner PP |title=Treatment of Crohn's Disease of Inflammatory, Stenotic, and Fistulizing Phenotypes |journal=Current Treatment Options in Gastroenterology |volume=6 |issue=3 |pages=183–200 |date=June 2003 |pmid=12744819 |doi=10.1007/s11938-003-0001-1 |s2cid=21302609}}</ref> |
|||
[[Corticosteroids|Steroids]] are often used to quickly induce remission and relieve symptoms in Crohn's disease, but they are ineffective for maintaining remission. Options include intravenous steroids, [[prednisone]], and [[budesonide]], with budesonide preferred for its safety, though it's limited to mild to moderate cases in the ileum and right colon. Patients on systemic steroids should switch to other medications for long-term remission, as prolonged use can cause adrenal issues, [[weight gain]], [[cataracts]], [[hypertension]], and [[Type 2 diabetes|diabetes]]. Additionally, systemic steroids may increase the risk of serious infections and mortality in moderate to severe Crohn's disease.<ref name="pmid27184635"/> |
|||
=== |
=== Conventional immunosuppressants === |
||
[[Thiopurine]]s, like [[azathioprine]] and [[6-mercaptopurine]], maintain remission in Crohn's disease but do not induce it initially. Since thiopurines take 6 to 12 weeks to work, steroids are often used to manage symptoms during this time. Before starting thiopurines, liver metabolism is assessed and [[Epstein-Barr]] virus is tested in patients under 25. Around 15% to 20% of patients stop thiopurines due to side effects, including low blood cell counts, liver problems, nausea, vomiting, allergic reactions, and acute pancreatitis. Thiopurines also raise the risk of certain cancers and serious conditions, necessitating regular lab monitoring.<ref name="pmid27184635"/> |
|||
A [[colonoscopy]] is the best test for making the diagnosis of Crohn's disease, as it allows direct visualization of the colon and the [[terminal ileum]], identifying the pattern of disease involvement. On occasion, the colonoscope can travel past the terminal ileum, but it varies from person to person. During the procedure, the [[gastroenterologist]] can also perform a biopsy, taking small samples of tissue for laboratory analysis, which may help confirm a diagnosis. As 30% of Crohn's disease involves only the ileum,<ref name="Baumgart2012" /> [[cannula]]tion of the terminal ileum is required in making the diagnosis. Finding a patchy distribution of disease, with involvement of the colon or ileum, but not the [[rectum]], is suggestive of Crohn's disease, as are other endoscopic stigmata.<ref name="Hara2006" /> |
|||
The utility of capsule endoscopy for this, however, is still uncertain.<ref>{{cite journal |vauthors=Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK |title=A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn's disease |journal=The American Journal of Gastroenterology |volume=101 |issue=5 |pages=954–64 |date=May 2006 |doi=10.1111/j.1572-0241.2006.00506.x |pmid=16696781 |s2cid=25684863}}</ref> |
|||
[[Methotrexate]] is used to induce and maintain remission in Crohn's disease, being slightly more effective than thiopurines and taking 8 to 16 weeks to work. About 17% of patients stop taking it due to side effects like nausea, vomiting, headaches, and fatigue. It can affect liver health and, rarely, lower blood cell counts, requiring regular blood tests. Methotrexate may also cause anemia and mouth sores, so daily folic acid is recommended. Additionally, it may increase the risk of certain [[skin cancer]]s and [[lymphoma]]. Methotrexate is discontinued during pregnancy due to the risks of [[miscarriage]] and [[birth defect]]s.<ref name="pmid27184635"/> |
|||
=== Radiologic tests === |
|||
A [[barium follow-through|small bowel follow-through]] may suggest the diagnosis of Crohn's disease and is useful when the disease involves only the small intestine. Because colonoscopy and [[Esophagogastroduodenoscopy|gastroscopy]] allow direct visualization of only the terminal ileum and beginning of the [[duodenum]], they cannot be used to evaluate the remainder of the small intestine. As a result, a barium follow-through X-ray, wherein [[barium sulfate]] suspension is ingested and [[fluoroscopy|fluoroscopic]] images of the bowel are taken over time, is useful for looking for inflammation and narrowing of the small bowel.<ref name="Hara2006">{{cite journal |vauthors=Hara AK, Leighton JA, Heigh RI, Sharma VK, Silva AC, De Petris G, Hentz JG, Fleischer DE |title=Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy |journal=Radiology |volume=238 |issue=1 |pages=128–34 |date=January 2006 |pmid=16373764 |doi=10.1148/radiol.2381050296}}</ref><ref>{{cite journal |vauthors=Dixon PM, Roulston ME, Nolan DJ |title=The small bowel enema: a ten year review |journal=Clinical Radiology |volume=47 |issue=1 |pages=46–8 |date=January 1993 |pmid=8428417 |doi=10.1016/S0009-9260(05)81213-9}}</ref> Barium enemas, in which barium is inserted into the rectum and fluoroscopy is used to image the bowel, are rarely used in the work-up of Crohn's disease due to the advent of colonoscopy. They remain useful for identifying anatomical abnormalities when strictures of the colon are too small for a colonoscope to pass through, or in the detection of colonic fistulae (in this case contrast should be performed with iodate substances).<ref>{{cite journal |vauthors=Carucci LR, Levine MS |title=Radiographic imaging of inflammatory bowel disease |journal=Gastroenterology Clinics of North America |volume=31 |issue=1 |pages=93–117, ix |date=March 2002 |pmid=12122746 |doi=10.1016/S0889-8553(01)00007-3}}</ref> |
|||
=== Biologics === |
|||
[[CT scan|CT]] and [[MRI]] scans are useful for evaluating the small bowel with [[enteroclysis]] protocols.<ref>{{cite journal |vauthors=Rajesh A, Maglinte DD |title=Multislice CT enteroclysis: technique and clinical applications |journal=Clinical Radiology |volume=61 |issue=1 |pages=31–9 |date=January 2006 |pmid=16356814 |doi=10.1016/j.crad.2005.08.006}}</ref> They are also useful for looking for intra-abdominal complications of Crohn's disease, such as abscesses, small bowel obstructions, or fistulae.<ref>{{cite journal |vauthors=Zissin R, Hertz M, Osadchy A, Novis B, Gayer G |title=Computed tomographic findings of abdominal complications of Crohn's disease--pictorial essay |journal=Canadian Association of Radiologists Journal |volume=56 |issue=1 |pages=25–35 |date=February 2005 |pmid=15835588 |url=https://www.proquest.com/openview/d36a211b9091938911c873b3ed55d0dc/1 |access-date=May 18, 2022 |archive-date=October 7, 2022 |archive-url=https://web.archive.org/web/20221007193456/https://www.proquest.com/openview/d36a211b9091938911c873b3ed55d0dc/1 |url-status=live}}</ref> Magnetic resonance imaging (MRI) is another option for imaging the [[small bowel]] as well as looking for complications, though it is more expensive and less readily available.<ref>{{cite journal |vauthors=Mackalski BA, Bernstein CN |title=New diagnostic imaging tools for inflammatory bowel disease |journal=Gut |volume=55 |issue=5 |pages=733–41 |date=May 2006 |pmid=16609136 |pmc=1856109 |doi=10.1136/gut.2005.076612}}</ref> MRI techniques such as diffusion-weighted imaging and high-resolution imaging are more sensitive in detecting ulceration and inflammation compared to CT.<ref>{{cite journal |vauthors=Sinha R, Rajiah P, Murphy P, Hawker P, Sanders S |title=Utility of high-resolution MR imaging in demonstrating transmural pathologic changes in Crohn disease |journal=Radiographics |volume=29 |issue=6 |pages=1847–67 |date=October 2009 |pmid=19959525 |doi=10.1148/rg.296095503}}</ref><ref>{{cite journal |vauthors=Sinha R, Rajiah P, Ramachandran I, Sanders S, Murphy PD |title=Diffusion-weighted MR imaging of the gastrointestinal tract: technique, indications, and imaging findings |journal=Radiographics |volume=33 |issue=3 |pages=655–76; discussion 676–80 |date=May 2013 |pmid=23674768 |doi=10.1148/rg.333125042 | doi-access = free}}</ref> |
|||
[[TNF inhibitor|Anti-TNF therapy]] is the most effective treatment for inducing and maintaining remission, with FDA-approved agents including [[infliximab]], [[adalimumab]], and [[certolizumab pegol]].<ref name="pmid27184635"/> It blocks the inflammatory protein [[TNF]] and induces cell death in activated [[T cells]].<ref name="pmid26896086">{{cite journal | vauthors = Levin AD, Wildenberg ME, van den Brink GR | title = Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease | journal = Journal of Crohn's and Colitis | volume = 10 | issue = 8 | pages = 989–997 | pmid = 29242771 | date = August 2016 | doi = 10.1093/ecco-jcc/jjw053 | doi-access = free | pmc = 5724577 }}</ref> Responses may occur within a week, but full effects can take up to six weeks. Loss of response can happen due to the development of [[Immunogenicity|antidrug antibodies]], necessitating a switch in agents or drug classes. Anti-TNF agents are often combined with thiopurines or methotrexate to minimize antibody development. Side effects include injection-site reactions, a higher risk of infection, a slight increase in melanoma risk, and rare cases of [[cytopenias]] and [[liver toxicity]].<ref name="pmid27184635"/> |
|||
[[Vedolizumab]] is the first treatment designed specifically for the gut in moderate to severe Crohn's disease. It blocks the molecule [[LPAM-1|α4β7]] that helps white blood cells enter the gut, reducing inflammation. Unlike [[natalizumab]], it does not carry a risk of the serious brain infection [[Progressive multifocal leukoencephalopathy|PML]]. While vedolizumab can induce remission, it works slowly, taking about 12 weeks to show effects, and its overall effectiveness is limited. However, patients who respond well can maintain remission for up to a year. Since it specifically targets the gut, it does not significantly increase the risk of serious side effects or infections, except for mild [[Sinusitis|nasal infections]].<ref name="pmid27184635"/> |
|||
=== Blood tests === |
|||
A [[complete blood count]] may reveal anemia, which commonly is caused by blood loss leading to iron deficiency or by [[Cyanocobalamin|vitamin B{{ssub|12}}]] deficiency, usually caused by ileal disease impairing vitamin B{{ssub|12}} absorption. Rarely autoimmune hemolysis may occur.<ref>{{cite journal |vauthors=Goh J, O'Morain CA |title=Review article: nutrition and adult inflammatory bowel disease |journal=Alimentary Pharmacology & Therapeutics |volume=17 |issue=3 |pages=307–20 |date=February 2003 |pmid=12562443 |doi=10.1046/j.1365-2036.2003.01482.x |s2cid=72099458 | doi-access = free}}</ref> [[Ferritin]] levels help assess if iron deficiency is contributing to the anemia. [[Erythrocyte sedimentation rate]] (ESR) and [[C-reactive protein]] help assess the degree of inflammation, which is important as ferritin can also be raised in inflammation.<ref>{{cite journal |vauthors=Chamouard P, Richert Z, Meyer N, Rahmi G, Baumann R |title=Diagnostic value of C-reactive protein for predicting activity level of Crohn's disease |journal=Clinical Gastroenterology and Hepatology |volume=4 |issue=7 |pages=882–7 |date=July 2006 |pmid=16630759 |doi=10.1016/j.cgh.2006.02.003}}</ref> |
|||
[[Ustekinumab]], approved for moderate to severe Crohn's disease in October 2016, has been FDA-approved for [[psoriasis]] since 2009.<ref name="pmid38877997">{{cite journal |vauthors=Gordon H, Minozzi S, Kopylov U, Verstockt B, Chaparro M, Buskens C, Warusavitarne J, Agrawal M, Allocca M, Atreya R, Battat R, Bettenworth D, Bislenghi G, Brown SR, Burisch J, Casanova MJ, Czuber-Dochan W, de Groof J, El-Hussuna A, Ellul P, Fidalgo C, Fiorino G, Gisbert JP, Sabino JG, Hanzel J, Holubar S, Iacucci M, Iqbal N, Kapizioni C, Karmiris K, Kobayashi T, Kotze PG, Luglio G, Maaser C, Moran G, Noor N, Papamichael K, Peros G, Reenaers C, Sica G, Sigall-Boneh R, Vavricka SR, Yanai H, Myrelid P, Adamina M, Raine T |title=ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment |journal=J Crohns Colitis |volume=18 |issue=10 |pages=1531–1555 |date=October 2024 |pmid=38877997 |doi=10.1093/ecco-jcc/jjae091 |url=}}</ref> It appears to be comparable to anti-TNF therapy in both the induction and maintenance of remission, functioning by blocking the inflammatory molecules [[interleukin-12|IL-12]] and [[interleukin-13|IL-23]]. The onset of action is similar to that of anti-TNF treatments, with responses typically observed within six weeks. Notably, Ustekinumab does not seem to increase the risk of serious infections, although the studies conducted in Crohn's disease have been relatively short-term.<ref name="pmid27184635"/> |
|||
Other causes of anemia include medication used in treatment of inflammatory bowel disease, like azathioprine, which can lead to cytopenia, and sulfasalazine, which can also result in [[folate deficiency]]. Testing for ''[[Saccharomyces cerevisiae]]'' antibodies (ASCA) and [[anti-neutrophil cytoplasmic antibody|antineutrophil cytoplasmic antibodies]] (ANCA) has been evaluated to identify inflammatory diseases of the intestine<ref>{{cite journal |vauthors=Kaila B, Orr K, Bernstein CN |title=The anti-Saccharomyces cerevisiae antibody assay in a province-wide practice: accurate in identifying cases of Crohn's disease and predicting inflammatory disease |journal=Canadian Journal of Gastroenterology |volume=19 |issue=12 |pages=717–21 |date=December 2005 |pmid=16341311 |doi=10.1155/2005/147681 | doi-access = free}}</ref> and to differentiate Crohn's disease from ulcerative colitis.<ref>{{cite journal |vauthors=Israeli E, Grotto I, Gilburd B, Balicer RD, Goldin E, Wiik A, Shoenfeld Y |title=Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease |journal=Gut |volume=54 |issue=9 |pages=1232–6 |date=September 2005 |pmid=16099791 |pmc=1774672 |doi=10.1136/gut.2004.060228}}</ref> Furthermore, increasing amounts and levels of serological antibodies such as ASCA, antilaminaribioside [Glc(β1,3)Glb(β); ALCA], antichitobioside [GlcNAc(β1,4)GlcNAc(β); ACCA], antimannobioside [Man(α1,3)Man(α)AMCA], antiLaminarin [(Glc(β1,3))3n(Glc(β1,6))n; anti-L] and antichitin [GlcNAc(β1,4)n; anti-C] associate with disease behavior and surgery, and may aid in the prognosis of Crohn's disease.<ref>{{cite journal |vauthors=Ferrante M, Henckaerts L, Joossens M, Pierik M, Joossens S, Dotan N, Norman GL, Altstock RT, Van Steen K, Rutgeerts P, Van Assche G, Vermeire S |title=New serological markers in inflammatory bowel disease are associated with complicated disease behaviour |journal=Gut |volume=56 |issue=10 |pages=1394–403 |date=October 2007 |pmid=17456509 |pmc=2000264 |doi=10.1136/gut.2006.108043 |first12=S}}</ref><ref>{{cite journal |vauthors=Papp M, Altorjay I, Dotan N, Palatka K, Foldi I, Tumpek J, Sipka S, Udvardy M, Dinya T, Lakatos L, Kovacs A, Molnar T, Tulassay Z, Miheller P, Norman GL, Szamosi T, Papp J, Lakatos PL |title=New serological markers for inflammatory bowel disease are associated with earlier age at onset, complicated disease behavior, risk for surgery, and NOD2/CARD15 genotype in a Hungarian IBD cohort |journal=The American Journal of Gastroenterology |volume=103 |issue=3 |pages=665–81 |date=March 2008 |doi=10.1111/j.1572-0241.2007.01652.x |pmid=18047543 |s2cid=6015339}}</ref><ref>{{cite journal |vauthors=Seow CH, Stempak JM, Xu W, Lan H, Griffiths AM, Greenberg GR, Steinhart AH, Dotan N, Silverberg MS |title=Novel anti-glycan antibodies related to inflammatory bowel disease diagnosis and phenotype |journal=The American Journal of Gastroenterology |volume=104 |issue=6 |pages=1426–34 |date=June 2009 |pmid=19491856 |doi=10.1038/ajg.2009.79 |s2cid=25021606}}</ref><ref>{{cite journal |vauthors=Dotan I |title=Serologic markers in inflammatory bowel disease: tools for better diagnosis and disease stratification |journal=Expert Review of Gastroenterology & Hepatology |volume=1 |issue=2 |pages=265–74 |date=December 2007 |pmid=19072419 |doi=10.1586/17474124.1.2.265 |s2cid=32035337}}</ref> |
|||
===[[Small molecule]]s=== |
|||
