Atom: Difference between revisions
No edit summary |
No edit summary Tags: Mobile edit Mobile web edit Advanced mobile edit |
||
| Line 1: | Line 1: | ||
{{Short description|Smallest unit of a chemical element}} |
|||
{{otheruses}} |
|||
{{Other uses}} |
|||
{| border="1" cellspacing="0" align="right" cellpadding="2" style="margin-left:1em" width=300 |
|||
{{pp-semi-indef|small=yes}} |
|||
|- |
|||
{{Use dmy dates|date=August 2024}} |
|||
! bgcolor=purple | '''''Helium atom''''' |
|||
{{Infobox |
|||
|- |
|||
| above = Atom |
|||
| align="left" | [[Image:Helium atom QM.svg|300px|right|Helium atom ground state]] |
|||
| abovestyle = background-color:#B5B5CC; |
|||
|- |
|||
| headerstyle = background-color:#B5B5CC; |
|||
| style="font-size: smaller; text-align: justify;" | An illustration of the [[helium]] atom, depicting the [[atomic nucleus|nucleus]] (pink) and the [[electron cloud]] distribution (black). The nucleus (upper right) is in reality spherically symmetric, although for more complicated nuclei this is not always the case. The black bar is one [[ångström]], equal to 10<sup>−10</sup> [[Metre|m]] or 100,000 [[Femtometre|fm]]. |
|||
| image = [[File:Helium atom QM.svg|300px|Helium atom ground state]] |
|||
|- |
|||
| caption = An illustration of the [[helium]] atom, depicting the [[atomic nucleus|nucleus]] (pink) and the [[electron cloud]] distribution (black). The nucleus (upper right) in helium-4 is in reality spherically symmetric and closely resembles the electron cloud, although for more complicated nuclei this is not always the case. The black bar is one [[angstrom]] ({{val|e=-10|u=m}} or {{val|100|ul=pm}}). |
|||
! bgcolor=gray | Classification |
|||
| header1 = Classification |
|||
|- |
|||
| data2 = Smallest recognized division of a chemical element |
|||
| |
|||
| header3 = Properties |
|||
{| align="center" |
|||
| label4 = [[atomic mass|Mass range]] |
|||
|- |
|||
| data4 = {{val|1.67|e=-27}} to {{val|4.52|e=-25|u=kg}} |
|||
| Smallest recognized division of a [[chemical element]] |
|||
| label5 = [[Electric charge]] |
|||
|} |
|||
| data5 = zero (neutral), or [[ion]] charge |
|||
|- |
|||
| label6 = [[Diameter]] range |
|||
! bgcolor=purple | Properties |
|||
| data6 = 62 pm ([[Helium|He]]) to 520 pm ([[Caesium|Cs]]) ([[Atomic radii of the elements (data page)|data page]]) |
|||
|- |
|||
| label7 = [[Subatomic particle|Components]] |
|||
| |
|||
| data7 = [[Electron]]s and a compact [[atomic nucleus|nucleus]] of [[proton]]s and [[neutron]]s |
|||
{| align="center" |
|||
}} |
|||
|- |
|||
| [[atomic mass|Mass range]]: || 1.67{{e|-24}} to 4.52{{e|-22}} [[g]] |
|||
|- |
|||
| [[Electric charge]]: || zero (neutral), or [[ion]] charge |
|||
|- |
|||
| [[Diameter]] range: || 31 [[Picometre|pm]] ([[Helium|He]]) to 520 pm ([[Caesium|Cs]]) ([[Atomic radii of the elements (data page)|data page]]) |
|||
|- |
|||
| [[Subatomic particle|Components]]: || [[Electron]]s and a compact [[atomic nucleus|nucleus]] of [[proton]]s and [[neutron]]s |
|||
|} |
|||
|} |
|||
'''Atoms''' are the basic particles of the [[chemical element]]s. An atom consists of a [[atomic nucleus|nucleus]] of [[proton]]s and generally [[neutron]]s, surrounded by an electromagnetically bound swarm of [[electron]]s. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is [[sodium]], and any atom that contains 29 protons is [[copper]]. Atoms with the same number of protons but a different number of neutrons are called [[isotope]]s of the same element. |
|||
The concept of the atom as an indivisible component of matter was first proposed by early [[India]]n and [[Ancient Greece|Greek]] philosophers. In the 17th and 18th centuries, [[chemist]]s provided a physical basis for this idea by showing that certain substances could not be further broken down by chemical methods. During the late 19th and the early 20th centuries, [[physicist]]s discovered subatomic components and structure inside the atom, thereby demonstrating that the 'atom' was not indivisible. The principles of [[quantum mechanics]], including the [[wave–particle duality]] of matter, were used to successfully [[Scientific modelling|model]] the atom.<ref>{{cite web |
|||
| first=Hans | last=Haubold | coauthors=Mathai, A. M. | year=1998 |
|||
| url=http://www.columbia.edu/~ah297/unesa/universe/universe-chapter3.html |
|||
| title=Microcosmos: From Leucippus to Yukawa |
|||
| work=Structure of the Universe |
|||
| publisher=Common Sense Science |
|||
| accessdate=2008-01-17 |
|||
}}</ref><ref>Harrison (2003).</ref> |
|||
Atoms are extremely small, typically around 100 [[picometer]]s across. A human hair is about a million carbon atoms wide. Atoms are smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. They are so small that accurately predicting their behavior using [[classical physics]] is not possible due to [[quantum mechanics|quantum effects]]. |
|||
Relative to everyday experience, atoms are minuscule objects with proportionately tiny masses that can only be observed individually using special instruments such as the [[scanning tunneling microscope]]. More than 99.9% of an atom's mass is concentrated in the nucleus,<ref>Most isotopes have more nucleons than electrons. In the corner case of hydrogen-1, with a single electron and nucleon, the proton is <math>\begin{smallmatrix}\frac{1836}{1837} \approx 0.9995\end{smallmatrix}</math>, or 99.95% of the total atomic mass.</ref> with protons and neutrons having about equal mass. In atoms with too many or too few neutrons relative to the number of protons, the nucleus is unstable and subject to [[radioactive decay]].<ref>{{cite web |
|||
| author=Staff | date=[[August 1]], [[2007]] |
|||
| url=http://www2.slac.stanford.edu/vvc/theory/nuclearstability.html |
|||
| title=Radioactive Decays |
|||
| publisher=Stanford Linear Accelerator Center, Stanford University |
|||
| accessdate=2007-01-02 }}</ref> The electrons surrounding the nucleus occupy a set of stable [[energy level]]s, or [[Atomic orbital|orbitals]], and they can transition between these states by the absorption or emission of [[photon]]s that match the energy differences between the levels. The electrons determine the chemical properties of an element, and strongly influence an atom's [[Magnetism|magnetic]] properties. |
|||
More than 99.9994%<ref>{{cite web |title=DOE Explains...Nuclei |url=https://www.energy.gov/science/doe-explainsnuclei |access-date=2024-11-05 |website=Energy.gov |language=en}}</ref> of an atom's [[mass]] is in the nucleus. Protons have a positive [[electric charge]] and neutrons have no charge, so the nucleus is positively charged. The electrons are negatively charged, and this opposing charge is what binds them to the nucleus. If the numbers of [[proton]]s and electrons are equal, as they normally are, then the atom is electrically neutral as a whole. If an atom has more electrons than protons, then it has an overall negative charge, and is called a negative [[ion]] (or anion). Conversely, if it has more protons than electrons, it has a positive charge, and is called a positive ion (or cation). |
|||
==History== |
|||
{{main|Atomic theory|Atomism}} |
|||
The electrons of an atom are attracted to the protons in an atomic nucleus by the [[electromagnetic force]]. The protons and neutrons in the nucleus are attracted to each other by the [[nuclear force]]. This force is usually stronger than the electromagnetic force that repels the positively charged protons from one another. Under certain circumstances, the repelling electromagnetic force becomes stronger than the nuclear force. In this case, the nucleus [[Nuclear fission|splits]] and [[Nuclear fission|leaves behind different elements]]. This is a form of [[nuclear decay]]. |
|||
The concept that matter is composed of [[wiktionary:discrete|discrete]] units and cannot be divided into arbitrarily tiny quantities has been around for millennia, but these ideas were founded in abstract, philosophical reasoning rather than experimentation and empirical observation. The nature of atoms in philosophy varied considerably over time and between cultures and schools, and often had spiritual elements. Nevertheless, the basic idea of the atom was adopted by scientists thousands of years later because it elegantly explained new discoveries in the field of chemistry.<ref name=Ponomarev>Ponomarev (1993).</ref> |
|||
Atoms can attach to one or more other atoms by [[chemical bond]]s to form [[chemical compound]]s such as [[molecule]]s or [[crystal]]s. The ability of atoms to attach and detach from each other is responsible for most of the physical changes observed in nature. [[Chemistry]] is the science that studies these changes. |
|||
The earliest references to the concept of atoms date back to [[History of India|ancient India]] in the 6th century [[BCE]].<ref>Gangopadhyaya (1981).</ref> The [[Nyaya]] and [[Vaisheshika]] schools developed elaborate [[Vaisheshika#The atomic theory|theories]] of how atoms combined into more complex objects (first in pairs, then trios of pairs).<ref>Teresi (2003: 213–214).</ref> The references to atoms in the West emerged a century later from [[Leucippus]] whose student, [[Democritus]], systemized his views. In approximately 450 BCE, Democritus coined the term ''átomos'' ([[Greek language|Greek]] ''ἄτομος''), which meant "uncuttable" or "the smallest indivisible particle of matter", i.e., something that cannot be divided. Although the Indian and Greek concepts of the atom were based purely on philosophy, modern science has retained the name coined by Democritus.<ref name=Ponomarev/> |
|||
== History of atomic theory == |
|||
Further progress in the understanding of atoms did not occur until the science of [[chemistry]] began to develop. In 1661, the [[Natural philosophy|natural philosopher]] [[Robert Boyle]] published ''[[The Sceptical Chymist]]'' in which he argued that matter was composed of various combinations of different "corpuscules" or atoms, rather than the [[classical element]]s of air, earth, fire and water.<ref>Siegfried (2002).</ref> In 1789 the term element was defined by the French nobleman and scientific researcher [[Antoine Lavoisier]] to mean basic substances that could not be further broken down by the methods of chemistry.<ref>{{cite web |
|||
{{Main|History of atomic theory}} |
|||
| url=http://web.lemoyne.edu/~GIUNTA/EA/LAVPREFann.HTML |
|||
| title=Lavoisier's Elements of Chemistry |
|||
| work=Elements and Atoms |
|||
| publisher=Le Moyne College, Department of Chemistry |
|||
| accessdate=2007-12-18 }}</ref> |
|||
=== In philosophy === |
|||
[[Image:A New System of Chemical Philosophy fp.jpg|left|thumb|Various atoms and molecules as depicted in [[John Dalton|John Dalton's]] ''A New System of Chemical Philosophy'' (1808)]] |
|||
{{Main|Atomism}} |
|||
In 1803, the Englishman [[John Dalton]], an instructor and natural philosopher, used the concept of atoms to explain why elements always reacted in a ratio of small [[Natural number|whole number]]s—the |
|||
The basic idea that matter is made up of tiny indivisible particles is an old idea that appeared in many ancient cultures. The word ''atom'' is derived from the [[ancient Greek]] word ''atomos'',{{efn|a combination of the negative term "a-" and "τομή," the term for "cut"}} which means "uncuttable". But this ancient idea was based in philosophical reasoning rather than scientific reasoning. Modern atomic theory is not based on these old concepts.<ref>{{cite book|last1=Pullman|first1=Bernard|title=The Atom in the History of Human Thought|date=1998|publisher=Oxford University Press|location=Oxford, England|isbn=978-0-19-515040-7|pages=31–33|url=https://books.google.com/books?id=IQs5hur-BpgC&q=Leucippus+Democritus+atom&pg=PA56|access-date=25 October 2020|archive-date=5 February 2021|archive-url=https://web.archive.org/web/20210205165029/https://books.google.com/books?id=IQs5hur-BpgC&q=Leucippus+Democritus+atom&pg=PA56|url-status=live}}</ref><ref>[[#refMelsen1952|Melsen (1952). ''From Atomos to Atom'', pp. 18–19]]</ref> In the early 19th century, the scientist [[John Dalton]] found evidence that matter really is composed of discrete units, and so applied the word ''atom'' to those units.<ref>[[#refPullman1998|Pullman (1998). ''The Atom in the History of Human Thought'', p. 201]]</ref> |
|||
[[law of multiple proportions]]—and why certain gases dissolved better in water than others. He proposed that each element consists of atoms of a single, unique type, and that these atoms could join to each other, to form chemical compounds.<ref>Wurtz (1881).</ref><ref>Dalton (1808).</ref> |
|||
=== Dalton's law of multiple proportions === |
|||
Additional validation of particle theory (and by extension [[atomic theory]]) occurred in 1827 when [[Botany|botanist]] [[Robert Brown (botanist)|Robert Brown]] used a [[microscope]] to look at dust grains floating in water and discovered that they moved about erratically—a phenomenon that became known as "[[Brownian motion]]". J. Desaulx suggested in 1877 that the phenomenon was caused by the thermal motion of water molecules, and in 1905 [[Albert Einstein]] produced the first mathematical analysis of the motion, thus confirming the hypothesis.<ref>Mazo (2002)</ref><ref>{{cite web |
|||
[[File:Daltons symbols.gif|thumb|right|Various atoms and molecules from ''A New System of Chemical Philosophy'' (John Dalton 1808).]] |
|||
| last=Lee | first=Y. K. | coauthors=Hoon, Kelvin |
|||
In the early 1800s, John Dalton compiled experimental data gathered by him and other scientists and discovered a pattern now known as the "[[law of multiple proportions]]". He noticed that in any group of chemical compounds which all contain two particular chemical elements, the amount of Element A per measure of Element B will differ across these compounds by ratios of small whole numbers. This pattern suggested that each element combines with other elements in multiples of a basic unit of weight, with each element having a unit of unique weight. Dalton decided to call these units "atoms".<ref>Pullman (1998). ''The Atom in the History of Human Thought'', p. 199: "The constant ratios, expressible in terms of integers, of the weights of the constituents in composite bodies could be construed as evidence on a macroscopic scale of interactions at the microscopic level between basic units with fixed weights. For Dalton, this agreement strongly suggested a corpuscular structure of matter, even though it did not constitute definite proof."</ref> |
|||
| year=1995 |
|||
| url=http://www.doc.ic.ac.uk/~nd/surprise_95/journal/vol4/ykl/report.html |
|||
| title=Brownian Motion | publisher=Imperial College, London |
|||
| accessdate=2007-12-18 }}</ref> |
|||
For example, there are two types of [[tin oxide (disambiguation)|tin oxide]]: one is a grey powder that is 88.1% tin and 11.9% oxygen, and the other is a white powder that is 78.7% tin and 21.3% oxygen. Adjusting these figures, in the grey powder there is about 13.5 g of oxygen for every 100 g of tin, and in the white powder there is about 27 g of oxygen for every 100 g of tin. 13.5 and 27 form a ratio of 1:2. Dalton concluded that in the grey oxide there is one atom of oxygen for every atom of tin, and in the white oxide there are two atoms of oxygen for every atom of tin ([[tin(II) oxide|SnO]] and [[tin dioxide|SnO<sub>2</sub>]]).<ref>[[#refDalton1817|Dalton (1817). ''A New System of Chemical Philosophy'' vol. 2, p. 36]]</ref><ref>[[#refMelsen1952|Melsen (1952). ''From Atomos to Atom'', p. 137]]</ref> |
|||
The physicist [[J. J. Thomson]], through his work on [[cathode ray]]s in 1897, discovered the electron and its subatomic nature, which destroyed the concept of atoms as being indivisible units.<ref name="nobel1096">{{cite web |
|||
| author=The Nobel Foundation | year=1906 |
|||
| url=http://nobelprize.org/nobel_prizes/physics/laureates/1906/thomson-bio.html |
|||
| title=J.J. Thomson | publisher=Nobelprize.org |
|||
| accessdate=2007-12-20 }}</ref> Thomson believed that the electrons were distributed throughout the atom, with their charge balanced by the presence of a uniform sea of positive charge (the [[plum pudding model]]). |
|||
Dalton also analyzed [[iron oxide]]s. There is one type of iron oxide that is a black powder which is 78.1% iron and 21.9% oxygen; and there is another iron oxide that is a red powder which is 70.4% iron and 29.6% oxygen. Adjusting these figures, in the black powder there is about 28 g of oxygen for every 100 g of iron, and in the red powder there is about 42 g of oxygen for every 100 g of iron. 28 and 42 form a ratio of 2:3. Dalton concluded that in these oxides, for every two atoms of iron, there are two or three atoms of oxygen respectively ([[iron(II) oxide|Fe<sub>2</sub>O<sub>2</sub>]] and [[iron(III) oxide|Fe<sub>2</sub>O<sub>3</sub>]]).{{efn|Iron(II) oxide's formula is written here as "Fe<sub>2</sub>O<sub>2</sub>" rather than the more conventional "FeO" because this better illustrates the explanation.}}<ref>[[#refDalton1817|Dalton (1817). ''A New System of Chemical Philosophy'' vol. 2, p. 28]]</ref><ref>[[#refMillington1906|Millington (1906). ''John Dalton'', p. 113]]</ref> |
|||
[[Image:Bohr Model.svg|right|thumb|200px|A Bohr model of the |
|||
hydrogen atom, showing an electron jumping between fixed orbits and emitting a [[photon]] |
|||
of energy with a specific frequency]] |
|||
As a final example: [[nitrous oxide]] is 63.3% nitrogen and 36.7% oxygen, [[nitric oxide]] is 44.05% nitrogen and 55.95% oxygen, and [[nitrogen dioxide]] is 29.5% nitrogen and 70.5% oxygen. Adjusting these figures, in nitrous oxide there is 80 g of oxygen for every 140 g of nitrogen, in nitric oxide there is about 160 g of oxygen for every 140 g of nitrogen, and in nitrogen dioxide there is 320 g of oxygen for every 140 g of nitrogen. 80, 160, and 320 form a ratio of 1:2:4. The respective formulas for these oxides are [[nitrous oxide|N<sub>2</sub>O]], [[nitric oxide|NO]], and [[nitrogen dioxide|NO<sub>2</sub>]].<ref>[[#refDalton1808|Dalton (1808). ''A New System of Chemical Philosophy'' vol. 1, pp. 316–319]]</ref><ref>[[#refHolbrowEtAl2010|Holbrow et al. (2010). ''Modern Introductory Physics'', pp. 65–66]]</ref> |
|||
However, in 1909, researchers under the direction of physicist [[Ernest Rutherford]] bombarded a sheet of gold foil with helium ions and discovered that a small percentage were deflected through much larger angles than was predicted using Thomson's proposal. Rutherford interpreted the [[gold foil experiment]] as suggesting that the positive charge of an atom and most of its mass was concentrated in a nucleus at the center of the atom (the [[Rutherford model]]), with the electrons orbiting it like planets around a sun. Positively charged helium ions passing close to this dense nucleus would then be deflected away at much sharper angles.<ref>{{cite journal |
|||
| last=Rutherford | first=E. |
|||
| title=The Scattering of α and β Particles by Matter and the Structure of the Atom |
|||
| journal=Philosophical Magazine |
|||
| year=1911 | volume=21 | pages=669–88 |
|||
| url=http://dbhs.wvusd.k12.ca.us/webdocs/Chem-History/Rutherford-1911/Rutherford-1911.html |
|||
| accessdate=2008-01-18 |
|||
}}</ref> |
|||
=== Discovery of the electron === |
|||
While experimenting with the products of [[radioactive decay]], in 1913 [[radiochemistry|radiochemist]] [[Frederick Soddy]] discovered that there appeared to be more than one type of atom at each position on the periodic table.<ref>{{cite web |
|||
In 1897, [[J. J. Thomson]] discovered that [[cathode ray]]s are not a form of light but made of negatively charged particles because they can be deflected by electric and magnetic fields.<ref>{{cite journal |author=J. J. Thomson |url=http://web.lemoyne.edu/~GIUNTA/thomson1897.html |title=Cathode rays |journal=Philosophical Magazine |volume=44 |issue=269 |page=293–316 |year=1897}}</ref> He measured these particles to be at least a thousand times lighter than [[hydrogen]] (the lightest atom).<ref>In his book ''The Corpuscular Theory of Matter'' (1907), Thomson estimates electrons to be 1/1700 the mass of hydrogen.</ref> He called these new particles ''corpuscles'' but they were later renamed ''[[electron]]s'' since these are the particles that carry electricity.<ref>[http://library.thinkquest.org/C0111709/English/DC-Circuts/mechanism.html "The Mechanism Of Conduction In Metals"] {{Webarchive|url=https://web.archive.org/web/20121025004809/http://library.thinkquest.org/ |date=25 October 2012 }}, Think Quest.</ref> Thomson also showed that electrons were identical to particles given off by [[Photoelectric effect|photoelectric]] and radioactive materials.<ref name="Thomson">{{cite journal|last=Thomson|first=J.J.|title=On bodies smaller than atoms|journal=The Popular Science Monthly|pages=323–335|date=August 1901|url=https://books.google.com/books?id=3CMDAAAAMBAJ&pg=PA323|access-date=21 June 2009|archive-date=1 December 2016|archive-url=https://web.archive.org/web/20161201152039/https://books.google.com/books?id=3CMDAAAAMBAJ&pg=PA323|url-status=live}}</ref> Thomson explained that an electric current is the passing of electrons from one atom to the next, and when there was no current the electrons embedded themselves in the atoms. This in turn meant that atoms were not indivisible as scientists thought. The atom was composed of electrons whose negative charge was balanced out by some source of positive charge to create an electrically neutral atom. Ions, Thomson explained, must be atoms which have an excess or shortage of electrons.<ref>J. J. Thomson (1907). ''On the Corpuscular Theory of Matter'', p. 26: "The simplest interpretation of these results is that the positive ions are the atoms or groups of atoms of various elements from which one or more corpuscles have been removed [...] while the negative electrified body is one with more corpuscles than the unelectrified one."</ref> |
|||
| url=http://nobelprize.org/nobel_prizes/chemistry/laureates/1921/soddy-bio.html |
|||
| title=Frederick Soddy, The Nobel Prize in Chemistry 1921 |
|||
| publisher=Nobel Foundation |
|||
| accessdate=2008-01-18 |
|||
}}</ref> The term [[isotope]] was coined by [[Margaret Todd (doctor)|Margaret Todd]] as a suitable name for different atoms that belong to the same element. J.J. Thomson created a technique for separating atom types through his work on ionized gases, which subsequently led to the discovery of stable isotopes.<ref>{{cite journal |
|||
| last=Thomson | first=Joseph John |
|||
| title=Rays of positive electricity |
|||
| journal=Proceedings of the Royal Society |
|||
| year=1913 | volume=A 89 | pages=1–20 |
|||
| url=http://web.lemoyne.edu/~giunta/canal.html |
|||
| accessdate=2007-01-18 }}</ref> |
|||
=== Discovery of the nucleus === |
|||
Meanwhile, in 1913, physicist [[Niels Bohr]] revised Rutherford's model by suggesting that the electrons were confined into clearly defined orbits, and could jump between these, but could not freely spiral inward or outward in intermediate states.<ref>{{cite web |
|||
[[File:Geiger-Marsden experiment expectation and result.svg|thumb|right|The [[Rutherford scattering experiments]]: The extreme scattering of some alpha particles suggested the existence of a nucleus of concentrated charge.]] |
|||
| last=Stern | first=David P. | date=[[May 16]], [[2005]] |
|||
{{Main|Rutherford scattering experiments}} |
|||
| url=http://www-spof.gsfc.nasa.gov/stargaze/Q5.htm |
|||
The electrons in the atom logically had to be balanced out by a commensurate amount of positive charge, but Thomson had no idea where this positive charge came from, so he tentatively proposed that it was everywhere in the atom, the atom being in the shape of a sphere. This was the mathematically simplest hypothesis to fit the available evidence, or lack thereof. Following from this, Thomson imagined that the balance of electrostatic forces would distribute the electrons throughout the sphere in a more or less even manner.<ref>J. J. Thomson (1907). ''The Corpuscular Theory of Matter'', p. 103: "In default of exact knowledge of the nature of the way in which positive electricity occurs in the atom, we shall consider a case in which the positive electricity is distributed in the way most amenable to mathematical calculation, i.e., when it occurs as a sphere of uniform density, throughout which the corpuscles are distributed."</ref> Thomson's model is popularly known as the [[plum pudding model]], though neither Thomson nor his colleagues used this analogy.<ref name=HonGoldstein2013>{{cite journal |author1=Giora Hon |author2=Bernard R. Goldstein |date=6 September 2013 |title=J. J. Thomson's plum-pudding atomic model: The making of a scientific myth |journal=Annalen der Physik |volume=525 |issue=8–9 |pages=A129–A133 |doi= 10.1002/andp.201300732 |bibcode=2013AnP...525A.129H |url=https://onlinelibrary.wiley.com/doi/10.1002/andp.201300732 | issn=0003-3804}}</ref> Thomson's model was incomplete, it was unable to predict any other properties of the elements such as [[emission spectra]] and [[valency (chemistry)|valencies]]. It was soon rendered obsolete by the discovery of the [[atomic nucleus]]. |
|||
| title=The Atomic Nucleus and Bohr's Early Model of the Atom |
|||
| publisher=NASA Goddard Space Flight Center |
|||
| accessdate=2007-12-20 }}</ref> An electron must absorb or emit specific amounts of energy to transition between these fixed orbits. When the [[light]] from a heated material is passed through a [[Prism (optics)|prism]], it produced a multi-colored [[spectrum]]. The appearance of fixed [[Spectral line|lines in this spectrum]] was successfully explained by the orbital transitions.<ref>{{cite web |
|||
| last=Bohr | first=Niels | date=[[December 11]], [[1922]] |
|||
| url=http://nobelprize.org/nobel_prizes/physics/laureates/1922/bohr-lecture.html |
|||
| title=Niels Bohr, The Nobel Prize in Physics 1922, Nobel Lecture |
|||
| publisher=The Nobel Foundation |
|||
| accessdate=2008-02-16 }}</ref> |
|||
Between 1908 and 1913, [[Ernest Rutherford]] and his colleagues [[Hans Geiger]] and [[Ernest Marsden]] performed a series of experiments in which they bombarded thin foils of metal with a beam of [[alpha particles]]. They did this to measure the scattering patterns of the alpha particles. They spotted a small number of alpha particles being deflected by angles greater than 90°. This shouldn't have been possible according to the Thomson model of the atom, whose charges were too diffuse to produce a sufficiently strong electric field. The deflections should have all been negligible. Rutherford proposed that the positive charge of the atom is concentrated in a tiny volume at the center of the atom and that the electrons surround this nucleus in a diffuse cloud. This nucleus carried almost all of the atom's mass, the electrons being so very light. Only such an intense concentration of charge, anchored by its high mass, could produce an electric field that could deflect the alpha particles so strongly.<ref name=Heilbron2003p64-68>[[#refHeilbron2003|Heilbron (2003). ''Ernest Rutherford and the Explosion of Atoms'', pp. 64–68]]</ref> |
|||
In 1926, [[Erwin Schrödinger]], using [[Louis de Broglie]]'s 1924 proposal that particles behave to an extent like waves, developed a mathematical model of the atom that described the electrons as three-dimensional [[waveform]]s, rather than point particles. A consequence of using waveforms to describe electrons is that it is mathematically impossible to obtain precise values for both the [[Point (geometry)|position]] and [[momentum]] of a particle at the same time; this became known as the [[uncertainty principle]]. In this concept, for each measurement of a position one could only obtain a range of probable values for momentum, and vice versa. Although this model was difficult to visually conceptualize, it was able to explain observations of atomic behavior that previous models could not, such as certain structural and [[Spectral line|spectral]] patterns of atoms larger than hydrogen. Thus, the planetary model of the atom was discarded in favor of one that described orbital zones around the nucleus where a given electron is most likely to exist.<ref>{{cite web |
|||
| last=Brown | first=Kevin | year=2007 |
|||
| url=http://www.mathpages.com/home/kmath538/kmath538.htm |
|||
| title=The Hydrogen Atom | publisher=MathPages |
|||
| accessdate=2007-12-21 |
|||
}}</ref><ref>{{cite web |
|||
| last=Harrison | first=David M. | date=March 2000 |
|||
| url=http://www.upscale.utoronto.ca/GeneralInterest/Harrison/DevelQM/DevelQM.html |
|||
| title=The Development of Quantum Mechanics |
|||
| publisher=University of Toronto |
|||
| accessdate=2007-12-21 }}</ref> |
|||
=== Bohr model === |
|||
[[Image:Mass spectrometer schematics.png|left|thumb|280px|Schematic diagram of a simple mass spectrometer]] |
|||
{{Main|Bohr model}} |
|||
The development of the [[mass spectrometry|mass spectrometer]] allowed the exact mass of atoms to be measured. The device uses a magnet to bend the trajectory of a beam of ions, and the amount of deflection is determined by the ratio of an atom's mass to its charge. The chemist [[Francis William Aston]] used this instrument to demonstrate that isotopes had different masses. The mass of these isotopes varied by integer amounts, called the [[whole number rule]].<ref>{{cite journal |
|||
[[File:Bohr atom animation 2.gif|right|thumb|The Bohr model of the atom, with an electron making instantaneous "quantum leaps" from one orbit to another with gain or loss of energy. This model of electrons in orbits is obsolete.]] |
|||
| title=The constitution of atmospheric neon |
|||
A problem in classical mechanics is that an accelerating charged particle radiates electromagnetic radiation, causing the particle to lose kinetic energy. Circular motion counts as acceleration, which means that an electron orbiting a central charge should spiral down into that nucleus as it loses speed. In 1913, the physicist [[Niels Bohr]] proposed a new model in which the electrons of an atom were assumed to orbit the nucleus but could only do so in a finite set of orbits, and could jump between these orbits only in discrete changes of energy corresponding to absorption or radiation of a photon.<ref name=stern20050516 /> This quantization was used to explain why the electrons' orbits are stable and why elements absorb and emit electromagnetic radiation in discrete spectra.<ref name=bohr19221211 /> Bohr's model could only predict the emission spectra of hydrogen, not atoms with more than one electron. |
|||
| journal=Philosophical Magazine | year=1920 |
|||
| first=Francis W. | last=Aston |
|||
| volume=39 | issue=6 | pages=449–55 }}</ref> The explanation for these different atomic isotopes awaited the discovery of the [[neutron]], a neutral-charged particle with a mass similar to the [[proton]], by the physicist [[James Chadwick]] in 1932. Isotopes were then explained as elements with the same number of protons, but different numbers of neutrons within the nucleus.<ref>{{cite web |
|||
| last=Chadwick | first=James | date=[[December 12]], [[1935]] |
|||
| url=http://nobelprize.org/nobel_prizes/physics/laureates/1935/chadwick-lecture.html |
|||
| title=Nobel Lecture: The Neutron and Its Properties |
|||
| publisher=Nobel Foundation |
|||
| accessdate=2007-12-21 }}</ref> |
|||
=== Discovery of protons and neutrons=== |
|||
In the 1950s, the development of improved [[particle accelerator]] and [[particle detector]]s allowed scientists to study the impacts of atoms moving at high energies.<ref>{{cite web |
|||
{{Main|Atomic nucleus|Discovery of the neutron}} |
|||
| last=Kullander | first=Sven | date=[[August 28]], [[2001]] |
|||
Back in 1815, [[William Prout]] observed that the atomic weights of many elements were multiples of hydrogen's atomic weight, which is in fact true for all of them if one takes [[isotopes]] into account. In 1898, [[J. J. Thomson]] found that the positive charge of a hydrogen ion is equal to the negative charge of an electron, and these were then the smallest known charged particles.<ref>{{cite journal |last=J. J. Thomson |date=1898 |title=On the Charge of Electricity carried by the Ions produced by Röntgen Rays |url=https://archive.org/details/londonedinburgh5461898lon/page/528/mode/2up |journal=The London, Edinburgh and Dublin Philosophical Magazine and Journal of Science |series=5 |volume=46 |issue=283 |pages=528–545 |doi=10.1080/14786449808621229}}</ref> Thomson later found that the positive charge in an atom is a positive multiple of an electron's negative charge.<ref>J. J. Thomson (1907). ''The Corpuscular Theory of Matter''. p. 26–27: "In an unelectrified atom there are as many units of positive electricity as there are of negative; an atom with a unit of positive charge is a neutral atom which has lost one corpuscle, while an atom with a unit of negative charge is a neutral atom to which an additional corpuscle has been attached."</ref> In 1913, [[Henry Moseley]] discovered that the frequencies of X-ray emissions from an [[excited state|excited]] atom were a mathematical function of its [[atomic number]] and hydrogen's nuclear charge. In 1919 [[Ernest Rutherford|Rutherford]] bombarded [[nitrogen]] gas with [[alpha particle]]s and detected [[hydrogen]] ions being emitted from the gas, and concluded that they were produced by alpha particles hitting and splitting the nuclei of the nitrogen atoms.<ref>{{cite journal|author=Rutherford, Ernest|url=http://web.lemoyne.edu/~GIUNTA/rutherford.html |title=Collisions of alpha Particles with Light Atoms. IV. An Anomalous Effect in Nitrogen|journal=Philosophical Magazine|year=1919|volume=37|page=581|doi=10.1080/14786440608635919|issue=222}}</ref> |
|||
| url=http://nobelprize.org/nobel_prizes/physics/articles/kullander/ |
|||
| title=Accelerators and Nobel Laureates |
|||
| publisher=The Nobel Foundation |
|||
| accessdate=2008-01-31 }}</ref> Neutrons and protons were found to be [[hadron]]s, or composites of smaller particles called [[quark]]s. Standard models of nuclear physics were developed that successfully explained the properties of the nucleus in terms of these sub-atomic particles and the forces that govern their interactions.<ref>{{cite web |
|||
| author=Staff | date=[[October 17]], [[1990]] |
|||
| url=http://nobelprize.org/nobel_prizes/physics/laureates/1990/press.html |
|||
| title=The Nobel Prize in Physics 1990 |
|||
| publisher=The Nobel Foundation |
|||
| accessdate=2008-01-31 }}</ref> |
|||
These observations led Rutherford to conclude that the hydrogen nucleus is a singular particle with a positive charge equal to the electron's negative charge.<ref>''The Development of the Theory of Atomic Structure'' (Rutherford 1936). Reprinted in ''Background to Modern Science: Ten Lectures at Cambridge arranged by the History of Science Committee 1936'':<br/>"In 1919 I showed that when light atoms were bombarded by α-particles they could be broken up with the emission of a proton, or hydrogen nucleus. We therefore presumed that a proton must be one of the units of which the nuclei of other atoms were composed..."</ref> He named this particle "[[proton]]" in 1920.<ref>{{cite journal |author=Orme Masson |date=1921 |title=The Constitution of Atoms |journal=The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science |volume=41 |issue=242 |pages=281–285 |doi=10.1080/14786442108636219 |url=https://archive.org/details/londonedinburg6411921lond/page/280/mode/2up }}<br/>Footnote by Ernest Rutherford: 'At the time of writing this paper in Australia, Professor Orme Masson was not aware that the name "proton" had already been suggested as a suitable name for the unit of mass nearly 1, in terms of oxygen 16, that appears to enter into the nuclear structure of atoms. The question of a suitable name for this unit was discussed at an informal meeting of a number of members of Section A of the British Association at Cardiff this year. The name "baron" suggested by Professor Masson was mentioned, but was considered unsuitable on account of the existing variety of meanings. Finally the name "proton" met with general approval, particularly as it suggests the original term "protyle " given by Prout in his well-known hypothesis that all atoms are built up of hydrogen. The need of a special name for the nuclear unit of mass 1 was drawn attention to by Sir Oliver Lodge at the Sectional meeting, and the writer then suggested the name "proton."'</ref> The number of protons in an atom (which Rutherford called the "[[atomic number]]"<ref>Eric Scerri (2020). ''The Periodic Table: Its Story and Its Significance'', p. 185</ref><ref>Helge Kragh (2012). ''Niels Bohr and the Quantum Atom'', p. 33</ref>) was found to be equal to the element's ordinal number on the [[periodic table]] and therefore provided a simple and clear-cut way of distinguishing the elements from each other. The atomic weight of each element is higher than its proton number, so Rutherford hypothesized that the surplus weight was carried by unknown particles with no electric charge and a mass equal to that of the proton. |
