Halogenation: Difference between revisions
"In chemistry, halogenation is a chemical reaction which introduces of one or more halogens into a chemical compound." Removed OF Tags: Mobile edit Mobile web edit |
|||
| (324 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical reaction which adds one or more halogen elements to a compound}} |
|||
'''Halogenation''' is a [[chemical reaction]] that replaces a [[hydrogen]] atom with a [[halogen]] atom. More specific descriptions exist that specify the type of halogen: fluorination, chlorination, bromination, and iodination. |
|||
{{redirect|Fluorination|the addition of fluoride to drinking water|Water fluoridation}} |
|||
{{for|the addition of chlorine, hypochlorite, etc. to drinking water|Water chlorination}} |
|||
In [[chemistry]], '''halogenation''' is a [[chemical reaction]] which introduces one or more [[halogen]]s into a [[chemical compound]]. [[Halide]]-containing compounds are pervasive, making this type of transformation important, e.g. in the production of [[polymers]], [[drugs]].<ref>{{cite book |doi=10.1002/9780470771723.ch3|chapter=Formation of Carbon-Halogen Bonds|title=Halides, Pseudo-Halides and Azides: Part 2 (1983)|year=1983|last1=Hudlicky|first1=Milos|last2=Hudlicky|first2=Tomas|pages=1021–1172|isbn=9780470771723|editor1=S. Patai|editor2=Z. Rappoport|series=PATAI's Chemistry of Functional Groups}}</ref> This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens ({{chem2|[[Fluorine|F2]], [[Chlorine|Cl2]], [[Bromine|Br2]], [[Iodine|I2]]}}). Halides are also commonly introduced using salts of the halides and halogen acids.{{cln|reason=What on this Earth is "halogen acids"???|date=July 2023}} Many specialized [[reagent]]s exist for and introducing halogens into diverse [[Substrate (chemistry)|substrates]], e.g. [[thionyl chloride]]. |
|||
In a [[Markovnikov's rule|Markovnikov addition]] reaction, a halogen like [[bromine]] is reacted with an [[alkene]] which causes the [[pi bond|π-bond]] to break forming an [[haloalkane]]. This makes the [[hydrocarbon]] more reactive and [[bromine]] as it turns out, is a good [[leaving group]] in further chemical reactions such as [[nucleophilic aliphatic substitution]] reactions and [[elimination reaction]]s |
|||
==Organic chemistry== |
|||
Several types of halogenation exist, including: |
|||
Several pathways exist for the halogenation of organic compounds, including [[free radical halogenation]], [[ketone halogenation]], [[electrophilic halogenation]], and [[halogen addition reaction]]. The nature of the [[Substrate (chemistry)|substrate]] determines the pathway. The facility of halogenation is influenced by the halogen. [[Fluorine]] and [[chlorine]] are more [[electrophilic]] and are more aggressive halogenating agents. [[Bromine]] is a weaker halogenating agent than both fluorine and chlorine, while [[iodine]] is the least reactive of them all. The facility of [[dehydrohalogenation]] follows the reverse trend: iodine is most easily removed from organic compounds, and [[organofluorine]] compounds are highly stable. |
|||
===Free radical halogenation === |
|||
Halogenation of [[saturated hydrocarbon]]s is a [[substitution reaction]]. The reaction typically involves [[free radical]] pathways. The [[regiochemistry]] of the halogenation of [[alkanes]] is largely determined by the relative weakness of the [[Carbon–hydrogen bond|C–H bonds]]. This trend is reflected by the faster reaction at [[Tertiary (chemistry)|tertiary]] and [[Secondary (chemistry)|secondary]] positions. |
|||
| ⚫ | |||
* [[Electrophilic halogenation]] |
|||
Free radical chlorination is used for the industrial production of some [[solvents]]:<ref name=Ullmann>{{Ullmann|doi=10.1002/14356007.a06_233.pub2}}</ref> |
|||
==Bromination== |
|||
:{{chem2|CH4 + Cl2 → CH3Cl + HCl}} |
|||
[[Image:Bromination.png|right|bromination reaction mechanism]] |
|||
