Gastroparesis: Difference between revisions
→Prokinetics: ce gloss, actually used as a rescue med and before procedures, could be noted... |
|||
| (36 intermediate revisions by 6 users not shown) | |||
| Line 32: | Line 32: | ||
==Signs and symptoms== |
==Signs and symptoms== |
||
Gastroparesis has been linked to [[vomiting]], [[bloating]], early [[satiety]], and [[epigastric pain]]. Symptoms of delayed gastric emptying tend to be exacerbated by eating, particularly after fatty foods and indigestible solids like salads and leafy vegetables.<ref name="Clinical Presentations of Gastroparesis">{{cite book |last1=Reddy |first1=Shilpa C. |last2=Wo |first2=John M. |editor1-last=Parkman |editor1-first=Henry P. |editor2-last=McCallum |editor2-first=Richard W. |title=Gastroparesis |date=August 24, 2011 |publisher=Humana Press |location=Totowa, NJ |isbn=978-1-60761-551-4 |pages=25–35 |url=https://link.springer.com/book/10.1007/978-1-60761-552-1 |access-date=20 November 2023 |chapter=Clinical Presentations of Gastroparesis|doi=10.1007/978-1-60761-552-1_3}}</ref> In general, [[nausea]] is the most commonly reported symptom, affecting up to 96% of gastroparesis patients. However, depending on the etiology, the predominant symptom reported can differ.<ref name="Bharadwaj Meka Tandon Rathur 2016 pp. 285–294">{{cite journal | last1=Bharadwaj | first1=Shishira | last2=Meka | first2=Krishna | last3=Tandon | first3=Parul | last4=Rathur | first4=Abdullah | last5=Rivas | first5=John M. | last6=Vallabh | first6=Hiren | last7=Jevenn | first7=Andrea | last8=Guirguis | first8=John | last9=Sunesara | first9=Imran | last10=Nischnick | first10=Amy | last11=Ukleja | first11=Andrew | title=Management of gastroparesis-associated malnutrition | journal=Journal of Digestive Diseases | publisher=Wiley | volume=17 | issue=5 | year=2016 | issn=1751-2972 | doi=10.1111/1751-2980.12344 | pages=285–294| pmid=27111029 | s2cid=26992080 |url=https://onlinelibrary.wiley.com/doi/10.1111/1751-2980.12344|access-date=20 November 2023 }}</ref> The severity of gastric emptying dysfunction does not correspond to the severity of symptoms.<ref name="Clinical Presentations of Gastroparesis"/> [[Heartburn]] and poor glycemic control may be the only symptoms of delayed gastric emptying in [[Diabetes|diabetic]] patients. Physical examination in patients with gastroparesis may be completely normal |
Gastroparesis has been linked to [[vomiting]], [[bloating]], early [[satiety]], and [[epigastric pain]]. Symptoms of delayed gastric emptying tend to be exacerbated by eating, particularly after fatty foods and indigestible solids like salads and leafy vegetables.<ref name="Clinical Presentations of Gastroparesis">{{cite book |last1=Reddy |first1=Shilpa C. |last2=Wo |first2=John M. |editor1-last=Parkman |editor1-first=Henry P. |editor2-last=McCallum |editor2-first=Richard W. |title=Gastroparesis |date=August 24, 2011 |publisher=Humana Press |location=Totowa, NJ |isbn=978-1-60761-551-4 |pages=25–35 |url=https://link.springer.com/book/10.1007/978-1-60761-552-1 |access-date=20 November 2023 |chapter=Clinical Presentations of Gastroparesis|doi=10.1007/978-1-60761-552-1_3}}</ref> In general, [[nausea]] is the most commonly reported symptom, affecting up to 96% of gastroparesis patients. However, depending on the etiology, the predominant symptom reported can differ.<ref name="Bharadwaj Meka Tandon Rathur 2016 pp. 285–294">{{cite journal | last1=Bharadwaj | first1=Shishira | last2=Meka | first2=Krishna | last3=Tandon | first3=Parul | last4=Rathur | first4=Abdullah | last5=Rivas | first5=John M. | last6=Vallabh | first6=Hiren | last7=Jevenn | first7=Andrea | last8=Guirguis | first8=John | last9=Sunesara | first9=Imran | last10=Nischnick | first10=Amy | last11=Ukleja | first11=Andrew | title=Management of gastroparesis-associated malnutrition | journal=Journal of Digestive Diseases | publisher=Wiley | volume=17 | issue=5 | year=2016 | issn=1751-2972 | doi=10.1111/1751-2980.12344 | pages=285–294| pmid=27111029 | s2cid=26992080 |url=https://onlinelibrary.wiley.com/doi/10.1111/1751-2980.12344|access-date=20 November 2023 }}</ref> The severity of gastric emptying dysfunction does not correspond to the severity of symptoms.<ref name="Clinical Presentations of Gastroparesis"/> [[Heartburn]] and poor glycemic control may be the only symptoms of delayed gastric emptying in [[Diabetes|diabetic]] patients. Physical examination in patients with gastroparesis may be completely normal or, in its more severe forms, [[dehydration]] and [[malnutrition]], as well as a [[succussion splash]], can be present.<ref name="Pace 2019 pp. 21–31">{{cite book | last=Pace | first=Laura A. | title=Gastroparesis | chapter=Etiology and Clinical Presentation of Gastroparesis | publisher=Springer International Publishing | publication-place=Cham | date=November 9, 2019 | isbn=978-3-030-28928-7 | doi=10.1007/978-3-030-28929-4_2 | pages=21–31| s2cid=209235384 |url=https://link.springer.com/book/10.1007/978-3-030-28929-4|access-date=20 November 2023}}</ref> |
||
Nausea in gastroparesis is usually [[postprandial]], but morning or persistent nausea may occur. Vomiting is characterized by [[retching]] and forceful evacuation of gastric contents from the [[stomach]] up to and out of the mouth. Some patients may experience retching without gastric contents being expelled.<ref name="Clinical Presentations of Gastroparesis"/> |
Nausea in gastroparesis is usually [[postprandial]], but morning or persistent nausea may occur. Vomiting is characterized by [[retching]] and forceful evacuation of gastric contents from the [[stomach]] up to and out of the mouth. Some patients may experience retching without gastric contents being expelled.<ref name="Clinical Presentations of Gastroparesis"/> |
||
| Line 53: | Line 53: | ||
Because of the debilitating symptoms, patients with gastroparesis are at risk of significant nutritional abnormalities. In one study, 305 patients with gastroparesis had their dietary intake and nutritional status evaluated, and the average caloric intake was 1168 kcal/day, which resulted in substantial nutritional deficiencies. Furthermore, 64% of gastroparesis patients consumed a calorie-deficient diet. Additionally, higher symptom scores were inversely proportional to caloric intake.<ref name="Parkman Yates Hasler Nguyan 2011 pp. 486–498.e7">{{cite journal | last1=Parkman | first1=Henry P. | last2=Yates | first2=Katherine P. | last3=Hasler | first3=William L. | last4=Nguyan | first4=Linda | last5=Pasricha | first5=Pankaj J. | last6=Snape | first6=William J. | last7=Farrugia | first7=Gianrico | last8=Calles | first8=Jorge | last9=Koch | first9=Kenneth L. | last10=Abell | first10=Thomas L. | last11=McCallum | first11=Richard W. | last12=Petito | first12=Dorothy | last13=Parrish | first13=Carol Rees | last14=Duffy | first14=Frank | last15=Lee | first15=Linda | last16=Unalp–Arida | first16=Aynur | last17=Tonascia | first17=James | last18=Hamilton | first18=Frank | title=Dietary Intake and Nutritional Deficiencies in Patients With Diabetic or Idiopathic Gastroparesis | journal=Gastroenterology | publisher=Elsevier BV | volume=141 | issue=2 | year=2011 | issn=0016-5085 | doi=10.1053/j.gastro.2011.04.045 | pages=486–498.e7| pmid=21684286 | pmc=3499101 }}</ref> Another study found that the severity of nutritional deficiencies was proportional to the duration of gastric emptying.<ref>{{cite journal |last1=Ogorek |first1=C P |last2=Davidson |first2=L |last3=Fisher |first3=R S |last4=Krevsky |first4=B |title=Idiopathic gastroparesis is associated with a multiplicity of severe dietary deficiencies |journal=The American Journal of Gastroenterology |date=1991 |volume=86 |issue=4 |pages=423–428 |pmid=2012043 |url=https://pubmed.ncbi.nlm.nih.gov/2012043/ |access-date=20 November 2023}}</ref> Minerals like [[iron]], [[fat-soluble vitamins]], [[thiamine]], and [[folate]] are commonly reported deficiencies. [[Iron deficiency]] is common in patients with gastroparesis.<ref name="Bharadwaj Meka Tandon Rathur 2016 pp. 285–294"/> |
Because of the debilitating symptoms, patients with gastroparesis are at risk of significant nutritional abnormalities. In one study, 305 patients with gastroparesis had their dietary intake and nutritional status evaluated, and the average caloric intake was 1168 kcal/day, which resulted in substantial nutritional deficiencies. Furthermore, 64% of gastroparesis patients consumed a calorie-deficient diet. Additionally, higher symptom scores were inversely proportional to caloric intake.<ref name="Parkman Yates Hasler Nguyan 2011 pp. 486–498.e7">{{cite journal | last1=Parkman | first1=Henry P. | last2=Yates | first2=Katherine P. | last3=Hasler | first3=William L. | last4=Nguyan | first4=Linda | last5=Pasricha | first5=Pankaj J. | last6=Snape | first6=William J. | last7=Farrugia | first7=Gianrico | last8=Calles | first8=Jorge | last9=Koch | first9=Kenneth L. | last10=Abell | first10=Thomas L. | last11=McCallum | first11=Richard W. | last12=Petito | first12=Dorothy | last13=Parrish | first13=Carol Rees | last14=Duffy | first14=Frank | last15=Lee | first15=Linda | last16=Unalp–Arida | first16=Aynur | last17=Tonascia | first17=James | last18=Hamilton | first18=Frank | title=Dietary Intake and Nutritional Deficiencies in Patients With Diabetic or Idiopathic Gastroparesis | journal=Gastroenterology | publisher=Elsevier BV | volume=141 | issue=2 | year=2011 | issn=0016-5085 | doi=10.1053/j.gastro.2011.04.045 | pages=486–498.e7| pmid=21684286 | pmc=3499101 }}</ref> Another study found that the severity of nutritional deficiencies was proportional to the duration of gastric emptying.<ref>{{cite journal |last1=Ogorek |first1=C P |last2=Davidson |first2=L |last3=Fisher |first3=R S |last4=Krevsky |first4=B |title=Idiopathic gastroparesis is associated with a multiplicity of severe dietary deficiencies |journal=The American Journal of Gastroenterology |date=1991 |volume=86 |issue=4 |pages=423–428 |pmid=2012043 |url=https://pubmed.ncbi.nlm.nih.gov/2012043/ |access-date=20 November 2023}}</ref> Minerals like [[iron]], [[fat-soluble vitamins]], [[thiamine]], and [[folate]] are commonly reported deficiencies. [[Iron deficiency]] is common in patients with gastroparesis.<ref name="Bharadwaj Meka Tandon Rathur 2016 pp. 285–294"/> |
||
Other complications include fluctuations in [[blood glucose]] due to unpredictable digestion times due to changes in rate and amount of food passing into the small bowel, a decrease in quality of life, since it can make keeping up with work and other responsibilities more difficult, and severe [[fatigue]] due to [[caloric deficit]].<ref name="mayoclinic.org">{{cite web |title=Gastroparesis Complications – Mayo Clinic |url=http://www.mayoclinic.org/diseases-conditions/gastroparesis/basics/complications/con-20023971 |website=[[Mayo Clinic]]}}</ref> |