|||
Around 1985, [[Steven Chu]] and co-workers at [[Bell Labs]] developed a technique for lowering the temperatures of atoms using [[laser]]s. In the same year, a team led by [[William Daniel Phillips|William D. Phillips]] managed to contain atoms of sodium in a [[Magnetic trap (atoms)|magnetic trap]]. The combination of these two techniques and a method based on the [[Doppler effect]], developed by [[Claude Cohen-Tannoudji]] and his group, allows small numbers of atoms to be cooled to several [[Kelvin|microkelvin]]. This allows the atoms to be studied with great precision, and later led to the discovery of [[Bose-Einstein condensation]].<ref>{{cite web |
|||
| author=Staff | date=[[October 15]], [[1997]] |
|||
| url=http://nobelprize.org/nobel_prizes/physics/laureates/1997/ |
|||
| title=The Nobel Prize in Physics 1997 |
|||
| publisher=Nobel Foundation |
|||
| accessdate=2008-02-10 }}</ref> |
|||
In 1928, [[Walter Bothe]] observed that [[beryllium]] emitted a highly penetrating, electrically neutral radiation when bombarded with alpha particles. It was later discovered that this radiation could knock hydrogen atoms out of [[paraffin wax]]. Initially it was thought to be high-energy [[gamma radiation]], since gamma radiation had a similar effect on electrons in metals, but [[James Chadwick]] found that the [[ionization]] effect was too strong for it to be due to electromagnetic radiation, so long as energy and momentum were conserved in the interaction. In 1932, Chadwick exposed various elements, such as hydrogen and nitrogen, to the mysterious "beryllium radiation", and by measuring the energies of the recoiling charged particles, he deduced that the radiation was actually composed of electrically neutral particles which could not be massless like the gamma ray, but instead were required to have a mass similar to that of a proton. Chadwick now claimed these particles as Rutherford's neutrons.<ref>{{cite journal|author=James Chadwick |year=1932|url=http://web.mit.edu/22.54/resources/Chadwick.pdf |archive-url=https://ghostarchive.org/archive/20221009/http://web.mit.edu/22.54/resources/Chadwick.pdf |archive-date=9 October 2022 |url-status=live |title=Possible Existence of a Neutron|doi=10.1038/129312a0|journal=Nature|page=312|volume=129|bibcode = 1932Natur.129Q.312C|issue=3252|s2cid=4076465|doi-access=free}}</ref> |
|||
Historically, single atoms have been prohibitively small for scientific applications. Recently, devices have been constructed that use a single metal atom connected through organic [[ligand]]s to construct a [[single electron transistor]].<ref>{{cite journal |
|||
| author=Park, Jiwoong ''et al'' | journal = Nature |
|||
| year = 2002 | volume = 417 | issue = 6890 | pages=722–25 |
|||
| title = Coulomb blockade and the Kondo effect in single-atom transistors |
|||
| url=http://adsabs.harvard.edu/abs/2002Natur.417..722P |
|||
| doi=10.1038/nature00791 | accessdate=2008-01-03 }}</ref> Experiments have been carried out by trapping and slowing single atoms using [[laser cooling]] in a cavity to gain a better physical understanding of matter.<ref>{{cite journal |
|||
| first=P. | last=Domokos | coauthors=Janszky, J.; Adam, P. |
|||
| title=Single-atom interference method for generating Fock states |
|||
| journal=Physical Review A | volume=50 |
|||
| pages=3340–44 | year=1994 | doi=10.1103/PhysRevA.50.3340 |
|||
| url=http://adsabs.harvard.edu/abs/1994PhRvA..50.3340D |
|||
| accessdate=2008-01-03 }}</ref> |
|||
=== The current consensus model === |
|||
==Components== |
|||
[[File:S-p-Orbitals.svg|thumb|right|The modern model of atomic orbitals draws zones where an electron is most likely to be found at any moment.]] |
|||
===Subatomic particles=== |
|||
In 1925, [[Werner Heisenberg]] published the first consistent mathematical formulation of quantum mechanics ([[matrix mechanics]]).<ref name="Pais">{{cite book|last=Pais|first=Abraham|year=1986|location=New York|title=Inward Bound: Of Matter and Forces in the Physical World|publisher=Oxford University Press|isbn=978-0-19-851971-3|pages=[https://archive.org/details/inwardboundofmat00pais_0/page/228 228–230]|url=https://archive.org/details/inwardboundofmat00pais_0/page/228}}</ref> One year earlier, [[Louis de Broglie]] had proposed that all particles behave like waves to some extent,<ref>{{cite book |title=Introducing Quantum Theory |author1=McEvoy, J. P. |author2=Zarate, Oscar |publisher=Totem Books |year=2004 |isbn=978-1-84046-577-8 |pages=110–114}}</ref> and in 1926 [[Erwin Schroedinger]] used this idea to develop the [[Schroedinger equation]], which describes electrons as three-dimensional [[waveform]]s rather than points in space.<ref>{{cite web |last=Kozłowski |first=Miroslaw |year=2019 |title=The Schrödinger equation A History |url=https://www.researchgate.net/publication/332241721}}</ref> A consequence of using waveforms to describe particles is that it is mathematically impossible to obtain precise values for both the [[point (geometry)|position]] and [[momentum]] of a particle at a given point in time. This became known as the [[uncertainty principle]], formulated by [[Werner Heisenberg]] in 1927.<ref name="Pais" /> In this concept, for a given accuracy in measuring a position one could only obtain a range of probable values for momentum, and vice versa.<ref>{{cite web|author=Chad Orzel|url=https://www.youtube.com/watch?v=TQKELOE9eY4|title=What is the Heisenberg Uncertainty Principle?|website=TED-Ed|date=16 September 2014|via=YouTube|archive-url=https://web.archive.org/web/20150913185956/https://www.youtube.com/watch?v=TQKELOE9eY4|archive-date=13 September 2015|url-status=live}}</ref> Thus, the planetary model of the atom was discarded in favor of one that described [[atomic orbital]] zones around the nucleus where a given electron is most likely to be found.<ref name=brown2007 /><ref name=harrison2000 /> This model was able to explain observations of atomic behavior that previous models could not, such as certain structural and [[Spectral line|spectral]] patterns of atoms larger than hydrogen. |
|||
{{main|Subatomic particle}} |
|||
Though the word ''atom'' originally denoted a particle that cannot be cut into smaller particles, in modern scientific usage the atom is composed of various [[subatomic particle]]s. The constituent particles of an atom consist of the [[electron]], the [[proton]] and, for atoms other than [[hydrogen|hydrogen-1]], the [[neutron]]. |
|||
== Structure == |
|||
The electron is by far the least massive of these particles at 9.11{{e|−28}} g, with a negative [[Electric charge|electrical charge]] and a size that is too small to be measured using available techniques.<ref>Demtröder (2002).</ref> Protons have a positive charge and a mass 1,836 times that of the electron, at 1.6726{{e|−24}} g, although this can be reduced by changes to the atomic [[binding energy]]. Neutrons have no electrical charge and have a free mass of 1,839 times the mass of electrons,<ref>Woan (2000).</ref> |
|||
=== Subatomic particles === |
|||
or 1.6929{{e|−24}} g. |
|||
{{Main|Subatomic particle}} |
|||
Neutrons and protons have comparable dimensions—on the order of 2.5{{e|−15}} [[Metre|m]]—although the 'surface' of these particles is not sharply defined.<ref>MacGregor (1992).</ref> |
|||
Though the word ''atom'' originally denoted a particle that cannot be cut into smaller particles, in modern scientific usage the atom is composed of various [[subatomic particle]]s. The constituent particles of an atom are the [[electron]], the [[proton]] and the [[neutron]]. |
|||
The electron is the least massive of these particles by four orders of magnitude at {{val|9.11|e=-31|u=kg}}, with a negative [[Electric charge|electrical charge]] and a size that is too small to be measured using available techniques.<ref>{{cite book|last=Demtröder|first=Wolfgang|year=2002|title=Atoms, Molecules and Photons: An Introduction to Atomic- Molecular- and Quantum Physics|url=https://archive.org/details/atomsmoleculesph00demt_277|url-access=limited|publisher=Springer|edition=1st|isbn=978-3-540-20631-6|oclc=181435713|pages=[https://archive.org/details/atomsmoleculesph00demt_277/page/n51 39]–42}}</ref> It was the lightest particle with a positive rest mass measured, until the discovery of [[neutrino]] mass. Under ordinary conditions, electrons are bound to the positively charged nucleus by the attraction created from opposite electric charges. If an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively charged as a whole; a charged atom is called an [[ion]]. Electrons have been known since the late 19th century, mostly thanks to [[J.J. Thomson]]; see [[history of subatomic physics]] for details. |
|||
In the [[Standard Model]] of physics, both protons and neutrons are composed of [[elementary particle]]s called [[quark]]s. The quark is a type of [[fermion]], one of the two basic constituents of matter—the other being the [[lepton]], of which the electron is an example. There are six types of quarks, and each has a fractional electric charge of either +2/3 or −1/3. Protons are composed of two [[up quark]]s and one [[down quark]], while a neutron consists of one up quark and two down quarks. This distinction accounts for the difference in mass and charge between the two particles. The quarks are held together by the [[strong nuclear force]], which is mediated by [[gluon]]s. The gluon is a member of the family of [[boson]]s, which are elementary particles that mediate physical [[force]]s.<ref>{{cite web |
|||
| author=Particle Data Group | year=2002 |
|||
| url=http://www.particleadventure.org/ |
|||
| title=The Particle Adventure |
|||
| publisher=Lawrence Berkeley Laboratory |
|||
| accessdate=2007-01-03 |
|||
}}</ref><ref>{{cite web |
|||
| first=James | last=Schombert |
|||
| date=[[April 18]], [[2006]] |
|||
| url=http://abyss.uoregon.edu/~js/ast123/lectures/lec07.html |
|||
| title=Elementary Particles |
|||
| publisher=University of Oregon |
|||
| accessdate=2007-01-03 |
|||
}}</ref> |
|||
Protons have a positive charge and a mass of {{val|1.6726|e=-27|u=kg}}. The number of protons in an atom is called its [[atomic number]]. [[Ernest Rutherford]] (1919) observed that nitrogen under alpha-particle bombardment ejects what appeared to be hydrogen nuclei. By 1920 he had accepted that the hydrogen nucleus is a distinct particle within the atom and named it [[proton]]. |
|||
===Nucleus=== |
|||
{{main|Atomic nucleus}} |
|||
All of the bound protons and neutrons in an atom make up a tiny [[atomic nucleus]], and are collectively called [[nucleon]]s. The radius of a nucleus is approximately equal to |
|||
<math>\begin{smallmatrix}1.07 \cdot \sqrt[3]{A}\end{smallmatrix}</math> fm, |
|||
where ''A'' is the total number of nucleons.<ref>Jevremovic (2005).</ref> This is much smaller than the radius of the atom, which is on the order of 10<sup>5</sup> fm. The nucleons are bound together by a short-ranged attractive potential called the [[residual strong force]]. At distances smaller than 2.5 [[femtometre|fm]], this force is much more powerful than the [[electrostatic force]] that causes positively charged protons to repel each other.<ref name=pfeffer>Pfeffer (2000).</ref> |
|||
Neutrons have no electrical charge and have a mass of {{val|1.6749|e=-27|u=kg}}.<ref>{{cite book|last=Woan|first=Graham|year=2000|title=The Cambridge Handbook of Physics|publisher=Cambridge University Press|isbn=978-0-521-57507-2|oclc=224032426|page=[https://archive.org/details/cambridgehandboo0000woan/page/8 8]|url=https://archive.org/details/cambridgehandboo0000woan/page/8}}</ref><ref name="2014 CODATA">Mohr, P.J.; Taylor, B.N. and Newell, D.B. (2014), [http://physics.nist.gov/constants "The 2014 CODATA Recommended Values of the Fundamental Physical Constants"] {{Webarchive|url=https://web.archive.org/web/20120211083747/http://physics.nist.gov/cuu/Constants/index.html |date=11 February 2012 }} (Web Version 7.0). The database was developed by J. Baker, M. Douma, and [[Svetlana Kotochigova|S. Kotochigova]]. (2014). National Institute of Standards and Technology, Gaithersburg, Maryland 20899.</ref> Neutrons are the heaviest of the three constituent particles, but their mass can be reduced by the [[nuclear binding energy]]. Neutrons and protons (collectively known as [[nucleon]]s) have comparable dimensions—on the order of {{val|2.5|e=-15|u=m}}—although the 'surface' of these particles is not sharply defined.<ref>{{cite book|last=MacGregor|first=Malcolm H.|year=1992|title=The Enigmatic Electron|publisher=Oxford University Press|isbn=978-0-19-521833-6|oclc=223372888|pages=[https://archive.org/details/astronomyencyclo0000unse/page/33 33–37]|url=https://archive.org/details/astronomyencyclo0000unse/page/33}}</ref> The neutron was discovered in 1932 by the English physicist [[James Chadwick]]. |
|||
Atoms of the same [[chemical element|element]] have the same number of protons, called the [[atomic number]]. Within a single element, the number of neutrons may vary, determining the [[isotope]] of that element. The number of neutrons relative to the protons determines the stability of the nucleus, with certain isotopes undergoing [[radioactive decay]].<ref>{{cite web |
|||
| last=Wenner | first=Jennifer M. | date=[[October 10]], [[2007]] |
|||
| url=http://serc.carleton.edu/quantskills/methods/quantlit/RadDecay.html |
|||
| title=How Does Radioactive Decay Work? |
|||
| publisher=Carleton College | accessdate=2008-01-09 }}</ref> |
|||
In the [[Standard Model]] of physics, electrons are truly elementary particles with no internal structure, whereas protons and neutrons are composite particles composed of [[elementary particle]]s called [[quark]]s. There are two types of quarks in atoms, each having a fractional electric charge. Protons are composed of two [[up quark]]s (each with charge +{{sfrac|2|3}}) and one [[down quark]] (with a charge of −{{sfrac|1|3}}). Neutrons consist of one up quark and two down quarks. This distinction accounts for the difference in mass and charge between the two particles.<ref name=pdg2002 /><ref name=schombert2006 /> |
|||
The neutron and the proton are different types of [[fermion]]s. The [[Pauli exclusion principle]] is a [[quantum mechanics|quantum mechanical]] effect that prohibits ''identical'' fermions (such as multiple protons) from occupying the same quantum physical state at the same time. Thus every proton in the nucleus must occupy a different state, with its own energy level, and the same rule applies to all of the neutrons. (This prohibition does not apply to a proton and neutron occupying the same quantum state.)<ref name="raymond"/> |
|||
The quarks are held together by the [[strong interaction]] (or strong force), which is mediated by [[gluon]]s. The protons and neutrons, in turn, are held to each other in the nucleus by the [[nuclear force]], which is a residuum of the strong force that has somewhat different range-properties (see the article on the nuclear force for more). The gluon is a member of the family of [[gauge boson]]s, which are elementary particles that mediate physical forces.<ref name=pdg2002 /><ref name=schombert2006 /> |
|||
A nucleus that has a different number of protons than neutrons can potentially drop to a lower energy state through a radioactive decay that causes the number of protons and neutrons to more closely match. As a result, atoms with matching numbers of protons and neutrons are more stable against decay. However, with increasing atomic number, the mutual repulsion of the protons requires an increasing proportion of neutrons to maintain the stability of the nucleus, which slightly modifies this trend of equal numbers of protons to neutrons.<ref name="raymond"/> |
|||
=== Nucleus === |
|||
[[Image:Wpdms physics proton proton chain 1.svg|right|thumb|200px|This diagram illustrates a nuclear fusion process that forms a deuterium nucleus, consisting of a proton and a neutron, from two protons. A [[positron]] (e<sup>+</sup>)—an [[antimatter]] electron—is emitted along with an electron [[neutrino]].]] |
|||
{{Main|Atomic nucleus}} |
|||
[[File:Binding energy curve - common isotopes.svg|thumb|The [[binding energy]] needed for a nucleon to escape the nucleus, for various isotopes]]<!-- A brief explanation is provided here because 'binding energy' is not explained until the end of the section. --> |
|||
All the bound protons and neutrons in an atom make up a tiny [[atomic nucleus]], and are collectively called [[nucleon]]s. The radius of a nucleus is approximately equal to <math>1.07 \sqrt[3]{A}</math> [[femtometre]]s, where <math>A</math> is the total number of nucleons.<ref>{{cite book|last=Jevremovic|first=Tatjana|year=2005|title=Nuclear Principles in Engineering|url=https://archive.org/details/nuclearprinciple00jevr_450|url-access=limited|publisher=Springer|isbn=978-0-387-23284-3|oclc=228384008|page=[https://archive.org/details/nuclearprinciple00jevr_450/page/n83 63]}}</ref> This is much smaller than the radius of the atom, which is on the order of 10<sup>5</sup> fm. The nucleons are bound together by a short-ranged attractive potential called the [[residual strong force]]. At distances smaller than 2.5 fm this force is much more powerful than the [[electrostatic force]] that causes positively charged protons to repel each other.<ref>{{cite book|last1=Pfeffer|first1=Jeremy I.|last2=Nir|first2=Shlomo|year=2000|title=Modern Physics: An Introductory Text|publisher=Imperial College Press|isbn=978-1-86094-250-1|oclc=45900880|pages=330–336}}</ref> |
|||
The number of protons and neutrons in the atomic nucleus can be modified, although this can require very high energies because of the strong force. [[Nuclear fusion]] occurs when multiple atomic particles join to form a heavier nucleus, such as through the energetic collision of two nuclei. At the core of the Sun, protons require energies of 3–10 KeV to overcome their mutual repulsion—the [[coulomb barrier]]—and |
|||
fuse together into a single nucleus.<ref>{{cite web |
|||
| last=Mihos | first=Chris | date=[[July 23]], [[2002]] |
|||
| url=http://burro.cwru.edu/Academics/Astr221/StarPhys/coulomb.html |
|||
| title=Overcoming the Coulomb Barrier |
|||
| publisher=Case Western Reserve University |
|||
| accessdate=2008-02-13 }}</ref> [[Nuclear fission]] is the opposite process, causing a nucleus to split into two smaller nuclei—usually through radioactive decay. The nucleus can also be modified through bombardment by high energy subatomic particles or photons. In such processes that change the number of protons in a nucleus, the atom becomes an atom of a different chemical element.<ref>{{cite web |
|||
| author=Staff | date=[[March 30]], [[2007]] |
|||
| url=http://www.lbl.gov/abc/Basic.html |
|||
| title=ABC's of Nuclear Science |
|||
| publisher=Lawrence Berkeley National Laboratory |
|||
| accessdate=2007-01-03 |
|||
}}</ref><ref>{{cite web |
|||
| first=Arjun | last=Makhijani | coauthors=Saleska, Scott |
|||
| date=[[March 2]], [[2001]] |
|||
| url=http://www.ieer.org/reports/n-basics.html |
|||
| title=Basics of Nuclear Physics and Fission |
|||
| publisher=Institute for Energy and Environmental Research |
|||
| accessdate=2007-01-03 |
|||
}}</ref> |
|||
Atoms of the same [[chemical element|element]] have the same number of protons, called the [[atomic number]]. Within a single element, the number of neutrons may vary, determining the [[isotope]] of that element. The total number of protons and neutrons determine the [[nuclide]]. The number of neutrons relative to the protons determines the stability of the nucleus, with certain isotopes undergoing [[radioactive decay]].<ref name=wenner2007 /> |
|||
The mass of the nucleus following a fusion reaction is less than the sum of the masses of the separate particles. The difference between these two values is emitted as energy, as described by [[Albert Einstein]]'s [[mass–energy equivalence]] formula, ''E'' = ''mc''², where ''m'' is the mass loss and ''c'' is the [[speed of light]]. This deficit is the [[binding energy]] of the nucleus.<ref>Shultis ''et al'' (2002).</ref> |
|||
The proton, the electron, and the neutron are classified as [[fermion]]s. Fermions obey the [[Pauli exclusion principle]] which prohibits ''[[identical particles|identical]]'' fermions, such as multiple protons, from occupying the same quantum state at the same time. Thus, every proton in the nucleus must occupy a quantum state different from all other protons, and the same applies to all neutrons of the nucleus and to all electrons of the electron cloud.<ref name="raymond" /> |
|||
The fusion of two nuclei that have lower atomic numbers than [[iron]] and [[nickel]] is an [[exothermic reaction|exothermic process]] that releases more energy than is required to bring them together.<ref>{{cite journal |
|||
| last = Fewell | first = M. P. |
|||
| title=The atomic nuclide with the highest mean binding energy |
|||
| journal=[[American Journal of Physics]] |
|||
| year=1995 | volume=63 | issue=7 | pages=653–58 |
|||
| url=http://adsabs.harvard.edu/abs/1995AmJPh..63..653F |
|||
| accessdate = 2007-02-01 }}</ref> It is this energy-releasing process that makes nuclear fusion in [[star]]s a self-sustaining reaction. For heavier nuclei, the total binding energy begins to decrease. That means fusion processes with nuclei that have higher atomic numbers is an [[endothermic reaction|endothermic process]]. These more massive nuclei can not undergo an energy-producing fusion reaction that can sustain the [[hydrostatic equilibrium]] of a star.<ref name="raymond">{{cite web |
|||
| last=Raymond | first=David | date=[[April 7]], [[2006]] |
|||
| url=http://physics.nmt.edu/~raymond/classes/ph13xbook/node216.html |
|||
| title=Nuclear Binding Energies |
|||
| publisher=New Mexico Tech |
|||
| accessdate=2007-01-03 }}</ref> |
|||
A nucleus that has a different number of protons than neutrons can potentially drop to a lower energy state through a radioactive decay that causes the number of protons and neutrons to more closely match. As a result, atoms with matching numbers of protons and neutrons are more stable against decay, but with increasing atomic number, the mutual repulsion of the protons requires an increasing proportion of neutrons to maintain the stability of the nucleus.<ref name="raymond" /> |
|||
===Electron cloud=== |
|||
{{main|Electron cloud}} |
|||
[[File:Wpdms physics proton proton chain 1.svg|right|thumb|upright|Illustration of a nuclear fusion process that forms a deuterium nucleus, consisting of a proton and a neutron, from two protons. A [[positron]] (e<sup>+</sup>)—an [[antimatter]] electron—is emitted along with an electron [[neutrino]].]] |
|||
[[Image:Potential energy well.svg|200px|right|thumb|This is an example of a potential well, showing the minimum energy ''V''(''x'') needed to reach each position ''x''. A particle with energy ''E'' is constrained to a range of positions between ''x''<sub>1</sub> and ''x''<sub>2</sub>.]] |
|||
The electrons in an atom are attracted to the protons in the nucleus by the [[electromagnetic force]]. This force binds the electrons inside an [[electrostatic]] [[potential well]] surrounding the smaller nucleus, which means that an external source of energy is needed in order for the electron to escape. The closer an electron is to the nucleus, the greater the attractive force. Hence electrons bound near the center of the potential well require more energy to escape than those at the exterior. |
|||
The number of protons and neutrons in the atomic nucleus can be modified, although this can require very high energies because of the strong force. [[Nuclear fusion]] occurs when multiple atomic particles join to form a heavier nucleus, such as through the energetic collision of two nuclei. For example, at the core of the Sun protons require energies of 3 to 10 keV to overcome their mutual repulsion—the [[coulomb barrier]]—and fuse together into a single nucleus.<ref name=mihos2002 /> [[Nuclear fission]] is the opposite process, causing a nucleus to split into two smaller nuclei—usually through radioactive decay. The nucleus can also be modified through bombardment by high energy subatomic particles or photons. If this modifies the number of protons in a nucleus, the atom changes to a different chemical element.<ref name=lbnl20070330 /><ref name=makhijani_saleska2001 /> |
|||
Electrons, like other particles, have properties of both a [[Wave–particle duality|particle and a wave]]. The electron cloud is a region inside the potential well where each electron forms a type of three-dimensional [[standing wave]]—a wave form that does not move relative to the nucleus. This behavior is defined by an [[atomic orbital]], a mathematical function that characterises the probability that an electron will appear to be at a particular location when its position is measured. Only a discrete (or [[Wiktionary:quantize|quantize]]d) set of these orbitals exist around the nucleus, as other possible wave patterns will rapidly decay into a more stable form.<ref name=Brucat>{{cite web |
|||
| last=Brucat | first=Philip J. | year=2008 |
|||
| url=http://www.chem.ufl.edu/~itl/2045/lectures/lec_10.html |
|||
| title=The Quantum Atom | publisher=University of Florida |
|||
| accessdate=2007-01-04 }}</ref> Orbitals can have one or more ring or node structures, and they differ from each other in size, shape and orientation.<ref>{{cite web |
|||
| last=Manthey | first=David | year=2001 |
|||
| url=http://www.orbitals.com/orb/ |
|||
| title=Atomic Orbitals | publisher=Orbital Central |
|||
| accessdate=2008-01-21 }}</ref> |
|||
If the mass of the nucleus following a fusion reaction is less than the sum of the masses of the separate particles, then the difference between these two values can be emitted as a type of usable energy (such as a [[gamma ray]], or the kinetic energy of a [[beta particle]]), as described by [[Albert Einstein]]'s [[mass–energy equivalence]] formula, ''E=mc<sup>2</sup>'', where ''m'' is the mass loss and ''c'' is the [[speed of light]]. This deficit is part of the [[binding energy]] of the new nucleus, and it is the non-recoverable loss of the energy that causes the fused particles to remain together in a state that requires this energy to separate.<ref>{{cite book|last1=Shultis|first1=J. Kenneth|last2=Faw|first2=Richard E.|title=Fundamentals of Nuclear Science and Engineering|year=2002|publisher=CRC Press|isbn=978-0-8247-0834-4|oclc=123346507|pages=10–17}}</ref> |
|||
[[Image:AOs-1s-2pz.png|left|250px|thumb|This illustration shows the wave functions of the first five atomic orbitals. Note how each of the three 2p orbitals display a single angular [[Node (physics)|node]] that has an orientation and a minimum at the center.]] |
|||
The fusion of two nuclei that create larger nuclei with lower atomic numbers than [[iron]] and [[nickel]]—a total nucleon number of about 60—is usually an [[exothermic reaction|exothermic process]] that releases more energy than is required to bring them together.<ref name=ajp63_7_653 /> It is this energy-releasing process that makes nuclear fusion in [[star]]s a self-sustaining reaction. For heavier nuclei, the binding energy per [[nucleon]] begins to decrease. That means that a fusion process producing a nucleus that has an atomic number higher than about 26, and a [[mass number]] higher than about 60, is an [[endothermic reaction|endothermic process]]. Thus, more massive nuclei cannot undergo an energy-producing fusion reaction that can sustain the [[hydrostatic equilibrium]] of a star.<ref name="raymond" /> |
|||
Each atomic orbital corresponds to a particular [[energy level]] of the electron. The electron can change its state to a higher energy level by absorbing a [[photon]] with sufficient energy to boost it into the new quantum state. Likewise, through [[spontaneous emission]], an electron in a higher energy state can drop to a lower energy state while radiating the excess energy as a photon. These characteristic energy values, defined by the differences in the energies of the quantum states, are responsible for [[atomic spectral line]]s.<ref name=Brucat/> |
|||
=== Electron cloud === |
|||
The amount of energy needed to remove or add an electron (the [[electron binding energy]]) is far less than the [[binding energy|binding energy of nucleons]]. For example, it requires only 13.6 eV to strip a [[Stationary state|ground-state]] electron from a Hydrogen atom.<ref>{{cite web |
|||
{{Main|Electron configuration|Electron shell|Atomic orbital}}{{See also|Electronegativity}}[[File:Potential energy well.svg|right|thumb|A potential well, showing, according to [[classical mechanics]], the minimum energy ''V''(''x'') needed to reach each position ''x''. Classically, a particle with energy ''E'' is constrained to a range of positions between ''x''<sub>1</sub> and ''x''<sub>2</sub>.]] |
|||
| last=Herter | first=Terry | year=2006 |
|||
The electrons in an atom are attracted to the protons in the nucleus by the [[electromagnetic force]]. This force binds the electrons inside an [[electrostatic]] [[potential well]] surrounding the smaller nucleus, which means that an external source of energy is needed for the electron to escape. The closer an electron is to the nucleus, the greater the attractive force. Hence electrons bound near the center of the potential well require more energy to escape than those at greater separations. |
|||
| url=http://instruct1.cit.cornell.edu/courses/astro101/lectures/lec08.htm |
|||
| title=Lecture 8: The Hydrogen Atom |
|||
| publisher=Cornell University | accessdate=2008-02-14 }}</ref> Atoms are [[electric charge|electrically]] neutral if they have an equal number of protons and electrons. Atoms that have either a deficit or a surplus of electrons are called [[ion]]s. Electrons that are farthest from the nucleus may be transferred to other nearby atoms or shared between atoms. By this mechanism, atoms are able to [[chemical bond|bond]] into [[molecule]]s and other types of [[chemical compound]]s like [[Ionic crystal|ionic]] and [[Covalent bond|covalent]] network [[Crystallization|crystals]].<ref>Smirnov (2003).</ref> |
|||
Electrons, like other particles, have properties of both a [[wave–particle duality|particle and a wave]]. The electron cloud is a region inside the potential well where each electron forms a type of three-dimensional [[standing wave]]—a wave form that does not move relative to the nucleus. This behavior is defined by an [[atomic orbital]], a mathematical function that characterises the probability that an electron appears to be at a particular location when its position is measured.<ref name=science157_3784_13 /> Only a discrete (or [[wikt:quantize|quantized]]) set of these orbitals exist around the nucleus, as other possible wave patterns rapidly decay into a more stable form.<ref name=Brucat2008 /> Orbitals can have one or more ring or node structures, and differ from each other in size, shape and orientation.<ref name=manthey2001 /> |
|||
==Properties== |
|||
By definition, any two atoms with an identical number of ''protons'' in their nuclei belong to the same [[chemical element]]. Atoms with the same number of protons but a different number of ''neutrons'' are different [[isotope]]s of the same element. Hydrogen atoms, for example, always have only a single proton, but isotopes exist with no neutrons ([[hydrogen atom|hydrogen-1]], sometimes called protium, by far the most common form), one neutron ([[deuterium]]) and two neutrons ([[tritium]]).<ref>{{cite web |
|||
| last=Matis | first=Howard S. | date=[[August 9]], [[2000]] |
|||
| url=http://www.lbl.gov/abc/wallchart/chapters/02/3.html |
|||
| title=The Isotopes of Hydrogen |
|||
| work=Guide to the Nuclear Wall Chart |
|||
| publisher=Lawrence Berkeley National Lab |
|||
| accessdate=2007-12-21 }}</ref> The known elements form a continuous range of atomic numbers from hydrogen with a single proton up to the 118-proton element [[ununoctium]].<ref>{{cite news |
|||
| last=Weiss | first=Rick | date=[[October 17]], [[2006]] |
|||
| title=Scientists Announce Creation of Atomic Element, the Heaviest Yet |
|||
| publisher=Washington Post |
|||
| url=http://www.washingtonpost.com/wp-dyn/content/article/2006/10/16/AR2006101601083.html |
|||
| accessdate=2007-12-21 }}</ref> All known isotopes of elements with atomic numbers greater than 82 are radioactive.<ref name=sills>Sills (2003).</ref><ref name=dume>{{cite news |
|||
| last=Dumé | first=Belle | date=[[April 23]], [[2003]] |
|||
| title=Bismuth breaks half-life record for alpha decay |
|||
| publisher=Physics World |
|||
| url=http://physicsworld.com/cws/article/news/17319 |
|||
| accessdate=2007-12-21 }}</ref> |
|||
[[File:Atomic-orbital-clouds spdf m0.png|thumb|upright=1.5|3D views of some [[Hydrogen-like atom|hydrogen-like]] atomic orbitals showing probability density and phase ('''g''' orbitals and higher are not shown)]] |
|||
===Mass=== |
|||
{{main|Atomic mass}} |
|||
Each atomic orbital corresponds to a particular [[energy level]] of the electron. The electron can change its state to a higher energy level by absorbing a [[photon]] with sufficient energy to boost it into the new quantum state. Likewise, through [[spontaneous emission]], an electron in a higher energy state can drop to a lower energy state while radiating the excess energy as a photon. These characteristic energy values, defined by the differences in the energies of the quantum states, are responsible for [[atomic spectral line]]s.<ref name=Brucat2008 /> |
|||
Because the large majority of an atom's mass comes from the protons and neutrons, the total number of these particles in an atom is called the [[mass number]]. The [[Invariant mass|mass of an atom at rest]] is often expressed using the [[Atomic mass unit|unified atomic mass unit]] (u), which is also called a Dalton (Da). This unit is defined as a twelfth of the mass of a free neutral atom of [[carbon-12]], which is approximately 1.66×10<sup>−24</sup> g.<ref name=iupac/> [[hydrogen atom|hydrogen-1]], the lightest isotope of hydrogen and the atom with the lowest mass, has an atomic weight of 1.007825 u.<ref>{{cite web |
|||
| last=Chieh | first=Chung |
|||
| date=[[January 22]], [[2001]] |
|||
| url=http://www.science.uwaterloo.ca/~cchieh/cact/nuctek/nuclideunstable.html |
|||
| title=Nuclide Stability |
|||
| publisher=University of Waterloo |
|||
| accessdate=2007-01-04 }}</ref> An atom has a mass approximately equal to the mass number times the atomic mass unit.<ref>{{cite web |
|||
| url=http://physics.nist.gov/cgi-bin/Compositions/stand_alone.pl?ele=&ascii=html&isotype=some |
|||
| title=Atomic Weights and Isotopic Compositions for All Elements |
|||
