Biodiesel: Difference between revisions
m link climate change |
|||
| Line 1: | Line 1: | ||
{{Short description|Fuel made from vegetable oils or animal fats}} |
|||
{{Three other uses|transesterified lipids|hydrogenated alkane renewable diesel|vegetable oil refining|biomass and organic waste to fuel production|Biomass to liquid|unmodified vegetable oil used as motor fuel|Vegetable oil used as fuel}} |
|||
{{redirect|Green diesel|dyed fuel|Fuel dyes}} |
|||
[[Image:Soybus.jpg|right|thumb|200px|Bus run by '''Biodiesel''']] |
|||
{{About|transesterified liquids|hydrogenated alkane renewable diesel|hydrotreated vegetable oil|biomass and organic waste-to-fuel production|Biomass to liquid|unmodified vegetable oil used as motor fuel|Vegetable oil fuel}} |
|||
[[Image:Methyl Linoleate.png|thumb|200px|Space-filling model of Methyl Linoleate, or Linoleic Acid Methyl Ester, a common Methyl Ester produced from Soybean or Canola oil and Methanol.]] [[Image:Ethyl Stearate.png|thumb|200px|Space-filling model of Ethyl Stearate, or Stearic Acid Ethyl Ester, an Ethyl Ester produced from Soybean or Canola oil and Ethanol.]] |
|||
{{Overly detailed|nosplit=nosplit|date=January 2023}} |
|||
{{broader|Biofuel}} |
|||
[[File:Régiolis Granville-Paris en gare d'Argentan (août 2019).JPG|thumb|Experimental French [[Régiolis]] Class train using biodiesel]] |
|||
[[File:Ethyl Stearate.png|thumb|Space-filling model of ethyl stearate, or stearic acid ethyl ester, an ethyl ester produced from soybean or canola oil and ethanol]] |
|||
[[File:BiodieselRoutes.svg|thumb|Two general pathways for biodiesels from a fat. The process starts with [[hydrogenation]] of backbone double bonds. [[Fatty acid methyl ester]]s can then be produced by transesterification. C16 and C18 diesel fuels arise by hydrogenolysis of the saturated fat.]] |
|||
'''Biodiesel''' is a [[renewable]] [[biofuel]], a form of [[diesel fuel]], derived from biological sources like vegetable oils, animal fats, or recycled greases, and consisting of long-chain [[fatty acid ester]]s. It is typically made from fats.<ref>{{cite book |doi=10.1002/0471238961.trigmurz.a01 |chapter=Triglycerides and Oils for Biofuels |title=Kirk-Othmer Encyclopedia of Chemical Technology |date=2012 |last1=Murzin |first1=Dmitry Yu. |last2=Mäki-Arvela |first2=Päivi |last3=Simakova |first3=Irina L. |pages=1–14 |isbn=978-0-471-48494-3 }}</ref><ref>{{cite book |doi=10.1002/0471238961.0621051211120119.a01.pub2 |chapter=Biomass Energy |title=Kirk-Othmer Encyclopedia of Chemical Technology |date=2003 |last1=Paisley |first1=Mark A. |isbn=978-0-471-48494-3 }}</ref><ref>{{cite journal |last1=Huang |first1=Daming |last2=Zhou |first2=Haining |last3=Lin |first3=Lin |title=Biodiesel: an Alternative to Conventional Fuel |journal=[[Energy Procedia]] |date=2012 |volume=16 |issue=Part C |pages=1874–1885 |doi=10.1016/j.egypro.2012.01.287 |doi-access=free}}</ref> |
|||
'''Biodiesel''' refers to a non-petroleum-based [[diesel]] fuel consisting of short chain [[alkyl]] ([[methyl]] or [[ethyl]]) [[ester]]s, typically made by [[transesterification]] of [[vegetable oil]]s or [[animal fat]]s, which can be used (alone, or blended with conventional petrodiesel) in unmodified [[diesel-engine]] vehicles. Biodiesel is distinguished from the ''[[straight vegetable oil]]'' (SVO) (aka "waste vegetable oil", "WVO", "unwashed biodiesel", "pure plant oil", "PPO") used (alone, or blended) as fuels in some ''converted'' diesel vehicles. "Biodiesel" is standardized as mono-alkyl esters and other non-diesel fuels of biological origin are not included.<ref name=NBB>{{cite web |
|||
|title=Biodiesel 101 - Biodiesel Definitions |
|||
The roots of biodiesel as a fuel source can be traced back to when J. Patrick and E. Duffy first conducted [[transesterification]] of vegetable oil in 1853, predating [[Rudolf Diesel]]'s development of the diesel engine.<ref>{{Cite journal |last=Demirbaş |first=Ayhan |date=2002-11-01 |title=Biodiesel from vegetable oils via transesterification in supercritical methanol |url=https://www.sciencedirect.com/science/article/pii/S0196890401001704 |journal=Energy Conversion and Management |volume=43 |issue=17 |pages=2349–2356 |doi=10.1016/S0196-8904(01)00170-4 |issn=0196-8904}}</ref> Diesel's engine, initially designed for mineral oil, successfully ran on peanut oil at the [[1900 Paris Exposition]]. This landmark event highlighted the potential of vegetable oils as an alternative fuel source. The interest in using vegetable oils as fuels resurfaced periodically, particularly during resource-constrained periods such as World War II. However, challenges such as high viscosity and resultant engine deposits were significant hurdles. The modern form of biodiesel emerged in the 1930s, when a method was found for transforming vegetable oils for fuel use, laying the groundwork for contemporary biodiesel production. |
|||
|publisher=National Biodiesel Board |
|||
|url=http://www.biodiesel.org/resources/definitions/default.shtm |
|||
The physical and chemical properties of biodiesel vary depending on its source and production method. The US [[National Biodiesel Board]] defines "biodiesel" as a mono-alkyl ester.<ref name=NBB>{{cite web|title=Biodiesel Basics|publisher=National Biodiesel Board|url=http://www.biodiesel.org/what-is-biodiesel/biodiesel-basics|format=?|access-date=2013-01-29|archive-date=2014-08-04|archive-url=https://web.archive.org/web/20140804155702/http://www.biodiesel.org/what-is-biodiesel/biodiesel-basics|url-status=live}}</ref> It has been experimented with in railway locomotives and power generators. Generally characterized by a higher boiling point and flash point than petrodiesel, biodiesel is slightly [[miscible]] with water and has distinct lubricating properties. Its calorific value is approximately 9% lower than that of standard diesel, impacting [[fuel efficiency]]. Biodiesel production has evolved significantly, with early methods including the direct use of vegetable oils, to more advanced processes like transesterification, which reduces viscosity and improves combustion properties. Notably, biodiesel production generates glycerol as a by-product, which has its own commercial applications. |
|||
|format=? |
|||
|accessdate=2008-2-16}}</ref> |
|||
Biodiesel's primary application is in transport. There have been efforts to make it a [[Drop-in biofuels|drop-in biofuel]], meaning compatible with existing diesel engines and distribution infrastructure. However, it is usually blended with [[petrodiesel]], typically to less than 10%, since most engines cannot run on pure biodiesel without modification.<ref name="sciencedirect.com">{{cite journal|last1=Omidvarborna|title=Characterization of particulate matter emitted from transit buses fueled with B20 in idle modes|journal=Journal of Environmental Chemical Engineering|volume=2|issue=4|pages=2335–2342|doi=10.1016/j.jece.2014.09.020|display-authors=etal|date=December 2014}}</ref><ref>{{Cite web |url=http://www.vtt.fi/inf/pdf/technology/2012/T46.pdf |title=Nylund.N-O & Koponen.K. 2013. Fuel and Technology Alternatives for Buses. Overall Energy Efficiency and Emission Performance. IEA Bioenergy Task 46 |access-date=2021-04-18 |archive-date=2020-02-16 |archive-url=https://web.archive.org/web/20200216193457/https://www.vtt.fi/inf/pdf/technology/2012/T46.pdf |url-status=live }}</ref> The blend percentage of biodiesel is indicated by a "B" factor. B100 represents pure biodiesel, while blends like B20 contain 20% of biodiesel, with the remainder being traditional petrodiesel. These blends offer a compromise between the environmental benefits of biodiesel and performance characteristics of standard diesel fuel. Biodiesel blends can be used as [[heating oil]]. |
|||
The environmental impact of biodiesel is complex and varies based on factors like feedstock type, land use changes, and production methods. While it can potentially reduce greenhouse gas emissions compared to fossil fuels, concerns about biodiesel include land use changes, deforestation, and the food vs. fuel debate. The debate centers on the impact of biodiesel production on food prices and availability, as well as its overall carbon footprint. Despite these challenges, biodiesel remains a key component in the global strategy to reduce reliance on fossil fuels and mitigate the impacts of [[climate change]]. |
|||
==Blends== |
==Blends== |
||
[[File:Biodiesel.JPG|upright|thumb|right|Biodiesel sample]] |
|||
Blends of biodiesel and conventional hydrocarbon based diesel are products most commonly distributed for use in the retail diesel fuel marketplace. Much of the world uses a system known as the "B" factor to state the amount of biodiesel in any fuel mix: fuel containing 20% biodiesel is labeled '''B20''', while pure biodiesel is referred to as '''B100'''. It is common to see '''B99''', since 1% petrodiesel is sufficiently toxic to retard mold. Blends of 20 percent biodiesel with 80 percent petroleum diesel (B20) can generally be used in unmodified diesel engines. Biodiesel can also be used in its pure form (B100), but may require certain engine modifications to avoid maintenance and performance problems. Blending B100 with petro diesel may be accomplished by A. Mixing in tanks at manufacturing point prior to delivery to tanker truck, B. Splash mixing in the tanker truck (adding specific percentages of Biodiesel and Petro Diesel) C. Inline mixing, two components arrive at tanker truck simultaneously. |
|||
Blends of biodiesel and conventional hydrocarbon-based diesel are most commonly distributed for use in the retail diesel fuel marketplace. Much of the world uses a system known as the "B" factor to state the amount of biodiesel in any fuel mix:<ref name="basics">{{cite web|url=http://www.biodiesel.org/what-is-biodiesel/biodiesel-basics|title=Biodiesel Basics - Biodiesel.org|work=biodiesel.org|year=2012|access-date=May 5, 2012|archive-date=August 4, 2014|archive-url=https://web.archive.org/web/20140804155702/http://www.biodiesel.org/what-is-biodiesel/biodiesel-basics|url-status=live}}</ref> |
|||
==Origin== |
|||
* 100% biodiesel is referred to as B100 |
|||
On [[August 31]], [[1937]], G. Chavanne of the University of Brussels (Belgium) was granted a patent for a 'Procedure for the transformation of vegetable oils for their uses as fuels' (fr. 'Procédé de Transformation d’Huiles Végétales en Vue de Leur Utilisation comme Carburants') Belgian Patent 422,877. This patent described the alcoholysis (often referred to as transesterification) of vegetable oils using ethanol (and mentions methanol) in order to separate the fatty acids from the glycerol by replacing the glycerol with short linear alcohols. This appears to be the first account of the production of what is known as 'biodiesel' today.<ref name=knothe>{{cite web |
|||
* 20% biodiesel, 80% petrodiesel is labeled B20<ref name="sciencedirect.com"/> |
|||
|last=Knothe |
|||
* 10% biodiesel, 90% petrodiesel is labeled B10 |
|||
|first=G. |
|||
* 7% biodiesel, 93% petrodiesel is labeled B7 |
|||
|title=Historical Perspectives on Vegetable Oil-Based Diesel Fuels |
|||
* 5% biodiesel, 95% petrodiesel is labeled B5 |
|||
|publisher=INFORM, Vol. 12(11), p. 1103-1107 (2001) |
|||
* 2% biodiesel, 98% petrodiesel is labeled B2 |
|||
|url=http://www.biodiesel.org/resources/reportsdatabase/reports/gen/20011101_gen-346.pdf |
|||
|format=PDF |
|||
|accessdate=2007-7-11}}</ref> |
|||
Blends of 20% biodiesel and lower can be used in diesel equipment with no, or only minor modifications,<ref>{{cite web|url=http://www.nrel.gov/vehiclesandfuels/pdfs/43672.pdf|title=Biodiesel Handling and Use Guide, Fourth Edition|access-date=2011-02-13|publisher=National Renewable Energy Laboratory|url-status=dead|archive-url=https://web.archive.org/web/20111110021554/http://www.nrel.gov/vehiclesandfuels/pdfs/43672.pdf|archive-date=2011-11-10}}</ref> although certain manufacturers do not extend warranty coverage if equipment is damaged by these blends. The B6 to B20 blends are covered by the [[ASTM International|ASTM]] D7467 specification.<ref>{{cite web|url=http://www.astm.org|title=American Society for Testing and Materials|access-date=2011-02-13|publisher=ASTM International|archive-date=2019-12-08|archive-url=https://web.archive.org/web/20191208173241/https://www.astm.org/|url-status=live}}</ref> Biodiesel can also be used in its pure form (B100), but may require certain engine modifications to avoid maintenance and performance problems.<ref>{{cite web|url=http://www.nrel.gov/docs/fy09osti/43672.pdf|title=Biodiesel Handling and Use Guide|work=nrel.gov|year=2009|access-date=December 21, 2011|archive-date=April 28, 2011|archive-url=https://web.archive.org/web/20110428043515/http://www.nrel.gov/docs/fy09osti/43672.pdf|url-status=live}}</ref> Blending B100 with petroleum diesel may be accomplished by: |
|||
==Applications== |
|||
* Mixing in tanks at manufacturing point prior to delivery to tanker truck |
|||
* Splash mixing in the tanker truck (adding specific percentages of biodiesel and petroleum diesel) |
|||
* In-line mixing, two components arrive at tanker truck simultaneously. |
|||
* Metered pump mixing, petroleum diesel and biodiesel meters are set to X total volume. |
|||
=== Technical standards === |
|||
Biodiesel can be used in pure form (B100) or may be blended with petroleum diesel at any concentration in most modern diesel engines. Biodiesel has different [[solvent]] properties than petrodiesel, and will degrade natural [[rubber]] [[gasket]]s and [[hose (tubing)|hose]]s in vehicles (mostly found in vehicles manufactured before 1992), although these tend to wear out naturally and most likely will have already been replaced with [[FKM]], which is nonreactive to biodiesel. Biodiesel has been known to break down deposits of residue in the fuel lines where petrodiesel has been used.<ref>{{cite web |
|||
{{Main|Biodiesel standard}} |
|||
|last=Tyson |
|||
|first=K.S. |
|||
|last=McCormick |
|||
|first=R.L. |
|||
|title=2006 Biodiesel Handling and Use Guide Third Edition |
|||
|url=http://www.nrel.gov/vehiclesandfuels/npbf/pdfs/40555.pdf |
|||
|format=PDF |
|||
|accessdate=2006-12-18}}</ref> As a result, [[fuel filter]]s may become clogged with particulates if a quick transition to pure biodiesel is made. Therefore, it is recommended to change the fuel filters on engines and heaters shortly after first switching to a biodiesel blend.{{Fact|date=February 2008}} |
|||
Biodiesel has a number of standards for its quality including European standard [[EN 14214]], ASTM International D6751, and National Standard of Canada CAN/CGSB-3.524. |
|||
===Distribution=== |
|||
ASTM D6751 (American Society for Testing and Materials) details standards and specifications for biodiesels blended with middle distillate fuels. This specification standard specifies various test methods to be used in the determination of certain properties for biodiesel blends. Some of the tests mentioned include flash point and kinematic viscosity.[https://web.archive.org/web/20210506124713/https://www.astm.org/Standards/D6751.htm] |
|||
Biodiesel use and production are increasing rapidly. Fueling stations make biodiesel readily available to [[consumer]]s across Europe, and increasingly in the USA and Canada, and a growing number of transport fleets use it as an additive in their fuel. Biodiesel is often more expensive to purchase than petroleum diesel but this is expected to diminish due to [[Economy of scale|economies of scale]] and [[agricultural subsidies]] versus the rising cost of petroleum as [[hubbert peak|reserves are depleted]]. |
|||
==Historical background== |
|||
===Vehicular use and manufacturer acceptance=== |
|||
[[File:Rudolf Diesel.jpg|thumb|upright|Rudolf Diesel]] |
|||
Research sponsored by petroleum producers has found petroleum diesel better for car engines than biodiesel{{Fact|date=February 2008}}. This has been disputed by independent bodies{{Fact|date=February 2008}}, including for example the Volkswagen environmental awareness division, who note that biodiesel reduces engine wear. Pure biodiesel produced 'at home' is in use by thousands of drivers who have not experienced failure{{Fact|date=February 2008}}. In spite of the fact that biodiesel sold to the public is held to high standards set by national standards bodies, biodiesel has been widely available at fuel stations for less than a decade, and hence is reasonably perceived to carry more risk than older fuels. |
|||
[[Transesterification]] of a [[vegetable oil]] was conducted as early as 1853 by Patrick Duffy, four decades before the first [[diesel engine]] became functional.<ref>{{cite journal|last1=Duffy|first1=Patrick|year=1853|title=XXV. On the constitution of stearine.|journal=Quarterly Journal of the Chemical Society of London|volume=5|issue=4|page=303|doi=10.1039/QJ8530500303|url=https://zenodo.org/record/2129460|access-date=2019-07-05|archive-date=2020-07-26|archive-url=https://web.archive.org/web/20200726233212/https://zenodo.org/record/2129460|url-status=live}}</ref><ref>{{cite journal|last1=Rob|year=1898|title=Über partielle Verseifung von Ölen und Fetten II.|journal=Zeitschrift für Angewandte Chemie|volume=11|issue=30|pages=697–702|doi=10.1002/ange.18980113003|bibcode=1898AngCh..11..697H|url=https://zenodo.org/record/1424409|access-date=2019-07-05|archive-date=2020-07-26|archive-url=https://web.archive.org/web/20200726233731/https://zenodo.org/record/1424409|url-status=live}}</ref> Earlier processes for making [[oil lamp|lamp]] oil, were patented (1810, Prague) but not published in peer-reviewed publications. [[Rudolf Diesel]]'s prime model, a single {{convert|10|ft|m|2|abbr=on}} iron cylinder with a flywheel at its base, ran on its own power for the first time in [[Augsburg]], Germany, on 10 August 1893 running on nothing but [[peanut oil]]. In remembrance of this event, 10 August has been declared "[https://web.archive.org/web/20130310072028/http://www.biodieselcommunity.org/international-biodiesel-day/ International Biodiesel Day]".<ref>{{cite web|url=http://www.daysoftheyear.com/days/biodiesel-day/|title=Biodiesel Day|work=Days Of The Year|access-date=30 May 2015|archive-date=25 February 2021|archive-url=https://web.archive.org/web/20210225214325/https://www.daysoftheyear.com/days/biodiesel-day/|url-status=live}}</ref> |
|||
It is often reported that Diesel designed his engine to run on peanut oil, but this is not the case. Diesel stated in his published papers, "at the Paris Exhibition in 1900 ([[Exposition Universelle (1900)|''Exposition Universelle'']]) there was shown by the Otto Company a small Diesel engine, which, at the request of the [[Cabinet of France|French government]] ran on [[arachide]] (earth-nut or pea-nut) oil (see biodiesel), and worked so smoothly that only a few people were aware of it. The engine was constructed for using mineral oil, and was then worked on vegetable oil without any alterations being made. The French Government at the time thought of testing the applicability to power production of the Arachide, or earth-nut, which grows in considerable quantities in their African colonies, and can easily be cultivated there." Diesel himself later conducted related tests and appeared supportive of the idea.<ref>The Biodiesel Handbook, Chapter 2 – The History of Vegetable Oil Based Diesel Fuels, by Gerhard Knothe, {{ISBN|978-1-893997-79-0}}</ref> In a 1912 speech Diesel said, "the use of vegetable oils for engine fuels may seem insignificant today but such oils may become, in the course of time, as important as petroleum and the [[coal-tar]] products of the present time." |
|||
Volkswagen, for example, may tell European customers that they have no problem with biodiesel, while dealers in the [[USA]] have posted notices that only blends with less than 5% biodiesel are permitted. In 2005, DaimlerChrysler released Jeep Liberty CRD diesels from the factory into the American market with 5% biodiesel blends, indicating at least partial acceptance of biodiesel as an acceptable diesel fuel additive.<ref>Kemp, William. Biodiesel: Basics and Beyond. Canada: Aztext Press, 2006.</ref> In 2007, DiamlerChrysler indicated intention to increase warranty coverage to 20% biodiesel blends if biofuel quality in the United States can be standardized. <ref>http://nbb.grassroots.com/07Releases/Incentive/</ref> |
|||
Despite the widespread use of petroleum-derived diesel fuels, interest in vegetable oils as fuels for internal combustion engines was reported in several countries during the 1920s and 30s and later during World War II. [[Belgium]], France, Italy, the United Kingdom, [[Portugal]], Germany, [[Brazil]], [[Argentina]], Japan and China were reported to have tested and used vegetable oils as diesel fuels during this time. Some operational problems were reported due to the high viscosity of vegetable oils compared to petroleum diesel fuel, which results in poor [[Aerosol|atomization]] of the fuel in the fuel spray and often leads to deposits and coking of the injectors, combustion chamber and valves. Attempts to overcome these problems included heating of the vegetable oil, blending it with petroleum-derived diesel fuel or ethanol, [[pyrolysis]] and [[Fluid catalytic cracking|cracking]] of the oils. |
|||
Pure biodiesel (B100) can be used in any petroleum [[diesel engine]], though it is more commonly used in lower concentrations. Some areas have mandated ultra-low sulfur petrodiesel, which reduces the natural viscosity and lubricity of the fuel due to the removal of sulfur and certain other materials. Additives are required to make ULSD properly flow in engines, making biodiesel one popular alternative. Ranges as low as 2% (B2) have been shown to restore lubricity. Many municipalities have started using 5% biodiesel (B5) in snow-removal equipment and other systems. |
|||
On 31 August 1937, Georges Chavanne of the University of Brussels (Belgium) was granted a patent for a "Procedure for the transformation of vegetable oils for their uses as fuels" (fr. "''Procédé de Transformation d’Huiles Végétales en Vue de Leur Utilisation comme Carburants''") Belgian Patent 422,877. This patent described the alcoholysis (often referred to as transesterification) of vegetable oils using ethanol (and mentions methanol) in order to separate the fatty acids from the glycerol by replacing the glycerol with short linear alcohols. This appears to be the first account of the production of what is known as "biodiesel" today.<ref name=knothe>{{cite web|last=Knothe|first=G.|title=Historical Perspectives on Vegetable Oil-Based Diesel Fuels|publisher=INFORM, Vol. 12(11), p. 1103-1107 (2001)|url=http://www.biodiesel.org/resources/reportsdatabase/reports/gen/20011101_gen-346.pdf|access-date=2007-07-11|archive-date=2018-10-04|archive-url=https://web.archive.org/web/20181004134307/http://biodiesel.org/resources/reportsdatabase/reports/gen/20011101_gen-346.pdf|url-status=live}}</ref> This is similar (copy) to the patented methods used in the 18th century to make lamp-oil, and may be inspired by some old historical oil lamps, in some places. |
|||
===Railroad use=== |
|||
The British businessman [[Richard Branson|Richard Branson's]] [[Virgin Voyager]] train, number 220007 ''Thames Voyager'' <ref>{{cite web |title=First UK biodiesel train launched| url=http://news.bbc.co.uk/1/hi/uk/6729115.stm |publisher=BBC | accessdate=2007-11-17}}</ref>, billed as the world's first "biodiesel train" was converted to run on 80% petrodiesel and only 20% biodiesel, and it is claimed it will save 14% on direct emissions. |
|||
More recently, in 1977, Brazilian scientist Expedito Parente invented and submitted for patent, the first industrial process for the production of biodiesel.<ref>{{cite web|url=https://www.nist.gov/oiaa/TECHBIO1.pdf|title=Lipofuels: Biodiesel and Biokerosene|publisher=www.nist.gov|access-date=2009-03-09|archive-date=2009-03-18|archive-url=https://web.archive.org/web/20090318183921/http://www.nist.gov/oiaa/TECHBIO1.pdf|url-status=live}}</ref> This process is classified as biodiesel by international norms, conferring a "standardized identity and quality. No other proposed biofuel has been validated by the motor industry."<ref>[http://www.tecbio.com.br/templates/loadpaginas.php?pagina=sobreobiodiesel_ing What is it? (biodiesel)] Quote from Tecbio website. {{webarchive|url=https://web.archive.org/web/20071020142934/http://www.tecbio.com.br/templates/loadpaginas.php?pagina=sobreobiodiesel_ing|date=October 20, 2007}}</ref> As of 2010, Parente's company [[Tecbio]] is working with [[Boeing]] and [[NASA]] to certify bioquerosene (bio-kerosene), another product produced and patented by the Brazilian scientist.<ref>{{cite web|url=http://www.defesanet.com.br/zz/energia_4.htm|title=O Globo newspaper interview in Portuguese|publisher=Defesanet.com.br|access-date=2010-03-15|archive-date=2010-10-29|archive-url=https://web.archive.org/web/20101029062051/http://www.defesanet.com.br/zz/energia_4.htm|url-status=live}}</ref> |
|||
===Aircraft use=== |
|||
Aircraft manufacturers are understandably even more cautious, but some test flights have been performed<ref name=trainer>[http://www.mytelus.com/money/news/article.do?pageID=ex_business/home&articleID=2868422 Soviet-era training jet flies on biodiesel]</ref> and commercial passenger jet testing has been announced<ref name=virgin>[http://www.aboutmyplanet.com/environment/virgin-atlantic/ Virgin Atlantic to Run Bio-diesel Test Flight]</ref> by Virgin Atlantic's Richard Branson. |
|||
Research into the use of transesterified [[sunflower oil]], and refining it to diesel fuel standards, was initiated in South Africa in 1979. By 1983, the process for producing fuel-quality, engine-tested biodiesel was completed and published internationally.<ref>SAE Technical Paper series no. 831356. SAE International Off Highway Meeting, Milwaukee, Wisconsin, USA, 1983</ref> An Austrian company, Gaskoks, obtained the technology from the South African Agricultural Engineers; the company erected the first biodiesel [[pilot plant]] in November 1987, and the first industrial-scale plant in April 1989 (with a capacity of 30,000 tons of [[rapeseed]] per annum). |
|||
The world's first biofuel-powered commercial aircraft took off from [[London Heathrow Airport|London's Heathrow Airport]] on [[February 24]] [[2008]] and touched down in [[Amsterdam]] on a demonstration flight hailed as a first step towards "cleaner" flying.<ref name=commercial>[http://edition.cnn.com/2008/BUSINESS/02/24/flight.biofuels/index.html Biofuel-powered jet to make test flight]</ref> |
|||
Throughout the 1990s, plants were opened in many European countries, including the [[Czech Republic]], Germany and [[Sweden]]. France launched local production of biodiesel fuel (referred to as ''diester'') from rapeseed oil, which is mixed into regular diesel fuel at a level of 5%, and into the diesel fuel used by some captive fleets (e.g. [[public transportation]]) at a level of 30%. [[Renault]], [[Peugeot]] and other manufacturers have certified truck engines for use with up to that level of partial biodiesel; experiments with 50% biodiesel are underway. During the same period, nations in other parts of the world also saw local production of biodiesel starting up: by 1998, the Austrian Biofuels Institute had identified 21 countries with commercial biodiesel projects. 100% biodiesel is now available at many normal service stations across Europe. |
|||
===As a heating oil=== |
|||
==Properties== |
|||
Biodiesel can also be used as a heating fuel in domestic and commercial boilers. Older furnaces may contain rubber parts that would be affected by biodiesel's solvent properties, but can otherwise burn biodiesel without any conversion required. Care must be taken at first, however, given that varnishes left behind by petrodiesel will be released and can clog pipes- fuel filtering and prompt filter replacement is required. Another approach is to start using biodiesel as blend, and decreasing the petroleum proportion over time can allow the varnishes to come off more gradually and be less likely to clog. Thanks to its strong solvent properties, however, the furnace is cleaned out and generally becomes more efficient. A technical research paper <ref> {{cite web |
|||
The color of biodiesel ranges from clear to golden to dark brown, depending on the production method and the feedstock used to make the fuel. This also changes the resulting fuel properties.<ref>{{cite web|url=https://www.nrel.gov/docs/fy03osti/31461.pdf|title=The Effect of Biodiesel Composition on Engine Emissions from a DDC Series 60 Diesel Engine|access-date=2022-12-13}}</ref> In general, biodiesel is slightly [[miscible]] with water, has a high [[boiling point]] and low [[vapor pressure]]. The [[flash point]] of biodiesel can exceed {{convert|130|C|F}},<ref>{{cite web|url=http://www.biodiesel.org/pdf_files/fuelfactsheets/MSDS.pdf|title=Generic biodiesel material safety data sheet (MSDS)|access-date=2010-03-15|archive-date=2009-12-22|archive-url=https://web.archive.org/web/20091222011136/http://www.biodiesel.org/pdf_files/fuelfactsheets/MSDS.pdf|url-status=live}}</ref> significantly higher than that of petroleum diesel which may be as low as {{convert|52|C|F}}.<ref name="Marathon MSDS">{{cite web|title=MSDS ID NO.: 0301MAR019|url=http://www.marathonpetroleum.com/content/documents/mpc/sds/0301MAR019.pdf|website=Marathon Petroleum|access-date=22 December 2017|pages=5, 7|date=7 December 2010|archive-url=https://web.archive.org/web/20171222105649/http://www.marathonpetroleum.com/content/documents/mpc/sds/0301MAR019.pdf|archive-date=2017-12-22|url-status=dead}}</ref><ref name="CITGO MSDS">{{cite web|title=Safety Data Sheet - CITGO No. 2 Diesel Fuel, Low Sulfur, All Grades|url=http://www.docs.citgo.com/msds_pi/AG2DF.pdf|website=CITGO|access-date=22 December 2017|page=7|date=29 July 2015|archive-date=16 October 2015|archive-url=https://web.archive.org/web/20151016172702/http://www.docs.citgo.com/msds_pi/AG2DF.pdf|url-status=live}}</ref> Biodiesel has a density around ~0.88 g/cm<sup>3</sup>, higher than petrodiesel (~0.85 g/cm<sup>3</sup>).<ref name="Marathon MSDS" /><ref name="CITGO MSDS" /> |
|||
| last = Robertson |
|||
| first = Andrew |
|||
| title =Biodiesel Heating Oil: Sustainable Heating for the future |
|||
| work = |
|||
| publisher =Institute of Plumbing and Heating Engineering |
|||
| date = |
|||
| url =http://www.iphe.org.uk/publications/tech_literature.html |
|||
| format = |
|||
| doi = |
|||
| accessdate = 2008-01-07 }} </ref> describes laboratory research and field trials project using pure biodiesel and biodiesel blends as a heating fuel in oil fired boilers. |
|||
During the Biodiesel Expo 2006 in the UK, Andrew J. Robertson presented his biodiesel heating oil research from his technical paper and suggested that B20 biodiesel could reduce UK household CO<sub>2</sub> emissions by 1.5 million tonnes per year |
|||
The [[calorific value]] of biodiesel is about 37.27 MJ/kg.<ref>[http://webarchive.nationalarchives.gov.uk/20091002060826/http%3A//www.berr.gov.uk/files/file14925.pdf Carbon and Energy Balances for a Range of Biofuels Options] Sheffield Hallam University</ref> This is 9% lower than regular Number 2 petrodiesel. Variations in biodiesel energy density is more dependent on the feedstock used than the production process. Still, these variations are less than for petrodiesel.<ref>{{cite conference|last=National Biodiesel Board |title=Energy Content |pages=1 |date=October 2005 |location=Jefferson City, USA |url=http://www.biodiesel.org/docs/ffs-basics/energy-content-final-oct-2005.pdf?sfvrsn=6 |access-date=2013-09-24 |format=PDF |url-status=dead |archive-url=https://web.archive.org/web/20130927234307/http://www.biodiesel.org/docs/ffs-basics/energy-content-final-oct-2005.pdf?sfvrsn=6 |archive-date=2013-09-27 }}</ref> It has been claimed biodiesel gives better lubricity and more complete combustion thus increasing the engine energy output and partially compensating for the higher energy density of petrodiesel.<ref>[http://www.unh.edu/p2/biodiesel/article_alge.html UNH Biodiesel Group] {{webarchive |url=https://web.archive.org/web/20040906074833/http://www.unh.edu/p2/biodiesel/article_alge.html |date=September 6, 2004 }}</ref> |
|||
==Historical background== |
|||
[[Transesterification]] of a [[vegetable oil]] was conducted as early as 1853 by scientists E. Duffy and J. Patrick, many years before the first [[diesel engine]] became functional. [[Rudolf Diesel]]'s prime model, a single 10 ft (3 m) iron cylinder with a flywheel at its base, ran on its own power for the first time in [[Augsburg]], [[Germany]], on [[August 10]], [[1893]]. In remembrance of this event, [[August 10]] has been declared "International Biodiesel Day". |
|||
Biodiesel also contains virtually no sulfur<ref>{{cite web|url=http://www.astm.org/COMMIT/E48_MacDonald.pdf|title=E48_MacDonald.pdf (application/pdf Object)|work=astm.org|year=2011|access-date=May 3, 2012|archive-date=November 20, 2012|archive-url=https://web.archive.org/web/20121120152629/http://www.astm.org/COMMIT/E48_MacDonald.pdf|url-status=live}}</ref> and although lacking sulfur compounds that in petrodiesel provide much of the lubricity, it has promising lubricating properties and [[cetane number|cetane ratings]] compared to low sulfur diesel fuels and often serves as an additive to [[ultra-low-sulfur diesel]] (ULSD) fuel to aid with lubrication.<ref>{{cite web |title=Lubricity Benefits |url=http://biodiesel.org/docs/ffs-performace_usage/lubricity-benefits.pdf |url-status=live |archive-url=https://web.archive.org/web/20170809000214/http://biodiesel.org/docs/ffs-performace_usage/lubricity-benefits.pdf |archive-date=2017-08-09 |access-date=2017-12-22 |website=National Biodiesel Board}}</ref> Biodiesel Fuels with higher lubricity may increase the usable life of high-pressure fuel injection equipment that relies on the fuel for its lubrication. Depending on the engine, this might include high pressure injection pumps, pump injectors (also called ''unit injectors'') and [[fuel injector]]s.[[File:Biodiesel 3.jpg|thumb|Older diesel Mercedes are popular for running on biodiesel.]] |
|||
Rudolf Diesel demonstrated a Diesel engine running on peanut oil (at the request of the French government) built by the French [[Otto Company]] at the [[World Fair]] in [[Paris]], [[France]] in 1900, where it received the ''Grand Prix'' (highest prize). |
|||
<ref>[http://www.biodiesel.org/resources/reportsdatabase/reports/gen/20011101_gen-346.pdf biodiesel.org report 246]</ref> |
|||
==Applications== |
|||
This engine stood as an example of Diesel's vision because it was powered by [[peanut]] oil — a [[biofuel]], though not ''biodiesel'', since it was not transesterified. He believed that the utilization of biomass fuel was the real future of his engine. In a 1912 speech Diesel said, "the use of vegetable oils for engine fuels may seem insignificant today but such oils may become, in the course of time, as important as petroleum and the [[coal-tar]] products of the present time." |
|||
[[File:Targray Biodiesel Railcar.png|thumb|Targray Biofuels railcar transporting Biodiesel.]] |
|||
Biodiesel can be used in pure form (B100) or may be blended with petroleum diesel at any concentration in most injection pump diesel engines. New extreme high-pressure (29,000 psi) [[common rail]] engines have strict factory limits of B5 or B20, depending on manufacturer.<ref>"[http://biodiesel.org/using-biodiesel/oem-information/oem-statement-summary-chart OEM Statement Summary Chart] {{Webarchive|url=http://webarchive.loc.gov/all/20160407054752/http://biodiesel.org/using%2Dbiodiesel/oem%2Dinformation/oem%2Dstatement%2Dsummary%2Dchart |date=2016-04-07 }}." Biodiesel.org. National Biodiesel Board, 1 Dec. 2014. Web. 19 Nov. 2015.</ref> Biodiesel has different [[solvent]] properties from petrodiesel, and will degrade natural rubber [[gasket]]s and [[hose (tubing)|hoses]] in vehicles (mostly vehicles manufactured before 1992), although these tend to wear out naturally and most likely will have already been replaced with [[FKM]], which is nonreactive to biodiesel. Biodiesel has been known to break down deposits of residue in the fuel lines where petrodiesel has been used.<ref>{{cite web|last=McCormick|first=R.L.|title=2006 Biodiesel Handling and Use Guide Third Edition|url=http://www.nrel.gov/vehiclesandfuels/npbf/pdfs/40555.pdf|access-date=2006-12-18|archive-url=https://web.archive.org/web/20061216051136/http://www.nrel.gov/vehiclesandfuels/npbf/pdfs/40555.pdf|archive-date=2006-12-16|url-status=dead}}</ref> As a result, [[fuel filter]]s may become clogged with particulates if a quick transition to pure biodiesel is made. Therefore, it is recommended to change the fuel filters on engines and heaters shortly after first switching to a biodiesel blend.<ref>{{cite web|title=US EPA Biodiesel Factsheet|url=http://www.epa.gov/smartway/growandgo/documents/factsheet-biodiesel.htm|url-status=dead|archive-url=https://web.archive.org/web/20080726004436/http://www.epa.gov/smartway/growandgo/documents/factsheet-biodiesel.htm|archive-date=July 26, 2008|date=2016-03-03}}</ref> |
|||
===Distribution=== |
|||
During the 1920s, diesel engine manufacturers altered their engines to utilize the lower [[viscosity]] of petrodiesel (a [[fossil fuel]]), rather than vegetable oil (a [[biomass fuel]]). The petroleum industries were able to make inroads in fuel markets because their fuel was much cheaper to produce than the [[biomass]] alternatives. The result, for many years, was a near elimination of the biomass fuel production [[infrastructure]]. Only recently, have environmental impact concerns and a decreasing price differential made biomass fuels such as biodiesel a growing alternative. |
|||
Since the passage of the [[Energy Policy Act of 2005]], biodiesel use has been increasing in the United States.<ref>{{cite web|title=Twenty In Ten: Strengthening America's Energy Security|url=https://georgewbush-whitehouse.archives.gov/stateoftheunion/2007/initiatives/energy.html|publisher=[[Whitehouse.gov]]|access-date=2008-09-10|archive-date=2009-09-06|archive-url=https://web.archive.org/web/20090906142725/http://georgewbush-whitehouse.archives.gov/stateoftheunion/2007/initiatives/energy.html|url-status=live}}</ref> In the UK, the [[Renewable Transport Fuel Obligation]] obliges suppliers to include 5% renewable fuel in all transport fuel sold in the UK by 2010. For road diesel, this effectively means 5% biodiesel (B5). |
