Titin: Difference between revisions
Restored revision 1253407200 by MacaroniPizzaHotDog (talk): Already covered in the linquistics section below |
|||
| Line 1: | Line 1: | ||
{{Short description|Largest known protein in human muscles}} |
|||
{{Distinguish|Tintin}} |
|||
{{distinguish|Titan (disambiguation){{!}}Titan|Tintin (disambiguation){{!}}Tintin|Titian}} |
|||
<!-- |
<!-- |
||
DO NOT ADD THE FULL CHEMICAL NAME OF TITIN |
DO NOT ADD THE FULL CHEMICAL NAME OF TITIN INTO THIS ARTICLE. |
||
----------------------------------------------------------------------------- |
|||
This has been discussed extensively on this article's talk page and the consensus is to *not* provide the full chemical name of titin here. Please see the talk page for details. |
|||
This comment has been discussed extensively on this article's talk page and the consensus is to NOT to provide the full chemical name of titin here. Please see the talk page for details. |
|||
--> |
--> |
||
{{Infobox_gene}} |
|||
[[File:Cardiac sarcomere structure.png|thumb|330x330px|[[Cardiac#Microanatomy|Cardiac sarcomere]] structure, featuring titin]] |
|||
{{PBB|geneid=7273}} |
|||
[[File:Mammalian Titin Structure from the relaxed thick filament.tif|thumb|Reconstruction of the thin (green) and thick filament from mammalian cardiac tissue. Myosin is in blue, MyBP-C is in yellow, and titin is in two shades of red (dark red for titin-alpha and light red for titin-beta).]] |
|||
'''Titin''', also known as '''connectin''', is the largest known [[protein]] that is important in the contraction of [[striated muscle]] tissues.<ref>{{OMIM|188840}}</ref><ref name= "Entrez_ 7273">{{cite web | title = Entrez Gene: TTN titin| url = http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=7273| accessdate = }}</ref> |
|||
'''Titin'''<ref name="Wang_1979"/> {{IPAc-en|ˈ|t|aɪ|t|ɪ|n}} (contraction for <u>Tit</u>an prote<u>in</u>) (also called '''connectin''') is a [[protein]] that in humans is encoded by the ''TTN'' [[gene]].<ref name="Entrez_ 7273">{{cite web |date=April 2018 |title=TTN, the human gene for Titin |url=https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=ShowDetailView&TermToSearch=7273 |url-status=live |access-date= |website=[[National Library of Medicine]]; [[National Center for Biotechnology Information]] |archive-date=2010-03-07 |archive-url=https://web.archive.org/web/20100307070431/http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=7273}}</ref><ref name="Labeit_1990">{{cite journal | vauthors = Labeit S, Barlow DP, Gautel M, Gibson T, Holt J, Hsieh CL, Francke U, Leonard K, Wardale J, Whiting A | display-authors = 6 | title = A regular pattern of two types of 100-residue motif in the sequence of titin | journal = Nature | volume = 345 | issue = 6272 | pages = 273–276 | date = May 1990 | pmid = 2129545 | doi = 10.1038/345273a0 | url = https://ui.adsabs.harvard.edu/abs/1990Natur.345..273L/abstract | access-date = 8 May 2022 | url-status = live | s2cid = 4240433 | bibcode = 1990Natur.345..273L | archive-url = https://web.archive.org/web/20211022223626/https://ui.adsabs.harvard.edu/abs/1990Natur.345..273L/abstract | archive-date = 22 October 2021 }}</ref> The protein, which is over 1 [[Micrometre|μm]] in length,<ref>{{cite web | vauthors = Lee EH |url=http://www.ks.uiuc.edu/Research/z1z2/ |title=The Chain-like Elasticity of Titin |publisher=Theoretical and Computational Biophysics Group, University of Illinois |access-date=25 September 2014 |archive-date=13 February 2021 |archive-url=https://web.archive.org/web/20210213032405/http://www.ks.uiuc.edu/Research/z1z2/ |url-status=live}}</ref> functions as a molecular [[Spring (device)|spring]] that is responsible for the passive elasticity of [[muscle]]. It comprises 244 individually folded [[protein domain]]s connected by unstructured [[peptide]] sequences.<ref name="Labeit_1995">{{cite journal | vauthors = Labeit S, Kolmerer B | title = Titins: giant proteins in charge of muscle ultrastructure and elasticity | journal = Science | volume = 270 | issue = 5234 | pages = 293–296 | date = October 1995 | pmid = 7569978 | doi = 10.1126/science.270.5234.293 | url = https://ui.adsabs.harvard.edu/abs/1995Sci...270..293L/abstract | access-date = 8 May 2022 | url-status = live | s2cid = 20470843 | bibcode = 1995Sci...270..293L | archive-url = https://web.archive.org/web/20210302130853/https://ui.adsabs.harvard.edu/abs/1995Sci...270..293L/abstract | archive-date = 2 March 2021 }}</ref> These domains [[denaturation (biochemistry)|unfold]] when the protein is stretched and [[protein folding|refold]] when the tension is removed.<ref name="Minajeva_2001">{{cite journal | vauthors = Minajeva A, Kulke M, Fernandez JM, Linke WA | title = Unfolding of titin domains explains the viscoelastic behavior of skeletal myofibrils | journal = Biophysical Journal | volume = 80 | issue = 3 | pages = 1442–1451 | date = March 2001 | pmid = 11222304 | pmc = 1301335 | doi = 10.1016/S0006-3495(01)76116-4 | bibcode = 2001BpJ....80.1442M | author-link2 = Matthew Kulke }}</ref> |
|||
Titin is important in the contraction of [[striated muscle tissue]]s. It connects the [[Myofibril#Appearance|Z disc]] to the [[Myofibril#Appearance|M line]] in the [[sarcomere]]. The protein contributes to force transmission at the Z disc and resting tension in the [[Myofibril#Appearance|I band]] region.<ref name="Itoh-Satoh_2002">{{cite journal | vauthors = Itoh-Satoh M, Hayashi T, Nishi H, Koga Y, Arimura T, Koyanagi T, Takahashi M, Hohda S, Ueda K, Nouchi T, Hiroe M, Marumo F, Imaizumi T, Yasunami M, Kimura A | display-authors = 6 | title = Titin mutations as the molecular basis for dilated cardiomyopathy | journal = Biochemical and Biophysical Research Communications | volume = 291 | issue = 2 | pages = 385–393 | date = February 2002 | pmid = 11846417 | doi = 10.1006/bbrc.2002.6448 }}</ref> It limits the range of motion of the sarcomere in tension, thus contributing to the passive stiffness of muscle. Variations in the sequence of titin between different types of striated muscle ([[Cardiac muscle|cardiac]] or [[Skeletal muscle|skeletal]]) have been correlated with differences in the mechanical properties of these muscles.<ref name= "Entrez_ 7273"/><ref>{{OMIM|188840}}</ref> |
|||
==Structure== |
|||
Titin is the largest known protein, consisting of 34,350 [[amino acid]]s. The [[molecular weight]] of the mature protein is approximately 2,993,442.763 [[atomic mass unit|Da]],<ref>[http://www.chemie.fu-berlin.de/cgi-bin/molform?C132983H211861N36149O40883S693 Result of Molecular Weight Calculation<!-- Bot generated title -->]</ref> and it has a theoretical [[isoelectric point]] of 6.01.<ref>{{cite web |url=http://us.expasy.org/cgi-bin/pi_tool1?Q10466@noft@ |title=ExPASy-calculated pI for titin |accessdate=2007-08-26 |format= |work=}}</ref> The protein's empirical [[chemical formula]] is C<sub>132983</sub>H<sub>211861</sub>N<sub>36149</sub>O<sub>40883</sub>S<sub>693</sub>. It has a theoretical [[instability index]] (II) of 39.69, indicating that it would be stable in a [[test tube]]. The protein's [[in vivo]] [[half-life]], the time it takes for half of the amount of protein in a cell to disappear after its synthesis in the cell, is predicted to be approximately 30 hours (in [[mammalian]] [[reticulocyte]]s).<ref>{{cite web|url=http://us.expasy.org/cgi-bin/niceprot.pl?Q10466|title=Swiss-Prot Protein knowledgebase, main entry|accessdate=2006-05-04}}</ref> |
|||
Titin is the third most abundant protein in muscle (after [[myosin]] and [[actin]]), and an adult human contains approximately 0.5 kg of titin.<ref name="Labeit_1997">{{cite journal | vauthors = Labeit S, Kolmerer B, Linke WA | title = The giant protein titin. Emerging roles in physiology and pathophysiology | journal = Circulation Research | volume = 80 | issue = 2 | pages = 290–294 | date = February 1997 | pmid = 9012751 | doi = 10.1161/01.RES.80.2.290 }}</ref> With its length of ~27,000 to ~35,000 [[amino acid]]s (depending on the [[Alternative splicing|splice isoform]]), titin is the largest known [[protein]].<ref name="Opitz_2003">{{cite journal | vauthors = Opitz CA, Kulke M, Leake MC, Neagoe C, Hinssen H, Hajjar RJ, Linke WA | title = Damped elastic recoil of the titin spring in myofibrils of human myocardium | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 100 | issue = 22 | pages = 12688–12693 | date = October 2003 | pmid = 14563922 | pmc = 240679 | doi = 10.1073/pnas.2133733100 | bibcode = 2003PNAS..10012688O | doi-access = free }}</ref> Furthermore, the gene for titin contains the largest number of [[exon]]s (363) discovered in any single gene,<ref name="Bang_2001">{{cite journal | vauthors = Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S | display-authors = 6 | title = The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-disc to I-band Linking system | journal = Circulation Research | volume = 89 | issue = 11 | pages = 1065–1072 | date = November 2001 | pmid = 11717165 | doi = 10.1161/hh2301.100981 | doi-access = free }}</ref> as well as the longest single exon (17,106 [[Base pair|bp]]). |
|||
==Function== |
|||
Titin is a large abundant protein of striated muscle. The protein is divided into two regions: |
|||
;[[N-terminus|N-terminal]] I-band: is the elastic part of the molecule, contains two regions of tandem [[immunoglobulin]] domains on either side of a PEVK region that is rich in proline, glutamate, valine and lysine |
|||
;[[C-terminus|C-terminal]] A-band: thought to act as a protein-ruler, contains a mixture of immunoglobulin and [[fibronectin]] repeats, and possesses [[kinase]] activity. |
|||
== Discovery == |
|||
A N-terminal Z-disc region and a C-terminal M-line region bind to the Z-line and M-line of the [[sarcomere]] respectively so that a |
|||
Titin interacts with many [[sarcomere|sarcomeric]] proteins including:<ref name="pmid11717165">{{cite journal | author = Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S | title = The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system | journal = Circ. Res. | volume = 89 | issue = 11 | pages = 1065–72 | year = 2001 | month = November | pmid = 11717165 | doi = 10.1161/hh2301.100981| url = http://circres.ahajournals.org/cgi/pmidlookup?view=long&pmid=11717165 }}</ref>your face |
|||
In 1954, Reiji Natori proposed the existence of an elastic structure in muscle fiber to account for the return to the resting state when muscles are stretched and then released.<ref name = "Natori_1954">{{cite journal |vauthors=Natori R |title=Skinned Fibres of Skeletal Muscle and the Mechanism of Muscle Contraction-A Chronological Account of the Author's Investigations into Muscle Physiology |journal=Jikeikai Medical Journal |year=1954 |volume=54 |issue=1 |url=http://ir.jikei.ac.jp/bitstream/10328/3410/1/54-1-51.pdf |access-date=2014-09-09 |archive-date=2016-06-03 |archive-url=https://web.archive.org/web/20160603072338/http://ir.jikei.ac.jp/bitstream/10328/3410/1/54-1-51.pdf |url-status=dead |