| publisher=National Institute of Standards and Technology |
|||
| accessdate=2007-01-04 }}</ref> The heaviest [[stable atom]] is lead-208,<ref name=sills/> with a mass of 207.9766521 u.<ref>{{cite journal |
|||
| last=Audi | first=G. | coauthors=Wapstra, A. H.; Thibault C. |
|||
| title=The Ame2003 atomic mass evaluation (II) |
|||
| journal=Nuclear Physics |
|||
| year=2003 | volume=A729 | pages=337–676 |
|||
| url=http://www.nndc.bnl.gov/amdc/web/masseval.html |
|||
| accessdate=2008-02-07 }}</ref> |
|||
The amount of energy needed to remove or add an electron—the [[electron binding energy]]—is far less than the [[binding energy|binding energy of nucleons]]. For example, it requires only 13.6 eV to strip a [[Stationary state|ground-state]] electron from a hydrogen atom,<ref name=herter_8 /> compared to 2.23 ''million'' eV for splitting a [[deuterium]] nucleus.<ref name=pr79_2_282 /> Atoms are [[electric charge|electrically]] neutral if they have an equal number of protons and electrons. Atoms that have either a deficit or a surplus of electrons are called [[ion]]s. Electrons that are farthest from the nucleus may be transferred to other nearby atoms or shared between atoms. By this mechanism, atoms are able to [[chemical bond|bond]] into [[molecule]]s and other types of [[chemical compound]]s like [[Ionic crystal|ionic]] and [[Covalent bond|covalent]] network [[Crystallization|crystals]].<ref>{{cite book|last=Smirnov|first=Boris M.|year=2003|title=Physics of Atoms and Ions|url=https://archive.org/details/physicsatomsions00smir|url-access=limited|publisher=Springer|isbn=978-0-387-95550-6|pages=[https://archive.org/details/physicsatomsions00smir/page/n262 249]–272}}</ref> |
|||
As even the most massive atoms are far too light to work with directly, chemists instead use the unit of [[Mole (unit)|mole]]s. The mole is defined such that one mole of any element will always have the same number of atoms (about [[Avogadro constant|6.022×10<sup>23</sup>]]). This number was chosen so that if an element has an atomic mass of 1 u, a mole of atoms of that element will have a mass of 1 g. [[Carbon]], for example, has an atomic mass of 12 u, so a mole of carbon atoms weighs 12 g.<ref name=iupac>Mills ''et al'' (1993).</ref> |
|||
== |
== Properties == |
||
=== Nuclear properties === |
|||
{{main|Atomic radius}} |
|||
{{Main|Isotope|Stable isotope|List of nuclides|List of elements by stability of isotopes}} |
|||
Atoms lack a well-defined outer boundary, so the dimensions are usually described in terms of the distances between two nuclei when the two atoms are joined in a [[chemical bond]]. The radius varies with the location of an atom on the atomic chart, the type of chemical bond, the number of neighboring atoms ([[coordination number]]) and a [[quantum mechanics|quantum mechanical]] property known as [[Spin (physics)|spin]].<ref>{{cite journal |
|||
By definition, any two atoms with an identical number of ''protons'' in their nuclei belong to the same [[chemical element]]. Atoms with equal numbers of protons but a different number of ''neutrons'' are different isotopes of the same element. For example, all hydrogen atoms admit exactly one proton, but isotopes exist with no neutrons ([[hydrogen-1]], by far the most common form,<ref name=matis2000 /> also called protium), one neutron ([[deuterium]]), two neutrons ([[tritium]]) and [[isotopes of hydrogen|more than two neutrons]]. The known elements form a set of atomic numbers, from the single-proton element [[hydrogen]] up to the 118-proton element [[oganesson]].<ref name=weiss20061017 /> All known isotopes of elements with atomic numbers greater than 82 are radioactive, although the radioactivity of element 83 ([[bismuth]]) is so slight as to be practically negligible.<ref name=s131>{{cite book|last=Sills|first=Alan D.|year=2003|title=Earth Science the Easy Way|publisher=Barron's Educational Series|isbn=978-0-7641-2146-3|oclc=51543743|pages=[https://archive.org/details/earthscienceeasy00alan/page/131 131–134]|url=https://archive.org/details/earthscienceeasy00alan/page/131}}</ref><ref name=dume20030423 /> |
|||
| last = Shannon | first = R. D. |
|||
| title=Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides |
|||
| journal=Acta Crystallographica, Section A |
|||
| year=1976 | volume=32 | pages=751 |
|||
| url=http://journals.iucr.org/a/issues/1976/05/00/issconts.html |
|||
| accessdate=2007-01-03 |
|||
| doi=10.1107/S0567739476001551 }}</ref> On the [[periodic table]] of the elements, atom size tends to increase when moving down columns, but decrease when moving across rows (left to right).<ref>{{cite web |
|||
| last=Dong | first=Judy | year=1998 |
|||
| url=http://hypertextbook.com/facts/MichaelPhillip.shtml |
|||
| title=Diameter of an Atom |
|||
| publisher=The Physics Factbook |
|||
| accessdate=2007-11-19 }}</ref> Consequently, the smallest atom is helium with a radius of 32 [[Picometre|pm]], while one of the largest is [[caesium]] at 225 pm.<ref>Zumdahl (2002).</ref> These dimensions are thousands of times smaller than the wavelengths of [[light]] (400–700 [[nanometre|nm]]) so they can not be viewed using an [[optical microscope]]. However, individual atoms can be observed using a [[scanning tunneling microscope]]. |
|||
About 339 nuclides occur naturally on [[Earth]],<ref name=lidsay20000730 /> of which 251 (about 74%) have not been observed to decay, and are referred to as "[[stable isotope]]s". Only 90 nuclides are stable [[list of nuclides|theoretically]], while another 161 (bringing the total to 251) have not been observed to decay, even though in theory it is energetically possible. These are also formally classified as "stable". An additional 35 radioactive nuclides have half-lives longer than 100 million years, and are long-lived enough to have been present since the birth of the [[Solar System]]. This collection of 286 nuclides are known as [[primordial nuclide]]s. Finally, an additional 53 short-lived nuclides are known to occur naturally, as daughter products of primordial nuclide decay (such as [[radium]] from [[uranium]]), or as products of natural energetic processes on Earth, such as cosmic ray bombardment (for example, carbon-14).<ref name=tuli2005 /><ref group=note>For more recent updates see [[Brookhaven National Laboratory]]'s [http://www.nndc.bnl.gov/chart Interactive Chart of Nuclides] ] {{Webarchive|url=https://web.archive.org/web/20200725182342/https://www.nndc.bnl.gov/nudat2/ |date=25 July 2020 }}.</ref><!-- See article [[list of nuclides]]. The numbers are derived by [[WP:CALC]] (counting the table), which is not [[WP:OR]]--> |
|||
Some examples will demonstrate the minuteness of the atom. A typical human hair is about 1 million carbon atoms in width.<ref>{{cite web |
|||
| author=Staff | year=2007 |
|||
| url=http://oregonstate.edu/terra/2007winter/features/nanotech.php |
|||
| title=Small Miracles: Harnessing nanotechnology |
|||
| publisher=Oregon State University |
|||
| accessdate=2007-01-07 }}—describes the width of a human hair as 10<sup>5</sup> nm and 10 carbon atoms as spanning 1 nm.</ref> A single drop of water contains about 2 [[sextillion]] (2{{e|21}}) atoms of oxygen, and twice the number of hydrogen atoms.<ref>Padilla ''et al'' (2002): Science textbook, Page 32: "There are 2,000,000,000,000,000,000,000 (that's 2 sextillion) atoms of oxygen in one drop of water—and twice as many atoms of hydrogen."</ref> A single [[Carat (mass)|carat]] [[diamond]] with a mass of 0.2 g contains about 10 [[sextillion]] atoms of [[carbon]].<ref>A carat is 200 milligrams. [[Atomic mass|By definition]], Carbon-12 has 12 grams per mole. The [[Avogadro constant]] defines 6×10<sup>23</sup> atoms per mole.</ref> If an apple was magnified to the size of the Earth, then the atoms in the apple would be approximately the size of the original apple.<ref>Feynman, Six Easy Pieces, (1995).</ref> |
|||
For 80 of the chemical elements, at least one [[stable isotope]] exists. As a rule, there is only a handful of stable isotopes for each of these elements, the average being 3.1 stable isotopes per element. Twenty-six "[[monoisotopic element]]s" have only a single stable isotope, while the largest number of stable isotopes observed for any element is ten, for the element [[tin]]. Elements [[technetium|43]], [[promethium|61]], and all elements numbered [[bismuth|83]] or higher have no stable isotopes.<ref name=CRC>CRC Handbook (2002).</ref>{{rp|1–12}} |
|||
===Radioactive decay=== |
|||
{{main|Radioactive decay}} |
|||
Stability of isotopes is affected by the ratio of protons to neutrons, and also by the presence of certain "magic numbers" of neutrons or protons that represent closed and filled quantum shells. These quantum shells correspond to a set of energy levels within the [[Nuclear shell model|shell model]] of the nucleus; filled shells, such as the filled shell of 50 protons for tin, confers unusual stability on the nuclide. Of the 251 known stable nuclides, only four have both an odd number of protons ''and'' odd number of neutrons: [[hydrogen-2]] ([[deuterium]]), [[lithium-6]], [[boron-10]], and [[nitrogen-14]]. ([[Tantalum-180m]] is odd-odd and observationally stable, but is predicted to decay with a very long half-life.) Also, only four naturally occurring, radioactive odd-odd nuclides have a half-life over a billion years: [[potassium-40]], [[vanadium-50]], [[lanthanum-138]], and [[lutetium-176]]. Most odd-odd nuclei are highly unstable with respect to [[beta decay]], because the decay products are even-even, and are therefore more strongly bound, due to [[Semi-empirical mass formula#Pairing term|nuclear pairing effects]].<ref>{{cite book |last=Krane |first=K. |year=1988 |title=Introductory Nuclear Physics |url=https://archive.org/details/introductorynucl00kran |url-access=limited |publisher=[[John Wiley & Sons]] |isbn=978-0-471-85914-7 |pages=[https://archive.org/details/introductorynucl00kran/page/n90 68]}}</ref> |
|||
[[Image:Isotopes and half-life 1.PNG|right|300px|thumb|This diagram shows the half-life (T<sub>½</sub>) in seconds of various isotopes with Z protons and N neutrons.]] |
|||
=== Mass === |
|||
Every element has one or more isotopes that have unstable nuclei that are subject to [[radioactive decay]], causing the nucleus to emit particles or electromagnetic radiation. Radioactivity can occur when the radius of a nucleus is large compared with the radius of the strong force, which only acts over distances on the order of 1 fm.<ref name=splung>{{cite web |
|||
{{Main|Atomic mass|mass number}} |
|||
| url=http://www.splung.com/content/sid/5/page/radioactivity |
|||
| title=Radioactivity | publisher=Splung.com |
|||
| accessdate=2007-12-19 }}</ref> |
|||
The large majority of an atom's mass comes from the protons and neutrons that make it up. The total number of these particles (called "nucleons") in a given atom is called the [[mass number]]. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. An example of use of a mass number is "carbon-12," which has 12 nucleons (six protons and six neutrons). |
|||
There are three primary forms of radioactive decay:<ref>'Annunziata<!-- Note: the single quote mark before the name is correct. --> (2003).</ref><ref>{{cite web |
|||
| last=Firestone | first=Richard B. | date=[[May 22]], [[2000]] |
|||
| url=http://isotopes.lbl.gov/education/decmode.html |
|||
| title=Radioactive Decay Modes |
|||
| publisher=Berkeley Laboratory |
|||
| accessdate=2007-01-07 }}</ref> |
|||
* [[Alpha decay]] is caused when the nucleus emits an alpha particle, which is a helium nucleus consisting of two protons and two neutrons. The result of the emission is a new element with a lower [[atomic number]]. |
|||
* [[Beta decay]] is regulated by the [[weak force]], and results from a transformation of a neutron into a proton, or a proton into a neutron. The first is accompanied by the emission of an electron and an [[antineutrino]], while the second causes the emission of a [[positron]] and a [[neutrino]]. The electron or positron emissions are called beta particles. Beta decay either increases or decreases the atomic number of the nucleus by one. |
|||
* [[Gamma decay]] results from a change in the energy level of the nucleus to a lower state, resulting in the emission of electromagnetic radiation. This can occur following the emission of an alpha or a beta particle from radioactive decay. |
|||
The actual [[Invariant mass|mass of an atom at rest]] is often expressed in [[dalton (unit)|daltons]] (Da), also called the unified atomic mass unit (u). This unit is defined as a twelfth of the mass of a free neutral atom of [[carbon-12]], which is approximately {{val|1.66|e=-27|u=kg}}.<ref name=iupac /> [[hydrogen atom|Hydrogen-1]] (the lightest isotope of hydrogen which is also the nuclide with the lowest mass) has an atomic weight of 1.007825 Da.<ref name=chieh2001 /> The value of this number is called the [[atomic mass]]. A given atom has an atomic mass approximately equal (within 1%) to its mass number times the atomic mass unit (for example the mass of a nitrogen-14 is roughly 14 Da), but this number will not be exactly an integer except (by definition) in the case of carbon-12.<ref name=nist_wc /> The heaviest [[stable atom]] is lead-208,<ref name=s131 /> with a mass of {{val|207.9766521|u=Da}}.<ref name=audi2003 /> |
|||
Each radioactive isotope has a characteristic decay time period—the [[half-life]]—that is determined by the amount of time needed for half of a sample to decay. This is an [[exponential decay]] process that steadily decreases the proportion of the remaining isotope by 50% every half life. Hence after two half-lives have passed only 25% of the isotope will be present, and so forth.<ref name=splung/> |
|||
As even the most massive atoms are far too light to work with directly, chemists instead use the unit of [[Mole (unit)|moles]]. One mole of atoms of any element always has the same number of atoms (about [[Avogadro constant|{{val|6.022|e=23}}]]). This number was chosen so that if an element has an atomic mass of 1 u, a mole of atoms of that element has a mass close to one gram. Because of the definition of the [[Atomic mass unit|unified atomic mass unit]], each carbon-12 atom has an atomic mass of exactly 12 Da, and so a mole of carbon-12 atoms weighs exactly 0.012 kg.<ref name=iupac>{{cite book |
|||
===Magnetic moment=== |
|||
|last=Mills |
|||
{{main|Electron magnetic dipole moment|Nuclear magnetic moment}} |
|||
|first=Ian |
|||
|author2=Cvitaš, Tomislav |
|||
|author3=Homann, Klaus |
|||
|author4=Kallay, Nikola |
|||
|author5=Kuchitsu, Kozo |
|||
|title=Quantities, Units and Symbols in Physical Chemistry |
|||
|publisher=[[International Union of Pure and Applied Chemistry]], Commission on Physiochemical Symbols Terminology and Units, Blackwell Scientific Publications |
|||
|location=Oxford |
|||
|edition=2nd |
|||
|year=1993 |
|||
|isbn=978-0-632-03583-0 |
|||
|oclc=27011505 |
|||
|url=https://archive.org/details/quantitiesunitss0000unse/page/70 |
|||
|page=[https://archive.org/details/quantitiesunitss0000unse/page/70 70] |
|||
}}</ref> |
|||
=== Shape and size === |
|||
Elementary particles possess an intrinsic quantum mechanical property known as [[Spin (physics)|spin]]. This is analogous to the [[angular momentum]] of an object that is spinning around its [[center of mass]], although strictly speaking these particles are believed to be point-like and cannot be said to be rotating. Spin is measured in units of the reduced [[Planck constant]] (<math>\hbar</math>), with electrons, protons and neutrons all having spin ½ <math>\hbar</math>, or "spin-½". In an atom, electrons in motion around the [[Atomic nucleus|nucleus]] possess [[orbital angular momentum]] in addition to their spin, while the nucleus itself possesses angular momentum due to its nuclear spin.<ref>{{cite web |
|||
{{Main|Atomic radius}} |
|||
| last=Hornak | first=J. P. | year=2006 |
|||
Atoms lack a well-defined outer boundary, so their dimensions are usually described in terms of an [[atomic radius]]. This is a measure of the distance out to which the electron cloud extends from the nucleus.<ref name=Ghosh02>{{cite journal | author = Ghosh, D.C. |author2= Biswas, R. | title = Theoretical calculation of Absolute Radii of Atoms and Ions. Part 1. The Atomic Radii | journal = Int. J. Mol. Sci. | volume = 3 |issue= 11 | pages = 87–113 | year = 2002 | doi=10.3390/i3020087| doi-access = free }}</ref> This assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Atomic radii may be derived from the distances between two nuclei when the two atoms are joined in a [[chemical bond]]. The radius varies with the location of an atom on the atomic chart, the type of chemical bond, the number of neighboring atoms ([[coordination number]]) and a [[quantum mechanics|quantum mechanical]] property known as [[Spin (physics)|spin]].<ref name=aca32_5_751 /> On the [[periodic table]] of the elements, atom size tends to increase when moving down columns, but decrease when moving across rows (left to right).<ref name=dong1998 /> Consequently, the smallest atom is helium with a radius of 32 [[Picometre|pm]], while one of the largest is [[caesium]] at 225 pm.<ref>{{cite book |
|||
| url=http://astro.rit.edu/htbooks/nmr/bnmr.htm |
|||
|last=Zumdahl|first=Steven S.|year=2002 |
|||
| title=Chapter 3: Spin Physics | work=The Basics of NMR |
|||
|title=Introductory Chemistry: A Foundation |
|||
| publisher=Rochester Institute of Technology |
|||
|edition=5th|publisher=Houghton Mifflin |
|||
| accessdate=2007-01-07 }}</ref> |
|||
|url=http://college.hmco.com/chemistry/intro/zumdahl/intro_chemistry/5e/students/protected/periodictables/pt/pt/pt_ar5.html |
|||
|isbn=978-0-618-34342-3 |
|||
|oclc=173081482| archive-url= https://web.archive.org/web/20080304155935/http://college.hmco.com/chemistry/intro/zumdahl/intro_chemistry/5e/students/protected/periodictables/pt/pt/pt_ar5.html| archive-date= 4 March 2008 | url-status= live}}</ref> |
|||
When subjected to external forces, like [[electrical field]]s, the shape of an atom may deviate from [[spherical symmetry]]. The deformation depends on the field magnitude and the orbital type of outer shell electrons, as shown by [[group theory|group-theoretical]] considerations. Aspherical deviations might be elicited for instance in [[crystal]]s, where large crystal-electrical fields may occur at [[crystal symmetry|low-symmetry]] lattice sites.<ref name= Bethe1929>{{cite journal|author = Bethe, Hans|title = Termaufspaltung in Kristallen|journal = Annalen der Physik|volume = 3|issue = 2|pages = 133–208|year = 1929|doi = 10.1002/andp.19293950202|bibcode = 1929AnP...395..133B }}</ref><ref name= ZPB1995a>{{cite journal | author = Birkholz, Mario | title = Crystal-field induced dipoles in heteropolar crystals – I. concept | journal = Z. Phys. B | volume = 96 | issue = 3 | pages = 325–332 | year = 1995 | doi = 10.1007/BF01313054 |bibcode = 1995ZPhyB..96..325B | url=https://www.researchgate.net/publication/227050494| citeseerx = 10.1.1.424.5632 | s2cid = 122527743 }}</ref> Significant [[ellipsoid]]al deformations have been shown to occur for sulfur ions<ref name=pssb2008>{{cite journal | author = Birkholz, M. | author2 = Rudert, R. | title = Interatomic distances in pyrite-structure disulfides – a case for ellipsoidal modeling of sulfur ions | journal = Physica Status Solidi B | volume = 245 | issue = 9 | pages = 1858–1864 | year = 2008 | url = https://www.mariobirkholz.de/pssb2008.pdf | doi = 10.1002/pssb.200879532 | bibcode = 2008PSSBR.245.1858B | s2cid = 97824066 | access-date = 2 May 2021 | archive-date = 2 May 2021 | archive-url = https://web.archive.org/web/20210502151542/https://www.mariobirkholz.de/pssb2008.pdf | url-status = live }}</ref> and [[chalcogen]] ions<ref name=mdpi2014>{{cite journal | author = Birkholz, M. | title = Modeling the Shape of Ions in Pyrite-Type Crystals| journal = Crystals | volume = 4 | issue = 3| pages = 390–403 | year = 2014 | doi = 10.3390/cryst4030390| doi-access = free}}</ref> in [[pyrite]]-type compounds. |
|||
The [[magnetic field]] produced by an atom—its [[magnetic moment]]—is determined by these various forms of angular momentum, just as a rotating charged object classically produces a magnetic field. However, the most dominant contribution comes from spin. Due to the nature of electrons to obey the [[Pauli exclusion principle]], in which no two electrons may be found in the same [[quantum state]], bound electrons pair up with each other, with one member of each pair in a spin up state and the other in the opposite, spin down state. Thus these spins cancel each other out, reducing the total magnetic dipole moment to zero in some atoms with even number of electrons.<ref name=schroeder>{{cite web |
|||
| last=Schroeder | first=Paul A. |
|||
| date=[[February 25]], [[2000]] |
|||
| url=http://www.gly.uga.edu/schroeder/geol3010/magnetics.html |
|||
| title=Magnetic Properties |
|||
| publisher=University of Georgia |
|||
| accessdate=2007-01-07 |
|||
}}</ref> |
|||
Atomic dimensions are thousands of times smaller than the wavelengths of [[light]] (400–700 [[nanometre|nm]]) so they cannot be viewed using an [[optical microscope]], although individual atoms can be observed using a [[scanning tunneling microscope]]. To visualize the minuteness of the atom, consider that a typical human hair is about 1 million carbon atoms in width.<ref name=osu2007 /> A single drop of water contains about 2 [[sextillion]] ({{val|2|e=21}}) atoms of oxygen, and twice the number of hydrogen atoms.<ref>{{cite book |
|||
In [[Ferromagnetism|ferromagnetic]] elements such as iron, an odd number of electrons leads to an unpaired electron and a net overall magnetic moment. The orbitals of neighboring atoms overlap and a lower energy state is achieved when the spins of unpaired electrons are aligned with each other, a process is known as an [[exchange interaction]]. When the magnetic moments of ferromagnetic atoms are lined up, the material can produce a measurable macroscopic field. [[Paramagnetism|Paramagnetic materials]] have atoms with magnetic moments that line up in random directions when no magnetic field is present, but the magnetic moments of the individual atoms line up in the presence of a field.<ref>{{cite web |
|||
|last=Padilla|first=Michael J. |
|||
|author2=Miaoulis, Ioannis|author3= Cyr, Martha|year = 2002 |
|||
| date=[[September 1]], [[2007]] |
|||
|title = Prentice Hall Science Explorer: Chemical Building Blocks |
|||
| url=http://www.vectorsite.net/tpqm_04.html |
|||
|publisher = Prentice-Hall, Inc. |
|||
| title=<nowiki>[4.3]</nowiki> Magnetic Properties of the Atom |
|||
|location = Upper Saddle River, New Jersey |
|||
| work=Elementary Quantum Physics |
|||
|isbn = 978-0-13-054091-1 |
|||
| publisher=In The Public Domain website |
|||
|oclc=47925884|page=32 |
|||
| accessdate=2007-01-07 |
|||
|quote=There are 2,000,000,000,000,000,000,000 (that's 2 sextillion) atoms of oxygen in one drop of water—and twice as many atoms of hydrogen.}}</ref> A single [[Carat (unit)|carat]] [[diamond]] with a mass of {{val|2|e=-4|u=kg}} contains about 10 sextillion (10<sup>22</sup>) atoms of [[carbon]].<ref group=note>A carat is 200 milligrams. [[Atomic mass unit|By definition]], carbon-12 has 0.012 kg per mole. The [[Avogadro constant]] defines {{val|6|e=23}} atoms per mole.</ref> If an apple were magnified to the size of the Earth, then the atoms in the apple would be approximately the size of the original apple.<ref>{{cite web |url=https://feynmanlectures.caltech.edu/I_01.html#Ch1-S2-p3 |title=The Feynman Lectures on Physics Vol. I Ch. 1: Atoms in Motion |access-date=3 May 2022 |archive-date=30 July 2022 |archive-url=https://web.archive.org/web/20220730092955/https://www.feynmanlectures.caltech.edu/I_01.html#Ch1-S2-p3 |url-status=live }}</ref> |
|||
}}</ref><ref name=schroeder/> |
|||
=== Radioactive decay === |
|||
The nucleus of an atom can also have a net spin. Normally these nuclei are aligned in random directions because of [[thermal equilibrium]]. However, for certain elements (such as [[xenon|xenon-129]]) it is possible to [[polarization|polarize]] a significant proportion of the nuclear spin states so that they are aligned in the same direction—a condition called [[hyperpolarization (physics)|hyperpolarization]]. This has important applications in [[magnetic resonance imaging]].<ref>{{cite journal |
|||
{{Main|Radioactive decay}} |
|||
| last=Yarris | first=Lynn | title=Talking Pictures |
|||
| journal=Berkeley Lab Research Review |
|||
| date=Spring 1997 |
|||
| url=http://www.lbl.gov/Science-Articles/Research-Review/Magazine/1997/story1.html |
|||
| accessdate=2008-01-09 |
|||
}}</ref><ref>Liang and Haacke (1999: 412–26).</ref> |
|||
[[File:Isotopes and half-life.svg|right|thumb|This diagram shows the [[half-life]] (T<sub>{{frac|1|2}}</sub>) of various isotopes with Z protons and N neutrons.]] |
|||
===Energy levels=== |
|||
{{main|Energy level|Atomic spectral line}} |
|||
Every element has one or more isotopes that have unstable nuclei that are subject to radioactive decay, causing the nucleus to emit particles or electromagnetic radiation. Radioactivity can occur when the radius of a nucleus is large compared with the radius of the strong force, which only acts over distances on the order of 1 fm.<ref name=splung /> |
|||
When an electron is bound to an atom, it has a [[potential energy]] that is inversely proportional to its distance from the nucleus. This is measured by the amount of energy needed to unbind the electron from the atom, and is usually given in units of [[electronvolt]]s (eV). In the quantum mechanical model, a bound electron can only occupy a set of states centered on the nucleus, and each state corresponds to a specific energy level. The lowest energy state of a bound electron is called the ground state, while an electron at a higher energy level is in an excited state.<ref>{{cite web |
|||
| last=Zeghbroeck | first=Bart J. Van | year=1998 |
|||
| url=http://physics.ship.edu/~mrc/pfs/308/semicon_book/eband2.htm |
|||
| title=Energy levels | publisher=Shippensburg University |
|||
| accessdate=2007-12-23 }}</ref> |
|||
The most common forms of radioactive decay are:<ref>{{cite book |
|||
In order for an electron to transition between two different states, it must absorb or emit a [[photon]] at an energy matching the difference in the potential energy of those levels. The energy of an emitted photon is proportional to its [[frequency]], so these specific energy levels appear as distinct bands in the [[electromagnetic spectrum]].<ref>Fowles (1989).</ref> Each element has a characteristic spectrum that can depend on the nuclear charge, subshells filled by electrons, the electromagnetic interactions between the electrons and other factors.<ref>{{cite web |
|||
|last=L'Annunziata<!-- Note: the single quote mark before the name is correct. --> |
|||
| last=Martin | first=W. C. |
|||
|first=Michael F. |
|||
| coauthors=Wiese, W. L. | date=May 2007 |
|||
|year=2003|title=Handbook of Radioactivity Analysis |
|||
| url=http://physics.nist.gov/Pubs/AtSpec/ |
|||
|url=https://archive.org/details/handbookradioact00lann|url-access=limited|publisher=Academic Press|isbn=978-0-12-436603-9 |
|||
| title=Atomic Spectroscopy: A Compendium of Basic Ideas, Notation, Data, and Formulas |
|||
|oclc=16212955|pages=[https://archive.org/details/handbookradioact00lann/page/n22 3]–56}}</ref><ref name=firestone20000522 /> |
|||
| publisher=National Institute of Standards and Technology |
|||
* [[Alpha decay]]: this process is caused when the nucleus emits an alpha particle, which is a helium nucleus consisting of two protons and two neutrons. The result of the emission is a new element with a lower [[atomic number]]. |
|||
| accessdate=2007-01-08 }}</ref> |
|||
* [[Beta decay]] (and [[electron capture]]): these processes are regulated by the [[weak force]], and result from a transformation of a neutron into a proton, or a proton into a neutron. The neutron to proton transition is accompanied by the emission of an electron and an [[antineutrino]], while proton to neutron transition (except in electron capture) causes the emission of a [[positron]] and a [[neutrino]]. The electron or positron emissions are called beta particles. Beta decay either increases or decreases the atomic number of the nucleus by one. Electron capture is more common than positron emission, because it requires less energy. In this type of decay, an electron is absorbed by the nucleus, rather than a positron emitted from the nucleus. A neutrino is still emitted in this process, and a proton changes to a neutron. |
|||
* [[Gamma decay]]: this process results from a change in the energy level of the nucleus to a lower state, resulting in the emission of electromagnetic radiation. The excited state of a nucleus which results in gamma emission usually occurs following the emission of an alpha or a beta particle. Thus, gamma decay usually follows alpha or beta decay. |
|||
Other more rare types of [[radioactive decay]] include ejection of neutrons or protons or clusters of [[nucleon]]s from a nucleus, or more than one [[beta particle]]. An analog of gamma emission which allows excited nuclei to lose energy in a different way, is [[internal conversion]]—a process that produces high-speed electrons that are not beta rays, followed by production of high-energy photons that are not gamma rays. A few large nuclei explode into two or more charged fragments of varying masses plus several neutrons, in a decay called [[Spontaneous fission|spontaneous nuclear fission]]. |
|||
[[Image:Fraunhofer lines.jpg|right|thumb|300px|An example of absorption lines in a spectrum]] |
|||
Each [[radioactive isotope]] has a characteristic decay time period—the [[half-life]]—that is determined by the amount of time needed for half of a sample to decay. This is an [[exponential decay]] process that steadily decreases the proportion of the remaining isotope by 50% every half-life. Hence after two half-lives have passed only 25% of the isotope is present, and so forth.<ref name=splung /> |
|||
When a continuous spectrum of energy is passed through a gas or plasma, some of the photons are absorbed by atoms, causing electrons to change their energy level. Those excited electrons that remain bound to their atom will spontaneously emit this energy as a photon, traveling in a random direction, and so drop back to lower energy levels. Thus the atoms behave like a filter that forms a series of dark [[absorption band]]s in the energy output. (An observer viewing the atoms from a different direction, which does not include the continuous spectrum in the background, will instead see a series of [[Spectral line|emission lines]] from the photons emitted by the atoms.) [[Spectroscopy|Spectroscopic]] measurements of the strength and width of [[spectral line]]s allow the composition and physical properties of a substance to be determined.<ref name=>{{cite web |
|||
| url=http://www.avogadro.co.uk/light/bohr/spectra.htm |
|||
| title=Atomic Emission Spectra - Origin of Spectral Lines |
|||
| publisher=Avogadro Web Site |
|||
| accessdate=2006-08-10 |
|||
}}</ref> |
|||
=== Magnetic moment === |
|||
Close examination of the spectral lines reveals that some display |
|||
{{Main|Electron magnetic moment|Nuclear magnetic moment}} |
|||
a [[fine structure]] splitting. This occurs because of |
|||
[[spin-orbit coupling]], which is an interaction between the |
|||
spin and motion of the outermost electron.<ref>{{cite web |
|||
| last=Fitzpatrick | first=Richard |
|||
| date=[[February 16]], [[2007]] |
|||
| url=http://farside.ph.utexas.edu/teaching/qm/lectures/node55.html |
|||
| title=Fine structure |
|||
| publisher=University of Texas at Austin |
|||
| accessdate=2008-02-14 }}</ref> When an atom is in an external magnetic field, spectral lines become split into three or more components; a phenomenon called the [[Zeeman effect]]. This is caused by the interaction of the magnetic field with the magnetic moment of the atom and its electrons. Some atoms can have multiple [[electron configuration]]s with the same energy level, which thus appear as a single spectral line. The interaction of the magnetic field with the atom shifts these electron configurations to slightly different energy levels, resulting in multiple spectral lines.<ref>{{cite web |
|||
| last=Weiss | first=Michael | year=2001 |
|||
| url=http://math.ucr.edu/home/baez/spin/node8.html |
|||
| title=The Zeeman Effect |
|||
| publisher=University of California-Riverside |
|||
| accessdate=2008-02-06 |
|||
}}</ref> The presence of an external [[electric field]] can cause a comparable splitting and shifting of spectral lines by modifying the electron energy levels, a phenomenon called the [[Stark effect]].<ref>Beyer (2003).</ref> |
|||
Elementary particles possess an intrinsic quantum mechanical property known as [[Spin (physics)|spin]]. This is analogous to the [[angular momentum]] of an object that is spinning around its [[center of mass]], although strictly speaking these particles are believed to be point-like and cannot be said to be rotating. Spin is measured in units of the reduced [[Planck constant]] (ħ), with electrons, protons and neutrons all having spin {{frac|1|2}} ħ, or "spin-{{frac|1|2}}". In an atom, electrons in motion around the [[Atomic nucleus|nucleus]] possess orbital [[angular momentum]] in addition to their spin, while the nucleus itself possesses angular momentum due to its nuclear spin.<ref name=hornak2006 /> |
|||
If a bound electron is in an excited state, an interacting photon with the proper energy can cause [[stimulated emission]] of a photon with a matching energy level. For this to occur, the electron must drop to a lower energy state that has an energy difference matching the energy of the interacting photon. The emitted photon and the interacting photon will then move off in parallel and with matching phases. That is, the wave patterns of the two photons will be synchronized. This physical property is used to make [[laser]]s, which can emit a coherent beam of light energy in a narrow frequency band.<ref>{{cite web |
|||
| last=Watkins |
|||
| first=Thayer |
|||
| url=http://www.sjsu.edu/faculty/watkins/stimem.htm |
|||
| title=Coherence in Stimulated Emission |
|||
| publisher=San José State University |
|||
| accessdate=2007-12-23 |
|||
}}</ref> |
|||
The [[magnetic field]] produced by an atom—its [[magnetic moment]]—is determined by these various forms of angular momentum, just as a rotating charged object classically produces a magnetic field, but the most dominant contribution comes from electron spin. Due to the nature of electrons to obey the [[Pauli exclusion principle]], in which no two electrons may be found in the same [[quantum state]], bound electrons pair up with each other, with one member of each pair in a spin up state and the other in the opposite, spin down state. Thus these spins cancel each other out, reducing the total magnetic dipole moment to zero in some atoms with even number of electrons.<ref name=schroeder2 /> |
|||
===Valence=== |
|||
{{main|Valence (chemistry)}} |
|||
In [[Ferromagnetism|ferromagnetic]] elements such as iron, cobalt and nickel, an odd number of electrons leads to an unpaired electron and a net overall magnetic moment. The orbitals of neighboring atoms overlap and a lower energy state is achieved when the spins of unpaired electrons are aligned with each other, a spontaneous process known as an [[exchange interaction]]. When the magnetic moments of ferromagnetic atoms are lined up, the material can produce a measurable macroscopic field. [[Paramagnetism|Paramagnetic materials]] have atoms with magnetic moments that line up in random directions when no magnetic field is present, but the magnetic moments of the individual atoms line up in the presence of a field.<ref name=schroeder2 /><ref name=goebel20070901 /> |