|||
===Vehicular use and manufacturer acceptance=== |
|||
Despite the widespread use of fossil petroleum-derived diesel fuels, interest in vegetable oils as fuels in internal combustion engines is reported in several countries during the 1920's and 1930's and later during [[World War II]]. [[Belgium]], [[France]], [[Italy]], the [[United Kingdom]], [[Portugal]], [[Germany]], [[Brazil]], [[Argentina]], [[Japan]] and [[China]] have been reported to have tested and used vegetable oils as diesel fuels during this time. Some operational problems were reported due to the high viscosity of vegetable oils compared to petroleum diesel fuel, which result in poor [[atomization]] of the fuel in the fuel spray and often leads to deposits and coking of the injectors, combustion chamber and valves. Attempts to overcome these problems included heating of the vegetable oil, blending it with petroleum-derived diesel fuel or ethanol, [[pyrolysis]] and cracking of the oils. |
|||
In 2005, Chrysler (then part of DaimlerChrysler) released the Jeep Liberty CRD diesels from the factory into the European market with 5% biodiesel blends, indicating at least partial acceptance of biodiesel as an acceptable diesel fuel additive.<ref>Kemp, William. Biodiesel: Basics and Beyond. Canada: Aztext Press, 2006.</ref> In 2007, DaimlerChrysler indicated its intention to increase warranty coverage to 20% biodiesel blends if biofuel quality in the United States can be standardized.<ref>{{cite web|url=http://nbb.grassroots.com/07Releases/Incentive/|title=National Biodiesel Board, 2007. Chrysler Supports Biodiesel Industry; Encourages Farmers, Refiners, Retailers and Customers to Drive New Diesels Running on Renewable Fuel.|publisher=Nbb.grassroots.com|date=2007-09-24|access-date=2010-03-15|url-status=dead|archive-url=https://web.archive.org/web/20100306182315/http://nbb.grassroots.com/07Releases/Incentive/|archive-date=2010-03-06}}</ref> |
|||
The [[Volkswagen Group]] has released a statement indicating that several of its vehicles are compatible with B5 and B100 made from [[rape seed]] oil and compatible with the [[EN 14214]] standard. The use of the specified biodiesel type in its cars will not void any warranty.<ref>{{cite web|url=http://www.volkswagen.co.uk/assets/common/pdf/general/biodiesel.pdf|title=Biodiesel statement|publisher=Volkswagen.co.uk|access-date=2011-08-04|archive-date=2011-09-27|archive-url=https://web.archive.org/web/20110927152249/http://www.volkswagen.co.uk/assets/common/pdf/general/biodiesel.pdf|url-status=live}}</ref> |
|||
On [[August 31]], [[1937]], G. Chavanne of the University of Brussels (Belgium) was granted a patent for a "Procedure for the transformation of vegetable oils for their uses as fuels" (fr. 'Procédé de Transformation d’Huiles Végétales en Vue de Leur Utilisation comme Carburants') Belgian Patent 422,877. This patent described the alcoholysis (often referred to as transesterification) of vegetable oils using methanol and ethanol in order to separate the fatty acids from the glycerol by replacing the glycerol by short linear alcohols. This appears to be the first account of the production of what is known as "biodiesel" today. |
|||
[[Mercedes-Benz]] does not allow diesel fuels containing greater than 5% biodiesel (B5) due to concerns about "production shortcomings".<ref>{{cite web |last=Mercedes-Benz |year=2010 |title=Biodiesel Information for Passenger Cars |url=http://www.mbusa.com/vcm/MB/DigitalAssets/pdfmb/serviceandparts/biodiesel_Brochure5.pdf |url-status=dead |archive-url=https://web.archive.org/web/20121028074915/http://www.mbusa.com/vcm/MB/DigitalAssets/pdfmb/serviceandparts/biodiesel_Brochure5.pdf |archive-date=October 28, 2012 |access-date=September 11, 2012 |work=mbusa.com}}</ref> Any damages caused by the use of such non-approved fuels will not be covered by the Mercedes-Benz Limited Warranty. |
|||
More recently, in 1977, Brazilian scientist Expedito Parente produced biodiesel using transesterification with ethanol, and again filed a patent for the same process. This process is classified as biodiesel by international norms, conferring a "standardized identity and quality. No other proposed biofuel has been validated by the motor industry."<ref>[http://www.tecbio.com.br/templates/loadpaginas.php?pagina=sobreobiodiesel_ing] Quote from Tecbio website </ref> Currently, Parente's company [[Tecbio]] is working with [[Boeing]] and [[NASA]] to certify bioquerosene (bio-kerosene), another product produced and patented by the Brazilian scientist.<ref>[http://www.defesanet.com.br/zz/energia_4.htm] O Globo newspaper interview in Portuguese]</ref> |
|||
Starting in 2004, the city of [[Halifax Regional Municipality|Halifax, Nova Scotia]] decided to update its bus system to allow the fleet of city buses to run entirely on a fish-oil based biodiesel. This caused the city some initial mechanical issues, but after several years of refining, the entire fleet had successfully been converted.<ref>{{cite web|url=http://www.biodieselinvesting.com/biodiesel-archives/2006/08/31/halifax-city-buses-to-run-on-biodiesel-again/|archive-url=https://web.archive.org/web/20061018135732/http://www.biodieselinvesting.com/biodiesel-archives/2006/08/31/halifax-city-buses-to-run-on-biodiesel-again/|url-status=dead|archive-date=2006-10-18|title=Halifax City Buses to Run on Biodiesel Again | Biodiesel and Ethanol Investing|publisher=Biodieselinvesting.com|date=2006-08-31|access-date=2009-10-17}}</ref><ref>{{cite web|url=http://www.halifax.ca/metrotransit/Biodiesel.html|title=Biodiesel|publisher=Halifax.ca|access-date=2009-10-17|url-status=dead|archive-url=https://web.archive.org/web/20101224042615/http://halifax.ca/metrotransit/Biodiesel.html|archive-date=2010-12-24}}</ref><ref>{{cite web|url=http://www.halifax.ca/metrotransit/news/10_2004-Biodiesel.html|title=Halifax Transit|publisher=Halifax.ca|date=2004-10-12|access-date=2013-12-04|url-status=dead|archive-url=https://web.archive.org/web/20140814103101/http://www.halifax.ca/metrotransit/news/10_2004-Biodiesel.html|archive-date=2014-08-14}}</ref> |
|||
Research into the use of transesterified [[sunflower oil]], and refining it to [[diesel fuel]] standards, was initiated in [[South Africa]] in 1979. By 1983, the process for producing fuel-quality, engine-tested biodiesel was completed and published internationally.<ref>SAE Technical Paper series no. 831356. SAE International Off Highway Meeting, Milwaukee, Wisconsin, USA, 1983</ref> An [[Austria]]n company, [[Gaskoks]], obtained the technology from the South African Agricultural Engineers; the company erected the first biodiesel [[pilot plant]] in November 1987, and the first industrial-scale plant in April 1989 (with a capacity of 30,000 tons of [[rapeseed]] per annum). |
|||
In 2007, McDonald's of UK announced it would start producing biodiesel from the waste oil byproduct of its restaurants. This fuel would be used to run its fleet.<ref>{{cite web|url=http://news.mongabay.com/2007/0709-green_mcdonalds.html |archive-url=https://archive.today/20120715203039/http://news.mongabay.com/2007/0709-green_mcdonalds.html |url-status=dead |archive-date=2012-07-15|title=McDonald's bolsters "green" credentials with recycled biodiesel oil|publisher=News.mongabay.com|date=2007-07-09|access-date=2009-10-17}}</ref> |
|||
Throughout the 1990s, plants were opened in many European countries, including the [[Czech Republic]], [[Germany]] and [[Sweden]]. [[France]] launched local production of biodiesel fuel (referred to as ''diester'') from rapeseed oil, which is mixed into regular diesel fuel at a level of 5%, and into the diesel fuel used by some captive fleets (e.g. [[public transportation]]) at a level of 30%. [[Renault]], [[Peugeot]] and other manufacturers have certified truck engines for use with up to that level of partial biodiesel; experiments with 50% biodiesel are underway. During the same period, nations in other parts of the world also saw local production of biodiesel starting up: by 1998, the Austrian Biofuels Institute had identified 21 countries with commercial biodiesel projects. 100% Biodiesel is now available at many normal service stations across Europe. |
|||
The 2014 Chevy Cruze Clean Turbo Diesel, direct from the factory, will be rated for up to B20 (blend of 20% biodiesel / 80% regular diesel) biodiesel compatibility<ref>{{cite web|url=http://media.gm.com/media/us/en/gm/news.detail.html/content/Pages/news/us/en/2013/Feb/13-chicago-show/0207-cruze-diese-engine.html|title=Cruze Clean Turbo Diesel Delivers Efficient Performance|date=2013-02-07|access-date=2013-08-05|archive-date=2013-08-10|archive-url=https://web.archive.org/web/20130810082038/http://media.gm.com/media/us/en/gm/news.detail.html/content/Pages/news/us/en/2013/Feb/13-chicago-show/0207-cruze-diese-engine.html|url-status=live}}</ref> |
|||
In September 2005 [[Minnesota]] became the first U.S. state to mandate that all diesel fuel sold in the state contain part biodiesel, requiring a content of at least 2% biodiesel.<ref>[http://www.biodiesel.org/resources/pressreleases/gen/20050929_mn_mandate_implemented.pdf] [[Minnesota]] regulations on biodiesel content</ref> |
|||
== |
===Railway usage=== |
||
[[File:Biodiesel locomotive at Mount Washington Cog Railway.JPG|thumb|Biodiesel locomotive and its external fuel tank at [[Mount Washington Cog Railway]]]] |
|||
Biodiesel has better [[lubricant|lubricity]] than that of today's diesel fuels. During the manufacture of these, to comply with low SO<sub>2</sub> engine emission limits set in modern standards, severe hydrotreatment is included. Biodiesel addition reduces wear<ref>[http://www.biodiesel.org/pdf_files/fuelfactsheets/Lubricity.PDF Biodiesel<!-- Bot generated title -->]</ref> increasing the life of the fuel injection equipment that relies on the fuel for its lubrication, such as high pressure injection pumps, pump injectors (also called ''unit injectors'') and [[fuel injector]]s.[[Image:Biodiesel 3.jpg|thumb|170px|Older diesel Mercedes are popular for running on biodiesel.]] |
|||
British [[train operating company]] [[Virgin Trains West Coast]] claimed to have run the UK's first "biodiesel train", when a [[British Rail Class 220|Class 220]] was converted to run on 80% petrodiesel and 20% biodiesel.<ref>{{cite news|title=First UK biodiesel train launched|url=http://news.bbc.co.uk/1/hi/uk/6729115.stm|publisher=BBC|access-date=2007-11-17|date=2007-06-07|archive-date=2008-02-13|archive-url=https://web.archive.org/web/20080213193255/http://news.bbc.co.uk/1/hi/uk/6729115.stm|url-status=live}}</ref><ref>Virgin launches trials with Britain's first biofuel train ''[[Rail (magazine)|Rail]]'' issue 568 20 June 2007 page 6</ref> |
|||
The [[British Royal Train]] on 15 September 2007 completed its first ever journey run on 100% biodiesel fuel supplied by Green Fuels Ltd. [[Prince Charles]] and Green Fuels managing director James Hygate were the first passengers on a train fueled entirely by biodiesel fuel. Since 2007, the Royal Train has operated successfully on B100 (100% biodiesel).<ref>{{cite web|url=http://www.ews-railway.co.uk/cmsnews/news_article.asp?guid=%7BD3A02A0C-1B28-4E08-8548-B3863CFF286C%7D|title=EWS Railway – News Room|publisher=www.ews-railway.co.uk|access-date=2009-06-12|archive-url=https://web.archive.org/web/20200219100207/https://uk.dbcargo.com/rail-uk-en?guid=%7BD3A02A0C-1B28-4E08-8548-B3863CFF286C%7D|archive-date=2020-02-19|url-status=dead}}</ref> A government white paper also proposed converting large portions of the UK railways to biodiesel but the proposal was subsequently dropped in favour of further electrification.<ref>{{Cite book|last=Great Britain. Parliament. House of Commons. Transport Committee|url=https://www.worldcat.org/oclc/273500097|title=Delivering a sustainable railway : a 30-year strategy for the railways? : tenth report of session 2007-08 : report, together with formal minutes, oral and written evidence|date=2008|publisher=Stationery Office|isbn=978-0-215-52222-1|location=London|oclc=273500097|access-date=2021-07-07|archive-date=2021-07-31|archive-url=https://web.archive.org/web/20210731013413/https://www.worldcat.org/title/delivering-a-sustainable-railway-a-30-year-strategy-for-the-railways-tenth-report-of-session-2007-08-report-together-with-formal-minutes-oral-and-written-evidence/oclc/273500097|url-status=live}}</ref> |
|||
The [[volumetric energy density]] of biodiesel is about 33 MJ/L. This is 9 % lower than regular Number 2 [[Diesel|petrodiesel]]. Variations in biodiesel energy density is more dependent on the feedstock used than the production process. Still these variations are less than for petrodiesel.<ref>{{cite conference |
|||
| first = |
|||
| last = National Biodiesel Board |
|||
| authorlink = |
|||
| coauthors = |
|||
| title = Energy Content |
|||
| booktitle = |
|||
| pages = 1 |
|||
| publisher = |
|||
| date = 2005-10 |
|||
| location = Jefferson City, USA |
|||
| url = http://www.biodiesel.org/pdf_files/fuelfactsheets/BTU_Content_Final_Oct2005.pdf |
|||
| doi = |
|||
| id = |
|||
| accessdate = 2007-11-20 }}</ref> It has been claimed biodiesel gives better lubricity and more complete combustion thus increasing the engine energy output and partially compensating for the higher energy density of petrodiesel.<ref>[http://www.unh.edu/p2/biodiesel/article_alge.html UNH Biodiesel Group<!-- Bot generated title -->]</ref> |
|||
Similarly, a state-owned [[short-line railroad]] in [[Eastern Washington]] ran a test of a 25% biodiesel / 75% petrodiesel blend during the summer of 2008, purchasing fuel from a biodiesel producer sited along the railroad tracks.<ref>{{cite news|title=Biodiesel will drive Eastern Wa. train during summerlong test|url=http://seattletimes.nwsource.com/html/localnews/2008011135_biodiesel22.html|newspaper=Seattle Times|access-date=2009-03-01|first=Shawn|last=Vestal|date=2008-06-22|archive-date=2009-02-02|archive-url=https://web.archive.org/web/20090202184016/http://seattletimes.nwsource.com/html/localnews/2008011135_biodiesel22.html|url-status=live}}</ref> The train will be powered by biodiesel made in part from [[canola]] grown in agricultural regions through which the short line runs. |
|||
Biodiesel is a liquid which varies in color — between golden and dark brown — depending on the production feedstock. It is [[miscible|immiscible]] with water, has a high [[boiling point]] and low [[vapor pressure]]. Typical methyl ester biodiesel has a [[flash point]] of ~ 150 °C (300 °F). Biodiesel has a density of ~ 0.88 g/cm³, less than that of water. |
|||
Also in 2007, Disneyland began running the park trains on B98 (98% biodiesel). The program was discontinued in 2008 due to storage issues, but in January 2009, it was announced that the park would then be running all trains on biodiesel manufactured from its own used cooking oils. This is a change from running the trains on soy-based biodiesel.<ref>{{cite web|url=http://www.upi.com/Top_News/2009/01/29/Disneyland_trains_running_on_biodiesel/UPI-10151233252145/|title=Disneyland trains running on biodiesel - UPI.com|publisher=www.upi.com|access-date=2009-03-16|archive-date=2009-01-30|archive-url=https://web.archive.org/web/20090130035705/http://www.upi.com/Top_News/2009/01/29/Disneyland_trains_running_on_biodiesel/UPI-10151233252145/|url-status=live}}</ref> |
|||
Biodiesel has a [[viscosity]] similar to [[diesel fuel|petrodiesel]], the current industry term for diesel produced from [[petroleum]]. Biodiesel has high [[lubricity]] and virtually no sulfur content, and it is often used as an additive to [[Ultra-Low Sulfur Diesel]] (ULSD) fuel. |
|||
In 2007, the historic [[Mount Washington Cog Railway|Mt. Washington Cog Railway]] added the first biodiesel locomotive to its all-steam locomotive fleet. The fleet has climbed up the western slopes of [[Mount Washington (New Hampshire)|Mount Washington]] in [[New Hampshire]] since 1868 with a peak vertical climb of 37.4 degrees.<ref>{{cite news|last=Kotrba|first=Ron|title='Name that Biodiesel Train' contest|url=http://www.biodieselmagazine.com/blog/article/2013/05/name-that-biodiesel-train-contest|access-date=8 May 2014|newspaper=Biodiesel Magazine|date=29 May 2013|archive-date=8 May 2014|archive-url=https://web.archive.org/web/20140508063652/http://www.biodieselmagazine.com/blog/article/2013/05/name-that-biodiesel-train-contest|url-status=live}}</ref> |
|||
==Technical standards== |
|||
[[Image:Biodiesel.JPG|thumb|Biodiesel sample]] |
|||
The common international standard for biodiesel is [[EN 14214]]. |
|||
In 2009, the [[Grand Canyon Railway]] started running engine [[Grand Canyon Railway 4960|4960]] on used cooking oil. |
|||
There are additional national specifications. [[ASTM D6751]] is the most common standard referenced in the United States and Canada. In Germany, the requirements for biodiesel are fixed in the [[DIN]] EN 14214 standard and in the UK the requirements for biodiesel is fixed in the [[British Standards|BS]] EN 14214 standard, although these last two standards are essentially the same as [[EN 14214]] and are just prefixed with the respective national standards institution codes. <br />There are standards for three different varieties of biodiesel, which are made of different oils: |
|||
*RME ([[rapeseed]] methyl [[ester]], according to DIN E 51606) |
|||
*PME (vegetable methyl ester, purely vegetable products, according to DIN E 51606) |
|||
*FME (fat methyl ester, vegetable and animal products, according to DIN V 51606) |
|||
On 8 July 2014,<ref>{{cite news|url=http://www.thehindu.com/news/national/railway-budget-201415-highlights/article6189594.ece|title=Railway Budget 2014–15: Highlights|author=PTI|newspaper=The Hindu|access-date=30 May 2015|date=2014-07-08|archive-date=2014-11-29|archive-url=https://web.archive.org/web/20141129183345/http://www.thehindu.com/news/national/railway-budget-201415-highlights/article6189594.ece|url-status=live}}</ref> the then Indian Railway Minister [[D.V. Sadananda Gowda]] announced in Railway Budget that 5% bio-diesel will be used in Indian Railways' Diesel Engines.<ref>{{cite web|url=http://pib.nic.in/newsite/PrintRelease.aspx?relid=111095|title=Indian Railways to go for Bio-Diesel in a Big Way – Gowda|access-date=30 May 2015|archive-date=14 April 2015|archive-url=https://web.archive.org/web/20150414215142/http://pib.nic.in/newsite/PrintRelease.aspx?relid=111095|url-status=live}}</ref> |
|||
The standards ensure that the following important factors in the fuel production process are satisfied: |
|||
*Complete reaction. |
|||
*Removal of [[glycerin]]. |
|||
*Removal of [[catalyst]]. |
|||
*Removal of [[alcohol]]. |
|||
===As a heating oil=== |
|||
*Absence of [[Fatty acid#Free fatty acids|free fatty acids]]. |
|||
{{Main|Bioliquids}} |
|||
*Low [[sulfur]] content. |
|||
Biodiesel can also be used as a heating fuel in domestic and commercial boilers, a mix of [[heating oil]] and [[biofuel]] which is standardized and taxed slightly differently from diesel fuel used for transportation. Bioheat fuel is a proprietary blend of biodiesel and traditional heating oil. Bioheat is a registered trademark of the [[National Biodiesel Board]] [NBB] and the [https://web.archive.org/web/20130701175827/http://www.nora-oilheat.org/site20/index.mv?screen=home National Oilheat Research Alliance] [NORA] in the United States, and Columbia Fuels in Canada.<ref>{{cite web|url=http://www.biodieselmagazine.com/articles/4096/environment-consumers-win-with-bioheat-trademark-victory/|title=Environment, consumers win with Bioheat trademark victory|work=biodieselmagazine.com|year=2011|access-date=October 27, 2011|archive-date=November 20, 2011|archive-url=https://web.archive.org/web/20111120073303/http://biodieselmagazine.com/articles/4096/environment-consumers-win-with-bioheat-trademark-victory|url-status=live}}</ref> Heating biodiesel is available in various blends. ASTM 396 recognizes blends of up to 5 percent biodiesel as equivalent to pure petroleum heating oil. Blends of higher levels of up to 20% biofuel are used by many consumers. Research is underway to determine whether such blends affect performance. |
|||
Older furnaces may contain rubber parts that would be affected by biodiesel's solvent properties, but can otherwise burn biodiesel without any conversion required. Care must be taken, given that varnishes left behind by petrodiesel will be released and can clog pipes—fuel filtering and prompt filter replacement is required. Another approach is to start using biodiesel as a blend, and decreasing the petroleum proportion over time can allow the varnishes to come off more gradually and be less likely to clog. Due to biodiesel's strong solvent properties, the furnace is cleaned out and generally becomes more efficient.<ref>{{cite web|url=http://www.mass.gov/eea/docs/eea/lbe/bioheat-report.pdf|title=The Massachusetts Bioheat Fuel Pilot Program|date=June 2007|access-date=2012-12-31|archive-date=2012-09-15|archive-url=https://web.archive.org/web/20120915055425/http://www.mass.gov/eea/docs/eea/lbe/bioheat-report.pdf|url-status=live}} Prepared for the Massachusetts Executive Office of Energy and Environmental Affairs</ref> |
|||
Basic industrial tests to determine whether the products conform to the standards typically include [[Chromatography#Gas chromatography|gas chromatography]], a test that verifies only the more important of the variables above. Tests that are more complete are more expensive. Fuel meeting the quality standards is very non-toxic, with a toxicity rating ([[LD50|LD<sub>50</sub>]]) of greater than 50 mL/kg. |
|||
A law passed under [[Massachusetts]] Governor [[Deval Patrick]] requires all home heating diesel in that state to be 2% biofuel by July 1, 2010, and 5% biofuel by 2013.<ref>Massachusetts Oil Heat Council (27 February 2008). [http://massoilheat.org/MOC%20BioHeat%20Press%20Release.pdf MA Oilheat Council Endorses BioHeat Mandate] {{webarchive |url=https://web.archive.org/web/20080511204114/http://massoilheat.org/MOC%20BioHeat%20Press%20Release.pdf |date=May 11, 2008 }}</ref> New York City has passed a similar law. |
|||
==Gelling== |
|||
The [[cloud point]], or temperature at which pure (B100) biodiesel starts to gel, varies significantly and depends upon the mix of esters and therefore the feedstock oil used to produce the biodiesel. For example, biodiesel produced from low [[erucic acid]] varieties of canola seed (RME) starts to gel at approximately −10 °C (14 °F). Biodiesel produced from tallow tends to gel at around +16 °C (68 °F). As of 2006, there are a very limited number of products that will significantly lower the gel point of straight biodiesel. A number of studies have shown that winter operation is possible with biodiesel blended with other fuel oils including #2 low [[sulfur]] [[diesel]] fuel and #1 diesel / [[kerosene]]. The exact blend depends on the operating environment: successful operations have run using a 65% LS #2, 30% K #1, and 5% bio blend. Other areas have run a 70% Low Sulfur #2, 20% Kerosene #1, and 10% bio blend or an 80% K#1, and 20% biodiesel blend. According to the National Biodiesel Board (NBB), B20 (20% biodiesel, 80% petrodiesel) does not need any treatment in addition to what is already taken with petrodiesel. |
|||
===Cleaning oil spills=== |
|||
To permit the use of biodiesel without mixing and without the possibility of gelling at low temperatures, some people modify their vehicles with a second fuel tank for biodiesel in addition to the standard fuel tank. Alternately, a vehicle with two tanks is chosen. The second fuel tank is [[Thermal insulation|insulated]] and a [[Heat exchanger|heating coil]] using [[antifreeze|engine coolant]] is run through the tank. When a temperature sensor indicates that the fuel is warm enough to burn, the driver switches from the petrodiesel tank to the biodiesel tank. This is similar to the method used for running straight vegetable oil. |
|||
With 80–90% of oil spill costs invested in shoreline cleanup, there is a search for more efficient and cost-effective methods to extract oil spills from the shorelines.<ref>{{cite journal|last1=French McCay|first1=D.|last2=Rowe|first2=J. J.|last3=Whittier|first3=N.|last4=Sankaranarayanan|first4=S.|last5=Schmidt Etkin|first5=D.|year=2004|title=Estimation of potential impacts and natural resource damages of oil|journal=J. Hazard. Mater.|volume=107|issue=1–2|pages=11–25|doi=10.1016/j.jhazmat.2003.11.013|pmid=15036639}}</ref> Biodiesel has displayed its capacity to significantly dissolve crude oil, depending on the source of the fatty acids. In a laboratory setting, oiled sediments that simulated polluted shorelines were sprayed with a single coat of biodiesel and exposed to simulated tides.<ref>{{cite journal|last1=Fernández-Ãlvarez|first1=P.|last2=Vila|first2=J.|last3=Garrido|first3=J. M.|last4=Grifoll|first4=M.|last5=Feijoo|first5=G.|last6=Lema|first6=J. M.|year=2007|title=Evaluation of biodiesel as bioremediation agent for the treatment of the shore affected by the heavy oil spill of the Prestige|journal=J. Hazard. Mater.|volume=147|issue=3|pages=914–922|doi=10.1016/j.jhazmat.2007.01.135|pmid=17360115}}</ref> Biodiesel is an effective solvent to oil due to its methyl ester component, which considerably lowers the viscosity of the crude oil. Additionally, it has a higher buoyancy than crude oil, which later aids in its removal. As a result, 80% of oil was removed from cobble and fine sand, 50% in coarse sand, and 30% in gravel. Once the oil is liberated from the shoreline, the oil-biodiesel mixture is manually removed from the water surface with skimmers. Any remaining mixture is easily broken down due to the high [[biodegradability]] of biodiesel, and the increased surface area exposure of the mixture. |
|||
===Biodiesel in generators=== |
|||
==Contamination by water== |
|||
[[File:Biodiesel rental generator.jpg|thumb|upright|Biodiesel is also used in rental generators]] |
|||
Biodiesel may contain small but problematic quantities of water. Although it is [[hydrophobic]] (non-miscible with water [[molecule]]s), it is said to be, at the same time, [[hygroscopy|hygroscopic]] to the point of attracting water molecules from [[atmosphere|atmospheric]] [[moisture]]<ref>{{cite web |
|||
In 2001, UC Riverside installed a 6-megawatt backup power system that is entirely fueled by biodiesel. Backup diesel-fueled generators allow companies to avoid damaging blackouts of critical operations at the expense of high pollution and emission rates. By using B100, these generators were able to essentially eliminate the byproducts that result in smog, ozone, and sulfur emissions.<ref>National Biodiesel Board Electrical Generation. http://www.biodiesel.org/using-biodiesel/market-segments/electrical-generation {{Webarchive|url=https://web.archive.org/web/20130410012410/http://www.biodiesel.org/using-biodiesel/market-segments/electrical-generation |date=2013-04-10 }} (accessed 20 January 2013)</ref> The use of these generators in residential areas around schools, hospitals, and the general public result in substantial reductions in poisonous carbon monoxide and particulate matter.<ref name="Monyem, A. 2001">{{cite journal|last1=Monyem|first1=A.|last2=Van Gerpen|first2=J.|year=2001|title=The effect of biodiesel oxidation on engine performance and emissions|journal=Biomass Bioenergy|volume=20|issue=4|pages=317–325|doi=10.1016/s0961-9534(00)00095-7|bibcode=2001BmBe...20..317M |url=https://lib.dr.iastate.edu/rtd/11950|access-date=2018-11-22|archive-date=2018-01-09|archive-url=https://web.archive.org/web/20180109031302/http://lib.dr.iastate.edu/rtd/11950/|url-status=live}}</ref> |
|||
|last= UFOP - Union zur Förderung von Oel |
|||
|title=Biodiesel FlowerPower: Facts * Arguments * Tips |
|||
|url=http://64.233.167.104/custom?q=cache:OVkS1z7K_jYJ:www.biodiesel.org/resources/reportsdatabase/reports/gen/20040101_gen-331.pdf+hygroscopic&hl=en&ct=clnk&cd=1&gl=us |
|||
|format=PDF |
|||
|accessdate=2007-06-13}}</ref>; one of the reasons biodiesel can absorb water is the persistence of mono and diglycerides left over from an incomplete reaction. These molecules can act as an emulsifier, allowing water to mix with the biodiesel.{{Fact|date=February 2008}} In addition, there may be water that is residual to processing or resulting from storage tank [[condensation]]. The presence of water is a problem because: |
|||
== Effects == |
|||
* Water reduces the heat of [[combustion]] of the bulk fuel. This means more [[smoke]], harder starting, less [[power (physics)|power]]. |
|||
===Fuel efficiency=== |
|||
* Water causes [[corrosion]] of vital fuel system components: fuel pumps, injector pumps, fuel lines, etc. |
|||
The power output of biodiesel depends on its blend, quality, and load conditions under which the fuel is burnt. The [[thermal efficiency]] for example of B100 as compared to B20 will vary due to the differing energy content of the various blends. Thermal efficiency of a fuel is based in part on fuel characteristics such as: [[viscosity]], [[specific density]], and [[flash point]]; these characteristics will change as the blends as well as the quality of biodiesel varies. The [[American Society for Testing and Materials]] has set standards in order to judge the quality of a given fuel sample.<ref> |
|||
* Water & microbes cause the paper element filters in the system to fail ( rot), which in turn results in premature failure of the fuel pump due to ingestion of large particles. |
|||
ASTM Standard D6751-12, 2003, "Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels," ASTM International, West Conshohocken, PA, 2003, {{doi|10.1520/C0033-03}}, astm.org.</ref> |
|||
* Water freezes to form ice crystals near 0 °C (32 °F). These crystals provide sites for [[nucleation]] and accelerate the gelling of the residual fuel. |
|||
* Water accelerates the growth of microbe colonies, which can plug up a fuel system. Biodiesel users who have heated fuel tanks therefore face a year-round microbe problem. |
|||
Previously, the amount of water contaminating biodiesel has been difficult to measure by taking samples, since water and oil separate. However, it is now possible to measure the water content using water in oil sensors. |
|||
* Additionally, water can cause pitting in the pistons on a diesel engine. |
|||
One study found that the brake [[thermal efficiency]] of B40 was superior to traditional petroleum counterpart at higher compression ratios (this higher brake thermal efficiency was recorded at compression ratios of 21:1). It was noted that, as the compression ratios increased, the efficiency of all fuel types – as well as blends being tested – increased; though it was found that a blend of B40 was the most economical at a compression ratio of 21:1 over all other blends. The study implied that this increase in efficiency was due to fuel density, viscosity, and heating values of the fuels.<ref>{{cite journal|last1=Muralidharan|first1=K. K.|last2=Vasudevan|first2=D. D.|year=2011|title=Performance, emission and combustion characteristics of a variable compression ratio engine using methyl esters of waste cooking oil and diesel blends|journal=Applied Energy|volume=88|issue=11|pages=3959–3968|doi=10.1016/j.apenergy.2011.04.014|bibcode=2011ApEn...88.3959M }}</ref> |
|||
Biodiesel can also be used as a heating fuel in domestic and commercial boilers. and would only require around 330,000 hectares of arable land for the required biodiesel for the UK heating oil sector. The paper also suggests that existing oil boilers can easily and cheaply be converted to biodiesel if B20 biodiesel is used. |
|||
===Combustion=== |
|||
==Availability and prices== |
|||
Fuel systems on some modern diesel engines were not designed to accommodate biodiesel, while many heavy duty engines are able to run with biodiesel blends up to B20.<ref name="sciencedirect.com"/> Traditional [[Fuel injection|direct injection]] fuel systems operate at roughly 3,000 psi at the injector tip while the modern [[common rail]] fuel system operates upwards of 30,000 PSI at the injector tip. Components are designed to operate at a great temperature range, from below freezing to over {{convert|1,000|F-change}}. Diesel fuel is expected to burn efficiently and produce as few emissions as possible. As emission standards are being introduced to diesel engines the need to control harmful emissions is being designed into the parameters of diesel engine fuel systems. The traditional inline injection system is more forgiving to poorer quality fuels as opposed to the common rail fuel system. The higher pressures and tighter tolerances of the common rail system allows for greater control over atomization and injection timing. This control of atomization as well as combustion allows for greater efficiency of modern diesel engines as well as greater control over emissions. Components within a diesel fuel system interact with the fuel in a way to ensure efficient operation of the fuel system and so the engine. If an out-of-specification fuel is introduced to a system that has specific parameters of operation, then the integrity of the overall fuel system may be compromised. Some of these parameters such as spray pattern and atomization are directly related to injection timing.<ref>{{cite journal|year=2009|title=Effect of Fuel Injection Timing and Injection Pressure on Combustion and Odorous Emissions in DI Diesel Engines|journal=Journal of Energy Resources Technology|volume=131|issue=3|page=032201|doi=10.1115/1.3185346|last1=Roy|first1=Murari Mohon}}</ref> |
|||
[[Image:Diesel prices.jpg|thumb|170px|In some countries biodiesel is less expensive than conventional diesel.]] |
|||
{{see details|Biodiesel around the World}} |
|||
One study found that during atomization, biodiesel and its blends produced droplets greater in diameter than the droplets produced by traditional petrodiesel. The smaller droplets were attributed to the lower viscosity and surface tension of traditional diesel fuel. It was found that droplets at the periphery of the spray pattern were larger in diameter than the droplets at the center. This was attributed to the faster pressure drop at the edge of the spray pattern; there was a proportional relationship between the droplet size and the distance from the injector tip. It was found that B100 had the greatest spray penetration, this was attributed to the greater density of B100.<ref>{{cite journal|last1=Chen|first1=P.|last2=Wang|first2=W.|last3=Roberts|first3=W. L.|last4=Fang|first4=T.|year=2013|title=Spray and atomization of diesel fuel and its alternatives from a single-hole injector using a common rail fuel injection system|journal=Fuel|volume=103|pages=850–861|doi=10.1016/j.fuel.2012.08.013}}</ref> Having a greater droplet size can lead to inefficiencies in the combustion, increased emissions, and decreased horse power. In another study it was found that there is a short injection delay when injecting biodiesel. This injection delay was attributed to the greater viscosity of Biodiesel. It was noted that the higher viscosity and the greater [[cetane rating]] of biodiesel over traditional petrodiesel lead to poor atomization, as well as mixture penetration with air during the ignition delay period.<ref>{{cite journal|last1=Hwang|first1=J.|last2=Qi|first2=D.|last3=Jung|first3=Y.|last4=Bae|first4=C.|year=2014|title=Effect of injection parameters on the combustion and emission characteristics in a common-rail direct injection diesel engine fueled with waste cooking oil biodiesel|journal=Renewable Energy|volume=63|pages=639–17|doi=10.1016/j.renene.2013.08.051}}</ref> Another study noted that this ignition delay may aid in a decrease of {{NOx|link=yes}} emission.<ref>{{cite journal|last1=McCarthy|first1=P. P.|last2=Rasul|first2=M. G.|last3=Moazzem|first3=S. S.|year=2011|title=Analysis and comparison of performance and emissions of an internal combustion engine fuelled with petroleum diesel and different bio-diesels|journal=Fuel|volume=90|issue=6|pages=2147–2157|doi=10.1016/j.fuel.2011.02.010}}</ref> |
|||
Global biodiesel production reached 3.8 million tons in 2005. Approximately 85% of biodiesel production came from the European Union. |
|||
===Emissions=== |
|||
In the United States, average retail (at the [[pump]]) prices, including Federal and state [[motor tax]]es, of B2/B5 are lower than [[petroleum]] diesel by about 12 cents, and B20 blends are the same as petrodiesel. <ref>[http://www.eere.energy.gov/afdc/pdfs/afpr_jul_07.pdf Clean Cities Alternative Fuel Price Report July 2007<!-- Bot generated title -->]</ref> B99 and B100 generally cost more than petrodiesel except where local governments provide a subsidy. |
|||
Emissions are inherent to the combustion of diesel fuels that are regulated by the U.S. Environmental Protection Agency ([[E.P.A.]]). As these emissions are a byproduct of the combustion process, in order to ensure E.P.A. compliance a fuel system must be capable of controlling the combustion of fuels as well as the mitigation of emissions. There are a number of new technologies being phased in to control the production of diesel emissions. The [[exhaust gas recirculation]] system, E.G.R., and the [[diesel particulate filter]], D.P.F., are both designed to mitigate the production of harmful emissions.<ref>United States Environmental Protection Agency. (2014, April 9). National Clean Diesel Campaign. Retrieved From the Environmental Protection Agency website: http://www.epa.gov/diesel/ {{Webarchive|url=https://web.archive.org/web/20140418220733/http://www.epa.gov/diesel/ |date=2014-04-18 }}</ref> |
|||
