Isoamyl acetate: Difference between revisions
rv; inaccurate chemistry |
mNo edit summary |
||
| (224 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound with banana odor}} |
|||
<!-- Here is a table of data; skip past it to edit the text. --> |
|||
{{chembox |
|||
{| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" |
|||
| Watchedfields = changed |
|||
! {{chembox header}}| '''{{{name|Isoamyl acetate}}}''' |
|||
| verifiedrevid = 477004274 |
|||
|- |
|||
| Name = Isoamyl acetate |
|||
| align="center" colspan="2" bgcolor="#ffffff" | [[Image:Isoamyl_acetate.png|200px|Isoamyl acetate]] |
|||
| ImageFile = |
|||
|- |
|||
| ImageFile1 = Isoamyl_acetate.svg |
|||
| [[IUPAC nomenclature|Chemical name]] |
|||
| ImageSize1 = 200px |
|||
| {{{IUPAC|3-methyl-1-butyl acetate}}} |
|||
| ImageName1 = Isoamyl acetate |
|||
|- |
|||
| ImageFile2 = Isoamyl-acetate-3D-balls.png |
|||
| Other names |
|||
| ImageSize2 = 200px |
|||
| isopentyl acetate<br>banana oil<br>juicy fruit smell<br>isopentyl ethanoate<br>pear essence<br>3-methylbutyl acetate |
|||
| ImageFile3 = Isopentyl acetate.jpg |
|||
|- |
|||
| ImageSize3 = 50px |
|||
| [[Chemical formula]] |
|||
| PIN = 3-Methylbutyl acetate |
|||
| {{{formula|C<sub>7</sub>H<sub>14</sub>O<sub>2</sub>}}} |
|||
| SystematicName = 3-Methylbutyl ethanoate |
|||
|- |
|||
| OtherNames = Isopentyl acetate<br />Isopentyl ethanoate<br />Isoamyl acetate<br />Banana oil<br />Pear essence |
|||
| [[Molecular mass]] |
|||
| IUPACName = |
|||
| {{{mol_mass|130.19}}} g/mol |
|||
| Section1 = {{Chembox Identifiers |
|||
|- |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| [[CAS registry number|CAS number]] |
|||
| ChemSpiderID = 29016 |
|||
| [{{{CAS|123-92-2}}}] |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
|- |
|||
| UNII = Z135787824 |
|||
| [[Density]] |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
| {{{density|0.876}}} g/cm<sup>3</sup> |
|||
| KEGG = C12296 |
|||
|- |
|||
| InChI = 1/C7H14O2/c1-6(2)4-5-9-7(3)8/h6H,4-5H2,1-3H3 |
|||
| [[Melting point]] |
|||
| InChIKey = MLFHJEHSLIIPHL-UHFFFAOYAI |
|||
| {{{melting_point|-78}}} °C |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
|- |
|||
| ChEMBL = 42013 |
|||
| [[Boiling point]] |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| {{{boiling_point|142}}} °C |
|||
| StdInChI = 1S/C7H14O2/c1-6(2)4-5-9-7(3)8/h6H,4-5H2,1-3H3 |
|||
|- |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = MLFHJEHSLIIPHL-UHFFFAOYSA-N |
|||
| CASNo_Ref = {{cascite|correct|CAS}} |
|||
| CASNo = 123-92-2 |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| ChEBI = 31725 |
|||
| PubChem = 31276 |
|||
| Beilstein = 1744750 |
|||
| Gmelin = 101452 |
|||
| EC_number = 204-662-3 |
|||
| RTECS = NS9800000 |
|||
| UNNumber = 1104 1993 |
|||
| SMILES = O=C(OCCC(C)C)C |
|||
}} |
|||
| Section2 = {{Chembox Properties |
|||
| C=7 | H =14 | O=2 |
|||
| Density = 0.876 g/cm<sup>3</sup> |
|||
| Appearance = Colorless liquid |
|||
| Odor = Banana-like<ref name=PGCH/> |
|||
| MeltingPtC = −78 |
|||
| BoilingPtC = 142<!--Ref Index: nD-20 = 1.4006--> |
|||
| Solubility = 0.3% (20{{nbsp}}°C)<ref name=PGCH/> |
|||
| VaporPressure = 4{{nbsp}}mmHg or 0.533{{nbsp}}kPa (20{{nbsp}}°C)<ref name=PGCH/> |
|||
| MagSus = −89.4·10<sup>−6</sup>{{nbsp}}cm<sup>3</sup>/mol |
|||
| RefractIndex =1.4020 at 20° |
|||
}} |
|||
| Section3 = {{Chembox Hazards |
|||
| NFPA-H = 1 |