|||
|hdl=10328/3410}}</ref> In 1977, Koscak Maruyama and coworkers isolated an elastic protein from muscle fiber that they called connectin.<ref name="Maruyama_1997">{{cite journal | vauthors = Maruyama K, Matsubara S, Natori R, Nonomura Y, Kimura S | title = Connectin, an elastic protein of muscle. Characterization and Function | journal = Journal of Biochemistry | volume = 82 | issue = 2 | pages = 317–337 | date = August 1977 | pmid = 914784 }}</ref> Two years later, [[Kuan Wang]] and coworkers identified a doublet band on [[gel electrophoresis|electrophoresis gel]] corresponding to a high molecular weight, elastic protein that they named titin.<ref name="Wang_1979">{{cite journal | vauthors = Wang K, McClure J, Tu A | title = Titin: major myofibrillar components of striated muscle | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 76 | issue = 8 | pages = 3698–3702 | date = August 1979 | pmid = 291034 | pmc = 383900 | doi = 10.1073/pnas.76.8.3698 | doi-access = free | bibcode = 1979PNAS...76.3698W }}</ref><ref name="Maruyama_1994">{{cite journal | vauthors = Maruyama K | title = Connectin, an elastic protein of striated muscle | journal = Biophysical Chemistry | volume = 50 | issue = 1–2 | pages = 73–85 | date = May 1994 | pmid = 8011942 | doi = 10.1016/0301-4622(94)85021-6 }}</ref> |
|||
In 1990, Siegfried Labeit isolated a partial [[cDNA]] clone of titin.<ref name="Labeit_1990"/> Five years later, Labeit and Bernhard Kolmerer determined the cDNA sequence of human cardiac titin.<ref name="Labeit_1995"/> In 2001, Labeit and colleagues determined the complete sequence of the human titin gene.<ref name="Bang_2001"/><ref name = "OMIM" >{{OMIM|188840|Titin}}</ref> |
|||
== Genetics == |
|||
The human gene encoding for titin is located on the long arm of chromosome 2 and contains 363 exons, which together code for 38,138 [[amino acid]] [[Residue (chemistry)#Biochemistry|residues]] (4200 kDa).<ref name="Bang_2001"/> Within the gene are found a large number of PEVK (proline-glutamate-valine-lysine -abundant [[structural motif]]s) exons 84 to 99 nucleotides in length, which code for conserved 28- to 33-residue motifs that may represent structural units of the titin PEVK spring. The number of PEVK motifs in the titin gene appears to have increased during evolution, apparently modifying the genomic region responsible for titin's spring properties.<ref>{{cite journal | vauthors = Freiburg A, Trombitas K, Hell W, Cazorla O, Fougerousse F, Centner T, Kolmerer B, Witt C, Beckmann JS, Gregorio CC, Granzier H, Labeit S | display-authors = 6 | title = Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity | journal = Circulation Research | volume = 86 | issue = 11 | pages = 1114–1121 | date = June 2000 | pmid = 10850961 | doi = 10.1161/01.res.86.11.1114 | doi-access = free }}</ref> |
|||
== Isoforms == |
|||
A number of titin [[protein isoform|isoform]]s are produced in different striated muscle tissues as a result of [[alternative splicing]].<ref name="url_UniProt_Q8WZ42)"/> All but one of these isoforms are in the range of ~27,000 to ~36,000 amino acid residues in length. The exception is the small cardiac novex-3 isoform, which is only 5,604 amino acid residues in length. The following table lists the known titin isoforms: |
|||
{| class="wikitable" |
|||
|- |
|||
! Isoform !! Alias/description !! Length ([[amino acid |aa]]) !! Molecular weight ([[atomic mass unit|Da]]) |
|||
|- |
|||
| Q8WZ42-1 || The "canonical" sequence || 34,350 || 3,816,030 |
|||
|- |
|||
| Q8WZ42-2 || || 34,258 || 3,805,708 |
|||
|- |
|||
| Q8WZ42-3 || Small cardiac N2-B || 26,926 || 2,992,939 |
|||
|- |
|||
| Q8WZ42-4 || Soleus || 33,445 || 3,716,027 |
|||
|- |
|||
| Q8WZ42-5 || || 32,900 || 3,653,085 |
|||
|- |
|||
| Q8WZ42-6 || Small cardiac novex-3 || 5,604 || 631,567 |
|||
|- |
|||
| Q8WZ42-7 || Cardiac novex-2 || 33,615 || 3,734,648 |
|||
|- |
|||
| Q8WZ42-8 || Cardiac novex-1 || 34,475 || 3,829,846 |
|||
|- |
|||
| Q8WZ42-9 || || 27,118 || 3,013,957 |
|||
|- |
|||
| Q8WZ42-10 || || 27,051 || 3,006,755 |
|||
|- |
|||
| Q8WZ42-11 || || 33,423 || 3,713,600 |
|||
|- |
|||
| Q8WZ42-12 || || 35,991 || 3,994,625 |
|||
|- |
|||
| Q8WZ42-13 || || 34,484 || 3,831,069 |
|||
|} |
|||
== Structure == |
|||
Titin is the largest known protein; its human variant consists of 34,350 [[amino acid]]s, with the [[molecular weight]] of the mature "canonical" isoform of the protein being approximately 3,816,030.05 [[atomic mass unit|Da]].<ref name="urlExPASy_human"/> Its mouse homologue is even larger, comprising 35,213 amino acids with a molecular weight of 3,906,487.6 [[atomic mass unit|Da]].<ref name="urlExPASy_mouse">{{cite web |url=http://www.expasy.org/cgi-bin/protparam1?A2ASS6@noft@ |title=ProtParam for mouse titin |work=ExPASy Proteomics Server |publisher=Swiss Institute of Bioinformatics |access-date=2010-05-06}}</ref> It has a theoretical [[isoelectric point]] of 6.02.<ref name="urlExPASy_human"/> The protein's [[empirical formula|empirical]] [[chemical formula]] is C<sub>169,719</sub>H<sub>270,466</sub>N<sub>45,688</sub>O<sub>52,238</sub>S<sub>911</sub>.<ref name="urlExPASy_human">{{cite web |url=http://web.expasy.org/cgi-bin/protparam/protparam1?Q8WZ42@1-34350@ |title=ProtParam for human titin |work=ExPASy Proteomics Server |publisher=Swiss Institute of Bioinformatics |access-date=2011-07-25 |archive-date=2019-09-18 |archive-url=https://web.archive.org/web/20190918231125/https://web.expasy.org/cgi-bin/protparam/protparam1?Q8WZ42@1-34350@ |url-status=live}}</ref> It has a theoretical [[instability index]] (II) of 42.38, classifying the protein as unstable.<ref name="urlExPASy_human"/> The protein's [[in vivo]] [[half-life]], the time it takes for half of the amount of protein in a cell to break down after its synthesis in the cell, is predicted to be approximately 30 hours (in [[mammalian]] [[reticulocyte]]s).<ref name="url_UniProt_Q8WZ42)">{{cite web |url=https://www.uniprot.org/uniprot/Q8WZ42 |title=Titin - Homo sapiens (Human) |work=Universal Protein Resource |publisher=UniProt Consortium |date=2010-10-05 |access-date=2010-10-15 |archive-date=2021-02-13 |archive-url=https://web.archive.org/web/20210213032327/https://www.uniprot.org/uniprot/Q8WZ42 |url-status=live}}</ref> |
|||
[[File:Titin_IG_Domains.jpg|thumb|256x256px|Titin Ig domains. a) Schematic of part of a sarcomere b) Structure of Ig domains c) Topology of Ig domains.<ref>{{cite journal | vauthors = Giganti D, Yan K, Badilla CL, Fernandez JM, Alegre-Cebollada J | title = Disulfide isomerization reactions in titin immunoglobulin domains enable a mode of protein elasticity | journal = Nature Communications | volume = 9 | issue = 1 | pages = 185 | date = January 2018 | pmid = 29330363 | pmc = 5766482 | doi = 10.1038/s41467-017-02528-7 | bibcode = 2018NatCo...9..185G }}</ref>|alt=]] |
|||
The Titin protein is located between the [[myosin]] thick filament and the Z disk.<ref name="Wang_1991">{{cite journal | vauthors = Wang K, McCarter R, Wright J, Beverly J, Ramirez-Mitchell R | title = Regulation of skeletal muscle stiffness and elasticity by titin isoforms: a test of the segmental extension model of resting tension | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 88 | issue = 16 | pages = 7101–7105 | date = August 1991 | pmid = 1714586 | pmc = 52241 | doi = 10.1073/pnas.88.16.7101 | doi-access = free | bibcode = 1991PNAS...88.7101W }}</ref> Titin consists primarily of a linear array of two types of modules, also referred to as [[protein domain]]s (244 copies in total): type I [[fibronectin type III domain]] (132 copies) and type II [[immunoglobulin domain]] (112 copies).<ref name="Labeit_1997" /><ref name="Labeit_1995"/> However, the exact number of these domains is different in different species. This linear array is further organized into two regions: |
|||
* [[N-terminus|N-terminal]] I-band: acts as the elastic part of the molecule and is composed mainly of type II modules. More specifically the I-band contains two regions of tandem type II immunoglobulin domains on either side of a ''PEVK region'' that is rich in [[proline]] (P), [[Glutamic acid|glutamate]] (E), [[valine]] (V) and [[lysine]] (K).<ref name="Wang_1991"/> |
|||
* [[C-terminus|C-terminal]] A-band: is thought to act as a protein-ruler and is composed of alternating type I (Fn3) and II (Ig) modules with super-repeat segments. These have been shown to align to the 43 nm axial repeats of myosin thick filaments with immunoglobulin domains correlating to myosin crowns.<ref name="Bennett_1996">{{cite journal | vauthors = Bennett PM, Gautel M | title = Titin domain patterns correlate with the axial disposition of myosin at the end of the thick filament | journal = Journal of Molecular Biology | volume = 259 | issue = 5 | pages = 896–903 | date = June 1996 | pmid = 8683592 | doi = 10.1006/jmbi.1996.0367 }}</ref> |
|||
The C-terminal region also contains a serine [[kinase]] domain<ref name="Mayans_1998" /><ref name="Higgins_1994">{{cite journal | vauthors = Higgins DG, Labeit S, Gautel M, Gibson TJ | title = The evolution of titin and related giant muscle proteins | journal = Journal of Molecular Evolution | volume = 38 | issue = 4 | pages = 395–404 | date = April 1994 | pmid = 8007007 | doi = 10.1007/BF00163156 | s2cid = 35756951 | bibcode = 1994JMolE..38..395H }}</ref> that is primarily known for adapting the muscle to mechanical strain.<ref name="Puchner_2008">{{cite journal | vauthors = Puchner EM, Alexandrovich A, Kho AL, Hensen U, Schäfer LV, Brandmeier B, Gräter F, Grubmüller H, Gaub HE, Gautel M | display-authors = 6 | title = Mechanoenzymatics of titin kinase | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 105 | issue = 36 | pages = 13385–13390 | date = September 2008 | pmid = 18765796 | pmc = 2527993 | doi = 10.1073/pnas.0805034105 | doi-access = free | bibcode = 2008PNAS..10513385P }}</ref> It is “stretch-sensitive” and helps repair overstretching of the sarcomere.<ref name="Myhre_2014">{{cite journal | vauthors = Myhre JL, Pilgrim D | title = A Titan but not necessarily a ruler: assessing the role of titin during thick filament patterning and assembly | journal = Anatomical Record | volume = 297 | issue = 9 | pages = 1604–1614 | date = September 2014 | pmid = 25125174 | doi = 10.1002/ar.22987 | s2cid = 32840140 | doi-access = free }}</ref> The N-terminal (the Z-disc end) contains a "Z repeat" that recognizes [[Actinin alpha 2]].<ref>{{cite web |title=Titin, Z repeat (IPR015129) < InterPro < EMBL-EBI |url=http://www.ebi.ac.uk/interpro/entry/IPR015129 |access-date=13 March 2019 |archive-date=13 February 2021 |archive-url=https://web.archive.org/web/20210213032409/http://www.ebi.ac.uk/interpro/entry/IPR015129 |url-status=live }}</ref> |
|||