The feedstock used to make the biodiesel fuel can significantly alter the resulting exhaust gas and particulate matter emissions,<ref>{{cite web|url=https://www.nrel.gov/docs/fy03osti/31461.pdf|title=The Effect of Biodiesel Composition on Engine Emissions from a DDC Series 60 Diesel Engine|access-date=2022-12-13}}</ref><ref>{{cite journal|last1=Landwehr|first1=K.R.|last2=Hillas|first2=J.|last3=Mead-Hunter|first3=R.|last4=Brooks|first4=P.|last5=King|first5=A.|last6=O'Leary|first6=R.A.|year=2021|title=Fuel feedstock determines biodiesel exhaust toxicity in a human airway epithelial cell exposure model|journal=J. Hazard. Mater.|volume=420|page=126637 |doi=10.1016/j.jhazmat.2021.126637|pmid=34329109|doi-access=free}}</ref> even when blended with commercial diesel fuel.<ref>{{cite journal|last1=Landwehr|first1=K.R.|last2=Hillas|first2=J.|last3=Mead-Hunter|first3=R.|last4=King|first4=A.|last5=O'Leary|first5=R.A.|last6=Kicic|first6=A.|year=2023|title=Biodiesel feedstock determines exhaust toxicity in 20% biodiesel: 80% mineral diesel blends|journal=J. Chemosphere|volume=310|page=136873 |doi=10.1016/j.chemosphere.2022.136873|pmid=36252896|bibcode=2023Chmsp.31036873L |s2cid=252938667 |doi-access=free|hdl=20.500.11937/94726|hdl-access=free}}</ref> A study performed by the [[Chonbuk National University]] concluded that a B30 biodiesel blend reduced [[carbon monoxide]] emissions by approximately 83% and [[particulate matter]] emissions by roughly 33%. {{NOx|link=yes}} emissions, however, were found to increase without the application of an E.G.R. system. The study also concluded that, with E.G.R, a B20 biodiesel blend considerably reduced the emissions of the engine.<ref>Sam, Yoon Ki, et al. "Effects Of Canola Oil Biodiesel Fuel Blends On Combustion, Performance, And Emissions Reduction In A Common Rail Diesel Engine." Energies (19961073) 7.12 (2014): 8132–8149. Academic Search Complete. Web. 14 Nov. 2015.</ref> Additionally, analysis by the [[California Air Resources Board]] found that biodiesel had the lowest carbon emissions of the fuels tested, those being [[ultra-low-sulfur diesel]], gasoline, corn-based [[Ethanol fuel|ethanol]], [[compressed natural gas]], and five types of biodiesel from varying feedstocks. Their conclusions also showed great variance in carbon emissions of biodiesel based on the feedstock used. Of [[Soybean oil|soy]], [[tallow]], [[canola]], [[corn oil|corn]], and [[Vegetable oil fuel|used cooking oil]], soy showed the highest carbon emissions, while used cooking oil produced the lowest.<ref>{{cite web|last1=Robinson|first1=Jessica|title=Nation's strictest regulatory board affirms biodiesel as lowest-carbon fuel|url=http://biodiesel.org/news/news-display/2015/09/28/nation's-strictest-regulatory-board-affirms-biodiesel-as-lowest-carbon-fuel|publisher=National Biodiesel Board|archive-url=https://web.archive.org/web/20170830004357/http://biodiesel.org/news/news-display/2015/09/28/nation's-strictest-regulatory-board-affirms-biodiesel-as-lowest-carbon-fuel|archive-date=August 30, 2017|date=September 28, 2015}}</ref> |
|||
==Production== |
|||
{{main|Biodiesel production}} |
|||
While studying the effect of biodiesel on [[diesel particulate filter]]s, it was found that though the presence of sodium and potassium carbonates aided in the catalytic conversion of ash, as the diesel particulates are catalyzed, they may congregate inside the D.P.F. and so interfere with the clearances of the filter.{{clarify|date=December 2017}} This may cause the filter to clog and interfere with the regeneration process.<ref>{{cite journal|last1=Hansen|first1=B.|last2=Jensen|first2=A.|last3=Jensen|first3=P.|year=2013|title=Performance of diesel particulate filter catalysts in the presence of biodiesel ash species|journal=Fuel|volume=106|pages=234–240|doi=10.1016/j.fuel.2012.11.038|s2cid=40883915 |url=https://backend.orbit.dtu.dk/ws/files/54330527/1_s2.0_S0016236112009362_main.pdf}}</ref> In a study on the impact of E.G.R. rates with blends of jathropa biodiesel it was shown that there was a decrease in [[fuel efficiency]] and torque output due to the use of biodiesel on a diesel engine designed with an E.G.R. system. It was found that [[Carbon monoxide|CO]] and {{CO2|link=yes}} emissions increased with an increase in exhaust gas recirculation but {{NOx|link=yes}} levels decreased. The opacity level of the jathropa blends was in an acceptable range, where traditional diesel was out of acceptable standards. It was shown that a decrease in Nox emissions could be obtained with an E.G.R. system. This study showed an advantage over traditional diesel within a certain operating range of the E.G.R. system.<ref>{{cite journal|last1=Gomaa|first1=M. M.|last2=Alimin|first2=A. J.|last3=Kamarudin|first3=K. A.|year=2011|title=The effect of EGR rates on NOX and smoke emissions of an IDI diesel engine fuelled with Jatropha biodiesel blends|journal=International Journal of Energy & Environment|volume=2|issue=3|pages=477–490}}</ref> |
|||
Chemically, transesterified biodiesel comprises a mix of mono-[[alkyl]] [[ester]]s of long chain [[fatty acid]]s. The most common form uses [[methanol]] (converted to sodium methoxide) to produce [[methyl]] esters as it is the cheapest alcohol available, though [[ethanol]] can be used to produce an ethyl ester biodiesel and higher alcohols such as isopropanol and butanol have also been used. Using alcohols of higher molecular weights improves the cold flow properties of the resulting ester, at the cost of a less efficient transesterification reaction. A [[lipid]] [[transesterification]] production process is used to convert the base oil to the desired esters. Any Free [[fatty acid]]s (FFAs) in the base oil are either converted to soap and removed from the process, or they are esterified (yielding more biodiesel) using an acidic catalyst. After this processing, unlike [[straight vegetable oil]], biodiesel has [[combustion]] properties very similar to those of petroleum diesel, and can replace it in most current uses. |
|||
As of 2017, blended biodiesel fuels (especially B5, B8, and B20) are regularly used in many heavy-duty vehicles, especially transit buses in US cities. Characterization of exhaust emissions showed significant emission reductions compared to regular diesel.<ref name="sciencedirect.com"/> |
|||
A byproduct of the transesterification process is the production of [[glycerol]]. For every 1 tonne of biodiesel that is manufactured, 100 kg of glycerol are produced. Originally, there was a valuable market for the glycerol, which assisted the economics of the process as a whole. However, with the increase in global biodiesel production, the market price for this crude glycerol (containing 20% water and catalyst residues) has crashed. Research is being conducted globally to use this glycerol as a chemical building block. One initiative in the UK is [http://www.theglycerolchallenge.org The Glycerol Challenge]. |
|||
===Material compatibility=== |
|||
Usually this crude glycerol has to be purified, typically by performing vacuum distillation. This is rather energy intensive. The refined glycerol (98%+ purity) can then be utilised directly, or converted into other products. The following announcements were made in 2007: A joint venture of [[Ashland Inc.]] and [[Cargill]] announced plans to make [[propylene glycol]] in Europe from [[glycerol]]<ref>chemweek's Business Daily, Tuesday [[May 8]], [[2007]]</ref> and [[Dow Chemical]] announced similar plans for North America.<ref>http://www.dow.com/propyleneglycol/news/20070315b.htm, accessed [[June 25]], [[2007]] </ref> [[Dow]] also plans to build a plant in [[China]] to make [[epichlorhydrin]] from [[glycerol]].<ref>http://epoxy.dow.com/epoxy/news/2007/20070326b.htm, accessed [[June 25]], [[2007]]</ref> [[Epichlorhydrin]] is a raw material for [[epoxy resins]]. |
|||
* Plastics: High-density polyethylene (HDPE) is compatible but [[polyvinyl chloride]] (PVC) is slowly degraded.<ref name="basics" /> Polystyrene is dissolved on contact with biodiesel. |
|||
* Metals: Biodiesel (like [[methanol]]) has an effect on copper-based materials (e.g. brass), and it also affects zinc, tin, lead, and cast iron.<ref name="basics" /> Stainless steels (316 and 304) and aluminum are unaffected. |
|||
* Rubber: Biodiesel also affects types of natural rubbers found in some older engine components. Studies have also found that fluorinated elastomers (FKM) cured with peroxide and base-metal oxides can be degraded when biodiesel loses its stability caused by oxidation. Commonly used synthetic rubbers FKM- GBL-S and FKM- GF-S found in modern vehicles were found to handle biodiesel in all conditions.<ref>[http://www.dupontelastomers.com/literature/viton/20E5483C5825D7398525736700470EB1.pdf Fluoroelastomer Compatibility with Biodiesel Fuels] {{Webarchive|url=https://web.archive.org/web/20141006070624/http://www.dupontelastomers.com/literature/viton/20E5483C5825D7398525736700470EB1.pdf |date=2014-10-06 }} Eric W. Thomas, Robert E. Fuller and Kenji Terauchi DuPont Performance Elastomers L.L.C. January 2007</ref> |
|||
== Production == |
|||
{{Further|Biodiesel production}}[[File:Bequer-B100-SOJA-SOYBEAM.jpg|upright|thumb|right|Pure biodiesel (B-100) made from soybeans]] |
|||
Biodiesel is commonly produced by the [[transesterification]] of the vegetable oil or animal fat feedstock, and other non-edible raw materials such as frying oil, etc. There are several methods for carrying out this transesterification reaction including the common batch process, heterogeneous catalysts,<ref>{{cite journal|last1=Hernández|first1=M.R.|last2=Reyes-Labarta|first2=J.A.|title=Reyes-Labarta|journal=Industrial & Engineering Chemistry Research|date=2010|volume=49|issue=19|pages=9068–9076|doi=10.1021/ie100978m}}</ref> supercritical processes, ultrasonic methods, and even microwave methods. |
|||
Chemically, transesterified biodiesel comprises a mix of mono-[[alkyl]] esters of long chain [[fatty acid]]s. The most common form uses [[methanol]] (converted to sodium methoxide) to produce [[methyl]] esters (commonly referred to as [[Fatty acid methyl ester|Fatty Acid Methyl Ester]] – FAME) as it is the cheapest alcohol available, though [[ethanol]] can be used to produce an ethyl ester (commonly referred to as Fatty Acid Ethyl Ester – FAEE) biodiesel and higher alcohols such as [[isopropanol]] and [[n-Butanol|butanol]] have also been used. Using alcohols of higher molecular weights improves the cold flow properties of the resulting ester, at the cost of a less efficient transesterification reaction. A [[lipid]] [[transesterification]] production process is used to convert the base oil to the desired esters. Any free fatty acids (FFAs) in the base oil are either [[saponification|converted to soap]] and removed from the process, or they are esterified (yielding more biodiesel) using an acidic catalyst. After this processing, unlike [[straight vegetable oil]], biodiesel has combustion properties very similar to those of petroleum diesel, and can replace it in most current uses. |
|||
The methanol used in most biodiesel production processes is made using fossil fuel inputs. However, there are sources of [[Carbon Recycling International|renewable methanol]] made using carbon dioxide or biomass as feedstock, making their production processes free of fossil fuels.<ref>{{cite web|url=http://cri.is/index.php?option=com_content&view=article&id=4&Itemid=3&lang=en|title=Products|access-date=13 July 2012|publisher=Carbon Recycling International|url-status=dead|archive-url=https://web.archive.org/web/20130729155610/http://cri.is/index.php?option=com_content&view=article&id=4&Itemid=3&lang=en|archive-date=29 July 2013}}</ref> |
|||
A by-product of the transesterification process is the production of [[glycerol]]. For every 1 tonne of biodiesel that is manufactured, 100 kg of glycerol are produced. Originally, there was a valuable market for the glycerol, which assisted the economics of the process as a whole. However, with the increase in global biodiesel production, the market price for this crude glycerol (containing 20% water and catalyst residues) has crashed. Research is being conducted globally to use this glycerol as a chemical building block (see [[Glycerol#Chemical intermediate|chemical intermediate]] under Wikipedia article "[[Glycerol]]"). One initiative in the UK is The Glycerol Challenge.<ref>{{cite web|title=Biofuels and Glycerol|url=http://www.theglycerolchallenge.org|publisher=theglycerolchallenge.org|access-date=2008-07-09|url-status=dead|archive-url=https://web.archive.org/web/20080523163346/http://theglycerolchallenge.org/|archive-date=2008-05-23}}</ref> |
|||
Usually this crude glycerol has to be purified, typically by performing vacuum distillation. This is rather energy intensive. The refined glycerol (98%+ purity) can then be utilised directly, or converted into other products. The following announcements were made in 2007: A joint venture of [[Ashland Inc.]] and [[Cargill]] announced plans to make [[propylene glycol]] in Europe from glycerol<ref>Chemweek's Business Daily, Tuesday May 8, 2007</ref> and [[Dow Chemical]] announced similar plans for North America.<ref>{{cite web|url=http://www.dow.com/propyleneglycol/news/20070315b.htm|title=Retrieved June 25, 2007|publisher=Dow.com|access-date=2010-03-15|archive-url=https://web.archive.org/web/20090916061724/http://www.dow.com/propyleneglycol/news/20070315b.htm|archive-date=2009-09-16|url-status=dead}}</ref> Dow also plans to build a plant in China to make [[epichlorhydrin]] from glycerol.<ref>{{cite web|url=http://epoxy.dow.com/epoxy/news/2007/20070326b.htm|title=Retrieved June 25, 2007|publisher=Epoxy.dow.com|access-date=2010-03-15|archive-date=2009-09-16|archive-url=https://web.archive.org/web/20090916061912/http://epoxy.dow.com/epoxy/news/2007/20070326b.htm|url-status=dead}}</ref> [[Epichlorhydrin]] is a raw material for [[epoxy resins]]. |
|||
Global [[biodiesel production]] reached 3.8 million tons in 2005. Approximately 85% of biodiesel production came from the European Union.{{Citation needed|date=January 2017}}<ref>{{Cite book |last1=Dasmohapatra |first1=Gourkrishna |url=https://books.google.com/books?id=jkJDDAAAQBAJ |title=Engineering Chemistry I (WBUT), 3rd Edition |publisher=Vikas Publishing House |isbn=9789325960039 |access-date=2017-01-13 |archive-url=https://web.archive.org/web/20200403125806/https://books.google.com/books?id=jkJDDAAAQBAJ&pg |archive-date=2020-04-03 |url-status=live}}</ref> |
|||
{{Further|Biodiesel around the world}} |
|||
===Production levels=== |
===Production levels=== |
||
{{Overly detailed|section|date=January 2023}} |
|||
Biodiesel production capacity is growing rapidly, with an average annual growth rate from 2002-2006 of over 40% <ref>{{cite web |
|||
{{Further | Biodiesel around the world}} |
|||
| last = Martinot (Lead Author) |
|||
| first = Eric |
|||
In 2007, biodiesel production capacity was growing rapidly, with an average annual growth rate from 2002 to 2006 of over 40%.<ref>{{cite web |last=Martinot |first=Eric |year=2008 |title=Renewables 2007. Global Status Report |url=http://www.martinot.info/RE2007_Global_Status_Report.pdf |url-status=live |archive-url=https://web.archive.org/web/20080410055942/http://www.martinot.info/RE2007_Global_Status_Report.pdf |archive-date=2008-04-10 |access-date=2008-04-03 |publisher=REN21 - Renewable Energy Policy Network for the 21st Century}}</ref> For the year 2006, the latest for which actual production figures could be obtained, total world biodiesel production was about 5–6 million tonnes, with 4.9 million tonnes processed in Europe (of which 2.7 million tonnes was from Germany) and most of the rest from the US. In 2008 production in Europe alone had risen to 7.8 million tonnes.<ref>{{cite web|title=Statistics. the EU biodiesel industry|publisher=European Biodiesel Board|date=2008-03-28|url=http://www.ebb-eu.org/stats.php#|access-date=2008-04-03|archive-date=2006-11-14|archive-url=https://web.archive.org/web/20061114025810/http://www.ebb-eu.org/stats.php|url-status=dead}}</ref> In July 2009, a duty was added to American imported biodiesel in the European Union in order to balance the competition from European, especially German producers.<ref>{{cite web|title=US Biodiesel Taxed in EU|publisher=Hadden Industries|url=http://www.haddenindustries.org|access-date=2009-08-28|archive-url=https://web.archive.org/web/20091011223539/http://www.haddenindustries.org/|archive-date=2009-10-11|url-status=dead}}</ref><ref>{{cite web|title=US Biodiesel Demand|work=Biodiesel: The official site of the National Biodiesel Board|publisher=NBB|url=http://www.biodiesel.org/pdf_files/fuelfactsheets/Production_Graph_Slide.pdf|access-date=2008-04-03|archive-date=2008-04-10|archive-url=https://web.archive.org/web/20080410055944/http://www.biodiesel.org/pdf_files/fuelfactsheets/Production_Graph_Slide.pdf|url-status=live}}</ref> The capacity for 2008 in Europe totalled 16 million tonnes. This compares with a total demand for diesel in the US and Europe of approximately 490 million tonnes (147 billion gallons).<ref>{{cite web|title=Biodiesel to drive up the price of cooking oil|publisher=Biopower London|year=2006|url=http://www.biopowerlondon.co.uk/news2.htm|access-date=2008-04-03|archive-url=https://web.archive.org/web/20080607201833/http://www.biopowerlondon.co.uk/news2.htm|archive-date=2008-06-07|url-status=dead}}</ref> Total world production of vegetable oil for all purposes in 2005–06 was about 110 million tonnes, with about 34 million tonnes each of [[palm oil]] and [[soybean oil]].<ref>{{cite web|title=Major Commodities|publisher=FEDIOL (EU Oil and Proteinmeal Industry)|url=http://www.fediol.be/2/index.php|access-date=2008-04-08|archive-url=https://web.archive.org/web/20080421171613/http://www.fediol.be/2/index.php|archive-date=2008-04-21|url-status=dead}}</ref> As of 2018, [[Indonesia]] is the world's top supplier of palmoil-based biofuel with annual production of 3.5 million tons,<ref>{{cite news|url=https://www.reuters.com/article/us-malaysia-palmoil-biodiesel/indonesia-to-boost-biodiesel-exports-malaysia-expects-to-lose-market-share-idUSKBN1HQ0OO|title=Indonesia to boost biodiesel exports, Malaysia expects to lose market share|newspaper=Reuters|access-date=31 August 2018|archive-date=31 August 2018|archive-url=https://web.archive.org/web/20180831141058/https://www.reuters.com/article/us-malaysia-palmoil-biodiesel/indonesia-to-boost-biodiesel-exports-malaysia-expects-to-lose-market-share-idUSKBN1HQ0OO|url-status=live}}</ref><ref>{{cite web|url=https://www.biofuelsdigest.com/bdigest/2018/03/12/indonesian-biodiesel-production-seen-jumping-to-3-5-million-tons-this-year/|title=Indonesian biodiesel production seen jumping to 3.5 million tonnes this year|date=12 March 2018 |access-date=31 August 2018|archive-date=31 August 2018|archive-url=https://web.archive.org/web/20180831104353/https://www.biofuelsdigest.com/bdigest/2018/03/12/indonesian-biodiesel-production-seen-jumping-to-3-5-million-tons-this-year/|url-status=live}}</ref> and expected to export about 1 million tonnes of biodiesel.<ref>{{cite news|url=https://af.reuters.com/article/commoditiesNews/idAFJ9N1TZ00S|archive-url=https://web.archive.org/web/20180830123456/https://af.reuters.com/article/commoditiesNews/idAFJ9N1TZ00S|url-status=dead|archive-date=30 August 2018|title=Indonesia's 2018 biodiesel exports seen at around 1 mln tonnes - assoc|newspaper=Reuters|access-date=31 August 2018}}</ref> |
|||
| title = Renewables 2007. Global Status Report |
|||
| work = |
|||
US biodiesel production in 2011 brought the industry to a new milestone. Under the EPA Renewable Fuel Standard, targets have been implemented for the biodiesel production plants in order to monitor and document production levels in comparison to total demand. According to the year-end data released by the EPA, biodiesel production in 2011 reached more than 1 billion gallons. This production number far exceeded the 800 million gallon target set by the EPA. The projected production for 2020 is nearly 12 billion gallons.<ref name="biodiesel.org">{{cite web|url=https://www.biodiesel.org/production/production-statistics|title=U.S. biodiesel production|author=National Biodiesel Board|year=2018|access-date=2019-07-11|archive-date=2020-04-03|archive-url=https://web.archive.org/web/20200403125833/https://www.biodiesel.org/production/production-statistics|url-status=live}}</ref> |
|||
| publisher = REN21 (Renewable Energy Policy Network for the 21stCentury |
|||
| date = 2008 |
|||
| url = http://www.martinot.info/RE2007_Global_Status_Report.pdf |
|||
| doi = |
|||
| accessdate = 2008-04-03}}</ref>. For the year 2006, the latest for which actual production figures could be obtained, total world biodiesel production was about 5-6 million tonnes, with 4.9 million tonnes coming from Europe <ref>{{cite web |
|||
| title = Statistics. the EU biodiesel industry |
|||
| publisher = European Biodiesel Board |
|||
| date = 2008-03-28 |
|||
| url = http://www.ebb-eu.org/stats.php# |
|||
| accessdate = 2008-04-03}}</ref> (of which 2.7 million tonnes was from Germany) and most of the rest from the USA. <ref>{{cite web |
|||
| title = US Biodiesel Demand |
|||
| work = Biodiesel: The official site of the National Biodiesel Board |
|||
| publisher = NBB |
|||
| url = http://www.biodiesel.org/pdf_files/fuelfactsheets/Production_Graph_Slide.pdf |
|||
| accessdate =2008-04-03 }}</ref> |
|||
The capacity for 2007 in Europe totalled 10.3 million tonnes. This compares with a total demand for diesel in the US and Europe of approximately 490 million tonnes (147 billion gallons)<ref>{{cite web |
|||
| title = Biodiesel to drive up the price of cooking oil |
|||
| work = |
|||
| publisher = Biopower London |
|||
| date = 2006 |
|||
| url = http://www.biopowerlondon.co.uk/news2.htm |
|||
| accessdate = 2008-04-03}}</ref> |
|||
===Biodiesel feedstocks=== |
===Biodiesel feedstocks=== |
||
{{Vegetable oils|image=Soybeanvarieties.jpg|caption=[[Soybean]]s are used as a source of biodiesel}} |
|||
{{Vegetable oils}} |
|||
A variety of oils can be used to produce biodiesel. These include: |
A variety of oils can be used to produce biodiesel. These include: |
||
*Virgin oil feedstock; [[rapeseed]] and [[soybean]] oils are most commonly used, soybean oil alone accounting for about ninety percent of all fuel stocks in the US. It also can be obtained from [[Thlaspi arvense|field pennycress]] and [[Jatropha]] other [[agriculture|crops]] such as [[Mustard plant|mustard]], [[flax]], [[sunflower]], [[palm oil]], [[hemp]] (see [[List of vegetable oils#Oils used for biofuel|List of vegetable oils]] for a more complete list); |
|||
*[[Waste vegetable oil]] (WVO); |
|||
*Animal [[fat]]s including [[tallow]], [[lard]], [[yellow grease]], chicken fat, <ref name="ChickenFat"> {{cite news |
|||
| url = http://www.washingtonpost.com/wp-dyn/content/article/2007/01/02/AR2007010201057.html |
|||
| title = Not a Tiger, but Maybe a Chicken in Your Tank |
|||
| publisher = Associated Press |
|||
| work = Washington Post |
|||
| last = Leonard |
|||
| first = Christopher |
|||
| page = D03 |
|||
| date = 2007-01-03 |
|||
| accessdate = 2007-12-04 |
|||
}}</ref> and the by-products of the production of [[Omega-3 fatty acids]] from fish oil. |
|||
*[[Algae fuel|Algae]], which [[algaculture|can be grown]] using waste materials such as sewage<ref name="Kiong">{{cite news |
|||
| author = Errol Kiong | title = NZ firm makes bio-diesel from sewage in world first |
|||
| url = http://www.nzherald.co.nz/section/story.cfm?c_id=1&ObjectID=10381404 |
|||
| publisher = The New Zealand Herald |
|||
| date = [[12 May]] [[2006]] | accessdate = 2007-01-10 |
|||
}}</ref> and without displacing land currently used for food production. |
|||
* Virgin oil feedstock – [[rapeseed]] and [[soybean oil]]s are most commonly used, soybean oil <ref name="sciencedirect.com" /> accounting for about half of U.S. production.<ref>{{cite web|last=U.S. Energy Information Administration|title=Monthly Biodiesel Production Reports|url=http://www.eia.gov/biofuels/biodiesel/production/|publisher=U.S. Department of Energy|access-date=27 February 2013|archive-date=13 March 2013|archive-url=https://web.archive.org/web/20130313093836/http://www.eia.gov/biofuels/biodiesel/production/|url-status=live}}</ref> It also can be obtained from [[Pongamia]], [[Thlaspi arvense|field pennycress]] and [[jatropha]] and other crops such as [[mustard plant|mustard]], [[jojoba]], [[flax]], [[sunflower]], [[palm oil]], [[coconut]] and [[hemp]] (see [[List of vegetable oils#Oils used for biofuel|list of vegetable oils for biofuel]] for more information); |
|||
Many advocates suggest that waste vegetable oil is the best source of oil to produce biodiesel, but since the available supply is drastically less than the amount of petroleum-based fuel that is burned for transportation and home heating in the world, this local solution does not scale well. |
|||
* [[Waste vegetable oil]] (WVO); |
|||
* Animal fats including [[tallow]], [[lard]], [[yellow grease]], chicken fat,<ref name="ChickenFat">{{cite news|url=https://www.washingtonpost.com/wp-dyn/content/article/2007/01/02/AR2007010201057.html|title=Not a Tiger, but Maybe a Chicken in Your Tank|agency=Associated Press|newspaper=The Washington Post|last=Leonard|first=Christopher|page=D03|date=2007-01-03|access-date=2007-12-04|archive-date=2012-11-04|archive-url=https://web.archive.org/web/20121104062906/http://www.washingtonpost.com/wp-dyn/content/article/2007/01/02/AR2007010201057.html|url-status=live}}</ref> and the by-products of the production of [[Omega-3 fatty acids]] from fish oil. |
|||
* [[Algae fuel|Algae]], which [[algaculture|can be grown]] using waste materials such as sewage<ref name="Kiong">{{cite news|first=Errol|last=Kiong|title=NZ firm makes bio-diesel from sewage in world first|url=http://www.nzherald.co.nz/section/story.cfm?c_id=1&ObjectID=10381404|archive-url=https://wayback.archive-it.org/all/20060602074304/http://www.nzherald.co.nz/section/story.cfm?c_id=1&ObjectID=10381404|url-status=dead|archive-date=June 2, 2006|newspaper=The New Zealand Herald|date=May 12, 2006|access-date=2007-01-10}}</ref> and without displacing land currently used for food production. |
|||
* Oil from [[halophyte]]s such as ''[[Salicornia#Industrial use (contemporary)|Salicornia bigelovii]]'', which can be grown using saltwater in coastal areas where conventional crops cannot be grown, with yields equal to the yields of soybeans and other oilseeds grown using freshwater irrigation<ref name="Glenn1998">{{cite journal|last=Glenn|first=Edward P.|author2=Brown, J. Jed|author3=O'Leary, James W.|date=August 1998|title=Irrigating Crops with Seawater|journal=[[Scientific American]]|volume=279|pages=76–81 [79]|issue=August 1998|url=http://www.miracosta.edu/home/kmeldahl/writing/..%5Carticles/crops.pdf|access-date=2008-11-17|doi=10.1038/scientificamerican0898-76|bibcode=1998SciAm.279b..76G|archive-date=2015-09-06|archive-url=https://web.archive.org/web/20150906075620/http://www.miracosta.edu/home/kmeldahl/articles/crops.pdf|url-status=live}}</ref> |
|||
* Sewage Sludge – The sewage-to-biofuel field is attracting interest from major companies like Waste Management and startups like InfoSpi, which are betting that renewable sewage biodiesel can become competitive with petroleum diesel on price.<ref>{{cite news|last=Casey|first=Tina|date=May 2010|title=The Smell of Change is in the Air with Renewable Biodiesel from Sewage|newspaper=[[Scientific American]]|url=http://www.scientificamerican.com/article.cfm?id=the-smell-of-change-is-in-the-air-w-2010-05}}</ref> |
|||
Many advocates suggest that waste vegetable oil is the best source of oil to produce biodiesel, but since the available supply is drastically less than the amount of petroleum-based fuel that is burned for transportation and home heating in the world, this local solution could not scale to the current rate of consumption. |
|||
Animal fats are a by-product of meat production and cooking. Although it would not be efficient to raise animals (or catch fish) simply for their fat, use of the by-product adds value to the livestock industry (hogs, cattle, poultry). Today, multi-feedstock biodiesel facilities are producing high quality animal-fat based biodiesel.<ref name="NRA1">{{cite web |year=2010 |title=Monthly US Raw Material Usage for US Biodiesel Production 2007-2009 |url=http://assets.nationalrenderers.org/Monthly_US_Raw_Material_Useage_for_US_Biodiesel_Production_2007_2009.pdf |url-status=live |archive-url=https://web.archive.org/web/20121019205836/http://assets.nationalrenderers.org/Monthly_US_Raw_Material_Useage_for_US_Biodiesel_Production_2007_2009.pdf |archive-date=October 19, 2012 |access-date=March 23, 2012 |work=assets.nationalrenderers.org}}</ref><ref name="AusBiofuels">{{cite web |last=O'Connell |first=Deborah |year=2008 |title=Biofuels in Australia: Issues and Prospects. A report for the Rural Industries Research and Development Corporation |url=http://www.bioenergy.org.nz/documents/liquidbiofuels/AustraliaBiofuels.pdf |url-status=dead |archive-url=https://web.archive.org/web/20120503205948/http://www.bioenergy.org.nz/documents/liquidbiofuels/AustraliaBiofuels.pdf |archive-date=3 May 2012 |access-date=23 March 2012 |work=bioenergy.org.nz}}</ref> Currently, a 5-million dollar plant is being built in the US, with the intent of producing 11.4 million litres (3 million gallons) biodiesel from some of the estimated 1 billion kg (2.2 billion pounds) of chicken fat<ref>{{cite web|title=Biodiesel from Animal Fat|publisher=E85.whipnet.net|url=http://www.whipnet.com/sites/e85.whipnet.net/alt.fuel/animal.fat.html|access-date=2021-01-16|archive-date=2021-01-23|archive-url=https://web.archive.org/web/20210123000905/http://www.whipnet.com/sites/e85.whipnet.net/alt.fuel/animal.fat.html|url-status=live}}</ref> produced annually at the local Tyson poultry plant.<ref name="ChickenFat" /> Similarly, some small-scale biodiesel factories use waste fish oil as feedstock.<ref>{{cite web|title=Biodiesel produced from "tra", "basa" catfish oil|publisher=governmental site|url=http://www.fistenet.gov.vn/details_e.asp?Object=2111609&news_id=4540732|access-date=2008-05-25|url-status=dead|archive-url=https://web.archive.org/web/20061004000259/http://www.fistenet.gov.vn/details_e.asp?Object=2111609&News_ID=4540732|archive-date=October 4, 2006}}</ref><ref>{{cite web|title=Demonstrating the value of a fishy biodiesel blend in Alaska's Aleutian Islands|publisher=Biodiesel america|url=http://www.biodieselamerica.org/files/articles/alaskafishoil_fs_3_18_02.pdf|access-date=2008-05-25|archive-url=https://web.archive.org/web/20070202184313/http://biodieselamerica.org/files/articles/alaskafishoil_fs_3_18_02.pdf|archive-date=February 2, 2007|url-status=dead}}</ref> An EU-funded project (ENERFISH) suggests that at a Vietnamese plant to produce biodiesel from [[catfish]] (basa, also known as pangasius), an output of 13 tons/day of biodiesel can be produced from 81 tons of fish waste (in turn resulting from 130 tons of fish). This project utilises the biodiesel to fuel a [[Cogeneration|CHP]] unit in the fish processing plant, mainly to power the fish freezing plant.<ref>{{cite web|title=Enerfish integrated energy solutions for seafood processing stations|publisher=VTT, Finland/Enerfish Consortium|url=http://www.enerfish.eu/index.php|access-date=2009-10-20|archive-url=https://web.archive.org/web/20091022094757/http://www.enerfish.eu/index.php|archive-date=2009-10-22|url-status=dead}}</ref> |
|||
Animal fats are similarly limited in supply, and it would not be efficient to raise animals simply for their fat. However, producing biodiesel with animal fat that would have otherwise been discarded could replace a small percentage of petroleum diesel usage. Currently, a 5-million dollar plant is being built in the USA, with the intent of producing 11.4 million litres (3 million gallons) biodiesel from some of the estimated 1 billion kg (2.3 billion pounds) of chicken fat<ref> {{cite web |
|||
| title =Biodiesel from Animal Fat |
|||
| publisher = E85.whipnet.net |
|||
| url =http://e85.whipnet.net/alt.fuel/animal.fat.html |
|||
| accessdate = 2008-01-07 }} </ref> produced annually the local Tyson poultry plant. <ref name="ChickenFat"/> |
|||
====Quantity of feedstocks required==== |
====Quantity of feedstocks required==== |
||
{{See also|Food vs. fuel}} |
|||
Worldwide production of vegetable oil and animal fat is not yet sufficient to replace liquid fossil fuel use. Furthermore, some object to the vast amount of [[agriculture|farming]] and the resulting [[fertilizer|fertilization]], [[pesticide]] use, and land use conversion that would be needed to produce the additional vegetable oil. The estimated transportation diesel fuel and home heating oil used in the United States is about 160 million tonnes (350 billion pounds) according to the [[Energy Information Administration]], [[US Department of Energy]] - <ref>http://tonto.eia.doe.gov/dnav/pet/pet_cons_821dst_dcu_nus_a.htm)</ref>. In the United States, estimated production of vegetable oil for all uses is about 11 million tonnes (24 billion pounds) and estimated production of animal fat is 5.3 million tonnes (12 billion pounds).<ref>{{cite web |
|||
| last = Van Gerpen |
|||
| first = John |
|||
| title = Business Management for Biodiesel Producers, August 2002 - January 2004 |
|||
| work = |
|||
| publisher = National Renewable Energy Laboratory |
|||
| date = 2004 - 07 |
|||
| url = http://www.nrel.gov/docs/fy04osti/36242.pdf |
|||
| format = |
|||
| doi = |
|||
| accessdate = 2008-01-07 }}</ref> |
|||
Current worldwide production of vegetable oil and animal fat is not sufficient to replace liquid fossil fuel use. Furthermore, some object to the vast amount of farming and the resulting [[fertilizer|fertilization]], [[pesticide]] use, and land use conversion that would be needed to produce the additional vegetable oil.<ref>[http://tonto.eia.doe.gov/dnav/pet/pet_cons_821dst_dcu_nus_a.htm)]{{dead link|date=December 2015}}</ref> The advantages of algae are that it can be grown on non-arable land such as deserts or in marine environments, and the potential oil yields are much higher than from plants. |
|||
If the entire arable land area of the USA (470 million acres, or 1.9 million square kilometers) were devoted to biodiesel production from soy, this would just about provide the 160 million tonnes required (assuming an optimistic 98 gpa of biodiesel). This land area could in principle be reduced significantly using algae, if the obstacles can be overcome. The [[United States Department of Energy|US DOE]] estimates that if algae fuel replaced all the petroleum fuel in the United States, it would require 15,000 square miles (38,849 [[square kilometer]]s), which is a few thousand square miles larger than [[Maryland]], or 1.3 Belgiums, <ref>[http://www.washingtonpost.com/wp-dyn/content/article/2008/01/03/AR2008010303907.html A Promising Oil Alternative: Algae Energy - washingtonpost.com]</ref> <ref name="Briggs2004">{{cite web |
|||
| url = http://www.unh.edu/p2/biodiesel/article_alge.html |
|||
| title = Widescale Biodiesel Production from Algae| author = Michael Briggs |
|||
| year = 2004| month = August| accessdate = 2007-01-02 |
|||
| publisher = UNH Biodiesel Group (University of New Hampshire) |
|||
}}</ref>assuming a yield of 15000 gpa. The advantages of algae are that it can be grown on non-arable land such as deserts or in marine environments, and the potential oil yields are much higher than from plants. |
|||
===Yield=== |
===Yield=== |
||
Feedstock yield efficiency per |
Feedstock yield efficiency per unit area affects the feasibility of ramping up production to the huge industrial levels required to power a significant percentage of vehicles. |
||
{| class="wikitable" |
|||
* Algae: 1800 gpa or more (est.- see soy figures and DOE quote below) |
|||
|- |
|||
* Palm oil: 508 gpa<ref name=gristmill>[http://gristmill.grist.org/story/2006/2/7/12145/81957 Biofuels: some numbers]</ref> |
|||
|+Some typical yields |
|||
* Coconut: 230 gpa<ref name=gristmill /> |
|||
|- |
|||
* Rapeseed: 102 gpa<ref name=gristmill /> |
|||
! rowspan="2" |Crop |
|||
* Soy: 59.2-98.6 gpa in Indiana<ref name=perdue.edu>[www.ces.purdue.edu/extmedia/ID/ID-337.pdf Purdue report ID-337]</ref> (Soy is used in 80% of USA biodiesel<ref name=Soy-improving_yield>[http://americanfuels.blogspot.com/2008/02/biodiesel-yields-even-higher-energy.html Biodiesel Yields Even Higher Energy Balance]</ref>) |
|||
! colspan="2" |Yield |
|||
* Peanut: 90 gpa<ref name=gristmill /> |
|||
|- |
|||
* Sunflower: 82 gpa<ref name=gristmill /> |
|||
!L/ha |
|||
!US gal/acre |
|||
|- |
|||
|'''Palm oil'''<ref name="gristmill" group="n">{{cite web|url=http://www.grist.org/article/biofuel-some-numbers|title=Biofuels: some numbers|publisher=Grist.org|access-date=2010-03-15|date=2006-02-08|archive-date=2010-03-01|archive-url=https://web.archive.org/web/20100301061733/http://www.grist.org/article/biofuel-some-numbers/|url-status=live}}</ref> |
|||
| align="right" | {{#expr:508*9.35395627round0}} |
|||
| align="right" | 508 |
|||
|- |
|||
|'''Coconut''' |
|||
| align="right" | {{#expr:230*9.35395627round0}} |
|||
| align="right" | 230 |
|||
|- |
|||
|'''[[Cyperus esculentus]]'''<ref group="n">Makareviciene et al., "Opportunities for the use of chufa sedge in biodiesel production", <br />Industrial Crops and Products, 50 (2013) p. 635, table 2.</ref> |
|||
| align="right" | {{#expr:174*9.35395627round0}} |
|||
| align="right" | 174 |
|||
|- |
|||
|'''Rapeseed'''<ref name="gristmill" group="n" /> |
|||
| align="right" | {{#expr:102*9.35395627round0}} |
|||
| align="right" | 102 |
|||
|- |
|||
|'''Soy (Indiana)'''<ref name="perdue.edu">{{cite web|url=http://www.ces.purdue.edu/extmedia/ID/ID-337.pdf|title=Purdue report ID-337|website=purdue.edu|access-date=9 July 2017|url-status=dead|archive-url=https://web.archive.org/web/20120301001245/http://www.ces.purdue.edu/extmedia/ID/ID-337.pdf|archive-date=1 March 2012}}</ref> |
|||
| align="right" | {{#expr:59.2*9.35395627round0}}-{{#expr:98.6*9.35395627round0}} |
|||
| align="right" | 59.2–98.6 |
|||
|- |
|||
|'''[[Chinese tallow]]'''<ref group="n">Klass, Donald, "Biomass for Renewable Energy, Fuels,<br />and Chemicals", page 341. Academic Press, 1998.</ref><ref group="n">Kitani, Osamu, "Volume V: Energy and Biomass Engineering,<br />CIGR Handbook of Agricultural Engineering", Amer Society of Agricultural, 1999.</ref> |
|||
| align="right" | {{#expr:97*9.35395627round0}} |
|||
| align="right" | 97 |
|||
|- |
|||
|'''Peanut'''<ref name="gristmill" group="n" /> |
|||
| align="right" | {{#expr:90*9.35395627round0}} |
|||
| align="right" | 90 |
|||
|- |
|||