|||
The outermost electron shell of an atom in its uncombined |
|||
state is known as the valence shell, and the electrons in |
|||
that shell are called [[valence electron]]s. The number of |
|||
valence electrons determines the [[chemical bond|bonding]] |
|||
behavior with other atoms. Atoms tend to [[Chemical reaction|chemically react]] with each other in a manner that will fill (or empty) their outer valence shells.<ref>{{cite web |
|||
| last=Reusch | first=William | date=[[July 16]], [[2007]] |
|||
| url=http://www.cem.msu.edu/~reusch/VirtualText/intro1.htm |
|||
| title=Virtual Textbook of Organic Chemistry |
|||
| publisher=Michigan State University |
|||
| accessdate=2008-01-11 }}</ref> |
|||
The nucleus of an atom will have no spin when it has even numbers of both neutrons and protons, but for other cases of odd numbers, the nucleus may have a spin. Normally nuclei with spin are aligned in random directions because of [[thermal equilibrium]], but for certain elements (such as [[xenon|xenon-129]]) it is possible to [[spin polarization|polarize]] a significant proportion of the nuclear spin states so that they are aligned in the same direction—a condition called [[hyperpolarization (physics)|hyperpolarization]]. This has important applications in [[magnetic resonance imaging]].<ref name=yarris1997 /><ref>{{cite book |
|||
The [[chemical element]]s are often displayed in a [[periodic table]] that is laid out to display recurring chemical properties, and elements with the same number of valence electrons form a group that is aligned in the same column of the table. (The horizontal rows correspond to the filling of a quantum shell of electrons.) The elements at the far right of the table have their outer shell completely filled with electrons, which results in chemically inert elements known as the [[noble gas]]es.<ref>{{cite web |
|||
|last1=Liang|first1=Z.-P.|last2=Haacke|first2=E.M. |
|||
| author=Husted, Robert et al | date=[[December 11]], [[2003]] |
|||
|editor=Webster, J.G.|year=1999 |
|||
| url=http://periodic.lanl.gov/default.htm |
|||
|volume=2 |
|||
| title=Periodic Table of the Elements |
|||
|title=Encyclopedia of Electrical and Electronics Engineering: Magnetic Resonance Imaging |
|||
| publisher=Los Alamos National Laboratory |
|||
|publisher=John Wiley & Sons |
|||
| accessdate=2008-01-11 }} |
|||
|isbn=978-0-471-13946-1|pages=412–426}}</ref> |
|||
</ref><ref>{{cite web |
|||
| first=Rudy | last=Baum | year=2003 |
|||
| url=http://pubs.acs.org/cen/80th/elements.html |
|||
| title=It's Elemental: The Periodic Table |
|||
| publisher=Chemical & Engineering News |
|||
| accessdate=2008-01-11 }}</ref> |
|||
=== |
=== Energy levels === |
||
[[File:Atomic orbital energy levels.svg|thumb|right|These electron's energy levels (not to scale) are sufficient for ground states of atoms up to [[cadmium]] (5s<sup>2</sup> 4d<sup>10</sup>) inclusively. Do not forget that even the top of the diagram is lower than an unbound electron state.]] |
|||
{{main|State of matter|Phase (matter)}} |
|||
The [[potential energy]] of an electron in an atom is [[negative number|negative]] relative to when the [[distance]] from the nucleus [[limit at infinity|goes to infinity]]; its dependence on the electron's [[position (vector)|position]] reaches the [[minimum]] inside the nucleus, roughly in [[inverse proportion]] to the distance. In the quantum-mechanical model, a bound electron can occupy only a set of [[quantum state|states]] centered on the nucleus, and each state corresponds to a specific [[energy level]]; see [[time-independent Schrödinger equation]] for a theoretical explanation. An energy level can be measured by the [[ionization potential|amount of energy needed to unbind]] the electron from the atom, and is usually given in units of [[electronvolt]]s (eV). The lowest energy state of a bound electron is called the ground state, i.e. [[stationary state]], while an electron transition to a higher level results in an excited state.<ref name=zeghbroeck1998 /> The electron's energy increases along with [[principal quantum number|''n'']] because the (average) distance to the nucleus increases. Dependence of the energy on [[azimuthal quantum number|{{ell}}]] is caused not by the [[electrostatic potential]] of the nucleus, but by interaction between electrons. |
|||
For an electron to [[atomic electron transition|transition between two different states]], e.g. [[ground state]] to first [[excited state]], it must absorb or emit a [[photon]] at an energy matching the difference in the potential energy of those levels, according to the [[Niels Bohr]] model, what can be precisely calculated by the [[Schrödinger equation]]. |
|||
[[Image:Bose Einstein condensate.png|left|250px|thumb|These snapshots illustrate the formation of a bose-einstein condensate.]] |
|||
Electrons jump between orbitals in a particle-like fashion. For example, if a single photon strikes the electrons, only a single electron changes states in response to the photon; see [[Atomic orbital|Electron properties]]. |
|||
Quantities of atoms are found in different states of matter that depend on the physical conditions, such as [[temperature]] and [[pressure]]. By varying the conditions, materials can transition between [[solid]]s, [[liquid]]s, [[gas]]es and [[plasma (physics)|plasmas]].<ref>Goodstein (2002).</ref> Within a state, a material can also exist in different phases. An example of this is solid carbon, which can exist as [[graphite]] or [[diamond]].<ref>{{cite journal |
|||
| last=Brazhkin | first=Vadim V. |
|||
| title=Metastable phases, phase transformations, and phase diagrams in physics and chemistry |
|||
| journal=Physics-Uspekhi |
|||
| year=2006 | volume=49 | pages=719–24 |
|||
| doi=10.1070/PU2006v049n07ABEH006013 }}</ref> |
|||
The energy of an emitted photon is proportional to its [[frequency]], so these specific energy levels appear as distinct bands in the [[electromagnetic spectrum]].<ref>{{cite book |
|||
At temperatures close to [[absolute zero]], atoms can form a [[Bose–Einstein condensate]], at which point quantum mechanical effects, which are normally only observed at the atomic scale, become apparent on a macroscopic scale.<ref>Myers (2003).</ref><ref>{{cite news |
|||
|last=Fowles|first=Grant R.|year=1989 |
|||
| author=Staff | date=[[October 9]], [[2001]] |
|||
|title=Introduction to Modern Optics |
|||
| title=Bose-Einstein Condensate: A New Form of Matter |
|||
|url=https://archive.org/details/introductiontomo00fowl_441|url-access=limited|publisher=Courier Dover Publications |
|||
| publisher=National Institute of Standards and Technology |
|||
|isbn=978-0-486-65957-2 |
|||
| url=http://www.nist.gov/public_affairs/releases/BEC_background.htm |
|||
|oclc=18834711|pages=[https://archive.org/details/introductiontomo00fowl_441/page/n233 227]–233}}</ref> Each element has a characteristic spectrum that can depend on the nuclear charge, subshells filled by electrons, the electromagnetic interactions between the electrons and other factors.<ref name=martin2007 /> |
|||
| accessdate=2008-01-16 }}</ref> This super-cooled collection of atoms |
|||
then behaves as a single [[Super Atom]], which may allow fundamental checks of quantum mechanical behavior.<ref>{{cite web |
|||
| last=Colton | first=Imogen | coauthors=Fyffe, Jeanette |
|||
| date=[[February 3]], [[1999]] |
|||
| url=http://www.ph.unimelb.edu.au/~ywong/poster/articles/bec.html |
|||
| title=Super Atoms from Bose-Einstein Condensation |
|||
| publisher=The University of Melbourne |
|||
| accessdate=2008-02-06 }}</ref> |
|||
[[File:Fraunhofer lines.svg|right|thumb|upright=1.5|An example of absorption lines in a spectrum]] |
|||
==Identification== |
|||
[[Image:Atomic resolution Au100.JPG|right|250px|thumb|This [[scanning tunneling microscope]] image clearly shows the individual atoms that make up this [[gold]]([[Miller index|100]]) surface. [[Surface reconstruction|Reconstruction]] causes the surface atoms to deviate from the bulk [[crystal structure]] and arrange in columns several atoms wide with pits between them.]] |
|||
When a continuous [[electromagnetic spectrum|spectrum of energy]] is passed through a gas or plasma, some of the photons are absorbed by atoms, causing electrons to change their energy level. Those excited electrons that remain bound to their atom spontaneously emit this energy as a photon, traveling in a random direction, and so drop back to lower energy levels. Thus the atoms behave like a filter that forms a series of dark [[absorption band]]s in the energy output. (An observer viewing the atoms from a view that does not include the continuous spectrum in the background, instead sees a series of [[emission line]]s from the photons emitted by the atoms.) [[Spectroscopy|Spectroscopic]] measurements of the strength and width of [[atomic spectral line]]s allow the composition and physical properties of a substance to be determined.<ref name=avogadro /> |
|||
The [[scanning tunneling microscope]] is a device for viewing surfaces at the atomic level. It uses the [[quantum tunneling]] phenomenon, which allows particles to pass through a barrier that would normally be insurmountable. Electrons tunnel through the vacuum between two planar metal electrodes, on each of which is an adsorbed atom, providing a tunneling-current density that can be measured. Scanning one atom (taken as the tip) as it moves past the other (the sample) permits plotting of tip displacement versus lateral separation for a constant current. The calculation shows the extent to which scanning-tunneling-microscope images of an individual atom are visible. It confirms that for low bias, the microscope images the space-averaged dimensions of the electron orbitals across closely packed energy levels—the [[Fermi level]] [[local density of states]].<ref>{{cite web |
|||
| last=Jacox | first=Marilyn | coauthors=Gadzuk, J. William |
|||
| url=http://physics.nist.gov/GenInt/STM/stm.html |
|||
| title=Scanning Tunneling Microscope |
|||
| publisher=National Institute of Standards and Technology |
|||
| date=November 1997 | accessdate=2008-01-11 |
|||
}}</ref><ref>{{cite web |
|||
| url=http://nobelprize.org/nobel_prizes/physics/laureates/1986/index.html |
|||
| title=The Nobel Prize in Physics 1986 |
|||
| publisher=The Nobel Foundation |
|||
| accessdate=2008-01-11 }}—in particular, see the Nobel lecture by G. Binnig and H. Rohrer.</ref> |
|||
Close examination of the spectral lines reveals that some display a [[fine structure]] splitting. This occurs because of [[spin–orbit interaction|spin–orbit coupling]], which is an interaction between the spin and motion of the outermost electron.<ref name=fitzpatrick20070216 /> When an atom is in an external magnetic field, spectral lines become split into three or more components; a phenomenon called the [[Zeeman effect]]. This is caused by the interaction of the magnetic field with the magnetic moment of the atom and its electrons. Some atoms can have multiple [[electron configuration]]s with the same energy level, which thus appear as a single spectral line. The interaction of the magnetic field with the atom shifts these electron configurations to slightly different energy levels, resulting in multiple spectral lines.<ref name=weiss2001 /> The presence of an external [[electric field]] can cause a comparable splitting and shifting of spectral lines by modifying the electron energy levels, a phenomenon called the [[Stark effect]].<ref>{{cite book |
|||
An atom can be [[ion]]ized by removing one of its electrons. The [[electric charge]] causes the trajectory of an atom to bend when it passes through a [[magnetic field]]. The radius by which the trajectory of a moving ion is turned by the magnetic field is determined by the mass of the atom. The [[Mass spectrometry|mass spectrometer]] uses this principle to measure the [[mass-to-charge ratio]] of ions. If a sample contains multiple isotopes, the mass spectrometer can determine the proportion of each isotope in the sample by measuring the intensity of the different beams of ions. Techniques to vaporize atoms include [[inductively coupled plasma atomic emission spectroscopy]] and [[inductively coupled plasma mass spectrometry]], both of which use a plasma to vaporize samples for analysis.<ref>{{cite journal |
|||
|last1=Beyer|first1=H.F. |
|||
| first=N. | last=Jakubowski |
|||
|last2=Shevelko|first2=V.P. |
|||
| coauthors = Moens, L.; Vanhaecke, F |
|||
|year=2003 |
|||
| title = Sector field mass spectrometers in ICP-MS |
|||
|title=Introduction to the Physics of Highly Charged Ions |
|||
| journal = Spectrochimica Acta Part B: Atomic Spectroscopy |
|||
|publisher=CRC Press|isbn=978-0-7503-0481-8 |
|||
| volume = 53 | issue = 13 | year = 1998 |
|||
|oclc=47150433|pages=232–236}}</ref> |
|||
If a bound electron is in an excited state, an interacting photon with the proper energy can cause [[stimulated emission]] of a photon with a matching energy level. For this to occur, the electron must drop to a lower energy state that has an energy difference matching the energy of the interacting photon. The emitted photon and the interacting photon then move off in parallel and with matching phases. That is, the wave patterns of the two photons are synchronized. This physical property is used to make [[laser]]s, which can emit a coherent beam of light energy in a narrow frequency band.<ref name=watkins_sjsu /> |
|||
A more area-selective method is [[electron energy loss spectroscopy]], which measures the energy loss of an [[electron beam]] within a [[transmission electron microscope]] when it interacts with a portion of a sample. The [[atom probe|atom-probe tomograph]] has sub-nanometer resolution in 3-D and can chemically identify individual atoms using time-of-flight mass spectrometry.<ref>{{cite journal |
|||
| last=Müller | first=Erwin W. |
|||
| authorlink=Erwin Müller |
|||
|coauthors=[[J. A. Panitz|Panitz, John A.]], [[S. Brooks McLane|McLane, S. Brooks]] |
|||
| year=1968 |
|||
| title=The Atom-Probe Field Ion Microscope |
|||
| journal=Review of Scientific Instruments |
|||
| volume=39 | issue=1 | pages=83–86 |
|||
| issn=0034-6748 | doi=10.1063/1.1683116 }}</ref> |
|||
=== Valence and bonding behavior === |
|||
Spectra of [[excited state]]s can be used to analyze the atomic composition of distant [[star]]s. Specific light [[wavelength]]s contained in the observed light from stars can be separated out and related to the quantized transitions in free gas atoms. These colors can be replicated using a [[gas-discharge lamp]] containing the same element.<ref>{{cite web |
|||
{{Main|Valence (chemistry)|Chemical bond}} |
|||
| last=Lochner | first=Jim |
|||
| coauthors=Gibb, Meredith; Newman, Phil |
|||
| date=[[April 30]], [[2007]] |
|||
| url=http://imagine.gsfc.nasa.gov/docs/science/how_l1/spectral_what.html |
|||
| title=What Do Spectra Tell Us? |
|||
| publisher=NASA/Goddard Space Flight Center |
|||
| accessdate=2008-01-03 }}</ref> [[Helium]] was discovered in this way in the spectrum of the Sun 23 years before it was found on Earth.<ref>{{cite web |
|||
| last=Winter | first=Mark | year=2007 |
|||
| url=http://www.webelements.com/webelements/elements/text/He/hist.html |
|||
| title=Helium | publisher=WebElements |
|||
| accessdate=2008-01-03 }}</ref> |
|||
Valency is the combining power of an element. It is determined by the number of bonds it can form to other atoms or groups.<ref>{{GoldBookRef|title=valence|file=V06588}}</ref> The outermost electron shell of an atom in its uncombined state is known as the [[valence shell]], and the electrons in |
|||
==Origin and current state== |
|||
that shell are called [[valence electron]]s. The number of valence electrons determines the [[chemical bond|bonding]] |
|||
Atoms form about 4% of the total mass density of the observable [[universe]], with an average density of about 0.25 atoms/m<sup>3</sup>.<ref>{{cite web |
|||
behavior with other atoms. Atoms tend to [[Chemical reaction|chemically react]] with each other in a manner that fills (or empties) their outer valence shells.<ref name=reusch20070716 /> For example, a transfer of a single electron between atoms is a useful approximation for bonds that form between atoms with one-electron more than a filled shell, and others that are one-electron short of a full shell, such as occurs in the compound [[sodium chloride]] and other chemical ionic salts. Many elements display multiple valences, or tendencies to share differing numbers of electrons in different compounds. Thus, [[chemical bond]]ing between these elements takes many forms of electron-sharing that are more than simple electron transfers. Examples include the element carbon and the [[organic compounds]].<ref name=chemguide /> |
|||
| last=Hinshaw | first=Gary |
|||
| date=[[February 10]], [[2006]] |
|||
| url=http://map.gsfc.nasa.gov/m_uni/uni_101matter.html |
|||
| title=What is the Universe Made Of? |
|||
| publisher=NASA/WMAP | accessdate=2008-01-07 }}</ref> Within a galaxy such as the [[Milky Way]], atoms have a much higher concentration, with the density of matter in the [[interstellar medium]] (ISM) ranging from 10<sup>5</sup> to 10<sup>9</sup> atoms/m<sup>3</sup>.<ref>Choppin ''et al'' (2001).</ref> The Sun is believed to be inside the [[Local Bubble]], a region of highly ionized gas, so the density in the solar neighborhood is only about 10<sup>3</sup> atoms/m<sup>3</sup>.<ref>{{cite journal |
|||
| last=Davidsen | first=Arthur F. |
|||
| title=Far-Ultraviolet Astronomy on the Astro-1 Space Shuttle Mission |
|||
| journal=Science | year=1993 | volume=259 |
|||
| issue=5093 | pages=327–34 |
|||
| url=http://www.sciencemag.org/cgi/content/abstract/259/5093/327 |
|||
| accessdate=2008-01-07 }}</ref> Stars form from dense clouds in the ISM, and the evolutionary processes of stars result in the steady enrichment of the ISM with elements more massive than hydrogen and helium. Up to 95% of the Milky Way's atoms are concentrated inside stars and the total mass of atoms forms about 10% of the mass of the galaxy.<ref>Lequeux (2005).</ref> (The remainder of the mass is an unknown [[dark matter]].<ref>{{cite web |
|||
| first=Nigel | last=Smith | date=[[January 6]], [[2000]] |
|||
| url=http://physicsworld.com/cws/article/print/809 |
|||
| title=The search for dark matter |
|||
| publisher=Physics World | accessdate = 2008-02-14 }}</ref>) |
|||
The [[chemical element]]s are often displayed in a [[periodic table]] that is laid out to display recurring chemical properties, and elements with the same number of valence electrons form a group that is aligned in the same column of the table. (The horizontal rows correspond to the filling of a quantum shell of electrons.) The elements at the far right of the table have their outer shell completely filled with electrons, which results in chemically inert elements known as the [[noble gas]]es.<ref name=husted20031211 /><ref name=baum2003 /> |
|||
===Nucleosynthesis=== |
|||
{{main|Nucleosynthesis}} |
|||
Stable protons and electrons appeared one second after the [[Big Bang]]. During the following three minutes, [[Big Bang nucleosynthesis]] produced most of the [[helium]], [[lithium]], and [[deuterium]] atoms in the universe, and perhaps some of the [[beryllium]] and [[boron]].<ref>{{cite journal |
|||
| last=Croswell | first=Ken |
|||
| title=Boron, bumps and the Big Bang: Was matter spread evenly when the Universe began? Perhaps not; the clues lie in the creation of the lighter elements such as boron and beryllium |
|||
| journal=New Scientist | year=1991 | issue=1794 | pages=42 |
|||
| url=http://space.newscientist.com/article/mg13217944.700-boron-bumps-and-the-big-bang-was-matter-spread-evenly-whenthe-universe-began-perhaps-not-the-clues-lie-in-the-creation-of-thelighter-elements-such-as-boron-and-beryllium.html |
|||
| accessdate=2008-01-14 }}</ref><ref>{{cite journal |
|||
| last=Copi | first=Craig J. |
|||
| coauthors=Schramm, David N.; Turner, Michael S |
|||
| title=Big-Bang Nucleosynthesis and the Baryon Density of the Universe |
|||
| journal=Science | year=1995 | volume=267 | pages=192–99 |
|||
| url=http://www.sciencemag.org/cgi/reprint/267/5195/192.pdf |
|||
| doi = 10.1126/science.7809624 <!--Retrieved from url by DOI bot--> |
|||
| format=PDF | accessdate=2008-01-13 |pmid=7809624 |
|||
}}</ref><ref>{{cite web |
|||
| last=Hinshaw | first=Gary |
|||
| date=[[December 15]], [[2005]] |
|||
| url=http://map.gsfc.nasa.gov/m_uni/uni_101bbtest2.html |
|||
| title=Tests of the Big Bang: The Light Elements |
|||
| publisher=NASA/WMAP | accessdate=2008-01-13 |
|||
}}</ref> The first atoms (complete with bound electrons) were theoretically created 380,000 years after the Big Bang—an epoch called [[Timeline of the Big Bang#Recombination: 380,000 years|recombination]], when the expanding universe cooled enough to allow electrons to become attached to nuclei.<ref>{{cite web |
|||
| last=Abbott | first=Brian | date=[[May 30]], [[2007]] |
|||
| url=http://www.haydenplanetarium.org/universe/duguide/exgg_wmap.php |
|||
| title=Microwave (WMAP) All-Sky Survey |
|||
| publisher=Hayden Planetarium | accessdate=2008-01-13 |
|||
}}</ref> Since then, atomic nuclei have been combined in [[star]]s through the process of [[nuclear fusion]] to produce elements up to iron.<ref>{{cite journal |
|||
| title=The synthesis of the elements from hydrogen |
|||
| author = F. Hoyle |
|||
| journal = [[Monthly Notices of the Royal Astronomical Society]] |
|||
| volume = 106 | pages = 343–83 | year=1946 |
|||
| url=http://adsabs.harvard.edu/abs/1946MNRAS.106..343H |
|||
| accessdate=2008-01-13 }}</ref> |
|||
=== States === |
|||
Isotopes such as lithium-6 are generated in space through [[cosmic ray spallation]].<ref>{{cite journal |
|||
{{Main|State of matter|Phase (matter)}} |
|||
| last=Knauth | first=D. C. |
|||
| coauthors=Federman, S. R.; Lambert, David L.; Crane, P. |
|||
| title=Newly synthesized lithium in the interstellar medium |
|||
| journal=Nature | year=2000 | volume=405 | pages=656–58 |
|||
| doi=10.1038/35015028 }}</ref> This occurs when a high-energy proton strikes an atomic nucleus, causing large numbers of nucleons to be ejected. Elements heavier than iron were produced in [[supernova]]e through the [[r-process]] and in [[Asymptotic giant branch|AGB stars]] through the [[s-process]], both of which involve the capture of neutrons by atomic nuclei.<ref>{{cite web |
|||
| last=Mashnik | first=Stepan G. |
|||
| title=On Solar System and Cosmic Rays Nucleosynthesis and Spallation Processes |
|||
| url=http://arxiv.org/abs/astro-ph/0008382 |
|||
| date=August 2000 | publisher=Cornell University |
|||
| accessdate=2008-01-14 }}</ref> Elements such as [[lead]] formed largely through the radioactive decay of heavier elements.<ref>{{cite web |
|||
| author=Kansas Geological Survey | date=[[May 4]], [[2005]] |
|||
| url=http://www.kgs.ku.edu/Extension/geotopics/earth_age.html |
|||
| title=Age of the Earth | publisher=University of Kansas |
|||
| accessdate=2008-01-14 }}</ref> |
|||
[[File:Bose Einstein condensate.png|right|thumb|Graphic illustrating the formation of a [[Bose–Einstein condensate]] ]] |
|||
===Earth=== |
|||
Quantities of atoms are found in different states of matter that depend on the physical conditions, such as [[temperature]] and [[pressure]]. By varying the conditions, materials can transition between [[solid]]s, [[liquid]]s, [[gas]]es and [[plasma (physics)|plasmas]].<ref> |
|||
{{cite book |
|||
|last=Goodstein|first=David L.|year=2002 |
|||
|title=States of Matter|url=https://archive.org/details/statesmatter00good_082|url-access=limited|publisher=Courier Dover Publications |
|||
|isbn=978-0-13-843557-8|pages=[https://archive.org/details/statesmatter00good_082/page/n445 436]–438 |
|||
}}</ref> Within a state, a material can also exist in different [[allotropes]]. An example of this is solid carbon, which can exist as [[graphite]] or [[diamond]].<ref name=pu49_7_719 /> Gaseous allotropes exist as well, such as [[dioxygen]] and [[ozone]]. |
|||
At temperatures close to [[absolute zero]], atoms can form a [[Bose–Einstein condensate]], at which point quantum mechanical effects, which are normally only observed at the atomic scale, become apparent on a macroscopic scale.<ref> |
|||
Most of the atoms that make up the Earth and its inhabitants were present in their current form in the [[nebula]] that collapsed out of a [[molecular cloud]] to form the solar system. The rest are the result of radioactive decay, and their relative proportion can be used to determine the [[age of the Earth]] through [[radiometric dating]].<ref name = "Manuel_2001">Manuel (2001).</ref><ref>{{cite journal |
|||
{{cite book |
|||
| last=Dalrymple | first=G. Brent |
|||
|last=Myers|first=Richard|year=2003 |
|||
| title=The age of the Earth in the twentieth century: a problem (mostly) solved |
|||
|title=The Basics of Chemistry|url=https://archive.org/details/basicschemistry00myer|url-access=limited|publisher=Greenwood Press |
|||
| journal=Geological Society, London, Special Publications |
|||
|isbn=978-0-313-31664-7 |
|||
| year=2001 | volume=190 | pages=205–21 |
|||
|oclc=50164580|page=[https://archive.org/details/basicschemistry00myer/page/n98 85] |
|||
| doi=10.1144/GSL.SP.2001.190.01.14 |
|||
}}</ref><ref name=nist_bec /> This super-cooled collection of atoms |
|||
| url=http://sp.lyellcollection.org/cgi/content/abstract/190/1/205 |
|||
then behaves as a single [[super atom]], which may allow fundamental checks of quantum mechanical behavior.<ref name=colton_fyffe1999 /> |
|||
| accessdate=2008-01-14 }}</ref> Most of the [[helium]] in the crust of the Earth (about 99% of the helium from gas wells, as shown by its lower abundance of [[helium-3]]) is a product of [[alpha decay]].<;;ref>{{cite web |
|||
{{clear}} |
|||
| last=Anderson | first=Don L. |
|||
| authorlink=Don L. Anderson |
|||
| coauthors=Foulger, G. R.; Meibom, Anders |
|||
| date=[[September 2]], [[2006]] |
|||
| url=http://www.mantleplumes.org/HeliumFundamentals.html |
|||
| title=Helium: Fundamental models |
|||
| publisher=MantlePlumes.org | accessdate=2007-01-14 }}</ref> |
|||
== Identification == |
|||
There are a few trace atoms on Earth that were not present at the beginning (i.e., not "primordial"), nor are results of radioactive decay. [[Carbon-14]] is continuously generated by cosmic rays in the atmosphere.<ref>{{cite news |
|||
[[File:Atomic resolution Au100.JPG|right|thumb|[[Scanning tunneling microscope]] image showing the individual atoms making up this [[gold]] ([[Miller index|100]]) surface. The surface atoms deviate from the bulk [[crystal structure]] and arrange in columns several atoms wide with pits between them (See [[surface reconstruction]]).]] |
|||
| last=Pennicott | first=Katie | date=[[May 10]], [[2001]] |
|||
While atoms are too small to be seen, devices such as the [[scanning tunneling microscope]] (STM) enable their visualization at the surfaces of solids. The microscope uses the [[quantum tunneling]] phenomenon, which allows particles to pass through a barrier that would be insurmountable in the classical perspective. Electrons tunnel through the vacuum between two [[Biasing|biased]] electrodes, providing a tunneling current that is exponentially dependent on their separation. One electrode is a sharp tip ideally ending with a single atom. At each point of the scan of the surface the tip's height is adjusted so as to keep the tunneling current at a set value. How much the tip moves to and away from the surface is interpreted as the height profile. For low bias, the microscope images the averaged electron orbitals across closely packed energy levels—the local [[density of states|density of the electronic states]] near the [[Fermi level]].<ref name=jacox1997 /><ref name=nf_physics1986 /> Because of the distances involved, both electrodes need to be extremely stable; only then periodicities can be observed that correspond to individual atoms. The method alone is not chemically specific, and cannot identify the atomic species present at the surface. |
|||
| title=Carbon clock could show the wrong time |
|||
| publisher=PhysicsWeb |
|||
| url=http://physicsworld.com/cws/article/news/2676 |
|||
| accessdate=2008-01-14 }}</ref> Some atoms on Earth have been artificially generated either deliberately or as by-products of nuclear reactors or explosions.<ref>{{cite news |
|||
| last=Yarris |
|||
| first=Lynn |
|||
| date=[[July 27]], [[2001]] |
|||
| title=New Superheavy Elements 118 and 116 Discovered at Berkeley Lab |
|||
| publisher=Berkeley Lab |
|||
| url=http://enews.lbl.gov/Science-Articles/Archive/elements-116-118.html |
|||
| accessdate=2008-01-14 |
|||
}}</ref><ref>{{cite journal |
|||
| author=Diamond, H. ''et al'' |
|||
| title=Heavy Isotope Abundances in Mike Thermonuclear Device |
|||
| journal=Physical Review |
|||
| year=1960 |
|||
| volume=119 |
|||
| pages=2000–04 |
|||
| url=http://prola.aps.org/abstract/PR/v119/i6/p2000_1 |
|||
| accessdate=2008-01-14 }}</ref> Of the [[Transuranium element|transuranic elements]]—those with atomic numbers greater than 92—only plutonium and [[neptunium]] occur naturally on Earth.<ref>{{cite web |
|||
| author=Poston Sr., John W. | date=[[March 23]], [[1998]] |
|||
| title=Do transuranic elements such as plutonium ever occur naturally? |
|||
| publisher=Scientific American |
|||
| url=http://www.sciam.com/chemistry/article/id/do-transuranic-elements-s/topicID/4/catID/3 |
|||
| accessdate=2008-01-15 |
|||
}}</ref><ref>{{cite journal |
|||
| last=Keller | first=C. |
|||
| title=Natural occurrence of lanthanides, actinides, and superheavy elements |
|||
| journal=Chemiker Zeitung |
|||
| year=1973 | volume=97 | issue=10 | pages=522–30 |
|||
| url=http://www.osti.gov/energycitations/product.biblio.jsp?osti_id=4353086 |
|||
| accessdate=2008-01-15 }}</ref> Transuranic elements have radioactive lifetimes shorter than the current age of the Earth<ref>Marco (2001).</ref> and thus identifiable quantities of these elements have long since decayed, with the exception of traces of [[plutonium-244]] possibly deposited by cosmic dust.<ref name = "Manuel_2001"/> Natural deposits of plutonium and neptunium are produced by [[neutron capture]] in uranium ore.<ref>{{cite web |
|||
| url=http://www.oklo.curtin.edu.au/index.cfm |
|||
| title=Oklo Fossil Reactors |
|||
| publisher=Curtin University of Technology |
|||
| accessdate=2008-01-15 }}</ref> |
|||
Atoms can be easily identified by their mass. If an atom is [[ion]]ized by removing one of its electrons, its trajectory when it passes through a [[magnetic field]] will bend. The radius by which the trajectory of a moving ion is turned by the magnetic field is determined by the mass of the atom. The [[Mass spectrometry|mass spectrometer]] uses this principle to measure the [[mass-to-charge ratio]] of ions. If a sample contains multiple isotopes, the mass spectrometer can determine the proportion of each isotope in the sample by measuring the intensity of the different beams of ions. Techniques to vaporize atoms include [[inductively coupled plasma atomic emission spectroscopy]] and [[inductively coupled plasma mass spectrometry]], both of which use a plasma to vaporize samples for analysis.<ref name=sab53_13_1739 /> |
|||
The Earth contains approximately 1.33{{e|50}} atoms.<ref>{{cite web |
|||
| last=Weisenberger | first=Drew |
|||
| url=http://education.jlab.org/qa/mathatom_05.html |
|||
| title=How many atoms are there in the world? |
|||
| publisher=Jefferson Lab |
|||
| accessdate=2008-01-16 }}</ref> In the planet's atmosphere, small numbers of independent atoms exist for the [[noble gas]]es, such as [[argon]] and [[neon]]. The remaining 99% of the atmosphere is bound in the form of molecules, including [[carbon dioxide]] and [[Diatomic molecule|diatomic]] [[oxygen]] and [[nitrogen]]. At the surface of the Earth, atoms combine to form various compounds, including [[water]], [[salt]], [[silicate]]s and [[oxide]]s. Atoms can also combine to create materials that do not consist of discrete molecules, including [[crystal]]s and liquid or solid [[metal]]s.<ref>{{cite web |
|||
| last=Pidwirny | first=Michael |
|||
| url=http://www.physicalgeography.net/fundamentals/contents.html |
|||
| title=Fundamentals of Physical Geography |
|||
| publisher=University of British Columbia Okanagan |
|||
| accessdate=2008-01-16 |
|||
}}</ref><ref>{{cite journal |
|||
| last=Anderson | first=Don L. |
|||
| title=The inner inner core of Earth |
|||
| journal=Proceedings of the National Academy of Science |
|||
| year=2002 | volume=99 | issue=22 | pages=13966–68 |
|||
| url=http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=137819 |
|||
| accessdate=2008-01-16 | doi=10.1073/pnas.232565899 }}</ref> This atomic matter forms networked arrangements that lack the particular type of small-scale interrupted order associated with molecular matter.<ref>Pauling (1960).</ref> |
|||
The [[atom probe|atom-probe tomograph]] has sub-nanometer resolution in 3-D and can chemically identify individual atoms using [[time-of-flight mass spectrometry]].<ref name=rsi39_1_83 /> |
|||
===Rare and theoretical forms=== |
|||
While isotopes with atomic numbers higher than [[lead]] (82) are known to be radioactive, an "[[island of stability]]" has been proposed for some elements with atomic numbers above 103. These [[superheavy element]]s may have a nucleus that is relatively stable against radioactive decay.<ref>{{cite journal |
|||
| title=Second postcard from the island of stability |
|||
| author=Anonymous | journal=CERN Courier |
|||
| date=[[October 2]], [[2001]] |
|||
| url=http://cerncourier.com/cws/article/cern/28509 |
|||
| accessdate=2008-01-14 }}</ref> The most likely candidate for a stable superheavy atom, [[unbihexium]], has 126 protons and 184 neutrons.<ref>{{cite journal |
|||
| last=Jacoby | first=Mitch |
|||
| title=As-yet-unsynthesized superheavy atom should form a stable diatomic molecule with fluorine |
|||
| journal=Chemical & Engineering News |
|||
| year=2006 | volume=84 | issue=10 | pages=19 |
|||
| url=http://pubs.acs.org/cen/news/84/i10/8410notw9.html |
|||
| accessdate=2008-01-14 }}</ref> |
|||
Electron emission techniques such as [[X-ray photoelectron spectroscopy]] (XPS) and [[Auger electron spectroscopy]] (AES), which measure the binding energies of the [[core electron]]s, are used to identify the atomic species present in a sample in a non-destructive way. With proper focusing both can be made area-specific. Another such method is [[electron energy loss spectroscopy]] (EELS), which measures the energy loss of an [[electron beam]] within a [[transmission electron microscope]] when it interacts with a portion of a sample. |
|||
Each particle of matter has a corresponding [[antimatter]] particle with the opposite electrical charge. Thus, the [[positron]] is a positively charged antielectron and the antiproton is a negatively charged equivalent of a proton. For unknown reasons, antimatter particles are rare in the universe, hence, no antimatter atoms have been discovered.<ref>{{cite news |
|||
| last=Koppes | first=Steve | date=[[March 1]], [[1999]] |
|||
| title=Fermilab Physicists Find New Matter-Antimatter Asymmetry |
|||
| publisher=University of Chicago |
|||
| url=http://www-news.uchicago.edu/releases/99/990301.ktev.shtml |
|||
| accessdate=2008-01-14 |
|||
}}</ref><ref>{{cite news |
|||
| last=Cromie | first=William J. | date=[[August 16]], [[2001]] |
|||
| title=A lifetime of trillionths of a second: Scientists explore antimatter |
|||
| publisher=Harvard University Gazette |
|||
| url=http://www.hno.harvard.edu/gazette/2001/08.16/antimatter.html |
|||
| accessdate=2008-01-14 |
|||
}}</ref> [[Antihydrogen]], the antimatter counterpart of hydrogen, was first produced at the [[CERN]] laboratory in [[Geneva]] in 1996.<ref>{{cite journal |
