Bismuth: Difference between revisions
m Reverted edits by 76.205.51.12 (talk) to last version by O.Koslowski |
Citation bot (talk | contribs) Added bibcode. | Use this bot. Report bugs. | Suggested by Abductive | #UCB_toolbar |
||
| (938 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical element with atomic number 83}} |
|||
{{About|the chemical element|other uses}} |
|||
{{Use dmy dates|date=January 2015}} |
|||
{{Good article}} |
|||
{{infobox bismuth}} |
{{infobox bismuth}} |
||
'''Bismuth''' is a [[chemical element]] with symbol |
'''Bismuth''' is a [[chemical element]] with the [[Symbol (chemistry)|symbol]] '''Bi''' and [[atomic number]] 83. It is a [[post-transition metal]] and one of the [[pnictogen]]s, with chemical properties resembling its lighter [[group 15]] siblings [[arsenic]] and [[antimony]]. Elemental bismuth occurs naturally, and its [[sulfide]] and [[oxide]] forms are important commercial [[ore]]s. The [[free element]] is 86% as dense as lead. It is a brittle metal with a silvery-white color when freshly produced. [[Passivation (chemistry)|Surface oxidation]] generally gives samples of the metal a somewhat rosy cast. Further oxidation under heat can give bismuth a vividly [[Iridescence|iridescent]] appearance due to [[thin-film interference]]. Bismuth is both the most [[Diamagnetism|diamagnetic]] element and one of the least [[Thermal conductivity|thermally conductive]] metals known. |
||
Bismuth used to be considered the element with the highest atomic mass whose nuclei do not spontaneously decay. However, in 2003 it was discovered to be extremely weakly [[radioactive]]. The metal's only [[primordial isotope]], [[bismuth-209]], undergoes [[alpha decay]] with a [[half-life]] about a billion times the estimated [[age of the universe]].<ref>{{cite news| url=http://physicsworld.com/cws/article/news/2003/apr/23/bismuth-breaks-half-life-record-for-alpha-decay| title=Bismuth breaks half-life record for alpha decay| date=23 April 2003| publisher=Physicsworld| first=Belle| last= Dumé}}</ref><ref name="Kean">{{cite book|last=Kean|first=Sam|title=The Disappearing Spoon (and other true tales of madness, love, and the history of the world from the Periodic Table of Elements)|publisher=Back Bay Books |location=New York/Boston|year=2011|pages=158–160|isbn=978-0-316-051637}}</ref> |
|||
Bismuth metal has been known from ancient times, although until the 18th century it was often confused with lead and tin, which share some physical properties. The etymology is uncertain, but possibly comes from Arabic ''bi ismid'', meaning having the properties of antimony<ref>[http://webmineral.com/data/Bismuth.shtml Bismuth]. WebMineral. Retrieved on 17 December 2011.</ref> or German words ''weisse masse'' or ''wismuth'' ("white mass"), translated in the mid sixteenth century to [[New Latin]] ''bisemutum''.<ref name=oet/> |
|||
Bismuth metal has been known since ancient times. Before modern analytical methods bismuth's metallurgical similarities to lead and [[tin]] often led it to be confused with those metals. The etymology of "bismuth" is uncertain. The name may come from mid-sixteenth century [[Neo-Latin]] translations of the German words {{lang|de|weiße Masse}} or {{lang|de|Wismuth}}, meaning 'white mass', which were rendered as {{lang|la|bisemutum}} or {{lang|la|bisemutium}}. |
|||
Bismuth has long been considered as the highest-atomic-mass element that is stable. However, it was recently discovered to be slightly radioactive: its only [[primordial isotope]] [[bismuth-209]] [[alpha decay]]s with a [[half life]] more than a [[1000000000 (number)|billion]] times the estimated [[age of the universe]].<ref>{{cite news| url=http://physicsworld.com/cws/article/news/2003/apr/23/bismuth-breaks-half-life-record-for-alpha-decay| title=Bismuth breaks half-life record for alpha decay| date=3 September 2012| publisher=Physicsworld| first=Belle| last= Dumé}}</ref> |
|||
Bismuth compounds account for about half the production of bismuth. They are used in cosmetics |
Bismuth compounds account for about half the global production of bismuth. They are used in cosmetics; [[pigment]]s; and a few pharmaceuticals, notably [[bismuth subsalicylate]], used to treat [[diarrhea]].<ref name="Kean" /> Bismuth's unusual propensity to expand as it solidifies is responsible for some of its uses, as in the casting of printing type.<ref name="Kean" /> Bismuth, when in its elemental form, has unusually low [[toxicity]] for a [[heavy metals|heavy metal]].<ref name="Kean" /> As the [[Lead poisoning|toxicity of lead]] and the cost of its [[environmental remediation]] became more apparent during the 20th century, suitable bismuth [[alloy]]s have gained popularity as replacements for lead. Presently, around a third of global bismuth production is dedicated to needs formerly met by lead. |
||
==History== |
== History and etymology== |
||
Bismuth metal has been known since ancient times and it was one of the first 10 metals to have been discovered. The name ''bismuth'' dates to around 1665 and is of uncertain etymology. The name possibly comes from obsolete German ''{{lang|de|Bismuth}}'', ''{{lang|de|Wismut}}'', ''{{lang|de|Wissmuth}}'' (early 16th century), perhaps related to [[Old High German]] ''{{lang|goh|hwiz}}'' ("white").<ref name="oet">{{OEtymD|bismuth}}</ref> The [[Neo-Latin]] ''{{lang|la|bisemutium}}'' (coined by [[Georgius Agricola]], who Latinized many German mining and technical words) is from the German ''{{lang|de|Wismuth}}'', itself perhaps from ''{{lang|de|weiße Masse}}'', meaning "white mass".<ref>[https://oxfordindex.oup.com/view/10.1093/acref/9780192830982.013.1517 ''Bismuth''] {{Webarchive|url=https://web.archive.org/web/20190828000111/https://oxfordindex.oup.com/view/10.1093/acref/9780192830982.013.1517 |date=28 August 2019 }}, The Concise Oxford Dictionary of English Etymology</ref><ref name="Norman">{{cite book |last=Norman |first=Nicholas C. |date=1998 |title=Chemistry of Arsenic, Antimony, and Bismuth |page=41 |publisher=Springer |isbn=978-0-7514-0389-3 |url=https://books.google.com/books?id=vVhpurkfeN4C&pg=PA41}}</ref> |
|||
The name ''bismuth'' is from ca. 1660s, and is of uncertain etymology. It is one of the first 10 metals to have been discovered. Bismuth appears in the 1660s, from obsolete [[German language|German]] ''Bismuth'', ''Wismut'', ''Wissmuth'' (early 16th century); perhaps related to [[Old High German]] ''hwiz'' ("white").<ref name=oet>{{OEtymD|bismuth}}</ref> The [[New Latin]] ''bisemutum'' (due to [[Georgius Agricola]], who Latinized many German mining and technical words) is from the German ''Wismuth'', perhaps from ''weiße Masse'', "white mass."<ref>{{cite book| url = http://books.google.com/books?id=vVhpurkfeN4C&pg=PA41| page = 41| title = Chemistry of arsenic, antimony, and bismuth| isbn = 978-0-7514-0389-3| author1 = Norman| first1 = Nicholas C.| year = 1998}}</ref> The element was confused in early times with [[tin]] and lead because of its resemblance to those elements. Bismuth has been known since ancient times, so no one person is credited with its discovery. [[Georgius Agricola|Agricola]], in [[De Natura Fossilium]] (ca. 1546) states that bismuth is a distinct metal in a family of metals including tin and lead. This was based on observation of the metals and their physical properties.<ref>{{cite book| author = Agricola, Georgious| title = De Natura Fossilium| location = New York| publisher = Mineralogical Society of America| year = 1546 (orig.); 1955 (trans)| page = 178}}</ref><!--http://books.google.com/books?id=9pxPAAAAcAAJ&pg=PA143--> Miners in the age of alchemy also gave bismuth the name ''tectum argenti,'' or "silver being made," in the sense of silver still in the process of being formed within the Earth.<ref>{{cite book| url = http://books.google.com/books?id=GL5PAAAAMAAJ&pg=PT181| page = 181| chapter = Bismuth| title = American edition of the British encyclopedia: Or, Dictionary of Arts and sciences ; comprising an accurate and popular view of the present improved state of human knowledge| author1 = Nicholson| first1 = William| year = 1819}}</ref><ref name="Weeks">{{cite journal|doi = 10.1021/ed009p11|title = The discovery of the elements. II. Elements known to the alchemists|year = 1932|last1 = Weeks|first1 = Mary Elvira|journal = Journal of Chemical Education|volume = 9|pages = 11|bibcode = 1932JChEd...9...11W}}</ref><ref>Giunta, Carmen J. [http://web.lemoyne.edu/~giunta/archems.html Glossary of Archaic Chemical Terms], [[Le Moyne College]]. See also for other terms for bismuth, including ''stannum glaciale'' (glacial tin or ice-tin).</ref> |
|||
The element was confused in early times with tin and lead because of its resemblance to those elements. Because bismuth has been known since ancient times, no one person is credited with its discovery. [[Georgius Agricola|Agricola]] (1546) states that bismuth is a distinct metal in a family of metals including tin and lead. This was based on observation of the metals and their physical properties.<ref>{{cite book |last=Agricola |first=Georgious |orig-year=1546 |date=1955 |title=De Natura Fossilium |location=New York |publisher=Mineralogical Society of America |page=178 |url=http://farlang.com/books/agricola-bandy-de-natura-fossilium |access-date=8 April 2019 |archive-date=14 May 2021 |archive-url=https://web.archive.org/web/20210514044323/http://farlang.com/books/agricola-bandy-de-natura-fossilium |url-status=dead }} <!-- https://books.google.com/books?id=9pxPAAAAcAAJ&pg=PA143 --></ref> |
|||
Beginning with [[Johann Heinrich Pott]] in 1738,<ref>{{cite book|url = http://books.google.com/books?id=eQVAAAAAcAAJ&pg=RA1-PA134| page=134| chapter = De Wismutho|title = Exercitationes chymicae|publisher=Berolini: Apud Johannem Andream Rüdigerum|author1 = Pott|first1 = Johann Heinrich|year = 1738}}</ref> [[Carl Wilhelm Scheele]] and [[Torbern Olof Bergman]] the distinctness of lead and bismuth became clear and [[Claude François Geoffroy]] demonstrated in 1753 that this metal is distinct from lead and tin.<ref name="Weeks"/><ref name=CRC/><ref>{{cite journal|url = http://gallica.bnf.fr/ark:/12148/bpt6k3551g/f197.image.r=royal.langEN|title = Sur Bismuth|page = 190|year = 1753|journal = Histoire de l'Académie royale des sciences ... avec les mémoires de mathématique & de physique ... tirez des registres de cette Académie|author = Geoffroy}}</ref> |
|||
<!--"Artificial bismuth" was commonly used in place of the actual metal. It was made by hammering tin into thin plates, and cementing them by a mixture of white tartar, [[Potassium nitrate|saltpeter]], and [[arsenic]], stratified in a [[crucible]] over an open fire.{{Citation needed|date=May 2009}}--> |
|||
Bismuth was also known to the [[Incas]] and used (along with the usual copper and tin) in a special [[Bismuth bronze|bronze alloy]] for knives.<ref>{{cite journal|jstor = 1692247|journal = Science|volume = 223|issue = 4636|pages = 585–586 |last1 = Gordon| first1= Robert B.|last2 = Rutledge| first2= John W.|title = Bismuth Bronze from Machu Picchu, Peru|doi = 10.1126/science.223.4636.585|pmid = 17749940|year = 1984|bibcode = 1984Sci...223..585G}}</ref> |
|||
Miners in the age of alchemy also gave bismuth the name ''{{lang|la|tectum argenti}},'' or "silver being made" in the sense of silver still in the process of being formed within the Earth.<ref>{{cite book |last=Nicholson |first=William |date=1819 |chapter=Bismuth |title=American edition of the British encyclopedia: Or, Dictionary of Arts and sciences; comprising an accurate and popular view of the present improved state of human knowledge |page=181 |chapter-url=https://books.google.com/books?id=GL5PAAAAMAAJ&pg=PT181}}</ref><ref name="Weeks">{{cite journal |last=Weeks |first=Mary Elvira |author-link=Mary Elvira Weeks |date=1932 |title=The discovery of the elements. II. Elements known to the alchemists |journal=Journal of Chemical Education |volume=9 |issue=1 |page=11 |doi=10.1021/ed009p11 |bibcode=1932JChEd...9...11W}}</ref><ref>{{cite web |last=Giunta |first=Carmen J. |url=http://web.lemoyne.edu/~giunta/archems.html |title=Glossary of Archaic Chemical Terms |publisher=[[Le Moyne College]]}} See also for other terms for bismuth, including ''stannum glaciale'' (glacial tin or ice-tin).</ref> |
|||
==Characteristics== |
|||
[[File:Bi-crystal.jpg|thumb|left|upright|Bismuth crystal illustrating the many iridescent refraction hues of its oxide surface]] |
|||
[[File:Wismut Kristall und 1cm3 Wuerfel.jpg|thumb|left|Artificially grown bismuth crystal illustrating the stair-step crystal structure, with a 1-cm cube of bismuth metal]] |
|||
Bismuth was also known to the [[Incas]] and used (along with the usual copper and tin) in a special [[Bismuth bronze|bronze alloy]] for knives.<ref>{{cite journal |last1 = Gordon | first1= Robert B. |last2 = Rutledge | first2= John W. |date = 1984 |title = Bismuth Bronze from Machu Picchu, Peru |journal = Science |volume = 223 |issue = 4636 |pages = 585–586 |doi = 10.1126/science.223.4636.585 |pmid = 17749940 |bibcode = 1984Sci...223..585G |s2cid = 206572055 |jstor = 1692247}}</ref> |
|||
===Physical characteristics=== |
|||
Bismuth is a brittle metal with a white, silver-pink hue, often occurring in its native form, with an [[Iridescence|iridescent]] [[Bismuth(III) oxide|oxide]] tarnish showing many colors from yellow to blue. The spiral, stair-stepped structure of bismuth crystals is the result of a higher growth rate around the outside edges than on the inside edges. The variations in the thickness of the oxide layer that forms on the surface of the crystal causes different wavelengths of light to interfere upon reflection, thus displaying a rainbow of colors. When [[combustion|burned]] in [[oxygen]], bismuth burns with a blue [[flame]] and [[bismuth oxide|its oxide]] forms yellow [[Vapor|fumes]].<ref name=CRC/> Its [[toxicity]] is much lower than that of its neighbors in the [[periodic table]], such as lead, [[antimony]], and [[polonium]]. |
