Neisseria meningitidis: Difference between revisions
m →Prevention: italics not required |
|||
| (566 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
{{short description|Species of bacterium that can cause meningitis}} |
|||
{{Taxobox |
|||
{{Speciesbox |
|||
| color = blue |
|||
| |
| image = Neisseria meningitidis Charles-Orszag 2018.png |
||
| image_alt = Scanning electron micrograph of a single "N. meningitidis" cell (colorized in blue) with its adhesive pili (colorized in yellow). The scale bar corresponds to 1 µm. |
|||
| image= Neisseria meningitidis.jpg |
|||
| image_caption = [[Scanning electron microscope|Scanning electron micrograph]] of a single ''N. meningitidis'' cell (colorized in blue) with its adhesive [[pilus|pili]] (colorized in yellow). The scale bar corresponds to 1 µm. |
|||
| image_caption=[[micrograph|Photomicrograph]] of ''N. meningitidis'' |
|||
| genus = Neisseria |
|||
| regnum = [[Bacterium|Bacteria]] |
|||
| species = meningitidis |
|||
| phylum = [[Proteobacteria]] |
|||
| authority = Albrecht & Ghon 1901 |
|||
| classis = [[Beta Proteobacteria]] |
|||
| synonyms = |
|||
| ordo = [[Neisseriaceae|Neisseriales]] |
|||
| synonyms_ref = |
|||
| familia = [[Neisseriaceae]] |
|||
| genus = ''[[Neisseria]]'' |
|||
| species = '''''N. meningitidis''''' |
|||
| binomial = ''Neisseria meningitidis'' |
|||
| binomial_authority = Albrecht & Ghon 1901 |
|||
}} |
}} |
||
{{commonscat}} |
|||
'''''Neisseria meningitidis''''' is a heterotrophic [[gram-negative]] [[diplococcus|diplococcal]] [[bacterium]] best known for its role in [[meningitis]]<ref name=Sherris>{{cite book | author = Ryan KJ, Ray CG (editors) | title = Sherris Medical Microbiology | edition = 4th | publisher = McGraw Hill | year = 2004 | pages = 329–333 | isbn= 0838585299 }}</ref> and other forms of [[meningococcal disease]] such as meningococcemia. ''N. meningitidis'' is a major cause of morbidity and mortality in childhood in industrialized countries and is responsible for epidemics in Africa and in Asia. |
|||
'''''Neisseria meningitidis''''', often referred to as the '''meningococcus''', is a [[Gram-negative bacterium]] that can cause [[meningitis]] and other forms of [[meningococcal disease]] such as [[meningococcemia]], a life-threatening [[sepsis]]. The bacterium is referred to as a [[coccus]] because it is round, and more specifically a [[diplococcus]] because of its tendency to form pairs. |
|||
About 10% of adults are carriers of the bacteria in their [[nasopharynx]].<ref name="Moelius_1993">{{cite book | vauthors = Hitchcock PJ, Robinson Jr EN, McGee ZA, Koomey JM |year=1993 |chapter=Neisseriae: Gonococcus and Meningococcus | veditors = Schaechter M, Medoff G, Eisenstein BI |editor1-link=Moselio Schaechter | title=Mechanisms of Microbial Disease |edition=2nd |location=Baltimore |publisher=Williams & Wilkins |page=231 |isbn=9780683076066}}</ref> As an exclusively human pathogen, it causes developmental impairment and death in about 10% of cases. It causes the only form of bacterial meningitis known to occur [[epidemic]]ally, mainly in Africa and Asia. It occurs worldwide in both epidemic and endemic form.<ref>{{cite web |url=https://www.cdc.gov/meningococcal/global.html |title=Meningococcal Disease in Other Countries |date=31 May 2019 |department=[[National Center for Immunization and Respiratory Diseases]] |website=Meningococcal Disease |publisher=Centers for Disease Control and Prevention |url-status=live |archive-url=https://web.archive.org/web/20201030193851/http://www.cdc.gov/meningococcal/global.html |archive-date=2020-10-30}}</ref> |
|||
Anton Weichselbaum in 1887 was first reported the disease from patients infected with meningococci <ref>van Deuren, M., Brandtzaeg, P., and van der Meer, J .W. .M. (2000). Update on meningoccal disease with emphasis on pathogenesis and clinical management. Clinical Microbiological Reviews. 13, 144-166</ref>. |
|||
''N. meningitidis'' is spread through saliva and respiratory secretions during coughing, sneezing, kissing, chewing on toys and through sharing a source of fresh water. It has also been reported to be [[Sexually transmitted infection|transmitted through oral sex]] and cause [[urethritis]] in men.<ref>{{cite journal | vauthors = Bazan JA, Peterson AS, Kirkcaldy RD, Briere EC, Maierhofer C, Turner AN, Licon DB, Parker N, Dennison A, Ervin M, Johnson L, Weberman B, Hackert P, Wang X, Kretz CB, Abrams AJ, Trees DL, Del Rio C, Stephens DS, Tzeng YL, DiOrio M, Roberts MW | display-authors = 6 | title = Notes from the Field: Increase in Neisseria meningitidis-Associated Urethritis Among Men at Two Sentinel Clinics - Columbus, Ohio, and Oakland County, Michigan, 2015 | journal = MMWR. Morbidity and Mortality Weekly Report | volume = 65 | issue = 21 | pages = 550–552 | date = June 2016 | pmid = 27254649 | pmc = 5390329 | doi = 10.15585/mmwr.mm6521a5 | publisher = Centers for Disease Control and Prevention | author-link18 = Carlos del Rio | doi-access = free }}</ref> It infects its host cells by sticking to them with long thin extensions called [[pilus|pili]] and the surface-exposed proteins Opa and Opc and has several [[virulence factors]]. |
|||
Meningococci only infect humans and have never been isolated from animals because the bacterium cannot get iron other than human source ([[transferrin]] and [[lactoferrin]]).<ref>Meningococcal Disease (2001) Humana Press, Andrew J. Pollard and Martin C.J. Maiden</ref> |
|||
==Signs and symptoms== |
|||
It exists as normal flora in the [[nasopharynx]] of up to 40% of adults. It causes the only form of bacterial meningitis known to cause [[epidemic]]s. |
|||
{{Main|Meningococcal disease}} |
|||
Meningococcus can cause [[meningitis]] and other forms of meningococcal disease.<ref name=Sherris/> It initially produces general symptoms like [[fatigue (medical)|fatigue]], fever, and headache and can rapidly progress to [[neck stiffness]], coma and death in 10% of cases. [[Petechia]]e occur in about 50% of cases. Chance of survival is highly correlated with blood cortisol levels, with lower levels prior to steroid administration corresponding with increased patient mortality.<ref>{{cite journal | vauthors = Riordan FA, Thomson AP, Ratcliffe JM, Sills JA, Diver MJ, Hart CA | title = Admission cortisol and adrenocorticotrophic hormone levels in children with meningococcal disease: evidence of adrenal insufficiency? | journal = Critical Care Medicine | volume = 27 | issue = 10 | pages = 2257–2261 | date = October 1999 | pmid = 10548217 | doi = 10.1097/00003246-199910000-00032 }}</ref> Symptoms of meningococcal meningitis are easily confused with those caused by other bacteria, such as ''[[Haemophilus influenzae]]'' and ''[[Streptococcus pneumoniae]]''.<ref name="Pollard_2001"/><ref name="Mola_2008"/> Suspicion of meningitis is a [[medical emergency]] and immediate medical assessment is recommended. Current guidance in the United Kingdom is that if a case of meningococcal meningitis or [[sepsis|septicaemia]] (infection of the blood) is suspected, intravenous antibiotics should be given and the ill person admitted to the hospital.<ref>{{cite web |url=https://www.gov.uk/government/publications/meningococcal-disease-guidance-on-public-health-management |title=Guidance for public health management of meningococcal disease in the UK |year=2019 |website=gov.uk |publisher=Public Health England |format=pdf |id=GW-599 |access-date=2021-01-31 |url-status=live |archive-url=https://web.archive.org/web/20201231014938/https://www.gov.uk/government/publications/meningococcal-disease-guidance-on-public-health-management |archive-date=2020-12-31}}</ref> This means that laboratory tests may be less likely to confirm the presence of ''Neisseria meningitidis'' as the antibiotics will dramatically lower the number of bacteria in the body. The UK guidance is based on the idea that the reduced ability to identify the bacteria is outweighed by reduced [[Mortality rate|chance of death]].{{cn|date=February 2023}} |
|||
Septicaemia caused by ''Neisseria meningitidis'' has received much less public attention than meningococcal meningitis even though septicaemia has been linked to infant deaths.<ref name="Pollard_2001">{{cite book | veditors = Pollard AJ, Maiden MC |title=Meningococcal Vaccines: Methods and Protocols |url=https://archive.org/details/meningococcalvac0000unse |url-access=limited |year=2001 |publisher=Humana Press |location= Totowa, NJ |isbn=978-0-89603-801-1}}</ref> Meningococcal septicaemia typically causes a [[purpuric]] rash, that does not lose its color when pressed with a glass slide ("[[non-blanching rash|non-blanching]]") and does not cause the classical symptoms of meningitis. This means the condition may be ignored by those not aware of the significance of the rash. Septicaemia carries an approximate 50% [[mortality rate]] over a few hours from initial onset.{{cn|date=February 2023}} |
|||
Meningococcus is spread through the exchange of saliva and other respiratory secretions during activities like coughing, kissing, and chewing on toys. Though it initially produces with general symptoms like [[fatigue (medical)|fatigue]], it can rapidly progress from fever, headache and [[neck stiffness]] to [[coma]] and death. [[Death]] occurs in approximately 10% of cases.<ref>Meningococcal Disease (2001) Humana Press, Andrew J. Pollard and Martin C.J. Maiden</ref> Those with impaired immunity may be at particular risk of meningococcus (e.g. those with [[nephrotic syndrome]] or [[splenectomy]]; vaccines are given in cases of [[asplenia|removed or non-functioning spleens]]). |
|||
Other severe complications include [[Waterhouse–Friderichsen syndrome]], a massive, usually bilateral, hemorrhage into the adrenal glands caused by [[wikt:fulminant|fulminant]] meningococcemia, [[adrenal insufficiency]], and [[disseminated intravascular coagulation]].<ref name="Mola_2008"/> |
|||
