Ethylenediaminetetraacetic acid: Difference between revisions
| (676 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
{{about|the chemical|the medication|Sodium calcium edetate}} |
|||
{{Otheruses}} |
|||
{{Redirect|EDTA}} |
|||
{{Redirect-distinguish|Versene|Versine}} |
|||
{{chembox |
{{chembox |
||
| |
|Verifiedfields = changed |
||
|Watchedfields = changed |
|||
| verifiedrevid = 265311426 |
|||
|verifiedrevid = 408562373 |
|||
| ImageFile1 = ethylenediaminetetraacetic.png |
|||
|ImageFile = EDTA.svg |
|||
| ImageSize1 = 200px |
|||
|ImageFile_Ref = {{chemboximage|correct|??}} |
|||
| ImageFile2 = EDTA-xtal-3D-balls.png |
|||
|ImageName = 3-dimensional formula of ethylenediaminetetraacetic acid |
|||
| ImageSize2 = 200px |
|||
|IUPACName = ''N'',''N''′-(Ethane-1,2-diyl)bis[''N''-(carboxymethyl)glycine]<ref name=iupac2013 /> |
|||
| ImageName2 = Ball and stick model of the EDTA molecule, in the zwitterionic form found in the solid state |
|||
|SystematicName = 2,2′,2′′,2′′′-(Ethane-1,2-diyldinitrilo)tetraacetic acid<ref name=iupac2013>{{cite book | title = Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book) | publisher = [[Royal Society of Chemistry|The Royal Society of Chemistry]] | date = 2014 | location = Cambridge | pages = 79, 123, 586, 754 | isbn = 978-0-85404-182-4 }}</ref> |
|||
| ImageName = EDTA |
|||
|OtherNames = {{Anchor|Names}} {{unbulleted list|EthyleneDiamineTetraAcetic acid|Diaminoethane-tetraacetic acid|Edetic acid ([[conjugate acid|conjugate base]] edetate) ([[international nonproprietary name|INN]], [[United States Adopted Name|USAN]])|Versene}} |
|||
| IUPACName = 2,2',2<nowiki>''</nowiki>,2<nowiki>'''</nowiki>-(ethane-1,2-diyldinitrilo)tetraacetic acid |
|||
|Section1 = {{Chembox Identifiers |
|||
| OtherNames = EDTA, Y, H<sub>4</sub>EDTA, Diaminoethanetetraacetic acid, Edetic acid, Edetate, Ethylenedinitrilotetraacetic acid, Versene, Ethylene diamine tetraacetic acid, ethylenediaminetetraacetate, 2-[2-(Bis(carboxymethyl)amino) ethyl-(carboxymethyl)amino]acetic acid |
|||
|Abbreviations = EDTA, H<sub>4</sub>EDTA |
|||
| Section1 = {{Chembox Identifiers |
|||
| |
|CASNo = 60-00-4 |
||
|CASNo_Ref = {{cascite|correct|CAS}} |
|||
| CASOther =<br> |
|||
|CASNo_Comment = (free acid) |
|||
{{Chembox_CASNo2|6381-92-6}} (disodium dihydrate),<br> |
|||
|CASNo2_Ref = {{cascite|correct|CAS}} |
|||
{{Chembox_CASNo2|139-33-3}} (disodium),<br> |
|||
|CASNo2 = 6381-92-6 |
|||
{{Chembox_CASNo2|64-02-8}} (tetrasodium),<br> |
|||
|CASNo2_Comment = (dihydrate disodium salt) <!--also verified against the Chemical Abstracts Service list --> |
|||
{{Chembox_CASNo2|62-33-9}} (calcium disodium) <!-- Also CAS verified --> |
|||
|PubChem = 6049 |
|||
| CASNo_Ref = {{cascite}} |
|||
| |
|ChemSpiderID = 5826 |
||
|ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| PubChem = 6049 |
|||
|DrugBank = DB00974 |
|||
| SMILES = OC(CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O)=O |
|||
|DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| RTECS = AH4025000 |
|||
|UNII = 9G34HU7RV0 |
|||
}} |
|||
|UNII_Ref = {{fdacite|correct|FDA}} |
|||
| Section2 = {{Chembox Properties |
|||
|UNII1_Ref = {{fdacite|correct|FDA}} |
|||
| C=10|H=16|N=2|O=8 |
|||
|UNII1 = 7FLD91C86K |
|||
| MolarMass = 292.24 |
|||
|UNII1_Comment = (dihydrate disodium salt) |
|||
| Density = 0.86 g/cm<sup>3</sup> |
|||
|EINECS = 200-449-4 |
|||
| MeltingPt = 237–245 °C (dec.) |
|||
|UNNumber = 3077 |
|||
| pKa = pK<sub>1</sub>=0.0 (CO<sub>2</sub>H) (µ=1.0)<br>pK<sub>2</sub>=1.5 (CO<sub>2</sub>H) (µ=0.1)<br>pK<sub>3</sub>=2.00 (CO<sub>2</sub>H) (µ=0.1)<br>pK<sub>4</sub>=2.69 (CO<sub>2</sub>H) (µ=0.1)<br>pK<sub>5</sub>=6.13 (NH<sup>+</sup>) (µ=0.1)<br>pK<sub>6</sub>=10.37 (NH<sup>+</sup>) (µ=0.1)<ref>Harris, D.C. "Quantitative Chemical Analysis", 7<sup>th</sup> ed., W. H. Freeman and Compagny, New York, 2007</ref> |
|||
|KEGG = D00052 |
|||
}} |
|||
|KEGG_Ref = {{keggcite|correct|kegg}} |
|||
| Section7 = {{Chembox Hazards |
|||
|MeSHName = Edetic+Acid |
|||
| ExternalMSDS = [http://ptcl.chem.ox.ac.uk/MSDS/ED/EDTA.html External MSDS] |
|||
|ChEBI = 4735 |
|||
| MainHazards = irritant |
|||
|ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| NFPA-H = 1 |
|||
|ChEMBL = 858 |
|||
| NFPA-F = 0 | Reactivity=0 |
|||
|ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
| NFPA-R = |
|||
|RTECS = AH4025000 |
|||
| RPhrases = {{R36}} |
|||
|Beilstein = 1716295 |
|||
| SPhrases = {{S26}} |
|||
|Gmelin = 144943 |
|||
}} |
|||
|SMILES = OC(=O)CN(CCN(CC(O)=O)CC(O)=O)CC(O)=O |
|||
|StdInChI = 1S/C10H16N2O8/c13-7(14)3-11(4-8(15)16)1-2-12(5-9(17)18)6-10(19)20/h1-6H2,(H,13,14)(H,15,16)(H,17,18)(H,19,20) |
|||
|StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
|StdInChIKey = KCXVZYZYPLLWCC-UHFFFAOYSA-N |
|||
|StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
}} |
}} |
||
|Section2 = {{Chembox Properties |
|||
|C=10 | H=16 | N=2 | O=8 |
|||
|Appearance = Colourless crystals |
|||
|Density = 0.860 g cm<sup>−3</sup> (at 20 °C) |
|||
|LogP = −0.836 |
|||
|pKa = 2.0, 2.7, 6.16, 10.26<ref>{{cite book | chapter-url=https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470110195.ch10 | doi=10.1002/9780470110195.ch10 | chapter=Applications of Metal Buffers and Metal Indicators in Biochemistry | title=Methods of Biochemical Analysis | date=1956 | last1=Raaflaub | first1=Jürg | volume=3 | pages=301–325 | pmid=13369167 | isbn=978-0-470-30492-1 }}</ref> |
|||
| pKb = <!-- see text --> |
|||
}} |
|||
|Section3 = {{Chembox Thermochemistry |
|||
|DeltaHf = −1765.4 to −1758.0 kJ mol<sup>−1</sup> |
|||
|DeltaHc = −4461.7 to −4454.5 kJ mol<sup>−1</sup> |
|||
}} |
|||
|Section4 = {{Chembox Pharmacology |
|||
|AdminRoutes = {{unbulleted list|Intramuscular|Intravenous}} |
|||
|ATCCode_prefix = S01 |
|||
|ATCCode_suffix = XA05 |
|||
|ATC_Supplemental = {{ATC|V03|AB03}} (salt) |
|||
}} |
|||
|Section5 = {{Chembox Hazards |
|||
|GHSPictograms = {{gHS exclamation mark}} |
|||
|GHSSignalWord = '''WARNING''' |
|||
|HPhrases = {{h-phrases|319}} |
|||
|PPhrases = {{p-phrases|305+351+338}} |
|||
|NFPA-H = 1 |
|||
|NFPA-F = 0 |
|||
|NFPA-R = 0 |
|||
|LD50 = 1000 mg/kg (oral, rat)<ref>[https://chem.nlm.nih.gov/chemidplus/rn/62-33-9 Substance Name: Sodium calcium edetate]. NIH.gov</ref> |
|||
}} |
|||
|Section6 = {{Chembox Related |
|||
|OtherFunction_label = alkanoic acids |
|||
|OtherFunction = {{unbulleted list|[[Daminozide]]|[[Octopine]]}} |
|||
|OtherCompounds = {{unbulleted list|[[Triethylenetetramine]]|[[Tetraacetylethylenediamine]]|[[PMDTA]]|[[Bis-tris propane]]}} |
|||
}} |
|||
}} |
|||
'''Ethylenediaminetetraacetic acid''' ('''EDTA'''), also called '''EDTA acid''', is an [[aminopolycarboxylic acid]] with the formula [CH<sub>2</sub>N(CH<sub>2</sub>CO<sub>2</sub>H)<sub>2</sub>]<sub>2</sub>. This white, slightly water-soluble solid is widely used to bind to iron (Fe<sup>2+</sup>/Fe<sup>3+</sup>) and calcium ions (Ca<sup>2+</sup>), forming water-soluble [[coordination complex|complexes]] even at neutral pH. It is thus used to dissolve Fe- and Ca-containing scale as well as to deliver iron ions under conditions where its oxides are insoluble. EDTA is available as several salts, notably '''disodium EDTA''', [[sodium calcium edetate]], and [[tetrasodium EDTA]], but these all function similarly.<ref name="Ullmann" /> |
|||
==Uses== |
|||
EDTA Is widely used in industry. It also has applications in food preservation, medicine, cosmetics, water softening, in laboratories, and other fields. |
|||
=== Industrial === |
|||
EDTA is mainly used to [[Sequestrant|sequester]] (bind or confine) metal ions in aqueous solution. In the [[Textile|textile industry]], it prevents metal ion impurities from modifying colours of dyed products. In the [[pulp and paper industry]], EDTA inhibits the ability of metal ions, especially [[manganese|Mn<sup>2+</sup>]], from catalysing the [[disproportionation]] of [[hydrogen peroxide]], which is used in [[totally chlorine free|chlorine-free bleaching]]. |
|||
==== Gas scrubbing ==== |
|||
Aqueous [Fe(EDTA)]<sup>−</sup> is used for removing ("[[Sulfur scrubbing|scrubbing]]") [[hydrogen sulfide]] from gas streams. This conversion is achieved by oxidising the hydrogen sulfide to elemental sulfur, which is non-volatile: |
|||
:2 [Fe(EDTA)]<sup>−</sup> + [[hydrogen sulfide|H<sub>2</sub>S]] → 2 [Fe(EDTA)]<sup>2−</sup> + [[sulfur|S]] + 2 H<sup>+</sup> |
|||
In this application, the iron(III) centre is [[Redox|reduced]] to its iron(II) derivative, which can then be reoxidised by air. In a similar manner, [[nitrogen oxide]]s are removed from gas streams using [Fe(EDTA)]<sup>2−</sup>. |
|||
=== Food === |
|||
In a similar manner, EDTA is added to some food as a [[preservative]] or stabiliser to prevent catalytic oxidative decolouration, which is catalysed by metal ions.<ref name="furia1964">{{cite journal | last=Furia | first=T. | title=EDTA in Foods – A technical review | journal=Food Technology | volume=18 | issue=12 | pages=1874–1882 | year=1964}}</ref> |
|||
=== Water softener === |
|||
The reduction of water hardness in laundry applications and the dissolution of scale in boilers both rely on EDTA and related [[complex (chemistry)|complexants]] to bind [[calcium|Ca<sup>2+</sup>]], [[magnesium|Mg<sup>2+</sup>]], as well as other metal ions. Once bound to EDTA, these metal complexes are less likely to form precipitates or to interfere with the action of the [[soap]]s and [[detergent]]s.{{Cn|date=June 2022}} For similar reasons, cleaning solutions often contain EDTA. In a similar manner EDTA is used in the cement industry for the determination of free [[Limestone|lime]] and free [[Magnesium oxide|magnesia]] in cement and [[Clinker (cement)|clinkers]].<ref name="Cement Chemistry, H. F. W. Taylor">{{Cite book |last=Taylor |first=H. F. W. |year=1990 |title=Cement Chemistry |publisher=Academic Press |isbn=978-0-12-683900-5}}</ref>{{page needed|date=October 2017}} |
