Teduglutide: Difference between revisions
Wouterstomp (talk | contribs) new page |
add CA |
||
| (36 intermediate revisions by 28 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound}} |
|||
'''Teduglutide''' (brand names '''Gattex''' and '''Revestive''') is a [[glucagon-like peptide-2]] analog that is used for the treatment of [[short bowel syndrome]]. It works by promoting [[mucosa]]l growth and possibly restoring [[gastric emptying]] and secretion. In Europe it is marketed under the brand Revestive by [[Nycomed]]. It was approved by the United States under the name Gattex on December 21, 2012. |
|||
{{Use dmy dates|date=March 2024}} |
|||
{{cs1 config |name-list-style=vanc |display-authors=6}} |
|||
{{Infobox drug |
|||
| Watchedfields = changed |
|||
| verifiedrevid = 443663022 |
|||
| image = teduglutide.png |
|||
| alt = |
|||
<!-- Clinical data --> |
|||
{{pharmacology-stub}} |
|||
| pronounce = |
|||
| tradename = Revestive, Gattex |
|||
| Drugs.com = {{drugs.com|monograph|teduglutide}} |
|||
| MedlinePlus = |
|||
| DailyMedID = Teduglutide |
|||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|||
| pregnancy_AU_comment = |
|||
| pregnancy_category = |
|||
| routes_of_administration = [[Subcutaneous injection]] |
|||
| class = |
|||
| ATC_prefix = A16 |
|||
| ATC_suffix = AX08 |
|||
| ATC_supplemental = |
|||
<!-- Legal status --> |
|||
| legal_AU = S4 |
|||
| legal_AU_comment = <ref>{{cite web | title=Prescription medicines: registration of new chemical entities in Australia, 2017 | website=Therapeutic Goods Administration (TGA) | date=21 June 2022 | url=https://www.tga.gov.au/resources/publication/publications/prescription-medicines-registration-new-chemical-entities-australia-2017 | access-date=9 April 2023}}</ref><ref>{{cite web | title=Prescription medicines and biologicals: TGA annual summary 2017 | website=Therapeutic Goods Administration (TGA) | date=21 June 2022 | url=https://www.tga.gov.au/resources/publication/publications/prescription-medicines-and-biologicals-tga-annual-summary-2017 | access-date=31 March 2024}}</ref> |
|||
| legal_BR = <!-- OTC, A1, A2, A3, B1, B2, C1, C2, C3, C4, C5, D1, D2, E, F --> |
|||
| legal_BR_comment = |
|||
| legal_CA = Rx-only |
|||
| legal_CA_comment = <ref>{{cite web | title=Health Canada New Drug Authorizations: 2015 Highlights | website=[[Health Canada]] | date=4 May 2016 | url=https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/health-canada-new-drug-authorizations-2015-highlights.html | access-date=7 April 2024}}</ref> |
|||
| legal_DE = <!-- Anlage I, II, III or Unscheduled --> |
|||
| legal_DE_comment = |
|||
| legal_NZ = <!-- Class A, B, C --> |
|||
| legal_NZ_comment = |
|||
| legal_UK = <!-- GSL, P, POM, CD, CD Lic, CD POM, CD No Reg POM, CD (Benz) POM, CD (Anab) POM or CD Inv POM / Class A, B, C --> |
|||
| legal_UK_comment = |
|||
| legal_US = Rx-only |
|||
| legal_US_comment = <ref name="Gattex FDA label" /> |
|||
| legal_EU = Rx-only |
|||
| legal_EU_comment = <ref name="Revestive EPAR" /><ref name="Revestive EU PI" /> |
|||
| legal_UN = <!-- N I, II, III, IV / P I, II, III, IV --> |
|||
| legal_UN_comment = |
|||
| legal_status = <!-- For countries not listed above --> |
|||
<!-- Pharmacokinetic data --> |
|||
| bioavailability = 88% |
|||
| protein_bound = |
|||
| metabolism = [[Proteolysis]] |
|||
| metabolites = |
|||
| onset = |
|||
| elimination_half-life = 2h |
|||
| duration_of_action = |
|||
| excretion = |
|||
<!-- Identifiers --> |
|||
| CAS_number = 197922-42-2 |