|||
| NFPA-F = 3 |
|||
| NFPA-R = 0 |
|||
| FlashPtC = 25 |
|||
| PEL = TWA 100{{nbsp}}ppm (525{{nbsp}}mg/m<sup>3</sup>)<ref name=PGCH>{{PGCH|0347}}</ref> |
|||
| ExploLimits = 1.0% (100{{nbsp}}°C) – 7.5%<ref name=PGCH/> |
|||
| REL = TWA 100{{nbsp}}ppm (525{{nbsp}}mg/m<sup>3</sup>)<ref name=PGCH/> |
|||
| IDLH = 1000{{nbsp}}ppm<ref name=PGCH/> |
|||
| LCLo = 6470{{nbsp}}ppm (cat)<ref name=IDLH>{{IDLH|123922|Isoamyl acetate}}</ref> |
|||
| LD50 = 7422{{nbsp}}mg/kg (rabbit, oral)<br/>16,600{{nbsp}}mg/kg (rat, oral)<ref name=IDLH/> |
|||
| GHSPictograms = {{GHS02}} |
|||
| GHSSignalWord = Danger |
|||
| HPhrases = {{H-phrases|226|315|319|335|336|372}} |
|||
| PPhrases = {{P-phrases|210|233|240|241|242|243|260|261|264|270|271|280|302+352|303+361+353|304+340|305+351+338|312|314|321|332+313|337+313|362|370+378|403+233|403+235|405|501}} |
|||
}} |
|||
| Section4 = {{Chembox Related |
|||
| OtherCompounds = [[Isoamyl formate]] |
|||
}} |
|||
}}<!-- |
|||
| [[Refractive index]] |
| [[Refractive index]] |
||
| {{{ |
| {{{Refractive index (nD20)|1.4020}}}--> |
||
|- |
|||
| [[Simplified molecular input line entry specification|SMILES]] |
|||
| {{{SMILES|CC(C)CCOC(C)=O}}} |
|||
|- |
|||
| {{chembox header}} | <small>[[wikipedia:Chemical infobox|Disclaimer and references]]</small> |
|||
|- |
|||
|} |
|||
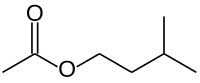
'''Isoamyl acetate''', also known as '''isopentyl acetate''', is an [[ester]] formed from [[isoamyl alcohol]] and [[acetic acid]], with the molecular formula {{chem2|C7H14O2}}. It is a colorless liquid that is only slightly soluble in water, but very soluble in most organic solvents. Isoamyl acetate has a strong odor which is described as similar to both [[banana]] and [[pear]].<ref>{{Cite web | url = http://www.chemicalland21.com/specialtychem/perchem/ISO-AMYL%20ACETATE.htm | title = Iso-amyl acetate | publisher = chemicalland21.com}}</ref> Pure isoamyl acetate, or mixtures of isoamyl acetate, [[amyl acetate]], and other flavors in [[ethanol]] may be referred to as '''banana oil'''<ref>Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg "Flavors and Fragrances" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2002. {{doi|10.1002/14356007.a11_141}}.</ref> or '''pear oil'''.<ref>{{cite web |title=T3DB: Isopentyl acetate |url=http://www.t3db.ca/toxins/T3D4851 |website=Toxin and Toxin Target Database (T3DB) |publisher=Canadian Institutes of Health Research, Canada Foundation for Innovation, and by The Metabolomics Innovation Centre (TMIC) |access-date=2 April 2023}}</ref> |
|||
'''Isoamyl acetate''' is an [[organic compound]] that is the [[ester]] formed from [[isoamyl alcohol]] and [[acetic acid]]. It is a clear colorless liquid that is only slightly soluble in water, but very soluble in most organic solvents. |
|||
==Natural occurrence== |
|||
Isoamyl acetate has a strong odor(similar to juicy fruit) which is also described as similar to both [[banana]] and [[pear]]. ''Banana oil'' is a term that is applied either to pure isoamyl acetate or to flavorings that are mixtures of isoamyl acetate, [[amyl acetate]], [[nitrocellulose]] and other flavors. ''Pear oil'' commonly refers to a solution of isoamyl acetate in [[ethanol]] that is used as an artificial flavor. |
|||