Other complications include fluctuations in [[blood glucose]] due to unpredictable digestion times due to changes in rate and amount of food passing into the small bowel, a decrease in [[quality of life]], since it can make keeping up with work and other responsibilities more difficult, and severe [[fatigue]] due to [[caloric deficit]].<ref name="mayoclinic.org">{{cite web |title=Gastroparesis Complications – Mayo Clinic |url=http://www.mayoclinic.org/diseases-conditions/gastroparesis/basics/complications/con-20023971 |website=[[Mayo Clinic]]}}</ref> |
||
==Causes== |
==Causes== |
||
There are many possible etiologies of gastroparesis. Many cases are idiopathic. Others are secondary to chronic or systemic conditions. Gastroparesis may be also acquired after an infection |
There are many possible etiologies of gastroparesis. Many cases are idiopathic. Others are secondary to chronic or systemic conditions. Gastroparesis may be also acquired after an infection or trauma, or it may be iatrogenic. |
||
People with gastroparesis are disproportionately female. One possible explanation for this finding is that women have an inherently slower stomach emptying time than men.<ref>{{cite web|url=https://online.epocrates.com/noFrame/showPage.do?method=diseases&MonographId=642&ActiveSectionId=23 |title=Epocrates article, registration required |publisher=Online.epocrates.com |access-date=2012-10-09}}</ref> A hormonal link has been suggested, as gastroparesis symptoms tend to worsen the week before menstruation when progesterone levels are highest.<ref name="autogenerated1">{{cite web |url=http://www.oley.org/lifeline/gastro.html |title=Summary for Oley Foundation by R. W. McCallum, MD |publisher=Oley.org |access-date=2012-10-09 |url-status=dead |archive-url=https://web.archive.org/web/20051212053857/http://www.oley.org/lifeline/gastro.html |archive-date=2005-12-12 }}{{MEDRS|date=January 2018}}</ref> |
People with gastroparesis are disproportionately female. One possible explanation for this finding is that women have an inherently slower stomach emptying time than men.<ref>{{cite web|url=https://online.epocrates.com/noFrame/showPage.do?method=diseases&MonographId=642&ActiveSectionId=23 |title=Epocrates article, registration required |publisher=Online.epocrates.com |access-date=2012-10-09}}</ref> A hormonal link has been suggested, as gastroparesis symptoms tend to worsen the week before menstruation when progesterone levels are highest.<ref name="autogenerated1">{{cite web |url=http://www.oley.org/lifeline/gastro.html |title=Summary for Oley Foundation by R. W. McCallum, MD |publisher=Oley.org |access-date=2012-10-09 |url-status=dead |archive-url=https://web.archive.org/web/20051212053857/http://www.oley.org/lifeline/gastro.html |archive-date=2005-12-12 }}{{MEDRS|date=January 2018}}</ref> |
||
| Line 90: | Line 90: | ||
===Differential diagnosis=== |
===Differential diagnosis=== |
||
More than 30% of patients with severe gastroparesis symptoms also experience severe constipation.<ref name=":AGA23">{{cite journal | vauthors = Moshiree B, Drossman D, Shaukat A | title = AGA Clinical Practice Update on Evaluation and Management of Belching, Abdominal Bloating, and Distention: Expert Review | journal = Gastroenterology | volume = 165 | issue = 3 | pages = |
More than 30% of patients with severe gastroparesis symptoms also experience severe constipation.<ref name=":AGA23">{{cite journal | vauthors = Moshiree B, Drossman D, Shaukat A | title = AGA Clinical Practice Update on Evaluation and Management of Belching, Abdominal Bloating, and Distention: Expert Review | journal = Gastroenterology | volume = 165 | issue = 3 | pages = 791–800.e3 | date = September 2023 | pmid = 37452811 | doi = 10.1053/j.gastro.2023.04.039 | doi-access = free }}</ref> This may be linked to delayed small bowel and colon transit.<ref name=":AGA23"/> If symptoms are [[Disease#Refractory disease|refractory]] (especially in the setting of weight loss), or if an intestinal neuromyopathic disorder is suspected, then motility may be evaluated via antroduodenal manometry, wireless motility capsules, whole gut scintigraphy, or radiopaque markers.<ref name=":AGA23"/> Extragastric dysmotility may also be treated with prokinetic medications.<ref name=":AGA23"/> |
||
===Testing overview=== |
===Testing overview=== |
||
| Line 144: | Line 144: | ||
===Pharmacotherapy=== |
===Pharmacotherapy=== |
||
==== |
==== Dopamine antagonists ==== |
||
[[Dopamine receptor|D<sub>2</sub> receptor]] antagonists are used to treat gastroparesis by reversing [[dopamine]]'s inhibition of [[acetylcholine]] (ACh) release.<ref name=CamProk>{{cite journal | author = Camilleri M, Atieh J | title = New Developments in Prokinetic Therapy for Gastric Motility Disorders | journal = Front Pharmacol | volume = 12 | date = 2021 | pmid = 34504426 | pmc = 8421525 | doi=10.3389/fphar.2021.711500| doi-access = free }}</ref> This increases gastrointestinal contractility and resting tone,<ref>{{Cite web|url=https://www.drugs.com/monograph/metoclopramide-hydrochloride.html|title=Metochlopramide Hydrochloride|website=Monograph|publisher=The American Society of Health-System Pharmacists.|access-date=23 March 2016}}</ref> and improves motility in the stomach and proximal small bowel.<ref name=CamProk/> Dopamine antagonist action in the [[central nervous system]] (CNS) also prevents nausea and vomiting.<ref>{{Cite book|title=Pharmacology |edition=5th | vauthors = Rang HP, Dale MM, Ritter JM, Moore PK |publisher=Churchill Livingstone|year=2003|isbn=0-443-07145-4|location=Edinburgh}}{{page needed|date=January 2018}}</ref> |
|||
[[Domperidone]] may be accessed by the US FDA's [[expanded access|Expanded Access]] program.<ref name=CamProk/><ref name=CamHiACG/> It is dosed 10–20 mg three times daily and at bedtime.<ref name=CamProk/> But it is associated with an increased risk of cardiac dysrhythmias, hence its restricted availability.<ref name=CamProk/> It is contraindicated in patients with corrected [[QT interval]]s of >470 ms in males or >450 ms in females.<ref name=CamProk/> |
|||
Similarly, the dopamine receptor antagonist [[domperidone]] may be used as an antiemetic to treat gastroparesis, though it is unavailable in some countries and is subject to a regulatory scheme for access in the United States.<ref name=CamHiACG/> |
|||
[[Metoclopramide]] has more CNS adverse effects than domperidone and carries a US FDA [[boxed warning]] for [[tardive dyskinesia]], a potentially irreversible condition.<ref name=CamProk/> There are clinical guidelines specifically to mitigate this risk in its use,<ref name=CamHiACG>{{cite journal | author = Camilleri, Michael | title = Highlights From the New ACG Clinical Guideline for Gastroparesis | journal = Gastroenterol Hepatol (NY) | volume = 18 | issue = 10 | pages = 586–588 | date = Oct 2022 | pmid = 36397928 | pmc = 9666798 }}</ref> with doses limited to 5–10 mg orally before meals and at bedtime.<ref name=CamProk/> A liquid formulation is recommended, and a new intranasal formulation is also available.<ref name=CamProk/> |
|||
=====Prokinetics===== |
|||
| ⚫ | Among [[motilin]] agonists, [[erythromycin]] is well known to improve emptying of the stomach. Its prolonged use is limited by the development of [[tachyphylaxis]]; therapeutic effects may wane after a few weeks of consistent use.<ref name=CamHiACG/> [[ |
||
==== Other prokinetics ==== |
|||
| ⚫ | |||
| ⚫ | Among [[motilin]] agonists, [[erythromycin]] is well known to improve emptying of the stomach. Its prolonged use is limited by the development of [[tachyphylaxis]]; therapeutic effects may wane after a few weeks of consistent use.<ref name=CamHiACG/> [[Clarithromycin]] and [[azithromycin]], though less studied for this indication, share the same [[mechanism of action]] and (limited) utility.<ref name=CamProk/> Azithromycin has the fewest drug interactions of the three.{{cn|date=October 2024}} |
||
| ⚫ | [[Prucalopride]], a [[5-HT4 receptor|5-HT<sub>4</sub> receptor]] agonist, may also be tried, particularly if there is chronic constipation<ref name=":AGA23"/> (for which it is labeled in Europe and US).<ref name=CamProk/> Some evidence suggests it may improve symptoms and quality of life in gastroparesis.<ref name=CamProk/> |
||
[[Pyridostigmine]], an [[acetylcholinesterase inhibitor]], may facilitate emptying by increasing [[acetylcholine]] in the gastrointestinal neuromusculature.<ref name=CamHiACG/> |
|||
[[Pyridostigmine]], an [[acetylcholinesterase inhibitor]], may facilitate emptying by increasing ACh in the gastrointestinal neuromusculature.<ref name=CamHiACG/> It lacks rigorous clinical trials for this indication, but there is clinical experience with its [[off-label use]] as a prokinetic in several gastrointestinal motility disorders.<ref name=CamProk/> |
|||
| ⚫ | |||
| ⚫ | The antidepressant [[mirtazapine]] has proven effective in the treatment of gastroparesis unresponsive to conventional treatment.<ref name=pmid24914393>{{cite journal | vauthors = Kundu S, Rogal S, Alam A, Levinthal DJ | title = Rapid improvement in post-infectious gastroparesis symptoms with mirtazapine | journal = World Journal of Gastroenterology | volume = 20 | issue = 21 | pages = 6671–6674 | date = June 2014 | pmid = 24914393 | pmc = 4047357 | doi = 10.3748/wjg.v20.i21.6671 | doi-access = free }}</ref> This may be due to its antiemetic and appetite stimulant properties. Mirtazapine acts on the same [[serotonin]] receptor ([[5-HT3]]) as |
||
| ⚫ | |||
| ⚫ | A variety of other central neuromodulators, including (but not limited to) other antidepressants, may be tried for symptoms like bloating and distention (and not only in the setting of gastroparesis, but also for disorders of [[Gut–brain axis|gut–brain]] interaction, or DGBI).<ref name=":AGA23"/> They may reduce [[visceral pain]] or hypersensitivity, especially of a postprandial nature.<ref name=":AGA23"/> They may also be selected to address any possible |
||
| ⚫ | The antidepressant [[mirtazapine]] has proven effective in the treatment of gastroparesis unresponsive to conventional treatment.<ref name=pmid24914393>{{cite journal | vauthors = Kundu S, Rogal S, Alam A, Levinthal DJ | title = Rapid improvement in post-infectious gastroparesis symptoms with mirtazapine | journal = World Journal of Gastroenterology | volume = 20 | issue = 21 | pages = 6671–6674 | date = June 2014 | pmid = 24914393 | pmc = 4047357 | doi = 10.3748/wjg.v20.i21.6671 | doi-access = free }}</ref> This may be due to its antiemetic and appetite stimulant properties. Mirtazapine acts on the same [[serotonin]] receptor ([[5-HT3]]) as the popular antiemetic [[ondansetron]].<ref name=pmid16959934>{{cite journal | vauthors = Kim SW, Shin IS, Kim JM, Kang HC, Mun JU, Yang SJ, Yoon JS | title = Mirtazapine for severe gastroparesis unresponsive to conventional prokinetic treatment | journal = Psychosomatics | volume = 47 | issue = 5 | pages = 440–442 | year = 2006 | pmid = 16959934 | doi = 10.1176/appi.psy.47.5.440 | doi-access = free }}</ref> |
