Butane: Difference between revisions
No edit summary |
Pikachu.447 (talk | contribs) No edit summary Tags: Mobile edit Mobile app edit Android app edit |
||
| Line 1: | Line 1: | ||
{{Short description|Flammable organic compound widely used as a fuel}} |
|||
{| align="right" width="300" border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse" |
|||
{{Distinguish|butene|butyne|Bhutan}} |
|||
|- style="border-top: 3px solid gray" |
|||
{{Use dmy dates|date=March 2021}} |
|||
! bgcolor="#ffddaa" colspan="2" | General |
|||
{{Chembox |
|||
|- |
|||
| Watchedfields = changed |
|||
| [[IUPAC nomenclature|Chemical Name]] || '''Butane''' <br /> ''n''-butane |
|||
| verifiedrevid = 464365466 |
|||
| ImageFileL1 = Butan Lewis.svg |
|||
| ImageFileL1_Ref = {{chemboximage|correct|??}} |
|||
| ImageSizeL1 = 130px |
|||
| ImageAltL1 = Skeletal formula of butane with all carbon and hydrogen atoms shown |
|||
| ImageFileR1 = Butane simple.svg |
|||
| ImageFileR1_Ref = {{chemboximage|correct|??}} |
|||
| ImageSizeR1 = 110px |
|||
| ImageAltR1 = Skeletal formula of butane with all implicit hydrogens shown |
|||
| ImageFileL2 = Butane-3D-balls.png |
|||
| ImageFileL2_Ref = {{chemboximage|correct|??}} |
|||
| ImageSizeL2 = 130px |
|||
| ImageAltL2 = Ball-and-stick model of the butane molecule |
|||
| ImageFileR2 = Butane-3D-space-filling.png |
|||
| ImageFileR2_Ref = {{chemboximage|correct|??}} |
|||
| ImageSizeR2 = 110px |
|||
| ImageAltR2 = Space-filling model of the butane molecule |
|||
| PIN = Butane<ref name=iupac2013>{{cite book |title=Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book) |chapter=General Principles, Rules, and Conventions |publisher=[[Royal Society of Chemistry|The Royal Society of Chemistry]] |date=2014 |location=Cambridge |at=P-12.1 |doi=10.1039/9781849733069-00001 |isbn=978-0-85404-182-4 |quote=Similarly, the retained names 'ethane', 'propane', and 'butane' were never replaced by systematic names 'dicarbane', 'tricarbane', and 'tetracarbane' as recommended for analogues of silane, 'disilane'; phosphane, 'triphosphane'; and sulfane, 'tetrasulfane'.}}</ref> |
|||
| SystematicName = Tetracarbane (never recommended<ref name=iupac2013 />) |
|||
| OtherNames = {{Unbulleted list|Butyl hydride<ref <ref name=PGCH/>|Quartane<ref name=quartane>{{Cite journal|title=I. On the action of trichloride of phosphorus on the salts of the aromatic monamines |author=August Wilhelm Von Hofmann |year=1867 |journal=Proceedings of the Royal Society of London |volume=15 |pages=54–62 |doi=10.1098/rspl.1866.0018 |s2cid=98496840 |url=https://books.google.com/books?id=w1BJAAAAcAAJ&pg=RA1-PA58}}</ref>|R600}} |
|||
|Section1={{Chembox Identifiers |
|||
| CASNo = 106-97-8 |
|||
| CASNo_Ref = {{cascite|correct|CAS}} |
|||
| PubChem = 7843 |
|||
| ChemSpiderID = 7555 |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| UNII = 6LV4FOR43R |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| EINECS = 203-448-7 |
|||
| UNNumber = 1011 |
|||
| KEGG = D03186 |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
| MeSHName = butane |
|||
| ChEBI = 37808 |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| ChEMBL = 134702 |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
| RTECS = EJ4200000 |
|||
| Beilstein = 969129 |
|||
| Gmelin = 1148 |
|||
| SMILES = CCCC |
|||
| StdInChI = 1S/C4H10/c1-3-4-2/h3-4H2,1-2H3 |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = IJDNQMDRQITEOD-UHFFFAOYSA-N |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
}} |
|||
|Section2={{Chembox Properties |
|||
| C=4 | H=10 |
|||
| Appearance = Colorless gas |
|||
| Odor = Gasoline-like or natural gas-like<ref name=PGCH/> |
|||
| Density = 2.48 kg/m<sup>3</sup> (at {{convert|15|C}}) |
|||
| MeltingPtK = 133 to 139 |
|||
| BoilingPtK = 272 to 274 |
|||
| Solubility = 61 mg/L (at {{convert|20|C}}) |
|||
| LogP = 2.745 |
|||
| MagSus = −57.4·10<sup>−6</sup> cm<sup>3</sup>/mol |
|||
| VaporPressure = ~170 kPa at 283 K <ref>{{Cite journal|title=Pressure-Volume-Temperature Relations for n-Butane|journal=Industrial & Engineering Chemistry|volume=32|issue=3|pages=358–360|author=W. B. Kay|doi=10.1021/ie50363a016|year=1940}}</ref> |
|||
| HenryConstant = 11 nmol Pa<sup>−1</sup> kg<sup>−1</sup> |
|||
}} |
|||
|Section3={{Chembox Thermochemistry |
|||
| DeltaHf = −126.3–−124.9 kJ/mol |
|||
| DeltaHc = −2.8781–−2.8769 MJ/mol |
|||
| HeatCapacity = 98.49 J/(K·mol) |
|||
}} |
|||
|Section4={{Chembox Hazards |
|||
| Hazards_ref = <ref name="chemadvisor">{{cite web|title=Safety Data Sheet, Material Name: N-Butane|url=http://www.chemadvisor.com/Matheson/database/msds/MAT15370000800003.PDF|publisher=Matheson Tri-Gas Incorporated|access-date=11 December 2011|location=USA|date=5 February 2011|url-status=dead|archive-url=https://web.archive.org/web/20111001074639/http://www.chemadvisor.com/Matheson/database/msds/MAT15370000800003.PDF|archive-date=1 October 2011}}</ref> |
|||
| GHSPictograms = {{GHS flame}} |
|||
| GHSSignalWord = '''DANGER''' |
|||
| HPhrases = {{H-phrases|220}} |
|||
| PPhrases = {{P-phrases|210}} |
|||
| NFPA-H = 1 |
|||
| NFPA-F = 4 |
|||
| NFPA-R = 0 |
|||
| NFPA-S = SA |
|||
| FlashPtC = −60 |
|||
| AutoignitionPtC = 405 |
|||
| ExploLimits = 1.8–8.4% |
|||
| PEL = none<ref name=PGCH>{{PGCH|0068}}</ref> |
|||
| IDLH = 1600 ppm<ref name=PGCH/> |
|||
| REL = TWA 800 ppm (1900 mg/m<sup>3</sup>)<ref name=PGCH/> |
|||
}} |
|||