Isoamyl acetate occurs naturally in many plants, including [[apple]], [[banana]], [[coffee]], [[grape]], [[guava]], [[lychee]], [[papaya]], [[peach]], [[pomegranate]], and [[tomato]].<ref name="ntp" /><ref>{{cite web |title=Isoamyl acetate Taxonomy |url=https://pubchem.ncbi.nlm.nih.gov/compound/Isoamyl-acetate#section=Taxonomy&fullscreen=true |website=PubChem |publisher=National Center for Biotechnology Information |access-date=2 April 2023 |language=en}}</ref> It is also released by fermentation processes, including those used for making [[beer]], [[sake]], [[cognac]], and [[whisky]].<ref name="ntp">{{cite web |author1=Technical Resources International, Inc |title=SUMMARY OF DATA FOR CHEMICAL SELECTION: Isoamyl Acetate |url=https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/isoamylacetate_508.pdf |website=National Toxicology Program |publisher=U.S. Department of Health and Human Services |access-date=2 April 2023 |date=November 1994}}</ref> |
|||
Isoamyl acetate is released by a [[honey bee]]'s [[stinger|sting]] apparatus where it serves as a [[Honey bee pheromones#Alarm pheromone|pheromone]] beacon to attract other bees and provoke them to sting.<ref>{{cite journal|author=Boch R|author2=Shearer DA |author3=Stone BC |date=September 8, 1962|title=Identification of isoamyl acetate as an active component in the sting pheromone of the honey bee |journal=Nature |volume=195 |issue=4845 |pages=1018–20 |publisher=Nature Publishing Group |location=England |pmid=13870346|doi=10.1038/1951018b0 |bibcode=1962Natur.195.1018B |s2cid=4224788 }}</ref> |
|||
Isoamyl acetate is also used to test the effectiveness of [[respirator]]s or [[gas mask]]s because it has a strong smell which is generally not experienced as unpleasant that can be detected at low concentrations, and has low toxicity. |
|||
==Production== |
|||
It is also used as a solvent for some varnishes and nitrocellulose lacquers, as well as being a honey bee [[pheromone]] and can be used to attract large groups of honeybees to a small area. |
|||
Isoamyl acetate is prepared by the acid-catalyzed reaction ([[Fischer esterification]]) between [[isoamyl alcohol]] and glacial [[acetic acid]] as shown in the reaction equation below. Typically, [[sulfuric acid]] is used as the [[catalyst]]. Alternatively, [[p-Toluenesulfonic acid|''p''-toluenesulfonic acid]] or an acidic ion exchange resin can be used as the catalyst. |
|||
:[[Image:Essigsäureisopentylester Reaktionsschemata.svg|550px]] |
|||
==External links== |
|||
{{ChemicalSources}} |
|||
It is also produced synthetically by the [[Rectification (chemical/process engineering)|rectification]] of [[amyl acetate]].<ref>{{cite web |title=Isoamyl acetate Methods of Manufacturing |url=https://pubchem.ncbi.nlm.nih.gov/compound/Isoamyl-acetate#section=Methods-of-Manufacturing |website=PubChem |publisher=National Center for Biotechnology Information |language=en}}</ref> |
|||
[[Category:Carboxylate esters]] |
|||
[[Category:Acetates]] |
|||
[[Category:Flavors]] |
|||
[[Category:Solvents]] |
|||
[[Category:Pheromones]] |
|||
==Applications== |
|||
[[fr:Acétate d'isoamyle]] |
|||
Isoamyl acetate is used to confer banana or pear flavor in foods such as [[circus peanut]]s, [[Juicy Fruit]] and [[pear drop]]s.<ref>{{cite web |title=Isoamyl acetate |url=https://www.acs.org/content/acs/en/molecule-of-the-week/archive/i/isoamyl-acetate.html |website=American Chemical Society |access-date=27 October 2022 |language=en}}</ref> ''Banana oil'' and ''pear oil'' commonly refer to a solution of isoamyl acetate in [[ethanol]] that is used as an [[artificial flavor]]. |