|||
[[File:Bismuth symbol.svg|thumb|upright=0.5|right|[[Alchemical symbol]] used by [[Torbern Bergman]] (1775)]] |
|||
No other metal is verified to be more naturally [[Diamagnetism|diamagnetic]] than bismuth.<ref name=CRC/><ref>Krüger, p. 171.</ref> ([[Superdiamagnetism]] is a different physical phenomenon.) Of any metal, it has one of the lowest values of [[thermal conductivity]] (after [[manganese]], and maybe [[neptunium]] and [[plutonium]]) and the highest [[Hall effect|Hall coefficient]].<ref>{{cite journal| doi = 10.1098/rspa.1936.0126 |jstor = 96773| title = The Theory of the Galvomagnetic Effects in Bismuth| year = 1936| last1 = Jones| first1 = H.| journal = Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences| volume = 155| issue = 886| page = 653|bibcode = 1936RSPSA.155..653J}}</ref> It has a high [[electrical resistance]].<ref name=CRC>{{cite book| first= C. R.|last= Hammond|page=4-1<!-- not a range --> |title = The Elements, in Handbook of Chemistry and Physics 81st edition| location = Boca Raton (FL, US)|publisher =CRC press| isbn = 0-8493-0485-7| year = 2004}}</ref> When deposited in sufficiently thin layers on a substrate, bismuth is a [[semiconductor]], rather than a [[poor metal]].<ref>{{cite journal| title = Semimetal-to-semiconductor transition in bismuth thin films||journal = Phys. Rev. B|volume =48|page =11431|year =1993|doi =10.1103/PhysRevB.48.11431|bibcode = 1993PhRvB..4811431H| issue = 15| last1 = Hoffman| first1 = C.| last2 = Meyer| first2 = J.| last3 = Bartoli| first3 = F.| last4 = Di Venere| first4 = A.| last5 = Yi| first5 = X.| last6 = Hou| first6 = C.| last7 = Wang| first7 = H.| last8 = Ketterson| first8 = J.| last9 = Wong| first9 = G.}}</ref> |
|||
Beginning with [[Johann Heinrich Pott]] in 1738,<ref>{{cite book |last=Pott |first=Johann Heinrich |date=1738 |chapter=De Wismutho |title=Exercitationes Chymicae |page=134 |publisher=Berolini: Apud Johannem Andream Rüdigerum |chapter-url=https://books.google.com/books?id=eQVAAAAAcAAJ&pg=RA1-PA134}}</ref> [[Carl Wilhelm Scheele]], and [[Torbern Olof Bergman]], the distinctness of lead and bismuth became clear, and [[Claude François Geoffroy]] demonstrated in 1753 that this metal is distinct from lead and tin.<ref name="Weeks" /><ref name="CRC" /><ref>{{cite journal |author-link=Claude François Geoffroy |last=Geoffroy |first=C.F. |title = Sur Bismuth |page = 190 |date = 1753 |journal = Histoire de l'Académie Royale des Sciences ... Avec les Mémoires de Mathématique & de Physique ... Tirez des Registres de Cette Académie |url = http://gallica.bnf.fr/ark:/12148/bpt6k3551g/f197.image.r=royal.langEN}}</ref> |
|||
== Characteristics == |
|||
Elemental bismuth is one of very few substances of which the liquid phase is [[density|denser]] than its solid phase (water being the best-known example). Bismuth expands 3.32% on solidification; therefore, it was long a component of low-melting [[typesetting]] [[alloy]]s, where it compensated for the contraction of the other alloying components,<ref name=CRC/><ref>{{cite book| url =http://books.google.com/books?id=XD-dhveegRYC| page = 268| title =Modern physical science| isbn =978-0-03-007381-6| author1 =Tracy| first1 =George R| last2 =Tropp| first2 =Harry E| last3 =Friedl| first3 =Alfred E.| year =1974}}</ref><ref>{{cite journal| doi = 10.1039/JS8682100071| title = IX.—Freezing of water and bismuth| year = 1868| last1 = Tribe| first1 = Alfred| journal = Journal of the Chemical Society| volume = 21| page = 71}}</ref><ref>{{cite book| url =http://books.google.com/books?id=n-fiyYg3iSIC&pg=PA82|page = 82| title =The Physics of Phase Transitions| isbn =978-3-540-33390-6| author1 =Papon| first1 =Pierre| last2 =Leblond| first2 =Jacques| last3 =Meijer| first3 =Paul Herman Ernst| year =2006}}</ref> to form almost isostatic [[Lead-bismuth eutectic|bismuth-lead eutectic]] alloys. |
|||
[[File:Wismut Kristall und 1cm3 Wuerfel.jpg|thumb|left|upright=1.2|Left: A bismuth [[hopper crystal]] exhibiting the stairstep crystal structure and [[Iridescence|iridescent]] colors, which are produced by [[thin-film interference|interference]] of light within the oxide film on its surface. Right: a 1 cm<sup>3</sup> cube of unoxidised bismuth metal]] |
|||
=== Physical characteristics === |
|||
Though virtually unseen in nature, high-purity bismuth can form distinctive, colorful [[hopper crystal]]s. It is relatively nontoxic and has a low melting point just above 271 °C, so crystals may be grown using a household stove, although the resulting crystals will tend to be lower quality than lab-grown crystals.<ref>{{cite book|url=http://books.google.com/books?id=FGaIhhZ8ivsC&pg=PA2|page=2|title=The science of crystallization: microscopic interfacial phenomena|last=Tiller| first = William A.|publisher=Cambridge University Press|year=1991|isbn=0-521-38827-9}}</ref> |
|||
[[File:Bi phase diagram.png|thumb|left|upright=1.2|Pressure-temperature phase diagram of bismuth. ''T''<sub>C</sub> refers to the superconducting transition temperature]] |
|||
Bismuth is a brittle metal with a dark, silver-pink hue, often with an [[Iridescence|iridescent]] [[Bismuth(III) oxide|oxide]] tarnish showing many colors from yellow to blue. The spiral, stair-stepped structure of bismuth crystals is the result of a higher growth rate around the outside edges than on the inside edges. The variations in the thickness of the oxide layer that forms on the surface of the crystal cause different wavelengths of light to interfere upon reflection, thus displaying a rainbow of colors. When [[combustion|burned]] in [[oxygen]], bismuth burns with a blue [[flame]] and [[bismuth oxide|its oxide]] forms yellow [[Vapor|fumes]].<ref name="CRC" /> Its [[toxicity]] is much lower than that of its neighbors in the [[periodic table]], such as lead and [[antimony]].<ref name=tox/> |
|||
No other metal is verified to be more naturally [[Diamagnetism|diamagnetic]] than bismuth.<ref name="CRC" /><ref>[[#Kruger|Krüger]], p. 171.</ref> ([[Superdiamagnetism]] is a different physical phenomenon.) Of any metal, it has one of the lowest values of [[thermal conductivity]] (after [[manganese]], [[neptunium]] and [[plutonium]]) and the highest [[Hall effect|Hall coefficient]].<ref>{{cite journal| doi = 10.1098/rspa.1936.0126 |jstor = 96773| title = The Theory of the Galvomagnetic Effects in Bismuth| date = 1936| last1 = Jones| first1 = H.| journal = Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences| volume = 155| issue = 886|pages = 653–663|bibcode = 1936RSPSA.155..653J| doi-access = free}}</ref> It has a high [[Electrical resistivity and conductivity|electrical resistivity]].<ref name="CRC">{{cite book| first = C. R.| last = Hammond| pages = 4.1|url=https://archive.org/details/crchandbookofche81lide/page/4| title = The Elements, in Handbook of Chemistry and Physics| edition = 81st| location = Boca Raton (FL, US)| publisher = CRC press| isbn = 978-0-8493-0485-9| date = 2004| url-access = registration}}</ref> When deposited in sufficiently thin layers on a substrate, bismuth is a [[semiconductor]], despite being a [[post-transition metal]].<ref>{{cite journal| title = Semimetal-to-semiconductor transition in bismuth thin films|journal = Phys. Rev. B|volume =48|date =1993|doi =10.1103/PhysRevB.48.11431|pmid = 10007465|bibcode = 1993PhRvB..4811431H| issue = 15|pages = 11431–11434| last1 = Hoffman| first1 = C.| last2 = Meyer| first2 = J.| last3 = Bartoli| first3 = F.| last4 = Di Venere| first4 = A.| last5 = Yi| first5 = X.| last6 = Hou| first6 = C.| last7 = Wang| first7 = H.| last8 = Ketterson| first8 = J.| last9 = Wong| first9 = G.}}</ref> Elemental bismuth is [[density|denser]] in the liquid phase than the solid, a characteristic it shares with [[germanium]], [[silicon]], [[gallium]], and water.<ref name="w768">[[#Wiberg|Wiberg]], p. 768.</ref> Bismuth expands 3.32% on solidification; therefore, it was long a component of low-melting [[typesetting]] [[alloy]]s, where it compensated for the contraction of the other alloying components<ref name="CRC" /><ref>{{cite book| url =https://books.google.com/books?id=XD-dhveegRYC| page = 268| title =Modern physical science| isbn =978-0-03-007381-6| last1 =Tracy| first1 =George R.| last2 =Tropp| first2 =Harry E.| last3 =Friedl| first3 =Alfred E.| date =1974| publisher = Holt, Rinehart and Winston}}</ref><ref>{{cite journal| doi = 10.1039/JS8682100071| title = IX.—Freezing of water and bismuth| date = 1868| last1 = Tribe| first1 = Alfred| journal = Journal of the Chemical Society| volume = 21| page = 71| url = https://zenodo.org/record/2000757}}</ref><ref>{{cite book| url =https://books.google.com/books?id=n-fiyYg3iSIC&pg=PA82|page = 82| title =The Physics of Phase Transitions| isbn =978-3-540-33390-6| last1 =Papon| first1 =Pierre| last2 =Leblond| first2 =Jacques| last3 =Meijer| first3 =Paul Herman Ernst| date =2006| publisher=Springer }}</ref> to form almost isostatic [[Lead-bismuth eutectic|bismuth-lead eutectic]] alloys. |
|||
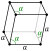
At ambient conditions bismuth crystallizes in the [[Trigonal crystal system|rhombohedral lattice]]<ref>Krüger, p. 172.</ref> ([[Pearson symbol]] hR6, [[space group]] R{{overline|3}}m No. 166), which is often classed into trigonal or hexagonal crystal systems.<ref name=str/> When compressed at room temperature, this Bi-I structure changes first to the [[monoclinic]] Bi-II at 2.55 GPa, then to the [[tetragonal]] Bi-III at 2.7 GPa, and finally to the [[body-centered cubic]] Bi-IV at 7.7 GPa. The corresponding transitions can be monitored via changes in electrical conductivity; they are rather reproducible and abrupt, and are therefore used for calibration of high-pressure equipment.<ref>{{cite book|author=Boldyreva, Elena |title=High-Pressure Crystallography: From Fundamental Phenomena to Technological Applications|url=http://books.google.com/books?id=pyN0dhHChzsC&pg=PA264|date=18 August 2010| publisher= Springer| isbn=978-90-481-9257-1|pages=264–265}}</ref><ref>{{cite book|last=Manghnani | first= Murli H.|title=Science and Technology of High Pressurs2|url=http://books.google.com/books?id=7OoZ9TN8ROQC&pg=PA1086|year=2000|publisher=Universities Press (India) Limited| isbn=978-81-7371-339-2|page=1086}}</ref> |
|||
Though virtually unseen in nature, high-purity bismuth can form distinctive, colorful [[hopper crystal]]s. It is relatively nontoxic and has a low melting point just above {{convert|271|C|F}}, so crystals may be grown using a household stove, although the resulting crystals will tend to be of lower quality than lab-grown crystals.<ref>{{cite book|url=https://books.google.com/books?id=FGaIhhZ8ivsC&pg=PA2|page=2|title=The science of crystallization: microscopic interfacial phenomena|last=Tiller| first = William A.|publisher=Cambridge University Press|date=1991|isbn=978-0-521-38827-6}}</ref> |
|||
===Chemical characteristics=== |
|||
Bismuth is stable to both dry and moist air at ordinary temperatures. When red-hot, it reacts with water to make bismuth(III) oxide.<ref name=s8/> |
|||
At ambient conditions, bismuth shares the same layered structure as the metallic forms of [[arsenic]] and [[antimony]],<ref name="w767">[[#Wiberg|Wiberg]], p. 767.</ref> crystallizing in the [[Trigonal crystal system|rhombohedral lattice]].<ref>[[#Kruger|Krüger]], p. 172.</ref> When compressed at room temperature, this Bi–I structure changes first to the [[monoclinic]] Bi-II at 2.55 GPa, then to the [[tetragonal]] Bi-III at 2.7 GPa, and finally to the [[body-centered cubic]] Bi-V at 7.7 GPa. The corresponding transitions can be monitored via changes in electrical conductivity; they are rather reproducible and abrupt and are therefore used for calibration of high-pressure equipment.<ref>{{cite book|author=Boldyreva, Elena |author-link1=Elena Boldyreva |title=High-Pressure Crystallography: From Fundamental Phenomena to Technological Applications|url=https://books.google.com/books?id=pyN0dhHChzsC&pg=PA264|date=2010| publisher= Springer| isbn=978-90-481-9257-1|pages=264–265}}</ref><ref>{{cite book|last=Manghnani | first= Murli H.|title=Science and Technology of High Pressure: Proceedings of the International Conference on High Pressure Science and Technology (AIRAPT-17) |location=Honolulu, Hawaii |date=25–30 July 1999|volume=2|url=https://books.google.com/books?id=7OoZ9TN8ROQC&pg=PA1086|publication-date=2000|publisher=Universities Press (India) | isbn=978-81-7371-339-2|page=1086}}</ref> |
|||
:2 Bi + 3 H<sub>2</sub>O → Bi<sub>2</sub>O<sub>3</sub> + 3 H<sub>2</sub> |
|||
=== Chemical characteristics === |
|||
It reacts with [[fluorine]] to make [[bismuth(V) fluoride]] at 500 °C or [[bismuth(III) fluoride]] at lower temperatures (typically from Bi melts); with other [[halogen]]s it yields only bismuth(III) halides.<ref name=w770>Wiberg, pp. 769–770.</ref><ref name=g559>Greenwood, pp. 559–561.</ref><ref name=k185/> The trihalides are corrosive and easily react with moisture, forming [[oxyhalides]] with the formula BiOX.<ref name=s9>Suzuki, p. 9.</ref> |
|||
Bismuth is stable to both dry and moist air at ordinary temperatures. When red-hot, it reacts with water to make bismuth(III) oxide.<ref name="s8" /> |
|||
: 2 Bi + 3 X<sub>2</sub> → 2 BiX<sub>3</sub> (X = F, Cl, Br, I) |
|||
: {{chem2|2 Bi + 3 H2O -> Bi2O3 + 3 H2}} |
|||
Bismuth dissolves in concentrated [[sulfuric acid]] to make [[bismuth(III) sulfate]] and [[sulfur dioxide]].<ref name=s8>Suzuki, p. 8.</ref> |
|||
It reacts with [[fluorine]] to form [[bismuth(V) fluoride]] at {{convert|500|C|F}} or [[bismuth(III) fluoride]] at lower temperatures (typically from Bi melts); with other [[halogen]]s it yields only bismuth(III) halides.<ref name="w770">[[#Wiberg|Wiberg]], pp. 769–770.</ref><ref name="g559">[[#Greenwood|Greenwood]], pp. 559–561.</ref><ref name="k185" /> The trihalides are corrosive and easily react with moisture, forming [[oxyhalides]] with the formula BiOX.<ref name="s9">[[#Suzuki|Suzuki]], p. 9.</ref> |
