Amiodarone: Difference between revisions
m →Excretion: in the last paragraph, I corrected "Although" to "Because" to make the statement internally consistent, ie, make sense. |
|||
| (492 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Antiarrhythmic medication used for various types of irregular heartbeats}} |
|||
{{Drugbox |
|||
{{Use dmy dates|date=December 2023}} |
|||
{{cs1 config |name-list-style=vanc |display-authors=6}} |
|||
{{Infobox drug |
|||
| Watchedfields = changed |
|||
| verifiedrevid = 464394155 |
| verifiedrevid = 464394155 |
||
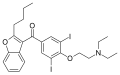
| image = Amiodarone structure.svg |
|||
| IUPAC_name = (2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-diiodophenoxy}ethyl)diethylamine |
|||
| alt = |
|||
| image = Amiodarone.png |
|||
| image2 = Amiodarone |
| image2 = Amiodarone-based-on-hydrochloride-xtal-3D-bs-17.png |
||
| alt2 = |
|||
<!--Clinical data--> |
<!-- Clinical data --> |
||
| pronounce = {{IPAc-en|ˌ|æ|m|i|ˈ|oʊ|d|ə|r|oʊ|n}} or {{IPAc-en|ə|ˈ|m|iː|oʊ-|d|ə|ˌ|r|oʊ|n}}<!--Major medical and pharmaceutical dictionaries disagree on which pronunciation is listed, not listed, preferred, or not preferred; as with many drug names, predictably variable [[spelling pronunciation]]s are a powerful force in the real-world usage of clinicians and pharmacists.--> |
|||
| tradename = Cordarone |
|||
| tradename = Cordarone, Nexterone, Pacerone, others |
|||
| Drugs.com = {{drugs.com|monograph|amiodarone-hydrochloride}} |
| Drugs.com = {{drugs.com|monograph|amiodarone-hydrochloride}} |
||
| MedlinePlus = a687009 |
| MedlinePlus = a687009 |
||
| DailyMedID = Amiodarone |
|||
| pregnancy_AU = |
|||
| |
| pregnancy_AU = C |
||
| pregnancy_category = |
| pregnancy_category = |
||
| routes_of_administration = [[Oral administration|By mouth]], [[intravenous]], [[intraosseous]] |
|||
| legal_status = Rx-only |
|||
| ATC_prefix = C01 |
|||
| routes_of_administration = [[Wiktionary:oral|oral]] or [[intravenous]] |
|||
| ATC_suffix = BD01 |
|||
| legal_AU = S4 |
|||
<!--Pharmacokinetic data--> |
|||
| |
| legal_CA = Rx-only |
||
| legal_US = Rx-only |
|||
| legal_US_comment = <ref name="DailyMed-Pacerone-2022">{{cite web | title=Pacerone- amiodarone hydrochloride tablet | website=DailyMed | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4c4ec9b0-13a5-49a8-b57b-b3f64e4316a7 | access-date=8 September 2021 | archive-date=29 December 2022 | archive-url=https://web.archive.org/web/20221229200210/https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4c4ec9b0-13a5-49a8-b57b-b3f64e4316a7 | url-status=live }}</ref><ref name="DailyMed-2018">{{cite web | title=Cordarone (amiodarone) tablets, for oral use Initial U.S. Approval: 1985 | website=DailyMed | date=30 October 2018 | url=https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=350496 | access-date=8 September 2021 | archive-date=29 December 2022 | archive-url=https://web.archive.org/web/20221229200207/https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=350496 | url-status=live }}</ref><ref name="DailyMed-Nexterone-2022">{{cite web | title=Nexterone- Amiodarone HCl injection, solution | website=DailyMed | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9d4d66f7-fa14-e832-e053-2a95a90a8b62 | access-date=8 September 2021 | archive-date=29 December 2022 | archive-url=https://web.archive.org/web/20221229200210/https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9d4d66f7-fa14-e832-e053-2a95a90a8b62 | url-status=live }}</ref> |
|||
<!-- Pharmacokinetic data --> |
|||
| bioavailability = 20–55% |
|||
| protein_bound = 96% |
|||
| metabolism = [[Liver]] |
| metabolism = [[Liver]] |
||
| elimination_half-life = 58 |
| elimination_half-life = 58 d (range 15–142 d) |
||
| excretion = Primarily |
| excretion = Primarily liver and bile |
||
<!--Identifiers--> |
<!-- Identifiers --> |
||
| CASNo_Ref = {{cascite|correct|CAS}} |
|||
| CAS_number_Ref = {{cascite|correct|??}} |
| CAS_number_Ref = {{cascite|correct|??}} |
||
| CAS_number = 1951-25-3 |
| CAS_number = 1951-25-3 |
||
| ATC_prefix = C01 |
|||
| ATC_suffix = BD01 |
|||
| PubChem = 2157 |
| PubChem = 2157 |
||
| IUPHAR_ligand = 2566 |
| IUPHAR_ligand = 2566 |
||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
||
| DrugBank = DB01118 |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| ChemSpiderID = 2072 |
| ChemSpiderID = 2072 |
||
| Line 42: | Line 52: | ||
| ChEMBL = 633 |
| ChEMBL = 633 |
||
<!--Chemical data--> |
<!-- Chemical data --> |
||
| IUPAC_name = (2-<nowiki/>{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-diiodophenoxy}ethyl)diethylamine |
|||
| C=25 | H=29 | I=2 | N=1 | O=3 |
|||
| C=25 | H=29 | I=2 | N=1 | O=3 |
|||
| molecular_weight = 645,31 g/mol |
|||
| |
| SMILES = CCN(CC)CCOc1c(I)cc(cc1I)C(=O)c2c3ccccc3oc2CCCC |
||
| InChI = 1/C25H29I2NO3/c1-4-7-11-22-23(18-10-8-9-12-21(18)31-22)24(29)17-15-19(26)25(20(27)16-17)30-14-13-28(5-2)6-3/h8-10,12,15-16H,4-7,11,13-14H2,1-3H3 |
|||
| InChIKey = IYIKLHRQXLHMJQ-UHFFFAOYAO |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChI = 1S/C25H29I2NO3/c1-4-7-11-22-23(18-10-8-9-12-21(18)31-22)24(29)17-15-19(26)25(20(27)16-17)30-14-13-28(5-2)6-3/h8-10,12,15-16H,4-7,11,13-14H2,1-3H3 |
| StdInChI = 1S/C25H29I2NO3/c1-4-7-11-22-23(18-10-8-9-12-21(18)31-22)24(29)17-15-19(26)25(20(27)16-17)30-14-13-28(5-2)6-3/h8-10,12,15-16H,4-7,11,13-14H2,1-3H3 |
||
| Line 54: | Line 62: | ||
}} |
}} |
||
<!-- Definition and medical uses --> |
|||
'''Amiodarone''' is an [[antiarrhythmic agent]] used for various types of [[cardiac dysrhythmia]]s, both [[Tachyarrhythmia#Ventricular|ventricular]] and [[Tachyarrhythmia#Atrial|atrial]]. It was discovered in 1961. Despite relatively common [[side-effects]], it is used in arrhythmias that are otherwise difficult to treat with medication. |
|||
'''Amiodarone''' is an [[antiarrhythmic medication]] used to treat and prevent a number of types of [[cardiac dysrhythmia]]s.<ref name=AHFS2016/> This includes [[ventricular tachycardia]], [[ventricular fibrillation]], and [[wide complex tachycardia]], [[atrial fibrillation]], and [[paroxysmal supraventricular tachycardia]].<ref name=AHFS2016/> Evidence in [[cardiac arrest]], however, is poor.<ref name=Ali2018>{{cite journal | vauthors = Ali MU, Fitzpatrick-Lewis D, Kenny M, Raina P, Atkins DL, Soar J, Nolan J, Ristagno G, Sherifali D | title = Effectiveness of antiarrhythmic drugs for shockable cardiac arrest: A systematic review | journal = Resuscitation | volume = 132 | pages = 63–72 | date = November 2018 | pmid = 30179691 | doi = 10.1016/j.resuscitation.2018.08.025 | s2cid = 52154562 | url = http://wrap.warwick.ac.uk/113491/1/WRAP-effectiveness-antiarrhythmic-drugs-cardiac-review-Nolan-2018.pdf | access-date = 17 December 2019 | archive-date = 5 March 2020 | archive-url = https://web.archive.org/web/20200305122730/http://wrap.warwick.ac.uk/113491/1/WRAP-effectiveness-antiarrhythmic-drugs-cardiac-review-Nolan-2018.pdf | url-status = live }}</ref> It can be given by mouth, [[intravenously]], or [[intraosseously]].<ref name=AHFS2016/> When used by mouth, it can take a few weeks for effects to begin.<ref name=AHFS2016/><ref name="Xavier-Research-Press-2018">{{cite book|isbn=978-1-7242-7798-5 |title=Review of the Medical Use of Amiodarone (Nexterone, Pacerone) |date=24 July 2018 |publisher=Xavier Research Press }}</ref> |
|||
<!-- Side effects and mechanism --> |
|||
==History== |
|||
Common side effects include feeling tired, tremor, nausea, and constipation.<ref name=AHFS2016/> As amiodarone can have serious side effects, it is mainly recommended only for significant ventricular arrhythmias.<ref name=AHFS2016/> Serious side effects include lung toxicity<ref name="pmid33262034">{{cite journal |vauthors=Feduska ET, Thoma BN, Torjman MC, Goldhammer JE |title=Acute Amiodarone Pulmonary Toxicity |journal=J Cardiothorac Vasc Anesth |volume=35 |issue=5 |pages=1485–1494 |date=May 2021 |pmid=33262034 |doi=10.1053/j.jvca.2020.10.060 |s2cid=227253264 |url=}}</ref> such as [[interstitial pneumonitis]], [[liver problems]], heart arrhythmias, vision problems, [[thyroid problems]], and death.<ref name=AHFS2016/> If taken during [[pregnancy]] or [[breastfeeding]] it can cause problems in the fetus or the infant.<ref name=AHFS2016/> It is a [[Antiarrhythmic agent#Class III agents|class III antiarrhythmic medication]].<ref name=AHFS2016/> It works partly by increasing the time before a heart cell can contract again.<ref name=AHFS2016>{{cite web|title=Amiodarone Hydrochloride|url=https://www.drugs.com/monograph/amiodarone-hydrochloride.html|publisher=The American Society of Health-System Pharmacists|access-date=22 August 2016|url-status=live|archive-url=https://web.archive.org/web/20160919085754/https://www.drugs.com/monograph/amiodarone-hydrochloride.html|archive-date=19 September 2016}}</ref><ref name="Xavier-Research-Press-2018"/> |
|||
The original observation that amiodarone's progenitor molecule, [[khellin]], had cardioactive properties, was made by the Lebanese physiologist [[Gleb von Anrep]] while working in Cairo. Khellin is a plant extract of [[Khella]] or [[Ammi visnaga]], a common plant in north Africa. Anrep noticed that one of his technicians had been cured of anginal symptoms after taking khellin, then used for various, non-cardiac ailments. This led to efforts by European pharmaceutical industries to isolate an active compound.{{citation needed|date=February 2012}} Amiodarone was initially developed in 1961 at the Labaz company, [[Belgium]], by chemists Tondeur and Binon, who were working on preparations derived from khellin. It became popular in Europe as a treatment for [[angina pectoris]].<ref>{{cite journal |author=Deltour G, Binon F, Tondeur R, ''et al.'' |title=[Studies in the benzofuran series. VI. Coronary-dilating activity of alkylated and aminoalkylated derivatives of 3-benzoylbenzofuran.] |language=French |journal=Archives internationales de pharmacodynamie et de thérapie |volume=139 |issue= |pages=247–54 |year=1962 |pmid=14026835 |doi=}}</ref><ref>{{cite journal |author=Charlier R, Deltour G, Tondeur R, Binon F |title=[Studies in the benzofuran series. VII. Preliminary pharmacological study of 2-butyl-3-(3,5-diiodo-4-beta-N-diethylaminoethoxybenzoyl)-benzofuran.] |language=French |journal=Archives internationales de pharmacodynamie et de thérapie |volume=139 |issue= |pages=255–64 |year=1962 |pmid=14020244 |doi=}}</ref> |
|||
<!-- History, society and culture --> |
|||
As a doctoral candidate at Oxford University, Dr. Bramah Singh determined that amiodarone and [[sotalol]] had antiarrhythmic properties and belonged to a new class of antiarrhythmic agents (what would become the class III antiarrhythmic agents).<ref>{{cite journal |author=Singh BN, Vaughan Williams EM |title=The effect of amiodarone, a new anti-anginal drug, on cardiac muscle |journal=Br. J. Pharmacol. |volume=39 |issue=4 |pages=657–67 |year=1970 |pmid=5485142 |doi= |pmc=1702721}}</ref> Today the mechanisms of action of amiodarone and sotalol have been investigated in more detail. Both drugs have been demonstrated to prolong the duration of the [[action potential]], prolonging the refractory period, by interacting among other cellular function with [[K+ channels]]. |
|||
Amiodarone was first made in 1961 and came into medical use in 1962 for [[angina|chest pain believed to be related to the heart]].<ref>{{cite book|title=Analytical Profiles of Drug Substances and Excipients|date=1992|publisher=Academic Press|isbn=978-0-08-086115-9|page=4|url=https://books.google.com/books?id=IMCH-05Z6-EC&pg=PA4|language=en|url-status=live|archive-url=https://web.archive.org/web/20170908192558/https://books.google.com/books?id=IMCH-05Z6-EC&pg=PA4|archive-date=8 September 2017}}</ref> It was pulled from the market in 1967 due to side effects.<ref name=Fis2005/> In 1974 it was found to be useful for arrhythmias and reintroduced.<ref name=Fis2005>{{cite book| vauthors = Fischer J, Ganellin CR |title=Analogue-based Drug Discovery |date=2005 |publisher=John Wiley & Sons |isbn=978-3-527-60749-5 |page=12 |url= https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA12 |language=en|url-status=live |archive-url= https://web.archive.org/web/20170908192558/https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA12 |archive-date=8 September 2017}}</ref> It is on the [[WHO Model List of Essential Medicines|World Health Organization's List of Essential Medicines]].<ref name="WHO23rd">{{cite book | vauthors = ((World Health Organization)) | title = The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023) | year = 2023 | hdl = 10665/371090 | author-link = World Health Organization | publisher = World Health Organization | location = Geneva | id = WHO/MHP/HPS/EML/2023.02 | hdl-access=free }}</ref> It is available as a [[generic medication]].<ref name=AHFS2016/> In 2022, it was the 237th most commonly prescribed medication in the United States, with more than 1{{nbsp}}million prescriptions.<ref>{{cite web | title=The Top 300 of 2022 | url=https://clincalc.com/DrugStats/Top300Drugs.aspx | website=ClinCalc | access-date=30 August 2024 | archive-date=30 August 2024 | archive-url=https://web.archive.org/web/20240830202410/https://clincalc.com/DrugStats/Top300Drugs.aspx | url-status=live }}</ref><ref>{{cite web | title = Amiodarone Drug Usage Statistics, United States, 2013 - 2022 | website = ClinCalc | url = https://clincalc.com/DrugStats/Drugs/Amiodarone | access-date = 30 August 2024 }}</ref> |
|||
==Medical uses== |
|||
Based on Singh's work, the [[Argentina|Argentinian]] physician Dr. Mauricio Rosenbaum began using amiodarone to treat his patients who suffered from supraventricular and ventricular arrhythmias, with impressive results. Based on papers written by Dr. Rosenbaum developing Singh's theories, physicians in the [[United States]] began prescribing amiodarone to their patients with potentially life-threatening arrhythmias in the late 1970s.<ref>{{cite journal |author=Rosenbaum MB, Chiale PA, Halpern MS, ''et al.'' |title=Clinical efficacy of amiodarone as an antiarrhythmic agent |journal=Am. J. Cardiol. |volume=38 |issue=7 |pages=934–44 |year=1976 |pmid=793369|doi=10.1016/0002-9149(76)90807-9}}</ref><ref>{{cite journal |author=Rosenbaum MB, Chiale PA, Haedo A, Lázzari JO, Elizari MV |title=Ten years of experience with amiodarone |journal=Am. Heart J. |volume=106 |issue=4 Pt 2 |pages=957–64 |year=1983 |pmid=6613843|doi=10.1016/0002-8703(83)90022-4}}</ref> By 1980, amiodarone was commonly prescribed throughout Europe for the treatment of arrhythmias, but in the U.S. amiodarone remained unapproved by the [[Food and Drug Administration]], and physicians were forced to directly obtain amiodarone from pharmaceutical companies in [[Canada]] and [[Europe]].{{Citation needed|date=October 2007}} |
|||
Amiodarone has been used both in the treatment of acute life-threatening arrhythmias as well as the long-term suppression of arrhythmias.<ref name="pmid32166725">{{cite journal |vauthors=Hamilton D, Nandkeolyar S, Lan H, Desai P, Evans J, Hauschild C, Choksi D, Abudayyeh I, Contractor T, Hilliard A |title=Amiodarone: A Comprehensive Guide for Clinicians |journal=Am J Cardiovasc Drugs |volume=20 |issue=6 |pages=549–558 |date=December 2020 |pmid=32166725 |doi=10.1007/s40256-020-00401-5 |s2cid=212682149 |url=}}</ref> Amiodarone is commonly used to treat different types of abnormal heart rhythms, such as atrial arrhythmias (supraventricular arrhythmias) and ventricular arrhythmias.<ref name="pmid32166725"/> |
|||
Atrial arrhythmias and supraventricular arrhythmias are terms often used interchangeably to refer to abnormal heart rhythms originating from the upper chambers of the heart, known as the atria. These types of arrhythmias include conditions such as atrial fibrillation, atrial flutter, and paroxysmal supraventricular tachycardia. They are collectively referred to as supraventricular or atrial arrhythmias because they occur above (supra) the ventricles in the electrical conduction system of the heart.<ref name="pmid30675928">{{cite journal |vauthors=Mori S, Tretter JT, Spicer DE, Bolender DL, Anderson RH |title=What is the real cardiac anatomy? |journal=Clin Anat |volume=32 |issue=3 |pages=288–309 |date=April 2019 |pmid=30675928 |pmc=6849845 |doi=10.1002/ca.23340 |url=}}</ref> |
|||
The FDA was reluctant to officially approve the use of amiodarone, since initial reports had shown increased incidence of serious pulmonary side-effects of the drug. In the mid 1980s, the European pharmaceutical companies began putting pressure on the FDA to approve amiodarone by threatening to cut the supply to American physicians if it were not approved. In December 1985, amiodarone was approved by the FDA for the treatment of arrhythmias.<ref>{{cite web |url=http://www.fda.gov/cder/foi/nda/pre96/18-972_Cardarone.htm |title=Drug Approval Package |accessdate=2007-09-30 |work=}} {{Dead link|date=September 2010|bot=H3llBot}}</ref> This makes amiodarone one of the few drugs approved by the FDA without rigorous randomized clinical trials.{{Citation needed|date=October 2007}} |
|||
Ventricular arrhythmias are abnormal heart rhythms that originate in the ventricles, which are the lower chambers of the heart. These arrhythmias can be potentially life-threatening and may disrupt the heart's ability to pump blood effectively.<ref name="pmid30675928"/> |
|||
==Dosing== |
|||
Amiodarone is available in oral and intravenous formulations. |
|||
Amiodarone can be effective in treating conditions like ventricular fibrillation (a rapid and irregular heartbeat), ventricular tachycardia (fast heartbeat originating from the lower chambers), and cardiac arrest due to shock-resistant ventricular fibrillation.<ref name="pmid32166725"/> |
|||
Orally, it is available under the trade names '''Pacerone''' (produced by Upsher-Smith Laboratories, Inc.) and '''Cordarone''' (produced by Wyeth-Ayerst Laboratories) in 200 [[milligram|mg]] and 400 mg tablets; It is also available under the trade name '''Aratac''' (produced by Alphapharm Pty Ltd) in 100 mg and 200 mg tablets in Australia and New Zealand. Also '''Arycor''' in South Africa (Produced by Winthrop Pharmaceuticals.) in doses of 100 mg and 200 mg scored tablets. In South America, it is known as '''Atlansil''' and is produced by Roemmers. |
|||
In cases where a patient is experiencing shock-resistant ventricular arrhythmias including stable ventricular tachycardia or unstable ventricular fibrillation, amiodarone may be used.<ref name="Larson_2022">{{cite journal | vauthors = Larson J, Rich L, Deshmukh A, Judge EC, Liang JJ | title = Pharmacologic Management for Ventricular Arrhythmias: Overview of Anti-Arrhythmic Drugs | journal = Journal of Clinical Medicine | volume = 11 | issue = 11 | pages = 3233 | date = June 2022 | pmid = 35683620 | pmc = 9181251 | doi = 10.3390/jcm11113233 | doi-access = free }}</ref> A recent study suggested that another antiarrhythmic, procainamide, may be more effective in stopping ventricular tachycardia – with less side effects and a higher survival rate in patients requiring multiple shocks.<ref name="Ortiz_2017">{{cite journal | vauthors = Ortiz M, Martín A, Arribas F, Coll-Vinent B, Del Arco C, Peinado R, Almendral J | title = Randomized comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: the PROCAMIO study | journal = European Heart Journal | volume = 38 | issue = 17 | pages = 1329–1335 | date = May 2017 | pmid = 27354046 | pmc = 5410924 | doi = 10.1093/eurheartj/ehw230 | url = }}</ref> However, due to a small sample size and lack of statistical significance, more evidence is required, and amiodarone remains the drug of choice in ventricular arrhythmias.<ref name="Larson_2022" /><ref name="Ortiz_2017" /> |
