Caesium acetate: Difference between revisions
m Typo fixing, use degree symbol, not masculine ordinal indicator or superscripted "o" using AWB (7852) |
m primitve fix |
||
| (34 intermediate revisions by 29 users not shown) | |||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
| verifiedrevid = |
| verifiedrevid = 409701857 |
||
| Reference = <ref>{{RubberBible62nd|page=B-91}}.</ref> |
| Name = |
||
| Reference = <ref>{{RubberBible62nd|page=B-91}}.</ref> |
|||
| ImageFile = cesium acetate. |
| ImageFile = cesium acetate.svg |
||
| |
| ImageSize = 120px |
||
| ImageCaption = Structural formula |
|||
| ⚫ | |||
| ImageFile1 = Caesiumacetat.png |
|||
| ⚫ | |||
| ImageFileL1 = Acetate-anion-3D-balls.png |
|||
| ⚫ | |||
| ImageFileR1 = Caesium-3D.png |
|||
| ⚫ | |||
| ImageSize1 = 240px |
|||
| ImageCaption1 = Unit cell of anhydrous caesium acetate. |
|||
| ImageAlt1 = anhydrous caesium acetate crystallizes in a hexagonal unit cell. |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ChemSpiderID = 141192 |
| ChemSpiderID = 141192 |
||
| InChI = 1/C2H4O2.Cs/c1-2(3)4;/h1H3,(H,3,4);/q;+1/p-1 |
| InChI = 1/C2H4O2.Cs/c1-2(3)4;/h1H3,(H,3,4);/q;+1/p-1 |
||
| Line 15: | Line 23: | ||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChIKey = ZOAIGCHJWKDIPJ-UHFFFAOYSA-M |
| StdInChIKey = ZOAIGCHJWKDIPJ-UHFFFAOYSA-M |
||
| CASNo_Ref = {{cascite|correct| |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
| CASNo = 3396-11-0 |
| CASNo = 3396-11-0 |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| ⚫ | |||
| UNII = M74D2UJ8C9 |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
}} |
}} |
||
| Section2 = {{Chembox Properties |
| Section2 = {{Chembox Properties |
||
| |
| C=2 | H=3 | O=2 | Cs=1 |
||
| |
| MolarMass = 191.949 g/mol |
||
| |
| Appearance = colourless, [[hygroscopic]] |
||
| |
| Density = 2.423 g/cm<sup>3</sup>, solid |
||
| |
| MeltingPtC = 194 |
||
| |
| BoilingPtC = 945 |
||
| |
| Solubility = 945.1 g/100 g (−2.5 °C)<br/>1345.5 g/100 ml (88.5 °C) |
||
}} |
}} |
||
| Section3 = |
|||
| ⚫ | |||
| Section4 = {{Chembox Structure |
|||
| EUIndex = not listed |
|||
| LattConst_ref = <ref name="crystal structure">{{Cite journal |doi = 10.1002/zaac.19936190823|title = Kristallstruktur von Caesiumacetat, Cs(CH3COO)|journal = Zeitschrift für Anorganische und Allgemeine Chemie|volume = 619|issue = 8|pages = 1462–1464|year = 1993|last1 = Lossin|first1 = Adalbert|last2 = Meyer|first2 = Gerd}}</ref> |
|||
| ⚫ | |||
| Structure_ref = <ref name="crystal structure" /> |
|||
| CrystalStruct = Primitive [[hexagonal]] |
|||
| LattConst_a = 1488.0 pm |
|||
| LattConst_c = 397.65 pm |
|||
| SpaceGroup = P6/m, No. 175 |
|||
| UnitCellFormulas = 6 |
|||
| UnitCellVolume = 76.542 cm<sup>3</sup>·mol<sup>−1</sup> |
|||
}} |
|||
| Section5 = |
|||
| Section6 = |
|||
| ⚫ | |||
| ⚫ | |||
}} |
}} |
||
| Section8 = {{Chembox Related |
| Section8 = {{Chembox Related |
||
| |
| OtherAnions = [[Caesium formate]] |
||
| |
| OtherCations = [[Lithium acetate]]<br/>[[Sodium acetate]]<br/>[[Potassium acetate]]<br/>[[Rubidium acetate]] |
||
}} |
}} |
||
}} |
}} |
||
'''Caesium acetate''' or '''cesium acetate''' is an [[ion]]ic [[caesium]] compound with the molecular formula CH<sub>3</sub> |
'''Caesium acetate''' or '''cesium acetate''' is an [[ion]]ic [[caesium]] compound with the molecular formula CH<sub>3</sub>COOCs. It is a white solid that may be formed by the reaction of [[caesium hydroxide]] or [[caesium carbonate]] with [[acetic acid]].<ref name="e-EROS">{{Citation|last=Yode|first=Ryan|title=Cesium Acetate|date=2015|url=https://onlinelibrary.wiley.com/doi/abs/10.1002/047084289X.rn01845|encyclopedia=Encyclopedia of Reagents for Organic Synthesis|pages=1–11|publisher=John Wiley & Sons|language=en|doi=10.1002/047084289x.rn01845|isbn=978-0-470-84289-8|access-date=2020-07-21}}</ref> |
||
== Uses == |
|||
It may be formed by the reaction of [[caesium hydroxide]] or [[caesium oxide]] with [[acetic acid]]. |
|||
It is used in organic synthesis. One example is in the [[Perkin synthesis]]: the formation of [[Saturated and unsaturated compounds|unsaturated]] [[cinnamic acid|cinnamic-type acids]] by the condensation of aromatic [[aldehyde]]s with [[fatty acid]]s. Replacement of the commonly used sodium acetate with caesium acetate has been shown to improve yields by up to 10 times.<ref name="e-EROS"/><ref>{{citation|last1=Koepp|first1=E.|title=Perkin-Synthese mit Cäsiumacetat|journal=Synthesis|volume=1987|issue=2|pages=177–179|year=1987|doi=10.1055/s-1987-27880|last2=Vögtle|first2=F.}}.</ref> |
|||