Suspicion of meningitis is a [[medical emergency]] and immediate medical assessment is recommended. Current guidance in the [[United Kingdom]] is that any doctor who suspects a case of meningococcal meningitis or [[sepsis|septicaemia]] (infection of the blood) should give intravenous antibiotics ([[penicillin|benzylpenicillin]] or [[Cefotaxime]]) and admit the ill person to the hospital.<ref>Health Protection Agency Meningococcus Forum (August 2006). Guidance for public health management of meningococcal disease in the UK. Available online at: http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1194947389261</ref> This means that laboratory tests may be less likely to confirm the presence of ''Neisseria meningitidis'' as the antibiotics will dramatically lower the number of bacteria in the body. The UK guidance is based on the idea that the reduced ability to identify the bacteria is outweighed by reduced [[Mortality rate|chance of death]]. |
|||
Not all instances of a purpura-like rash are due to meningococcal septicaemia; other possible causes, such as [[idiopathic thrombocytopenic purpura]] (ITP; a [[platelet]] disorder) and [[Henoch–Schönlein purpura]], also need prompt investigation.{{cn|date=February 2023}} |
|||
== Microbiology == |
|||
[[Septicaemia]] caused by ''Neisseria meningitidis'' has received much less public attention than meningococcal meningitis even though septicaemia has been linked to infant deaths. <ref>Meningococcal Vaccines (2001) Humana Press, Andrew J. Pollard and Martin C.J. Maiden</ref> Meningococcal septicaemia typically causes a [[purpuric]] rash that does not lose its colour when pressed with a glass ("non-blanching") and does not cause the classical symptoms of meningitis. This means the condition may be ignored by those not aware of the significance of the rash. Septicaemia carries an approximate 50% mortality rate over a few hours from initial onset. Many health organizations advise anyone with a non-blanching rash to go to a hospital [[emergency room]] as soon as possible.{{Fact|date=April 2007}} Note that not all cases of a [[purpura]]-like rash are due to meningococcal septicaemia; however, other possible causes need prompt investigation as well (e.g. [[Idiopathic thrombocytopenic purpura|ITP]] a [[platelet]] disorder or [[Henoch-Schönlein purpura]]). |
|||
''N. meningitidis'' is a [[Gram-negative]] diplococcus since it has an outer and inner membranes with a thin layer of [[peptidoglycan]] in between. It is 0.6–1.0 micrometers in size. It tests positive for the enzyme [[cytochrome c oxidase]].<ref>{{cite web |url=https://www.cdc.gov/std/gonorrhea/lab/Nmen.htm |title=Neisseria meningitidis |date=31 March 2017 |website=Characteristics of N. gonorrhoeae and Related Species |department=[[National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention]] |publisher=Centers for Disease Control and Prevention |url-status=live |archive-url=https://web.archive.org/web/20201020023623/http://www.cdc.gov/std/gonorrhea/lab/Nmen.htm |archive-date=2020-10-20}}</ref> |
|||
===Habitat=== |
|||
''[[Waterhouse-Friderichsen syndrome]]'' is a massive, usually bilateral, hemorrhage into the adrenal glands caused by [[Wiktionary:fulminant|fulminant]] meningococcemia. |
|||
''N. meningitidis'' is a part of the normal [[wikt:nonpathogenic|nonpathogenic]] flora in the [[nasopharynx]] of up to 8–25% of adults.<ref name="Stephens_2007">{{cite journal | vauthors = Stephens DS, Greenwood B, Brandtzaeg P | title = Epidemic meningitis, meningococcaemia, and Neisseria meningitidis | journal = Lancet | volume = 369 | issue = 9580 | pages = 2196–2210 | date = June 2007 | pmid = 17604802 | doi = 10.1016/S0140-6736(07)61016-2 | author-link2 = Brian Greenwood | s2cid = 16951072 | citeseerx = 10.1.1.324.1736 }}</ref> It colonizes and infects only humans, and has never been isolated from other animals. This is thought to result from the bacterium's inability to get iron from sources other than human [[transferrin]] and [[lactoferrin]].<ref name="Pollard_2001"/> |
|||
== |
=== Subtypes === |
||
Disease-causing strains are classified according to the [[antigen]]ic structure of their [[polysaccharide]] capsule.<ref> |
|||
{{cite book |year=2015 |chapter=Meningococcal Disease |chapter-url=https://www.cdc.gov/vaccines/pubs/pinkbook/mening.html | veditors = Hamborsky J, Kroger A, Wolfe S |title=Epidemiology and Prevention of Vaccine-Preventable Diseases |url=https://purl.access.gpo.gov/GPO/LPS227 |edition=13th |publisher=Centers for Disease Control and Prevention |access-date=2021-01-31}}</ref> Serotype distribution varies markedly around the world.<ref name="Stephens_2007"/> Among the 13 identified capsular types of ''N. meningitidis'', six (A, B, C, W135, X, and Y) account for most disease cases worldwide.<ref name="Harrison_2010">{{cite journal | vauthors = Harrison LH | title = Epidemiological profile of meningococcal disease in the United States | journal = Clinical Infectious Diseases | volume = 50 | issue = Suppl 2 | pages = S37–S44 | date = March 2010 | pmid = 20144015 | pmc = 2820831 | doi = 10.1086/648963 }}</ref> Type A has been the most prevalent in Africa and Asia, but is rare/practically absent in North America. In the United States, serogroup B is the predominant cause of disease and mortality, followed by serogroup C. The multiple subtypes have hindered development of a universal vaccine for meningococcal disease.{{cn|date=February 2023}} |
|||
== Pathogenesis == |
|||
Lipopolysaccharide (LPS) is a component of the cell wall of ''N. meningitidis'' which acts as an [[endotoxin]]. Other virulence factors include a polysaccharide [[capsule (microbiology)|capsule]] which prevents host [[phagocytosis]] and aids in evasion of the host immune response; and [[fimbriae]] which mediate attachment of the bacterium to the epithelial cells of the [[nasopharynx]]. |
|||
== |
=== Virulence === |
||
[[Lipooligosaccharide]] (LOS) is a component of the [[Bacterial outer membrane|outer membrane]] of ''N. meningitidis''. This acts as an [[endotoxin]] and is responsible for [[septic shock]] and hemorrhage due to the destruction of red blood cells.<ref>{{cite journal | vauthors = Griffiss JM, Schneider H, Mandrell RE, Yamasaki R, Jarvis GA, Kim JJ, Gibson BW, Hamadeh R, Apicella MA | display-authors = 6 | title = Lipooligosaccharides: the principal glycolipids of the neisserial outer membrane | journal = Reviews of Infectious Diseases | volume = 10 | issue = Suppl 2 | pages = S287–S295 | year = 1988 | pmid = 2460911 | doi = 10.1093/cid/10.supplement_2.s287 | jstor = 4454586 }}</ref> Other virulence factors include a polysaccharide [[capsule (microbiology)|capsule]] which prevents host [[phagocytosis]] and aids in evasion of the host immune response. Adhesion is another key virulence strategy to successfully invade host cell. There are several known proteins that are involved in adhesion and invasion, or mediate interactions with specific host cell receptors. These include the Type IV pilin adhesin which mediates attachment of the bacterium to the [[epithelial cells]] of the [[Pharynx|nasopharynx]],<ref name="Jarrell_2009">{{cite book | veditors = Jarrell KF |year=2009 |title=Pili and Flagella: Current Research and Future Trends |publisher=Caister Academic Press |isbn=978-1-904455-48-6}}</ref><ref name= "Ullrich_2009">{{cite book | veditors = Ullrich M |year=2009 |title=Bacterial Polysaccharides: Current Innovations and Future Trends |publisher=Caister Academic Press |isbn=978-1-904455-45-5}}</ref> surface-exposed Opa and Opc proteins which mediate interactions with specific host cell receptors,<ref name="Hill_2010">{{cite journal | vauthors = Hill DJ, Griffiths NJ, Borodina E, Virji M | title = Cellular and molecular biology of Neisseria meningitidis colonization and invasive disease | journal = Clinical Science | volume = 118 | issue = 9 | pages = 547–564 | date = February 2010 | pmid = 20132098 | pmc = 2830671 | doi = 10.1042/CS20090513 }}</ref> and NadA which is involved in adhesion.<ref name = "Caugant_2020">{{cite journal | vauthors = Caugant DA, Brynildsrud OB | title = Neisseria meningitidis: using genomics to understand diversity, evolution and pathogenesis | journal = Nature Reviews. Microbiology | volume = 18 | issue = 2 | pages = 84–96 | date = February 2020 | pmid = 31705134 | doi = 10.1038/s41579-019-0282-6 | s2cid = 207960642 }}</ref> |
|||
Pathogenic meningococci that have invaded into the bloodstream must be able to survive in the new niche, this is facilitated by acquisition and utilisation of iron (FetA and Hmbr), resisting intracellular oxidative killing by producing [[catalase]] and [[superoxide dismutase]] and ability to avoid [[Complement system|complement]] mediated killing (fHbp).<ref name="Hill_2010" /> Meningococci produce an IgA protease, an enzyme that cleaves IgA class antibodies and thus allows the bacteria to evade a subclass of the humoral immune system.{{cn|date=March 2023}} |
|||
A [[Cerebrospinal fluid|CSF]] specimen is sent to the laboratory hi immediately for identification of the organism. Diagnosis relies on culturing the organism on a [[chocolate agar]] plate. Further testing to differentiate the species includes testing for [[oxidase]] (all ''Neisseria'' show a positive reaction) and the [[carbohydrates]] [[maltose]], [[sucrose]], and [[glucose]] test in which ''N. meningitidis'' will oxidize (that is, utilize) the glucose and maltose. Serology determines the group of the isolated organism. |
|||
A hypervirulent strain was discovered in China. Its impact is yet to be determined.<ref name="Mola_2008"/> |
|||
If the organism reaches the circulation, then [[blood cultures]] should be drawn and processed accordingly. |
|||
===Complement inhibition=== |
|||
Quintain NS and RMIT University have developed a rapid diagnostic test for meningococcal disease, which will ultimately provide results in under 15 minutes. |
|||