Low serum levels of vitamin D are associated with Crohn's disease.<ref name="IBD2015">{{cite journal |vauthors=Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F |title=Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis |journal=Inflammatory Bowel Diseases |volume=21 |issue=11 |pages=2708–17 |date=November 2015 |pmid=26348447 |pmc=4615394 |doi=10.1097/MIB.0000000000000546}}</ref> Further studies are required to determine the significance of this association.<ref name="IBD2015" /> |
|||
The [[JAK inhibitor]] such as [[upadacitinib]] is approved for treatment of moderate to severe Crohn's disease, with a large multi-centre [[randomized control trial]] demonstrating its effectiveness in induction and maintenance of disease.<ref name=pmid38877997 /><ref name="pmid37224198">{{cite journal| author=Loftus EV, Panés J, Lacerda AP, Peyrin-Biroulet L, D'Haens G, Panaccione R | display-authors=etal| title=Upadacitinib Induction and Maintenance Therapy for Crohn's Disease. | journal=N Engl J Med | year= 2023 | volume= 388 | issue= 21 | pages= 1966–1980 | pmid=37224198 | doi=10.1056/NEJMoa2212728 | pmc= | hdl=2268/304716| url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=37224198 | hdl-access=free }} [https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=&cmd=prlinks&id=37665995 Review in: Ann Intern Med. 2023 Sep;176(9):JC103. doi: 10.7326/J23-0069]</ref> |
|||
=== Surgery === |
|||
=== Comparison with ulcerative colitis === |
|||
[[Image:ResectedIleum.jpg|thumb|right|Resected ileum from a person with Crohn's disease]] |
|||
The most common disease that mimics the symptoms of Crohn's disease is ulcerative colitis, as both are inflammatory bowel diseases that can affect the colon with similar symptoms. It is important to differentiate these diseases, since the course of the diseases and treatments may be different. In some cases, however, it may not be possible to tell the difference, in which case the disease is classified as indeterminate colitis.<ref name="Baumgart2012" /><ref name="emed" /><ref name="Podolsky" /> |
|||
{{Findings in CD vs. UC}} |
|||
Many individuals with Crohn's disease may require a [[bowel resection]] to remove part of the intestine due to blockages, lesions, infections, or ineffective medications. Since surgery is not a cure, the goal is to preserve as much of the small bowel as possible,<ref name="pmid27184635"/> and extensive resections can lead to [[short bowel syndrome]].<ref name="pmid34239262">{{cite journal | vauthors = Aksan A, Farrag K, Blumenstein I, Schröder O, Dignass AU, Stein J | title = Chronic intestinal failure and short bowel syndrome in Crohn's disease | journal = World Journal of Gastroenterology | volume = 27 | issue = 24 | pages = 3440–3465 | pmid = 34239262 | pmc = 8240052 | date = June 2021 | doi = 10.3748/wjg.v27.i24.3440 | doi-access = free }}</ref> In cases with widespread strictures, only the most prominent stricture is typically resected, while minor strictures may be dilated through [[strictureplasty]]. After a resection, the healthy ends of the intestine are rejoined in a primary [[surgical anastomosis|anastomosis]].<ref name="pmid27184635"/> |
|||
=== Differential diagnosis === |
|||
Other conditions with similar symptoms as Crohn's disease includes intestinal [[tuberculosis]], [[Behçet's disease]], [[ulcerative colitis]], [[nonsteroidal anti-inflammatory drug]] enteropathy, [[irritable bowel syndrome]] and [[celiac disease]].<ref name="WGO-IBD">{{cite web |url=http://www.worldgastroenterology.org/UserFiles/file/guidelines/inflammatory-bowel-disease-english-2015.pdf |title=Inflammatory Bowel Disease |date=August 2015 |publisher=World Gastroenterology Organization|access-date=March 13, 2016|url-status=dead|archive-url=https://web.archive.org/web/20160314014940/http://www.worldgastroenterology.org/UserFiles/file/guidelines/inflammatory-bowel-disease-english-2015.pdf|archive-date=March 14, 2016}}</ref> Irritable bowel syndrome is excluded when there are inflammatory changes.<ref name="WGO-IBD" /> Celiac disease cannot be excluded if specific antibodies ([[Anti-transglutaminase antibodies#Anti-tissue transglutaminase|anti-transglutaminase antibodies]]) are negative,<ref>{{cite journal |vauthors=Lewis NR, Scott BB |title=Systematic review: the use of serology to exclude or diagnose coeliac disease (a comparison of the endomysial and tissue transglutaminase antibody tests) |journal=Alimentary Pharmacology & Therapeutics |volume=24 |issue=1 |pages=47–54 |date=July 2006 |pmid=16803602 |doi=10.1111/j.1365-2036.2006.02967.x |s2cid=16823218 |type=Review |quote=Both the endomysial antibody and tissue transglutaminase antibody have very high sensitivities (93% for both) and specificities (>99% and >98% respectively) for the diagnosis of typical coeliac disease with villous atrophy. (...) As the detection of at least partial villous atrophy was used to make a diagnosis of coeliac disease in the vast majority of studies, we can't assume that the same LRs apply to coeliac patients with lesser abnormality such as an increase in intraepithelial lymphocytes or electron-microscopic changes only. In fact, if such lesser abnormalities were used as criteria for diagnosing (and excluding) coeliac disease, the sensitivity of the tests could be lower (i.e. more false negatives), especially since a number of studies suggest that the EMA and tTG antibody tests are less sensitive with lesser degrees of mucosal abnormality |doi-access=free}}</ref><ref>{{cite journal |vauthors=Rodrigo L, Garrote JA, Vivas S |title=[Celiac disease] |language=es |journal=Medicina Clinica |volume=131 |issue=7 |pages=264–70 |date=September 2008 |pmid=18775218 |doi=10.1016/S0025-7753(08)72247-4 |url=http://www.elsevier.es/es-revista-medicina-clinica-2-articulo-enfermedad-celiaca-13125306 |type=Review |archive-url = https://web.archive.org/web/20160319224000/http://www.elsevier.es/es-revista-medicina-clinica-2-articulo-enfermedad-celiaca-13125306 |url-status=dead |archive-date=March 19, 2016 |quote=Estos marcadores presentan en general una elevada sensibilidad y especificidad (cercanas al 90%) en presencia de atrofia marcada de las vellosidades intestinales. Sin embargo, muestran una notable disminución de la sensibilidad (del orden del 40-50%) en casos con atrofia vellositaria leve o cambios mínimos. ''These markers generally have high sensitivity and specificity (around 90%) in the presence of marked atrophy of the villi. However, they show a marked decrease in sensitivity (of the order of 40-50%) in cases with mild villous atrophy or minimal changes''. |access-date=March 13, 2016}}</ref> nor in absence of [[intestinal villi]] atrophy.<ref>{{cite journal |vauthors=Rostami Nejad M, Hogg-Kollars S, Ishaq S, Rostami K |title=Subclinical celiac disease and gluten sensitivity |journal=Gastroenterology and Hepatology from Bed to Bench |volume=4 |issue=3 |pages=102–8 |date=2011 |pmid=24834166 |pmc=4017418 |type=Review}}</ref><ref>{{cite journal |vauthors=Bold J, Rostami K |title=Gluten tolerance; potential challenges in treatment strategies |journal=Gastroenterology and Hepatology from Bed to Bench |volume=4 |issue=2 |pages=53–7 |date=2011 |pmid=24834157 |pmc=4017406 |type=Review}}</ref> |
|||
Approximately six to twelve months after surgery, patients usually undergo a colonoscopy to check for inflammation, using the [[Rutgeerts scoring system]] to assess the likelihood of recurrence. About 50% may experience a return of symptoms within five years, and nearly 40% may need a second surgery within ten years,<ref name="pmid27184635"/> often due to inflammation near the anastomosis.<ref name="pmid21088749">{{cite journal | vauthors = Lewis RT, Maron DJ | title = Efficacy and complications of surgery for Crohn's disease | journal = Gastroenterology and Hepatology | volume = 6 | issue = 9 | pages = 587–596 | pmid = 21088749 | pmc = 2976865 | date = September 2010 }}</ref> While drug therapy aims to prevent recurrences, its effectiveness remains uncertain.<ref name="pmid27184635"/> |
|||
== Management == |
|||
{{Main|Management of Crohn's disease}} |
|||
{{Treatment in CD vs. UC}} |
|||
There is no cure for Crohn's disease and [[remission (medicine)|remission]] may not be possible or prolonged if achieved. In cases where remission is possible, [[relapse]] can be prevented and [[symptom]]s controlled with medication, lifestyle and dietary changes, changes to eating habits (eating smaller amounts more often), reduction of stress, moderate activity, and exercise. Surgery is generally contraindicated and has not been shown to prevent relapse. Adequately controlled, Crohn's disease may not significantly restrict daily living.<ref name="WebMD">{{cite web |url=http://www.webmd.com/digestive-disorders/features/crohns-disease-54-tips-to-help-you-manage |title=Crohn's Disease: 54 Tips to Help You Manage |website=[[WebMD]] |vauthors=Fries WS, Nazario B |date=May 16, 2007 |access-date=February 14, 2008 |url-status=live |archive-url = https://web.archive.org/web/20080208234633/http://www.webmd.com/digestive-disorders/features/crohns-disease-54-tips-to-help-you-manage |archive-date=February 8, 2008}}</ref> Treatment for Crohn's disease involves first treating the [[acute (medical)|acute]] problem and its symptoms, then maintaining remission of the disease. |
|||
=== |
=== Diet === |
||
* [[Enteral nutrition]], which administers nutrients as a powder or liquid, is the primary method for inducing remission in children with Crohn's disease. It can induce remission in up to 80% of cases. Outside of Japan, it is not used to treat Crohn's disease in adults due to unpalatable formulations.<ref name="pmid31540038">{{cite journal | vauthors = Di Caro S, Fragkos KC, Keetarut K, Koo HF, Sebepos-Rogers G, Saravanapavan H, Barragry J, Rogers J, Mehta SJ, Rahman F | title = Enteral Nutrition in Adult Crohn's Disease: Toward a Paradigm Shift | journal = Nutrients | volume = 11 | issue = 4 | pages = 2222 | doi = 10.3390/nu11092222 | pmid = 31540038 | pmc = 6770416 | date = September 2019 | doi-access = free }}</ref> |
|||
Certain lifestyle changes can reduce symptoms, including [[Diet (nutrition)|dietary]] adjustments, [[elemental diet]], proper [[Drinking water|hydration]], and [[smoking cessation]]. Recent reviews underlined the importance to adopt diets that are best supported by evidence, even if little is known about the impact of diets on these patients.<ref>{{cite journal |vauthors=Roncoroni L, Gori R, Elli L, Tontini GE, Doneda L, Norsa L, Cuomo M, Lombardo V, Scricciolo A, Caprioli F, Costantino A, Scaramella L, Vecchi M |title=Nutrition in Patients with Inflammatory Bowel Diseases: A Narrative Review |journal=Nutrients |volume=14 |issue=4 |pages=751 |date=February 2022 |pmid=35215401 |pmc=8879392 |doi=10.3390/nu14040751 |doi-access=free }}</ref><ref>{{cite journal |vauthors=Ananthakrishnan AN, Kaplan GG, Bernstein CN, Burke KE, Lochhead PJ, Sasson AN, Agrawal M, Tiong JH, Steinberg J, Kruis W, Steinwurz F, Ahuja V, Ng SC, Rubin DT, Colombel JF, Gearry R |title=Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus |journal=Lancet Gastroenterol Hepatol |volume=7 |issue=7 |pages=666–678 |date=July 2022 |pmid=35487235 |doi=10.1016/S2468-1253(22)00021-8 }}</ref> |
|||
* [[Parenteral nutrition]], which administers nutrients directly into the bloodstream, may be used to supply nutrients to patients when there is extensive inflammation that impairs absorption. Parenteral nutrition has the same effectiveness as enteral nutrition in inducing remission.<ref name="pmid31766687">{{cite journal | vauthors = Comeche JM, Comino I, Altavilla C, Tuells J, Gutierrez-Hervas A, Caballero P | title = Parenteral Nutrition in Patients with Inflammatory Bowel Disease Systematic Review, Meta-Analysis and Meta-Regression | journal = Nutrients | volume = 11 | issue = 12 | pages = 2865 | doi = 10.3390/nu11122865 | pmid = 31766687 | pmc = 6950216 | date = November 2019 | doi-access = free }}</ref> |
|||
Diets that include higher levels of fiber and fruit are associated with reduced risk, while diets rich in total fats, [[polyunsaturated fatty acid]]s, meat, and [[omega-6 fatty acid]]s may increase the risk of Crohn's.<ref>{{cite journal |vauthors=Hou JK, Abraham B, El-Serag H |title=Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature |journal=The American Journal of Gastroenterology |volume=106 |issue=4 |pages=563–73 |date=April 2011 |pmid=21468064 |doi=10.1038/ajg.2011.44 |s2cid=10337669}}</ref> Maintaining a balanced diet with proper portion control can help manage symptoms of the disease. Eating small meals frequently instead of big meals may also help with a low appetite. A [[food diary]] may help with identifying foods that trigger symptoms. Despite the recognized importance of dietary fiber for intestinal health, some people should follow a [[Low-fiber/low-residue diet|low residue diet]] to control acute symptoms especially if foods high in [[insoluble fiber]] cause symptoms, e.g., due to obstruction or irritation of the bowel.<ref name="WebMD" /> Some find relief in eliminating [[casein]] (a protein found in cow's milk) and [[gluten]] (a protein found in wheat, rye and barley) from their diets. They may have specific dietary intolerances (not allergies), for example, [[lactose]].<ref>{{cite book |vauthors=Escott-Stump S |title=Nutrition and Diagnosis-Related Care, 7th edition |year=2008 |publisher=Lippincott Williams & Wilkins |location=Baltimore, MD |isbn=978-1-60831-017-3 |pages=1020 (pp 431)}}</ref> [[Fatigue (medical)|Fatigue]] can be helped with regular exercise, a healthy diet, and enough sleep, and for those with malabsorption of [[vitamin B12|vitamin B<sub>12</sub>]] due to disease or surgical resection of the [[terminal ileum]], cobalamin injections. Smoking may worsen symptoms and the course of the disease, and stopping is recommended. Alcohol consumption can also worsen symptoms, and moderation or cessation is advised.<ref name="WebMD" /> |
|||
* The [[Mediterranean diet]], rich in fruits, vegetables, unsaturated fats, and lean protein, is beneficial to general health but has not been found to reduce the rate of flares in Crohn's disease.<ref name="pmid38276922">{{cite journal | vauthors = Hashash JG, Elkins J, Lewis JD, Binion DG | title = AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients With Inflammatory Bowel Disease: Expert Review | journal = Gastroenterology | volume = 166 | issue = 3 | pages = 521–532 | doi = 10.1053/j.gastro.2023.11.303 | pmid = 38276922 | date = January 2024 | doi-access = free }}</ref> |
|||
* Cooking and processing fruits and vegetables to reduce fibrousness increases tolerance of those foods in people with intestinal strictures.<ref name="pmid38276922"/> |
|||
=== |
=== Other treatments === |
||
* [[Mesalamine]] is not effective for inducing or maintaining remission in Crohn's disease, but is nevertheless commonly used as a treatment due to its perceived benefits and low risk of side effects.<ref name="pmid32242028"/> |
|||
Acute treatment uses medications to treat any infection (normally [[antibiotic]]s) and to reduce inflammation (normally [[aminosalicylic acid|aminosalicylate]] anti-inflammatory drugs and [[corticosteroid]]s). When symptoms are in remission, treatment enters maintenance, with a goal of avoiding the recurrence of symptoms. Prolonged use of corticosteroids has significant [[Adverse effect (medicine)|side-effects]]; as a result, they are, in general, not used for long-term treatment. Alternatives include aminosalicylates alone, though only a minority are able to maintain the treatment, and many require immunosuppressive drugs.<ref name="HanauerCrohns" /> It has been also suggested that antibiotics change the enteric flora, and their continuous use may pose the risk of overgrowth with pathogens such as ''[[Clostridium difficile (bacteria)|Clostridium difficile]]''.<ref name="Shanahan">{{cite journal |vauthors=Shanahan F |title=Crohn's disease |journal=Lancet |volume=359 |issue=9300 |pages=62–9 |date=January 2002 |pmid=11809204 |doi=10.1016/S0140-6736(02)07284-7 |s2cid=743620}}</ref> |