|||
In '''India''' amiodarone is marketed(produced by Cipla Pharmaceutical) under the brand name '''Tachyra''' available in 100 mg &200 mg and intravenous ampules. |
|||
Amiodarone is also commonly used as the first-line therapy for patients who receive shocks from implantable cardioverter defibrillators caused by ventricular arrhythmias. Combining amiodarone with beta-blockers has been shown to reduce the likelihood of experiencing inappropriate shocks from implantable cardioverter defibrillators.<ref name="pmid32166725"/> |
|||
It is also available in intravenous ampules and vials, typically in 150 mg increments. |
|||
===Cardiac arrest=== |
|||
The dose of amiodarone administered is tailored to the individual and the dysrhythmia that is being treated. When administered orally, the [[bioavailability]] of amiodarone is quite variable. Absorption ranges from 22 to 95%, with better absorption when it is given with food.<ref>{{cite journal |author=Siddoway LA |title=Amiodarone: guidelines for use and monitoring |journal=American Family Physician |volume=68 |issue=11 |pages=2189–96 |year=2003 |pmid=14677664 |url=http://www.aafp.org/afp/20031201/2189.html}}</ref> |
|||
[[Defibrillation]] is the treatment of choice for [[ventricular fibrillation]] and pulseless [[ventricular tachycardia]] resulting in [[cardiac arrest]]. While amiodarone has been used in shock-refractory cases, evidence of benefit is poor.<ref name=Ali2018/> Although amiodarone does not appear to improve survival in those who had a cardiac arrest in-hospital,<ref>{{cite journal | vauthors = Laina A, Karlis G, Liakos A, Georgiopoulos G, Oikonomou D, Kouskouni E, Chalkias A, Xanthos T | title = Amiodarone and cardiac arrest: Systematic review and meta-analysis | journal = International Journal of Cardiology | volume = 221 | pages = 780–788 | date = October 2016 | pmid = 27434349 | doi = 10.1016/j.ijcard.2016.07.138 }}</ref> some studies suggested that early administration of amiodarone was associated with better survival and positive outcomes for people who had a cardiac arrest out-of-hospital.<ref>{{cite journal | vauthors = Lupton JR, Neth MR, Sahni R, Jui J, Wittwer L, Newgard CD, Daya MR | title = Survival by time-to-administration of amiodarone, lidocaine, or placebo in shock-refractory out-of-hospital cardiac arrest | journal = Academic Emergency Medicine | volume = 30 | issue = 9 | pages = 906–917 | date = September 2023 | pmid = 36869657 | doi = 10.1111/acem.14716 }}</ref><ref>{{cite journal | vauthors = Perry E, Nehme E, Stub D, Anderson D, Nehme Z | title = The impact of time to amiodarone administration on survival from out-of-hospital cardiac arrest | journal = Resuscitation Plus | volume = 14 | pages = 100405 | date = June 2023 | pmid = 37303855 | pmc = 10250159 | doi = 10.1016/j.resplu.2023.100405 }}</ref> |
|||
===Ventricular tachycardia=== |
|||
Amiodarone is fat-soluble, and tends to concentrate in tissues including fat, muscle, liver, lungs, and skin. This confers a high [[volume of distribution]] (5000 liters in a 70 kg adult) and a long half-life. Due to the long half-life of amiodarone, oral loading typically takes days to weeks. |
|||
Amiodarone may be used in the treatment of ventricular tachycardia in certain instances.<ref name="pmid36818930">{{cite journal |vauthors=Medić F, Bakula M, Alfirević M, Bakula M, Mucić K, Marić N |title=Amiodarone and Thyroid Dysfunction |journal=Acta Clin Croat |volume=61 |issue=2 |pages=327–341 |date=August 2022 |pmid=36818930 |pmc=9934045 |doi=10.20471/acc.2022.61.02.20 |url=}}</ref> Individuals with hemodynamically ''unstable'' ventricular tachycardia ''should not'' initially receive amiodarone. These individuals should be [[cardioversion|cardioverted]]. |
|||
Amiodarone can be used in individuals with hemodynamically stable ventricular tachycardia. In these cases, amiodarone can be used regardless of the individual's underlying heart function and the type of ventricular tachycardia; it can be used in individuals with [[monomorphic ventricular tachycardia]], but is contraindicated in individuals with [[polymorphic ventricular tachycardia]] as it is associated with a prolonged [[QT interval]] which will be made worse with anti-arrhythmic drugs.<ref>{{cite web | work = Resuscitation Council (UK) | url = https://www.resus.org.uk/resuscitation-guidelines/ | title = Peri-arrest arrhythmias – Tachycardia algorithm] | archive-url = https://web.archive.org/web/20160103224521/https://www.resus.org.uk/resuscitation-guidelines/ | archive-date=3 January 2016 | access-date = 25 January 2016 }}</ref> |
|||
An oral loading dose is typically a total of 10 grams, divided over one to two weeks but there are many other dosing regimens. Once an individual is loaded, a typical maintenance dose of amiodarone is 100 or 200 mg either once or twice daily. |
|||
===Atrial fibrillation=== |
|||
An intravenous loading dose is typically 300 mg in 20-30cc 5% Dextrose solution (D5W) for cardiac arrest. The loading infusion for dysrhythmias is typically 150 mg in a 100cc bag of 5% Dextrose solution (D5W) given over 10 minutes. Both can be followed by a 360 mg slow infusion over 6 hours then a maintenance infusion of 540 mg over 18 hours. |
|||
Individuals who have undergone [[coronary artery bypass surgery|open heart surgery]] are at an increased risk of developing [[atrial fibrillation]] (or AF) in the first few days post-procedure.<ref name="pmid32166725"/><ref>{{cite web | url=https://www.uptodate.com/contents/atrial-fibrillation-and-flutter-after-cardiac-surgery | title=UpToDate | access-date=5 February 2024 | archive-date=5 February 2024 | archive-url=https://web.archive.org/web/20240205195136/https://www.uptodate.com/contents/atrial-fibrillation-and-flutter-after-cardiac-surgery | url-status=live }}</ref><ref>{{cite web | url=https://www.health.harvard.edu/heart-health/atrial-fibrillation-after-surgery-common-and-undertreated | title=Atrial fibrillation after surgery: Common and undertreated? | date=October 2022 | access-date=5 February 2024 | archive-date=5 February 2024 | archive-url=https://web.archive.org/web/20240205195136/https://www.health.harvard.edu/heart-health/atrial-fibrillation-after-surgery-common-and-undertreated | url-status=live }}</ref> In the ARCH trial, [[intravenous]] amiodarone (2 g administered over 2 d) has been shown to reduce the incidence of atrial fibrillation after open heart surgery when compared to placebo.<ref>{{cite web | url=https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2010/02/22/19/19/ARCH | title=Amiodarone Reduction in Coronary Heart Trial | access-date=5 February 2024 | archive-date=5 February 2024 | archive-url=https://web.archive.org/web/20240205195137/https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2010/02/22/19/19/ARCH | url-status=live }}</ref><ref>{{cite journal | vauthors = Guarnieri T, Nolan S, Gottlieb SO, Dudek A, Lowry DR | title = Intravenous amiodarone for the prevention of atrial fibrillation after open heart surgery: the Amiodarone Reduction in Coronary Heart (ARCH) trial | journal = Journal of the American College of Cardiology | volume = 34 | issue = 2 | pages = 343–347 | date = August 1999 | pmid = 10440143 | doi = 10.1016/S0735-1097(99)00212-0 | s2cid = 24714524 }}</ref> However, clinical studies have failed to demonstrate long-term efficacy and have shown potentially fatal side effects such as pulmonary toxicities. While amiodarone is not approved for AF by the [[Food and Drug Administration|US Food and Drug Administration]] (FDA), it is a commonly prescribed off-label treatment due to the lack of equally effective treatment alternatives.<ref name="pmid14677664">{{cite journal | url=https://www.aafp.org/pubs/afp/issues/2003/1201/p2189.html | title=Amiodarone: Guidelines for Use and Monitoring | journal=American Family Physician | date=December 2003 | volume=68 | issue=11 | pages=2189–2197 | pmid=14677664 | vauthors=Siddoway LA | access-date=5 February 2024 | archive-date=5 February 2024 | archive-url=https://web.archive.org/web/20240205195136/https://www.aafp.org/pubs/afp/issues/2003/1201/p2189.html | url-status=live }}</ref><ref>{{cite journal | url=https://www.aafp.org/pubs/afp/issues/2005/0401/p1434.html | title=Practice Guideline Briefs | journal=American Family Physician | date=April 2005 | volume=71 | issue=7 | pages=1434 | vauthors=Neff MJ | access-date=5 February 2024 | archive-date=5 February 2024 | archive-url=https://web.archive.org/web/20240205195136/https://www.aafp.org/pubs/afp/issues/2005/0401/p1434.html | url-status=live }}</ref> |
|||
So-called 'acute onset atrial fibrillation', defined by the North American Society of Pacing and Electrophysiology (NASPE) in 2003, responds well to short-duration treatment with amiodarone.<ref name="pmid14677664" /><ref>{{cite journal | url=https://www.aafp.org/pubs/afp/issues/2002/0715/p249.html | title=Acute Management of Atrial Fibrillation: Part I. Rate and Rhythm Control | journal=American Family Physician | date=15 July 2002 | volume=66 | issue=2 | pages=249–257 | pmid=12152960 | vauthors=King DE, Dickerson LM, Sack JL | access-date=5 February 2024 | archive-date=5 February 2024 | archive-url=https://web.archive.org/web/20240205195136/https://www.aafp.org/pubs/afp/issues/2002/0715/p249.html | url-status=live }}</ref> This has been demonstrated in seventeen randomized controlled trials, of which five included a placebo arm. The incidence of severe side effects in this group is low.<ref>{{cite journal | url=https://bmjopen.bmj.com/content/10/3/e034774 | pmid=32209631 | date=2020 | title=Managing new-onset atrial fibrillation in critically ill patients: A systematic narrative review | journal=BMJ Open | volume=10 | issue=3 | pages=e034774 | doi=10.1136/bmjopen-2019-034774 | pmc=7202704 | vauthors=O'Bryan LJ, Redfern OC, Bedford J, Petrinic T, Young JD, Watkinson PJ | access-date=5 February 2024 | archive-date=5 February 2024 | archive-url=https://web.archive.org/web/20240205195136/https://bmjopen.bmj.com/content/10/3/e034774 | url-status=live }}</ref><ref>{{cite journal | url=https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/215367 | doi=10.1001/archinte.163.7.777 | title=Effectiveness of Amiodarone for Conversion of Atrial Fibrillation to Sinus Rhythm | date=2003 | journal=Archives of Internal Medicine | volume=163 | issue=7 | pages=777–785 | pmid=12695268 | vauthors=Letelier LM, Udol K, Ena J, Weaver B, Guyatt GH | url-access=subscription | access-date=5 February 2024 | archive-date=5 February 2024 | archive-url=https://web.archive.org/web/20240205195136/https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/215367 | url-status=live }}</ref><ref>{{cite web | url=https://www.nice.org.uk/guidance/ng196/chapter/Recommendations | title=Recommendations | Atrial fibrillation: Diagnosis and management | Guidance | NICE | date=27 April 2021 | access-date=5 February 2024 | archive-date=5 February 2024 | archive-url=https://web.archive.org/web/20240205195137/https://www.nice.org.uk/guidance/ng196/chapter/Recommendations | url-status=live }}</ref> |
|||
==Mechanism of action== |
|||
Amiodarone is categorized as a class III [[antiarrhythmic agent]], and prolongs phase 3 of the [[cardiac action potential]], the repolarization phase where there is normally decreased calcium permeability and increased potassium permeability. It has numerous other effects however, including actions that are similar to those of antiarrhythmic classes Ia, II, and IV. |
|||
Amiodarone is an effective, antiarrhythmic-of-choice in achieving cardioversion to sinus rhythm in critical care populations with new onset atrial fibrillation (NOAF). However, other anti-arrhythmic agents may exert superior rhythm control, rate control and lower mortality rate which may be more favourable than amiodarone in specific cases.<ref>Johnston BW, Chean CS, Duarte R, Hill R, Blackwood B, McAuley DF, Welters ID. Management of new onset atrial fibrillation in critically unwell adult patients: a systematic review and narrative synthesis. British Journal of Anaesthesia. 2022 May 1;128(5):759-71.</ref> |
|||
Amiodarone shows [[beta blocker]]-like and [[potassium channel blocker]]-like actions on the [[SA node|SA]] and [[AV node]]s, increases the refractory period via sodium- and potassium-channel effects, and slows intra-cardiac conduction of the [[cardiac action potential]], via sodium-channel effects. |
|||
==Contraindications== |
|||
Amiodarone resembles T4 thyroid hormone, and its binding to the nuclear thyroid receptor might contribute to some of its pharmacologic and toxic actions <ref>Goodman & Gilman's The Pharmacological Basis of Therapeutics, 11th Edition.</ref> |
|||
Women who are [[pregnant]] or may become pregnant are strongly advised not to take amiodarone. Since amiodarone can be expressed in breast milk, women taking the drug are advised to stop nursing. |
|||
It is contraindicated in individuals with [[sinus node|sinus nodal]] [[bradycardia]], [[atrioventricular node|atrioventricular]] block, and second or third-degree heart block who do not have an [[artificial pacemaker]]. |
|||
==Indications for use== |
|||
Because amiodarone has a low incidence of pro-arrhythmic effects, it has been used both in the treatment of acute life-threatening arrhythmias as well as the chronic suppression of arrhythmias. It is useful both in supraventricular arrhythmias and ventricular arrhythmias. |
|||
Individuals with baseline depressed lung function should be monitored closely if amiodarone therapy is to be initiated. |
|||
===Ventricular fibrillation=== |
|||
The treatment of choice for [[ventricular fibrillation]] (VF) is electrical defibrillation. However, amiodarone can be useful in shock-refractory VF. In the ARREST trial, amiodarone was shown to improve survival to [[hospital]] admission (when compared to [[placebo]]) in individuals who suffer [[cardiac arrest]] with shock-refractory VF.<ref>{{cite journal |author=Kudenchuk PJ, Cobb LA, Copass MK, ''et al.'' |title=Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation |journal=N. Engl. J. Med. |volume=341 |issue=12 |pages=871–8 |year=1999 |pmid=10486418|doi=10.1056/NEJM199909163411203}}</ref> It is on the basis of this study that the guidelines created by the [[American Heart Association]] for the treatment of VF include amiodarone as a second line agent (after [[epinephrine]] or [[vasopressin]]). ARREST was not adequately powered to demonstrate survival to hospital discharge. |
|||
Formulations of amiodarone that contain benzyl alcohol should not be given to neonates, because the benzyl alcohol may cause the potentially fatal "gasping syndrome".<ref>{{cite journal | title = Neonatal deaths associated with use of benzyl alcohol--United States | journal = MMWR. Morbidity and Mortality Weekly Report | volume = 31 | issue = 22 | pages = 290–291 | date = June 1982 | pmid = 6810084 | url = https://www.cdc.gov/mmwr/preview/mmwrhtml/00001109.htm | url-status = live | archive-url = https://web.archive.org/web/20120830225243/http://www.cdc.gov/mmwr/preview/mmwrhtml/00001109.htm | archive-date = 30 August 2012 | author1 = Centers for Disease Control (CDC) }}</ref> |
|||
===Ventricular tachycardia=== |
|||
Amiodarone may be used in the treatment of ventricular tachycardia in certain instances. Individuals with hemodynamically ''unstable'' ventricular tachycardia ''should not'' initially receive amiodarone. These individuals should be [[cardioversion|cardioverted]] out of their unstable rhythm. |
|||
Amiodarone can worsen the cardiac arrhythmia brought on by [[digitalis]] toxicity. |
|||
Amiodarone can be used in individuals with hemodynamically stable ventricular tachycardia. In these cases, amiodarone can be used regardless of the individual's underlying heart function and the type of ventricular tachycardia; it can be used in individuals with [[monomorphic ventricular tachycardia]], but is contraindicated in individuals with [[polymorphic ventricular tachycardia]] as it is associated with a prolonged QT interval which will be made worse with anti-arrhythmic drugs. The dose of amiodarone is 150 [[milligram|mg]] [[intravenous|IV]] administered over 10 minutes. |
|||
Contraindications of amiodarone also include: |
|||
===Atrial fibrillation=== |
|||
* hypersensitivity to amiodarone or any of its components;<ref name="pmid32166725"/> |
|||
Individuals who have undergone [[coronary artery bypass surgery|open heart surgery]] are at an increased risk of developing [[atrial fibrillation]] (or AF) in the first few days post-procedure. In the ARCH trial, [[intravenous]] amiodarone (2 [[gram]]s administered over 2 days) has been shown to reduce the incidence of atrial fibrillation after open heart surgery when compared to placebo.<ref>{{cite journal |author=Guarnieri T, Nolan S, Gottlieb SO, Dudek A, Lowry DR |title=Intravenous amiodarone for the prevention of atrial fibrillation after open heart surgery: the Amiodarone Reduction in Coronary Heart (ARCH) trial |journal=J. Am. Coll. Cardiol. |volume=34 |issue=2 |pages=343–7 |year=1999 |pmid=10440143|doi=10.1016/S0735-1097(99)00212-0}}</ref> However, clinical studies have failed to demonstrate long-term efficacy and have shown potentially fatal side effects such as pulmonary toxicities. While amiodarone is not approved for AF by the FDA, it is a commonly prescribed off-label treatment due to the lack of efficacious treatment alternatives. |
|||
* severe [[hepatic impairment]];<ref name="pmid32166725" /> |
|||
* [[sinus node dysfunction]], including severe [[sinus bradycardia]] or [[sinoatrial block]], since amiodarone can cause significant [[bradycardia]] and sinus nodal arrest;<ref name="pmid32166725" /> |
|||
* second- or third-degree [[Atrioventricular block|atrioventricular (AV) block]], due to its negative [[chronotropic]] (affecting the heart rate) and [[dromotropic]] (affecting the conductivity) effects on the AV conduction system, unless a [[Artificial cardiac pacemaker|pacemaker]] is implanted;<ref name="pmid32166725" /> |
|||
* [[thyrotoxicosis]] that cannot be controlled by conventional means, such as [[Graves' disease]].<ref name="pmid32166725"/> |
|||
There are no specific guidelines for endurance or high-intensity exercise while taking amiodarone. However, since amiodarone may cause bradycardia and QTc prolongation which can affect exercise capacity and increase the risk of arrhythmias during intense exercise, it would generally be advisable for patients taking this medication to consult their healthcare provider before engaging in high-intensity physical activities such as strenuous endurance exercises.<ref name="pmid32166725"/> |
|||
So called 'acute onset atrial fibrillation', defined by the North American Society of Pacing and Electrophysiology (NASPE) in 2003, responds well to short duration treatment with amiodarone. This has been demonstrated in seventeen randomised controlled trials, of which five included a placebo arm. The incidence of severe side effects in this group is low. |
|||
== Side effects == |
|||
The benefit of amiodarone in the treatment of atrial fibrillation in the critical care population has yet to be determined but it may prove to be the agent of choice where the patient is haemodynamically unstable and unsuitable for DC cardioversion. It is recommended in such a role by the UK government's [[National Institute for Health and Clinical Excellence]] (NICE). |