||
| ⚫ | A variety of other central neuromodulators, including (but not limited to) other antidepressants, may be tried for symptoms like bloating and distention (and not only in the setting of gastroparesis, but also for disorders of [[Gut–brain axis|gut–brain]] interaction, or DGBI).<ref name=":AGA23"/> They may reduce [[visceral pain]] or hypersensitivity, especially of a postprandial nature.<ref name=":AGA23"/> They may also be selected to address any possible psychiatric comorbidities.<ref name=":AGA23"/> |
||
====Other agents==== |
====Other agents==== |
||
[[Sildenafil]], which increases blood flow to the genital area in men, is being used by some practitioners to stimulate the gastrointestinal tract in cases of diabetic gastroparesis.<ref>{{cite journal | vauthors = Gottlieb S | title = Sildenafil may help diabetic patients | journal = BMJ | volume = 321 | issue = 7258 | pages = 401A | date = August 2000 | pmid = 10938040 | pmc = 1127789 }}</ref> |
[[Sildenafil]], which increases blood flow to the genital area in men, is being used by some practitioners to stimulate the gastrointestinal tract in cases of diabetic gastroparesis.<ref>{{cite journal | vauthors = Gottlieb S | title = Sildenafil may help diabetic patients | journal = BMJ | volume = 321 | issue = 7258 | pages = 401A | date = August 2000 | pmid = 10938040 | pmc = 1127789 }}</ref> |
||
=====Experimental agents===== |
|||
[[Camicinal]] is a [[motilin]] agonist being tested for the treatment of gastroparesis.{{cn|date=October 2024}} |
|||
====Deprescribing==== |
====Deprescribing==== |
||
Gastroparesis presents with symptoms like those of slow gastric emptying caused by some opioid medications, some antidepressants, some allergy medications, some weight loss medications, and some [[antihypertensive]]s. For patients |
Gastroparesis presents with symptoms like those of slow gastric emptying caused by some opioid medications, some antidepressants, some allergy medications, some weight loss medications, and some [[antihypertensive]]s. For gastroparesis patients, these medications may make the condition worse.<ref>{{Cite web|url=https://www.mayoclinic.org/diseases-conditions/gastroparesis/symptoms-causes/syc-20355787|title=Overview|website=[[Mayo Clinic]]}}</ref> |
||
====Drug development==== |
|||
[[Camicinal]] was an investigational motilin agonist that did not improve enteral feeding intolerance.<ref name=camicinalfail>{{cite journal | vauthors = Deane AM, Lamontagne F, Dukes GE, Neil D, Vasist L, Barton ME, Hacquoil K, Ou X, Richards D, Stelfox HT, Mehta S, Day AG, Chapman MJ, Heyland DK | title = Nutrition Adequacy Therapeutic Enhancement in the Critically Ill: A Randomized Double-Blind, Placebo-Controlled Trial of the Motilin Receptor Agonist Camicinal (GSK962040): The NUTRIATE Study | journal = Journal of Parenteral and Enteral Nutrition| volume = 42 | issue = 5 | pages = 949–459 | year = 2018 | pmid = 29957868 | doi = 10.1002/jpen.1038 | hdl = 11343/294086 }}</ref> |
|||
===Other therapeutic interventions=== |
===Other therapeutic interventions=== |
||
| Line 175: | Line 177: | ||
Medically refractory gastroparesis may also be treated with a pyloromyotomy, which widens the gastric outlet by cutting the circular pylorus muscle. This can be done laparoscopically or endoscopically (called G-POEM). Vertical [[sleeve gastrectomy]], a procedure in which a part or all of the affected portion of the stomach is removed, has been shown to have some success in the treatment of gastroparesis in obese patients, even curing it in some instances. Further studies have been recommended due to the limited sample size of previous studies.<ref>{{cite journal |doi=10.1089/bari.2015.0052 |title=The Effect of Sleeve Gastrectomy on Gastroparesis: A Short Clinical Review |journal=Bariatric Surgical Practice and Patient Care |volume=11 |issue=2 |pages=84–9 |year=2016 | vauthors = Samuel B, Atiemo K, Cohen P, Czerniach D, Kelly J, Perugini R }}</ref><ref>{{Cite conference | vauthors = Bagloo M, Besseler M, Ude A | title = Sleeve gastrectomy for the treatment of diabetic gastroparesis. | conference = Proceedings 12th world congress of endoscopic surgery | date = April 2010 | pages = 14–17)|url=https://www.sages.org/meetings/annual-meeting/abstracts-archive/sleeve-gastrectomy-for-the-treatment-of-diabetic-gastroparesis/ }}</ref> |
Medically refractory gastroparesis may also be treated with a pyloromyotomy, which widens the gastric outlet by cutting the circular pylorus muscle. This can be done laparoscopically or endoscopically (called G-POEM). Vertical [[sleeve gastrectomy]], a procedure in which a part or all of the affected portion of the stomach is removed, has been shown to have some success in the treatment of gastroparesis in obese patients, even curing it in some instances. Further studies have been recommended due to the limited sample size of previous studies.<ref>{{cite journal |doi=10.1089/bari.2015.0052 |title=The Effect of Sleeve Gastrectomy on Gastroparesis: A Short Clinical Review |journal=Bariatric Surgical Practice and Patient Care |volume=11 |issue=2 |pages=84–9 |year=2016 | vauthors = Samuel B, Atiemo K, Cohen P, Czerniach D, Kelly J, Perugini R }}</ref><ref>{{Cite conference | vauthors = Bagloo M, Besseler M, Ude A | title = Sleeve gastrectomy for the treatment of diabetic gastroparesis. | conference = Proceedings 12th world congress of endoscopic surgery | date = April 2010 | pages = 14–17)|url=https://www.sages.org/meetings/annual-meeting/abstracts-archive/sleeve-gastrectomy-for-the-treatment-of-diabetic-gastroparesis/ }}</ref> |
||
== |
==Prognosis== |
||
Long-term studies in gastroparesis patients show that it is not a benign disease and has significant [[morbidity]] and a poor prognosis due to the limited options for treatment.<ref name="Hyett Martinez Gill Mehra 2009 pp. 445–452">{{cite journal | last1=Hyett | first1=Brian | last2=Martinez | first2=Fernando J. | last3=Gill | first3=Brian M. | last4=Mehra | first4=Shilpa | last5=Lembo | first5=Anthony | last6=Kelly | first6=Ciaran P. | last7=Leffler | first7=Daniel A. | title=Delayed Radionucleotide Gastric Emptying Studies Predict Morbidity in Diabetics With Symptoms of Gastroparesis | journal=Gastroenterology | publisher=Elsevier BV | volume=137 | issue=2 | year=2009 | issn=0016-5085 | doi=10.1053/j.gastro.2009.04.055 | pages=445–452| pmid=19410575 }}</ref> The [[mortality rate]] is highest in patients with [[Decompensation|decompensated]] gastroparesis who are more likely to develop complications. For example, one study discovered that over a 6-year period, 7% of those with gastroparesis died, with 22% requiring long-term [[Enteral feeding|enteral]] or [[Parenteral nutrition|parenteral]] feeding. 26% of these patients failed to respond to medical treatment, and 6% had [[gastric electrical stimulation]]. The 10 patients who died succumbed to [[metabolic]] issues, [[Cardiovascular disease|cardiac]] complications, [[renal failure]], [[suicide]], and [[bowel ischemia]] caused by adhesions.<ref name="Soykan 1998 pp. 2398–2404">{{cite journal | last=Soykan | first=Irfan | title=Demography, Clinical Characteristics, Psychological and Abuse Profiles, Treatment, and Long-Term Follow-up of Patients with Gastroparesis | journal=Digestive Diseases and Sciences | publisher=Springer Science and Business Media LLC | volume=43 | issue=11 | year=1998 | issn=0163-2116 | doi=10.1023/a:1026665728213 | pages=2398–2404| pmid=9824125 | s2cid=7643584 }}</ref> |
Long-term studies in gastroparesis patients show that it is not a benign disease and has significant [[morbidity]] and a poor prognosis due to the limited options for treatment.<ref name="Hyett Martinez Gill Mehra 2009 pp. 445–452">{{cite journal | last1=Hyett | first1=Brian | last2=Martinez | first2=Fernando J. | last3=Gill | first3=Brian M. | last4=Mehra | first4=Shilpa | last5=Lembo | first5=Anthony | last6=Kelly | first6=Ciaran P. | last7=Leffler | first7=Daniel A. | title=Delayed Radionucleotide Gastric Emptying Studies Predict Morbidity in Diabetics With Symptoms of Gastroparesis | journal=Gastroenterology | publisher=Elsevier BV | volume=137 | issue=2 | year=2009 | issn=0016-5085 | doi=10.1053/j.gastro.2009.04.055 | pages=445–452| pmid=19410575 }}</ref> The [[mortality rate]] is highest in patients with [[Decompensation|decompensated]] gastroparesis who are more likely to develop complications. For example, one study discovered that over a 6-year period, 7% of those with gastroparesis died, with 22% requiring long-term [[Enteral feeding|enteral]] or [[Parenteral nutrition|parenteral]] feeding. 26% of these patients failed to respond to medical treatment, and 6% had [[gastric electrical stimulation]]. The 10 patients who died succumbed to [[metabolic]] issues, [[Cardiovascular disease|cardiac]] complications, [[renal failure]], [[suicide]], and [[bowel ischemia]] caused by adhesions.<ref name="Soykan 1998 pp. 2398–2404">{{cite journal | last=Soykan | first=Irfan | title=Demography, Clinical Characteristics, Psychological and Abuse Profiles, Treatment, and Long-Term Follow-up of Patients with Gastroparesis | journal=Digestive Diseases and Sciences | publisher=Springer Science and Business Media LLC | volume=43 | issue=11 | year=1998 | issn=0163-2116 | doi=10.1023/a:1026665728213 | pages=2398–2404| pmid=9824125 | s2cid=7643584 }}</ref> |
||
| Line 183: | Line 185: | ||
==Prevalence== |
==Prevalence== |
||
Post-infectious gastroparesis, which constitutes the majority of idiopathic gastroparesis cases, affects up to 4% of the |
Post-infectious gastroparesis, which constitutes the majority of idiopathic gastroparesis cases, affects up to 4% of the US population.{{Citation needed|reason=Hye-Kyung's paper in the citation list gives a prevalence of less than 40 in 100,000 for women and 10 in 100,000 for men|date=February 2023}} Women in their 20s and 30s seem to be susceptible. One study of 146 US gastroparesis patients found the mean age of patients was 34 years with 82% affected being women, while another study found the patients were young or middle aged and up to 90% were women.<ref name=Thorn2010/> |
||