|||
| last=Hijmans | first=Tom W. |
|||
| title=Particle physics: Cold antihydrogen |
|||
| journal=Nature | year=2002 | volume=419 |
|||
| pages=439–40 | doi=10.1038/419439a |
|||
}}</ref><ref>{{cite news |
|||
| author=Staff | date=[[October 30]], [[2002]] |
|||
| title=Researchers 'look inside' antimatter | publisher=BBC News |
|||
| url=http://news.bbc.co.uk/2/hi/science/nature/2375717.stm |
|||
| accessdate=2008-01-14 }}</ref> |
|||
Spectra of [[excited state]]s can be used to analyze the atomic composition of distant [[star]]s. Specific light [[wavelength]]s contained in the observed light from stars can be separated out and related to the quantized transitions in free gas atoms. These colors can be replicated using a [[gas-discharge lamp]] containing the same element.<ref name=lochner2007 /> [[Helium]] was discovered in this way in the spectrum of the Sun 23 years before it was found on Earth.<ref name=winter2007 /> |
|||
Other [[exotic atom]]s have been created by replacing one of the protons, neutrons or electrons with other particles that have the same charge. For example, an electron can be replaced by a more massive [[muon]], forming a [[muonic atom]]. These types of atoms can be used to test the fundamental predictions of physics.<ref>{{cite journal |
|||
| last=Barrett | first=Roger |
|||
| coauthors=Jackson, Daphne; Mweene, Habatwa |
|||
| title=The Strange World of the Exotic Atom |
|||
| journal=New Scientist |
|||
| year=1990 | issue=1728 | pages=77–115 |
|||
| url=http://media.newscientist.com/article/mg12717284.600-the-strange-world-of-the-exotic-atom-physicists-can-nowmake-atoms-and-molecules-containing-negative-particles-other-than-electronsand-use-them-not-just-to-test-theories-but-also-to-fight-cancer-.html |
|||
| accessdate=2008-01-04 }} |
|||
</ref><ref>{{cite journal |
|||
| last=Indelicato | first=Paul |
|||
| title=Exotic Atoms | journal=Physica Scripta |
|||
| year=2004 | volume=T112 | pages=20–26 |
|||
| doi=10.1238/Physica.Topical.112a00020 }} |
|||
</ref><ref>{{cite web |
|||
| last=Ripin | first=Barrett H. | date=July 1998 |
|||
| url=http://www.aps.org/publications/apsnews/199807/experiment.cfm |
|||
| title=Recent Experiments on Exotic Atoms |
|||
| publisher=American Physical Society |
|||
| accessdate=2008-02-15 }}</ref> |
|||
== Origin and current state == |
|||
==See also== |
|||
[[Baryonic matter]] forms about 4% of the total energy density of the [[observable universe]], with an average density of about 0.25 particles/m<sup>3</sup> (mostly [[proton]]s and electrons).<ref name=hinshaw20060210 /> Within a galaxy such as the [[Milky Way]], particles have a much higher concentration, with the density of matter in the [[interstellar medium]] (ISM) ranging from 10<sup>5</sup> to 10<sup>9</sup> atoms/m<sup>3</sup>.<ref> |
|||
{{col-start}} |
|||
{{cite book |
|||
{{col-break}} |
|||
|last1=Choppin|first1=Gregory R. |
|||
* [[Introduction to quantum mechanics]] |
|||
|last2=Liljenzin|first2=Jan-Olov|last3=Rydberg|first3=Jan |
|||
|year=2001|title=Radiochemistry and Nuclear Chemistry |
|||
|publisher=Elsevier|isbn=978-0-7506-7463-8 |
|||
|oclc=162592180|page=441 |
|||
}}</ref> The Sun is believed to be inside the [[Local Bubble]], so the density in the [[solar neighborhood]] is only about 10<sup>3</sup> atoms/m<sup>3</sup>.<ref name=science259_5093_327 /> Stars form from dense clouds in the ISM, and the evolutionary processes of stars result in the steady enrichment of the ISM with elements more massive than hydrogen and helium. |
|||
Up to 95% of the Milky Way's baryonic matter are concentrated inside stars, where conditions are unfavorable for atomic matter. The total baryonic mass is about 10% of the mass of the galaxy;<ref> |
|||
{{cite book |
|||
|last=Lequeux|first=James|year=2005 |
|||
|title=The Interstellar Medium |
|||
|url=https://archive.org/details/interstellarmedi00ryte|url-access=limited|publisher=Springer|isbn=978-3-540-21326-0 |
|||
|oclc=133157789|page=[https://archive.org/details/interstellarmedi00ryte/page/n411 4] |
|||
}}</ref> the remainder of the mass is an unknown [[dark matter]].<ref name=nigel2000 /> High [[temperature]] inside stars makes most "atoms" fully ionized, that is, separates ''all'' electrons from the nuclei. In [[stellar remnant]]s—with exception of their surface layers—an immense [[pressure]] make electron shells impossible. |
|||
=== Formation === |
|||
{{Main|Nucleosynthesis}} |
|||
[[File:Nucleosynthesis periodic table.svg|thumb|600px|Periodic table showing the origin of each element. Elements from carbon up to sulfur may be made in small stars by the [[alpha process]]. Elements beyond iron are made in large stars with slow neutron capture ([[s-process]]). Elements heavier than iron may be made in neutron star mergers or supernovae after the [[r-process]].]] |
|||
Electrons are thought to exist in the Universe since early stages of the [[Big Bang]]. Atomic nuclei forms in [[nucleosynthesis]] reactions. In about three minutes [[Big Bang nucleosynthesis]] produced most of the [[helium]], [[lithium]], and [[deuterium]] in the Universe, and perhaps some of the [[beryllium]] and [[boron]].<ref name=ns1794_42 /><ref name=science267_5195_192 /><ref name=hinshaw20051215 /> |
|||
Ubiquitousness and stability of atoms relies on their [[binding energy]], which means that an atom has a lower energy than an unbound system of the nucleus and electrons. Where the [[temperature]] is much higher than [[ionization potential]], the matter exists in the form of [[plasma (physics)|plasma]]—a gas of positively charged ions (possibly, bare nuclei) and electrons. When the temperature drops below the ionization potential, atoms become [[statistical physics|statistically]] favorable. Atoms (complete with bound electrons) became to dominate over [[charged particle]]s 380,000 years after the Big Bang—an epoch called [[recombination (cosmology)|recombination]], when the expanding Universe cooled enough to allow electrons to become attached to nuclei.<ref name=abbott20070530 /> |
|||
Since the Big Bang, which produced no [[carbon]] or [[atomic number|heavier elements]], atomic nuclei have been combined in [[star]]s through the process of [[nuclear fusion]] to produce more of the element [[helium]], and (via the [[triple-alpha process]]) the sequence of elements from carbon up to [[iron]];<ref name=mnras106_343 /> see [[stellar nucleosynthesis]] for details. |
|||
Isotopes such as lithium-6, as well as some beryllium and boron are generated in space through [[cosmic ray spallation]].<ref name=nature405_656 /> This occurs when a high-energy proton strikes an atomic nucleus, causing large numbers of nucleons to be ejected. |
|||
Elements heavier than iron were produced in [[supernova]]e and colliding [[neutron star]]s through the [[r-process]], and in [[Asymptotic giant branch|AGB stars]] through the [[s-process]], both of which involve the capture of neutrons by atomic nuclei.<ref name=mashnik2000 /> Elements such as [[lead]] formed largely through the radioactive decay of heavier elements.<ref name=kgs20050504 /> |
|||
=== Earth === |
|||
Most of the atoms that make up the [[Earth]] and its inhabitants were present in their current form in the [[nebula]] that collapsed out of a [[molecular cloud]] to form the [[Solar System]]. The rest are the result of radioactive decay, and their relative proportion can be used to determine the [[age of the Earth]] through [[radiometric dating]].<ref name=Manuel2001pp511-519>[[#refManuel2001|Manuel (2001). ''Origin of Elements in the Solar System'', pp. 40–430, 511–519]]</ref><ref name=gs190_1_205 /> Most of the [[helium]] in the crust of the Earth (about 99% of the helium from gas wells, as shown by its lower abundance of [[helium-3]]) is a product of [[alpha decay]].<ref name=anderson_foulger_meibom2006 /> |
|||
There are a few trace atoms on Earth that were not present at the beginning (i.e., not "primordial"), nor are results of radioactive decay. [[Carbon-14]] is continuously generated by cosmic rays in the atmosphere.<ref name=pennicott2001 /> Some atoms on Earth have been artificially generated either deliberately or as by-products of nuclear reactors or explosions.<ref name=yarris2001 /><ref name=pr119_6_2000 /> Of the [[Transuranium element|transuranic elements]]—those with atomic numbers greater than 92—only [[plutonium]] and [[neptunium]] occur naturally on Earth.<ref name=poston1998 /><ref name=cz97_10_522 /> Transuranic elements have radioactive lifetimes shorter than the current age of the Earth<ref>{{cite book|last1=Zaider|first1=Marco|last2=Rossi|first2=Harald H.|year=2001|title=Radiation Science for Physicians and Public Health Workers|publisher=Springer|isbn=978-0-306-46403-4|oclc=44110319|page=[https://archive.org/details/radiationscience0000zaid/page/17 17]|url=https://archive.org/details/radiationscience0000zaid/page/17}}</ref> and thus identifiable quantities of these elements have long since decayed, with the exception of traces of [[plutonium-244]] possibly deposited by cosmic dust.<ref name=Manuel2001pp511-519 /> Natural deposits of plutonium and neptunium are produced by [[neutron capture]] in uranium ore.<ref name=ofr_cut /> |
|||
The Earth contains approximately {{val|1.33|e=50}} atoms.<ref name=weisenberger /> Although small numbers of independent atoms of [[noble gas]]es exist, such as [[argon]], [[neon]], and [[helium]]<!-- note that noble gases exist not only in the atmosphere -->, 99% of [[Earth's atmosphere|the atmosphere]] is bound in the form of molecules, including [[carbon dioxide]] and [[Diatomic molecule|diatomic]] [[oxygen]] and [[nitrogen]]. At the surface of the Earth, an overwhelming majority of atoms combine to form various compounds, including [[water]], [[salt]], [[silicate]]s and [[oxide]]s. Atoms can also combine to create materials that do not consist of discrete molecules, including [[crystal]]s and liquid or solid [[metal]]s.<ref name=pidwirnyf /><ref name=pnas99_22_13966 /> This atomic matter forms networked arrangements that lack the particular type of small-scale interrupted order associated with molecular matter.<ref>{{cite book |
|||
|last=Pauling|first=Linus|year=1960 |
|||
|title=The Nature of the Chemical Bond |
|||
|publisher=Cornell University Press |
|||
|isbn=978-0-8014-0333-0 |
|||
|oclc=17518275|pages=5–10}}</ref> |
|||
=== Rare and theoretical forms === |
|||
==== Superheavy elements ==== |
|||
{{Main|Superheavy element}} |
|||
All nuclides with atomic numbers higher than 82 ([[lead]]) are known to be radioactive. No nuclide with an atomic number exceeding 92 ([[uranium]]) exists on Earth as a [[primordial nuclide]], and heavier elements generally have shorter half-lives. Nevertheless, an "[[island of stability]]" encompassing relatively long-lived isotopes of superheavy elements<ref name=cern28509 /> with atomic numbers [[darmstadtium|110]] to [[flerovium|114]] might exist.<ref name=KarpovSHE>{{cite journal|last1=Karpov|first1=A. V.|last2=Zagrebaev|first2=V. I.|last3=Palenzuela|first3=Y. M.|last4=Ruiz|first4=L. F.|last5=Greiner|first5=W.|title=Decay properties and stability of the heaviest elements|journal=International Journal of Modern Physics E|date=2012|volume=21|issue=2|pages=1250013-1–1250013-20<!-- Deny Citation Bot-->|doi=10.1142/S0218301312500139|url=http://nrv.jinr.ru/karpov/publications/Karpov12_IJMPE.pdf|bibcode=2012IJMPE..2150013K|display-authors=3|access-date=24 March 2020|archive-date=3 December 2016|archive-url=https://web.archive.org/web/20161203230540/http://nrv.jinr.ru/karpov/publications/Karpov12_IJMPE.pdf|url-status=live}}</ref> Predictions for the half-life of the most stable nuclide on the island range from a few minutes to millions of years.<ref name=physorg>{{cite web |url=http://newscenter.lbl.gov/2009/09/24/114-confirmed/ |title=Superheavy Element 114 Confirmed: A Stepping Stone to the Island of Stability |date=2009 |publisher=[[Lawrence Berkeley National Laboratory|Berkeley Lab]] |access-date=24 March 2020 |archive-date=20 July 2019 |archive-url=https://web.archive.org/web/20190720200414/https://newscenter.lbl.gov/2009/09/24/114-confirmed/ |url-status=live }}</ref> In any case, superheavy elements (with ''Z'' > 104) would not exist due to increasing [[Coulomb]] repulsion (which results in [[spontaneous fission]] with increasingly short half-lives) in the absence of any stabilizing effects.<ref name=liquiddrop>{{cite journal |last=Möller |first=P. |date=2016 |title=The limits of the nuclear chart set by fission and alpha decay |journal=EPJ Web of Conferences |volume=131 |pages=03002-1–03002-8<!-- Deny Citation Bot--> |url=http://inspirehep.net/record/1502715/files/epjconf-NS160-03002.pdf |doi=10.1051/epjconf/201613103002 |bibcode=2016EPJWC.13103002M |doi-access=free |access-date=24 March 2020 |archive-date=11 March 2020 |archive-url=https://web.archive.org/web/20200311130852/http://inspirehep.net/record/1502715/files/epjconf-NS160-03002.pdf |url-status=live }}</ref> |
|||
==== Exotic matter ==== |
|||
{{Main|1=Exotic matter}} |
|||
Each particle of matter has a corresponding [[antimatter]] particle with the opposite electrical charge. Thus, the [[positron]] is a positively charged [[antielectron]] and the [[antiproton]] is a negatively charged equivalent of a [[proton]]. When a matter and corresponding antimatter particle meet, they annihilate each other. Because of this, along with an imbalance between the number of matter and antimatter particles, the latter are rare in the universe. The first causes of this imbalance are not yet fully understood, although theories of [[baryogenesis]] may offer an explanation. As a result, no antimatter atoms have been discovered in nature.<ref name=koppes1999 /><ref name=cromie20010816 /> In 1996, the antimatter counterpart of the hydrogen atom ([[antihydrogen]]) was synthesized at the [[CERN]] laboratory in [[Geneva]].<ref name=nature419_6906_439 /><ref name=BBC20021030 /> |
|||
Other [[exotic atom]]s have been created by replacing one of the protons, neutrons or electrons with other particles that have the same charge. For example, an electron can be replaced by a more massive [[muon]], forming a [[muonic atom]]. These types of atoms can be used to test fundamental predictions of physics.<ref name=ns1728_77 /><ref name=psT112_1_20 /><ref name=ripin1998 /> |
|||
== See also == |
|||
{{Portal|Physics|Chemistry}} |
|||
{{cmn|colwidth=21em| |
|||
* [[History of quantum mechanics]] |
* [[History of quantum mechanics]] |
||
* [[Infinite divisibility]] |
* [[Infinite divisibility]] |
||
* [[ |
* [[Outline of chemistry]] |
||
* [[Motion]] |
|||
{{col-break}} |
|||
* [[ |
* [[Timeline of atomic and subatomic physics]] |
||
* [[Nuclear model]] |
* [[Nuclear model]] |
||
* [[ |
* [[Radionuclide]] |
||
}} |
|||
* [[Transuranium element]] |
|||
{{col-end}} |
|||
== |
== Notes == |
||
{{reflist|group="note"}} |
|||
===Notes=== |
|||
{{notelist}} |
|||
== References == |
|||
<!-- this 'empty' section displays references defined elsewhere --> |
<!-- this 'empty' section displays references defined elsewhere --> |
||
{{reflist| |
{{reflist|30em|refs= |
||
<!-- unused <ref name=adp322_8_549>{{cite journal|last=Einstein|first=Albert|year=1905|title=Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen|journal=[[Annalen der Physik]]|volume=322|issue=8|pages=549–560|language=de|url=http://www.zbp.univie.ac.at/dokumente/einstein2.pdf|doi=10.1002/andp.19053220806|bibcode=1905AnP...322..549E|archive-url=https://web.archive.org/web/20070718202731/http://www.zbp.univie.ac.at/dokumente/einstein2.pdf|archive-date=18 July 2007|url-status=live|doi-access=free}}</ref> --> |
|||
===Book references=== |
|||
*{{cite book |
|||
<!-- unused <ref name=lee_hoon1995>{{cite web|last=Lee|first=Y.K.|year=1995|author2=Hoon, K.|title=Brownian Motion|url=http://www.doc.ic.ac.uk/~nd/surprise_95/journal/vol4/ykl/report.html|publisher=[[Imperial College]]|archive-url=https://web.archive.org/web/20071218061408/http://www.doc.ic.ac.uk/~nd/surprise_95/journal/vol4/ykl/report.html|archive-date=18 December 2007|url-status=dead}}</ref> --> |
|||
| last=L'Annunziata<!-- Note: the single quote mark before the name is correct. --> |
|||
| first=Michael F. |
|||
<!-- unused <ref name=e31_2_50>{{cite journal|last=Patterson|first=G.|year=2007 |title=Jean Perrin and the triumph of the atomic doctrine|journal=[[Endeavour (journal)|Endeavour]]|volume=31|issue=2|pages=50–53|doi=10.1016/j.endeavour.2007.05.003|pmid=17602746}}</ref> --> |
|||
| year=2003 | title=Handbook of Radioactivity Analysis |
|||
| publisher=Academic Press | id=ISBN 0124366031 }} |
|||
<!-- UNUSED REF <ref name=npc1921>{{cite web|url=http://nobelprize.org/nobel_prizes/chemistry/laureates/1921/soddy-bio.html|title=Frederick Soddy, The Nobel Prize in Chemistry 1921|publisher=[[Nobel Foundation]]|access-date=18 January 2008|archive-url=https://web.archive.org/web/20080409210519/http://nobelprize.org/nobel_prizes/chemistry/laureates/1921/soddy-bio.html|archive-date=9 April 2008|url-status=live}}</ref> --> |
|||
*{{cite book |
|||
| last=Beyer | first=H. F. | coauthors=Shevelko, V. P. |
|||
<!-- UNUSED REF <ref name=prsA_89_1_1913>{{cite journal|doi=10.1098/rspa.1913.0057|last=Thomson|first=Joseph John|title=Rays of positive electricity|url=http://web.lemoyne.edu/~giunta/canal.html|journal=[[Proceedings of the Royal Society]]|year=1913|volume=89|issue=607|pages=1–20|bibcode=1913RSPSA..89....1T|doi-access=free|archive-url=https://web.archive.org/web/20161104174348/http://web.lemoyne.edu/~giunta/canal.html|archive-date=4 November 2016|url-status=live}}</ref> --> |
|||
| year=2003 |
|||
| title=Introduction to the Physics of Highly Charged Ions |
|||
<ref name=stern20050516>{{cite web|last=Stern|first=David P.|date=16 May 2005|title=The Atomic Nucleus and Bohr's Early Model of the Atom|url=http://www-spof.gsfc.nasa.gov/stargaze/Q5.htm|publisher=[[NASA]]/[[Goddard Space Flight Center]]|archive-url=https://web.archive.org/web/20070820084047/http://www-spof.gsfc.nasa.gov/stargaze/Q5.htm|archive-date=20 August 2007|url-status=live}}</ref> |
|||
| publisher=CRC Press | id=ISBN 0750304812 }} |
|||
*{{cite book |
|||
<ref name=bohr19221211>{{cite web|last=Bohr|first=Niels|date=11 December 1922|title=Niels Bohr, The Nobel Prize in Physics 1922, Nobel Lecture|url=http://nobelprize.org/nobel_prizes/physics/laureates/1922/bohr-lecture.html|publisher=[[Nobel Foundation]]|archive-url=https://web.archive.org/web/20080415183736/http://nobelprize.org/nobel_prizes/physics/laureates/1922/bohr-lecture.html|archive-date=15 April 2008|url-status=live}}</ref> |
|||
| last=Choppin | first=Gregory R. |
|||
| coauthors=Liljenzin, Jan-Olov; Rydberg, Jan |
|||
<!-- <ref name=jacs38_4_762>{{cite journal|last=Lewis|first=Gilbert N.|year=1916|title=The Atom and the Molecule|journal=[[Journal of the American Chemical Society]]|volume=38|issue=4|pages=762–786|doi=10.1021/ja02261a002|s2cid=95865413 |url=https://zenodo.org/record/1429068|archive-url=https://web.archive.org/web/20190825132554/https://zenodo.org/record/1429068/files/article.pdf|archive-date=25 August 2019|url-status=live}}</ref> |
|||
| year=2001 | title=Radiochemistry and Nuclear Chemistry |
|||
<ref name=jacs41_6_868>{{cite journal|last=Langmuir|first=Irving|year=1919|title=The Arrangement of Electrons in Atoms and Molecules|journal=[[Journal of the American Chemical Society]]|volume=41|issue=6|pages=868–934|doi=10.1021/ja02227a002|url=https://zenodo.org/record/1429026|archive-url=https://web.archive.org/web/20190621192330/https://zenodo.org/record/1429026|archive-date=21 June 2019|url-status=live}}</ref>--> |
|||
| publisher=Elsevier | id=ISBN 0750674636 }} |
|||
*{{cite book |
|||
<!-- |
|||
| first=J. | last=Dalton |
|||
<ref name=fop17_6_575>{{cite journal|last=Scully|first=Marlan O. |author2=Lamb, Willis E.|author3= Barut, Asim|year=1987|title=On the theory of the Stern-Gerlach apparatus|journal=[[Foundations of Physics]]|volume=17|issue=6|pages=575–583|doi=10.1007/BF01882788|bibcode = 1987FoPh...17..575S |s2cid=122529426 }}</ref> |
|||
| authorlink=John Dalton | year=1808 |
|||
--> |
|||
| title=A New System of Chemical Philosophy, Part 1 |
|||
| publisher=S. Russell |
|||
<ref name=brown2007>{{cite web|last=Brown|first=Kevin|year=2007|url=http://www.mathpages.com/home/kmath538/kmath538.htm|title=The Hydrogen Atom|publisher=MathPages|archive-url=https://archive.today/20120905172648/http://www.mathpages.com/home/kmath538/kmath538.htm|archive-date=5 September 2012|url-status=live}}</ref> |
|||
| location=London and Manchester }} |
|||
*{{cite book |
|||
<ref name=harrison2000>{{cite web|last=Harrison|first=David M.|year=2000|title=The Development of Quantum Mechanics|url=http://www.upscale.utoronto.ca/GeneralInterest/Harrison/DevelQM/DevelQM.html|publisher=[[University of Toronto]]| archive-url= https://web.archive.org/web/20071225095938/http://www.upscale.utoronto.ca/GeneralInterest/Harrison/DevelQM/DevelQM.html| archive-date= 25 December 2007 | url-status= live}}</ref> |
|||
| last=Demtröder | first=Wolfgang | year=2002 |
|||
| title=Atoms, Molecules and Photons: An Introduction to Atomic- Molecular- and Quantum Physics |
|||
<!-- UNUSED REF <ref name=pm39_6_449>{{cite journal|last=Aston|first=Francis W.|year=1920|title=The constitution of atmospheric neon|journal=[[Philosophical Magazine]]|volume=39|issue=6|pages=449–455|doi=10.1080/14786440408636058|url=https://zenodo.org/record/1430720|access-date=25 October 2020|archive-date=27 April 2021|archive-url=https://web.archive.org/web/20210427095842/https://zenodo.org/record/1430720|url-status=live}}</ref> --> |
|||
| publisher=Springer | edition=1st Edition |
|||
| id=ISBN 3540206310 }} |
|||
<!-- UNUSED REF <ref name=chadwick1935>{{cite web|last=Chadwick|first=James|date=12 December 1935|title=Nobel Lecture: The Neutron and Its Properties|url=http://nobelprize.org/nobel_prizes/physics/laureates/1935/chadwick-lecture.html|publisher=[[Nobel Foundation]]|archive-url=https://web.archive.org/web/20071012100351/http://nobelprize.org/nobel_prizes/physics/laureates/1935/chadwick-lecture.html|archive-date=12 October 2007|url-status=live}}</ref> --> |
|||
*{{cite book |
|||
| last=Feynman | first=Richard | year=1995 |
|||
<!-- UNUSED REF <ref name="CHF">{{cite web|title=Otto Hahn, Lise Meitner, and Fritz Strassmann|url=https://www.sciencehistory.org/historical-profile/otto-hahn-lise-meitner-and-fritz-strassmann|website=Science History Institute|date=June 2016|archive-url=https://web.archive.org/web/20180321130950/https://www.sciencehistory.org/historical-profile/otto-hahn-lise-meitner-and-fritz-strassmann|archive-date=21 March 2018|url-status=live}}</ref> --> |
|||
| title=Six Easy Pieces | publisher=The Penguin Group |
|||
| id=ISBN 978-0-140-27666-4}} |
|||
<!-- UNUSED REF <ref name=Bowden>{{cite book|last1=Bowden|first1=Mary Ellen|title=Chemical achievers : the human face of the chemical sciences|date=1997|publisher=Chemical Heritage Foundation|location=Philadelphia, PA|isbn=978-0-941901-12-3|chapter=Otto Hahn, Lise Meitner, and Fritz Strassmann|pages=[https://archive.org/details/chemicalachiever0000bowd/page/76 76–80, 125]|chapter-url=https://archive.org/details/chemicalachiever0000bowd/page/76}}</ref> --> |
|||
*{{cite book |
|||
| last=Fowles | first=Grant R. | year=1989 |
|||
<!-- UNUSED REF <ref name=nature143_3615_239>{{cite journal |last1=Meitner|first1=Lise|last2=Frisch|first2=Otto Robert|year=1939|title=Disintegration of uranium by neutrons: a new type of nuclear reaction|journal=[[Nature (journal)|Nature]]|volume=143 |issue=3615 |pages=239–240 |doi=10.1038/143239a0 |bibcode = 1939Natur.143..239M |s2cid=4113262}}</ref> --> |
|||
| title=Introduction to Modern Optics |
|||
| publisher=Courier Dover Publications |
|||
<!-- UNUSED REF <ref name=schroeder>{{cite web|last=Schroeder|first=M.|title=Lise Meitner – Zur 125. Wiederkehr Ihres Geburtstages|url=http://www.physik3.gwdg.de/~mrs/Vortraege/Lise_Meitner-Vortrag-20031106/|language=de|access-date=4 June 2009|url-status=dead|archive-url=https://web.archive.org/web/20110719034227/http://www.physik3.gwdg.de/~mrs/Vortraege/Lise_Meitner-Vortrag-20031106/|archive-date=19 July 2011}}</ref> --> |
|||
| id=ISBN 0486659577 }} |
|||
*{{cite book |
|||
<ref name=schroeder2>{{cite web |last=Schroeder|first=Paul A. |
|||
| last=Gangopadhyaya | first=Mrinalkanti |
|||
|date=25 February 2000 |url=http://www.gly.uga.edu/schroeder/geol3010/magnetics.html |title=Magnetic Properties |publisher=University of Georgia|archive-url = https://web.archive.org/web/20070429150216/http://www.gly.uga.edu/schroeder/geol3010/magnetics.html |archive-date = 29 April 2007}}</ref> |
|||
| title=Indian Atomism: History and Sources |
|||
| publisher=Humanities Press | year=1981 |
|||
<!-- UNUSED REF <ref name=pt50_9_26>{{cite journal|last1=Crawford |first1=E.| year=1997|title=A Nobel tale of postwar injustice|url=https://www.researchgate.net/publication/260861491|journal=[[Physics Today]]|volume=50|issue=9|pages=26–32|doi=10.1063/1.881933|last2=Sime|first2=Ruth Lewin|author-link2=Ruth Lewin Sime|last3=Walker|first3=Mark |bibcode = 1997PhT....50i..26C }}</ref> --> |
|||
| location=Atlantic Highlands, New Jersey |
|||
| isbn=0-391-02177-X }} |
|||
<!-- UNUSED REF <ref name=kullander2001>{{cite web|last=Kullander|first=Sven|date=28 August 2001|title=Accelerators and Nobel Laureates|url=http://nobelprize.org/nobel_prizes/physics/articles/kullander/|publisher=[[Nobel Foundation]]|archive-url=https://web.archive.org/web/20080413064924/http://nobelprize.org/nobel_prizes/physics/articles/kullander/|archive-date=13 April 2008|url-status=live}}</ref> --> |
|||
*{{cite book |
|||
| last=Goodstein | first=David L. | year=2002 |
|||
<!-- UNUSED REF <ref name=npp1990>{{cite web|date=17 October 1990|title=The Nobel Prize in Physics 1990|url=http://nobelprize.org/nobel_prizes/physics/laureates/1990/press.html|publisher=[[Nobel Foundation]]|archive-url=https://web.archive.org/web/20080514100040/http://nobelprize.org/nobel_prizes/physics/laureates/1990/press.html|archive-date=14 May 2008|url-status=live}}</ref> --> |
|||
| title=States of Matter | publisher=Courier Dover Publications |
|||
| id=ISBN 048649506X }} |
|||
<ref name=pdg2002>{{cite web|author=Particle Data Group|year=2002|url=http://www.particleadventure.org/|title=The Particle Adventure|publisher=Lawrence Berkeley Laboratory| archive-url= https://web.archive.org/web/20070104075936/http://www.particleadventure.org/| archive-date= 4 January 2007 | url-status= live}}</ref> |
|||
*{{cite book |
|||
| last=Harrison | first=Edward Robert | year=2003 |
|||
<ref name=schombert2006>{{cite web|first=James|last=Schombert|date=18 April 2006|url=http://abyss.uoregon.edu/~js/ast123/lectures/lec07.html|title=Elementary Particles|publisher=University of Oregon|archive-url=https://web.archive.org/web/20110830212645/http://abyss.uoregon.edu/~js/ast123/lectures/lec07.html|archive-date=30 August 2011|url-status=live}}</ref> |
|||
| title=Masks of the Universe: Changing Ideas on the Nature of the Cosmos |
|||
| publisher=Cambridge University Press |
|||
<ref name=wenner2007>{{cite web|last=Wenner|first=Jennifer M.|date=10 October 2007|url=http://serc.carleton.edu/quantskills/methods/quantlit/RadDecay.html|title=How Does Radioactive Decay Work?|publisher=Carleton College|archive-url=https://web.archive.org/web/20080511173156/http://serc.carleton.edu/quantskills/methods/quantlit/RadDecay.html|archive-date=11 May 2008|url-status=live}}</ref> |
|||
| id=ISBN 0521773512 }} |
|||
*{{cite book |
|||
<ref name=mihos2002>{{cite web|last=Mihos|first=Chris|date=23 July 2002|url=http://burro.cwru.edu/Academics/Astr221/StarPhys/coulomb.html|title=Overcoming the Coulomb Barrier|publisher=Case Western Reserve University|archive-url=https://web.archive.org/web/20060912013620/http://burro.cwru.edu/Academics/Astr221/StarPhys/coulomb.html|archive-date=12 September 2006|url-status=live}}</ref> |
|||
| last=Jevremovic | first=Tatjana | year=2005 |
|||
| title=Nuclear Principles in Engineering |
|||
<ref name=lbnl20070330>{{cite web|author=Staff|date=30 March 2007|url=http://www.lbl.gov/abc/Basic.html|title=ABC's of Nuclear Science|publisher=Lawrence Berkeley National Laboratory| archive-url= https://web.archive.org/web/20061205215708/http://www.lbl.gov/abc/Basic.html| archive-date= 5 December 2006 | url-status= live}}</ref> |
|||
| publisher=Springer | id=ISBN 0387232842 }} |
|||
*{{cite book |
|||
<ref name=makhijani_saleska2001>{{cite web|first=Arjun|last=Makhijani|author2=Saleska, Scott|date=2 March 2001|url=http://www.ieer.org/reports/n-basics.html|title=Basics of Nuclear Physics and Fission|publisher=Institute for Energy and Environmental Research| archive-url= https://web.archive.org/web/20070116045217/http://www.ieer.org/reports/n-basics.html| archive-date= 16 January 2007 | url-status= live}}</ref> |
|||
| last=Lequeux | first=James | year=2005 |
|||
| title=The Interstellar Medium |
|||
<ref name=ajp63_7_653>{{cite journal|last=Fewell|first=M.P.|title=The atomic nuclide with the highest mean binding energy|journal=[[American Journal of Physics]]|year=1995|volume=63|issue=7|pages=653–658|bibcode=1995AmJPh..63..653F|doi=10.1119/1.17828}}</ref> |
|||
| publisher=Springer | id=ISBN 3540213260 }} |
|||
*{{cite book |
|||
<ref name="raymond">{{cite web|last=Raymond |first=David |date=7 April 2006 |url=http://physics.nmt.edu/~raymond/classes/ph13xbook/node216.html |archive-url=https://web.archive.org/web/20021201030437/http://physics.nmt.edu/~raymond/classes/ph13xbook/node216.html |url-status=dead |archive-date=1 December 2002 |title=Nuclear Binding Energies |publisher=New Mexico Tech}}</ref> |
|||
| last=Liang | first=Z.-P. | coauthors=Haacke, E. M. |
|||
| editor=Webster, J. G. | year=1999 |
|||
<ref name=science157_3784_13>{{cite journal|last=Mulliken|first=Robert S.|title=Spectroscopy, Molecular Orbitals, and Chemical Bonding|journal=[[Science (journal)|Science]] |year=1967|volume=157|issue=3784|pages=13–24|doi=10.1126/science.157.3784.13|pmid=5338306|bibcode = 1967Sci...157...13M }}</ref> |
|||
| volume=vol. 2 | pages=pp. 412–26 |
|||
| title=Encyclopedia of Electrical and Electronics Engineering: Magnetic Resonance Imaging |
|||
<ref name=Brucat2008>{{cite web|last=Brucat |first=Philip J. |year=2008 |url=http://www.chem.ufl.edu/~itl/2045/lectures/lec_10.html |title=The Quantum Atom |publisher=University of Florida|archive-url=https://web.archive.org/web/20061207032136/http://www.chem.ufl.edu/~itl/2045/lectures/lec_10.html |archive-date=7 December 2006 |url-status=dead }}</ref> |
|||
| publisher=John Wiley & Sons |
|||
| url=http://ieeexplore.ieee.org/iel5/8734/27658/01233976.pdf?arnumber=1233976 |
|||
<ref name=herter_8>{{cite web|last=Herter |first=Terry |year=2006 |url=http://astrosun2.astro.cornell.edu/academics/courses/astro101/herter/lectures/lec08.htm |title=Lecture 8: The Hydrogen Atom |publisher=Cornell University|url-status=dead |archive-url=https://web.archive.org/web/20120222062433/http://astrosun2.astro.cornell.edu/academics/courses/astro101/herter/lectures/lec08.htm |archive-date=22 February 2012 }}</ref> |
|||
| format=PDF | accessdate=2008-01-09 | id=ISBN 0471139467 }} |
|||
*{{cite book |
|||
<ref name=pr79_2_282>{{cite journal|last1=Bell|first1=R.E.|title=Gamma-Rays from the Reaction H<sup>1</sup>(n,γ)D<sup>2</sup> and the Binding Energy of the Deuteron|journal=[[Physical Review]]|year=1950|volume=79|issue=2|pages=282–285|doi=10.1103/PhysRev.79.282|last2=Elliott|first2=L.G.|bibcode = 1950PhRv...79..282B }}</ref> |
|||
| last=MacGregor | first=Malcolm H. | year=1992 |
|||
| title=The Enigmatic Electron |
|||
<ref name=matis2000>{{cite web|last=Matis|first=Howard S.|date=9 August 2000|url=http://www.lbl.gov/abc/wallchart/chapters/02/3.html|title=The Isotopes of Hydrogen|website=Guide to the Nuclear Wall Chart|publisher=Lawrence Berkeley National Lab| archive-url= https://web.archive.org/web/20071218153548/http://www.lbl.gov/abc/wallchart/chapters/02/3.html| archive-date= 18 December 2007 | url-status= live}}</ref> |
|||
| publisher=Oxford University Press | id=ISBN 0195218337 }} |
|||
*{{cite book |
|||
<ref name=weiss20061017>{{cite news|last=Weiss|first=Rick|date=17 October 2006|title=Scientists Announce Creation of Atomic Element, the Heaviest Yet|newspaper=Washington Post|url=https://www.washingtonpost.com/wp-dyn/content/article/2006/10/16/AR2006101601083.html|archive-url=https://web.archive.org/web/20110820082130/http://www.washingtonpost.com/wp-dyn/content/article/2006/10/16/AR2006101601083.html|archive-date=20 August 2011|url-status=live}}</ref> |
|||
| last=Manuel | first=Oliver | year=2001 |
|||
| title=Origin of Elements in the Solar System: Implications of Post-1957 Observations |
|||
<ref name=dume20030423>{{cite news|last=Dumé|first=Belle|date=23 April 2003|title=Bismuth breaks half-life record for alpha decay|publisher=Physics World|url=http://physicsworld.com/cws/article/news/17319| archive-url= https://web.archive.org/web/20071214151450/http://physicsworld.com/cws/article/news/17319| archive-date= 14 December 2007 | url-status= live}}</ref> |
|||
| publisher=Springer | id=ISBN 0306465620 }} |
|||
*{{cite book |
|||
<ref name=lidsay20000730>{{cite web|last=Lindsay|first=Don|date=30 July 2000|url=http://www.don-lindsay-archive.org/creation/isotope_list.html|title=Radioactives Missing From The Earth|publisher=Don Lindsay Archive| archive-url= https://web.archive.org/web/20070428225550/http://www.don-lindsay-archive.org/creation/isotope_list.html| archive-date= 28 April 2007 | url-status= live}}</ref> |
|||
| last=Mazo | first=Robert M. | year=2002 |
|||
| title=Brownian Motion: Fluctuations, Dynamics, and Applications |
|||
<ref name=manthey2001>{{cite web|last=Manthey|first=David|year=2001|url=http://www.orbitals.com/orb/|title=Atomic Orbitals|publisher=Orbital Central| archive-url= https://web.archive.org/web/20080110102801/http://www.orbitals.com/orb/| archive-date= 10 January 2008 | url-status= live}}</ref> |
|||
| publisher=Oxford University Press | id=ISBN 0198515677 }} |
|||
*{{cite book |
|||
<ref name=tuli2005>{{cite web|first=Jagdish K.|last=Tuli|date=April 2005|title=Nuclear Wallet Cards|publisher=National Nuclear Data Center, Brookhaven National Laboratory|url=http://nucleus.iaea.org/CIR/CIR/NuclearWalletCards.html|archive-url=https://web.archive.org/web/20111003185243/http://nucleus.iaea.org/CIR/CIR/NuclearWalletCards.html|archive-date=3 October 2011|url-status=live}}</ref> |
|||
| last=Mills | first=Ian |
|||
| coauthors=Cvitaš, Tomislav; Homann, Klaus; Kallay, Nikola; Kuchitsu, Kozo |
|||
<ref name=chieh2001>{{cite web|last=Chieh|first=Chung|date=22 January 2001|url=http://www.science.uwaterloo.ca/~cchieh/cact/nuctek/nuclideunstable.html|title=Nuclide Stability|publisher=University of Waterloo|archive-url=https://web.archive.org/web/20070830110015/http://www.science.uwaterloo.ca/~cchieh/cact/nuctek/nuclideunstable.html|archive-date=30 August 2007|url-status=dead}}</ref> |
|||
| title=Quantities, Units and Symbols in Physical Chemistry |
|||
| publisher=[[International Union of Pure and Applied Chemistry]], Commission on Physiochemical Symbols Terminology and Units, Blackwell Scientific Publications |
|||
<ref name=nist_wc>{{cite web |
|||
| location=Oxford | edition=2nd edition | date=1993 |
|||
|url=http://physics.nist.gov/cgi-bin/Compositions/stand_alone.pl?ele=&ascii=html&isotype=some |
|||
| url=http://www.iupac.org/publications/books/gbook/green_book_2ed.pdf | format=PDF |
|||
|title=Atomic Weights and Isotopic Compositions for All Elements |
|||
| id=ISBN 0-632-03583-8 | accessdate=2007-12-17 }} |
|||
|publisher=National Institute of Standards and Technology |
|||
*{{cite book |
|||
|access-date=4 January 2007| archive-url= https://web.archive.org/web/20061231212733/http://physics.nist.gov/cgi-bin/Compositions/stand_alone.pl?ele=&ascii=html&isotype=some| archive-date= 31 December 2006 | url-status= live}}</ref> |