|||
The solubilisation of [[iron|Fe<sup>3+</sup>]] ions at or below near neutral [[pH]] can be accomplished using EDTA. This property is useful in [[agriculture]] including hydroponics. However, given the pH dependence of ligand formation, EDTA is not helpful for improving iron solubility in above neutral soils.<ref>{{Cite journal | doi = 10.2136/sssaj1969.03615995003300010024x| title = Reactions of EDTA Complexes of Fe, Zn, Mn, and Cu with Soils| journal = Soil Science Society of America Journal| volume = 33| issue = 1| page = 86| year = 1969| last1 = Norvell | first1 = W. A.| last2 = Lindsay | first2 = W. L.| bibcode = 1969SSASJ..33...86N}}</ref> Otherwise, at near-neutral pH and above, iron(III) forms insoluble salts, which are less [[bioavailability|bioavailable]] to susceptible plant species. |
|||
=== Ion-exchange chromatography === |
|||
EDTA was used in separation of the [[lanthanide metal]]s by [[ion-exchange chromatography]]. Perfected by F. H. Spedding ''et al''. in 1954, the method relies on the steady increase in [[Stability constants of complexes|stability constant]] of the lanthanide EDTA complexes with [[atomic number]].<ref>{{Cite tech report |last1=Powell |first1=J. E. |last2=Spedding |first2=F. H. |date=1956 |title=Basic Principles Involved in the Macro-Separation of Adjacent Rare Earths from Each Other by Means of Ion Exchange |publisher=Iowa State College |doi=10.2172/4289324 |doi-access=free |s2cid=93195586 |osti=4289324 |osti-access=free}}</ref> Using [[sulfonate]]d [[polystyrene]] beads and [[copper|Cu<sup>2+</sup>]] as a retaining ion, EDTA causes the lanthanides to migrate down the column of resin while separating into bands of pure lanthanides. The lanthanides elute in order of decreasing atomic number. Due to the expense of this method, relative to [[countercurrent solvent extraction]], ion exchange is now used only to obtain the highest purities of lanthanides (typically greater than 99.99%).{{Citation needed|date=June 2009}} |
|||
===Medicine=== |
|||
[[Sodium calcium edetate]], an EDTA derivative, is used to bind metal ions in the practice of [[chelation therapy]], such as for treating [[mercury poisoning|mercury]] and [[lead poisoning]].<ref name=r1>{{cite web | last = DeBusk | first = Ruth | title = Ethylenediaminetetraacetic acid (EDTA) | year = 2002 | url=http://www.umm.edu/altmed/articles/ethylenediaminetetraacetic-acid-000302.htm | archive-url=https://web.archive.org/web/20070504081119/http://www.umm.edu/altmed/articles/ethylenediaminetetraacetic-acid-000302.htm| archive-date=2007-05-04 | publisher=University of Maryland Medical Center|display-authors=etal}}</ref> It is used in a similar manner to remove excess [[iron]] from the body. This therapy is used to treat the complication of repeated [[blood transfusion]]s, as would be applied to treat [[thalassaemia]]. |
|||
==== In testing ==== |
|||
In [[medical diagnosis]] and organ function tests (here, [[kidney function]] test), the [[chromium|chromium(III)]] complex [Cr(EDTA)]<sup>−</sup> (as radioactive [[chromium-51]] (<sup>51</sup>Cr)) is administered [[intravenously]] and its filtration into the [[urine]] is monitored. This method is useful for evaluating [[Renal function#Measurement with radioactive tracers|glomerular filtration rate]] (GFR) in [[nuclear medicine]].<ref>{{cite journal|last1=Soveri|first1=Inga|last2=Berg|first2=Ulla B.|last3=Björk|first3=Jonas|last4=Elinder|first4=Carl-Gustaf|last5=Grubb|first5=Anders|last6=Mejare|first6=Ingegerd|last7=Sterner|first7=Gunnar|last8=Bäck|first8=Sten-Erik|title=Measuring GFR: A Systematic Review|journal=American Journal of Kidney Diseases|date=September 2014|volume=64|issue=3|pages=411–424|doi=10.1053/j.ajkd.2014.04.010|pmid=24840668}}</ref> |
|||
EDTA is used extensively in the analysis of blood. It is an [[anticoagulant]] for blood samples for [[Complete blood count|CBC/FBC]]s, where the EDTA chelates the calcium present in the blood specimen, arresting the coagulation process and preserving blood cell morphology.<ref>{{cite journal |pmid=17484616 |year=2007 |last1=Banfi |first1=G |title=The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes |journal=Clinical Chemistry and Laboratory Medicine |volume=45 |issue=5 |pages=565–76 |last2=Salvagno |first2=G. L |last3=Lippi |first3=G |doi=10.1515/CCLM.2007.110 |s2cid=23824484 }}</ref> Tubes containing EDTA are marked with [[Lavender (color)|lavender]] (purple) or pink tops.<ref name="mi">{{cite web |title=Order of draw for multiple tube collections |url=http://mlabs.umich.edu/files/pdfs/PRC%20-%20Order_Draw_Multiple.pdf |publisher=Michigan Medicine Laboratories |date=2019-09-15 |access-date=2020-03-27 |archive-date=2019-11-26 |archive-url=https://web.archive.org/web/20191126160736/http://mlabs.umich.edu/files/pdfs/PRC%20-%20Order_Draw_Multiple.pdf }}</ref> EDTA is also in tan top tubes for lead testing and can be used in royal blue top tubes for trace metal testing.<ref name="mi" /> |
|||
EDTA is a slime dispersant, and has been found to be highly effective in reducing bacterial growth during implantation of [[intraocular lens]]es (IOLs).<ref>{{Cite journal | doi = 10.1093/jac/dkn533| pmid = 19147522| title = Impact of slime dispersants and anti-adhesives on in vitro biofilm formation of Staphylococcus epidermidis on intraocular lenses and on antibiotic activities| journal = Journal of Antimicrobial Chemotherapy| volume = 63| issue = 3| pages = 480–4| year = 2009| last1 = Kadry | first1 = A. A.| last2 = Fouda | first2 = S. I.| last3 = Shibl | first3 = A. M.| last4 = Abu El-Asrar | first4 = A. A.| doi-access = }}</ref> |
|||
==== Dentistry ==== |
|||
[[Dentist]]s and [[endodontist]]s use EDTA solutions to remove inorganic debris ([[smear layer]]) and lubricate the [[root canal]]s in endodontics. This procedure helps prepare root canals for [[obturation]]. Furthermore, EDTA solutions with the addition of a [[surfactant]] loosen up [[calcification]]s inside a root canal and allow instrumentation (canal shaping) and facilitate apical advancement of a file in a tight or calcified root canal towards the apex. |
|||
==== Eyedrops ==== |
|||
It serves as a [[preservative]] (usually to enhance the action of another preservative such as [[benzalkonium chloride]] or [[thiomersal]]) in ocular preparations and [[eyedrops]]. |
|||
===Alternative medicine=== |
|||
Some [[Alternative medicine|alternative practitioners]] believe EDTA acts as an [[antioxidant]], preventing [[free radical]]s from injuring [[blood vessel]] walls, therefore reducing [[atherosclerosis]].<ref>{{Cite journal | pmc=1282574| doi = 10.1186/1471-2261-5-32| pmid = 19147522| title = EDTA chelation therapy for cardiovascular disease: a systematic review| journal = BMC Cardiovasc Disord| volume = 5| issue = 32| year = 2005| last1 = Seely | first1 = D. M.| last2 = Wu | first2 = P.| last3 = Mills | first3 = E. J.| pages = 480–484| doi-access = free}}</ref> These ideas are unsupported by scientific studies, and seem to contradict some currently accepted principles.<ref>{{cite web|url=http://www.quackwatch.org/01QuackeryRelatedTopics/chelationimp.html|title=EDTA Chelation Therapy for Atherosclerosis And Degenerative Diseases: Implausibility and Paradoxical Oxidant Effects|last1=Green|first1=Saul|first2=Wallace|last2=Sampson|date= December 14, 2002|work=Quackwatch|access-date=16 December 2009}}</ref> The [[Food and Drug Administration|U.S. FDA]] has not approved it for the treatment of atherosclerosis.<ref>{{cite web|url=https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm113738.htm|title=Postmarket Drug Safety Information for Patients and Providers – Questions and Answers on Edetate Disodium (marketed as Endrate and generic products)|work=U.S. Food and Drug Administration}}</ref> |
|||
===Cosmetics=== |
|||
In [[shampoo]]s, cleaners, and other personal care products, EDTA salts are used as a sequestering agent to improve their stability in air.<ref name="lanigan2002" /> |
|||
===Laboratory applications=== |
|||
In the laboratory, EDTA is widely used for scavenging metal ions: In [[biochemistry]] and [[molecular biology]], ion depletion is commonly used to deactivate [[metalloenzyme|metal-dependent enzyme]]s, either as an assay for their reactivity or to suppress damage to [[DNA]], [[protein]]s, and [[polysaccharide]]s.<ref>{{cite journal |title=A novel nuclease activity that is activated by Ca<sup>2+</sup> chelated to EGTA |last1=Domínguez |first1=K. |last2=Ward |first2=W. S. |journal=[[Systems Biology in Reproductive Medicine]] |date=December 2009 |volume=55 |issue=5–6|doi=10.3109/19396360903234052 |pmid=19938954 |pages=193–199|pmc=2865586 }}</ref> EDTA also acts as a selective [[Enzyme inhibitor|inhibitor]] against dNTP hydrolyzing enzymes ([[Taq polymerase]], [[DUTP diphosphatase|dUTPase]], MutT),<ref>{{Cite journal|last1=Lopata|first1=Anna|last2=Jójárt|first2=Balázs|last3=Surányi|first3=Éva V.|last4=Takács|first4=Enikő|last5=Bezúr|first5=László|last6=Leveles|first6=Ibolya|last7=Bendes|first7=Ábris Á|last8=Viskolcz|first8=Béla|last9=Vértessy|first9=Beáta G.|last10=Tóth|first10=Judit|date=October 2019|title=Beyond Chelation: EDTA Tightly Binds Taq DNA Polymerase, MutT and dUTPase and Directly Inhibits dNTPase Activity|journal=Biomolecules|volume=9|issue=10|page=621|doi=10.3390/biom9100621|pmid=31627475|pmc=6843921|doi-access=free}}</ref> liver [[arginase]]<ref>{{Cite journal|last1=Carvajal|first1=Nelson|last2=Orellana|first2=María S|last3=Bórquez|first3=Jessica|last4=Uribe|first4=Elena|last5=López|first5=Vasthi|last6=Salas|first6=Mónica|date=2004-08-01|title=Non-chelating inhibition of the H101N variant of human liver arginase by EDTA|journal=Journal of Inorganic Biochemistry|volume=98|issue=8|pages=1465–1469|doi=10.1016/j.jinorgbio.2004.05.005|pmid=15271525|issn=0162-0134}}</ref> and [[horseradish peroxidase]]<ref>{{Cite journal|last1=Bhattacharyya|first1=D K|last2=Adak|first2=S|last3=Bandyopadhyay|first3=U|last4=Banerjee|first4=R K|date=1994-03-01|title=Mechanism of inhibition of horseradish peroxidase-catalysed iodide oxidation by EDTA.|journal=Biochemical Journal|volume=298|issue=Pt 2|pages=281–288|issn=0264-6021|pmc=1137937|pmid=8135732|doi=10.1042/bj2980281}}</ref> independently of metal ion [[chelation]]. These findings urge the rethinking of the utilisation of EDTA as a biochemically inactive metal ion scavenger in enzymatic experiments. In analytical chemistry, EDTA is used in [[complexometric titration]]s and analysis of [[water hardness]] or as a [[masking agent]] to sequester metal ions that would interfere with the analyses. |