The [[reaction mechanism]] for an alkene bromination can be described as follows. The [[bromine]] - bromine [[covalent bond]] attracts the attention of the π-bonding [[electrons]], the π-bonds being [[electron]] dense and Bromine being very [[electronegativity|electronegative]]. This leads to a weakening and eventual break where heat, plays a crucial role in driving the break forward of the [[pi bond|π-bond]]. Once the [[pi bond|π-bond]] has been broken, its [[electrons]] are transfered to Br<sub>2</sub>, causing the Br-Br bond to be severed when the bonding [[electrons]] are transfered to the other [[bromine]]. At this stage there is the positively charged intermediate and the loose bromine ion (Br<sup>-</sup>). The bonding of [[Bromine]] is special in this intermediate, due to its relatively large size compared to [[carbon]], the bromide [[ion]] is capable to latching onto both carbons which once formed the [[pi bond|π-bond]], making a three-membered ring. Coming from the opposite direction as the Br<sub>2</sub>, the Br<sup>-</sup> loosens one of the C-Br Bonds, leaving the final brominated product, a brominated [[alkane]]. |
|||
Naturally-occurring [[organobromine compound]]s are usually produced by free radical pathway [[catalyzed]] by the [[enzyme]] [[bromoperoxidase]]. The reaction requires [[bromide]] in combination with [[oxygen]] as an [[oxidant]]. The [[oceans]] are estimated to release 1–2 [[million]] tons of [[bromoform]] and 56,000 tons of [[bromomethane]] annually.<ref>{{cite journal|doi=10.1039/a900201d|title=The diversity of naturally occurring organobromine compounds|year=1999|last1=Gribble|first1=Gordon W.|journal=Chemical Society Reviews|volume=28|issue=5|pages=335–346}}</ref>{{cln|reason=Which ton??? Short ton, long ton or metric ton???|date=July 2023}} |
|||
The [[iodoform reaction]], which involves degradation of [[methyl ketone]]s, proceeds by the free radical iodination. |
|||
===Fluorination=== |
|||
Because of its extreme reactivity, fluorine ({{chem2|F2}}) represents a special category with respect to halogenation. Most organic compounds, saturated or otherwise, burn upon contact with {{chem2|F2}}, ultimately yielding [[carbon tetrafluoride]]. By contrast, the heavier halogens are far less reactive toward saturated hydrocarbons. |
|||
Highly specialised conditions and apparatus are required for fluorinations with elemental [[fluorine]]. Commonly, fluorination reagents are employed instead of {{chem2|F2}}. Such reagents include [[cobalt trifluoride]], [[chlorine trifluoride]], and [[iodine pentafluoride]].<ref>{{cite book |doi=10.1002/14356007.a11_307 |chapter=Fluorine Compounds, Inorganic |title=Ullmann's Encyclopedia of Industrial Chemistry |date=2000 |last1=Aigueperse |first1=Jean |last2=Mollard |first2=Paul |last3=Devilliers |first3=Didier |last4=Chemla |first4=Marius |last5=Faron |first5=Robert |last6=Romano |first6=René |last7=Cuer |first7=Jean Pierre |isbn=3-527-30673-0 }}</ref> |
|||
The method [[electrochemical fluorination]] is used commercially for the production of [[perfluorinated compound]]s. It generates small amounts of elemental fluorine [[in situ]] from [[hydrogen fluoride]]. The method avoids the hazards of handling fluorine gas. Many commercially important [[organic compounds]] are fluorinated using this technology. |
|||
=== Addition of halogens to alkenes and alkynes === |
|||
[[File:96. Адиција на хлор на етин.ogg|thumb|right|Double-addition of [[chlorine gas]] to [[ethyne]]]] |
|||
[[Unsaturated compound]]s, especially [[alkenes]] and [[alkynes]], ''add'' halogens: |
|||
:{{chem2|R\sCH\dCH\sR' + X2 → R\sCHX\sCHX\sR'}} |
|||
In [[oxychlorination]], the combination of [[hydrogen chloride]] and [[oxygen]] serves as the equivalent of [[chlorine]], as illustrated by this route to [[1,2-dichloroethane]]: |
|||
:{{chem2|4 HCl + 2 CH2\dCH2 + O2 → 2 Cl\sCH2\sCH2\sCl + 2 H2O}} |
|||