Factor H binding protein (fHbp) that is exhibited in ''N. meningitidis'' and some commensal species is the main inhibitor of the [[alternative complement pathway]]. fHbp protects meningococci from complement-mediated death in human serum experiments, but has also been shown to protect meningococci from antimicrobial peptides ''in vitro''. Factor H binding protein is key to the pathogenesis of ''N. meningitidis'', and is, therefore, important as a potential vaccine candidate.<ref>{{cite journal | vauthors = McNeil LK, Zagursky RJ, Lin SL, Murphy E, Zlotnick GW, Hoiseth SK, Jansen KU, Anderson AS | display-authors = 6 | title = Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease | journal = Microbiology and Molecular Biology Reviews | volume = 77 | issue = 2 | pages = 234–252 | date = June 2013 | pmid = 23699256 | pmc = 3668674 | doi = 10.1128/MMBR.00056-12 | doi-access = free | author7-link = Kathrin Jansen }}</ref> Porins are also an important factor for complement inhibition for both pathogenic and commensal species. Porins are important for nutrient acquisition. Porins are also recognized by [[TLR2]], they bind complement factors ([[C3b]], [[C4b]], [[factor H]], and [[C4b-binding protein|C4bp]] (complement factor 4b-binding protein)). Cooperation with pili for CR3-mediated internalization is another function of porins. Ability to translocate into host cells and modulate [[reactive oxygen species]] production and apoptosis is made possible by porins, as well. Strains of the same species can express different porins.{{cn|date=February 2023}} |
|||
Clinical tests that are used currently for the diagnosis of meningococcal disease take between 2 and 48 hours and often rely on the culturing of bacteria from either blood or cerebrospinal fluid (CSF) samples. As the disease has a fatality risk approaching 15% within 12 hours of infection, early diagnosis and antibiotic treatment is crucial. |
|||
== Genome == |
|||
Quintain is working with Melbourne-based company Charlwood Design, to produce a prototype clinical device that will incorporate a mechanism for safe sample handling and delivery. It is expected that the diagnostic test will be available within 2-3 years, with the nanoparticulate gold diagnostic platform adapted for a range of other clinically important diseases shortly thereafter. |
|||
At least 8 complete genomes of ''Neisseria meningitidis'' strains have been determined which encode about 2,100 to 2,500 proteins.<ref name="Piet_2011">{{cite journal | vauthors = Piet JR, Huis in 't Veld RA, van Schaik BD, van Kampen AH, Baas F, van de Beek D, Pannekoek Y, van der Ende A | display-authors = 6 | title = Genome sequence of Neisseria meningitidis serogroup B strain H44/76 | journal = Journal of Bacteriology | volume = 193 | issue = 9 | pages = 2371–2372 | date = May 2011 | pmid = 21378179 | pmc = 3133077 | doi = 10.1128/JB.01331-10 | doi-access = free }}</ref> |
|||
The genome of '''strain MC58''' (serogroup B) has 2,272,351 base pairs. When sequenced in 2000, it was found to contain 2158 [[open reading frames]] (ORFs). Of these, a biological function was predicted for 1158 (53.7%). There were three major islands of [[Horizontal gene transfer|horizontal DNA transfer]] found. Two encode proteins involved in pathogenicity. The third island only codes for hypothetical proteins. They also found more genes that undergo [[phase variation]] than any pathogen then known. Phase variation is a mechanism that helps the pathogen to evade the [[immune system]] of the host.<ref name="Tettelin_2000"> |
|||
== Vaccines == |
|||
{{cite journal | vauthors = Tettelin H, Saunders NJ, Heidelberg J, Jeffries AC, Nelson KE, Eisen JA, Ketchum KA, Hood DW, Peden JF, Dodson RJ, Nelson WC, Gwinn ML, DeBoy R, Peterson JD, Hickey EK, Haft DH, Salzberg SL, White O, Fleischmann RD, Dougherty BA, Mason T, Ciecko A, Parksey DS, Blair E, Cittone H, Clark EB, Cotton MD, Utterback TR, Khouri H, Qin H, Vamathevan J, Gill J, Scarlato V, Masignani V, Pizza M, Grandi G, Sun L, Smith HO, Fraser CM, Moxon ER, Rappuoli R, Venter JC | display-authors = 6 | title = Complete genome sequence of Neisseria meningitidis serogroup B strain MC58 | journal = Science | volume = 287 | issue = 5459 | pages = 1809–1815 | date = March 2000 | pmid = 10710307 | doi = 10.1126/science.287.5459.1809 | author41-link = Rino Rappuoli | author39-link = Claire M. Fraser | author38-link = Hamilton O. Smith | bibcode = 2000Sci...287.1809. | jstor = 3074600 | author6-link = Jonathan Eisen | author5-link = Karen E. Nelson | author17-link = Steven Salzberg | author18-link = Owen White | author42-link = Craig Venter }}</ref> |
|||
The genome size of '''strain H44/76''' is 2.18 Mb, and encodes 2,480 open reading frames (ORFs), compared to 2.27 Mb and 2,465 ORFs for MC58.<ref name="Piet_2011"/> Both strains have a GC content of 51.5%.<ref name="Piet_2011"/> A comparison with MC58 showed that four genes are uniquely present in H44/76 and nine genes are only present in MC58. Of all ORFs in H44/76, 2,317 (93%) show more than 99% sequence identity.<ref name="Piet_2011"/> |
|||
The complete genome sequence of '''strain NMA510612''' (serogroup A) consists of one circular chromosome with a size of 2,188,020 bp, and the average GC content is 51.5%. The chromosome is predicted to possess 4 rRNA operons, 163 insertion elements (IS), 59 tRNAs, and 2,462 ORFs.<ref name="Zhang_2014">{{cite journal | vauthors = Zhang Y, Yang J, Xu L, Zhu Y, Liu B, Shao Z, Zhang X, Jin Q | display-authors = 6 | title = Complete Genome Sequence of Neisseria meningitidis Serogroup A Strain NMA510612, Isolated from a Patient with Bacterial Meningitis in China | journal = Genome Announcements | volume = 2 | issue = 3 | pages = e00360–14 | date = May 2014 | pmid = 24812217 | pmc = 4014685 | doi = 10.1128/genomeA.00360-14 }}</ref> |
|||
There is a public database available for ''N. meningitidis'' core genome [[multilocus sequence typing | Multilocus sequence typing]] (cgMLST). Available at: [https://pubmlst.org/bigsdb?db=pubmlst_neisseria_seqdef Neisseria typing] |
|||
==Genetic transformation== |
|||
Genetic [[Transformation (genetics)|transformation]] is the process by which a recipient bacterial cell takes up DNA from a neighboring cell and integrates this DNA into the recipient's genome by [[Genetic recombination|recombination]]. In ''N. meningitidis'', DNA transformation requires the presence of short DNA sequences (9–10 mers residing in [[coding region]]s) of the donor DNA. These sequences are called [[Uptake signal sequence|DNA uptake sequences (DUSs)]]. Specific recognition of these sequences is mediated by a type IV [[pilin]].<ref name="Cehovin_2013">{{cite journal | vauthors = Cehovin A, Simpson PJ, McDowell MA, Brown DR, Noschese R, Pallett M, Brady J, Baldwin GS, Lea SM, Matthews SJ, Pelicic V | display-authors = 6 | title = Specific DNA recognition mediated by a type IV pilin | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 110 | issue = 8 | pages = 3065–3070 | date = February 2013 | pmid = 23386723 | pmc = 3581936 | doi = 10.1073/pnas.1218832110 | doi-access = free | bibcode = 2013PNAS..110.3065C }}</ref> In ''N. meningitidis'' DUSs occur at a significantly higher density in genes involved in [[DNA repair]] and [[Genetic recombination|recombination]] (as well as in [[restriction modification system|restriction-modification]] and [[DNA replication|replication]]) than in other annotated gene groups. The over-representation of DUS in DNA repair and recombination genes may reflect the benefit of maintaining the integrity of the DNA repair and recombination machinery by preferentially taking up genome maintenance genes, that could replace their damaged counterparts in the recipient cell.<ref name="Davidsen_2004">{{cite journal | vauthors = Davidsen T, Rødland EA, Lagesen K, Seeberg E, Rognes T, Tønjum T | title = Biased distribution of DNA uptake sequences towards genome maintenance genes | journal = Nucleic Acids Research | volume = 32 | issue = 3 | pages = 1050–1058 | year = 2004 | pmid = 14960717 | pmc = 373393 | doi = 10.1093/nar/gkh255 | doi-access = free }}</ref> |
|||
''N. meningititis'' colonizes the nasopharyngeal [[mucous membrane|mucosa]], which is rich in [[macrophage]]s. Upon their activation, macrophages produce [[superoxide]] (O<sub>2</sub><sup>−</sup>) and [[hydrogen peroxide]] (H<sub>2</sub>O<sub>2</sub>). Thus ''N. meningitidis'' is likely to encounter [[oxidative stress]] during its life cycle.<ref name="pmid16417521">{{cite journal | vauthors = Dyet K, Moir J | title = Effect of combined oxidative and nitrosative stress on Neisseria meningitidis | journal = Biochemical Society Transactions | volume = 34 | issue = Pt 1 | pages = 197–199 | date = February 2006 | pmid = 16417521 | doi = 10.1042/BST0340197 | s2cid = 10045867 }}</ref> Consequently, an important benefit of genetic [[transformation (genetics)|transformation]] to ''N. meningitidis'' may be the maintenance of the recombination and repair machinery of the cell that removes oxidative DNA damages such as those caused by [[reactive oxygen species|reactive oxygen]]. This is consistent with the more general idea that transformation benefits bacterial [[pathogen]]s by facilitating repair of DNA damages produced by the oxidative defenses of the host during infection.<ref name="pmid18295550">{{cite journal | vauthors = Michod RE, Bernstein H, Nedelcu AM | title = Adaptive value of sex in microbial pathogens | journal = Infection, Genetics and Evolution | volume = 8 | issue = 3 | pages = 267–285 | date = May 2008 | pmid = 18295550 | doi = 10.1016/j.meegid.2008.01.002 | citeseerx = 10.1.1.211.2248 }}</ref> |
|||