|||
| last=Myers | first=Richard | year=2003 |
|||
| title=The Basics of Chemistry | publisher=Greenwood Press |
|||
<ref name=audi2003>{{cite journal |last1=Audi |first1=G. |title=The Ame2003 atomic mass evaluation (II) |journal=[[Nuclear Physics A]] |year=2003 |volume=729 |issue=1 |pages=337–676 |url=http://amdc.in2p3.fr/masstables/Ame2003/Ame2003b.pdf |doi=10.1016/j.nuclphysa.2003.11.003 |bibcode=2003NuPhA.729..337A |last2=Wapstra |first2=A.H. |last3=Thibault |first3=C.|archive-url=https://web.archive.org/web/20051016185841/http://amdc.in2p3.fr/masstables/Ame2003/Ame2003b.pdf |archive-date=16 October 2005 |url-status=live }}</ref> |
|||
| id=ISBN 0313316643 }} |
|||
*{{cite book |
|||
<ref name=aca32_5_751>{{cite journal|last=Shannon|first=R.D.|title=Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides|journal=[[Acta Crystallographica A]]|year=1976|volume=32|issue=5|pages=751–767|doi=10.1107/S0567739476001551|bibcode=1976AcCrA..32..751S|url=http://journals.iucr.org/a/issues/1976/05/00/a12967/a12967.pdf|access-date=25 August 2019|archive-date=14 August 2020|archive-url=https://web.archive.org/web/20200814154832/https://journals.iucr.org/a/issues/1976/05/00/a12967/a12967.pdf|url-status=live}}</ref> |
|||
| last=Padilla | first=Michael J. |
|||
| coauthors=Miaoulis, Ioannis; Cyr, Martha | year = 2002 |
|||
<ref name=dong1998>{{cite web|last=Dong|first=Judy|year=1998 |url=http://hypertextbook.com/facts/MichaelPhillip.shtml|title=Diameter of an Atom|publisher=The Physics Factbook| archive-url= https://web.archive.org/web/20071104160920/http://hypertextbook.com/facts/MichaelPhillip.shtml| archive-date= 4 November 2007 | url-status= live}}</ref> |
|||
| title = Prentice Hall Science Explorer: Chemical Building Blocks |
|||
| publisher = Prentice-Hall, Inc. |
|||
<ref name=osu2007>{{cite web |
|||
| location = Upper Saddle River, New Jersey USA |
|||
|author=Staff |
|||
| id = ISBN 0-13-054091-9}} |
|||
|year=2007 |
|||
*{{cite book |
|||
|url=http://oregonstate.edu/terra/2007/02/small-miracles/ |
|||
| last=Pauling | first=Linus | year=1960 |
|||
|title=Small Miracles: Harnessing nanotechnology |
|||
| title=The Nature of the Chemical Bond |
|||
|publisher=Oregon State University |
|||
|archive-url=https://web.archive.org/web/20110521145107/http://oregonstate.edu/terra/2007/02/small-miracles/ |
|||
| id=ISBN 0801403332 }} |
|||
|archive-date=21 May 2011 |
|||
*{{cite book |
|||
|url-status=live |
|||
| last=Pfeffer | first=Jeremy I. |
|||
}} – describes the width of a human hair as {{val|e=5|u=nm}} and 10 carbon atoms as spanning 1 nm.</ref> |
|||
| coauthor=Nir, Shlomo | year=2000 |
|||
| title=Modern Physics: An Introductory Text |
|||
<ref name=splung>{{cite web |url=http://www.splung.com/content/sid/5/page/radioactivity |title=Radioactivity |publisher=Splung.com |access-date=19 December 2007| archive-url= https://web.archive.org/web/20071204135150/http://www.splung.com/content/sid/5/page/radioactivity| archive-date= 4 December 2007 | url-status= live}}</ref> |
|||
| publisher=Imperial College Press | id=ISBN 1860942504 }} |
|||
*{{cite book |
|||
<ref name=firestone20000522>{{cite web|last=Firestone |first=Richard B. |date=22 May 2000 |url=http://isotopes.lbl.gov/education/decmode.html |title=Radioactive Decay Modes |publisher=Berkeley Laboratory|url-status=dead |archive-url=https://web.archive.org/web/20060929111801/http://isotopes.lbl.gov/education/decmode.html |archive-date=29 September 2006 }}</ref> |
|||
| last=Ponomarev | first=Leonid Ivanovich | year=1993 |
|||
| title=The Quantum Dice | publisher=CRC Press |
|||
<ref name=hornak2006>{{cite web |last=Hornak|first=J.P.|year=2006 |url=http://www.cis.rit.edu/htbooks/nmr/chap-3/chap-3.htm |title=Chapter 3: Spin Physics|website=The Basics of NMR |publisher=Rochester Institute of Technology| archive-url= https://web.archive.org/web/20070203044312/http://www.cis.rit.edu/htbooks/nmr/chap-3/chap-3.htm| archive-date= 3 February 2007 | url-status= live}}</ref> |
|||
| id=ISBN 0750302518 }} |
|||
*{{cite book |
|||
<ref name=goebel20070901>{{cite web |last=Goebel |first=Greg |date=1 September 2007 |url=http://www.vectorsite.net/tpqm_04.html |title=<nowiki>[4.3]</nowiki> Magnetic Properties of the Atom |website=Elementary Quantum Physics |publisher=In The Public Domain website|archive-url=https://web.archive.org/web/20110629143026/http://www.vectorsite.net/tpqm_04.html |archive-date=29 June 2011 |url-status=dead}}</ref> |
|||
| last=Shultis | first=J. Kenneth | coauthors=Faw, Richard E. |
|||
| title=Fundamentals of Nuclear Science and Engineering |
|||
<ref name=yarris1997>{{cite journal |last=Yarris|first=Lynn|title=Talking Pictures |journal=Berkeley Lab Research Review |date=Spring 1997 |url=http://www.lbl.gov/Science-Articles/Research-Review/Magazine/1997/story1.html| archive-url= https://web.archive.org/web/20080113104939/http://www.lbl.gov/Science-Articles/Research-Review/Magazine/1997/story1.html| archive-date= 13 January 2008 | url-status= live}}</ref> |
|||
| year=2002 | publisher=CRC Press | id=ISBN 0824708342 }} |
|||
*{{cite book |
|||
<ref name=zeghbroeck1998>{{cite web |
|||
| last=Siegfried | first=Robert | year=2002 |
|||
|last=Zeghbroeck |
|||
| title=From Elements to Atoms: A History of Chemical Composition |
|||
|first=Bart J. Van |
|||
| publisher=DIANE | id=ISBN 0871699249 }} |
|||
|year=1998 |
|||
*{{cite book |
|||
|url=http://physics.ship.edu/~mrc/pfs/308/semicon_book/eband2.htm |
|||
| last=Sills | first=Alan D. | year=2003 |
|||
|archive-url=https://web.archive.org/web/20050115030639/http://physics.ship.edu/~mrc/pfs/308/semicon_book/eband2.htm |
|||
| title=Earth Science the Easy Way |
|||
|url-status=dead |
|||
| publisher=Barron's Educational Series | id=ISBN 0764121464 }} |
|||
|archive-date=15 January 2005 |
|||
*{{cite book |
|||
|title=Energy levels |
|||
| last=Smirnov | first=Boris M. | year=2003 |
|||
|publisher=Shippensburg University}}</ref> |
|||
| title=Physics of Atoms and Ions |
|||
| publisher=Springer | id=ISBN 038795550X }} |
|||
<ref name=martin2007>{{cite web |last=Martin|first=W.C. |author2=Wiese, W.L.|date=May 2007 |url=http://physics.nist.gov/Pubs/AtSpec/ |title=Atomic Spectroscopy: A Compendium of Basic Ideas, Notation, Data, and Formulas |publisher=National Institute of Standards and Technology| archive-url= https://web.archive.org/web/20070208113156/http://physics.nist.gov/Pubs/AtSpec/| archive-date= 8 February 2007 | url-status= live}}</ref> |
|||
*{{cite book |
|||
| last=Teresi | first=Dick | publisher = Simon & Schuster |
|||
<ref name=avogadro>{{cite web|url=http://www.avogadro.co.uk/light/bohr/spectra.htm |title=Atomic Emission Spectra – Origin of Spectral Lines |publisher=Avogadro Web Site |access-date=10 August 2006 |url-status=dead |archive-url=https://web.archive.org/web/20060228231025/http://www.avogadro.co.uk/light/bohr/spectra.htm |archive-date=28 February 2006 }}</ref> |
|||
| title = Lost Discoveries: The Ancient Roots of Modern Science |
|||
| date=2003 | isbn=074324379X |
|||
<ref name=fitzpatrick20070216>{{cite web |last=Fitzpatrick |first=Richard |date=16 February 2007 |url=http://farside.ph.utexas.edu/teaching/qm/lectures/node55.html |title=Fine structure |publisher=University of Texas at Austin|archive-url=https://web.archive.org/web/20110927021402/http://farside.ph.utexas.edu/teaching/qm/lectures/node55.html |archive-date=27 September 2011 |url-status=live }}</ref> |
|||
| url = http://books.google.com/books?id=pheL_ubbXD0C&dq |
|||
| pages = 213–214}} |
|||
<ref name=weiss2001>{{cite web |last=Weiss|first=Michael|year=2001 |url=http://math.ucr.edu/home/baez/spin/node8.html |title=The Zeeman Effect |publisher=University of California-Riverside| archive-url= https://web.archive.org/web/20080202143147/http://math.ucr.edu/home/baez/spin/node8.html| archive-date= 2 February 2008 | url-status= live}}</ref> |
|||
*{{cite book |
|||
| last=Woan | first=Graham | year=2000 |
|||
<ref name=watkins_sjsu>{{cite web |last=Watkins |first=Thayer |url=http://www.sjsu.edu/faculty/watkins/stimem.htm |title=Coherence in Stimulated Emission |publisher=San José State University |access-date=23 December 2007 | archive-url= https://web.archive.org/web/20080112234014/http://www.sjsu.edu/faculty/watkins/stimem.htm| archive-date= 12 January 2008 | url-status= live}}</ref> |
|||
| title=The Cambridge Handbook of Physics |
|||
| publisher=Cambridge University Press | id=ISBN 0521575079 }} |
|||
<ref name=reusch20070716>{{cite web|last=Reusch |first=William |date=16 July 2007 |url=http://www.cem.msu.edu/~reusch/VirtualText/intro1.htm |title=Virtual Textbook of Organic Chemistry |publisher=Michigan State University|url-status=dead |archive-url=https://web.archive.org/web/20071029211245/http://www.cem.msu.edu/~reusch/VirtualText/intro1.htm |archive-date=29 October 2007 }}</ref> |
|||
*{{cite book |
|||
| first=Charles Adolphe | last=Wurtz | year=1881 |
|||
<ref name=chemguide>{{cite web |url=http://www.chemguide.co.uk/atoms/bonding/covalent.html |title=Covalent bonding – Single bonds |publisher=chemguide |year=2000|archive-url=https://web.archive.org/web/20081101203444/http://www.chemguide.co.uk/atoms/bonding/covalent.html |archive-date=1 November 2008 |url-status=live }}</ref> |
|||
| title=The Atomic Theory |
|||
| publisher=D. Appleton and company |
|||
<ref name=husted20031211>{{cite web |last=Husted | first=Robert |date=11 December 2003 |url=http://periodic.lanl.gov/default.htm |title=Periodic Table of the Elements |publisher=Los Alamos National Laboratory| archive-url= https://web.archive.org/web/20080110103232/http://periodic.lanl.gov/default.htm| archive-date= 10 January 2008 | url-status= live|display-authors=etal}}</ref> |
|||
| location=New York }} |
|||
*{{cite book |
|||
<ref name=baum2003>{{cite magazine |first=Rudy |last=Baum |year=2003 |url=http://pubs.acs.org/cen/80th/elements.html |title=It's Elemental: The Periodic Table |magazine=Chemical & Engineering News|archive-url=https://web.archive.org/web/20110406121140/http://pubs.acs.org/cen/80th/elements.html |archive-date=6 April 2011 |url-status=live }}</ref> |
|||
| last=Zaider | first=Marco | coauthors=Rossi, Harald H. | year=2001 |
|||
| title=Radiation Science for Physicians and Public Health Workers |
|||
<ref name=pu49_7_719>{{cite journal |last=Brazhkin|first=Vadim V. |title=Metastable phases, phase transformations, and phase diagrams in physics and chemistry |journal=Physics-Uspekhi |year=2006|volume=49 |issue=7|pages=719–724 |doi=10.1070/PU2006v049n07ABEH006013 |bibcode = 2006PhyU...49..719B |s2cid=93168446 }}</ref> |
|||
| publisher=Springer | id=ISBN 0306464039 }} |
|||
*{{cite book |
|||
<ref name=nist_bec>{{cite news |author=Staff|date=9 October 2001 |title=Bose–Einstein Condensate: A New Form of Matter |publisher=National Institute of Standards and Technology |url=https://www.nist.gov/public_affairs/releases/bec_background.cfm| archive-url= https://web.archive.org/web/20080103192918/https://www.nist.gov/public_affairs/releases/BEC_background.htm| archive-date= 3 January 2008 | url-status= live}}</ref> |
|||
| last=Zumdahl | first=Steven S. | year=2002 |
|||
| title=Introductory Chemistry: A Foundation |
|||
<ref name=colton_fyffe1999>{{cite web |last=Colton|first=Imogen|author2=Fyffe, Jeanette |
|||
| edition=5th edition | publisher=Houghton Mifflin |
|||
|date=3 February 1999 |url=http://www.ph.unimelb.edu.au/~ywong/poster/articles/bec.html |title=Super Atoms from Bose–Einstein Condensation |publisher=The University of Melbourne| archive-url = https://web.archive.org/web/20070829200820/http://www.ph.unimelb.edu.au/~ywong/poster/articles/bec.html| archive-date = 29 August 2007}}</ref> |
|||
| url=http://college.hmco.com/chemistry/intro/zumdahl/intro_chemistry/5e/students/protected/periodictables/pt/pt/pt_ar5.html |
|||
| accessdate=2008-02-05 | id=ISBN 0-618-34342-3 }} |
|||
<ref name=jacox1997>{{cite web |last=Jacox|first=Marilyn|author2=Gadzuk, J. William |url=http://physics.nist.gov/GenInt/STM/stm.html |title=Scanning Tunneling Microscope |publisher=National Institute of Standards and Technology |date=November 1997| archive-url= https://web.archive.org/web/20080107133132/http://physics.nist.gov/GenInt/STM/stm.html| archive-date= 7 January 2008 | url-status= live}}</ref> |
|||
<ref name=nf_physics1986>{{cite web |url=http://nobelprize.org/nobel_prizes/physics/laureates/1986/index.html |title=The Nobel Prize in Physics 1986 |publisher=The Nobel Foundation |access-date=11 January 2008 |archive-url=https://web.archive.org/web/20080917103215/http://nobelprize.org/nobel_prizes/physics/laureates/1986/index.html |archive-date=17 September 2008 |url-status=live }} In particular, see the Nobel lecture by G. Binnig and H. Rohrer.</ref> |
|||
<ref name=sab53_13_1739>{{cite journal |first1=N.|last1=Jakubowski |title = Sector field mass spectrometers in ICP-MS |journal = Spectrochimica Acta Part B: Atomic Spectroscopy |volume = 53|issue = 13|year = 1998 |doi=10.1016/S0584-8547(98)00222-5|pages = 1739–1763|bibcode = 1998AcSpB..53.1739J |last2=Moens |first2=Luc |last3=Vanhaecke |first3=Frank }}</ref> |
|||
<ref name=rsi39_1_83>{{cite journal |last1=Müller |first1=Erwin W. |author-link1=Erwin Wilhelm Müller |last2=Panitz |first2=John A. |author-link2=J.A. Panitz |last3=McLane |first3=S. Brooks |author-link3=S. Brooks McLane |year=1968 |title=The Atom-Probe Field Ion Microscope |journal=[[Review of Scientific Instruments]] |volume=39 |issue=1 |pages=83–86 |doi=10.1063/1.1683116 |
|||
|bibcode = 1968RScI...39...83M }}</ref> |
|||
<ref name=lochner2007>{{cite web |last=Lochner|first=Jim |author2=Gibb, Meredith|author3= Newman, Phil |date=30 April 2007 |url=http://imagine.gsfc.nasa.gov/docs/science/how_l1/spectral_what.html |title=What Do Spectra Tell Us? |publisher=NASA/Goddard Space Flight Center| archive-url= https://web.archive.org/web/20080116035542/http://imagine.gsfc.nasa.gov/docs/science/how_l1/spectral_what.html| archive-date= 16 January 2008 | url-status= live}}</ref> |
|||
<ref name=winter2007>{{cite web |last=Winter|first=Mark|year=2007 |url=http://www.webelements.com/webelements/elements/text/He/hist.html |title=Helium|publisher=WebElements| archive-url= https://web.archive.org/web/20071230182148/http://www.webelements.com/webelements/elements/text/He/hist.html| archive-date= 30 December 2007 | url-status= live}}</ref> |
|||
<ref name=hinshaw20060210>{{cite web |last=Hinshaw|first=Gary |date=10 February 2006 |url=http://map.gsfc.nasa.gov/m_uni/uni_101matter.html |title=What is the Universe Made Of? |publisher=NASA/WMAP| archive-url= https://web.archive.org/web/20071231143948/http://map.gsfc.nasa.gov/m_uni/uni_101matter.html| archive-date= 31 December 2007 | url-status= live}}</ref> |
|||
<ref name=science259_5093_327>{{cite journal |last=Davidsen|first=Arthur F. |title=Far-Ultraviolet Astronomy on the Astro-1 Space Shuttle Mission |journal=[[Science (journal)|Science]] |year=1993|volume=259 |issue=5093|pages=327–334 |doi=10.1126/science.259.5093.327 |pmid=17832344|bibcode = 1993Sci...259..327D |s2cid=28201406 }}</ref> |
|||
<ref name=nigel2000>{{cite web |first=Nigel|last=Smith|date=6 January 2000 |url=http://physicsworld.com/cws/article/print/809 |title=The search for dark matter |publisher=Physics World| archive-url= https://web.archive.org/web/20080216185952/http://physicsworld.com/cws/article/print/809| archive-date= 16 February 2008 | url-status= live}}</ref> |
|||
<ref name=ns1794_42>{{cite journal|last=Croswell |first=Ken |title=Boron, bumps and the Big Bang: Was matter spread evenly when the Universe began? Perhaps not; the clues lie in the creation of the lighter elements such as boron and beryllium |journal=New Scientist |year=1991 |issue=1794 |page=42 |url=https://www.newscientist.com/article/mg13217944.700-boron-bumps-and-the-big-bang-was-matter-spread-evenly-whenthe-universe-began-perhaps-not-the-clues-lie-in-the-creation-of-thelighter-elements-such-as-boron-and-beryllium.html|archive-url=https://web.archive.org/web/20080207065342/http://space.newscientist.com/article/mg13217944.700-boron-bumps-and-the-big-bang-was-matter-spread-evenly-whenthe-universe-began-perhaps-not-the-clues-lie-in-the-creation-of-thelighter-elements-such-as-boron-and-beryllium.html |archive-date=7 February 2008 |url-status=dead }}</ref> |
|||
<ref name=science267_5195_192>{{cite journal |last1=Copi |first1=Craig J. |last2=Schramm |first2=DN |last3=Turner |first3=MS |year=1995 |title=Big-Bang Nucleosynthesis and the Baryon Density of the Universe |journal=[[Science (journal)|Science]] |volume=267 |issue=5195 |pages=192–199 |doi=10.1126/science.7809624 |pmid=7809624 |arxiv=astro-ph/9407006 |bibcode=1995Sci...267..192C |s2cid=15613185 |url=https://cds.cern.ch/record/265576 |type=Submitted manuscript|archive-url=https://web.archive.org/web/20190814070006/https://cds.cern.ch/record/265576 |archive-date=14 August 2019 |url-status=live }}</ref> |
|||
<ref name=hinshaw20051215>{{cite web |last=Hinshaw|first=Gary|date=15 December 2005 |url=http://map.gsfc.nasa.gov/m_uni/uni_101bbtest2.html |title=Tests of the Big Bang: The Light Elements |publisher=NASA/WMAP| archive-url= https://web.archive.org/web/20080117021252/http://map.gsfc.nasa.gov/m_uni/uni_101bbtest2.html| archive-date= 17 January 2008 | url-status= live}}</ref> |
|||
<ref name=abbott20070530>{{cite web|last=Abbott |first=Brian |date=30 May 2007 |url=http://www.haydenplanetarium.org/universe/duguide/exgg_wmap.php |title=Microwave (WMAP) All-Sky Survey |publisher=Hayden Planetarium|url-status=dead |archive-url=https://web.archive.org/web/20130213023246/http://www.haydenplanetarium.org/universe/duguide/exgg_wmap.php |archive-date=13 February 2013 }}</ref> |
|||
<ref name=mnras106_343>{{cite journal |title=The synthesis of the elements from hydrogen |first=F. |last=Hoyle |journal=[[Monthly Notices of the Royal Astronomical Society]] |volume=106|issue=5 |pages=343–383|year=1946 |bibcode=1946MNRAS.106..343H |doi=10.1093/mnras/106.5.343|doi-access=free }}</ref> |
|||
<ref name=nature405_656>{{cite journal |last1=Knauth|first1=D.C. |title=Newly synthesized lithium in the interstellar medium |journal=[[Nature (journal)|Nature]] |year=2000|volume=405|pages=656–658 |doi=10.1038/35015028 |last2=Knauth|first2=D.C. |last3=Lambert|first3=David L. |last4=Crane|first4=P. |pmid=10864316 |issue=6787|bibcode = 2000Natur.405..656K |s2cid=4397202 }}</ref> |
|||
<ref name=mashnik2000>{{cite arXiv |last=Mashnik|first=Stepan G. |title=On Solar System and Cosmic Rays Nucleosynthesis and Spallation Processes |eprint=astro-ph/0008382 |year=2000 }}</ref> |
|||
<ref name=kgs20050504>{{cite web |author=Kansas Geological Survey |date=4 May 2005 |title=Age of the Earth |url=http://www.kgs.ku.edu/Extension/geotopics/earth_age.html |publisher=University of Kansas|archive-url=https://web.archive.org/web/20080705052359/http://www.kgs.ku.edu/Extension/geotopics/earth_age.html |archive-date=5 July 2008 |url-status=dead}}</ref> |
|||
<ref name=gs190_1_205>{{cite journal |last=Dalrymple |first=G. Brent |title=The age of the Earth in the twentieth century: a problem (mostly) solved |journal=Geological Society, London, Special Publications |year=2001 |volume=190 |issue=1 |pages=205–221 |doi=10.1144/GSL.SP.2001.190.01.14 |url=http://sp.lyellcollection.org/cgi/content/abstract/190/1/205|bibcode=2001GSLSP.190..205D |s2cid=130092094 |archive-url=https://web.archive.org/web/20071111141237/http://sp.lyellcollection.org/cgi/content/abstract/190/1/205 |archive-date=11 November 2007 |url-status=live }}</ref> |
|||
<ref name=anderson_foulger_meibom2006>{{cite web |last=Anderson|first=Don L. |author-link=Don L. Anderson |author2=Foulger, G.R.|author3= Meibom, Anders |date=2 September 2006 |url=http://www.mantleplumes.org/HeliumFundamentals.html |title=Helium: Fundamental models |publisher=MantlePlumes.org| archive-url= https://web.archive.org/web/20070208194933/http://www.mantleplumes.org/HeliumFundamentals.html| archive-date= 8 February 2007 | url-status= live}}</ref> |
|||
<ref name=pennicott2001>{{cite news |last=Pennicott|first=Katie|date=10 May 2001 |title=Carbon clock could show the wrong time |publisher=PhysicsWeb |url=http://physicsworld.com/cws/article/news/2676| archive-url= https://web.archive.org/web/20071215103132/http://physicsworld.com/cws/article/news/2676| archive-date= 15 December 2007 | url-status= live}}</ref> |
|||
<ref name=yarris2001>{{cite news |last=Yarris |first=Lynn |date=27 July 2001 |title=New Superheavy Elements 118 and 116 Discovered at Berkeley Lab |publisher=Berkeley Lab |url=http://enews.lbl.gov/Science-Articles/Archive/elements-116-118.html|archive-url=https://web.archive.org/web/20080109103538/http://enews.lbl.gov/Science-Articles/Archive/elements-116-118.html |archive-date=9 January 2008 |url-status=dead}}</ref> |
|||
<ref name=pr119_6_2000>{{cite journal |author=Diamond, H |year=1960 |title=Heavy Isotope Abundances in Mike Thermonuclear Device |journal=[[Physical Review]] |volume=119 |issue=6 |pages=2000–2004 |doi=10.1103/PhysRev.119.2000 |bibcode = 1960PhRv..119.2000D |display-authors=etal}}</ref> |
|||
<ref name=poston1998>{{cite magazine |last=Poston |first=John W. Sr. |date=23 March 1998 |title=Do transuranic elements such as plutonium ever occur naturally? |magazine=Scientific American |url=http://www.scientificamerican.com/article/do-transuranic-elements-s/|archive-url=https://web.archive.org/web/20150327001605/http://www.scientificamerican.com/article/do-transuranic-elements-s/ |archive-date=27 March 2015 |url-status=live }}</ref> |
|||
<ref name=cz97_10_522>{{cite journal |last=Keller|first=C. |title=Natural occurrence of lanthanides, actinides, and superheavy elements |journal=Chemiker Zeitung |year=1973|volume=97|issue=10|pages=522–530 |osti=4353086 }}</ref> |
|||
<ref name=ofr_cut>{{cite web|url=http://www.oklo.curtin.edu.au/index.cfm |title=Oklo Fossil Reactors |publisher=Curtin University of Technology |access-date=15 January 2008 |archive-url=https://web.archive.org/web/20071218194159/http://www.oklo.curtin.edu.au/index.cfm |archive-date=18 December 2007 |url-status=dead }}</ref> |
|||
<ref name=weisenberger>{{cite web |last=Weisenberger |first=Drew |url=http://education.jlab.org/qa/mathatom_05.html |title=How many atoms are there in the world? |publisher=Jefferson Lab |access-date=16 January 2008 |archive-url=https://web.archive.org/web/20071022185850/http://education.jlab.org/qa/mathatom_05.html |archive-date=22 October 2007 |url-status=live }}</ref> |
|||
<ref name=pidwirnyf>{{cite web |last=Pidwirny|first=Michael |url=http://www.physicalgeography.net/fundamentals/contents.html |title=Fundamentals of Physical Geography |publisher=University of British Columbia Okanagan |access-date=16 January 2008 | archive-url= https://web.archive.org/web/20080121080709/http://www.physicalgeography.net/fundamentals/contents.html| archive-date= 21 January 2008 | url-status= live}}</ref> |
|||
<ref name=pnas99_22_13966>{{cite journal |last=Anderson|first=Don L. |title=The inner inner core of Earth |journal=[[Proceedings of the National Academy of Sciences]] |year=2002|volume=99|issue=22|pages=13966–13968 |doi=10.1073/pnas.232565899 |pmid=12391308 |pmc=137819|bibcode = 2002PNAS...9913966A |doi-access=free }}</ref> |
|||
<ref name=cern28509>{{cite journal |title=Second postcard from the island of stability |author=Anonymous|journal=CERN Courier |date=2 October 2001 |url=http://cerncourier.com/cws/article/cern/28509| archive-url= https://web.archive.org/web/20080203031237/http://cerncourier.com/cws/article/cern/28509| archive-date= 3 February 2008 | url-status= live}}</ref> |
|||
<ref name=koppes1999>{{cite news |last=Koppes |first=Steve |date=1 March 1999 |title=Fermilab Physicists Find New Matter-Antimatter Asymmetry |publisher=University of Chicago |url=http://www-news.uchicago.edu/releases/99/990301.ktev.shtml|archive-url=https://web.archive.org/web/20080719211849/http://www-news.uchicago.edu/releases/99/990301.ktev.shtml |archive-date=19 July 2008 |url-status=live }}</ref> |
|||
<ref name=cromie20010816>{{cite web |last=Cromie |first=William J. |date=16 August 2001 |title=A lifetime of trillionths of a second: Scientists explore antimatter |website=Harvard University Gazette |url=http://news.harvard.edu/gazette/2001/08.16/antimatter.html|archive-url=https://web.archive.org/web/20060903172020/http://www.news.harvard.edu/gazette/2001/08.16/antimatter.html |archive-date=3 September 2006 |url-status=live }}</ref> |
|||
<ref name=nature419_6906_439>{{cite journal |last=Hijmans|first=Tom W. |title=Particle physics: Cold antihydrogen |journal=[[Nature (journal)|Nature]] |year=2002|volume=419 |pages=439–440|doi=10.1038/419439a |
|||
|pmid=12368837 |issue=6906 |bibcode = 2002Natur.419..439H |doi-access=free}}</ref> |
|||
<ref name=BBC20021030>{{cite news|author=Staff|date=30 October 2002|title=Researchers 'look inside' antimatter|work=BBC News|url=http://news.bbc.co.uk/2/hi/science/nature/2375717.stm|archive-url=https://web.archive.org/web/20070222204339/http://news.bbc.co.uk/2/hi/science/nature/2375717.stm|archive-date=22 February 2007|url-status=live}}</ref> |
|||
<ref name=ns1728_77>{{cite journal|last=Barrett |first=Roger |title=The Strange World of the Exotic Atom |journal=New Scientist |year=1990 |issue=1728 |pages=77–115 |url=http://media.newscientist.com/article/mg12717284.600-the-strange-world-of-the-exotic-atom-physicists-can-nowmake-atoms-and-molecules-containing-negative-particles-other-than-electronsand-use-them-not-just-to-test-theories-but-also-to-fight-cancer-.html|archive-url=https://web.archive.org/web/20071221164440/http://media.newscientist.com/article/mg12717284.600-the-strange-world-of-the-exotic-atom-physicists-can-nowmake-atoms-and-molecules-containing-negative-particles-other-than-electronsand-use-them-not-just-to-test-theories-but-also-to-fight-cancer-.html |archive-date=21 December 2007 |url-status=dead }}</ref> |
|||
<ref name=psT112_1_20>{{cite journal|last=Indelicato|first=Paul|title=Exotic Atoms|journal=[[Physica Scripta]]|year=2004|volume=T112|issue=1|pages=20–26|doi=10.1238/Physica.Topical.112a00020|arxiv=physics/0409058|bibcode=2004PhST..112...20I|s2cid=11134265|url=https://hal.archives-ouvertes.fr/hal-00002825|archive-url=https://web.archive.org/web/20181104170051/https://hal.archives-ouvertes.fr/hal-00002825|archive-date=4 November 2018|url-status=live}}</ref> |
|||
<ref name=ripin1998>{{cite web |last=Ripin|first=Barrett H.|date=July 1998 |url=http://www.aps.org/publications/apsnews/199807/experiment.cfm.html |archive-url=https://archive.today/20120723135110/http://www.aps.org/publications/apsnews/199807/experiment.cfm.html |url-status=dead |archive-date=23 July 2012 |title=Recent Experiments on Exotic Atoms |publisher=American Physical Society}}</ref> |
|||
}} |
|||
== Bibliography == |
|||
* {{cite book |
|||
|author=Oliver Manuel |year=2001 |
|||
|title=Origin of Elements in the Solar System: Implications of Post-1957 Observations |
|||
|publisher=Springer|isbn=978-0-306-46562-8 |
|||
|oclc=228374906 |ref=refManuel2001 |
|||
}} |
|||
* {{cite book |author=Andrew G. van Melsen |translator=Henry J. Koren |year=2004 |orig-year=1952 |title=From Atomos to Atom: The History of the Concept Atom |publisher=Dover Publications |isbn=0-486-49584-1 |ref=refMelsen1952}} |
|||
* {{cite book |author=J.P. Millington |year=1906 |title=John Dalton |publisher=J. M. Dent & Co. (London); E. P. Dutton & Co. (New York) |url=https://archive.org/details/in.ernet.dli.2015.30924/ |ref=refMillington1906}} |
|||
* {{cite book|author1=Charles H. Holbrow |author2=James N. Lloyd |author3=Joseph C. Amato |author4=Enrique Galvez |author5=M. Elizabeth Parks |year=2010 |title=Modern Introductory Physics |publisher=Springer Science & Business Media |isbn=978-0-387-79079-4 |ref=refHolbrowEtAl2010}} |
|||
* {{cite book |
|||
|author=John Dalton |
|||
|year=1808 |
|||
|title=A New System of Chemical Philosophy vol. 1 |
|||
|url=https://library.si.edu/digital-library/book/newsystemofchemi12dalt |
|||
|ref=refDalton1808 |
|||
}} |
|||
* {{cite book |
|||
|author=John Dalton |
|||
|year=1817 |
|||
|title=A New System of Chemical Philosophy vol. 2 |
|||
|url=https://library.si.edu/digital-library/book/newsystemofchemi21dalt |
|||
|ref=refDalton1817 |
|||
}} |
|||
* {{cite book |
|||
|author=John L. Heilbron |
|||
|year=2003 |
|||
|title=Ernest Rutherford and the Explosion of Atoms |
|||
|publisher=[[Oxford University Press]] |
|||
|isbn=0-19-512378-6 |
|||
|ref=refHeilbron2003 |
|||
}} |
|||
* {{cite book |
|||
|author=Jaume Navarro |
|||
|year=2012 |
|||
|title=A History of the Electron: J. J. and G. P. Thomson |
|||
|publisher=Cambridge University Press |
|||
|isbn=978-1-107-00522-8 |
|||
|ref=refNavarro2012 |
|||
}} |
|||
* {{cite book|author=Bernard Pullman |translator=Axel Reisinger |year=1998 |title=The Atom in the History of Human Thought |publisher=Oxford University Press |isbn=0-19-511447-7 |ref=refPullman1998}} |
|||
* {{cite book |
|||
|author=Jean Perrin |
|||
|year=1910 |
|||
|orig-year=1909 |
|||
|title=Brownian Movement and Molecular Reality |
|||
|translator=F. Soddy |
|||
|publisher=Taylor and Francis |
|||
|url=https://archive.org/details/brownianmovement00perr |
|||
|ref=refPerrin1909 |
|||
}} |
|||
* {{cite book|author-link=Eric Scerri |author=Eric R. Scerri |year=2020 |title=The Periodic Table, Its Story and Its Significance |edition=2nd |publisher=Oxford University Press |location=New York |isbn=978-0-190-91436-3}} |
|||
== Further reading == |
|||
{{refbegin}} |
|||
* {{cite book |
|||
|last=Gangopadhyaya|first=Mrinalkanti |
|||
|title=Indian Atomism: History and Sources |
|||
|publisher=Humanities Press|year=1981 |
|||
|location=Atlantic Highlands, New Jersey |
|||
|isbn=978-0-391-02177-8 |
|||
|oclc=10916778 |
|||
}} |
|||
* {{cite book |
|||
|last=Iannone|first=A. Pablo|year=2001 |
|||
|title=Dictionary of World Philosophy |
|||
|publisher=Routledge|isbn=978-0-415-17995-9 |
|||
|oclc=44541769 |
|||
}} |
|||
* {{cite book |
|||
|last=King|first=Richard|year=1999 |
|||
|title=Indian philosophy: an introduction to Hindu and Buddhist thought |
|||
|publisher=Edinburgh University Press |
|||
|isbn=978-0-7486-0954-3 |
|||
}} |
|||
* {{cite book |
|||
|last=McEvilley|first=Thomas |
|||
|title=The shape of ancient thought: comparative studies in Greek and Indian philosophies |
|||
|publisher=Allworth Press|year=2002 |
|||
|isbn=978-1-58115-203-6 |
|||
}} |
|||
* {{cite book |
|||
|last=Siegfried|first=Robert|year=2002 |
|||
|title=From Elements to Atoms: A History of Chemical Composition |
|||
|publisher=Diane|isbn=978-0-87169-924-4 |
|||
|oclc=186607849 |
|||
}} |
|||
* {{cite book|last=Teresi|first=Dick|publisher=Simon & Schuster|title=Lost Discoveries: The Ancient Roots of Modern Science|year=2003|isbn=978-0-7432-4379-7|url=https://books.google.com/books?id=pheL_ubbXD0C|pages=213–214|access-date=25 October 2020|archive-date=4 August 2020|archive-url=https://web.archive.org/web/20200804145606/https://books.google.com/books?id=pheL_ubbXD0C|url-status=live}} |
|||
* {{cite book |
|||
|last=Wurtz|first=Charles Adolphe|year=1881 |
|||
|title=The Atomic Theory |
|||
|publisher=D. Appleton and company |
|||
|location=New York |
|||
|isbn=978-0-559-43636-9 |
|||
}} |
|||
{{refend}} |
|||
== External links == |
== External links == |
||
{{Sister project links|voy=no|wikt=atom|v=The Atom|n=no|q=Atom|s=The New Student's Reference Work}} |
|||
{{Wikisource1914NSRW|Atom}} |
|||
* [https://www.feynmanlectures.caltech.edu/I_01.html Atoms in Motion – The Feynman Lectures on Physics] |
|||
{{Commons|Atom}} |
|||
* {{cite web|first=Tim|last=Sharp|title=What is an Atom?|url=https://www.livescience.com/37206-atom-definition.html|publisher=Live Science|date=8 August 2017}} |
|||
*{{cite web |
|||
| last=Francis | first=Eden | year=2002 |
|||
| url=http://dl.clackamas.cc.or.us/ch104-07/atomic_size.htm |
|||
| title=Atomic Size | publisher=Clackamas Community College |
|||
| accessdate=2007-01-09 }} |
|||
*{{cite web |
|||
| last=Freudenrich | first=Craig C |
|||
| url=http://dl.clackamas.cc.or.us/ch104-07/atomic_size.htm |
|||
| title=How Atoms Work | publisher=How Stuff Works |
|||
| accessdate=2007-01-09 }} |
|||
*{{cite web |
|||
| url=http://en.wikibooks.org/wiki/FHSST_Physics_Atom:The_Atom |
|||
| work=Free High School Science Texts: Physics |
|||
| title=Atom:The Atom | publisher=Wikibooks |
|||
| accessdate=2007-01-09 }} |
|||
*{{cite web |
|||
| author=Anonymous | year=2007 |
|||
| url=http://www.scienceaid.co.uk/chemistry/basics/theatom.html |
|||
| title=The atom | publisher=Science aid+ |
|||
| accessdate=2007-01-09 }}—a guide to the atom for teens. |
|||
*{{cite web |
|||
| author=Anonymous | date=[[January 3]], [[2006]] |
|||
| url=http://www.bbc.co.uk/dna/h2g2/A6672963 |
|||
| title=Atoms and Atomic Structure |
|||
| publisher=BBC | accessdate=2007-01-11 }} |
|||
*{{cite web |
|||
| author=Various | date=[[January 3]], [[2006]] |
|||
| url=http://www.colorado.edu/physics/2000/index.pl?Type=TOC |
|||
| title=Physics 2000, Table of Contents |
|||
| publisher=University of Colorado | accessdate=2008-01-11 }} |
|||
{{Composition}} |
|||
{{particles}} |
{{particles}} |
||
{{Branches of chemistry}} |
|||
{{biological organisation}} |
|||
{{ |
{{Authority control}} |
||
[[Category:Atoms| ]] |
[[Category:Atoms| ]] |
||
[[Category: |
[[Category:Chemistry]] |
||
[[Category: |
[[Category:Articles containing video clips]] |
||
[[af:Atoom]] |
|||
[[ar:ذرة]] |
|||
[[an:Atomo]] |
|||
[[ast:Átomu]] |
|||
[[az:Atom]] |
|||
[[bn:পরমাণু]] |
|||
[[zh-min-nan:Goân-chú]] |
|||
[[be:Атам]] |
|||
[[bs:Atom]] |
|||
[[br:Atom]] |
|||
[[bg:Атом]] |
|||
[[ca:Àtom]] |
|||
[[cs:Atom]] |
|||
[[cy:Atom]] |
|||
[[da:Atom]] |
|||
[[de:Atom]] |
|||
[[dsb:Atom]] |
|||
[[et:Aatom]] |
|||
[[el:Άτομο]] |
|||
[[es:Átomo]] |
|||
[[eo:Atomo]] |
|||
[[eu:Atomo]] |
|||
[[fa:اتم]] |
|||
[[fo:Atom]] |
|||
[[fr:Atome]] |
|||
[[ga:Adamh]] |
|||
[[gl:Átomo]] |
|||
[[ko:원자]] |
|||
[[hsb:Atom]] |
|||
[[hr:Atom]] |
|||
[[io:Atomo]] |
|||
[[id:Atom]] |
|||
[[ia:Atomo]] |
|||
[[is:Frumeind]] |
|||
[[it:Atomo]] |
|||
[[he:אטום]] |
|||
[[kn:ಅಣು]] |
|||
[[ka:ატომი]] |
|||
[[sw:Atomi]] |
|||
[[ht:Atòm]] |
|||
[[la:Atomus]] |
|||
[[lv:Atoms]] |
|||
[[lb:Atom]] |
|||
[[lt:Atomas]] |
|||
[[ln:Atome]] |
|||
[[jbo:ratni]] |
|||
[[hu:Atom]] |
|||
[[mk:Атом]] |
|||
[[ml:അണു]] |
|||
[[mr:अणू]] |
|||
[[ms:Atom]] |
|||
[[mn:Атом]] |
|||
[[nl:Atoom]] |
|||
[[ne:अणु]] |
|||
[[new:अणु]] |
|||
[[ja:原子]] |
|||
[[pih:Etem]] |
|||
[[no:Atom]] |
|||
[[nn:Atom]] |
|||
[[nrm:Atôme]] |
|||
[[nov:Atome]] |
|||
[[uz:Atom]] |
|||
[[nds:Atom]] |
|||
[[pl:Atom]] |
|||
[[pt:Átomo]] |
|||
[[ro:Atom]] |
|||
[[qu:Iñuku]] |
|||
[[ru:Атом]] |
|||
[[sco:Atom]] |
|||
[[sq:Atomi]] |
|||
[[scn:Àtumu]] |
|||
[[simple:Atom]] |
|||
[[sk:Atóm]] |
|||
[[sl:Atom]] |
|||
[[sr:Атом]] |
|||
[[sh:Atom]] |
|||
[[su:Atom]] |
|||
[[fi:Atomi]] |
|||
[[sv:Atom]] |
|||
[[tl:Atomo]] |
|||
[[ta:அணு]] |
|||
[[th:อะตอม]] |
|||
[[vi:Nguyên tử]] |
|||
[[tg:Атом]] |
|||
[[tr:Atom]] |
|||
[[bug:Atong]] |
|||
[[uk:Атом]] |
|||
[[yi:אטאם]] |
|||
[[yo:Átọ́mù]] |
|||
[[zh-yue:原子]] |
|||
[[zh:原子]] |
|||
Latest revision as of 18:34, 28 December 2024
| Atom | |
|---|---|
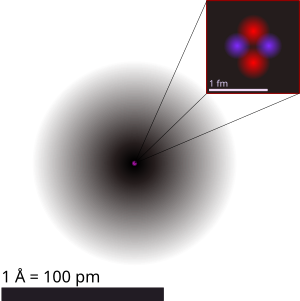 An illustration of the helium atom, depicting the nucleus (pink) and the electron cloud distribution (black). The nucleus (upper right) in helium-4 is in reality spherically symmetric and closely resembles the electron cloud, although for more complicated nuclei this is not always the case. The black bar is one angstrom (10−10 m or 100 pm). | |
| Classification | |
| Smallest recognized division of a chemical element | |
| Properties | |
| Mass range | 1.67×10−27 to 4.52×10−25 kg |
| Electric charge | zero (neutral), or ion charge |
| Diameter range | 62 pm (He) to 520 pm (Cs) (data page) |
| Components | Electrons and a compact nucleus of protons and neutrons |
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element.