[[File:Biadamantylidene-bromonium-ion-from-xtal-1994-2D-skeletal.png|170px|thumb|right|Structure of a [[bromonium ion]]]] |
|||
The addition of halogens to alkenes proceeds via [[Reaction intermediate|intermediate]] [[halonium ion]]s. In special cases, such intermediates have been isolated.<ref>{{cite journal | journal = [[Chem. Commun.]] | year = 1998 | pages = 927–928 | doi = 10.1039/a709063c | title = X-Ray structure of bridged 2,2′-bi(adamant-2-ylidene) chloronium cation and comparison of its reactivity with a singly bonded chloroarenium cation |author1=T. Mori |author2=R. Rathore | issue = 8 }}</ref> |
|||
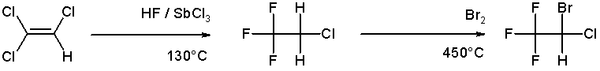
Bromination is more [[chemical selectivity|selective]] than chlorination because the reaction is less [[exothermic]]. Illustrative of the bromination of an alkene is the route to the [[anesthetic]] [[halothane]] from [[trichloroethylene]]:<ref>''Synthesis of Essential Drugs'', Ruben Vardanyan, Victor Hruby; Elsevier 2005 {{ISBN|0-444-52166-6}}</ref> |
|||
:[[Image:Halothane synthesis.png|600px|Halothane synthesis]] |
|||
Iodination and bromination can be effected by the addition of [[iodine]] and [[bromine]] to alkenes. The reaction, which conveniently proceeds with the discharge of the color of {{chem2|I2 and Br2}}, is the basis of the [[analytical method]]. The [[iodine number]] and [[bromine number]] are measures of the [[degree of unsaturation]] for [[fat]]s and other organic compounds. |
|||
===Halogenation of aromatic compounds=== |
|||
{{main|Aryl halide}} |
|||
[[Aromatic compound]]s are subject to [[electrophilic halogenation]]: |
|||
:{{chem2|R\sC6H5 + X2 → HX + R\sC6H4\sX}} |
|||
This kind of reaction typically works well for [[chlorine]] and [[bromine]]. Often a [[Lewis acid]]ic [[catalyst]] is used, such as [[ferric chloride]].<ref name=PhCl>{{cite book |doi=10.1002/14356007.o06_o03 |chapter=Chlorinated Benzenes and Other Nucleus-Chlorinated Aromatic Hydrocarbons |title=Ullmann's Encyclopedia of Industrial Chemistry |year=2011 |last1=Beck |first1=Uwe |last2=Löser |first2=Eckhard |isbn=978-3527306732 }}</ref> Many detailed procedures are available.<ref>Organic chemistry by Jonathan Clayden, Nick Grieves, Stuart Warren, Oxford University Press</ref><ref>{{OrgSynth|author=Edward R. Atkinson, Donald M. Murphy, and James E. Lufkin |year=1951|title=''dl''-4,4′,6,6′-Tetrachlorodiphenic Acid|volume=31| page=96|doi=10.15227/orgsyn.031.0096}}</ref> |
|||
Because [[fluorine]] is so [[Reactivity (chemistry)|reactive]], other methods, such as the [[Balz–Schiemann reaction]], are used to prepare fluorinated aromatic compounds. |
|||
===Other halogenation methods=== |
|||
In the [[Hunsdiecker reaction]], [[carboxylic acids]] are converted to [[organic halide]], whose [[carbon chain]] is shortened by one [[carbon]] atom with respect to the carbon chain of the particular carboxylic acid. The carboxylic acid is first converted to its [[silver]] salt, which is then oxidized with [[halogen]]: |
|||
:{{chem2|R\sCOO−Ag+ + [[Bromine|Br2]] → R\sBr + [[carbon dioxide|CO2]] + [[Silver bromide|Ag+Br−]]}} |
|||
:{{chem2|[[Silver acetate|CH3\sCOO−Ag+]] + Br2 → [[Bromomethane|CH3\sBr]] + CO2 + Ag+Br−}} |
|||
Many [[organometallic compound]]s react with halogens to give the organic halide: |
|||
:{{chem2|RM + X2 → RX + MX}} |
|||
:{{chem2|[[n-Butyllithium|CH3CH2CH2CH2Li]] + [[Chlorine|Cl2]] → [[1-chlorobutane|CH3CH2CH2CH2Cl]] + [[Lithium chloride|LiCl]]}} |
|||
==Inorganic chemistry== |
|||
All [[Chemical element|elements]] aside from [[argon]], [[neon]], and [[helium]] form [[fluorides]] by direct reaction with [[fluorine]]. [[Chlorine]] is slightly more selective, but still reacts with most [[metals]] and heavier [[nonmetals]]. Following the usual trend, [[bromine]] is less [[Reactivity (chemistry)|reactive]] and [[iodine]] least of all. Of the many reactions possible, illustrative is the formation of [[gold(III) chloride]] by the chlorination of [[gold]]. The chlorination of metals is usually not very important industrially since the [[chlorides]] are more easily made from the [[oxides]] and [[hydrogen chloride]]. Where chlorination of [[inorganic compounds]] is practiced on a relatively large scale is for the production of [[phosphorus trichloride]] and [[disulfur dichloride]].<ref>{{Greenwood&Earnshaw2nd}}</ref> |