|Section5={{Chembox Related |
|||
| OtherFunction_label = alkanes |
|||
| OtherFunction = {{Unbulleted list|[[Propane]]|[[Isobutane]]|[[Pentane]]}} |
|||
| OtherCompounds = [[Perfluorobutane]] |
|||
}} |
|||
}} |
|||
'''Butane''' ({{IPAc-en|ˈ|b|juː|t|eɪ|n}}) is an [[alkane]] with the formula C<sub>4</sub>H<sub>10</sub>. Butane exists as two isomers, ''n''-butane with connectivity {{chem2|CH3CH2CH2CH3}} and iso-butane with the formula {{chem2|(CH3)3CH}}. Both isomers are highly flammable, colorless, easily [[liquefy|liquefied]] [[gas]]es that quickly vaporize at room temperature and pressure. Butanes are a trace components of [[natural gas]]es (NG gases). The other hydrocarbons in NG include [[propane]], [[ethane]], and especially [[methane]], which are more abundant. [[Liquefied petroleum gas]] is a mixture of propane and some butanes.<ref>{{cite book |doi=10.1002/14356007.a17_073.pub2 |chapter=Natural Gas |title=Ullmann's Encyclopedia of Industrial Chemistry |date=2006 |last1=Hammer |first1=Georg |last2=Lübcke |first2=Torsten |last3=Kettner |first3=Roland |last4=Pillarella |first4=Mark R. |last5=Recknagel |first5=Herta |last6=Commichau |first6=Axel |last7=Neumann |first7=Hans-Joachim |last8=Paczynska-Lahme |first8=Barbara |isbn=978-3-527-30385-4 }}</ref> |
|||
The name butane comes from the root [[IUPAC nomenclature of organic chemistry#Alkanes|but-]] (from [[butyric acid]], named after the Greek word for [[butter]]) and the suffix [[alkanes|-ane]] (for organic compounds). |
|||
== History == |
|||
The first synthesis of butane was accidentally achieved by British chemist [[Edward Francland]] in 1849 from [[ethyl iodide]] and [[zinc]], but he had not realized that the [[ethyl radical]] dimerized and misidentified the substance.<ref>{{cite journal |doi=10.1021/om010439f |title=Zinc Alkyls, Edward Frankland, and the Beginnings of Main-Group Organometallic Chemistry |year=2001 |last1=Seyferth |first1=Dietmar |journal=Organometallics |volume=20 |issue=14 |pages=2940–2955 |doi-access=free}}</ref> |
|||
It was discovered in crude petroleum in 1864 by [[Edmund Ronalds]], who was the first to describe its properties,<ref name="Watts">{{cite book | last1=Watts | first1=H. | last2=Muir | first2=M. M. P. | last3=Morley | first3=H. F. | title=Watts' Dictionary of Chemistry | publisher=Longmans, Green | volume=4 | year=1894 | url=https://books.google.com/books?id=J3kPAQAAIAAJ |page=385}}</ref><ref>{{Cite journal |last=Maybery |first=C. F. |date=1896 |title=On the Composition of the Ohio and Canadian Sulphur Petroleums |journal=Proceedings of the American Academy of Arts and Sciences |volume=31 |pages=1–66 |doi=10.2307/20020618 |jstor=20020618}}</ref> which he named "hydride of [[butyl]]",<ref>{{cite web | url=https://books.google.com/books?id=YyO3j9Yi3nEC&pg=PA54 | title=Journal of the Chemical Society | year=1865 }}</ref> based on the naming for the then-known [[Butyric Acid|butyric acid]], which had been named and described by the French chemist [[Michel Eugène Chevreul]]<ref>Chevreul (1817) [https://books.google.com/books?id=y1E3AAAAYAAJ&pg=PA79 "Extrait d'une lettre de M. Chevreul à MM. les Rédacteurs du Journal de Pharmacie"] (Extract of a letter from Mr. Chevreul to the editors of the Journal of Pharmacy), ''Journal de Pharmacie et des sciences accessoires'', '''3''' : 79–81. On p. 81, he named butyric acid: ''"Ce principe, que j'ai appelé depuis acid butérique, … "'' (This principle [i.e., constituent], which I have since named "butyric acid", … )</ref> 40 years earlier. Other names arose in the 1860s: "butyl hydride",<ref>{{cite web | url=https://books.google.com/books?id=6ss-AAAAcAAJ&pg=PA41 | title=Petroleum and Its Products: An Accoumt of the Properties, Uses, and Commercial Value Etc., of Petroleum, the Methods Employed in Refining it and the Properties, Uses, Etc., of Its Product | last1=Norman Tate | first1=A. | year=1863 }}</ref> "hydride of tetryl"<ref>{{cite web | url=https://books.google.com/books?id=6YvPAAAAMAAJ&pg=PA181 | title=A Dictionary of Chemistry | last1=Watts | first1=Henry | year=1865 }}</ref> and "tetryl hydride",<ref>{{cite web | url=https://books.google.com/books?id=Q7YHAAAAIAAJ&pg=PA277 | title=Elements of chemistry pt. 3 1867 | last1=Miller | first1=William Allen | year=1867 }}</ref> "diethyl" or "ethyl ethylide"<ref>{{cite web | url=https://books.google.com/books?id=b7ktAAAAYAAJ&pg=PA266 | title=Elements of Chemistry: Theoretical and Practical: Organic chemistry | last1=Miller | first1=William Allen | year=1869 }}</ref> and others. [[August Wilhelm von Hofmann]], in his 1866 systemic nomenclature, proposed the name "quartane",<ref name=quartane/> and the modern name was introduced to English from German around 1874.<ref>{{cite web | url=https://books.google.com/books?id=w9MJAAAAMAAJ&pg=PA154 | title=A Manual of the Chemistry of the Carbon Compounds: Or, Organic Chemistry | last1=Schorlemmer | first1=Carl | year=1874 }}</ref> |
|||
Butane did not have much practical use until the 1910s, when W. Snelling identified butane and propane as components in gasoline. He found that if they were cooled, they could be stored in a volume-reduced liquified state in pressurized containers. In 1911, Snelling's liquified petroleum gas was publicly available, and his process for producing the mixture was patented in 1913.<ref>{{Cite web |url=http://blog.texaspropane.com/history-propane/ | title=The History of Propane | author=Texas Propane | year=2022}}</ref> Butane is one of the most produced industrial chemicals in the 21st century, with around 80-90 billion [[Pound (mass)|lbs]] (40 million US tons, 36 million [[Tonne|metric tons]]) produced by the [[United States]] every year.<ref>{{cite web | url=https://www.epa.gov/chemical-data-reporting/chemical-production-data | title=Chemical Production Data |year=2024 }}</ref> |