|||
:6 H<sub>2</sub>SO<sub>4</sub> + 2 Bi → 6 H<sub>2</sub>O + Bi<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub> + 3 SO<sub>2</sub> |
|||
: {{chem2|4 Bi + 6 X2 -> 4 BiX3}} (X = F, Cl, Br, I) |
|||
It reacts with [[nitric acid]] to make [[bismuth(III) nitrate]]. |
|||
: {{chem2|4 BiX3 + 2 O2 -> 4 BiOX + 4 X2}} |
|||
Bismuth dissolves in concentrated [[sulfuric acid]] to make bismuth(III) sulfate and [[sulfur dioxide]].<ref name="s8">[[#Suzuki|Suzuki]], p. 8.</ref> |
|||
:Bi + 6 HNO<sub>3</sub> → 3 H<sub>2</sub>O + 3 NO<sub>2</sub> + Bi(NO<sub>3</sub>)<sub>3</sub> |
|||
: {{chem2|6 H2SO4 + 2 Bi -> 6 H2O + Bi2(SO4)3 + 3 SO2}} |
|||
It also dissolves in [[hydrochloric acid]], but only with oxygen present.<ref name=s8/> |
|||
It reacts with [[nitric acid]] to make [[bismuth(III) nitrate]] (which decomposes into [[nitrogen dioxide]] when heated<ref name = "Ollevier">{{cite book |last1=Krabbe |first1=S.W. |last2=Mohan |first2=R.S. |editor-first=Thierry |editor-last=Ollevier |title=Topics in Current chemistry 311, Bismuth-Mediated Organic Reactions |publisher=Springer |year=2012 |pages=100–110 |chapter=Environmentally friendly organic synthesis using Bi(III) compounds |isbn=978-3-642-27239-4}}</ref>).<ref>{{cite book |last=Rich |first=Ronald |date=2007 |title=Inorganic Reactions in Water (e-book) |publisher= Springer |isbn=978-3-540-73962-3 }}</ref> |
|||
:4 Bi + 3 O<sub>2</sub> + 12 HCl → 4 BiCl<sub>3</sub> + 6 H<sub>2</sub>O |
|||
: {{chem2|Bi + 6 HNO3 -> 3 H2O + 3 NO2 + Bi(NO3)3}} |
|||
It is used as a [[Transmetalation|transmetalating]] agent in the synthesis of alkaline-earth metal complexes: |
|||
It also dissolves in [[hydrochloric acid]], but only with oxygen present.<ref name="s8" /> |
|||
:3 Ba + 2 BiPh<sub>3</sub> → 3 BaPh<sub>2</sub> + 2 Bi |
|||
: {{chem2|4 Bi + 3 O2 + 12 HCl -> 4 BiCl3 + 6 H2O}} |
|||
===Isotopes=== |
|||
{{main|Isotopes of bismuth}} |
|||
The only primordial [[isotope]] of bismuth, [[bismuth-209]], was traditionally regarded as the heaviest stable isotope, but it had long been suspected<ref>{{cite journal|doi = 10.1007/BF02824346|title = Alpha-activity of209Bi|year = 1972|last1 = Carvalho|first1 = H. G.|last2 = Penna|first2 = M.|journal = Lettere al Nuovo Cimento|volume = 3|issue = 18|pages = 720}}</ref> to be unstable on theoretical grounds. This was finally demonstrated in 2003, when researchers at the Institut d'Astrophysique Spatiale in [[Orsay]], France, measured the [[Alpha decay|alpha emission]] [[half-life]] of {{SimpleNuclide2|Bismuth|209|link=yes}} to be {{val|1.9|e=19|u=years}},<ref>{{cite journal|last = Marcillac|first = Pierre de| coauthors = Noël Coron, Gérard Dambier, Jacques Leblanc, and Jean-Pierre Moalic|year = 2003|month = April|title = Experimental detection of α-particles from the radioactive decay of natural bismuth|journal = Nature|volume = 422|pages = 876–878|pmid=12712201|doi = 10.1038/nature01541|issue = 6934| bibcode= 2003Natur.422..876D}}</ref> over a [[1000000000 (number)|billion]] times longer than the current estimated [[age of the universe]]. Owing to its extraordinarily long half-life, for all presently known medical and industrial applications, bismuth can be treated as if it is stable and nonradioactive. The radioactivity is of academic interest because bismuth is one of few elements whose radioactivity was suspected and theoretically predicted, before being detected in the laboratory. Bismuth has the longest known alpha decay half-life, although [[Isotopes of tellurium|tellurium-128]] has a double beta decay half-life of over {{val|2.2|e=24|u=years}}.<ref name="NUBASE">{{cite journal| first = Audi| last = Georges|title = The NUBASE Evaluation of Nuclear and Decay Properties| journal = Nuclear Physics A| volume = 729| pages = 3–128| publisher = Atomic Mass Data Center| year = 2003| doi=10.1016/j.nuclphysa.2003.11.001| bibcode= 2003NuPhA.729....3A| last2 = Bersillon| first2 = O.| last3 = Blachot| first3 = J.| last4 = Wapstra| first4 = A.H.}}</ref> |
|||
=== Isotopes === |
|||
Several isotopes of bismuth with short half-lives occur within the radioactive disintegration chains of [[actinium]], [[radium]], and [[thorium]], and more have been synthesized experimentally. Bismuth-213 is also found on the decay chain of [[uranium-233]].<ref>{{cite book |url=http://books.google.com/books?id=ZAHJkrJlwbYC&pg=PA78|page =78 |title=Modern Nuclear Chemistry |isbn=978-0-471-11532-8 |last1= Loveland |first1=Walter D |last2=Morrissey |first2=David J |last3=Seaborg |first3=Glenn Theodore |year=2006}}</ref> |
|||
{{Main|Isotopes of bismuth}} |
|||
The only primordial [[isotope]] of bismuth, [[bismuth-209]], was regarded as the heaviest stable nuclide, but it had long been suspected<ref>{{cite journal |doi = 10.1007/BF02824346 |title = Alpha-activity of {{sup|209}}Bi |date = 1972 |last1 = Carvalho |first1 = H. G. |last2 = Penna |first2 = M. |journal = Lettere al Nuovo Cimento |volume = 3 |issue = 18|page = 720|s2cid = 120952231 }}</ref> to be unstable on theoretical grounds. This was finally demonstrated in 2003, when researchers at the ''[[Institut d'astrophysique spatiale]]'' in [[Orsay]], France, measured the [[alpha decay|alpha (α) decay]] [[half-life]] of {{sup|209}}Bi to be {{val|2.01|e=19|u=years}} (3 [[Becquerel|Bq]]/Mg),<ref>{{cite journal |last = Marcillac |first = Pierre de |author2 = Noël Coron |author3 = Gérard Dambier |author4 = Jacques Leblanc |author5 = Jean-Pierre Moalic |name-list-style = amp |date=2003 |title = Experimental detection of α-particles from the radioactive decay of natural bismuth |journal = Nature |volume = 422 |pages = 876–878 |pmid=12712201 |doi = 10.1038/nature01541 |issue = 6934 |bibcode= 2003Natur.422..876D|s2cid = 4415582 }}</ref>{{NUBASE2016|name}} over {{val||e=9}} times longer than the estimated [[age of the universe]].<ref name="Kean" /> Due to its hugely long half-life, for all known medical and industrial applications, bismuth can be treated as stable. The radioactivity is of academic interest because bismuth is one of a few elements whose radioactivity was suspected and theoretically predicted before being detected in the laboratory.<ref name="Kean" /> Bismuth has the longest known α-decay half-life, though [[tellurium-128]] has a [[double beta decay]] half-life of over {{val|2.2|e=24|u=years}}.{{NUBASE2016|ref}} |
|||
Six isotopes of bismuth with short half-lives (210–215 inclusive) occur in the natural radioactive [[decay chain]]s of [[actinium]], [[radium]], [[thorium]], and [[neptunium]]; and more have been synthesized. (Though all primordial {{sup|237}}Np has long since decayed, it is continually regenerated by (n,2n) knockout reactions on natural {{sup|238}}U.)<ref>{{cite book |url=https://books.google.com/books?id=ZAHJkrJlwbYC&pg=PA78|page =78 |title=Modern Nuclear Chemistry |isbn=978-0-471-11532-8 |last1= Loveland |first1=Walter D. |last2=Morrissey |first2=David J. |last3=Seaborg |first3=Glenn T. |date=2006|publisher =John Wiley & Sons |bibcode =2005mnc..book.....L }}</ref><ref>{{cite journal |last1=Peppard |first1=D. F. |last2=Mason |first2=G. W. |last3=Gray |first3=P. R. |last4=Mech |first4=J. F. |title=Occurrence of the (4n + 1) series in nature |journal=Journal of the American Chemical Society |date=1952 |volume=74 |issue=23 |pages=6081–6084 |doi=10.1021/ja01143a074 |bibcode=1952JAChS..74.6081P |url=https://digital.library.unt.edu/ark:/67531/metadc172698/m2/1/high_res_d/metadc172698.pdf }}</ref> |
|||
Commercially, the radioactive isotope bismuth-213 can be produced by bombarding [[radium]] with [[bremsstrahlung]] photons from a [[linear particle accelerator]]. In 1997, an antibody conjugate with bismuth-213, which has a 45-minute half-life and decays with the emission of an alpha particle, was used to treat patients with leukemia. This isotope has also been tried in cancer treatment, for example, in the targeted alpha therapy (TAT) program.<ref>{{cite journal|doi=10.1016/S0360-3016(01)01585-1| title=Advancements in cancer therapy with alpha-emitters: a review|year=2001| last1=Imam|first1=S| journal=International Journal of Radiation Oncology Biology Physics|volume=51|page=271}}</ref><ref>{{cite book |url=http://books.google.com/books?id=Y6haWM6lFkYC&pg=PT520|page =520 |title=Issues in Cancer Epidemiology and Research |year= 2011 |isbn=978-1-4649-6352-0 |last= Acton | first= Ashton}}</ref> |
|||
Commercially, bismuth-213 can be produced by bombarding [[radium]] with [[bremsstrahlung]] photons from a [[linear particle accelerator]]. In 1997, an antibody conjugate with bismuth-213, which has a 45-minute half-life and α-decays, was used to treat leukemia patients. This isotope has also been tried in cancer treatment, for example, in the [[targeted alpha-particle therapy|targeted alpha therapy]] (TAT) program.<ref>{{cite journal|doi=10.1016/S0360-3016(01)01585-1| pmid=11516878| title=Advancements in cancer therapy with alpha-emitters: a review|date=2001| last1=Imam|first1=S.| journal=International Journal of Radiation Oncology, Biology, Physics|volume=51| issue=1| pages=271–8}}</ref><ref>{{cite book |url=https://books.google.com/books?id=Y6haWM6lFkYC&pg=PT520|page =520 |title=Issues in Cancer Epidemiology and Research |date= 2011 |isbn=978-1-4649-6352-0 |last= Acton | first= Ashton|publisher =ScholarlyEditions }}</ref> |
|||
==Chemical compounds== |
|||
{{category see also|Bismuth compounds}} |
|||
== Chemical compounds == |
|||
Bismuth forms trivalent and pentavalent compounds, the trivalent ones being more common. Many of its chemical properties are similar to those of [[arsenic]] and [[antimony]], although they are less toxic than derivatives of those lighter elements. |
|||
{{Main|Bismuth compounds}} |
|||
[[File:Bismuth(III)_oxide_2.jpg|thumb|right|Bismuth(III) oxide powder]] |
|||
Chemically, bismuth resembles [[arsenic]] and [[antimony]], but is much less toxic.<ref name=tox>{{cite book|chapter=Coordination Chemistry of the s, p, and f Metals|author1=Levason, W. |author2=Reid, G. |title= Comprehensive Coordination Chemistry II|year= 2003|doi=10.1016/B0-08-043748-6/02023-5| publisher=Elsevier Pergamon | place=Amsterdam | isbn=0-08-043748-6}}</ref> In almost all known compounds, bismuth has [[oxidation state]] +3; a few have states +5 or −3. |
|||
===Oxides and sulfides=== |
|||
At elevated temperatures, the vapors of the metal combine rapidly with oxygen, forming the yellow trioxide, [[Bismuth(III) oxide|{{chem|Bi|2|O|3}}]].<ref name=w768>Wiberg, p. 768.</ref><ref name=g553>Greenwood, p. 553.</ref> When molten, at temperatures above 710 °C, this oxide corrodes any metal oxide, and even platinum.<ref name=k185>Krüger, p. 185</ref> On reaction with base, it forms two series of [[oxyanion]]s: BiO{{su|b=2|p=−}}, which is polymeric and forms linear chains, and BiO{{su|b=3|p=3−}}. The anion in {{chem|Li|3|BiO|3}} is actually a cubic octameric anion, {{chem|Bi|8|O}}{{su|b=24|p=24−}}, whereas the anion in {{chem|Na|3|BiO|3}} is tetrameric.<ref name="norman1" /> |
|||
The [[bismuth trioxide|trioxide]]<ref name="w768" /><ref name="g553">[[#Greenwood|Greenwood]], p. 553.</ref> and [[bismuth trisulfide|trisulfide]] can both be made from the elements,<ref name=Forgot>{{cite book|title=An Introduction to the Study of Chemistry|url=https://books.google.com/books?id=lGjTyw9gYfYC|publisher=Forgotten Books|isbn=978-1-4400-5235-4|page=363}}</ref><ref name="g559" /> although the trioxide is extremely corrosive at high temperatures.<ref name="k185">[[#Kruger|Krüger]], p. 185</ref> The pentoxide is not stable at room temperature, and will evolve [[oxygen|{{chem|O|2}}]] gas if heated.<ref>{{cite book |
|||
The dark red bismuth(V) oxide, {{chem|Bi|2|O|5}}, is unstable, liberating [[oxygen|{{chem|O|2}}]] gas upon heating.<ref>{{cite book |
|||
| title = Concise encyclopedia chemistry |
| title = Concise encyclopedia chemistry |
||
| url = https://archive.org/details/conciseencyclope0000unse_x5a9 |
|||
| url-access = registration |
|||
| first1 = Thomas|last1 = Scott |
| first1 = Thomas|last1 = Scott |
||
| first2 = Mary|last2 = Eagleson |
| first2 = Mary|last2 = Eagleson |
||
| publisher = Walter de Gruyter |
| publisher = Walter de Gruyter |
||
| |
| date = 1994 |
||
| isbn = 3-11-011451- |
| isbn = 978-3-11-011451-5 |
||
| page = [https://archive.org/details/conciseencyclope0000unse_x5a9/page/136/ 136] |
|||
| page = 136 |
|||
}}</ref> Both oxides form [[complex anion]]s,<ref name="norman1" /><ref name="g578"/> and [[sodium bismuthate|NaBiO<sub>3</sub>]] is a strong oxidising agent.<ref name="g578">[[#Greenwood|Greenwood]], p. 578.</ref> The trisulfide is common in bismuth [[ore]].<ref name=Forgot /> |
|||
}}</ref> |
|||
Similarly, bismuth forms all possible trihalides, but the only pentahalide is BiF<sub>5</sub>. All are [[Lewis acid]]s.<ref name="s8" /> Bismuth forms several formally-Bi<sup>I</sup> halides; these are complex salts with unusually-structured polyatomic cations and anions.<ref name="norman1" /><ref name="gillespie1">{{cite book |
|||
Bismuth sulfide, [[Bismuth(III) sulfide|{{chem|Bi|2|S|3}}]], occurs naturally in bismuth ores.<ref>{{cite book|title=An Introduction to the Study of Chemistry|url=http://books.google.com/books?id=lGjTyw9gYfYC&pg=PA363|accessdate=21 June 2012|publisher=Forgotten Books|isbn=978-1-4400-5235-4|page=363}}</ref> It is also produced by the combination of molten bismuth and sulfur.<ref name=g553/> |
|||
| title = Advances in Inorganic Chemistry and Radiochemistry |
|||
| url = https://archive.org/details/isbn_0120236176/page/n87 |
|||
| url-access = limited |
|||
| first1 = R. J. |last1 = Gillespie |
|||
| first2 = J. |last2 = Passmore |
|||
| editor = Emeléus, H. J. |
|||
| editor2 = Sharp A. G. |
|||