|||
* [[Antibiotics]] are not effective in inducing or maintaining remission in Crohn's disease. However, they are effective in treating perianal fistulas and inflammation when used alongside anti-TNF therapy.<ref name="pmid32242028"/> |
|||
* [[Fecal microbiota transplants]] have shown potential effectiveness in preliminary studies. However, larger studies are needed to verify its efficacy.<ref name="pmid34169565">{{cite journal | vauthors = Fehily SR, Basnayake C, Wright EK, Kamm MA | title = Fecal microbiota transplantation therapy in Crohn's disease: Systematic review | journal = Journal of Gastroenterology and Hepatology | volume = 36 | issue = 10 | pages = 2672–2686 | doi = 10.1111/jgh.15598 | pmid = 34169565 | date = July 2021 | hdl = 11343/298722 }}</ref> |
|||
* [[Acupuncture]] influences the immune system by stimulating the [[vagus nerve]]. Early studies have shown efficacy in improving clinical symptoms, but larger studies are needed.<ref name="pmid30535303">{{cite journal | vauthors = Song G, Fiocchi C, Achkar JP | title = Acupuncture in Inflammatory Bowel Disease | journal = Inflammatory Bowel Diseases | volume = 25 | issue = 7 | pages = 1129–1139 | doi = 10.1093/ibd/izy371 | pmid = 30535303 | date = June 2019 }}</ref> |
|||
* [[Cannabis]] is a potential therapy for Crohn's disease by targeting the endocannabinoid system. Although it has shown benefits in animal models, its efficacy as a treatment is uncertain.<ref>{{cite journal |vauthors=Kafil TS, Nguyen TM, MacDonald JK, Chande N |title=Cannabis for the treatment of Crohn's disease |journal=The Cochrane Database of Systematic Reviews |volume=11 |pages=CD012853 |date=November 2018 |issue=11 |pmid=30407616 |pmc=6517156 |doi=10.1002/14651858.CD012853.pub2}}</ref> |
|||
* [[Cognitive behavioral therapy]] has shown short-term positive psychological effects in people with Crohn's disease, but it has no long-term effect on physical or psychological health.<ref name="pmid34911469">{{cite journal | vauthors = Chen J, Chen X, Sun Y, Xie Y, Wang X, Li R, Hesketh T | title = The physiological and psychological effects of cognitive behavior therapy on patients with inflammatory bowel disease before COVID-19: a systematic review | journal = BMC Gastroenterology | volume = 21 | issue = 1 | pages = 469 | date = December 2021 | pmid = 34911469 | pmc = 8672154 | doi = 10.1186/s12876-021-02003-0| doi-access = free }}</ref> |
|||
== Outlook == |
|||
Medications used to treat the symptoms of Crohn's disease include [[mesalazine|5-aminosalicylic acid]] (5-ASA) formulations, [[prednisone]], immunomodulators such as [[azathioprine]] (given as the prodrug for [[6-mercaptopurine]]), [[methotrexate]],<ref name="pmid29338679">{{cite journal |vauthors=Djurić Z, Šaranac L, Budić I, Pavlović V, Djordjević J |title=Therapeutic role of methotrexate in pediatric Crohn's disease |journal=Bosnian Journal of Basic Medical Sciences |volume=18 |issue=3 |pages=211–216 |date=August 2018 |pmid=29338679 |pmc=6087553 |doi=10.17305/bjbms.2018.2792}}</ref> and [[anti-TNF]] therapies and [[monoclonal antibodies]], such as [[infliximab]], [[adalimumab]],<ref name="Podolsky">{{cite journal |vauthors=Podolsky DK |title=Inflammatory bowel disease |journal=The New England Journal of Medicine |volume=347 |issue=6 |pages=417–29 |date=August 2002 |pmid=12167685 |doi=10.1056/NEJMra020831 |url=http://gut.bmj.com/cgi/content/short/39/Suppl_1/A15 |type=Submitted manuscript |access-date=September 4, 2018 |archive-date=April 28, 2021 |archive-url=https://web.archive.org/web/20210428151606/https://gut.bmj.com/cgi/content/short/39/Suppl_1/A15 |url-status=live}}</ref> [[certolizumab]],<ref>{{cite press release |url=https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116882.htm |title=FDA Approves Cimzia to Treat Crohn's Disease |publisher=[[Food and Drug Administration]] (FDA) |date=April 22, 2008 |access-date=November 5, 2009 |url-status=live |archive-url=https://web.archive.org/web/20091020111256/https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116882.htm |archive-date=October 20, 2009}}</ref> [[vedolizumab]], [[ustekinumab]],<ref>{{cite web |title=Prescribing information ustekinumab |url=https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125261s147lbl.pdf |website=FDA |access-date=May 23, 2019 |archive-date=October 18, 2020 |archive-url=https://web.archive.org/web/20201018151109/https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125261s147lbl.pdf |url-status=live}}</ref> [[natalizumab]],<ref>{{cite journal |vauthors=Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S, van Deventer S, Goldblum R, Despain D, Hogge GS, Rutgeerts P |title=Natalizumab induction and maintenance therapy for Crohn's disease |journal=The New England Journal of Medicine |volume=353 |issue=18 |pages=1912–25 |date=November 2005 |pmid=16267322 |doi=10.1056/NEJMoa043335 |collaboration=International Efficacy of Natalizumab as Active Crohn's Therapy (ENACT-1) Trial Group; Evaluation of Natalizumab as Continuous Therapy (ENACT-2) Trial Group|doi-access=free}}</ref><ref>{{cite journal |vauthors=Nelson SM, Nguyen TM, McDonald JW, MacDonald JK |title=Natalizumab for induction of remission in Crohn's disease |journal=The Cochrane Database of Systematic Reviews |volume=2018 |pages=CD006097 |date=August 2018 |issue=8 |pmid=30068022 |pmc=6513248 |doi=10.1002/14651858.CD006097.pub3}}</ref>[[Risankizumab|risankizumab-rzaa]], and [[upadacitinib]]<ref>{{Cite web |title=Discover RINVOQ® (upadacitinib) |url=https://www.rinvoq.com/ |access-date=2023-05-29 |website=RINVOQ |language=en |archive-date=May 18, 2023 |archive-url=https://web.archive.org/web/20230518231109/https://www.rinvoq.com/ |url-status=live}}</ref> [[Hydrocortisone]] should be used in severe attacks of Crohn's disease.<ref name="OHCM">{{cite book |vauthors=Longmore M, Wilkinson I, Turmezei T, Cheung CK |title=Oxford Handbook of Clinical Medicine |edition=7th |publisher=[[Oxford University Press]] |year=2007 |pages=266–7 |isbn=978-0-19-856837-7}}</ref> [[Biological therapy for inflammatory bowel disease|Biological therapies]] are medications used to avoid long-term steroid use, decrease inflammation, and treat people who have fistulas with abscesses.<ref name="ustekinumab" /> The monoclonal antibody ustekinumab appears to be a safe treatment option, and may help people with moderate to severe active Crohn's disease.<ref name="MacD2016">{{cite journal |vauthors=MacDonald JK, Nguyen TM, Khanna R, Timmer A |title=Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease |journal=The Cochrane Database of Systematic Reviews |volume=2016 |pages=CD007572 |date=November 2016 |issue=11 |pmid=27885650 |pmc=6464484 |doi=10.1002/14651858.CD007572.pub3}}</ref> The long term safety and effectiveness of monoclonal antibody treatment is not known.<ref name="MacD2016" /> The monoclonal antibody [[briakinumab]] is not effective for people with active Crohn's disease and it is no longer being manufactured.<ref name="MacD2016" /> |
|||
Crohn's disease is a chronic condition requiring ongoing management, as there is currently no cure. Inflammation is typically controlled through medications such as [[corticosteroids|steroids]] and [[immunosuppressants]], and in severe cases, [[bowel resection|surgery]] may be necessary. The clinical course of the disease is classified into four patterns:<ref name="pmid34456186">{{cite journal | vauthors = Cho CW, You MW, Oh CH, Lee CK, Moon SK | title = Long-term Disease Course of Crohn's Disease: Changes in Disease Location, Phenotype, Activities, and Predictive Factors | journal = Gut and Liver | volume = 16 | issue = 2 | pages = 157–170 | pmid = 34456186 | pmc = 8924800 | date = March 2022 | doi = 10.5009/gnl210118 | doi-access = free }}</ref> |
|||
* Remission: Severity decreases in response to treatment, leading to sustained remission.<ref name="pmid34456186"/> |
|||
The gradual loss of blood from the gastrointestinal tract, as well as chronic inflammation, often leads to anemia, and professional guidelines suggest routinely monitoring for this.<ref name="Mowat2011">{{cite journal |vauthors=Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho GT, Satsangi J, Bloom S |title=Guidelines for the management of inflammatory bowel disease in adults |journal=Gut |volume=60 |issue=5 |pages=571–607 |date=May 2011 |pmid=21464096 |doi=10.1136/gut.2010.224154 |s2cid=8269837 |collaboration=IBD Section of the British Society of Gastroenterology}}</ref><ref name="ReferenceA">{{cite journal |vauthors=Goddard AF, James MW, McIntyre AS, Scott BB |title=Guidelines for the management of iron deficiency anaemia |journal=Gut |volume=60 |issue=10 |pages=1309–16 |date=October 2011 |pmid=21561874 |doi=10.1136/gut.2010.228874 | doi-access = free}}</ref><ref name="ReferenceB">{{cite journal |vauthors=Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, Gomollon F, Hjortswang H, Koutroubakis I, Kulnigg S, Oldenburg B, Rampton D, Schroeder O, Stein J, Travis S, Van Assche G |title=Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases |journal=Inflammatory Bowel Diseases |volume=13 |issue=12 |pages=1545–53 |date=December 2007 |pmid=17985376 |doi=10.1002/ibd.20285 |doi-access=free}}</ref> |
|||
* Improved and Stable: Severity lessens, but mild inflammation persists.<ref name="pmid34456186"/> |
|||
* Relapsing: The disease fluctuates between periods of remission and severe inflammation.<ref name="pmid34456186"/> |
|||
* Refractory: Severe inflammation continues without respite.<ref name="pmid34456186"/> |
|||
Approximately 40% to 56% of individuals with Crohn's disease achieve clinical remission after one year of [[infliximab]] treatment, increasing to 56% to 58% when combined with an immunosuppressant. Furthermore, 16% to 39% attain both clinical and endoscopic remission, showing no signs of inflammation in the intestine. Once in remission, individuals have an 80% chance of maintaining this state for the following year. Conversely, 10% to 15% of individuals may experience ongoing active disease without remission.<ref name="pmid34456186"/> |
|||
=== Immunosuppressant therapies, infection risks and vaccinations === |
|||
Chronic inflammation from Crohn's disease increases the risk of heart problems, [[cancer]]s, [[arthritis]], [[osteoporosis]] (weakened bones), and mental health issues. Some medications can also raise the chances of infections and cancers. Because of these combined risks, people with Crohn's disease tend to have a shorter lifespan compared to those who are healthy. In Canada, studies show that women affected with Crohn's disease live about 7.7 years less than unaffected women, and affected men live about 7.7 years less than otherwise expected.<ref name="pmid33168761">{{cite journal | vauthors = Kuenzig ME, Manuel DG, Donelle J, Benchimol EI | title = Life expectancy and health-adjusted life expectancy in people with inflammatory bowel disease Factors | journal = Canadian Medical Association Journal | volume = 192 | issue = 45 | pages = E1394–E1402 | pmid = 33168761 | pmc = 7669301 | date = November 2020 | doi = 10.1503/cmaj.190976 | doi-access = free }}</ref> |
|||
Many patients affected by Crohn's disease need immunosuppressant therapies, which are known to be associated with a higher risk of contracting opportunistic infectious diseases and of pre-neoplastic or neoplastic lesions such as cervical high-grade dysplasia and cancer.<ref>{{cite journal |vauthors=Beaugerie L, Itzkowitz SH |title=Cancers complicating inflammatory bowel disease |journal=N Engl J Med |volume=372 |issue=15 |pages=1441–52 |date=April 2015 |pmid=25853748 |doi=10.1056/NEJMra1403718 }}</ref><ref>{{cite journal |vauthors=Toruner M, Loftus EV, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, Colombel JF, Egan LJ |title=Risk factors for opportunistic infections in patients with inflammatory bowel disease |journal=Gastroenterology |volume=134 |issue=4 |pages=929–36 |date=April 2008 |pmid=18294633 |doi=10.1053/j.gastro.2008.01.012 }}</ref> |
|||
Many of these potentially harmful diseases, such as [[Hepatitis B]], [[Influenza]], [[Varicella zoster virus|herpes zoster virus]], [[pneumococcal pneumonia]], or [[Human papillomavirus infection|human papilloma virus]], can be prevented by vaccines. Each drug used in the treatment of IBD should be classified according to the degree of immunosuppression induced in the patient. Several guidelines suggest investigating patients’ vaccination status before starting any treatment and performing vaccinations against [[Vaccine-preventable disease]] when required.<ref>{{cite journal |vauthors=Farraye FA, Melmed GY, Lichtenstein GR, Kane SV |title=ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease |journal=Am J Gastroenterol |volume=112 |issue=2 |pages=241–258 |date=February 2017 |pmid=28071656 |doi=10.1038/ajg.2016.537 }}</ref> |
|||
Compared to the rest of the population, patients affected by IBD are known to be at higher risk of contracting some [[vaccine-preventable diseases]] such as flu and pneumonia.<ref>{{cite journal |vauthors=Ananthakrishnan AN, McGinley EL |title=Infection-related hospitalizations are associated with increased mortality in patients with inflammatory bowel diseases |journal=J Crohns Colitis |volume=7 |issue=2 |pages=107–12 |date=March 2013 |pmid=22440891 |doi=10.1016/j.crohns.2012.02.015 }}</ref> |
|||
Nevertheless, despite the increased risk of infections, vaccination rates in IBD patients are known to be suboptimal and may also be lower than vaccination rates in the general population.<ref>{{cite journal |vauthors=Malhi G, Rumman A, Thanabalan R, Croitoru K, Silverberg MS, Hillary Steinhart A, Nguyen GC |title=Vaccination in inflammatory bowel disease patients: attitudes, knowledge, and uptake |journal=J Crohns Colitis |volume=9 |issue=6 |pages=439–44 |date=June 2015 |pmid=25908717 |doi=10.1093/ecco-jcc/jjv064 }}</ref><ref>{{cite journal |vauthors=Costantino A, Michelon M, Noviello D, Macaluso FS, Leone S, Bonaccorso N, Costantino C, Vecchi M, Caprioli F |title=Attitudes towards Vaccinations in a National Italian Cohort of Patients with Inflammatory Bowel Disease |journal=Vaccines |volume=11 |issue=10 |pages=1591 |date=October 2023 |pmid=37896993 |pmc=10611209 |doi=10.3390/vaccines11101591 |doi-access=free }}</ref> |
|||
== Epidemiology == |
|||
Crohn's disease is most prevalent in [[North America]] and [[Western Europe]], particularly among [[Ashkenazi jew]]s and possibly more common in women.<ref name="pmid27184635"/> The annual incidence in North America is 0–20.2 new cases per 100,000 people, while incidence in Europe is 0.3–12.7 per 100,000. The prevalence of Crohn's disease is 322 per 100,000 in [[Germany]], 319 per 100,000 in [[Canada]],<ref name="pmid32242028"/> and 300 per 100,000 in the [[United States]].<ref name="pmid37223580">{{cite journal | vauthors = Weisman MH, Stens O, Kim SH, Hou JK, Miller FW, Dillon CF | title = Inflammatory Bowel Disease Prevalence: Surveillance data from the U.S. National Health and Nutrition Examination Survey | journal = Preventive Medicine Reports | volume = 9 | issue = 33 | pages = 102173 | date = March 2023 | pmid = 37223580 | pmc = 10201824 | doi = 10.1016/j.pmedr.2023.102173 | doi-access = free}}</ref> The prevalence of Crohn's disease has risen in newly industrialized countries, with rates of 18.6 per 100,000 in [[Hong Kong]] and 3.9 per 100,000 in [[Taiwan]].<ref name="pmid32242028"/> |
|||