The elasticity of the PEVK region has both [[entropic]] and [[enthalpic]] contributions and is characterized by a polymer [[persistence length]] and a [[Young's modulus|stretch modulus]].<ref>{{cite journal | vauthors = Linke WA, Ivemeyer M, Mundel P, Stockmeier MR, Kolmerer B | title = Nature of PEVK-titin elasticity in skeletal muscle | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 95 | issue = 14 | pages = 8052–8057 | date = July 1998 | pmid = 9653138 | pmc = 20927 | doi = 10.1073/pnas.95.14.8052 | doi-access = free | bibcode = 1998PNAS...95.8052L }}</ref> At low to moderate extensions PEVK elasticity can be modeled with a standard [[worm-like chain]] (WLC) model of [[entropic elasticity of an ideal chain|entropic elasticity]]. At high extensions PEVK stretching can be modeled with a modified WLC model that incorporates enthalpic elasticity. The difference between low-and high- stretch elasticity is due to electrostatic stiffening and [[hydrophobic effect]]s. |
|||
Embedded between the PEVK and Ig residues are N2A domains.<ref name="Buck_2014">{{cite journal | vauthors = Buck D, Smith JE, Chung CS, Ono Y, Sorimachi H, Labeit S, Granzier HL | title = Removal of immunoglobulin-like domains from titin's spring segment alters titin splicing in mouse skeletal muscle and causes myopathy | journal = The Journal of General Physiology | volume = 143 | issue = 2 | pages = 215–230 | date = February 2014 | pmid = 24470489 | pmc = 4001778 | doi = 10.1085/jgp.201311129 }}</ref> |
|||
== Evolution == |
|||
The titin domains have evolved from a common ancestor through many gene duplication events.<ref>{{cite journal | vauthors = Tskhovrebova L, Trinick J | title = Properties of titin immunoglobulin and fibronectin-3 domains | journal = The Journal of Biological Chemistry | volume = 279 | issue = 45 | pages = 46351–46354 | date = November 2004 | pmid = 15322090 | doi = 10.1074/jbc.r400023200 | url = http://www.jbc.org/content/279/45/46351 | access-date = 2018-12-16 | url-status = live | doi-access = free | archive-url = https://web.archive.org/web/20180603172142/http://www.jbc.org/content/279/45/46351 | archive-date = 2018-06-03 }}</ref> Domain duplication was facilitated by the fact that most domains are encoded by single exons. Other giant sarcomeric proteins made out of Fn3/Ig repeats include [[obscurin]] and [[MYOM1|myomesin]]. Throughout evolution, titin mechanical strength appears to decrease through the loss of disulfide bonds as the organism becomes heavier.<ref>{{cite journal | vauthors = Manteca A, Schönfelder J, Alonso-Caballero A, Fertin MJ, Barruetabeña N, Faria BF, Herrero-Galán E, Alegre-Cebollada J, De Sancho D, Perez-Jimenez R | display-authors = 6 | title = Mechanochemical evolution of the giant muscle protein titin as inferred from resurrected proteins | journal = Nature Structural & Molecular Biology | volume = 24 | issue = 8 | pages = 652–657 | date = August 2017 | pmid = 28671667 | doi = 10.1038/nsmb.3426 | s2cid = 54482436 | hdl = 20.500.12105/9931 | hdl-access = free }}</ref> |
|||
Titin A-band has homologs in invertebrates, such as twitchin (unc-22) and projectin, which also contain Ig and FNIII repeats and a protein kinase domain.<ref name="Myhre_2014" /> The gene duplication events took place independently but were from the same ancestral Ig and FNIII domains. It is said that the protein titin was the first to diverge out of the family.<ref name="Higgins_1994" /> ''Drosophila'' projectin, officially known as bent (''bt''), is associated with lethality by failing to escape the egg in some mutations as well as dominant changes in wing angles.<ref>{{cite journal | vauthors = Fyrberg CC, Labeit S, Bullard B, Leonard K, Fyrberg E | title = Drosophila projectin: relatedness to titin and twitchin and correlation with lethal(4) 102 CDa and bent-dominant mutants | journal = Proceedings. Biological Sciences | volume = 249 | issue = 1324 | pages = 33–40 | date = July 1992 | pmid = 1359548 | doi = 10.1098/rspb.1992.0080 | s2cid = 34408190 | bibcode = 1992RSPSB.249...33F }}</ref><ref>{{cite web |title=bent phenotype |url=http://cgslab.com/cgs1/phenotypes.cgi?n=17 |website=Classical Genetics Simulator |access-date=13 March 2019 |archive-date=11 February 2019 |archive-url=https://web.archive.org/web/20190211134649/http://www.cgslab.com/cgs1/phenotypes.cgi?n=17 |url-status=live}}</ref><ref>{{cite web |title=FlyBase Gene Report: Dmel\bt |url=https://flybase.org/reports/FBgn0005666 |website=flybase.org |access-date=13 March 2019 |archive-date=13 March 2019 |archive-url=https://web.archive.org/web/20190313224757/http://flybase.org/reports/FBgn0005666 |url-status=live}}</ref> |
|||
{{Pfam box|Pfam=PF06582|Name=Titin repeat|Symbol=Titin_Ig-rpts|InterPro=IPR010939}} |
|||
''Drosophila'' Titin, also known as Kettin or [[wikt:sallimus|sallimus]] (''sls''), is kinase-free. It has roles in the elasticity of both muscle and chromosomes. It is homologous to vertebrate titin I-band and contains Ig PEVK domains, the many repeats being a hot target for splicing.<ref>{{cite journal | vauthors = Machado C, Andrew DJ | title = D-Titin: a giant protein with dual roles in chromosomes and muscles | journal = The Journal of Cell Biology | volume = 151 | issue = 3 | pages = 639–652 | date = October 2000 | pmid = 11062264 | pmc = 2185597 | doi = 10.1083/jcb.151.3.639 | url = http://jcb.rupress.org/content/jcb/151/3/639.full.pdf | access-date = 2019-09-04 | url-status = live | archive-url = https://web.archive.org/web/20190904063833/http://jcb.rupress.org/content/jcb/151/3/639.full.pdf | archive-date = 2019-09-04 }}</ref> There also exists a titin homologue, ''ttn-1'', in ''[[C. elegans]]''.<ref>{{cite web |title=ttn-1 (gene) |url=https://wormbase.org/species/c_elegans/gene/WBGene00006436 |website=WormBase: Nematode Information Resource |access-date=13 March 2019 |archive-date=27 March 2018 |archive-url=https://web.archive.org/web/20180327031428/http://www.wormbase.org/species/c_elegans/gene/WBGene00006436 |url-status=live }}</ref> It has a kinase domain, some Ig/Fn3 repeats, and PEVT repeats that are similarly elastic.<ref>{{cite journal | vauthors = Forbes JG, Flaherty DB, Ma K, Qadota H, Benian GM, Wang K | title = Extensive and modular intrinsically disordered segments in C. elegans TTN-1 and implications in filament binding, elasticity and oblique striation | journal = Journal of Molecular Biology | volume = 398 | issue = 5 | pages = 672–689 | date = May 2010 | pmid = 20346955 | pmc = 2908218 | doi = 10.1016/j.jmb.2010.03.032 }}</ref> |
|||
== Function == |
|||
[[File:Sarcomere.svg|thumb|200px|Sliding filament model of muscle contraction. (Titin labeled at upper right.)]] |
|||
Titin is a large abundant protein of striated muscle. Titin's primary functions are to stabilize the thick filament, center it between the thin filaments, prevent overstretching of the sarcomere, and to recoil the sarcomere like a spring after it is stretched.<ref>{{cite book |vauthors=Saladin K |title=Anatomy & Physiology |date=2015 |edition=7th |publisher=McGraw Hill |page=401 |isbn=978-0-07-340371-7}}</ref> An N-terminal Z-disc region and a C-terminal M-line region bind to the Z-line and M-line of the [[sarcomere]], respectively, so that a single titin molecule spans half the length of a sarcomere. Titin also contains binding sites for muscle-associated proteins so it serves as an adhesion template for the assembly of contractile machinery in muscle cells. It has also been identified as a structural protein for [[chromosome]]s.<ref name="Machado_1998">{{cite journal | vauthors = Machado C, Sunkel CE, Andrew DJ | title = Human autoantibodies reveal titin as a chromosomal protein | journal = The Journal of Cell Biology | volume = 141 | issue = 2 | pages = 321–333 | date = April 1998 | pmid = 9548712 | pmc = 2148454 | doi = 10.1083/jcb.141.2.321 }}</ref><ref name="Machado_2000">{{cite book |vauthors=Machado C, Andrew DJ |chapter=Titin as a Chromosomal Protein |series=Advances in Experimental Medicine and Biology |title=Elastic Filaments of the Cell |volume=481 |pages=221–32; discussion 232–6 |year=2000 |pmid=10987075 |doi=10.1007/978-1-4615-4267-4_13 |isbn=978-1-4613-6916-5}}</ref> Considerable variability exists in the I-band, the M-line and the Z-disc regions of titin. Variability in the I-band region contributes to the differences in elasticity of different titin isoforms and, therefore, to the differences in elasticity of different muscle types. Of the many titin variants identified, five are described with complete transcript information available.<ref name= "Entrez_ 7273"/><ref name="Labeit_1990" /> |
|||
[[Dominance (genetics)|Dominant]] mutation in TTN causes predisposition to [[hernia]]s.<ref name="Mihailov_2017">{{cite journal | vauthors = Mihailov E, Nikopensius T, Reigo A, Nikkolo C, Kals M, Aruaas K, Milani L, Seepter H, Metspalu A | display-authors = 6 | title = Whole-exome sequencing identifies a potential TTN mutation in a multiplex family with inguinal hernia | journal = Hernia | volume = 21 | issue = 1 | pages = 95–100 | date = February 2017 | pmid = 27115767 | pmc = 5281683 | doi = 10.1007/s10029-016-1491-9 }}</ref> |
|||
Titin interacts with many [[sarcomere|sarcomeric]] proteins including:<ref name="Bang_2001"/> |
|||
* Z line region: [[TCAP (gene)|telethonin]] and [[ACTN1|alpha-actinin]] |
* Z line region: [[TCAP (gene)|telethonin]] and [[ACTN1|alpha-actinin]] |
||
* I band region: [[CAPN3|calpain-3]] and [[OBSCN|obscurin]] |
* I band region: [[CAPN3|calpain-3]] and [[OBSCN|obscurin]] |
||
* M line region: [[MYBPC3|myosin-binding protein C]], [[calmodulin 1]], [[CAPN3]], and [[TRIM63|MURF1]] |
* M line region: [[MYBPC3|myosin-binding protein C]], [[calmodulin 1]], [[CAPN3]], and [[TRIM63|MURF1]] |
||
== Clinical relevance == |
|||
your face |
|||