|||
EDTA finds many specialised uses in the biomedical labs, such as in [[veterinary]] [[ophthalmology]] as an [[collagenase|anticollagenase]] to prevent the worsening of [[corneal ulcers in animals]]. In [[tissue culture]], EDTA is used as a chelating agent that binds to [[calcium]] and prevents joining of [[cadherins]] between cells, preventing clumping of cells grown in liquid suspension, or detaching adherent cells for [[passaging]]. In [[histopathology]], EDTA can be used as a decalcifying agent making it possible to cut sections using a [[microtome]] once the tissue sample is demineralised. |
|||
EDTA is also known to inhibit a range of [[Metalloproteinase|metallopeptidases]], the method of inhibition occurs via the [[chelation]] of the metal ion required for catalytic activity.<ref>{{Cite book| pmid = 7674923| year = 1995| last1 = Auld| first1 = D. S.| chapter = Removal and replacement of metal ions in metallopeptidases| title = Proteolytic Enzymes: Aspartic and Metallo Peptidases| series = Methods in Enzymology| volume = 248| pages = 228–242 | doi = 10.1016/0076-6879(95)48016-1 | isbn = 978-0-12-182149-4}}</ref> EDTA can also be used to test for [[bioavailability]] of heavy metals in [[sediment]]s. However, it may ''influence'' the bioavailability of metals in solution, which may pose concerns regarding its effects in the environment, especially given its widespread uses and applications. |
|||
===Other=== |
|||
The oxidising properties of [Fe(EDTA)]<sup>−</sup> are used in [[photography]] to solubilise [[silver]] particles.<ref name="Ullmann" /> |
|||
EDTA is also used to remove crud (corroded metals) from fuel rods in nuclear reactors.<ref>{{cite journal |url=https://www.sciencedirect.com/science/article/pii/B9780124058972000203 |first1=Gregory |last1=Choppin |first2=Jan-Olov |last2=Liljenzin |first3=Jan |last3=Rydberg |first4=Christian |last4=Ekberg |date=2013 |title=Chapter 20 - Nuclear Power Reactors |journal=Radiochemistry and Nuclear Chemistry |edition=Fourth |pages=655–684 |doi=10.1016/B978-0-12-405897-2.00020-3|isbn=978-0-12-405897-2 }}</ref> |
|||
==Side effects== |
|||
EDTA exhibits low acute toxicity with {{LD50}} (rat) of 2.0 g/kg to 2.2 g/kg.<ref name="Ullmann"/> It has been found to be both [[Cytotoxicity|cytotoxic]] and weakly [[Genotoxicity|genotoxic]] in laboratory animals. Oral exposures have been noted to cause reproductive and developmental effects.<ref name="lanigan2002">{{cite journal|last1=Lanigan |first1=R. S. |last2=Yamarik |first2=T. A. | title=Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA | journal=International Journal of Toxicology |volume='''21''' Suppl. 2 |pages=95–142 |year=2002 |pmid=12396676|doi=10.1080/10915810290096522 | issue=5|s2cid=83388249 }}</ref> The same study<ref name="lanigan2002" /> also found that both dermal exposure to EDTA in most cosmetic formulations and inhalation exposure to EDTA in [[aerosol]]ised cosmetic formulations would produce exposure levels below those seen to be toxic in oral dosing studies. |
|||
==Synthesis== |
|||
The compound was first described in 1935 by [[Ferdinand Münz]],<ref>{{cite journal |last=Paolieri |first=Matteo |date=December 2017 |title= Ferdinand Münz: EDTA and 40 years of inventions |url=https://www.researchgate.net/publication/321552574 |journal=Bull. Hist. Chem. |publisher=ACS | volume=42 |issue=2 |pages=133–140}}</ref> who prepared the compound from [[ethylenediamine]] and [[chloroacetic acid]].<ref>{{cite patent|inventor-last=Münz|inventor-first=Ferdinand|inventor-link=Ferdinand_Münz|pubdate=1938-09-20|title=Polyamino carboxylic acids and process of making same|assign1=[[American IG|General Aniline Works Ltd.]]|country=US|number=2130505}}. Also {{cite patent|inventor-last=Münz|inventor-first=Ferdinand|assign1=[[IG Farben|I. G. Farbenindustrie]]|country=DE|number=718981|pubdate=1938-09-20|title=Verfahren zum Unschädlichmachen der Härtebildner des Wassers [Process for rendering the hardness components of water harmless]|inventor-link=Ferdinand_Münz}}</ref> Today, EDTA is mainly synthesised from [[ethylenediamine]] (1,2-diaminoethane), [[formaldehyde]], and [[sodium cyanide]].<ref>{{cite web|url=http://www.chm.bris.ac.uk/motm/edta/synthesis_of_edta.htm|title=Industrial Synthesis of EDTA|publisher=University of Bristol}}</ref> This route yields the tetrasodium EDTA, which is converted in a subsequent step into the acid forms: |
|||
:H<sub>2</sub>NCH<sub>2</sub>CH<sub>2</sub>NH<sub>2</sub> + 4 [[formaldehyde|CH<sub>2</sub>O]] + 4 [[sodium cyanide|NaCN]] + 4 H<sub>2</sub>O → (NaO<sub>2</sub>CCH<sub>2</sub>)<sub>2</sub>NCH<sub>2</sub>CH<sub>2</sub>N(CH<sub>2</sub>CO<sub>2</sub>Na)<sub>2</sub> + 4 [[ammonia|NH<sub>3</sub>]] |
|||
:(NaO<sub>2</sub>CCH<sub>2</sub>)<sub>2</sub>NCH<sub>2</sub>CH<sub>2</sub>N(CH<sub>2</sub>CO<sub>2</sub>Na)<sub>2</sub> + 4 [[hydrochloric acid|HCl]] → (HO<sub>2</sub>CCH<sub>2</sub>)<sub>2</sub>NCH<sub>2</sub>CH<sub>2</sub>N(CH<sub>2</sub>CO<sub>2</sub>H)<sub>2</sub> + 4 [[sodium chloride|NaCl]] |
|||
This process is used to produce about 80,000 tonnes of EDTA each year. Impurities cogenerated by this route include [[glycine]] and [[nitrilotriacetic acid]]; they arise from reactions of the [[ammonia]] coproduct.<ref name="Ullmann">{{Ullmann|last=Hart|first=J. Roger|date=2005|title=Ethylenediaminetetraacetic Acid and Related Chelating Agents|doi=10.1002/14356007.a10_095}}</ref> |
|||
==Nomenclature== |
|||
To describe EDTA and its various [[Protonation|protonated forms]], chemists distinguish between EDTA<sup>4−</sup>, the [[conjugate base]] that is the [[ligand]], and H<sub>4</sub>EDTA, the [[precursor (chemistry)|precursor]] to that ligand. At very low pH (very acidic conditions) the fully protonated H<sub>6</sub>EDTA<sup>2+</sup> form predominates, whereas at very high pH or very basic condition, the fully deprotonated EDTA<sup>4−</sup> form is prevalent. In this article, the term EDTA is used to mean H<sub>4−''x''</sub>EDTA<sup>''x''−</sup>, whereas in its complexes EDTA<sup>4−</sup> stands for the tetraanion ligand. |
|||
==Coordination chemistry principles== |
|||
[[Image:Metal-EDTA.svg|thumb|Metal–EDTA [[chelate]] as found in Co(III) complexes]] |
|||
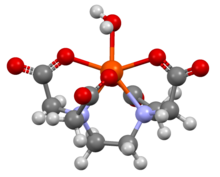
[[File:SFEDTD01.png|thumb|Structure of [Fe(EDTA)(H<sub>2</sub>O)]<sup>−</sup>, showing that the EDTA<sup>4−</sup> ligand does not fully encapsulate [[iron|Fe(III)]], which is seven-coordinate<ref>{{cite journal|last1=Solans |first1=X. |last2=Font Altaba |first2=M. |last3=García Oricain |first3=J.|title=Crystal Structures of Ethylenediaminetetraacetato Metal Complexes. V. Structures Containing the [Fe(C<sub>10</sub>H<sub>12</sub>N<sub>2</sub>O<sub>8</sub>)(H<sub>2</sub>O)]<sup>−</sup> Anion|journal=Acta Crystallographica Section C|year=1984|volume=40|issue=4 |pages=635–638|doi=10.1107/S0108270184005151}}</ref>]] |
|||
In [[Coordination complex|coordination chemistry]], EDTA<sup>4−</sup> is a member of the [[aminopolycarboxylic acid]] family of ligands. EDTA<sup>4−</sup> usually binds to a metal cation through its two amines and four carboxylates, i.e., it is a [[hexadentate]] ("six-toothed") [[chelating agent]]. Many of the resulting [[complex (chemistry)|coordination compound]]s adopt [[octahedral geometry]]. Although of little consequence for its applications, these octahedral complexes are [[Chirality (chemistry)|chiral]]. The [[cobalt|cobalt(III)]] anion [Co(EDTA)]<sup>−</sup> has been resolved into [[enantiomer]]s.<ref>{{Cite book | last1 = Kirchner | first1 = S. | last2 = Gyarfas | first2 = Eleonora C. | chapter = Barium (Ethylenediaminetetraacetato)cobaltate(III) 4-Hydrate | year = 1957 | title = Inorganic Syntheses | volume = 5 | pages = 186–188 | doi = 10.1002/9780470132364.ch52 | isbn = 978-0-470-13236-4 }}</ref> Many complexes of EDTA<sup>4−</sup> adopt more complex structures due to either the formation of an additional bond to water, ''i.e.'' seven-coordinate complexes, or the displacement of one carboxylate arm by water. The [[iron|iron(III)]] [[Ferric EDTA|complex]] of EDTA is seven-coordinate.<ref>{{cite journal | last1 = López Alcalá | first1 = J. M. | last2 = Puerta Vizcaíno | first2 = M. C. | last3 = González Vílchez | first3 = F. | last4 = Duesler | first4 = E. N. | last5 = Tapscott | first5 = R. E. | year = 1984 | title = A redetermination of sodium aqua[ethylenediaminetetraacetato(4−)]ferrate(III) dihydrate, Na[Fe(C<sub>10</sub>H<sub>12</sub>N<sub>2</sub>O<sub>8</sub>)(H<sub>2</sub>O)]·2H<sub>2</sub>O| journal = Acta Crystallogr C | volume = 40 | issue = 6 | pages = 939–941 | doi = 10.1107/S0108270184006338 | doi-access = | bibcode = 1984AcCrC..40..939L }}</ref> Early work on the development of EDTA was undertaken by [[Gerold Schwarzenbach]] in the 1940s.<ref>{{cite web|last=Sinex |first=Scott A. |url=http://www.chm.bris.ac.uk/motm/edta/edtah.htm |title=EDTA – A Molecule with a Complex Story |publisher=University of Bristol}}</ref> EDTA forms especially strong complexes with [[manganese|Mn(II)]], [[copper|Cu(II)]], Fe(III), [[lead|Pb(II)]] and Co(III).<ref>{{cite book | last1 = Holleman | first1 = A. F. |last2=Wiberg |first2=E. | title = Inorganic Chemistry | publisher = Academic Press | location = San Diego | year = 2001 | isbn = 978-0-12-352651-9}}</ref>{{page needed|date=March 2018}} |