Meningococci population is extensively diverse genetically, this is due to [[horizontal gene transfer]]s while in the nasophanrynx. Gene transfer can occur within and between genomes of ''[[Neisseria]]'' species, and it is the main mechanism of acquiring new traits.<ref>{{cite journal | vauthors = Hanage WP, Fraser C, Spratt BG | title = Fuzzy species among recombinogenic bacteria | journal = BMC Biology | volume = 3 | issue = 1 | pages = 6 | date = March 2005 | pmid = 15752428 | pmc = 554772 | doi = 10.1186/1741-7007-3-6 | doi-access = free }}</ref> This is facilitated by the natural competence of the meningococci to take up foreign DNA.<ref name = "Caugant_2020" /> The commensal species of ''Neisseria'' can act as a reservoir of genes that can be acquired; for example, this is how capsule switching can occur as a means of hiding from the immune system.<ref name = "Caugant_2020" /> An invasive ''N. meningitidis'' strain of serogroup C broke out in Nigeria in 2013 – the strain was a new sequence type, ST-10217 determined by [[multilocus sequence typing]].<ref name = "Brynildsrud_2018">{{cite journal | vauthors = Brynildsrud OB, Eldholm V, Bohlin J, Uadiale K, Obaro S, Caugant DA | title = Acquisition of virulence genes by a carrier strain gave rise to the ongoing epidemics of meningococcal disease in West Africa | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 115 | issue = 21 | pages = 5510–5515 | date = May 2018 | pmid = 29735685 | doi = 10.1073/pnas.1802298115 | pmc = 6003489 | doi-access = free }}</ref> It was determined that a commensal strain of ''N. meningitidis'' acquired an 8-kb prophage, the meningococcal disease-associated island (MDAΦ), previously associated with hyper-invasiveness;<ref name = "Caugant_2020" /> and the full serogroup C capsule operon, thus becoming a hypervirulent strain. This illustrates how hypervirulent strains can arise from non-pathgenic strains due to the high propensity of gene transfers and DNA uptake by ''N. meningitidis''.<ref name = "Brynildsrud_2018" /> |
|||
==Diagnosis== |
|||
[[File:Neisseria meningitidis Colonies growth on New York City Medium Agar.jpg|thumb|right|The growth of ''Neisseria meningitidis'' colonies on [[New York City agar]] ]] |
|||
[[File:Neisseria meningitidis CSF Gram 1000.jpg|thumb|''Neisseria meningitidis'' in cerebrospinal fluid (CSF) seen by Gram stain at 1000× magnification]] |
|||
A small amount of [[cerebrospinal fluid]] (CSF) is sent to the laboratory as soon as possible for analysis. The diagnosis is suspected, when [[Gram-negative]] diplococci are seen on [[Gram stain]] of a centrifuged sample of CSF; sometimes they are located inside [[white blood cells]]. The microscopic identification takes around 1–2 hours after specimen arrival in the laboratory.<ref name=Sherris>{{cite book | veditors = Ryan KJ, Ray CG |title=Sherris Medical Microbiology |edition=4th |publisher=McGraw-Hill |location=New York |year=2004 |pages=329–333 |isbn=978-0-8385-8529-0 }}</ref> |
|||
The [[Gold standard (test)|gold standard]] of diagnosis is microbiological isolation of ''N. meningitidis'' by growth from a sterile body fluid, which could be CSF or blood.<ref name="Mola_2008"/> Diagnosis is confirmed when the organism has grown, most often on a [[chocolate agar]] plate, but also on [[Thayer–Martin agar]]. To differentiate any bacterial growth from other species a small amount of a [[bacterial colony]] is Gram stained and tested for [[oxidase]] and [[catalase]]. Gram negative diplococci that are oxidase and catalase positive are then tested for fermentation of the following [[carbohydrates]]: [[maltose]], [[sucrose]], and [[glucose]]. ''N. meningitidis'' will ferment glucose and maltose. Finally, [[serology]] determines the [[serotype|subgroup]] of the ''N. meningitidis'', which is important for [[epidemiological surveillance]] purposes; this may often only be done in specialized laboratories.{{cn|date=February 2023}} |
|||
The above tests take a minimum of 48–72 hours [[turnaround time]] for growing the organism, and up to a week more for serotyping. Growth can and often does fail, either because antibiotics have been given preemptively, or because specimens have been inappropriately transported, as the organism is extremely susceptible to antibiotics and [[fastidious]] in its temperature, {{CO2}} and growth medium requirements.{{cn|date=February 2023}} |
|||
[[Polymerase chain reaction]] (PCR) tests where available, mostly in industrialized countries, have been increasingly used; PCR can rapidly identify the organism, and works even after antibiotics have been given.<ref name="Mola_2008"/> |
|||
== Prevention == |
|||
All recent contacts of the infected patient over the seven days before onset should receive medication to prevent them from contracting the infection. This especially includes young children and their child caregivers or nursery-school contacts, as well as anyone who had direct exposure to the patient through kissing, sharing utensils, or medical interventions such as [[mouth-to-mouth resuscitation]]. Anyone who frequently ate, slept or stayed at the patient's home during the seven days before the onset of symptom, or those who sat beside the patient on an airplane flight or classroom for eight hours or longer, should also receive [[chemoprophylaxis]]. The agent of choice is usually oral [[rifampicin]] for a few days.<ref name="Mola_2008"/> |
|||
Receiving a dose of the [[meningococcal vaccine]] before traveling to a country in the "meningitis belt" or having a booster meningitis vaccine, normally five years apart could prevent someone from getting an infection from the pathogen.<ref>{{cite web |url=https://www.cdc.gov/meningococcal/about/prevention.html |title=Prevention |date=31 May 2019 |department=[[National Center for Immunization and Respiratory Diseases]] |website=Meningococcal Disease |publisher=Centers for Disease Control and Prevention |url-status=live |archive-url=https://web.archive.org/web/20201030194706/http://www.cdc.gov/meningococcal/about/prevention.html |archive-date=2020-10-30}}</ref> |
|||
===Vaccination=== |
|||
{{main|Meningococcal vaccine}} |
{{main|Meningococcal vaccine}} |
||
There are currently two vaccines available in the US to prevent meningococcal disease. '''[[Menactra]]''' is licensed for use in people aged 11 to 55, while '''[[Menomune]]''' is used for people outside of this age group and for travellers. |
|||
====United States==== |
|||
A number of vaccines are available in the U.S. to prevent meningococcal disease. Some of the vaccines cover serogroup B, while others cover A, C, W, and Y.<ref name=CDCVaccinationPublic>{{cite web |url=https://www.cdc.gov/vaccines/vpd/mening/public/index.html |date=29 July 2019 |title=Meningococcal Vaccination: What Everyone Should Know |department=[[National Center for Immunization and Respiratory Diseases]] |publisher=U.S. Centers for Disease Control and Prevention |url-status=live |archive-url=https://web.archive.org/web/20210123155330/http://www.cdc.gov/vaccines/vpd/mening/public/index.html |archive-date=2021-01-23}}</ref> The [[Centers for Disease Control and Prevention]] (CDC) recommends all teenagers receive MenACWY vaccine and booster, with optional MenB. MenACWY and MenB are also recommended for people of other ages with various medical conditions and social risk factors.<ref name=CDCVaccinationPublic/> |
|||
A meningococcal polysaccharide vaccine (MPSV4) has been available since the 1970s and is the only meningococcal vaccine licensed for people older than 55. MPSV4 may be used in people 2–55 years old if the MCV4 vaccines are not available or contraindicated. Two meningococcal [[conjugate vaccine]]s (MCV4) are licensed for use in the U.S. The first conjugate vaccine was licensed in 2005, the second in 2010. Conjugate vaccines are the preferred vaccine for people 2 through 55 years of age. It is indicated in those with impaired immunity, such as [[nephrotic syndrome]] or [[splenectomy]].{{cn|date=February 2023}} |
|||
In June 2012, the U.S. [[Food and Drug Administration]] (FDA) approved a combination vaccine against two types of meningococcal diseases and [[Haemophilus influenzae|Hib]] disease for infants and children 6 weeks to 18 months old. The vaccine, [[Meningococcal vaccine#Bivalent (Serogroups C and Y)|Menhibrix]], was designed to prevent disease caused by ''Neisseria meningitidis'' serogroups C and Y, and ''Haemophilus influenzae'' type b (Hib). It was the first meningococcal vaccine that could be given to infants as young as six weeks old.<ref>{{cite press release |date=14 June 2012 |title=FDA approves new combination vaccine that protects children against two bacterial diseases |url=https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm308350.htm |publisher=U.S. Food and Drug Administration |url-status=unfit |archive-url=https://web.archive.org/web/20170119035233/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm308350.htm |archive-date=2017-01-19}}</ref> |
|||
In October 2014 the FDA approved the first vaccine effective against serogroup B, named [[Trumenba]], for use in 10- to 25-year-old individuals.<ref>{{cite press release |date=29 October 2014 |title=First vaccine approved by FDA to prevent serogroup B Meningococcal disease |url=https://www.fda.gov/news-events/press-announcements/first-vaccine-approved-fda-prevent-serogroup-b-meningococcal-disease |publisher=U.S. Food and Drug Administration |url-status=live |archive-url=https://web.archive.org/web/20210122162301/http://www.fda.gov/news-events/press-announcements/first-vaccine-approved-fda-prevent-serogroup-b-meningococcal-disease |archive-date=2021-01-22}}</ref> |
|||
====Africa==== |
|||
In 2010, the [[Meningitis Vaccine Project]] introduced a vaccine called [[MenAfriVac]] in the [[African meningitis belt]]. It was made by generic drug maker [[Serum Institute of India]] and cost 50 U.S. cents per injection. Beginning in [[Burkina Faso]] in 2010, it has been given to 215 million people across [[Benin]], [[Cameroon]], [[Chad]], [[Ivory Coast]], [[Ethiopia]], [[Ghana]], [[Mali]], [[Niger]], [[Mauritania]], [[Nigeria]], [[Senegal]], [[Sudan]], [[Togo]] and [[Gambia]].<ref name= "Kelland_2015">{{cite news | vauthors = Kelland K |title=Tailor-made vaccine set to banish Africa's meningitis epidemics |url=https://www.reuters.com/article/us-health-meningitis-vaccine-idUSKBN0KI14520150109 |access-date=31 January 2021 |work=Reuters |publisher=Thomson Reuters |date=9 January 2015 |url-status=live |archive-url=https://web.archive.org/web/20191218112628/https://www.reuters.com/article/us-health-meningitis-vaccine-idUSKBN0KI14520150109 |archive-date=2019-12-18}}</ref> The vaccination campaign has resulted in near-elimination of serogroup A meningitis from the participating countries.<ref>{{cite press release |date=22 February 2016 |title=Meningitis A nearly eliminated in Africa through vaccination, reaching more than 235 million people |url=http://immunizationinafrica2016.org/releases/2016/2/23/as-meningitis-nears |url-status=live |type=Press release |publisher=Ministerial Conference on Immunization in Africa |archive-url=https://web.archive.org/web/20170202004714/http://immunizationinafrica2016.org/releases/2016/2/23/as-meningitis-nears |archive-date=2017-02-02}}</ref> |