|'''Sunflower'''<ref name="gristmill" group="n" /> |
|||
| align="right" | {{#expr:82*9.35395627round0}} |
|||
| align="right" | 82 |
|||
|- |
|||
|'''Hemp'''{{Citation needed|date=September 2009}} |
|||
| align="right" | 242 |
|||
| align="right" | {{#expr:242/9.35395623round0}} |
|||
|- |
|||
| colspan="3" |{{Refbegin}} |
|||
<references group=n/> |
|||
{{Refend}} |
|||
|} |
|||
Algae fuel yields have not yet been accurately determined, but DOE is reported as saying that algae yield 30 times more energy per acre than land crops such as soybeans.<ref name="washingtonpost-algae">{{cite news|url=https://www.washingtonpost.com/wp-dyn/content/article/2008/01/03/AR2008010303907.html|title=DOE quoted by Washington Post in "A Promising Oil Alternative: Algae Energy"|work=Washingtonpost.com|date=2008-01-06|access-date=2010-03-15|archive-date=2011-05-14|archive-url=https://web.archive.org/web/20110514091059/http://www.washingtonpost.com/wp-dyn/content/article/2008/01/03/AR2008010303907.html|url-status=live}}</ref> Yields of 36 tonnes/hectare are considered practical by Ami Ben-Amotz of the Institute of Oceanography in [[Haifa]], who has been farming Algae commercially for over 20 years.<ref>{{cite journal|last=Strahan|first=David|title=Green Fuel for the Airline Industry|journal=New Scientist|volume=199|issue=2669|pages=34–37|date=13 August 2008|url=https://www.newscientist.com/channel/tech/mg19926691.700-green-fuel-for-the-airline-industry.html|access-date=2008-09-23|doi=10.1016/S0262-4079(08)62067-9|archive-date=2021-07-31|archive-url=https://web.archive.org/web/20210731013356/https://www.newscientist.com/article/mg19926691-700-green-fuel-for-the-airline-industry/?ignored=irrelevant|url-status=live}}</ref> |
|||
|title=Algae for Liquid Fuel Production |
|||
|publisher=Oakhaven Permaculture Center |
|||
|author=Thomas F. Riesing, Ph.D. |
|||
|date=Spring 2006 |accessdate=2006-12-18 |
|||
|url=http://oakhavenpc.org/cultivating_algae.htm}} Note: originally published in issue #59 of ''Permaculture Activist''</ref> |
|||
[[Jatropha# |
[[Jatropha#Vegoil and biodiesel|Jatropha]] has been cited as a high-yield source of biodiesel but yields are highly dependent on climatic and soil conditions. The estimates at the low end put the yield at about 200 US gal/acre (1.5-2 tonnes per hectare) per crop; in more favorable climates two or more crops per year have been achieved.<ref name="jatrophaex">{{cite news|url=http://findarticles.com/p/articles/mi_m0CYH/is_15_7/ai_107215410|title=India's jatropha plant biodiesel yield termed wildly exaggerated|publisher=Findarticles.com|date=2003-08-18|access-date=2010-03-15|archive-date=2009-10-02|archive-url=https://web.archive.org/web/20091002102739/http://findarticles.com/p/articles/mi_m0CYH/is_15_7/ai_107215410/|url-status=live}}</ref> It is grown in the [[Philippines]], [[Mali]] and [[India]], is drought-resistant, and can [[intercropping|share space]] with other cash crops such as coffee, sugar, fruits and vegetables.<ref name="reuk">{{cite web|url=http://www.reuk.co.uk/Jatropha-for-Biodiesel-Figures.htm|title=Jatropha for biodiesel|publisher=Reuk.co.uk|access-date=2010-03-15|archive-date=2009-09-04|archive-url=https://web.archive.org/web/20090904003238/http://www.reuk.co.uk/Jatropha-for-Biodiesel-Figures.htm|url-status=live}}</ref> It is well-suited to semi-arid lands and can contribute to slow down [[desertification]], according to its advocates.<ref>Weed's biofuel potential sparks African land grab, Washington Times, February 21, 2007, Karen Palmer</ref> |
||
===Efficiency and economic arguments=== |
=== Efficiency and economic arguments === |
||
[[File:Diesel prices.jpg|thumb|upright=0.6|In some countries biodiesel is less expensive than conventional diesel]] |
|||
Transitioning fully to biofuels could require immense tracts of land if traditional food crops are used (although [[non food crop]]s can be utilized). The problem would be especially severe for nations with large economies, since energy consumption scales with economic output.<ref name="Energy and the Economy">{{cite web|title=Looking Forward: Energy and the Economy |url=http://www.dallasfed.org/news/educate/2004/04ecsummit-brown.pdf |archive-url=https://web.archive.org/web/20060310194203/http://www.dallasfed.org/news/educate/2004/04ecsummit-brown.pdf |url-status=dead |archive-date=2006-03-10 |access-date=2006-08-29 }} |
|||
According to a study written by Drs. Van Dyne and Raymer for the [[Tennessee Valley Authority]], the average US farm consumes fuel at the rate of 82 [[litre]]s per [[hectare]] (8.75 US [[gallon]]s per [[acre]]) of land to produce one crop. However, average crops of rapeseed produce oil at an average rate of 1,029 L/ha (110 US gal/acre), and high-yield rapeseed fields produce about 1,356 L/ha (145 US gal/acre). The ratio of input to output in these cases is roughly 1:12.5 and 1:16.5. Photosynthesis is known to have an efficiency rate of about 3-6% of total solar radiation<ref name="www.fao.org">{{cite paper | author = Kazuhisa Miyamoto |
|||
</ref> |
|||
| title = Renewable biological systems for alternative sustainable energy production (FAO Agricultural Services Bulletin - 128) |
|||
| version = Final | date= 1997 |
|||
For [[third world]] countries, biodiesel sources that use marginal land could make more sense; e.g., [[Millettia pinnata|pongam oiltree]] nuts grown along roads or [[jatropha]] grown along rail lines.<ref name="www.tve.org.813">{{cite web|title=Hands On: Power Pods – India |url=http://www.tve.org/ho/doc.cfm?aid=1433&lang=English |access-date=2005-10-24 |url-status=dead |archive-url=https://web.archive.org/web/20120426092429/http://tve.org/ho/doc.cfm?aid=1433&lang=English |archive-date=2012-04-26 }} |
|||
| publisher = FAO - Food and Agriculture Organization of the United Nations |
|||
</ref> |
|||
| url = http://www.fao.org/docrep/w7241e/w7241e05.htm | format = HTML | accessdate = 2007-03-18}} |
|||
</ref> |
|||
In tropical regions, such as Malaysia and Indonesia, plants that produce palm oil are being planted at a rapid pace to supply growing biodiesel demand in Europe and other markets. Scientists have shown that the removal of rainforest for palm plantations is not ecologically sound since the expansion of oil palm plantations poses a threat to natural rainforest and biodiversity.<ref name="Wilcove and Koh 2010">{{cite journal|title=Addressing the threats to biodiversity from oil-palm agriculture|journal=Biodiversity and Conservation|volume=19|issue=4|pages=999–1007|doi=10.1007/s10531-009-9760-x|year=2010|last1=Wilcove|first1=David S.|last2=Koh|first2=Lian Pin|bibcode=2010BiCon..19..999W |s2cid=10728423}}</ref> |
|||
and if the entire mass of a crop is utilized for energy production, the overall efficiency of this chain is currently about 1%<ref name="petroleum.berkeley.edu">{{cite web | author = Tad Patzek |
|||
| title = Thermodynamics of the Corn-Ethanol Biofuel Cycle (section 3.11 Solar Energy Input into Corn Production) |
|||
It has been estimated in Germany that [[palm oil diesel]] has less than one third of the production costs of rapeseed biodiesel.<ref name="Palm Oil Based Biodiesel">{{cite web|title=Palm Oil Based Biodiesel Has Higher Chances Of Survival|url=http://www.bernama.com.my/bernama/v3/news_business.php?id=157856|access-date=2006-12-20|archive-url=https://web.archive.org/web/20070929102644/http://www.bernama.com.my/bernama/v3/news_business.php?id=157856|archive-date=2007-09-29|url-status=dead}}</ref> |
|||
| date = 2006-07-22 |
|||
| publisher = Berkeley; Critical Reviews in Plant Sciences, 23(6):519-567 (2004) |
|||
In the US, the production of biodiesel was reported in 2018 to support more than 64,000 jobs.<ref name="biodiesel.org"/> The growth in biodiesel also helps significantly increase GDP. In 2011, biodiesel created more than $3 billion in GDP.<ref name="NBBEPA">{{cite news|first=Ben|last=Evans|title=National Biodiesel Board Statement on EPA Renewable Fuels Rule|url=http://www.biodiesel.org/news/biodiesel-news/news-display/2011/12/27/national-biodiesel-board-statement-on-epa-renewable-fuels-rule|date=December 27, 2011|access-date=2012-04-10|archive-date=2020-04-03|archive-url=https://web.archive.org/web/20200403125818/http://www.biodiesel.org/news/biodiesel-news/news-display/2011/12/27/national-biodiesel-board-statement-on-epa-renewable-fuels-rule|url-status=live}}</ref> |
|||
| url = http://petroleum.berkeley.edu/papers/patzek/CRPS416-Patzek-Web.pdf | format = PDF | accessdate = 2008-03-03}}</ref> While this may compare unfavorably to [[solar cells]] combined with an electric drive train, biodiesel is less costly to deploy (solar cells cost approximately US$1,000 per square meter) and transport (electric vehicles require batteries which currently have a much lower energy density than liquid fuels). |
|||
==Energy security== |
|||
One of the main drivers for adoption of biodiesel is [[energy security]]. This means that a nation's dependence on oil is reduced, and substituted with use of locally available sources, such as coal, gas, or renewable sources. Thus a country can benefit from adoption of biofuels, without a reduction in greenhouse gas emissions. While the total energy balance is debated, it is clear that the dependence on oil is reduced. One example is the energy used to manufacture fertilizers, which could come from a variety of sources other than petroleum. The US National Renewable Energy Laboratory (NREL) states that energy security is the number one driving force behind the US biofuels programme,<ref name = "NREL biodiesel algae">{{cite journal|first1=John|last1=Sheehan|first2=Terri|last2=Dunahay|first3=John|last3=Benemann|first4=Paul|last4=Roessler|title=A look back at the U.S. Department of Energy's Aquatic Species Program: Biodiesel from Algae|version=Close-out Report|publisher=United States Department of Energy|date=July 1998|access-date=2007-01-02|url=http://www.nrel.gov/docs/legosti/fy98/24190.pdf|format=PDF (3.7 Mb)|journal=|archive-date=2020-04-23|archive-url=https://web.archive.org/web/20200423092435/https://www.nrel.gov/docs/legosti/fy98/24190.pdf|url-status=live}}</ref> and a White House "Energy Security for the 21st Century" paper makes it clear that energy security is a major reason for promoting biodiesel.<ref>{{cite web|title=Energy Security for the 21st Century|publisher=The White House|date=2008-03-05|url=https://georgewbush-whitehouse.archives.gov/infocus/energy/|access-date=2008-04-15|archive-date=2019-09-14|archive-url=https://web.archive.org/web/20190914150733/https://georgewbush-whitehouse.archives.gov/infocus/energy/|url-status=live}}</ref> The former EU commission president, Jose Manuel Barroso, speaking at a recent EU biofuels conference, stressed that properly managed biofuels have the potential to reinforce the EU's security of supply through diversification of energy sources.<ref>{{cite web|title=International Biofuels Conference|publisher=HGCA|url=http://www.hgca.com/content.output/2369/2369/Markets/Analysis/International%20Biofuel%20Conference.mspx|access-date=2008-04-15|archive-url=https://web.archive.org/web/20081211211937/http://www.hgca.com/content.output/2369/2369/Markets/Analysis/International%20Biofuel%20Conference.mspx|archive-date=2008-12-11|url-status=dead}}</ref> |
|||
== Global biofuel policies == |
|||
Many countries around the world are involved in the growing use and production of biofuels, such as biodiesel, as an alternative energy source to fossil fuels and oil. To foster the biofuel industry, governments have implemented legislations and laws as incentives to reduce oil dependency and to increase the use of renewable energies.<ref name="Sorda, G. 2010">{{cite journal|last1=Sorda|first1=G.|last2=Banse|first2=M.|last3=Kemfert|first3=C.|year=2010|title=An Overview of Biofuel Policies Across the World|journal=Energy Policy|volume=38|issue=11|pages=6977–6988|doi=10.1016/j.enpol.2010.06.066}}</ref> Many countries have their own independent policies regarding the taxation and rebate of biodiesel use, import, and production. |
|||
=== Canada === |
|||
It was required by the Canadian Environmental Protection Act Bill C-33 that by 2010, gasoline contained 5% renewable content and that by 2013, diesel and heating oil contained 2% renewable content.<ref name="Sorda, G. 2010"/> The EcoENERGY for Biofuels Program subsidized the production of biodiesel, among other biofuels, via an incentive rate of CAN$0.20 per liter from 2008 to 2010. A decrease of $0.04 will be applied every year following, until the incentive rate reaches $0.06 in 2016. Individual provinces also have specific legislative measures in regards to biofuel use and production.<ref>Dessureault, D., 2009. Canada Biofuels Annual. USDA Foreign Agricultural Service, GAIN Report Number CA9037, approved by U.S. Embassy, 30.06.2009</ref> |
|||
=== United States === |
|||
The [[United States biofuel policies#Internal Revenue Service (IRS)|Volumetric Ethanol Excise Tax Credit]] (VEETC) was the main source of financial support for biofuels, but was scheduled to expire in 2010. Through this act, biodiesel production guaranteed a tax credit of US$1 per gallon produced from virgin oils, and $0.50 per gallon made from recycled oils.<ref>Kuplow, D. Biofuels – At What Cost? Government support for ethanol and biodiesel in the United States. Cambridge, MA, 2007</ref> |
|||
Currently soybean oil is being used to produce soybean biodiesel for many commercial purposes such as blending fuel for transportation sectors.<ref name="sciencedirect.com"/> |
|||
=== European Union === |
|||
The European Union is the greatest producer of biodiesel, with [[France]] and [[Germany]] being the top producers. To increase the use of biodiesel, there are policies requiring the blending of biodiesel into fuels, including penalties if those rates are not reached. In France, the goal was to reach 10% integration but plans for that stopped in 2010.<ref name="Sorda, G. 2010"/> As an incentive for the European Union countries to continue the production of the biofuel, there are tax rebates for specific quotas of biofuel produced. In Germany, the minimum percentage of biodiesel in transport diesel is set at 7% so called "B7". |
|||
=== Malaysia === |
|||
[[Malaysia]] plans to implement its nationwide adoption of the B20 palm oil biofuel programme by the end of 2022. The mandate to manufacture biofuel with a 20% palm oil component - known as B20 - for the transport sector was first rolled out in January 2020 but faced delays due to movement curbs imposed to contain coronavirus outbreaks.<ref>{{Cite news|date=2022-01-05|title=Malaysia aims to fully implement B20 biodiesel mandate by year-end|language=en|work=Reuters|url=https://www.reuters.com/business/energy/malaysia-aims-fully-implement-b20-biodiesel-mandate-by-year-end-2022-01-05/|access-date=2022-01-05}}</ref> |
|||
== Issues and concerns == |
|||
=== Food, land and water vs. fuel === |
|||
{{Main|Food vs. fuel}} |
|||
Up to 40% of corn produced in the United States is used to make ethanol,<ref>{{cite news |title=Food vs fuel: Ukraine war sharpens debate on use of crops for energy |url=https://www.ft.com/content/b424067e-f56b-4e49-ac34-5b3de07e7f08 |work=Financial Times |date=12 June 2022}}</ref> and worldwide 10% of all grain is turned into biofuel.<ref>{{cite news |title=Guest view: Global hunger fight means no biofuel |url=https://www.reuters.com/breakingviews/guest-view-global-hunger-fight-means-no-biofuel-2022-06-06/ |work=Reuters |date=6 June 2022}}</ref> A 50% reduction in grain used for biofuels in the US and Europe would replace all of [[Ukraine]]'s grain exports.<ref>{{cite news |title=Cutting biofuels can help avoid global food shock from Ukraine war |url=https://www.newscientist.com/article/2312151-cutting-biofuels-can-help-avoid-global-food-shock-from-ukraine-war/ |work=New Scientist |date=14 March 2022}}</ref> |
|||
In some poor countries the rising price of vegetable oil is causing problems.<ref>{{cite web|url=http://www.abc.net.au/news/stories/2007/07/19/1982450.htm|title=Biofuel demand makes fried food expensive in Indonesia – ABC News (Australian Broadcasting Corporation)|publisher=Abc.net.au|date=2007-07-19|access-date=2010-03-15|archive-date=2011-03-20|archive-url=https://web.archive.org/web/20110320032446/http://www.abc.net.au/news/stories/2007/07/19/1982450.htm|url-status=live}}</ref><ref>{{cite web|url=https://www.nytimes.com/|title=Breaking News, World News & Multimedia|website=[[The New York Times]]|access-date=9 July 2017|archive-date=14 February 2008|archive-url=https://web.archive.org/web/20080214141140/http://www.iht.com/articles/1996/06/19/white.t_2.php|url-status=live}}</ref> Some propose that fuel only be made from non-edible vegetable oils such as [[camelina]], [[jatropha]] or [[seashore mallow]]<ref>{{cite web|url=http://www.biodiesel.org/resources/sustainability/pdfs/Food%20and%20FuelApril162008.pdf|title=Biodiesel Brings a Lot to the Table|access-date=30 May 2015|archive-date=2012-02-12|archive-url=https://web.archive.org/web/20120212120249/http://www.biodiesel.org/resources/sustainability/pdfs/Food%20and%20FuelApril162008.pdf|url-status=dead|date=April 2008}}</ref> which can thrive on marginal agricultural land where many trees and crops will not grow, or would produce only low yields. |
|||
Others argue that the problem is more fundamental. Farmers may switch from producing food crops to producing biofuel crops to make more money, even if the new crops are not edible.<ref>{{cite web|first=Esmarie|last=Swanepoel|url=http://www.engineeringnews.co.za/article.php?a_id=119281|title=Food versus fuel debate escalates|publisher=Engineeringnews.co.za|access-date=2010-03-15|archive-date=2008-03-24|archive-url=https://web.archive.org/web/20080324022853/http://www.engineeringnews.co.za/article.php?a_id=119281|url-status=live}}</ref><ref>{{cite web|first=Lester|last=Brown|url=http://www.theglobalist.com/StoryId.aspx?StoryId=5077|title=How Food and Fuel Compete for Land by Lester Brown – The Globalist > > Global Energy|publisher=The Globalist|access-date=2010-03-15|url-status=dead|archive-url=https://web.archive.org/web/20100112174512/http://www.theglobalist.com/StoryId.aspx?StoryId=5077|archive-date=2010-01-12}}</ref> The [[law of supply and demand]] predicts that if fewer farmers are producing food the price of food will rise. It may take some time, as farmers can take some time to change which things they are growing, but increasing demand for [[first generation biofuel]]s is likely to result in price increases for many kinds of food. Some have pointed out that there are poor farmers and poor countries who are making more money because of the higher price of vegetable oil.<ref>{{cite news|url=http://www.economist.com/research/articlesBySubject/displaystory.cfm?subjectid=7216688&story_id=10252015|newspaper=The Economist|title=The End Of Cheap Food|date=2007-12-06|access-date=2008-02-29|archive-date=2018-08-26|archive-url=https://web.archive.org/web/20180826045344/http://www.economist.com/research/articlesBySubject/displaystory.cfm?subjectid=7216688&story_id=10252015|url-status=live}}</ref> |
|||
Biodiesel from sea algae would not necessarily displace terrestrial land currently used for food production and new [[algaculture]] jobs could be created. |
|||
By comparison it should be mentioned that the production of [[biogas]] utilizes agricultural waste to generate a [[biofuel]] known as biogas, and also produces [[compost]], thereby enhancing agriculture, sustainability and food production. |
|||
=== Environmental impact of biodiesel === |
|||
{{update|section|date=October 2022|inaccurate=y}} |
|||
[[File:Riau palm oil 2007.jpg|thumb|[[Deforestation in Indonesia]], to make way for an [[oil palm]] plantation.]] |
|||
The surge of interest in biodiesels has highlighted a number of [[List of environmental issues|environmental effects]] associated with its use. These potentially include reductions in [[greenhouse gas emissions]],<ref> |
|||
{{cite journal |year=2003 |title=Biodiesel – Just the Basics |url=http://www.eere.energy.gov/vehiclesandfuels/pdfs/basics/jtb_biodiesel.pdf |url-status=dead |version=Final |publisher=United States Department of Energy |archive-url=https://web.archive.org/web/20070918122719/http://www1.eere.energy.gov/vehiclesandfuels/pdfs/basics/jtb_biodiesel.pdf |archive-date=2007-09-18 |access-date=2007-08-24}} |
|||
</ref> [[deforestation]], pollution and the rate of [[biodegradation]]. |
|||
According to the [https://web.archive.org/web/20110202202402/http://www.epa.gov/otaq/renewablefuels/420r10006.pdf Renewable Fuel Standards Program Regulatory Impact Analysis], released by the [[Environmental Protection Agency]] (EPA) of the United States in February 2010, biodiesel from soy oil results, on average, in a 57% reduction in greenhouse gases compared to petroleum diesel, and biodiesel produced from waste grease results in an 86% reduction. See chapter 2.6 of [https://web.archive.org/web/20110202202402/http://www.epa.gov/otaq/renewablefuels/420r10006.pdf the EPA report] for more detailed information. |
|||
However, environmental organizations, for example, [[Rettet den Regenwald|Rainforest Rescue]]<ref>{{cite web |title=Achievement – Biofuel: Shell backs out of indigenous territory – Rainforest Rescue |url=http://www.rainforest-rescue.org/achievements/4432/biofuel-shell-backs-out-of-indigenous-territory |url-status=live |archive-url=https://web.archive.org/web/20150531021709/https://www.rainforest-rescue.org/achievements/4432/biofuel-shell-backs-out-of-indigenous-territory |archive-date=31 May 2015 |access-date=30 May 2015}}</ref> and [[Greenpeace]],<ref>{{cite web |title=End of the road for dirty biofuels |url=http://www.greenpeace.org/international/en/news/Blogs/makingwaves/end-of-the-road-for-dirty-biofuels/blog/38904/ |url-status=live |archive-url=https://web.archive.org/web/20200403125835/https://wayback.archive-it.org/9650/http:/p3-raw.greenpeace.org/international/en/news/Blogs/makingwaves/end-of-the-road-for-dirty-biofuels/blog/38904/ |archive-date=3 April 2020 |access-date=30 May 2015 |work=Greenpeace International}}</ref> criticize the cultivation of plants used for biodiesel production, e.g., oil palms, soybeans and sugar cane. The deforestation of rainforests exacerbates climate change and sensitive ecosystems are destroyed to clear land for oil palm, soybean and sugar cane plantations. Moreover, that biofuels contribute to world hunger, since arable land is no longer used for growing foods. The Environmental Protection Agency published data in January 2012, showing that biofuels made from palm oil will not count towards the renewable fuels mandate of the United States as they are not climate-friendly.<ref name="news.mongabay.com">{{cite web |date=2012-01-27 |title=Palm oil does not meet U.S. renewable fuels standard, rules EPA |url=http://news.mongabay.com/2012/0127-no_palm_oil_epa.html |url-status=live |archive-url=https://web.archive.org/web/20150530224247/http://news.mongabay.com/2012/0127-no_palm_oil_epa.html |archive-date=2015-05-30 |access-date=30 May 2015 |work=Mongabay}}</ref> Environmentalists welcome the conclusion because the growth of oil palm plantations has driven tropical deforestation, for example, in Indonesia and Malaysia.<ref name="news.mongabay.com" /><ref>{{cite web |date=2012-01-26 |title=EPA: Palm oil flunks the climate test |url=https://thehill.com/policy/energy-environment/104068-epa-palm-oil-flunks-the-climate-test/ |url-status=live |archive-url=https://web.archive.org/web/20130605071141/http://thehill.com/blogs/e2-wire/e2-wire/206781-epa-palm-oil-based-fuels-flunk-the-climate-test |archive-date=2013-06-05 |access-date=30 May 2015 |work=TheHill}}</ref> |
|||
[[Indonesia]] produces biodiesel primarily from [[palm oil]]. Since agricultural land is limited, in order to plant [[monoculture]]s of [[Elaeis|oil palms]], land used for other cultivations or the tropical forest need to be cleared. A major environmental threat is then the [[Rainforest destruction|destruction of rainforests]] in Indonesia.<ref>{{cite news |date=8 December 2021 |title=Indonesia's biodiesel drive is leading to deforestation |url=https://www.bbc.com/news/59387191 |work=BBC News}}</ref> |
|||
The environmental impact of biodiesel is diverse and not clearcut. An often mentioned incentive for using biodiesel is its capacity to lower [[greenhouse gas emissions]] compared to those of [[fossil fuels]]. Whether this is true or not depends on many factors. |
|||
==== Greenhouse gas emissions ==== |
|||
However, these statistics by themselves are not enough to show whether such a change makes economic sense. |
|||
[[Image:BioDieselFootprint.jpg|right|thumb|350px|Calculation of [[Carbon Intensity]] of Soy biodiesel grown in the US and burnt in the UK, using figures calculated by the UK government for the purposes of the [[Renewable transport fuel obligation]].<ref name="UKRTFO">{{cite web |date=January 2008 |title=Carbon and Sustainability Reporting Within the Renewable Transport Fuel Obligation |url=http://www.dft.gov.uk/pgr/roads/environment/rtfo/govrecrfa.pdf |url-status=dead |archive-url=https://web.archive.org/web/20080410055943/http://www.dft.gov.uk/pgr/roads/environment/rtfo/govrecrfa.pdf |archive-date=2008-04-10 |access-date=2008-04-29 |work=UK Department for Transport |format=PDF 1.41 MB}}</ref>]] |
|||
Additional factors must be taken into account, such as: the fuel equivalent of the energy required for processing, the yield of fuel from raw oil, the return on cultivating food, the effect biodiesel will have of food prices and the relative cost of biodiesel versus petrodiesel. |
|||
[[Image:BiodieselsCountryOfOrigin.jpg|right|thumb|350px|Graph of UK figures for the [[Carbon Intensity]] of Biodiesels and [[fossil fuels]]. This graph assumes that all biodiesel is used in its country of origin. It also assumes that the diesel is produced from pre-existing croplands rather than by changing land use<ref name="UKBioDiesel">Graph derived from information found in UK government document.[http://www.dft.gov.uk/pgr/roads/environment/rtfo/govrecrfa.pdf Carbon and Sustainability Reporting Within the Renewable Transport Fuel Obligation] {{webarchive|url=https://web.archive.org/web/20080625044948/http://www.dft.gov.uk/pgr/roads/environment/rtfo/govrecrfa.pdf|date=June 25, 2008}}</ref>]] |
|||
The debate over the [[energy balance]] of biodiesel is ongoing. Transitioning fully to biofuels could require immense tracts of land if traditional food crops are used (although [[non food crop]]s can be utilized). The problem would be especially severe for nations with large economies, since energy consumption scales with economic output.<ref name="Energy and the Economy">{{cite web | title=Looking Forward: Energy and the Economy | url=http://www.dallasfed.org/news/educate/2004/04ecsummit-brown.pdf | format=PDF | accessmonthday=August 29 | accessyear=2006 }} |
|||
</ref> |
|||
A general critique against biodiesel is the [[land use]] change, which have potential to cause even more emissions than what would be caused by using fossil fuels alone.<ref name="Science, Land Use Change">{{cite journal |last=Fargione |first=Joseph |author2=Jason Hill |author3=David Tilman |author4=Stephen Polasky |author5=Peter Hawthorne |date=2008-02-29 |title=Land Clearing and the Biofuel Carbon Debt |url=http://www.sciencemag.org/cgi/content/abstract/1152747 |url-status=dead |format=fee required |journal=[[Science (journal)|Science]] |volume=319 |issue=5867 |pages=1235–8 |bibcode=2008Sci...319.1235F |doi=10.1126/science.1152747 |pmid=18258862 |s2cid=206510225 |archive-url=https://web.archive.org/web/20080413151143/http://www.sciencemag.org/cgi/content/abstract/1152747 |archive-date=April 13, 2008 |access-date=2008-04-29}} |
|||
If using only traditional food plants, most such nations do not have sufficient arable land to produce biofuel for the nation's vehicles. Nations with smaller economies (hence less energy consumption) and more arable land may be in better situations, although many regions cannot afford to divert land away from food production. |
|||
* {{cite press release |title=New Study Raises Major Questions on Biofuels |publisher=The Nature Conservancy in Minnesota |date=2008-02-07 |url=http://www.nature.org/wherewework/northamerica/states/minnesota/press/press3348.html |access-date=2008-04-29 |archive-url=https://web.archive.org/web/20080513183927/http://www.nature.org/wherewework/northamerica/states/minnesota/press/press3348.html |archive-date=2008-05-13 |url-status=dead}}</ref> Yet this problem would be fixed with [[algal biofuel]] which can use land unsuitable for agriculture. |
|||
For [[third world]] countries, biodiesel sources that use marginal land could make more sense, e.g. [[honge oil]] nuts<ref name="www.tve.org.813">{{cite web | title=Hands On: Power Pods - India | url=http://www.tve.org/ho/doc.cfm?aid=1433&lang=English | accessmonthday=October 24 |accessyear=2005 }} |
|||
</ref> |
|||
grown along roads or [[jatropha]] grown along rail lines. |
|||
[[Carbon dioxide]] is one of the major [[greenhouse gas]]es. Although the burning of biodiesel produces carbon dioxide emissions similar to those from ordinary fossil fuels, the [[plant]] feedstock used in the production absorbs carbon dioxide from the atmosphere when it grows. Plants absorb carbon dioxide through a process known as [[photosynthesis]] which allows it to store energy from sunlight in the form of sugars and starches. After the [[biomass]] is converted into biodiesel and burned as fuel the energy and carbon is released again. Some of that energy can be used to power an engine while the carbon dioxide is released back into the atmosphere. |
|||
In tropical regions, such as Malaysia and Indonesia, oil palm is being planted at a rapid pace to supply growing biodiesel demand in Europe and other markets. It has been estimated in Germany that palm oil biodiesel has less than 1/3 the production costs of rapeseed biodiesel.<ref name="Palm Oil Based Biodiesel">{{cite web | title=Palm Oil Based Biodiesel Has Higher Chances Of Survival | url=http://www.bernama.com.my/bernama/v3/news_business.php?id=157856 | accessmonthday=December 20 | accessyear=2006 }}</ref> The direct source of the energy content of biodiesel is solar energy captured by plants during [[photosynthesis]]. Regarding the positive energy balance of biodiesel{{Fact|date=March 2008}}: |
|||
:When straw was left in the field, biodiesel production was strongly energy positive, yielding 1 [[1 E9 J|GJ]] biodiesel for every 0.561 GJ of energy input (a yield/cost ratio of 1.78). |
|||
:When straw was burned as fuel and oilseed rapemeal was used as a fertilizer, the yield/cost ratio for biodiesel production was even better (3.71). In other words, for every unit of energy input to produce biodiesel, the output was 3.71 units (the difference of 2.71 units would be from solar energy). |
|||
When considering the total amount of greenhouse gas emissions it is therefore important to consider the whole production process and what indirect effects such production might cause. The effect on carbon dioxide emissions is highly dependent on production methods and the type of feedstock used. Calculating the [[carbon intensity]] of biofuels is a complex and inexact process, and is highly dependent on the assumptions made in the calculation. A calculation usually includes: |
|||
Biodiesel is becoming of interest to companies interested in commercial scale production as well as the more usual home brew biodiesel user and the user of [[straight vegetable oil]] or waste vegetable oil in diesel engines. Homemade [[biodiesel processor]]s are many and varied. |
|||
* Emissions from growing the feedstock (e.g. Petrochemicals used in fertilizers) |
|||
==Environmental effects== |
|||
* Emissions from transporting the feedstock to the factory |
|||
[[Image:BioDieselFootprint.jpg|right|thumb|500px|Calculation of [[Carbon Intensity]] of Soy biodiesel grown in the US and burnt in the UK, using UK government calculation <ref name="UKBioDiesel">Graph derived from information found in UK government document.[http://www.dft.gov.uk/pgr/roads/environment/rtfo/govrecrfa.pdf Carbon and Sustainability Reporting Within the Renewable Transport Fuel Obligation]</ref>]][[Image:BiodieselsCountryOfOrigin.jpg|right|thumb|500px|Graph of UK figures for the [[Carbon Intensity]] of Biodiesels and [[fossil fuels]]. This graph assumes that all biodiesels are burnt in their country of origin<ref name="UKBioDiesel"/>]] |
|||
* Emissions from processing the feedstock into biodiesel |
|||
===Carbon dioxide production=== |
|||
* Absorption of CO<sub>2</sub> Emissions from growing the feedstock |
|||
Making and burning Biodiesel contributes to atmospheric [[Carbon Dioxide|carbon dioxide]] to a smaller extent than burning [[Fossil Fuels|fossil fuels]]. The calculation of exactly how much Carbon Dioxide is produced is a complex and inexact process, and is highly dependant on the method by which the biofuel is produced and the assumptions made in the calculation. A calculation should include: |
|||
Other factors can be very significant but are sometimes not considered. These include: |
|||
* The '''cost''' of growing the feedstock |
|||
* Emissions from the change in land use of the area where the fuel feedstock is grown. |
|||
* The '''cost''' of transporting the feedstock to the factory |
|||
* Emissions from transportation of the biodiesel from the factory to its point of use |
|||
* The '''cost''' of processing the feedstock into biodiesel |
|||
Such a calculation may or may not consider the following effects: |
|||
* The '''cost''' of the change in land use of the area where the fuel feedstock is grown. |
|||
* The '''cost''' of transportation of the biodiesel from the factory to its point of use |
|||
* The efficiency of the biodiesel compared with standard diesel |
* The efficiency of the biodiesel compared with standard diesel |
||
* The amount of Carbon Dioxide produced at the tail pipe. (Biodiesel can produce 4.7% more) |
* The amount of Carbon Dioxide produced at the tail pipe. (Biodiesel can produce 4.7% more){{Citation needed|date=May 2013}} |
||
* The |
* The benefits due to the production of useful by-products, such as cattle feed or [[glycerine]] |
||
The graphs on the right shows figures calculated by the UK government for the purposes of the [[Renewable transport fuel obligation]]<ref>.[http://www.dft.gov.uk/pgr/roads/environment/rtfo/govrecrfa.pdf Carbon and Sustainability Reporting Within the Renewable Transport Fuel Obligation]</ref> |
|||
If land use change is not considered and assuming today's production methods, biodiesel from rapeseed and sunflower oil produce 45%-65% lower greenhouse gas emissions than petrodiesel.<ref name="SHU"> |
|||
===Pollution=== |
|||
{{cite web |last=Mortimer |first=N. D. |author2=P. Cormack |author3=M. A. Elsayed |author4=R. E. Horne |date=January 2003 |title=Evaluation of the comparative energy, global warming and socio-economic costs and benefits of biodiesel |url=http://sciencesearch.defra.gov.uk/Document.aspx?Document=NF0422_488_FRP.pdf |access-date=2008-05-01 |work=[[Sheffield Hallam University]] |publisher=UK [[Department for Environment, Food and Rural Affairs]] (DEFRA) |format=PDF 763 KB}} |
|||
*In the United States, biodiesel is the only alternative fuel to have successfully completed the Health Effects Testing requirements (Tier I and Tier II) of the [[Clean Air Act (1990)]]. |
|||
*Biodiesel contains fewer [[aromatic hydrocarbon]]s: benzofluoranthene: 56% reduction; Benzopyrenes: 71% reduction.{{Fact|date=February 2007}} |
|||
*Biodiesel can reduce by as much as 20% the direct (tailpipe) emission of [[particulate]]s, small particles of solid combustion products, on vehicles with particulate filters, compared with low-sulfur (<50 ppm) diesel. Particulate emissions as the result of production are reduced by around 50%, compared with fossil-sourced diesel. (Beer et al, 2004). |
|||
*Biodiesel has a higher [[cetane rating]] than petrodiesel, which can improve performance and clean up emissions compared to crude petro-diesel (with cetane lower than 40). |
|||
* ''Summary:'' {{cite web |title=Biodiesel Life Cycle Assessment |url=http://www.esru.strath.ac.uk/EandE/Web_sites/02-03/biofuels/why_lca.htm |access-date=2008-05-01}} |
|||
===Non-toxic to humans=== |
|||
</ref><ref> |
|||
The potential health effects of inhalation are negligible, as are those of skin contact and ingestion. Contact with eyes could cause irritation.<ref>[http://www.biodiesel.org/pdf_files/fuelfactsheets/MSDS.pdf Generic biodiesel material safety data sheet (MSDS)]</ref> |
|||
{{cite web |date=March 2007 |title=Well-to-Wheels analysis of future automotive fuels and powertrains in the European context |url=http://ies.jrc.ec.europa.eu/wtw.html |url-status=dead |archive-url=https://web.archive.org/web/20080207180809/http://ies.jrc.ec.europa.eu/wtw.html |archive-date=2008-02-07 |access-date=2008-05-01 |work=[[Joint Research Centre (European Commission)]], EUCAR & CONCAWE}} |
|||
</ref><ref name="EEA report">{{Cite book |author=European Environment Agency. |url=http://reports.eea.europa.eu/eea_report_2006_3/en/term_2005.pdf |title=Transport and environment : facing a dilemma : TERM 2005: indicators tracking transport and environment in the European Union |publisher=[[European Environment Agency]]; Luxembourg : Office for Official Publications of the European Communities |year=2006 |isbn=92-9167-811-2 |location=[[Copenhagen]] |format=PDF 3.87 MB |issn=1725-9177 |access-date=2008-05-01 |archive-url=http://webarchive.loc.gov/all/20060719210750/http://reports.eea.europa.eu/eea_report_2006_3/en/term_2005.pdf |archive-date=July 19, 2006 |url-status=dead}} |
|||