| publisher = Academic Press |
|||
| date = 1975 |
|||
| isbn = 978-0-12-023617-6 |
|||
| pages = 77–78 |
|||
}}</ref> |
|||
[[File:MatlockiteStructure.png|thumb|Bismuth oxychloride (BiOCl) structure (mineral [[bismoclite]]). Bismuth atoms shown as grey, oxygen red, chlorine green.]] |
[[File:MatlockiteStructure.png|thumb|upright|Bismuth oxychloride (BiOCl) structure (mineral [[bismoclite]]). Bismuth atoms are shown as grey, oxygen red, chlorine green.]] |
||
In strongly acidic [[aqueous]] solution, the Bi{{su|p=3+}} ion solvates to form {{chem|Bi(H|2|O)|8|3+}}.<ref name="Persson2010">{{cite journal|last1=Persson|first1=Ingmar|title=Hydrated metal ions in aqueous solution: How regular are their structures?|journal=Pure and Applied Chemistry|volume=82|issue=10|date=2010|pages=1901–1917|doi=10.1351/PAC-CON-09-10-22|doi-access=free}}</ref> As pH increases, the cations polymerize until the octahedral [[bismuthyl]] complex {{awrap|[{{chem|Bi|6|O|4|(OH)|4}}]{{su|p=6+}}}},<ref name="NäslundPersson2000">{{cite journal|last1=Näslund|first1=Jan|last2=Persson|first2=Ingmar|last3=Sandström|first3=Magnus|title=Solvation of the Bismuth(III) Ion by Water, Dimethyl Sulfoxide, N,N'-Dimethylpropyleneurea, and N,N-Dimethylthioformamide. An EXAFS, Large-Angle X-ray Scattering, and Crystallographic Structural Study|journal=Inorganic Chemistry|volume=39|issue=18|date=2000|pages=4012–4021|doi=10.1021/ic000022m|pmid=11198855}}</ref> often abbreviated BiO<sup>+</sup>. Although [[bismuth oxychloride]] and [[bismuth oxynitrate]] have stoichiometries suggesting the ion, they are [[double salt]]s instead.<ref name="k184" /> [[Bismuth nitrate]] (not ''oxy''nitrate) is one of the few aqueous-insoluble [[nitrate]] salts.{{citation needed|date=November 2024}} |
|||
[[Bismuth oxychloride]] (BiOCl, see figure at right) and [[bismuth oxynitate]] (BiONO<sub>3</sub>) stoichiometrically appear as simple anionic salts of the bismuthyl(III) cation (BiO<sup>+</sup>) which commonly occurs in aqueous bismuth compounds. However, in the case of BiOCl, the salt crystal forms in a structure of alternating plates of Bi, O, and Cl atoms, with each oxygen coordinating with four bismuth atoms in the adjacent plane. This mineral compound is used as a pigment and cosmetic (see below).<ref name=k184/> |
|||
Bismuth forms very few stable [[bismuthide]]s, [[intermetallic]] compounds in which it attains oxidation state −3.<ref>{{Cite journal |last=Okamoto |first=H. |date=2002-03-01 |title=Bi-Nd (Bismuth-Neodymium) |journal=Journal of Phase Equilibria |volume=23 |issue=2 |pages=191 |doi=10.1361/1054971023604224 }}</ref> The [[hydride]] spontaneously decomposes at room temperature and stabilizes only below {{convert|−60|C|F}}.<ref name="norman1">{{cite book |
|||
===Bismuthine and bismuthides=== |
|||
Unlike earlier members of group 15 elements such as nitrogen, phosphorus, and arsenic, and similar to the previous group 15 element [[antimony]], bismuth does not form a stable [[hydride]]. Bismuth hydride, [[bismuthine]] ({{chem|BiH|3}}), is an [[endothermic]] compound that spontaneously decomposes at room temperature. It is stable only below −60 °C.<ref name="norman1">{{cite book |
|||
| title = Chemistry of arsenic, antimony, and bismuth |
| title = Chemistry of arsenic, antimony, and bismuth |
||
| first1 = S. M. |last1 =Godfrey |
| first1 = S. M. |last1 =Godfrey |
||
| Line 93: | Line 109: | ||
| first3 = A. G. |last3 =Mackie |
| first3 = A. G. |last3 =Mackie |
||
| first4 = R. G. |last4 =Pritchard |
| first4 = R. G. |last4 =Pritchard |
||
| editor = Nicholas C. Norman |
| editor-first = Nicholas C. |editor-last =Norman |
||
| publisher = Springer |
| publisher = Springer |
||
| |
| date = 1998 |
||
| isbn = 0-7514-0389- |
| isbn = 978-0-7514-0389-3 |
||
| pages = 67–84 |
| pages = 67–84 |
||
}}</ref> Sodium bismuthide has interest as a [[topological insulator|topological]] [[Dirac semimetal|Dirac insulator]].<ref name="k1401">{{cite web |url=https://www.kurzweilai.net/3d-counterpart-to-graphene-discovered |title=3D counterpart to graphene discovered [UPDATE] |date=20 January 2014|publisher=KurzweilAI |access-date=28 January 2014}}</ref><ref>{{Cite journal | last1 = Liu | first1 = Z. K. | last2 = Zhou | first2 = B. | last3 = Zhang | first3 = Y. | last4 = Wang | first4 = Z. J. | last5 = Weng | first5 = H. M. | last6 = Prabhakaran | first6 = D. | last7 = Mo | first7 = S. K. | last8 = Shen | first8 = Z. X. | last9 = Fang | first9 = Z. | last10 = Dai | first10 = X. | last11 = Hussain | first11 = Z. | last12 = Chen | first12 = Y. L. | title = Discovery of a Three-Dimensional Topological Dirac Semimetal, Na<sub>3</sub>Bi | doi = 10.1126/science.1245085 | journal = Science | year = 2014 |arxiv=1310.0391| pmid = 24436183| volume=343 | issue = 6173 | pages=864–7|bibcode = 2014Sci...343..864L | s2cid = 206552029 }}</ref> |
|||
}}</ref> Bismuthides are [[intermetallic]] compounds between bismuth and other metals. |
|||
== Occurrence and production == |
|||
===Halides=== |
|||
{{See also|List of countries by bismuth production}} |
|||
The [[halide]]s of bismuth in low oxidation states have been shown to adopt unusual structures. What was originally thought to be bismuth(I) chloride, BiCl, turns out to be a complex compound consisting of Bi{{su|b=9|p=5+}} cations and BiCl{{su|b=5|p=2−}} and Bi{{su|b=2}}Cl{{su|b=8|p=2−}} anions.<ref name="norman1" /><ref name="gillespie1">{{cite book |
|||
| title = Advances in Inorganic Chemistry and Radiochemistry |
|||
| first1 = R. J. |last1 = Gillespie |
|||
| first2 = J. |last2 = Passmore |
|||
| editor = Emeléus, H. J.; Sharp A. G. |
|||
| publisher = Academic Press |
|||
| year = 1975 |
|||
| isbn = 0-12-023617-6 |
|||
| pages = 77–78 |
|||
}}</ref> The Bi{{su|b=9|p=5+}} cation has a distorted tricapped [[trigonal prism]]atic molecular geometry, and is also found in {{chem|Bi|10|Hf|3|Cl|18}}, which is prepared by reducing a mixture of [[hafnium(IV) chloride]] and [[bismuth chloride]] with elemental bismuth, having the structure {{chem|[Bi|+|] [Bi|9|5+|] [HfCl|6|2-|]|3}}.<ref name="norman1"/>{{rp|50}} Other polyatomic bismuth cations are also known, such as Bi{{su|b=8|p=2+}}, found in {{chem|Bi|8|(AlCl|4|)|2}}.<ref name="gillespie1" /> Bismuth also forms a low-valence bromide with the same structure as "BiCl". There is a ''true'' monoiodide, BiI, which contains chains of {{chem|Bi|4|I|4}} units. BiI decomposes upon heating to the triiodide, [[Bismuth(III) iodide|{{chem|BiI|3}}]], and elemental bismuth. A monobromide of the same structure also exists.<ref name="norman1" /> |
|||
In oxidation state +3, bismuth forms trihalides with all of the halogens: {{chem|BiF|3}}, {{chem|BiCl|3}}, {{chem|BiBr|3}}, and {{chem|BiI|3}}. All of these except {{chem|BiF|3}} are [[hydrolyze]]d by water to form the ''bismuthyl'' cation, BiO{{su|p=+}}, the commonly encountered bismuth (III) oxycation noted above.<ref name="norman1" /> |
|||
Bismuth(III) chloride reacts with [[hydrogen chloride]] in [[ether]] solution to produce the acid {{chem|HBiCl|4}}.<ref name=s8/> |
|||
The oxidation state +5 is less frequently encountered. One such compound is [[bismuth pentafluoride|{{chem|BiF|5}}]], a powerful oxidizing and fluorinating agent. It is also a strong fluoride acceptor, reacting with [[xenon tetrafluoride]] to form the {{chem|XeF|3|+}} cation:<ref name=s8/> |
|||
[[File:Bismite.jpg|thumb|upright|[[Bismite]] mineral]] |
|||
: {{chem|BiF|5}} + {{chem|XeF|4}} → {{chem|XeF|3|+|BiF|6|-}} |
|||
[[File:BrokenBismuthIngot.jpg|thumb|upright|Chunk of a broken bismuth ingot]] |
|||
The reported abundance of bismuth in the Earth's crust varies significantly by source from 180ppb (similar to that of silver) to 8ppb (twice as common as gold). The most important [[ore]]s of bismuth are [[bismuthinite]] and [[bismite]].<ref name="CRC" /> [[Native element mineral|Native]] bismuth is known from Australia, Bolivia, and China.<ref name="arizona1">{{cite book|editor=Anthony, John W.|editor2=Bideaux, Richard A.|editor3=Bladh, Kenneth W.|editor4=Nichols, Monte C. |title=Handbook of Mineralogy: Elements, Sulfides, Sulfosalts |date=15 April 1990 |publisher=Mineralogical Society of America |place=Chantilly, VA, US |chapter-url=http://rruff.geo.arizona.edu/doclib/hom/bismuth.pdf |section=Bismuth |access-date=5 December 2011 |isbn=978-0-9622097-0-3}}</ref><ref>[[#Kruger|Krüger]], pp. 172–173.</ref> |
|||
===Aqueous species=== |
|||
In [[aqueous]] solution, the Bi{{su|p=3+}} ion exists in various states of hydration, depending on the [[pH]]: |
|||
{| class=wikitable style="text-align:right" |
|||
|+ World bismuth production, 2022, in tonnes |
|||
!pH range |
|||
! Country |
|||
!Species |
|||
! Refining<ref>{{cite web|url=https://pubs.usgs.gov/periodicals/mcs2023/mcs2023-bismuth.pdf|title=2023 USGS Minerals Yearbook: Bismuth| publisher = United States Geological Survey|last=Merrill|first= Adam M. }}</ref> |
|||
|- |
|- |
||
!China |
|||
! <3 |
|||
|16,000 |
|||
| {{chem|Bi(H|2|O)|6|3+}} |
|||
|- |
|- |
||
!Laos |
|||
!0-4 |
|||
|2,000 |
|||
| {{chem|Bi(H|2|O)|5|OH|2+}} |
|||
|- |
|- |
||
!South Korea |
|||
!1-5 |
|||
|950 |
|||
| {{chem|Bi(H|2|O)|4|(OH)|2|+}} |
|||
|- |
|- |
||
!Japan |
|||
!5-14 |
|||
| 480 |
|||
| {{chem|Bi(H|2|O)|3|(OH)|3}} |
|||
|- |
|- |
||
!Kazakhstan |
|||
! >11 |
|||
|220 |
|||
| {{chem|Bi(H|2|O)|2|(OH)|4|-}} |
|||
|- |
|||
!Other |
|||
|350 |
|||
|- |
|||
!Total |
|||
|20,000 |
|||
|} |
|} |
||
These mononuclear species are in equilibrium. Polynuclear species also exist, the most important of which is BiO{{su|p=+}}, which exists in hexameric form as the octahedral complex [{{chem|Bi|6|O|4|(OH)|4}}]{{su|p=6+}} (or 6 [BiO{{su|p=+}}]·2 {{chem|H|2|O}}).<ref>Wiberg, p. 771.</ref> |
|||
According to the [[United States Geological Survey]] (USGS), 10,200 tonnes of bismuth were produced worldwide by mining and 17,100 tonnes by refining in 2016. Since then, USGS does not provide mining data for bismuth, considering them unreliable. Globally, bismuth is mostly produced by refining, as a byproduct of extraction of other metals such as lead, copper, [[tin]], [[molybdenum]] and [[tungsten]], though the refining-to-mining ratio depends on the country.<ref>[[#Kruger|Krüger]], p. 173.</ref><ref name="Oje" /><ref>{{cite journal |doi = 10.1016/0891-3919(57)90180-8 |title = The preparation of bismuth for use in a liquid-metal fuelled reactor |date = 1957 |last1 = Horsley |first1 = G. W. |journal = Journal of Nuclear Energy |volume = 6 |issue = 1–2 |page = 41}}</ref><ref>{{cite journal |doi = 10.1134/S0020168511020166 |title = Pb distribution in multistep bismuth refining products |date = 2011 |last1 = Shevtsov |first1 = Yu. V. |last2 = Beizel’ |first2 = N. F. |journal = Inorganic Materials |volume = 47 |issue = 2 |page = 139|s2cid = 96931735 }}</ref> |
|||
==Occurrence and production== |
|||
[[File:Bismite.jpg|thumb|left|upright|[[Bismite]] mineral]] |
|||
<!-- Deleted image removed: [[File:Bismuth (mined)2.PNG|thumb|290px|Bismuth output in 2005]] --> |
|||
Bismuth travels in crude lead bullion (which can contain up to 10% bismuth) through several stages of refining, until it is removed by the [[Kroll-Betterton process]] which separates the impurities as slag, or the electrolytic [[Betts process]]. Bismuth will behave similarly with another of its major metals, copper.<ref name="Oje">{{cite journal |doi = 10.1007/BF03222821 |title = Bismuth—Production, properties, and applications |date = 1992 |last1 = Ojebuoboh |first1 = Funsho K. |journal = JOM |volume = 44 |issue = 4 |pages = 46–49|bibcode = 1992JOM....44d..46O|s2cid = 52993615 }}</ref> The raw bismuth metal from both processes contains still considerable amounts of other metals, foremost lead. By reacting the molten mixture with chlorine gas the metals are converted to their chlorides while bismuth remains unchanged. Impurities can also be removed by various other methods for example with fluxes and treatments yielding high-purity bismuth metal (over 99% Bi).<ref name=usgs2/> |
|||
In the Earth's crust, bismuth is about [[Abundances of the elements (data page)|twice as abundant as gold]]. The most important [[ore]]s of bismuth are [[bismuthinite]] and [[bismite]].<ref name=CRC/> Native bismuth is known from Australia, Bolivia, and China.<ref name="arizona1">{{cite book|editor=Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W. and Nichols, Monte C. |title=Handbook of Mineralogy |publisher=Mineralogical Society of America |place=Chantilly, VA, US |volume=I (Elements, Sulfides, Sulfosalts) |url=http://rruff.geo.arizona.edu/doclib/hom/bismuth.pdf |format=PDF |chapter=Bismuth |accessdate=5 December 2011 |isbn=0-9622097-0-8}}</ref><ref>Krüger, pp. 172–173.</ref> |
|||
=== Price === |
|||
According to the [[United States Geological Survey]], the world mining production of bismuth in 2010 was 8,900 tonnes, with the major contributions from China (6,500 tonnes), Peru (1,100 tonnes) and Mexico (850 tonnes). The refinery production was 16,000 tonnes, of which China produced 13,000, Mexico 850 and Belgium 800 tonnes.<ref name=usgs2010>{{cite web|url=http://minerals.usgs.gov/minerals/pubs/commodity/bismuth/myb1-2010-bismu.pdf|title=2010 USGS Minerals Yearbook: Bismuth|format = PDF| accessdate =9 September 2010|publisher = United States Geological Survey|last=Carlin|first= James F., Jr.}}</ref> |
|||
[[File:BiPrice.png|thumb|upright=1.3|World mine production and annual averages of bismuth price (New York, not adjusted for inflation).<ref name="usgs" />]] |
|||
The price for pure bismuth metal was relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.<ref name="usgs" /> |
|||