Crohn's cannot be cured by [[surgery]], as the disease eventually recurs, though it is used in the case of partial or full blockage of the intestine.<ref>{{cite journal |vauthors=Kristo I, Stift A, Bergmann M, Riss S |title=Surgical recurrence in Crohn's disease: Are we getting better? |journal=World Journal of Gastroenterology |volume=21 |issue=20 |pages=6097–100 |date=May 2015 |pmid=26034346 |pmc=4445088 |doi=10.3748/wjg.v21.i20.6097 |doi-access=free}}</ref> Surgery may also be required for complications such as obstructions, fistulas, or abscesses, or if the disease does not respond to drugs. After the first surgery, Crohn's usually comes back at the site where the diseased intestine was removed and the healthy ends were rejoined; it can also come back in other locations. After a resection, scar tissue builds up, which can cause [[stenosis|strictures]], which form when the intestines become too small to allow excrement to pass through easily, which can lead to a blockage. After the first resection, another resection may be necessary within five years.<ref>{{cite web |url=http://ibdcrohns.about.com/od/surgeryprocedures/a/resectioncrohns.htm |title=Resection Surgery for Crohn's Disease |publisher=[[About.com]] |access-date=February 14, 2008 |date=January 12, 2007 |vauthors=Tresca AJ |url-status=live |archive-url=https://web.archive.org/web/20071113142856/http://ibdcrohns.about.com/od/surgeryprocedures/a/resectioncrohns.htm |archive-date=November 13, 2007}}</ref> For patients with an obstruction due to a stricture, two options for treatment are [[strictureplasty]] and resection of that portion of bowel. There is no [[statistical significance]] between strictureplasty alone versus strictureplasty and resection in cases of duodenal involvement. In these cases, re-operation rates were 31% and 27%, respectively, indicating that strictureplasty is a safe and effective treatment for selected people with duodenal involvement.<ref name="pmid8918424">{{cite journal |vauthors=Ozuner G, Fazio VW, Lavery IC, Milsom JW, Strong SA |title=Reoperative rates for Crohn's disease following strictureplasty. Long-term analysis |journal=Diseases of the Colon and Rectum |volume=39 |issue=11 |pages=1199–203 |date=November 1996 |pmid=8918424 |doi=10.1007/BF02055108 |s2cid=33628350}}</ref> |
|||
The typical age of onset is between 20 and 30 years, with a smaller peak around 50 years, leading to a median onset age of 30.<ref name="pmid27184635"/> About 20 to 25% of patients presenting with [[inflammatory bowel disease]] are children under 18 years old, while 80% are adolescents. Additionally, the incidence of Crohn's disease in children is on the rise, with 2.5–11.4 new cases per 100,000 and a prevalence of 58 per 100,000.<ref name="pmid29487490">{{cite journal | vauthors = von Allmen D | title = ediatric Crohn's Disease | journal = Clinics in Colon and Rectal Surgery | volume = 31 | issue = 2 | pages = 80–88 | date = February 2018 | pmid = 29487490 | pmc = 5825885 | doi = 10.1055/s-0037-1609022 | doi-access = free}}</ref> |
|||
Postsurgical recurrence of Crohn's disease is relatively common. Crohn's lesions are nearly always found at the site of the resected bowel. The join (or [[Surgical anastomosis|anastomosis]]) after surgery may be inspected, usually during a colonoscopy, and disease activity graded. The "Rutgeerts score" is an endoscopic scoring system for postoperative disease recurrence in Crohn's disease. Postsurgical remission per the Rutgeerts score is graded as i0; while mild postsurgical recurrences are graded i1 and i2, and moderate to severe recurrences are graded i3 and i4.<ref name="pmid2394349">{{cite journal |vauthors=Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M |title=Predictability of the postoperative course of Crohn's disease |journal=Gastroenterology |volume=99 |issue=4 |pages=956–63 |date=October 1990 |pmid=2394349 |doi=10.1016/0016-5085(90)90613-6|doi-access=free}}</ref> Fewer lesions result in a lower grade. Based on the score, treatment plans can be designed to give the patient the best chance of managing the recurrence of the disease.<ref name="pmid24917974">{{cite journal |vauthors=Yamamoto T, Bamba T, Umegae S, Matsumoto K |title=The impact of early endoscopic lesions on the clinical course of patients following ileocolonic resection for Crohn's disease: A 5-year prospective cohort study |journal=United European Gastroenterology Journal |volume=1 |issue=4 |pages=294–8 |date=August 2013 |pmid=24917974 |pmc=4040796 |doi=10.1177/2050640613495197}}</ref> |
|||
== History == |
|||
[[Short bowel syndrome]] (SBS, also short gut syndrome or simply short gut) is caused by the surgical removal of part of the small intestine. It usually develops in those patients who have had half or more of their small intestines removed.<ref>{{cite web |title=Short Bowel Syndrome |website=[[National Institute of Diabetes and Digestive and Kidney Diseases]] (NIDDK) |date=July 2015 |url=https://www.niddk.nih.gov/health-information/digestive-diseases/short-bowel-syndrome |archive-url=https://web.archive.org/web/20191209002943/https://www.niddk.nih.gov/health-information/digestive-diseases/short-bowel-syndrome |archive-date=December 9, 2019 |url-status=dead |access-date=December 8, 2019}}</ref> [[Diarrhea]] is the main symptom, but others may include weight loss, cramping, bloating, and [[heartburn]]. Short bowel syndrome is treated with changes in diet, intravenous feeding, vitamin and mineral supplements, and treatment with medications. In some cases of SBS, [[intestinal transplant surgery]] may be considered; though the number of transplant centres offering this procedure is quite small and it comes with a high risk due to the chance of infection and rejection of the transplanted intestine.<ref>{{cite web |url=http://www.revolutionhealth.com/conditions/digestive/crohns-disease/surgery/intestinal-transplant |title=Intestinal transplant for Crohn's disease |vauthors=Rhodes M |date=October 24, 2006 |publisher=Everyday Health|access-date=March 22, 2009|url-status=live|archive-url=https://web.archive.org/web/20081008034121/http://www.revolutionhealth.com/conditions/digestive/crohns-disease/surgery/intestinal-transplant|archive-date=October 8, 2008}}</ref> |
|||
[[Giovanni Battista Morgagni]], often referred to as the father of [[anatomic pathology]], provided one of the earliest detailed accounts of the disease in his 1761 treatise, noting specific autopsy findings in a young patient who suffered from severe gastrointestinal symptoms.<ref name="pmid24094598">{{cite journal | vauthors = Mulder DJ, Noble AJ, Justinich CJ, Duffin JM | title = A tale of two diseases: the history of inflammatory bowel disease | journal = Journal of Crohn's and Colitis | volume = 8 | issue = 5 | pages = 341–348 | pmid = 24094598 | date = May 2014 | doi = 10.1016/j.crohns.2013.09.009 | doi-access = free }}</ref> |
|||
The first notable series of cases of Crohn's disease was reported by Polish surgeon [[Antoni Leśniowski]] in 1903,<ref name="pmid31701137">{{cite journal | vauthors = Van Hootegem P, Travis S | title = Is Crohn's Disease a Rightly Used Eponym? | journal = Journal of Crohn's and Colitis | volume = 14 | issue = 6 | pages = 867–871 | pmid = 31701137 | date = July 2020 | doi = 10.1093/ecco-jcc/jjz183 | doi-access = free }}</ref> followed by Scottish surgeon [[Thomas Kennedy Dalziel]] in 1913, who described nine patients exhibiting significant pathological features treated by [[bowel resection|surgical resection]]. However, the disease only gained widespread recognition with a landmark 1932 article by [[Burrill B. Crohn]], [[Leon Ginzburg]], and [[Gordon D. Oppenheimer]]. In this publication, they introduced the term "regional ileitis" based on their observations of chronic inflammation in the terminal [[ileum]] of 14 patients.<ref name="pmid24094598"/> |
|||
[[Bile acid diarrhea]] is another complication following surgery for Crohn's disease in which the [[Ileum|terminal ileum]] has been removed. This leads to the development of excessive watery diarrhea. It is usually thought to be due to an inability of the ileum to reabsorb bile acids after resection of the terminal ileum and was the first type of [[bile acid malabsorption]] recognized.<ref>{{cite journal |vauthors=Hofmann AF |title=The syndrome of ileal disease and the broken enterohepatic circulation: cholerheic enteropathy |journal=Gastroenterology |volume=52 |issue=4 |pages=752–7 |date=April 1967 |pmid=5337211 |doi=10.1016/S0016-5085(67)80140-9 |doi-access=free}}</ref> |
|||
Over the following decades, Crohn's disease was recognized as affecting various parts of the gastrointestinal tract, with reports of involvement from the [[esophagus]] to the [[large intestine|colon]]. This period also marked the identification of skip lesions—areas of healthy bowel between diseased sections—adding to the understanding of the disease's pathology. Public awareness of Crohn's disease increased significantly after [[President Eisenhower]] underwent surgery for the condition in 1956, which highlighted its impact on quality of life and encouraged discussions about the disease.<ref name="pmid24094598"/> |
|||
=== Microbiome modification === |
|||
The use of oral [[probiotic]] supplements to modify the composition and behaviour of the gastrointestinal [[microbiome]] has been researched to understand whether it may help to improve remission rate in people with Crohn's disease. However, only two [[controlled trials]] were available in 2020, with no clear overall evidence of higher remission nor lower adverse effects, in people with Crohn's disease receiving probiotic supplementation.<ref>{{Cite journal |last1=Limketkai |first1=Berkeley N |last2=Akobeng |first2=Anthony K |last3=Gordon |first3=Morris |last4=Adepoju |first4=Akinlolu Adedayo |date=2020-07-17 |editor-last=Cochrane Gut Group |title=Probiotics for induction of remission in Crohn's disease |journal=Cochrane Database of Systematic Reviews |language=en |volume=2020 |issue=7 |pages=CD006634 |doi=10.1002/14651858.CD006634.pub3 |pmc=7389339 |pmid=32678465}}</ref> |
|||
In 1960, [[ulcerative colitis]] and Crohn's colitis were officially classified as distinct diseases, despite lingering beliefs that Crohn's disease could not manifest in the colon. During this decade, advancements such as [[fiberscope|fiberoptic]] [[colonoscopy]] and the capability to perform [[biopsies]] significantly enhanced the diagnosis and management of Crohn's disease, facilitating improved visualization of the gastrointestinal tract and more accurate assessments of disease severity. Subsequent decades saw the testing of various medications for Crohn's disease in clinical trials, including the identification of [[methotrexate]]'s efficacy in 1989.<ref name="pmid24094598"/> |
|||
=== Mental health === |
|||
Crohn's may result in [[anxiety disorders|anxiety]] or [[mood disorders]], especially in young people who may have stunted growth or embarrassment from [[fecal incontinence]].<ref name="Szigethy">{{cite journal |vauthors=Szigethy E, McLafferty L, Goyal A |title=Inflammatory bowel disease |journal=Child and Adolescent Psychiatric Clinics of North America |volume=19 |issue=2 |pages=301–18, ix |date=April 2010 |pmid=20478501 |doi=10.1016/j.chc.2010.01.007 |url=http://gut.bmj.com/cgi/content/short/39/Suppl_1/A15 |type=Submitted manuscript |access-date=September 4, 2018 |archive-date=April 28, 2021 |archive-url=https://web.archive.org/web/20210428151606/https://gut.bmj.com/cgi/content/short/39/Suppl_1/A15 |url-status=live}}</ref> Counselling as well as [[antidepressant]] or [[anxiolytic]] medication may help some people manage.<ref name="Szigethy" /> |
|||
In the 1990s, the focus of treatment for Crohn's disease began to shift towards [[biologics|biologic therapies]], particularly [[TNF inhibitor|anti-TNF agents]]. Concurrently, [[Clinical nutrition|nutritional therapy]] gained prominence in managing pediatric cases and instances of malnutrition. The introduction of [[Magnetic resonance enterography|MRI enterography]] emerged as a safe and effective method for monitoring disease activity. This was further augmented by the FDA's approval of [[capsule endoscopy]] in 2001, which allowed for improved imaging of the small intestine. Since the inception of [[genome-wide association studies]] in 2005, several genetic markers associated with Crohn's disease have been identified, contributing to a deeper understanding of the condition.<ref name="pmid24094598"/> |
|||
{{as of|2017}} there is a small amount of research looking at [[Mindfulness#Therapy programs|mindfulness-based therapies]], hypnotherapy, and [[cognitive behavioural therapy]].<ref>{{cite journal |vauthors=Ballou S, Keefer L |title=Psychological Interventions for Irritable Bowel Syndrome and Inflammatory Bowel Diseases |journal=Clinical and Translational Gastroenterology |volume=8 |issue=1 |pages=e214 |date=January 2017 |pmid=28102860 |pmc=5288603 |doi=10.1038/ctg.2016.69}}</ref> A meta analysis of interventions to improve mood (including talking therapy, [[Antidepressant|antidepressants]], and exercise) in people with inflammatory bowel disease found that they reduced inflammatory markers such as [[C-reactive protein]] and [[faecal calprotectin]]. Psychological therapies reduced inflammation more than antidepressants or exercise.<ref>{{Cite journal |last1=Seaton |first1=Natasha |last2=Hudson |first2=Joanna |last3=Harding |first3=Sophie |last4=Norton |first4=Sam |last5=Mondelli |first5=Valeria |last6=Jones |first6=Annie S.K. |last7=Moss-Morris |first7=Rona |date=1 February 2024 |title=Do interventions for mood improve inflammatory biomarkers in inflammatory bowel disease?: a systematic review and meta-analysis |url=https://doi.org/10.1016/j.ebiom.2023.104910 |journal=eBioMedicine |volume=100 |pages=104910 |doi=10.1016/j.ebiom.2023.104910 |issn=2352-3964 |pmc=10878994 |pmid=38272759}}</ref><ref>{{Cite journal |date=17 July 2024 |title=Improving mood reduces inflammation in inflammatory bowel disease |url=https://evidence.nihr.ac.uk/alert/improving-mood-reduces-inflammation-in-inflammatory-bowel-disease/ |journal=NIHR Evidence|doi=10.3310/nihrevidence_63192 }}</ref> |
|||
Support organizations such as the [[Crohn's & Colitis Foundation]] have also emerged, providing resources and community for patients, helping to raise awareness and funding for research initiatives.<ref name="pmid24094598"/> Today, Crohn's disease continues to be a focus of extensive research, aiming to improve treatment outcomes and enhance the quality of life for those affected.<ref name="pmid24094598"/> |
|||
=== Alternative medicine === |
|||
It is common for people with Crohn's disease to try [[complementary or alternative medicine|complementary or alternative therapy]].<ref name="Caprilli_2006">{{cite journal |vauthors=Caprilli R, Gassull MA, Escher JC, Moser G, Munkholm P, Forbes A, Hommes DW, Lochs H, Angelucci E, Cocco A, Vucelic B, Hildebrand H, Kolacek S, Riis L, Lukas M, de Franchis R, Hamilton M, Jantschek G, Michetti P, O'Morain C, Anwar MM, Freitas JL, Mouzas IA, Baert F, Mitchell R, Hawkey CJ |collaboration=European Crohn's Colitis Organisation |title=European evidence based consensus on the diagnosis and management of Crohn's disease: special situations |journal=Gut |volume=55 |issue=Suppl 1 |pages=i36–58 |date=March 2006 |pmid=16481630 |pmc=1859996 |doi=10.1136/gut.2005.081950c |quote=the colitis activity index fell significantly in the treatment group compared to the sham acupuncture group. However, recruitment did not reach its target and the number of patients was small.}}</ref> These include diets, [[probiotics]], fish oil, and other [[Herbalism|herbal]] and nutritional supplements. |
|||
* [[Acupuncture]] is used to treat inflammatory bowel disease in [[China]], and is being used more frequently in [[Western world|Western society]].<ref>{{cite journal |vauthors=Joos S, Brinkhaus B, Maluche C, Maupai N, Kohnen R, Kraehmer N, Hahn EG, Schuppan D |title=Acupuncture and moxibustion in the treatment of active Crohn's disease: a randomized controlled study |journal=Digestion |volume=69 |issue=3 |pages=131–9 |year=2004 |pmid=15114043 |doi=10.1159/000078151 |s2cid=7852406}}</ref> At this time, evidence is insufficient to recommend the use of acupuncture.<ref name="Caprilli_2006" /> |
|||