</ref><ref>{{cite web |title=Biodiesel |url=http://www.energysavingtrust.org.uk/fleet/technology/alternativefuels/biodiesel/ |url-status=dead |archive-url=https://web.archive.org/web/20200622003040/https://energysavingtrust.org.uk/fleet/technology/alternativefuels/biodiesel/ |archive-date=2020-06-22 |access-date=2008-05-01 |work=[[Energy Saving Trust]] |quote=[B]iodiesel is considered a renewable fuel. It gives a 60 per cent reduction in CO2 well to wheel}}</ref> However, there is ongoing research to improve the efficiency of the production process.<ref name="SHU" /><ref name="EEA report" /> Biodiesel produced from used cooking oil or other waste fat could reduce CO<sub>2</sub> emissions by as much as 85%.<ref name="UKRTFO" /> As long as the feedstock is grown on existing cropland, land use change has little or no effect on greenhouse gas emissions. However, there is concern that increased feedstock production directly affects the rate of deforestation. Such [[clearcutting]] cause carbon stored in the forest, soil and [[peat]] layers to be released. The amount of greenhouse gas emissions from deforestation is so large that the benefits from lower emissions (caused by biodiesel use alone) would be negligible for hundreds of years.<ref name="UKRTFO" /><ref name="Science, Land Use Change" /> Biofuel produced from feedstock such as palm oil could therefore cause much higher carbon dioxide emissions than some types of fossil fuels.<ref name="greenpeace">{{cite book |url=http://www.greenpeace.org/raw/content/international/press/reports/cooking-the-climate-full.pdf |title=How the palm oil industry is cooking the climate |date=November 2007 |publisher=[[Greenpeace|Greenpeace International]] |format=PDF 10.48 MB |quote=The main areas remaining for new extensive plantations are the large tracts of tropical peatlands – until recently virgin rainforest areas. Over 50% of new plantations are planned in these peatland areas |access-date=2008-04-30 |archive-url=https://web.archive.org/web/20110303024245/http://www.greenpeace.org/raw/content/international/press/reports/cooking-the-climate-full.pdf |archive-date=2011-03-03 |url-status=dead}}</ref> |
|||
=== |
==== Pollution ==== |
||
In the United States, biodiesel is the only [[alternative fuel]] to have successfully completed the Health Effects Testing requirements (Tier I and Tier II) of the [[Clean Air Act (1990)]]. |
|||
*Biodiesel is considered readily biodegradable under ideal conditions and non-toxic. A [[University of Idaho]] study compared biodegradation rates of biodiesel, neat vegetable oils, biodiesel and petroleum diesel blends, and neat 2-D diesel fuel. Using low concentrations of the product to be degraded (10 ppm) in nutrient and sewage sludge amended solutions, they demonstrated that biodiesel degraded at the same rate as a dextrose control and 5 times as quickly as petroleum diesel over a period of 28 days, and that biodiesel blends doubled the rate of petroleum diesel degradation through co-metabolism <ref name=uidaho04>[http://www.uidaho.edu/bioenergy/BiodieselEd/publication/04.pdf University Of Idaho bioenergy]</ref>. The same study examined soil degradation using 10 000 ppm of biodiesel and petroleum diesel, and found biodiesel degraded at twice the rate of petroleum diesel in soil. In all cases, it was determined biodiesel also degraded more completely than petroleum diesel, which produced poorly degradable undetermined intermediates. Toxicity studies for the same project demonstrated no mortalities and few toxic effects on rats and rabbits with up to 5000 mg/kg of biodiesel. Petroleum diesel showed no mortalities at the same concentration either, however toxic effects such as hair loss and urinary discolouring were noted with concentrations of >2000 mg/l in rabbits. |
|||
Biodiesel can reduce the direct tailpipe-emission of [[particulate]]s, small particles of solid combustion products, on vehicles with particulate filters by as much as 20 percent compared with low-sulfur (< 50 ppm) diesel. Particulate emissions as the result of production are reduced by around 50 percent compared with fossil-sourced diesel.{{sfn|Beer et al.|2004}} |
|||
===Flammability=== |
|||
*The [[flash point]] of biodiesel (>130 °C)<ref>[http://www.biodiesel.org/pdf_files/fuelfactsheets/MSDS.pdf Generic biodiesel material safety data sheet (MSDS)]</ref> is significantly higher than that of petroleum diesel (64 °C) or gasoline (−45 °C). |
|||
== |
==== Biodegradation ==== |
||
A [[University of Idaho]] study compared [[biodegradation]] rates of biodiesel, neat vegetable oils, biodiesel and petroleum diesel blends, and neat 2-D diesel fuel. Using low concentrations of the product to be degraded (10 ppm) in nutrient and sewage sludge amended solutions, they demonstrated that biodiesel degraded at the same rate as a dextrose control and 5 times as quickly as petroleum diesel over a period of 28 days, and that biodiesel blends doubled the rate of petroleum diesel degradation through [[co-metabolism]].<ref name="uidaho04">{{cite web |date=2004-12-03 |title=Biodegradability, BOD<sub>5</sub>, COD and Toxicity of Biodiesel Fuels |url=http://www.uidaho.edu/bioenergy/BiodieselEd/publication/04.pdf |url-status=dead |archive-url=https://web.archive.org/web/20080410055942/http://www.uidaho.edu/bioenergy/BiodieselEd/publication/04.pdf |archive-date=April 10, 2008 |access-date=2008-04-30 |work=National Biodiesel Education Program, University of Idaho |format=PDF 64 KB}}</ref> |
|||
The same study examined [[soil retrogression and degradation|soil degradation]] using 10 000 ppm of biodiesel and petroleum diesel, and found biodiesel degraded at twice the rate of petroleum diesel in soil. In all cases, it was determined biodiesel also degraded more completely than petroleum diesel, which produced poorly degradable undetermined intermediates. Toxicity studies for the same project demonstrated no mortalities and few toxic effects on rats and rabbits with up to 5000 mg/kg of biodiesel. Petroleum diesel showed no mortalities at the same concentration either; however, toxic effects such as hair loss and urinary discolouring were noted with concentrations of >2000 mg/L in rabbits.:<ref>{{cite web |title=Biodiesel |url=http://www.solarnavigator.net/bio_diesel_biodiesel.htm |access-date=2012-04-18 |publisher=solar navigator}}</ref> |
|||
===== In aquatic environments ===== |
|||
{{main|Food vs fuel}} |
|||
As biodiesel becomes more widely used, it is important to consider how consumption affects water quality and aquatic ecosystems. Research examining the [[biodegradability]] of different biodiesel fuels found that all of the biofuels studied (including Neat Rapeseed oil, Neat Soybean oil, and their modified ester products) were “readily biodegradable” compounds, and had a relatively high biodegradation rate in water.<ref>Zhang, X.; Peterson, C. L.; Reece, D.; Moller, G.; Haws, R. Biodegradability of Biodiesel in the Aquatic Environment. ASABE 1998, 41(5), 1423-1430</ref> Additionally, the presence of biodiesel can increase the rate of diesel biodegradation via co-metabolism. As the ratio of biodiesel is increased in biodiesel/diesel mixtures, the faster the diesel is degraded. Another study using controlled experimental conditions also showed that fatty acid methyl esters, the primary molecules in biodiesel, degraded much faster than petroleum diesel in sea water.<ref>DeMello, J. A.; Carmichael, C. A.; Peacock, E. E.; Nelson, R. K.; Arey, J. S.; Reddy, C. M. Biodegradation and Environmental Behavior of Biodiesel Mixtures in the Sea: An Initial Study. Marine Poll. Bull. 2007, 54, 894-904</ref> |
|||
==== Carbonyl emissions ==== |
|||
Food quality vegetable oil has become so expensive there is no longer a profit viability for its use. Food grade vegetable oil pricing is on a similar upward ramp as food in general. Accessing food stuffs in poor countries has always been problematic for the inhabitants. Non food grade vegetable feed stocks are under use or consideration for use to make biodiesel and have been so during the entire history of biodiesel. |
|||
When considering the emissions from fossil fuel and biofuel use, research typically focuses on major pollutants such as hydrocarbons. It is generally recognized that using biodiesel in place of diesel results in a substantial reduction in regulated gas emissions, but there has been a lack of information in research literature about the non-regulated compounds which also play a role in air pollution.<ref name="Tan, J. 2009">He, C.; Ge, Y.; Tan, J.; You, K.; Han, X.; Wang, J.; You, Q.; Shah, A. N. Comparison of Carbonyl Compounds Emissions from Diesel Engine Fueled with Biodiesel and Diesel. Atmos. Environ. 2009, 43, 3657-3661</ref> One study focused on the emissions of non-criteria carbonyl compounds from the burning of pure diesel and biodiesel blends in heavy-duty diesel engines. The results found that carbonyl emissions of formaldehyde, acetaldehyde, acrolein, acetone, propionaldehyde and butyraldehyde, were higher in biodiesel mixtures than emissions from pure diesel. Biodiesel use results in higher carbonyl emissions but lower total hydrocarbon emissions, which may be better as an alternative fuel source. Other studies have been done which conflict with these results, but comparisons are difficult to make due to various factors that differ between studies (such as types of fuel and engines used). In a paper which compared 12 research articles on carbonyl emissions from biodiesel fuel use, it found that 8 of the papers reported increased carbonyl compound emissions while 4 showed the opposite.<ref name="Tan, J. 2009" /> This is evidence that there is still much research required on these compounds. |
|||
=== Mechanical concerns === |
|||
In some poor countries the rising price of vegetable oil is causing problems. <ref>[http://www.abc.net.au/news/stories/2007/07/19/1982450.htm Biofuel demand makes fried food expensive in Indonesia - ABC News (Australian Broadcasting Corporation)<!-- Bot generated title -->]</ref> <ref>[http://www.iht.com/articles/2008/01/19/business/palmoil.php The other oil shock: Vegetable oil prices soar - International Herald Tribune<!-- Bot generated title -->]</ref> There are those that say using a food crop for fuel sets up competition between food in poor countries and fuel in rich countries. Some propose that fuel only be made from non-edible vegetable oils like [[jatropha oil]]. Others argue that the problem is more fundamental. Farmers can switch from producing food crops to producing biofuel crops to make more money, even if the new crops are not edible. |
|||
{{Main|Issues relating to biofuels}} |
|||
<ref>[http://www.engineeringnews.co.za/article.php?a_id=119281 Food versus fuel debate escalates<!-- Bot generated title -->]</ref> <ref>[http://www.theglobalist.com/StoryId.aspx?StoryId=5077 How Food and Fuel Compete for Land by Lester Brown - The Globalist > > Global Energy<!-- Bot generated title -->]</ref> The [[law of supply and demand]] predicts that if less farmers are producing food the price of food will rise. It may take some time, as farmers can take some time to change which things they are growing, but increasing demand for biofuels is likely to result in price increases for many kinds of food. Some have pointed out that there are poor farmers and poor countries making more money because of the higher price of vegetable oil. <ref>{{cite web |url= http://www.economist.com/research/articlesBySubject/displaystory.cfm?subjectid=7216688&story_id=10252015 | title= The Economist – The End Of Cheap Food}}</ref> |
|||
==== Engine wear ==== |
|||
==Environmental concerns== |
|||
Lubricity of fuel plays an important role in wear that occurs in an engine. A diesel engine relies on its fuel to provide lubricity for the metal components that are constantly in contact with each other.<ref name="Fazal, M. A. 2011">{{cite journal |last1=Fazal |first1=M. A. |last2=Haseeb |first2=A. S. M.A. |last3=Masiuki |year=2011 |title=An evaluation of material compatibility; performance; emission and engine durability |journal=Renewable and Sustainable Energy Reviews |volume=15 |pages=1314–1324 |doi=10.1016/j.rser.2010.10.004}}</ref> Biodiesel is a much better lubricant compared with fossil petroleum diesel due to the presence of esters. Tests have shown that the addition of a small amount of biodiesel to diesel can significantly increase the lubricity of the fuel in short term.<ref>Masjuki HH, Maleque MA. The effect of palm oil diesel fuel contaminated lubricant on sliding wear of cast irons against mild steel. Wear. 1996, 198, 293–9</ref> However, over a longer period of time{{clarify|reason=use or storage?|date=September 2023}} (2–4 years), studies show that biodiesel loses its lubricity.<ref>Clark, S.J.; Wagner, L.; Schrock, M.D.; Piennaar, P.G. Methyl and ethyl soybean esters as renewable fuels for diesel engines. JAOCS. 1984, 61, 1632–8</ref>{{failed verification|reason=This one says 200 hours! Maybe ditch it and just keep Monyem?|date=September 2023}} This could be because of enhanced corrosion over time due to oxidation of the unsaturated molecules or increased water content in biodiesel from moisture absorption.<ref name="Monyem, A. 2001" /> |
|||
==== Fuel viscosity ==== |
|||
The locations where oil-producing plants are grown is of increasing concern. Mono-culture plantations [[Clearfelling|clear cut]] large areas of [[tropical forest]] in order to grow such oil rich crops such as [[oil palm]]. In the [[Philippines]] and [[Indonesia]] such forest clearing is already underway for the production of palm oil. In Indonesia, for example, deforestation has caused displacement of indigenous peoples. Also, in some areas use of pesticides for biofuel crops are disrupting clean water supplies.<ref name="indigenous">[http://www.guardian.co.uk/environment/2008/feb/11/biofuels.energy Biofuel demand leading to human rights abuses, report claims] Jessica Aldred, ''guardian.co.uk'', [[February 11]] [[2008]] Retrieved [[February 11]] [[2008]]</ref> [[Habitat destruction|Loss of habitat]] on such a scale could [[Endangered species|endanger]] numerous species of plants and animals. A particular concern which has received considerable attention is the threat to the already-shrinking populations of [[orangutan]]s on the Indonesian islands of [[Borneo]] and [[Sumatra]], which face possible [[extinction]].<ref>{{cite paper |
|||
One of the main concerns regarding biodiesel is its viscosity. The viscosity of diesel is 2.5–3.2 cSt at 40 °C and the viscosity of biodiesel made from soybean oil is between 4.2 and 4.6 cSt<ref name="Tat, M.E. 1999">Tat, M.E.; Van Gerpan, J.H. The Kinematic Viscosity of Biodiesel and its Blends with Diesel Fuel. JAOCS. 1999, 76, 1511–1513</ref> The viscosity of diesel must be high enough to provide sufficient lubrication for the engine parts but low enough to flow at operational temperature. High viscosity can plug the fuel filter and injection system in engines.<ref name="Tat, M.E. 1999" /> Vegetable oil is composed of lipids with long chains of hydrocarbons, to reduce its viscosity the lipids are broken down into smaller molecules of esters. This is done by converting vegetable oil and animal fats into alkyl esters using transesterification to reduce their viscosity<ref>{{cite journal |last1=Altin |first1=R. |last2=Cetinkaya |first2=S. |last3=Yucesu |first3=H.S. |year=2001 |title=The potential of using vegetable oil fuels as fuel for diesel engines |journal=Energy Conversion and Management |volume=42 |issue=5 |pages=529–538 |doi=10.1016/s0196-8904(00)00080-7}}</ref> Nevertheless, biodiesel viscosity remains higher than that of diesel, and the engine may not be able to use the fuel at low temperatures due to the slow flow through the fuel filter.<ref>{{cite journal |last1=Schmidt |first1=W. S. |year=2007 |title=Biodiesel: Cultivating Alternative Fuels |journal=Environmental Health Perspectives |volume=115 |issue=2 |pages=87–91 |doi=10.1289/ehp.115-a86 |pmc=1817719 |pmid=17384754}}</ref> |
|||
| author = Helen Buckland, Ed Matthew (ed.) |
|||
| title = The Oil for Ape Scandal: How palm oil is threatening the orang-utan | version = Summary |
|||
| publisher = Friends of the Earth Trust | date = [[19 September]] [[2005]] |
|||
| url = http://www.foe.co.uk/resource/reports/oil_for_ape_summary.pdf | format = PDF (458 Kb) |
|||
| accessdate = 2007-01-02 |
|||
}}</ref><ref name="IUCN Pongo abelii">¶ |
|||
{{Citation¶ |
|||
| last1 = Singleton | first1 = I.¶ |
|||
| last2 = Wich | first2 = S.A.¶ |
|||
| last3 = Griffiths | first3 = M.¶ |
|||
| year = 2007¶ |
|||
| title = Pongo abelii. In: IUCN 2007. 2007 IUCN Red List of Threatened Species. <www.iucnredlist.org>¶ |
|||
| url = http://www.iucnredlist.org/search/details.php/39780/all¶ |
|||
| accessdate = 2008-04-02 }}¶ |
|||
</ref><ref name="IUCN Pongo pygmaeus">¶ |
|||
{{Citation¶ |
|||
| last1 = Ancrenaz | first1 = M.¶ |
|||
| last2 = Marshall | first2 = A.¶ |
|||
| last3 = Goossens | first3 = B.¶ |
|||
| last4 = van Schaik | first4 = C.¶ |
|||
| last5 = Sugardjito | first5 = J.¶ |
|||
| last6 = Gumal | first6 = M.¶ |
|||
| last7 = Wich | first7 = S.¶ |
|||
| year = 2007¶ |
|||
| title = Pongo pygmaeus. In: IUCN 2007. 2007 IUCN Red List of Threatened Species. <www.iucnredlist.org>¶ |
|||
| url = http://www.iucnredlist.org/search/details.php/17975/all¶ |
|||
| accessdate = 2008-04-02 }}¶ |
|||
</ref> |
|||
=== |
==== Engine performance ==== |
||
Biodiesel has higher brake-specific fuel consumption compared to diesel, which means more biodiesel fuel consumption is required for the same torque. However, B20 biodiesel blend has been found to provide maximum increase in thermal efficiency, lowest brake-specific energy consumption, and lower harmful emissions.<ref name="sciencedirect.com" /><ref name="Monyem, A. 2001" /><ref name="Fazal, M. A. 2011" /> The engine performance depends on the properties of the fuel, as well as on combustion, injector pressure and many other factors.<ref>Knothe, G. Biodiesel and renewable diesel: A comparison. Process in energy and Combustion Science. 2010, 36, 364–373</ref> Since there are various blends of biodiesel, that may account for the contradicting reports as regards engine performance. |
|||
Biodiesel and feedstock oils produced in Asia, South America and Africa are currently less expensive than those produced in Europe and North America suggesting that imports to these wealthier nations are likely to increase in future. Like all petroleum based fuels, biodiesel also requires a significant investment of energy before it arrives at petrol pumps, thus fair comparisons among fuels require full lifecycle analyses for each fuel type. If [[deforestation]], and [[monoculture]] farming techniques were used to grow biofuel crops, biodiesel is predicted to become a serious threat to the environment. These problems could be exacerbated as biodiesel becomes more popular unless stringent laws are introduced and enforced to control biodiesel production. [[Non-food energy crop]]s and lipid rich algaes with vastly greater oil yields may also replace low-yield annual food crops such as soybeans, skirting the deforestation risk associated with widespread uptake of biodiesel.GuJi |
|||
==== Exhaust emissions ==== |
|||
As non-food crops also facilitate the use{{Fact|date=February 2008}} of degraded lands, wastewater, processed sewage, and other waste streams, the benefits of such crops go well beyond their greater yields{{Fact|date=February 2008}}. Moreover, select non-food crops such as jatropha and castorbean can be grown in polycultures, in non-till agricultural applications, and they scale well from the standpoint of production, storage, and processing. As such, these crops might benefit small-scale farmers throughout tropical and temperate latitudes, providing a cash crop option which can also displace local demand for imported petroleum.{{Fact|date=February 2008}} |
|||
The feedstock used to make the biodiesel alters the fuel’s properties by changing the average carbon chain length and number of double bonds present in the fatty acid methyl esters.<ref>{{cite journal |last1=Altin |first1=R. |last2=Cetinkaya |first2=S. |last3=Yucesu |first3=H.S. |year=2001 |title=Effect of Fatty Acid Profiles and Molecular Structures of Nine New Source of Biodiesel on Combustion and Emission |journal=Energy Conversion and Management |volume=42 |issue=5 |pages=529–538 |doi=10.1016/s0196-8904(00)00080-7}}</ref> |
|||
==== Low temperature gelling ==== |
|||
===NO<sub>x</sub> emissions=== |
|||
When biodiesel is cooled below a certain point, some of the molecules aggregate and form crystals. The fuel starts to appear cloudy once the crystals become larger than one quarter of the wavelengths of [[visible spectrum|visible light]] – this is the [[cloud point]] (CP). As the fuel is cooled further these crystals become larger. The lowest temperature at which fuel can pass through a 45 micrometre filter is the [[cold filter plugging point]] (CFPP).<ref>{{cite web |author1=袁明豪 |author2=陳奕宏 |date=2017-01-12 |editor=蔡美瑛 |title=生質柴油的冰與火之歌 |url=https://scitechvista.nat.gov.tw/c/hCy1.htm |url-status=dead |archive-url=https://web.archive.org/web/20210322122136/https://scitechvista.nat.gov.tw/c/hCy1.htm |archive-date=2021-03-22 |access-date=2017-06-22 |publisher=[[Ministry of Science and Technology (Taiwan)|Ministry of Science and Technology]] |language=zh |location=Taiwan}}</ref> As biodiesel is cooled further it will gel and then solidify. Within Europe, there are differences in the CFPP requirements between countries. This is reflected in the different national standards of those countries. The temperature at which pure (B100) biodiesel starts to gel varies significantly and depends upon the mix of esters and therefore the feedstock oil used to produce the biodiesel. For example, biodiesel produced from low [[erucic acid]] varieties of canola seed (RME) starts to gel at approximately {{convert|-10|°C|°F}}. Biodiesel produced from beef [[tallow]] and [[palm oil]] tends to gel at around {{convert|16|°C|°F}} and {{convert|13|°C|°F}} respectively.<ref>Sanford, S.D., et al., "Feedstock and Biodiesel Characteristics Report," [[Renewable Energy Group|Renewable Energy Group, Inc.]], www.regfuel.com (2009).</ref> There are a number of commercially available additives that will significantly lower the pour point and cold filter plugging point of pure biodiesel. Winter operation is also possible by blending biodiesel with other fuel oils including #2 low [[sulfur]] diesel fuel and #1 diesel / [[kerosene]]. |
|||
If burned without additives, Biodiesel (B100) is estimated to produce about 10% more [[nitrogen oxide]] NO<sub>x</sub> tailpipe-emissions than [http://www.epa.gov/SmartwayLogistics/growandgo/documents/factsheet-biodiesel.htm petrodiesel]. As biodiesel has a low sulfur content, NO<sub>x</sub> emissions can be reduced through the use of [[catalytic converter]]s to less than the NO<sub>x</sub> emissions from conventional diesel engines. Moreover, as a transportation fuel, biodiesel is in its infancy in terms of additives which are capable of improving energy density, resistance to gelling, and NO<sub>x</sub> emissions. Debate continues over NO<sub>x</sub>, particulates, smog, and greenhouse gas emissions from biodiesel and all other new transportation fuels, biofuels in particular. Ultimately, greater clarity on the fundamental distinctions between smog and other local pollution issues vs. greenhouse gas emissions will be essential for both well founded public policy as well as well informed consumer choices. In February 2006 a Navy biodiesel expert claimed NO<sub>x</sub> emissions in practice were actually lower than baseline. Further research is needed. |
|||
Another approach to facilitate the use of biodiesel in cold conditions is by employing a second fuel tank for biodiesel in addition to the standard diesel fuel tank. The second fuel tank can be [[Thermal insulation|insulated]] and a [[heat exchanger|heating coil]] using [[antifreeze|engine coolant]] is run through the tank. The fuel tanks can be switched over when the fuel is sufficiently warm. A similar method can be used to operate diesel vehicles using straight vegetable oil. |
|||
Recent advances in the use of [[cerium oxide]] help eliminate NO<sub>x</sub> emissions from both petrodiesel and biodiesel,<ref>{{cite web |
|||
| author = Catherine Foster |
|||
| title = New catalyst helps eliminate NOx from diesel exhaust |
|||
| publisher = Argonne National Laboratory | date = [[27 April]] [[2007]] |
|||
| url = http://www.anl.gov/Media_Center/News/2007/CMT070427.html | format = HTML |
|||
| accessdate = 2007-05-05 |
|||
}}</ref> and diesel fuel additives based on cerium oxide can improve fuel consumption by 11%{{Fact|date=February 2008}} in unmodified diesel engines. |
|||
==== Contamination by water ==== |
|||
==Current research== |
|||
Biodiesel may contain small but problematic quantities of water. Although it is only slightly miscible with water it is [[hygroscopy|hygroscopic]].<ref>{{cite web |last=UFOP – Union zur Förderung von Oel |title=Biodiesel FlowerPower: Facts * Arguments * Tips |url=http://www.biodiesel.org/resources/reportsdatabase/reports/gen/20040101_gen-331.pdf |url-status=live |archive-url=https://web.archive.org/web/20070714023830/http://www.biodiesel.org/resources/reportsdatabase/reports/gen/20040101_gen-331.pdf |archive-date=2007-07-14 |access-date=2007-06-13}}</ref> One of the reasons biodiesel can absorb water is the persistence of mono and diglycerides left over from an incomplete reaction. These molecules can act as an emulsifier, allowing water to mix with the biodiesel.{{Citation needed|date=February 2008}} In addition, there may be water that is residual to processing or resulting from storage tank [[condensation]]. The presence of water is a problem because: |
|||
There is ongoing research into finding more suitable crops and improving oil yield. Using the current yields, vast amounts of land and fresh water would be needed to produce enough oil to completely replace fossil fuel usage. It would require twice the land area of the US to be devoted to soybean production, or two-thirds to be devoted to rapeseed production, to meet current US heating and transportation needs. {{Fact|date=November 2007}} |
|||
* Water reduces the heat of fuel [[combustion]], causing smoke, harder starting, and reduced [[power (physics)|power]]. |
|||
* Water causes [[corrosion]] of fuel system components (pumps, fuel lines, etc.) |
|||
* Microbes in water cause the paper-element filters in the system to rot and fail, causing failure of the fuel pump due to ingestion of large particles. |
|||
* Water freezes to form ice crystals that provide sites for [[nucleation]], accelerating gelling of the fuel. |
|||
* Water causes pitting in pistons. |
|||
Previously, the amount of water contaminating biodiesel has been difficult to measure by taking samples, since water and oil separate. However, it is now possible to measure the water content using water-in-oil sensors.<ref>{{Cite news |title=Detecting and Controlling Water in Oil |url=http://www.machinerylubrication.com/Read/787/detecting-water-in-oil |url-status=dead |archive-url=https://web.archive.org/web/20161024090036/http://www.machinerylubrication.com/Read/787/detecting-water-in-oil |archive-date=2016-10-24 |access-date=2016-10-23}}</ref> |
|||
Specially bred mustard varieties can produce reasonably high oil yields, and have the added benefit that the meal leftover after the oil has been pressed out can act as an effective and biodegradable [[pesticide]].{{Fact|date=November 2007}} |
|||
Water contamination is also a potential problem when using certain chemical [[catalyst]]s involved in the production process, substantially reducing catalytic efficiency of base (high pH) catalysts such as [[potassium hydroxide]]. However, the super-critical methanol production methodology, whereby the transesterification process of oil feedstock and methanol is effectuated under high temperature and pressure, has been shown to be largely unaffected by the presence of water contamination during the production phase |
|||
===Algaculture=== |
|||
{{main article|Algaculture}} |
|||
From 1978 to 1996, the [[National Renewable Energy Laboratory|U.S. National Renewable Energy Laboratory]] experimented with using algae as a biodiesel source in the "[[Aquatic Species Program]]".<ref>{{cite paper |
|||
| author = John Sheehan, Terri Dunahay, John Benemann, Paul Roessler |
|||
| title = A look back at the U.S. Department of Energy's Aquatic Species Program: Biodiesel from Algae | version = Close-out Report |
|||
| publisher = United States Department of Energy | date= July 1998 | accessdate = 2007-01-02 |
|||
| url = http://www.nrel.gov/docs/legosti/fy98/24190.pdf | format = PDF (3.7 Mb) |
|||
}}</ref> |
|||
A self-published article by Michael Briggs, at the [[University of New Hampshire|UNH]] Biodiesel Group, offers estimates for the realistic replacement of all [[motor vehicle|vehicular]] fuel with biodiesel by utilizing algae that have a natural oil content greater than 50%, which Briggs suggests can be grown on algae ponds at [[wastewater treatment]] plants.<ref name="Briggs2004"/> This oil-rich algae can then be extracted from the system and processed into biodiesel, with the dried remainder further reprocessed to create [[ethanol]]. |
|||
==Research== |
|||
The production of algae to harvest oil for biodiesel has not yet been undertaken on a commercial scale, but [[feasibility study|feasibility studies]] have been conducted to arrive at the above yield estimate. In addition to its projected high yield, algaculture — unlike [[agriculture|crop-based]] [[biofuels]] — does not entail a decrease in [[food production]], since it requires neither [[Farmland (farming)|farmland]] nor [[fresh water]]. Some companies[http://www.greenfuelonline.com/technology.htm][http://www.valcent.net/t/news_detailf62c.html?id=36] are pursuing algae bio-reactors for various purposes, including biodiesel production. |
|||
There was research into finding more suitable crops and improving oil yield. Other sources are possible including human [[fecal]] matter, with [[Ghana]] building its first "fecal sludge-fed biodiesel plant."<ref>{{cite journal|url=http://www.csmonitor.com/Environment/2012/1003/Ghana-s-best-shot-at-going-green-sewage-power|title=Ghana's best shot at going green: sewage power|author=The Christian Science Monitor|journal=The Christian Science Monitor|access-date=30 May 2015|date=2012-10-03|archive-date=2015-05-30|archive-url=https://web.archive.org/web/20150530205644/http://www.csmonitor.com/Environment/2012/1003/Ghana-s-best-shot-at-going-green-sewage-power|url-status=live}}</ref> |
|||
Specially bred mustard varieties can produce reasonably high oil yields and are very useful in [[crop rotation]] with cereals, and have the added benefit that the meal leftover after the oil has been pressed out can act as an effective and biodegradable pesticide.<ref>{{cite web|url=http://www1.eere.energy.gov/biomass/pdfs/mustard_hybrids.pdf|title=Mustard Hybrids for Low-Cost Biodiesel and Organic Pesticides|access-date=2010-03-15|url-status=dead|archive-url=https://web.archive.org/web/20110726174434/http://www1.eere.energy.gov/biomass/pdfs/mustard_hybrids.pdf|archive-date=2011-07-26}}</ref> |
|||
On [[May 11]], [[2006]] the Aquaflow Bionomic Corporation in [[Marlborough, New Zealand]] announced that it had produced its first sample of bio-diesel fuel made from algae found in [[sewage]] ponds.<ref name="Kiong" /> |
|||
Unlike previous attempts, the algae was naturally grown in pond [[Effluent|discharge]] from the Marlborough District Council's [[sewage treatment]] works. |
|||
The [[NFESC]], with [[Santa Barbara, California|Santa Barbara]]-based Biodiesel Industries is working to develop biodiesel technologies for the US navy and military, one of the largest diesel fuel users in the world.<ref>{{cite web|url=http://www.futureenergies.com/modules.php?op=modload&name=News&file=article&sid=770|title=PORT HUENEME, Calif: U.S. Navy to Produce its Own Biodiesel :: Future Energies :: The future of energy|publisher=Future Energies|date=2003-10-30|access-date=2009-10-17|archive-url=https://web.archive.org/web/20110711055612/http://www.futureenergies.com/modules.php?op=modload&name=News&file=article&sid=770|archive-date=2011-07-11|url-status=dead}}</ref> |
|||
A group of Spanish developers working for a company called [http://agnux.wordpress.com/tag/ecofasa/ Ecofasa] announced a new biofuel made from trash. The fuel is created from general urban waste which is treated by bacteria to produce fatty acids, which can be used to make biodiesel.<ref>{{cite web|url=http://lele.newsvine.com/_news/2008/10/18/2014473-ecofasa-turns-waste-to-biodiesel-using-bacteria-|title=Newsvine – Ecofasa turns waste to biodiesel using bacteria|publisher=Lele.newsvine.com|date=2008-10-18|access-date=2009-10-17|archive-date=2008-11-03|archive-url=https://web.archive.org/web/20081103042959/http://lele.newsvine.com/_news/2008/10/18/2014473-ecofasa-turns-waste-to-biodiesel-using-bacteria-|url-status=live}}</ref> |
|||
Another approach that does not require the use of chemical for the production involves the use of genetically modified microbes.<ref>{{cite web|url=http://newscenter.lbl.gov/news-releases/2010/01/27/microbes-produce-biofuels/|title=Microbes Produce Fuels Directly from Biomass|work=News Center|access-date=30 May 2015|date=2010-01-27|archive-date=2014-02-17|archive-url=https://web.archive.org/web/20140217184536/http://newscenter.lbl.gov/news-releases/2010/01/27/microbes-produce-biofuels/|url-status=live}}</ref><ref>{{cite web|url=http://cheme.berkeley.edu/faculty/keasling/|title=Faculty & Research|access-date=30 May 2015|archive-date=26 October 2011|archive-url=https://web.archive.org/web/20111026222929/http://cheme.berkeley.edu/faculty/keasling/|url-status=live}}</ref> |
|||
===Algal biodiesel=== |
|||
{{Main|Algaculture|Algal fuel}} |
|||
From 1978 to 1996, the [[National Renewable Energy Laboratory|U.S. NREL]] experimented with using algae as a biodiesel source in the "[[Aquatic Species Program]]".<ref name = "NREL biodiesel algae"/> |
|||
A self-published article by Michael Briggs, at the [[University of New Hampshire|UNH]] Biodiesel Group, offers estimates for the realistic replacement of all [[motor vehicle|vehicular]] fuel with biodiesel by utilizing algae that have a natural oil content greater than 50%, which Briggs suggests can be grown on algae ponds at [[wastewater treatment]] plants.<ref name="Briggs2004">{{cite web |last=Briggs |first=Michael |date=August 2004 |title=Widescale Biodiesel Production from Algae |url=http://www.unh.edu/p2/biodiesel/article_alge.html |url-status=dead |archive-url=https://web.archive.org/web/20060324084858/http://www.unh.edu/p2/biodiesel/article_alge.html |archive-date=March 24, 2006 |access-date=2007-01-02 |publisher=UNH Biodiesel Group (University of New Hampshire)}}</ref> This oil-rich algae can then be extracted from the system and processed into biodiesel, with the dried remainder further reprocessed to create ethanol. |
|||
The production of algae to harvest oil for biodiesel has not yet been undertaken on a commercial scale, but [[feasibility study|feasibility studies]] have been conducted to arrive at the above yield estimate. In addition to its projected high yield, algaculture — unlike [[agriculture|crop-based]] biofuels — does not entail a decrease in [[food production]], since it requires neither [[farmland (farming)|farmland]] nor [[fresh water]]. Many companies are pursuing algae bio-reactors for various purposes, including scaling up biodiesel production to commercial levels.<ref>{{cite web|url=http://www.valcent.net/t/news_detailf62c.html?id=36|title=Valcent Products Inc. Develops "Clean Green" Vertical Bio-Reactor|access-date=2008-07-09|publisher=Valcent Products|url-status=dead|archive-url=https://web.archive.org/web/20080618163304/http://www.valcent.net/t/news_detailf62c.html?id=36|archive-date=2008-06-18}}</ref><ref> |
|||
{{cite web|url=http://www.greenfuelonline.com/technology.htm|title=Technology: High Yield Carbon Recycling|publisher=[[GreenFuel Technologies Corporation]]|archive-url=https://web.archive.org/web/20080921095341/http://www.greenfuelonline.com/technology.html|archive-date=2008-09-21|access-date=2015-06-14|url-status=dead}}</ref> |
|||
Biodiesel lipids could be extracted from wet algae using a simple and economical reaction in [[ionic liquids]].<ref>{{cite journal|title=Energy-efficient extraction of fuel and chemical feedstocks from algae|journal=[[Green Chemistry (journal)|Green Chemistry]]|volume=14|issue=2|year=2012|pages=419–427|author=R. E. Teixeira|doi=10.1039/C2GC16225C}}</ref> |
|||
===Pongamia=== |
|||
{{Main|Millettia pinnata|Pongamia oil}} |
|||
''Millettia pinnata'', also known as the Pongam Oiltree or Pongamia, is a leguminous, oilseed-bearing tree that has been identified as a candidate for non-edible vegetable oil production. |
|||
Pongamia plantations for biodiesel production have a two-fold environmental benefit. The trees both store carbon and produce fuel oil. Pongamia grows on marginal land not fit for food crops and does not require nitrate fertilizers. The oil producing tree has the highest yield of oil producing plant (approximately 40% by weight of the seed is oil) while growing in malnourished soils with high levels of salt. It is becoming a main focus in a number of biodiesel research organizations.<ref>{{cite web|url=http://www.cilr.uq.edu.au/UserImages/File/factsheets/Pongamia%20Binder1.pdf|title=Pongamia Factsheet|access-date=2013-10-02|archive-date=2013-05-01|archive-url=https://web.archive.org/web/20130501195229/http://www.cilr.uq.edu.au/UserImages/File/factsheets/Pongamia%20Binder1.pdf|url-status=live}}</ref> The main advantages of Pongamia are a higher recovery and quality of oil than other crops and no direct competition with food crops. However, growth on marginal land can lead to lower oil yields which could cause competition with food crops for better soil. |
|||
===Jatropha=== |
|||
{{Main|Jatropha|Jatropha Oil}} |
|||
[[File:Jatropha Biodiesel - DRDO - Pride of India - Exhibition - 100th Indian Science Congress - Kolkata 2013-01-03 2579.JPG|thumb|150px|Jatropha Biodiesel from [[Defence Research and Development Organisation|DRDO]], India.]] |
|||