Atoms are extremely small, typically around 100 picometers across. A human hair is about a million carbon atoms wide. Atoms are smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. They are so small that accurately predicting their behavior using classical physics is not possible due to quantum effects.
More than 99.9994%[1] of an atom's mass is in the nucleus. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. The electrons are negatively charged, and this opposing charge is what binds them to the nucleus. If the numbers of protons and electrons are equal, as they normally are, then the atom is electrically neutral as a whole. If an atom has more electrons than protons, then it has an overall negative charge, and is called a negative ion (or anion). Conversely, if it has more protons than electrons, it has a positive charge, and is called a positive ion (or cation).
The electrons of an atom are attracted to the protons in an atomic nucleus by the electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by the nuclear force. This force is usually stronger than the electromagnetic force that repels the positively charged protons from one another. Under certain circumstances, the repelling electromagnetic force becomes stronger than the nuclear force. In this case, the nucleus splits and leaves behind different elements. This is a form of nuclear decay.
Atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules or crystals. The ability of atoms to attach and detach from each other is responsible for most of the physical changes observed in nature. Chemistry is the science that studies these changes.
History of atomic theory
In philosophy
The basic idea that matter is made up of tiny indivisible particles is an old idea that appeared in many ancient cultures. The word atom is derived from the ancient Greek word atomos,[a] which means "uncuttable". But this ancient idea was based in philosophical reasoning rather than scientific reasoning. Modern atomic theory is not based on these old concepts.[2][3] In the early 19th century, the scientist John Dalton found evidence that matter really is composed of discrete units, and so applied the word atom to those units.[4]
Dalton's law of multiple proportions

In the early 1800s, John Dalton compiled experimental data gathered by him and other scientists and discovered a pattern now known as the "law of multiple proportions". He noticed that in any group of chemical compounds which all contain two particular chemical elements, the amount of Element A per measure of Element B will differ across these compounds by ratios of small whole numbers. This pattern suggested that each element combines with other elements in multiples of a basic unit of weight, with each element having a unit of unique weight. Dalton decided to call these units "atoms".[5]
For example, there are two types of tin oxide: one is a grey powder that is 88.1% tin and 11.9% oxygen, and the other is a white powder that is 78.7% tin and 21.3% oxygen. Adjusting these figures, in the grey powder there is about 13.5 g of oxygen for every 100 g of tin, and in the white powder there is about 27 g of oxygen for every 100 g of tin. 13.5 and 27 form a ratio of 1:2. Dalton concluded that in the grey oxide there is one atom of oxygen for every atom of tin, and in the white oxide there are two atoms of oxygen for every atom of tin (SnO and SnO2).[6][7]
Dalton also analyzed iron oxides. There is one type of iron oxide that is a black powder which is 78.1% iron and 21.9% oxygen; and there is another iron oxide that is a red powder which is 70.4% iron and 29.6% oxygen. Adjusting these figures, in the black powder there is about 28 g of oxygen for every 100 g of iron, and in the red powder there is about 42 g of oxygen for every 100 g of iron. 28 and 42 form a ratio of 2:3. Dalton concluded that in these oxides, for every two atoms of iron, there are two or three atoms of oxygen respectively (Fe2O2 and Fe2O3).[b][8][9]
As a final example: nitrous oxide is 63.3% nitrogen and 36.7% oxygen, nitric oxide is 44.05% nitrogen and 55.95% oxygen, and nitrogen dioxide is 29.5% nitrogen and 70.5% oxygen. Adjusting these figures, in nitrous oxide there is 80 g of oxygen for every 140 g of nitrogen, in nitric oxide there is about 160 g of oxygen for every 140 g of nitrogen, and in nitrogen dioxide there is 320 g of oxygen for every 140 g of nitrogen. 80, 160, and 320 form a ratio of 1:2:4. The respective formulas for these oxides are N2O, NO, and NO2.[10][11]
Discovery of the electron
In 1897, J. J. Thomson discovered that cathode rays are not a form of light but made of negatively charged particles because they can be deflected by electric and magnetic fields.[12] He measured these particles to be at least a thousand times lighter than hydrogen (the lightest atom).[13] He called these new particles corpuscles but they were later renamed electrons since these are the particles that carry electricity.[14] Thomson also showed that electrons were identical to particles given off by photoelectric and radioactive materials.[15] Thomson explained that an electric current is the passing of electrons from one atom to the next, and when there was no current the electrons embedded themselves in the atoms. This in turn meant that atoms were not indivisible as scientists thought. The atom was composed of electrons whose negative charge was balanced out by some source of positive charge to create an electrically neutral atom. Ions, Thomson explained, must be atoms which have an excess or shortage of electrons.[16]
Discovery of the nucleus

The electrons in the atom logically had to be balanced out by a commensurate amount of positive charge, but Thomson had no idea where this positive charge came from, so he tentatively proposed that it was everywhere in the atom, the atom being in the shape of a sphere. This was the mathematically simplest hypothesis to fit the available evidence, or lack thereof. Following from this, Thomson imagined that the balance of electrostatic forces would distribute the electrons throughout the sphere in a more or less even manner.[17] Thomson's model is popularly known as the plum pudding model, though neither Thomson nor his colleagues used this analogy.[18] Thomson's model was incomplete, it was unable to predict any other properties of the elements such as emission spectra and valencies. It was soon rendered obsolete by the discovery of the atomic nucleus.
Between 1908 and 1913, Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden performed a series of experiments in which they bombarded thin foils of metal with a beam of alpha particles. They did this to measure the scattering patterns of the alpha particles. They spotted a small number of alpha particles being deflected by angles greater than 90°. This shouldn't have been possible according to the Thomson model of the atom, whose charges were too diffuse to produce a sufficiently strong electric field. The deflections should have all been negligible. Rutherford proposed that the positive charge of the atom is concentrated in a tiny volume at the center of the atom and that the electrons surround this nucleus in a diffuse cloud. This nucleus carried almost all of the atom's mass, the electrons being so very light. Only such an intense concentration of charge, anchored by its high mass, could produce an electric field that could deflect the alpha particles so strongly.[19]
Bohr model

A problem in classical mechanics is that an accelerating charged particle radiates electromagnetic radiation, causing the particle to lose kinetic energy. Circular motion counts as acceleration, which means that an electron orbiting a central charge should spiral down into that nucleus as it loses speed. In 1913, the physicist Niels Bohr proposed a new model in which the electrons of an atom were assumed to orbit the nucleus but could only do so in a finite set of orbits, and could jump between these orbits only in discrete changes of energy corresponding to absorption or radiation of a photon.[20] This quantization was used to explain why the electrons' orbits are stable and why elements absorb and emit electromagnetic radiation in discrete spectra.[21] Bohr's model could only predict the emission spectra of hydrogen, not atoms with more than one electron.
Discovery of protons and neutrons
Back in 1815, William Prout observed that the atomic weights of many elements were multiples of hydrogen's atomic weight, which is in fact true for all of them if one takes isotopes into account. In 1898, J. J. Thomson found that the positive charge of a hydrogen ion is equal to the negative charge of an electron, and these were then the smallest known charged particles.[22] Thomson later found that the positive charge in an atom is a positive multiple of an electron's negative charge.[23] In 1913, Henry Moseley discovered that the frequencies of X-ray emissions from an excited atom were a mathematical function of its atomic number and hydrogen's nuclear charge. In 1919 Rutherford bombarded nitrogen gas with alpha particles and detected hydrogen ions being emitted from the gas, and concluded that they were produced by alpha particles hitting and splitting the nuclei of the nitrogen atoms.[24]
These observations led Rutherford to conclude that the hydrogen nucleus is a singular particle with a positive charge equal to the electron's negative charge.[25] He named this particle "proton" in 1920.[26] The number of protons in an atom (which Rutherford called the "atomic number"[27][28]) was found to be equal to the element's ordinal number on the periodic table and therefore provided a simple and clear-cut way of distinguishing the elements from each other. The atomic weight of each element is higher than its proton number, so Rutherford hypothesized that the surplus weight was carried by unknown particles with no electric charge and a mass equal to that of the proton.
In 1928, Walter Bothe observed that beryllium emitted a highly penetrating, electrically neutral radiation when bombarded with alpha particles. It was later discovered that this radiation could knock hydrogen atoms out of paraffin wax. Initially it was thought to be high-energy gamma radiation, since gamma radiation had a similar effect on electrons in metals, but James Chadwick found that the ionization effect was too strong for it to be due to electromagnetic radiation, so long as energy and momentum were conserved in the interaction. In 1932, Chadwick exposed various elements, such as hydrogen and nitrogen, to the mysterious "beryllium radiation", and by measuring the energies of the recoiling charged particles, he deduced that the radiation was actually composed of electrically neutral particles which could not be massless like the gamma ray, but instead were required to have a mass similar to that of a proton. Chadwick now claimed these particles as Rutherford's neutrons.[29]
The current consensus model

In 1925, Werner Heisenberg published the first consistent mathematical formulation of quantum mechanics (matrix mechanics).[30] One year earlier, Louis de Broglie had proposed that all particles behave like waves to some extent,[31] and in 1926 Erwin Schroedinger used this idea to develop the Schroedinger equation, which describes electrons as three-dimensional waveforms rather than points in space.[32] A consequence of using waveforms to describe particles is that it is mathematically impossible to obtain precise values for both the position and momentum of a particle at a given point in time. This became known as the uncertainty principle, formulated by Werner Heisenberg in 1927.[30] In this concept, for a given accuracy in measuring a position one could only obtain a range of probable values for momentum, and vice versa.[33] Thus, the planetary model of the atom was discarded in favor of one that described atomic orbital zones around the nucleus where a given electron is most likely to be found.[34][35] This model was able to explain observations of atomic behavior that previous models could not, such as certain structural and spectral patterns of atoms larger than hydrogen.
Structure
Subatomic particles
Though the word atom originally denoted a particle that cannot be cut into smaller particles, in modern scientific usage the atom is composed of various subatomic particles. The constituent particles of an atom are the electron, the proton and the neutron.
The electron is the least massive of these particles by four orders of magnitude at 9.11×10−31 kg, with a negative electrical charge and a size that is too small to be measured using available techniques.[36] It was the lightest particle with a positive rest mass measured, until the discovery of neutrino mass. Under ordinary conditions, electrons are bound to the positively charged nucleus by the attraction created from opposite electric charges. If an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively charged as a whole; a charged atom is called an ion. Electrons have been known since the late 19th century, mostly thanks to J.J. Thomson; see history of subatomic physics for details.
Protons have a positive charge and a mass of 1.6726×10−27 kg. The number of protons in an atom is called its atomic number. Ernest Rutherford (1919) observed that nitrogen under alpha-particle bombardment ejects what appeared to be hydrogen nuclei. By 1920 he had accepted that the hydrogen nucleus is a distinct particle within the atom and named it proton.
Neutrons have no electrical charge and have a mass of 1.6749×10−27 kg.[37][38] Neutrons are the heaviest of the three constituent particles, but their mass can be reduced by the nuclear binding energy. Neutrons and protons (collectively known as nucleons) have comparable dimensions—on the order of 2.5×10−15 m—although the 'surface' of these particles is not sharply defined.[39] The neutron was discovered in 1932 by the English physicist James Chadwick.
In the Standard Model of physics, electrons are truly elementary particles with no internal structure, whereas protons and neutrons are composite particles composed of elementary particles called quarks. There are two types of quarks in atoms, each having a fractional electric charge. Protons are composed of two up quarks (each with charge +2/3) and one down quark (with a charge of −1/3). Neutrons consist of one up quark and two down quarks. This distinction accounts for the difference in mass and charge between the two particles.[40][41]
The quarks are held together by the strong interaction (or strong force), which is mediated by gluons. The protons and neutrons, in turn, are held to each other in the nucleus by the nuclear force, which is a residuum of the strong force that has somewhat different range-properties (see the article on the nuclear force for more). The gluon is a member of the family of gauge bosons, which are elementary particles that mediate physical forces.[40][41]
Nucleus

All the bound protons and neutrons in an atom make up a tiny atomic nucleus, and are collectively called nucleons. The radius of a nucleus is approximately equal to femtometres, where is the total number of nucleons.[42] This is much smaller than the radius of the atom, which is on the order of 105 fm. The nucleons are bound together by a short-ranged attractive potential called the residual strong force. At distances smaller than 2.5 fm this force is much more powerful than the electrostatic force that causes positively charged protons to repel each other.[43]
Atoms of the same element have the same number of protons, called the atomic number. Within a single element, the number of neutrons may vary, determining the isotope of that element. The total number of protons and neutrons determine the nuclide. The number of neutrons relative to the protons determines the stability of the nucleus, with certain isotopes undergoing radioactive decay.[44]
The proton, the electron, and the neutron are classified as fermions. Fermions obey the Pauli exclusion principle which prohibits identical fermions, such as multiple protons, from occupying the same quantum state at the same time. Thus, every proton in the nucleus must occupy a quantum state different from all other protons, and the same applies to all neutrons of the nucleus and to all electrons of the electron cloud.[45]
A nucleus that has a different number of protons than neutrons can potentially drop to a lower energy state through a radioactive decay that causes the number of protons and neutrons to more closely match. As a result, atoms with matching numbers of protons and neutrons are more stable against decay, but with increasing atomic number, the mutual repulsion of the protons requires an increasing proportion of neutrons to maintain the stability of the nucleus.[45]

The number of protons and neutrons in the atomic nucleus can be modified, although this can require very high energies because of the strong force. Nuclear fusion occurs when multiple atomic particles join to form a heavier nucleus, such as through the energetic collision of two nuclei. For example, at the core of the Sun protons require energies of 3 to 10 keV to overcome their mutual repulsion—the coulomb barrier—and fuse together into a single nucleus.[46] Nuclear fission is the opposite process, causing a nucleus to split into two smaller nuclei—usually through radioactive decay. The nucleus can also be modified through bombardment by high energy subatomic particles or photons. If this modifies the number of protons in a nucleus, the atom changes to a different chemical element.[47][48]
If the mass of the nucleus following a fusion reaction is less than the sum of the masses of the separate particles, then the difference between these two values can be emitted as a type of usable energy (such as a gamma ray, or the kinetic energy of a beta particle), as described by Albert Einstein's mass–energy equivalence formula, E=mc2, where m is the mass loss and c is the speed of light. This deficit is part of the binding energy of the new nucleus, and it is the non-recoverable loss of the energy that causes the fused particles to remain together in a state that requires this energy to separate.[49]
The fusion of two nuclei that create larger nuclei with lower atomic numbers than iron and nickel—a total nucleon number of about 60—is usually an exothermic process that releases more energy than is required to bring them together.[50] It is this energy-releasing process that makes nuclear fusion in stars a self-sustaining reaction. For heavier nuclei, the binding energy per nucleon begins to decrease. That means that a fusion process producing a nucleus that has an atomic number higher than about 26, and a mass number higher than about 60, is an endothermic process. Thus, more massive nuclei cannot undergo an energy-producing fusion reaction that can sustain the hydrostatic equilibrium of a star.[45]
Electron cloud

The electrons in an atom are attracted to the protons in the nucleus by the electromagnetic force. This force binds the electrons inside an electrostatic potential well surrounding the smaller nucleus, which means that an external source of energy is needed for the electron to escape. The closer an electron is to the nucleus, the greater the attractive force. Hence electrons bound near the center of the potential well require more energy to escape than those at greater separations.
Electrons, like other particles, have properties of both a particle and a wave. The electron cloud is a region inside the potential well where each electron forms a type of three-dimensional standing wave—a wave form that does not move relative to the nucleus. This behavior is defined by an atomic orbital, a mathematical function that characterises the probability that an electron appears to be at a particular location when its position is measured.[51] Only a discrete (or quantized) set of these orbitals exist around the nucleus, as other possible wave patterns rapidly decay into a more stable form.[52] Orbitals can have one or more ring or node structures, and differ from each other in size, shape and orientation.[53]

Each atomic orbital corresponds to a particular energy level of the electron. The electron can change its state to a higher energy level by absorbing a photon with sufficient energy to boost it into the new quantum state. Likewise, through spontaneous emission, an electron in a higher energy state can drop to a lower energy state while radiating the excess energy as a photon. These characteristic energy values, defined by the differences in the energies of the quantum states, are responsible for atomic spectral lines.[52]
The amount of energy needed to remove or add an electron—the electron binding energy—is far less than the binding energy of nucleons. For example, it requires only 13.6 eV to strip a ground-state electron from a hydrogen atom,[54] compared to 2.23 million eV for splitting a deuterium nucleus.[55] Atoms are electrically neutral if they have an equal number of protons and electrons. Atoms that have either a deficit or a surplus of electrons are called ions. Electrons that are farthest from the nucleus may be transferred to other nearby atoms or shared between atoms. By this mechanism, atoms are able to bond into molecules and other types of chemical compounds like ionic and covalent network crystals.[56]
Properties
Nuclear properties
By definition, any two atoms with an identical number of protons in their nuclei belong to the same chemical element. Atoms with equal numbers of protons but a different number of neutrons are different isotopes of the same element. For example, all hydrogen atoms admit exactly one proton, but isotopes exist with no neutrons (hydrogen-1, by far the most common form,[57] also called protium), one neutron (deuterium), two neutrons (tritium) and more than two neutrons. The known elements form a set of atomic numbers, from the single-proton element hydrogen up to the 118-proton element oganesson.[58] All known isotopes of elements with atomic numbers greater than 82 are radioactive, although the radioactivity of element 83 (bismuth) is so slight as to be practically negligible.[59][60]
About 339 nuclides occur naturally on Earth,[61] of which 251 (about 74%) have not been observed to decay, and are referred to as "stable isotopes". Only 90 nuclides are stable theoretically, while another 161 (bringing the total to 251) have not been observed to decay, even though in theory it is energetically possible. These are also formally classified as "stable". An additional 35 radioactive nuclides have half-lives longer than 100 million years, and are long-lived enough to have been present since the birth of the Solar System. This collection of 286 nuclides are known as primordial nuclides. Finally, an additional 53 short-lived nuclides are known to occur naturally, as daughter products of primordial nuclide decay (such as radium from uranium), or as products of natural energetic processes on Earth, such as cosmic ray bombardment (for example, carbon-14).[62][note 1]
For 80 of the chemical elements, at least one stable isotope exists. As a rule, there is only a handful of stable isotopes for each of these elements, the average being 3.1 stable isotopes per element. Twenty-six "monoisotopic elements" have only a single stable isotope, while the largest number of stable isotopes observed for any element is ten, for the element tin. Elements 43, 61, and all elements numbered 83 or higher have no stable isotopes.[63]: 1–12
Stability of isotopes is affected by the ratio of protons to neutrons, and also by the presence of certain "magic numbers" of neutrons or protons that represent closed and filled quantum shells. These quantum shells correspond to a set of energy levels within the shell model of the nucleus; filled shells, such as the filled shell of 50 protons for tin, confers unusual stability on the nuclide. Of the 251 known stable nuclides, only four have both an odd number of protons and odd number of neutrons: hydrogen-2 (deuterium), lithium-6, boron-10, and nitrogen-14. (Tantalum-180m is odd-odd and observationally stable, but is predicted to decay with a very long half-life.) Also, only four naturally occurring, radioactive odd-odd nuclides have a half-life over a billion years: potassium-40, vanadium-50, lanthanum-138, and lutetium-176. Most odd-odd nuclei are highly unstable with respect to beta decay, because the decay products are even-even, and are therefore more strongly bound, due to nuclear pairing effects.[64]
Mass
The large majority of an atom's mass comes from the protons and neutrons that make it up. The total number of these particles (called "nucleons") in a given atom is called the mass number. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. An example of use of a mass number is "carbon-12," which has 12 nucleons (six protons and six neutrons).
The actual mass of an atom at rest is often expressed in daltons (Da), also called the unified atomic mass unit (u). This unit is defined as a twelfth of the mass of a free neutral atom of carbon-12, which is approximately 1.66×10−27 kg.[65] Hydrogen-1 (the lightest isotope of hydrogen which is also the nuclide with the lowest mass) has an atomic weight of 1.007825 Da.[66] The value of this number is called the atomic mass. A given atom has an atomic mass approximately equal (within 1%) to its mass number times the atomic mass unit (for example the mass of a nitrogen-14 is roughly 14 Da), but this number will not be exactly an integer except (by definition) in the case of carbon-12.[67] The heaviest stable atom is lead-208,[59] with a mass of 207.9766521 Da.[68]
As even the most massive atoms are far too light to work with directly, chemists instead use the unit of moles. One mole of atoms of any element always has the same number of atoms (about 6.022×1023). This number was chosen so that if an element has an atomic mass of 1 u, a mole of atoms of that element has a mass close to one gram. Because of the definition of the unified atomic mass unit, each carbon-12 atom has an atomic mass of exactly 12 Da, and so a mole of carbon-12 atoms weighs exactly 0.012 kg.[65]
Shape and size
Atoms lack a well-defined outer boundary, so their dimensions are usually described in terms of an atomic radius. This is a measure of the distance out to which the electron cloud extends from the nucleus.[69] This assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Atomic radii may be derived from the distances between two nuclei when the two atoms are joined in a chemical bond. The radius varies with the location of an atom on the atomic chart, the type of chemical bond, the number of neighboring atoms (coordination number) and a quantum mechanical property known as spin.[70] On the periodic table of the elements, atom size tends to increase when moving down columns, but decrease when moving across rows (left to right).[71] Consequently, the smallest atom is helium with a radius of 32 pm, while one of the largest is caesium at 225 pm.[72]
When subjected to external forces, like electrical fields, the shape of an atom may deviate from spherical symmetry. The deformation depends on the field magnitude and the orbital type of outer shell electrons, as shown by group-theoretical considerations. Aspherical deviations might be elicited for instance in crystals, where large crystal-electrical fields may occur at low-symmetry lattice sites.[73][74] Significant ellipsoidal deformations have been shown to occur for sulfur ions[75] and chalcogen ions[76] in pyrite-type compounds.
Atomic dimensions are thousands of times smaller than the wavelengths of light (400–700 nm) so they cannot be viewed using an optical microscope, although individual atoms can be observed using a scanning tunneling microscope. To visualize the minuteness of the atom, consider that a typical human hair is about 1 million carbon atoms in width.[77] A single drop of water contains about 2 sextillion (2×1021) atoms of oxygen, and twice the number of hydrogen atoms.[78] A single carat diamond with a mass of 2×10−4 kg contains about 10 sextillion (1022) atoms of carbon.[note 2] If an apple were magnified to the size of the Earth, then the atoms in the apple would be approximately the size of the original apple.[79]
Radioactive decay
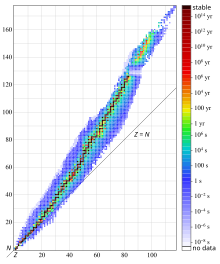
Every element has one or more isotopes that have unstable nuclei that are subject to radioactive decay, causing the nucleus to emit particles or electromagnetic radiation. Radioactivity can occur when the radius of a nucleus is large compared with the radius of the strong force, which only acts over distances on the order of 1 fm.[80]
The most common forms of radioactive decay are:[81][82]
- Alpha decay: this process is caused when the nucleus emits an alpha particle, which is a helium nucleus consisting of two protons and two neutrons. The result of the emission is a new element with a lower atomic number.
- Beta decay (and electron capture): these processes are regulated by the weak force, and result from a transformation of a neutron into a proton, or a proton into a neutron. The neutron to proton transition is accompanied by the emission of an electron and an antineutrino, while proton to neutron transition (except in electron capture) causes the emission of a positron and a neutrino. The electron or positron emissions are called beta particles. Beta decay either increases or decreases the atomic number of the nucleus by one. Electron capture is more common than positron emission, because it requires less energy. In this type of decay, an electron is absorbed by the nucleus, rather than a positron emitted from the nucleus. A neutrino is still emitted in this process, and a proton changes to a neutron.
- Gamma decay: this process results from a change in the energy level of the nucleus to a lower state, resulting in the emission of electromagnetic radiation. The excited state of a nucleus which results in gamma emission usually occurs following the emission of an alpha or a beta particle. Thus, gamma decay usually follows alpha or beta decay.
Other more rare types of radioactive decay include ejection of neutrons or protons or clusters of nucleons from a nucleus, or more than one beta particle. An analog of gamma emission which allows excited nuclei to lose energy in a different way, is internal conversion—a process that produces high-speed electrons that are not beta rays, followed by production of high-energy photons that are not gamma rays. A few large nuclei explode into two or more charged fragments of varying masses plus several neutrons, in a decay called spontaneous nuclear fission.
Each radioactive isotope has a characteristic decay time period—the half-life—that is determined by the amount of time needed for half of a sample to decay. This is an exponential decay process that steadily decreases the proportion of the remaining isotope by 50% every half-life. Hence after two half-lives have passed only 25% of the isotope is present, and so forth.[80]
Magnetic moment
Elementary particles possess an intrinsic quantum mechanical property known as spin. This is analogous to the angular momentum of an object that is spinning around its center of mass, although strictly speaking these particles are believed to be point-like and cannot be said to be rotating. Spin is measured in units of the reduced Planck constant (ħ), with electrons, protons and neutrons all having spin 1⁄2 ħ, or "spin-1⁄2". In an atom, electrons in motion around the nucleus possess orbital angular momentum in addition to their spin, while the nucleus itself possesses angular momentum due to its nuclear spin.[83]
The magnetic field produced by an atom—its magnetic moment—is determined by these various forms of angular momentum, just as a rotating charged object classically produces a magnetic field, but the most dominant contribution comes from electron spin. Due to the nature of electrons to obey the Pauli exclusion principle, in which no two electrons may be found in the same quantum state, bound electrons pair up with each other, with one member of each pair in a spin up state and the other in the opposite, spin down state. Thus these spins cancel each other out, reducing the total magnetic dipole moment to zero in some atoms with even number of electrons.[84]
In ferromagnetic elements such as iron, cobalt and nickel, an odd number of electrons leads to an unpaired electron and a net overall magnetic moment. The orbitals of neighboring atoms overlap and a lower energy state is achieved when the spins of unpaired electrons are aligned with each other, a spontaneous process known as an exchange interaction. When the magnetic moments of ferromagnetic atoms are lined up, the material can produce a measurable macroscopic field. Paramagnetic materials have atoms with magnetic moments that line up in random directions when no magnetic field is present, but the magnetic moments of the individual atoms line up in the presence of a field.[84][85]
The nucleus of an atom will have no spin when it has even numbers of both neutrons and protons, but for other cases of odd numbers, the nucleus may have a spin. Normally nuclei with spin are aligned in random directions because of thermal equilibrium, but for certain elements (such as xenon-129) it is possible to polarize a significant proportion of the nuclear spin states so that they are aligned in the same direction—a condition called hyperpolarization. This has important applications in magnetic resonance imaging.[86][87]
Energy levels

The potential energy of an electron in an atom is negative relative to when the distance from the nucleus goes to infinity; its dependence on the electron's position reaches the minimum inside the nucleus, roughly in inverse proportion to the distance. In the quantum-mechanical model, a bound electron can occupy only a set of states centered on the nucleus, and each state corresponds to a specific energy level; see time-independent Schrödinger equation for a theoretical explanation. An energy level can be measured by the amount of energy needed to unbind the electron from the atom, and is usually given in units of electronvolts (eV). The lowest energy state of a bound electron is called the ground state, i.e. stationary state, while an electron transition to a higher level results in an excited state.[88] The electron's energy increases along with n because the (average) distance to the nucleus increases. Dependence of the energy on ℓ is caused not by the electrostatic potential of the nucleus, but by interaction between electrons.
For an electron to transition between two different states, e.g. ground state to first excited state, it must absorb or emit a photon at an energy matching the difference in the potential energy of those levels, according to the Niels Bohr model, what can be precisely calculated by the Schrödinger equation. Electrons jump between orbitals in a particle-like fashion. For example, if a single photon strikes the electrons, only a single electron changes states in response to the photon; see Electron properties.
The energy of an emitted photon is proportional to its frequency, so these specific energy levels appear as distinct bands in the electromagnetic spectrum.[89] Each element has a characteristic spectrum that can depend on the nuclear charge, subshells filled by electrons, the electromagnetic interactions between the electrons and other factors.[90]

When a continuous spectrum of energy is passed through a gas or plasma, some of the photons are absorbed by atoms, causing electrons to change their energy level. Those excited electrons that remain bound to their atom spontaneously emit this energy as a photon, traveling in a random direction, and so drop back to lower energy levels. Thus the atoms behave like a filter that forms a series of dark absorption bands in the energy output. (An observer viewing the atoms from a view that does not include the continuous spectrum in the background, instead sees a series of emission lines from the photons emitted by the atoms.) Spectroscopic measurements of the strength and width of atomic spectral lines allow the composition and physical properties of a substance to be determined.[91]
Close examination of the spectral lines reveals that some display a fine structure splitting. This occurs because of spin–orbit coupling, which is an interaction between the spin and motion of the outermost electron.[92] When an atom is in an external magnetic field, spectral lines become split into three or more components; a phenomenon called the Zeeman effect. This is caused by the interaction of the magnetic field with the magnetic moment of the atom and its electrons. Some atoms can have multiple electron configurations with the same energy level, which thus appear as a single spectral line. The interaction of the magnetic field with the atom shifts these electron configurations to slightly different energy levels, resulting in multiple spectral lines.[93] The presence of an external electric field can cause a comparable splitting and shifting of spectral lines by modifying the electron energy levels, a phenomenon called the Stark effect.[94]
If a bound electron is in an excited state, an interacting photon with the proper energy can cause stimulated emission of a photon with a matching energy level. For this to occur, the electron must drop to a lower energy state that has an energy difference matching the energy of the interacting photon. The emitted photon and the interacting photon then move off in parallel and with matching phases. That is, the wave patterns of the two photons are synchronized. This physical property is used to make lasers, which can emit a coherent beam of light energy in a narrow frequency band.[95]
Valence and bonding behavior
Valency is the combining power of an element. It is determined by the number of bonds it can form to other atoms or groups.[96] The outermost electron shell of an atom in its uncombined state is known as the valence shell, and the electrons in that shell are called valence electrons. The number of valence electrons determines the bonding behavior with other atoms. Atoms tend to chemically react with each other in a manner that fills (or empties) their outer valence shells.[97] For example, a transfer of a single electron between atoms is a useful approximation for bonds that form between atoms with one-electron more than a filled shell, and others that are one-electron short of a full shell, such as occurs in the compound sodium chloride and other chemical ionic salts. Many elements display multiple valences, or tendencies to share differing numbers of electrons in different compounds. Thus, chemical bonding between these elements takes many forms of electron-sharing that are more than simple electron transfers. Examples include the element carbon and the organic compounds.[98]
The chemical elements are often displayed in a periodic table that is laid out to display recurring chemical properties, and elements with the same number of valence electrons form a group that is aligned in the same column of the table. (The horizontal rows correspond to the filling of a quantum shell of electrons.) The elements at the far right of the table have their outer shell completely filled with electrons, which results in chemically inert elements known as the noble gases.[99][100]
States

Quantities of atoms are found in different states of matter that depend on the physical conditions, such as temperature and pressure. By varying the conditions, materials can transition between solids, liquids, gases and plasmas.[101] Within a state, a material can also exist in different allotropes. An example of this is solid carbon, which can exist as graphite or diamond.[102] Gaseous allotropes exist as well, such as dioxygen and ozone.
At temperatures close to absolute zero, atoms can form a Bose–Einstein condensate, at which point quantum mechanical effects, which are normally only observed at the atomic scale, become apparent on a macroscopic scale.[103][104] This super-cooled collection of atoms then behaves as a single super atom, which may allow fundamental checks of quantum mechanical behavior.[105]
Identification

While atoms are too small to be seen, devices such as the scanning tunneling microscope (STM) enable their visualization at the surfaces of solids. The microscope uses the quantum tunneling phenomenon, which allows particles to pass through a barrier that would be insurmountable in the classical perspective. Electrons tunnel through the vacuum between two biased electrodes, providing a tunneling current that is exponentially dependent on their separation. One electrode is a sharp tip ideally ending with a single atom. At each point of the scan of the surface the tip's height is adjusted so as to keep the tunneling current at a set value. How much the tip moves to and away from the surface is interpreted as the height profile. For low bias, the microscope images the averaged electron orbitals across closely packed energy levels—the local density of the electronic states near the Fermi level.[106][107] Because of the distances involved, both electrodes need to be extremely stable; only then periodicities can be observed that correspond to individual atoms. The method alone is not chemically specific, and cannot identify the atomic species present at the surface.
Atoms can be easily identified by their mass. If an atom is ionized by removing one of its electrons, its trajectory when it passes through a magnetic field will bend. The radius by which the trajectory of a moving ion is turned by the magnetic field is determined by the mass of the atom. The mass spectrometer uses this principle to measure the mass-to-charge ratio of ions. If a sample contains multiple isotopes, the mass spectrometer can determine the proportion of each isotope in the sample by measuring the intensity of the different beams of ions. Techniques to vaporize atoms include inductively coupled plasma atomic emission spectroscopy and inductively coupled plasma mass spectrometry, both of which use a plasma to vaporize samples for analysis.[108]
The atom-probe tomograph has sub-nanometer resolution in 3-D and can chemically identify individual atoms using time-of-flight mass spectrometry.[109]
Electron emission techniques such as X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy (AES), which measure the binding energies of the core electrons, are used to identify the atomic species present in a sample in a non-destructive way. With proper focusing both can be made area-specific. Another such method is electron energy loss spectroscopy (EELS), which measures the energy loss of an electron beam within a transmission electron microscope when it interacts with a portion of a sample.
Spectra of excited states can be used to analyze the atomic composition of distant stars. Specific light wavelengths contained in the observed light from stars can be separated out and related to the quantized transitions in free gas atoms. These colors can be replicated using a gas-discharge lamp containing the same element.[110] Helium was discovered in this way in the spectrum of the Sun 23 years before it was found on Earth.[111]
Origin and current state
Baryonic matter forms about 4% of the total energy density of the observable universe, with an average density of about 0.25 particles/m3 (mostly protons and electrons).[112] Within a galaxy such as the Milky Way, particles have a much higher concentration, with the density of matter in the interstellar medium (ISM) ranging from 105 to 109 atoms/m3.[113] The Sun is believed to be inside the Local Bubble, so the density in the solar neighborhood is only about 103 atoms/m3.[114] Stars form from dense clouds in the ISM, and the evolutionary processes of stars result in the steady enrichment of the ISM with elements more massive than hydrogen and helium.
Up to 95% of the Milky Way's baryonic matter are concentrated inside stars, where conditions are unfavorable for atomic matter. The total baryonic mass is about 10% of the mass of the galaxy;[115] the remainder of the mass is an unknown dark matter.[116] High temperature inside stars makes most "atoms" fully ionized, that is, separates all electrons from the nuclei. In stellar remnants—with exception of their surface layers—an immense pressure make electron shells impossible.