|||
| CAS_supplemental = |
|||
| PubChem = 16139605 |
|||
| IUPHAR_ligand = 7049 |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| DrugBank = |
|||
| ChemSpiderID = 17296109 |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 7M19191IKG |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
| KEGG = D06053 |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| ChEBI = 72305 |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
| ChEMBL = |
|||
| NIAID_ChemDB = |
|||
| PDB_ligand = |
|||
| synonyms = |
|||
<!-- Chemical and physical data --> |
|||
| IUPAC_name = L-histidylglycyl-L-α-aspartylglycyl-L-seryl-L-phenylalanyl-L-seryl-L-α-aspartyl-L-α-glutamyl-L-methionyl-L-asparaginyl-L-threonyl-L-isoleucyl-L-leucyl-L-α-aspartyl-L-asparaginyl-L-leucyl-L-alanyl-L-alanyl-L-arginyl-L-α-aspartyl-L-phenylalanyl-L-isoleucyl-L-asparaginyl-L-tryptophyl-L-leucyl-L-isoleucyl-L-glutaminyl-L-threonyl-L-lysyl-L-isoleucyl-L-threonyl-L-aspartic acid |
|||
| C=164 | H=252 | N=44 | O=55 |S=1 |
|||
| SMILES = CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)[C@@H](C)CC)[C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/C164H252N44O55S/c1-21-77(11)126(156(255)187-95(44-46-114(167)214)141(240)206-130(83(17)211)160(259)186-93(42-33-34-49-165)140(239)202-129(80(14)24-4)159(258)208-131(84(18)212)161(260)200-111(163(262)263)66-125(230)231)203-151(250)100(54-76(9)10)189-145(244)103(57-88-67-175-92-41-32-31-40-90(88)92)192-147(246)105(60-116(169)216)199-157(256)127(78(12)22-2)204-152(251)102(56-87-38-29-26-30-39-87)190-149(248)109(64-123(226)227)195-137(236)94(43-35-50-174-164(171)172)183-134(233)82(16)179-133(232)81(15)180-142(241)98(52-74(5)6)188-146(245)104(59-115(168)215)194-150(249)110(65-124(228)229)196-143(242)99(53-75(7)8)198-158(257)128(79(13)23-3)205-162(261)132(85(19)213)207-153(252)106(61-117(170)217)193-139(238)97(48-51-264-20)185-138(237)96(45-47-120(220)221)184-148(247)108(63-122(224)225)197-155(254)113(72-210)201-144(243)101(55-86-36-27-25-28-37-86)191-154(253)112(71-209)182-119(219)70-177-136(235)107(62-121(222)223)181-118(218)69-176-135(234)91(166)58-89-68-173-73-178-89/h25-32,36-41,67-68,73-85,91,93-113,126-132,175,209-213H,21-24,33-35,42-66,69-72,165-166H2,1-20H3,(H2,167,214)(H2,168,215)(H2,169,216)(H2,170,217)(H,173,178)(H,176,234)(H,177,235)(H,179,232)(H,180,241)(H,181,218)(H,182,219)(H,183,233)(H,184,247)(H,185,237)(H,186,259)(H,187,255)(H,188,245)(H,189,244)(H,190,248)(H,191,253)(H,192,246)(H,193,238)(H,194,249)(H,195,236)(H,196,242)(H,197,254)(H,198,257)(H,199,256)(H,200,260)(H,201,243)(H,202,239)(H,203,250)(H,204,251)(H,205,261)(H,206,240)(H,207,252)(H,208,258)(H,220,221)(H,222,223)(H,224,225)(H,226,227)(H,228,229)(H,230,231)(H,262,263)(H4,171,172,174)/t77-,78-,79-,80-,81-,82-,83+,84+,85+,91-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,126-,127-,128-,129-,130-,131-,132-/m0/s1 |
|||
| StdInChI_comment = |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = CILIXQOJUNDIDU-ASQIGDHWSA-N |
|||
| density = |
|||
| density_notes = |
|||
| melting_point = |
|||
| melting_high = |
|||
| melting_notes = |
|||
| boiling_point = |
|||
| boiling_notes = |
|||
| solubility = |
|||
| sol_units = |
|||
| specific_rotation = |
|||
}} |
|||