|||
Several features of EDTA's complexes are relevant to its applications. First, because of its high [[denticity]], this ligand has a high affinity for metal cations: |
|||
:[Fe(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup> + H<sub>4</sub>EDTA {{eqm}} [Fe(EDTA)]<sup>−</sup> + 6 H<sub>2</sub>O + 4 H<sup>+</sup> {{pad|2em}}[[Equilibrium constant|''K''<sub>eq</sub>]] = 10<sup>25.1</sup> |
|||
Written in this way, the [[Stability constants of complexes|equilibrium quotient]] shows that metal ions compete with protons for binding to EDTA. Because metal ions are extensively enveloped by EDTA, their [[catalysis|catalytic properties]] are often suppressed. Finally, since complexes of EDTA<sup>4−</sup> are [[anion]]ic, they tend to be highly soluble in water. For this reason, EDTA is able to dissolve deposits of [[metal oxide]]s and [[carbonate]]s. |
|||
The [[Acid dissociation constant|p''K''<sub>a</sub> values]] of free EDTA are 0, 1.5, 2, 2.66 ([[deprotonation]] of the four [[carboxyl group]]s) and 6.16, 10.24 (deprotonation of the two [[amino group]]s).<ref name="Latscha">Hans Peter Latscha: ''Analytische Chemie.'' Springer-Verlag, 2013, {{ISBN|978-3-642-18493-2}}, p. 303.</ref> |
|||
==Environmental concerns== |
|||
===Abiotic degradation=== |
|||
EDTA is in such widespread use that questions have been raised whether it is a [[persistent organic pollutant]]. While EDTA serves many positive functions in different industrial, pharmaceutical and other avenues, the longevity of EDTA can pose serious issues in the environment. The degradation of EDTA is slow. It mainly occurs [[abiotic]]ally in the presence of sunlight.<ref name=Bucheli>{{citation|last1=Bucheli-Witschel |first1=M. |last2=Egli |first2=T. | journal= FEMS Microbiology Reviews| title=DAB: Environmental Fate and Microbial Degradation of Aminopolycarboxylic Acids| volume=25| issue= 1| pages=69–106| year=2001| doi=10.1111/j.1574-6976.2001.tb00572.x| pmid= 11152941 | doi-access=free}}</ref> |
|||
The most important process for the elimination of EDTA from surface waters is direct [[photolysis]] at wavelengths below 400 nm.<ref>{{Cite thesis |last= Kari |first= F. G.|title= Umweltverhalten von Ethylenediaminetetraacetate (EDTA) under spezieller Berucksuchtigung des photochemischen Ab-baus.|type=PhD |year= 1994 |publisher= Swiss Federal Institute of Technology}}</ref> Depending on the light conditions, the photolysis [[half-life|half-lives]] of iron(III) EDTA in surface waters can range as low as 11.3 minutes up to more than 100 hours.<ref>{{cite journal |last1= Frank|first1= R.|last2= Rau|first2=H. |year=1989 |title= Photochemical transformation in aqueous solution and possible environmental fate of Ethylenediaminetetraacetatic acid (EDTA)|journal= Ecotoxicology and Environmental Safety |volume= 19 |issue= 1|pages=55–63 |doi=10.1016/0147-6513(90)90078-j|pmid= 2107071}}</ref> Degradation of FeEDTA, but not EDTA itself, produces iron complexes of the triacetate (ED3A), diacetate (EDDA), and monoacetate (EDMA) – 92% of EDDA and EDMA biodegrades in 20 hours while ED3A displays significantly higher resistance. Many environmentally-abundant EDTA species (such as [[magnesium|Mg<sup>2+</sup>]] and [[calcium|Ca<sup>2+</sup>]]) are more persistent. |
|||
===Biodegradation=== |
|||
In many [[industrial wastewater treatment]] plants, EDTA elimination can be achieved at about 80% using [[microorganisms]].<ref>{{cite journal |last1= Kaluza|first1= U.|last2= Klingelhofer|first2=P. |first3 = Taeger |last3=K. |year=1998 |title= Microbial degradation of EDTA in an industrial wastewater treatment plant |journal= Water Research |volume= 32 |issue= 9|pages=2843–2845 |doi=10.1016/S0043-1354(98)00048-7|bibcode= 1998WatRe..32.2843K}}</ref> Resulting byproducts are ED3A and [[iminodiacetic acid]] (IDA) – suggesting that both the backbone and acetyl groups were attacked. Some microorganisms have even been discovered to form nitrates out of EDTA, but they function optimally at moderately alkaline conditions of pH 9.0–9.5.<ref>{{cite journal |last1= VanGinkel|first1= C. G. |last2= Vandenbroucke|first2=K. L. |first3 = Troo |last3=C. A. |year=1997 |title= Biological removal of EDTA in conventional activated-sludge plants operated under alkaline conditions |journal= Bioresource Technology |volume= 32 |issue= 2–3 |pages=2843–2845 |doi=10.1016/S0960-8524(96)00158-7|bibcode= 1997BiTec..59..151V }}</ref> |
|||
Several bacterial strains isolated from sewage treatment plants efficiently degrade EDTA. Specific strains include ''[[Agrobacterium radiobacter]]'' ATCC 55002<ref>{{cite journal |last1= Lauff|first1= J. J. |last2= Steele|first2=D. B. |last3 = Coogan |first3=L. A. |last4 = Breitfeller |first4=J. M.|year=1990 |title= Degradation of the ferric chelate of EDTA by a pure culture of an ''Agrobacterium'' sp. |pmid=16348340|pmc=184952 |journal= Applied and Environmental Microbiology |volume= 56 |pages=3346–3353 |issue=11|doi= 10.1128/AEM.56.11.3346-3353.1990 |bibcode= 1990ApEnM..56.3346L }}</ref> and the sub-branches of [[Pseudomonadota]] like BNC1, BNC2,<ref name="Nortemannl 1992 671–676">{{cite journal |last= Nortemannl|first= B |year=1992 |title= Total degradation of EDTA by mixed culturesand a bacterial isolate |journal= Applied and Environmental Microbiology |volume= 58 |pages=671–676 |issue=2|doi= 10.1128/AEM.58.2.671-676.1992 |pmid= 16348653 |pmc= 195300 |bibcode= 1992ApEnM..58..671N }}</ref> and strain DSM 9103.<ref>{{cite speech |title=Degradation of EDTA by a bacterial isolate. Poster presented at the 45th Annual Meeting of the Swiss Society for Microbiology |last1=Witschel |first1=M. |last2=Weilemann |first2=H.-U. |last3=Egli |first3=T. |year=1995 |location= Lugano, Switzerland}}</ref> The three strains share similar properties of [[aerobic respiration]] and are classified as [[gram-negative bacteria]]. Unlike photolysis, the chelated species is not exclusive to iron(III) in order to be degraded. Rather, each strain uniquely consumes varying metal–EDTA complexes through several enzymatic pathways. Agrobacterium radiobacter only degrades Fe(III) EDTA<ref name="Nortemannl 1992 671–676"/> while BNC1 and DSM 9103 are not capable of degrading iron(III) EDTA and are more suited for [[calcium]], [[barium]], [[magnesium]] and [[manganese|manganese(II)]] complexes.<ref>{{cite journal |last1= Hennekenl|first1= L. |last2=Nortemann |first2=B. |last3 = Hempel |first3=D. C. |year=1995 |title= Influence of physiological conditions on EDTA degradation |journal= Applied and Environmental Microbiology |volume= 44 |issue= 1–2 |pages=190–197 |doi=10.1007/bf00164501|s2cid= 30072817 }}</ref> EDTA complexes require dissociation before degradation. |
|||
==Alternatives to EDTA== |
|||
Interest in environmental safety has raised concerns about biodegradability of [[aminopolycarboxylates]] such as EDTA. These concerns incentivize the investigation of alternative aminopolycarboxylates.<ref name=Bucheli/> Candidate chelating agents include [[nitrilotriacetic acid]] (NTA), iminodisuccinic acid (IDS), [[polyaspartic acid]], [[EDDS|''S,S''-ethylenediamine-''N'',''N''′-disuccinic acid (EDDS)]], methylglycinediacetic acid (MGDA), and <small>L</small>-Glutamic acid ''N'',''N''-diacetic acid, tetrasodium salt (GLDA).<ref>{{cite journal|doi=10.1021/es0348750|pmid=14968886|title=Extraction of Heavy Metals from Soils Using Biodegradable Chelating Agents|journal=Environmental Science & Technology|volume=38|issue=3|pages=937–944|year=2004|last1=Tandy|first1=Susan|last2=Bossart|first2=Karin|last3=Mueller|first3=Roland|last4=Ritschel|first4=Jens|last5=Hauser|first5=Lukas|last6=Schulin|first6=Rainer|last7=Nowack|first7=Bernd|bibcode=2004EnST...38..937T}}</ref> |
|||
===Iminodisuccinic acid (IDS)=== |
|||
Commercially used since 1998, [[Tetrasodium iminodisuccinate|iminodisuccinic acid]] (IDS) biodegrades by about 80% after only 7 days. IDS binds to calcium exceptionally well and forms stable compounds with other heavy metal ions. In addition to having a lower toxicity after chelation, IDS is degraded by ''[[Agrobacterium tumefaciens]]'' (BY6), which can be harvested on a large scale. The enzymes involved, [[IDS epimerase]] and C−N [[lyase]], do not require any [[Cofactor (biochemistry)|cofactors]].<ref>{{citation|last1=Cokesa |first1=Z. |last2=Knackmuss |first2=H. |last3=Rieger |first3=P. | journal= Applied and Environmental Microbiology| title=Biodegradation of All Stereoisomers of the EDTA Substitute Iminodisuccinate by Agrobacterium Tumefaciens BY6 Requires an Epimerase and a Stereoselective C−N Lyase| volume=70| issue= 7| pages=3941–3947| year=2004| doi=10.1128/aem.70.7.3941-3947.2004| pmid= 15240267| pmc= 444814 |bibcode=2004ApEnM..70.3941C }}</ref> |
|||
===Polyaspartic acid=== |
|||