|||
==See also== |
==See also== |
||
{{wikiquote}} |
|||
*[[Alkyl halide|Halogenoalkanes]] (Alkyl halides) |
|||
{{col div|colwidth=30em}} |
|||
| ⚫ | |||
*[[Dehalogenation]] |
|||
*[[Haloalkane]] (Alkyl halide) |
|||
| ⚫ | |||
| ⚫ | |||
*[[Haloketone]] |
|||
*[[Electrophilic substitution]] |
*[[Electrophilic substitution]] |
||
{{colend}} |
|||
==References== |
|||
{{Reflist|2}} |
|||
{{Authority control}} |
|||
[[Category:Halogenation reactions]] |
|||
[[Category:Organic reactions]] |
[[Category:Organic reactions]] |
||
[[Category:Inorganic reactions]] |
[[Category:Inorganic reactions]] |
||
[[Category:Halogens]] |
|||
[[zh:卤化]] |
|||
Latest revision as of 15:24, 12 June 2024
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs.[1] This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens (F2, Cl2, Br2, I2). Halides are also commonly introduced using salts of the halides and halogen acids.[clarification needed] Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
Organic chemistry
[edit]Several pathways exist for the halogenation of organic compounds, including free radical halogenation, ketone halogenation, electrophilic halogenation, and halogen addition reaction. The nature of the substrate determines the pathway. The facility of halogenation is influenced by the halogen. Fluorine and chlorine are more electrophilic and are more aggressive halogenating agents. Bromine is a weaker halogenating agent than both fluorine and chlorine, while iodine is the least reactive of them all. The facility of dehydrohalogenation follows the reverse trend: iodine is most easily removed from organic compounds, and organofluorine compounds are highly stable.
Free radical halogenation
[edit]Halogenation of saturated hydrocarbons is a substitution reaction. The reaction typically involves free radical pathways. The regiochemistry of the halogenation of alkanes is largely determined by the relative weakness of the C–H bonds. This trend is reflected by the faster reaction at tertiary and secondary positions.
Free radical chlorination is used for the industrial production of some solvents:[2]
- CH4 + Cl2 → CH3Cl + HCl
Naturally-occurring organobromine compounds are usually produced by free radical pathway catalyzed by the enzyme bromoperoxidase. The reaction requires bromide in combination with oxygen as an oxidant. The oceans are estimated to release 1–2 million tons of bromoform and 56,000 tons of bromomethane annually.[3][clarification needed]
The iodoform reaction, which involves degradation of methyl ketones, proceeds by the free radical iodination.
Fluorination
[edit]Because of its extreme reactivity, fluorine (F2) represents a special category with respect to halogenation. Most organic compounds, saturated or otherwise, burn upon contact with F2, ultimately yielding carbon tetrafluoride. By contrast, the heavier halogens are far less reactive toward saturated hydrocarbons.
Highly specialised conditions and apparatus are required for fluorinations with elemental fluorine. Commonly, fluorination reagents are employed instead of F2. Such reagents include cobalt trifluoride, chlorine trifluoride, and iodine pentafluoride.[4]
The method electrochemical fluorination is used commercially for the production of perfluorinated compounds. It generates small amounts of elemental fluorine in situ from hydrogen fluoride. The method avoids the hazards of handling fluorine gas. Many commercially important organic compounds are fluorinated using this technology.