It is often used to invert secondary alcohols. After converting the alcohol to a good leaving group, such as a [[mesylate]], direct [[SN2 reaction|S<sub>N</sub>2 substitution]] with the acetate produces the O-acetate with inverted stereochemistry, which can be converted back to a hydroxyl group.<ref name="e-EROS"/> |
|||
Caesium acetate is occasionally used instead of caesium formate in petroleum drilling fluids.{{cn|date=October 2013}} |
|||
==References== |
==References== |
||
| Line 47: | Line 74: | ||
==Further reading== |
==Further reading== |
||
*{{citation | last1 = Torisawa | first1 = Yasuhiro | last2 = Okabe | first2 = Hiromitsu | last3 = Ikegami | first3 = Shiro | title = Efficient Inversions of Secondary Alcohols using Cesium Acetate and 18-Crown-6 | journal = Chem. Lett. | year = 1984 | volume = 13 | issue = 9 | pages = 1555–56 | doi = 10.1246/cl.1984.1555}}. |
*{{citation | last1 = Torisawa | first1 = Yasuhiro | last2 = Okabe | first2 = Hiromitsu | last3 = Ikegami | first3 = Shiro | title = Efficient Inversions of Secondary Alcohols using Cesium Acetate and 18-Crown-6 | journal = Chem. Lett. | year = 1984 | volume = 13 | issue = 9 | pages = 1555–56 | doi = 10.1246/cl.1984.1555| doi-access = free }}. |
||
==External links== |
==External links== |
||
*[ |
*[https://web.archive.org/web/20080511192739/http://specialmetals.chemetall.com/pdf/Cesium_Acetate_pure.pdf Caesium acetate factsheet from Chemetall GmbH] |
||
{{Organic-compound-stub}} |
|||
{{Caesium compounds}} |
{{Caesium compounds}} |
||
{{Acetates}} |
|||
[[Category:Caesium compounds]] |
[[Category:Caesium compounds]] |
||
[[Category:Acetates]] |
[[Category:Acetates]] |
||
[[Category:Reagents for organic chemistry]] |
[[Category:Reagents for organic chemistry]] |
||
[[ar:خلات السيزيوم]] |
|||
[[de:Caesiumacetat]] |
|||
[[nl:Cesiumacetaat]] |
|||
Latest revision as of 08:46, 7 September 2024
 Structural formula
| |||
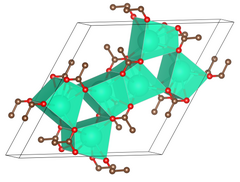 Unit cell of anhydrous caesium acetate.
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Caesium acetate | |||
| Other names
Cesium acetate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.020.226 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H3CsO2 | |||
| Molar mass | 191.949 g/mol | ||
| Appearance | colourless, hygroscopic | ||
| Density | 2.423 g/cm3, solid | ||
| Melting point | 194 °C (381 °F; 467 K) | ||
| Boiling point | 945 °C (1,733 °F; 1,218 K) | ||
| 945.1 g/100 g (−2.5 °C) 1345.5 g/100 ml (88.5 °C) | |||
| Structure[2] | |||
| Primitive hexagonal | |||
| P6/m, No. 175 | |||
a = 1488.0 pm, c = 397.65 pm[2]
| |||
Lattice volume (V)
|
76.542 cm3·mol−1 | ||
Formula units (Z)
|
6 | ||
| Hazards | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions
|
Caesium formate | ||
Other cations
|
Lithium acetate Sodium acetate Potassium acetate Rubidium acetate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Caesium acetate or cesium acetate is an ionic caesium compound with the molecular formula CH3COOCs. It is a white solid that may be formed by the reaction of caesium hydroxide or caesium carbonate with acetic acid.[3]
Uses
[edit]It is used in organic synthesis. One example is in the Perkin synthesis: the formation of unsaturated cinnamic-type acids by the condensation of aromatic aldehydes with fatty acids. Replacement of the commonly used sodium acetate with caesium acetate has been shown to improve yields by up to 10 times.[3][4]
It is often used to invert secondary alcohols. After converting the alcohol to a good leaving group, such as a mesylate, direct SN2 substitution with the acetate produces the O-acetate with inverted stereochemistry, which can be converted back to a hydroxyl group.[3]
Caesium acetate is occasionally used instead of caesium formate in petroleum drilling fluids.[citation needed]
References
[edit]- ^ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. B-91. ISBN 0-8493-0462-8..
- ^ a b Lossin, Adalbert; Meyer, Gerd (1993). "Kristallstruktur von Caesiumacetat, Cs(CH3COO)". Zeitschrift für Anorganische und Allgemeine Chemie. 619 (8): 1462–1464. doi:10.1002/zaac.19936190823.
- ^ a b c Yode, Ryan (2015), "Cesium Acetate", Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, pp. 1–11, doi:10.1002/047084289x.rn01845, ISBN 978-0-470-84289-8, retrieved 2020-07-21
- ^ Koepp, E.; Vögtle, F. (1987), "Perkin-Synthese mit Cäsiumacetat", Synthesis, 1987 (2): 177–179, doi:10.1055/s-1987-27880.
Further reading
[edit]- Torisawa, Yasuhiro; Okabe, Hiromitsu; Ikegami, Shiro (1984), "Efficient Inversions of Secondary Alcohols using Cesium Acetate and 18-Crown-6", Chem. Lett., 13 (9): 1555–56, doi:10.1246/cl.1984.1555.