* A 2006 survey in Germany found that about half of people with IBD used some form of alternative medicine, with the most common being [[homeopathy]], and a study in France found that about 30% used alternative medicine.<ref>{{cite journal |vauthors=Joos S |title=Review on efficacy and health services research studies of complementary and alternative medicine in inflammatory bowel disease |journal=Chinese Journal of Integrative Medicine |volume=17 |issue=6 |pages=403–9 |date=June 2011 |pmid=21660673 |doi=10.1007/s11655-011-0758-3 |s2cid=207298246}}</ref> [[Homeopathic preparations]] are not proven with this or any other condition,<ref>{{cite journal |vauthors=Smith K |title=Homeopathy is Unscientific and Unethical |journal=Bioethics |volume=26 |issue=9 |doi=10.1111/j.1467-8519.2011.01956.x |pages=508–512 |year=2012 |s2cid=143067523 |url=https://zenodo.org/record/1035885 |access-date=October 28, 2017 |archive-date=October 29, 2017 |archive-url=https://web.archive.org/web/20171029012949/https://zenodo.org/record/1035885 |url-status=live}}</ref><ref>{{cite book |vauthors=Ladyman J |chapter=Towards a Demarcation of Science from Pseudoscience |pages=45–59 |chapter-url=https://books.google.com/books?id=Pc4OAAAAQBAJ&pg=PA45 |veditors=Pigliucci M, Boudry M |year=2013 |title=Philosophy of Pseudoscience: Reconsidering the Demarcation Problem |publisher=[[University of Chicago Press]] |isbn=978-0-226-05182-6 |quote=Yet homeopathy is a paradigmatic example of pseudoscience. It is neither simply bad science nor science fraud, but rather profoundly departs from scientific method and theories while being described as scientific by some of its adherents (often sincerely).}}</ref><ref>{{cite book |doi=10.1007/978-1-4614-8541-4_2 |chapter=Science, Pseudoscience, and Not Science: How do They Differ? |chapter-url=https://books.google.com/books?id=7RIJAgAAQBAJ&pg=PA19 |title=Healthcare and Biomedical Technology in the 21st Century |year=2014 |vauthors=Baran GR, Kiani MF, Samuel SP |pages=19–57 |isbn=978-1-4614-8540-7 |quote=within the traditional medical community it is considered to be quackery}}</ref> with large-scale studies finding them to be no more effective than a [[placebo]].<ref name="pmid12492603">{{cite journal |vauthors=Ernst E |title=A systematic review of systematic reviews of homeopathy |journal=British Journal of Clinical Pharmacology |volume=54 |issue=6 |pages=577–82 |date=December 2002 |pmid=12492603 |pmc=1874503 |doi=10.1046/j.1365-2125.2002.01699.x |author-link = Edzard Ernst}}</ref><ref name="shang">{{cite journal |vauthors=Shang A, Huwiler-Müntener K, Nartey L, Jüni P, Dörig S, Sterne JA, Pewsner D, Egger M |title=Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo-controlled trials of homoeopathy and allopathy |journal=Lancet |volume=366 |issue=9487 |pages=726–32 |year=2005 |pmid=16125589 |doi=10.1016/S0140-6736(05)67177-2 |s2cid=17939264}}</ref><ref>{{cite web |date=February 22, 2010 |title=Evidence Check 2: Homeopathy - Science and Technology Committee |publisher=[[House of Commons of the United Kingdom|British House of Commons]] Science and Technology Committee |url=https://publications.parliament.uk/pa/cm200910/cmselect/cmsctech/45/4504.htm |access-date=April 5, 2014 |archive-date=September 19, 2015 |archive-url=https://web.archive.org/web/20150919021452/http://www.publications.parliament.uk/pa/cm200910/cmselect/cmsctech/45/4504.htm |url-status=live}}</ref> |
|||
* There are contradicting studies regarding the effect of [[medical cannabis]] on [[inflammatory bowel disease]],<ref name="Naftali2014">{{cite journal |vauthors=Naftali T, Mechulam R, Lev LB, Konikoff FM |title=Cannabis for inflammatory bowel disease |journal=Digestive Diseases |volume=32 |issue=4 |pages=468–74 |date=2014 |pmid=24969296 |doi=10.1159/000358155 |s2cid=25309621}}</ref> and its effects on management are uncertain.<ref>{{cite journal |vauthors=Kafil TS, Nguyen TM, MacDonald JK, Chande N |title=Cannabis for the treatment of Crohn's disease |journal=The Cochrane Database of Systematic Reviews |volume=11 |pages=CD012853 |date=November 2018 |issue=11 |pmid=30407616 |pmc=6517156 |doi=10.1002/14651858.CD012853.pub2}}</ref> |
|||
== |
=== Etymology === |
||
Crohn's disease is named after Dr. Burrill Crohn, though its eponymous association arose from complex circumstances. Initially, researchers Ginzburg and Oppenheimer identified a pattern of the disease and compiled 12 cases, all linked to surgeon A. A. Berg. However, Berg declined authorship due to his lack of prior involvement. Ginzburg and Oppenheimer then connected with Crohn, who received the manuscript, which was later published with his name listed first and two additional cases included.<ref name="pmid24094598"/> |
|||
Crohn's disease is a [[Chronic (medicine)|chronic]] condition for which there is no known cure. It is characterised by periods of improvement followed by episodes when symptoms flare up. With treatment, most people achieve a healthy weight, and the mortality rate for the disease is relatively low. It can vary from being benign to very severe, and people with CD could experience just one episode or have continuous symptoms. It usually reoccurs, although some people can remain disease-free for years or decades. Up to 80% of people with Crohn's disease are hospitalized at some point during the course of their disease, with the highest rate occurring in the first year after diagnosis.<ref name="ACG_Guideline">{{cite journal |vauthors=Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE |title=ACG Clinical Guideline: Management of Crohn's Disease in Adults |journal=The American Journal of Gastroenterology |volume=113 |issue=4 |pages=481–517 |date=April 2018 |pmid=29610508 |doi=10.1038/ajg.2018.27 |s2cid=4568430}}</ref> Most people with Crohn's live a normal lifespan.<ref>{{cite web |url=http://www.umm.edu/patiented/articles/who_gets_crohns_disease_000103_5.htm |title=Crohn's disease - Prognosis |publisher=[[University of Maryland Medical Center|University of Maryland Medical Centre]]|access-date=October 19, 2012|url-status=live|archive-url=https://web.archive.org/web/20120829165909/http://www.umm.edu/patiented/articles/who_gets_crohns_disease_000103_5.htm|archive-date=August 29, 2012}}</ref> However, Crohn's disease is associated with a small increase in risk of small bowel and colorectal carcinoma (bowel cancer).<ref>{{cite journal |vauthors=Canavan C, Abrams KR, Mayberry J |title=Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn's disease |journal=Alimentary Pharmacology & Therapeutics |volume=23 |issue=8 |pages=1097–104 |date=April 2006 |pmid=16611269 |doi=10.1111/j.1365-2036.2006.02854.x |s2cid=25193522 |doi-access=free}}</ref> |
|||
== Epidemiology == |
|||
The percentage of people with Crohn's disease has been determined in [[Norway]] and the [[United States]] and is similar at 6 to 7.1:100,000. The [[Crohn's & Colitis Foundation of America]] cites this number as approx 149:100,000; NIH cites 28 to 199 per 100,000.<ref name="Hiatt">{{cite journal |vauthors=Hiatt RA, Kaufman L |title=Epidemiology of inflammatory bowel disease in a defined northern California population |journal=The Western Journal of Medicine |volume=149 |issue=5 |pages=541–6 |date=November 1988 |pmid=3250100 |pmc=1026530}}</ref><ref>{{cite journal |vauthors=Moum B, Vatn MH, Ekbom A, Aadland E, Fausa O, Lygren I, Stray N, Sauar J, Schulz T |title=Incidence of Crohn's disease in four counties in southeastern Norway, 1990-93. A prospective population-based study. The Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists |journal=Scandinavian Journal of Gastroenterology |volume=31 |issue=4 |pages=355–61 |date=April 1996 |pmid=8726303 |doi=10.3109/00365529609006410}}</ref> Crohn's disease is more common in northern countries, and with higher rates still in the northern areas of these countries.<ref>{{cite journal |vauthors=Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, van Blankenstein M |title=Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD) |journal=Gut |volume=39 |issue=5 |pages=690–7 |date=November 1996 |pmid=9014768 |pmc=1383393 |doi=10.1136/gut.39.5.690}}</ref> The incidence of Crohn's disease is thought to be similar in [[Europe]] but lower in [[Asia]] and [[Africa]].<ref name="Hiatt" /> It also has a higher incidence in [[Ashkenazi Jews]]<ref name="Baumgart2012" /><ref>{{cite journal |vauthors=Yang H, McElree C, Roth MP, Shanahan F, Targan SR, Rotter JI |title=Familial empirical risks for inflammatory bowel disease: differences between Jews and non-Jews |journal=Gut |volume=34 |issue=4 |pages=517–24 |date=April 1993 |pmid=8491401 |pmc=1374314 |doi=10.1136/gut.34.4.517}}</ref> and smokers.<ref name="pmid19067428">{{cite journal |vauthors=Seksik P, Nion-Larmurier I, Sokol H, Beaugerie L, Cosnes J |title=Effects of light smoking consumption on the clinical course of Crohn's disease |journal=Inflammatory Bowel Diseases |volume=15 |issue=5 |pages=734–41 |date=May 2009 |pmid=19067428 |doi=10.1002/ibd.20828 |s2cid=10988974|doi-access=free}}</ref> |
|||
Crohn's disease begins most commonly in people in their teens and 20s, and people in their 50s through to their 70s.<ref name="Baumgart2012" /><ref name="emed" /><ref name="eMedicineHealth" /> It is rarely diagnosed in early childhood. It usually affects female children more severely than males.<ref>{{cite web |url=http://www.ccfa.org/reuters/ibdboysgirls |title=Crohn's disease manifests differently in boys and girls |publisher=[[Crohn's and Colitis Foundation of America]] |url-status=dead |archive-url=https://web.archive.org/web/20080216141623/http://www.ccfa.org/reuters/ibdboysgirls |archive-date=February 16, 2008}}</ref> However, only slightly more women than men have Crohn's disease.<ref>{{cite web |url=http://www.webmd.com/hw-popup/who-is-affected-by-crohns-disease |title=Who is affected by Crohn's disease |publisher=Healthwise |url-status=dead |archive-url=https://web.archive.org/web/20090123043717/http://www.webmd.com/hw-popup/who-is-affected-by-crohns-disease |archive-date=January 23, 2009}}</ref> Parents, siblings or children of people with Crohn's disease are 3 to 20 times more likely to develop the disease.<ref>{{cite journal |vauthors=Satsangi J, Jewell DP, Bell JI |title=The genetics of inflammatory bowel disease |journal=Gut |volume=40 |issue=5 |pages=572–4 |date=May 1997 |pmid=9203931 |pmc=1027155 |doi=10.1136/gut.40.5.572}}</ref> Twin studies find that if one has the disease there is a 55% chance the other will too.<ref>{{cite journal |vauthors=Tysk C, Lindberg E, Järnerot G, Flodérus-Myrhed B |title=Ulcerative colitis and Crohn's disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking |journal=Gut |volume=29 |issue=7 |pages=990–6 |date=July 1988 |pmid=3396969 |pmc=1433769 |doi=10.1136/gut.29.7.990}}</ref> |
|||
The incidence of Crohn's disease is increasing in Europe<ref name="BurischJess2013">{{cite journal |vauthors=Burisch J, Jess T, Martinato M, Lakatos PL |title=The burden of inflammatory bowel disease in Europe |journal=Journal of Crohn's & Colitis |volume=7 |issue=4 |pages=322–37 |date=May 2013 |pmid=23395397 |doi=10.1016/j.crohns.2013.01.010 |doi-access=free}}</ref> and in newly industrialised countries.<ref name="Ng2017">{{cite journal |vauthors=Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JC, Chan FK, Sung JJ, Kaplan GG |title=Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies |journal=Lancet |volume=390 |issue=10114 |pages=2769–2778 |date=December 2018 |pmid=29050646 |doi=10.1016/S0140-6736(17)32448-0 |s2cid=32940 |doi-access=free}}</ref> For example, in Brazil, there has been an annual increase of 11% in the incidence of Crohn's disease since 1990.<ref name="Ng2017" /> |
|||
== History == |
|||
{{main|List of people diagnosed with Crohn's disease}} |
|||
Inflammatory bowel diseases were described by [[Giovanni Battista Morgagni]] (1682–1771) and by Scottish physician [[Thomas Kennedy Dalziel]] in 1913.<ref>{{cite journal |vauthors=Kirsner JB |title=Historical aspects of inflammatory bowel disease |journal=Journal of Clinical Gastroenterology |volume=10 |issue=3 |pages=286–97 |date=June 1988 |pmid=2980764 |doi=10.1097/00004836-198806000-00012}}</ref> |
|||
Originally, the disease was referred to as "regional ileitis," reflecting the findings of the time, but subsequent reports revealed its presence throughout the gastrointestinal tract, leading to the adoption of the eponym.<ref name="pmid24094598"/> In Poland, it was historically called “Lesniowski-Crohn's disease.”<ref name="pmid31701137"/> There has been growing criticism of medical eponyms for their inaccuracies, prompting a movement towards using non-possessive forms, such as "Crohn disease," which has gained traction in recent years among academic and medical publications.<ref name="pmid24094598"/> |
|||
''Ileitis terminalis'' was first described by Polish surgeon [[Antoni Leśniowski]] in 1904, although it was not conclusively distinguished from intestinal tuberculosis.<ref>{{cite journal |vauthors=Lichtarowicz AM, Mayberry JF |title=Antoni Lésniowski and his contribution to regional enteritis (Crohn's disease) |journal=Journal of the Royal Society of Medicine |volume=81 |issue=8 |pages=468–70 |date=August 1988 |pmid=3047387 |pmc=1291720 |doi=10.1177/014107688808100817}}</ref> In Poland, it is still called Leśniowski-Crohn's disease ({{lang-pl|choroba Leśniowskiego-Crohna}}). [[Burrill Bernard Crohn]], an American gastroenterologist at [[New York City]]'s [[Mount Sinai Hospital, New York|Mount Sinai Hospital]], described fourteen cases in 1932, and submitted them to the [[American Medical Association]] under the rubric of "Terminal ileitis: A new clinical entity". Later that year, he, along with colleagues [[Leon Ginzburg]] and Gordon Oppenheimer, published the case series "Regional ileitis: a pathologic and clinical entity". However, due to the precedence of Crohn's name in the alphabet, it later became known in the worldwide literature as Crohn's disease.<ref name="CrohnBB" /> |
|||
== References == |
== References == |
||
Latest revision as of 20:18, 26 December 2024
Crohn's disease is a chronic inflammatory bowel disease characterized by recurrent episodes of intestinal inflammation, primarily manifesting as diarrhea and abdominal pain. Unlike ulcerative colitis, inflammation can occur anywhere in the gastrointestinal tract, though it most frequently affects the ileum and colon, involving all layers of the intestinal wall. Symptoms may be non-specific and progress gradually, often delaying diagnosis. About one-third of patients have colonic disease, another third have ileocolic disease, and the remaining third have isolated ileal disease. Systemic symptoms such as chronic fatigue, weight loss, and low-grade fevers are common. Organs such as the skin and joints can also be affected. Complications can include bowel obstructions, fistulas, nutrition problems, and an increased risk of intestinal cancers.[1]
Crohn's disease is influenced by genetic, environmental, and immunological factors. Smoking is a major modifiable risk factor, especially in Western countries, where it doubles the likelihood of developing the disease. Dietary shifts from high-fiber to processed foods may reduce microbiota diversity and increase risk, while high-fiber diets can offer some protection. Genetic predisposition plays a significant role, with first-degree relatives facing a five-fold increased risk, particularly due to mutations in genes like NOD2 that affect immune response. The condition results from a dysregulated immune response to gut bacteria and increased intestinal permeability, alongside changes in the gut microbiome.[1]
Diagnosing Crohn's disease can be complex due to symptom overlap with other gastrointestinal disorders. It typically involves a combination of clinical history, physical examination, and various diagnostic tests. Key methods include ileocolonoscopy, which identifies the disease in about 90% of cases, and imaging techniques like CT and MRI enterography, which help assess the extent of the disease and its complications. Histological examination of biopsy samples is the most reliable method for confirming diagnosis.[1]