|||
== Density == |
|||
The density of butane is highly dependent on temperature and pressure in the reservoir.<ref name="Zivenko-2019">{{Cite journal|last=Zivenko|first=Oleksiy|title=LPG Accounting Specificity During ITS Storage and Transportation |date=2019|journal=Measuring Equipment and Metrology|language=en|volume=80|issue=3|pages=21–27|doi=10.23939/istcmtm2019.03.021|s2cid=211776025 |issn=0368-6418|doi-access=free}}</ref> For example, the density of liquid butane is 571.8±1 kg/m<sup>3</sup> (for pressures up to 2 MPa and temperature 27±0.2 °C), while the density of liquid butane is 625.5±0.7 kg/m<sup>3</sup> (for pressures up to 2 MPa and temperature −13±0.2 °C). |
|||
== Isomers == |
|||
{| class="wikitable" style="text-align:center" |
|||
|- |
|- |
||
| style="background:#def;"|Common name |
|||
| bgcolor="#ffeedd" | [[Chemical formula]] |
|||
|'''normal butane'''<br />'''unbranched butane'''<br />'''''n''-butane''' |
|||
| [[Carbon|C]][[Hydrogen|H]]<sub>3</sub>([[Carbon|C]][[Hydrogen|H]]<sub>2</sub>)<sub>2</sub>[[Carbon|C]][[Hydrogen|H]]<sub>3</sub> |
|||
|'''[[isobutane]]'''<br />'''''i''-butane''' |
|||
|- |
|- |
||
| style="background:#def;"|IUPAC name |
|||
| bgcolor="#ffeedd" | [[Molecular weight]] |
|||
|'''butane''' |
|||
| 58.1 g/mol |
|||
|'''methylpropane''' |
|||
|- |
|- |
||
| style="background:#def;"|Molecular<br />diagram |
|||
| bgcolor="#ffeedd" | [[CAS number]] |
|||
|[[Image:Butan Lewis.svg|150px]] |
|||
| 106-97-8 |
|||
|[[Image:Isobutane 1.svg|120px]] |
|||
|- |
|- |
||
| style="background:#def;"|Skeletal<br />diagram |
|||
| bgcolor="#ffeedd" | [[MSDS]] |
|||
|[[Image:Butane simple.svg|120px]] |
|||
| [[Wikisource:{{PAGENAME}} MSDS|{{PAGENAME}} MSDS]] |
|||
|[[Image:I-Butane-2D-Skeletal.svg|100px]] |
|||
|- |
|||
! bgcolor="#ffddaa" colspan="2" | Physical properties |
|||
|- |
|||
| bgcolor="#ffeedd" | [[pH]] (10% [[solution]] with [[water]]) |
|||
| 7.0 |
|||
|- |
|||
! bgcolor="#ffddaa" colspan="2" | Phase behavior |
|||
|- |
|||
| bgcolor="#ffeedd" | [[Melting point]] |
|||
| -138.3 °C (134.9 K) |
|||
|- |
|||
| bgcolor="#ffeedd" | [[Boiling point]] |
|||
| -0.5 °C (272.7 K) |
|||
|- |
|||
| bgcolor="#ffeedd" | [[Heat of vaporization]]<br />(Δ<sub >vap</sub >H) |
|||
| 21 kJ/mol |
|||
|- |
|||
! bgcolor="#ffddaa" colspan="2" | Safety |
|||
|- |
|||
| bgcolor="#ffeedd" | [[Flash point]] |
|||
| -60 °C |
|||
|- |
|||
! bgcolor="#ffeedd" colspan="2" | Precautions |
|||
|- |
|||
| colspan="2" | |
|||
* '''Hazards''': |
|||
** Extremely flammable |
|||
* '''Personal protection''': |
|||
** ? |
|||
* '''Reacts with''': |
|||
** ? |
|||
* '''Storage''': |
|||
** Keep container in a well ventilated place |
|||
|- |
|||
! bgcolor="#ffddaa" colspan="2" | [[Gas]] properties |
|||
|- |
|||
| bgcolor="#ffeedd" | Δ<sub >f</sub >H<sup >0</sup ><sub >gas</sub > |
|||
| -126 kJ/mol |
|||
|- |
|||
| bgcolor="#ffeedd" | S<sup >0</sup ><sub >gas</sub > |
|||
| 310 J/mol·K |
|||
|- |
|||
| bgcolor="#ffeedd" | C<sub >p</sub > |
|||
| 97 J/mol·K |
|||
|- |
|||
| height="50" colspan=2 | |
|||
|- |
|||
! bgcolor="#ffddaa" colspan="2" | General |
|||
|- |
|||
| [[IUPAC nomenclature|Chemical Name]] || '''2-methylpropane''' <br /> Isobutane |
|||
|- |
|||
| bgcolor="#ffeedd" | [[Chemical formula]] |
|||
| [[Carbon|C]][[Hydrogen|H]]<sub>3</sub>[[Carbon|C]][[Hydrogen|H]]([[Carbon|C]][[Hydrogen|H]]<sub>3</sub>)<sub>2</sub> |
|||
|- |
|||
| bgcolor="#ffeedd" | [[Molecular weight]] |
|||
| 58.1 g/mol |
|||
|- |
|||
! bgcolor="#ffddaa" colspan="2" | Phase behavior |
|||
|- |
|||
| bgcolor="#ffeedd" | [[Melting point]] |
|||
| -159.6 °C (113.6 K) |
|||
|- |
|||
| bgcolor="#ffeedd" | [[Boiling point]] |
|||
| -11.7 °C (261.5 K) |
|||
|- |
|||
| bgcolor="#ffddaa" colspan="2" align="center" | |
|||
<small > |
|||
'''Except where noted, all data was produced under conditions of [[standard temperature and pressure]].''' |
|||
</small > |
|||
|} |
|} |
||
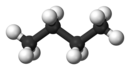
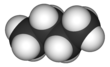
[[Rotation]] about the central C−C [[Chemical bond|bond]] produces two different [[conformational isomerism|conformations]] (''trans'' and ''gauche'') for ''n''-butane.<ref name=Balabin_2009>{{cite journal |journal=[[J. Phys. Chem. A]] |volume = 113 |issue = 6 |pages = 1012–9 |doi=10.1021/jp809639s |title=Enthalpy Difference between Conformations of Normal Alkanes: Raman Spectroscopy Study of ''n''-Pentane and ''n''-Butane |year=2009 |author=Roman M. Balabin|pmid=19152252|bibcode = 2009JPCA..113.1012B |author-link = Roman Balabin }}</ref> |
|||
== Reactions == |
|||
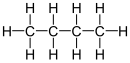
'''Butane''', also called '''''n''-butane''', is the unbranched alkane with four carbon atoms, CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub>. ''Butane'' is also used as a collective term for ''n''-butane together with its only other isomer, '''''iso''-butane''' (also called '''''i''-butane''', '''isobutane''', or '''2-methylpropane'''), CH<sub>3</sub>CH(CH<sub>3</sub>)<sub>2</sub>. |