|||
== Treatment == |
|||
Persons with confirmed ''N. meningitidis'' infection should be hospitalized immediately for treatment with antibiotics. Because meningococcal disease can disseminate very rapidly, a single dose of intramuscular antibiotic is often given at the earliest possible opportunity, even before hospitalization, if disease symptoms look suspicious enough.<ref name="Mola_2008"/> Third-generation [[cephalosporin]] antibiotics (i.e. [[cefotaxime]], [[ceftriaxone]]) should be used to treat a suspected or culture-proven meningococcal infection before antibiotic susceptibility results are available.<ref name="Tunkel_2004">{{cite journal | vauthors = Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ | title = Practice guidelines for the management of bacterial meningitis | journal = Clinical Infectious Diseases | volume = 39 | issue = 9 | pages = 1267–1284 | date = November 2004 | pmid = 15494903 | doi = 10.1086/425368 | doi-access = free }}</ref> [[Clinical practice guideline]]s endorse [[empirical treatment]] in the event a [[lumbar puncture]] to collect [[cerebrospinal fluid]] (CSF) for laboratory testing cannot first be performed.<ref name= Tunkel_2004/><ref>{{cite book | vauthors = Valles J, Ferrer R, Fernández-Viladrich P |year=2005 |chapter=Bloodstream Infections Including Endocarditis and Meningitis | veditors = van Saene HK, Silvestri L, de la Cal MA |title=Infection Control in the Intensive Care Unit |url=https://archive.org/details/infectioncontrol0000unse |url-access=limited |edition=2nd |location=Milan; New York |publisher=Springer |pages=337–377 |isbn=88-470-0185-4}}</ref> Antibiotic treatment may affect the results of microbiology tests, but a diagnosis may be made on the basis of blood-cultures and clinical examination.<ref name="Coant_1992">{{cite journal | vauthors = Coant PN, Kornberg AE, Duffy LC, Dryja DM, Hassan SM | title = Blood culture results as determinants in the organism identification of bacterial meningitis | journal = Pediatric Emergency Care | volume = 8 | issue = 4 | pages = 200–205 | date = August 1992 | pmid = 1381091 | doi = 10.1097/00006565-199208000-00006 }}</ref> |
|||
==Epidemiology== |
|||
''N. meningitidis'' is a major cause of illness, developmental impairment and death during childhood in industrialized countries and has been responsible for epidemics in Africa and in Asia. Every year, about 2,500 to 3,500 people become infected with ''N. meningitidis'' in the US, with a frequency of about 1 in 100,000. Children younger than five years are at greatest risk, followed by teenagers of high school age. Rates in the [[African meningitis belt]] were as high as 1 in 1,000 to 1 in 100 before introduction of a vaccine in 2010.<ref name="Mola_2008">{{cite journal | vauthors = Mola SJ, Nield LS, Weisse ME |year=2008 |title=Treatment and Prevention of ''N. meningitidis'' Infection |journal=Infections in Medicine |volume=25 |issue=3 |pages=128–133 |issn=0749-6524}}</ref> The [[incidence (epidemiology)|incidence]] of meningococcal disease is highest among infants (children younger than one year old) whose immune system is relatively immature. In industrialized countries there is a second peak of incidence in young adults, who are congregating closely, living in dormitories or smoking.<ref name=GencoWetzler>{{cite book | veditors = Genco CA, Wetzler L | year = 2010 |title=Neisseria: Molecular Mechanisms of Pathogenesis |publisher=Caister Academic Press |isbn=978-1-904455-51-6}}</ref> Vaccine development is ongoing.<ref name="Baarda_2015">{{cite journal | vauthors = Baarda BI, Sikora AE | title = Proteomics of Neisseria gonorrhoeae: the treasure hunt for countermeasures against an old disease | journal = Frontiers in Microbiology | volume = 6 | pages = 1190 | year = 2015 | pmid = 26579097 | pmc = 4620152 | doi = 10.3389/fmicb.2015.01190 | doi-access = free }}</ref> |
|||
It is spread through saliva and other respiratory secretions during coughing, sneezing, kissing, and chewing on toys. Inhalation of respiratory droplets from a [[Asymptomatic carrier|carrier]] which may be someone who is themselves in the early stages of disease can [[transmission (medicine)|transmit]] the bacteria. Close contact with a carrier is the predominant [[risk factor]]. Other risk factors include a weakened general or local immune response, such as a recent upper respiratory infection, smoking, and [[complement deficiency]]. The [[incubation period]] is short, from 2 to 10 days. In susceptible individuals, ''N. meningitidis'' may invade the bloodstream and cause a [[systemic infection]], sepsis, [[disseminated intravascular coagulation]], breakdown of circulation, and [[septic shock]].{{cn|date=February 2023}} |
|||
==History== |
|||
In 1884 [[Ettore Marchiafava]] and [[Angelo Celli]] first observed the bacterium inside cells in the [[cerebral spinal fluid]] (CSF).<ref name="Stephens_2009">{{cite journal | vauthors = Stephens DS | title = Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis | journal = Vaccine | volume = 27 | issue = Suppl 2 | pages = B71–B77 | date = June 2009 | pmid = 19477055 | pmc = 2712446 | doi = 10.1016/j.vaccine.2009.04.070 }}</ref> In 1887 [[Anton Weichselbaum]] isolated the bacterium from the CSF of patients with bacterial meningitis.<ref name="Bhalla_2006">{{cite journal | vauthors = Manchanda V, Gupta S, Bhalla P | title = Meningococcal disease: history, epidemiology, pathogenesis, clinical manifestations, diagnosis, antimicrobial susceptibility and prevention | journal = Indian Journal of Medical Microbiology | volume = 24 | issue = 1 | pages = 7–19 | date = January 2006 | pmid = 16505549 | doi = 10.1016/S0255-0857(21)02464-6 | doi-access = free }}</ref> He named the bacterium ''Diplococcus intracellularis meningitidis''.<ref name="Stephens_2009"/> |
|||
==Biotechnology== |
|||
Components from ''Neisseria meningitidis'' are being harnessed in biotechnology. Its Cas9 enzyme is a useful tool in [[CRISPR gene editing]] because the enzyme is small and has distinct targeting features to the commonly used enzyme from ''[[Streptococcus pyogenes]]''.<ref name="Hou_2013">{{cite journal | vauthors = Hou Z, Zhang Y, Propson NE, Howden SE, Chu LF, Sontheimer EJ, Thomson JA | title = Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 110 | issue = 39 | pages = 15644–15649 | date = September 2013 | pmid = 23940360 | pmc = 3785731 | doi = 10.1073/pnas.1313587110 | doi-access = free | bibcode = 2013PNAS..11015644H }}</ref> The cell-surface protein FrpC from ''Neisseria meningitidis'' has been engineered to allow covalent coupling between proteins, because it generates a reactive anhydride when exposed to calcium.<ref name="Scheu_2021">{{cite journal | vauthors = Scheu AH, Lim SY, Metzner FJ, Mohammed S, Howarth M | title = NeissLock provides an inducible protein anhydride for covalent targeting of endogenous proteins | journal = Nature Communications | volume = 12 | issue = 1 | pages = 717 | date = January 2021 | pmid = 33514717 | pmc = 7846742 | doi = 10.1038/s41467-021-20963-5 | bibcode = 2021NatCo..12..717S }}</ref> The bacterium also expresses unique enzymes able to cleave [[IgA]] antibodies.<ref name="Spoerry_2021">{{cite journal | vauthors = Spoerry C, Karlsson J, Aschtgen MS, Loh E | title = ''Neisseria meningitidis'' IgA1-specific serine protease exhibits novel cleavage activity against IgG3 | journal = Virulence | volume = 12 | issue = 1 | pages = 389–403 | date = December 2021 | pmid = 33459578 | pmc = 7834093 | doi = 10.1080/21505594.2021.1871822 }}</ref> |
|||
== See also == |
|||
* [[DNA uptake sequence]] DNA taken up by Neisseria |
|||
* [[NmVac4-A/C/Y/W-135]] polysaccharide vaccine |
|||
* [[Sara Branham Matthews]] microbiologist |
|||
* [[Shwartzman phenomenon]] |
|||
* [[Sepsis]] |
|||
{{Clear}} |
|||
== References == |
== References == |
||
{{ |
{{Reflist|30em}} |
||
{{Gram-negative bacterial diseases}} |
|||
== External links == |
|||
[[Category:Proteobacteria]] |
|||
* {{cite web |title=''Neisseria meningitidis'' |work=NCBI Taxonomy Browser |url=https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=487 |id=487}} |
|||
* [https://bacdive.dsmz.de/strain/10473 Type strain of ''Neisseria meningitidis'' at Bac''Dive'' - the Bacterial Diversity Metadatabase] |
|||
{{Authority control}} |
|||
{{Portal bar|Biology}} |
|||
{{Taxonbar|from=Q154625}} |
|||
[[Category:Gram-negative bacteria]] |
|||
[[cs:Neisseria meningitidis]] |
|||
[[Category:Pathogenic bacteria]] |
|||
[[de:Meningokokken]] |
|||
[[Category:Polysaccharide encapsulated bacteria]] |
|||
[[es:Neisseria meningitidis]] |
|||
[[Category:Neisseriales]] |
|||
[[fr:Neisseria meningitidis]] |
|||
[[Category:Bacteria described in 1901]] |
|||
[[id:Meningokokus]] |
|||
[[it:Neisseria meningitidis]] |
|||
[[nl:Meningokok]] |
|||
[[ja:髄膜炎菌]] |
|||
[[no:Meningokokk]] |
|||
[[pl:Dwoinka zapalenia opon mózgowo-rdzeniowych]] |
|||
[[pt:Neisseria meningitidis]] |
|||
[[ru:Менингококк]] |
|||
[[fi:Meningokokki]] |
|||
[[vi:Meningococcus]] |
|||
[[tr:Neisseria meningitidis]] |
|||
[[zh:腦膜炎雙球菌]] |
|||
Latest revision as of 00:18, 14 November 2024
| Neisseria meningitidis | |
|---|---|
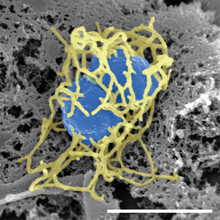
| |
| Scanning electron micrograph of a single N. meningitidis cell (colorized in blue) with its adhesive pili (colorized in yellow). The scale bar corresponds to 1 µm. | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Betaproteobacteria |
| Order: | Neisseriales |
| Family: | Neisseriaceae |
| Genus: | Neisseria |
| Species: | N. meningitidis
|
| Binomial name | |
| Neisseria meningitidis Albrecht & Ghon 1901
| |
Neisseria meningitidis, often referred to as the meningococcus, is a Gram-negative bacterium that can cause meningitis and other forms of meningococcal disease such as meningococcemia, a life-threatening sepsis. The bacterium is referred to as a coccus because it is round, and more specifically a diplococcus because of its tendency to form pairs.