|||
At oral doses of 400 mg per day or higher, amiodarone can have serious, varied [[side effect]]s, including [[toxicity]] to the [[thyroid]] gland,<ref name="pmid37731073"/> liver, lung, and [[retina]]l functions, requiring clinical surveillance and regular laboratory testing.<ref name="Drugs.com-2022">{{cite web |title=Amiodarone |url=https://www.drugs.com/amiodarone.html |publisher=Drugs.com |access-date=25 July 2022 |date=18 May 2022 |archive-date=25 July 2022 |archive-url=https://web.archive.org/web/20220725164122/https://www.drugs.com/amiodarone.html |url-status=live }}</ref><ref name="pmid32368381"/> [[Allergic reaction]]s to amiodarone may occur.<ref name="Drugs.com-2022"/> Most individuals administered amiodarone on a chronic basis will experience at least one side effect.<ref name="pmid32368381"/> In some people, daily use of amiodarone at 100 mg oral doses can be effective for arrhythmia control with no or minimal side effects.<ref name="pmid32368381"/> |
|||
Some common side effects include: |
|||
==Contraindications== |
|||
* nausea and vomiting;<ref name="pmid32166725" /> |
|||
Individuals who are [[pregnant]] or may become pregnant are strongly advised to not take amiodarone. Since amiodarone can be expressed in breast milk, women taking amiodarone are advised to stop nursing. |
|||
* taste disturbances (changes in taste perception, often described as a metallic or bitter taste in the mouth);<ref name="pmid32166725" /> |
|||
* [[Photodermatitis|photosensitivity]] of the skin, also known as [[photodermatitis]], where exposure to sunlight or ultraviolet radiation may lead to skin reactions such as rashes or sunburn-like symptoms;<ref name="pmid32166725" /> |
|||
* corneal microdeposits (deposits may accumulate on the [[cornea]] over time, resulting in blurred vision or visual halos{{--}}bright circles or rings around a light source, such as headlights; still, these corneal deposits typically do not affect vision significantly);<ref name="pmid32166725" /><ref name="pmid31447894">{{cite journal |vauthors=Colunga Biancatelli RM, Congedo V, Calvosa L, Ciacciarelli M, Polidoro A, Iuliano L |title=Adverse reactions of Amiodarone |journal=J Geriatr Cardiol |volume=16 |issue=7 |pages=552–566 |date=July 2019 |pmid=31447894 |pmc=6689516 |doi=10.11909/j.issn.1671-5411.2019.07.004 |doi-broken-date=2 November 2024 |url=}}</ref> |
|||
* thyroid dysfunction<ref name="pmid37547257">{{cite journal |vauthors=Gašparini D, Raljević D, Pehar-Pejčinović V, Klarica Gembić T, Peršić V, Turk Wensveen T |title=When amiodarone-induced thyroiditis meets cardiomyopathy with excessive trabeculation: a case report |journal=[[Front Cardiovasc Med]] |volume=10 |issue= |pages=1212965 |date=2023 |pmid=37547257 |pmc=10401478 |doi=10.3389/fcvm.2023.1212965 |doi-access=free }}</ref> (in approximately 15-20% of patients, amiodarone treatment results in thyroid dysfunction, either amiodarone-induced hypothyroidism or amiodarone-induced thyrotoxicosis; the drug can lead to both hypo- and hyperthyroidism);<ref name="pmid37731073"/> |
|||
* pulmonary toxicity<ref name="pmid37123694">{{cite journal |vauthors=Scaramozzino MU, Sapone G, Plastina UR, Nucara M |title=Amiodarone-Induced Lung Toxicity: A Case Initially Not Correctly Framed |journal=[[Cureus]] |volume=15 |issue=3 |pages=e36818 |date=March 2023 |pmid=37123694 |pmc=10146449 |doi=10.7759/cureus.36818 |doi-access=free |url=}}</ref><ref name="pmid37249923">{{cite journal |vauthors=Tsai IL, Huang LT, Yu YT, Lee CT, Huang TH |title=Variable radiographic and histologic presentations of amiodarone-related interstitial lung disease and the importance of avoiding re-exposure |journal=Respirol Case Rep |volume=11 |issue=6 |pages=e01165 |date=June 2023 |pmid=37249923 |pmc=10209837 |doi=10.1002/rcr2.1165 |url=}}</ref><ref name="pmid36553223">{{cite journal |vauthors=Budin CE, Cocuz IG, Sabău AH, Niculescu R, Ianosi IR, Ioan V, Cotoi OS |title=Pulmonary Fibrosis Related to Amiodarone-Is It a Standard Pathophysiological Pattern? A Case-Based Literature Review |journal=[[Diagnostics (journal)|Diagnostics]] |volume=12 |issue=12 |date=December 2022 |page=3217 |pmid=36553223 |pmc=9777900 |doi=10.3390/diagnostics12123217 |doi-access=free }}</ref><ref name="pmid36176494">{{cite journal |vauthors=Mitrofan CE, Cretu A, Mitrofan C, Bar C, Ghiciuc CM |title=Amiodarone induced lung disease |journal=Arch Clin Cases |volume=9 |issue=3 |pages=126–132 |date=2022 |pmid=36176494 |pmc=9512125 |doi=10.22551/2022.36.0903.10217 |url=}}</ref> (lung problems such as pulmonary fibrosis or interstitial lung disease may occur rarely but have the potential for serious consequences if left untreated);<ref name="pmid33262034"/><ref name="pmid32166725" /> |
|||
* liver abnormalities (liver damage, including elevated liver enzymes ([[Aspartate transaminase|AST]]/[[Alanine transaminase|ALT]]) and hepatotoxicity, although severe cases are rare);<ref name="pmid32166725" /> |
|||
* bradycardia and heart block (since it slows down heart rate by affecting the sinus node function and AV conduction system, it can increase the risk of heart block);<ref name="pmid32166725" /> |
|||
* QT Interval prolongation.<ref name="pmid32166725" /> |
|||
Amiodarone can potentially cause renal toxicity, but solid studies on whether amiodarone may be toxic to the kidneys are lacking.<ref name="pmid37743903">{{cite journal |vauthors=Duineveld MD, Kers J, Vleming LJ |title=Case report of progressive renal dysfunction as a consequence of amiodarone-induced phospholipidosis |journal=[[Eur Heart J Case Rep]] |volume=7 |issue=9 |pages=ytad457 |date=September 2023 |pmid=37743903 |pmc=10516635 |doi=10.1093/ehjcr/ytad457 |url=}}</ref> |
|||
It is contraindicated in individuals with [[sinus node|sinus nodal]] [[bradycardia]], [[atrioventricular node|atrioventricular]] block, and second or third degree heart block who do not have an [[artificial pacemaker]]. |
|||
===Lung=== |
|||
Individuals with baseline depressed lung function should be monitored closely if amiodarone therapy is to be initiated. |
|||
[[Image:IPF amiodarone.JPG|thumb|upright=1.3|A chest X-ray demonstrating [[pulmonary fibrosis]] due to amiodarone.]] |
|||
Side effects of oral amiodarone at doses of 400 mg or higher include various [[pulmonary]] effects.<ref name="Drugs.com-2021">{{cite web | url = https://www.drugs.com/sfx/amiodarone-side-effects.html#professional | title = Amiodarone Side Effects | publisher = Drugs.com | date = 25 April 2021 | access-date = 25 July 2022 | archive-date = 24 February 2016 | archive-url = https://web.archive.org/web/20160224012131/http://www.drugs.com/sfx/amiodarone-side-effects.html#professional | url-status = live }}</ref> The most serious reaction is [[interstitial lung disease]]. Risk factors include high cumulative dose, more than 400 milligrams per day, duration over two months, increased age, and preexisting pulmonary disease. Some individuals were noted to develop [[pulmonary fibrosis]] after a week of treatment, while others did not develop it after years of continuous use.<ref name="Drugs.com-2021"/> Common practice is to avoid the agent if possible in individuals with decreased lung function. |
|||
The most specific test of pulmonary toxicity due to amiodarone is a dramatically decreased [[Dlco|DL<sub>CO</sub>]] noted on [[pulmonary function test]]ing. |
|||
=== Thyroid === |
|||
Induced abnormalities in [[thyroid]] function are common.<ref name="pmid37547257"/><ref name="Drugs.com-2022"/> In approximately 15-20% of patients, amiodarone treatment results in thyroid dysfunction, either amiodarone-induced hypothyroidism or amiodarone-induced thyrotoxicosis.<ref name="pmid19584973">{{cite journal |vauthors=Tsang W, Houlden RL |title=Amiodarone-induced thyrotoxicosis: a review |journal=Can J Cardiol |volume=25 |issue=7 |pages=421–4 |date=July 2009 |pmid=19584973 |pmc=2723027 |doi=10.1016/s0828-282x(09)70512-4}}</ref><ref name="pmid29489285"/><ref name="pmid37731073">{{cite journal |vauthors=Cappellani D, Bartalena L, Bogazzi F |title=Short review: novel concepts in the approach to patients with amiodarone-induced thyrotoxicosis |journal=J Endocrinol Invest |volume= 47|issue= 2|pages= 275–283|date=September 2023 |pmid=37731073 |doi=10.1007/s40618-023-02168-3 |s2cid=262088052 |doi-access=free |pmc=10859339 }}</ref><ref name="pmid36818930"/> Both under- and overactivity of the thyroid may occur.<ref name="Drugs.com-2022"/> |
|||
Amiodarone is structurally similar to [[thyroxine]] and also contains [[iodine]]. Both of these factors contribute to the effects of amiodarone on thyroid function.<ref name="pmid36818930"/><ref name="pmid19584973"/><ref>{{cite journal | vauthors = Lombardi A, Inabnet WB, Owen R, Farenholtz KE, Tomer Y | title = Endoplasmic reticulum stress as a novel mechanism in amiodarone-induced destructive thyroiditis | journal = The Journal of Clinical Endocrinology and Metabolism | volume = 100 | issue = 1 | pages = E1-10 | date = January 2015 | pmid = 25295624 | pmc = 4283007 | doi = 10.1210/jc.2014-2745 }}</ref><ref>{{cite book| vauthors = Hall GM, Hunter JM, Cooper MS |title=Core Topics in Endocrinology in Anaesthesia and Critical Care|date=2010|publisher=Cambridge University Press|isbn=978-1-139-48612-5|page=170|url=https://books.google.com/books?id=3xd9O-W1HE8C&pg=PA170|language=en|url-status=live|archive-url=https://web.archive.org/web/20170908192558/https://books.google.com/books?id=3xd9O-W1HE8C&pg=PA170|archive-date=8 September 2017}}</ref> Amiodarone also causes an anti-thyroid action, via [[Plummer effect|Plummer]] and [[Wolff–Chaikoff effect]]s, due its large amount of iodine in its molecule, which causes a particular "cardiac hypothyroidism" with bradycardia and arrhythmia.<ref>{{cite journal| vauthors = Venturi S |title=Evolutionary Significance of Iodine|journal=Current Chemical Biology|volume=5 |pages=155–162|year=2011|issn=1872-3136|doi=10.2174/187231311796765012|issue=3|doi-broken-date=17 November 2024 }}</ref><ref>{{cite journal| vauthors = Venturi S |title=Iodine, PUFAs and Iodolipids in Health and Disease: An Evolutionary Perspective|journal=Human Evolution|volume= 29 |issue= 1–3|pages=185–205|year=2014|issn=0393-9375}}</ref> |
|||
Thyroid function should be checked at least every six months.<ref name="pmid29594056">{{cite journal | vauthors = Bartalena L, Bogazzi F, Chiovato L, Hubalewska-Dydejczyk A, Links TP, Vanderpump M | title = 2018 European Thyroid Association (ETA) Guidelines for the Management of Amiodarone-Associated Thyroid Dysfunction | journal = European Thyroid Journal | volume = 7 | issue = 2 | pages = 55–66 | date = March 2018 | pmid = 29594056 | pmc = 5869486 | doi = 10.1159/000486957 }}</ref> |
|||
* [[Hypothyroidism]] (slowing of the thyroid) occurs frequently; in the SAFE trial, which compared amiodarone with other medications for the treatment of atrial fibrillation, biochemical hypothyroidism (as defined by a TSH level of 4.5–10 mU/L) occurred in 25.8% of the amiodarone-treated group as opposed to 6.6% of the control group (taking placebo or [[sotalol]]). Overt hypothyroidism (defined as TSH >10 mU/L) occurred at 5.0% compared to 0.3%; most of these (>90%) were detected within the first six months of amiodarone treatment.<ref name="pmid17904459">{{cite journal | vauthors = Batcher EL, Tang XC, Singh BN, Singh SN, Reda DJ, Hershman JM | title = Thyroid function abnormalities during amiodarone therapy for persistent atrial fibrillation | journal = The American Journal of Medicine | volume = 120 | issue = 10 | pages = 880–885 | date = October 2007 | pmid = 17904459 | doi = 10.1016/j.amjmed.2007.04.022 | url = https://zenodo.org/record/1258726 | access-date = 27 August 2020 | archive-date = 9 August 2020 | archive-url = https://web.archive.org/web/20200809085816/https://zenodo.org/record/1258726 | url-status = live }}</ref> |
|||
* [[Amiodarone induced thyrotoxicosis]] (AIT), can be caused due to the high iodine content in the drug via the [[Jod-Basedow phenomenon|Jod-Basedow effect]]. This is known as Type 1 AIT, and usually occurs in patients with an underlying predisposition to hyperthyroidism such as [[Graves' disease]], within weeks to months after starting amiodarone. Type 1 AIT is usually treated with anti-thyroid drugs or [[thyroidectomy]]. Type 2 AIT is caused by a destructive [[thyroiditis]] due to a direct toxic effect of amiodarone on thyroid follicular epithelial cells.<ref name="pmid19584973"/><ref name=Ylli/> Type 2 AIT can occur even years after starting amiodarone, is usually self-limited and responds to [[anti-inflammatory]] treatment such as [[corticosteroid]]s.<ref name=Ylli>{{cite journal | vauthors = Ylli D, Wartofsky L, Burman KD | title = Evaluation and Treatment of Amiodarone-Induced Thyroid Disorders | journal = The Journal of Clinical Endocrinology and Metabolism | volume = 106 | issue = 1 | pages = 226–236 | date = January 2021 | pmid = 33159436 | doi = 10.1210/clinem/dgaa686 | s2cid = 226275566 | doi-access = }}</ref> In practice, often the type of AIT is undetermined or presumed as mixed with both treatments combined.<ref name=Ylli/> Thyroid uptake measurements (I-123 or I-131), which are used to differentiate causes of hyperthyroidism, are generally unreliable in patients who have been taking amiodarone. Because of the high iodine content of amiodarone, the thyroid gland is effectively saturated, thus preventing further uptake of isotopes of iodine. However, positive radioactive iodine can be used to rule in type 1AIT .{{Citation needed|date=September 2007}} |
|||
<gallery> |
|||
Amiodarone structure.svg|Amiodarone |
|||
Thyroxine.svg|[[Thyroxine]] |
|||
</gallery> |
|||
=== Eye === |
|||
[[Cornea]]l micro-deposits ([[cornea verticillata]],<ref name="pmid7116220">{{cite journal | vauthors = Chew E, Ghosh M, McCulloch C | title = Amiodarone-induced cornea verticillata | journal = Canadian Journal of Ophthalmology. Journal Canadien d'Ophtalmologie | volume = 17 | issue = 3 | pages = 96–99 | date = June 1982 | pmid = 7116220 }}</ref> also called vortex or whorl keratopathy) are almost universally present (over 90%) in individuals taking amiodarone longer than 6 months, especially doses greater than 400 mg/day. These deposits typically do not cause any symptoms. About 1 in 10 individuals may complain of a bluish halo. Anterior subcapsular lens deposits are relatively common (50%) in higher doses (greater than 600 mg/day) after 6 months of treatment. |
|||
[[Optic neuropathy]], nonarteritic anterior ischemic optic neuropathy (N-AION), occurs in 1–2% of people and is not dosage dependent.<ref>{{cite journal | vauthors = Passman RS, Bennett CL, Purpura JM, Kapur R, Johnson LN, Raisch DW, West DP, Edwards BJ, Belknap SM, Liebling DB, Fisher MJ, Samaras AT, Jones LG, Tulas KM, McKoy JM | title = Amiodarone-associated optic neuropathy: a critical review | journal = The American Journal of Medicine | volume = 125 | issue = 5 | pages = 447–453 | date = May 2012 | pmid = 22385784 | pmc = 3322295 | doi = 10.1016/j.amjmed.2011.09.020 }}</ref> Bilateral optic disc swelling and mild and reversible visual field defects can also occur. |
|||
[[Madarosis|Loss of eyelashes]] has been linked to amiodarone use.<ref>{{cite book| vauthors = Roy FH |title=Ocular differential diagnosis |date=2012 |publisher=Jaypee Highlights Medical Publishers|location=Panama City, Panama |isbn=978-93-5025-571-1 |page=94 |edition=9th |url=https://books.google.com/books?id=94WZpuXSTasC&pg=PA94|url-status=live |archive-url=https://web.archive.org/web/20170908192558/https://books.google.com/books?id=94WZpuXSTasC&pg=PA94|archive-date=8 September 2017}}</ref> |
|||
===Liver=== |
|||
Abnormal [[liver enzyme]] results are common in people taking amiodarone.<ref name="Drugs.com-2022"/> Much rarer are [[jaundice]], [[hepatomegaly]] (liver enlargement), and [[hepatitis]] (inflammation of the liver).<ref>{{cite journal | vauthors = Flaharty KK, Chase SL, Yaghsezian HM, Rubin R | title = Hepatotoxicity associated with amiodarone therapy | journal = Pharmacotherapy | volume = 9 | issue = 1 | pages = 39–44 | year = 1989 | pmid = 2646621 | doi = 10.1002/j.1875-9114.1989.tb04102.x | s2cid = 37972060 }}</ref> |
|||
In clinical observations, it has been noted that the administration of amiodarone, even at lower therapeutic doses, has been associated with the development of a condition mimicking alcoholic cirrhosis. This condition, often referred to as pseudo-alcoholic cirrhosis, presents with similar histopathological features to those observed in patients with alcoholic cirrhosis.<ref>{{cite journal | vauthors = Singhal A, Ghosh P, Khan SA | title = Low dose amiodarone causing pseudo-alcoholic cirrhosis | journal = Age and Ageing | volume = 32 | issue = 2 | pages = 224–225 | date = March 2003 | pmid = 12615569 | doi = 10.1093/ageing/32.2.224 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Puli SR, Fraley MA, Puli V, Kuperman AB, Alpert MA | title = Hepatic cirrhosis caused by low-dose oral amiodarone therapy | journal = The American Journal of the Medical Sciences | volume = 330 | issue = 5 | pages = 257–261 | date = November 2005 | pmid = 16284489 | doi = 10.1097/00000441-200511000-00012 }}</ref> However, this extreme adverse event manifestation—pseudo-alcoholic cirrhosis caused by low dose amiodarone—is very rare.<ref name="pmid32368381">{{cite journal |vauthors=Chokesuwattanaskul R, Shah N, Chokesuwattanaskul S, Liu Z, Thakur R |title=Low-dose Amiodarone Is Safe: A Systematic Review and Meta-analysis |journal=J Innov Card Rhythm Manag |volume=11 |issue=4 |pages=4054–4061 |date=April 2020 |pmid=32368381 |pmc=7192149 |doi=10.19102/icrm.2020.110403 |url=}}</ref> |
|||
===Skin=== |
|||
Long-term administration of amiodarone (usually more than eighteen months) is associated with a light-sensitive blue-grey discoloration of the skin, sometimes called [[ceruloderma]]; such patients should avoid exposure to the sun and use [[sunscreen]] that protects against [[ultraviolet]]-A and -B. The discoloration will slowly improve upon cessation of the medication, however, the skin color may not return completely.<ref>{{cite journal | vauthors = Murphy RP, Canavan M | title = Skin Discoloration from Amiodarone | journal = The New England Journal of Medicine | volume = 382 | issue = 3 | pages = e5 | date = January 2020 | pmid = 31940702 | doi = 10.1056/NEJMicm1906774 | s2cid = 210333420 }}</ref> |
|||
===Pregnancy and breastfeeding=== |
|||
Use during pregnancy may result in a number of problems in the infant including thyroid problems, heart problems, neurological problems, and preterm birth.<ref name=AmiPreg>{{cite web |title=Amiodarone Pregnancy and Breastfeeding Warnings |url=https://www.drugs.com/pregnancy/amiodarone.html |website=Drugs.com |access-date=8 December 2021 |language=en |archive-date=15 October 2020 |archive-url=https://web.archive.org/web/20201015165300/https://www.drugs.com/pregnancy/amiodarone.html |url-status=live }}</ref> Use during breastfeeding is generally not recommended though one dose may be okay.<ref name=AmiPreg/> |
|||
===Other=== |
|||
Long-term use of amiodarone has been associated with [[peripheral neuropathies]].<ref>{{cite journal | vauthors = Fraser AG, McQueen IN, Watt AH, Stephens MR | title = Peripheral neuropathy during long-term high-dose amiodarone therapy | journal = Journal of Neurology, Neurosurgery, and Psychiatry | volume = 48 | issue = 6 | pages = 576–578 | date = June 1985 | pmid = 2989436 | pmc = 1028375 | doi = 10.1136/jnnp.48.6.576 }}</ref> |