|||
{{stack| |
|||
[[File:Spectrum of blue flame - intensity corrected.png|thumb|upright=1.15|Spectrum of the blue flame from a [[butane torch]] showing CH molecular [[Radical (chemistry)|radical]] band emission and C<sub>2</sub> [[Swan band]]s]] |
|||
}} |
|||
When oxygen is plentiful, butane undergoes [[complete combustion]] to form [[carbon dioxide]] and [[water vapor]]; when oxygen is limited, due to [[incomplete combustion]], carbon ([[soot]]) or [[carbon monoxide]] may be formed instead of carbon dioxide. Butane is denser than air. |
|||
Butanes are highly flammable, colorless, easily [[liquefy|liquefied]] [[gas]]es. |
|||
When there is sufficient oxygen: |
|||
==Chemical structure== |
|||
: 2 C<sub>4</sub>H<sub>10</sub> + 13 O<sub>2</sub> → 8 CO<sub>2</sub> + 10 H<sub>2</sub>O |
|||
When oxygen is limited: |
|||
: 2 C<sub>4</sub>H<sub>10</sub> + 9 O<sub>2</sub> → 8 CO + 10 H<sub>2</sub>O |
|||
By weight, butane contains about {{convert|49.5|MJ/kg|kWh/kg MJ/lb Btu/lb|lk=on|abbr=on|sp=us}} or by liquid volume {{convert|29.7|MJ/L|kWh/L MJ/usgal Btu/usgal|abbr=out|lk=in|sp=us}}. |
|||
''n''-Butane has the following [[chemical structure]]: |
|||
The maximum [[adiabatic flame]] temperature of butane with air is {{convert|2243|K}}. |
|||
H H H H |
|||
| | | | |
|||
H - C - C - C - C - H |
|||
| | | | |
|||
H H H H |
|||
''n''-Butane is the feedstock for [[DuPont]]'s catalytic process for the preparation of [[maleic anhydride]]: |
|||
Isobutane, on the other hand, has a branched-chain structure: |
|||
:2 CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub> + 7 O<sub>2</sub> → 2 C<sub>2</sub>H<sub>2</sub>(CO)<sub>2</sub>O + 8 H<sub>2</sub>O |
|||
''n''-Butane, like all [[Hydrocarbon|hydrocarbons]], undergoes [[free radical]] chlorination providing both 1-chloro- and 2-chlorobutanes, as well as more highly chlorinated derivatives. The relative rates of the chlorinations are partially explained by the differing [[bond dissociation energies]]: 425 and 411 [[joule|kJ]]/[[Mole (unit)|mol]] for the two types of C-H bonds. |
|||
[[Image:N&i-butane.png]] |
|||
== |
== Uses == |
||
Normal butane can be used for [[gasoline]] blending, as a fuel gas, fragrance extraction solvent, either alone or in a mixture with [[propane]], and as a feedstock for the manufacture of [[ethylene]] and [[butadiene]], a key ingredient of [[synthetic rubber]]. [[Isobutane]] is primarily used by [[refineries]] to enhance (increase) the [[octane]] number of motor gasoline.<ref>[https://www.sec.gov/Archives/edgar/data/1166036/000104746912001759/a2207469z10-k.htm MarkWest Energy Partners, L.P. Form 10-K]. Sec.gov.</ref><ref>[https://www.sec.gov/Archives/edgar/data/1297067/000119312512089552/d270993d10k.htm Copano Energy, L.L.C. Form 10-K]. Sec.gov. Retrieved on 2012-12-03.</ref><ref>[https://www.sec.gov/Archives/edgar/data/1379661/000138917012000005/form10-k.htm Targa Resources Partners LP Form10-k]. Sec.gov. Retrieved on 2012-12-03.</ref><ref>[https://www.sec.gov/Archives/edgar/data/1179060/000104746912001738/a2207540z10-k.htm Crosstex Energy, L.P. FORM 10-K]. Sec.gov.</ref> |
|||
For gasoline blending, n-butane is the main component used to manipulate the [[Reid vapor pressure]] (RVP). Since winter fuels require much higher vapor pressure for engines to start, refineries raise the RVP by blending more butane into the fuel.<ref>{{Cite web|last=Maurice Stewart, Ken Arnold|title=Reid Vapour Pressure|url=https://www.sciencedirect.com/topics/engineering/reid-vapour-pressure|url-status=live|website=Science Direct|archive-url=https://web.archive.org/web/20200613130216/https://www.sciencedirect.com/topics/engineering/reid-vapour-pressure |archive-date=13 June 2020 }}</ref> n-Butane has a relatively high [[Octane rating#Research_Octane_Number_(RON)|research octane number]] (RON) and [[Octane rating#Motor_Octane_Number_(MON)|motor octane number]] (MON), which are 93 and 92 respectively.<ref>{{Cite web|last=Jechura|first=John|title=octane rating|url=https://inside.mines.edu/~jjechura/Refining/11_Blending_Optimization.pdf|url-status=live|website=Colorado School of Mines|archive-url=https://web.archive.org/web/20150501041749/http://inside.mines.edu:80/~jjechura/Refining/11_Blending_Optimization.pdf |archive-date=1 May 2015 }}</ref> |
|||
When air is plentiful, butane burns to form carbon dioxide and steam: |
|||
When blended with [[propane]] and other hydrocarbons, the mixture may be referred to commercially as [[liquefied petroleum gas]] (LPG). It is used as a petrol component, as a feedstock for the production of base [[petrochemicals]] in [[steam cracking]], as fuel for cigarette [[lighter]]s and as a [[propellant]] in [[aerosol spray]]s such as [[deodorant]]s.<ref>[http://eprinc.org/?p=300 A Primer on Gasoline Blending] {{Webarchive|url=https://web.archive.org/web/20130630221725/http://eprinc.org/?p=300 |date=30 June 2013 }}. An EPRINC Briefing Memorandum.</ref> |
|||
butane + oxygen -> carbon dioxide + steam |
|||
Pure forms of butane, especially isobutane, are used as [[refrigerant]]s and have largely replaced the [[Ozone depletion|ozone-layer-depleting]] [[halomethane]]s in refrigerators, freezers, and air conditioning systems. The operating pressure for butane is lower than operating pressures for halomethanes such as [[Dichlorodifluoromethane|Freon-12]] (R-12). Hence, R-12 systems, such as those in automotive air conditioning systems, when converted to pure butane, will function poorly. Instead, a mixture of isobutane and propane is used to give cooling system performance comparable to R-12.<ref>{{Cite web |title=R600a {{!}} Product Information |url=https://www.agas.com/au/products-services/refrigerants/r600a/ |access-date=2023-12-01 |website=www.agas.com |language=en-AU}}</ref> |