[[Polyaspartic acid]], like IDS, binds to calcium and other heavy metal ions. It has many practical applications including corrosion inhibitors, wastewater additives, and agricultural polymers. A Polyaspartic acid-based [[laundry detergent]] was the first laundry detergent in the world to receive the [[EU Ecolabel|EU flower ecolabel]].<ref name ="Roweton 1997" >{{Ullmann|chapter=Polyaspartates and Polysuccinimide |author=Thomas Klein |author2=Ralf-Johann Moritz |author3=René Graupner |year= 2008|doi=10.1002/14356007.l21_l01}}</ref> Calcium binding ability of polyaspartic acid has been exploited for targeting of drug-loaded nanocarriers to bone.<ref>{{Cite journal|last1=Adelnia|first1=Hossein|last2=Tran|first2=Huong D.N.|last3=Little|first3=Peter J.|last4=Blakey|first4=Idriss|last5=Ta|first5=Hang T.|date=2021-06-14|title=Poly(aspartic acid) in Biomedical Applications: From Polymerization, Modification, Properties, Degradation, and Biocompatibility to Applications|journal=ACS Biomaterials Science & Engineering|volume=7|issue=6|pages=2083–2105|doi=10.1021/acsbiomaterials.1c00150|pmid=33797239|hdl=10072/404497 |s2cid=232761877|hdl-access=free}}</ref> Preparation of [[hydrogel]]s based on polyaspartic acid, in a variety of physical forms ranging from [[fiber]] to [[particle]], can potentially enable facile separation of the chelated ions from a solution.<ref>{{Cite journal|last1=Adelnia|first1=Hossein|last2=Blakey|first2=Idriss|last3=Little|first3=Peter J.|last4=Ta|first4=Hang T.|date=2019|title=Hydrogels Based on Poly(aspartic acid): Synthesis and Applications|journal=Frontiers in Chemistry|volume=7|page=755|language=English|doi=10.3389/fchem.2019.00755|pmid=31799235|pmc=6861526|bibcode=2019FrCh....7..755A|issn=2296-2646|doi-access=free}}</ref> Therefore, despite being weaker than EDTA, polyaspartic acid can still be regarded as a viable alternative due to these features as well as [[biocompatibility]], and [[Biodegradation|biodegradability]].<ref>{{Cite journal|last1=Hasson|first1=David|last2=Shemer|first2=Hilla|last3=Sher|first3=Alexander|date=2011-06-15|title=State of the Art of Friendly "Green" Scale Control Inhibitors: A Review Article|journal=Industrial & Engineering Chemistry Research|volume=50|issue=12|pages=7601–7607|doi=10.1021/ie200370v|issn=0888-5885}}</ref> |
|||
===''S'',''S''-Ethylenediamine-''N'',''N''′-disuccinic acid (EDDS)=== |
|||
A [[structural isomer]] of EDTA, [[EDDS|ethylenediamine-''N'',''N''′-disuccinic acid]] (EDDS) is readily biodegradable at high rate in its ''S'',''S'' form.<ref>{{cite journal |author1=Tandy, S. |author2=Ammann, A. |author3=Schulin, R. |author-link3=Rainer Schulin |author4=Nowack, B. |year=2006 |title=Biodegredation and speciation of residual SS-ethylenediaminedisuccinic acid (EDDS) in soil solution left after soil washing |journal=Environmental Pollution |volume=142 |issue=2 |pages=191–199 |doi=10.1016/j.envpol.2005.10.013 |pmid=16338042|bibcode=2006EPoll.142..191T }}</ref> |
|||
===Methylglycinediacetic acid (MGDA)=== |
|||
[[Trisodium dicarboxymethyl alaninate]], also known as methylglycinediacetic acid (MGDA), has a high rate of biodegradation at over 68%, but unlike many other chelating agents can degrade without the assistance of adapted bacteria. Additionally, unlike EDDS or IDS, MGDA can withstand higher temperatures while maintaining a high stability as well as the entire pH range.{{Citation needed|date=December 2019|reason=removed citation to predatory publisher content}} MGDA has been shown to be an effective chelating agent, with a capacity for mobilization comparable with that of [[nitrilotriacetic acid]] (NTA), with application to water for industrial use and for the removal of [[calcium oxalate]] from urine from patients with [[kidney stone]]s.<ref>{{cite journal |last1=Bretti |first1=Clemente |last2=Cigala |first2=Rosalia Maria |last3=De Stefano |first3=Concetta |last4=Lando |first4=Gabriele |last5=Sammartano |first5=Silvio |title=Thermodynamic solution properties of a biodegradable chelant (MGDA) and its interaction with the major constituents of natural fluids |journal=Fluid Phase Equilibria |date=2017 |volume=434 |pages=63–73 |doi=10.1016/j.fluid.2016.11.027|bibcode=2017FlPEq.434...63B }}</ref> |
|||
==Methods of detection and analysis== |
|||
The most sensitive method of detecting and measuring EDTA in biological samples is selected reaction monitoring [[capillary electrophoresis]] [[liquid chromatography-mass spectrometry|mass spectrometry]] (SRM-CE/MS), which has a [[detection limit]] of 7.3 ng/mL in human plasma and a [[Detection limit|quantitation limit]] of 15 ng/mL.<ref name="sheppard">{{Cite journal | doi = 10.1021/ac971726p| pmid = 9253241| title = Peer Reviewed: Determining EDTA in Blood| journal = Analytical Chemistry| volume = 69| issue = 15| pages = 477A–480A| year = 1997| last1 = Sheppard | first1 = R. L. | last2 = Henion | first2 = J. }}</ref> This method works with sample volumes as small as 7–8 nL.<ref name="sheppard" /> |
|||
EDTA has also been measured in non-alcoholic beverages using [[High-performance liquid chromatography|high performance liquid chromatography]] (HPLC) at a level of 2.0 μg/mL.<ref>{{Cite journal | doi = 10.1007/s002160051422| title = Optimisation of the analytical detection of EDTA by HPLC in natural waters| journal = Fresenius' Journal of Analytical Chemistry| volume = 364| issue = 8| page = 727| year = 1999| last1 = Loyaux-Lawniczak | first1 = S. | last2 = Douch | first2 = J. | last3 = Behra | first3 = P.| s2cid = 95648833}}</ref><ref>{{Cite journal | doi = 10.1016/j.jfca.2006.05.008| title = Development and validation of a method for the determination of EDTA in non-alcoholic drinks by HPLC| journal = Journal of Food Composition and Analysis| volume = 20| issue = 3–4| page = 248| year = 2007| last1 = Cagnasso | first1 = C. E. | last2 = López | first2 = L. B. | last3 = Rodríguez | first3 = V. G. | last4 = Valencia | first4 = M. E. }}</ref> |
|||
== In popular culture == |
|||
'''EDTA''' is a widely used initialism for the [[chemical compound]] '''ethylenediaminetetraacetic acid''' (which has many other names, see Table). EDTA is a [[polyamino carboxylic acid]] with the [[chemical formula|formula]] [CH<sub>2</sub>N(CH<sub>2</sub>CO<sub>2</sub>H)<sub>2</sub>]<sub>2</sub>. This colourless, water-soluble solid is widely used to dissolve scale. Its usefulness arises because of its role as a [[chelating agent]], i.e. its ability to "sequester" [[metal]] [[ion]]s such as Ca<sup>2+</sup> and Fe<sup>3+</sup>. After being bound by EDTA, metal ions remain in solution but exhibit diminished reactivity. EDTA is produced as several salts, notably disodium EDTA and calcium disodium EDTA. |
|||
In the movie ''[[Blade (1998 film)|Blade]]'' (1998), EDTA is used as a weapon to kill vampires, exploding when in contact with vampire blood.<ref>{{cite web |title=Blade (1998) |website=Internet Movie Database ([[IMDb]])|url=https://imdb.com/title/tt0120611/goofs/#:~:text=when%20a,grenade |access-date=2022-11-14}}</ref> |
|||
No I did |
|||
==References== |
|||
No I did |
|||
{{reflist|30em}} |
|||
==External links== |
|||
... |
|||
* [http://www.chm.bris.ac.uk/motm/edta/edtah.htm EDTA: Molecule of the Month] |
|||
* [https://web.arhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhchive.org/web/20061029103725/http://www.chem.utk.edu/~chem319/Experiments/Exp6.pdf EDTA Determination of Total Water Hardness] |
|||
*{{cite journal |doi=10.1590/S0100-40422003000600020 |title=EDTA: The chelating agent under environmental scrutiny |journal=Química Nova |volume=26 |issue=6 |pages=901–905 |year=2003 |last1=Oviedo |first1=Claudia |last2=Rodríguez |first2=Jaime |doi-access=free }} |
|||
{{Antidotes}} |
|||
== Notes & References== |
|||
{{ |
{{antithrombotics}} |
||
{{Chelating agents}} |
|||
{{Endodontology}} |
|||
{{Consumer Food Safety}} |
|||
{{DEFAULTSORT:Ethylenediaminetetraacetic Acid}} |
|||
... |
|||
[[Category:Acetic acids]] |
|||
[[Category:Diamines]] |
|||
[[Category:Antidotes]] |
|||
[[Category:Chelating agents]] |
|||
[[Category:Photographic chemicals]] |
|||
[[Category:Preservatives]] |
|||
[[Category:E-number additives]] |
|||
[[Category:Hexadentate ligands]] |
|||
[[Category:Ophthalmology drugs]] |
|||
Latest revision as of 00:59, 24 December 2024
| |
| Names | |
|---|---|
| IUPAC name
N,N′-(Ethane-1,2-diyl)bis[N-(carboxymethyl)glycine][1]
| |
| Systematic IUPAC name
2,2′,2′′,2′′′-(Ethane-1,2-diyldinitrilo)tetraacetic acid[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | EDTA, H4EDTA |
| 1716295 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.409 |
| EC Number |
|
| 144943 | |
| KEGG | |
| MeSH | Edetic+Acid |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| UN number | 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H16N2O8 | |
| Molar mass | 292.244 g·mol−1 |
| Appearance | Colourless crystals |
| Density | 0.860 g cm−3 (at 20 °C) |
| log P | −0.836 |
| Acidity (pKa) | 2.0, 2.7, 6.16, 10.26[2] |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−1765.4 to −1758.0 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−4461.7 to −4454.5 kJ mol−1 |
| Pharmacology | |
| S01XA05 (WHO) V03AB03 (WHO) (salt) | |
| |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H319 | |
| P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1000 mg/kg (oral, rat)[3] |
| Related compounds | |
Related alkanoic acids
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ethylenediaminetetraacetic acid (EDTA), also called EDTA acid, is an aminopolycarboxylic acid with the formula [CH2N(CH2CO2H)2]2. This white, slightly water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes even at neutral pH. It is thus used to dissolve Fe- and Ca-containing scale as well as to deliver iron ions under conditions where its oxides are insoluble. EDTA is available as several salts, notably disodium EDTA, sodium calcium edetate, and tetrasodium EDTA, but these all function similarly.[4]
Uses
[edit]EDTA Is widely used in industry. It also has applications in food preservation, medicine, cosmetics, water softening, in laboratories, and other fields.