Before World War II, demand for bismuth was small and mainly pharmaceutical—bismuth compounds were used to treat such conditions as digestive disorders, [[sexually transmitted diseases]] and burns. Minor amounts of bismuth metal were consumed in fusible alloys for [[fire sprinkler]] systems and [[Fuse (electrical)|fuse wire]]. During World War II bismuth was considered a [[strategic material]], used for solders, fusible alloys, medications and atomic research. To stabilize the market, the producers set the price at $1.25 per pound ($2.75 /kg) during the war and at $2.25 per pound ($4.96 /kg) from 1950 until 1964.<ref name="usgs" /> |
|||
The difference between world bismuth mine and refinery production reflects bismuth's status as a byproduct of extraction of other metals such as lead, copper, tin, molybdenum and tungsten.<ref>Krüger, p. 173.</ref> Bismuth travels in crude lead bullion (which can contain up to 10% bismuth) through several stages of refining, until it is removed by the [[Kroll-Betterton process]] which separates the impurities as slag, or the electrolytic [[Betts process]]. Bismuth will behave similarly with another of its major metals, copper.<ref name="Oje">{{cite journal |doi = 10.1007/BF03222821 |title = Bismuth—Production, properties, and applications |year = 1992 |last1 = Ojebuoboh |first1 = Funsho K. |journal = JOM |volume = 44 |issue = 4 |pages = 46–49|bibcode = 1992JOM....44d..46O}}</ref> |
|||
In the early 1970s, the price rose rapidly due to increasing demand for bismuth as a metallurgical additive to aluminium, iron and steel. This was followed by a decline owing to increased world production, stabilized consumption, and the recessions of 1980 and 1981–1982. In 1984, the price began to climb as consumption increased worldwide, especially in the United States and Japan. In the early 1990s, research began on the evaluation of bismuth as a nontoxic replacement for lead in ceramic glazes, fishing sinkers, food-processing equipment, free-machining [[brass]]es for plumbing applications, lubricating greases, and shot for [[waterfowl hunting]].<ref name="s14">[[#Suzuki|Suzuki]], p. 14.</ref> Growth in these areas remained slow during the middle 1990s, in spite of the backing of lead replacement by the United States federal government, but intensified around 2005. This resulted in a rapid and continuing increase in price.<ref name="usgs">[http://minerals.usgs.gov/minerals/pubs/commodity/bismuth/ Bismuth Statistics and Information]. see "Metal Prices in the United States through 1998" for a price summary and "Historical Statistics for Mineral and Material Commodities in the United States" for production. USGS.</ref> |
|||
The raw bismuth metal from both processes contains still considerable amounts of other metals, foremost lead. By reacting the molten mixture with chlorine gas the metals are converted to their chlorides while bismuth remains unchanged. Impurities can also be removed by various other methods for example with fluxes and treatments yielding high-purity bismuth metal (over 99% Bi). World bismuth production from refineries is a more complete and reliable statistic.<ref name="Oje"/><ref>{{cite journal |doi = 10.1016/0891-3919(57)90180-8 |title = The preparation of bismuth for use in a liquid-metal fuelled reactor |year = 1957 |last1 = Horsley |first1 = G.W. |journal = Journal of Nuclear Energy (1954) |volume = 6 |pages = 41}}</ref><ref>{{cite journal |doi = 10.1134/S0020168511020166 |title = Pb distribution in multistep bismuth refining products |year = 2011 |last1 = Shevtsov |first1 = Yu. V. |last2 = Beizel' |first2 = N. F. |journal = Inorganic Materials |volume = 47 |issue = 2 |pages = 139}}</ref><!--patent US 2955931--> |
|||
=== |
=== Recycling === |
||
Most bismuth is produced as a byproduct of other metal-extraction processes including the smelting of lead, and also of tungsten and copper. Its [[sustainability]] is dependent on increased recycling, which is problematic.<ref>{{Cite book |url=https://data.europa.eu/doi/10.2873/167813 |title=Report on critical raw materials and the circular economy |date=2018 |publisher=European Commission. Directorate General for Internal Market, Industry, Entrepreneurship and SMEs |doi=10.2873/167813|isbn=9789279946264 |author1=European Commission. Directorate General for Internal Market, Industry, Entrepreneurship and SMEs. }}</ref> |
|||
[[File:BiPrice.png|thumb|290px|World mine production and annual averages of bismuth price (New York, not adjusted for inflation).<ref name=usgs/>]] |
|||
The price for pure bismuth metal has been relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price, usually reflected the cost of recovery and the balance between production and demand.<ref name=usgs/> |
|||
It was once believed that bismuth could be practically recycled from the soldered joints in electronic equipment. Recent efficiencies in solder application in electronics mean there is substantially less solder deposited, and thus less to recycle. While recovering the silver from silver-bearing solder may remain economic, recovering bismuth is substantially less so.<ref>{{cite web|url = http://leadfree.ipc.org/files/RoHS_15.pdf|author = Warburg, N|publisher = University of Stuttgart|title = IKP, Department of Life-Cycle Engineering|access-date = 5 May 2009|url-status = dead|archive-url = https://web.archive.org/web/20090225155540/http://leadfree.ipc.org/files/RoHS_15.pdf|archive-date = 25 February 2009|df = dmy-all}}</ref> |
|||
Demand for bismuth was small prior to World War II and was pharmaceutical – bismuth compounds were used to treat such conditions as digestive disorders, [[sexually transmitted disease]]s and burns. Minor amounts of bismuth metal were consumed in fusible alloys for [[fire sprinkler]] systems and [[Fuse (electrical)|fuse wire]]. During World War II bismuth was considered a strategic material, used for solders, fusible alloys, medications and atomic research. To stabilize the market, the producers set the price at $1.25 per pound (2.75 $/kg) during the war and at $2.25 per pound (4.96 $/kg) from 1950 until 1964.<ref name=usgs/> |
|||
Dispersed bismuth is used in certain stomach medicines ([[bismuth subsalicylate]]), paints ([[bismuth vanadate]]), [[pearlescent]] cosmetics ([[bismuth oxychloride]]), and bismuth-containing bullets. Recycling bismuth from these uses is impractical.<ref name=usgs2/> |
|||
In the early 1970s, the price grew rapidly due to increasing demand for bismuth as a metallurgical additive to aluminium, iron and steel. This was followed by a decline owing to increased world production, stabilized consumption, and the recessions of 1980 and 1981–82. In 1984, the price began to climb as consumption increased worldwide, especially in the United States and Japan. In the early 1990s, research began on the evaluation of bismuth as a nontoxic replacement for lead in ceramic glazes, fishing sinkers, food-processing equipment, free-machining [[brass]]es for plumbing applications, lubricating greases, and shot for [[waterfowl hunting]].<ref name=s14>Suzuki, p. 14.</ref> Growth in these areas remained slow during the middle 1990s, in spite of the backing of lead replacement by the US Government, but intensified around 2005. This resulted in a rapid and continuing increase in price.<ref name=usgs>[http://minerals.usgs.gov/minerals/pubs/commodity/bismuth/ Bismuth Statistics and Information]. see "Metal Prices in the United States through 1998" for a price summary and "Historical Statistics for Mineral and Material Commodities in the United States" for production. USGS.</ref> |
|||
== |
== Applications == |
||
[[File:Processing of bismuth. Etching. Wellcome V0023568.jpg|alt=Black and white engraving of two men extracting and working bismuth, hammering and pouring on a hillside.|thumb|18th-century engraving of bismuth processing. During this era, bismuth was used to treat some digestive complaints.]] |
|||
Whereas bismuth is most available today as a byproduct, its [[sustainability]] is more dependent on recycling. Bismuth is mostly a byproduct of lead smelting, along with silver, [[zinc]], [[antimony]], and other metals, and also of [[tungsten]] production, along with [[molybdenum]] and [[tin]], and also of copper production. Recycling bismuth is difficult in many of its end uses, primarily because of scattering. |
|||
Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.<ref name=usgs2>{{cite web|url=https://pubs.usgs.gov/myb/vol1/2018/myb1-2018-bismuth.pdf|title=2018 USGS Minerals Yearbook: Bismuth|publisher = United States Geological Survey| author1=Singerling, Sheryl A. |author2=Callaghan, Robert M. }}</ref> |
|||
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.<ref name=usgs2/> |
|||
Probably the easiest to recycle would be bismuth-containing fusible alloys in the form of larger objects, then larger soldered objects. Half of the world's solder consumption is in [[electronics]] (i.e., circuit boards).<ref>{{cite book| last = Taylor|first = Harold A.|title = Bismuth. Financial Times Executive Commodity Reports|page = 17|isbn = 1-84083-326-2| year = 2000| publisher = Financial Times Energy| location = London}}</ref> As the soldered objects get smaller or contain little solder or little bismuth, the recovery gets progressively more difficult and less economic, although solder with a higher silver content will be more worthwhile recovering. Next in recycling feasibility would be sizeable catalysts with a fair bismuth content, perhaps as [[bismuth phosphomolybdate]], and then bismuth used in galvanizing and as a free-machining metallurgical additive. |
|||
=== Medicines === |
|||
Bismuth in uses where it is dispersed most widely include stomach medicines ([[bismuth subsalicylate]]), paints ([[bismuth vanadate]]) on a dry surface, [[pearlescent]] cosmetics ([[bismuth oxychloride]]), and bismuth-containing bullets that have been fired. The bismuth scattered in these uses is unrecoverable with present technology. |
|||
Bismuth is an ingredient in some pharmaceuticals,<ref name="Kean" /> although the use of some of these substances is declining.<ref name="k184" /> |
|||
The most important sustainability fact about bismuth is its byproduct status, which can either improve sustainability (i.e., [[vanadium]] or [[manganese nodules]]) or, for bismuth from lead ore, constrain it; bismuth is constrained. The extent that the constraint on bismuth can be ameliorated or not is going to be tested by the future of the lead storage battery, since 90% of the world market for lead is in storage batteries for gasoline or diesel-powered motor vehicles. |
|||
* [[Bismuth subsalicylate]] is used to treat [[diarrhea]];<ref name="Kean" /> it is the [[active ingredient]] in such "pink bismuth" preparations as [[Pepto-Bismol]], as well as the 2004 reformulation of [[Kaopectate]]. It is also used to treat some other gastro-intestinal diseases like [[shigellosis]]<ref>[https://www.cdc.gov/shigella/diagnosistreatment.html CDC, shigellosis].</ref> and [[cadmium poisoning]].<ref name="Kean" /> The mechanism of action of this substance is still not well documented, although an [[oligodynamic effect]] (toxic effect of small doses of heavy metal ions on microbes) may be involved in at least some cases. [[Salicylic acid]] from [[hydrolysis]] of the compound is antimicrobial for toxogenic ''E. coli,'' an important pathogen in [[traveler's diarrhea]].<ref>{{cite journal|author=Sox TE|author2= Olson CA|title=Binding and killing of bacteria by bismuth subsalicylate|journal =Antimicrob Agents Chemother|date= 1989|volume=33|issue=12|pages=2075–82|pmid=2694949|pmc=172824|doi=10.1128/AAC.33.12.2075}}</ref> |
|||
* A combination of [[bismuth subsalicylate]] and [[bismuth subcitrate]] is used to treat [[Helicobacter pylori|the bacteria]] causing [[peptic ulcer]]s.<ref>{{cite web|url=http://www.ema.europa.eu/docs/en_GB/document_library/PIP_decision/WC500005327.pdf|title=P/74/2009: European Medicines Agency decision of 20 April 2009 on the granting of a product specific waiver for Bismuth subcitrate potassium / Metronidazole / Tetracycline hydrochloride (EMEA-000382-PIP01-08) in accordance with Regulation (EC) No 1901/2006 of the European Parliament and of the Council as amended|publisher=[[European Medicines Agency]]|date=2009-06-10|access-date=13 August 2022|archive-date=24 October 2017|archive-url=https://web.archive.org/web/20171024205050/http://www.ema.europa.eu/docs/en_GB/document_library/PIP_decision/WC500005327.pdf|url-status=dead}}</ref><ref>{{cite journal | vauthors = Urgesi R, Cianci R, Riccioni ME | title = Update on triple therapy for eradication of Helicobacter pylori: current status of the art | journal = Clinical and Experimental Gastroenterology | volume = 5 | pages = 151–7 | year = 2012 | pmid = 23028235 | pmc = 3449761 | doi = 10.2147/CEG.S25416 | doi-access = free }}</ref> |
|||
* [[Bibrocathol]] is an organic bismuth-containing compound used to treat eye infections.<ref>{{cite book | vauthors = Gurtler L | chapter = Chapter 2: The Eye and Conjunctiva as Target of Entry for Infectious Agents: Prevention by Protection and by Antiseptic Prophylaxis | veditors = Kramer A, Behrens-Baumann W |title=Antiseptic prophylaxis and therapy in ocular infections: principles, clinical practice, and infection control | series = Developments in Ophthalmology |date= January 2002 | volume = 33 | pages = 9–13 |publisher=Karger |location=Basel |isbn=978-3-8055-7316-0 | doi = 10.1159/000065934 | pmid = 12236131 | chapter-url = https://books.google.com/books?id=JkQOSAnOIhoC&dq=Bibrocathol&pg=PA96 }}</ref> |
|||
* [[Bismuth subgallate]], the [[active ingredient]] in Devrom, is used as an internal deodorant to treat malodor from [[flatulence]] and [[feces]].<ref>{{cite journal | vauthors = Gorbach SL | title = Bismuth therapy in gastrointestinal diseases | journal = Gastroenterology | volume = 99 | issue = 3 | pages = 863–75 | date = September 1990 | pmid = 2199292 | doi = 10.1016/0016-5085(90)90983-8 | author-link = Sherwood Gorbach }}</ref><ref>{{cite journal | vauthors = Sparberg M | title = Correspondence: Bismuth subgallate as an effective means for the control of ileostomy odor: a double blind study | journal = Gastroenterology | volume = 66 | issue = 3 | pages = 476 | date = March 1974 | pmid = 4813513 | doi = 10.1016/S0016-5085(74)80150-2 | doi-access = free }}</ref> |