|||
Amiodarone is sometimes responsible for [[epididymitis]]. Amiodarone accumulates in the head of the organ and can cause unilateral or bilateral inflammation. It tends to resolve if amiodarone is stopped.<ref>{{cite journal | vauthors = Thomas A, Woodard C, Rovner ES, Wein AJ | title = Urologic complications of neurologic medications | journal = The Urologic Clinics of North America | volume = 30 | issue = 1 | pages = 123–131 | date = February 2003 | pmid = 12580564 | doi = 10.1016/S0094-0143(02)00111-8 }}</ref> |
|||
Some cases of [[gynecomastia]] have been reported in men on amiodarone.<ref name="pmid15242307">{{cite journal | vauthors = Bembo SA, Carlson HE | title = Gynecomastia: its features, and when and how to treat it | journal = Cleveland Clinic Journal of Medicine | volume = 71 | issue = 6 | pages = 511–7 | date = June 2004 | pmid = 15242307 | doi = 10.3949/ccjm.71.6.511 | doi-broken-date = 24 November 2024 | url = http://www.ccjm.org/content/71/6/511.full.pdf | archive-url = https://web.archive.org/web/20090709184316/http://www.ccjm.org/content/71/6/511.full.pdf| archive-date=9 July 2009 }}</ref> |
|||
A retrospective cohort study found an increased risk of digestive, liver, head and neck and liver cancers amongst male patients exposed to amiodarone versus female participants in the same study and the general population.<ref name="Su_2013">{{cite journal |vauthors=Su VY, Hu YW, Chou KT, Ou SM, Lee YC, Lin EY, Chen TJ, Tzeng CH, Liu CJ |date=May 2013 |title=Amiodarone and the risk of cancer: a nationwide population-based study |journal=Cancer |volume=119 |issue=9 |pages=1699–1705 |doi=10.1002/cncr.27881 |pmid=23568847 |s2cid=24144312 |doi-access=free}}</ref> This study also identified that the Standardized Incidence Ratio of cancer occurrence increased significantly in males aged 20-59 and >80 years old who were exposed to a higher dose of Amiodarone in comparison to those exposed to a lower dose. This suggests that there is a dose-effect relationship.<ref name="Su_2013" /> These results should be interpreted with caution due to limitations of the study design and care should be taken prior to altering current clinical and prescribing practices. Amiodarone and its effect on cancer is still a topic that requires more robust research. |
|||
== Drug-drug interactions == |
|||
The [[pharmacokinetics]] of numerous [[medication|drugs]], including many that are commonly administered to individuals with [[heart]] disease, are affected by amiodarone.<ref>{{cite journal | vauthors = Lesko LJ | title = Pharmacokinetic drug interactions with amiodarone | journal = Clinical Pharmacokinetics | volume = 17 | issue = 2 | pages = 130–140 | date = August 1989 | pmid = 2673606 | doi = 10.2165/00003088-198917020-00005 }}</ref><ref name="pmid6137140">{{cite journal | vauthors = Marcus FI | title = Drug interactions with amiodarone | journal = American Heart Journal | volume = 106 | issue = 4 Pt 2 | pages = 924–930 | date = October 1983 | pmid = 6137140 | doi = 10.1016/0002-8703(83)90017-0 }}</ref><ref name="Goodman">{{cite book|isbn=978-1264258079|date=1 November 2022 |title=Goodman & Gilman's the Pharmacological Basis of Therapeutics | vauthors = Brunton LL, Knollmann BC |publisher=McGraw Hill }}</ref> |
|||
Amiodarone has particularly important interactions with the following drugs: |
|||
The injection should not be given to neonates, because the benzyl alcohol it contains may cause the fatal "gasping syndrome". |
|||
* [[class I antiarrhythmic]]s (amiodarone should not be combined with other class I antiarrhythmic drugs, such as [[disopyramide]], [[flecainide]], [[procainamide]], [[quinidine]], etc., due to an increased risk of QTc prolongation and potential arrhythmias);<ref name="pmid32166725"/> |
|||
* [[beta blocker]]s and [[calcium channel blocker]]s (combining amiodarone with beta-blockers or calcium channel blockers, such as [[sotalol]], can further slow down heart rate and cause bradycardia or heart block);<ref name="pmid32166725"/> |
|||
* [[digoxin]] (amiodarone inhibits a protein called P-glycoprotein (P-gp), which transports digoxin out of cells in the gut, liver, and kidneys, therefore, concurrent use of these medications increases [[digoxin]] levels in the body, potentially leading to digoxin toxicity);<ref name="pmid32166725"/> |
|||
* [[statin]]s (amiodarone can inhibit enzymes in the liver responsible for metabolizing certain statins, such as [[simvastatin]], [[atorvastatin]], etc., therefore interaction elevates plasma concentrations of these [[statin]]s, increasing the risk of [[myopathy]], that is muscle damage, or [[rhabdomyolysis]], that is severe muscle breakdown);<ref name="pmid32166725"/> |
|||
* [[warfarin]] (since the [[Anticoagulant|anticoagulation]] effects of [[warfarin]] depend on metabolism of warfarin by both cytochromes [[CYP2C9]] and [[CYP3A4]], coadministation leads to rise in [[International Normalized Ratio|international normalized ratio]] (INR)—the amount of time taken for the blood to form a clot—placing patient at higher bleeding risks);<ref name="pmid32166725"/><ref name="pmid32929996">{{cite journal |vauthors=Tisdale JE, Chung MK, Campbell KB, Hammadah M, Joglar JA, Leclerc J, Rajagopalan B |title=Drug-Induced Arrhythmias: A Scientific Statement From the American Heart Association |journal=Circulation |volume=142 |issue=15 |pages=e214–e233 |date=October 2020 |pmid=32929996 |doi=10.1161/CIR.0000000000000905 |url=|doi-access=free }}</ref> Amiodarone potentiates the action of [[warfarin]] by inhibiting the clearance of both (S) and (R) warfarin. Individuals taking both of these medications should have their warfarin doses adjusted based on their dosing of amiodarone and have their anticoagulation status (measured as [[prothrombin time]] (PT) and [[international normalized ratio]] (INR)) measured more frequently.<ref name="pmid22315259">{{cite journal |vauthors=Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, Svensson PJ, Veenstra DL, Crowther M, Guyatt GH |title=Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines |journal=Chest |volume=141 |issue=2 Suppl |pages=e152S–e184S |date=February 2012 |pmid=22315259 |doi=10.1378/chest.11-2295|pmc=3278055 }}</ref><ref>{{cite web | url=https://www.uptodate.com/contents/warfarin-drug-information | title=UpToDate: Warfarin Drug Information | access-date=17 November 2024 | archive-date=10 September 2024 | archive-url=https://web.archive.org/web/20240910182616/https://www.uptodate.com/contents/warfarin-drug-information? | url-status=live }}</ref> Dose reduction of warfarin is as follows: 40% reduction if the amiodarone dose is {{Val|400|u=mg}} daily, 35% reduction if the amiodarone dose is {{Val|300|u=mg}} daily, 30% reduction if the amiodarone dose is {{Val|200|u=mg}} daily, and 25% reduction if amiodarone dose is {{Val|100|u=mg}} daily.<ref name="pmid22315259"/><ref name="fda-drug-safety"/> The effect of amiodarone on the warfarin concentrations can be as early as a few days after initiation of treatment; however, the interaction may not peak for up to seven weeks;<ref name="pmid22315259"/><ref name="fda-drug-safety">{{cite web | url=https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/amiodarone-hydrochloride-marketed-cordarone-and-pacerone-information | title=Amiodarone hydrochloride (Marketed as Cordarone and Pacerone) Information | work=FDA | date=18 June 2019 | access-date=17 November 2024 | archive-date=7 October 2024 | archive-url=https://web.archive.org/web/20241007003132/https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/amiodarone-hydrochloride-marketed-cordarone-and-pacerone-information | url-status=live }}</ref> |
|||
* [[anti-HIV medications]] (several [[HIV]] medications, such as [[ritonavir]], [[indinavir]], etc., interact with amiodarone by inhibiting [[CYP3A4]] enzyme hence leading to decreased clearance of amiodarone, i.e., increasing the concentration of amiodarone in the organism).<ref name="pmid32166725"/><ref name="FDA-2010">{{cite web|url=https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018972s042lbl.pdf|title=Cordarone (amiodarone HCl) tables|publisher=FDA|year=2010|access-date=22 March 2024|archive-date=3 August 2023|archive-url=https://web.archive.org/web/20230803071828/http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018972s042lbl.pdf|url-status=live}}</ref> |
|||
Amiodarone inhibits the action of the [[cytochrome P450]] [[isozyme]] family; such inhibition reduces the clearance of many drugs, including the following:<ref name="Goodman"/><ref>{{cite book|isbn=978-1585287000|date=27 August 2021|title=Clinical Pharmacokinetics|publisher=American Society of Health-System Pharmacists|author=John E. Murphy}}</ref><ref>{{cite book|isbn=978-1574393644|date=12 April 2014 |title=Drug Interaction Analysis and Management 2014 | vauthors = Hansten PD, Horn JR |publisher=Wolters Kluwer Health }}</ref> |
|||
Amiodarone can worsen the cardiac arrhythmia brought on by [[Digitalis|digitalis]] toxicity. |
|||
* [[ciclosporin]],<ref name="Goodman"/> |
|||
* [[digoxin]],<ref name="Goodman"/> |
|||
* [[flecainide]],<ref name="Goodman"/> |
|||
* [[procainamide]],<ref name="Goodman"/> |
|||
* [[quinidine]],<ref name="Goodman"/> |
|||
* [[sildenafil]],<ref name="Goodman"/> |
|||
* [[simvastatin]],<ref name="Goodman"/> |
|||
* [[theophylline]],<ref name="Goodman"/> |
|||
* [[warfarin]].<ref name="Goodman"/> |
|||
In 2015, [[Gilead Sciences]] warned healthcare providers about people who began taking the hepatitis C drugs [[ledipasvir/sofosbuvir]] or [[sofosbuvir]] along with amiodarone, who developed abnormally slow heartbeats or died of [[cardiac arrest]].<ref>{{cite news | vauthors = West S | url = https://www.bloomberg.com/news/articles/2015-03-21/gilead-warns-after-hepatitis-patient-on-heart-drug-dies | title = Gilead Warns After Hepatitis Patient on Heart Drug Dies | newspaper = Bloomberg | archive-url = https://web.archive.org/web/20170322104145/https://www.bloomberg.com/news/articles/2015-03-21/gilead-warns-after-hepatitis-patient-on-heart-drug-dies | archive-date=22 March 2017 | date = 21 March 2015 }}</ref> |
|||
Not to be given with [[Lidocaine|lidocaine]] → increases risk of asystole {{Citation needed|reason=interaction not in BNF, please provide reference|date=September 2010}} |
|||
==Metabolism== |
==Metabolism== |
||
Amiodarone is extensively metabolized in the liver by CYP3A4, a member of the cytochrome P450 superfamily of enzymes, therefore, amiodarone and can affect the metabolism of numerous other [[medication|drugs]] that depend on cytochrome P450, such as [[digoxin]], [[phenytoin]], [[warfarin]], etc.<ref name="pmid14677664"/><ref name="Amiodarone-Deranged-Physiology">{{cite web | url=https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20967/amiodarone | title=Amiodarone | Deranged Physiology | access-date=22 March 2024 | archive-date=5 December 2023 | archive-url=https://web.archive.org/web/20231205075036/https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20967/amiodarone | url-status=live }}</ref><ref>{{cite journal|doi=10.2165/00003088-198917020-00005 |title=Pharmacokinetic Drug Interactions with Amiodarone |date=1989 |journal=Clinical Pharmacokinetics |volume=17 |issue=2 |pages=130–140 |pmid=2673606 | vauthors = Lesko L }}</ref><ref name="pmid29489285">{{cite book|pmid=29489285|id={{NCBIBook|NBK482154}} |date=2024 |title=Amiodarone | vauthors = Florek JB, Lucas A, Girzadas D }}</ref> |
|||
Amiodarone is extensively metabolized in the liver by cytochrome P450 3A4, and can affect the metabolism of numerous other [[medication|drugs]]. It interacts with digoxin, warfarin, phenytoin and others. The major metabolite of amiodarone is desethylamiodarone (DEA), which also has antiarrhythmic properties. The metabolism of amiodarone is inhibited by [[grapefruit]] juice, leading to elevated [[blood plasma|serum]] levels of amiodarone. |
|||
The major metabolite of amiodarone is desethylamiodarone (DEA), which also has antiarrhythmic properties.<ref name="pmid14677664"/> |
|||
On August 8, 2008 FDA issued a warning of the risk of [[rhabdomyolysis]], which can lead to [[kidney failure]] or death, when [[simvastatin]] is used with amiodarone. This interaction is dose-dependent with simvastatin doses exceeding 20 mg. This drug combination especially with higher doses of simvastatin should be avoided.<ref name="urlInformation on Simvastatin/Amiodarone">{{cite web |url=http://www.fda.gov/cder/drug/infopage/simvastatin_amiodarone/default.htm |title=Information on Simvastatin/Amiodarone |work= |accessdate=2008-09-21| archiveurl= http://web.archive.org/web/20080921230357/http://www.fda.gov/Cder/drug/infopage/simvastatin_amiodarone/default.htm| archivedate= 21 September 2008 <!--DASHBot-->| deadurl= no}} {{Dead link|date=September 2010|bot=H3llBot}}</ref> |
|||
The metabolism of amiodarone is inhibited by [[Grapefruit–drug interactions|grapefruit]], leading to elevated [[blood plasma|serum]] levels of amiodarone.<ref name="pmid20142570">{{cite journal | author=Bressler R | title=Grapefruit juice and drug interactions. Exploring mechanisms of this interaction and potential toxicity for certain drugs | journal=Geriatrics | volume=61 | issue=11 | pages=12–18 | year=2006 | pmid=17112309 }}</ref> |
|||
===Interactions with other drugs=== |
|||
The [[pharmacokinetics]] of numerous [[medication|drugs]], including many that are commonly administered to individuals with [[heart]] disease, are affected by amiodarone. Particularly, doses of [[digoxin]] should be halved in individuals taking amiodarone. |
|||
On 8 August 2008, the [[Food and Drug Administration|US Food and Drug Administration]] (FDA) issued a warning of the risk of [[rhabdomyolysis]], which can lead to [[kidney failure]] or death, when [[simvastatin]] is used with amiodarone. This interaction is dose-dependent with simvastatin doses exceeding 20 mg. This drug combination, especially with higher doses of simvastatin, should be avoided.<ref name="FDA-Simvastin-2008">{{cite web |url=https://www.fda.gov/cder/drug/infopage/simvastatin_amiodarone/default.htm |title=Information on Simvastatin/Amiodarone |website=[[Food and Drug Administration]] |access-date=21 September 2008| archive-url= https://web.archive.org/web/20080921230357/https://www.fda.gov/Cder/drug/infopage/simvastatin_amiodarone/default.htm| archive-date= 21 September 2008 | url-status= live}}</ref> |
|||
Amiodarone potentiates the action of [[warfarin]]. Individuals taking both of these medications should have their warfarin dose halved and their anticoagulation status (measured as [[prothrombin time]] (PT) and [[international normalized ratio]] (INR)) measured more frequently. The effect of amiodarone in the warfarin concentration can be as early as a few days after initiation of treatment, or can be delayed a few weeks. |
|||
Amiodarone is extensively metabolized in the liver. The primary metabolic pathway of amiodarone is by cytochrome P450 (CYP) enzymes, particularly CYP3A4 and CYP2C8.<ref name="FDA-2010"/><ref name="pmid29489285"/><ref name="Goodman"/><ref name="pmid24516494">{{cite journal | vauthors = Palleria C, Di Paolo A, Giofrè C, Caglioti C, Leuzzi G, Siniscalchi A, De Sarro G, Gallelli L | title = Pharmacokinetic drug-drug interaction and their implication in clinical management | journal = Journal of Research in Medical Sciences | volume = 18 | issue = 7 | pages = 601–610 | date = July 2013 | pmid = 24516494 | pmc = 3897029 }}</ref><ref name="pmid20047147">{{cite journal | vauthors = VandenBrink BM, Isoherranen N | title = The role of metabolites in predicting drug-drug interactions: focus on irreversible cytochrome P450 inhibition | journal = Current Opinion in Drug Discovery & Development | volume = 13 | issue = 1 | pages = 66–77 | date = January 2010 | pmid = 20047147 | pmc = 2898504 }}</ref> The metabolism of amiodaron can be characterized by two phases:<ref name="Latini-1984">{{cite journal | vauthors = Latini R, Tognoni G, Kates RE | title = Clinical pharmacokinetics of amiodarone | journal = Clinical Pharmacokinetics | volume = 9 | issue = 2 | pages = 136–156 | date = April 1984 | pmid = 6370540 | doi = 10.2165/00003088-198409020-00002 }}</ref> |
|||
Amiodarone inhibits the action of the [[cytochrome P450]] [[isozyme]] family. This reduces the clearance of many drugs, including the following: - |
|||
* [[Cyclosporine]] |
|||
* phase I metabolism, when amiodarone undergoes oxidative processes mainly mediated by CYP3A4 and to a lesser extent by CYP2C8; these reactions result in the formation of several active metabolites, including desethylamiodarone (DEA) and di-desethylamiodarone (DDEA); DEA is the most abundant metabolite and exhibits similar pharmacological effects as amiodarone;<ref name="Latini-1984"/><ref name="pmid28643175">{{cite journal |vauthors=Haverkamp W, Israel C, Parwani A |title=[Clinical aspects of treatment with amiodarone] |language=German |journal=Herzschrittmacherther Elektrophysiol |volume=28 |issue=3 |pages=307–316 |date=September 2017 |pmid=28643175 |doi=10.1007/s00399-017-0516-0 |url=}}</ref> |
|||
* [[Digoxin]] |
|||
* phase II metabolism, when both amiodarone and its major metabolite DEA can undergo conjugation reactions with glucuronic acid; this process increases water solubility of these compounds for their efficient elimination from the body.<ref name="pmid33415501">{{cite journal |vauthors=Di L, Balesano A, Jordan S, Shi SM |title=The Role of Alcohol Dehydrogenase in Drug Metabolism: Beyond Ethanol Oxidation |journal=AAPS J |volume=23 |issue=1 |pages=20 |date=January 2021 |pmid=33415501 |doi=10.1208/s12248-020-00536-y}}</ref> |
|||
* [[Flecainide]] |
|||
* [[Procainamide]] |
|||
Amiodarone has an exceptionally long half-life due to a combination of several factors:<ref name="pmid32166725"/> |
|||
* [[Quinidine]] |
|||
* high lipid solubility, given that amiodarone has high lipid solubility, which allows it to distribute throughout various tissues in the body rapidly; the extensive tissue distribution of amiodarone contributes to a large volume of distribution that leads to slow clearance from plasma compartments; |
|||
* [[Sildenafil]] |
|||
* extensive tissue binding, so that amiodarone extensively binds to different tissues, including fat deposits, muscles, heart tissue, and other organs; this binding creates reservoirs where drug release can occur slowly over time, resulting in an extended duration of action even after stopping the therapy; |
|||
* [[Simvastatin]] |
|||
* enterohepatic recycling, meaning that amiodarone undergoes enterohepatic recycling, where it is reabsorbed from the intestines after being excreted into bile, which contributes to its prolonged presence.<ref name="pmid33658939">{{cite journal |vauthors=Shleghm MR, Mircioiu C, Voicu VA, Mircioiu I, Anuta V |title=Estimation of the In Vivo Release of Amiodarone From the Pharmacokinetics of Its Active Metabolite and Correlation With Its In Vitro Release |journal=Front Pharmacol |volume=11 |pages=621667 |date=2020 |pmid=33658939 |pmc=7917713 |doi=10.3389/fphar.2020.621667 |doi-access=free}}</ref> |
|||
* [[Theophylline]] |
|||
* [[Warfarin]] |
|||
=== Excretion === |
=== Excretion === |
||
Excretion is primarily |