Management of Crohn's disease is individualized, focusing on disease severity and location to achieve mucosal healing and improve long-term outcomes. Treatment may include corticosteroids for quick symptom relief, immunosuppressants for maintaining remission, and biologics like anti-TNF therapies, which are effective for both induction and maintenance. Surgery may be necessary for complications such as blockages. Despite ongoing treatment, Crohn's disease is a chronic condition with no cure, often leading to a higher risk of related health issues and reduced life expectancy.[1]
The disease is most prevalent in North America and Western Europe, particularly among Ashkenazi Jews, with prevalence rates of 322 per 100,000 in Germany, 319 in Canada,[1] and 300 in the United States.[5] There is also a rising prevalence in newly industrialized countries, such as 18.6 per 100,000 in Hong Kong and 3.9 in Taiwan. The typical age of onset is between 20 and 30 years, with an increasing number of cases among children.[1]
Signs and symptoms
[edit]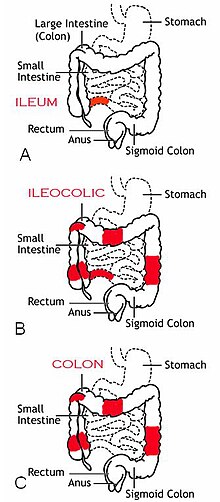
Crohn's disease is characterized by recurring flares of intestinal inflammation, with diarrhea and abdominal pain as the primary symptoms. Symptoms may be non-specific and progress gradually, and many people have symptoms for years before diagnosis. Unlike ulcerative colitis, inflammation can occur anywhere in the gastrointestinal tract, most often in the ileum and colon, and can involve all layers of the intestine. Disease location tends to be stable, with a third of patients having colonic disease, a third having ileocolic disease, and a third having ileal disease. The disease may also involve perianal, upper gastrointestinal, and extraintestinal organs.[1]
Gastrointestinal
[edit]- Diarrhea affects 82% of people at the onset of Crohn's disease, with severity ranging from mild to severe enough to require substitution of water and electrolytes. With Crohn's disease, diarrhea is frequent and urgent rather than voluminous.[6]
- Abdominal pain affects at least 70% of people during the course of Crohn's disease. It can result directly from intestinal inflammation, or from complications such as strictures and fistulas.[7] Pain most commonly occurs in the lower right abdomen.[8]
- Rectal bleeding is less common than in ulcerative colitis, and is more likely to occur with inflammation in the colon or rectum. Bleeding in the colon or rectum is bright red, whereas bleeding in higher segments causes dark or black stools.[9]
- Bloating, flatus, and other symptoms of irritable bowel syndrome occur in 41% of people in remission.[10]
- Perianal involvement occurs in 18–43% of cases, more frequently if the colon and rectum are inflamed, and can cause fistulas, skin tags, hemorrhoids, fissures, ulcers, and strictures.[11]
- Upper gastrointestinal involvement is rare, occurring in 0.5-16% of cases, and may cause symptoms such as pain while swallowing, difficulty swallowing, vomiting, and nausea.[12]
Systemic
[edit]Crohn's disease often presents with systemic symptoms, including:
- Chronic fatigue, which lasts for at least 6 months and cannot be cured by rest, occurs in 80% of people with Crohn's disease, including 30% of people who are in remission.[13]
- Fevers, typically low-grade, are often reported as initial symptoms of Crohn's disease. High-grade fevers are often a result of abscesses.[14]
- Weight loss often occurs due to diarrhea and reduced appetite.[14]
Extraintestinal
[edit]Extraintestinal manifestations occur in 21–47% of cases, and include symptoms such as:[1]
- Mouth ulcers, such as canker sores.[15]
- Eye inflammation, such as uveitis, scleritis, and episcleritis.[1]
- Skin inflammation, such as erythema nodosum and pyoderma gangrenosum.[1]
- Blood conditions such as portal hypertension, thromboembolism, thrombosis, pulmonary embolism.[1]
- Joint inflammation such as arthritis, ankylosing spondylitis, sacroiliitis.[1]
- Respiratory conditions such as obstructive sleep apnea and chest infections.[1]
- Liver, bile duct, and gallbladder conditions such as primary sclerosing cholangitis and cirrhosis.[1]
- Mental disorders such as depression and anxiety.[16]
- Metabolic bone disease.[1]
Complications
[edit]
Bowel damage due to inflammation occurs in half of cases within 10 years of diagnosis, and can lead to stricturing or penetrating disease forms. This can cause complications such as:
- Bowel obstructions may occur due to strictures, particularly in the small intestine, and may require surgical removal of the affected segment (bowel resection) or surgical dilation (stricturoplasty).[2]
- Fistulas, openings in the gut, result from penetrating disease and can cause diarrhea, urinary tract infections, and stool leakage to the vagina or skin. It is treated by bowel resection or fistulotomy[2]
- Abscesses, infected pockets, can also result from penetrating disease, causing abdominal pain, fever, and chills. It may be treated by surgical drainage.[2]
Malnutrition occurs in 38.9% of people in remission and 82.8% of people with active disease due to malabsorption in the small intestine, reduced appetite, and drug interactions.[17] This can cause complications such as:
- Anemia occurs in 6–74% of cases as a result of iron deficiency and blood loss. It is treated by oral iron or intravenous iron depending on disease activity.[17]
- Vitamin D deficiency is prevalent and can cause osteoporosis, and is treated by oral supplementation.[17]
- Folic acid, vitamin B12, zinc, magnesium, and selenium deficiencies may also occur, and are treated through oral supplementation.[17]
- Impaired growth and nutritional deficiency occur in 65–85% of children with Crohn's disease.[18]
Intestinal cancers may develop as a result of prolonged or severe inflammation.[19] This includes:
- Colorectal cancer has a prevalence of 7% at 30 years after diagnosis and accounts for 15% of deaths in people with Crohn's. Risk is higher if the disease occurs in most of the colon. Endoscopic surveillance is performed to detect and remove polyps, while surgery is required for dysplasia beyond the mucosal surface.[19]
- Small bowel cancer has a prevalence of 1.6%, at least 12-times greater in people with Crohn's disease. Unlike colorectal cancer, endoscopic surveillance is ineffective and not recommended for small bowel cancer.[20]
Causes
[edit]Risk factors
[edit]Smoking is a major modifiable risk factor for Crohn's disease, particularly in Western countries, where it doubles the risk. This risk is higher in females and varies with age. Smoking is also linked to earlier disease onset, increased need for immunosuppression, more surgeries, and higher recurrence rates. Ethnic differences have been noted, with studies in Japan linking passive smoking to the disease.[1] Proposed mechanisms for smoking's effects include impaired autophagy, direct toxicity to immune cells, and changes in the microbiome.[3]
Diet may influence the development of Crohn's disease by affecting the gut microbiome. The shift from high-fiber, low-fat foods to processed foods reduces microbiota diversity, increasing the risk of Crohn's disease.[1] Conversely, high-fiber diets may reduce risk by up to 40%, likely due to the production of anti-inflammatory short-chain fatty acids from fiber metabolism by gut bacteria.[3] The Mediterranean diet is also linked to a lower risk of later-onset Crohn's disease. Since diet's effect on the microbiome is temporary, its role in gut dysbiosis is controversial.[1]
Childhood antibiotic exposure is linked to a higher risk of Crohn's disease due to changes in the intestinal microbiome, which shapes the immune system in early life. Other medications, like oral contraceptives, aspirin, and NSAIDs, may also increase risk by up to two-fold. Conversely, breastfeeding and statin use may reduce risk, though breastfeeding's effects are inconsistent. Early life factors such as mode of delivery, pet exposure, and infections—related to the hygiene hypothesis—also significantly influence risk, likely due to influences on the microbiome.[3]
Genetics
[edit]Genetics significantly influences the risk of Crohn's disease. First-degree relatives of affected individuals have a five-fold increased risk, while identical twins have a 38–50% risk if one twin is affected. Genome-wide association studies have identified around 200 loci linked to Crohn's, most found in non-coding regions that regulate gene expression and overlap with other immune-related conditions, such as ankylosing spondylitis and psoriasis.[3] While genetics can predict disease location, it does not determine complications like stricturing. A substantial portion of inherited risk is attributed to a few key polymorphisms.[1]
- NOD2 mutations are the primary genetic risk factor for ileal Crohn's disease, impairing the function of immune cells, particularly Paneth cells. These mutations are found in 10–27% of individuals with Crohn's disease, predominantly in Caucasian populations. Heterozygotes (one mutated copy) have a three-fold risk, while homozygotes (two copies) have a 20–40 fold risk.[21]
- ATG16L1 mutations impair autophagy and immune defense, and are more common in Caucasians.[1]
- IL23R mutations increase inflammatory signaling of the interleukin-23 pathway, and are more common in Caucasians.[1]
- TNFSF15 mutations are the primary genetic risk factor in Asian populations.[1]
- IL10RA mutations impair the anti-inflammatory signaling of interleukin-10, causing early-onset Crohn's disease with high penetrance.
Mechanism
[edit]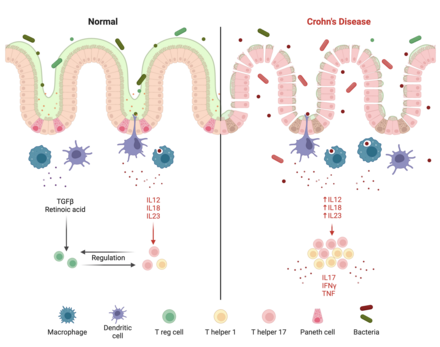
Crohn's disease is believed to be caused by a dysregulated immune response to gut bacteria, though the exact mechanism is unknown. This is evidenced by the disease's links to genes involved in bacteria defense and its occurrence in the ileum and colon, the most bacteria-dense segments of the intestine.[23] In Crohn's disease, a permeable intestinal barrier and a deficient innate immune response enable bacteria to enter intestinal tissue, causing an excessive inflammatory response from T helper 1 (Th1) and T helper 17 (Th17) cells. An altered microbiome may also be causatory and serve as the link to environmental factors.[3]
Intestinal barrier
[edit]The epithelial barrier is a single layer of epithelial cells covered in antimicrobial mucus that protects the intestine from gut bacteria.[23] Epithelial cells are joined by tight junction proteins, which are reduced by Crohn's-linked polymorphisms. In particular, claudin-5 and claudin-8 are reduced, while pore-forming claudin-2 is increased, causing intestinal permeability. Epithelial cells under stress emit inflammatory signals such as the unfolded protein response to stimulate the immune system, and Crohn's-linked polymorphisms to the ATG16L1 gene lower the threshold at which this response is triggered.[1]
In a functional state, the intestinal epithelium and IgA dimers work together to manage and keep the luminal microflora distinct from the mucosal immune system.[24] Paneth cells exist in the epithelial barrier of the small intestine and secrete α-defensins to prevent bacteria from entering gut tissue.[23] Genetic polymorphisms associated with Crohn's disease can impair this ability and lead to Crohn's disease in the ileum. NOD2 is a receptor produced by Paneth cells to sense bacteria, and mutations to NOD2 can inhibit the antimicrobial activity of Paneth cells. ATG16L1, IRGM, and LRRK2 are proteins involved in selective autophagy, the mechanism by which Paneth cells secrete α-defensins, and mutations to these genes also impair the antimicrobial activity of Paneth cells.[1]
Intraepithelial lymphocytes (IELs) are immune cells that exist in the epithelial barrier, consisting mostly of activated T cells. They interact with gut bacteria directly and emit signals to regulate the intestinal immune system. IELs in Crohn's disease produce increased levels of inflammatory cytokines IL-17, IFNγ, and TNF.[1] It is hypothesized that inflammatory signals from the immune system and alterations to the gut microbiome influence IELs to produce inflammatory signals, contributing to Crohn's disease.[25]
Immune system
[edit]Normally, intestinal macrophages have reduced inflammatory behavior while retaining their ability to consume and destroy pathogens. In Crohn's disease, the number and activity of macrophages is reduced, enabling the entrance of pathogens into intestinal tissue.[3] Macrophages degrade internal pathogens through autophagy, which is impaired by Crohn's-linked polymorphisms in genes such as NOD2 and ATG16L1.[1] Additionally, people with Crohn's tend to have a separate abnormal population of macrophages that secrete proinflammatory cytokines such as TNF and IL-6.[3]
Neutrophils are recruited from the bloodstream in response to inflammatory signals, and defend tissue by secreting antimicrobial substances and consuming pathogens.[23] In Crohn's disease, neutrophil recruitment is delayed and autophagy is impaired, allowing bacteria to survive in intestinal tissue.[3] Dysfunction in neutrophil secretion of reactive oxygen species, which are toxic to bacteria, is associated with very early onset Crohn's disease. Although neutrophils are important in bacterial defense, their subsequent accumulation in Crohn's disease damages the epithelial barrier and perpetuates inflammation.[1]
Innate lymphoid cells (ILCs) consist of subtypes including ILC1s, ILC2s, and ILC3s. ILC3s are particularly important for regenerating the epithelial barrier through secretion of IL-17 by NCR- ILC3s and IL-22 by NCR+ ILC3s. During Crohn's disease, inflammatory signals from antigen-presenting cells, such as IL-23, cause excessive IL-17 and IL-22 secretion. Although these cytokines protect the intestinal barrier, excessive production damages the barrier through increased inflammation and neutrophil recruitment. Additionally, IL-12 from activated dendritic cells influence NCR+ ILC3s to transform into inflammatory IFNγ-producing ILC1s.[26]
Naive T cells are activated primarily by dendritic cells, which then differentiate into anti-inflammatory T regulatory cells (Tregs) or inflammatory T helper cells to maintain balance. In Crohn's disease, macrophages and antigen-presenting cells secrete IL-12, IL-18, and IL-23 in response to pathogens, increasing Th1 and T17 differentiation and promoting inflammation via IL-17, IFNγ and TNF. IL-23 is particularly important, and IL-23 receptor polymorphisms that increase activity are linked with Crohn's disease. Tregs suppress inflammation via IL-10, and mutations to IL-10 and its receptor cause very early onset Crohn's disease.[1]
Microbiome
[edit]People with Crohn's disease tend to have altered microbiomes, although no disease-specific microorganisms have been identified. An altered microbiome may link environmental factors with Crohn's, though causality is uncertain. Firmicutes tend to be reduced, particularly Faecalibacterium prausnitzii, which produces short-chain fatty acids that reduce inflammation. Bacteroidetes and proteobacteria tend to be increased, particularly adherent-invasive E. coli, which attaches to intestinal epithelial cells. Additionally, mucolytic and sulfate-reducing bacteria are elevated, contributing to damage to the intestinal barrier.[3]
Alterations in gut viral and fungal communities may contribute to Crohn's disease. Caudovirales bacteriophage sequences found in children with Crohn's suggest a potential biomarker for early-onset disease. A meta-analysis showed lower viral diversity in Crohn's patients compared to healthy individuals, with increased Synechococcus phage S CBS1 and Retroviridae viruses. Additionally, a Japanese study found that the fungal microbiota in Crohn's patients differs significantly from that of healthy individuals, particularly with an abundance of Candida.[1]
Diagnosis
[edit]Diagnosis of Crohn's disease may be challenging since its symptoms overlap with other gastrointestinal diseases. An accurate diagnosis requires a combined assessment of clinical history, physical examination, and diagnostic tests.