|||
When air is limited, carbon (soot) or carbon monoxide may also be formed. |
|||
Butane is also used as lighter fuel for common [[lighter]]s or [[butane torch]]es, and is sold bottled as a fuel for cooking, barbecues and camping stoves. In the 20th century, the [[Braun (company)|Braun]] company of [[Germany]] made a cordless hair styling device product that used butane as its heat source to produce [[steam]].<ref name="braun">{{cite news |title=Braun C 100 TS Styling Iron User Manual Type 3589 |url=http://personalcare.manualsonline.com/manuals/mfg/braun/c_100_ts_1.html |publisher=Inmar-OIQ, LLC |date=n.d.}}</ref> |
|||
Butane gas is sold bottled as a fuel for cooking and camping, in which case it is referred to commercially as [[Liquified_petroleum_gas|LPG]], or, in the UK, ''calor gas''. It is also used as a petrol component, as a feedstock for the production of base petrochemicals in steam cracking, as fuel for [[cigarette lighter]]s and as a [[propellant]] in [[aerosol spray]]s. |
|||
As fuel, butane is often mixed with small amounts of [[mercaptan]]s to give the unburned gas an offensive smell easily detected by the human nose. In this way, butane leaks can easily be identified. While [[hydrogen sulfide]] and mercaptans are toxic, they are present in levels so low that [[suffocation]] and [[fire hazard]] by the butane becomes a concern far before [[toxicity]].<ref name="Gresham 2019">{{cite web | last=Gresham | first=Chip | title=Hydrogen Sulfide Toxicity: Practice Essentials, Pathophysiology, Etiology | website=Medscape Reference | date=16 November 2019 | url=https://emedicine.medscape.com/article/815139 | access-date=22 March 2021 |url-access=registration}}</ref><ref name="Toxicology2013">{{cite book |author1=Committee on Acute Exposure Guideline Levels |author2=Committee on Toxicology |author3=Board on Environmental Studies and Toxicology |author4=Division on Earth and Life Studies |author5=National Research Council |title=2. Methyl Mercaptan Acute Exposure Guideline Levels |via=NCBI Bookshelf |date=26 September 2013 |publisher=National Academies Press (US) |url=https://www.ncbi.nlm.nih.gov/books/NBK201324/ }}</ref> Most commercially available butane also contains some contaminant oil, which can be removed by filtration. If not removed, it will otherwise leave a deposit at the point of ignition and may eventually block the uniform flow of gas.<ref>{{Cite web |title=BHO Mystery Oil |date=2013-08-26 |website=Skunk Pharm Research |url=https://skunkpharmresearch.com/bho-mystery-oil/ |access-date=2019-12-05}}</ref> |
|||
Recent concerns with depletion of the [[ozone layer]] by [[freon]] gases have led to increased use of isobutane as a gas for [[refrigeration]] systems, especially in domestic [[refrigerator]]s and [[freezer]]s. |
|||
When used as a [[refrigerant]], isobutane is also known as [[R600a]]. |
|||
The butane used as a solvent for fragrance extraction does not contain these contaminants.<ref name="SAGE">{{cite journal |title=Final Report of the Safety Assessment of Isobutane, Isopentane, n-Butane, and Propane |journal=Journal of the American College of Toxicology |publisher=SAGE Publications |volume=1 |issue=4 |year=1982 |issn=0730-0913 |doi=10.3109/10915818209021266 |pages=127–142| s2cid=208503534}}</ref> Butane gas can cause [[Gas explosion|gas explosions]] in poorly ventilated areas if leaks go unnoticed and are ignited by spark or flame.<ref name="chemadvisor"/> Purified butane is used as a solvent in the industrial extraction of cannabis oils. |
|||
== See also == |
|||
<gallery mode="packed" heights="140"> |
|||
File:Photo D2.jpg | Butane fuel canisters for use in camping stoves |
|||
File:The Green Lighter 1 cropped.jpg | Butane lighter, showing liquid butane reservoir |
|||
File:Aerosol.png | An aerosol spray can, which may be using butane as a propellant |
|||
File:ButaneGasCylinder WhiteBack.jpg | Butane gas cylinder used for cooking |
|||
</gallery> |
|||
== Health effects == |
|||
[[File:HarmCausedByDrugsTable.svg|thumb|upright=1.35|Table from the 2010 ISCD study ranking various drugs (legal and illegal) based on statements by drug-harm experts. Butane was found to be the 14th overall most dangerous drug.<ref name="Nutt_2010">{{cite journal | vauthors = Nutt DJ, King LA, Phillips LD | title = Drug harms in the UK: a multicriteria decision analysis | journal = Lancet | volume = 376 | issue = 9752 | pages = 1558–1565 | date = November 2010 | pmid = 21036393 | doi = 10.1016/S0140-6736(10)61462-6 | s2cid = 5667719 | citeseerx = 10.1.1.690.1283 }}</ref>]] |
|||