There has only been one true epidemiological study of idiopathic gastroparesis which was completed by the Rochester Epidemiology Project.<ref name="Jung Choung Locke Schleck 2009" /> They looked at patients from 1996 to 2006 who were seeking medical attention instead of a random population sample and found that the prevalence of delayed gastric emptying was fourfold higher in women. It is difficult for medical professionals and researchers to collect enough data and provide accurate numbers since studying gastroparesis requires specialized laboratories and equipment.<ref>{{cite journal | vauthors = Bharucha AE | title = Epidemiology and natural history of gastroparesis | journal = Gastroenterology Clinics of North America | volume = 44 | issue = 1 | pages = 9–19 | date = March 2015 | pmid = 25667019 | pmc = 4323583 | doi = 10.1016/j.gtc.2014.11.002 }}</ref> |
There has only been one true epidemiological study of idiopathic gastroparesis which was completed by the Rochester Epidemiology Project.<ref name="Jung Choung Locke Schleck 2009" /> They looked at patients from 1996 to 2006 who were seeking medical attention instead of a random population sample and found that the prevalence of delayed gastric emptying was fourfold higher in women. It is difficult for medical professionals and researchers to collect enough data and provide accurate numbers since studying gastroparesis requires specialized laboratories and equipment.<ref>{{cite journal | vauthors = Bharucha AE | title = Epidemiology and natural history of gastroparesis | journal = Gastroenterology Clinics of North America | volume = 44 | issue = 1 | pages = 9–19 | date = March 2015 | pmid = 25667019 | pmc = 4323583 | doi = 10.1016/j.gtc.2014.11.002 }}</ref> |
||
| Line 239: | Line 241: | ||
[[Category:Congenital disorders of digestive system]] |
[[Category:Congenital disorders of digestive system]] |
||
[[Category:Gastrointestinal motility disorders]] |
|||
[[Category:Stomach disorders]] |
[[Category:Stomach disorders]] |
||
Latest revision as of 08:32, 3 December 2024
| Gastroparesis | |
|---|---|
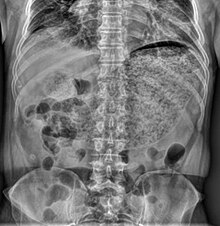 | |
| Simple abdominal X-ray shows gastric distension with a large amount of material in the stomach, suggesting severe gastric hypomotility | |
| Pronunciation |
|
| Specialty | Gastroenterology |
| Symptoms | Nausea, vomiting, abdominal pain, feeling full after consuming very little ("early satiety") |
| Complications | Malnutrition, fatigue, weight loss, vitamin deficiencies, intestinal obstruction due to bezoars, small intestinal bacterial overgrowth |
| Causes | Damage to the vagus nerve,[2] chemotherapy-induced neuropathy,[3] autonomic neuropathy[4] |
| Risk factors | Diabetes, abdominal or esophageal surgery, infection, certain medications that slow the rate of stomach emptying, scleroderma, nervous system diseases, hypothyroidism[2] |
| Diagnostic method | Barium swallow X-ray, barium beefsteak meal, radioisotope gastric-emptying scan (GES), wireless motility capsule (WMC), serial X-ray after ingesting radiopaque markers (ROM), gastric manometry, esophagogastroduodenoscopy (EGD), stable isotope breath test |
| Differential diagnosis | Peptic ulcer disease, gastric outlet obstruction, functional dyspepsia |
| Treatment | Dietary modifications, medications to stimulate gastric emptying, medications to reduce vomiting[5] gastric electrical stimulation[6] |
Gastroparesis (gastro- from Ancient Greek γαστήρ – gaster, "stomach"; and -paresis, πάρεσις – "partial paralysis") is a medical disorder of ineffective neuromuscular contractions (peristalsis) of the stomach, resulting in food and liquid remaining in the stomach for a prolonged period of time. Stomach contents thus exit more slowly into the duodenum of the digestive tract, a medical sign called delayed gastric emptying. The opposite of this, where stomach contents exit quickly into the duodenum, is called dumping syndrome.
Symptoms include nausea, vomiting, abdominal pain, feeling full soon after beginning to eat (early satiety), abdominal bloating, and heartburn. Many or most cases are idiopathic. The most common known cause is autonomic neuropathy of the vagus nerve, which innervates the stomach. Uncontrolled diabetes mellitus is a frequent cause of this nerve damage, but trauma to the vagus nerve is also possible. Some cases may be considered post-infectious.
Diagnosis is via one or more of the following: barium swallow X-ray, barium beefsteak meal, radioisotope gastric-emptying scan, gastric manometry, esophagogastroduodenoscopy (EGD), and a stable isotope breath test. Complications include malnutrition, fatigue, weight loss, vitamin deficiencies, intestinal obstruction due to bezoars, and small intestinal bacterial overgrowth. There may also be poor glycemic control and irregular absorption of nutrients, particularly in the setting of diabetes.[7][8]
Treatment includes dietary modification, medications to stimulate gastric emptying (including some prokinetic agents), medications to reduce vomiting (including some antiemetics), and surgical approaches.[5] Additionally, gastric electrical stimulation (GES; approved on a humanitarian device exemption) can be used as treatment.[6] Nutrition may be managed variously, ranging from oral dietary modification to jejunostomy feeding tube (if oral intake is inadequate).[6] A gastroparesis diagnosis is associated with poor outcomes, and survival is generally lower among patients than in the general population.[9]
Signs and symptoms
[edit]Gastroparesis has been linked to vomiting, bloating, early satiety, and epigastric pain. Symptoms of delayed gastric emptying tend to be exacerbated by eating, particularly after fatty foods and indigestible solids like salads and leafy vegetables.[10] In general, nausea is the most commonly reported symptom, affecting up to 96% of gastroparesis patients. However, depending on the etiology, the predominant symptom reported can differ.[11] The severity of gastric emptying dysfunction does not correspond to the severity of symptoms.[10] Heartburn and poor glycemic control may be the only symptoms of delayed gastric emptying in diabetic patients. Physical examination in patients with gastroparesis may be completely normal or, in its more severe forms, dehydration and malnutrition, as well as a succussion splash, can be present.[12]
Nausea in gastroparesis is usually postprandial, but morning or persistent nausea may occur. Vomiting is characterized by retching and forceful evacuation of gastric contents from the stomach up to and out of the mouth. Some patients may experience retching without gastric contents being expelled.[10]
Postprandial fullness is an unpleasant feeling of stomach fullness that occurs after eating. Patients might characterize postprandial fullness as a feeling of food remaining in the stomach for an extended period of time. Satiation is a lack of hunger after eating. It is the inverse of hunger and appetite. Early satiety is the disappearance of appetite before nutrient absorption during food ingestion. Early satiation may be described by patients with gastroparesis as a loss of appetite or disappearance of appetite while eating. Early satiety is the sensation of stomach fullness that occurs shortly after beginning to eat and is out of proportion to the meal.[10]
Bloating is a highly subjective feeling of increased abdominal pressure. Bloating without eating should be distinguished from postprandial fullness. It is sometimes, but not always, associated with food consumption.[10]
Abdominal discomfort or pain is common, affecting 90% of gastroparesis patients. Idiopathic gastroparesis patients may experience more abdominal pain than diabetic gastroparesis patients.[13] Physicians believe that postprandial epigastric pain is the most common symptom of gastroparesis.[14] Abdominal pain has a wide range of symptoms. Around 40% of patients have localized epigastric pain, but it can be diffuse in some cases. Pain is usually classified as postprandial, but it can also occur at night and interfere with sleep. The severity of abdominal pain is unrelated to the impairment of gastric emptying.[13]
Complications
[edit]Gastroparesis can lead to difficult glycemic control (which exacerbates gastric dysmotility), aspiration, bezoar formation, abnormalities in fluid and electrolyte balance, and inadequate nutrition intake resulting in weight loss.[15]
Some patients may experience severe nausea and vomiting, which can lead to dehydration, as evidenced by orthostatic hypotension as well as acute renal insufficiency. Some patients with severe gastroparesis lose a significant amount of weight and suffer from nutritional deficiencies, necessitating small bowel feeding access to bypass the stomach.[10]
Individuals with gastroparesis are also more likely to develop gastric bezoars. Bezoars are large masses of foreign substances and food that have become trapped in the GI tract, especially in the stomach.[11] The incidence of bezoar formation in gastroparesis patients has been estimated to be approximately six percent based on a barium study.[16]
There is a strong link between gastroparesis and the development of small intestinal bacterial overgrowth (SIBO). One study examined 50 gastroparesis patients using a glucose breath test and discovered that SIBO was present in 60% of their cohort. Furthermore, longer episodes of gastroparesis symptoms increase the risk of SIBO. Poor gastrointestinal motility (see enteric nervous system) and gastric acid production are believed to allow bacteria to colonize the small intestine. Furthermore, many individuals with gastroparesis are treated with acid-suppressive drugs, which significantly impair the GI tract's innate bactericidal activity. SIBO causes small bowel inflammation, impairing absorption and worsening nutritional deficiencies in gastroparesis.[17]
Because of the debilitating symptoms, patients with gastroparesis are at risk of significant nutritional abnormalities. In one study, 305 patients with gastroparesis had their dietary intake and nutritional status evaluated, and the average caloric intake was 1168 kcal/day, which resulted in substantial nutritional deficiencies. Furthermore, 64% of gastroparesis patients consumed a calorie-deficient diet. Additionally, higher symptom scores were inversely proportional to caloric intake.[18] Another study found that the severity of nutritional deficiencies was proportional to the duration of gastric emptying.[19] Minerals like iron, fat-soluble vitamins, thiamine, and folate are commonly reported deficiencies. Iron deficiency is common in patients with gastroparesis.[11]
Other complications include fluctuations in blood glucose due to unpredictable digestion times due to changes in rate and amount of food passing into the small bowel, a decrease in quality of life, since it can make keeping up with work and other responsibilities more difficult, and severe fatigue due to caloric deficit.[20]
Causes
[edit]There are many possible etiologies of gastroparesis. Many cases are idiopathic. Others are secondary to chronic or systemic conditions. Gastroparesis may be also acquired after an infection or trauma, or it may be iatrogenic.