About 10% of adults are carriers of the bacteria in their nasopharynx.[1] As an exclusively human pathogen, it causes developmental impairment and death in about 10% of cases. It causes the only form of bacterial meningitis known to occur epidemically, mainly in Africa and Asia. It occurs worldwide in both epidemic and endemic form.[2]
N. meningitidis is spread through saliva and respiratory secretions during coughing, sneezing, kissing, chewing on toys and through sharing a source of fresh water. It has also been reported to be transmitted through oral sex and cause urethritis in men.[3] It infects its host cells by sticking to them with long thin extensions called pili and the surface-exposed proteins Opa and Opc and has several virulence factors.
Signs and symptoms
[edit]Meningococcus can cause meningitis and other forms of meningococcal disease.[4] It initially produces general symptoms like fatigue, fever, and headache and can rapidly progress to neck stiffness, coma and death in 10% of cases. Petechiae occur in about 50% of cases. Chance of survival is highly correlated with blood cortisol levels, with lower levels prior to steroid administration corresponding with increased patient mortality.[5] Symptoms of meningococcal meningitis are easily confused with those caused by other bacteria, such as Haemophilus influenzae and Streptococcus pneumoniae.[6][7] Suspicion of meningitis is a medical emergency and immediate medical assessment is recommended. Current guidance in the United Kingdom is that if a case of meningococcal meningitis or septicaemia (infection of the blood) is suspected, intravenous antibiotics should be given and the ill person admitted to the hospital.[8] This means that laboratory tests may be less likely to confirm the presence of Neisseria meningitidis as the antibiotics will dramatically lower the number of bacteria in the body. The UK guidance is based on the idea that the reduced ability to identify the bacteria is outweighed by reduced chance of death.[citation needed]
Septicaemia caused by Neisseria meningitidis has received much less public attention than meningococcal meningitis even though septicaemia has been linked to infant deaths.[6] Meningococcal septicaemia typically causes a purpuric rash, that does not lose its color when pressed with a glass slide ("non-blanching") and does not cause the classical symptoms of meningitis. This means the condition may be ignored by those not aware of the significance of the rash. Septicaemia carries an approximate 50% mortality rate over a few hours from initial onset.[citation needed]
Other severe complications include Waterhouse–Friderichsen syndrome, a massive, usually bilateral, hemorrhage into the adrenal glands caused by fulminant meningococcemia, adrenal insufficiency, and disseminated intravascular coagulation.[7] Not all instances of a purpura-like rash are due to meningococcal septicaemia; other possible causes, such as idiopathic thrombocytopenic purpura (ITP; a platelet disorder) and Henoch–Schönlein purpura, also need prompt investigation.[citation needed]
Microbiology
[edit]N. meningitidis is a Gram-negative diplococcus since it has an outer and inner membranes with a thin layer of peptidoglycan in between. It is 0.6–1.0 micrometers in size. It tests positive for the enzyme cytochrome c oxidase.[9]
Habitat
[edit]N. meningitidis is a part of the normal nonpathogenic flora in the nasopharynx of up to 8–25% of adults.[10] It colonizes and infects only humans, and has never been isolated from other animals. This is thought to result from the bacterium's inability to get iron from sources other than human transferrin and lactoferrin.[6]
Subtypes
[edit]Disease-causing strains are classified according to the antigenic structure of their polysaccharide capsule.[11] Serotype distribution varies markedly around the world.[10] Among the 13 identified capsular types of N. meningitidis, six (A, B, C, W135, X, and Y) account for most disease cases worldwide.[12] Type A has been the most prevalent in Africa and Asia, but is rare/practically absent in North America. In the United States, serogroup B is the predominant cause of disease and mortality, followed by serogroup C. The multiple subtypes have hindered development of a universal vaccine for meningococcal disease.[citation needed]
Pathogenesis
[edit]Virulence
[edit]Lipooligosaccharide (LOS) is a component of the outer membrane of N. meningitidis. This acts as an endotoxin and is responsible for septic shock and hemorrhage due to the destruction of red blood cells.[13] Other virulence factors include a polysaccharide capsule which prevents host phagocytosis and aids in evasion of the host immune response. Adhesion is another key virulence strategy to successfully invade host cell. There are several known proteins that are involved in adhesion and invasion, or mediate interactions with specific host cell receptors. These include the Type IV pilin adhesin which mediates attachment of the bacterium to the epithelial cells of the nasopharynx,[14][15] surface-exposed Opa and Opc proteins which mediate interactions with specific host cell receptors,[16] and NadA which is involved in adhesion.[17]
Pathogenic meningococci that have invaded into the bloodstream must be able to survive in the new niche, this is facilitated by acquisition and utilisation of iron (FetA and Hmbr), resisting intracellular oxidative killing by producing catalase and superoxide dismutase and ability to avoid complement mediated killing (fHbp).[16] Meningococci produce an IgA protease, an enzyme that cleaves IgA class antibodies and thus allows the bacteria to evade a subclass of the humoral immune system.[citation needed]
A hypervirulent strain was discovered in China. Its impact is yet to be determined.[7]
Complement inhibition
[edit]Factor H binding protein (fHbp) that is exhibited in N. meningitidis and some commensal species is the main inhibitor of the alternative complement pathway. fHbp protects meningococci from complement-mediated death in human serum experiments, but has also been shown to protect meningococci from antimicrobial peptides in vitro. Factor H binding protein is key to the pathogenesis of N. meningitidis, and is, therefore, important as a potential vaccine candidate.[18] Porins are also an important factor for complement inhibition for both pathogenic and commensal species. Porins are important for nutrient acquisition. Porins are also recognized by TLR2, they bind complement factors (C3b, C4b, factor H, and C4bp (complement factor 4b-binding protein)). Cooperation with pili for CR3-mediated internalization is another function of porins. Ability to translocate into host cells and modulate reactive oxygen species production and apoptosis is made possible by porins, as well. Strains of the same species can express different porins.[citation needed]
Genome
[edit]At least 8 complete genomes of Neisseria meningitidis strains have been determined which encode about 2,100 to 2,500 proteins.[19]
The genome of strain MC58 (serogroup B) has 2,272,351 base pairs. When sequenced in 2000, it was found to contain 2158 open reading frames (ORFs). Of these, a biological function was predicted for 1158 (53.7%). There were three major islands of horizontal DNA transfer found. Two encode proteins involved in pathogenicity. The third island only codes for hypothetical proteins. They also found more genes that undergo phase variation than any pathogen then known. Phase variation is a mechanism that helps the pathogen to evade the immune system of the host.[20]
The genome size of strain H44/76 is 2.18 Mb, and encodes 2,480 open reading frames (ORFs), compared to 2.27 Mb and 2,465 ORFs for MC58.[19] Both strains have a GC content of 51.5%.[19] A comparison with MC58 showed that four genes are uniquely present in H44/76 and nine genes are only present in MC58. Of all ORFs in H44/76, 2,317 (93%) show more than 99% sequence identity.[19]
The complete genome sequence of strain NMA510612 (serogroup A) consists of one circular chromosome with a size of 2,188,020 bp, and the average GC content is 51.5%. The chromosome is predicted to possess 4 rRNA operons, 163 insertion elements (IS), 59 tRNAs, and 2,462 ORFs.[21]
There is a public database available for N. meningitidis core genome Multilocus sequence typing (cgMLST). Available at: Neisseria typing
Genetic transformation
[edit]Genetic transformation is the process by which a recipient bacterial cell takes up DNA from a neighboring cell and integrates this DNA into the recipient's genome by recombination. In N. meningitidis, DNA transformation requires the presence of short DNA sequences (9–10 mers residing in coding regions) of the donor DNA. These sequences are called DNA uptake sequences (DUSs). Specific recognition of these sequences is mediated by a type IV pilin.[22] In N. meningitidis DUSs occur at a significantly higher density in genes involved in DNA repair and recombination (as well as in restriction-modification and replication) than in other annotated gene groups. The over-representation of DUS in DNA repair and recombination genes may reflect the benefit of maintaining the integrity of the DNA repair and recombination machinery by preferentially taking up genome maintenance genes, that could replace their damaged counterparts in the recipient cell.[23]
N. meningititis colonizes the nasopharyngeal mucosa, which is rich in macrophages. Upon their activation, macrophages produce superoxide (O2−) and hydrogen peroxide (H2O2). Thus N. meningitidis is likely to encounter oxidative stress during its life cycle.[24] Consequently, an important benefit of genetic transformation to N. meningitidis may be the maintenance of the recombination and repair machinery of the cell that removes oxidative DNA damages such as those caused by reactive oxygen. This is consistent with the more general idea that transformation benefits bacterial pathogens by facilitating repair of DNA damages produced by the oxidative defenses of the host during infection.[25]
Meningococci population is extensively diverse genetically, this is due to horizontal gene transfers while in the nasophanrynx. Gene transfer can occur within and between genomes of Neisseria species, and it is the main mechanism of acquiring new traits.[26] This is facilitated by the natural competence of the meningococci to take up foreign DNA.[17] The commensal species of Neisseria can act as a reservoir of genes that can be acquired; for example, this is how capsule switching can occur as a means of hiding from the immune system.[17] An invasive N. meningitidis strain of serogroup C broke out in Nigeria in 2013 – the strain was a new sequence type, ST-10217 determined by multilocus sequence typing.[27] It was determined that a commensal strain of N. meningitidis acquired an 8-kb prophage, the meningococcal disease-associated island (MDAΦ), previously associated with hyper-invasiveness;[17] and the full serogroup C capsule operon, thus becoming a hypervirulent strain. This illustrates how hypervirulent strains can arise from non-pathgenic strains due to the high propensity of gene transfers and DNA uptake by N. meningitidis.[27]