Inhalation of butane can cause [[euphoria]], [[drowsiness]], [[unconsciousness]], [[asphyxia]], [[cardiac arrhythmia]], fluctuations in blood pressure and temporary memory loss, when abused directly from a highly pressurized container, and can result in death from [[asphyxiation]] and [[ventricular fibrillation]]. Butane enters the blood supply, and within seconds, leads to intoxication.<ref>{{cite web |title=Neurotoxic Effects from Butane Gas |date=19 Dec 2009 |website=thcfarmer.com |url=https://www.thcfarmer.com/community/threads/neurotoxic-effects-from-butane-gas.15291/ |access-date=3 October 2016}}</ref> Butane is the most commonly abused [[Volatile substance abuse|volatile]] substance in the UK, and was the cause of 52% of solvent related deaths in 2000.<ref>{{cite web |title=Trends in death Associated with Abuse of Volatile Substances 1971–2004 |vauthors=Field-Smith M, Bland JM, Taylor JC |publisher=Department of Public Health Sciences. London: St George’s Medical School |url=http://www.sgul.ac.uk/dms/AF54AFD9D207A9A41D353717989DC4E0.pdf |display-authors=etal |url-status=dead |archive-url=https://web.archive.org/web/20070327161634/http://www.sgul.ac.uk/dms/AF54AFD9D207A9A41D353717989DC4E0.pdf |archive-date=March 27, 2007}}</ref> By spraying butane directly into the throat, the jet of fluid can cool rapidly to {{convert|−20|C}} by expansion, causing prolonged [[laryngospasm]].<ref name="multiple">{{cite journal |vauthors=Ramsey J, Anderson HR, Bloor K |display-authors=etal |title=An introduction to the practice, prevalence and chemical toxicology of volatile substance abuse |journal=Hum Toxicol |year=1989 |volume=8 |pages=261–269 |doi=10.1177/096032718900800403 |pmid=2777265 |issue=4| s2cid=19617950}}</ref> [[Intoxicative inhalant#Sudden sniffing death syndrome|"Sudden sniffer's death"]] syndrome, first described by Bass in 1970,<ref>{{cite journal |vauthors=Bass M |title=Sudden sniffing death |journal=JAMA |year=1970 |volume=212 |issue=12 |pages=2075–2079 |doi=10.1001/jama.1970.03170250031004 |pmid=5467774}}</ref> is the most common single cause of solvent related deaths, resulting in 55% of known fatal cases.<ref name="multiple"/> |
|||
== See also == |
|||
* [[Cyclobutane]] |
|||
* [[Volatile substance abuse]] |
* [[Volatile substance abuse]] |
||
* [[Butane (data page)]] |
|||
* [[Industrial gas]] |
|||
== References == |
|||
{{Reflist}} |
|||
== External links == |
== External links == |
||
{{commons}} |
|||
* [http://www.inchem.org/documents/icsc/icsc/eics0232.htm International Chemical Safety Card 0232] |
|||
* [https://www.cdc.gov/niosh/npg/npgd0068.html NIOSH Pocket Guide to Chemical Hazards] |
|||
{{Alkanes}} |
|||
* [http://www.it.swin.edu.au/personal/fwang/Mom/Mom_butane.html ''n''-Butane], [http://www.chm.bris.ac.uk/motm/motm.htm Molecule of the Month]. |
|||
{{Hydrides by group}} |
|||
{{E number infobox 930-949}} |
|||
{{alkanes}} |
|||
{{GABAAR PAMs}} |
|||
{{Authority control}} |
|||
[[Category:Butane| ]] |
|||
[[Category:Alkanes]] |
[[Category:Alkanes]] |
||
[[Category:Fuel gas]] |
|||
[[Category:Refrigerants]] |
|||
[[ca:Butà]] |
|||
[[Category:GABAA receptor positive allosteric modulators]] |
|||
[[de:Butan]] |
|||
[[Category:E-number additives]] |
|||
[[es:Butano]] |
|||
[[eo:Butano]] |
|||
[[fr:Butane]] |
|||
[[nl:Butaan]] |
|||
[[pt:Butano]] |
|||
[[ja:ブタン]] |
|||
[[ru:Бутан (вещество)]] |
|||
[[sl:Butan (plin)]] |
|||
[[sv:Butan]] |
|||
Latest revision as of 07:41, 22 December 2024
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Butane[3] | |||
| Systematic IUPAC name
Tetracarbane (never recommended[3]) | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 969129 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.136 | ||
| EC Number |
| ||
| E number | E943a (glazing agents, ...) | ||
| 1148 | |||
| KEGG | |||
| MeSH | butane | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1011 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H10 | |||
| Molar mass | 58.124 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Gasoline-like or natural gas-like[1] | ||
| Density | 2.48 kg/m3 (at 15 °C (59 °F)) | ||
| Melting point | −140 to −134 °C; −220 to −209 °F; 133 to 139 K | ||
| Boiling point | −1 to 1 °C; 30 to 34 °F; 272 to 274 K | ||
| 61 mg/L (at 20 °C (68 °F)) | |||
| log P | 2.745 | ||
| Vapor pressure | ~170 kPa at 283 K [4] | ||
Henry's law
constant (kH) |
11 nmol Pa−1 kg−1 | ||
| −57.4·10−6 cm3/mol | |||
| Thermochemistry | |||
Heat capacity (C)
|
98.49 J/(K·mol) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−126.3–−124.9 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−2.8781–−2.8769 MJ/mol | ||
| Hazards[5] | |||
| GHS labelling: | |||

| |||
| Danger | |||
| H220 | |||
| P210 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −60 °C (−76 °F; 213 K) | ||
| 405 °C (761 °F; 678 K) | |||
| Explosive limits | 1.8–8.4% | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[1] | ||
REL (Recommended)
|
TWA 800 ppm (1900 mg/m3)[1] | ||
IDLH (Immediate danger)
|
1600 ppm[1] | ||
| Related compounds | |||
Related alkanes
|
|||
Related compounds
|
Perfluorobutane | ||
| Supplementary data page | |||
| Butane (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Butane (/ˈbjuːteɪn/) is an alkane with the formula C4H10. Butane exists as two isomers, n-butane with connectivity CH3CH2CH2CH3 and iso-butane with the formula (CH3)3CH. Both isomers are highly flammable, colorless, easily liquefied gases that quickly vaporize at room temperature and pressure. Butanes are a trace components of natural gases (NG gases). The other hydrocarbons in NG include propane, ethane, and especially methane, which are more abundant. Liquefied petroleum gas is a mixture of propane and some butanes.[6]
The name butane comes from the root but- (from butyric acid, named after the Greek word for butter) and the suffix -ane (for organic compounds).