Excretion is primarily via the liver and the bile duct with almost no elimination via the kidney and it is not dialyzable.<ref name="DailyMed-Pacerone-2022" /> Elimination half-life average of 58 days (ranging from 25 to 100 days [Remington: The Science and Practice of Pharmacy 21st edition]) for amiodarone and 36 days for the active metabolite, desethylamiodarone (DEA).<ref name="DailyMed-Pacerone-2022" /> There is 10-50% transfer of amiodarone and DEA in the placenta as well as a presence in breast milk.<ref name="DailyMed-Pacerone-2022" /> Accumulation of amiodarone and DEA occurs in adipose tissue and highly perfused organs (i.e. liver, lungs),<ref name="DailyMed-Pacerone-2022" /> therefore, if an individual was taking amiodarone on a chronic basis if it is stopped it will remain in the system for weeks to months.<ref name="DailyMed-Pacerone-2022" /> |
||
Whereas amiodarone is primarily eliminated from the body through hepatic metabolism and biliary excretion, a very small portion of amiodarone and its metabolites are excreted unchanged in urine or feces.<ref name="FDA-2010"/><ref name="pmid29489285"/> |
|||
== Side effects == |
|||
Amiodarone has numerous side effects. Most individuals administered amiodarone on a chronic basis will experience at least one side effect. |
|||
The liver plays a significant role in the elimination of amiodarone. After being extensively metabolized by cytochrome P450 enzymes, particularly [[CYP3A4]] and [[CYP2C8]], amiodarone is transported into bile via multidrug-resistant protein 2 (MRP2) transporter. Bile containing amiodarone and its metabolites is then released into the gastrointestinal tract.{{medical citation needed|date=March 2024}} |
|||
===Lung=== |
|||
[[Image:IPF amiodarone.JPG|thumb|A chest X-ray demonstrating [[pulmonary fibrosis]] due to amiodarone.]] |
|||
The most serious reaction that is due to amiodarone is [[interstitial lung disease]]. Risk factors include high cumulative dose, more than 400 milligrams per day, duration over two months, increased age, and preexisting pulmonary disease. Some individuals were noted to develop [[pulmonary fibrosis]] after a week of treatment, while others did not develop it after years of continuous use. Common practice is to avoid the agent if possible in individuals with decreased lung function. |
|||
Some of these compounds can be reabsorbed back into systemic circulation through enterohepatic recirculation, where they may undergo additional rounds of metabolism before eventually being excreted again into bile.{{medical citation needed|date=March 2024}} |
|||
The most specific test of pulmonary toxicity due to amiodarone is a dramatically decreased [[Dlco|DL<sub>CO</sub>]] noted on [[pulmonary function test]]ing. |
|||
Because renal excretion contributes only minimally to the elimination of amiodarone, dose adjustment based on kidney function is generally not necessary. This is because most patients with normal renal function can adequately clear the drug through hepatic metabolism and biliary elimination pathways.<ref name="pmid32166725"/> |
|||
=== Thyroid === |
|||
[[Image:Thyroxine.svg|thumb|[[Thyroxine]] and amiodarone have similar structures.]] |
|||
Due to the iodine content of the agent (37.3% by weight), abnormalities in [[thyroid]] function are common. Amiodarone is structurally similar to [[thyroxine]] (a thyroid hormone), which contributes to the effects of amiodarone on thyroid function.{{Citation needed|date=October 2011}} Both under- and overactivity of the thyroid may occur on amiodarone treatment. Measurement of free [[thyroxine]] (FT4) alone may be unreliable in detecting these problems and [[thyroid-stimulating hormone]] (TSH) should therefore also be checked every 6 months.<ref>[[British National Formulary]] guidance on thyroid function monitoring ([http://www.bnf.org/bnf/bnf/49/openat/2417.htm?q=%22amiodarone%22 BNF Amiodarone])</ref> |
|||
==Pharmacology== |
|||
* [[Hypothyroidism]] (slowing of the thyroid, due to the [[Wolff-Chaikoff effect]]) occurs frequently; in the SAFE trial, which compared amiodarone with other medications for the treatment of atrial fibrillation, biochemical hypothyroidism (as defined by a TSH level of 4.5-10 mU/l) occurred in 25.8% of the amiodarone-treated group as opposed to 6.6% of the control group (taking placebo or [[sotalol]]). Overt hypothyroidism (defined as TSH >10 mU/l) occurred at 5.0% compared to 0.3%; most of these (>90%) were detected within the first six months of amiodarone treatment.<ref name=Batcher>{{cite journal |author=Batcher EL, Tang XC, Singh BN, Singh SN, Reda DJ, Hershman JM |year=2007 |month=October |title=Thyroid function abnormalities during amiodarone therapy for persistent atrial fibrillation |journal=Am J Med |volume=120 |issue=10 |pages=880–85 |url=http://www.amjmed.com/article/PIIS0002934307004718/abstract | doi =10.1016/j.amjmed.2007.04.022 |pmid=17904459}}</ref> |
|||
Amiodarone is categorized as a class III [[antiarrhythmic agent]], and prolongs phase 3 of the [[cardiac action potential]], the repolarization phase where there is normally decreased calcium permeability and increased potassium permeability. It has numerous other effects, however, including actions that are similar to those of antiarrhythmic classes Ia, II, and IV.{{medical citation needed|date=March 2024}} |
|||
Amiodarone is a blocker of [[voltage gated potassium channel|voltage gated potassium]] ([[KCNH2]]) and [[voltage gated calcium channel]]s ([[CACNA2D2]]).<ref>{{cite web|url=https://www.drugbank.ca/drugs/DB01118|publisher=Drugbank|title=Amiodarone|access-date=28 May 2019|archive-date=23 May 2019|archive-url=https://web.archive.org/web/20190523212530/https://www.drugbank.ca/drugs/DB01118|url-status=live}}</ref> |
|||
* [[Hyperthyroidism]] (an overactive thyroid, due to the [[Jod-Basedow phenomenon|Jod-Basedow Effect]]) can also occur. However, in the SAFE trial, the increased rate of hyperthyroidism (5.3% compared to 2.4%) was not statistically significant. Most hyperthyroid patients (defined as TSH <0.35 mU/l) were asymptomatic.<ref name=Batcher/> |
|||
Amiodarone slows the conduction rate and prolongs the refractory period of the SA and AV nodes.<ref name = harris>{{cite book| veditors = Harris L, Williams RR |title=Amiodarone: pharmacology, pharmacokinetics, toxicology, clinical effects|date=1986|publisher=Médecine et sciences internationales|location=Paris|isbn=978-2-86439-125-8|page=12}}</ref> It also prolongs the refractory periods of the ventricles, bundles of His, and the Purkinje fibers without exhibiting any effects on the conduction rate.<ref name = harris/> Amiodarone has been shown to prolong the myocardial cell action potential duration and refractory period and is a non-competitive β-adrenergic inhibitor.<ref>{{cite web|title=FDA Drug Label|url=https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=11249|url-status=live|archive-url=https://web.archive.org/web/20170327171522/https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=11249|archive-date=27 March 2017}}</ref> |
|||
Thyroid uptake measurements (I-123 or I-131), which are used to differentiate causes of hyperthyroidism, are generally unreliable in patients who have been taking amiodarone. Because of the high iodine content of amiodarone, the thyroid gland is effectively saturated, thus preventing further uptake of isotopes of iodine. However, the radioactive iodine uptake (nuclear thyroid uptake test) may still be helpful in the diagnosis and management of amiodarone-induced hyperthyroidism.{{Citation needed|date=September 2007}} |
|||
It also shows [[beta blocker]]-like and [[calcium channel blocker]]-like actions on the [[SA node|SA]] and [[AV node]]s, increases the refractory period via sodium- and potassium-channel effects, and slows intra-cardiac conduction of the [[cardiac action potential]], via sodium-channel effects. It is suggested that amiodarone may also exacerbate the phenotype associated with Long QT-3 syndrome causing mutations such as ∆KPQ. This effect is due to a combination of blocking the peak sodium current, but also contributing to an increased persistent sodium current.<ref name=pmid26973526 >{{cite journal | vauthors = Ghovanloo MR, Abdelsayed M, Ruben PC | title = Effects of Amiodarone and N-desethylamiodarone on Cardiac Voltage-Gated Sodium Channels | journal = Frontiers in Pharmacology | volume = 7 | pages = 39 | year = 2016 | pmid = 26973526 | pmc = 4771766 | doi = 10.3389/fphar.2016.00039 | doi-access = free }}</ref> |
|||
=== Eye === |
|||
[[Cornea]]l micro-deposits (Corneal verticillata, also called vortex or whorl keratopathy) are almost universally present (over 90%) in individuals taking amiodarone longer than 6 months, especially doses greater than 400 mg/day. These deposits typically do not cause any symptoms. About 1 in 10 individuals may complain of a bluish halo. Anterior subcapsular lens deposits are relatively common (50%) in higher doses (greater than 600 mg/day) after 6 months of treatment. |
|||
Optic neuropathy, nonarteritic anterior ischemic optic neuropathy (N-AION), occurs in 1-2% of people and is not dosage dependent.<ref>Passman RS, Bennett CL, Purpura JM, Kapur R, et al. (2012)"Amiodarone-associated Optic Neuropathy: A Critical Review" Am J of Med, '''125''':5, (447-53)</ref> Bilateral optic disc swelling and mild and reversible visual field defects can also occur. |
|||
Amiodarone chemically resembles [[thyroxine]] (thyroid hormone), and its binding to the nuclear thyroid receptor might contribute to some of its pharmacologic and toxic actions.<ref>{{cite book | veditors = Brunton LL, Lazo JS, Parker K |title=Goodman & Gilman's The Pharmacological Basis of Therapeutics |edition=11th |publisher=McGraw-Hill |location=New York |year=2005 | isbn=0-07-142280-3|title-link=Goodman & Gilman's The Pharmacological Basis of Therapeutics }}{{page needed|date=July 2022}}</ref> |
|||
===Gastrointestinal system and liver=== |
|||
The mechanisms of action of amiodarone include blocking potassium ion channels (prolonging repolarization), blocking sodium ion channels, and antagonizing alpha- and beta-adrenergic receptors.<ref name="pmid32166725"/> |
|||
Abnormal [[liver enzyme]] results are common in patients on amiodarone. Much rarer are [[jaundice]], [[hepatomegaly]] (liver enlargement), and [[hepatitis]] (inflammation of the liver).<ref>{{cite journal |author=Flaharty KK, Chase SL, Yaghsezian HM, Rubin R |title=Hepatotoxicity associated with amiodarone therapy |journal=Pharmacotherapy |volume=9 |issue=1 |pages=39–44 |year=1989 |pmid=2646621 |doi=}}</ref> |
|||
The action of amiodarone can be characterized by the following effects:<ref name="pmid32166725"/> |
|||
* potassium channel blockade, since amiodarone blocks potassium channels involved in cardiac repolarization during phase 3 of the action potential, so that this blockade prolongs the duration of cardiac action potentials, resulting in an increased refractory period and decreased excitability;<ref name="pmid32166725"/> |
|||
* sodium channel blockade, characterized by inhibiting sodium ion influx through voltage-gated sodium channels, so that amiodarone reduces the conduction velocity of electrical impulses in cardiac tissue that leads to a slowed heart rate and improved rhythm control;<ref name="pmid32166725"/> |
|||
* calcium channel blockade, by inhibiting L-type calcium channels in myocardial cells, decreasing intracellular calcium concentration during ventricular contraction;<ref name="pmid32166725"/> |
|||
* noncompetitive adrenergic receptor antagonism, meaning that amiodarone has both alpha- and beta-adrenergic receptor antagonistic effects, which help reduce sympathetic stimulation on the heart.<ref name="pmid32166725"/> |
|||
==History== |
|||
Low-dose amiodarone has been reported to cause pseudo-alcoholic cirrhosis.<ref>{{cite journal |author=Singhal A, Ghosh P, Khan SA |title=Low dose amiodarone causing pseudo-alcoholic cirrhosis |journal=Age and ageing |volume=32 |issue=2 |pages=224–5 |year=2003 |pmid=12615569|doi=10.1093/ageing/32.2.224}}</ref><ref>{{cite journal |author=Puli SR, Fraley MA, Puli V, Kuperman AB, Alpert MA |title=Hepatic cirrhosis caused by low-dose oral amiodarone therapy |journal=Am. J. Med. Sci. |volume=330 |issue=5 |pages=257–61 |year=2005 |pmid=16284489|doi=10.1097/00000441-200511000-00012}}</ref> |
|||
The original observation that amiodarone's progenitor molecule, [[khellin]], had cardioactive properties, was made by the Russian physiologist [[Anrep effect|Gleb von Anrep]] while working in Cairo in 1946.<ref>{{cite journal | vauthors = Anrep GV, Barsoum GS, Kenawy MR, Misrahy G | title = Ammi Visnaga in the Treatment of the Anginal Syndrome | journal = British Heart Journal | volume = 8 | issue = 4 | pages = 171–177 | date = October 1946 | pmid = 18610042 | pmc = 503580 | doi = 10.1136/hrt.8.4.171 }}</ref> Khellin is obtained from a plant extract of [[Khella]] or ''[[Ammi visnaga]]'', a common plant in north Africa. Anrep noticed that one of his technicians had been cured of anginal symptoms after taking khellin, then used for various, non-cardiac ailments. This led to efforts by European pharmaceutical industries to isolate an active compound.{{citation needed|date=February 2012}} Amiodarone was initially developed in 1961 at the Labaz company, [[Belgium]], by chemists Tondeur and Binon, who were working on preparations derived from khellin. It became popular in Europe as a treatment for [[angina pectoris]].<ref>{{cite journal | vauthors = Deltour G, Binon F, Tondeur R, Goldenberg C, Henaux F, Sion R, Deray E, Charlier R | title = [Studies in the benzofuran series. VI. Coronary-dilating activity of alkylated and aminoalkylated derivatives of 3-benzoylbenzofuran] | language = fr | journal = Archives Internationales de Pharmacodynamie et de Therapie | volume = 139 | pages = 247–254 | date = September 1962 | pmid = 14026835 }}</ref><ref>{{cite journal | vauthors = Charlier R, Deltour G, Tondeur R, Binon F | title = [Studies in the benzofuran series. VII. Preliminary pharmacological study of 2-butyl-3-(3,5-diiodo-4-beta-N-diethylaminoethoxybenzoyl)-benzofuran] | language = fr | journal = Archives Internationales de Pharmacodynamie et de Therapie | volume = 139 | pages = 255–264 | date = September 1962 | pmid = 14020244 }}</ref><ref name="Books-LLC-Antiarrhythmic-Agents">{{cite book|isbn=978-1-156-39374-1|title=Antiarrhythmic Agents|publisher=Books LLC}}</ref> |
|||
As a doctoral candidate at Oxford University, Bramah Singh determined that amiodarone and [[sotalol]] had antiarrhythmic properties and belonged to a new class of antiarrhythmic agents (what would become the class III antiarrhythmic agents).<ref>{{cite journal | vauthors = Singh BN, Vaughan Williams EM | title = The effect of amiodarone, a new anti-anginal drug, on cardiac muscle | journal = British Journal of Pharmacology | volume = 39 | issue = 4 | pages = 657–667 | date = August 1970 | pmid = 5485142 | pmc = 1702721 | doi = 10.1111/j.1476-5381.1970.tb09891.x }}</ref> Today the mechanisms of action of amiodarone and sotalol have been investigated in more detail. Both drugs have been demonstrated to prolong the duration of the [[action potential]], prolonging the refractory period, by interacting among other cellular functions with [[K+ channels]].<ref name="Books-LLC-Antiarrhythmic-Agents"/> |
|||
===Skin=== |
|||
Long-term administration of amiodarone is associated with a blue-grey discoloration of the skin. This is more commonly seen in individuals with lighter skin tones. The discoloration may revert upon cessation of the drug. However, the skin color may not return completely to normal. |
|||
Based on Singh's work, the [[Argentina|Argentinian]] physician Mauricio Rosenbaum began using amiodarone to treat his patients who have supraventricular and ventricular arrhythmias, with impressive results. Based on papers written by Rosenbaum developing Singh's theories, physicians in the [[United States]] began prescribing amiodarone to their patients with potentially life-threatening arrhythmias in the late 1970s.<ref>{{cite journal | vauthors = Rosenbaum MB, Chiale PA, Halpern MS, Nau GJ, Przybylski J, Levi RJ, Lázzari JO, Elizari MV | title = Clinical efficacy of amiodarone as an antiarrhythmic agent | journal = The American Journal of Cardiology | volume = 38 | issue = 7 | pages = 934–944 | date = December 1976 | pmid = 793369 | doi = 10.1016/0002-9149(76)90807-9 }}</ref><ref>{{cite journal | vauthors = Rosenbaum MB, Chiale PA, Haedo A, Lázzari JO, Elizari MV | title = Ten years of experience with amiodarone | journal = American Heart Journal | volume = 106 | issue = 4 Pt 2 | pages = 957–964 | date = October 1983 | pmid = 6613843 | doi = 10.1016/0002-8703(83)90022-4 }}</ref> |
|||
Individuals taking amiodarone may become more sensitive to the harmful effects of [[ultraviolet|UV-A]] light. Using sunblock that also blocks UV-A rays appears to prevent this side effect. |
|||
The US [[Food and Drug Administration]] (FDA) was reluctant to officially approve the use of amiodarone since initial reports had shown an increased incidence of serious pulmonary side effects of the drug. In the mid-1980s, the European pharmaceutical companies began putting pressure on the FDA to approve amiodarone by threatening to cut the supply to American physicians if it was not approved. In December 1985, amiodarone was approved by the FDA for the treatment of arrhythmias.<ref name="DailyMed-2018" /><ref>{{cite web|title=Drug Approval Package: Cordarone (Amiodarone Hydrochloride) Tablets. NDA #018972|url=http://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/18-972_Cardarone.cfm|publisher=U.S. Food and Drug Administration|access-date=6 February 2014|url-status=live|archive-url=https://web.archive.org/web/20140221092003/http://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/18-972_Cardarone.cfm|archive-date=21 February 2014}}</ref> |
|||
===Neurological=== |
|||
Long-term administration of amiodarone has been associated with peripheral neuropathies.<ref>{{cite journal |author=A G Fraser, I N McQueen, A H Watt, and M R Stephens |title=Peripheral neuropathy during longterm high-dose amiodarone therapy |journal=J Neurol Neurosurg Psychiatry |volume=48 |issue=6 |pages=576–578 |year=1985 |month=June |pmc=1028375 |url=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1028375/ |pmid=2989436 |doi=10.1136/jnnp.48.6.576 }}</ref> |
|||
=== |
=== Name === |
||
Amiodarone may be an acronym{{Citation needed|date=May 2021}} for its IUPAC name (2-butyl-1-benzofuran-3-yl)-[4-[2-(diethyl'''<u>am</u>'''ino)ethoxy]-3,5-di<u>'''iod'''</u>o'''<u>phen</u>'''yl]methan'''<u>one</u>''',<ref>{{cite web|url=https://pubchem.ncbi.nlm.nih.gov/compound/amiodarone#section=3D-Conformer|title=Compound summary for CID 2157|publisher=pubchem.ncbi.nil.nih.gov|url-status=live|archive-url=https://web.archive.org/web/20160324044226/https://pubchem.ncbi.nlm.nih.gov/compound/amiodarone#section=3D-Conformer|archive-date=24 March 2016}}</ref> where '''<u>[[Aryl|ar]]</u>''' is a placeholder for phenyl. This is partially supported by [[dronedarone]] which is noniodinated benzofuran derivative of amiodarone, where the <u>'''ar'''</u>ylmethan'''<u>one</u>''' is conserved.{{Citation needed|date=May 2021}} |
|||
==Dosing== |
|||
Amiodarone is sometimes responsible for [[epididymitis]], a condition of the scrotum normally associated with bacterial infections but which can also occur as a non-bacterial inflammatory condition. Amiodarone accumulates in the head of the organ and can cause unilateral or bilateral inflammation. It tends to resolve if amiodarone is stopped.<ref>{{cite journal |author=Thomas A, Woodard C, Rovner ES, Wein AJ |title=Urologic complications of nonurologic medications |journal=Urol. Clin. North Am. |volume=30 |issue=1 |pages=123–31 |year=2003 |month=February |pmid=12580564 |doi=10.1016/S0094-0143(02)00111-8}}</ref> |