Endoscopy
[edit]

Ileocolonoscopy is the primary procedure for diagnosing Crohn's disease in the ileum and colon, accurately identifying it in about 90% of cases.[27] During this exam, doctors closely examine the intestinal lining and take small tissue samples for further testing. Signs of Crohn's disease include uneven inflammation and 'skip lesions', which are patches of inflammation separated by healthy tissue. The ulcers can be small (less than 5 mm) or larger (over 5 mm), often appearing cobblestone-like. Their depth helps determine disease severity. Unlike ulcerative colitis, Crohn's disease usually does not affect the rectum or cause continuous inflammation around the bowel.[1]
In certain cases, such as disease in the upper small bowel, standard colonoscopy may be ineffective. Physicians may then opt for device-assisted enteroscopy or capsule endoscopy. While capsule endoscopy is effective in detecting abnormalities, it may not reliably diagnose Crohn's disease and carries a risk of retention, which is about 1.6% when Crohn's disease is suspected and increases to 13% if already diagnosed. To reduce this risk, physicians typically perform small-bowel imaging and use a patency capsule that disintegrates within 48 to 72 hours. Once the patency capsule has passed through the intestine, capsule endoscopy may be performed.[2]
Device-assisted enteroscopy is not typically the first choice for diagnosing small-bowel Crohn's disease due to its invasiveness and higher costs.[1] The procedure closely examines the small intestine using specialized tools, such as longer endoscopes or balloon-assisted devices, making it easier for doctors to visualize and treat issues.[28] It often requires sedation and is generally reserved for patients needing a tissue sample or immediate treatment.[1]
Cross-sectional Imaging
[edit]
Cross-sectional imaging techniques, like bowel ultrasonography (BUS), CT enterography (CTE), and MRI enterography (MRE), are essential for understanding how extensive Crohn's disease is and whether there are any complications, like blockages or abnormal connections between organs. All three methods are quite accurate for diagnosing Crohn's disease and spotting these complications.[1]
- CTE involves radiation and requires the use of contrast agents (substances that help show details in images). Despite this, CTE is very effective, with over 80% accuracy in diagnosing the disease and its complications, including blockages and fistulas (abnormal connections).[1]
- MRE is particularly good for detecting intestinal narrowing, with 89% sensitivity and 94% specificity. It is the preferred option for examining fistulas and abscesses in the pelvic area.[1]
- BUS is a non-invasive method that doesn't involve radiation and is effective for assessing the intestinal wall and related issues, such as fistulas and abscesses. Despite its limitations, it can accurately identify signs of Crohn's disease when the bowel wall thickens to more than 3 mm, achieving high accuracy rates (88–100%) when also considering factors like fistulas and abscesses.[1]
Histology
[edit]
The most reliable way to confirm a diagnosis of Crohn's disease is through a histological examination of biopsy samples or tissue removed during surgery. This process helps distinguish Crohn's disease from ulcerative colitis and other types of colitis, particularly infections. While no features are unique to Crohn's disease, typical signs include patchy chronic inflammation, irregularities in the intestinal lining, granulomas (not related to tissue injury), and abnormal villi structure in the terminal ileum. A pathologist specializing in inflammatory bowel disease is important for accurate Crohn's disease diagnoses. Even if biopsy results are unclear, doctors can still suggest a Crohn's disease diagnosis based on clinical symptoms, endoscopic findings, and imaging results.[1]
Disease activity indexes
[edit]The Crohn's Disease Activity Index (CDAI) is a scoring system to assess the symptoms associated with Crohn's disease. It assigns a score based on eight clinical factors, including overall well-being, frequency of loose stools, abdominal pain, presence of abdominal masses, changes in weight, low hemoglobin levels, and use of opiates for diarrhea. The CDAI is primarily used in clinical trials to evaluate the effectiveness of treatments and to determine whether the disease is in remission. This is particularly significant, as approximately 50% of patients who report feeling well may still exhibit signs of active disease in the intestine, while some patients with symptoms may present with normal intestinal findings.[1]
The Harvey–Bradshaw Index (HBI) provides a more streamlined approach by assessing only clinical factors, thus eliminating the need for laboratory tests. Neither the CDAI nor the HBI incorporates diagnostic procedures such as endoscopies or imaging studies; instead, they focus exclusively on symptom tracking. The HBI is generally considered easier to apply than the CDAI and may be more suitable for certain clinical trials and routine practice due to its simplicity in calculation and reduced reliance on patient recall of symptoms.[1]
The Crohn's Disease Endoscopic Index of Severity (CDEIS) is a scoring system used during endoscopy to evaluate Crohn's disease severity. It assesses six factors: deep and shallow ulcers, nonulcerated and ulcerated stenosis, the area covered by ulcers, and the overall disease-affected area across five intestinal sections. Scores range from 0 to 44, with higher scores indicating more severe disease. While often seen as the standard for measuring severity, CDEIS can be complex to calculate and may underestimate severity if only one segment, particularly the ileum, is affected. There are also no clear score cutoffs for specific outcomes or treatment responses, limiting its effectiveness in determining remission.[29]
The Simple Endoscopic Score for Crohn's Disease (SES-CD) offers a more straightforward approach than the CDEIS scoring system, using four key factors to evaluate Crohn's disease during an endoscopy. These factors include the presence and size of ulcers, the area affected by ulcers, the overall extent of the disease, and any narrowing of the intestine (stenosis). The first three factors are scored from 0 to 3 in each of the five sections of the intestine, with a maximum score of 15 for each section. Stenosis is scored separately, ranging from 0 to 11. This results in a total SES-CD score that can range from 0 to 56, with higher scores indicating more severe disease.[29]
Laboratory testing
[edit]While no lab test can definitively confirm or rule out Crohn's disease, results from serum and stool tests can help support the diagnosis:[2]
- The antimicrobial antibody ASCA is a well-known blood test marker used in the diagnosis of Crohn's disease. Approximately 60–70% of individuals with Crohn's disease test positive for ASCA, while only 10–15% of those with ulcerative colitis and less than 5% of patients with other types of colitis have positive results.[1]
- The autoantibody pANCA is found in 10–15% of Crohn's disease cases, in 60–70% of ulcerative colitis cases, and in less than 5% of patients with other types of colitis that aren't inflammatory bowel disease. Additionally, patients with Crohn's disease who test positive for pANCA often show symptoms similar to those of ulcerative colitis.[1]
- C-reactive protein (CRP) is a blood marker that indicates inflammation and can help monitor Crohn's disease activity. However, about one third of patients with active disease may have normal CRP levels, while one third with high levels of CRP have inactive disease. Moreover, CRP's ability to predict disease progression is not well established.[1]
- Fecal calprotectin is a stool test used to differentiate inflammatory bowel disease, like Crohn's disease, from irritable bowel syndrome. While there are no official cut-off values, it is commonly used to assess disease activity in Crohn's.[1] Elevated levels may also indicate other intestinal infections or inflammatory conditions, not just Crohn's disease.[2]
Differential diagnosis
[edit]Crohn's disease has similar endoscopic, radiographic and histological features with other inflammatory or infectious diseases. 10% of people with Crohn's disease are initially diagnosed with indeterminate colitis.[1]
- Behçet’s disease can cause intestinal inflammation, primarily featuring single ulcers and symptoms outside the intestines, which differ from Crohn's disease. Recurrent sores in the mouth and genitals raise suspicion for Behçet's. A pathergy test, where the skin is lightly pricked to check for a red bump or ulcer, can help confirm the diagnosis. Eye inflammation (uveitis) and skin issues are also common in Behçet's disease.[1]
- Intestinal lymphoma lacks symptoms that differentiate it from other conditions; diagnosis can only be confirmed through histological examination of tissue samples.[1]
- Intestinal tuberculosis can cause symptoms such as fever, night sweats, and ulcers in the transverse colon, along with a swollen ileocecal valve. Key tissue changes include granulomas, which may be caseating, confluent, or large. A positive smear test for acid-fast bacillus and imaging that detects necrotic lymph nodes are also important indicators of the disease.[1]
- Ischemic colitis is also a possible alternative diagnosis to Crohn's disease. It often shows swelling and redness of the inner lining of the colon, while the rectum usually remains unaffected.[1]
Classification
[edit]The Montreal classification system is a widely used framework for categorizing the phenotypes of Crohn's disease. It considers three primary factors: the age at diagnosis (divided into three groups: less than 16 years, 17 to 40 years, and over 40 years), the location of the disease (which can be ileal, colonic, ileocolonic, or isolated upper), and the behavior of the disease (including non-stricturing/non-penetrating, stricturing, penetrating, and perianal types).[30]
Management
[edit]The management of Crohn's disease is customized based on the severity, location, and behavior of the disease. Providers also assess the risk of aggressive disease to determine the need for more intensive treatment. Risk factors include diagnosis before age 30, extensive disease involvement, perianal complications, deep ulcers, and history of surgery. A key goal of treatment is to achieve mucosal healing, which restores the intestinal lining. Mucosal healing is linked to better outcomes, such as fewer flare-ups, reduced hospitalizations, steroid-free remission, and a longer interval without surgery.[1]
Corticosteroids
[edit]Steroids are often used to quickly induce remission and relieve symptoms in Crohn's disease, but they are ineffective for maintaining remission. Options include intravenous steroids, prednisone, and budesonide, with budesonide preferred for its safety, though it's limited to mild to moderate cases in the ileum and right colon. Patients on systemic steroids should switch to other medications for long-term remission, as prolonged use can cause adrenal issues, weight gain, cataracts, hypertension, and diabetes. Additionally, systemic steroids may increase the risk of serious infections and mortality in moderate to severe Crohn's disease.[29]
Conventional immunosuppressants
[edit]Thiopurines, like azathioprine and 6-mercaptopurine, maintain remission in Crohn's disease but do not induce it initially. Since thiopurines take 6 to 12 weeks to work, steroids are often used to manage symptoms during this time. Before starting thiopurines, liver metabolism is assessed and Epstein-Barr virus is tested in patients under 25. Around 15% to 20% of patients stop thiopurines due to side effects, including low blood cell counts, liver problems, nausea, vomiting, allergic reactions, and acute pancreatitis. Thiopurines also raise the risk of certain cancers and serious conditions, necessitating regular lab monitoring.[29]
Methotrexate is used to induce and maintain remission in Crohn's disease, being slightly more effective than thiopurines and taking 8 to 16 weeks to work. About 17% of patients stop taking it due to side effects like nausea, vomiting, headaches, and fatigue. It can affect liver health and, rarely, lower blood cell counts, requiring regular blood tests. Methotrexate may also cause anemia and mouth sores, so daily folic acid is recommended. Additionally, it may increase the risk of certain skin cancers and lymphoma. Methotrexate is discontinued during pregnancy due to the risks of miscarriage and birth defects.[29]
Biologics
[edit]Anti-TNF therapy is the most effective treatment for inducing and maintaining remission, with FDA-approved agents including infliximab, adalimumab, and certolizumab pegol.[29] It blocks the inflammatory protein TNF and induces cell death in activated T cells.[31] Responses may occur within a week, but full effects can take up to six weeks. Loss of response can happen due to the development of antidrug antibodies, necessitating a switch in agents or drug classes. Anti-TNF agents are often combined with thiopurines or methotrexate to minimize antibody development. Side effects include injection-site reactions, a higher risk of infection, a slight increase in melanoma risk, and rare cases of cytopenias and liver toxicity.[29]
Vedolizumab is the first treatment designed specifically for the gut in moderate to severe Crohn's disease. It blocks the molecule α4β7 that helps white blood cells enter the gut, reducing inflammation. Unlike natalizumab, it does not carry a risk of the serious brain infection PML. While vedolizumab can induce remission, it works slowly, taking about 12 weeks to show effects, and its overall effectiveness is limited. However, patients who respond well can maintain remission for up to a year. Since it specifically targets the gut, it does not significantly increase the risk of serious side effects or infections, except for mild nasal infections.[29]
Ustekinumab, approved for moderate to severe Crohn's disease in October 2016, has been FDA-approved for psoriasis since 2009.[32] It appears to be comparable to anti-TNF therapy in both the induction and maintenance of remission, functioning by blocking the inflammatory molecules IL-12 and IL-23. The onset of action is similar to that of anti-TNF treatments, with responses typically observed within six weeks. Notably, Ustekinumab does not seem to increase the risk of serious infections, although the studies conducted in Crohn's disease have been relatively short-term.[29]
The JAK inhibitor such as upadacitinib is approved for treatment of moderate to severe Crohn's disease, with a large multi-centre randomized control trial demonstrating its effectiveness in induction and maintenance of disease.[32][33]
Surgery
[edit]
Many individuals with Crohn's disease may require a bowel resection to remove part of the intestine due to blockages, lesions, infections, or ineffective medications. Since surgery is not a cure, the goal is to preserve as much of the small bowel as possible,[29] and extensive resections can lead to short bowel syndrome.[34] In cases with widespread strictures, only the most prominent stricture is typically resected, while minor strictures may be dilated through strictureplasty. After a resection, the healthy ends of the intestine are rejoined in a primary anastomosis.[29]
Approximately six to twelve months after surgery, patients usually undergo a colonoscopy to check for inflammation, using the Rutgeerts scoring system to assess the likelihood of recurrence. About 50% may experience a return of symptoms within five years, and nearly 40% may need a second surgery within ten years,[29] often due to inflammation near the anastomosis.[35] While drug therapy aims to prevent recurrences, its effectiveness remains uncertain.[29]
Diet
[edit]- Enteral nutrition, which administers nutrients as a powder or liquid, is the primary method for inducing remission in children with Crohn's disease. It can induce remission in up to 80% of cases. Outside of Japan, it is not used to treat Crohn's disease in adults due to unpalatable formulations.[36]
- Parenteral nutrition, which administers nutrients directly into the bloodstream, may be used to supply nutrients to patients when there is extensive inflammation that impairs absorption. Parenteral nutrition has the same effectiveness as enteral nutrition in inducing remission.[37]
- The Mediterranean diet, rich in fruits, vegetables, unsaturated fats, and lean protein, is beneficial to general health but has not been found to reduce the rate of flares in Crohn's disease.[38]
- Cooking and processing fruits and vegetables to reduce fibrousness increases tolerance of those foods in people with intestinal strictures.[38]
Other treatments
[edit]- Mesalamine is not effective for inducing or maintaining remission in Crohn's disease, but is nevertheless commonly used as a treatment due to its perceived benefits and low risk of side effects.[1]
- Antibiotics are not effective in inducing or maintaining remission in Crohn's disease. However, they are effective in treating perianal fistulas and inflammation when used alongside anti-TNF therapy.[1]
- Fecal microbiota transplants have shown potential effectiveness in preliminary studies. However, larger studies are needed to verify its efficacy.[39]
- Acupuncture influences the immune system by stimulating the vagus nerve. Early studies have shown efficacy in improving clinical symptoms, but larger studies are needed.[40]
- Cannabis is a potential therapy for Crohn's disease by targeting the endocannabinoid system. Although it has shown benefits in animal models, its efficacy as a treatment is uncertain.[41]
- Cognitive behavioral therapy has shown short-term positive psychological effects in people with Crohn's disease, but it has no long-term effect on physical or psychological health.[42]
Outlook
[edit]Crohn's disease is a chronic condition requiring ongoing management, as there is currently no cure. Inflammation is typically controlled through medications such as steroids and immunosuppressants, and in severe cases, surgery may be necessary. The clinical course of the disease is classified into four patterns:[43]
- Remission: Severity decreases in response to treatment, leading to sustained remission.[43]
- Improved and Stable: Severity lessens, but mild inflammation persists.[43]
- Relapsing: The disease fluctuates between periods of remission and severe inflammation.[43]
- Refractory: Severe inflammation continues without respite.[43]
Approximately 40% to 56% of individuals with Crohn's disease achieve clinical remission after one year of infliximab treatment, increasing to 56% to 58% when combined with an immunosuppressant. Furthermore, 16% to 39% attain both clinical and endoscopic remission, showing no signs of inflammation in the intestine. Once in remission, individuals have an 80% chance of maintaining this state for the following year. Conversely, 10% to 15% of individuals may experience ongoing active disease without remission.[43]
Chronic inflammation from Crohn's disease increases the risk of heart problems, cancers, arthritis, osteoporosis (weakened bones), and mental health issues. Some medications can also raise the chances of infections and cancers. Because of these combined risks, people with Crohn's disease tend to have a shorter lifespan compared to those who are healthy. In Canada, studies show that women affected with Crohn's disease live about 7.7 years less than unaffected women, and affected men live about 7.7 years less than otherwise expected.[4]
Epidemiology
[edit]Crohn's disease is most prevalent in North America and Western Europe, particularly among Ashkenazi jews and possibly more common in women.[29] The annual incidence in North America is 0–20.2 new cases per 100,000 people, while incidence in Europe is 0.3–12.7 per 100,000. The prevalence of Crohn's disease is 322 per 100,000 in Germany, 319 per 100,000 in Canada,[1] and 300 per 100,000 in the United States.[5] The prevalence of Crohn's disease has risen in newly industrialized countries, with rates of 18.6 per 100,000 in Hong Kong and 3.9 per 100,000 in Taiwan.[1]
The typical age of onset is between 20 and 30 years, with a smaller peak around 50 years, leading to a median onset age of 30.[29] About 20 to 25% of patients presenting with inflammatory bowel disease are children under 18 years old, while 80% are adolescents. Additionally, the incidence of Crohn's disease in children is on the rise, with 2.5–11.4 new cases per 100,000 and a prevalence of 58 per 100,000.[44]
History
[edit]Giovanni Battista Morgagni, often referred to as the father of anatomic pathology, provided one of the earliest detailed accounts of the disease in his 1761 treatise, noting specific autopsy findings in a young patient who suffered from severe gastrointestinal symptoms.[45]
The first notable series of cases of Crohn's disease was reported by Polish surgeon Antoni Leśniowski in 1903,[46] followed by Scottish surgeon Thomas Kennedy Dalziel in 1913, who described nine patients exhibiting significant pathological features treated by surgical resection. However, the disease only gained widespread recognition with a landmark 1932 article by Burrill B. Crohn, Leon Ginzburg, and Gordon D. Oppenheimer. In this publication, they introduced the term "regional ileitis" based on their observations of chronic inflammation in the terminal ileum of 14 patients.[45]
Over the following decades, Crohn's disease was recognized as affecting various parts of the gastrointestinal tract, with reports of involvement from the esophagus to the colon. This period also marked the identification of skip lesions—areas of healthy bowel between diseased sections—adding to the understanding of the disease's pathology. Public awareness of Crohn's disease increased significantly after President Eisenhower underwent surgery for the condition in 1956, which highlighted its impact on quality of life and encouraged discussions about the disease.[45]
In 1960, ulcerative colitis and Crohn's colitis were officially classified as distinct diseases, despite lingering beliefs that Crohn's disease could not manifest in the colon. During this decade, advancements such as fiberoptic colonoscopy and the capability to perform biopsies significantly enhanced the diagnosis and management of Crohn's disease, facilitating improved visualization of the gastrointestinal tract and more accurate assessments of disease severity. Subsequent decades saw the testing of various medications for Crohn's disease in clinical trials, including the identification of methotrexate's efficacy in 1989.[45]
In the 1990s, the focus of treatment for Crohn's disease began to shift towards biologic therapies, particularly anti-TNF agents. Concurrently, nutritional therapy gained prominence in managing pediatric cases and instances of malnutrition. The introduction of MRI enterography emerged as a safe and effective method for monitoring disease activity. This was further augmented by the FDA's approval of capsule endoscopy in 2001, which allowed for improved imaging of the small intestine. Since the inception of genome-wide association studies in 2005, several genetic markers associated with Crohn's disease have been identified, contributing to a deeper understanding of the condition.[45]
Support organizations such as the Crohn's & Colitis Foundation have also emerged, providing resources and community for patients, helping to raise awareness and funding for research initiatives.[45] Today, Crohn's disease continues to be a focus of extensive research, aiming to improve treatment outcomes and enhance the quality of life for those affected.[45]
Etymology
[edit]Crohn's disease is named after Dr. Burrill Crohn, though its eponymous association arose from complex circumstances. Initially, researchers Ginzburg and Oppenheimer identified a pattern of the disease and compiled 12 cases, all linked to surgeon A. A. Berg. However, Berg declined authorship due to his lack of prior involvement. Ginzburg and Oppenheimer then connected with Crohn, who received the manuscript, which was later published with his name listed first and two additional cases included.[45]
Originally, the disease was referred to as "regional ileitis," reflecting the findings of the time, but subsequent reports revealed its presence throughout the gastrointestinal tract, leading to the adoption of the eponym.[45] In Poland, it was historically called “Lesniowski-Crohn's disease.”[46] There has been growing criticism of medical eponyms for their inaccuracies, prompting a movement towards using non-possessive forms, such as "Crohn disease," which has gained traction in recent years among academic and medical publications.[45]
References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk Roda G, Chien Ng S, Kotze PG, Argollo M, Panaccione R, Spinelli A, et al. (April 2020). "Crohn's Disease". Nature Reviews Disease Primers. 6 (1): 22. doi:10.1038/s41572-020-0156-2. PMID 32242028.