|||
* Bismuth compounds (including sodium bismuth tartrate) were formerly used to treat syphilis.<ref>{{cite journal|author=Parnell, R. J. G. |title=Bismuth in the Treatment of Syphilis|journal=Journal of the Royal Society of Medicine|date=1924|volume=17|pages=19–26|pmc=2201253|issue=War section|pmid=19984212|doi=10.1177/003591572401702604}}</ref><ref>Giemsa, Gustav (1924) {{US patent|1540117}} "Manufacture of bismuth tartrates"</ref> [[Arsenic]] combined with either bismuth or mercury was a mainstay of syphilis treatment from the 1920s until the advent of penicillin in 1943.<ref>{{cite journal |last1=Frith |first1=John |title=Syphilis – Its Early History and Treatment Until Penicillin, and the Debate on its Origins |journal=Journal of Military and Veterans' Health |date=November 2012 |volume=20 |issue=4 |page=54 |url=https://jmvh.org/article/syphilis-its-early-history-and-treatment-until-penicillin-and-the-debate-on-its-origins/ |access-date=30 January 2022}}</ref> |
|||
* "Milk of bismuth" (an aqueous suspension of [[bismuth hydroxide]] and [[bismuth subcarbonate]]) was marketed as an alimentary cure-all in the early 20th century, and has been used to treat [[gastrointestinal disorders]].<ref>{{Cite web |url=http://www.medilexicon.com/medicaldictionary.php?t=55448 |title=Milk of Bismuth |access-date=2022-08-13 |archive-date=2013-06-04 |archive-url=https://web.archive.org/web/20130604170722/http://www.medilexicon.com/medicaldictionary.php?t=55448 |url-status=dead }}</ref> |
|||
* [[Bismuth subnitrate]] (Bi<sub>5</sub>O(OH)<sub>9</sub>(NO<sub>3</sub>)<sub>4</sub>) and [[bismuth subcarbonate]] (Bi<sub>2</sub>O<sub>2</sub>(CO<sub>3</sub>)) are also used in medicine.<ref name="CRC" /> |
|||
=== Cosmetics and pigments === |
|||
The life-cycle assessment of bismuth will focus on solders, one of the major uses of bismuth, and the one with the most complete information. The average primary energy use for solders is around 200 MJ per kg, with the high-bismuth solder (58% Bi) only 20% of that value, and three low-bismuth solders (2% to 5% Bi) running very close to the average. The [[global warming]] potential averaged 10 to 14 kg [[carbon dioxide]], with the high-bismuth solder about two-thirds of that and the low-bismuth solders about average. The acidification potential for the solders is around 0.9 to 1.1 kg [[sulfur dioxide]] equivalent, with the high-bismuth solder and one low-bismuth solder only one-tenth of the average and the other low-bismuth solders about average.<ref>{{cite web|url = http://leadfree.ipc.org/files/RoHS_15.pdf|author=Warburg, N |publisher=University of Stuttgart| title = IKP, Department of Life-Cycle Engineering| accessdate =5 May 2009}}</ref> There is very little life-cycle information on other bismuth alloys or compounds. |
|||
[[Bismuth oxychloride]] (BiOCl) is sometimes used in cosmetics, as a pigment in paint for eye shadows, hair sprays and nail polishes.<ref name="Kean" /><ref name="k184" /><ref name="Effp">{{cite journal|doi = 10.1016/j.porgcoat.2005.07.003|title = Effect pigments—past, present and future|date = 2005|last1 = Maile|first1 = Frank J.|last2 = Pfaff|first2 = Gerhard|last3 = Reynders|first3 = Peter|journal = Progress in Organic Coatings|volume = 54|issue = 3|page = 150}}</ref><ref name="Paff">{{cite book|url = https://books.google.com/books?id=Q1Pc0aY-vg4C&pg=PA36| page = 36|title = Special effect pigments: Technical basics and applications|isbn = 978-3-86630-905-0|last1 = Pfaff|first1 = Gerhard|date = 2008|publisher=Vincentz Network GmbH}}</ref> This compound is found as the mineral bismoclite and in crystal form contains layers of atoms (see figure above) that refract light chromatically, resulting in an [[iridescent]] appearance similar to [[nacre]] of pearl. It was used as a cosmetic in [[ancient Egypt]] and in many places since. ''Bismuth white'' (also "Spanish white") can refer to either bismuth oxychloride or [[bismuth oxynitrate]] (BiONO<sub>3</sub>), when used as a white pigment.<ref>{{cite book |last=Sadler |first=Peter J|editor-first=A.G. |editor-last=Sykes |title=ADVANCES IN INORGANIC CHEMISTRY, Volume 36 |publisher=Academic Press |date=1991 |chapter=Chapter 1|isbn=0-12-023636-2}}</ref> [[Bismuth vanadate]] is used as a light-stable non-reactive paint pigment (particularly for artists' paints), often as a replacement for the more toxic cadmium sulfide yellow and orange-yellow pigments. The most common variety in artists' paints is a lemon yellow, visually indistinguishable from its cadmium-containing alternative.<ref>{{cite book | last=Weldon | first=Dwight G. | title=Failure analysis of paints and coatings | publisher=Wiley | publication-place=Chichester, U.K. | date=2009 | isbn=978-1-61583-267-5 | oclc=608477934 | page=40}}</ref> |
|||
==Applications== |
|||
Bismuth has few commercial applications, none of which is particularly large. Taking the US as an example, 884 tonnes of bismuth were consumed in 2010, of which 63% went into chemicals (including pharmaceuticals, pigments, and cosmetics), 26% into metallurgical additives for casting and galvanizing,<ref>{{cite journal|doi = 10.1016/j.matlet.2006.06.029|title = The effect of bismuth on the structure of zinc hot-dip galvanized coatings|year = 2007|last1 = Pistofidis|first1 = N.|last2 = Vourlias|first2 = G.|last3 = Konidaris|first3 = S.|last4 = Pavlidou|first4 = El.|last5 = Stergiou|first5 = A.|last6 = Stergioudis|first6 = G.|journal = Materials Letters|volume = 61|issue = 4–5|page = 994}}</ref> 7% into bismuth alloys, solders and ammunition, and the balance into research and other uses.<ref name=usgs2010/> |
|||
=== Metal and alloys === |
|||
Some manufacturers use bismuth as a substitute in equipment for potable water systems such as valves to meet "lead-free" mandates in the U.S. (starts in 2014). This is a fairly large application since it covers all residential and commercial building construction. |
|||
Bismuth is used in alloys with other metals such as tin and lead. [[Wood's metal]], an alloy of bismuth, lead, tin, and cadmium is used in automatic sprinkler systems for fires. It forms the largest part (50%) of [[Rose's metal]], a [[fusible alloy]], which also contains 25–28% lead and 22–25% tin. It was also used to make [[bismuth bronze]], which was used during the [[Bronze Age]], having been found in Inca knives at [[Machu Picchu]].<ref>{{cite journal|jstor=1692247 |title=Bismuth Bronze from Machu Picchu, Peru |publisher=American Association for the Advancement of Science |last1=Gordon |first1=Robert B. |last2=Rutledge |first2=John W. |journal=Science |year=1984 |volume=223 |issue=4636 |pages=585–586 |doi=10.1126/science.223.4636.585 |pmid=17749940 |bibcode=1984Sci...223..585G |s2cid=206572055 }}</ref> |
|||
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications. |
|||
=== |
==== Lead replacement ==== |
||
The density difference between lead (11.32 g/cm<sup>3</sup>) and bismuth (9.78 g/cm<sup>3</sup>) is small enough that for many [[ballistics]] and weighting applications, bismuth can substitute for lead. For example, it can replace lead as a dense material in [[fishing sinker]]s. It has been used as a replacement for lead in [[Shot (pellet)|shot]], bullets and [[less-lethal]] [[riot gun]] ammunition. The Netherlands, Denmark, England, Wales, the United States, and many other countries now prohibit the use of lead shot for the hunting of wetland birds, as many birds are prone to [[lead poisoning]] owing to mistaken ingestion of lead (instead of small stones and grit) to aid digestion, or even prohibit the use of lead for all hunting, such as in the Netherlands. Bismuth-tin alloy shot is one alternative that provides similar ballistic performance to lead.<ref name=usgs2/> |
|||
Bismuth is an ingredient in some pharmaceuticals, although the use of some of these substances is declining.<ref name=k184/> |
|||
* [[Bismuth subsalicylate]] is used as an [[diarrhea|antidiarrheal]]; it is the [[active ingredient]] in such "Pink Bismuth" preparations as [[Pepto-Bismol]], as well as the 2004 reformulation of [[Kaopectate]]. It is also used to treat some other gastro-intestinal diseases. The mechanism of action of this substance is still not well documented, although an [[oligodynamic effect]] (toxic effect of small doses of heavy metal ions on microbes) may be involved in at least some cases. [[Salicylic acid]] from hydrolysis of the compound is antimicrobial for toxogenic ''E. coli,'' an important pathogen in [[traveler's diarrhea]].<ref>{{cite journal|author=Sox TE, Olson CA|title=Binding and killing of bacteria by bismuth subsalicylate|journal =Antimicrob Agents Chemother|year= 1989|volume=33|issue=12|pages=2075–82|pmid=2694949|pmc=172824|doi=10.1128/AAC.33.12.2075}}</ref> |
|||
* a combination of [[bismuth subsalicylate]] and [[bismuth subcitrate]] are used to treat [[peptic ulcer]]s. |
|||
* [[Bibrocathol]] is an organic bismuth-containing compound used to treat eye infections. |
|||
* [[Bismuth subgallate]], the [[active ingredient]] in Devrom, is used as an internal deodorant to treat malodor from [[flatulence]] ("gas") and [[feces]]. |
|||
* Bismuth compounds (including [[sodium bismuth tartrate]]) were formerly used to treat syphilis<ref>{{cite journal|author=R. J. G. Parnell, Surgeon-Commander, Royal Navy|title=Bismuth in the Treatment of Syphillis|journal=Journal of the Royal Society of Medicine|year=1924|volume=17(War section)|pages=19–26|pmc=2201253|issue=War Sect}}</ref><ref>{{cite patent|country=usa|number=1540117|title=manufacture of bismuth tartrates|inventor=gustav giemsa}}></ref> |
|||
* "Milk of bismuth" (an aqueous solution of [[bismuth hydroxide]] and [[bismuth subcarbonate]]) was marketed as an ailimentary cureall in the early 20th century |
|||
* [[Bismuth subnitrate]] (Bi<sub>5</sub>O(OH)<sub>9</sub>(NO<sub>3</sub>)<sub>4</sub>) and [[bismuth subcarbonate]] (Bi<sub>2</sub>O<sub>2</sub>(CO<sub>3</sub>)) are also used in medicine.<ref name=CRC/> |
|||
Bismuth, as a dense element of high atomic weight, is used in bismuth-impregnated latex shields to shield from X-ray in medical examinations, such as [[X-ray computed tomography|CTs]], mostly as it is considered non-toxic.<ref>{{cite journal|author=Hopper KD|author2=King SH|author3=Lobell ME|author4=TenHave TR|author5=Weaver JS|title= The breast: inplane x-ray protection during diagnostic thoracic CT—shielding with bismuth radioprotective garments|pmid=9393547|date=1997|volume=205|issue=3|pages=853–8|journal=Radiology|doi=10.1148/radiology.205.3.9393547}}</ref> |
|||
===Cosmetics and pigments=== |
|||
[[Bismuth oxychloride]] (BiOCl) is sometimes used in cosmetics, as a pigment in paint for eye shadows, hair sprays and nail polishes.<ref name=k184/><ref name ="Effp">{{cite journal|doi = 10.1016/j.porgcoat.2005.07.003|title = Effect pigments—past, present and future|year = 2005|last1 = Maile|first1 = Frank J.|last2 = Pfaff|first2 = Gerhard|last3 = Reynders|first3 = Peter|journal = Progress in Organic Coatings|volume = 54|issue = 3|page = 150}}</ref><ref name="Paff">{{cite book|url = http://books.google.com/books?id=Q1Pc0aY-vg4C&pg=PA36| page = 36|title = Special effect pigments: Technical basics and applications|isbn = 978-3-86630-905-0|author1 = Pfaff|first1 = Gerhard|year = 2008|publisher=Vincentz Network GmbH}}</ref> This compound is found as the mineral bismoclite and in crystal form contains layers of atoms (see figure above) that refract light chromatically, resulting in an [[iridescent]] appearance similar to [[nacre]] of pearl. It was used as a cosmetic in ancient Egypt and in many places since. ''Bismuth white'' (also "Spanish white") can refer to either bismuth oxychloride or [[bismuth oxynitrate]] (BiONO<sub>3</sub>), when used as a white pigment. |
|||
The European Union's [[Restriction of Hazardous Substances Directive]] (RoHS) for reduction of lead has broadened bismuth's use in electronics as a component of low-melting point solders, as a replacement for traditional tin-lead solders.<ref name=usgs2 /> Its low toxicity will be especially important for solders to be used in food processing equipment and copper water pipes, although it can also be used in other applications including those in the automobile industry, in the European Union, for example.<ref name="lohse">{{cite web|first1 = Joachim |last1 = Lohse|first2 = Stéphanie |last2 = Zangl|first3=Rita|last3=Groß|first4=Carl-Otto|last4=Gensch|first5=Otmar|last5=Deubzer|url = http://ec.europa.eu/environment/waste/pdf/description_layout.pdf| access-date =11 September 2009|title = Adaptation to Scientific and Technical Progress of Annex II Directive 2000/53/EC|publisher=European Commission|date=September 2007}}</ref> |
|||
===Metal and alloys=== |
|||
Bismuth has been evaluated as a replacement for lead in free-machining [[brass]]es for plumbing applications,<ref>{{cite journal|doi = 10.1016/j.matchar.2006.02.005|title = Compositional distributions in classical and lead-free brasses|date = 2006|last1 = La Fontaine|first1 = A.|last2 = Keast|first2 = V. J.|journal = Materials Characterization|volume = 57|issue = 4–5|page = 424}}</ref> although it does not equal the performance of leaded steels.<ref name="lohse" /> |
|||
====Lead replacement==== |
|||