Diagnosis
[edit]

A small amount of cerebrospinal fluid (CSF) is sent to the laboratory as soon as possible for analysis. The diagnosis is suspected, when Gram-negative diplococci are seen on Gram stain of a centrifuged sample of CSF; sometimes they are located inside white blood cells. The microscopic identification takes around 1–2 hours after specimen arrival in the laboratory.[4]
The gold standard of diagnosis is microbiological isolation of N. meningitidis by growth from a sterile body fluid, which could be CSF or blood.[7] Diagnosis is confirmed when the organism has grown, most often on a chocolate agar plate, but also on Thayer–Martin agar. To differentiate any bacterial growth from other species a small amount of a bacterial colony is Gram stained and tested for oxidase and catalase. Gram negative diplococci that are oxidase and catalase positive are then tested for fermentation of the following carbohydrates: maltose, sucrose, and glucose. N. meningitidis will ferment glucose and maltose. Finally, serology determines the subgroup of the N. meningitidis, which is important for epidemiological surveillance purposes; this may often only be done in specialized laboratories.[citation needed]
The above tests take a minimum of 48–72 hours turnaround time for growing the organism, and up to a week more for serotyping. Growth can and often does fail, either because antibiotics have been given preemptively, or because specimens have been inappropriately transported, as the organism is extremely susceptible to antibiotics and fastidious in its temperature, CO2 and growth medium requirements.[citation needed]
Polymerase chain reaction (PCR) tests where available, mostly in industrialized countries, have been increasingly used; PCR can rapidly identify the organism, and works even after antibiotics have been given.[7]
Prevention
[edit]All recent contacts of the infected patient over the seven days before onset should receive medication to prevent them from contracting the infection. This especially includes young children and their child caregivers or nursery-school contacts, as well as anyone who had direct exposure to the patient through kissing, sharing utensils, or medical interventions such as mouth-to-mouth resuscitation. Anyone who frequently ate, slept or stayed at the patient's home during the seven days before the onset of symptom, or those who sat beside the patient on an airplane flight or classroom for eight hours or longer, should also receive chemoprophylaxis. The agent of choice is usually oral rifampicin for a few days.[7]
Receiving a dose of the meningococcal vaccine before traveling to a country in the "meningitis belt" or having a booster meningitis vaccine, normally five years apart could prevent someone from getting an infection from the pathogen.[28]
Vaccination
[edit]United States
[edit]A number of vaccines are available in the U.S. to prevent meningococcal disease. Some of the vaccines cover serogroup B, while others cover A, C, W, and Y.[29] The Centers for Disease Control and Prevention (CDC) recommends all teenagers receive MenACWY vaccine and booster, with optional MenB. MenACWY and MenB are also recommended for people of other ages with various medical conditions and social risk factors.[29]
A meningococcal polysaccharide vaccine (MPSV4) has been available since the 1970s and is the only meningococcal vaccine licensed for people older than 55. MPSV4 may be used in people 2–55 years old if the MCV4 vaccines are not available or contraindicated. Two meningococcal conjugate vaccines (MCV4) are licensed for use in the U.S. The first conjugate vaccine was licensed in 2005, the second in 2010. Conjugate vaccines are the preferred vaccine for people 2 through 55 years of age. It is indicated in those with impaired immunity, such as nephrotic syndrome or splenectomy.[citation needed]
In June 2012, the U.S. Food and Drug Administration (FDA) approved a combination vaccine against two types of meningococcal diseases and Hib disease for infants and children 6 weeks to 18 months old. The vaccine, Menhibrix, was designed to prevent disease caused by Neisseria meningitidis serogroups C and Y, and Haemophilus influenzae type b (Hib). It was the first meningococcal vaccine that could be given to infants as young as six weeks old.[30]
In October 2014 the FDA approved the first vaccine effective against serogroup B, named Trumenba, for use in 10- to 25-year-old individuals.[31]
Africa
[edit]In 2010, the Meningitis Vaccine Project introduced a vaccine called MenAfriVac in the African meningitis belt. It was made by generic drug maker Serum Institute of India and cost 50 U.S. cents per injection. Beginning in Burkina Faso in 2010, it has been given to 215 million people across Benin, Cameroon, Chad, Ivory Coast, Ethiopia, Ghana, Mali, Niger, Mauritania, Nigeria, Senegal, Sudan, Togo and Gambia.[32] The vaccination campaign has resulted in near-elimination of serogroup A meningitis from the participating countries.[33]
Treatment
[edit]Persons with confirmed N. meningitidis infection should be hospitalized immediately for treatment with antibiotics. Because meningococcal disease can disseminate very rapidly, a single dose of intramuscular antibiotic is often given at the earliest possible opportunity, even before hospitalization, if disease symptoms look suspicious enough.[7] Third-generation cephalosporin antibiotics (i.e. cefotaxime, ceftriaxone) should be used to treat a suspected or culture-proven meningococcal infection before antibiotic susceptibility results are available.[34] Clinical practice guidelines endorse empirical treatment in the event a lumbar puncture to collect cerebrospinal fluid (CSF) for laboratory testing cannot first be performed.[34][35] Antibiotic treatment may affect the results of microbiology tests, but a diagnosis may be made on the basis of blood-cultures and clinical examination.[36]
Epidemiology
[edit]N. meningitidis is a major cause of illness, developmental impairment and death during childhood in industrialized countries and has been responsible for epidemics in Africa and in Asia. Every year, about 2,500 to 3,500 people become infected with N. meningitidis in the US, with a frequency of about 1 in 100,000. Children younger than five years are at greatest risk, followed by teenagers of high school age. Rates in the African meningitis belt were as high as 1 in 1,000 to 1 in 100 before introduction of a vaccine in 2010.[7] The incidence of meningococcal disease is highest among infants (children younger than one year old) whose immune system is relatively immature. In industrialized countries there is a second peak of incidence in young adults, who are congregating closely, living in dormitories or smoking.[37] Vaccine development is ongoing.[38]
It is spread through saliva and other respiratory secretions during coughing, sneezing, kissing, and chewing on toys. Inhalation of respiratory droplets from a carrier which may be someone who is themselves in the early stages of disease can transmit the bacteria. Close contact with a carrier is the predominant risk factor. Other risk factors include a weakened general or local immune response, such as a recent upper respiratory infection, smoking, and complement deficiency. The incubation period is short, from 2 to 10 days. In susceptible individuals, N. meningitidis may invade the bloodstream and cause a systemic infection, sepsis, disseminated intravascular coagulation, breakdown of circulation, and septic shock.[citation needed]
History
[edit]In 1884 Ettore Marchiafava and Angelo Celli first observed the bacterium inside cells in the cerebral spinal fluid (CSF).[39] In 1887 Anton Weichselbaum isolated the bacterium from the CSF of patients with bacterial meningitis.[40] He named the bacterium Diplococcus intracellularis meningitidis.[39]
Biotechnology
[edit]Components from Neisseria meningitidis are being harnessed in biotechnology. Its Cas9 enzyme is a useful tool in CRISPR gene editing because the enzyme is small and has distinct targeting features to the commonly used enzyme from Streptococcus pyogenes.[41] The cell-surface protein FrpC from Neisseria meningitidis has been engineered to allow covalent coupling between proteins, because it generates a reactive anhydride when exposed to calcium.[42] The bacterium also expresses unique enzymes able to cleave IgA antibodies.[43]
See also
[edit]- DNA uptake sequence DNA taken up by Neisseria
- NmVac4-A/C/Y/W-135 polysaccharide vaccine
- Sara Branham Matthews microbiologist
- Shwartzman phenomenon
- Sepsis
References
[edit]- ^ Hitchcock PJ, Robinson Jr EN, McGee ZA, Koomey JM (1993). "Neisseriae: Gonococcus and Meningococcus". In Schaechter M, Medoff G, Eisenstein BI (eds.). Mechanisms of Microbial Disease (2nd ed.). Baltimore: Williams & Wilkins. p. 231. ISBN 9780683076066.
- ^ "Meningococcal Disease in Other Countries". National Center for Immunization and Respiratory Diseases. Meningococcal Disease. Centers for Disease Control and Prevention. 31 May 2019. Archived from the original on 2020-10-30.
- ^ Bazan JA, Peterson AS, Kirkcaldy RD, Briere EC, Maierhofer C, Turner AN, et al. (June 2016). "Notes from the Field: Increase in Neisseria meningitidis-Associated Urethritis Among Men at Two Sentinel Clinics - Columbus, Ohio, and Oakland County, Michigan, 2015". MMWR. Morbidity and Mortality Weekly Report. 65 (21). Centers for Disease Control and Prevention: 550–552. doi:10.15585/mmwr.mm6521a5. PMC 5390329. PMID 27254649.
- ^ a b Ryan KJ, Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). New York: McGraw-Hill. pp. 329–333. ISBN 978-0-8385-8529-0.
- ^ Riordan FA, Thomson AP, Ratcliffe JM, Sills JA, Diver MJ, Hart CA (October 1999). "Admission cortisol and adrenocorticotrophic hormone levels in children with meningococcal disease: evidence of adrenal insufficiency?". Critical Care Medicine. 27 (10): 2257–2261. doi:10.1097/00003246-199910000-00032. PMID 10548217.
- ^ a b c Pollard AJ, Maiden MC, eds. (2001). Meningococcal Vaccines: Methods and Protocols. Totowa, NJ: Humana Press. ISBN 978-0-89603-801-1.
- ^ a b c d e f g h Mola SJ, Nield LS, Weisse ME (2008). "Treatment and Prevention of N. meningitidis Infection". Infections in Medicine. 25 (3): 128–133. ISSN 0749-6524.