- ^ a b c d e f g Feuerstein JD, Cheifetz AS (June 2017). "Crohn Disease: Epidemiology, Diagnosis, and Management". Mayo Clinic Proceedings. 92 (7): 1088–1103. doi:10.1016/j.mayocp.2017.04.010. PMID 28601423.
- ^ a b c d e f g h i j k Ramos GP, Papadakis KA (January 2019). "Mechanisms of Disease: Inflammatory Bowel Diseases". Mayo Clinic Proceedings. 94 (1): 155–165. doi:10.1016/j.mayocp.2018.09.013. PMC 6386158. PMID 30611442.
- ^ a b Kuenzig ME, Manuel DG, Donelle J, Benchimol EI (November 2020). "Life expectancy and health-adjusted life expectancy in people with inflammatory bowel disease Factors". Canadian Medical Association Journal. 192 (45): E1394–E1402. doi:10.1503/cmaj.190976. PMC 7669301. PMID 33168761.
- ^ a b Weisman MH, Stens O, Kim SH, Hou JK, Miller FW, Dillon CF (March 2023). "Inflammatory Bowel Disease Prevalence: Surveillance data from the U.S. National Health and Nutrition Examination Survey". Preventive Medicine Reports. 9 (33): 102173. doi:10.1016/j.pmedr.2023.102173. PMC 10201824. PMID 37223580.
- ^ Wenzl HH (September 2012). "Diarrhea in chronic inflammatory bowel diseases". Gastroenterology Clinics of North America. 41 (3): 651–675. doi:10.1016/j.gtc.2012.06.006. PMID 22917170.
- ^ Coates MD, Clarke K, Williams E, Jeganathan N, Yadav S, Giampetro D, et al. (September 2023). "Abdominal Pain in Inflammatory Bowel Disease: An Evidence-Based, Multidisciplinary Review". Crohns Colitis 360. 5 (4): otad055. doi:10.1093/crocol/otad055. PMC 10588456. PMID 37867930.
- ^ "What I need to know about Crohn's Disease". www.niddk.nih.gov. Archived from the original on November 21, 2015. Retrieved December 11, 2015.
- ^ "Bleeding and Blood in the Stool". crohn's and colitis. Retrieved October 16, 2024.
- ^ Barros LL, Farias AQ, Rezaie A (August 2019). "Gastrointestinal motility and absorptive disorders in patients with inflammatory bowel diseases: Prevalence, diagnosis and treatment". World Journal of Gastroenterology. 25 (31): 4414–4426. doi:10.3748/wjg.v25.i31.4414. PMC 6710178. PMID 31496621.
- ^ Pogacnik JS, Salgado G (September 2019). "Perianal Crohn's Disease". Clinics in Colon and Rectal Surgery. 32 (5): 377–385. doi:10.1055/s-0039-1687834. PMC 6731113. PMID 31507348.
- ^ Laube R, Liu K, Schifter M, Yang JL, Suen MK, Leong RW (February 2018). "Oral and upper gastrointestinal Crohn's disease". Journal of Gastroenterology and Hepatology. 33 (2): 355–364. doi:10.1111/jgh.13866. ISSN 0815-9319. PMID 28708248.
- ^ Włodarczyk M, Makaro A, Prusisz M, Włodarczyk J, Nowocień M, Maryńczak K, et al. (August 2023). "The Role of Chronic Fatigue in Patients with Crohn's Disease". Life (Basel). 13 (8): 1692. Bibcode:2023Life...13.1692W. doi:10.3390/life13081692. PMC 10455565. PMID 37629549.
- ^ a b Ranasinghe IR, Tian C, Hsu R (2024). "Crohn Disease". StatPearls Publishing. PMID 28613792. Retrieved October 18, 2024.
- ^ Antonelli E, Bassotti G, Tramontana M, Hansel K, Stingeni L, Ardizzone S, et al. (January 2021). "Dermatological Manifestations in Inflammatory Bowel Diseases". J Clin Med. 10 (2): 364. doi:10.3390/jcm10020364. PMC 7835974. PMID 33477990.
- ^ Bisgaard TH, Allin KH, Keefer L, Ananthakrishnan AN, Jess T (March 2023). "Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment". Nature Reviews Gastroenterology & Hepatology. 19 (11): 717–726. doi:10.1038/s41575-022-00634-6. PMID 35732730.
- ^ a b c d Jabłońska B, Mrowiec S (April 2023). "Nutritional Status and Its Detection in Patients with Inflammatory Bowel Diseases". Nutrients. 15 (8): 1991. doi:10.3390/nu15081991. PMC 10143611. PMID 37111210.
- ^ Gasparetto M, Guariso G (October 2014). "Crohn's disease and growth deficiency in children and adolescents". World Journal of Gastroenterology. 20 (37): 13219–13233. doi:10.3748/wjg.v20.i37.13219. PMC 4188880. PMID 25309059.
- ^ a b Sato Y, Tsujinaka S, Miura T, Kitamura Y, Suzuki H, Shibata C (August 2023). "Inflammatory Bowel Disease and Colorectal Cancer: Epidemiology, Etiology, Surveillance, and Management". Cancers (Basel). 15 (16): 4154. doi:10.3390/cancers15164154. PMC 10452690. PMID 37627182.
- ^ Bhatt H, Mathis KL (March 2023). "Small Bowel Carcinoma in the Setting of Inflammatory Bowel Disease". Clinics in Colon and Rectal Surgery. 37 (1): 46–52. doi:10.1055/s-0043-1762929. PMC 10769580. PMID 38188070.
- ^ Kayali S, Fantasia S, Gaiani F, Cavallaro LG, de'Angelis GL, Laghi L (April 2024). "NOD2 and Crohn's Disease Clinical Practice: From Epidemiology to Diagnosis and Therapy, Rewired". Inflammatory Bowel Diseases. doi:10.1093/ibd/izae075. PMID 38582044.
- ^ Plitas G, Rudensky AY (September 2016). "Regulatory T Cells: Differentiation and Function". Cancer Immunology Research. 4 (9): 721–725. doi:10.1158/2326-6066.CIR-16-0193. PMC 5026325. PMID 27590281.
- ^ a b c d Mowat AM, Agace WW (October 2014). "Regional specialization within the intestinal immune system". Nature Reviews Immunology. 14 (10): 667–85. doi:10.1038/nri3738. PMID 25234148.
- ^ Torres J, Mehandru S, Colombel J, Peyrin-Biroulet L (2017). "Crohn's disease". The Lancet. 389 (10080): 1741–1755. doi:10.1016/S0140-6736(16)31711-1. PMID 27914655.
- ^ Hu MD, Edelblum KL (August 2017). "Sentinels at the frontline: the role of intraepithelial lymphocytes in inflammatory bowel disease". Current Pharmacology Reports. 3 (6): 321–334. doi:10.1007/s40495-017-0105-2. PMC 5724577. PMID 29242771.
- ^ Zeng B, Shi S, Ashworth G, Dong C, Liu J, Xing F (April 2019). "ILC3 function as a double-edged sword in inflammatory bowel diseases". Cell Death & Disease. 10 (4): 315. doi:10.1038/s41419-019-1540-2. PMC 6453898. PMID 30962426.
- ^ Passos MA, Chaves FC, Chaves-Junior N (2018). "The Importance of Colonoscopy in Inflammatory Bowel Diseases". Arquivos Brasileiros de Cirurgia Digestiva. 31 (2): e1374. doi:10.1590/0102-672020180001e1374. PMC 6044200. PMID 29972402.
- ^ Schneider M, Höllerich J, Beyna T (July 2019). "Device-assisted enteroscopy: A review of available techniques and upcoming new technologies". World Journal of Gastroenterology. 25 (27): 3538–3545. doi:10.3748/wjg.v25.i27.3538. PMC 6658397. PMID 31367155.
- ^ a b c d e f g h i j k l m n o Koutroumpakis E, Katsanos KH (May 2016). "Implementation of the simple endoscopic activity score in crohn's disease". Saudi Journal of Gastroenterology. 22 (3): 183–191. doi:10.4103/1319-3767.182455. PMC 4898086. PMID 31367155.
- ^ Cockburn E, Kamal S, Chan A, Rao V, Liu T, Huang JY, et al. (November 2023). "Crohn's disease: an update". Clinical Medicine (London). 23 (6): 549–557. doi:10.7861/clinmed.2023-0493. PMC 11298500. PMID 38065612.
- ^ Levin AD, Wildenberg ME, van den Brink GR (August 2016). "Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease". Journal of Crohn's and Colitis. 10 (8): 989–997. doi:10.1093/ecco-jcc/jjw053. PMC 5724577. PMID 29242771.
- ^ a b Gordon H, Minozzi S, Kopylov U, Verstockt B, Chaparro M, Buskens C, et al. (October 2024). "ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment". J Crohns Colitis. 18 (10): 1531–1555. doi:10.1093/ecco-jcc/jjae091. PMID 38877997.
- ^ Loftus EV, Panés J, Lacerda AP, Peyrin-Biroulet L, D'Haens G, Panaccione R, et al. (2023). "Upadacitinib Induction and Maintenance Therapy for Crohn's Disease". N Engl J Med. 388 (21): 1966–1980. doi:10.1056/NEJMoa2212728. hdl:2268/304716. PMID 37224198.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Review in: Ann Intern Med. 2023 Sep;176(9):JC103. doi: 10.7326/J23-0069 - ^ Aksan A, Farrag K, Blumenstein I, Schröder O, Dignass AU, Stein J (June 2021). "Chronic intestinal failure and short bowel syndrome in Crohn's disease". World Journal of Gastroenterology. 27 (24): 3440–3465. doi:10.3748/wjg.v27.i24.3440. PMC 8240052. PMID 34239262.
- ^ Lewis RT, Maron DJ (September 2010). "Efficacy and complications of surgery for Crohn's disease". Gastroenterology and Hepatology. 6 (9): 587–596. PMC 2976865. PMID 21088749.
- ^ Di Caro S, Fragkos KC, Keetarut K, Koo HF, Sebepos-Rogers G, Saravanapavan H, et al. (September 2019). "Enteral Nutrition in Adult Crohn's Disease: Toward a Paradigm Shift". Nutrients. 11 (4): 2222. doi:10.3390/nu11092222. PMC 6770416. PMID 31540038.
- ^ Comeche JM, Comino I, Altavilla C, Tuells J, Gutierrez-Hervas A, Caballero P (November 2019). "Parenteral Nutrition in Patients with Inflammatory Bowel Disease Systematic Review, Meta-Analysis and Meta-Regression". Nutrients. 11 (12): 2865. doi:10.3390/nu11122865. PMC 6950216. PMID 31766687.
- ^ a b Hashash JG, Elkins J, Lewis JD, Binion DG (January 2024). "AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients With Inflammatory Bowel Disease: Expert Review". Gastroenterology. 166 (3): 521–532. doi:10.1053/j.gastro.2023.11.303. PMID 38276922.
- ^ Fehily SR, Basnayake C, Wright EK, Kamm MA (July 2021). "Fecal microbiota transplantation therapy in Crohn's disease: Systematic review". Journal of Gastroenterology and Hepatology. 36 (10): 2672–2686. doi:10.1111/jgh.15598. hdl:11343/298722. PMID 34169565.
- ^ Song G, Fiocchi C, Achkar JP (June 2019). "Acupuncture in Inflammatory Bowel Disease". Inflammatory Bowel Diseases. 25 (7): 1129–1139. doi:10.1093/ibd/izy371. PMID 30535303.
- ^ Kafil TS, Nguyen TM, MacDonald JK, Chande N (November 2018). "Cannabis for the treatment of Crohn's disease". The Cochrane Database of Systematic Reviews. 11 (11): CD012853. doi:10.1002/14651858.CD012853.pub2. PMC 6517156. PMID 30407616.
- ^ Chen J, Chen X, Sun Y, Xie Y, Wang X, Li R, et al. (December 2021). "The physiological and psychological effects of cognitive behavior therapy on patients with inflammatory bowel disease before COVID-19: a systematic review". BMC Gastroenterology. 21 (1): 469. doi:10.1186/s12876-021-02003-0. PMC 8672154. PMID 34911469.
- ^ a b c d e f Cho CW, You MW, Oh CH, Lee CK, Moon SK (March 2022). "Long-term Disease Course of Crohn's Disease: Changes in Disease Location, Phenotype, Activities, and Predictive Factors". Gut and Liver. 16 (2): 157–170. doi:10.5009/gnl210118. PMC 8924800. PMID 34456186.
- ^ von Allmen D (February 2018). "ediatric Crohn's Disease". Clinics in Colon and Rectal Surgery. 31 (2): 80–88. doi:10.1055/s-0037-1609022. PMC 5825885. PMID 29487490.
- ^ a b c d e f g h i j Mulder DJ, Noble AJ, Justinich CJ, Duffin JM (May 2014). "A tale of two diseases: the history of inflammatory bowel disease". Journal of Crohn's and Colitis. 8 (5): 341–348. doi:10.1016/j.crohns.2013.09.009. PMID 24094598.
- ^ a b Van Hootegem P, Travis S (July 2020). "Is Crohn's Disease a Rightly Used Eponym?". Journal of Crohn's and Colitis. 14 (6): 867–871. doi:10.1093/ecco-jcc/jjz183. PMID 31701137.
Further reading
[edit]- Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE (April 2018). "ACG Clinical Guideline: Management of Crohn's Disease in Adults". Am. J. Gastroenterol. 113 (4): 481–517. doi:10.1038/ajg.2018.27. PMID 29610508.
External links
[edit]- "Crohn's disease". MedlinePlus. U.S. National Library of Medicine.
 Media related to Crohn's disease at Wikimedia Commons
Media related to Crohn's disease at Wikimedia Commons