|||
Amiodarone is available in oral and intravenous formulations. |
|||
Orally, it is available under the brand names Pacerone (produced by [[Upsher-Smith Laboratories|Upsher-Smith Laboratories, Inc.]]) and Cordarone (produced by Wyeth-Ayerst Laboratories).<ref name="DailyMed-Pacerone-2022" /><ref name="DailyMed-2018" /> It is also available under the brand name Aratac (produced by Alphapharm Pty Ltd) in Australia and New Zealand, and further in Australia under the brands Cardinorm and Rithmik as well as a number of generic brands. Also Arycor in South Africa (Produced by Winthrop Pharmaceuticals.). In South America, it is known as Atlansil and is produced by Roemmers. |
|||
===Gynecomastia=== |
|||
Some cases of [[gynecomastia]] have been reported with men on amiodarone.<ref name="Gynecomastia: Its features, and when and how to treat it">[http://www.ccjm.org/content/71/6/511.full.pdf] Gynecomastia: Its features, and when and how to treat it</ref> |
|||
In India, amiodarone is marketed (produced by Cipla Pharmaceutical) under the brand name Tachyra. It is also available in intravenous ampules and vials. |
|||
== See also == |
|||
* [[Advanced cardiac life support]] (ACLS) |
|||
The dose of amiodarone administered is tailored to the individual and the dysrhythmia that is being treated. When administered orally, the [[bioavailability]] of amiodarone is quite variable. Absorption ranges from 22 to 95%, with better absorption when it is given with food.<ref name="pmid14677664"/> |
|||
* [[Antiarrhythmic agents]] |
|||
* [[Atrial fibrillation]] |
|||
* [[Cardiac action potential]] |
|||
* [[Dronedarone]] (similar drug under investigation) |
|||
* [[Ventricular tachycardia]] |
|||
== References == |
== References == |
||
{{reflist |
{{reflist}} |
||
== External links == |
|||
* {{cite journal |author=Siddoway LA |title=Amiodarone: guidelines for use and monitoring |journal=Am Fam Physician |volume=68 |issue=11 |pages=2189–96 |year=2003 |month=December |pmid=14677664 |doi= |url=http://www.aafp.org/afp/20031201/2189.html}} |
|||
* [http://www.medicinenet.com/amiodarone/article.htm Amiodarone (MedicineNet.com)] |
|||
* [http://www.fpnotebook.com/CV/Pharm/Amdrn.htm Amiodarone (FamilyPracticeNotebook.com)] |
|||
* [http://www.anaesthetist.com/icu/manage/drugs/heart/amiodarone.htm Amiodarone (The WorldWide Intensivist)] |
|||
* [http://druginfo.nlm.nih.gov/drugportal/dpdirect.jsp?name=Amiodarone U.S. National Library of Medicine: Drug Information Portal - Amiodarone] |
|||
* [http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm118073.htm Amiodarone (FDA MedWatch)] |
|||
{{Potassium channel blockers}} |
|||
{{Antiarrhythmic agents}} |
{{Antiarrhythmic agents}} |
||
{{Emergency medicine}} |
{{Emergency medicine}} |
||
{{Ion channel modulators}} |
|||
{{Monoamine reuptake inhibitors}} |
|||
{{Xenobiotic-sensing receptor modulators}} |
|||
{{Portal bar | Medicine}} |
|||
{{Authority control}} |
|||
[[Category: |
[[Category:Diethylamino compounds]] |
||
[[Category:Antiarrhythmic agents]] |
[[Category:Antiarrhythmic agents]] |
||
[[Category:Diarylketones]] |
|||
[[Category:Chemical substances for emergency medicine]] |
|||
[[Category:CYP2D6 inhibitors]] |
|||
[[Category:CYP3A4 inhibitors]] |
|||
[[Category:Hepatotoxins]] |
|||
[[Category:HERG blocker]] |
|||
[[Category:Iodobenzene derivatives]] |
|||
[[Category:Piceol ethers]] |
|||
[[Category:2-Phenoxyethanamines]] |
|||
[[Category:Potassium channel blockers]] |
|||
[[Category:VMAT inhibitors]] |
|||
[[Category:World Health Organization essential medicines]] |
|||
[[Category:Wikipedia medicine articles ready to translate]] |
|||
[[Category:Butyl compounds]] |
|||
[[Category:Benzofurans]] |
[[Category:Benzofurans]] |
||
[[Category:Organoiodides]] |
|||
[[Category:Aromatic ketones]] |
|||
[[Category:Phenol ethers]] |
|||
[[Category:Potassium channel blockers]] |
|||
[[de:Amiodaron]] |
|||
[[es:Amiodarona]] |
|||
[[fa:آمیودارون]] |
|||
[[fr:Amiodarone]] |
|||
[[hr:Amiodaron]] |
|||
[[it:Amiodarone]] |
|||
[[he:אמיודארון]] |
|||
[[nl:Amiodaron]] |
|||
[[ja:アミオダロン]] |
|||
[[no:Amiodaron]] |
|||
[[pl:Amiodaron]] |
|||
[[pt:Amiodarona]] |
|||
[[ru:Амиодарон]] |
|||
[[sv:Amiodaron]] |
|||
[[zh:胺碘酮]] |
|||
Latest revision as of 21:40, 14 December 2024
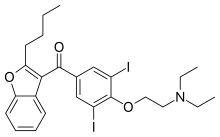 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌæmiˈoʊdəroʊn/ or /əˈmiːoʊdəˌroʊn/ |
| Trade names | Cordarone, Nexterone, Pacerone, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a687009 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, intraosseous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 20–55% |
| Protein binding | 96% |
| Metabolism | Liver |
| Elimination half-life | 58 d (range 15–142 d) |
| Excretion | Primarily liver and bile |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.157 |
| Chemical and physical data | |
| Formula | C25H29I2NO3 |
| Molar mass | 645.320 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Amiodarone is an antiarrhythmic medication used to treat and prevent a number of types of cardiac dysrhythmias.[4] This includes ventricular tachycardia, ventricular fibrillation, and wide complex tachycardia, atrial fibrillation, and paroxysmal supraventricular tachycardia.[4] Evidence in cardiac arrest, however, is poor.[5] It can be given by mouth, intravenously, or intraosseously.[4] When used by mouth, it can take a few weeks for effects to begin.[4][6]
Common side effects include feeling tired, tremor, nausea, and constipation.[4] As amiodarone can have serious side effects, it is mainly recommended only for significant ventricular arrhythmias.[4] Serious side effects include lung toxicity[7] such as interstitial pneumonitis, liver problems, heart arrhythmias, vision problems, thyroid problems, and death.[4] If taken during pregnancy or breastfeeding it can cause problems in the fetus or the infant.[4] It is a class III antiarrhythmic medication.[4] It works partly by increasing the time before a heart cell can contract again.[4][6]
Amiodarone was first made in 1961 and came into medical use in 1962 for chest pain believed to be related to the heart.[8] It was pulled from the market in 1967 due to side effects.[9] In 1974 it was found to be useful for arrhythmias and reintroduced.[9] It is on the World Health Organization's List of Essential Medicines.[10] It is available as a generic medication.[4] In 2022, it was the 237th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[11][12]
Medical uses
[edit]Amiodarone has been used both in the treatment of acute life-threatening arrhythmias as well as the long-term suppression of arrhythmias.[13] Amiodarone is commonly used to treat different types of abnormal heart rhythms, such as atrial arrhythmias (supraventricular arrhythmias) and ventricular arrhythmias.[13]
Atrial arrhythmias and supraventricular arrhythmias are terms often used interchangeably to refer to abnormal heart rhythms originating from the upper chambers of the heart, known as the atria. These types of arrhythmias include conditions such as atrial fibrillation, atrial flutter, and paroxysmal supraventricular tachycardia. They are collectively referred to as supraventricular or atrial arrhythmias because they occur above (supra) the ventricles in the electrical conduction system of the heart.[14]
Ventricular arrhythmias are abnormal heart rhythms that originate in the ventricles, which are the lower chambers of the heart. These arrhythmias can be potentially life-threatening and may disrupt the heart's ability to pump blood effectively.[14]
Amiodarone can be effective in treating conditions like ventricular fibrillation (a rapid and irregular heartbeat), ventricular tachycardia (fast heartbeat originating from the lower chambers), and cardiac arrest due to shock-resistant ventricular fibrillation.[13]
In cases where a patient is experiencing shock-resistant ventricular arrhythmias including stable ventricular tachycardia or unstable ventricular fibrillation, amiodarone may be used.[15] A recent study suggested that another antiarrhythmic, procainamide, may be more effective in stopping ventricular tachycardia – with less side effects and a higher survival rate in patients requiring multiple shocks.[16] However, due to a small sample size and lack of statistical significance, more evidence is required, and amiodarone remains the drug of choice in ventricular arrhythmias.[15][16]
Amiodarone is also commonly used as the first-line therapy for patients who receive shocks from implantable cardioverter defibrillators caused by ventricular arrhythmias. Combining amiodarone with beta-blockers has been shown to reduce the likelihood of experiencing inappropriate shocks from implantable cardioverter defibrillators.[13]
Cardiac arrest
[edit]Defibrillation is the treatment of choice for ventricular fibrillation and pulseless ventricular tachycardia resulting in cardiac arrest. While amiodarone has been used in shock-refractory cases, evidence of benefit is poor.[5] Although amiodarone does not appear to improve survival in those who had a cardiac arrest in-hospital,[17] some studies suggested that early administration of amiodarone was associated with better survival and positive outcomes for people who had a cardiac arrest out-of-hospital.[18][19]
Ventricular tachycardia
[edit]Amiodarone may be used in the treatment of ventricular tachycardia in certain instances.[20] Individuals with hemodynamically unstable ventricular tachycardia should not initially receive amiodarone. These individuals should be cardioverted.
Amiodarone can be used in individuals with hemodynamically stable ventricular tachycardia. In these cases, amiodarone can be used regardless of the individual's underlying heart function and the type of ventricular tachycardia; it can be used in individuals with monomorphic ventricular tachycardia, but is contraindicated in individuals with polymorphic ventricular tachycardia as it is associated with a prolonged QT interval which will be made worse with anti-arrhythmic drugs.[21]
Atrial fibrillation
[edit]Individuals who have undergone open heart surgery are at an increased risk of developing atrial fibrillation (or AF) in the first few days post-procedure.[13][22][23] In the ARCH trial, intravenous amiodarone (2 g administered over 2 d) has been shown to reduce the incidence of atrial fibrillation after open heart surgery when compared to placebo.[24][25] However, clinical studies have failed to demonstrate long-term efficacy and have shown potentially fatal side effects such as pulmonary toxicities. While amiodarone is not approved for AF by the US Food and Drug Administration (FDA), it is a commonly prescribed off-label treatment due to the lack of equally effective treatment alternatives.[26][27]
So-called 'acute onset atrial fibrillation', defined by the North American Society of Pacing and Electrophysiology (NASPE) in 2003, responds well to short-duration treatment with amiodarone.[26][28] This has been demonstrated in seventeen randomized controlled trials, of which five included a placebo arm. The incidence of severe side effects in this group is low.[29][30][31]
Amiodarone is an effective, antiarrhythmic-of-choice in achieving cardioversion to sinus rhythm in critical care populations with new onset atrial fibrillation (NOAF). However, other anti-arrhythmic agents may exert superior rhythm control, rate control and lower mortality rate which may be more favourable than amiodarone in specific cases.[32]
Contraindications
[edit]Women who are pregnant or may become pregnant are strongly advised not to take amiodarone. Since amiodarone can be expressed in breast milk, women taking the drug are advised to stop nursing.
It is contraindicated in individuals with sinus nodal bradycardia, atrioventricular block, and second or third-degree heart block who do not have an artificial pacemaker.
Individuals with baseline depressed lung function should be monitored closely if amiodarone therapy is to be initiated.
Formulations of amiodarone that contain benzyl alcohol should not be given to neonates, because the benzyl alcohol may cause the potentially fatal "gasping syndrome".[33]
Amiodarone can worsen the cardiac arrhythmia brought on by digitalis toxicity.
Contraindications of amiodarone also include:
- hypersensitivity to amiodarone or any of its components;[13]
- severe hepatic impairment;[13]
- sinus node dysfunction, including severe sinus bradycardia or sinoatrial block, since amiodarone can cause significant bradycardia and sinus nodal arrest;[13]
- second- or third-degree atrioventricular (AV) block, due to its negative chronotropic (affecting the heart rate) and dromotropic (affecting the conductivity) effects on the AV conduction system, unless a pacemaker is implanted;[13]
- thyrotoxicosis that cannot be controlled by conventional means, such as Graves' disease.[13]
There are no specific guidelines for endurance or high-intensity exercise while taking amiodarone. However, since amiodarone may cause bradycardia and QTc prolongation which can affect exercise capacity and increase the risk of arrhythmias during intense exercise, it would generally be advisable for patients taking this medication to consult their healthcare provider before engaging in high-intensity physical activities such as strenuous endurance exercises.[13]
Side effects
[edit]At oral doses of 400 mg per day or higher, amiodarone can have serious, varied side effects, including toxicity to the thyroid gland,[34] liver, lung, and retinal functions, requiring clinical surveillance and regular laboratory testing.[35][36] Allergic reactions to amiodarone may occur.[35] Most individuals administered amiodarone on a chronic basis will experience at least one side effect.[36] In some people, daily use of amiodarone at 100 mg oral doses can be effective for arrhythmia control with no or minimal side effects.[36]
Some common side effects include:
- nausea and vomiting;[13]
- taste disturbances (changes in taste perception, often described as a metallic or bitter taste in the mouth);[13]
- photosensitivity of the skin, also known as photodermatitis, where exposure to sunlight or ultraviolet radiation may lead to skin reactions such as rashes or sunburn-like symptoms;[13]
- corneal microdeposits (deposits may accumulate on the cornea over time, resulting in blurred vision or visual halos—bright circles or rings around a light source, such as headlights; still, these corneal deposits typically do not affect vision significantly);[13][37]
- thyroid dysfunction[38] (in approximately 15-20% of patients, amiodarone treatment results in thyroid dysfunction, either amiodarone-induced hypothyroidism or amiodarone-induced thyrotoxicosis; the drug can lead to both hypo- and hyperthyroidism);[34]
- pulmonary toxicity[39][40][41][42] (lung problems such as pulmonary fibrosis or interstitial lung disease may occur rarely but have the potential for serious consequences if left untreated);[7][13]
- liver abnormalities (liver damage, including elevated liver enzymes (AST/ALT) and hepatotoxicity, although severe cases are rare);[13]
- bradycardia and heart block (since it slows down heart rate by affecting the sinus node function and AV conduction system, it can increase the risk of heart block);[13]
- QT Interval prolongation.[13]
Amiodarone can potentially cause renal toxicity, but solid studies on whether amiodarone may be toxic to the kidneys are lacking.[43]
Lung
[edit]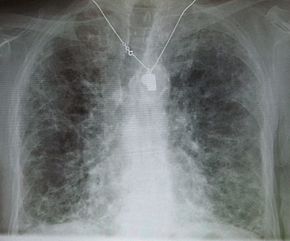
Side effects of oral amiodarone at doses of 400 mg or higher include various pulmonary effects.[44] The most serious reaction is interstitial lung disease. Risk factors include high cumulative dose, more than 400 milligrams per day, duration over two months, increased age, and preexisting pulmonary disease. Some individuals were noted to develop pulmonary fibrosis after a week of treatment, while others did not develop it after years of continuous use.[44] Common practice is to avoid the agent if possible in individuals with decreased lung function.
The most specific test of pulmonary toxicity due to amiodarone is a dramatically decreased DLCO noted on pulmonary function testing.
Thyroid
[edit]Induced abnormalities in thyroid function are common.[38][35] In approximately 15-20% of patients, amiodarone treatment results in thyroid dysfunction, either amiodarone-induced hypothyroidism or amiodarone-induced thyrotoxicosis.[45][46][34][20] Both under- and overactivity of the thyroid may occur.[35]
Amiodarone is structurally similar to thyroxine and also contains iodine. Both of these factors contribute to the effects of amiodarone on thyroid function.[20][45][47][48] Amiodarone also causes an anti-thyroid action, via Plummer and Wolff–Chaikoff effects, due its large amount of iodine in its molecule, which causes a particular "cardiac hypothyroidism" with bradycardia and arrhythmia.[49][50]
Thyroid function should be checked at least every six months.[51]
- Hypothyroidism (slowing of the thyroid) occurs frequently; in the SAFE trial, which compared amiodarone with other medications for the treatment of atrial fibrillation, biochemical hypothyroidism (as defined by a TSH level of 4.5–10 mU/L) occurred in 25.8% of the amiodarone-treated group as opposed to 6.6% of the control group (taking placebo or sotalol). Overt hypothyroidism (defined as TSH >10 mU/L) occurred at 5.0% compared to 0.3%; most of these (>90%) were detected within the first six months of amiodarone treatment.[52]
- Amiodarone induced thyrotoxicosis (AIT), can be caused due to the high iodine content in the drug via the Jod-Basedow effect. This is known as Type 1 AIT, and usually occurs in patients with an underlying predisposition to hyperthyroidism such as Graves' disease, within weeks to months after starting amiodarone. Type 1 AIT is usually treated with anti-thyroid drugs or thyroidectomy. Type 2 AIT is caused by a destructive thyroiditis due to a direct toxic effect of amiodarone on thyroid follicular epithelial cells.[45][53] Type 2 AIT can occur even years after starting amiodarone, is usually self-limited and responds to anti-inflammatory treatment such as corticosteroids.[53] In practice, often the type of AIT is undetermined or presumed as mixed with both treatments combined.[53] Thyroid uptake measurements (I-123 or I-131), which are used to differentiate causes of hyperthyroidism, are generally unreliable in patients who have been taking amiodarone. Because of the high iodine content of amiodarone, the thyroid gland is effectively saturated, thus preventing further uptake of isotopes of iodine. However, positive radioactive iodine can be used to rule in type 1AIT .[citation needed]
-
Amiodarone
Eye
[edit]Corneal micro-deposits (cornea verticillata,[54] also called vortex or whorl keratopathy) are almost universally present (over 90%) in individuals taking amiodarone longer than 6 months, especially doses greater than 400 mg/day. These deposits typically do not cause any symptoms. About 1 in 10 individuals may complain of a bluish halo. Anterior subcapsular lens deposits are relatively common (50%) in higher doses (greater than 600 mg/day) after 6 months of treatment. Optic neuropathy, nonarteritic anterior ischemic optic neuropathy (N-AION), occurs in 1–2% of people and is not dosage dependent.[55] Bilateral optic disc swelling and mild and reversible visual field defects can also occur.