Addition of halogens to alkenes and alkynes
[edit]Unsaturated compounds, especially alkenes and alkynes, add halogens:
- R−CH=CH−R' + X2 → R−CHX−CHX−R'
In oxychlorination, the combination of hydrogen chloride and oxygen serves as the equivalent of chlorine, as illustrated by this route to 1,2-dichloroethane:
- 4 HCl + 2 CH2=CH2 + O2 → 2 Cl−CH2−CH2−Cl + 2 H2O

The addition of halogens to alkenes proceeds via intermediate halonium ions. In special cases, such intermediates have been isolated.[5]
Bromination is more selective than chlorination because the reaction is less exothermic. Illustrative of the bromination of an alkene is the route to the anesthetic halothane from trichloroethylene:[6]
Iodination and bromination can be effected by the addition of iodine and bromine to alkenes. The reaction, which conveniently proceeds with the discharge of the color of I2 and Br2, is the basis of the analytical method. The iodine number and bromine number are measures of the degree of unsaturation for fats and other organic compounds.
Halogenation of aromatic compounds
[edit]Aromatic compounds are subject to electrophilic halogenation:
- R−C6H5 + X2 → HX + R−C6H4−X
This kind of reaction typically works well for chlorine and bromine. Often a Lewis acidic catalyst is used, such as ferric chloride.[7] Many detailed procedures are available.[8][9] Because fluorine is so reactive, other methods, such as the Balz–Schiemann reaction, are used to prepare fluorinated aromatic compounds.
Other halogenation methods
[edit]In the Hunsdiecker reaction, carboxylic acids are converted to organic halide, whose carbon chain is shortened by one carbon atom with respect to the carbon chain of the particular carboxylic acid. The carboxylic acid is first converted to its silver salt, which is then oxidized with halogen:
- R−COO−Ag+ + Br2 → R−Br + CO2 + Ag+Br−
- CH3−COO−Ag+ + Br2 → CH3−Br + CO2 + Ag+Br−
Many organometallic compounds react with halogens to give the organic halide:
- RM + X2 → RX + MX
- CH3CH2CH2CH2Li + Cl2 → CH3CH2CH2CH2Cl + LiCl
Inorganic chemistry
[edit]All elements aside from argon, neon, and helium form fluorides by direct reaction with fluorine. Chlorine is slightly more selective, but still reacts with most metals and heavier nonmetals. Following the usual trend, bromine is less reactive and iodine least of all. Of the many reactions possible, illustrative is the formation of gold(III) chloride by the chlorination of gold. The chlorination of metals is usually not very important industrially since the chlorides are more easily made from the oxides and hydrogen chloride. Where chlorination of inorganic compounds is practiced on a relatively large scale is for the production of phosphorus trichloride and disulfur dichloride.[10]
See also
[edit]- Dehalogenation
- Haloalkane (Alkyl halide)
- Halogenoarene (Aryl halide)
- Free radical halogenation
- Haloketone
- Electrophilic substitution
References
[edit]- ^ Hudlicky, Milos; Hudlicky, Tomas (1983). "Formation of Carbon-Halogen Bonds". In S. Patai; Z. Rappoport (eds.). Halides, Pseudo-Halides and Azides: Part 2 (1983). PATAI's Chemistry of Functional Groups. pp. 1021–1172. doi:10.1002/9780470771723.ch3. ISBN 9780470771723.
- ^ Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_233.pub2. ISBN 978-3527306732.
- ^ Gribble, Gordon W. (1999). "The diversity of naturally occurring organobromine compounds". Chemical Society Reviews. 28 (5): 335–346. doi:10.1039/a900201d.
- ^ Aigueperse, Jean; Mollard, Paul; Devilliers, Didier; Chemla, Marius; Faron, Robert; Romano, René; Cuer, Jean Pierre (2000). "Fluorine Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a11_307. ISBN 3-527-30673-0.
- ^ T. Mori; R. Rathore (1998). "X-Ray structure of bridged 2,2′-bi(adamant-2-ylidene) chloronium cation and comparison of its reactivity with a singly bonded chloroarenium cation". Chem. Commun. (8): 927–928. doi:10.1039/a709063c.
- ^ Synthesis of Essential Drugs, Ruben Vardanyan, Victor Hruby; Elsevier 2005 ISBN 0-444-52166-6
- ^ Beck, Uwe; Löser, Eckhard (2011). "Chlorinated Benzenes and Other Nucleus-Chlorinated Aromatic Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.o06_o03. ISBN 978-3527306732.
- ^ Organic chemistry by Jonathan Clayden, Nick Grieves, Stuart Warren, Oxford University Press
- ^ Edward R. Atkinson, Donald M. Murphy, and James E. Lufkin (1951). "dl-4,4′,6,6′-Tetrachlorodiphenic Acid". Organic Syntheses. 31: 96. doi:10.15227/orgsyn.031.0096
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.