Buphenine: Difference between revisions
Appearance
Content deleted Content added
stub |
Adding page to Category:Cerebral vasodilators as requested at WP:AFC/C (afcrc-helper) |
||
| (44 intermediate revisions by 35 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Medication}} |
|||
{{Drugbox |
{{Drugbox |
||
| image = Buphenine.svg |
|||
| IUPAC_name = |
|||
| |
| width = |
||
| ⚫ | |||
<!-- Clinical data --> |
|||
| ⚫ | |||
| tradename = Arlidin |
|||
| ⚫ | |||
| Drugs.com = {{drugs.com|international|buphenine}} |
|||
| ⚫ | |||
| |
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
||
| ⚫ | |||
| chemical_formula = C19H25NO2 |
|||
| ⚫ | |||
| molecular_weight = 299.4073 |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| legal_status = |
|||
| excretion = |
|||
| ⚫ | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|||
| ⚫ | |||
<!-- Pharmacokinetic data --> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| |
| excretion = |
||
| ⚫ | |||
<!-- Identifiers --> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ATC_supplemental = {{ATC|G02|CA02}} |
|||
| ⚫ | |||
| DrugBank = |
|||
| ChemSpiderID = 4407 |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 695DKH33EI |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
| ChEMBL = 114655 |
|||
| synonyms = Nylidrin |
|||
<!-- Chemical data --> |
|||
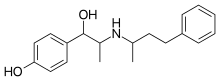
| IUPAC_name = 4-<nowiki>{1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]propyl}phenol</nowiki> |
|||
| C=19 | H=25 | N=1 | O=2 |
|||
| SMILES = OC(c1ccc(O)cc1)C(NC(C)CCc2ccccc2)C |
|||
}} |
}} |
||
'''Buphenine''' (or '''nylidrin''') is a [[sympathomimetic]]. |
|||
'''Buphenine''', also known as '''nylidrin''' and sold under the brand name '''Arlidin''', is a [[Beta2-adrenergic agonist|β<sub>2</sub> adrenoreceptor agonist]]<ref name="pmid2857689">{{cite journal |vauthors=Mittag TW, Tormay A, Messenger M, Podos SM |title=Ocular hypotension in the rabbit. Receptor mechanisms of pirbuterol and nylidrin |journal=Invest Ophthalmol Vis Sci |volume=26 |issue=2 |pages=163–9 |date=February 1985 |pmid=2857689 |url=http://www.iovs.org/cgi/pmidlookup?view=long&pmid=2857689 |url-status=dead |archiveurl=https://archive.today/20130415011509/http://www.iovs.org/cgi/pmidlookup?view=long&pmid=2857689 |archivedate=2013-04-15 }}</ref> that acts as a [[vasodilator]].<ref>{{cite journal| vauthors=Freedman L| title=Arlidin: a new vasodilative sympathomimetic drug | journal=Angiology | year= 1955 | volume= 6 | issue= 1 | pages= 52–8 | pmid=14350296 | doi=10.1177/000331975500600106| s2cid=46317963 }}</ref> |
|||
{{chem-stub}} |
|||
It was developed as a chemical derivative of [[oxilofrine]], and first reported in the literature in 1950.<ref>{{cite journal| vauthors=Külz F, Schneider M| title=Über neue gefäßerweiternde Sympathomimetika |language=German |trans-title=On new vasodilative sympathomimetics | journal=Klin Wochenschr | year= 1950 | volume= 28 | issue=31–32 | pages= 535–7| doi=10.1007/BF01481535 | pmid=14775050 }}</ref> |
|||
==See also== |
|||
* [[Isoxsuprine]] |
|||
==References== |
|||
{{Reflist}} |
|||
{{Other gynecologicals}} |
{{Other gynecologicals}} |
||
{{Peripheral vasodilators}} |
|||
{{Adrenergic receptor modulators}} |
|||
{{Ionotropic glutamate receptor modulators}} |
|||
[[Category:Beta-Hydroxyamphetamines]] |
|||
[[Category:Beta2-adrenergic agonists]] |
|||
[[Category:NMDA receptor antagonists]] |
|||
[[Category:4-Hydroxyphenyl compounds]] |
|||
[[Category:Tocolytics]] |
|||
{{cardiovascular-drug-stub}} |
|||
{{genito-urinary-drug-stub}} |
|||
[[Category:Cerebral vasodilators]] |
|||
Latest revision as of 14:37, 24 October 2024
 | |
| Clinical data | |
|---|---|
| Trade names | Arlidin |
| Other names | Nylidrin |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.531 |
| Chemical and physical data | |
| Formula | C19H25NO2 |
| Molar mass | 299.414 g·mol−1 |
| 3D model (JSmol) | |
| |
Buphenine, also known as nylidrin and sold under the brand name Arlidin, is a β2 adrenoreceptor agonist[1] that acts as a vasodilator.[2]
It was developed as a chemical derivative of oxilofrine, and first reported in the literature in 1950.[3]
See also
[edit]References
[edit]- ^ Mittag TW, Tormay A, Messenger M, Podos SM (February 1985). "Ocular hypotension in the rabbit. Receptor mechanisms of pirbuterol and nylidrin". Invest Ophthalmol Vis Sci. 26 (2): 163–9. PMID 2857689. Archived from the original on 2013-04-15.
- ^ Freedman L (1955). "Arlidin: a new vasodilative sympathomimetic drug". Angiology. 6 (1): 52–8. doi:10.1177/000331975500600106. PMID 14350296. S2CID 46317963.
- ^ Külz F, Schneider M (1950). "Über neue gefäßerweiternde Sympathomimetika" [On new vasodilative sympathomimetics]. Klin Wochenschr (in German). 28 (31–32): 535–7. doi:10.1007/BF01481535. PMID 14775050.