Several groups in various sectors are conducting research on ''[[Jatropha curcas]]'', a poisonous shrub-like tree that produces seeds considered by many to be a viable source of biodiesel feedstock oil.<ref>{{cite journal|title=Biology and genetic improvement of Jatropha curcas L.: A review|journal=Applied Energy|volume=87|issue=3|year=2010|pages=732–742|author1=B.N. Divakara|author2=H.D. Upadhyaya|author3=S.P. Wani|author4=C.L. Laxmipathi Gowda|doi=10.1016/j.apenergy.2009.07.013|bibcode=2010ApEn...87..732D |url=http://oar.icrisat.org/174/1/nset10.pdf|access-date=2019-07-05|archive-date=2020-03-05|archive-url=https://web.archive.org/web/20200305164222/http://oar.icrisat.org/174/1/nset10.pdf|url-status=live}}</ref> Much of this research focuses on improving the overall per acre oil yield of Jatropha through advancements in genetics, soil science, and horticultural practices. |
|||
[[SG Biofuels]], a San Diego-based Jatropha developer, has used molecular breeding and biotechnology to produce elite hybrid seeds of Jatropha that show significant yield improvements over first generation varieties.<ref>{{cite web|url=http://www.biofuelsdigest.com/bdigest/2011/05/16/jatropha-blooms-again-sg-biofuels-secures-250k-acres-for-hybrids|title=Jatropha blooms again: SG Biofuels secures 250K acres for hybrids|publisher=Biofuels Digest|date=2011-05-16|access-date=2012-03-08|archive-date=2021-02-25|archive-url=https://web.archive.org/web/20210225040011/https://www.biofuelsdigest.com/bdigest/2011/05/16/jatropha-blooms-again-sg-biofuels-secures-250k-acres-for-hybrids/|url-status=live}}</ref> [[SG Biofuels]] also claims that additional benefits have arisen from such strains, including improved flowering synchronicity, higher resistance to pests and disease, and increased cold weather tolerance.<ref>{{cite web|url=http://www.sgfuel.com/pages/hybrid-seeds-and-services/jMax-hybrid-seeds.php|title=Jmax Hybrid Seeds|publisher=SG Biofuels|date=2012-03-08|access-date=2012-03-08|url-status=dead|archive-url=https://web.archive.org/web/20111218030507/http://www.sgfuel.com/pages/hybrid-seeds-and-services/jMax-hybrid-seeds.php|archive-date=2011-12-18}}</ref> |
|||
Plant Research International, a department of the [[Wageningen University and Research Centre]] in the Netherlands, maintains an ongoing Jatropha Evaluation Project (JEP) that examines the feasibility of large scale Jatropha cultivation through field and laboratory experiments.<ref>{{cite web|author=Plant Research International|url=http://www.pri.wur.nl/UK/research/plant-based_raw_materials/jatropha/JATROPT|title=JATROPT (Jatropha curcas): Applied and technical research into plant properties|publisher=Plant Research International|date=2012-03-08|access-date=2012-03-08|archive-date=2017-06-28|archive-url=https://web.archive.org/web/20170628182441/http://www.pri.wur.nl/UK/research/plant-based_raw_materials/jatropha/JATROPT|url-status=live}}</ref> |
|||
The [[Center for Sustainable Energy Farming]] (CfSEF) is a Los Angeles-based non-profit research organization dedicated to Jatropha research in the areas of plant science, agronomy, and horticulture. Successful exploration of these disciplines is projected to increase Jatropha farm production yields by 200–300% in the next ten years.<ref>{{cite web|url=http://www.biodieselmagazine.com/articles/7743/energy-farming-methods-mature-improve|title=Energy Farming Methods Mature, Improve|work=Biodiesel Magazine|date=2011-04-11|access-date=2012-03-08|archive-date=2012-04-06|archive-url=https://web.archive.org/web/20120406184600/http://www.biodieselmagazine.com/articles/7743/energy-farming-methods-mature-improve|url-status=live}}</ref> |
|||
=== FOG from sewage === |
|||
So-called [[fats, oils and grease]] (FOG), recovered from [[sewage]] can also be turned into biodiesel.<ref>{{Cite web |title=Argent biodiesel |url=https://argentenergy.com/our-fuels/argent-biodiesel |url-status=dead |archive-url=https://web.archive.org/web/20190422074639/https://argentenergy.com/our-fuels/argent-biodiesel |archive-date=2019-04-22 |access-date=2019-07-31 |website=Argent Energy}}</ref> |
|||
===Fungi=== |
|||
A group at the [[Russian Academy of Sciences]] in Moscow published a paper in 2008, stating that they had isolated large amounts of lipids from single-celled fungi and turned it into biodiesel in an economically efficient manner.<ref>{{Cite journal|last1=Sergeeva|first1=Y. E.|last2=Galanina|first2=L. A.|last3=Andrianova|first3=D. A.|last4=Feofilova|first4=E. P.|title=Lipids of filamentous fungi as a material for producing biodiesel fuel|journal=Applied Biochemistry and Microbiology|volume=44|issue=5|year=2008|pages=576–581|doi=10.1134/S0003683808050128|pmid=18822779|s2cid=12731382}}</ref> |
|||
The recent discovery of a variant of the fungus ''[[Gliocladium roseum]]'' points toward the production of so-called [[myco-diesel]] from cellulose. This organism was recently discovered in the rainforests of northern [[Patagonia]] and has the unique capability of converting cellulose into medium length hydrocarbons typically found in diesel fuel.<ref>{{Cite journal|last1=Strobel|first1=G.|last2=Knighton|first2=B.|last3=Kluck|first3=K.|last4=Ren|first4=Y.|last5=Livinghouse|first5=T.|last6=Griffin|first6=M.|last7=Spakowicz|first7=D.|last8=Sears|first8=J.|title=The production of myco-diesel hydrocarbons and their derivatives by the endophytic fungus Gliocladium roseum (NRRL 50072)|journal=Microbiology|volume=154|issue=Pt 11|pages=3319–3328|year=2008|pmid=18957585|doi=10.1099/mic.0.2008/022186-0|url=http://scholarworks.montana.edu/xmlui/bitstream/1/9612/1/Strobel_MicBio_2010_A1b.pdf|doi-access=free|access-date=2018-04-20|archive-date=2021-07-31|archive-url=https://web.archive.org/web/20210731013354/http://scholarworks.montana.edu/xmlui/bitstream/handle/1/9612/Strobel_MicBio_2010_A1b.pdf;jsessionid=ADA8D6AF330696AAE7A3BEB87BAF879F?sequence=1|url-status=live}}</ref> |
|||
===Biodiesel from used coffee grounds=== |
|||
Researchers at the [[University of Nevada, Reno]], have successfully produced biodiesel from oil derived from [[used coffee grounds]]. Their analysis of the used grounds showed a 10% to 15% oil content (by weight). Once the oil was extracted, it underwent conventional processing into biodiesel. It is estimated that finished biodiesel could be produced for about one US dollar per gallon. Further, it was reported that "the technique is not difficult" and that "there is so much coffee around that several hundred million gallons of biodiesel could potentially be made annually." However, even if all the coffee grounds in the world were used to make fuel, the amount produced would be less than 1 percent of the diesel used in the United States annually. "It won’t solve the world’s energy problem," Dr. Misra said of his work.<ref>{{cite news|url=https://www.nytimes.com/2008/12/16/science/16objava.html|title=Diesel made Simply From Coffee Grounds|access-date=2008-12-15|newspaper=The New York Times|first=Henry|last=Fountain|date=2008-12-15|archive-date=2008-12-17|archive-url=https://web.archive.org/web/20081217143150/http://www.nytimes.com/2008/12/16/science/16objava.html|url-status=live}}</ref> |
|||
=== Biodiesel to hydrogen-cell power === |
|||
A microreactor has been developed to convert biodiesel into hydrogen steam to power fuel cells.<ref>{{cite journal|last1=Irving|first1=P. M.|last2=Pickles|first2=J. S.|year=2007|title=Operational Requirements for a Multi-fuel Processor that Generates Hydrogen from Bio- and Petroleum-Based Fuels for Both SOFC and PEM Fuel Cells|journal=ECS Transactions|volume=5|issue=1|pages=665–671|doi=10.1149/1.2729047|bibcode=2007ECSTr...5a.665I|s2cid=137810875}}</ref> |
|||
'''Steam reforming''', also known as '''fossil fuel reforming''' is a process which produces hydrogen gas from hydrocarbon fuels, most notably biodiesel due to its efficiency. A **microreactor**, or reformer, is the processing device in which water vapour reacts with the liquid fuel under high temperature and pressure. Under temperatures ranging from 700 – 1100 °C, a nickel-based catalyst enables the production of carbon monoxide and hydrogen:<ref>{{cite journal|last1=Park|first1=G.|last2=Seo|first2=D. J.|last3=Park|first3=S.|last4=Yoon|first4=Y.|last5=Kim|first5=C.|last6=Yoon|first6=W.|year=2004|title=Development of microchannel methanol steam reformer|journal=Chem. Eng. J.|volume=101|issue=1–3|pages=87–92|doi=10.1016/j.cej.2004.01.007}}</ref> |
|||
'' Hydrocarbon + {{chem|H|2|O}} ⇌ CO + 3{{chem|H|2}} (Highly endothermic)'' |
|||
Furthermore, a higher yield of hydrogen gas can be harnessed by further oxidizing carbon monoxide to produce more hydrogen and carbon dioxide: |
|||
'' CO + {{chem|H|2|O}} → {{CO2}} + {{chem|H|2}} (Mildly exothermic)'' |
|||
===Safflower oil=== |
|||
{{as of|2020}}, researchers at Australia's [[CSIRO]] have been studying [[safflower]] oil from a specially-bred variety as an engine [[lubricant]], and researchers at [[Montana State University]]'s Advanced Fuel Centre in the US have been studying the oil's performance in a large [[diesel engine]], with results described as a "game-changer".<ref>{{cite web | title=Safflower oil hailed by scientists as possible recyclable, biodegradable replacement for petroleum | website=ABC News | series=Landline | publisher=Australian Broadcasting Corporation | first=Tim | last=Lee | date=7 June 2020 | url=https://www.abc.net.au/news/2020-06-07/safflower-oil-new-biofuel-to-replace-petroleum/12321028 | access-date=7 June 2020 | archive-date=7 June 2020 | archive-url=https://web.archive.org/web/20200607012058/https://www.abc.net.au/news/2020-06-07/safflower-oil-new-biofuel-to-replace-petroleum/12321028 | url-status=live }}</ref> |
|||
==See also== |
==See also== |
||
* [[Civic amenity site]]; collection point for [[Waste vegetable oil|WVO]] |
|||
{{Portal|Sustainable development|Sustainable development.svg}} |
|||
* [[EcoJet concept car]] |
|||
*[[Biodiesel around the World]] |
|||
* [[Food, Conservation, and Energy Act of 2008]] |
|||
**[[Biodiesel in the United States]] |
|||
* [[Fuel (film)]] |
|||
**[[Biodiesel in the United Kingdom]] |
|||
* [[Gasoline gallon equivalent]] |
|||
*[[Biodiesel production]] |
|||
* [[Indirect land use change impacts of biofuels]] |
|||
*[[Bioenergy]] |
|||
*[[ |
* [[MY Ady Gil]] |
||
* [[Sustainable biofuel]] |
|||
*[[NExBTL]] is a paraffinic, rather than transesterified renewable diesel |
|||
* [[Table of biodiesel crop yields]] |
|||
*[[National Biodiesel Board]] |
|||
*[[Tonne of oil equivalent]] |
* [[Tonne of oil equivalent]] |
||
* [[United States vs. Imperial Petroleum]] |
|||
*[[Vegetable oil economy]] |
|||
*[[Vegetable |
* [[Vegetable oils as alternative energy]] |
||
* [[Vegetable oil fuel]] |
|||
* [[Issues relating to biofuels]] |
|||
* [[Low-carbon economy]] |
|||
==References== |
==References== |
||
{{Reflist}} |
|||
===Footnotes=== |
|||
* ''An Overview of Biodiesel and Petroleum Diesel Lifecycles'', May 1998, Sheehan, ''et al.'' NREL [http://www.nrel.gov/docs/legosti/fy98/24772.pdf (60pp pdf file)] |
|||
{{reflist}} |
|||
* ''Business Management for Biodiesel Producers'', January 2004, Jon Von Gerpen, Iowa State University under contract with the National Renewable Energy Laboratory (NREL) [http://www.nrel.gov/docs/fy04osti/36242.pdf (210pp pdf file)] |
|||
* ''[https://web.archive.org/web/20010108231400/http://www.biodiesel.co.uk/levington.htm Energy balances in the growth of oilseed rape for biodiesel and of wheat for bioethanol]'', June 2000, I.R. Richards |
|||
* ''Life Cycle Inventory of Biodiesel and Petroleum Diesel for Use in an Urban Bus'', 1998, Sheehan, ''et al.'' NREL [http://www.nrel.gov/docs/legosti/fy98/24089.pdf (314pp pdf file)] |
|||
* ''[http://www.csmonitor.com/2006/0111/p01s03-sten.html Algae – like a breath mint for smokestacks]'', January 11, 2006, Mark Clayton, ''[[The Christian Science Monitor]]'' |
|||
* {{cite web|last=Tyson|first=R.L.|title=2006 Biodiesel Handling and Use Guide Third Edition|url=http://www.nrel.gov/vehiclesandfuels/npbf/pdfs/40555.pdf|archive-url=https://web.archive.org/web/20061216051136/http://www.nrel.gov/vehiclesandfuels/npbf/pdfs/40555.pdf|archive-date=2006-12-16|url-status=dead}} |
|||
* [https://web.archive.org/web/20070606084122/http://www.wfs.org/futcontja07.htm Biodiesel's Bright Future] from the July–August issue of THE FUTURIST magazine. {{Data missing|date=July 2021}} |
|||
{{cite report |ref={{harvid|Beer et al.|2004}} |author1=Tom Beer |author2=Tim Grant |author3=Harry Watson |author4=Doina Olaru |others=CSIRO |title=Life-Cycle Emissions Analysis of Fuels for Light Vehicles |publisher=Australian Greenhouse Office |id=HA93A-C837/1/F5.2E |url=https://p2infohouse.org/ref/37/36498.pdf |date=2004}} |
|||
=== Other references === |
|||
*''An Overview of Biodiesel and Petroleum Diesel Lifecycles'', May 1998, Sheehan, ''et al.'' NREL [http://www.nrel.gov/docs/legosti/fy98/24772.pdf (60pp pdf file)] |
|||
*''Business Management for Biodiesel Producers'', January 2004, Jon Von Gerpen, Iowa State University under contract with the National Renewable Energy Laboratory (NREL) [http://www.nrel.gov/docs/fy04osti/36242.pdf (210pp pdf file)] |
|||
*''[http://www.biodiesel.co.uk/levington.htm Energy balances in the growth of oilseed rape for biodiesel and of wheat for bioethanol]'', June 2000, I.R. Richards |
|||
*''Life Cycle Inventory of Biodiesel and Petroleum Diesel for Use in an Urban Bus'', 1998, Sheehan, ''et al.'' NREL [http://www.nrel.gov/docs/legosti/fy98/24089.pdf (314pp pdf file)] |
|||
*''[http://www.csmonitor.com/2006/0111/p01s03-sten.html Algae - like a breath mint for smokestacks]'', [[January 11]], [[2006]], Mark Clayton, [[Christian Science Monitor]] |
|||
* {{cite web |
|||
| last = Tyson |
|||
| first = K.S. |
|||
| last = McCormick |
|||
| first = R.L. |
|||
| title = "2006 Biodiesel Handling and Use Guide Third Edition" |
|||
| url= http://www.nrel.gov/vehiclesandfuels/npbf/pdfs/40555.pdf |
|||
| accessdate =}} |
|||
*[http://www.wfs.org/futcontja07.htm Biodiesel's Bright Future] from the July-August issue of THE FUTURIST magazine. |
|||
==External links== |
==External links== |
||
{{Commons}} |
|||
{{commons|Biodiesel|Biodiesel}} |
|||
{{Wikibooks|Do-It-Yourself}} |
{{Wikibooks|Do-It-Yourself}} |
||
<!--Links should not be added unless they are substantiated on talk page first. If you don't first get support from an uninvolved contributor BEFORE adding the link, it will be reverted. Already too many links, Wikipedia is not a linkfarm ----> |
|||
{{Wikinews|Portal:Environment}} |
|||
* [http://www.srsbiodiesel.com/information/benefits-of-biodiesel Benefits of Biodiesel] |
|||
<!--Because this article is a magnet for everyone wanting to get their favorite link in here, links should not be added unless they are substantiated on the talk page first. If you don't first get support from an uninvolved contributor BEFORE adding the link, it will be reverted. There are already too many links, and Wikipedia is not a link farm. ----> |
|||
* [http://www.ebb-eu.org European Biodiesel Board] website – European Biodiesel Industry. |
|||
*{{dmoz|/Science/Technology/Energy/Renewable/Biomass_and_Biofuels/Biodiesel/|Biodiesel}} |
|||
* [https://web.archive.org/web/20100305140144/http://sustainablebiodieselalliance.com/dev/ Sustainable Biodiesel Alliance] |
|||
*[http://www.ebb-eu.org European Biodiesel Board] website - European Biodiesel Industry. |
|||
* {{webarchive |url=https://web.archive.org/web/20110104210054/http://www.iea.org/textbase/nppdf/free/2004/biofuels2004.pdf |date=January 4, 2011 |title=International Energy Agency: Biofuels for Transport – An International Perspective }} |
|||
*[http://www.gmo-safety.eu/en/oilseed_rape/agriculture/50.docu.html Renewable raw materials: Biodiesel leads to more rape (rapeseed) cultivation] |
|||
* [http://www.biodieseleducation.org National Biodiesel Education Program, University of Idaho]—unbiased, science-based information on biodiesel for biodiesel producers and distributors, fleet operators, farmers and feedstock producers, policy makers, and consumers. |
|||
*[http://www.unh.edu/p2/biodiesel/article_biodiesel_vs_hydrogen.html UNH Biodiesel Group's comparison of Biodiesel vs. Hydrogen] |
|||
* [https://web.archive.org/web/20091122133933/http://www.unep.fr/scp/rpanel/pdf/Assessing_Biofuels_Full_Report.pdf Towards Sustainable Production and Use of Resources: Assessing Biofuels] by the [[United Nations Environment Programme]], October 2009. |
|||
*[http://www.biodiesel.org Biodiesel.org] |
|||
* [https://web.archive.org/web/20100816053206/http://www.extension.org/pages/Farm_Energy_Biodiesel_Table_of_Contents Biodiesel Articles on eXtension]—eXtension (pronounced "E-Extension") is a wiki for extension professors and agents across the United States. The Farm Energy section contains over 30 articles on biodiesel, from the basics to more technical information. |
|||
*[http://www.biodieselcommunity.org/ Collaborative Biodiesel Tutorial] |
|||
* [http://pubs.cas.psu.edu/freepubs/pdfs/agrs103.pdf Biodiesel Safety and Best Management Practices for Small-Scale Noncommercial Use and Production] {{Webarchive|url=https://web.archive.org/web/20140211214657/http://pubs.cas.psu.edu/freepubs/pdfs/agrs103.pdf |date=2014-02-11 }} |
|||
*[http://www.oliomap.com/ Oliomap.com The Global Vegetable Fuel Resources Map] |
|||
{{Portal inline|Environment}} |
|||
[[Category:Alternative propulsion]] |
|||
{{Portal inline|Renewable Energy}} |
|||
[[Category:Biodiesel| ]] |
|||
[[Category:Direct biofuels]] |
|||
[[Category:Renewable energy]] |
|||
[[Category:Sustainable technologies]] |
|||
[[Category:Sustainable transport]] |
|||
[[Category:Diesel substitutes]] |
|||
{{ |
{{Bioenergy}} |
||
{{Alternative propulsion}} |
|||
[[af:Biodiesel]] |
|||
{{Automobile configuration}} |
|||
[[ar:بيوديزل]] |
|||
{{Human impact on the environment}} |
|||
[[bn:বায়োডিজেল]] |
|||
{{Palm oil}} |
|||
[[bg:Биодизел]] |
|||
[[ca:Biodièsel]] |
|||
{{Authority control}} |
|||
[[cs:Bionafta]] |
|||
[[de:Biodiesel]] |
|||
[[ |
[[Category:Biodiesel| ]] |
||
[[Category:Liquid fuels]] |
|||
[[el:Βιοντίζελ]] |
|||
[[Category:Commodity chemicals]] |
|||
[[es:Biodiésel]] |
|||
[[Category:Environmental impact of the energy industry|Biodiesel]] |
|||
[[eo:Biodizelo]] |
|||
[[fr:Biodiesel]] |
|||
[[gl:Biodiésel]] |
|||
[[ko:바이오디젤]] |
|||
[[hi:बायोडिजल]] |
|||
[[hr:Biodizel]] |
|||
[[id:Biodiesel]] |
|||
[[is:Lífdísill]] |
|||
[[it:Biodiesel]] |
|||
[[he:ביו דיזל]] |
|||
[[hu:Biodízel]] |
|||
[[nl:Biodiesel]] |
|||
[[ja:バイオディーゼル]] |
|||
[[no:Biodiesel]] |
|||
[[pl:Biodiesel]] |
|||
[[pt:Biodiesel]] |
|||
[[ro:Biodiesel]] |
|||
[[ru:Биодизель]] |
|||
[[simple:Biodiesel]] |
|||
[[sl:Biodizel]] |
|||
[[sr:Биодизел]] |
|||
[[fi:Biodiesel]] |
|||
[[sv:Biodiesel]] |
|||
[[th:ไบโอดีเซล]] |
|||
[[vi:Diesel sinh học]] |
|||
[[tr:Biodizel]] |
|||
[[uk:Біодизель]] |
|||
[[zh:生物柴油]] |
|||
Latest revision as of 22:27, 15 November 2024
This article may contain an excessive amount of intricate detail that may interest only a particular audience. (January 2023) |


Biodiesel is a renewable biofuel, a form of diesel fuel, derived from biological sources like vegetable oils, animal fats, or recycled greases, and consisting of long-chain fatty acid esters. It is typically made from fats.[1][2][3]
The roots of biodiesel as a fuel source can be traced back to when J. Patrick and E. Duffy first conducted transesterification of vegetable oil in 1853, predating Rudolf Diesel's development of the diesel engine.[4] Diesel's engine, initially designed for mineral oil, successfully ran on peanut oil at the 1900 Paris Exposition. This landmark event highlighted the potential of vegetable oils as an alternative fuel source. The interest in using vegetable oils as fuels resurfaced periodically, particularly during resource-constrained periods such as World War II. However, challenges such as high viscosity and resultant engine deposits were significant hurdles. The modern form of biodiesel emerged in the 1930s, when a method was found for transforming vegetable oils for fuel use, laying the groundwork for contemporary biodiesel production.
The physical and chemical properties of biodiesel vary depending on its source and production method. The US National Biodiesel Board defines "biodiesel" as a mono-alkyl ester.[5] It has been experimented with in railway locomotives and power generators. Generally characterized by a higher boiling point and flash point than petrodiesel, biodiesel is slightly miscible with water and has distinct lubricating properties. Its calorific value is approximately 9% lower than that of standard diesel, impacting fuel efficiency. Biodiesel production has evolved significantly, with early methods including the direct use of vegetable oils, to more advanced processes like transesterification, which reduces viscosity and improves combustion properties. Notably, biodiesel production generates glycerol as a by-product, which has its own commercial applications.
Biodiesel's primary application is in transport. There have been efforts to make it a drop-in biofuel, meaning compatible with existing diesel engines and distribution infrastructure. However, it is usually blended with petrodiesel, typically to less than 10%, since most engines cannot run on pure biodiesel without modification.[6][7] The blend percentage of biodiesel is indicated by a "B" factor. B100 represents pure biodiesel, while blends like B20 contain 20% of biodiesel, with the remainder being traditional petrodiesel. These blends offer a compromise between the environmental benefits of biodiesel and performance characteristics of standard diesel fuel. Biodiesel blends can be used as heating oil.
The environmental impact of biodiesel is complex and varies based on factors like feedstock type, land use changes, and production methods. While it can potentially reduce greenhouse gas emissions compared to fossil fuels, concerns about biodiesel include land use changes, deforestation, and the food vs. fuel debate. The debate centers on the impact of biodiesel production on food prices and availability, as well as its overall carbon footprint. Despite these challenges, biodiesel remains a key component in the global strategy to reduce reliance on fossil fuels and mitigate the impacts of climate change.
Blends
[edit]
Blends of biodiesel and conventional hydrocarbon-based diesel are most commonly distributed for use in the retail diesel fuel marketplace. Much of the world uses a system known as the "B" factor to state the amount of biodiesel in any fuel mix:[8]
- 100% biodiesel is referred to as B100
- 20% biodiesel, 80% petrodiesel is labeled B20[6]
- 10% biodiesel, 90% petrodiesel is labeled B10
- 7% biodiesel, 93% petrodiesel is labeled B7
- 5% biodiesel, 95% petrodiesel is labeled B5
- 2% biodiesel, 98% petrodiesel is labeled B2
Blends of 20% biodiesel and lower can be used in diesel equipment with no, or only minor modifications,[9] although certain manufacturers do not extend warranty coverage if equipment is damaged by these blends. The B6 to B20 blends are covered by the ASTM D7467 specification.[10] Biodiesel can also be used in its pure form (B100), but may require certain engine modifications to avoid maintenance and performance problems.[11] Blending B100 with petroleum diesel may be accomplished by:
- Mixing in tanks at manufacturing point prior to delivery to tanker truck
- Splash mixing in the tanker truck (adding specific percentages of biodiesel and petroleum diesel)
- In-line mixing, two components arrive at tanker truck simultaneously.
- Metered pump mixing, petroleum diesel and biodiesel meters are set to X total volume.
Technical standards
[edit]Biodiesel has a number of standards for its quality including European standard EN 14214, ASTM International D6751, and National Standard of Canada CAN/CGSB-3.524.
ASTM D6751 (American Society for Testing and Materials) details standards and specifications for biodiesels blended with middle distillate fuels. This specification standard specifies various test methods to be used in the determination of certain properties for biodiesel blends. Some of the tests mentioned include flash point and kinematic viscosity.[2]
Historical background
[edit]
Transesterification of a vegetable oil was conducted as early as 1853 by Patrick Duffy, four decades before the first diesel engine became functional.[12][13] Earlier processes for making lamp oil, were patented (1810, Prague) but not published in peer-reviewed publications. Rudolf Diesel's prime model, a single 10 ft (3.05 m) iron cylinder with a flywheel at its base, ran on its own power for the first time in Augsburg, Germany, on 10 August 1893 running on nothing but peanut oil. In remembrance of this event, 10 August has been declared "International Biodiesel Day".[14]
It is often reported that Diesel designed his engine to run on peanut oil, but this is not the case. Diesel stated in his published papers, "at the Paris Exhibition in 1900 (Exposition Universelle) there was shown by the Otto Company a small Diesel engine, which, at the request of the French government ran on arachide (earth-nut or pea-nut) oil (see biodiesel), and worked so smoothly that only a few people were aware of it. The engine was constructed for using mineral oil, and was then worked on vegetable oil without any alterations being made. The French Government at the time thought of testing the applicability to power production of the Arachide, or earth-nut, which grows in considerable quantities in their African colonies, and can easily be cultivated there." Diesel himself later conducted related tests and appeared supportive of the idea.[15] In a 1912 speech Diesel said, "the use of vegetable oils for engine fuels may seem insignificant today but such oils may become, in the course of time, as important as petroleum and the coal-tar products of the present time."
Despite the widespread use of petroleum-derived diesel fuels, interest in vegetable oils as fuels for internal combustion engines was reported in several countries during the 1920s and 30s and later during World War II. Belgium, France, Italy, the United Kingdom, Portugal, Germany, Brazil, Argentina, Japan and China were reported to have tested and used vegetable oils as diesel fuels during this time. Some operational problems were reported due to the high viscosity of vegetable oils compared to petroleum diesel fuel, which results in poor atomization of the fuel in the fuel spray and often leads to deposits and coking of the injectors, combustion chamber and valves. Attempts to overcome these problems included heating of the vegetable oil, blending it with petroleum-derived diesel fuel or ethanol, pyrolysis and cracking of the oils.
On 31 August 1937, Georges Chavanne of the University of Brussels (Belgium) was granted a patent for a "Procedure for the transformation of vegetable oils for their uses as fuels" (fr. "Procédé de Transformation d’Huiles Végétales en Vue de Leur Utilisation comme Carburants") Belgian Patent 422,877. This patent described the alcoholysis (often referred to as transesterification) of vegetable oils using ethanol (and mentions methanol) in order to separate the fatty acids from the glycerol by replacing the glycerol with short linear alcohols. This appears to be the first account of the production of what is known as "biodiesel" today.[16] This is similar (copy) to the patented methods used in the 18th century to make lamp-oil, and may be inspired by some old historical oil lamps, in some places.
More recently, in 1977, Brazilian scientist Expedito Parente invented and submitted for patent, the first industrial process for the production of biodiesel.[17] This process is classified as biodiesel by international norms, conferring a "standardized identity and quality. No other proposed biofuel has been validated by the motor industry."[18] As of 2010, Parente's company Tecbio is working with Boeing and NASA to certify bioquerosene (bio-kerosene), another product produced and patented by the Brazilian scientist.[19]
Research into the use of transesterified sunflower oil, and refining it to diesel fuel standards, was initiated in South Africa in 1979. By 1983, the process for producing fuel-quality, engine-tested biodiesel was completed and published internationally.[20] An Austrian company, Gaskoks, obtained the technology from the South African Agricultural Engineers; the company erected the first biodiesel pilot plant in November 1987, and the first industrial-scale plant in April 1989 (with a capacity of 30,000 tons of rapeseed per annum).
Throughout the 1990s, plants were opened in many European countries, including the Czech Republic, Germany and Sweden. France launched local production of biodiesel fuel (referred to as diester) from rapeseed oil, which is mixed into regular diesel fuel at a level of 5%, and into the diesel fuel used by some captive fleets (e.g. public transportation) at a level of 30%. Renault, Peugeot and other manufacturers have certified truck engines for use with up to that level of partial biodiesel; experiments with 50% biodiesel are underway. During the same period, nations in other parts of the world also saw local production of biodiesel starting up: by 1998, the Austrian Biofuels Institute had identified 21 countries with commercial biodiesel projects. 100% biodiesel is now available at many normal service stations across Europe.
Properties
[edit]The color of biodiesel ranges from clear to golden to dark brown, depending on the production method and the feedstock used to make the fuel. This also changes the resulting fuel properties.[21] In general, biodiesel is slightly miscible with water, has a high boiling point and low vapor pressure. The flash point of biodiesel can exceed 130 °C (266 °F),[22] significantly higher than that of petroleum diesel which may be as low as 52 °C (126 °F).[23][24] Biodiesel has a density around ~0.88 g/cm3, higher than petrodiesel (~0.85 g/cm3).[23][24]
The calorific value of biodiesel is about 37.27 MJ/kg.[25] This is 9% lower than regular Number 2 petrodiesel. Variations in biodiesel energy density is more dependent on the feedstock used than the production process. Still, these variations are less than for petrodiesel.[26] It has been claimed biodiesel gives better lubricity and more complete combustion thus increasing the engine energy output and partially compensating for the higher energy density of petrodiesel.[27]
Biodiesel also contains virtually no sulfur[28] and although lacking sulfur compounds that in petrodiesel provide much of the lubricity, it has promising lubricating properties and cetane ratings compared to low sulfur diesel fuels and often serves as an additive to ultra-low-sulfur diesel (ULSD) fuel to aid with lubrication.[29] Biodiesel Fuels with higher lubricity may increase the usable life of high-pressure fuel injection equipment that relies on the fuel for its lubrication. Depending on the engine, this might include high pressure injection pumps, pump injectors (also called unit injectors) and fuel injectors.

Applications
[edit]
Biodiesel can be used in pure form (B100) or may be blended with petroleum diesel at any concentration in most injection pump diesel engines. New extreme high-pressure (29,000 psi) common rail engines have strict factory limits of B5 or B20, depending on manufacturer.[30] Biodiesel has different solvent properties from petrodiesel, and will degrade natural rubber gaskets and hoses in vehicles (mostly vehicles manufactured before 1992), although these tend to wear out naturally and most likely will have already been replaced with FKM, which is nonreactive to biodiesel. Biodiesel has been known to break down deposits of residue in the fuel lines where petrodiesel has been used.[31] As a result, fuel filters may become clogged with particulates if a quick transition to pure biodiesel is made. Therefore, it is recommended to change the fuel filters on engines and heaters shortly after first switching to a biodiesel blend.[32]
Distribution
[edit]Since the passage of the Energy Policy Act of 2005, biodiesel use has been increasing in the United States.[33] In the UK, the Renewable Transport Fuel Obligation obliges suppliers to include 5% renewable fuel in all transport fuel sold in the UK by 2010. For road diesel, this effectively means 5% biodiesel (B5).
Vehicular use and manufacturer acceptance
[edit]In 2005, Chrysler (then part of DaimlerChrysler) released the Jeep Liberty CRD diesels from the factory into the European market with 5% biodiesel blends, indicating at least partial acceptance of biodiesel as an acceptable diesel fuel additive.[34] In 2007, DaimlerChrysler indicated its intention to increase warranty coverage to 20% biodiesel blends if biofuel quality in the United States can be standardized.[35]
The Volkswagen Group has released a statement indicating that several of its vehicles are compatible with B5 and B100 made from rape seed oil and compatible with the EN 14214 standard. The use of the specified biodiesel type in its cars will not void any warranty.[36]
Mercedes-Benz does not allow diesel fuels containing greater than 5% biodiesel (B5) due to concerns about "production shortcomings".[37] Any damages caused by the use of such non-approved fuels will not be covered by the Mercedes-Benz Limited Warranty.
Starting in 2004, the city of Halifax, Nova Scotia decided to update its bus system to allow the fleet of city buses to run entirely on a fish-oil based biodiesel. This caused the city some initial mechanical issues, but after several years of refining, the entire fleet had successfully been converted.[38][39][40]
In 2007, McDonald's of UK announced it would start producing biodiesel from the waste oil byproduct of its restaurants. This fuel would be used to run its fleet.[41]
The 2014 Chevy Cruze Clean Turbo Diesel, direct from the factory, will be rated for up to B20 (blend of 20% biodiesel / 80% regular diesel) biodiesel compatibility[42]
Railway usage
[edit]
British train operating company Virgin Trains West Coast claimed to have run the UK's first "biodiesel train", when a Class 220 was converted to run on 80% petrodiesel and 20% biodiesel.[43][44]
The British Royal Train on 15 September 2007 completed its first ever journey run on 100% biodiesel fuel supplied by Green Fuels Ltd. Prince Charles and Green Fuels managing director James Hygate were the first passengers on a train fueled entirely by biodiesel fuel. Since 2007, the Royal Train has operated successfully on B100 (100% biodiesel).[45] A government white paper also proposed converting large portions of the UK railways to biodiesel but the proposal was subsequently dropped in favour of further electrification.[46]
Similarly, a state-owned short-line railroad in Eastern Washington ran a test of a 25% biodiesel / 75% petrodiesel blend during the summer of 2008, purchasing fuel from a biodiesel producer sited along the railroad tracks.[47] The train will be powered by biodiesel made in part from canola grown in agricultural regions through which the short line runs.
Also in 2007, Disneyland began running the park trains on B98 (98% biodiesel). The program was discontinued in 2008 due to storage issues, but in January 2009, it was announced that the park would then be running all trains on biodiesel manufactured from its own used cooking oils. This is a change from running the trains on soy-based biodiesel.[48]
In 2007, the historic Mt. Washington Cog Railway added the first biodiesel locomotive to its all-steam locomotive fleet. The fleet has climbed up the western slopes of Mount Washington in New Hampshire since 1868 with a peak vertical climb of 37.4 degrees.[49]
In 2009, the Grand Canyon Railway started running engine 4960 on used cooking oil.
On 8 July 2014,[50] the then Indian Railway Minister D.V. Sadananda Gowda announced in Railway Budget that 5% bio-diesel will be used in Indian Railways' Diesel Engines.[51]
As a heating oil
[edit]Biodiesel can also be used as a heating fuel in domestic and commercial boilers, a mix of heating oil and biofuel which is standardized and taxed slightly differently from diesel fuel used for transportation. Bioheat fuel is a proprietary blend of biodiesel and traditional heating oil. Bioheat is a registered trademark of the National Biodiesel Board [NBB] and the National Oilheat Research Alliance [NORA] in the United States, and Columbia Fuels in Canada.[52] Heating biodiesel is available in various blends. ASTM 396 recognizes blends of up to 5 percent biodiesel as equivalent to pure petroleum heating oil. Blends of higher levels of up to 20% biofuel are used by many consumers. Research is underway to determine whether such blends affect performance.
Older furnaces may contain rubber parts that would be affected by biodiesel's solvent properties, but can otherwise burn biodiesel without any conversion required. Care must be taken, given that varnishes left behind by petrodiesel will be released and can clog pipes—fuel filtering and prompt filter replacement is required. Another approach is to start using biodiesel as a blend, and decreasing the petroleum proportion over time can allow the varnishes to come off more gradually and be less likely to clog. Due to biodiesel's strong solvent properties, the furnace is cleaned out and generally becomes more efficient.[53]
A law passed under Massachusetts Governor Deval Patrick requires all home heating diesel in that state to be 2% biofuel by July 1, 2010, and 5% biofuel by 2013.[54] New York City has passed a similar law.
Cleaning oil spills
[edit]With 80–90% of oil spill costs invested in shoreline cleanup, there is a search for more efficient and cost-effective methods to extract oil spills from the shorelines.[55] Biodiesel has displayed its capacity to significantly dissolve crude oil, depending on the source of the fatty acids. In a laboratory setting, oiled sediments that simulated polluted shorelines were sprayed with a single coat of biodiesel and exposed to simulated tides.[56] Biodiesel is an effective solvent to oil due to its methyl ester component, which considerably lowers the viscosity of the crude oil. Additionally, it has a higher buoyancy than crude oil, which later aids in its removal. As a result, 80% of oil was removed from cobble and fine sand, 50% in coarse sand, and 30% in gravel. Once the oil is liberated from the shoreline, the oil-biodiesel mixture is manually removed from the water surface with skimmers. Any remaining mixture is easily broken down due to the high biodegradability of biodiesel, and the increased surface area exposure of the mixture.