Industrial
[edit]EDTA is mainly used to sequester (bind or confine) metal ions in aqueous solution. In the textile industry, it prevents metal ion impurities from modifying colours of dyed products. In the pulp and paper industry, EDTA inhibits the ability of metal ions, especially Mn2+, from catalysing the disproportionation of hydrogen peroxide, which is used in chlorine-free bleaching.
Gas scrubbing
[edit]Aqueous [Fe(EDTA)]− is used for removing ("scrubbing") hydrogen sulfide from gas streams. This conversion is achieved by oxidising the hydrogen sulfide to elemental sulfur, which is non-volatile:
In this application, the iron(III) centre is reduced to its iron(II) derivative, which can then be reoxidised by air. In a similar manner, nitrogen oxides are removed from gas streams using [Fe(EDTA)]2−.
Food
[edit]In a similar manner, EDTA is added to some food as a preservative or stabiliser to prevent catalytic oxidative decolouration, which is catalysed by metal ions.[5]
Water softener
[edit]The reduction of water hardness in laundry applications and the dissolution of scale in boilers both rely on EDTA and related complexants to bind Ca2+, Mg2+, as well as other metal ions. Once bound to EDTA, these metal complexes are less likely to form precipitates or to interfere with the action of the soaps and detergents.[citation needed] For similar reasons, cleaning solutions often contain EDTA. In a similar manner EDTA is used in the cement industry for the determination of free lime and free magnesia in cement and clinkers.[6][page needed]
The solubilisation of Fe3+ ions at or below near neutral pH can be accomplished using EDTA. This property is useful in agriculture including hydroponics. However, given the pH dependence of ligand formation, EDTA is not helpful for improving iron solubility in above neutral soils.[7] Otherwise, at near-neutral pH and above, iron(III) forms insoluble salts, which are less bioavailable to susceptible plant species.
Ion-exchange chromatography
[edit]EDTA was used in separation of the lanthanide metals by ion-exchange chromatography. Perfected by F. H. Spedding et al. in 1954, the method relies on the steady increase in stability constant of the lanthanide EDTA complexes with atomic number.[8] Using sulfonated polystyrene beads and Cu2+ as a retaining ion, EDTA causes the lanthanides to migrate down the column of resin while separating into bands of pure lanthanides. The lanthanides elute in order of decreasing atomic number. Due to the expense of this method, relative to countercurrent solvent extraction, ion exchange is now used only to obtain the highest purities of lanthanides (typically greater than 99.99%).[citation needed]
Medicine
[edit]Sodium calcium edetate, an EDTA derivative, is used to bind metal ions in the practice of chelation therapy, such as for treating mercury and lead poisoning.[9] It is used in a similar manner to remove excess iron from the body. This therapy is used to treat the complication of repeated blood transfusions, as would be applied to treat thalassaemia.
In testing
[edit]In medical diagnosis and organ function tests (here, kidney function test), the chromium(III) complex [Cr(EDTA)]− (as radioactive chromium-51 (51Cr)) is administered intravenously and its filtration into the urine is monitored. This method is useful for evaluating glomerular filtration rate (GFR) in nuclear medicine.[10]
EDTA is used extensively in the analysis of blood. It is an anticoagulant for blood samples for CBC/FBCs, where the EDTA chelates the calcium present in the blood specimen, arresting the coagulation process and preserving blood cell morphology.[11] Tubes containing EDTA are marked with lavender (purple) or pink tops.[12] EDTA is also in tan top tubes for lead testing and can be used in royal blue top tubes for trace metal testing.[12]
EDTA is a slime dispersant, and has been found to be highly effective in reducing bacterial growth during implantation of intraocular lenses (IOLs).[13]
Dentistry
[edit]Dentists and endodontists use EDTA solutions to remove inorganic debris (smear layer) and lubricate the root canals in endodontics. This procedure helps prepare root canals for obturation. Furthermore, EDTA solutions with the addition of a surfactant loosen up calcifications inside a root canal and allow instrumentation (canal shaping) and facilitate apical advancement of a file in a tight or calcified root canal towards the apex.
Eyedrops
[edit]It serves as a preservative (usually to enhance the action of another preservative such as benzalkonium chloride or thiomersal) in ocular preparations and eyedrops.
Alternative medicine
[edit]Some alternative practitioners believe EDTA acts as an antioxidant, preventing free radicals from injuring blood vessel walls, therefore reducing atherosclerosis.[14] These ideas are unsupported by scientific studies, and seem to contradict some currently accepted principles.[15] The U.S. FDA has not approved it for the treatment of atherosclerosis.[16]
Cosmetics
[edit]In shampoos, cleaners, and other personal care products, EDTA salts are used as a sequestering agent to improve their stability in air.[17]
Laboratory applications
[edit]In the laboratory, EDTA is widely used for scavenging metal ions: In biochemistry and molecular biology, ion depletion is commonly used to deactivate metal-dependent enzymes, either as an assay for their reactivity or to suppress damage to DNA, proteins, and polysaccharides.[18] EDTA also acts as a selective inhibitor against dNTP hydrolyzing enzymes (Taq polymerase, dUTPase, MutT),[19] liver arginase[20] and horseradish peroxidase[21] independently of metal ion chelation. These findings urge the rethinking of the utilisation of EDTA as a biochemically inactive metal ion scavenger in enzymatic experiments. In analytical chemistry, EDTA is used in complexometric titrations and analysis of water hardness or as a masking agent to sequester metal ions that would interfere with the analyses.
EDTA finds many specialised uses in the biomedical labs, such as in veterinary ophthalmology as an anticollagenase to prevent the worsening of corneal ulcers in animals. In tissue culture, EDTA is used as a chelating agent that binds to calcium and prevents joining of cadherins between cells, preventing clumping of cells grown in liquid suspension, or detaching adherent cells for passaging. In histopathology, EDTA can be used as a decalcifying agent making it possible to cut sections using a microtome once the tissue sample is demineralised.
EDTA is also known to inhibit a range of metallopeptidases, the method of inhibition occurs via the chelation of the metal ion required for catalytic activity.[22] EDTA can also be used to test for bioavailability of heavy metals in sediments. However, it may influence the bioavailability of metals in solution, which may pose concerns regarding its effects in the environment, especially given its widespread uses and applications.
Other
[edit]The oxidising properties of [Fe(EDTA)]− are used in photography to solubilise silver particles.[4]
EDTA is also used to remove crud (corroded metals) from fuel rods in nuclear reactors.[23]
Side effects
[edit]EDTA exhibits low acute toxicity with LD50 (rat) of 2.0 g/kg to 2.2 g/kg.[4] It has been found to be both cytotoxic and weakly genotoxic in laboratory animals. Oral exposures have been noted to cause reproductive and developmental effects.[17] The same study[17] also found that both dermal exposure to EDTA in most cosmetic formulations and inhalation exposure to EDTA in aerosolised cosmetic formulations would produce exposure levels below those seen to be toxic in oral dosing studies.
Synthesis
[edit]The compound was first described in 1935 by Ferdinand Münz,[24] who prepared the compound from ethylenediamine and chloroacetic acid.[25] Today, EDTA is mainly synthesised from ethylenediamine (1,2-diaminoethane), formaldehyde, and sodium cyanide.[26] This route yields the tetrasodium EDTA, which is converted in a subsequent step into the acid forms:
This process is used to produce about 80,000 tonnes of EDTA each year. Impurities cogenerated by this route include glycine and nitrilotriacetic acid; they arise from reactions of the ammonia coproduct.[4]
Nomenclature
[edit]To describe EDTA and its various protonated forms, chemists distinguish between EDTA4−, the conjugate base that is the ligand, and H4EDTA, the precursor to that ligand. At very low pH (very acidic conditions) the fully protonated H6EDTA2+ form predominates, whereas at very high pH or very basic condition, the fully deprotonated EDTA4− form is prevalent. In this article, the term EDTA is used to mean H4−xEDTAx−, whereas in its complexes EDTA4− stands for the tetraanion ligand.