[[Mutation]]s anywhere within the unusually long sequence of this gene can cause [[premature stop codon]]s or other defects. Titin mutations are associated with hereditary [[myopathy]] with early respiratory failure,<ref>{{cite journal | vauthors = Pfeffer G, Elliott HR, Griffin H, Barresi R, Miller J, Marsh J, Evilä A, Vihola A, Hackman P, Straub V, Dick DJ, Horvath R, Santibanez-Koref M, Udd B, Chinnery PF | display-authors = 6 | title = Titin mutation segregates with hereditary myopathy with early respiratory failure | journal = Brain | volume = 135 | issue = Pt 6 | pages = 1695–1713 | date = June 2012 | pmid = 22577215 | pmc = 3359754 | doi = 10.1093/brain/aws102 }}</ref><ref>{{cite journal | vauthors = Ohlsson M, Hedberg C, Brådvik B, Lindberg C, Tajsharghi H, Danielsson O, Melberg A, Udd B, Martinsson T, Oldfors A | display-authors = 6 | title = Hereditary myopathy with early respiratory failure associated with a mutation in A-band titin | journal = Brain | volume = 135 | issue = Pt 6 | pages = 1682–1694 | date = June 2012 | pmid = 22577218 | doi = 10.1093/brain/aws103 | url = https://lup.lub.lu.se/search/ws/files/1591356/3900864.pdf | access-date = 2021-09-11 | url-status = live | archive-url = https://web.archive.org/web/20210911003000/https://lup.lub.lu.se/search/ws/files/1591356/3900864.pdf | archive-date = 2021-09-11 }}</ref> early-onset myopathy with fatal [[cardiomyopathy]],<ref>{{cite journal | vauthors = Carmignac V, Salih MA, Quijano-Roy S, Marchand S, Al Rayess MM, Mukhtar MM, Urtizberea JA, Labeit S, Guicheney P, Leturcq F, Gautel M, Fardeau M, Campbell KP, Richard I, Estournet B, Ferreiro A | display-authors = 6 | title = C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy | journal = Annals of Neurology | volume = 61 | issue = 4 | pages = 340–351 | date = April 2007 | pmid = 17444505 | doi = 10.1002/ana.21089 | s2cid = 6042810 }}</ref> core myopathy with heart disease, [[centronuclear myopathy]], [[limb-girdle muscular dystrophy]] type 2J,<ref name="Udd_2005"/> [[family|familial]] [[dilated cardiomyopathy]] 9,<ref name="Itoh-Satoh_2002"/><ref name="Siu_1999">{{cite journal | vauthors = Siu BL, Niimura H, Osborne JA, Fatkin D, MacRae C, Solomon S, Benson DW, Seidman JG, Seidman CE | display-authors = 6 | title = Familial dilated cardiomyopathy locus maps to chromosome 2q31 | journal = Circulation | volume = 99 | issue = 8 | pages = 1022–1026 | date = March 1999 | pmid = 10051295 | doi = 10.1161/01.cir.99.8.1022 | doi-access = free }}</ref> [[hypertrophic cardiomyopathy]] and [[distal muscular dystrophy|tibial muscular dystrophy]].<ref name="Richard_2002">{{cite journal | vauthors = Hackman P, Vihola A, Haravuori H, Marchand S, Sarparanta J, De Seze J, Labeit S, Witt C, Peltonen L, Richard I, Udd B | display-authors = 6 | title = Tibial muscular dystrophy is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal-muscle protein titin | journal = American Journal of Human Genetics | volume = 71 | issue = 3 | pages = 492–500 | date = September 2002 | pmid = 12145747 | pmc = 379188 | doi = 10.1086/342380 }}</ref> Further research also suggests that no genetically linked form of any [[dystrophy]] or myopathy can be safely excluded from being caused by a mutation on the TTN gene.<ref name="Udd_2005">{{cite journal | vauthors = Udd B, Vihola A, Sarparanta J, Richard I, Hackman P | title = Titinopathies and extension of the M-line mutation phenotype beyond distal myopathy and LGMD2J | journal = Neurology | volume = 64 | issue = 4 | pages = 636–642 | date = February 2005 | pmid = 15728284 | doi = 10.1212/01.WNL.0000151853.50144.82 | s2cid = 28801620 }}</ref> Truncating mutations in dilated cardiomyopathy patients are most commonly found in the A region; although truncations in the upstream I region might be expected to prevent translation of the A region entirely, [[alternative splicing]] creates some transcripts that do not encounter the premature stop codon, ameliorating its effect.<ref name="Hinson_2015">{{cite journal | vauthors = Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, Gorham J, Yang L, Schafer S, Sheng CC, Haghighi A, Homsy J, Hubner N, Church G, Cook SA, Linke WA, Chen CS, Seidman JG, Seidman CE | display-authors = 6 | title = HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy | journal = Science | volume = 349 | issue = 6251 | pages = 982–986 | date = August 2015 | pmid = 26315439 | pmc = 4618316 | doi = 10.1126/science.aaa5458 }}</ref> [[Splicing (genetics)|mRNA splicing factors]] such as RBM20 and [[KHDRBS3|SLM2]] ([[KHDRBS3]]) were shown to mediated alternative mRNA splicing of titin mRNA contributing to the development of [[heart failure]] due to [[Cardiomyopathy|cardiomyopathies]].<ref>{{cite journal | vauthors = Li S, Guo W, Dewey CN, Greaser ML | title = Rbm20 regulates titin alternative splicing as a splicing repressor | journal = Nucleic Acids Research | volume = 41 | issue = 4 | pages = 2659–2672 | date = February 2013 | pmid = 23307558 | pmc = 3575840 | doi = 10.1093/nar/gks1362 }}</ref><ref>{{cite journal | vauthors = Boeckel JN, Möbius-Winkler M, Müller M, Rebs S, Eger N, Schoppe L, Tappu R, Kokot KE, Kneuer JM, Gaul S, Bordalo DM, Lai A, Haas J, Ghanbari M, Drewe-Boss P, Liss M, Katus HA, Ohler U, Gotthardt M, Laufs U, Streckfuss-Bömeke K, Meder B | display-authors = 6 | title = SLM2 Is A Novel Cardiac Splicing Factor Involved in Heart Failure due to Dilated Cardiomyopathy | journal = Genomics, Proteomics & Bioinformatics | volume = 20 | issue = 1 | pages = 129–146 | date = February 2022 | pmid = 34273561 | pmc = 9510876 | doi = 10.1016/j.gpb.2021.01.006 | doi-access = free }}</ref> |
|||
==Linguistic significance== |
|||
Autoantibodies to titin are produced in patients with the autoimmune disease [[Myasthenia gravis]].<ref name="Skeie et al 2006">{{cite journal | vauthors = Skeie GO, Aarli JA, Gilhus NE | title = Titin and ryanodine receptor antibodies in myasthenia gravis | journal = Acta Neurologica Scandinavica. Supplementum | volume = 183 | issue = | pages = 19–23 | date = 2006 | pmid = 16637922 | doi = 10.1111/j.1600-0404.2006.00608.x | s2cid = 24972330 }}</ref> |
|||
== Interactions == |
|||
Titin has been shown to [[Protein-protein interaction|interact]] with: |
|||
{{div col|colwidth=20em}} |
|||
* [[ANK1]],<ref name="Kontrogianni-Konstantopoulos_2003">{{cite journal | vauthors = Kontrogianni-Konstantopoulos A, Bloch RJ | title = The hydrophilic domain of small ankyrin-1 interacts with the two N-terminal immunoglobulin domains of titin | journal = The Journal of Biological Chemistry | volume = 278 | issue = 6 | pages = 3985–3991 | date = February 2003 | pmid = 12444090 | doi = 10.1074/jbc.M209012200 | doi-access = free }}</ref> |
|||
* [[ANKRD1]],<ref name="Miller_2003">{{cite journal | vauthors = Miller MK, Bang ML, Witt CC, Labeit D, Trombitas C, Watanabe K, Granzier H, McElhinny AS, Gregorio CC, Labeit S | display-authors = 6 | title = The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules | journal = Journal of Molecular Biology | volume = 333 | issue = 5 | pages = 951–964 | date = November 2003 | pmid = 14583192 | doi = 10.1016/j.jmb.2003.09.012 }}</ref> |
|||
* [[ANKRD23]]<ref name= Miller_2003/> |
|||
* [[CAPN3]],<ref name="Ono_1998">{{cite journal | vauthors = Ono Y, Shimada H, Sorimachi H, Richard I, Saido TC, Beckmann JS, Ishiura S, Suzuki K | display-authors = 6 | title = Functional defects of a muscle-specific calpain, p94, caused by mutations associated with limb-girdle muscular dystrophy type 2A | journal = The Journal of Biological Chemistry | volume = 273 | issue = 27 | pages = 17073–17078 | date = July 1998 | pmid = 9642272 | doi = 10.1074/jbc.273.27.17073 | doi-access = free }}</ref><ref name="Sorimachi_1995">{{cite journal | vauthors = Sorimachi H, Kinbara K, Kimura S, Takahashi M, Ishiura S, Sasagawa N, Sorimachi N, Shimada H, Tagawa K, Maruyama K | display-authors = 6 | title = Muscle-specific calpain, p94, responsible for limb girdle muscular dystrophy type 2A, associates with connectin through IS2, a p94-specific sequence | journal = The Journal of Biological Chemistry | volume = 270 | issue = 52 | pages = 31158–31162 | date = December 1995 | pmid = 8537379 | doi = 10.1074/jbc.270.52.31158 | doi-access = free }}</ref> |
|||
* [[FHL2]],<ref name="Lange_2002">{{cite journal | vauthors = Lange S, Auerbach D, McLoughlin P, Perriard E, Schäfer BW, Perriard JC, Ehler E | title = Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2 | journal = Journal of Cell Science | volume = 115 | issue = Pt 24 | pages = 4925–4936 | date = December 2002 | pmid = 12432079 | doi = 10.1242/jcs.00181 | doi-access = free }}</ref> |
|||
* [[OBSCN]],<ref name="Young_2001">{{cite journal | vauthors = Young P, Ehler E, Gautel M | title = Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly | journal = The Journal of Cell Biology | volume = 154 | issue = 1 | pages = 123–136 | date = July 2001 | pmid = 11448995 | pmc = 2196875 | doi = 10.1083/jcb.200102110 }}</ref> |
|||
* [[Telethonin|TCAP]],<ref name="Gregorio_1998">{{cite journal | vauthors = Gregorio CC, Trombitás K, Centner T, Kolmerer B, Stier G, Kunke K, Suzuki K, Obermayr F, Herrmann B, Granzier H, Sorimachi H, Labeit S | display-authors = 6 | title = The NH2 terminus of titin spans the Z-disc: its interaction with a novel 19-kD ligand (T-cap) is required for sarcomeric integrity | journal = The Journal of Cell Biology | volume = 143 | issue = 4 | pages = 1013–1027 | date = November 1998 | pmid = 9817758 | pmc = 2132961 | doi = 10.1083/jcb.143.4.1013 }}</ref><ref name="Mayans_1998">{{cite journal | vauthors = Mayans O, van der Ven PF, Wilm M, Mues A, Young P, Fürst DO, Wilmanns M, Gautel M | display-authors = 6 | title = Structural basis for activation of the titin kinase domain during myofibrillogenesis | journal = Nature | volume = 395 | issue = 6705 | pages = 863–869 | date = October 1998 | pmid = 9804419 | doi = 10.1038/27603 | s2cid = 4426977 | bibcode = 1998Natur.395..863M }}</ref><ref name="Zou_2003">{{cite journal | vauthors = Zou P, Gautel M, Geerlof A, Wilmanns M, Koch MH, Svergun DI | title = Solution scattering suggests cross-linking function of telethonin in the complex with titin | journal = The Journal of Biological Chemistry | volume = 278 | issue = 4 | pages = 2636–2644 | date = January 2003 | pmid = 12446666 | doi = 10.1074/jbc.M210217200 | doi-access = free }}</ref><ref name="Mues_1998">{{cite journal | vauthors = Mues A, van der Ven PF, Young P, Fürst DO, Gautel M | title = Two immunoglobulin-like domains of the Z-disc portion of titin interact in a conformation-dependent way with telethonin | journal = FEBS Letters | volume = 428 | issue = 1–2 | pages = 111–114 | date = May 1998 | pmid = 9645487 | doi = 10.1016/S0014-5793(98)00501-8 | s2cid = 11786578 | doi-access = free }}</ref> and |
|||
* [[TRIM63]].<ref name="Centner_2001">{{cite journal | vauthors = Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S | display-authors = 6 | title = Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain | journal = Journal of Molecular Biology | volume = 306 | issue = 4 | pages = 717–726 | date = March 2001 | pmid = 11243782 | doi = 10.1006/jmbi.2001.4448 }}</ref> |
|||
{{Div col end}} |
|||
== Linguistic significance == |
|||
<!-- |
<!-- |
||
DO NOT ADD THE FULL CHEMICAL NAME OF TITIN TO THIS ARTICLE |
DO NOT ADD THE FULL CHEMICAL NAME OF TITIN TO THIS ARTICLE |
||
This has been discussed extensively on this article's talk page and the consensus is to *not* provide the full chemical name of titin here. Please see the talk page for details. |
This has been discussed extensively on this article's talk page and the consensus is to *not* provide the full chemical name of titin here. Please see the talk page for details. |
||
--> |
--> |
||
The name titin is derived from the Greek [[Titan (mythology)|Titan]] (a giant deity, anything of great size).<ref name="Wang_1979"/> |
|||