'''Teduglutide''', sold under the brand names '''Revestive''' (EU) and '''Gattex''' (US), is a 33-membered poly[[peptide]] and [[glucagon-like peptide-2]] (GLP-2) analog that is used for the treatment of [[short bowel syndrome]]. It works by promoting [[mucosa]]l growth and possibly restoring [[gastric emptying]] and secretion.<ref name="pmid22570676">{{cite journal | vauthors = Jeppesen PB | title = Teduglutide, a novel glucagon-like peptide 2 analog, in the treatment of patients with short bowel syndrome | journal = Therapeutic Advances in Gastroenterology | volume = 5 | issue = 3 | pages = 159–71 | date = May 2012 | pmid = 22570676 | pmc = 3342570 | doi = 10.1177/1756283X11436318 }}</ref> It was approved in both the European Union<ref name="Revestive EPAR">{{cite web | title=Revestive EPAR | website=[[European Medicines Agency]] (EMA) | date=11 December 2001 | url=https://www.ema.europa.eu/en/medicines/human/EPAR/revestive | access-date=20 March 2024}}</ref> and the United States in 2012.<ref name="Gattex FDA label">{{cite web | title=Gattex- teduglutide injection, powder, lyophilized, for solution; Gattex- teduglutide kit | website=DailyMed | date=28 February 2024 | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=66b69c1e-b25c-44d3-b5ff-1c1de9a516fa | access-date=20 March 2024}}</ref> |
|||
==Medical uses== |
|||
Up to a certain point, the gut can adapt to partial [[Bowel resection|resection]]s that result in short bowel syndrome. Still, [[parenteral]] substitution of water, minerals and vitamins (depending on which part of the gut has been removed) is often necessary. Teduglutide may reduce or shorten the necessity of such infusions by improving the [[intestinal mucosa]] and possibly by other mechanisms.<ref name="Klement">{{cite journal| vauthors = Klement A | date = 5 January 2015| title = Das Kurzdarmsyndrom ist erstmals behandelbar: Revestive| journal = Österreichische Apothekerzeitung| issue = 1/2015| page = 20f| language = German}}</ref> |
|||
==Adverse effects== |
|||
Common adverse effects in clinical studies included abdominal discomfort (49% of patients), [[respiratory infection]]s (28%), nausea (27%) and vomiting (14%), local reactions at the injection site (21%), and headache (17%).<ref name="Klement" /> |
|||
==Chemistry and mechanism of action== |
|||
Teduglutide differs from natural GLP-2 by a single [[amino acid]]: an [[alanine]] is replaced with a [[glycine]]. This blocks breaking down of the molecule by [[dipeptidyl peptidase]] and increases its half-life from seven minutes (GLP-2) to about two hours, while retaining its biological actions. These include maintenance of the intestinal mucosa, increasing intestinal blood flow, reducing [[gastrointestinal motility]] and [[gastrointestinal secretion|secretion]] of gastric acid.<ref name="Klement" /> |
|||
== Society and culture == |
|||
=== Legal status === |
|||
It was approved in both the European Union (brand name Revestive)<ref name="Revestive EPAR" /><ref name="Revestive EU PI">{{cite web | title=Revestive Product information | website=Union Register of medicinal products | date=4 September 2012 | url=https://ec.europa.eu/health/documents/community-register/html/h787.htm | access-date=20 March 2024}}</ref> and the United States (brand name Gattex) in 2012.<ref name="Gattex FDA label" /> It was granted [[orphan drug]] designation by the [[European Medicines Agency]] (EMA).<ref name="Revestive EPAR" /><ref name="Revestive EU PI" /> |
|||
== References == |
|||
{{reflist}} |
|||
{{Other alimentary tract and metabolism products}} |
|||
{{Portal bar | Medicine}} |
|||
{{Authority control}} |
|||
[[Category:Drugs acting on the gastrointestinal system and metabolism]] |
|||
[[Category:Orphan drugs]] |
|||
[[Category:Peptides]] |
|||