Coordination chemistry principles
[edit]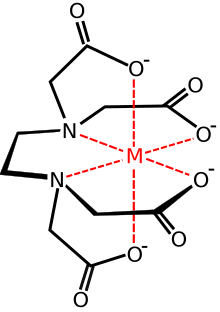
In coordination chemistry, EDTA4− is a member of the aminopolycarboxylic acid family of ligands. EDTA4− usually binds to a metal cation through its two amines and four carboxylates, i.e., it is a hexadentate ("six-toothed") chelating agent. Many of the resulting coordination compounds adopt octahedral geometry. Although of little consequence for its applications, these octahedral complexes are chiral. The cobalt(III) anion [Co(EDTA)]− has been resolved into enantiomers.[28] Many complexes of EDTA4− adopt more complex structures due to either the formation of an additional bond to water, i.e. seven-coordinate complexes, or the displacement of one carboxylate arm by water. The iron(III) complex of EDTA is seven-coordinate.[29] Early work on the development of EDTA was undertaken by Gerold Schwarzenbach in the 1940s.[30] EDTA forms especially strong complexes with Mn(II), Cu(II), Fe(III), Pb(II) and Co(III).[31][page needed]
Several features of EDTA's complexes are relevant to its applications. First, because of its high denticity, this ligand has a high affinity for metal cations:
- [Fe(H2O)6]3+ + H4EDTA ⇌ [Fe(EDTA)]− + 6 H2O + 4 H+ Keq = 1025.1
Written in this way, the equilibrium quotient shows that metal ions compete with protons for binding to EDTA. Because metal ions are extensively enveloped by EDTA, their catalytic properties are often suppressed. Finally, since complexes of EDTA4− are anionic, they tend to be highly soluble in water. For this reason, EDTA is able to dissolve deposits of metal oxides and carbonates.
The pKa values of free EDTA are 0, 1.5, 2, 2.66 (deprotonation of the four carboxyl groups) and 6.16, 10.24 (deprotonation of the two amino groups).[32]
Environmental concerns
[edit]Abiotic degradation
[edit]EDTA is in such widespread use that questions have been raised whether it is a persistent organic pollutant. While EDTA serves many positive functions in different industrial, pharmaceutical and other avenues, the longevity of EDTA can pose serious issues in the environment. The degradation of EDTA is slow. It mainly occurs abiotically in the presence of sunlight.[33]
The most important process for the elimination of EDTA from surface waters is direct photolysis at wavelengths below 400 nm.[34] Depending on the light conditions, the photolysis half-lives of iron(III) EDTA in surface waters can range as low as 11.3 minutes up to more than 100 hours.[35] Degradation of FeEDTA, but not EDTA itself, produces iron complexes of the triacetate (ED3A), diacetate (EDDA), and monoacetate (EDMA) – 92% of EDDA and EDMA biodegrades in 20 hours while ED3A displays significantly higher resistance. Many environmentally-abundant EDTA species (such as Mg2+ and Ca2+) are more persistent.
Biodegradation
[edit]In many industrial wastewater treatment plants, EDTA elimination can be achieved at about 80% using microorganisms.[36] Resulting byproducts are ED3A and iminodiacetic acid (IDA) – suggesting that both the backbone and acetyl groups were attacked. Some microorganisms have even been discovered to form nitrates out of EDTA, but they function optimally at moderately alkaline conditions of pH 9.0–9.5.[37]
Several bacterial strains isolated from sewage treatment plants efficiently degrade EDTA. Specific strains include Agrobacterium radiobacter ATCC 55002[38] and the sub-branches of Pseudomonadota like BNC1, BNC2,[39] and strain DSM 9103.[40] The three strains share similar properties of aerobic respiration and are classified as gram-negative bacteria. Unlike photolysis, the chelated species is not exclusive to iron(III) in order to be degraded. Rather, each strain uniquely consumes varying metal–EDTA complexes through several enzymatic pathways. Agrobacterium radiobacter only degrades Fe(III) EDTA[39] while BNC1 and DSM 9103 are not capable of degrading iron(III) EDTA and are more suited for calcium, barium, magnesium and manganese(II) complexes.[41] EDTA complexes require dissociation before degradation.
Alternatives to EDTA
[edit]Interest in environmental safety has raised concerns about biodegradability of aminopolycarboxylates such as EDTA. These concerns incentivize the investigation of alternative aminopolycarboxylates.[33] Candidate chelating agents include nitrilotriacetic acid (NTA), iminodisuccinic acid (IDS), polyaspartic acid, S,S-ethylenediamine-N,N′-disuccinic acid (EDDS), methylglycinediacetic acid (MGDA), and L-Glutamic acid N,N-diacetic acid, tetrasodium salt (GLDA).[42]
Iminodisuccinic acid (IDS)
[edit]Commercially used since 1998, iminodisuccinic acid (IDS) biodegrades by about 80% after only 7 days. IDS binds to calcium exceptionally well and forms stable compounds with other heavy metal ions. In addition to having a lower toxicity after chelation, IDS is degraded by Agrobacterium tumefaciens (BY6), which can be harvested on a large scale. The enzymes involved, IDS epimerase and C−N lyase, do not require any cofactors.[43]
Polyaspartic acid
[edit]Polyaspartic acid, like IDS, binds to calcium and other heavy metal ions. It has many practical applications including corrosion inhibitors, wastewater additives, and agricultural polymers. A Polyaspartic acid-based laundry detergent was the first laundry detergent in the world to receive the EU flower ecolabel.[44] Calcium binding ability of polyaspartic acid has been exploited for targeting of drug-loaded nanocarriers to bone.[45] Preparation of hydrogels based on polyaspartic acid, in a variety of physical forms ranging from fiber to particle, can potentially enable facile separation of the chelated ions from a solution.[46] Therefore, despite being weaker than EDTA, polyaspartic acid can still be regarded as a viable alternative due to these features as well as biocompatibility, and biodegradability.[47]
S,S-Ethylenediamine-N,N′-disuccinic acid (EDDS)
[edit]A structural isomer of EDTA, ethylenediamine-N,N′-disuccinic acid (EDDS) is readily biodegradable at high rate in its S,S form.[48]
Methylglycinediacetic acid (MGDA)
[edit]Trisodium dicarboxymethyl alaninate, also known as methylglycinediacetic acid (MGDA), has a high rate of biodegradation at over 68%, but unlike many other chelating agents can degrade without the assistance of adapted bacteria. Additionally, unlike EDDS or IDS, MGDA can withstand higher temperatures while maintaining a high stability as well as the entire pH range.[citation needed] MGDA has been shown to be an effective chelating agent, with a capacity for mobilization comparable with that of nitrilotriacetic acid (NTA), with application to water for industrial use and for the removal of calcium oxalate from urine from patients with kidney stones.[49]
Methods of detection and analysis
[edit]The most sensitive method of detecting and measuring EDTA in biological samples is selected reaction monitoring capillary electrophoresis mass spectrometry (SRM-CE/MS), which has a detection limit of 7.3 ng/mL in human plasma and a quantitation limit of 15 ng/mL.[50] This method works with sample volumes as small as 7–8 nL.[50]
EDTA has also been measured in non-alcoholic beverages using high performance liquid chromatography (HPLC) at a level of 2.0 μg/mL.[51][52]
In popular culture
[edit]In the movie Blade (1998), EDTA is used as a weapon to kill vampires, exploding when in contact with vampire blood.[53]
References
[edit]- ^ a b Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 79, 123, 586, 754. ISBN 978-0-85404-182-4.
- ^ Raaflaub, Jürg (1956). "Applications of Metal Buffers and Metal Indicators in Biochemistry". Methods of Biochemical Analysis. Vol. 3. pp. 301–325. doi:10.1002/9780470110195.ch10. ISBN 978-0-470-30492-1. PMID 13369167.
- ^ Substance Name: Sodium calcium edetate. NIH.gov
- ^ a b c d Hart, J. Roger (2005). "Ethylenediaminetetraacetic Acid and Related Chelating Agents". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_095. ISBN 978-3527306732.
- ^ Furia, T. (1964). "EDTA in Foods – A technical review". Food Technology. 18 (12): 1874–1882.
- ^ Taylor, H. F. W. (1990). Cement Chemistry. Academic Press. ISBN 978-0-12-683900-5.
- ^ Norvell, W. A.; Lindsay, W. L. (1969). "Reactions of EDTA Complexes of Fe, Zn, Mn, and Cu with Soils". Soil Science Society of America Journal. 33 (1): 86. Bibcode:1969SSASJ..33...86N. doi:10.2136/sssaj1969.03615995003300010024x.
- ^ Powell, J. E.; Spedding, F. H. (1956). Basic Principles Involved in the Macro-Separation of Adjacent Rare Earths from Each Other by Means of Ion Exchange (Technical report). Iowa State College. doi:10.2172/4289324. OSTI 4289324. S2CID 93195586.
- ^ DeBusk, Ruth; et al. (2002). "Ethylenediaminetetraacetic acid (EDTA)". University of Maryland Medical Center. Archived from the original on 2007-05-04.
- ^ Soveri, Inga; Berg, Ulla B.; Björk, Jonas; Elinder, Carl-Gustaf; Grubb, Anders; Mejare, Ingegerd; Sterner, Gunnar; Bäck, Sten-Erik (September 2014). "Measuring GFR: A Systematic Review". American Journal of Kidney Diseases. 64 (3): 411–424. doi:10.1053/j.ajkd.2014.04.010. PMID 24840668.
- ^ Banfi, G; Salvagno, G. L; Lippi, G (2007). "The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes". Clinical Chemistry and Laboratory Medicine. 45 (5): 565–76. doi:10.1515/CCLM.2007.110. PMID 17484616. S2CID 23824484.
- ^ a b "Order of draw for multiple tube collections" (PDF). Michigan Medicine Laboratories. 2019-09-15. Archived from the original (PDF) on 2019-11-26. Retrieved 2020-03-27.
- ^ Kadry, A. A.; Fouda, S. I.; Shibl, A. M.; Abu El-Asrar, A. A. (2009). "Impact of slime dispersants and anti-adhesives on in vitro biofilm formation of Staphylococcus epidermidis on intraocular lenses and on antibiotic activities". Journal of Antimicrobial Chemotherapy. 63 (3): 480–4. doi:10.1093/jac/dkn533. PMID 19147522.
- ^ Seely, D. M.; Wu, P.; Mills, E. J. (2005). "EDTA chelation therapy for cardiovascular disease: a systematic review". BMC Cardiovasc Disord. 5 (32): 480–484. doi:10.1186/1471-2261-5-32. PMC 1282574. PMID 19147522.
- ^ Green, Saul; Sampson, Wallace (December 14, 2002). "EDTA Chelation Therapy for Atherosclerosis And Degenerative Diseases: Implausibility and Paradoxical Oxidant Effects". Quackwatch. Retrieved 16 December 2009.
- ^ "Postmarket Drug Safety Information for Patients and Providers – Questions and Answers on Edetate Disodium (marketed as Endrate and generic products)". U.S. Food and Drug Administration.
- ^ a b c Lanigan, R. S.; Yamarik, T. A. (2002). "Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA". International Journal of Toxicology. 21 Suppl. 2 (5): 95–142. doi:10.1080/10915810290096522. PMID 12396676. S2CID 83388249.
- ^ Domínguez, K.; Ward, W. S. (December 2009). "A novel nuclease activity that is activated by Ca2+ chelated to EGTA". Systems Biology in Reproductive Medicine. 55 (5–6): 193–199. doi:10.3109/19396360903234052. PMC 2865586. PMID 19938954.