As the largest known protein, titin also has the longest [[International Union of Pure and Applied Chemistry nomenclature|IUPAC name]]. The full chemical name, which starts ''[[Methionyl]]...'' and ends ''...[[isoleucine]]'', contains 189,819 letters and is sometimes stated to be the [[Longest word in English|longest word in the English language]], or [[longest words|any language]].<ref name="CliffsNotes">{{cite web |url=http://www.cliffsnotes.com/WileyCDA/Section/id-305408,articleId-113603.html |title=What is the longest word in the English language? |format= |work=CliffsNotes.com |accessdate=2009-05-26}}</ref> However, professional dictionary writers regard generic names of [[chemical compound]]s as ''verbal [[Chemical formula|formulae]]'' rather than English words.<ref>{{cite web |
|||
| author = Oxford Word and Language Service team |
|||
| title = Ask the experts - What is the longest English word? |
|||
| publisher = AskOxford.com / [[Oxford University Press]] |
|||
| url = http://www.askoxford.com/asktheexperts/faq/aboutwords/longestword |
|||
| accessdate = 2008-01-13 }}</ref> |
|||
As the largest known protein, titin also has the longest [[IUPAC nomenclature of organic chemistry|IUPAC name]] of a protein. [[wikt:Appendix:List of protologisms/Long words/Titin|The full chemical name]] of the human canonical form of titin, which starts ''[[Methionine|methionyl]]...'' and ends ''...[[isoleucine]]'', contains 189,819 letters and is sometimes stated to be the [[Longest word in English|longest word in the English language]], or [[longest words|of any language]].<ref name="urlSarah McCulloch">{{cite web | url = http://www.sarahmcculloch.com/luminary-uprise/2009/longest-word/ | title = Longest word in English | vauthors = McCulloch S | work = Sarah McCulloch.com | date = December 2009 |archive-url=https://web.archive.org/web/20100114221953/http://www.sarahmcculloch.com/luminaryuprise/longest-word.html |archive-date=2010-01-14 | access-date = 2016-10-12 }}</ref> However, [[List of lexicographers|lexicographers]] regard generic names of [[chemical compound]]s as ''verbal [[Chemical formula|formulae]]'' rather than English words.<ref>{{cite web | author = Oxford Word and Language Service team | title = Ask the experts - What is the longest English word? | publisher = AskOxford.com / [[Oxford University Press]] | url = http://www.askoxford.com/asktheexperts/faq/aboutwords/longestword | access-date = 2008-01-13 | archive-url = https://web.archive.org/web/20080913173417/http://www.askoxford.com/asktheexperts/faq/aboutwords/longestword | archive-date = 2008-09-13 | url-status = dead}}</ref> |
|||
==Additional images== |
|||
<gallery> |
|||
Image:Sarcomere.svg|Sliding filament model of muscle contraction. (Titin labeled at upper right.) |
|||
</gallery> |
|||
== |
== See also == |
||
{{Reflist|2}} |
|||
* [[PKZILLA-1]] - new largest known protein with 45,212 amino acids |
|||
==Further reading== |
|||
{{refbegin | 2}} |
|||
{{PBB_Further_reading |
|||
| citations = |
|||
*{{cite journal | author=Kinbara K, Sorimachi H, Ishiura S, Suzuki K |title=Skeletal muscle-specific calpain, p49: structure and physiological function |journal=Biochem. Pharmacol. |volume=56 |issue= 4 |pages= 415–20 |year= 1998 |pmid= 9763216 |doi= }} |
|||
*{{cite journal | author=Kolmerer B, Witt CC, Freiburg A, ''et al.'' |title=The titin cDNA sequence and partial genomic sequences: insights into the molecular genetics, cell biology and physiology of the titin filament system |journal=Rev. Physiol. Biochem. Pharmacol. |volume=138 |issue= |pages= 19–55 |year= 1999 |pmid= 10396137 |doi=10.1007/BF02346659 }} |
|||
*{{cite journal | author=Trinick J, Tskhovrebova L |title=Titin: a molecular control freak |journal=Trends Cell Biol. |volume=9 |issue= 10 |pages= 377–80 |year= 1999 |pmid= 10481174 |doi=10.1016/S0962-8924(99)01641-4 }} |
|||
*{{cite journal | author=Sorimachi H, Ono Y, Suzuki K |title=Skeletal muscle-specific calpain, p94, and connectin/titin: their physiological functions and relationship to limb-girdle muscular dystrophy type 2A |journal=Adv. Exp. Med. Biol. |volume=481 |issue= |pages= 383–95; discussion 395–7 |year= 2000 |pmid= 10987085 |doi= }} |
|||
*{{cite journal | author=Tskhovrebova L, Trinick J |title=Role of titin in vertebrate striated muscle |journal=Philos. Trans. R. Soc. Lond., B, Biol. Sci. |volume=357 |issue= 1418 |pages= 199–206 |year= 2002 |pmid= 11911777 |doi= 10.1098/rstb.2001.1028 }} |
|||
*{{cite journal | author=Sela BA |title=[Titin: some aspects of the largest protein in the body] |journal=Harefuah |volume=141 |issue= 7 |pages= 631–5, 665 |year= 2002 |pmid= 12187564 |doi= }} |
|||
*{{cite journal | author=Tskhovrebova L, Trinick J |title=Properties of titin immunoglobulin and fibronectin-3 domains |journal=J. Biol. Chem. |volume=279 |issue= 45 |pages= 46351–4 |year= 2004 |pmid= 15322090 |doi= 10.1074/jbc.R400023200 }} |
|||
*{{cite journal | author=Wu Y, Labeit S, Lewinter MM, Granzier H |title=Titin: an endosarcomeric protein that modulates myocardial stiffness in DCM |journal=J. Card. Fail. |volume=8 |issue= 6 Suppl |pages= S276–86 |year= 2003 |pmid= 12555133 |doi= 10.1054/jcaf.2002.129278 }} |
|||
== References == |
|||
}} |
|||
{{Reflist|32em}} |
|||
{{reflist|group=Titin}} |
|||
== Further reading == |
|||
{{refbegin|32em}} |
|||
* {{cite journal | vauthors = Tskhovrebova L, Trinick J | title = Titin: properties and family relationships | journal = Nature Reviews. Molecular Cell Biology | volume = 4 | issue = 9 | pages = 679–689 | date = September 2003 | pmid = 14506471 | doi = 10.1038/nrm1198 | s2cid = 12293932 }} |
|||
* {{cite journal | vauthors = Kinbara K, Sorimachi H, Ishiura S, Suzuki K | title = Skeletal muscle-specific calpain, p49: structure and physiological function | journal = Biochemical Pharmacology | volume = 56 | issue = 4 | pages = 415–420 | date = August 1998 | pmid = 9763216 | doi = 10.1016/S0006-2952(98)00095-1 }} |
|||
* {{cite journal | vauthors = Kolmerer B, Witt CC, Freiburg A, Millevoi S, Stier G, Sorimachi H, Pelin K, Carrier L, Schwartz K, Labeit D, Gregorio CC, Linke WA, Labeit S | display-authors = 6 | title = The titin cDNA sequence and partial genomic sequences: insights into the molecular genetics, cell biology and physiology of the titin filament system | journal = Reviews of Physiology, Biochemistry and Pharmacology | volume = 138 | pages = 19–55 | year = 1999 | pmid = 10396137 | doi = 10.1007/BF02346659 }} |
|||
* {{cite journal | vauthors = Trinick J, Tskhovrebova L | title = Titin: a molecular control freak | journal = Trends in Cell Biology | volume = 9 | issue = 10 | pages = 377–380 | date = October 1999 | pmid = 10481174 | doi = 10.1016/S0962-8924(99)01641-4 }} |
|||
* {{cite book | vauthors = Sorimachi H, Ono Y, Suzuki K | chapter = Skeletal Muscle-Specific Calpain, p94, and Connectin/Titin: Their Physiological Functions and Relationship to Limb-Girdle Muscular Dystrophy Type 2A | series = Advances in Experimental Medicine and Biology | title = Elastic Filaments of the Cell | volume = 481 | pages = 383–95; discussion 395–7 | year = 2000 | pmid = 10987085 | doi = 10.1007/978-1-4615-4267-4_23 | isbn = 978-1-4613-6916-5 }} |
|||
* {{cite journal | vauthors = Tskhovrebova L, Trinick J | title = Role of titin in vertebrate striated muscle | journal = Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences | volume = 357 | issue = 1418 | pages = 199–206 | date = February 2002 | pmid = 11911777 | pmc = 1692937 | doi = 10.1098/rstb.2001.1028 }} |
|||
* {{cite journal | vauthors = Sela BA | title = [Titin: some aspects of the largest protein in the body] | journal = Harefuah | volume = 141 | issue = 7 | pages = 631–5, 665 | date = July 2002 | pmid = 12187564 }} |
|||
* {{cite journal | vauthors = Tskhovrebova L, Trinick J | title = Properties of titin immunoglobulin and fibronectin-3 domains | journal = The Journal of Biological Chemistry | volume = 279 | issue = 45 | pages = 46351–46354 | date = November 2004 | pmid = 15322090 | doi = 10.1074/jbc.R400023200 | doi-access = free }} |
|||
* {{cite journal | vauthors = Wu Y, Labeit S, Lewinter MM, Granzier H | title = Titin: an endosarcomeric protein that modulates myocardial stiffness in DCM | journal = Journal of Cardiac Failure | volume = 8 | issue = 6 Suppl | pages = S276–S286 | date = December 2002 | pmid = 12555133 | doi = 10.1054/jcaf.2002.129278 }} |
|||
{{refend}} |
{{refend}} |
||
==External links== |
== External links == |
||
{{Wiktionary pipe|Appendix:List of protologisms/Long words/Titin|the full chemical name of titin}} |
|||
* [https://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=hyper-card GeneReviews/NIH/NCBI/UW entry on Familial Hypertrophic Cardiomyopathy Overview] |
|||
* [https://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=udd GeneReviews/NCBI/NIH/UW entry on Udd Distal Myopathy, Tibial Muscular Dystrophy, Udd Myopathy] |
|||
* [https://www.ncbi.nlm.nih.gov/books/NBK83297/ GeneReviews/NIH/NCBI/UW entry on Salih Myopathy or Early-Onset Myopathy with Fatal Cardiomyopathy] |
|||
* [https://www.ebi.ac.uk/interpro/protein/Q8WZ42 InterPro domain organization of titin] |
|||
{{PDB Gallery|geneid=7273}} |
|||
{{Cytoskeletal proteins}} |
|||
{{Muscle tissue}} |
{{Muscle tissue}} |
||
{{Serine/threonine-specific protein kinases}} |
{{Serine/threonine-specific protein kinases}} |
||
{{Enzymes}} |
|||
{{Portal bar|Biology|border=no}} |
|||
{{NLM content}} |
{{NLM content}} |
||
[[Category:Structural proteins]] |
|||
<!-- The PBB_Controls template provides controls for Protein Box Bot, please see Template:PBB_Controls for details. --> |
|||
[[Category:Genes on human chromosome 2]] |
|||
{{PBB_Controls |
|||
[[Category:EC 2.7.11]] |
|||
| update_page = yes |
|||
[[Category:Muscular system]] |
|||
| require_manual_inspection = no |
|||
[[Category:Long words]] |
|||
| update_protein_box = yes |
|||
| update_summary = no |
|||
| update_citations = yes |
|||
}} |
|||
[[Category:Proteins]] |
|||
[[de:Titin]] |
|||
[[fr:Titine (protéine)]] |
|||
[[he:טיטין]] |
|||
[[ja:チチン]] |
|||
[[pl:Tytyna]] |
|||
[[pt:Titina]] |
|||
[[sv:Titin]] |
|||
[[vi:Titin]] |
|||
[[tr:Titin]] |
|||
[[uk:Тітін]] |
|||
Latest revision as of 16:56, 1 December 2024


Titin[5] /ˈtaɪtɪn/ (contraction for Titan protein) (also called connectin) is a protein that in humans is encoded by the TTN gene.[6][7] The protein, which is over 1 μm in length,[8] functions as a molecular spring that is responsible for the passive elasticity of muscle. It comprises 244 individually folded protein domains connected by unstructured peptide sequences.[9] These domains unfold when the protein is stretched and refold when the tension is removed.[10]
Titin is important in the contraction of striated muscle tissues. It connects the Z disc to the M line in the sarcomere. The protein contributes to force transmission at the Z disc and resting tension in the I band region.[11] It limits the range of motion of the sarcomere in tension, thus contributing to the passive stiffness of muscle. Variations in the sequence of titin between different types of striated muscle (cardiac or skeletal) have been correlated with differences in the mechanical properties of these muscles.[6][12]