Biodiesel in generators
[edit]
In 2001, UC Riverside installed a 6-megawatt backup power system that is entirely fueled by biodiesel. Backup diesel-fueled generators allow companies to avoid damaging blackouts of critical operations at the expense of high pollution and emission rates. By using B100, these generators were able to essentially eliminate the byproducts that result in smog, ozone, and sulfur emissions.[57] The use of these generators in residential areas around schools, hospitals, and the general public result in substantial reductions in poisonous carbon monoxide and particulate matter.[58]
Effects
[edit]Fuel efficiency
[edit]The power output of biodiesel depends on its blend, quality, and load conditions under which the fuel is burnt. The thermal efficiency for example of B100 as compared to B20 will vary due to the differing energy content of the various blends. Thermal efficiency of a fuel is based in part on fuel characteristics such as: viscosity, specific density, and flash point; these characteristics will change as the blends as well as the quality of biodiesel varies. The American Society for Testing and Materials has set standards in order to judge the quality of a given fuel sample.[59]
One study found that the brake thermal efficiency of B40 was superior to traditional petroleum counterpart at higher compression ratios (this higher brake thermal efficiency was recorded at compression ratios of 21:1). It was noted that, as the compression ratios increased, the efficiency of all fuel types – as well as blends being tested – increased; though it was found that a blend of B40 was the most economical at a compression ratio of 21:1 over all other blends. The study implied that this increase in efficiency was due to fuel density, viscosity, and heating values of the fuels.[60]
Combustion
[edit]Fuel systems on some modern diesel engines were not designed to accommodate biodiesel, while many heavy duty engines are able to run with biodiesel blends up to B20.[6] Traditional direct injection fuel systems operate at roughly 3,000 psi at the injector tip while the modern common rail fuel system operates upwards of 30,000 PSI at the injector tip. Components are designed to operate at a great temperature range, from below freezing to over 1,000 °F (560 °C). Diesel fuel is expected to burn efficiently and produce as few emissions as possible. As emission standards are being introduced to diesel engines the need to control harmful emissions is being designed into the parameters of diesel engine fuel systems. The traditional inline injection system is more forgiving to poorer quality fuels as opposed to the common rail fuel system. The higher pressures and tighter tolerances of the common rail system allows for greater control over atomization and injection timing. This control of atomization as well as combustion allows for greater efficiency of modern diesel engines as well as greater control over emissions. Components within a diesel fuel system interact with the fuel in a way to ensure efficient operation of the fuel system and so the engine. If an out-of-specification fuel is introduced to a system that has specific parameters of operation, then the integrity of the overall fuel system may be compromised. Some of these parameters such as spray pattern and atomization are directly related to injection timing.[61]
One study found that during atomization, biodiesel and its blends produced droplets greater in diameter than the droplets produced by traditional petrodiesel. The smaller droplets were attributed to the lower viscosity and surface tension of traditional diesel fuel. It was found that droplets at the periphery of the spray pattern were larger in diameter than the droplets at the center. This was attributed to the faster pressure drop at the edge of the spray pattern; there was a proportional relationship between the droplet size and the distance from the injector tip. It was found that B100 had the greatest spray penetration, this was attributed to the greater density of B100.[62] Having a greater droplet size can lead to inefficiencies in the combustion, increased emissions, and decreased horse power. In another study it was found that there is a short injection delay when injecting biodiesel. This injection delay was attributed to the greater viscosity of Biodiesel. It was noted that the higher viscosity and the greater cetane rating of biodiesel over traditional petrodiesel lead to poor atomization, as well as mixture penetration with air during the ignition delay period.[63] Another study noted that this ignition delay may aid in a decrease of NOx emission.[64]
Emissions
[edit]Emissions are inherent to the combustion of diesel fuels that are regulated by the U.S. Environmental Protection Agency (E.P.A.). As these emissions are a byproduct of the combustion process, in order to ensure E.P.A. compliance a fuel system must be capable of controlling the combustion of fuels as well as the mitigation of emissions. There are a number of new technologies being phased in to control the production of diesel emissions. The exhaust gas recirculation system, E.G.R., and the diesel particulate filter, D.P.F., are both designed to mitigate the production of harmful emissions.[65]
The feedstock used to make the biodiesel fuel can significantly alter the resulting exhaust gas and particulate matter emissions,[66][67] even when blended with commercial diesel fuel.[68] A study performed by the Chonbuk National University concluded that a B30 biodiesel blend reduced carbon monoxide emissions by approximately 83% and particulate matter emissions by roughly 33%. NOx emissions, however, were found to increase without the application of an E.G.R. system. The study also concluded that, with E.G.R, a B20 biodiesel blend considerably reduced the emissions of the engine.[69] Additionally, analysis by the California Air Resources Board found that biodiesel had the lowest carbon emissions of the fuels tested, those being ultra-low-sulfur diesel, gasoline, corn-based ethanol, compressed natural gas, and five types of biodiesel from varying feedstocks. Their conclusions also showed great variance in carbon emissions of biodiesel based on the feedstock used. Of soy, tallow, canola, corn, and used cooking oil, soy showed the highest carbon emissions, while used cooking oil produced the lowest.[70]
While studying the effect of biodiesel on diesel particulate filters, it was found that though the presence of sodium and potassium carbonates aided in the catalytic conversion of ash, as the diesel particulates are catalyzed, they may congregate inside the D.P.F. and so interfere with the clearances of the filter.[clarification needed] This may cause the filter to clog and interfere with the regeneration process.[71] In a study on the impact of E.G.R. rates with blends of jathropa biodiesel it was shown that there was a decrease in fuel efficiency and torque output due to the use of biodiesel on a diesel engine designed with an E.G.R. system. It was found that CO and CO2 emissions increased with an increase in exhaust gas recirculation but NOx levels decreased. The opacity level of the jathropa blends was in an acceptable range, where traditional diesel was out of acceptable standards. It was shown that a decrease in Nox emissions could be obtained with an E.G.R. system. This study showed an advantage over traditional diesel within a certain operating range of the E.G.R. system.[72]
As of 2017, blended biodiesel fuels (especially B5, B8, and B20) are regularly used in many heavy-duty vehicles, especially transit buses in US cities. Characterization of exhaust emissions showed significant emission reductions compared to regular diesel.[6]
Material compatibility
[edit]- Plastics: High-density polyethylene (HDPE) is compatible but polyvinyl chloride (PVC) is slowly degraded.[8] Polystyrene is dissolved on contact with biodiesel.
- Metals: Biodiesel (like methanol) has an effect on copper-based materials (e.g. brass), and it also affects zinc, tin, lead, and cast iron.[8] Stainless steels (316 and 304) and aluminum are unaffected.
- Rubber: Biodiesel also affects types of natural rubbers found in some older engine components. Studies have also found that fluorinated elastomers (FKM) cured with peroxide and base-metal oxides can be degraded when biodiesel loses its stability caused by oxidation. Commonly used synthetic rubbers FKM- GBL-S and FKM- GF-S found in modern vehicles were found to handle biodiesel in all conditions.[73]
Production
[edit]
Biodiesel is commonly produced by the transesterification of the vegetable oil or animal fat feedstock, and other non-edible raw materials such as frying oil, etc. There are several methods for carrying out this transesterification reaction including the common batch process, heterogeneous catalysts,[74] supercritical processes, ultrasonic methods, and even microwave methods.
Chemically, transesterified biodiesel comprises a mix of mono-alkyl esters of long chain fatty acids. The most common form uses methanol (converted to sodium methoxide) to produce methyl esters (commonly referred to as Fatty Acid Methyl Ester – FAME) as it is the cheapest alcohol available, though ethanol can be used to produce an ethyl ester (commonly referred to as Fatty Acid Ethyl Ester – FAEE) biodiesel and higher alcohols such as isopropanol and butanol have also been used. Using alcohols of higher molecular weights improves the cold flow properties of the resulting ester, at the cost of a less efficient transesterification reaction. A lipid transesterification production process is used to convert the base oil to the desired esters. Any free fatty acids (FFAs) in the base oil are either converted to soap and removed from the process, or they are esterified (yielding more biodiesel) using an acidic catalyst. After this processing, unlike straight vegetable oil, biodiesel has combustion properties very similar to those of petroleum diesel, and can replace it in most current uses.
The methanol used in most biodiesel production processes is made using fossil fuel inputs. However, there are sources of renewable methanol made using carbon dioxide or biomass as feedstock, making their production processes free of fossil fuels.[75]
A by-product of the transesterification process is the production of glycerol. For every 1 tonne of biodiesel that is manufactured, 100 kg of glycerol are produced. Originally, there was a valuable market for the glycerol, which assisted the economics of the process as a whole. However, with the increase in global biodiesel production, the market price for this crude glycerol (containing 20% water and catalyst residues) has crashed. Research is being conducted globally to use this glycerol as a chemical building block (see chemical intermediate under Wikipedia article "Glycerol"). One initiative in the UK is The Glycerol Challenge.[76]
Usually this crude glycerol has to be purified, typically by performing vacuum distillation. This is rather energy intensive. The refined glycerol (98%+ purity) can then be utilised directly, or converted into other products. The following announcements were made in 2007: A joint venture of Ashland Inc. and Cargill announced plans to make propylene glycol in Europe from glycerol[77] and Dow Chemical announced similar plans for North America.[78] Dow also plans to build a plant in China to make epichlorhydrin from glycerol.[79] Epichlorhydrin is a raw material for epoxy resins.
Global biodiesel production reached 3.8 million tons in 2005. Approximately 85% of biodiesel production came from the European Union.[citation needed][80]
Production levels
[edit]This section may contain an excessive amount of intricate detail that may interest only a particular audience. (January 2023) |
In 2007, biodiesel production capacity was growing rapidly, with an average annual growth rate from 2002 to 2006 of over 40%.[81] For the year 2006, the latest for which actual production figures could be obtained, total world biodiesel production was about 5–6 million tonnes, with 4.9 million tonnes processed in Europe (of which 2.7 million tonnes was from Germany) and most of the rest from the US. In 2008 production in Europe alone had risen to 7.8 million tonnes.[82] In July 2009, a duty was added to American imported biodiesel in the European Union in order to balance the competition from European, especially German producers.[83][84] The capacity for 2008 in Europe totalled 16 million tonnes. This compares with a total demand for diesel in the US and Europe of approximately 490 million tonnes (147 billion gallons).[85] Total world production of vegetable oil for all purposes in 2005–06 was about 110 million tonnes, with about 34 million tonnes each of palm oil and soybean oil.[86] As of 2018, Indonesia is the world's top supplier of palmoil-based biofuel with annual production of 3.5 million tons,[87][88] and expected to export about 1 million tonnes of biodiesel.[89]
US biodiesel production in 2011 brought the industry to a new milestone. Under the EPA Renewable Fuel Standard, targets have been implemented for the biodiesel production plants in order to monitor and document production levels in comparison to total demand. According to the year-end data released by the EPA, biodiesel production in 2011 reached more than 1 billion gallons. This production number far exceeded the 800 million gallon target set by the EPA. The projected production for 2020 is nearly 12 billion gallons.[90]
Biodiesel feedstocks
[edit]| Plant oils |
|---|
 |
| Types |
| Uses |
| Components |
A variety of oils can be used to produce biodiesel. These include:
- Virgin oil feedstock – rapeseed and soybean oils are most commonly used, soybean oil [6] accounting for about half of U.S. production.[91] It also can be obtained from Pongamia, field pennycress and jatropha and other crops such as mustard, jojoba, flax, sunflower, palm oil, coconut and hemp (see list of vegetable oils for biofuel for more information);
- Waste vegetable oil (WVO);
- Animal fats including tallow, lard, yellow grease, chicken fat,[92] and the by-products of the production of Omega-3 fatty acids from fish oil.
- Algae, which can be grown using waste materials such as sewage[93] and without displacing land currently used for food production.
- Oil from halophytes such as Salicornia bigelovii, which can be grown using saltwater in coastal areas where conventional crops cannot be grown, with yields equal to the yields of soybeans and other oilseeds grown using freshwater irrigation[94]
- Sewage Sludge – The sewage-to-biofuel field is attracting interest from major companies like Waste Management and startups like InfoSpi, which are betting that renewable sewage biodiesel can become competitive with petroleum diesel on price.[95]
Many advocates suggest that waste vegetable oil is the best source of oil to produce biodiesel, but since the available supply is drastically less than the amount of petroleum-based fuel that is burned for transportation and home heating in the world, this local solution could not scale to the current rate of consumption.
Animal fats are a by-product of meat production and cooking. Although it would not be efficient to raise animals (or catch fish) simply for their fat, use of the by-product adds value to the livestock industry (hogs, cattle, poultry). Today, multi-feedstock biodiesel facilities are producing high quality animal-fat based biodiesel.[96][97] Currently, a 5-million dollar plant is being built in the US, with the intent of producing 11.4 million litres (3 million gallons) biodiesel from some of the estimated 1 billion kg (2.2 billion pounds) of chicken fat[98] produced annually at the local Tyson poultry plant.[92] Similarly, some small-scale biodiesel factories use waste fish oil as feedstock.[99][100] An EU-funded project (ENERFISH) suggests that at a Vietnamese plant to produce biodiesel from catfish (basa, also known as pangasius), an output of 13 tons/day of biodiesel can be produced from 81 tons of fish waste (in turn resulting from 130 tons of fish). This project utilises the biodiesel to fuel a CHP unit in the fish processing plant, mainly to power the fish freezing plant.[101]
Quantity of feedstocks required
[edit]Current worldwide production of vegetable oil and animal fat is not sufficient to replace liquid fossil fuel use. Furthermore, some object to the vast amount of farming and the resulting fertilization, pesticide use, and land use conversion that would be needed to produce the additional vegetable oil.[102] The advantages of algae are that it can be grown on non-arable land such as deserts or in marine environments, and the potential oil yields are much higher than from plants.
Yield
[edit]Feedstock yield efficiency per unit area affects the feasibility of ramping up production to the huge industrial levels required to power a significant percentage of vehicles.
| Crop | Yield | |
|---|---|---|
| L/ha | US gal/acre | |
| Palm oil[n 1] | 4752 | 508 |
| Coconut | 2151 | 230 |
| Cyperus esculentus[n 2] | 1628 | 174 |
| Rapeseed[n 1] | 954 | 102 |
| Soy (Indiana)[103] | 554-922 | 59.2–98.6 |
| Chinese tallow[n 3][n 4] | 907 | 97 |
| Peanut[n 1] | 842 | 90 |
| Sunflower[n 1] | 767 | 82 |
| Hemp[citation needed] | 242 | 26 |
| ||
Algae fuel yields have not yet been accurately determined, but DOE is reported as saying that algae yield 30 times more energy per acre than land crops such as soybeans.[104] Yields of 36 tonnes/hectare are considered practical by Ami Ben-Amotz of the Institute of Oceanography in Haifa, who has been farming Algae commercially for over 20 years.[105]
Jatropha has been cited as a high-yield source of biodiesel but yields are highly dependent on climatic and soil conditions. The estimates at the low end put the yield at about 200 US gal/acre (1.5-2 tonnes per hectare) per crop; in more favorable climates two or more crops per year have been achieved.[106] It is grown in the Philippines, Mali and India, is drought-resistant, and can share space with other cash crops such as coffee, sugar, fruits and vegetables.[107] It is well-suited to semi-arid lands and can contribute to slow down desertification, according to its advocates.[108]
Efficiency and economic arguments
[edit]
Transitioning fully to biofuels could require immense tracts of land if traditional food crops are used (although non food crops can be utilized). The problem would be especially severe for nations with large economies, since energy consumption scales with economic output.[109]
For third world countries, biodiesel sources that use marginal land could make more sense; e.g., pongam oiltree nuts grown along roads or jatropha grown along rail lines.[110]
In tropical regions, such as Malaysia and Indonesia, plants that produce palm oil are being planted at a rapid pace to supply growing biodiesel demand in Europe and other markets. Scientists have shown that the removal of rainforest for palm plantations is not ecologically sound since the expansion of oil palm plantations poses a threat to natural rainforest and biodiversity.[111]
It has been estimated in Germany that palm oil diesel has less than one third of the production costs of rapeseed biodiesel.[112]
In the US, the production of biodiesel was reported in 2018 to support more than 64,000 jobs.[90] The growth in biodiesel also helps significantly increase GDP. In 2011, biodiesel created more than $3 billion in GDP.[113]
Energy security
[edit]One of the main drivers for adoption of biodiesel is energy security. This means that a nation's dependence on oil is reduced, and substituted with use of locally available sources, such as coal, gas, or renewable sources. Thus a country can benefit from adoption of biofuels, without a reduction in greenhouse gas emissions. While the total energy balance is debated, it is clear that the dependence on oil is reduced. One example is the energy used to manufacture fertilizers, which could come from a variety of sources other than petroleum. The US National Renewable Energy Laboratory (NREL) states that energy security is the number one driving force behind the US biofuels programme,[114] and a White House "Energy Security for the 21st Century" paper makes it clear that energy security is a major reason for promoting biodiesel.[115] The former EU commission president, Jose Manuel Barroso, speaking at a recent EU biofuels conference, stressed that properly managed biofuels have the potential to reinforce the EU's security of supply through diversification of energy sources.[116]
Global biofuel policies
[edit]Many countries around the world are involved in the growing use and production of biofuels, such as biodiesel, as an alternative energy source to fossil fuels and oil. To foster the biofuel industry, governments have implemented legislations and laws as incentives to reduce oil dependency and to increase the use of renewable energies.[117] Many countries have their own independent policies regarding the taxation and rebate of biodiesel use, import, and production.
Canada
[edit]It was required by the Canadian Environmental Protection Act Bill C-33 that by 2010, gasoline contained 5% renewable content and that by 2013, diesel and heating oil contained 2% renewable content.[117] The EcoENERGY for Biofuels Program subsidized the production of biodiesel, among other biofuels, via an incentive rate of CAN$0.20 per liter from 2008 to 2010. A decrease of $0.04 will be applied every year following, until the incentive rate reaches $0.06 in 2016. Individual provinces also have specific legislative measures in regards to biofuel use and production.[118]
United States
[edit]The Volumetric Ethanol Excise Tax Credit (VEETC) was the main source of financial support for biofuels, but was scheduled to expire in 2010. Through this act, biodiesel production guaranteed a tax credit of US$1 per gallon produced from virgin oils, and $0.50 per gallon made from recycled oils.[119] Currently soybean oil is being used to produce soybean biodiesel for many commercial purposes such as blending fuel for transportation sectors.[6]
European Union
[edit]The European Union is the greatest producer of biodiesel, with France and Germany being the top producers. To increase the use of biodiesel, there are policies requiring the blending of biodiesel into fuels, including penalties if those rates are not reached. In France, the goal was to reach 10% integration but plans for that stopped in 2010.[117] As an incentive for the European Union countries to continue the production of the biofuel, there are tax rebates for specific quotas of biofuel produced. In Germany, the minimum percentage of biodiesel in transport diesel is set at 7% so called "B7".
Malaysia
[edit]Malaysia plans to implement its nationwide adoption of the B20 palm oil biofuel programme by the end of 2022. The mandate to manufacture biofuel with a 20% palm oil component - known as B20 - for the transport sector was first rolled out in January 2020 but faced delays due to movement curbs imposed to contain coronavirus outbreaks.[120]
Issues and concerns
[edit]Food, land and water vs. fuel
[edit]Up to 40% of corn produced in the United States is used to make ethanol,[121] and worldwide 10% of all grain is turned into biofuel.[122] A 50% reduction in grain used for biofuels in the US and Europe would replace all of Ukraine's grain exports.[123]
In some poor countries the rising price of vegetable oil is causing problems.[124][125] Some propose that fuel only be made from non-edible vegetable oils such as camelina, jatropha or seashore mallow[126] which can thrive on marginal agricultural land where many trees and crops will not grow, or would produce only low yields.
Others argue that the problem is more fundamental. Farmers may switch from producing food crops to producing biofuel crops to make more money, even if the new crops are not edible.[127][128] The law of supply and demand predicts that if fewer farmers are producing food the price of food will rise. It may take some time, as farmers can take some time to change which things they are growing, but increasing demand for first generation biofuels is likely to result in price increases for many kinds of food. Some have pointed out that there are poor farmers and poor countries who are making more money because of the higher price of vegetable oil.[129]
Biodiesel from sea algae would not necessarily displace terrestrial land currently used for food production and new algaculture jobs could be created.
By comparison it should be mentioned that the production of biogas utilizes agricultural waste to generate a biofuel known as biogas, and also produces compost, thereby enhancing agriculture, sustainability and food production.
Environmental impact of biodiesel
[edit]This section's factual accuracy may be compromised due to out-of-date information. (October 2022) |

The surge of interest in biodiesels has highlighted a number of environmental effects associated with its use. These potentially include reductions in greenhouse gas emissions,[130] deforestation, pollution and the rate of biodegradation.
According to the Renewable Fuel Standards Program Regulatory Impact Analysis, released by the Environmental Protection Agency (EPA) of the United States in February 2010, biodiesel from soy oil results, on average, in a 57% reduction in greenhouse gases compared to petroleum diesel, and biodiesel produced from waste grease results in an 86% reduction. See chapter 2.6 of the EPA report for more detailed information.
However, environmental organizations, for example, Rainforest Rescue[131] and Greenpeace,[132] criticize the cultivation of plants used for biodiesel production, e.g., oil palms, soybeans and sugar cane. The deforestation of rainforests exacerbates climate change and sensitive ecosystems are destroyed to clear land for oil palm, soybean and sugar cane plantations. Moreover, that biofuels contribute to world hunger, since arable land is no longer used for growing foods. The Environmental Protection Agency published data in January 2012, showing that biofuels made from palm oil will not count towards the renewable fuels mandate of the United States as they are not climate-friendly.[133] Environmentalists welcome the conclusion because the growth of oil palm plantations has driven tropical deforestation, for example, in Indonesia and Malaysia.[133][134]
Indonesia produces biodiesel primarily from palm oil. Since agricultural land is limited, in order to plant monocultures of oil palms, land used for other cultivations or the tropical forest need to be cleared. A major environmental threat is then the destruction of rainforests in Indonesia.[135]
The environmental impact of biodiesel is diverse and not clearcut. An often mentioned incentive for using biodiesel is its capacity to lower greenhouse gas emissions compared to those of fossil fuels. Whether this is true or not depends on many factors.
Greenhouse gas emissions
[edit]
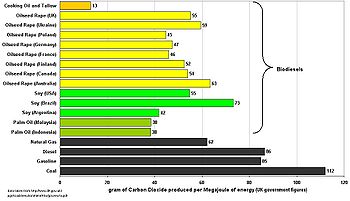
A general critique against biodiesel is the land use change, which have potential to cause even more emissions than what would be caused by using fossil fuels alone.[138] Yet this problem would be fixed with algal biofuel which can use land unsuitable for agriculture.
Carbon dioxide is one of the major greenhouse gases. Although the burning of biodiesel produces carbon dioxide emissions similar to those from ordinary fossil fuels, the plant feedstock used in the production absorbs carbon dioxide from the atmosphere when it grows. Plants absorb carbon dioxide through a process known as photosynthesis which allows it to store energy from sunlight in the form of sugars and starches. After the biomass is converted into biodiesel and burned as fuel the energy and carbon is released again. Some of that energy can be used to power an engine while the carbon dioxide is released back into the atmosphere.
When considering the total amount of greenhouse gas emissions it is therefore important to consider the whole production process and what indirect effects such production might cause. The effect on carbon dioxide emissions is highly dependent on production methods and the type of feedstock used. Calculating the carbon intensity of biofuels is a complex and inexact process, and is highly dependent on the assumptions made in the calculation. A calculation usually includes:
- Emissions from growing the feedstock (e.g. Petrochemicals used in fertilizers)
- Emissions from transporting the feedstock to the factory
- Emissions from processing the feedstock into biodiesel
- Absorption of CO2 Emissions from growing the feedstock
Other factors can be very significant but are sometimes not considered. These include:
- Emissions from the change in land use of the area where the fuel feedstock is grown.
- Emissions from transportation of the biodiesel from the factory to its point of use
- The efficiency of the biodiesel compared with standard diesel
- The amount of Carbon Dioxide produced at the tail pipe. (Biodiesel can produce 4.7% more)[citation needed]
- The benefits due to the production of useful by-products, such as cattle feed or glycerine
If land use change is not considered and assuming today's production methods, biodiesel from rapeseed and sunflower oil produce 45%-65% lower greenhouse gas emissions than petrodiesel.[139][140][141][142] However, there is ongoing research to improve the efficiency of the production process.[139][141] Biodiesel produced from used cooking oil or other waste fat could reduce CO2 emissions by as much as 85%.[136] As long as the feedstock is grown on existing cropland, land use change has little or no effect on greenhouse gas emissions. However, there is concern that increased feedstock production directly affects the rate of deforestation. Such clearcutting cause carbon stored in the forest, soil and peat layers to be released. The amount of greenhouse gas emissions from deforestation is so large that the benefits from lower emissions (caused by biodiesel use alone) would be negligible for hundreds of years.[136][138] Biofuel produced from feedstock such as palm oil could therefore cause much higher carbon dioxide emissions than some types of fossil fuels.[143]
Pollution
[edit]In the United States, biodiesel is the only alternative fuel to have successfully completed the Health Effects Testing requirements (Tier I and Tier II) of the Clean Air Act (1990).
Biodiesel can reduce the direct tailpipe-emission of particulates, small particles of solid combustion products, on vehicles with particulate filters by as much as 20 percent compared with low-sulfur (< 50 ppm) diesel. Particulate emissions as the result of production are reduced by around 50 percent compared with fossil-sourced diesel.[144]
Biodegradation
[edit]A University of Idaho study compared biodegradation rates of biodiesel, neat vegetable oils, biodiesel and petroleum diesel blends, and neat 2-D diesel fuel. Using low concentrations of the product to be degraded (10 ppm) in nutrient and sewage sludge amended solutions, they demonstrated that biodiesel degraded at the same rate as a dextrose control and 5 times as quickly as petroleum diesel over a period of 28 days, and that biodiesel blends doubled the rate of petroleum diesel degradation through co-metabolism.[145] The same study examined soil degradation using 10 000 ppm of biodiesel and petroleum diesel, and found biodiesel degraded at twice the rate of petroleum diesel in soil. In all cases, it was determined biodiesel also degraded more completely than petroleum diesel, which produced poorly degradable undetermined intermediates. Toxicity studies for the same project demonstrated no mortalities and few toxic effects on rats and rabbits with up to 5000 mg/kg of biodiesel. Petroleum diesel showed no mortalities at the same concentration either; however, toxic effects such as hair loss and urinary discolouring were noted with concentrations of >2000 mg/L in rabbits.:[146]
In aquatic environments
[edit]As biodiesel becomes more widely used, it is important to consider how consumption affects water quality and aquatic ecosystems. Research examining the biodegradability of different biodiesel fuels found that all of the biofuels studied (including Neat Rapeseed oil, Neat Soybean oil, and their modified ester products) were “readily biodegradable” compounds, and had a relatively high biodegradation rate in water.[147] Additionally, the presence of biodiesel can increase the rate of diesel biodegradation via co-metabolism. As the ratio of biodiesel is increased in biodiesel/diesel mixtures, the faster the diesel is degraded. Another study using controlled experimental conditions also showed that fatty acid methyl esters, the primary molecules in biodiesel, degraded much faster than petroleum diesel in sea water.[148]
Carbonyl emissions
[edit]When considering the emissions from fossil fuel and biofuel use, research typically focuses on major pollutants such as hydrocarbons. It is generally recognized that using biodiesel in place of diesel results in a substantial reduction in regulated gas emissions, but there has been a lack of information in research literature about the non-regulated compounds which also play a role in air pollution.[149] One study focused on the emissions of non-criteria carbonyl compounds from the burning of pure diesel and biodiesel blends in heavy-duty diesel engines. The results found that carbonyl emissions of formaldehyde, acetaldehyde, acrolein, acetone, propionaldehyde and butyraldehyde, were higher in biodiesel mixtures than emissions from pure diesel. Biodiesel use results in higher carbonyl emissions but lower total hydrocarbon emissions, which may be better as an alternative fuel source. Other studies have been done which conflict with these results, but comparisons are difficult to make due to various factors that differ between studies (such as types of fuel and engines used). In a paper which compared 12 research articles on carbonyl emissions from biodiesel fuel use, it found that 8 of the papers reported increased carbonyl compound emissions while 4 showed the opposite.[149] This is evidence that there is still much research required on these compounds.
Mechanical concerns
[edit]Engine wear
[edit]Lubricity of fuel plays an important role in wear that occurs in an engine. A diesel engine relies on its fuel to provide lubricity for the metal components that are constantly in contact with each other.[150] Biodiesel is a much better lubricant compared with fossil petroleum diesel due to the presence of esters. Tests have shown that the addition of a small amount of biodiesel to diesel can significantly increase the lubricity of the fuel in short term.[151] However, over a longer period of time[clarification needed] (2–4 years), studies show that biodiesel loses its lubricity.[152][failed verification] This could be because of enhanced corrosion over time due to oxidation of the unsaturated molecules or increased water content in biodiesel from moisture absorption.[58]
Fuel viscosity
[edit]One of the main concerns regarding biodiesel is its viscosity. The viscosity of diesel is 2.5–3.2 cSt at 40 °C and the viscosity of biodiesel made from soybean oil is between 4.2 and 4.6 cSt[153] The viscosity of diesel must be high enough to provide sufficient lubrication for the engine parts but low enough to flow at operational temperature. High viscosity can plug the fuel filter and injection system in engines.[153] Vegetable oil is composed of lipids with long chains of hydrocarbons, to reduce its viscosity the lipids are broken down into smaller molecules of esters. This is done by converting vegetable oil and animal fats into alkyl esters using transesterification to reduce their viscosity[154] Nevertheless, biodiesel viscosity remains higher than that of diesel, and the engine may not be able to use the fuel at low temperatures due to the slow flow through the fuel filter.[155]
Engine performance
[edit]Biodiesel has higher brake-specific fuel consumption compared to diesel, which means more biodiesel fuel consumption is required for the same torque. However, B20 biodiesel blend has been found to provide maximum increase in thermal efficiency, lowest brake-specific energy consumption, and lower harmful emissions.[6][58][150] The engine performance depends on the properties of the fuel, as well as on combustion, injector pressure and many other factors.[156] Since there are various blends of biodiesel, that may account for the contradicting reports as regards engine performance.
Exhaust emissions
[edit]The feedstock used to make the biodiesel alters the fuel’s properties by changing the average carbon chain length and number of double bonds present in the fatty acid methyl esters.[157]
Low temperature gelling
[edit]When biodiesel is cooled below a certain point, some of the molecules aggregate and form crystals. The fuel starts to appear cloudy once the crystals become larger than one quarter of the wavelengths of visible light – this is the cloud point (CP). As the fuel is cooled further these crystals become larger. The lowest temperature at which fuel can pass through a 45 micrometre filter is the cold filter plugging point (CFPP).[158] As biodiesel is cooled further it will gel and then solidify. Within Europe, there are differences in the CFPP requirements between countries. This is reflected in the different national standards of those countries. The temperature at which pure (B100) biodiesel starts to gel varies significantly and depends upon the mix of esters and therefore the feedstock oil used to produce the biodiesel. For example, biodiesel produced from low erucic acid varieties of canola seed (RME) starts to gel at approximately −10 °C (14 °F). Biodiesel produced from beef tallow and palm oil tends to gel at around 16 °C (61 °F) and 13 °C (55 °F) respectively.[159] There are a number of commercially available additives that will significantly lower the pour point and cold filter plugging point of pure biodiesel. Winter operation is also possible by blending biodiesel with other fuel oils including #2 low sulfur diesel fuel and #1 diesel / kerosene.
Another approach to facilitate the use of biodiesel in cold conditions is by employing a second fuel tank for biodiesel in addition to the standard diesel fuel tank. The second fuel tank can be insulated and a heating coil using engine coolant is run through the tank. The fuel tanks can be switched over when the fuel is sufficiently warm. A similar method can be used to operate diesel vehicles using straight vegetable oil.
Contamination by water
[edit]Biodiesel may contain small but problematic quantities of water. Although it is only slightly miscible with water it is hygroscopic.[160] One of the reasons biodiesel can absorb water is the persistence of mono and diglycerides left over from an incomplete reaction. These molecules can act as an emulsifier, allowing water to mix with the biodiesel.[citation needed] In addition, there may be water that is residual to processing or resulting from storage tank condensation. The presence of water is a problem because:
- Water reduces the heat of fuel combustion, causing smoke, harder starting, and reduced power.
- Water causes corrosion of fuel system components (pumps, fuel lines, etc.)
- Microbes in water cause the paper-element filters in the system to rot and fail, causing failure of the fuel pump due to ingestion of large particles.
- Water freezes to form ice crystals that provide sites for nucleation, accelerating gelling of the fuel.
- Water causes pitting in pistons.
Previously, the amount of water contaminating biodiesel has been difficult to measure by taking samples, since water and oil separate. However, it is now possible to measure the water content using water-in-oil sensors.[161]
Water contamination is also a potential problem when using certain chemical catalysts involved in the production process, substantially reducing catalytic efficiency of base (high pH) catalysts such as potassium hydroxide. However, the super-critical methanol production methodology, whereby the transesterification process of oil feedstock and methanol is effectuated under high temperature and pressure, has been shown to be largely unaffected by the presence of water contamination during the production phase
Research
[edit]There was research into finding more suitable crops and improving oil yield. Other sources are possible including human fecal matter, with Ghana building its first "fecal sludge-fed biodiesel plant."[162]
Specially bred mustard varieties can produce reasonably high oil yields and are very useful in crop rotation with cereals, and have the added benefit that the meal leftover after the oil has been pressed out can act as an effective and biodegradable pesticide.[163]
The NFESC, with Santa Barbara-based Biodiesel Industries is working to develop biodiesel technologies for the US navy and military, one of the largest diesel fuel users in the world.[164]
A group of Spanish developers working for a company called Ecofasa announced a new biofuel made from trash. The fuel is created from general urban waste which is treated by bacteria to produce fatty acids, which can be used to make biodiesel.[165]
Another approach that does not require the use of chemical for the production involves the use of genetically modified microbes.[166][167]
Algal biodiesel
[edit]From 1978 to 1996, the U.S. NREL experimented with using algae as a biodiesel source in the "Aquatic Species Program".[114] A self-published article by Michael Briggs, at the UNH Biodiesel Group, offers estimates for the realistic replacement of all vehicular fuel with biodiesel by utilizing algae that have a natural oil content greater than 50%, which Briggs suggests can be grown on algae ponds at wastewater treatment plants.[168] This oil-rich algae can then be extracted from the system and processed into biodiesel, with the dried remainder further reprocessed to create ethanol.
The production of algae to harvest oil for biodiesel has not yet been undertaken on a commercial scale, but feasibility studies have been conducted to arrive at the above yield estimate. In addition to its projected high yield, algaculture — unlike crop-based biofuels — does not entail a decrease in food production, since it requires neither farmland nor fresh water. Many companies are pursuing algae bio-reactors for various purposes, including scaling up biodiesel production to commercial levels.[169][170] Biodiesel lipids could be extracted from wet algae using a simple and economical reaction in ionic liquids.[171]
Pongamia
[edit]Millettia pinnata, also known as the Pongam Oiltree or Pongamia, is a leguminous, oilseed-bearing tree that has been identified as a candidate for non-edible vegetable oil production.
Pongamia plantations for biodiesel production have a two-fold environmental benefit. The trees both store carbon and produce fuel oil. Pongamia grows on marginal land not fit for food crops and does not require nitrate fertilizers. The oil producing tree has the highest yield of oil producing plant (approximately 40% by weight of the seed is oil) while growing in malnourished soils with high levels of salt. It is becoming a main focus in a number of biodiesel research organizations.[172] The main advantages of Pongamia are a higher recovery and quality of oil than other crops and no direct competition with food crops. However, growth on marginal land can lead to lower oil yields which could cause competition with food crops for better soil.
Jatropha
[edit]
Several groups in various sectors are conducting research on Jatropha curcas, a poisonous shrub-like tree that produces seeds considered by many to be a viable source of biodiesel feedstock oil.[173] Much of this research focuses on improving the overall per acre oil yield of Jatropha through advancements in genetics, soil science, and horticultural practices.
SG Biofuels, a San Diego-based Jatropha developer, has used molecular breeding and biotechnology to produce elite hybrid seeds of Jatropha that show significant yield improvements over first generation varieties.[174] SG Biofuels also claims that additional benefits have arisen from such strains, including improved flowering synchronicity, higher resistance to pests and disease, and increased cold weather tolerance.[175]
Plant Research International, a department of the Wageningen University and Research Centre in the Netherlands, maintains an ongoing Jatropha Evaluation Project (JEP) that examines the feasibility of large scale Jatropha cultivation through field and laboratory experiments.[176]
The Center for Sustainable Energy Farming (CfSEF) is a Los Angeles-based non-profit research organization dedicated to Jatropha research in the areas of plant science, agronomy, and horticulture. Successful exploration of these disciplines is projected to increase Jatropha farm production yields by 200–300% in the next ten years.[177]
FOG from sewage
[edit]So-called fats, oils and grease (FOG), recovered from sewage can also be turned into biodiesel.[178]
Fungi
[edit]A group at the Russian Academy of Sciences in Moscow published a paper in 2008, stating that they had isolated large amounts of lipids from single-celled fungi and turned it into biodiesel in an economically efficient manner.[179]
The recent discovery of a variant of the fungus Gliocladium roseum points toward the production of so-called myco-diesel from cellulose. This organism was recently discovered in the rainforests of northern Patagonia and has the unique capability of converting cellulose into medium length hydrocarbons typically found in diesel fuel.[180]
Biodiesel from used coffee grounds
[edit]Researchers at the University of Nevada, Reno, have successfully produced biodiesel from oil derived from used coffee grounds. Their analysis of the used grounds showed a 10% to 15% oil content (by weight). Once the oil was extracted, it underwent conventional processing into biodiesel. It is estimated that finished biodiesel could be produced for about one US dollar per gallon. Further, it was reported that "the technique is not difficult" and that "there is so much coffee around that several hundred million gallons of biodiesel could potentially be made annually." However, even if all the coffee grounds in the world were used to make fuel, the amount produced would be less than 1 percent of the diesel used in the United States annually. "It won’t solve the world’s energy problem," Dr. Misra said of his work.[181]
Biodiesel to hydrogen-cell power
[edit]A microreactor has been developed to convert biodiesel into hydrogen steam to power fuel cells.[182]
Steam reforming, also known as fossil fuel reforming is a process which produces hydrogen gas from hydrocarbon fuels, most notably biodiesel due to its efficiency. A **microreactor**, or reformer, is the processing device in which water vapour reacts with the liquid fuel under high temperature and pressure. Under temperatures ranging from 700 – 1100 °C, a nickel-based catalyst enables the production of carbon monoxide and hydrogen:[183]
Hydrocarbon + H
2O ⇌ CO + 3H
2 (Highly endothermic)
Furthermore, a higher yield of hydrogen gas can be harnessed by further oxidizing carbon monoxide to produce more hydrogen and carbon dioxide:
CO + H
2O → CO2 + H
2 (Mildly exothermic)
Safflower oil
[edit]As of 2020[update], researchers at Australia's CSIRO have been studying safflower oil from a specially-bred variety as an engine lubricant, and researchers at Montana State University's Advanced Fuel Centre in the US have been studying the oil's performance in a large diesel engine, with results described as a "game-changer".[184]
See also
[edit]- Civic amenity site; collection point for WVO
- EcoJet concept car
- Food, Conservation, and Energy Act of 2008
- Fuel (film)
- Gasoline gallon equivalent
- Indirect land use change impacts of biofuels
- MY Ady Gil
- Sustainable biofuel
- Table of biodiesel crop yields
- Tonne of oil equivalent
- United States vs. Imperial Petroleum
- Vegetable oils as alternative energy
- Vegetable oil fuel
- Issues relating to biofuels
- Low-carbon economy
References
[edit]- ^ Murzin, Dmitry Yu.; Mäki-Arvela, Päivi; Simakova, Irina L. (2012). "Triglycerides and Oils for Biofuels". Kirk-Othmer Encyclopedia of Chemical Technology. pp. 1–14. doi:10.1002/0471238961.trigmurz.a01. ISBN 978-0-471-48494-3.
- ^ Paisley, Mark A. (2003). "Biomass Energy". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0621051211120119.a01.pub2. ISBN 978-0-471-48494-3.
- ^ Huang, Daming; Zhou, Haining; Lin, Lin (2012). "Biodiesel: an Alternative to Conventional Fuel". Energy Procedia. 16 (Part C): 1874–1885. doi:10.1016/j.egypro.2012.01.287.