The density difference between lead (density 11.32 g·cm<sup>−3</sup>) and bismuth (density 9.78 g·cm<sup>−3</sup>) is small enough that for many ballistics and weighting applications, bismuth can substitute for lead. For example, it can replace lead as a dense material in fishing sinkers. It has been used as a replacement for lead in [[shotgun|shot]], bullets and [[less-lethal]] [[riot gun]] ammunition. The Netherlands, Denmark, England, Wales, the US, and many other countries now prohibit the use of lead shot for the hunting of wetland birds, as many birds are prone to [[lead poisoning]] owing to mistaken ingestion of lead (instead of small stones and grit) to aid digestion, or even prohibit the use of lead for all hunting, such as in the Netherlands. Bismuth-tin alloy shot is one alternative that provides similar ballistic performance to lead. (Another less expensive but also more poorly performing alternative is "steel" shot, which is actually soft iron.) Bismuth's lack of [[malleability]] does, however, make it unsuitable for use in expanding hunting bullets. |
|||
==== Other metal uses and specialty alloys ==== |
|||
Bismuth, as a dense element of high atomic weight, is used in bismuth-impregnated latex shields to shield from X-ray in medical examinations, such as [[X-ray computed tomography|CTs]], mostly as it is considered non-toxic.<ref>{{cite journal|author=Hopper KD, King SH, Lobell ME, TenHave TR, Weaver JS|title= The breast: inplane x-ray protection during diagnostic thoracic CT—shielding with bismuth radioprotective garments|pmid=9393547|year=1997|volume=205|issue=3|pages=853–8|journal=Radiology}}</ref> |
|||
<!-- *The high diamagnetic susceptibility of bismuth is used for [[Magnetic levitation|diamagnetic levitation]].<ref>{{cite web|url=http://www.instructables.com/id/Magnetic-Levitation-Sculpture/ |title=Magnetic Levitation Sculpture |publisher=Instructables.com |date=30 August 2011 |access-date=14 April 2012}}</ref> --><!-- Hardly a significant application --><!-- *Diamagnetic levitation with bismuth uses blocks of this metal as a shield to provide the levitation of very strong small magnets (typically neodymium, iron, boron compositions). The system must be very well adjusted including many times the action of external strong magnets. Practical examples of experiments can be found in the Internet under the clue "Bismuth Magnetic Levitation" --> |
|||
The [[European Union]]'s [[Restriction of Hazardous Substances Directive]] (RoHS) for reduction of lead has broadened bismuth's use in electronics as a component of low-melting point solders, as a replacement for traditional tin-lead solders.<ref name=usgs2010/> Its low toxicity will be especially important for solders to be used in food processing equipment and copper water pipes, although it can also be used in other applications including those in the automobile industry, in the EU for example.<ref name=lohse>{{cite web|first1 = Joachim |last1 = Lohse|first2 = Stéphanie |last2 = Zangl|first3=Rita|last3=Groß|first4=Carl-Otto|last4=Gensch|first5=Otmar|last5=Deubzer|url = http://ec.europa.eu/environment/waste/pdf/description_layout.pdf|format = PDF| accessdate =11 September 2009|title = Adaptation to Scientific and Technical Progress of Annex II Directive 2000/53/EC|publisher=European Commission|date=September 2007}}</ref> |
|||
Many bismuth [[alloy]]s have low [[melting point]]s and are found in specialty applications such as [[solder]]s. Many automatic sprinklers, electric fuses, and safety devices in fire detection and suppression systems contain the eutectic In<sub>19.1</sub>-Cd<sub>5.3</sub>-Pb<sub>22.6</sub>-Sn<sub>8.3</sub>-Bi<sub>44.7</sub> alloy that melts at {{convert|47|C}}<ref name="CRC" /> This is a convenient temperature since it is unlikely to be exceeded in normal living conditions. Low-melting alloys, such as Bi-Cd-Pb-Sn alloy which melts at {{convert|70|C|F}}, are also used in automotive and aviation industries. Before deforming a thin-walled metal part, it is filled with a melt or covered with a thin layer of the alloy to reduce the chance of breaking. Then the alloy is removed by submerging the part in boiling water.<ref name="k183">[[#Kruger|Krüger]], p. 183.</ref> |
|||
Bismuth is used to make [[free-machining steel]]s and free-machining aluminium alloys for precision machining properties. It has similar effect to lead and improves the chip breaking during machining. The shrinking on solidification in lead and the expansion of bismuth compensate each other and therefore lead and bismuth are often used in similar quantities.<ref>{{cite book|url = https://books.google.com/books?id=Wl1azjcJblIC&pg=PA239|page = 239|title = Steels: Metallurgy and applications|isbn = 978-0-7506-3757-2|last1 = Llewellyn|first1 = D. T.|last2 = Hudd|first2 = Roger C.|date = 1998|publisher=Butterworth-Heinemann}}</ref><ref>{{cite book|url =https://books.google.com/books?id=Lskj5k3PSIcC&pg=PA41| page = 41|title =Aluminum and Aluminum Alloys|isbn =978-0-87170-496-2|last1 =Davis |first1 =J. R.|date =1993|publisher=ASM International}}</ref> Similarly, alloys containing comparable parts of bismuth and lead exhibit a very small change (on the order 0.01%) upon melting, solidification or aging. Such alloys are used in high-precision casting, e.g. in dentistry, to create models and molds.<ref name="k183" /> Bismuth is also used as an alloying agent in production of malleable irons<ref name=usgs2 /> and as a [[thermocouple]] material.<ref name="CRC" /> |
|||
Bismuth has been evaluated as a replacement for lead in free-machining [[brass]]es for [[plumbing]] applications,<ref>{{cite journal|doi = 10.1016/j.matchar.2006.02.005|title = Compositional distributions in classical and lead-free brasses|year = 2006|last1 = La Fontaine|first1 = A.|last2 = Keast|first2 = V.J.|journal = Materials Characterization|volume = 57|issue = 4–5|page = 424}}</ref> although it does not equal the performance of leaded steels.<ref name=lohse/> |
|||
Bismuth is also used in aluminium-silicon cast alloys to refine silicon morphology. However, it indicated a poisoning effect on modification of [[strontium]].<ref>{{cite journal|last=Farahany|first=Saeed|author2=A. Ourdjini|author3=M.H. Idris|author4=L.T. Thai|title=Poisoning effect of bismuth on modification behavior of strontium in LM25 alloy|journal=Journal of Bulletin of Materials Science|date=2011|volume=34|issue=6|pages=1223–1231|doi=10.1007/s12034-011-0239-5|doi-access=free}}</ref><ref>{{cite journal|last=Farahany|first=Saeed|author2=A. Ourdjini|author3=M. H. Idris|author4=L.T. Thai|title=Effect of bismuth on the microstructure of unmodified and Sr-modified Al-7%Si-0.4Mg alloy|journal=Journal of Transactions of Nonferrous Metals Society of China|date=2011|volume=21|issue=7|pages=1455–1464|doi=10.1016/S1003-6326(11)60881-9|s2cid=73719425 }}</ref> Some bismuth alloys, such as Bi<sub>35</sub>-Pb<sub>37</sub>-Sn<sub>25</sub>, are combined with non-sticking materials such as [[mica]], glass and [[Vitreous enamel|enamels]] because they easily wet them allowing to make joints to other parts. Addition of bismuth to caesium enhances the quantum yield of caesium cathodes.<ref name="k184">[[#Kruger|Krüger]], p. 184.</ref> [[Sintering]] of bismuth and manganese powders at {{convert|300|C|F}} produces a permanent magnet and [[magnetostrictive]] material, which is used in ultrasonic generators and receivers working in the 10–100 kHz range and in magnetic and [[Holographic data storage|holographic]] memory devices.<ref name="s15">[[#Suzuki|Suzuki]], p. 15.</ref> |
|||
====Other metal uses and specialty alloys==== |
|||
<!--*The high diamagnetic susceptibility of bismuth is used for [[Magnetic levitation|diamagnetic levitation]].<ref>{{cite web|url=http://www.instructables.com/id/Magnetic-Levitation-Sculpture/ |title=Magnetic Levitation Sculpture |publisher=Instructables.com |date=2011-08-30 |accessdate=2012-04-14}}</ref> --><!-- Hardly a significant application--> |
|||
Many bismuth [[alloy]]s have low [[melting point]]s and are found in specialty applications such as [[solder]]s. Many automatic sprinklers, electric fuses, and safety devices in fire detection and suppression systems contain the eutectic In19.1-Cd5.3-Pb22.6-Sn8.3-Bi44.7 alloy that melts at {{convert|47|C}}<ref name=CRC/> This is a convenient temperature since it unlikely to be exceeded in normal living conditions. Low-melting alloys, such as Bi-Cd-Pb-Sn alloy which melts at 70 °C, are also used in automotive and aviation industries. Before deforming a thin-walled metal part, it is filled with a melt or covered with a thin layer of the alloy to reduce the chance of breaking. Then the alloy is removed by submerging the part in boiling water.<ref name=k183>Krüger, p. 183.</ref> |
|||
=== Other uses as compounds === |
|||
Bismuth is used to make [[free-machining steel]]s and free-machining aluminium alloys for precision machining properties. It has similar effect to lead and improves the chip breaking during machining. The shrinking on solidification in lead and the expansion of bismuth compensates each other and therefore lead and bismuth are often used in similar quantities.<ref>{{cite book|url = http://books.google.com/books?id=Wl1azjcJblIC&pg=PA239|page = 239|title = Steels: Metallurgy and applications|isbn = 978-0-7506-3757-2|author1 = Llewellyn|first1 = D. T|last2 = Hudd|first2 = Roger C|year = 1998|publisher=Butterworth-Heinemann}}</ref><ref>{{cite book|url =http://books.google.com/books?id=Lskj5k3PSIcC&pg=PA41| page = 41|title =Aluminum and Aluminum Alloys|isbn =978-0-87170-496-2|author1 =Davis & Associates|first1 =J.R|last2 =Handbook Committee|first2 =ASM International|year =1993}}</ref> Similarly, alloys containing comparable parts of bismuth and exhibit a very small change (on the order 0.01%) upon melting, solidification or aging. Such alloys are used in high-precision casting, e.g. in dentistry, to create models and molds.<ref name=k183/> Bismuth is also used as an alloying agent in production of malleable irons and as a [[thermocouple]] material.<ref name=CRC/><ref name=usgs2010/> |
|||
[[File:Bismuthvanadat.jpg|thumb|[[Bismuth vanadate]], a yellow pigment]] |
|||
Some bismuth alloys, such as Bi35-Pb37-Sn25, are combined with non-sticking materials such as [[mica]], glass and [[Vitreous enamel|enamels]] because they easily wet them allowing to make joints to other parts. Addition of bismuth to caesium enhances the quantum yield of Cs cathodes.<ref name=k184>Krüger, p. 184.</ref> [[Sintering]] of bismuth and manganese powders at 300 °C produces a permanent magnet and [[magnetostrictive]] material, which is used in ultrasonic generators and receivers working in the 10–100 kHz range and in magnetic memory devices.<ref name=s15>Suzuki, p. 15.</ref> |
|||
* Bismuth is included in [[BSCCO]] (bismuth strontium calcium copper oxide), which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.<ref>{{cite web|publisher = National High Magnetic Field Laboratory|title = BSCCO|url = http://www.magnet.fsu.edu/magnettechnology/research/asc/research/bscco.html|access-date = 18 January 2010|archive-url = https://web.archive.org/web/20130412234316/http://www.magnet.fsu.edu/magnettechnology/research/asc/research/bscco.html|archive-date = 12 April 2013|url-status = dead}}</ref><!-- 9780199565917Oxford University Press, 2009Laszlo Solymar, Donald WalshElectrical Properties of Materials https://books.google.com/books?id=AiWyp0NQW6UC&pg=PA389 --> |
|||
* [[Bismuth telluride]] is a semiconductor and an excellent [[thermoelectric effect|thermoelectric]] material.<ref name="k184" /><ref>{{cite book |url = https://books.google.com/books?id=jO3nzAbzAWYC&pg=PA12|page = 12 |title = Recent trends in thermoelectric materials research |isbn = 978-0-12-752178-7 |last1 = Tritt |first1 = Terry M. |date = 2000|publisher=Academic Press}}</ref> Bi<sub>2</sub>Te<sub>3</sub> diodes are used in mobile refrigerators, [[CPU]] coolers, and as detectors in [[infrared]] spectrophotometers.<ref name="k184" /> |
|||
* [[Bismuth oxide]], in its delta form, is a solid electrolyte for oxygen. This form normally breaks down below a high-temperature threshold, but can be electrodeposited well below this temperature in a highly alkaline solution.<ref>{{cite book | last1=Maric | first1=Radenka | last2=Mirshekari | first2=Gholamreza | title=Solid oxide fuel cells : from fundamental principles to complete systems | publication-place=Boca Raton | date=2020 | isbn=978-0-429-52784-5 | oclc=1228350036 | page=70}}</ref> |
|||
* [[Bismuth germanate]] is a scintillator, widely used in X-ray and gamma ray detectors.<ref>{{cite book | last=Saha | first=Gopal B. | title=Physics and radiobiology of nuclear medicine | publisher=Springer | publication-place=New York | date=2006 | isbn=978-0-387-36281-6 | oclc=655784658 | page=82}}</ref> |
|||
* [[Bismuth vanadate]] is an opaque yellow pigment used by some artists' oil, [[Acrylic paint|acrylic]], and watercolor paint companies, primarily as a replacement for the more toxic [[cadmium sulfide]] yellows in the greenish-yellow (lemon) to orange-toned yellow range. It performs practically identically to the cadmium pigments, such as in terms of resistance to degradation from UV exposure, opacity, tinting strength, and lack of reactivity when mixed with other pigments. The most commonly-used variety by artists' paint makers is lemon in color. In addition to being a replacement for several cadmium yellows, it also serves as a non-toxic visual replacement for the older chromate pigments made with zinc, lead, and strontium. If a green pigment and barium sulfate (for increased transparency) are added it can also serve as a replacement for [[barium chromate]], which possesses a more greenish cast than the others. In comparison with [[lead chromate]], it does not blacken due to [[hydrogen sulfide]] in the air (a process accelerated by UV exposure) and possesses a particularly brighter color than them, especially the lemon, which is the most translucent, dull, and fastest to blacken due to the higher percentage of lead sulfate required to produce that shade. It is also used, on a limited basis due to its cost, as a vehicle paint pigment.<ref>{{cite journal|doi = 10.1016/j.dyepig.2005.08.027|title = The photochromic effect of bismuth vanadate pigments: Investigations on the photochromic mechanism|date = 2007|last1 = Tücks|first1 = Andreas|last2 = Beck|first2 = Horst P.|journal = Dyes and Pigments|volume = 72|issue = 2|page = 163}}</ref><ref>{{cite book|chapter-url = https://books.google.com/books?id=WZV_hX9u0yIC&pg=PA92|chapter = Yellow pigments|pages = 91–93|title = Coloring of plastics: Fundamentals, colorants, preparations|isbn = 978-1-56990-352-0|author = Müller, Albrecht|publisher=Hanser Verlag|date = 2003}}</ref> |