Formation

Electrons are thought to exist in the Universe since early stages of the Big Bang. Atomic nuclei forms in nucleosynthesis reactions. In about three minutes Big Bang nucleosynthesis produced most of the helium, lithium, and deuterium in the Universe, and perhaps some of the beryllium and boron.[117][118][119]
Ubiquitousness and stability of atoms relies on their binding energy, which means that an atom has a lower energy than an unbound system of the nucleus and electrons. Where the temperature is much higher than ionization potential, the matter exists in the form of plasma—a gas of positively charged ions (possibly, bare nuclei) and electrons. When the temperature drops below the ionization potential, atoms become statistically favorable. Atoms (complete with bound electrons) became to dominate over charged particles 380,000 years after the Big Bang—an epoch called recombination, when the expanding Universe cooled enough to allow electrons to become attached to nuclei.[120]
Since the Big Bang, which produced no carbon or heavier elements, atomic nuclei have been combined in stars through the process of nuclear fusion to produce more of the element helium, and (via the triple-alpha process) the sequence of elements from carbon up to iron;[121] see stellar nucleosynthesis for details.
Isotopes such as lithium-6, as well as some beryllium and boron are generated in space through cosmic ray spallation.[122] This occurs when a high-energy proton strikes an atomic nucleus, causing large numbers of nucleons to be ejected.
Elements heavier than iron were produced in supernovae and colliding neutron stars through the r-process, and in AGB stars through the s-process, both of which involve the capture of neutrons by atomic nuclei.[123] Elements such as lead formed largely through the radioactive decay of heavier elements.[124]
Earth
Most of the atoms that make up the Earth and its inhabitants were present in their current form in the nebula that collapsed out of a molecular cloud to form the Solar System. The rest are the result of radioactive decay, and their relative proportion can be used to determine the age of the Earth through radiometric dating.[125][126] Most of the helium in the crust of the Earth (about 99% of the helium from gas wells, as shown by its lower abundance of helium-3) is a product of alpha decay.[127]
There are a few trace atoms on Earth that were not present at the beginning (i.e., not "primordial"), nor are results of radioactive decay. Carbon-14 is continuously generated by cosmic rays in the atmosphere.[128] Some atoms on Earth have been artificially generated either deliberately or as by-products of nuclear reactors or explosions.[129][130] Of the transuranic elements—those with atomic numbers greater than 92—only plutonium and neptunium occur naturally on Earth.[131][132] Transuranic elements have radioactive lifetimes shorter than the current age of the Earth[133] and thus identifiable quantities of these elements have long since decayed, with the exception of traces of plutonium-244 possibly deposited by cosmic dust.[125] Natural deposits of plutonium and neptunium are produced by neutron capture in uranium ore.[134]
The Earth contains approximately 1.33×1050 atoms.[135] Although small numbers of independent atoms of noble gases exist, such as argon, neon, and helium, 99% of the atmosphere is bound in the form of molecules, including carbon dioxide and diatomic oxygen and nitrogen. At the surface of the Earth, an overwhelming majority of atoms combine to form various compounds, including water, salt, silicates and oxides. Atoms can also combine to create materials that do not consist of discrete molecules, including crystals and liquid or solid metals.[136][137] This atomic matter forms networked arrangements that lack the particular type of small-scale interrupted order associated with molecular matter.[138]
Rare and theoretical forms
Superheavy elements
All nuclides with atomic numbers higher than 82 (lead) are known to be radioactive. No nuclide with an atomic number exceeding 92 (uranium) exists on Earth as a primordial nuclide, and heavier elements generally have shorter half-lives. Nevertheless, an "island of stability" encompassing relatively long-lived isotopes of superheavy elements[139] with atomic numbers 110 to 114 might exist.[140] Predictions for the half-life of the most stable nuclide on the island range from a few minutes to millions of years.[141] In any case, superheavy elements (with Z > 104) would not exist due to increasing Coulomb repulsion (which results in spontaneous fission with increasingly short half-lives) in the absence of any stabilizing effects.[142]
Exotic matter
Each particle of matter has a corresponding antimatter particle with the opposite electrical charge. Thus, the positron is a positively charged antielectron and the antiproton is a negatively charged equivalent of a proton. When a matter and corresponding antimatter particle meet, they annihilate each other. Because of this, along with an imbalance between the number of matter and antimatter particles, the latter are rare in the universe. The first causes of this imbalance are not yet fully understood, although theories of baryogenesis may offer an explanation. As a result, no antimatter atoms have been discovered in nature.[143][144] In 1996, the antimatter counterpart of the hydrogen atom (antihydrogen) was synthesized at the CERN laboratory in Geneva.[145][146]
Other exotic atoms have been created by replacing one of the protons, neutrons or electrons with other particles that have the same charge. For example, an electron can be replaced by a more massive muon, forming a muonic atom. These types of atoms can be used to test fundamental predictions of physics.[147][148][149]
See also
Notes
- ^ For more recent updates see Brookhaven National Laboratory's Interactive Chart of Nuclides ] Archived 25 July 2020 at the Wayback Machine.
- ^ A carat is 200 milligrams. By definition, carbon-12 has 0.012 kg per mole. The Avogadro constant defines 6×1023 atoms per mole.
References
- ^ "DOE Explains...Nuclei". Energy.gov. Retrieved 5 November 2024.
- ^ Pullman, Bernard (1998). The Atom in the History of Human Thought. Oxford, England: Oxford University Press. pp. 31–33. ISBN 978-0-19-515040-7. Archived from the original on 5 February 2021. Retrieved 25 October 2020.
- ^ Melsen (1952). From Atomos to Atom, pp. 18–19
- ^ Pullman (1998). The Atom in the History of Human Thought, p. 201
- ^ Pullman (1998). The Atom in the History of Human Thought, p. 199: "The constant ratios, expressible in terms of integers, of the weights of the constituents in composite bodies could be construed as evidence on a macroscopic scale of interactions at the microscopic level between basic units with fixed weights. For Dalton, this agreement strongly suggested a corpuscular structure of matter, even though it did not constitute definite proof."
- ^ Dalton (1817). A New System of Chemical Philosophy vol. 2, p. 36
- ^ Melsen (1952). From Atomos to Atom, p. 137
- ^ Dalton (1817). A New System of Chemical Philosophy vol. 2, p. 28
- ^ Millington (1906). John Dalton, p. 113
- ^ Dalton (1808). A New System of Chemical Philosophy vol. 1, pp. 316–319
- ^ Holbrow et al. (2010). Modern Introductory Physics, pp. 65–66
- ^ J. J. Thomson (1897). "Cathode rays". Philosophical Magazine. 44 (269): 293–316.
- ^ In his book The Corpuscular Theory of Matter (1907), Thomson estimates electrons to be 1/1700 the mass of hydrogen.
- ^ "The Mechanism Of Conduction In Metals" Archived 25 October 2012 at the Wayback Machine, Think Quest.
- ^ Thomson, J.J. (August 1901). "On bodies smaller than atoms". The Popular Science Monthly: 323–335. Archived from the original on 1 December 2016. Retrieved 21 June 2009.
- ^ J. J. Thomson (1907). On the Corpuscular Theory of Matter, p. 26: "The simplest interpretation of these results is that the positive ions are the atoms or groups of atoms of various elements from which one or more corpuscles have been removed [...] while the negative electrified body is one with more corpuscles than the unelectrified one."
- ^ J. J. Thomson (1907). The Corpuscular Theory of Matter, p. 103: "In default of exact knowledge of the nature of the way in which positive electricity occurs in the atom, we shall consider a case in which the positive electricity is distributed in the way most amenable to mathematical calculation, i.e., when it occurs as a sphere of uniform density, throughout which the corpuscles are distributed."
- ^ Giora Hon; Bernard R. Goldstein (6 September 2013). "J. J. Thomson's plum-pudding atomic model: The making of a scientific myth". Annalen der Physik. 525 (8–9): A129 – A133. Bibcode:2013AnP...525A.129H. doi:10.1002/andp.201300732. ISSN 0003-3804.
- ^ Heilbron (2003). Ernest Rutherford and the Explosion of Atoms, pp. 64–68
- ^ Stern, David P. (16 May 2005). "The Atomic Nucleus and Bohr's Early Model of the Atom". NASA/Goddard Space Flight Center. Archived from the original on 20 August 2007.
- ^ Bohr, Niels (11 December 1922). "Niels Bohr, The Nobel Prize in Physics 1922, Nobel Lecture". Nobel Foundation. Archived from the original on 15 April 2008.
- ^ J. J. Thomson (1898). "On the Charge of Electricity carried by the Ions produced by Röntgen Rays". The London, Edinburgh and Dublin Philosophical Magazine and Journal of Science. 5. 46 (283): 528–545. doi:10.1080/14786449808621229.
- ^ J. J. Thomson (1907). The Corpuscular Theory of Matter. p. 26–27: "In an unelectrified atom there are as many units of positive electricity as there are of negative; an atom with a unit of positive charge is a neutral atom which has lost one corpuscle, while an atom with a unit of negative charge is a neutral atom to which an additional corpuscle has been attached."
- ^ Rutherford, Ernest (1919). "Collisions of alpha Particles with Light Atoms. IV. An Anomalous Effect in Nitrogen". Philosophical Magazine. 37 (222): 581. doi:10.1080/14786440608635919.
- ^ The Development of the Theory of Atomic Structure (Rutherford 1936). Reprinted in Background to Modern Science: Ten Lectures at Cambridge arranged by the History of Science Committee 1936:
"In 1919 I showed that when light atoms were bombarded by α-particles they could be broken up with the emission of a proton, or hydrogen nucleus. We therefore presumed that a proton must be one of the units of which the nuclei of other atoms were composed..." - ^ Orme Masson (1921). "The Constitution of Atoms". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 41 (242): 281–285. doi:10.1080/14786442108636219.
Footnote by Ernest Rutherford: 'At the time of writing this paper in Australia, Professor Orme Masson was not aware that the name "proton" had already been suggested as a suitable name for the unit of mass nearly 1, in terms of oxygen 16, that appears to enter into the nuclear structure of atoms. The question of a suitable name for this unit was discussed at an informal meeting of a number of members of Section A of the British Association at Cardiff this year. The name "baron" suggested by Professor Masson was mentioned, but was considered unsuitable on account of the existing variety of meanings. Finally the name "proton" met with general approval, particularly as it suggests the original term "protyle " given by Prout in his well-known hypothesis that all atoms are built up of hydrogen. The need of a special name for the nuclear unit of mass 1 was drawn attention to by Sir Oliver Lodge at the Sectional meeting, and the writer then suggested the name "proton."' - ^ Eric Scerri (2020). The Periodic Table: Its Story and Its Significance, p. 185
- ^ Helge Kragh (2012). Niels Bohr and the Quantum Atom, p. 33
- ^ James Chadwick (1932). "Possible Existence of a Neutron" (PDF). Nature. 129 (3252): 312. Bibcode:1932Natur.129Q.312C. doi:10.1038/129312a0. S2CID 4076465. Archived (PDF) from the original on 9 October 2022.
- ^ a b Pais, Abraham (1986). Inward Bound: Of Matter and Forces in the Physical World. New York: Oxford University Press. pp. 228–230. ISBN 978-0-19-851971-3.
- ^ McEvoy, J. P.; Zarate, Oscar (2004). Introducing Quantum Theory. Totem Books. pp. 110–114. ISBN 978-1-84046-577-8.
- ^ Kozłowski, Miroslaw (2019). "The Schrödinger equation A History".
- ^ Chad Orzel (16 September 2014). "What is the Heisenberg Uncertainty Principle?". TED-Ed. Archived from the original on 13 September 2015 – via YouTube.
- ^ Brown, Kevin (2007). "The Hydrogen Atom". MathPages. Archived from the original on 5 September 2012.
- ^ Harrison, David M. (2000). "The Development of Quantum Mechanics". University of Toronto. Archived from the original on 25 December 2007.
- ^ Demtröder, Wolfgang (2002). Atoms, Molecules and Photons: An Introduction to Atomic- Molecular- and Quantum Physics (1st ed.). Springer. pp. 39–42. ISBN 978-3-540-20631-6. OCLC 181435713.
- ^ Woan, Graham (2000). The Cambridge Handbook of Physics. Cambridge University Press. p. 8. ISBN 978-0-521-57507-2. OCLC 224032426.
- ^ Mohr, P.J.; Taylor, B.N. and Newell, D.B. (2014), "The 2014 CODATA Recommended Values of the Fundamental Physical Constants" Archived 11 February 2012 at the Wayback Machine (Web Version 7.0). The database was developed by J. Baker, M. Douma, and S. Kotochigova. (2014). National Institute of Standards and Technology, Gaithersburg, Maryland 20899.
- ^ MacGregor, Malcolm H. (1992). The Enigmatic Electron. Oxford University Press. pp. 33–37. ISBN 978-0-19-521833-6. OCLC 223372888.
- ^ a b Particle Data Group (2002). "The Particle Adventure". Lawrence Berkeley Laboratory. Archived from the original on 4 January 2007.
- ^ a b Schombert, James (18 April 2006). "Elementary Particles". University of Oregon. Archived from the original on 30 August 2011.
- ^ Jevremovic, Tatjana (2005). Nuclear Principles in Engineering. Springer. p. 63. ISBN 978-0-387-23284-3. OCLC 228384008.
- ^ Pfeffer, Jeremy I.; Nir, Shlomo (2000). Modern Physics: An Introductory Text. Imperial College Press. pp. 330–336. ISBN 978-1-86094-250-1. OCLC 45900880.
- ^ Wenner, Jennifer M. (10 October 2007). "How Does Radioactive Decay Work?". Carleton College. Archived from the original on 11 May 2008.
- ^ a b c Raymond, David (7 April 2006). "Nuclear Binding Energies". New Mexico Tech. Archived from the original on 1 December 2002.
- ^ Mihos, Chris (23 July 2002). "Overcoming the Coulomb Barrier". Case Western Reserve University. Archived from the original on 12 September 2006.
- ^ Staff (30 March 2007). "ABC's of Nuclear Science". Lawrence Berkeley National Laboratory. Archived from the original on 5 December 2006.
- ^ Makhijani, Arjun; Saleska, Scott (2 March 2001). "Basics of Nuclear Physics and Fission". Institute for Energy and Environmental Research. Archived from the original on 16 January 2007.
- ^ Shultis, J. Kenneth; Faw, Richard E. (2002). Fundamentals of Nuclear Science and Engineering. CRC Press. pp. 10–17. ISBN 978-0-8247-0834-4. OCLC 123346507.
- ^ Fewell, M.P. (1995). "The atomic nuclide with the highest mean binding energy". American Journal of Physics. 63 (7): 653–658. Bibcode:1995AmJPh..63..653F. doi:10.1119/1.17828.
- ^ Mulliken, Robert S. (1967). "Spectroscopy, Molecular Orbitals, and Chemical Bonding". Science. 157 (3784): 13–24. Bibcode:1967Sci...157...13M. doi:10.1126/science.157.3784.13. PMID 5338306.
- ^ a b Brucat, Philip J. (2008). "The Quantum Atom". University of Florida. Archived from the original on 7 December 2006.
- ^ Manthey, David (2001). "Atomic Orbitals". Orbital Central. Archived from the original on 10 January 2008.
- ^ Herter, Terry (2006). "Lecture 8: The Hydrogen Atom". Cornell University. Archived from the original on 22 February 2012.
- ^ Bell, R.E.; Elliott, L.G. (1950). "Gamma-Rays from the Reaction H1(n,γ)D2 and the Binding Energy of the Deuteron". Physical Review. 79 (2): 282–285. Bibcode:1950PhRv...79..282B. doi:10.1103/PhysRev.79.282.
- ^ Smirnov, Boris M. (2003). Physics of Atoms and Ions. Springer. pp. 249–272. ISBN 978-0-387-95550-6.
- ^ Matis, Howard S. (9 August 2000). "The Isotopes of Hydrogen". Guide to the Nuclear Wall Chart. Lawrence Berkeley National Lab. Archived from the original on 18 December 2007.
- ^ Weiss, Rick (17 October 2006). "Scientists Announce Creation of Atomic Element, the Heaviest Yet". Washington Post. Archived from the original on 20 August 2011.
- ^ a b Sills, Alan D. (2003). Earth Science the Easy Way. Barron's Educational Series. pp. 131–134. ISBN 978-0-7641-2146-3. OCLC 51543743.
- ^ Dumé, Belle (23 April 2003). "Bismuth breaks half-life record for alpha decay". Physics World. Archived from the original on 14 December 2007.
- ^ Lindsay, Don (30 July 2000). "Radioactives Missing From The Earth". Don Lindsay Archive. Archived from the original on 28 April 2007.
- ^ Tuli, Jagdish K. (April 2005). "Nuclear Wallet Cards". National Nuclear Data Center, Brookhaven National Laboratory. Archived from the original on 3 October 2011.
- ^ CRC Handbook (2002).
- ^ Krane, K. (1988). Introductory Nuclear Physics. John Wiley & Sons. pp. 68. ISBN 978-0-471-85914-7.
- ^ a b Mills, Ian; Cvitaš, Tomislav; Homann, Klaus; Kallay, Nikola; Kuchitsu, Kozo (1993). Quantities, Units and Symbols in Physical Chemistry (2nd ed.). Oxford: International Union of Pure and Applied Chemistry, Commission on Physiochemical Symbols Terminology and Units, Blackwell Scientific Publications. p. 70. ISBN 978-0-632-03583-0. OCLC 27011505.
- ^ Chieh, Chung (22 January 2001). "Nuclide Stability". University of Waterloo. Archived from the original on 30 August 2007.
- ^ "Atomic Weights and Isotopic Compositions for All Elements". National Institute of Standards and Technology. Archived from the original on 31 December 2006. Retrieved 4 January 2007.
- ^ Audi, G.; Wapstra, A.H.; Thibault, C. (2003). "The Ame2003 atomic mass evaluation (II)" (PDF). Nuclear Physics A. 729 (1): 337–676. Bibcode:2003NuPhA.729..337A. doi:10.1016/j.nuclphysa.2003.11.003. Archived (PDF) from the original on 16 October 2005.
- ^ Ghosh, D.C.; Biswas, R. (2002). "Theoretical calculation of Absolute Radii of Atoms and Ions. Part 1. The Atomic Radii". Int. J. Mol. Sci. 3 (11): 87–113. doi:10.3390/i3020087.
- ^ Shannon, R.D. (1976). "Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides" (PDF). Acta Crystallographica A. 32 (5): 751–767. Bibcode:1976AcCrA..32..751S. doi:10.1107/S0567739476001551. Archived (PDF) from the original on 14 August 2020. Retrieved 25 August 2019.
- ^ Dong, Judy (1998). "Diameter of an Atom". The Physics Factbook. Archived from the original on 4 November 2007.
- ^ Zumdahl, Steven S. (2002). Introductory Chemistry: A Foundation (5th ed.). Houghton Mifflin. ISBN 978-0-618-34342-3. OCLC 173081482. Archived from the original on 4 March 2008.
- ^ Bethe, Hans (1929). "Termaufspaltung in Kristallen". Annalen der Physik. 3 (2): 133–208. Bibcode:1929AnP...395..133B. doi:10.1002/andp.19293950202.
- ^ Birkholz, Mario (1995). "Crystal-field induced dipoles in heteropolar crystals – I. concept". Z. Phys. B. 96 (3): 325–332. Bibcode:1995ZPhyB..96..325B. CiteSeerX 10.1.1.424.5632. doi:10.1007/BF01313054. S2CID 122527743.
- ^ Birkholz, M.; Rudert, R. (2008). "Interatomic distances in pyrite-structure disulfides – a case for ellipsoidal modeling of sulfur ions" (PDF). Physica Status Solidi B. 245 (9): 1858–1864. Bibcode:2008PSSBR.245.1858B. doi:10.1002/pssb.200879532. S2CID 97824066. Archived (PDF) from the original on 2 May 2021. Retrieved 2 May 2021.
- ^ Birkholz, M. (2014). "Modeling the Shape of Ions in Pyrite-Type Crystals". Crystals. 4 (3): 390–403. doi:10.3390/cryst4030390.
- ^ Staff (2007). "Small Miracles: Harnessing nanotechnology". Oregon State University. Archived from the original on 21 May 2011. – describes the width of a human hair as 105 nm and 10 carbon atoms as spanning 1 nm.
- ^ Padilla, Michael J.; Miaoulis, Ioannis; Cyr, Martha (2002). Prentice Hall Science Explorer: Chemical Building Blocks. Upper Saddle River, New Jersey: Prentice-Hall, Inc. p. 32. ISBN 978-0-13-054091-1. OCLC 47925884.
There are 2,000,000,000,000,000,000,000 (that's 2 sextillion) atoms of oxygen in one drop of water—and twice as many atoms of hydrogen.
- ^ "The Feynman Lectures on Physics Vol. I Ch. 1: Atoms in Motion". Archived from the original on 30 July 2022. Retrieved 3 May 2022.
- ^ a b "Radioactivity". Splung.com. Archived from the original on 4 December 2007. Retrieved 19 December 2007.
- ^ L'Annunziata, Michael F. (2003). Handbook of Radioactivity Analysis. Academic Press. pp. 3–56. ISBN 978-0-12-436603-9. OCLC 16212955.
- ^ Firestone, Richard B. (22 May 2000). "Radioactive Decay Modes". Berkeley Laboratory. Archived from the original on 29 September 2006.
- ^ Hornak, J.P. (2006). "Chapter 3: Spin Physics". The Basics of NMR. Rochester Institute of Technology. Archived from the original on 3 February 2007.
- ^ a b Schroeder, Paul A. (25 February 2000). "Magnetic Properties". University of Georgia. Archived from the original on 29 April 2007.
- ^ Goebel, Greg (1 September 2007). "[4.3] Magnetic Properties of the Atom". Elementary Quantum Physics. In The Public Domain website. Archived from the original on 29 June 2011.
- ^ Yarris, Lynn (Spring 1997). "Talking Pictures". Berkeley Lab Research Review. Archived from the original on 13 January 2008.
- ^ Liang, Z.-P.; Haacke, E.M. (1999). Webster, J.G. (ed.). Encyclopedia of Electrical and Electronics Engineering: Magnetic Resonance Imaging. Vol. 2. John Wiley & Sons. pp. 412–426. ISBN 978-0-471-13946-1.
- ^ Zeghbroeck, Bart J. Van (1998). "Energy levels". Shippensburg University. Archived from the original on 15 January 2005.
- ^ Fowles, Grant R. (1989). Introduction to Modern Optics. Courier Dover Publications. pp. 227–233. ISBN 978-0-486-65957-2. OCLC 18834711.
- ^ Martin, W.C.; Wiese, W.L. (May 2007). "Atomic Spectroscopy: A Compendium of Basic Ideas, Notation, Data, and Formulas". National Institute of Standards and Technology. Archived from the original on 8 February 2007.
- ^ "Atomic Emission Spectra – Origin of Spectral Lines". Avogadro Web Site. Archived from the original on 28 February 2006. Retrieved 10 August 2006.
- ^ Fitzpatrick, Richard (16 February 2007). "Fine structure". University of Texas at Austin. Archived from the original on 27 September 2011.
- ^ Weiss, Michael (2001). "The Zeeman Effect". University of California-Riverside. Archived from the original on 2 February 2008.
- ^ Beyer, H.F.; Shevelko, V.P. (2003). Introduction to the Physics of Highly Charged Ions. CRC Press. pp. 232–236. ISBN 978-0-7503-0481-8. OCLC 47150433.
- ^ Watkins, Thayer. "Coherence in Stimulated Emission". San José State University. Archived from the original on 12 January 2008. Retrieved 23 December 2007.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "valence". doi:10.1351/goldbook.V06588
- ^ Reusch, William (16 July 2007). "Virtual Textbook of Organic Chemistry". Michigan State University. Archived from the original on 29 October 2007.
- ^ "Covalent bonding – Single bonds". chemguide. 2000. Archived from the original on 1 November 2008.
- ^ Husted, Robert; et al. (11 December 2003). "Periodic Table of the Elements". Los Alamos National Laboratory. Archived from the original on 10 January 2008.
- ^ Baum, Rudy (2003). "It's Elemental: The Periodic Table". Chemical & Engineering News. Archived from the original on 6 April 2011.
- ^ Goodstein, David L. (2002). States of Matter. Courier Dover Publications. pp. 436–438. ISBN 978-0-13-843557-8.
- ^ Brazhkin, Vadim V. (2006). "Metastable phases, phase transformations, and phase diagrams in physics and chemistry". Physics-Uspekhi. 49 (7): 719–724. Bibcode:2006PhyU...49..719B. doi:10.1070/PU2006v049n07ABEH006013. S2CID 93168446.
- ^ Myers, Richard (2003). The Basics of Chemistry. Greenwood Press. p. 85. ISBN 978-0-313-31664-7. OCLC 50164580.
- ^ Staff (9 October 2001). "Bose–Einstein Condensate: A New Form of Matter". National Institute of Standards and Technology. Archived from the original on 3 January 2008.
- ^ Colton, Imogen; Fyffe, Jeanette (3 February 1999). "Super Atoms from Bose–Einstein Condensation". The University of Melbourne. Archived from the original on 29 August 2007.
- ^ Jacox, Marilyn; Gadzuk, J. William (November 1997). "Scanning Tunneling Microscope". National Institute of Standards and Technology. Archived from the original on 7 January 2008.
- ^ "The Nobel Prize in Physics 1986". The Nobel Foundation. Archived from the original on 17 September 2008. Retrieved 11 January 2008. In particular, see the Nobel lecture by G. Binnig and H. Rohrer.
- ^ Jakubowski, N.; Moens, Luc; Vanhaecke, Frank (1998). "Sector field mass spectrometers in ICP-MS". Spectrochimica Acta Part B: Atomic Spectroscopy. 53 (13): 1739–1763. Bibcode:1998AcSpB..53.1739J. doi:10.1016/S0584-8547(98)00222-5.
- ^ Müller, Erwin W.; Panitz, John A.; McLane, S. Brooks (1968). "The Atom-Probe Field Ion Microscope". Review of Scientific Instruments. 39 (1): 83–86. Bibcode:1968RScI...39...83M. doi:10.1063/1.1683116.
- ^ Lochner, Jim; Gibb, Meredith; Newman, Phil (30 April 2007). "What Do Spectra Tell Us?". NASA/Goddard Space Flight Center. Archived from the original on 16 January 2008.
- ^ Winter, Mark (2007). "Helium". WebElements. Archived from the original on 30 December 2007.
- ^ Hinshaw, Gary (10 February 2006). "What is the Universe Made Of?". NASA/WMAP. Archived from the original on 31 December 2007.
- ^ Choppin, Gregory R.; Liljenzin, Jan-Olov; Rydberg, Jan (2001). Radiochemistry and Nuclear Chemistry. Elsevier. p. 441. ISBN 978-0-7506-7463-8. OCLC 162592180.
- ^ Davidsen, Arthur F. (1993). "Far-Ultraviolet Astronomy on the Astro-1 Space Shuttle Mission". Science. 259 (5093): 327–334. Bibcode:1993Sci...259..327D. doi:10.1126/science.259.5093.327. PMID 17832344. S2CID 28201406.
- ^ Lequeux, James (2005). The Interstellar Medium. Springer. p. 4. ISBN 978-3-540-21326-0. OCLC 133157789.
- ^ Smith, Nigel (6 January 2000). "The search for dark matter". Physics World. Archived from the original on 16 February 2008.
- ^ Croswell, Ken (1991). "Boron, bumps and the Big Bang: Was matter spread evenly when the Universe began? Perhaps not; the clues lie in the creation of the lighter elements such as boron and beryllium". New Scientist (1794): 42. Archived from the original on 7 February 2008.
- ^ Copi, Craig J.; Schramm, DN; Turner, MS (1995). "Big-Bang Nucleosynthesis and the Baryon Density of the Universe". Science (Submitted manuscript). 267 (5195): 192–199. arXiv:astro-ph/9407006. Bibcode:1995Sci...267..192C. doi:10.1126/science.7809624. PMID 7809624. S2CID 15613185. Archived from the original on 14 August 2019.
- ^ Hinshaw, Gary (15 December 2005). "Tests of the Big Bang: The Light Elements". NASA/WMAP. Archived from the original on 17 January 2008.
- ^ Abbott, Brian (30 May 2007). "Microwave (WMAP) All-Sky Survey". Hayden Planetarium. Archived from the original on 13 February 2013.
- ^ Hoyle, F. (1946). "The synthesis of the elements from hydrogen". Monthly Notices of the Royal Astronomical Society. 106 (5): 343–383. Bibcode:1946MNRAS.106..343H. doi:10.1093/mnras/106.5.343.
- ^ Knauth, D.C.; Knauth, D.C.; Lambert, David L.; Crane, P. (2000). "Newly synthesized lithium in the interstellar medium". Nature. 405 (6787): 656–658. Bibcode:2000Natur.405..656K. doi:10.1038/35015028. PMID 10864316. S2CID 4397202.
- ^ Mashnik, Stepan G. (2000). "On Solar System and Cosmic Rays Nucleosynthesis and Spallation Processes". arXiv:astro-ph/0008382.
- ^ Kansas Geological Survey (4 May 2005). "Age of the Earth". University of Kansas. Archived from the original on 5 July 2008.
- ^ a b Manuel (2001). Origin of Elements in the Solar System, pp. 40–430, 511–519
- ^ Dalrymple, G. Brent (2001). "The age of the Earth in the twentieth century: a problem (mostly) solved". Geological Society, London, Special Publications. 190 (1): 205–221. Bibcode:2001GSLSP.190..205D. doi:10.1144/GSL.SP.2001.190.01.14. S2CID 130092094. Archived from the original on 11 November 2007.
- ^ Anderson, Don L.; Foulger, G.R.; Meibom, Anders (2 September 2006). "Helium: Fundamental models". MantlePlumes.org. Archived from the original on 8 February 2007.
- ^ Pennicott, Katie (10 May 2001). "Carbon clock could show the wrong time". PhysicsWeb. Archived from the original on 15 December 2007.
- ^ Yarris, Lynn (27 July 2001). "New Superheavy Elements 118 and 116 Discovered at Berkeley Lab". Berkeley Lab. Archived from the original on 9 January 2008.
- ^ Diamond, H; et al. (1960). "Heavy Isotope Abundances in Mike Thermonuclear Device". Physical Review. 119 (6): 2000–2004. Bibcode:1960PhRv..119.2000D. doi:10.1103/PhysRev.119.2000.
- ^ Poston, John W. Sr. (23 March 1998). "Do transuranic elements such as plutonium ever occur naturally?". Scientific American. Archived from the original on 27 March 2015.
- ^ Keller, C. (1973). "Natural occurrence of lanthanides, actinides, and superheavy elements". Chemiker Zeitung. 97 (10): 522–530. OSTI 4353086.
- ^ Zaider, Marco; Rossi, Harald H. (2001). Radiation Science for Physicians and Public Health Workers. Springer. p. 17. ISBN 978-0-306-46403-4. OCLC 44110319.
- ^ "Oklo Fossil Reactors". Curtin University of Technology. Archived from the original on 18 December 2007. Retrieved 15 January 2008.
- ^ Weisenberger, Drew. "How many atoms are there in the world?". Jefferson Lab. Archived from the original on 22 October 2007. Retrieved 16 January 2008.
- ^ Pidwirny, Michael. "Fundamentals of Physical Geography". University of British Columbia Okanagan. Archived from the original on 21 January 2008. Retrieved 16 January 2008.
- ^ Anderson, Don L. (2002). "The inner inner core of Earth". Proceedings of the National Academy of Sciences. 99 (22): 13966–13968. Bibcode:2002PNAS...9913966A. doi:10.1073/pnas.232565899. PMC 137819. PMID 12391308.
- ^ Pauling, Linus (1960). The Nature of the Chemical Bond. Cornell University Press. pp. 5–10. ISBN 978-0-8014-0333-0. OCLC 17518275.
- ^ Anonymous (2 October 2001). "Second postcard from the island of stability". CERN Courier. Archived from the original on 3 February 2008.
- ^ Karpov, A. V.; Zagrebaev, V. I.; Palenzuela, Y. M.; et al. (2012). "Decay properties and stability of the heaviest elements" (PDF). International Journal of Modern Physics E. 21 (2): 1250013-1 – 1250013-20. Bibcode:2012IJMPE..2150013K. doi:10.1142/S0218301312500139. Archived (PDF) from the original on 3 December 2016. Retrieved 24 March 2020.
- ^ "Superheavy Element 114 Confirmed: A Stepping Stone to the Island of Stability". Berkeley Lab. 2009. Archived from the original on 20 July 2019. Retrieved 24 March 2020.
- ^ Möller, P. (2016). "The limits of the nuclear chart set by fission and alpha decay" (PDF). EPJ Web of Conferences. 131: 03002-1 – 03002-8. Bibcode:2016EPJWC.13103002M. doi:10.1051/epjconf/201613103002. Archived (PDF) from the original on 11 March 2020. Retrieved 24 March 2020.
- ^ Koppes, Steve (1 March 1999). "Fermilab Physicists Find New Matter-Antimatter Asymmetry". University of Chicago. Archived from the original on 19 July 2008.
- ^ Cromie, William J. (16 August 2001). "A lifetime of trillionths of a second: Scientists explore antimatter". Harvard University Gazette. Archived from the original on 3 September 2006.
- ^ Hijmans, Tom W. (2002). "Particle physics: Cold antihydrogen". Nature. 419 (6906): 439–440. Bibcode:2002Natur.419..439H. doi:10.1038/419439a. PMID 12368837.
- ^ Staff (30 October 2002). "Researchers 'look inside' antimatter". BBC News. Archived from the original on 22 February 2007.
- ^ Barrett, Roger (1990). "The Strange World of the Exotic Atom". New Scientist (1728): 77–115. Archived from the original on 21 December 2007.
- ^ Indelicato, Paul (2004). "Exotic Atoms". Physica Scripta. T112 (1): 20–26. arXiv:physics/0409058. Bibcode:2004PhST..112...20I. doi:10.1238/Physica.Topical.112a00020. S2CID 11134265. Archived from the original on 4 November 2018.
- ^ Ripin, Barrett H. (July 1998). "Recent Experiments on Exotic Atoms". American Physical Society. Archived from the original on 23 July 2012.
Bibliography
- Oliver Manuel (2001). Origin of Elements in the Solar System: Implications of Post-1957 Observations. Springer. ISBN 978-0-306-46562-8. OCLC 228374906.
- Andrew G. van Melsen (2004) [1952]. From Atomos to Atom: The History of the Concept Atom. Translated by Henry J. Koren. Dover Publications. ISBN 0-486-49584-1.
- J.P. Millington (1906). John Dalton. J. M. Dent & Co. (London); E. P. Dutton & Co. (New York).
- Charles H. Holbrow; James N. Lloyd; Joseph C. Amato; Enrique Galvez; M. Elizabeth Parks (2010). Modern Introductory Physics. Springer Science & Business Media. ISBN 978-0-387-79079-4.
- John Dalton (1808). A New System of Chemical Philosophy vol. 1.
- John Dalton (1817). A New System of Chemical Philosophy vol. 2.
- John L. Heilbron (2003). Ernest Rutherford and the Explosion of Atoms. Oxford University Press. ISBN 0-19-512378-6.
- Jaume Navarro (2012). A History of the Electron: J. J. and G. P. Thomson. Cambridge University Press. ISBN 978-1-107-00522-8.
- Bernard Pullman (1998). The Atom in the History of Human Thought. Translated by Axel Reisinger. Oxford University Press. ISBN 0-19-511447-7.
- Jean Perrin (1910) [1909]. Brownian Movement and Molecular Reality. Translated by F. Soddy. Taylor and Francis.
- Eric R. Scerri (2020). The Periodic Table, Its Story and Its Significance (2nd ed.). New York: Oxford University Press. ISBN 978-0-190-91436-3.
Further reading
- Gangopadhyaya, Mrinalkanti (1981). Indian Atomism: History and Sources. Atlantic Highlands, New Jersey: Humanities Press. ISBN 978-0-391-02177-8. OCLC 10916778.
- Iannone, A. Pablo (2001). Dictionary of World Philosophy. Routledge. ISBN 978-0-415-17995-9. OCLC 44541769.
- King, Richard (1999). Indian philosophy: an introduction to Hindu and Buddhist thought. Edinburgh University Press. ISBN 978-0-7486-0954-3.
- McEvilley, Thomas (2002). The shape of ancient thought: comparative studies in Greek and Indian philosophies. Allworth Press. ISBN 978-1-58115-203-6.
- Siegfried, Robert (2002). From Elements to Atoms: A History of Chemical Composition. Diane. ISBN 978-0-87169-924-4. OCLC 186607849.
- Teresi, Dick (2003). Lost Discoveries: The Ancient Roots of Modern Science. Simon & Schuster. pp. 213–214. ISBN 978-0-7432-4379-7. Archived from the original on 4 August 2020. Retrieved 25 October 2020.
- Wurtz, Charles Adolphe (1881). The Atomic Theory. New York: D. Appleton and company. ISBN 978-0-559-43636-9.
External links
- Atoms in Motion – The Feynman Lectures on Physics
- Sharp, Tim (8 August 2017). "What is an Atom?". Live Science.

![{\displaystyle 1.07{\sqrt[{3}]{A}}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/a74a6ca6998768195969eef75ca046e8431c29d3)