People with gastroparesis are disproportionately female. One possible explanation for this finding is that women have an inherently slower stomach emptying time than men.[21] A hormonal link has been suggested, as gastroparesis symptoms tend to worsen the week before menstruation when progesterone levels are highest.[22]
Idiopathic
[edit]Idiopathic gastroparesis (gastroparesis with no known cause) accounts for a third (or more[citation needed]) of all chronic cases. It is thought that many of these cases are due to an autoimmune response, perhaps triggered by an acute viral infection.[2] Gastroenteritis, infectious mononucleosis, and other ailments have been anecdotally linked to the onset of the condition, but no systematic study has proven a link.[23] In cases of post-infectious gastroparesis, patients have symptoms and go undiagnosed for an average of 3 weeks to 6 months before their illness is identified correctly and treatment begins.[5]
Chronic conditions
[edit]It is frequently caused by autonomic neuropathy, occurring in about 30–50% of people with long-standing type 1 or type 2 diabetes.[4] In fact, diabetes mellitus has been named as the most common known cause of gastroparesis, as high levels of blood glucose may effect chemical changes in the nerves.[24] The vagus nerve becomes damaged by years of high blood glucose or insufficient transport of glucose into cells resulting in gastroparesis.[2] Adrenal and thyroid gland problems could also be a cause.[25]
Gastroparesis has also been associated with connective tissue diseases such as scleroderma and Ehlers–Danlos syndrome, and neurological conditions such as Parkinson's disease and multiple system atrophy.[26] It may occur as part of a mitochondrial disease. Chronic gastroparesis can be caused by other types of damage to the vagus nerve, such as abdominal surgery.[27]
Other causes
[edit]Transient gastroparesis may arise in acute illness of any kind, as a consequence of certain cancer treatments or other drugs which affect digestive action, or due to abnormal eating patterns. Heavy cigarette smoking is also a plausible cause since smoking causes damage to the stomach lining.[citation needed]
Patients with cancer may develop gastroparesis because of chemotherapy-induced peripheral neuropathy, immunosuppression followed by viral infections involving the GI tract, procedures such as celiac blocks, paraneoplastic neuropathy or myopathy, or after an allogeneic bone marrow transplant via graft-versus-host disease.[3]
An analysis by University Hospitals Cleveland Medical Center of records from the TriNetX database found that the number of patients diagnosed with gastroparesis after being prescribed a GLP-1 receptor agonist (0.1% of the patients) was 250% greater than the number of patients diagnosed with gastroparesis who did not take a GLP-1 medication (0.04%).[28]
Physiology and mechanism
[edit]The symptoms of gastroparesis are best understood in the context of the physiology of gastric emptying (GE). The stomach functions as a reservoir for food and nutritional content, which are broken down to produce chyme. Chyme is then released into the duodenum at a controlled rate to allow for maximum nutrient absorption. The controlled rate of chyme released is regulated by feedback mechanisms from the stomach and small intestines, which activate the vagus nerve and other hormones. The delay of any of the factors in gastric emptying causes disorganization or reduced frequency of antral contractions and thus delayed GE.[29]
On the molecular level, it is thought that gastroparesis can be caused by the loss of neuronal nitric oxide expression since the cells in the GI tract secrete nitric oxide. This important signaling molecule has various responsibilities in the GI tract and in muscles throughout the body. When nitric oxide levels are low, the smooth muscle and other organs may not be able to function properly.[30] Other important components of the stomach are the interstitial cells of Cajal (ICC) which act as a pacemaker since they transduce signals from motor neurons to produce an electrical rhythm in the smooth muscle cells.[31] Lower nitric oxide levels also correlate with loss of ICC cells, which can ultimately lead to the loss of function in the smooth muscle in the stomach, as well as in other areas of the gastrointestinal tract.[30]
Pathogenesis of symptoms in diabetic gastroparesis include:
- Loss of gastric neurons containing nitric oxide synthase (NOS) is responsible for defective accommodation reflex, which leads to early satiety and postprandial fullness.[4]
- Impaired electromechanical activity in the myenteric plexus is responsible for delayed gastric emptying, resulting in nausea and vomiting.[4]
- Sensory neuropathy in the gastric wall may be responsible for epigastric pain.[4]
- Abnormal pacemaker activity (tachybradyarrhythmia) may generate a noxious signal transmitted to the CNS to evoke nausea and vomiting.[4]
Diagnosis
[edit]Gastroparesis is suspected in patients who have abdominal pain, nausea, vomiting, or bloating, or when these symptoms occur after eating. Once an upper endoscopy has been performed to exclude peptic ulcer disease or gastric outlet obstruction as the root of their symptoms, those patients should be tested for gastroparesis.
Differential diagnosis
[edit]More than 30% of patients with severe gastroparesis symptoms also experience severe constipation.[32] This may be linked to delayed small bowel and colon transit.[32] If symptoms are refractory (especially in the setting of weight loss), or if an intestinal neuromyopathic disorder is suspected, then motility may be evaluated via antroduodenal manometry, wireless motility capsules, whole gut scintigraphy, or radiopaque markers.[32] Extragastric dysmotility may also be treated with prokinetic medications.[32]
Testing overview
[edit]There are several tests available to diagnose gastroparesis. Gastric emptying scintigraphy (GES) is the current gold standard.[33] But it often cannot distinguish gastroparesis from functional dyspepsia, both of which feature bloating, and both of which may be part of a spectrum of gastric neuromuscular dysfunction.[32] In addition, symptom severity may not correspond to the extent of delayed gastric emptying as measured by scintigraphy.[32]
Alternatives include stable isotope breath tests with carbon-13, which is unreliable in the setting of many diseases; wireless motility capsules, which may permit a more thorough examination; and antroduodenal manometry, which is invasive but may provide some information as to the etiopathogenesis.
Other imaging modalities are rarely used. MRI is expensive. Ultrasound requires expertise and is challenging in the setting of obesity.
Gastric emptying scintigraphy
[edit]Griffith et al. first described GES in 1966,[34] and it has since become the gold standard for diagnosing gastroparesis. Following an overnight fast, the patient consumes a standardized, radiotracer-bound, low-fat meal within 10 minutes of this test. A longer ingestion time may alter the results.