History
[edit]The first synthesis of butane was accidentally achieved by British chemist Edward Francland in 1849 from ethyl iodide and zinc, but he had not realized that the ethyl radical dimerized and misidentified the substance.[7]
It was discovered in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its properties,[8][9] which he named "hydride of butyl",[10] based on the naming for the then-known butyric acid, which had been named and described by the French chemist Michel Eugène Chevreul[11] 40 years earlier. Other names arose in the 1860s: "butyl hydride",[12] "hydride of tetryl"[13] and "tetryl hydride",[14] "diethyl" or "ethyl ethylide"[15] and others. August Wilhelm von Hofmann, in his 1866 systemic nomenclature, proposed the name "quartane",[2] and the modern name was introduced to English from German around 1874.[16]
Butane did not have much practical use until the 1910s, when W. Snelling identified butane and propane as components in gasoline. He found that if they were cooled, they could be stored in a volume-reduced liquified state in pressurized containers. In 1911, Snelling's liquified petroleum gas was publicly available, and his process for producing the mixture was patented in 1913.[17] Butane is one of the most produced industrial chemicals in the 21st century, with around 80-90 billion lbs (40 million US tons, 36 million metric tons) produced by the United States every year.[18]
Density
[edit]The density of butane is highly dependent on temperature and pressure in the reservoir.[19] For example, the density of liquid butane is 571.8±1 kg/m3 (for pressures up to 2 MPa and temperature 27±0.2 °C), while the density of liquid butane is 625.5±0.7 kg/m3 (for pressures up to 2 MPa and temperature −13±0.2 °C).
Isomers
[edit]| Common name | normal butane unbranched butane n-butane |
isobutane i-butane |
| IUPAC name | butane | methylpropane |
| Molecular diagram |

|

|
| Skeletal diagram |

|
Rotation about the central C−C bond produces two different conformations (trans and gauche) for n-butane.[20]
Reactions
[edit]
When oxygen is plentiful, butane undergoes complete combustion to form carbon dioxide and water vapor; when oxygen is limited, due to incomplete combustion, carbon (soot) or carbon monoxide may be formed instead of carbon dioxide. Butane is denser than air.
When there is sufficient oxygen:
- 2 C4H10 + 13 O2 → 8 CO2 + 10 H2O
When oxygen is limited:
- 2 C4H10 + 9 O2 → 8 CO + 10 H2O
By weight, butane contains about 49.5 MJ/kg (13.8 kWh/kg; 22.5 MJ/lb; 21,300 Btu/lb) or by liquid volume 29.7 megajoules per liter (8.3 kWh/L; 112 MJ/U.S. gal; 107,000 Btu/U.S. gal).
The maximum adiabatic flame temperature of butane with air is 2,243 K (1,970 °C; 3,578 °F).
n-Butane is the feedstock for DuPont's catalytic process for the preparation of maleic anhydride:
- 2 CH3CH2CH2CH3 + 7 O2 → 2 C2H2(CO)2O + 8 H2O
n-Butane, like all hydrocarbons, undergoes free radical chlorination providing both 1-chloro- and 2-chlorobutanes, as well as more highly chlorinated derivatives. The relative rates of the chlorinations are partially explained by the differing bond dissociation energies: 425 and 411 kJ/mol for the two types of C-H bonds.
Uses
[edit]Normal butane can be used for gasoline blending, as a fuel gas, fragrance extraction solvent, either alone or in a mixture with propane, and as a feedstock for the manufacture of ethylene and butadiene, a key ingredient of synthetic rubber. Isobutane is primarily used by refineries to enhance (increase) the octane number of motor gasoline.[21][22][23][24]
For gasoline blending, n-butane is the main component used to manipulate the Reid vapor pressure (RVP). Since winter fuels require much higher vapor pressure for engines to start, refineries raise the RVP by blending more butane into the fuel.[25] n-Butane has a relatively high research octane number (RON) and motor octane number (MON), which are 93 and 92 respectively.[26]
When blended with propane and other hydrocarbons, the mixture may be referred to commercially as liquefied petroleum gas (LPG). It is used as a petrol component, as a feedstock for the production of base petrochemicals in steam cracking, as fuel for cigarette lighters and as a propellant in aerosol sprays such as deodorants.[27]
Pure forms of butane, especially isobutane, are used as refrigerants and have largely replaced the ozone-layer-depleting halomethanes in refrigerators, freezers, and air conditioning systems. The operating pressure for butane is lower than operating pressures for halomethanes such as Freon-12 (R-12). Hence, R-12 systems, such as those in automotive air conditioning systems, when converted to pure butane, will function poorly. Instead, a mixture of isobutane and propane is used to give cooling system performance comparable to R-12.[28]
Butane is also used as lighter fuel for common lighters or butane torches, and is sold bottled as a fuel for cooking, barbecues and camping stoves. In the 20th century, the Braun company of Germany made a cordless hair styling device product that used butane as its heat source to produce steam.[29]
As fuel, butane is often mixed with small amounts of mercaptans to give the unburned gas an offensive smell easily detected by the human nose. In this way, butane leaks can easily be identified. While hydrogen sulfide and mercaptans are toxic, they are present in levels so low that suffocation and fire hazard by the butane becomes a concern far before toxicity.[30][31] Most commercially available butane also contains some contaminant oil, which can be removed by filtration. If not removed, it will otherwise leave a deposit at the point of ignition and may eventually block the uniform flow of gas.[32]
The butane used as a solvent for fragrance extraction does not contain these contaminants.[33] Butane gas can cause gas explosions in poorly ventilated areas if leaks go unnoticed and are ignited by spark or flame.[5] Purified butane is used as a solvent in the industrial extraction of cannabis oils.
-
Butane fuel canisters for use in camping stoves
-
Butane lighter, showing liquid butane reservoir
-
An aerosol spray can, which may be using butane as a propellant
-
Butane gas cylinder used for cooking
Health effects
[edit]
Inhalation of butane can cause euphoria, drowsiness, unconsciousness, asphyxia, cardiac arrhythmia, fluctuations in blood pressure and temporary memory loss, when abused directly from a highly pressurized container, and can result in death from asphyxiation and ventricular fibrillation. Butane enters the blood supply, and within seconds, leads to intoxication.[35] Butane is the most commonly abused volatile substance in the UK, and was the cause of 52% of solvent related deaths in 2000.[36] By spraying butane directly into the throat, the jet of fluid can cool rapidly to −20 °C (−4 °F) by expansion, causing prolonged laryngospasm.[37] "Sudden sniffer's death" syndrome, first described by Bass in 1970,[38] is the most common single cause of solvent related deaths, resulting in 55% of known fatal cases.[37]
See also
[edit]References
[edit]- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0068". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b August Wilhelm Von Hofmann (1867). "I. On the action of trichloride of phosphorus on the salts of the aromatic monamines". Proceedings of the Royal Society of London. 15: 54–62. doi:10.1098/rspl.1866.0018. S2CID 98496840.
- ^ a b "General Principles, Rules, and Conventions". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. P-12.1. doi:10.1039/9781849733069-00001. ISBN 978-0-85404-182-4.