|||
[[it:Acetato di isoamile]] |
|||
[[ja:酢酸イソアミル]] |
|||
It is also used as a [[solvent]] for some [[varnish]]es, [[oil paint]]s, and [[nitrocellulose lacquer]]s. As a solvent and carrier for materials such as [[nitrocellulose]], it was extensively used in the aircraft industry for stiffening and wind-proofing fabric flying surfaces, where it and its derivatives were generally known as '[[aircraft dope]]'. Now that most aircraft wings are made of metal, such use is mostly limited to historically accurate reproductions and scale models. |
|||
[[fi:Isoamyyliasetaatti]] |
|||
Because of its intense, pleasant odor and its low toxicity, isoamyl acetate is used to test the effectiveness of [[respirators]] or [[gas masks]].<ref>{{Cite web|url=https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=9780&p_table=STANDARDS|title=Fit Testing Procedures (Mandatory). - 1910.134 App A {{!}} Occupational Safety and Health Administration|website=www.osha.gov|access-date=2020-02-04}}</ref> |
|||
== References == |
|||
{{Reflist}} |
|||
{{Esters}} |
|||
{{DEFAULTSORT:Isoamyl Acetate}} |
|||
[[Category:Flavors]] |
|||
[[Category:Insect pheromones]] |
|||
[[Category:Ester solvents]] |
|||
[[Category:Acetate esters]] |
|||
[[Category:Isoamyl esters|Acetate, Isoamyl]] |
|||
[[Category:Sweet-smelling chemicals]] |
|||
Latest revision as of 12:11, 3 September 2024
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Methylbutyl acetate | |
| Systematic IUPAC name
3-Methylbutyl ethanoate | |
| Other names
Isopentyl acetate
Isopentyl ethanoate Isoamyl acetate Banana oil Pear essence | |
| Identifiers | |
3D model (JSmol)
|
|
| 1744750 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.240 |
| EC Number |
|
| 101452 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1104 1993 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H14O2 | |
| Molar mass | 130.187 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Banana-like[1] |
| Density | 0.876 g/cm3 |
| Melting point | −78 °C (−108 °F; 195 K) |
| Boiling point | 142 °C (288 °F; 415 K) |
| 0.3% (20 °C)[1] | |
| Vapor pressure | 4 mmHg or 0.533 kPa (20 °C)[1] |
| −89.4·10−6 cm3/mol | |
Refractive index (nD)
|
1.4020 at 20° |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H226, H315, H319, H335, H336, H372 | |
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P312, P314, P321, P332+P313, P337+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 25 °C (77 °F; 298 K) |
| Explosive limits | 1.0% (100 °C) – 7.5%[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
7422 mg/kg (rabbit, oral) 16,600 mg/kg (rat, oral)[2] |
LCLo (lowest published)
|
6470 ppm (cat)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 100 ppm (525 mg/m3)[1] |
REL (Recommended)
|
TWA 100 ppm (525 mg/m3)[1] |
IDLH (Immediate danger)
|
1000 ppm[1] |
| Related compounds | |
Related compounds
|
Isoamyl formate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isoamyl acetate, also known as isopentyl acetate, is an ester formed from isoamyl alcohol and acetic acid, with the molecular formula C7H14O2. It is a colorless liquid that is only slightly soluble in water, but very soluble in most organic solvents. Isoamyl acetate has a strong odor which is described as similar to both banana and pear.[3] Pure isoamyl acetate, or mixtures of isoamyl acetate, amyl acetate, and other flavors in ethanol may be referred to as banana oil[4] or pear oil.[5]