- ^ "Guidance for public health management of meningococcal disease in the UK" (pdf). gov.uk. Public Health England. 2019. GW-599. Archived from the original on 2020-12-31. Retrieved 2021-01-31.
- ^ "Neisseria meningitidis". National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Characteristics of N. gonorrhoeae and Related Species. Centers for Disease Control and Prevention. 31 March 2017. Archived from the original on 2020-10-20.
- ^ a b Stephens DS, Greenwood B, Brandtzaeg P (June 2007). "Epidemic meningitis, meningococcaemia, and Neisseria meningitidis". Lancet. 369 (9580): 2196–2210. CiteSeerX 10.1.1.324.1736. doi:10.1016/S0140-6736(07)61016-2. PMID 17604802. S2CID 16951072.
- ^ Hamborsky J, Kroger A, Wolfe S, eds. (2015). "Meningococcal Disease". Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Centers for Disease Control and Prevention. Retrieved 2021-01-31.
- ^ Harrison LH (March 2010). "Epidemiological profile of meningococcal disease in the United States". Clinical Infectious Diseases. 50 (Suppl 2): S37–S44. doi:10.1086/648963. PMC 2820831. PMID 20144015.
- ^ Griffiss JM, Schneider H, Mandrell RE, Yamasaki R, Jarvis GA, Kim JJ, et al. (1988). "Lipooligosaccharides: the principal glycolipids of the neisserial outer membrane". Reviews of Infectious Diseases. 10 (Suppl 2): S287–S295. doi:10.1093/cid/10.supplement_2.s287. JSTOR 4454586. PMID 2460911.
- ^ Jarrell KF, ed. (2009). Pili and Flagella: Current Research and Future Trends. Caister Academic Press. ISBN 978-1-904455-48-6.
- ^ Ullrich M, ed. (2009). Bacterial Polysaccharides: Current Innovations and Future Trends. Caister Academic Press. ISBN 978-1-904455-45-5.
- ^ a b Hill DJ, Griffiths NJ, Borodina E, Virji M (February 2010). "Cellular and molecular biology of Neisseria meningitidis colonization and invasive disease". Clinical Science. 118 (9): 547–564. doi:10.1042/CS20090513. PMC 2830671. PMID 20132098.
- ^ a b c d Caugant DA, Brynildsrud OB (February 2020). "Neisseria meningitidis: using genomics to understand diversity, evolution and pathogenesis". Nature Reviews. Microbiology. 18 (2): 84–96. doi:10.1038/s41579-019-0282-6. PMID 31705134. S2CID 207960642.
- ^ McNeil LK, Zagursky RJ, Lin SL, Murphy E, Zlotnick GW, Hoiseth SK, et al. (June 2013). "Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease". Microbiology and Molecular Biology Reviews. 77 (2): 234–252. doi:10.1128/MMBR.00056-12. PMC 3668674. PMID 23699256.
- ^ a b c d Piet JR, Huis in 't Veld RA, van Schaik BD, van Kampen AH, Baas F, van de Beek D, et al. (May 2011). "Genome sequence of Neisseria meningitidis serogroup B strain H44/76". Journal of Bacteriology. 193 (9): 2371–2372. doi:10.1128/JB.01331-10. PMC 3133077. PMID 21378179.
- ^ Tettelin H, Saunders NJ, Heidelberg J, Jeffries AC, Nelson KE, Eisen JA, et al. (March 2000). "Complete genome sequence of Neisseria meningitidis serogroup B strain MC58". Science. 287 (5459): 1809–1815. Bibcode:2000Sci...287.1809.. doi:10.1126/science.287.5459.1809. JSTOR 3074600. PMID 10710307.
- ^ Zhang Y, Yang J, Xu L, Zhu Y, Liu B, Shao Z, et al. (May 2014). "Complete Genome Sequence of Neisseria meningitidis Serogroup A Strain NMA510612, Isolated from a Patient with Bacterial Meningitis in China". Genome Announcements. 2 (3): e00360–14. doi:10.1128/genomeA.00360-14. PMC 4014685. PMID 24812217.
- ^ Cehovin A, Simpson PJ, McDowell MA, Brown DR, Noschese R, Pallett M, et al. (February 2013). "Specific DNA recognition mediated by a type IV pilin". Proceedings of the National Academy of Sciences of the United States of America. 110 (8): 3065–3070. Bibcode:2013PNAS..110.3065C. doi:10.1073/pnas.1218832110. PMC 3581936. PMID 23386723.
- ^ Davidsen T, Rødland EA, Lagesen K, Seeberg E, Rognes T, Tønjum T (2004). "Biased distribution of DNA uptake sequences towards genome maintenance genes". Nucleic Acids Research. 32 (3): 1050–1058. doi:10.1093/nar/gkh255. PMC 373393. PMID 14960717.
- ^ Dyet K, Moir J (February 2006). "Effect of combined oxidative and nitrosative stress on Neisseria meningitidis". Biochemical Society Transactions. 34 (Pt 1): 197–199. doi:10.1042/BST0340197. PMID 16417521. S2CID 10045867.
- ^ Michod RE, Bernstein H, Nedelcu AM (May 2008). "Adaptive value of sex in microbial pathogens". Infection, Genetics and Evolution. 8 (3): 267–285. CiteSeerX 10.1.1.211.2248. doi:10.1016/j.meegid.2008.01.002. PMID 18295550.
- ^ Hanage WP, Fraser C, Spratt BG (March 2005). "Fuzzy species among recombinogenic bacteria". BMC Biology. 3 (1): 6. doi:10.1186/1741-7007-3-6. PMC 554772. PMID 15752428.
- ^ a b Brynildsrud OB, Eldholm V, Bohlin J, Uadiale K, Obaro S, Caugant DA (May 2018). "Acquisition of virulence genes by a carrier strain gave rise to the ongoing epidemics of meningococcal disease in West Africa". Proceedings of the National Academy of Sciences of the United States of America. 115 (21): 5510–5515. doi:10.1073/pnas.1802298115. PMC 6003489. PMID 29735685.
- ^ "Prevention". National Center for Immunization and Respiratory Diseases. Meningococcal Disease. Centers for Disease Control and Prevention. 31 May 2019. Archived from the original on 2020-10-30.
- ^ a b "Meningococcal Vaccination: What Everyone Should Know". National Center for Immunization and Respiratory Diseases. U.S. Centers for Disease Control and Prevention. 29 July 2019. Archived from the original on 2021-01-23.
- ^ "FDA approves new combination vaccine that protects children against two bacterial diseases" (Press release). U.S. Food and Drug Administration. 14 June 2012. Archived from the original on 2017-01-19.
{{cite press release}}: CS1 maint: unfit URL (link) - ^ "First vaccine approved by FDA to prevent serogroup B Meningococcal disease" (Press release). U.S. Food and Drug Administration. 29 October 2014. Archived from the original on 2021-01-22.
- ^ Kelland K (9 January 2015). "Tailor-made vaccine set to banish Africa's meningitis epidemics". Reuters. Thomson Reuters. Archived from the original on 2019-12-18. Retrieved 31 January 2021.
- ^ "Meningitis A nearly eliminated in Africa through vaccination, reaching more than 235 million people" (Press release). Ministerial Conference on Immunization in Africa. 22 February 2016. Archived from the original on 2017-02-02.
- ^ a b Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ (November 2004). "Practice guidelines for the management of bacterial meningitis". Clinical Infectious Diseases. 39 (9): 1267–1284. doi:10.1086/425368. PMID 15494903.
- ^ Valles J, Ferrer R, Fernández-Viladrich P (2005). "Bloodstream Infections Including Endocarditis and Meningitis". In van Saene HK, Silvestri L, de la Cal MA (eds.). Infection Control in the Intensive Care Unit (2nd ed.). Milan; New York: Springer. pp. 337–377. ISBN 88-470-0185-4.
- ^ Coant PN, Kornberg AE, Duffy LC, Dryja DM, Hassan SM (August 1992). "Blood culture results as determinants in the organism identification of bacterial meningitis". Pediatric Emergency Care. 8 (4): 200–205. doi:10.1097/00006565-199208000-00006. PMID 1381091.
- ^ Genco CA, Wetzler L, eds. (2010). Neisseria: Molecular Mechanisms of Pathogenesis. Caister Academic Press. ISBN 978-1-904455-51-6.
- ^ Baarda BI, Sikora AE (2015). "Proteomics of Neisseria gonorrhoeae: the treasure hunt for countermeasures against an old disease". Frontiers in Microbiology. 6: 1190. doi:10.3389/fmicb.2015.01190. PMC 4620152. PMID 26579097.
- ^ a b Stephens DS (June 2009). "Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis". Vaccine. 27 (Suppl 2): B71–B77. doi:10.1016/j.vaccine.2009.04.070. PMC 2712446. PMID 19477055.
- ^ Manchanda V, Gupta S, Bhalla P (January 2006). "Meningococcal disease: history, epidemiology, pathogenesis, clinical manifestations, diagnosis, antimicrobial susceptibility and prevention". Indian Journal of Medical Microbiology. 24 (1): 7–19. doi:10.1016/S0255-0857(21)02464-6. PMID 16505549.
- ^ Hou Z, Zhang Y, Propson NE, Howden SE, Chu LF, Sontheimer EJ, Thomson JA (September 2013). "Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis". Proceedings of the National Academy of Sciences of the United States of America. 110 (39): 15644–15649. Bibcode:2013PNAS..11015644H. doi:10.1073/pnas.1313587110. PMC 3785731. PMID 23940360.
- ^ Scheu AH, Lim SY, Metzner FJ, Mohammed S, Howarth M (January 2021). "NeissLock provides an inducible protein anhydride for covalent targeting of endogenous proteins". Nature Communications. 12 (1): 717. Bibcode:2021NatCo..12..717S. doi:10.1038/s41467-021-20963-5. PMC 7846742. PMID 33514717.
- ^ Spoerry C, Karlsson J, Aschtgen MS, Loh E (December 2021). "Neisseria meningitidis IgA1-specific serine protease exhibits novel cleavage activity against IgG3". Virulence. 12 (1): 389–403. doi:10.1080/21505594.2021.1871822. PMC 7834093. PMID 33459578.
External links
[edit]- "Neisseria meningitidis". NCBI Taxonomy Browser. 487.
- Type strain of Neisseria meningitidis at BacDive - the Bacterial Diversity Metadatabase