- ^ Lopata, Anna; Jójárt, Balázs; Surányi, Éva V.; Takács, Enikő; Bezúr, László; Leveles, Ibolya; Bendes, Ábris Á; Viskolcz, Béla; Vértessy, Beáta G.; Tóth, Judit (October 2019). "Beyond Chelation: EDTA Tightly Binds Taq DNA Polymerase, MutT and dUTPase and Directly Inhibits dNTPase Activity". Biomolecules. 9 (10): 621. doi:10.3390/biom9100621. PMC 6843921. PMID 31627475.
- ^ Carvajal, Nelson; Orellana, María S; Bórquez, Jessica; Uribe, Elena; López, Vasthi; Salas, Mónica (2004-08-01). "Non-chelating inhibition of the H101N variant of human liver arginase by EDTA". Journal of Inorganic Biochemistry. 98 (8): 1465–1469. doi:10.1016/j.jinorgbio.2004.05.005. ISSN 0162-0134. PMID 15271525.
- ^ Bhattacharyya, D K; Adak, S; Bandyopadhyay, U; Banerjee, R K (1994-03-01). "Mechanism of inhibition of horseradish peroxidase-catalysed iodide oxidation by EDTA". Biochemical Journal. 298 (Pt 2): 281–288. doi:10.1042/bj2980281. ISSN 0264-6021. PMC 1137937. PMID 8135732.
- ^ Auld, D. S. (1995). "Removal and replacement of metal ions in metallopeptidases". Proteolytic Enzymes: Aspartic and Metallo Peptidases. Methods in Enzymology. Vol. 248. pp. 228–242. doi:10.1016/0076-6879(95)48016-1. ISBN 978-0-12-182149-4. PMID 7674923.
- ^ Choppin, Gregory; Liljenzin, Jan-Olov; Rydberg, Jan; Ekberg, Christian (2013). "Chapter 20 - Nuclear Power Reactors". Radiochemistry and Nuclear Chemistry (Fourth ed.): 655–684. doi:10.1016/B978-0-12-405897-2.00020-3. ISBN 978-0-12-405897-2.
- ^ Paolieri, Matteo (December 2017). "Ferdinand Münz: EDTA and 40 years of inventions". Bull. Hist. Chem. 42 (2). ACS: 133–140.
- ^ US 2130505, Münz, Ferdinand, "Polyamino carboxylic acids and process of making same", published 1938-09-20, assigned to General Aniline Works Ltd.. Also DE 718981, Münz, Ferdinand, "Verfahren zum Unschädlichmachen der Härtebildner des Wassers [Process for rendering the hardness components of water harmless]", published 1938-09-20, assigned to I. G. Farbenindustrie
- ^ "Industrial Synthesis of EDTA". University of Bristol.
- ^ Solans, X.; Font Altaba, M.; García Oricain, J. (1984). "Crystal Structures of Ethylenediaminetetraacetato Metal Complexes. V. Structures Containing the [Fe(C10H12N2O8)(H2O)]− Anion". Acta Crystallographica Section C. 40 (4): 635–638. doi:10.1107/S0108270184005151.
- ^ Kirchner, S.; Gyarfas, Eleonora C. (1957). "Barium (Ethylenediaminetetraacetato)cobaltate(III) 4-Hydrate". Inorganic Syntheses. Vol. 5. pp. 186–188. doi:10.1002/9780470132364.ch52. ISBN 978-0-470-13236-4.
- ^ López Alcalá, J. M.; Puerta Vizcaíno, M. C.; González Vílchez, F.; Duesler, E. N.; Tapscott, R. E. (1984). "A redetermination of sodium aqua[ethylenediaminetetraacetato(4−)]ferrate(III) dihydrate, Na[Fe(C10H12N2O8)(H2O)]·2H2O". Acta Crystallogr C. 40 (6): 939–941. Bibcode:1984AcCrC..40..939L. doi:10.1107/S0108270184006338.
- ^ Sinex, Scott A. "EDTA – A Molecule with a Complex Story". University of Bristol.
- ^ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 978-0-12-352651-9.
- ^ Hans Peter Latscha: Analytische Chemie. Springer-Verlag, 2013, ISBN 978-3-642-18493-2, p. 303.
- ^ a b Bucheli-Witschel, M.; Egli, T. (2001), "DAB: Environmental Fate and Microbial Degradation of Aminopolycarboxylic Acids", FEMS Microbiology Reviews, 25 (1): 69–106, doi:10.1111/j.1574-6976.2001.tb00572.x, PMID 11152941
- ^ Kari, F. G. (1994). Umweltverhalten von Ethylenediaminetetraacetate (EDTA) under spezieller Berucksuchtigung des photochemischen Ab-baus (PhD). Swiss Federal Institute of Technology.
- ^ Frank, R.; Rau, H. (1989). "Photochemical transformation in aqueous solution and possible environmental fate of Ethylenediaminetetraacetatic acid (EDTA)". Ecotoxicology and Environmental Safety. 19 (1): 55–63. doi:10.1016/0147-6513(90)90078-j. PMID 2107071.
- ^ Kaluza, U.; Klingelhofer, P.; K., Taeger (1998). "Microbial degradation of EDTA in an industrial wastewater treatment plant". Water Research. 32 (9): 2843–2845. Bibcode:1998WatRe..32.2843K. doi:10.1016/S0043-1354(98)00048-7.
- ^ VanGinkel, C. G.; Vandenbroucke, K. L.; C. A., Troo (1997). "Biological removal of EDTA in conventional activated-sludge plants operated under alkaline conditions". Bioresource Technology. 32 (2–3): 2843–2845. Bibcode:1997BiTec..59..151V. doi:10.1016/S0960-8524(96)00158-7.
- ^ Lauff, J. J.; Steele, D. B.; Coogan, L. A.; Breitfeller, J. M. (1990). "Degradation of the ferric chelate of EDTA by a pure culture of an Agrobacterium sp". Applied and Environmental Microbiology. 56 (11): 3346–3353. Bibcode:1990ApEnM..56.3346L. doi:10.1128/AEM.56.11.3346-3353.1990. PMC 184952. PMID 16348340.
- ^ a b Nortemannl, B (1992). "Total degradation of EDTA by mixed culturesand a bacterial isolate". Applied and Environmental Microbiology. 58 (2): 671–676. Bibcode:1992ApEnM..58..671N. doi:10.1128/AEM.58.2.671-676.1992. PMC 195300. PMID 16348653.
- ^ Witschel, M.; Weilemann, H.-U.; Egli, T. (1995). Degradation of EDTA by a bacterial isolate. Poster presented at the 45th Annual Meeting of the Swiss Society for Microbiology (Speech). Lugano, Switzerland.
- ^ Hennekenl, L.; Nortemann, B.; Hempel, D. C. (1995). "Influence of physiological conditions on EDTA degradation". Applied and Environmental Microbiology. 44 (1–2): 190–197. doi:10.1007/bf00164501. S2CID 30072817.
- ^ Tandy, Susan; Bossart, Karin; Mueller, Roland; Ritschel, Jens; Hauser, Lukas; Schulin, Rainer; Nowack, Bernd (2004). "Extraction of Heavy Metals from Soils Using Biodegradable Chelating Agents". Environmental Science & Technology. 38 (3): 937–944. Bibcode:2004EnST...38..937T. doi:10.1021/es0348750. PMID 14968886.
- ^ Cokesa, Z.; Knackmuss, H.; Rieger, P. (2004), "Biodegradation of All Stereoisomers of the EDTA Substitute Iminodisuccinate by Agrobacterium Tumefaciens BY6 Requires an Epimerase and a Stereoselective C−N Lyase", Applied and Environmental Microbiology, 70 (7): 3941–3947, Bibcode:2004ApEnM..70.3941C, doi:10.1128/aem.70.7.3941-3947.2004, PMC 444814, PMID 15240267
- ^ Thomas Klein; Ralf-Johann Moritz; René Graupner (2008). "Polyaspartates and Polysuccinimide". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.l21_l01. ISBN 978-3527306732.
- ^ Adelnia, Hossein; Tran, Huong D.N.; Little, Peter J.; Blakey, Idriss; Ta, Hang T. (2021-06-14). "Poly(aspartic acid) in Biomedical Applications: From Polymerization, Modification, Properties, Degradation, and Biocompatibility to Applications". ACS Biomaterials Science & Engineering. 7 (6): 2083–2105. doi:10.1021/acsbiomaterials.1c00150. hdl:10072/404497. PMID 33797239. S2CID 232761877.
- ^ Adelnia, Hossein; Blakey, Idriss; Little, Peter J.; Ta, Hang T. (2019). "Hydrogels Based on Poly(aspartic acid): Synthesis and Applications". Frontiers in Chemistry. 7: 755. Bibcode:2019FrCh....7..755A. doi:10.3389/fchem.2019.00755. ISSN 2296-2646. PMC 6861526. PMID 31799235.
- ^ Hasson, David; Shemer, Hilla; Sher, Alexander (2011-06-15). "State of the Art of Friendly "Green" Scale Control Inhibitors: A Review Article". Industrial & Engineering Chemistry Research. 50 (12): 7601–7607. doi:10.1021/ie200370v. ISSN 0888-5885.
- ^ Tandy, S.; Ammann, A.; Schulin, R.; Nowack, B. (2006). "Biodegredation and speciation of residual SS-ethylenediaminedisuccinic acid (EDDS) in soil solution left after soil washing". Environmental Pollution. 142 (2): 191–199. Bibcode:2006EPoll.142..191T. doi:10.1016/j.envpol.2005.10.013. PMID 16338042.
- ^ Bretti, Clemente; Cigala, Rosalia Maria; De Stefano, Concetta; Lando, Gabriele; Sammartano, Silvio (2017). "Thermodynamic solution properties of a biodegradable chelant (MGDA) and its interaction with the major constituents of natural fluids". Fluid Phase Equilibria. 434: 63–73. Bibcode:2017FlPEq.434...63B. doi:10.1016/j.fluid.2016.11.027.
- ^ a b Sheppard, R. L.; Henion, J. (1997). "Peer Reviewed: Determining EDTA in Blood". Analytical Chemistry. 69 (15): 477A – 480A. doi:10.1021/ac971726p. PMID 9253241.
- ^ Loyaux-Lawniczak, S.; Douch, J.; Behra, P. (1999). "Optimisation of the analytical detection of EDTA by HPLC in natural waters". Fresenius' Journal of Analytical Chemistry. 364 (8): 727. doi:10.1007/s002160051422. S2CID 95648833.
- ^ Cagnasso, C. E.; López, L. B.; Rodríguez, V. G.; Valencia, M. E. (2007). "Development and validation of a method for the determination of EDTA in non-alcoholic drinks by HPLC". Journal of Food Composition and Analysis. 20 (3–4): 248. doi:10.1016/j.jfca.2006.05.008.
- ^ "Blade (1998)". Internet Movie Database (IMDb). Retrieved 2022-11-14.
External links
[edit]- EDTA: Molecule of the Month
- EDTA Determination of Total Water Hardness
- Oviedo, Claudia; Rodríguez, Jaime (2003). "EDTA: The chelating agent under environmental scrutiny". Química Nova. 26 (6): 901–905. doi:10.1590/S0100-40422003000600020.