Most medical facilities use 99mTc sulfur colloid-labeled egg sandwiches or Egg Beaters egg whites with 1–2 slices of bread, strawberry jam, and water.[35] Previously, studies labeled both the solid and liquid phases of a meal; however, present standard tests just label the solid phase of a meal, since liquid emptying only becomes delayed in the most advanced stages of gastroparesis. However, when assessing for postsurgical anatomic issues or ruling out dumping syndrome in postsurgical patients, testing liquid emptying is valuable.[36]
Following ingestion, the patient undergoes standard imaging of the gastric area while standing, and the percentage of radioactivity left in the stomach is recorded using computerized software and normalized to the baseline value at 1, 2, and 4 hours postprandially.[37] Gastric emptying is considered delayed if there is more than 60% retention at 2 hours and/or more than 10% retention at 4 hours.[38]
Stable isotope breath tests with carbon-13
[edit]The stable isotope breath test involves using the stable isotope carbon-13 (13C) in a medium-chain fatty acid substrate such as octanoic acid. After that, the 13C-labeled substrate is attached to a food that can be digested, like muffins, or to Spirulina platensis, a blue-green algae that is 50–60% protein, 30% starch, and 10% lipids.[33]
Following an overnight fast, pre-meal breath samples are taken, and then meals are consumed. 13C-octanoate is absorbed in the duodenum and liquefies to chyme after feeding and after the stomach has been emptied. It is then transported to the liver via the portal circulation and metabolized to 13-carbon dioxide (13CO2) before being exhaled during expiration.[33]
Because stomach emptying is the testing process's rate-limiting step, the amount of 13CO2 present in an exhaled breath test represents gastric emptying. Every 30 minutes, post-meal breath samples are collected and analyzed using isotope-ratio mass spectrometry. For a total of 4–6 hours, samples are collected every 30 minutes.[39]
The stable isotope breath test is unreliable for individuals with small bowel diseases like celiac disease, exocrine pancreatic insufficiency, liver disease,[40] or lung disease because it involves duodenal absorption, 13C metabolism in the liver, and pulmonary exhalation of 13CO2. Physical activity is another factor that can influence CO2 excretion.[41]
Wireless motility capsule
[edit]The US Food and Drug Administration has approved the wireless motility capsule (WMC) for the evaluation of gastric emptying as well as colonic transit time for individuals with suspected slow transit constipation.[42] The capsule is 26.8 mm long and 11.7 mm wide, and it contains three sensors for temperature, pH, and pressure. Once ingested, the WMC continuously records measurements of the three variables as it moves through the gastrointestinal tract, and the information is wirelessly and in real-time transmitted to a receiver that the patient wears on their waist for the duration of the study.[43]
Patients consume a standardized meal that includes a nutrient bar accompanied by 50 cc of water on the day of testing. Patients must fast for 6 hours following consuming a meal. For the duration of the study, they are asked to press the EVENT button, record specific events in a diary, and then the receiver is gathered and the data is downloaded for analysis.[43]
Gastric emptying time is regarded as delayed if it is 5 hours or longer and is defined as the time required for the capsule to reach the duodenum, as determined by a pH increase of more than 3 units. Small bowel transit time is normally 2.5–6 hours and is calculated from the time the pH increases by more than three units to the time it drops by more than one unit and is sustained for at least 30 minutes. This drop denotes the capsule's passage to the cecum. The colon transit period (normal is 59 hours or less) is calculated from the time the WMC enters the cecum till it is expelled from the body, as indicated by a sudden drop in temperature or signal loss.[43]
Recent studies have also shown that luminal pressure measurements can be used to differentiate diabetic gastroparesis, which is characterized by a reduced amount of contractions and motility indices when compared to healthy individuals.[44] The ability to examine extragastric motility with a single test is another advantage of using WMC in the diagnosis of gastroparesis. This is useful because extragastric impaired motility occurs in more than 40% of those with suspected gastroparesis, and gastrointestinal symptoms do not correlate well with the gastrointestinal segment affected. Assessing the rest of the gastrointestinal tract in addition to gastric emptying provides information about motility in various segments of the gut, which can change management and improve symptoms.[45]
Antroduodenal manometry
[edit]Antroduodenal manometry involves endoscopically or under radiographic fluoroscopy inserting a manometry catheter or transducer with pressure sensors into the pyloric channel in order to obtain information about gastric and duodenal contractions.[46] Fasting and postprandial states are used to measure the pressure of the antral, pyloric, and duodenal contraction waves. The test can be performed in a stationary setting for 5–8 hours or in an ambulatory setting for 24 hours to evaluate duodenal motor function. Antroduodenal manometry reveals a decreased antral motility index in gastroparesis.[47]
Antroduodenal manometry aids in differentiating between myopathic (scleroderma, amyloidosis) and neuropathic (diabetes mellitus) causes of impaired motility. The test shows a decreased frequency and amplitude of migrating motor complexes in patients with a myopathic condition. The migrating motor complexes in patients whose disease has a neuropathic etiology have a normal amplitude, but they are ill-coordinated and cannot propagate.[47] This test is not widely available, and more validation research is required.[48] It is an invasive test that necessitates expertise to perform and comprehend the results. Furthermore, it is technically challenging, and the catheter may move from the pylorus while an individual is fed and the stomach dilates.[49]
Other imaging tests
[edit]Although transabdominal ultrasonography and magnetic resonance imaging (MRI) have been proposed as noninvasive diagnostic tools for gastroparesis, their use is currently restricted to research.[33]
By measuring changes in the antral area, two-dimensional ultrasonography can provide information about gastric emptying, and complete gastric emptying is determined when the antral area goes back to its preprandial baseline. Three-dimensional ultrasound can provide information on meal distribution and stomach volume.[50] It has also been proposed to use duplex sonography to examine transpyloric flow as well as liquid contents. While ultrasound appears to be an appealing safe technique, its use in the clinical setting is limited due to the significant expertise required and inadequate outcomes in obese patients.[36]
Another appealing tool is MRI, which uses transaxial abdominal images to gauge gastric accommodation and emptying every 15 minutes.[51] It can also distinguish between gastric meal and air and thus provide data on gastric emptying and secretions.[52] It is, however, costly and necessitates specialized equipment; with the exception of research, it is not standardized across centers, limiting its use to research only.[36]
Treatment
[edit]Treatment includes dietary modifications, medications to stimulate gastric emptying, medications to reduce vomiting, and surgical approaches.[5]
Diet
[edit]Dietary treatment involves low-fiber/low-residue diets and, in some cases, restrictions on fat or solids. Eating smaller meals, spaced two to three hours apart has proved helpful. Avoiding foods like rice or beef that cause the individual problems such as pain in the abdomen or constipation will help avoid symptoms.[53]
Pharmacotherapy
[edit]Dopamine antagonists
[edit]D2 receptor antagonists are used to treat gastroparesis by reversing dopamine's inhibition of acetylcholine (ACh) release.[54] This increases gastrointestinal contractility and resting tone,[55] and improves motility in the stomach and proximal small bowel.[54] Dopamine antagonist action in the central nervous system (CNS) also prevents nausea and vomiting.[56]
Domperidone may be accessed by the US FDA's Expanded Access program.[54][57] It is dosed 10–20 mg three times daily and at bedtime.[54] But it is associated with an increased risk of cardiac dysrhythmias, hence its restricted availability.[54] It is contraindicated in patients with corrected QT intervals of >470 ms in males or >450 ms in females.[54]
Metoclopramide has more CNS adverse effects than domperidone and carries a US FDA boxed warning for tardive dyskinesia, a potentially irreversible condition.[54] There are clinical guidelines specifically to mitigate this risk in its use,[57] with doses limited to 5–10 mg orally before meals and at bedtime.[54] A liquid formulation is recommended, and a new intranasal formulation is also available.[54]
Other prokinetics
[edit]Among motilin agonists, erythromycin is well known to improve emptying of the stomach. Its prolonged use is limited by the development of tachyphylaxis; therapeutic effects may wane after a few weeks of consistent use.[57] Clarithromycin and azithromycin, though less studied for this indication, share the same mechanism of action and (limited) utility.[54] Azithromycin has the fewest drug interactions of the three.[citation needed]
Prucalopride, a 5-HT4 receptor agonist, may also be tried, particularly if there is chronic constipation[32] (for which it is labeled in Europe and US).[54] Some evidence suggests it may improve symptoms and quality of life in gastroparesis.[54]
Pyridostigmine, an acetylcholinesterase inhibitor, may facilitate emptying by increasing ACh in the gastrointestinal neuromusculature.[57] It lacks rigorous clinical trials for this indication, but there is clinical experience with its off-label use as a prokinetic in several gastrointestinal motility disorders.[54]
Other neuromodulators and antiemetics
[edit]The antidepressant mirtazapine has proven effective in the treatment of gastroparesis unresponsive to conventional treatment.[58] This may be due to its antiemetic and appetite stimulant properties. Mirtazapine acts on the same serotonin receptor (5-HT3) as the popular antiemetic ondansetron.[59]
A variety of other central neuromodulators, including (but not limited to) other antidepressants, may be tried for symptoms like bloating and distention (and not only in the setting of gastroparesis, but also for disorders of gut–brain interaction, or DGBI).[32] They may reduce visceral pain or hypersensitivity, especially of a postprandial nature.[32] They may also be selected to address any possible psychiatric comorbidities.[32]
Other agents
[edit]Sildenafil, which increases blood flow to the genital area in men, is being used by some practitioners to stimulate the gastrointestinal tract in cases of diabetic gastroparesis.[60]
Deprescribing
[edit]Gastroparesis presents with symptoms like those of slow gastric emptying caused by some opioid medications, some antidepressants, some allergy medications, some weight loss medications, and some antihypertensives. For gastroparesis patients, these medications may make the condition worse.[61]
Drug development
[edit]Camicinal was an investigational motilin agonist that did not improve enteral feeding intolerance.[62]
Other therapeutic interventions
[edit]In specific cases where treatment of chronic nausea and vomiting proves resistant to drugs, implantable gastric stimulation may be used. A medical device is implanted that applies neurostimulation to the muscles of the lower stomach to reduce the symptoms. This is only done in refractory cases that have failed all medical management (usually at least two years of treatment).[53]
Medically refractory gastroparesis may also be treated with a pyloromyotomy, which widens the gastric outlet by cutting the circular pylorus muscle. This can be done laparoscopically or endoscopically (called G-POEM). Vertical sleeve gastrectomy, a procedure in which a part or all of the affected portion of the stomach is removed, has been shown to have some success in the treatment of gastroparesis in obese patients, even curing it in some instances. Further studies have been recommended due to the limited sample size of previous studies.[63][64]
Prognosis
[edit]Long-term studies in gastroparesis patients show that it is not a benign disease and has significant morbidity and a poor prognosis due to the limited options for treatment.[65] The mortality rate is highest in patients with decompensated gastroparesis who are more likely to develop complications. For example, one study discovered that over a 6-year period, 7% of those with gastroparesis died, with 22% requiring long-term enteral or parenteral feeding. 26% of these patients failed to respond to medical treatment, and 6% had gastric electrical stimulation. The 10 patients who died succumbed to metabolic issues, cardiac complications, renal failure, suicide, and bowel ischemia caused by adhesions.[66]
Other research indicates that diabetic gastroparesis is associated with an increased risk of morbidity but not mortality.[67] Olmsted County residents who had definite gastroparesis symptoms, as well as diagnostic testing for gastroparesis, had a 5-year estimated survival rate of 67%, which was significantly lower than the population average. Old age at the time of diagnosis has been linked to a lower chance of survival. Nondiabetic gastroparesis has been linked to a higher survival rate than diabetic gastroparesis.[68]
Some evidence suggests that post-viral gastroparesis has a better prognosis and lasts less time than idiopathic gastroparesis.[69] Cases of post-infectious gastroparesis are self-limiting, with recovery within 12 months of initial symptoms, although some cases last well over 2 years. In children, the duration tends to be shorter and the disease course milder than in adolescents and adults.[5]
Prevalence
[edit]Post-infectious gastroparesis, which constitutes the majority of idiopathic gastroparesis cases, affects up to 4% of the US population.[citation needed] Women in their 20s and 30s seem to be susceptible. One study of 146 US gastroparesis patients found the mean age of patients was 34 years with 82% affected being women, while another study found the patients were young or middle aged and up to 90% were women.[5]
There has only been one true epidemiological study of idiopathic gastroparesis which was completed by the Rochester Epidemiology Project.[9] They looked at patients from 1996 to 2006 who were seeking medical attention instead of a random population sample and found that the prevalence of delayed gastric emptying was fourfold higher in women. It is difficult for medical professionals and researchers to collect enough data and provide accurate numbers since studying gastroparesis requires specialized laboratories and equipment.[70]
See also
[edit]- Autoimmune gastrointestinal dysmotility
- Enteric nervous system
- Intestinal pseudo-obstruction, or enteric neuropathy
References
[edit]- ^ "How to pronounce gastroparesis in English". dictionary.cambridge.org.
- ^ a b c d "Gastroparesis Causes – Mayo Clinic". Mayo Clinic.