Titin is the third most abundant protein in muscle (after myosin and actin), and an adult human contains approximately 0.5 kg of titin.[13] With its length of ~27,000 to ~35,000 amino acids (depending on the splice isoform), titin is the largest known protein.[14] Furthermore, the gene for titin contains the largest number of exons (363) discovered in any single gene,[15] as well as the longest single exon (17,106 bp).
Discovery
[edit]In 1954, Reiji Natori proposed the existence of an elastic structure in muscle fiber to account for the return to the resting state when muscles are stretched and then released.[16] In 1977, Koscak Maruyama and coworkers isolated an elastic protein from muscle fiber that they called connectin.[17] Two years later, Kuan Wang and coworkers identified a doublet band on electrophoresis gel corresponding to a high molecular weight, elastic protein that they named titin.[5][18]
In 1990, Siegfried Labeit isolated a partial cDNA clone of titin.[7] Five years later, Labeit and Bernhard Kolmerer determined the cDNA sequence of human cardiac titin.[9] In 2001, Labeit and colleagues determined the complete sequence of the human titin gene.[15][19]
Genetics
[edit]The human gene encoding for titin is located on the long arm of chromosome 2 and contains 363 exons, which together code for 38,138 amino acid residues (4200 kDa).[15] Within the gene are found a large number of PEVK (proline-glutamate-valine-lysine -abundant structural motifs) exons 84 to 99 nucleotides in length, which code for conserved 28- to 33-residue motifs that may represent structural units of the titin PEVK spring. The number of PEVK motifs in the titin gene appears to have increased during evolution, apparently modifying the genomic region responsible for titin's spring properties.[20]
Isoforms
[edit]A number of titin isoforms are produced in different striated muscle tissues as a result of alternative splicing.[21] All but one of these isoforms are in the range of ~27,000 to ~36,000 amino acid residues in length. The exception is the small cardiac novex-3 isoform, which is only 5,604 amino acid residues in length. The following table lists the known titin isoforms:
| Isoform | Alias/description | Length (aa) | Molecular weight (Da) |
|---|---|---|---|
| Q8WZ42-1 | The "canonical" sequence | 34,350 | 3,816,030 |
| Q8WZ42-2 | 34,258 | 3,805,708 | |
| Q8WZ42-3 | Small cardiac N2-B | 26,926 | 2,992,939 |
| Q8WZ42-4 | Soleus | 33,445 | 3,716,027 |
| Q8WZ42-5 | 32,900 | 3,653,085 | |
| Q8WZ42-6 | Small cardiac novex-3 | 5,604 | 631,567 |
| Q8WZ42-7 | Cardiac novex-2 | 33,615 | 3,734,648 |
| Q8WZ42-8 | Cardiac novex-1 | 34,475 | 3,829,846 |
| Q8WZ42-9 | 27,118 | 3,013,957 | |
| Q8WZ42-10 | 27,051 | 3,006,755 | |
| Q8WZ42-11 | 33,423 | 3,713,600 | |
| Q8WZ42-12 | 35,991 | 3,994,625 | |
| Q8WZ42-13 | 34,484 | 3,831,069 |
Structure
[edit]Titin is the largest known protein; its human variant consists of 34,350 amino acids, with the molecular weight of the mature "canonical" isoform of the protein being approximately 3,816,030.05 Da.[22] Its mouse homologue is even larger, comprising 35,213 amino acids with a molecular weight of 3,906,487.6 Da.[23] It has a theoretical isoelectric point of 6.02.[22] The protein's empirical chemical formula is C169,719H270,466N45,688O52,238S911.[22] It has a theoretical instability index (II) of 42.38, classifying the protein as unstable.[22] The protein's in vivo half-life, the time it takes for half of the amount of protein in a cell to break down after its synthesis in the cell, is predicted to be approximately 30 hours (in mammalian reticulocytes).[21]
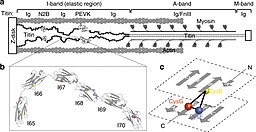
The Titin protein is located between the myosin thick filament and the Z disk.[25] Titin consists primarily of a linear array of two types of modules, also referred to as protein domains (244 copies in total): type I fibronectin type III domain (132 copies) and type II immunoglobulin domain (112 copies).[13][9] However, the exact number of these domains is different in different species. This linear array is further organized into two regions:
- N-terminal I-band: acts as the elastic part of the molecule and is composed mainly of type II modules. More specifically the I-band contains two regions of tandem type II immunoglobulin domains on either side of a PEVK region that is rich in proline (P), glutamate (E), valine (V) and lysine (K).[25]
- C-terminal A-band: is thought to act as a protein-ruler and is composed of alternating type I (Fn3) and II (Ig) modules with super-repeat segments. These have been shown to align to the 43 nm axial repeats of myosin thick filaments with immunoglobulin domains correlating to myosin crowns.[26]
The C-terminal region also contains a serine kinase domain[27][28] that is primarily known for adapting the muscle to mechanical strain.[29] It is “stretch-sensitive” and helps repair overstretching of the sarcomere.[30] The N-terminal (the Z-disc end) contains a "Z repeat" that recognizes Actinin alpha 2.[31]
The elasticity of the PEVK region has both entropic and enthalpic contributions and is characterized by a polymer persistence length and a stretch modulus.[32] At low to moderate extensions PEVK elasticity can be modeled with a standard worm-like chain (WLC) model of entropic elasticity. At high extensions PEVK stretching can be modeled with a modified WLC model that incorporates enthalpic elasticity. The difference between low-and high- stretch elasticity is due to electrostatic stiffening and hydrophobic effects.
Embedded between the PEVK and Ig residues are N2A domains.[33]
Evolution
[edit]The titin domains have evolved from a common ancestor through many gene duplication events.[34] Domain duplication was facilitated by the fact that most domains are encoded by single exons. Other giant sarcomeric proteins made out of Fn3/Ig repeats include obscurin and myomesin. Throughout evolution, titin mechanical strength appears to decrease through the loss of disulfide bonds as the organism becomes heavier.[35]
Titin A-band has homologs in invertebrates, such as twitchin (unc-22) and projectin, which also contain Ig and FNIII repeats and a protein kinase domain.[30] The gene duplication events took place independently but were from the same ancestral Ig and FNIII domains. It is said that the protein titin was the first to diverge out of the family.[28] Drosophila projectin, officially known as bent (bt), is associated with lethality by failing to escape the egg in some mutations as well as dominant changes in wing angles.[36][37][38]
| Titin repeat | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Titin_Ig-rpts | ||||||||
| Pfam | PF06582 | ||||||||
| InterPro | IPR010939 | ||||||||
| |||||||||
Drosophila Titin, also known as Kettin or sallimus (sls), is kinase-free. It has roles in the elasticity of both muscle and chromosomes. It is homologous to vertebrate titin I-band and contains Ig PEVK domains, the many repeats being a hot target for splicing.[39] There also exists a titin homologue, ttn-1, in C. elegans.[40] It has a kinase domain, some Ig/Fn3 repeats, and PEVT repeats that are similarly elastic.[41]
Function
[edit]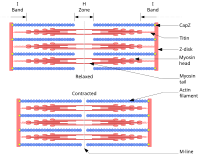
Titin is a large abundant protein of striated muscle. Titin's primary functions are to stabilize the thick filament, center it between the thin filaments, prevent overstretching of the sarcomere, and to recoil the sarcomere like a spring after it is stretched.[42] An N-terminal Z-disc region and a C-terminal M-line region bind to the Z-line and M-line of the sarcomere, respectively, so that a single titin molecule spans half the length of a sarcomere. Titin also contains binding sites for muscle-associated proteins so it serves as an adhesion template for the assembly of contractile machinery in muscle cells. It has also been identified as a structural protein for chromosomes.[43][44] Considerable variability exists in the I-band, the M-line and the Z-disc regions of titin. Variability in the I-band region contributes to the differences in elasticity of different titin isoforms and, therefore, to the differences in elasticity of different muscle types. Of the many titin variants identified, five are described with complete transcript information available.[6][7]
Dominant mutation in TTN causes predisposition to hernias.[45]
Titin interacts with many sarcomeric proteins including:[15]
- Z line region: telethonin and alpha-actinin
- I band region: calpain-3 and obscurin
- M line region: myosin-binding protein C, calmodulin 1, CAPN3, and MURF1
Clinical relevance
[edit]Mutations anywhere within the unusually long sequence of this gene can cause premature stop codons or other defects. Titin mutations are associated with hereditary myopathy with early respiratory failure,[46][47] early-onset myopathy with fatal cardiomyopathy,[48] core myopathy with heart disease, centronuclear myopathy, limb-girdle muscular dystrophy type 2J,[49] familial dilated cardiomyopathy 9,[11][50] hypertrophic cardiomyopathy and tibial muscular dystrophy.[51] Further research also suggests that no genetically linked form of any dystrophy or myopathy can be safely excluded from being caused by a mutation on the TTN gene.[49] Truncating mutations in dilated cardiomyopathy patients are most commonly found in the A region; although truncations in the upstream I region might be expected to prevent translation of the A region entirely, alternative splicing creates some transcripts that do not encounter the premature stop codon, ameliorating its effect.[52] mRNA splicing factors such as RBM20 and SLM2 (KHDRBS3) were shown to mediated alternative mRNA splicing of titin mRNA contributing to the development of heart failure due to cardiomyopathies.[53][54]
Autoantibodies to titin are produced in patients with the autoimmune disease Myasthenia gravis.[55]
Interactions
[edit]Titin has been shown to interact with:
Linguistic significance
[edit]The name titin is derived from the Greek Titan (a giant deity, anything of great size).[5]
As the largest known protein, titin also has the longest IUPAC name of a protein. The full chemical name of the human canonical form of titin, which starts methionyl... and ends ...isoleucine, contains 189,819 letters and is sometimes stated to be the longest word in the English language, or of any language.[66] However, lexicographers regard generic names of chemical compounds as verbal formulae rather than English words.[67]
See also
[edit]- PKZILLA-1 - new largest known protein with 45,212 amino acids
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000155657 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000051747 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c Wang K, McClure J, Tu A (August 1979). "Titin: major myofibrillar components of striated muscle". Proceedings of the National Academy of Sciences of the United States of America. 76 (8): 3698–3702. Bibcode:1979PNAS...76.3698W. doi:10.1073/pnas.76.8.3698. PMC 383900. PMID 291034.