Similarly, the retained names 'ethane', 'propane', and 'butane' were never replaced by systematic names 'dicarbane', 'tricarbane', and 'tetracarbane' as recommended for analogues of silane, 'disilane'; phosphane, 'triphosphane'; and sulfane, 'tetrasulfane'.
- ^ W. B. Kay (1940). "Pressure-Volume-Temperature Relations for n-Butane". Industrial & Engineering Chemistry. 32 (3): 358–360. doi:10.1021/ie50363a016.
- ^ a b "Safety Data Sheet, Material Name: N-Butane" (PDF). USA: Matheson Tri-Gas Incorporated. 5 February 2011. Archived from the original (PDF) on 1 October 2011. Retrieved 11 December 2011.
- ^ Hammer, Georg; Lübcke, Torsten; Kettner, Roland; Pillarella, Mark R.; Recknagel, Herta; Commichau, Axel; Neumann, Hans-Joachim; Paczynska-Lahme, Barbara (2006). "Natural Gas". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a17_073.pub2. ISBN 978-3-527-30385-4.
- ^ Seyferth, Dietmar (2001). "Zinc Alkyls, Edward Frankland, and the Beginnings of Main-Group Organometallic Chemistry". Organometallics. 20 (14): 2940–2955. doi:10.1021/om010439f.
- ^ Watts, H.; Muir, M. M. P.; Morley, H. F. (1894). Watts' Dictionary of Chemistry. Vol. 4. Longmans, Green. p. 385.
- ^ Maybery, C. F. (1896). "On the Composition of the Ohio and Canadian Sulphur Petroleums". Proceedings of the American Academy of Arts and Sciences. 31: 1–66. doi:10.2307/20020618. JSTOR 20020618.
- ^ "Journal of the Chemical Society". 1865.
- ^ Chevreul (1817) "Extrait d'une lettre de M. Chevreul à MM. les Rédacteurs du Journal de Pharmacie" (Extract of a letter from Mr. Chevreul to the editors of the Journal of Pharmacy), Journal de Pharmacie et des sciences accessoires, 3 : 79–81. On p. 81, he named butyric acid: "Ce principe, que j'ai appelé depuis acid butérique, … " (This principle [i.e., constituent], which I have since named "butyric acid", … )
- ^ Norman Tate, A. (1863). "Petroleum and Its Products: An Accoumt of the Properties, Uses, and Commercial Value Etc., of Petroleum, the Methods Employed in Refining it and the Properties, Uses, Etc., of Its Product".
- ^ Watts, Henry (1865). "A Dictionary of Chemistry".
- ^ Miller, William Allen (1867). "Elements of chemistry pt. 3 1867".
- ^ Miller, William Allen (1869). "Elements of Chemistry: Theoretical and Practical: Organic chemistry".
- ^ Schorlemmer, Carl (1874). "A Manual of the Chemistry of the Carbon Compounds: Or, Organic Chemistry".
- ^ Texas Propane (2022). "The History of Propane".
- ^ "Chemical Production Data". 2024.
- ^ Zivenko, Oleksiy (2019). "LPG Accounting Specificity During ITS Storage and Transportation". Measuring Equipment and Metrology. 80 (3): 21–27. doi:10.23939/istcmtm2019.03.021. ISSN 0368-6418. S2CID 211776025.
- ^ Roman M. Balabin (2009). "Enthalpy Difference between Conformations of Normal Alkanes: Raman Spectroscopy Study of n-Pentane and n-Butane". J. Phys. Chem. A. 113 (6): 1012–9. Bibcode:2009JPCA..113.1012B. doi:10.1021/jp809639s. PMID 19152252.
- ^ MarkWest Energy Partners, L.P. Form 10-K. Sec.gov.
- ^ Copano Energy, L.L.C. Form 10-K. Sec.gov. Retrieved on 2012-12-03.
- ^ Targa Resources Partners LP Form10-k. Sec.gov. Retrieved on 2012-12-03.
- ^ Crosstex Energy, L.P. FORM 10-K. Sec.gov.
- ^ Maurice Stewart, Ken Arnold. "Reid Vapour Pressure". Science Direct. Archived from the original on 13 June 2020.
- ^ Jechura, John. "octane rating" (PDF). Colorado School of Mines. Archived (PDF) from the original on 1 May 2015.
- ^ A Primer on Gasoline Blending Archived 30 June 2013 at the Wayback Machine. An EPRINC Briefing Memorandum.
- ^ "R600a | Product Information". www.agas.com. Retrieved 1 December 2023.
- ^ "Braun C 100 TS Styling Iron User Manual Type 3589". Inmar-OIQ, LLC. n.d.
- ^ Gresham, Chip (16 November 2019). "Hydrogen Sulfide Toxicity: Practice Essentials, Pathophysiology, Etiology". Medscape Reference. Retrieved 22 March 2021.
- ^ Committee on Acute Exposure Guideline Levels; Committee on Toxicology; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies; National Research Council (26 September 2013). 2. Methyl Mercaptan Acute Exposure Guideline Levels. National Academies Press (US) – via NCBI Bookshelf.
- ^ "BHO Mystery Oil". Skunk Pharm Research. 26 August 2013. Retrieved 5 December 2019.
- ^ "Final Report of the Safety Assessment of Isobutane, Isopentane, n-Butane, and Propane". Journal of the American College of Toxicology. 1 (4). SAGE Publications: 127–142. 1982. doi:10.3109/10915818209021266. ISSN 0730-0913. S2CID 208503534.
- ^ Nutt DJ, King LA, Phillips LD (November 2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–1565. CiteSeerX 10.1.1.690.1283. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. S2CID 5667719.
- ^ "Neurotoxic Effects from Butane Gas". thcfarmer.com. 19 December 2009. Retrieved 3 October 2016.
- ^ Field-Smith M, Bland JM, Taylor JC, et al. "Trends in death Associated with Abuse of Volatile Substances 1971–2004" (PDF). Department of Public Health Sciences. London: St George’s Medical School. Archived from the original (PDF) on 27 March 2007.
- ^ a b Ramsey J, Anderson HR, Bloor K, et al. (1989). "An introduction to the practice, prevalence and chemical toxicology of volatile substance abuse". Hum Toxicol. 8 (4): 261–269. doi:10.1177/096032718900800403. PMID 2777265. S2CID 19617950.
- ^ Bass M (1970). "Sudden sniffing death". JAMA. 212 (12): 2075–2079. doi:10.1001/jama.1970.03170250031004. PMID 5467774.