- ^ a b Davis MP, Weller R, Regel S (2018). "Gastroparesis and Cancer-Related Gastroparesis". In MacLeod RD, van den Block L (eds.). Textbook of Palliative Care. Springer International Publishing. pp. 1–15. doi:10.1007/978-3-319-31738-0_114-1. ISBN 978-3-319-31738-0.
- ^ a b c d e f Owyang C (October 2011). "Phenotypic switching in diabetic gastroparesis: mechanism directs therapy". Gastroenterology. 141 (4): 1134–1137. doi:10.1053/j.gastro.2011.08.014. PMID 21875587.
- ^ a b c d e f Thorn AR (March 2010). "Not just another case of nausea and vomiting: a review of postinfectious gastroparesis". Journal of the American Academy of Nurse Practitioners. 22 (3): 125–133. doi:10.1111/j.1745-7599.2009.00485.x. PMID 20236395. S2CID 12354903.
- ^ a b c Camilleri M, Kuo B, Nguyen L, Vaughn VM, Petrey J, Greer K, et al. (August 2022). "ACG Clinical Guideline: Gastroparesis". The American Journal of Gastroenterology. 117 (8): 1197–1220. doi:10.14309/ajg.0000000000001874. PMC 9373497. PMID 35926490.
- ^ . In: Simpson, Kathleen Rice, Creehan, Patricia A. eds. AWHONN's Perinatal Nursing. 4th Edition. 530 Walnut Street, Philadelphia, PA 19106 USA:Lippincott Williams & Wilkins; 2014. Available from: Books@Ovid at [1]. Retrieved November 09, 2020.
- ^ Fuglsang J, Ovesen PG (2015). "Pregnancy and Delivery in a Woman with Type 1 Diabetes, Gastroparesis, and a Gastric Neurostimulator". Diabetes Care. 38 (5): e75. doi:10.2337/dc14-2959. PMID 25908160. S2CID 34451324.
- ^ a b Jung HK, Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Szarka LA, et al. (April 2009). "The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006". Gastroenterology. 136 (4). Elsevier BV: 1225–1233. doi:10.1053/j.gastro.2008.12.047. PMC 2705939. PMID 19249393.
- ^ a b c d e f Reddy SC, Wo JM (August 24, 2011). "Clinical Presentations of Gastroparesis". In Parkman HP, McCallum RW (eds.). Gastroparesis. Totowa, NJ: Humana Press. pp. 25–35. doi:10.1007/978-1-60761-552-1_3. ISBN 978-1-60761-551-4. Retrieved 20 November 2023.
- ^ a b c Bharadwaj S, Meka K, Tandon P, Rathur A, Rivas JM, Vallabh H, et al. (2016). "Management of gastroparesis-associated malnutrition". Journal of Digestive Diseases. 17 (5). Wiley: 285–294. doi:10.1111/1751-2980.12344. ISSN 1751-2972. PMID 27111029. S2CID 26992080. Retrieved 20 November 2023.
- ^ Pace LA (November 9, 2019). "Etiology and Clinical Presentation of Gastroparesis". Gastroparesis. Cham: Springer International Publishing. pp. 21–31. doi:10.1007/978-3-030-28929-4_2. ISBN 978-3-030-28928-7. S2CID 209235384. Retrieved 20 November 2023.
- ^ a b Cherian D, Sachdeva P, Fisher RS, Parkman HP (2010). "Abdominal Pain Is a Frequent Symptom of Gastroparesis". Clinical Gastroenterology and Hepatology. 8 (8). Elsevier BV: 676–681. doi:10.1016/j.cgh.2010.04.027. ISSN 1542-3565. PMID 20472097.
- ^ Briley LC, Harrell SP, Woosley A, Eversmann J, Wo JM (2011). "National Survey of Physicians' Perception of the Cause, Complications, and Management of Gastroparesis". Southern Medical Journal. 104 (6). Southern Medical Association: 412–417. doi:10.1097/smj.0b013e318215fa5a. ISSN 0038-4348. PMID 21886030. S2CID 42779814. Retrieved 20 November 2023.
- ^ Bouras EP, Vazquez Roque MI, Aranda-Michel J (June 24, 2013). "Gastroparesis". Nutrition in Clinical Practice. 28 (4). Wiley: 437–447. doi:10.1177/0884533613491982. ISSN 0884-5336. PMID 23797376.
- ^ Levin A, Levine M, Rubesin S, Laufer I (2008). "An 8-year review of barium studies in the diagnosis of gastroparesis". Clinical Radiology. 63 (4). Elsevier BV: 407–414. doi:10.1016/j.crad.2007.10.007. ISSN 0009-9260. PMID 18325361.
- ^ Reddymasu SC, McCallum RW (2010). "Small Intestinal Bacterial Overgrowth in Gastroparesis". Journal of Clinical Gastroenterology. 44 (1). Ovid Technologies (Wolters Kluwer Health): e8–e13. doi:10.1097/mcg.0b013e3181aec746. ISSN 0192-0790. PMID 20027008. S2CID 12058906.
- ^ Parkman HP, Yates KP, Hasler WL, Nguyan L, Pasricha PJ, Snape WJ, et al. (2011). "Dietary Intake and Nutritional Deficiencies in Patients With Diabetic or Idiopathic Gastroparesis". Gastroenterology. 141 (2). Elsevier BV: 486–498.e7. doi:10.1053/j.gastro.2011.04.045. ISSN 0016-5085. PMC 3499101. PMID 21684286.
- ^ Ogorek CP, Davidson L, Fisher RS, Krevsky B (1991). "Idiopathic gastroparesis is associated with a multiplicity of severe dietary deficiencies". The American Journal of Gastroenterology. 86 (4): 423–428. PMID 2012043. Retrieved 20 November 2023.
- ^ "Gastroparesis Complications – Mayo Clinic". Mayo Clinic.
- ^ "Epocrates article, registration required". Online.epocrates.com. Retrieved 2012-10-09.
- ^ "Summary for Oley Foundation by R. W. McCallum, MD". Oley.org. Archived from the original on 2005-12-12. Retrieved 2012-10-09.[unreliable medical source?]
- ^ Naftali T, Yishai R, Zangen T, Levine A (August 15, 2007). "Post-infectious gastroparesis: Clinical and electerogastrographic aspects". Journal of Gastroenterology and Hepatology. 22 (9). Wiley: 1423–1428. doi:10.1111/j.1440-1746.2006.04738.x. ISSN 0815-9319. PMID 17716347. S2CID 26506859.
- ^ "Spotlight on gastroparesis," unauthored article, Balance (magazine of Diabetes UK, no. 246, May–June 2012, p. 43.
- ^ "Gastroparesis". Jackson Siegelbaum Gastroenterology. 2011-11-08. Retrieved 2019-11-06.
- ^ "Gastroparesis – Your Guide to Gastroparesis – Causes of Gastroparesis". Heartburn.about.com. Archived from the original on 2012-03-23. Retrieved 2012-02-10.
- ^ "Gastroparesis: Causes". MayoClinic.com. 2012-01-04. Retrieved 2012-10-09.
- ^ Goodman B (20 May 2024). "People using popular drugs for weight loss, diabetes are more likely to be diagnosed with stomach paralysis, studies find". CNN.com. Retrieved 2024-05-20.
- ^ Sullivan A, Temperley L, Ruban A (30 April 2020). "Pathophysiology, Aetiology and Treatment of Gastroparesis". Digestive Diseases and Sciences. 65 (6): 1615–1631. doi:10.1007/s10620-020-06287-2. hdl:10044/1/79544. PMID 32350720. S2CID 216649955.
- ^ a b Oh JH, Pasricha PJ (January 2013). "Recent advances in the pathophysiology and treatment of gastroparesis". Journal of Neurogastroenterology and Motility. 19 (1): 18–24. doi:10.5056/jnm.2013.19.1.18. PMC 3548121. PMID 23350043.
- ^ Al-Shboul OA (2013). "The importance of interstitial cells of cajal in the gastrointestinal tract". Saudi Journal of Gastroenterology. 19 (1): 3–15. doi:10.4103/1319-3767.105909. PMC 3603487. PMID 23319032.
- ^ a b c d e f g h i j Moshiree B, Drossman D, Shaukat A (September 2023). "AGA Clinical Practice Update on Evaluation and Management of Belching, Abdominal Bloating, and Distention: Expert Review". Gastroenterology. 165 (3): 791–800.e3. doi:10.1053/j.gastro.2023.04.039. PMID 37452811.
- ^ a b c d Cline M, Rouphael C (November 9, 2019). "Diagnostic Evaluation of Gastroparesis". Gastroparesis. Cham: Springer International Publishing. pp. 33–41. doi:10.1007/978-3-030-28929-4_3. ISBN 978-3-030-28928-7. S2CID 209258902.
- ^ Griffith G, Owen G, Kirkman S, Shields R (1966). "Measurement of Rate of Gastric Emptying Using Chromium-51". The Lancet. 287 (7449). Elsevier BV: 1244–1245. doi:10.1016/s0140-6736(66)90247-9. ISSN 0140-6736. PMID 4161213.
- ^ Parkman HP, Harris AD, Krevsky B, Urbain JL, Maurer AH, Fisher RS (June 1995). "Gastroduodenal motility and dysmotility: an update on techniques available for evaluation". The American Journal of Gastroenterology. 90 (6): 869–892. PMID 7771415. Retrieved 20 November 2023.
- ^ a b c Parkman HP, Hasler WL, Fisher RS (2004). "American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis". Gastroenterology. 127 (5). Elsevier BV: 1592–1622. doi:10.1053/j.gastro.2004.09.055. ISSN 0016-5085. PMID 15521026.
- ^ Tang DM, Friedenberg FK (2011). "Gastroparesis: Approach, Diagnostic Evaluation, and Management". Disease-a-Month. 57 (2). Elsevier BV: 74–101. doi:10.1016/j.disamonth.2010.12.007. ISSN 0011-5029. PMID 21329779.
- ^ Tougas G, Eaker EY, Abell TL, Abrahamsson H, Boivin M, Chen J, et al. (2000). "Assessment of gastric emptying using a low fat meal: establishment of international control values". The American Journal of Gastroenterology. 95 (6). Ovid Technologies (Wolters Kluwer Health): 1456–1462. doi:10.1111/j.1572-0241.2000.02076.x. ISSN 0002-9270. PMID 10894578. S2CID 40523729.