- ^ a b c "TTN, the human gene for Titin". National Library of Medicine; National Center for Biotechnology Information. April 2018. Archived from the original on 2010-03-07.
- ^ a b c Labeit S, Barlow DP, Gautel M, Gibson T, Holt J, Hsieh CL, et al. (May 1990). "A regular pattern of two types of 100-residue motif in the sequence of titin". Nature. 345 (6272): 273–276. Bibcode:1990Natur.345..273L. doi:10.1038/345273a0. PMID 2129545. S2CID 4240433. Archived from the original on 22 October 2021. Retrieved 8 May 2022.
- ^ Lee EH. "The Chain-like Elasticity of Titin". Theoretical and Computational Biophysics Group, University of Illinois. Archived from the original on 13 February 2021. Retrieved 25 September 2014.
- ^ a b c Labeit S, Kolmerer B (October 1995). "Titins: giant proteins in charge of muscle ultrastructure and elasticity". Science. 270 (5234): 293–296. Bibcode:1995Sci...270..293L. doi:10.1126/science.270.5234.293. PMID 7569978. S2CID 20470843. Archived from the original on 2 March 2021. Retrieved 8 May 2022.
- ^ Minajeva A, Kulke M, Fernandez JM, Linke WA (March 2001). "Unfolding of titin domains explains the viscoelastic behavior of skeletal myofibrils". Biophysical Journal. 80 (3): 1442–1451. Bibcode:2001BpJ....80.1442M. doi:10.1016/S0006-3495(01)76116-4. PMC 1301335. PMID 11222304.
- ^ a b Itoh-Satoh M, Hayashi T, Nishi H, Koga Y, Arimura T, Koyanagi T, et al. (February 2002). "Titin mutations as the molecular basis for dilated cardiomyopathy". Biochemical and Biophysical Research Communications. 291 (2): 385–393. doi:10.1006/bbrc.2002.6448. PMID 11846417.
- ^ Online Mendelian Inheritance in Man (OMIM): 188840
- ^ a b Labeit S, Kolmerer B, Linke WA (February 1997). "The giant protein titin. Emerging roles in physiology and pathophysiology". Circulation Research. 80 (2): 290–294. doi:10.1161/01.RES.80.2.290. PMID 9012751.
- ^ Opitz CA, Kulke M, Leake MC, Neagoe C, Hinssen H, Hajjar RJ, Linke WA (October 2003). "Damped elastic recoil of the titin spring in myofibrils of human myocardium". Proceedings of the National Academy of Sciences of the United States of America. 100 (22): 12688–12693. Bibcode:2003PNAS..10012688O. doi:10.1073/pnas.2133733100. PMC 240679. PMID 14563922.
- ^ a b c d Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, et al. (November 2001). "The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-disc to I-band Linking system". Circulation Research. 89 (11): 1065–1072. doi:10.1161/hh2301.100981. PMID 11717165.
- ^ Natori R (1954). "Skinned Fibres of Skeletal Muscle and the Mechanism of Muscle Contraction-A Chronological Account of the Author's Investigations into Muscle Physiology" (PDF). Jikeikai Medical Journal. 54 (1). hdl:10328/3410. Archived from the original (PDF) on 2016-06-03. Retrieved 2014-09-09.
- ^ Maruyama K, Matsubara S, Natori R, Nonomura Y, Kimura S (August 1977). "Connectin, an elastic protein of muscle. Characterization and Function". Journal of Biochemistry. 82 (2): 317–337. PMID 914784.
- ^ Maruyama K (May 1994). "Connectin, an elastic protein of striated muscle". Biophysical Chemistry. 50 (1–2): 73–85. doi:10.1016/0301-4622(94)85021-6. PMID 8011942.
- ^ Online Mendelian Inheritance in Man (OMIM): Titin - 188840
- ^ Freiburg A, Trombitas K, Hell W, Cazorla O, Fougerousse F, Centner T, et al. (June 2000). "Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity". Circulation Research. 86 (11): 1114–1121. doi:10.1161/01.res.86.11.1114. PMID 10850961.
- ^ a b "Titin - Homo sapiens (Human)". Universal Protein Resource. UniProt Consortium. 2010-10-05. Archived from the original on 2021-02-13. Retrieved 2010-10-15.
- ^ a b c d "ProtParam for human titin". ExPASy Proteomics Server. Swiss Institute of Bioinformatics. Archived from the original on 2019-09-18. Retrieved 2011-07-25.
- ^ "ProtParam for mouse titin". ExPASy Proteomics Server. Swiss Institute of Bioinformatics. Retrieved 2010-05-06.
- ^ Giganti D, Yan K, Badilla CL, Fernandez JM, Alegre-Cebollada J (January 2018). "Disulfide isomerization reactions in titin immunoglobulin domains enable a mode of protein elasticity". Nature Communications. 9 (1): 185. Bibcode:2018NatCo...9..185G. doi:10.1038/s41467-017-02528-7. PMC 5766482. PMID 29330363.
- ^ a b Wang K, McCarter R, Wright J, Beverly J, Ramirez-Mitchell R (August 1991). "Regulation of skeletal muscle stiffness and elasticity by titin isoforms: a test of the segmental extension model of resting tension". Proceedings of the National Academy of Sciences of the United States of America. 88 (16): 7101–7105. Bibcode:1991PNAS...88.7101W. doi:10.1073/pnas.88.16.7101. PMC 52241. PMID 1714586.
- ^ Bennett PM, Gautel M (June 1996). "Titin domain patterns correlate with the axial disposition of myosin at the end of the thick filament". Journal of Molecular Biology. 259 (5): 896–903. doi:10.1006/jmbi.1996.0367. PMID 8683592.
- ^ a b Mayans O, van der Ven PF, Wilm M, Mues A, Young P, Fürst DO, et al. (October 1998). "Structural basis for activation of the titin kinase domain during myofibrillogenesis". Nature. 395 (6705): 863–869. Bibcode:1998Natur.395..863M. doi:10.1038/27603. PMID 9804419. S2CID 4426977.
- ^ a b Higgins DG, Labeit S, Gautel M, Gibson TJ (April 1994). "The evolution of titin and related giant muscle proteins". Journal of Molecular Evolution. 38 (4): 395–404. Bibcode:1994JMolE..38..395H. doi:10.1007/BF00163156. PMID 8007007. S2CID 35756951.
- ^ Puchner EM, Alexandrovich A, Kho AL, Hensen U, Schäfer LV, Brandmeier B, et al. (September 2008). "Mechanoenzymatics of titin kinase". Proceedings of the National Academy of Sciences of the United States of America. 105 (36): 13385–13390. Bibcode:2008PNAS..10513385P. doi:10.1073/pnas.0805034105. PMC 2527993. PMID 18765796.
- ^ a b Myhre JL, Pilgrim D (September 2014). "A Titan but not necessarily a ruler: assessing the role of titin during thick filament patterning and assembly". Anatomical Record. 297 (9): 1604–1614. doi:10.1002/ar.22987. PMID 25125174. S2CID 32840140.
- ^ "Titin, Z repeat (IPR015129) < InterPro < EMBL-EBI". Archived from the original on 13 February 2021. Retrieved 13 March 2019.
- ^ Linke WA, Ivemeyer M, Mundel P, Stockmeier MR, Kolmerer B (July 1998). "Nature of PEVK-titin elasticity in skeletal muscle". Proceedings of the National Academy of Sciences of the United States of America. 95 (14): 8052–8057. Bibcode:1998PNAS...95.8052L. doi:10.1073/pnas.95.14.8052. PMC 20927. PMID 9653138.
- ^ Buck D, Smith JE, Chung CS, Ono Y, Sorimachi H, Labeit S, Granzier HL (February 2014). "Removal of immunoglobulin-like domains from titin's spring segment alters titin splicing in mouse skeletal muscle and causes myopathy". The Journal of General Physiology. 143 (2): 215–230. doi:10.1085/jgp.201311129. PMC 4001778. PMID 24470489.
- ^ Tskhovrebova L, Trinick J (November 2004). "Properties of titin immunoglobulin and fibronectin-3 domains". The Journal of Biological Chemistry. 279 (45): 46351–46354. doi:10.1074/jbc.r400023200. PMID 15322090. Archived from the original on 2018-06-03. Retrieved 2018-12-16.
- ^ Manteca A, Schönfelder J, Alonso-Caballero A, Fertin MJ, Barruetabeña N, Faria BF, et al. (August 2017). "Mechanochemical evolution of the giant muscle protein titin as inferred from resurrected proteins". Nature Structural & Molecular Biology. 24 (8): 652–657. doi:10.1038/nsmb.3426. hdl:20.500.12105/9931. PMID 28671667. S2CID 54482436.
- ^ Fyrberg CC, Labeit S, Bullard B, Leonard K, Fyrberg E (July 1992). "Drosophila projectin: relatedness to titin and twitchin and correlation with lethal(4) 102 CDa and bent-dominant mutants". Proceedings. Biological Sciences. 249 (1324): 33–40. Bibcode:1992RSPSB.249...33F. doi:10.1098/rspb.1992.0080. PMID 1359548. S2CID 34408190.
- ^ "bent phenotype". Classical Genetics Simulator. Archived from the original on 11 February 2019. Retrieved 13 March 2019.
- ^ "FlyBase Gene Report: Dmel\bt". flybase.org. Archived from the original on 13 March 2019. Retrieved 13 March 2019.
- ^ Machado C, Andrew DJ (October 2000). "D-Titin: a giant protein with dual roles in chromosomes and muscles" (PDF). The Journal of Cell Biology. 151 (3): 639–652. doi:10.1083/jcb.151.3.639. PMC 2185597. PMID 11062264. Archived (PDF) from the original on 2019-09-04. Retrieved 2019-09-04.