- ^ Demirbaş, Ayhan (2002-11-01). "Biodiesel from vegetable oils via transesterification in supercritical methanol". Energy Conversion and Management. 43 (17): 2349–2356. doi:10.1016/S0196-8904(01)00170-4. ISSN 0196-8904.
- ^ "Biodiesel Basics" (?). National Biodiesel Board. Archived from the original on 2014-08-04. Retrieved 2013-01-29.
- ^ a b c d e f g Omidvarborna; et al. (December 2014). "Characterization of particulate matter emitted from transit buses fueled with B20 in idle modes". Journal of Environmental Chemical Engineering. 2 (4): 2335–2342. doi:10.1016/j.jece.2014.09.020.
- ^ "Nylund.N-O & Koponen.K. 2013. Fuel and Technology Alternatives for Buses. Overall Energy Efficiency and Emission Performance. IEA Bioenergy Task 46" (PDF). Archived (PDF) from the original on 2020-02-16. Retrieved 2021-04-18.
- ^ a b c "Biodiesel Basics - Biodiesel.org". biodiesel.org. 2012. Archived from the original on August 4, 2014. Retrieved May 5, 2012.
- ^ "Biodiesel Handling and Use Guide, Fourth Edition" (PDF). National Renewable Energy Laboratory. Archived from the original (PDF) on 2011-11-10. Retrieved 2011-02-13.
- ^ "American Society for Testing and Materials". ASTM International. Archived from the original on 2019-12-08. Retrieved 2011-02-13.
- ^ "Biodiesel Handling and Use Guide" (PDF). nrel.gov. 2009. Archived (PDF) from the original on April 28, 2011. Retrieved December 21, 2011.
- ^ Duffy, Patrick (1853). "XXV. On the constitution of stearine". Quarterly Journal of the Chemical Society of London. 5 (4): 303. doi:10.1039/QJ8530500303. Archived from the original on 2020-07-26. Retrieved 2019-07-05.
- ^ Rob (1898). "Über partielle Verseifung von Ölen und Fetten II". Zeitschrift für Angewandte Chemie. 11 (30): 697–702. Bibcode:1898AngCh..11..697H. doi:10.1002/ange.18980113003. Archived from the original on 2020-07-26. Retrieved 2019-07-05.
- ^ "Biodiesel Day". Days Of The Year. Archived from the original on 25 February 2021. Retrieved 30 May 2015.
- ^ The Biodiesel Handbook, Chapter 2 – The History of Vegetable Oil Based Diesel Fuels, by Gerhard Knothe, ISBN 978-1-893997-79-0
- ^ Knothe, G. "Historical Perspectives on Vegetable Oil-Based Diesel Fuels" (PDF). INFORM, Vol. 12(11), p. 1103-1107 (2001). Archived (PDF) from the original on 2018-10-04. Retrieved 2007-07-11.
- ^ "Lipofuels: Biodiesel and Biokerosene" (PDF). www.nist.gov. Archived (PDF) from the original on 2009-03-18. Retrieved 2009-03-09.
- ^ What is it? (biodiesel) Quote from Tecbio website. Archived October 20, 2007, at the Wayback Machine
- ^ "O Globo newspaper interview in Portuguese". Defesanet.com.br. Archived from the original on 2010-10-29. Retrieved 2010-03-15.
- ^ SAE Technical Paper series no. 831356. SAE International Off Highway Meeting, Milwaukee, Wisconsin, USA, 1983
- ^ "The Effect of Biodiesel Composition on Engine Emissions from a DDC Series 60 Diesel Engine" (PDF). Retrieved 2022-12-13.
- ^ "Generic biodiesel material safety data sheet (MSDS)" (PDF). Archived (PDF) from the original on 2009-12-22. Retrieved 2010-03-15.
- ^ a b "MSDS ID NO.: 0301MAR019" (PDF). Marathon Petroleum. 7 December 2010. pp. 5, 7. Archived from the original (PDF) on 2017-12-22. Retrieved 22 December 2017.
- ^ a b "Safety Data Sheet - CITGO No. 2 Diesel Fuel, Low Sulfur, All Grades" (PDF). CITGO. 29 July 2015. p. 7. Archived (PDF) from the original on 16 October 2015. Retrieved 22 December 2017.
- ^ Carbon and Energy Balances for a Range of Biofuels Options Sheffield Hallam University
- ^ National Biodiesel Board (October 2005). Energy Content (PDF). Jefferson City, USA. p. 1. Archived from the original (PDF) on 2013-09-27. Retrieved 2013-09-24.
- ^ UNH Biodiesel Group Archived September 6, 2004, at the Wayback Machine
- ^ "E48_MacDonald.pdf (application/pdf Object)" (PDF). astm.org. 2011. Archived (PDF) from the original on November 20, 2012. Retrieved May 3, 2012.
- ^ "Lubricity Benefits" (PDF). National Biodiesel Board. Archived (PDF) from the original on 2017-08-09. Retrieved 2017-12-22.
- ^ "OEM Statement Summary Chart Archived 2016-04-07 at the Library of Congress Web Archives." Biodiesel.org. National Biodiesel Board, 1 Dec. 2014. Web. 19 Nov. 2015.
- ^ McCormick, R.L. "2006 Biodiesel Handling and Use Guide Third Edition" (PDF). Archived from the original (PDF) on 2006-12-16. Retrieved 2006-12-18.
- ^ "US EPA Biodiesel Factsheet". 2016-03-03. Archived from the original on July 26, 2008.
- ^ "Twenty In Ten: Strengthening America's Energy Security". Whitehouse.gov. Archived from the original on 2009-09-06. Retrieved 2008-09-10.
- ^ Kemp, William. Biodiesel: Basics and Beyond. Canada: Aztext Press, 2006.
- ^ "National Biodiesel Board, 2007. Chrysler Supports Biodiesel Industry; Encourages Farmers, Refiners, Retailers and Customers to Drive New Diesels Running on Renewable Fuel". Nbb.grassroots.com. 2007-09-24. Archived from the original on 2010-03-06. Retrieved 2010-03-15.
- ^ "Biodiesel statement" (PDF). Volkswagen.co.uk. Archived (PDF) from the original on 2011-09-27. Retrieved 2011-08-04.
- ^ Mercedes-Benz (2010). "Biodiesel Information for Passenger Cars" (PDF). mbusa.com. Archived from the original (PDF) on October 28, 2012. Retrieved September 11, 2012.
- ^ "Halifax City Buses to Run on Biodiesel Again | Biodiesel and Ethanol Investing". Biodieselinvesting.com. 2006-08-31. Archived from the original on 2006-10-18. Retrieved 2009-10-17.
- ^ "Biodiesel". Halifax.ca. Archived from the original on 2010-12-24. Retrieved 2009-10-17.
- ^ "Halifax Transit". Halifax.ca. 2004-10-12. Archived from the original on 2014-08-14. Retrieved 2013-12-04.
- ^ "McDonald's bolsters "green" credentials with recycled biodiesel oil". News.mongabay.com. 2007-07-09. Archived from the original on 2012-07-15. Retrieved 2009-10-17.
- ^ "Cruze Clean Turbo Diesel Delivers Efficient Performance". 2013-02-07. Archived from the original on 2013-08-10. Retrieved 2013-08-05.
- ^ "First UK biodiesel train launched". BBC. 2007-06-07. Archived from the original on 2008-02-13. Retrieved 2007-11-17.
- ^ Virgin launches trials with Britain's first biofuel train Rail issue 568 20 June 2007 page 6
- ^ "EWS Railway – News Room". www.ews-railway.co.uk. Archived from the original on 2020-02-19. Retrieved 2009-06-12.
- ^ Great Britain. Parliament. House of Commons. Transport Committee (2008). Delivering a sustainable railway : a 30-year strategy for the railways? : tenth report of session 2007-08 : report, together with formal minutes, oral and written evidence. London: Stationery Office. ISBN 978-0-215-52222-1. OCLC 273500097. Archived from the original on 2021-07-31. Retrieved 2021-07-07.
- ^ Vestal, Shawn (2008-06-22). "Biodiesel will drive Eastern Wa. train during summerlong test". Seattle Times. Archived from the original on 2009-02-02. Retrieved 2009-03-01.
- ^ "Disneyland trains running on biodiesel - UPI.com". www.upi.com. Archived from the original on 2009-01-30. Retrieved 2009-03-16.
- ^ Kotrba, Ron (29 May 2013). "'Name that Biodiesel Train' contest". Biodiesel Magazine. Archived from the original on 8 May 2014. Retrieved 8 May 2014.
- ^ PTI (2014-07-08). "Railway Budget 2014–15: Highlights". The Hindu. Archived from the original on 2014-11-29. Retrieved 30 May 2015.
- ^ "Indian Railways to go for Bio-Diesel in a Big Way – Gowda". Archived from the original on 14 April 2015. Retrieved 30 May 2015.
- ^ "Environment, consumers win with Bioheat trademark victory". biodieselmagazine.com. 2011. Archived from the original on November 20, 2011. Retrieved October 27, 2011.
- ^ "The Massachusetts Bioheat Fuel Pilot Program" (PDF). June 2007. Archived (PDF) from the original on 2012-09-15. Retrieved 2012-12-31. Prepared for the Massachusetts Executive Office of Energy and Environmental Affairs
- ^ Massachusetts Oil Heat Council (27 February 2008). MA Oilheat Council Endorses BioHeat Mandate Archived May 11, 2008, at the Wayback Machine
- ^ French McCay, D.; Rowe, J. J.; Whittier, N.; Sankaranarayanan, S.; Schmidt Etkin, D. (2004). "Estimation of potential impacts and natural resource damages of oil". J. Hazard. Mater. 107 (1–2): 11–25. doi:10.1016/j.jhazmat.2003.11.013. PMID 15036639.
- ^ Fernández-Ãlvarez, P.; Vila, J.; Garrido, J. M.; Grifoll, M.; Feijoo, G.; Lema, J. M. (2007). "Evaluation of biodiesel as bioremediation agent for the treatment of the shore affected by the heavy oil spill of the Prestige". J. Hazard. Mater. 147 (3): 914–922. doi:10.1016/j.jhazmat.2007.01.135. PMID 17360115.
- ^ National Biodiesel Board Electrical Generation. http://www.biodiesel.org/using-biodiesel/market-segments/electrical-generation Archived 2013-04-10 at the Wayback Machine (accessed 20 January 2013)
- ^ a b c Monyem, A.; Van Gerpen, J. (2001). "The effect of biodiesel oxidation on engine performance and emissions". Biomass Bioenergy. 20 (4): 317–325. Bibcode:2001BmBe...20..317M. doi:10.1016/s0961-9534(00)00095-7. Archived from the original on 2018-01-09. Retrieved 2018-11-22.
- ^ ASTM Standard D6751-12, 2003, "Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels," ASTM International, West Conshohocken, PA, 2003, doi:10.1520/C0033-03, astm.org.
- ^ Muralidharan, K. K.; Vasudevan, D. D. (2011). "Performance, emission and combustion characteristics of a variable compression ratio engine using methyl esters of waste cooking oil and diesel blends". Applied Energy. 88 (11): 3959–3968. Bibcode:2011ApEn...88.3959M. doi:10.1016/j.apenergy.2011.04.014.
- ^ Roy, Murari Mohon (2009). "Effect of Fuel Injection Timing and Injection Pressure on Combustion and Odorous Emissions in DI Diesel Engines". Journal of Energy Resources Technology. 131 (3): 032201. doi:10.1115/1.3185346.
- ^ Chen, P.; Wang, W.; Roberts, W. L.; Fang, T. (2013). "Spray and atomization of diesel fuel and its alternatives from a single-hole injector using a common rail fuel injection system". Fuel. 103: 850–861. doi:10.1016/j.fuel.2012.08.013.
- ^ Hwang, J.; Qi, D.; Jung, Y.; Bae, C. (2014). "Effect of injection parameters on the combustion and emission characteristics in a common-rail direct injection diesel engine fueled with waste cooking oil biodiesel". Renewable Energy. 63: 639–17. doi:10.1016/j.renene.2013.08.051.
- ^ McCarthy, P. P.; Rasul, M. G.; Moazzem, S. S. (2011). "Analysis and comparison of performance and emissions of an internal combustion engine fuelled with petroleum diesel and different bio-diesels". Fuel. 90 (6): 2147–2157. doi:10.1016/j.fuel.2011.02.010.
- ^ United States Environmental Protection Agency. (2014, April 9). National Clean Diesel Campaign. Retrieved From the Environmental Protection Agency website: http://www.epa.gov/diesel/ Archived 2014-04-18 at the Wayback Machine
- ^ "The Effect of Biodiesel Composition on Engine Emissions from a DDC Series 60 Diesel Engine" (PDF). Retrieved 2022-12-13.
- ^ Landwehr, K.R.; Hillas, J.; Mead-Hunter, R.; Brooks, P.; King, A.; O'Leary, R.A. (2021). "Fuel feedstock determines biodiesel exhaust toxicity in a human airway epithelial cell exposure model". J. Hazard. Mater. 420: 126637. doi:10.1016/j.jhazmat.2021.126637. PMID 34329109.
- ^ Landwehr, K.R.; Hillas, J.; Mead-Hunter, R.; King, A.; O'Leary, R.A.; Kicic, A. (2023). "Biodiesel feedstock determines exhaust toxicity in 20% biodiesel: 80% mineral diesel blends". J. Chemosphere. 310: 136873. Bibcode:2023Chmsp.31036873L. doi:10.1016/j.chemosphere.2022.136873. hdl:20.500.11937/94726. PMID 36252896. S2CID 252938667.
- ^ Sam, Yoon Ki, et al. "Effects Of Canola Oil Biodiesel Fuel Blends On Combustion, Performance, And Emissions Reduction In A Common Rail Diesel Engine." Energies (19961073) 7.12 (2014): 8132–8149. Academic Search Complete. Web. 14 Nov. 2015.
- ^ Robinson, Jessica (September 28, 2015). "Nation's strictest regulatory board affirms biodiesel as lowest-carbon fuel". National Biodiesel Board. Archived from the original on August 30, 2017.
- ^ Hansen, B.; Jensen, A.; Jensen, P. (2013). "Performance of diesel particulate filter catalysts in the presence of biodiesel ash species" (PDF). Fuel. 106: 234–240. doi:10.1016/j.fuel.2012.11.038. S2CID 40883915.
- ^ Gomaa, M. M.; Alimin, A. J.; Kamarudin, K. A. (2011). "The effect of EGR rates on NOX and smoke emissions of an IDI diesel engine fuelled with Jatropha biodiesel blends". International Journal of Energy & Environment. 2 (3): 477–490.
- ^ Fluoroelastomer Compatibility with Biodiesel Fuels Archived 2014-10-06 at the Wayback Machine Eric W. Thomas, Robert E. Fuller and Kenji Terauchi DuPont Performance Elastomers L.L.C. January 2007
- ^ Hernández, M.R.; Reyes-Labarta, J.A. (2010). "Reyes-Labarta". Industrial & Engineering Chemistry Research. 49 (19): 9068–9076. doi:10.1021/ie100978m.
- ^ "Products". Carbon Recycling International. Archived from the original on 29 July 2013. Retrieved 13 July 2012.
- ^ "Biofuels and Glycerol". theglycerolchallenge.org. Archived from the original on 2008-05-23. Retrieved 2008-07-09.
- ^ Chemweek's Business Daily, Tuesday May 8, 2007
- ^ "Retrieved June 25, 2007". Dow.com. Archived from the original on 2009-09-16. Retrieved 2010-03-15.
- ^ "Retrieved June 25, 2007". Epoxy.dow.com. Archived from the original on 2009-09-16. Retrieved 2010-03-15.
- ^ Dasmohapatra, Gourkrishna. Engineering Chemistry I (WBUT), 3rd Edition. Vikas Publishing House. ISBN 9789325960039. Archived from the original on 2020-04-03. Retrieved 2017-01-13.
- ^ Martinot, Eric (2008). "Renewables 2007. Global Status Report" (PDF). REN21 - Renewable Energy Policy Network for the 21st Century. Archived (PDF) from the original on 2008-04-10. Retrieved 2008-04-03.
- ^ "Statistics. the EU biodiesel industry". European Biodiesel Board. 2008-03-28. Archived from the original on 2006-11-14. Retrieved 2008-04-03.
- ^ "US Biodiesel Taxed in EU". Hadden Industries. Archived from the original on 2009-10-11. Retrieved 2009-08-28.
- ^ "US Biodiesel Demand" (PDF). Biodiesel: The official site of the National Biodiesel Board. NBB. Archived (PDF) from the original on 2008-04-10. Retrieved 2008-04-03.
- ^ "Biodiesel to drive up the price of cooking oil". Biopower London. 2006. Archived from the original on 2008-06-07. Retrieved 2008-04-03.
- ^ "Major Commodities". FEDIOL (EU Oil and Proteinmeal Industry). Archived from the original on 2008-04-21. Retrieved 2008-04-08.
- ^ "Indonesia to boost biodiesel exports, Malaysia expects to lose market share". Reuters. Archived from the original on 31 August 2018. Retrieved 31 August 2018.
- ^ "Indonesian biodiesel production seen jumping to 3.5 million tonnes this year". 12 March 2018. Archived from the original on 31 August 2018. Retrieved 31 August 2018.
- ^ "Indonesia's 2018 biodiesel exports seen at around 1 mln tonnes - assoc". Reuters. Archived from the original on 30 August 2018. Retrieved 31 August 2018.
- ^ a b National Biodiesel Board (2018). "U.S. biodiesel production". Archived from the original on 2020-04-03. Retrieved 2019-07-11.
- ^ U.S. Energy Information Administration. "Monthly Biodiesel Production Reports". U.S. Department of Energy. Archived from the original on 13 March 2013. Retrieved 27 February 2013.
- ^ a b Leonard, Christopher (2007-01-03). "Not a Tiger, but Maybe a Chicken in Your Tank". The Washington Post. Associated Press. p. D03. Archived from the original on 2012-11-04. Retrieved 2007-12-04.
- ^ Kiong, Errol (May 12, 2006). "NZ firm makes bio-diesel from sewage in world first". The New Zealand Herald. Archived from the original on June 2, 2006. Retrieved 2007-01-10.
- ^ Glenn, Edward P.; Brown, J. Jed; O'Leary, James W. (August 1998). "Irrigating Crops with Seawater" (PDF). Scientific American. 279 (August 1998): 76–81 [79]. Bibcode:1998SciAm.279b..76G. doi:10.1038/scientificamerican0898-76. Archived (PDF) from the original on 2015-09-06. Retrieved 2008-11-17.
- ^ Casey, Tina (May 2010). "The Smell of Change is in the Air with Renewable Biodiesel from Sewage". Scientific American.
- ^ "Monthly US Raw Material Usage for US Biodiesel Production 2007-2009" (PDF). assets.nationalrenderers.org. 2010. Archived (PDF) from the original on October 19, 2012. Retrieved March 23, 2012.
- ^ O'Connell, Deborah (2008). "Biofuels in Australia: Issues and Prospects. A report for the Rural Industries Research and Development Corporation" (PDF). bioenergy.org.nz. Archived from the original (PDF) on 3 May 2012. Retrieved 23 March 2012.
- ^ "Biodiesel from Animal Fat". E85.whipnet.net. Archived from the original on 2021-01-23. Retrieved 2021-01-16.
- ^ "Biodiesel produced from "tra", "basa" catfish oil". governmental site. Archived from the original on October 4, 2006. Retrieved 2008-05-25.
- ^ "Demonstrating the value of a fishy biodiesel blend in Alaska's Aleutian Islands" (PDF). Biodiesel america. Archived from the original (PDF) on February 2, 2007. Retrieved 2008-05-25.
- ^ "Enerfish integrated energy solutions for seafood processing stations". VTT, Finland/Enerfish Consortium. Archived from the original on 2009-10-22. Retrieved 2009-10-20.
- ^ [1][dead link]
- ^ "Purdue report ID-337" (PDF). purdue.edu. Archived from the original (PDF) on 1 March 2012. Retrieved 9 July 2017.
- ^ "DOE quoted by Washington Post in "A Promising Oil Alternative: Algae Energy"". Washingtonpost.com. 2008-01-06. Archived from the original on 2011-05-14. Retrieved 2010-03-15.
- ^ Strahan, David (13 August 2008). "Green Fuel for the Airline Industry". New Scientist. 199 (2669): 34–37. doi:10.1016/S0262-4079(08)62067-9. Archived from the original on 2021-07-31. Retrieved 2008-09-23.
- ^ "India's jatropha plant biodiesel yield termed wildly exaggerated". Findarticles.com. 2003-08-18. Archived from the original on 2009-10-02. Retrieved 2010-03-15.
- ^ "Jatropha for biodiesel". Reuk.co.uk. Archived from the original on 2009-09-04. Retrieved 2010-03-15.
- ^ Weed's biofuel potential sparks African land grab, Washington Times, February 21, 2007, Karen Palmer
- ^ "Looking Forward: Energy and the Economy" (PDF). Archived from the original (PDF) on 2006-03-10. Retrieved 2006-08-29.
- ^ "Hands On: Power Pods – India". Archived from the original on 2012-04-26. Retrieved 2005-10-24.
- ^ Wilcove, David S.; Koh, Lian Pin (2010). "Addressing the threats to biodiversity from oil-palm agriculture". Biodiversity and Conservation. 19 (4): 999–1007. Bibcode:2010BiCon..19..999W. doi:10.1007/s10531-009-9760-x. S2CID 10728423.
- ^ "Palm Oil Based Biodiesel Has Higher Chances Of Survival". Archived from the original on 2007-09-29. Retrieved 2006-12-20.
- ^ Evans, Ben (December 27, 2011). "National Biodiesel Board Statement on EPA Renewable Fuels Rule". Archived from the original on 2020-04-03. Retrieved 2012-04-10.
- ^ a b Sheehan, John; Dunahay, Terri; Benemann, John; Roessler, Paul (July 1998). "A look back at the U.S. Department of Energy's Aquatic Species Program: Biodiesel from Algae" (PDF (3.7 Mb)). Close-out Report. United States Department of Energy. Archived (PDF) from the original on 2020-04-23. Retrieved 2007-01-02.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "Energy Security for the 21st Century". The White House. 2008-03-05. Archived from the original on 2019-09-14. Retrieved 2008-04-15.
- ^ "International Biofuels Conference". HGCA. Archived from the original on 2008-12-11. Retrieved 2008-04-15.
- ^ a b c Sorda, G.; Banse, M.; Kemfert, C. (2010). "An Overview of Biofuel Policies Across the World". Energy Policy. 38 (11): 6977–6988. doi:10.1016/j.enpol.2010.06.066.
- ^ Dessureault, D., 2009. Canada Biofuels Annual. USDA Foreign Agricultural Service, GAIN Report Number CA9037, approved by U.S. Embassy, 30.06.2009
- ^ Kuplow, D. Biofuels – At What Cost? Government support for ethanol and biodiesel in the United States. Cambridge, MA, 2007
- ^ "Malaysia aims to fully implement B20 biodiesel mandate by year-end". Reuters. 2022-01-05. Retrieved 2022-01-05.
- ^ "Food vs fuel: Ukraine war sharpens debate on use of crops for energy". Financial Times. 12 June 2022.
- ^ "Guest view: Global hunger fight means no biofuel". Reuters. 6 June 2022.
- ^ "Cutting biofuels can help avoid global food shock from Ukraine war". New Scientist. 14 March 2022.
- ^ "Biofuel demand makes fried food expensive in Indonesia – ABC News (Australian Broadcasting Corporation)". Abc.net.au. 2007-07-19. Archived from the original on 2011-03-20. Retrieved 2010-03-15.
- ^ "Breaking News, World News & Multimedia". The New York Times. Archived from the original on 14 February 2008. Retrieved 9 July 2017.
- ^ "Biodiesel Brings a Lot to the Table" (PDF). April 2008. Archived from the original (PDF) on 2012-02-12. Retrieved 30 May 2015.
- ^ Swanepoel, Esmarie. "Food versus fuel debate escalates". Engineeringnews.co.za. Archived from the original on 2008-03-24. Retrieved 2010-03-15.
- ^ Brown, Lester. "How Food and Fuel Compete for Land by Lester Brown – The Globalist > > Global Energy". The Globalist. Archived from the original on 2010-01-12. Retrieved 2010-03-15.
- ^ "The End Of Cheap Food". The Economist. 2007-12-06. Archived from the original on 2018-08-26. Retrieved 2008-02-29.
- ^
"Biodiesel – Just the Basics" (PDF). Final. United States Department of Energy. 2003. Archived from the original (PDF) on 2007-09-18. Retrieved 2007-08-24.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "Achievement – Biofuel: Shell backs out of indigenous territory – Rainforest Rescue". Archived from the original on 31 May 2015. Retrieved 30 May 2015.
- ^ "End of the road for dirty biofuels". Greenpeace International. Archived from the original on 3 April 2020. Retrieved 30 May 2015.
- ^ a b "Palm oil does not meet U.S. renewable fuels standard, rules EPA". Mongabay. 2012-01-27. Archived from the original on 2015-05-30. Retrieved 30 May 2015.
- ^ "EPA: Palm oil flunks the climate test". TheHill. 2012-01-26. Archived from the original on 2013-06-05. Retrieved 30 May 2015.
- ^ "Indonesia's biodiesel drive is leading to deforestation". BBC News. 8 December 2021.
- ^ a b c "Carbon and Sustainability Reporting Within the Renewable Transport Fuel Obligation" (PDF). UK Department for Transport. January 2008. Archived from the original (PDF 1.41 MB) on 2008-04-10. Retrieved 2008-04-29.
- ^ Graph derived from information found in UK government document.Carbon and Sustainability Reporting Within the Renewable Transport Fuel Obligation Archived June 25, 2008, at the Wayback Machine
- ^ a b Fargione, Joseph; Jason Hill; David Tilman; Stephen Polasky; Peter Hawthorne (2008-02-29). "Land Clearing and the Biofuel Carbon Debt". Science. 319 (5867): 1235–8. Bibcode:2008Sci...319.1235F. doi:10.1126/science.1152747. PMID 18258862. S2CID 206510225. Archived from the original (fee required) on April 13, 2008. Retrieved 2008-04-29.
- "New Study Raises Major Questions on Biofuels" (Press release). The Nature Conservancy in Minnesota. 2008-02-07. Archived from the original on 2008-05-13. Retrieved 2008-04-29.
- ^ a b
Mortimer, N. D.; P. Cormack; M. A. Elsayed; R. E. Horne (January 2003). "Evaluation of the comparative energy, global warming and socio-economic costs and benefits of biodiesel" (PDF 763 KB). Sheffield Hallam University. UK Department for Environment, Food and Rural Affairs (DEFRA). Retrieved 2008-05-01.
- Summary: "Biodiesel Life Cycle Assessment". Retrieved 2008-05-01.
- ^ "Well-to-Wheels analysis of future automotive fuels and powertrains in the European context". Joint Research Centre (European Commission), EUCAR & CONCAWE. March 2007. Archived from the original on 2008-02-07. Retrieved 2008-05-01.
- ^ a b European Environment Agency. (2006). Transport and environment : facing a dilemma : TERM 2005: indicators tracking transport and environment in the European Union (PDF). Copenhagen: European Environment Agency; Luxembourg : Office for Official Publications of the European Communities. ISBN 92-9167-811-2. ISSN 1725-9177. Archived from the original (PDF 3.87 MB) on July 19, 2006. Retrieved 2008-05-01.
- ^ "Biodiesel". Energy Saving Trust. Archived from the original on 2020-06-22. Retrieved 2008-05-01.
[B]iodiesel is considered a renewable fuel. It gives a 60 per cent reduction in CO2 well to wheel
- ^ How the palm oil industry is cooking the climate (PDF). Greenpeace International. November 2007. Archived from the original (PDF 10.48 MB) on 2011-03-03. Retrieved 2008-04-30.
The main areas remaining for new extensive plantations are the large tracts of tropical peatlands – until recently virgin rainforest areas. Over 50% of new plantations are planned in these peatland areas
- ^ Beer et al. 2004.
- ^ "Biodegradability, BOD5, COD and Toxicity of Biodiesel Fuels" (PDF). National Biodiesel Education Program, University of Idaho. 2004-12-03. Archived from the original (PDF 64 KB) on April 10, 2008. Retrieved 2008-04-30.
- ^ "Biodiesel". solar navigator. Retrieved 2012-04-18.
- ^ Zhang, X.; Peterson, C. L.; Reece, D.; Moller, G.; Haws, R. Biodegradability of Biodiesel in the Aquatic Environment. ASABE 1998, 41(5), 1423-1430
- ^ DeMello, J. A.; Carmichael, C. A.; Peacock, E. E.; Nelson, R. K.; Arey, J. S.; Reddy, C. M. Biodegradation and Environmental Behavior of Biodiesel Mixtures in the Sea: An Initial Study. Marine Poll. Bull. 2007, 54, 894-904
- ^ a b He, C.; Ge, Y.; Tan, J.; You, K.; Han, X.; Wang, J.; You, Q.; Shah, A. N. Comparison of Carbonyl Compounds Emissions from Diesel Engine Fueled with Biodiesel and Diesel. Atmos. Environ. 2009, 43, 3657-3661
- ^ a b Fazal, M. A.; Haseeb, A. S. M.A.; Masiuki (2011). "An evaluation of material compatibility; performance; emission and engine durability". Renewable and Sustainable Energy Reviews. 15: 1314–1324. doi:10.1016/j.rser.2010.10.004.
- ^ Masjuki HH, Maleque MA. The effect of palm oil diesel fuel contaminated lubricant on sliding wear of cast irons against mild steel. Wear. 1996, 198, 293–9
- ^ Clark, S.J.; Wagner, L.; Schrock, M.D.; Piennaar, P.G. Methyl and ethyl soybean esters as renewable fuels for diesel engines. JAOCS. 1984, 61, 1632–8
- ^ a b Tat, M.E.; Van Gerpan, J.H. The Kinematic Viscosity of Biodiesel and its Blends with Diesel Fuel. JAOCS. 1999, 76, 1511–1513
- ^ Altin, R.; Cetinkaya, S.; Yucesu, H.S. (2001). "The potential of using vegetable oil fuels as fuel for diesel engines". Energy Conversion and Management. 42 (5): 529–538. doi:10.1016/s0196-8904(00)00080-7.
- ^ Schmidt, W. S. (2007). "Biodiesel: Cultivating Alternative Fuels". Environmental Health Perspectives. 115 (2): 87–91. doi:10.1289/ehp.115-a86. PMC 1817719. PMID 17384754.
- ^ Knothe, G. Biodiesel and renewable diesel: A comparison. Process in energy and Combustion Science. 2010, 36, 364–373
- ^ Altin, R.; Cetinkaya, S.; Yucesu, H.S. (2001). "Effect of Fatty Acid Profiles and Molecular Structures of Nine New Source of Biodiesel on Combustion and Emission". Energy Conversion and Management. 42 (5): 529–538. doi:10.1016/s0196-8904(00)00080-7.
- ^ 袁明豪; 陳奕宏 (2017-01-12). 蔡美瑛 (ed.). "生質柴油的冰與火之歌" (in Chinese). Taiwan: Ministry of Science and Technology. Archived from the original on 2021-03-22. Retrieved 2017-06-22.
- ^ Sanford, S.D., et al., "Feedstock and Biodiesel Characteristics Report," Renewable Energy Group, Inc., www.regfuel.com (2009).
- ^ UFOP – Union zur Förderung von Oel. "Biodiesel FlowerPower: Facts * Arguments * Tips" (PDF). Archived (PDF) from the original on 2007-07-14. Retrieved 2007-06-13.
- ^ "Detecting and Controlling Water in Oil". Archived from the original on 2016-10-24. Retrieved 2016-10-23.
- ^ The Christian Science Monitor (2012-10-03). "Ghana's best shot at going green: sewage power". The Christian Science Monitor. Archived from the original on 2015-05-30. Retrieved 30 May 2015.
- ^ "Mustard Hybrids for Low-Cost Biodiesel and Organic Pesticides" (PDF). Archived from the original (PDF) on 2011-07-26. Retrieved 2010-03-15.
- ^ "PORT HUENEME, Calif: U.S. Navy to Produce its Own Biodiesel :: Future Energies :: The future of energy". Future Energies. 2003-10-30. Archived from the original on 2011-07-11. Retrieved 2009-10-17.
- ^ "Newsvine – Ecofasa turns waste to biodiesel using bacteria". Lele.newsvine.com. 2008-10-18. Archived from the original on 2008-11-03. Retrieved 2009-10-17.
- ^ "Microbes Produce Fuels Directly from Biomass". News Center. 2010-01-27. Archived from the original on 2014-02-17. Retrieved 30 May 2015.
- ^ "Faculty & Research". Archived from the original on 26 October 2011. Retrieved 30 May 2015.
- ^ Briggs, Michael (August 2004). "Widescale Biodiesel Production from Algae". UNH Biodiesel Group (University of New Hampshire). Archived from the original on March 24, 2006. Retrieved 2007-01-02.
- ^ "Valcent Products Inc. Develops "Clean Green" Vertical Bio-Reactor". Valcent Products. Archived from the original on 2008-06-18. Retrieved 2008-07-09.
- ^ "Technology: High Yield Carbon Recycling". GreenFuel Technologies Corporation. Archived from the original on 2008-09-21. Retrieved 2015-06-14.
- ^ R. E. Teixeira (2012). "Energy-efficient extraction of fuel and chemical feedstocks from algae". Green Chemistry. 14 (2): 419–427. doi:10.1039/C2GC16225C.
- ^ "Pongamia Factsheet" (PDF). Archived (PDF) from the original on 2013-05-01. Retrieved 2013-10-02.
- ^ B.N. Divakara; H.D. Upadhyaya; S.P. Wani; C.L. Laxmipathi Gowda (2010). "Biology and genetic improvement of Jatropha curcas L.: A review" (PDF). Applied Energy. 87 (3): 732–742. Bibcode:2010ApEn...87..732D. doi:10.1016/j.apenergy.2009.07.013. Archived (PDF) from the original on 2020-03-05. Retrieved 2019-07-05.
- ^ "Jatropha blooms again: SG Biofuels secures 250K acres for hybrids". Biofuels Digest. 2011-05-16. Archived from the original on 2021-02-25. Retrieved 2012-03-08.
- ^ "Jmax Hybrid Seeds". SG Biofuels. 2012-03-08. Archived from the original on 2011-12-18. Retrieved 2012-03-08.
- ^ Plant Research International (2012-03-08). "JATROPT (Jatropha curcas): Applied and technical research into plant properties". Plant Research International. Archived from the original on 2017-06-28. Retrieved 2012-03-08.
- ^ "Energy Farming Methods Mature, Improve". Biodiesel Magazine. 2011-04-11. Archived from the original on 2012-04-06. Retrieved 2012-03-08.
- ^ "Argent biodiesel". Argent Energy. Archived from the original on 2019-04-22. Retrieved 2019-07-31.
- ^ Sergeeva, Y. E.; Galanina, L. A.; Andrianova, D. A.; Feofilova, E. P. (2008). "Lipids of filamentous fungi as a material for producing biodiesel fuel". Applied Biochemistry and Microbiology. 44 (5): 576–581. doi:10.1134/S0003683808050128. PMID 18822779. S2CID 12731382.
- ^ Strobel, G.; Knighton, B.; Kluck, K.; Ren, Y.; Livinghouse, T.; Griffin, M.; Spakowicz, D.; Sears, J. (2008). "The production of myco-diesel hydrocarbons and their derivatives by the endophytic fungus Gliocladium roseum (NRRL 50072)" (PDF). Microbiology. 154 (Pt 11): 3319–3328. doi:10.1099/mic.0.2008/022186-0. PMID 18957585. Archived from the original on 2021-07-31. Retrieved 2018-04-20.
- ^ Fountain, Henry (2008-12-15). "Diesel made Simply From Coffee Grounds". The New York Times. Archived from the original on 2008-12-17. Retrieved 2008-12-15.
- ^ Irving, P. M.; Pickles, J. S. (2007). "Operational Requirements for a Multi-fuel Processor that Generates Hydrogen from Bio- and Petroleum-Based Fuels for Both SOFC and PEM Fuel Cells". ECS Transactions. 5 (1): 665–671. Bibcode:2007ECSTr...5a.665I. doi:10.1149/1.2729047. S2CID 137810875.
- ^ Park, G.; Seo, D. J.; Park, S.; Yoon, Y.; Kim, C.; Yoon, W. (2004). "Development of microchannel methanol steam reformer". Chem. Eng. J. 101 (1–3): 87–92. doi:10.1016/j.cej.2004.01.007.
- ^ Lee, Tim (7 June 2020). "Safflower oil hailed by scientists as possible recyclable, biodegradable replacement for petroleum". ABC News. Landline. Australian Broadcasting Corporation. Archived from the original on 7 June 2020. Retrieved 7 June 2020.
- An Overview of Biodiesel and Petroleum Diesel Lifecycles, May 1998, Sheehan, et al. NREL (60pp pdf file)
- Business Management for Biodiesel Producers, January 2004, Jon Von Gerpen, Iowa State University under contract with the National Renewable Energy Laboratory (NREL) (210pp pdf file)
- Energy balances in the growth of oilseed rape for biodiesel and of wheat for bioethanol, June 2000, I.R. Richards
- Life Cycle Inventory of Biodiesel and Petroleum Diesel for Use in an Urban Bus, 1998, Sheehan, et al. NREL (314pp pdf file)
- Algae – like a breath mint for smokestacks, January 11, 2006, Mark Clayton, The Christian Science Monitor
- Tyson, R.L. "2006 Biodiesel Handling and Use Guide Third Edition" (PDF). Archived from the original (PDF) on 2006-12-16.
- Biodiesel's Bright Future from the July–August issue of THE FUTURIST magazine. [data missing]
Tom Beer; Tim Grant; Harry Watson; Doina Olaru (2004). Life-Cycle Emissions Analysis of Fuels for Light Vehicles (PDF) (Report). CSIRO. Australian Greenhouse Office. HA93A-C837/1/F5.2E.
External links
[edit]- Benefits of Biodiesel
- European Biodiesel Board website – European Biodiesel Industry.
- Sustainable Biodiesel Alliance
- International Energy Agency: Biofuels for Transport – An International Perspective at the Wayback Machine (archived January 4, 2011)
- National Biodiesel Education Program, University of Idaho—unbiased, science-based information on biodiesel for biodiesel producers and distributors, fleet operators, farmers and feedstock producers, policy makers, and consumers.
- Towards Sustainable Production and Use of Resources: Assessing Biofuels by the United Nations Environment Programme, October 2009.
- Biodiesel Articles on eXtension—eXtension (pronounced "E-Extension") is a wiki for extension professors and agents across the United States. The Farm Energy section contains over 30 articles on biodiesel, from the basics to more technical information.
- Biodiesel Safety and Best Management Practices for Small-Scale Noncommercial Use and Production Archived 2014-02-11 at the Wayback Machine