Loss of eyelashes has been linked to amiodarone use.[56]
Liver
[edit]Abnormal liver enzyme results are common in people taking amiodarone.[35] Much rarer are jaundice, hepatomegaly (liver enlargement), and hepatitis (inflammation of the liver).[57]
In clinical observations, it has been noted that the administration of amiodarone, even at lower therapeutic doses, has been associated with the development of a condition mimicking alcoholic cirrhosis. This condition, often referred to as pseudo-alcoholic cirrhosis, presents with similar histopathological features to those observed in patients with alcoholic cirrhosis.[58][59] However, this extreme adverse event manifestation—pseudo-alcoholic cirrhosis caused by low dose amiodarone—is very rare.[36]
Skin
[edit]Long-term administration of amiodarone (usually more than eighteen months) is associated with a light-sensitive blue-grey discoloration of the skin, sometimes called ceruloderma; such patients should avoid exposure to the sun and use sunscreen that protects against ultraviolet-A and -B. The discoloration will slowly improve upon cessation of the medication, however, the skin color may not return completely.[60]
Pregnancy and breastfeeding
[edit]Use during pregnancy may result in a number of problems in the infant including thyroid problems, heart problems, neurological problems, and preterm birth.[61] Use during breastfeeding is generally not recommended though one dose may be okay.[61]
Other
[edit]Long-term use of amiodarone has been associated with peripheral neuropathies.[62]
Amiodarone is sometimes responsible for epididymitis. Amiodarone accumulates in the head of the organ and can cause unilateral or bilateral inflammation. It tends to resolve if amiodarone is stopped.[63]
Some cases of gynecomastia have been reported in men on amiodarone.[64]
A retrospective cohort study found an increased risk of digestive, liver, head and neck and liver cancers amongst male patients exposed to amiodarone versus female participants in the same study and the general population.[65] This study also identified that the Standardized Incidence Ratio of cancer occurrence increased significantly in males aged 20-59 and >80 years old who were exposed to a higher dose of Amiodarone in comparison to those exposed to a lower dose. This suggests that there is a dose-effect relationship.[65] These results should be interpreted with caution due to limitations of the study design and care should be taken prior to altering current clinical and prescribing practices. Amiodarone and its effect on cancer is still a topic that requires more robust research.
Drug-drug interactions
[edit]The pharmacokinetics of numerous drugs, including many that are commonly administered to individuals with heart disease, are affected by amiodarone.[66][67][68]
Amiodarone has particularly important interactions with the following drugs:
- class I antiarrhythmics (amiodarone should not be combined with other class I antiarrhythmic drugs, such as disopyramide, flecainide, procainamide, quinidine, etc., due to an increased risk of QTc prolongation and potential arrhythmias);[13]
- beta blockers and calcium channel blockers (combining amiodarone with beta-blockers or calcium channel blockers, such as sotalol, can further slow down heart rate and cause bradycardia or heart block);[13]
- digoxin (amiodarone inhibits a protein called P-glycoprotein (P-gp), which transports digoxin out of cells in the gut, liver, and kidneys, therefore, concurrent use of these medications increases digoxin levels in the body, potentially leading to digoxin toxicity);[13]
- statins (amiodarone can inhibit enzymes in the liver responsible for metabolizing certain statins, such as simvastatin, atorvastatin, etc., therefore interaction elevates plasma concentrations of these statins, increasing the risk of myopathy, that is muscle damage, or rhabdomyolysis, that is severe muscle breakdown);[13]
- warfarin (since the anticoagulation effects of warfarin depend on metabolism of warfarin by both cytochromes CYP2C9 and CYP3A4, coadministation leads to rise in international normalized ratio (INR)—the amount of time taken for the blood to form a clot—placing patient at higher bleeding risks);[13][69] Amiodarone potentiates the action of warfarin by inhibiting the clearance of both (S) and (R) warfarin. Individuals taking both of these medications should have their warfarin doses adjusted based on their dosing of amiodarone and have their anticoagulation status (measured as prothrombin time (PT) and international normalized ratio (INR)) measured more frequently.[70][71] Dose reduction of warfarin is as follows: 40% reduction if the amiodarone dose is 400 mg daily, 35% reduction if the amiodarone dose is 300 mg daily, 30% reduction if the amiodarone dose is 200 mg daily, and 25% reduction if amiodarone dose is 100 mg daily.[70][72] The effect of amiodarone on the warfarin concentrations can be as early as a few days after initiation of treatment; however, the interaction may not peak for up to seven weeks;[70][72]
- anti-HIV medications (several HIV medications, such as ritonavir, indinavir, etc., interact with amiodarone by inhibiting CYP3A4 enzyme hence leading to decreased clearance of amiodarone, i.e., increasing the concentration of amiodarone in the organism).[13][73]
Amiodarone inhibits the action of the cytochrome P450 isozyme family; such inhibition reduces the clearance of many drugs, including the following:[68][74][75]
- ciclosporin,[68]
- digoxin,[68]
- flecainide,[68]
- procainamide,[68]
- quinidine,[68]
- sildenafil,[68]
- simvastatin,[68]
- theophylline,[68]
- warfarin.[68]
In 2015, Gilead Sciences warned healthcare providers about people who began taking the hepatitis C drugs ledipasvir/sofosbuvir or sofosbuvir along with amiodarone, who developed abnormally slow heartbeats or died of cardiac arrest.[76]
Metabolism
[edit]Amiodarone is extensively metabolized in the liver by CYP3A4, a member of the cytochrome P450 superfamily of enzymes, therefore, amiodarone and can affect the metabolism of numerous other drugs that depend on cytochrome P450, such as digoxin, phenytoin, warfarin, etc.[26][77][78][46]
The major metabolite of amiodarone is desethylamiodarone (DEA), which also has antiarrhythmic properties.[26]
The metabolism of amiodarone is inhibited by grapefruit, leading to elevated serum levels of amiodarone.[79]
On 8 August 2008, the US Food and Drug Administration (FDA) issued a warning of the risk of rhabdomyolysis, which can lead to kidney failure or death, when simvastatin is used with amiodarone. This interaction is dose-dependent with simvastatin doses exceeding 20 mg. This drug combination, especially with higher doses of simvastatin, should be avoided.[80]
Amiodarone is extensively metabolized in the liver. The primary metabolic pathway of amiodarone is by cytochrome P450 (CYP) enzymes, particularly CYP3A4 and CYP2C8.[73][46][68][81][82] The metabolism of amiodaron can be characterized by two phases:[83]
- phase I metabolism, when amiodarone undergoes oxidative processes mainly mediated by CYP3A4 and to a lesser extent by CYP2C8; these reactions result in the formation of several active metabolites, including desethylamiodarone (DEA) and di-desethylamiodarone (DDEA); DEA is the most abundant metabolite and exhibits similar pharmacological effects as amiodarone;[83][84]
- phase II metabolism, when both amiodarone and its major metabolite DEA can undergo conjugation reactions with glucuronic acid; this process increases water solubility of these compounds for their efficient elimination from the body.[85]
Amiodarone has an exceptionally long half-life due to a combination of several factors:[13]
- high lipid solubility, given that amiodarone has high lipid solubility, which allows it to distribute throughout various tissues in the body rapidly; the extensive tissue distribution of amiodarone contributes to a large volume of distribution that leads to slow clearance from plasma compartments;
- extensive tissue binding, so that amiodarone extensively binds to different tissues, including fat deposits, muscles, heart tissue, and other organs; this binding creates reservoirs where drug release can occur slowly over time, resulting in an extended duration of action even after stopping the therapy;
- enterohepatic recycling, meaning that amiodarone undergoes enterohepatic recycling, where it is reabsorbed from the intestines after being excreted into bile, which contributes to its prolonged presence.[86]
Excretion
[edit]Excretion is primarily via the liver and the bile duct with almost no elimination via the kidney and it is not dialyzable.[1] Elimination half-life average of 58 days (ranging from 25 to 100 days [Remington: The Science and Practice of Pharmacy 21st edition]) for amiodarone and 36 days for the active metabolite, desethylamiodarone (DEA).[1] There is 10-50% transfer of amiodarone and DEA in the placenta as well as a presence in breast milk.[1] Accumulation of amiodarone and DEA occurs in adipose tissue and highly perfused organs (i.e. liver, lungs),[1] therefore, if an individual was taking amiodarone on a chronic basis if it is stopped it will remain in the system for weeks to months.[1]
Whereas amiodarone is primarily eliminated from the body through hepatic metabolism and biliary excretion, a very small portion of amiodarone and its metabolites are excreted unchanged in urine or feces.[73][46]
The liver plays a significant role in the elimination of amiodarone. After being extensively metabolized by cytochrome P450 enzymes, particularly CYP3A4 and CYP2C8, amiodarone is transported into bile via multidrug-resistant protein 2 (MRP2) transporter. Bile containing amiodarone and its metabolites is then released into the gastrointestinal tract.[medical citation needed]
Some of these compounds can be reabsorbed back into systemic circulation through enterohepatic recirculation, where they may undergo additional rounds of metabolism before eventually being excreted again into bile.[medical citation needed]
Because renal excretion contributes only minimally to the elimination of amiodarone, dose adjustment based on kidney function is generally not necessary. This is because most patients with normal renal function can adequately clear the drug through hepatic metabolism and biliary elimination pathways.[13]
Pharmacology
[edit]Amiodarone is categorized as a class III antiarrhythmic agent, and prolongs phase 3 of the cardiac action potential, the repolarization phase where there is normally decreased calcium permeability and increased potassium permeability. It has numerous other effects, however, including actions that are similar to those of antiarrhythmic classes Ia, II, and IV.[medical citation needed]
Amiodarone is a blocker of voltage gated potassium (KCNH2) and voltage gated calcium channels (CACNA2D2).[87]
Amiodarone slows the conduction rate and prolongs the refractory period of the SA and AV nodes.[88] It also prolongs the refractory periods of the ventricles, bundles of His, and the Purkinje fibers without exhibiting any effects on the conduction rate.[88] Amiodarone has been shown to prolong the myocardial cell action potential duration and refractory period and is a non-competitive β-adrenergic inhibitor.[89]
It also shows beta blocker-like and calcium channel blocker-like actions on the SA and AV nodes, increases the refractory period via sodium- and potassium-channel effects, and slows intra-cardiac conduction of the cardiac action potential, via sodium-channel effects. It is suggested that amiodarone may also exacerbate the phenotype associated with Long QT-3 syndrome causing mutations such as ∆KPQ. This effect is due to a combination of blocking the peak sodium current, but also contributing to an increased persistent sodium current.[90]
Amiodarone chemically resembles thyroxine (thyroid hormone), and its binding to the nuclear thyroid receptor might contribute to some of its pharmacologic and toxic actions.[91] The mechanisms of action of amiodarone include blocking potassium ion channels (prolonging repolarization), blocking sodium ion channels, and antagonizing alpha- and beta-adrenergic receptors.[13] The action of amiodarone can be characterized by the following effects:[13]
- potassium channel blockade, since amiodarone blocks potassium channels involved in cardiac repolarization during phase 3 of the action potential, so that this blockade prolongs the duration of cardiac action potentials, resulting in an increased refractory period and decreased excitability;[13]
- sodium channel blockade, characterized by inhibiting sodium ion influx through voltage-gated sodium channels, so that amiodarone reduces the conduction velocity of electrical impulses in cardiac tissue that leads to a slowed heart rate and improved rhythm control;[13]
- calcium channel blockade, by inhibiting L-type calcium channels in myocardial cells, decreasing intracellular calcium concentration during ventricular contraction;[13]
- noncompetitive adrenergic receptor antagonism, meaning that amiodarone has both alpha- and beta-adrenergic receptor antagonistic effects, which help reduce sympathetic stimulation on the heart.[13]
History
[edit]The original observation that amiodarone's progenitor molecule, khellin, had cardioactive properties, was made by the Russian physiologist Gleb von Anrep while working in Cairo in 1946.[92] Khellin is obtained from a plant extract of Khella or Ammi visnaga, a common plant in north Africa. Anrep noticed that one of his technicians had been cured of anginal symptoms after taking khellin, then used for various, non-cardiac ailments. This led to efforts by European pharmaceutical industries to isolate an active compound.[citation needed] Amiodarone was initially developed in 1961 at the Labaz company, Belgium, by chemists Tondeur and Binon, who were working on preparations derived from khellin. It became popular in Europe as a treatment for angina pectoris.[93][94][95]
As a doctoral candidate at Oxford University, Bramah Singh determined that amiodarone and sotalol had antiarrhythmic properties and belonged to a new class of antiarrhythmic agents (what would become the class III antiarrhythmic agents).[96] Today the mechanisms of action of amiodarone and sotalol have been investigated in more detail. Both drugs have been demonstrated to prolong the duration of the action potential, prolonging the refractory period, by interacting among other cellular functions with K+ channels.[95]
Based on Singh's work, the Argentinian physician Mauricio Rosenbaum began using amiodarone to treat his patients who have supraventricular and ventricular arrhythmias, with impressive results. Based on papers written by Rosenbaum developing Singh's theories, physicians in the United States began prescribing amiodarone to their patients with potentially life-threatening arrhythmias in the late 1970s.[97][98]
The US Food and Drug Administration (FDA) was reluctant to officially approve the use of amiodarone since initial reports had shown an increased incidence of serious pulmonary side effects of the drug. In the mid-1980s, the European pharmaceutical companies began putting pressure on the FDA to approve amiodarone by threatening to cut the supply to American physicians if it was not approved. In December 1985, amiodarone was approved by the FDA for the treatment of arrhythmias.[2][99]
Name
[edit]Amiodarone may be an acronym[citation needed] for its IUPAC name (2-butyl-1-benzofuran-3-yl)-[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone,[100] where ar is a placeholder for phenyl. This is partially supported by dronedarone which is noniodinated benzofuran derivative of amiodarone, where the arylmethanone is conserved.[citation needed]
Dosing
[edit]Amiodarone is available in oral and intravenous formulations.
Orally, it is available under the brand names Pacerone (produced by Upsher-Smith Laboratories, Inc.) and Cordarone (produced by Wyeth-Ayerst Laboratories).[1][2] It is also available under the brand name Aratac (produced by Alphapharm Pty Ltd) in Australia and New Zealand, and further in Australia under the brands Cardinorm and Rithmik as well as a number of generic brands. Also Arycor in South Africa (Produced by Winthrop Pharmaceuticals.). In South America, it is known as Atlansil and is produced by Roemmers.
In India, amiodarone is marketed (produced by Cipla Pharmaceutical) under the brand name Tachyra. It is also available in intravenous ampules and vials.
The dose of amiodarone administered is tailored to the individual and the dysrhythmia that is being treated. When administered orally, the bioavailability of amiodarone is quite variable. Absorption ranges from 22 to 95%, with better absorption when it is given with food.[26]
References
[edit]- ^ a b c d e f g "Pacerone- amiodarone hydrochloride tablet". DailyMed. Archived from the original on 29 December 2022. Retrieved 8 September 2021.
- ^ a b c "Cordarone (amiodarone) tablets, for oral use Initial U.S. Approval: 1985". DailyMed. 30 October 2018. Archived from the original on 29 December 2022. Retrieved 8 September 2021.
- ^ "Nexterone- Amiodarone HCl injection, solution". DailyMed. Archived from the original on 29 December 2022. Retrieved 8 September 2021.
- ^ a b c d e f g h i j k "Amiodarone Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 19 September 2016. Retrieved 22 August 2016.
- ^ a b Ali MU, Fitzpatrick-Lewis D, Kenny M, Raina P, Atkins DL, Soar J, et al. (November 2018). "Effectiveness of antiarrhythmic drugs for shockable cardiac arrest: A systematic review" (PDF). Resuscitation. 132: 63–72. doi:10.1016/j.resuscitation.2018.08.025. PMID 30179691. S2CID 52154562. Archived (PDF) from the original on 5 March 2020. Retrieved 17 December 2019.
- ^ a b Review of the Medical Use of Amiodarone (Nexterone, Pacerone). Xavier Research Press. 24 July 2018. ISBN 978-1-7242-7798-5.
- ^ a b Feduska ET, Thoma BN, Torjman MC, Goldhammer JE (May 2021). "Acute Amiodarone Pulmonary Toxicity". J Cardiothorac Vasc Anesth. 35 (5): 1485–1494. doi:10.1053/j.jvca.2020.10.060. PMID 33262034. S2CID 227253264.
- ^ Analytical Profiles of Drug Substances and Excipients. Academic Press. 1992. p. 4. ISBN 978-0-08-086115-9. Archived from the original on 8 September 2017.
- ^ a b Fischer J, Ganellin CR (2005). Analogue-based Drug Discovery. John Wiley & Sons. p. 12. ISBN 978-3-527-60749-5. Archived from the original on 8 September 2017.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Amiodarone Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag Hamilton D, Nandkeolyar S, Lan H, Desai P, Evans J, Hauschild C, et al. (December 2020). "Amiodarone: A Comprehensive Guide for Clinicians". Am J Cardiovasc Drugs. 20 (6): 549–558. doi:10.1007/s40256-020-00401-5. PMID 32166725. S2CID 212682149.
- ^ a b Mori S, Tretter JT, Spicer DE, Bolender DL, Anderson RH (April 2019). "What is the real cardiac anatomy?". Clin Anat. 32 (3): 288–309. doi:10.1002/ca.23340. PMC 6849845. PMID 30675928.
- ^ a b Larson J, Rich L, Deshmukh A, Judge EC, Liang JJ (June 2022). "Pharmacologic Management for Ventricular Arrhythmias: Overview of Anti-Arrhythmic Drugs". Journal of Clinical Medicine. 11 (11): 3233. doi:10.3390/jcm11113233. PMC 9181251. PMID 35683620.
- ^ a b Ortiz M, Martín A, Arribas F, Coll-Vinent B, Del Arco C, Peinado R, et al. (May 2017). "Randomized comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: the PROCAMIO study". European Heart Journal. 38 (17): 1329–1335. doi:10.1093/eurheartj/ehw230. PMC 5410924. PMID 27354046.
- ^ Laina A, Karlis G, Liakos A, Georgiopoulos G, Oikonomou D, Kouskouni E, et al. (October 2016). "Amiodarone and cardiac arrest: Systematic review and meta-analysis". International Journal of Cardiology. 221: 780–788. doi:10.1016/j.ijcard.2016.07.138. PMID 27434349.
- ^ Lupton JR, Neth MR, Sahni R, Jui J, Wittwer L, Newgard CD, et al. (September 2023). "Survival by time-to-administration of amiodarone, lidocaine, or placebo in shock-refractory out-of-hospital cardiac arrest". Academic Emergency Medicine. 30 (9): 906–917. doi:10.1111/acem.14716. PMID 36869657.
- ^ Perry E, Nehme E, Stub D, Anderson D, Nehme Z (June 2023). "The impact of time to amiodarone administration on survival from out-of-hospital cardiac arrest". Resuscitation Plus. 14: 100405. doi:10.1016/j.resplu.2023.100405. PMC 10250159. PMID 37303855.
- ^ a b c Medić F, Bakula M, Alfirević M, Bakula M, Mucić K, Marić N (August 2022). "Amiodarone and Thyroid Dysfunction". Acta Clin Croat. 61 (2): 327–341. doi:10.20471/acc.2022.61.02.20. PMC 9934045. PMID 36818930.
- ^ "Peri-arrest arrhythmias – Tachycardia algorithm]". Resuscitation Council (UK). Archived from the original on 3 January 2016. Retrieved 25 January 2016.
- ^ "UpToDate". Archived from the original on 5 February 2024. Retrieved 5 February 2024.
- ^ "Atrial fibrillation after surgery: Common and undertreated?". October 2022. Archived from the original on 5 February 2024. Retrieved 5 February 2024.
- ^ "Amiodarone Reduction in Coronary Heart Trial". Archived from the original on 5 February 2024. Retrieved 5 February 2024.