Natural occurrence
[edit]Isoamyl acetate occurs naturally in many plants, including apple, banana, coffee, grape, guava, lychee, papaya, peach, pomegranate, and tomato.[6][7] It is also released by fermentation processes, including those used for making beer, sake, cognac, and whisky.[6]
Isoamyl acetate is released by a honey bee's sting apparatus where it serves as a pheromone beacon to attract other bees and provoke them to sting.[8]
Production
[edit]Isoamyl acetate is prepared by the acid-catalyzed reaction (Fischer esterification) between isoamyl alcohol and glacial acetic acid as shown in the reaction equation below. Typically, sulfuric acid is used as the catalyst. Alternatively, p-toluenesulfonic acid or an acidic ion exchange resin can be used as the catalyst.
It is also produced synthetically by the rectification of amyl acetate.[9]
Applications
[edit]Isoamyl acetate is used to confer banana or pear flavor in foods such as circus peanuts, Juicy Fruit and pear drops.[10] Banana oil and pear oil commonly refer to a solution of isoamyl acetate in ethanol that is used as an artificial flavor.
It is also used as a solvent for some varnishes, oil paints, and nitrocellulose lacquers. As a solvent and carrier for materials such as nitrocellulose, it was extensively used in the aircraft industry for stiffening and wind-proofing fabric flying surfaces, where it and its derivatives were generally known as 'aircraft dope'. Now that most aircraft wings are made of metal, such use is mostly limited to historically accurate reproductions and scale models.
Because of its intense, pleasant odor and its low toxicity, isoamyl acetate is used to test the effectiveness of respirators or gas masks.[11]
References
[edit]- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0347". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Isoamyl acetate". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "Iso-amyl acetate". chemicalland21.com.
- ^ Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg "Flavors and Fragrances" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a11_141.
- ^ "T3DB: Isopentyl acetate". Toxin and Toxin Target Database (T3DB). Canadian Institutes of Health Research, Canada Foundation for Innovation, and by The Metabolomics Innovation Centre (TMIC). Retrieved 2 April 2023.
- ^ a b Technical Resources International, Inc (November 1994). "SUMMARY OF DATA FOR CHEMICAL SELECTION: Isoamyl Acetate" (PDF). National Toxicology Program. U.S. Department of Health and Human Services. Retrieved 2 April 2023.
- ^ "Isoamyl acetate Taxonomy". PubChem. National Center for Biotechnology Information. Retrieved 2 April 2023.
- ^ Boch R; Shearer DA; Stone BC (September 8, 1962). "Identification of isoamyl acetate as an active component in the sting pheromone of the honey bee". Nature. 195 (4845). England: Nature Publishing Group: 1018–20. Bibcode:1962Natur.195.1018B. doi:10.1038/1951018b0. PMID 13870346. S2CID 4224788.
- ^ "Isoamyl acetate Methods of Manufacturing". PubChem. National Center for Biotechnology Information.
- ^ "Isoamyl acetate". American Chemical Society. Retrieved 27 October 2022.
- ^ "Fit Testing Procedures (Mandatory). - 1910.134 App A | Occupational Safety and Health Administration". www.osha.gov. Retrieved 2020-02-04.