- ^ "ttn-1 (gene)". WormBase: Nematode Information Resource. Archived from the original on 27 March 2018. Retrieved 13 March 2019.
- ^ Forbes JG, Flaherty DB, Ma K, Qadota H, Benian GM, Wang K (May 2010). "Extensive and modular intrinsically disordered segments in C. elegans TTN-1 and implications in filament binding, elasticity and oblique striation". Journal of Molecular Biology. 398 (5): 672–689. doi:10.1016/j.jmb.2010.03.032. PMC 2908218. PMID 20346955.
- ^ Saladin K (2015). Anatomy & Physiology (7th ed.). McGraw Hill. p. 401. ISBN 978-0-07-340371-7.
- ^ Machado C, Sunkel CE, Andrew DJ (April 1998). "Human autoantibodies reveal titin as a chromosomal protein". The Journal of Cell Biology. 141 (2): 321–333. doi:10.1083/jcb.141.2.321. PMC 2148454. PMID 9548712.
- ^ Machado C, Andrew DJ (2000). "Titin as a Chromosomal Protein". Elastic Filaments of the Cell. Advances in Experimental Medicine and Biology. Vol. 481. pp. 221–32, discussion 232–6. doi:10.1007/978-1-4615-4267-4_13. ISBN 978-1-4613-6916-5. PMID 10987075.
- ^ Mihailov E, Nikopensius T, Reigo A, Nikkolo C, Kals M, Aruaas K, et al. (February 2017). "Whole-exome sequencing identifies a potential TTN mutation in a multiplex family with inguinal hernia". Hernia. 21 (1): 95–100. doi:10.1007/s10029-016-1491-9. PMC 5281683. PMID 27115767.
- ^ Pfeffer G, Elliott HR, Griffin H, Barresi R, Miller J, Marsh J, et al. (June 2012). "Titin mutation segregates with hereditary myopathy with early respiratory failure". Brain. 135 (Pt 6): 1695–1713. doi:10.1093/brain/aws102. PMC 3359754. PMID 22577215.
- ^ Ohlsson M, Hedberg C, Brådvik B, Lindberg C, Tajsharghi H, Danielsson O, et al. (June 2012). "Hereditary myopathy with early respiratory failure associated with a mutation in A-band titin" (PDF). Brain. 135 (Pt 6): 1682–1694. doi:10.1093/brain/aws103. PMID 22577218. Archived (PDF) from the original on 2021-09-11. Retrieved 2021-09-11.
- ^ Carmignac V, Salih MA, Quijano-Roy S, Marchand S, Al Rayess MM, Mukhtar MM, et al. (April 2007). "C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy". Annals of Neurology. 61 (4): 340–351. doi:10.1002/ana.21089. PMID 17444505. S2CID 6042810.
- ^ a b Udd B, Vihola A, Sarparanta J, Richard I, Hackman P (February 2005). "Titinopathies and extension of the M-line mutation phenotype beyond distal myopathy and LGMD2J". Neurology. 64 (4): 636–642. doi:10.1212/01.WNL.0000151853.50144.82. PMID 15728284. S2CID 28801620.
- ^ Siu BL, Niimura H, Osborne JA, Fatkin D, MacRae C, Solomon S, et al. (March 1999). "Familial dilated cardiomyopathy locus maps to chromosome 2q31". Circulation. 99 (8): 1022–1026. doi:10.1161/01.cir.99.8.1022. PMID 10051295.
- ^ Hackman P, Vihola A, Haravuori H, Marchand S, Sarparanta J, De Seze J, et al. (September 2002). "Tibial muscular dystrophy is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal-muscle protein titin". American Journal of Human Genetics. 71 (3): 492–500. doi:10.1086/342380. PMC 379188. PMID 12145747.
- ^ Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, et al. (August 2015). "HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy". Science. 349 (6251): 982–986. doi:10.1126/science.aaa5458. PMC 4618316. PMID 26315439.
- ^ Li S, Guo W, Dewey CN, Greaser ML (February 2013). "Rbm20 regulates titin alternative splicing as a splicing repressor". Nucleic Acids Research. 41 (4): 2659–2672. doi:10.1093/nar/gks1362. PMC 3575840. PMID 23307558.
- ^ Boeckel JN, Möbius-Winkler M, Müller M, Rebs S, Eger N, Schoppe L, et al. (February 2022). "SLM2 Is A Novel Cardiac Splicing Factor Involved in Heart Failure due to Dilated Cardiomyopathy". Genomics, Proteomics & Bioinformatics. 20 (1): 129–146. doi:10.1016/j.gpb.2021.01.006. PMC 9510876. PMID 34273561.
- ^ Skeie GO, Aarli JA, Gilhus NE (2006). "Titin and ryanodine receptor antibodies in myasthenia gravis". Acta Neurologica Scandinavica. Supplementum. 183: 19–23. doi:10.1111/j.1600-0404.2006.00608.x. PMID 16637922. S2CID 24972330.
- ^ Kontrogianni-Konstantopoulos A, Bloch RJ (February 2003). "The hydrophilic domain of small ankyrin-1 interacts with the two N-terminal immunoglobulin domains of titin". The Journal of Biological Chemistry. 278 (6): 3985–3991. doi:10.1074/jbc.M209012200. PMID 12444090.
- ^ a b Miller MK, Bang ML, Witt CC, Labeit D, Trombitas C, Watanabe K, et al. (November 2003). "The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules". Journal of Molecular Biology. 333 (5): 951–964. doi:10.1016/j.jmb.2003.09.012. PMID 14583192.
- ^ Ono Y, Shimada H, Sorimachi H, Richard I, Saido TC, Beckmann JS, et al. (July 1998). "Functional defects of a muscle-specific calpain, p94, caused by mutations associated with limb-girdle muscular dystrophy type 2A". The Journal of Biological Chemistry. 273 (27): 17073–17078. doi:10.1074/jbc.273.27.17073. PMID 9642272.
- ^ Sorimachi H, Kinbara K, Kimura S, Takahashi M, Ishiura S, Sasagawa N, et al. (December 1995). "Muscle-specific calpain, p94, responsible for limb girdle muscular dystrophy type 2A, associates with connectin through IS2, a p94-specific sequence". The Journal of Biological Chemistry. 270 (52): 31158–31162. doi:10.1074/jbc.270.52.31158. PMID 8537379.
- ^ Lange S, Auerbach D, McLoughlin P, Perriard E, Schäfer BW, Perriard JC, Ehler E (December 2002). "Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2". Journal of Cell Science. 115 (Pt 24): 4925–4936. doi:10.1242/jcs.00181. PMID 12432079.
- ^ Young P, Ehler E, Gautel M (July 2001). "Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly". The Journal of Cell Biology. 154 (1): 123–136. doi:10.1083/jcb.200102110. PMC 2196875. PMID 11448995.
- ^ Gregorio CC, Trombitás K, Centner T, Kolmerer B, Stier G, Kunke K, et al. (November 1998). "The NH2 terminus of titin spans the Z-disc: its interaction with a novel 19-kD ligand (T-cap) is required for sarcomeric integrity". The Journal of Cell Biology. 143 (4): 1013–1027. doi:10.1083/jcb.143.4.1013. PMC 2132961. PMID 9817758.
- ^ Zou P, Gautel M, Geerlof A, Wilmanns M, Koch MH, Svergun DI (January 2003). "Solution scattering suggests cross-linking function of telethonin in the complex with titin". The Journal of Biological Chemistry. 278 (4): 2636–2644. doi:10.1074/jbc.M210217200. PMID 12446666.
- ^ Mues A, van der Ven PF, Young P, Fürst DO, Gautel M (May 1998). "Two immunoglobulin-like domains of the Z-disc portion of titin interact in a conformation-dependent way with telethonin". FEBS Letters. 428 (1–2): 111–114. doi:10.1016/S0014-5793(98)00501-8. PMID 9645487. S2CID 11786578.
- ^ Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, et al. (March 2001). "Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain". Journal of Molecular Biology. 306 (4): 717–726. doi:10.1006/jmbi.2001.4448. PMID 11243782.
- ^ McCulloch S (December 2009). "Longest word in English". Sarah McCulloch.com. Archived from the original on 2010-01-14. Retrieved 2016-10-12.
- ^ Oxford Word and Language Service team. "Ask the experts - What is the longest English word?". AskOxford.com / Oxford University Press. Archived from the original on 2008-09-13. Retrieved 2008-01-13.
Further reading
[edit]- Tskhovrebova L, Trinick J (September 2003). "Titin: properties and family relationships". Nature Reviews. Molecular Cell Biology. 4 (9): 679–689. doi:10.1038/nrm1198. PMID 14506471. S2CID 12293932.
- Kinbara K, Sorimachi H, Ishiura S, Suzuki K (August 1998). "Skeletal muscle-specific calpain, p49: structure and physiological function". Biochemical Pharmacology. 56 (4): 415–420. doi:10.1016/S0006-2952(98)00095-1. PMID 9763216.
- Kolmerer B, Witt CC, Freiburg A, Millevoi S, Stier G, Sorimachi H, et al. (1999). "The titin cDNA sequence and partial genomic sequences: insights into the molecular genetics, cell biology and physiology of the titin filament system". Reviews of Physiology, Biochemistry and Pharmacology. 138: 19–55. doi:10.1007/BF02346659. PMID 10396137.
- Trinick J, Tskhovrebova L (October 1999). "Titin: a molecular control freak". Trends in Cell Biology. 9 (10): 377–380. doi:10.1016/S0962-8924(99)01641-4. PMID 10481174.
- Sorimachi H, Ono Y, Suzuki K (2000). "Skeletal Muscle-Specific Calpain, p94, and Connectin/Titin: Their Physiological Functions and Relationship to Limb-Girdle Muscular Dystrophy Type 2A". Elastic Filaments of the Cell. Advances in Experimental Medicine and Biology. Vol. 481. pp. 383–95, discussion 395–7. doi:10.1007/978-1-4615-4267-4_23. ISBN 978-1-4613-6916-5. PMID 10987085.
- Tskhovrebova L, Trinick J (February 2002). "Role of titin in vertebrate striated muscle". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 357 (1418): 199–206. doi:10.1098/rstb.2001.1028. PMC 1692937. PMID 11911777.
- Sela BA (July 2002). "[Titin: some aspects of the largest protein in the body]". Harefuah. 141 (7): 631–5, 665. PMID 12187564.
- Tskhovrebova L, Trinick J (November 2004). "Properties of titin immunoglobulin and fibronectin-3 domains". The Journal of Biological Chemistry. 279 (45): 46351–46354. doi:10.1074/jbc.R400023200. PMID 15322090.
- Wu Y, Labeit S, Lewinter MM, Granzier H (December 2002). "Titin: an endosarcomeric protein that modulates myocardial stiffness in DCM". Journal of Cardiac Failure. 8 (6 Suppl): S276–S286. doi:10.1054/jcaf.2002.129278. PMID 12555133.
External links
[edit]- GeneReviews/NIH/NCBI/UW entry on Familial Hypertrophic Cardiomyopathy Overview
- GeneReviews/NCBI/NIH/UW entry on Udd Distal Myopathy, Tibial Muscular Dystrophy, Udd Myopathy
- GeneReviews/NIH/NCBI/UW entry on Salih Myopathy or Early-Onset Myopathy with Fatal Cardiomyopathy
- InterPro domain organization of titin
This article incorporates text from the United States National Library of Medicine, which is in the public domain.