- ^ Guarnieri T, Nolan S, Gottlieb SO, Dudek A, Lowry DR (August 1999). "Intravenous amiodarone for the prevention of atrial fibrillation after open heart surgery: the Amiodarone Reduction in Coronary Heart (ARCH) trial". Journal of the American College of Cardiology. 34 (2): 343–347. doi:10.1016/S0735-1097(99)00212-0. PMID 10440143. S2CID 24714524.
- ^ a b c d e Siddoway LA (December 2003). "Amiodarone: Guidelines for Use and Monitoring". American Family Physician. 68 (11): 2189–2197. PMID 14677664. Archived from the original on 5 February 2024. Retrieved 5 February 2024.
- ^ Neff MJ (April 2005). "Practice Guideline Briefs". American Family Physician. 71 (7): 1434. Archived from the original on 5 February 2024. Retrieved 5 February 2024.
- ^ King DE, Dickerson LM, Sack JL (15 July 2002). "Acute Management of Atrial Fibrillation: Part I. Rate and Rhythm Control". American Family Physician. 66 (2): 249–257. PMID 12152960. Archived from the original on 5 February 2024. Retrieved 5 February 2024.
- ^ O'Bryan LJ, Redfern OC, Bedford J, Petrinic T, Young JD, Watkinson PJ (2020). "Managing new-onset atrial fibrillation in critically ill patients: A systematic narrative review". BMJ Open. 10 (3): e034774. doi:10.1136/bmjopen-2019-034774. PMC 7202704. PMID 32209631. Archived from the original on 5 February 2024. Retrieved 5 February 2024.
- ^ Letelier LM, Udol K, Ena J, Weaver B, Guyatt GH (2003). "Effectiveness of Amiodarone for Conversion of Atrial Fibrillation to Sinus Rhythm". Archives of Internal Medicine. 163 (7): 777–785. doi:10.1001/archinte.163.7.777. PMID 12695268. Archived from the original on 5 February 2024. Retrieved 5 February 2024.
- ^ "Recommendations | Atrial fibrillation: Diagnosis and management | Guidance | NICE". 27 April 2021. Archived from the original on 5 February 2024. Retrieved 5 February 2024.
- ^ Johnston BW, Chean CS, Duarte R, Hill R, Blackwood B, McAuley DF, Welters ID. Management of new onset atrial fibrillation in critically unwell adult patients: a systematic review and narrative synthesis. British Journal of Anaesthesia. 2022 May 1;128(5):759-71.
- ^ Centers for Disease Control (CDC) (June 1982). "Neonatal deaths associated with use of benzyl alcohol--United States". MMWR. Morbidity and Mortality Weekly Report. 31 (22): 290–291. PMID 6810084. Archived from the original on 30 August 2012.
- ^ a b c Cappellani D, Bartalena L, Bogazzi F (September 2023). "Short review: novel concepts in the approach to patients with amiodarone-induced thyrotoxicosis". J Endocrinol Invest. 47 (2): 275–283. doi:10.1007/s40618-023-02168-3. PMC 10859339. PMID 37731073. S2CID 262088052.
- ^ a b c d e "Amiodarone". Drugs.com. 18 May 2022. Archived from the original on 25 July 2022. Retrieved 25 July 2022.
- ^ a b c d Chokesuwattanaskul R, Shah N, Chokesuwattanaskul S, Liu Z, Thakur R (April 2020). "Low-dose Amiodarone Is Safe: A Systematic Review and Meta-analysis". J Innov Card Rhythm Manag. 11 (4): 4054–4061. doi:10.19102/icrm.2020.110403. PMC 7192149. PMID 32368381.
- ^ Colunga Biancatelli RM, Congedo V, Calvosa L, Ciacciarelli M, Polidoro A, Iuliano L (July 2019). "Adverse reactions of Amiodarone". J Geriatr Cardiol. 16 (7): 552–566. doi:10.11909/j.issn.1671-5411.2019.07.004 (inactive 2 November 2024). PMC 6689516. PMID 31447894.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ a b Gašparini D, Raljević D, Pehar-Pejčinović V, Klarica Gembić T, Peršić V, Turk Wensveen T (2023). "When amiodarone-induced thyroiditis meets cardiomyopathy with excessive trabeculation: a case report". Front Cardiovasc Med. 10: 1212965. doi:10.3389/fcvm.2023.1212965. PMC 10401478. PMID 37547257.
- ^ Scaramozzino MU, Sapone G, Plastina UR, Nucara M (March 2023). "Amiodarone-Induced Lung Toxicity: A Case Initially Not Correctly Framed". Cureus. 15 (3): e36818. doi:10.7759/cureus.36818. PMC 10146449. PMID 37123694.
- ^ Tsai IL, Huang LT, Yu YT, Lee CT, Huang TH (June 2023). "Variable radiographic and histologic presentations of amiodarone-related interstitial lung disease and the importance of avoiding re-exposure". Respirol Case Rep. 11 (6): e01165. doi:10.1002/rcr2.1165. PMC 10209837. PMID 37249923.
- ^ Budin CE, Cocuz IG, Sabău AH, Niculescu R, Ianosi IR, Ioan V, et al. (December 2022). "Pulmonary Fibrosis Related to Amiodarone-Is It a Standard Pathophysiological Pattern? A Case-Based Literature Review". Diagnostics. 12 (12): 3217. doi:10.3390/diagnostics12123217. PMC 9777900. PMID 36553223.
- ^ Mitrofan CE, Cretu A, Mitrofan C, Bar C, Ghiciuc CM (2022). "Amiodarone induced lung disease". Arch Clin Cases. 9 (3): 126–132. doi:10.22551/2022.36.0903.10217. PMC 9512125. PMID 36176494.
- ^ Duineveld MD, Kers J, Vleming LJ (September 2023). "Case report of progressive renal dysfunction as a consequence of amiodarone-induced phospholipidosis". Eur Heart J Case Rep. 7 (9): ytad457. doi:10.1093/ehjcr/ytad457. PMC 10516635. PMID 37743903.
- ^ a b "Amiodarone Side Effects". Drugs.com. 25 April 2021. Archived from the original on 24 February 2016. Retrieved 25 July 2022.
- ^ a b c Tsang W, Houlden RL (July 2009). "Amiodarone-induced thyrotoxicosis: a review". Can J Cardiol. 25 (7): 421–4. doi:10.1016/s0828-282x(09)70512-4. PMC 2723027. PMID 19584973.
- ^ a b c d Florek JB, Lucas A, Girzadas D (2024). Amiodarone. PMID 29489285. NCBI NBK482154.
- ^ Lombardi A, Inabnet WB, Owen R, Farenholtz KE, Tomer Y (January 2015). "Endoplasmic reticulum stress as a novel mechanism in amiodarone-induced destructive thyroiditis". The Journal of Clinical Endocrinology and Metabolism. 100 (1): E1-10. doi:10.1210/jc.2014-2745. PMC 4283007. PMID 25295624.
- ^ Hall GM, Hunter JM, Cooper MS (2010). Core Topics in Endocrinology in Anaesthesia and Critical Care. Cambridge University Press. p. 170. ISBN 978-1-139-48612-5. Archived from the original on 8 September 2017.
- ^ Venturi S (2011). "Evolutionary Significance of Iodine". Current Chemical Biology. 5 (3): 155–162. doi:10.2174/187231311796765012 (inactive 17 November 2024). ISSN 1872-3136.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Venturi S (2014). "Iodine, PUFAs and Iodolipids in Health and Disease: An Evolutionary Perspective". Human Evolution. 29 (1–3): 185–205. ISSN 0393-9375.
- ^ Bartalena L, Bogazzi F, Chiovato L, Hubalewska-Dydejczyk A, Links TP, Vanderpump M (March 2018). "2018 European Thyroid Association (ETA) Guidelines for the Management of Amiodarone-Associated Thyroid Dysfunction". European Thyroid Journal. 7 (2): 55–66. doi:10.1159/000486957. PMC 5869486. PMID 29594056.
- ^ Batcher EL, Tang XC, Singh BN, Singh SN, Reda DJ, Hershman JM (October 2007). "Thyroid function abnormalities during amiodarone therapy for persistent atrial fibrillation". The American Journal of Medicine. 120 (10): 880–885. doi:10.1016/j.amjmed.2007.04.022. PMID 17904459. Archived from the original on 9 August 2020. Retrieved 27 August 2020.
- ^ a b c Ylli D, Wartofsky L, Burman KD (January 2021). "Evaluation and Treatment of Amiodarone-Induced Thyroid Disorders". The Journal of Clinical Endocrinology and Metabolism. 106 (1): 226–236. doi:10.1210/clinem/dgaa686. PMID 33159436. S2CID 226275566.
- ^ Chew E, Ghosh M, McCulloch C (June 1982). "Amiodarone-induced cornea verticillata". Canadian Journal of Ophthalmology. Journal Canadien d'Ophtalmologie. 17 (3): 96–99. PMID 7116220.
- ^ Passman RS, Bennett CL, Purpura JM, Kapur R, Johnson LN, Raisch DW, et al. (May 2012). "Amiodarone-associated optic neuropathy: a critical review". The American Journal of Medicine. 125 (5): 447–453. doi:10.1016/j.amjmed.2011.09.020. PMC 3322295. PMID 22385784.
- ^ Roy FH (2012). Ocular differential diagnosis (9th ed.). Panama City, Panama: Jaypee Highlights Medical Publishers. p. 94. ISBN 978-93-5025-571-1. Archived from the original on 8 September 2017.
- ^ Flaharty KK, Chase SL, Yaghsezian HM, Rubin R (1989). "Hepatotoxicity associated with amiodarone therapy". Pharmacotherapy. 9 (1): 39–44. doi:10.1002/j.1875-9114.1989.tb04102.x. PMID 2646621. S2CID 37972060.
- ^ Singhal A, Ghosh P, Khan SA (March 2003). "Low dose amiodarone causing pseudo-alcoholic cirrhosis". Age and Ageing. 32 (2): 224–225. doi:10.1093/ageing/32.2.224. PMID 12615569.
- ^ Puli SR, Fraley MA, Puli V, Kuperman AB, Alpert MA (November 2005). "Hepatic cirrhosis caused by low-dose oral amiodarone therapy". The American Journal of the Medical Sciences. 330 (5): 257–261. doi:10.1097/00000441-200511000-00012. PMID 16284489.
- ^ Murphy RP, Canavan M (January 2020). "Skin Discoloration from Amiodarone". The New England Journal of Medicine. 382 (3): e5. doi:10.1056/NEJMicm1906774. PMID 31940702. S2CID 210333420.
- ^ a b "Amiodarone Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 15 October 2020. Retrieved 8 December 2021.
- ^ Fraser AG, McQueen IN, Watt AH, Stephens MR (June 1985). "Peripheral neuropathy during long-term high-dose amiodarone therapy". Journal of Neurology, Neurosurgery, and Psychiatry. 48 (6): 576–578. doi:10.1136/jnnp.48.6.576. PMC 1028375. PMID 2989436.
- ^ Thomas A, Woodard C, Rovner ES, Wein AJ (February 2003). "Urologic complications of neurologic medications". The Urologic Clinics of North America. 30 (1): 123–131. doi:10.1016/S0094-0143(02)00111-8. PMID 12580564.
- ^ Bembo SA, Carlson HE (June 2004). "Gynecomastia: its features, and when and how to treat it" (PDF). Cleveland Clinic Journal of Medicine. 71 (6): 511–7. doi:10.3949/ccjm.71.6.511 (inactive 24 November 2024). PMID 15242307. Archived from the original (PDF) on 9 July 2009.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ a b Su VY, Hu YW, Chou KT, Ou SM, Lee YC, Lin EY, et al. (May 2013). "Amiodarone and the risk of cancer: a nationwide population-based study". Cancer. 119 (9): 1699–1705. doi:10.1002/cncr.27881. PMID 23568847. S2CID 24144312.
- ^ Lesko LJ (August 1989). "Pharmacokinetic drug interactions with amiodarone". Clinical Pharmacokinetics. 17 (2): 130–140. doi:10.2165/00003088-198917020-00005. PMID 2673606.
- ^ Marcus FI (October 1983). "Drug interactions with amiodarone". American Heart Journal. 106 (4 Pt 2): 924–930. doi:10.1016/0002-8703(83)90017-0. PMID 6137140.
- ^ a b c d e f g h i j k l Brunton LL, Knollmann BC (1 November 2022). Goodman & Gilman's the Pharmacological Basis of Therapeutics. McGraw Hill. ISBN 978-1264258079.
- ^ Tisdale JE, Chung MK, Campbell KB, Hammadah M, Joglar JA, Leclerc J, et al. (October 2020). "Drug-Induced Arrhythmias: A Scientific Statement From the American Heart Association". Circulation. 142 (15): e214 – e233. doi:10.1161/CIR.0000000000000905. PMID 32929996.
- ^ a b c Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, et al. (February 2012). "Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines". Chest. 141 (2 Suppl): e152S–e184S. doi:10.1378/chest.11-2295. PMC 3278055. PMID 22315259.
- ^ "UpToDate: Warfarin Drug Information". Archived from the original on 10 September 2024. Retrieved 17 November 2024.
- ^ a b "Amiodarone hydrochloride (Marketed as Cordarone and Pacerone) Information". FDA. 18 June 2019. Archived from the original on 7 October 2024. Retrieved 17 November 2024.
- ^ a b c "Cordarone (amiodarone HCl) tables" (PDF). FDA. 2010. Archived (PDF) from the original on 3 August 2023. Retrieved 22 March 2024.
- ^ John E. Murphy (27 August 2021). Clinical Pharmacokinetics. American Society of Health-System Pharmacists. ISBN 978-1585287000.
- ^ Hansten PD, Horn JR (12 April 2014). Drug Interaction Analysis and Management 2014. Wolters Kluwer Health. ISBN 978-1574393644.
- ^ West S (21 March 2015). "Gilead Warns After Hepatitis Patient on Heart Drug Dies". Bloomberg. Archived from the original on 22 March 2017.
- ^ "Amiodarone | Deranged Physiology". Archived from the original on 5 December 2023. Retrieved 22 March 2024.
- ^ Lesko L (1989). "Pharmacokinetic Drug Interactions with Amiodarone". Clinical Pharmacokinetics. 17 (2): 130–140. doi:10.2165/00003088-198917020-00005. PMID 2673606.
- ^ Bressler R (2006). "Grapefruit juice and drug interactions. Exploring mechanisms of this interaction and potential toxicity for certain drugs". Geriatrics. 61 (11): 12–18. PMID 17112309.
- ^ "Information on Simvastatin/Amiodarone". Food and Drug Administration. Archived from the original on 21 September 2008. Retrieved 21 September 2008.
- ^ Palleria C, Di Paolo A, Giofrè C, Caglioti C, Leuzzi G, Siniscalchi A, et al. (July 2013). "Pharmacokinetic drug-drug interaction and their implication in clinical management". Journal of Research in Medical Sciences. 18 (7): 601–610. PMC 3897029. PMID 24516494.
- ^ VandenBrink BM, Isoherranen N (January 2010). "The role of metabolites in predicting drug-drug interactions: focus on irreversible cytochrome P450 inhibition". Current Opinion in Drug Discovery & Development. 13 (1): 66–77. PMC 2898504. PMID 20047147.
- ^ a b Latini R, Tognoni G, Kates RE (April 1984). "Clinical pharmacokinetics of amiodarone". Clinical Pharmacokinetics. 9 (2): 136–156. doi:10.2165/00003088-198409020-00002. PMID 6370540.
- ^ Haverkamp W, Israel C, Parwani A (September 2017). "[Clinical aspects of treatment with amiodarone]". Herzschrittmacherther Elektrophysiol (in German). 28 (3): 307–316. doi:10.1007/s00399-017-0516-0. PMID 28643175.
- ^ Di L, Balesano A, Jordan S, Shi SM (January 2021). "The Role of Alcohol Dehydrogenase in Drug Metabolism: Beyond Ethanol Oxidation". AAPS J. 23 (1): 20. doi:10.1208/s12248-020-00536-y. PMID 33415501.
- ^ Shleghm MR, Mircioiu C, Voicu VA, Mircioiu I, Anuta V (2020). "Estimation of the In Vivo Release of Amiodarone From the Pharmacokinetics of Its Active Metabolite and Correlation With Its In Vitro Release". Front Pharmacol. 11: 621667. doi:10.3389/fphar.2020.621667. PMC 7917713. PMID 33658939.
- ^ "Amiodarone". Drugbank. Archived from the original on 23 May 2019. Retrieved 28 May 2019.
- ^ a b Harris L, Williams RR, eds. (1986). Amiodarone: pharmacology, pharmacokinetics, toxicology, clinical effects. Paris: Médecine et sciences internationales. p. 12. ISBN 978-2-86439-125-8.
- ^ "FDA Drug Label". Archived from the original on 27 March 2017.
- ^ Ghovanloo MR, Abdelsayed M, Ruben PC (2016). "Effects of Amiodarone and N-desethylamiodarone on Cardiac Voltage-Gated Sodium Channels". Frontiers in Pharmacology. 7: 39. doi:10.3389/fphar.2016.00039. PMC 4771766. PMID 26973526.
- ^ Brunton LL, Lazo JS, Parker K, eds. (2005). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. ISBN 0-07-142280-3.[page needed]
- ^ Anrep GV, Barsoum GS, Kenawy MR, Misrahy G (October 1946). "Ammi Visnaga in the Treatment of the Anginal Syndrome". British Heart Journal. 8 (4): 171–177. doi:10.1136/hrt.8.4.171. PMC 503580. PMID 18610042.
- ^ Deltour G, Binon F, Tondeur R, Goldenberg C, Henaux F, Sion R, et al. (September 1962). "[Studies in the benzofuran series. VI. Coronary-dilating activity of alkylated and aminoalkylated derivatives of 3-benzoylbenzofuran]". Archives Internationales de Pharmacodynamie et de Therapie (in French). 139: 247–254. PMID 14026835.
- ^ Charlier R, Deltour G, Tondeur R, Binon F (September 1962). "[Studies in the benzofuran series. VII. Preliminary pharmacological study of 2-butyl-3-(3,5-diiodo-4-beta-N-diethylaminoethoxybenzoyl)-benzofuran]". Archives Internationales de Pharmacodynamie et de Therapie (in French). 139: 255–264. PMID 14020244.
- ^ a b Antiarrhythmic Agents. Books LLC. ISBN 978-1-156-39374-1.
- ^ Singh BN, Vaughan Williams EM (August 1970). "The effect of amiodarone, a new anti-anginal drug, on cardiac muscle". British Journal of Pharmacology. 39 (4): 657–667. doi:10.1111/j.1476-5381.1970.tb09891.x. PMC 1702721. PMID 5485142.
- ^ Rosenbaum MB, Chiale PA, Halpern MS, Nau GJ, Przybylski J, Levi RJ, et al. (December 1976). "Clinical efficacy of amiodarone as an antiarrhythmic agent". The American Journal of Cardiology. 38 (7): 934–944. doi:10.1016/0002-9149(76)90807-9. PMID 793369.
- ^ Rosenbaum MB, Chiale PA, Haedo A, Lázzari JO, Elizari MV (October 1983). "Ten years of experience with amiodarone". American Heart Journal. 106 (4 Pt 2): 957–964. doi:10.1016/0002-8703(83)90022-4. PMID 6613843.
- ^ "Drug Approval Package: Cordarone (Amiodarone Hydrochloride) Tablets. NDA #018972". U.S. Food and Drug Administration. Archived from the original on 21 February 2014. Retrieved 6 February 2014.
- ^ "Compound summary for CID 2157". pubchem.ncbi.nil.nih.gov. Archived from the original on 24 March 2016.
- Diethylamino compounds
- Antiarrhythmic agents
- Diarylketones
- Chemical substances for emergency medicine
- CYP2D6 inhibitors
- CYP3A4 inhibitors
- Hepatotoxins
- HERG blocker
- Iodobenzene derivatives
- Piceol ethers
- 2-Phenoxyethanamines
- Potassium channel blockers
- VMAT inhibitors
- World Health Organization essential medicines
- Butyl compounds
- Benzofurans