[[Category:Drugs developed by Takeda Pharmaceutical Company]] |
|||
Latest revision as of 06:30, 7 April 2024
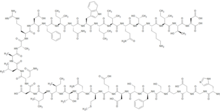 | |
| Clinical data | |
|---|---|
| Trade names | Revestive, Gattex |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | Subcutaneous injection |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 88% |
| Metabolism | Proteolysis |
| Elimination half-life | 2h |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C164H252N44O55S |
| Molar mass | 3752.13 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Teduglutide, sold under the brand names Revestive (EU) and Gattex (US), is a 33-membered polypeptide and glucagon-like peptide-2 (GLP-2) analog that is used for the treatment of short bowel syndrome. It works by promoting mucosal growth and possibly restoring gastric emptying and secretion.[7] It was approved in both the European Union[5] and the United States in 2012.[4]
Medical uses
[edit]Up to a certain point, the gut can adapt to partial resections that result in short bowel syndrome. Still, parenteral substitution of water, minerals and vitamins (depending on which part of the gut has been removed) is often necessary. Teduglutide may reduce or shorten the necessity of such infusions by improving the intestinal mucosa and possibly by other mechanisms.[8]
Adverse effects
[edit]Common adverse effects in clinical studies included abdominal discomfort (49% of patients), respiratory infections (28%), nausea (27%) and vomiting (14%), local reactions at the injection site (21%), and headache (17%).[8]
Chemistry and mechanism of action
[edit]Teduglutide differs from natural GLP-2 by a single amino acid: an alanine is replaced with a glycine. This blocks breaking down of the molecule by dipeptidyl peptidase and increases its half-life from seven minutes (GLP-2) to about two hours, while retaining its biological actions. These include maintenance of the intestinal mucosa, increasing intestinal blood flow, reducing gastrointestinal motility and secretion of gastric acid.[8]
Society and culture
[edit]Legal status
[edit]It was approved in both the European Union (brand name Revestive)[5][6] and the United States (brand name Gattex) in 2012.[4] It was granted orphan drug designation by the European Medicines Agency (EMA).[5][6]
References
[edit]- ^ "Prescription medicines: registration of new chemical entities in Australia, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 9 April 2023.
- ^ "Prescription medicines and biologicals: TGA annual summary 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 31 March 2024.
- ^ "Health Canada New Drug Authorizations: 2015 Highlights". Health Canada. 4 May 2016. Retrieved 7 April 2024.
- ^ a b c "Gattex- teduglutide injection, powder, lyophilized, for solution; Gattex- teduglutide kit". DailyMed. 28 February 2024. Retrieved 20 March 2024.
- ^ a b c d "Revestive EPAR". European Medicines Agency (EMA). 11 December 2001. Retrieved 20 March 2024.
- ^ a b c "Revestive Product information". Union Register of medicinal products. 4 September 2012. Retrieved 20 March 2024.
- ^ Jeppesen PB (May 2012). "Teduglutide, a novel glucagon-like peptide 2 analog, in the treatment of patients with short bowel syndrome". Therapeutic Advances in Gastroenterology. 5 (3): 159–71. doi:10.1177/1756283X11436318. PMC 3342570. PMID 22570676.
- ^ a b c Klement A (5 January 2015). "Das Kurzdarmsyndrom ist erstmals behandelbar: Revestive". Österreichische Apothekerzeitung (in German) (1/2015): 20f.