- ^ Clegg ME, Shafat A (May 6, 2010). "Procedures in the13C octanoic acid breath test for measurement of gastric emptying: analysis using Bland–Altman methods". Scandinavian Journal of Gastroenterology. 45 (7–8). Informa UK Limited: 852–861. doi:10.3109/00365521.2010.483740. ISSN 0036-5521. PMID 20443742. S2CID 20920554.
- ^ Van de Casteele M, Luypaerts A, Geypens B, Fevery J, Ghoos Y, Nevens F (2003). "Oxidative breakdown of octanoic acid is maintained in patients with cirrhosis despite advanced disease". Neurogastroenterology & Motility. 15 (2). Wiley: 113–120. doi:10.1046/j.1365-2982.2003.00397.x. ISSN 1350-1925. PMID 12680910. S2CID 36962495.
- ^ Shin AS, Camilleri M (July 17, 2013). "Diagnostic Assessment of Diabetic Gastroparesis". Diabetes. 62 (8). American Diabetes Association: 2667–2673. doi:10.2337/db12-1706. ISSN 0012-1797. PMC 3717858. PMID 23881199.
- ^ Rao SS, Camilleri M, Hasler WL, Maurer AH, Parkman HP, Saad R, et al. (December 7, 2010). "Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies". Neurogastroenterology & Motility. 23 (1). Wiley: 8–23. doi:10.1111/j.1365-2982.2010.01612.x. hdl:2027.42/79321. ISSN 1350-1925. PMID 21138500.
- ^ a b c Saad RJ, Hasler WL (2011). "A Technical Review and Clinical Assessment of the Wireless Motility Capsule". Gastroenterology & Hepatology. 7 (12). Millenium Medical Publishing: 795–804. PMC 3280411. PMID 22347818.
- ^ Kloetzer L, Chey WD, McCallum RW, Koch KL, Wo JM, Sitrin M, et al. (2010). "Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule". Neurogastroenterology & Motility. 22 (5). Wiley: 527–33, e117. doi:10.1111/j.1365-2982.2010.01468.x. hdl:2027.42/78624. ISSN 1350-1925. PMID 20122128. S2CID 22852106.
- ^ Rouphael C, Arora Z, Thota PN, Lopez R, Santisi J, Funk C, et al. (April 26, 2017). "Role of wireless motility capsule in the assessment and management of gastrointestinal dysmotility in patients with diabetes mellitus". Neurogastroenterology & Motility. 29 (9). Wiley. doi:10.1111/nmo.13087. ISSN 1350-1925. PMID 28444862. S2CID 13661563.
- ^ Soffer E, Thongsawat S (1996). "Clinical value of duodenojejunal manometry". Digestive Diseases and Sciences. 41 (5). Springer Science and Business Media LLC: 859–863. doi:10.1007/bf02091523. ISSN 0163-2116. PMID 8625755. S2CID 23912562.
- ^ a b Fraser RJ, Horowitz M, Maddox AF, Dent J (February 1, 1994). "Postprandial antropyloroduodenal motility and gastric emptying in gastroparesis—effects of cisapride". Gut. 35 (2). BMJ: 172–178. doi:10.1136/gut.35.2.172. ISSN 0017-5749. PMC 1374490. PMID 8307466.
- ^ Patcharatrakul T, Gonlachanvit S (July 31, 2013). "Technique of Functional and Motility Test: How to Perform Antroduodenal Manometry". Journal of Neurogastroenterology and Motility. 19 (3). The Korean Society of Neurogastroenterology and Motility: 395–404. doi:10.5056/jnm.2013.19.3.395. ISSN 2093-0879. PMC 3714419. PMID 23875108.
- ^ Quigley EM, Donovan JP, Lane MJ, Gallagher TF (1992). "Antroduodenal manometry". Digestive Diseases and Sciences. 37 (1). Springer Science and Business Media LLC: 20–28. doi:10.1007/bf01308337. ISSN 0163-2116. PMID 1728526. S2CID 29751595.
- ^ Hausken T, Ødegaard S, Matre K, Berstad A (1992). "Antroduodenal motility and movements of luminal contents studied by duplex sonography". Gastroenterology. 102 (5). Elsevier BV: 1583–1590. doi:10.1016/0016-5085(92)91717-i. ISSN 0016-5085. PMID 1568568.
- ^ Kim DY, Myung SJ, Camilleri M (2000). "Novel Testing of Human Gastric Motor and Sensory Functions: Rationale, Methods, and Potential Applications in Clinical Practice". American Journal of Gastroenterology. 95 (12). Ovid Technologies (Wolters Kluwer Health): 3365–3373. doi:10.1111/j.1572-0241.2000.03346.x. ISSN 0002-9270. PMID 11151863. S2CID 23758295.
- ^ Schwizer W, Maecke H, Michael F (1992). "Measurement of gastric emptying by magnetic resonance imaging in humans". Gastroenterology. 103 (2). Elsevier BV: 369–376. doi:10.1016/0016-5085(92)90823-h. ISSN 0016-5085. PMID 1634055.
- ^ a b "Treatment Options for Gastroparesis". Medtronic.com. Medtronic. 29 September 2014. Archived from the original on 10 March 2016. Retrieved 9 March 2016.
- ^ a b c d e f g h i j k l m Camilleri M, Atieh J (2021). "New Developments in Prokinetic Therapy for Gastric Motility Disorders". Front Pharmacol. 12. doi:10.3389/fphar.2021.711500. PMC 8421525. PMID 34504426.
- ^ "Metochlopramide Hydrochloride". Monograph. The American Society of Health-System Pharmacists. Retrieved 23 March 2016.
- ^ Rang HP, Dale MM, Ritter JM, Moore PK (2003). Pharmacology (5th ed.). Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4.[page needed]
- ^ a b c d Camilleri, Michael (Oct 2022). "Highlights From the New ACG Clinical Guideline for Gastroparesis". Gastroenterol Hepatol (NY). 18 (10): 586–588. PMC 9666798. PMID 36397928.
- ^ Kundu S, Rogal S, Alam A, Levinthal DJ (June 2014). "Rapid improvement in post-infectious gastroparesis symptoms with mirtazapine". World Journal of Gastroenterology. 20 (21): 6671–6674. doi:10.3748/wjg.v20.i21.6671. PMC 4047357. PMID 24914393.
- ^ Kim SW, Shin IS, Kim JM, Kang HC, Mun JU, Yang SJ, et al. (2006). "Mirtazapine for severe gastroparesis unresponsive to conventional prokinetic treatment". Psychosomatics. 47 (5): 440–442. doi:10.1176/appi.psy.47.5.440. PMID 16959934.
- ^ Gottlieb S (August 2000). "Sildenafil may help diabetic patients". BMJ. 321 (7258): 401A. PMC 1127789. PMID 10938040.
- ^ "Overview". Mayo Clinic.
- ^ Deane AM, Lamontagne F, Dukes GE, Neil D, Vasist L, Barton ME, et al. (2018). "Nutrition Adequacy Therapeutic Enhancement in the Critically Ill: A Randomized Double-Blind, Placebo-Controlled Trial of the Motilin Receptor Agonist Camicinal (GSK962040): The NUTRIATE Study". Journal of Parenteral and Enteral Nutrition. 42 (5): 949–459. doi:10.1002/jpen.1038. hdl:11343/294086. PMID 29957868.
- ^ Samuel B, Atiemo K, Cohen P, Czerniach D, Kelly J, Perugini R (2016). "The Effect of Sleeve Gastrectomy on Gastroparesis: A Short Clinical Review". Bariatric Surgical Practice and Patient Care. 11 (2): 84–9. doi:10.1089/bari.2015.0052.
- ^ Bagloo M, Besseler M, Ude A (April 2010). Sleeve gastrectomy for the treatment of diabetic gastroparesis. Proceedings 12th world congress of endoscopic surgery. pp. 14–17).
- ^ Hyett B, Martinez FJ, Gill BM, Mehra S, Lembo A, Kelly CP, et al. (2009). "Delayed Radionucleotide Gastric Emptying Studies Predict Morbidity in Diabetics With Symptoms of Gastroparesis". Gastroenterology. 137 (2). Elsevier BV: 445–452. doi:10.1053/j.gastro.2009.04.055. ISSN 0016-5085. PMID 19410575.
- ^ Soykan I (1998). "Demography, Clinical Characteristics, Psychological and Abuse Profiles, Treatment, and Long-Term Follow-up of Patients with Gastroparesis". Digestive Diseases and Sciences. 43 (11). Springer Science and Business Media LLC: 2398–2404. doi:10.1023/a:1026665728213. ISSN 0163-2116. PMID 9824125. S2CID 7643584.
- ^ Kong MF, Horowitz M, Jones KL, Wishart JM, Harding PE (March 1, 1999). "Natural history of diabetic gastroparesis". Diabetes Care. 22 (3). American Diabetes Association: 503–507. doi:10.2337/diacare.22.3.503. ISSN 0149-5992. PMID 10097936.
- ^ Jung HK, Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Szarka LA, et al. (2009). "The Incidence, Prevalence, and Outcomes of Patients With Gastroparesis in Olmsted County, Minnesota, From 1996 to 2006". Gastroenterology. 136 (4). Elsevier BV: 1225–1233. doi:10.1053/j.gastro.2008.12.047. ISSN 0016-5085. PMC 2705939. PMID 19249393.
- ^ Bityutskiy LP, Soykan I, McCallum RW (September 1997). "Viral gastroparesis: a subgroup of idiopathic gastroparesis—clinical characteristics and long-term outcomes". The American Journal of Gastroenterology. 92 (9): 1501–1504. PMID 9317072. Retrieved 20 November 2023.
- ^ Bharucha AE (March 2015). "Epidemiology and natural history of gastroparesis". Gastroenterology Clinics of North America. 44 (1): 9–19. doi:10.1016/j.gtc.2014.11.002. PMC 4323583. PMID 25667019.
Further reading
[edit]- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L (January 2013). "Clinical guideline: management of gastroparesis". The American Journal of Gastroenterology. 108 (1): 18–37, quiz 38. doi:10.1038/ajg.2012.373. PMC 3722580. PMID 23147521.
- Parkman HP, Fass R, Foxx-Orenstein AE (June 2010). "Treatment of patients with diabetic gastroparesis". Gastroenterology & Hepatology. 6 (6): 1–16. PMC 2920593. PMID 20733935.
- Kim BJ, Kuo B (January 30, 2019). "Gastroparesis and Functional Dyspepsia: A Blurring Distinction of Pathophysiology and Treatment". Journal of Neurogastroenterology and Motility. 25 (1). The Korean Society of Neurogastroenterology and Motility: 27–35. doi:10.5056/jnm18162. ISSN 2093-0879. PMC 6326193. PMID 30509017.