|||
* A [[catalyst]] for making [[acrylic fibers]].<ref name="CRC" /> |
|||
* As an [[electrocatalyst]] in the conversion of CO<sub>2</sub> to CO.<ref>{{Cite journal|title=Selective conversion of CO<sub>2</sub> to CO with high efficiency using an bismuth-based electrocatalyst|author=DiMeglio, John L.|author2=Rosenthal, Joel |date=2013 |journal=Journal of the American Chemical Society|volume=135 |issue=24 |pages=8798–8801|doi=10.1021/ja4033549|pmid=23735115|pmc=3725765}}</ref> |
|||
* Ingredient in [[lubrication|lubricating]] [[grease (lubricant)|greases]].<ref>{{cite book |url =https://books.google.com/books?id=YTa5TsL0KnIC&pg=PA430|page = 430 |title =Chemistry and Technology of Lubricants |isbn =978-1-4020-8661-8 |last1 =Mortier |first1 =Roy M. |last2 =Fox |first2 =Malcolm F. |last3 =Orszulik |first3 =Stefan T. |date =2010|publisher=Springer|bibcode = 2010ctl..book.....M }}</ref> |
|||
* In crackling microstars ([[dragon's egg]]s) in [[pyrotechnics]], as the [[Bismuth(III) oxide|oxide]], [[Bismuth subcarbonate|subcarbonate]] or subnitrate.<ref>{{cite journal|doi = 10.1016/j.atmosenv.2010.05.048|title = Emission factors and exposures from ground-level pyrotechnics|date = 2010|last1 = Croteau|first1 = Gerry|last2 = Dills|first2 = Russell|last3 = Beaudreau|first3 = Marc|last4 = Davis|first4 = Mac|journal = Atmospheric Environment|volume = 44|issue = 27|page = 3295|bibcode = 2010AtmEn..44.3295C }}</ref><ref>{{cite book|url = https://books.google.com/books?id=370UwG8CuNwC&pg=PA518|title = The Preparatory Manual of Black Powder and Pyrotechnics|isbn = 978-1-4116-8574-1|last1 = Ledgard|first1 = Jared|date = 2006|publisher=Lulu|pages=207, 319, 370, 518, search}}</ref> |
|||
*As catalyst for the fluorination of arylboronic pinacol esters through a Bi(III)/Bi(V) catalytic cycle, mimicking transition metals in electrophilic fluorination.<ref>{{cite journal|doi =10.1126/science.aaz2258 |title = Fluorination of arylboronic esters enabled by bismuth redox catalysis|date = 2020|last1 = Planas|first1 = Oriol|last2 = Wang|first2 = Feng|last3 = Leutzsch|first3 = Markus|last4 = Cornella|first4 = Josep|journal = Science|volume = 367|issue = 6475|pages = 313–317|pmid = 31949081|bibcode = 2020Sci...367..313P|s2cid = 210698047|doi-access = free|hdl = 21.11116/0000-0005-DB57-3|hdl-access = free}}</ref> |
|||
== Toxicology and ecotoxicology == |
|||
===Other uses as compounds=== |
|||
:''See also [[bismuthia]], a rare dermatological condition that results from the prolonged use of bismuth.'' |
|||
[[File:Bismuthvanadat.jpg|thumb|right| Bismuth vanadate, a yellow pigment]] |
|||
* Bismuth is included in [[BSCCO]] (bismuth strontium calcium copper oxide) which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.<ref>{{cite web|accessdate =19 January 2010|publisher = National High Magnetic Field Laboratory|title = BSCCO| url = http://www.magnet.fsu.edu/magnettechnology/research/asc/research/bscco.html}}</ref><!--9780199565917Oxford University Press, 2009Laszlo Solymar, Donald WalshElectrical Properties of Materials http://books.google.com/books?id=AiWyp0NQW6UC&pg=PA389--> |
|||
* [[Bismuth subnitrate]] is a component of [[ceramic glaze|glazes]] that produces an [[iridescence]] and is used as a pigment in paint. |
|||
* [[Bismuth telluride]] is a semiconductor and an excellent [[thermoelectric effect|thermoelectric]] material.<ref name=k184/><ref>{{cite book |url = http://books.google.com/books?id=jO3nzAbzAWYC&pg=PA12|page = 12 |title = Recent trends in thermoelectric materials research |isbn = 978-0-12-752178-7 |author1 = Tritt |first1 = Terry M |year = 2000|publisher=Academic Press}}</ref> Bi<sub>2</sub>Te<sub>3</sub> diodes are used in mobile refrigerators, [[CPU]] coolers, and as detectors in [[infrared]] spectrophotometers.<ref name=k184/> |
|||
* [[Bismuth oxide]], in its delta form, is a solid electrolyte for oxygen. This form normally only exists above{{clarify|date=August 2012|reason=temp not given}} and breaks down below a high-temperature threshold, but can be electrodeposited well below this temperature in a highly alkaline solution. |
|||
* [[Bismuth vanadate]] is an opaque yellow pigment in artists' oil and acrylic paint. This compound is a non-toxic lightfast substitute for lemon yellow pigments such as the cadmium sulfides and the lead/strontium/barium [[chromate]]s. Unlike lead chromate+lead sulfate lemon, bismuth vanadate does not readily blacken with UV exposure.<ref>{{cite journal|doi = 10.1016/j.dyepig.2005.08.027|title = The photochromic effect of bismuth vanadate pigments: Investigations on the photochromic mechanism|year = 2007|last1 = Tücks|first1 = Andreas|last2 = Beck|first2 = Horst P.|journal = Dyes and Pigments|volume = 72|issue = 2|page = 163}}</ref><ref>{{cite book|url = http://books.google.com/books?id=WZV_hX9u0yIC&pg=PA92|chapter = Yellow pigments|pages = 91–93|title = Coloring of plastics: Fundamentals, colorants, preparations|isbn = 978-1-56990-352-0|author = Müller, Albrecht|publisher=Hanser Verlag|year = 2003}}</ref> |
|||
* A catalyst for making acrylic fibers.<ref name=CRC/> |
|||
* Ingredient in [[lubrication|lubricating]] [[grease (lubricant)|greases]].<ref>{{cite book |url =http://books.google.com/books?id=YTa5TsL0KnIC&pg=PA430|page = 430 |title =Chemistry and Technology of Lubricants |isbn =978-1-4020-8661-8 |author1 =Mortier |first1 =Roy M |last2 =Fox |first2 =Malcolm F |last3 =Orszulik |first3 =Stefan T |year =2010|publisher=Springer}}</ref> |
|||
* In crackling microstars ([[dragon's eggs]]) in [[pyrotechnics]], as the [[Bismuth(III) oxide|oxide]], [[Bismuth subcarbonate|subcarbonate]] or subnitrate.<ref>{{cite journal|doi = 10.1016/j.atmosenv.2010.05.048|title = Emission factors and exposures from ground-level pyrotechnics|year = 2010|last1 = Croteau|first1 = Gerry|last2 = Dills|first2 = Russell|last3 = Beaudreau|first3 = Marc|last4 = Davis|first4 = Mac|journal = Atmospheric Environment|volume = 44|issue = 27|page = 3295}}</ref><ref>{{cite book|url = http://books.google.com/books?id=370UwG8CuNwC&pg=PA518|title = The Preparatory Manual of Black Powder and Pyrotechnics|isbn = 978-1-4116-8574-1|author1 = Ledgard|first1 = Jared|year = 2006|publisher=Lulu|pages=207, 319, 370, 518, search}}</ref> |
|||
Scientific literature indicates that some of the compounds of bismuth are less toxic to humans via ingestion than other heavy metals (lead, arsenic, antimony, etc.)<ref name="Kean" /> presumably due to the comparatively low solubility of bismuth salts.<ref name=":0" /> Its [[biological half-life]] for whole-body retention is reported to be 5 days but it can remain in the kidney for years in people treated with bismuth compounds.<ref name="Fowler">{{cite book |chapter-url = https://books.google.com/books?id=nKulgztuzL8C&pg=PA433| pages = 433 ff|chapter=Bismuth|title = Handbook on the toxicology of metals|publisher=Academic Press |isbn = 978-0-12-369413-3 |author = Fowler, B.A. |author2 = Sexton M.J. |name-list-style = amp |editor=Nordberg, Gunnar |date= 2007}}</ref> |
|||
==Toxicology and ecotoxicology== |
|||
Scientific literature concurs that bismuth and most of its compounds are less toxic compared to other heavy metals (lead, antimony, etc.) and that it is not bioaccumulative. They have low solubilities in the blood, are easily removed with urine, and showed no [[carcinogenic]], [[mutagenic]] or [[teratogenic]] effects in long-term tests on animals (up to 2 years).<ref>Suzuki, pp. 19–20.</ref> Its biological half-life for whole-body retention is 5 days but it can remain in the kidney for years in patients treated with bismuth compounds.<ref name=Fowler/> |
|||
Bismuth poisoning |
Bismuth poisoning can occur and has according to some reports been common in relatively recent times.<ref name=":0">{{Cite journal|last=DiPalma|first=Joseph R.|year=2001|title=Bismuth Toxicity, Often Mild, Can Result in Severe Poisonings|journal=Emergency Medicine News|volume=23 |issue=3|page=16|doi=10.1097/00132981-200104000-00012}}</ref><ref name="Lenntech">[http://www.lenntech.com/periodic/elements/bi.htm Data on Bismuth's health and environmental effects]. Lenntech.com. Retrieved on 17 December 2011.</ref> As with lead, bismuth poisoning can result in the formation of a black deposit on the [[gingiva]], known as a bismuth line.<ref name="biline">[http://medical-dictionary.thefreedictionary.com/bismuth+line "Bismuth line"] in ''TheFreeDictionary's Medical dictionary''. Farlex, Inc.</ref><ref>{{cite journal|doi = 10.1111/j.1365-2133.1973.tb01932.x|title = Drug induced changes in pigmentation|date = 1973|last1 = Levantine|first1 = Ashley|last2 = Almeyda|first2 = John|journal = British Journal of Dermatology|volume = 89|pages = 105–12|pmid = 4132858|issue = 1|s2cid = 7175799}}</ref><ref>[[#Kruger|Krüger]], pp. 187–188.</ref> Poisoning may be treated with [[dimercaprol]]; however, evidence for benefit is unclear.<ref name="WHO2008">{{cite book | title = WHO Model Formulary 2008 | year = 2009 | isbn = 9789241547659 | vauthors = ((World Health Organization)) | veditors = Stuart MC, Kouimtzi M, Hill SR | hdl = 10665/44053 | author-link = World Health Organization | publisher = World Health Organization | hdl-access=free | page=62 }}</ref><ref name="AHFS2016">{{cite web|title=Dimercaprol|url=https://www.drugs.com/monograph/dimercaprol.html|publisher=The American Society of Health-System Pharmacists|access-date= 8 December 2016}}</ref> |
||
Bismuth's environmental impacts are not well known; it may be less likely to bioaccumulate than some other heavy metals, and this is an area of active research.<ref>{{Cite journal|last=Boriova|display-authors=et al|year=2015|title=Bismuth(III) Volatilization and Immobilization by Filamentous Fungus ''Aspergillus clavatus'' During Aerobic Incubation|journal=Archives of Environmental Contamination and Toxicology|volume=68 |issue=2|pages=405–411|doi=10.1007/s00244-014-0096-5|pmid=25367214|bibcode=2015ArECT..68..405B |s2cid=30197424}}</ref><ref>{{Cite journal|last=Boriova|display-authors=et al|year=2013|title=Bioaccumulation and biosorption of bismuth Bi (III) by filamentous fungus ''Aspergillus clavatus''|url=http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/44/078/44078300.pdf|journal=Student Scientific Conference PriF UK 2013. Proceedings of Reviewed Contributions}}</ref> |
|||
Bismuth's environmental impacts are not very well known. It is considered that its environmental impact is small, due in part to the low solubility of its compounds.<ref name = Lenntech>[http://www.lenntech.com/periodic/elements/bi.htm Data on Bismuth's health and environmental effects]. Lenntech.com. Retrieved on 17 December 2011.</ref> Limited information however means that a close eye should be kept on its impact.<ref name=Fowler>{{cite book |url = http://books.google.com/books?id=nKulgztuzL8C&pg=PA433| pages = 433 ff.|chapter=Bismuth|title = Handbook on the toxicology of metals|publisher=Academic Press |isbn = 978-0-12-369413-3 |author = Fowler, B.A. and Sexton M.J. |editor=Nordberg, Gunnar |year= 2007}}</ref><ref>{{cite book |url =http://books.google.com/books?id=5UMGDcCbJ-MC&pg=PA504|page = 504 |title =Organometallics in Environment and Toxicology |isbn =978-1-84755-177-1 |author1 =Sigel |first1 =Astrid |year =2010|publisher=Royal Society of Chemistry|place=London}}</ref><ref>{{cite journal|doi = 10.1111/j.1365-2710.1989.tb00268.x|title = Bismuth Toxicity—A Reassessment|year = 1989|last1 = Bradley|first1 = B.|last2 = Singleton|first2 = M.|last3 = Po|first3 = A. Li Wan|journal = Journal of Clinical Pharmacy and Therapeutics|volume = 14|issue = 6|pages = 423–41|pmid = 2693474}}</ref> |
|||
<!-- |
|||
== See also == |
|||
<ref >{{cite journal|pmid =392661|year =1979|last1 =Serfontein|first1 =WJ|last2 =Mekel|first2 =R|title =Bismuth toxicity in man II. Review of bismuth blood and urine levels in patients after administration of therapeutic bismuth formulations in relation to the problem of bismuth toxicity in man|volume =26|issue =2|pages =391–411|journal =Research communications in chemical pathology and pharmacology}}</ref> |
|||
<ref >{{cite journal|pmid = 523778|year = 1979|last1 = Serfontein|first1 = WJ|last2 = Mekel|first2 = R|last3 = Bank|first3 = S|last4 = Barbezat|first4 = G|last5 = Novis|first5 = B|title = Bismuth toxicity in man – I. Bismuth blood and urine levels in patients after administration of a bismuth protein complex (Bicitropeptide)|volume = 26|issue = 2|pages = 383–9|journal = Research communications in chemical pathology and pharmacology}}</ref> |
|||
<ref >{{cite journal|pmid = 2682129|year = 1989|last1 = Slikkerveer|first1 = A|last2 = De Wolff|first2 = FA|title = Pharmacokinetics and toxicity of bismuth compounds|volume = 4|issue = 5|pages = 303–23|journal = Medical toxicology and adverse drug experience|doi = 10.1007/BF03259915}}</ref> |
|||
<ref >{{cite journal|pmid = 3189127|year = 1988|last1 = Dipalma|first1 = JR|title = Bismuth toxicity|volume = 38|issue = 5|pages = 244–6|journal = American family physician}}</ref>--> |
|||
==See also== |
|||
{{Wikipedia books|Bismuth}} |
|||
* [[Lead-bismuth eutectic]] |
|||
* [[:Category:Bismuth minerals|Bismuth minerals]] |
* [[:Category:Bismuth minerals|Bismuth minerals]] |
||
* [[Arsenic-bismuth]] |
|||
==Notes== |
|||
{{Notelist}} |
|||
==References== |
== References == |
||
{{Reflist| |
{{Reflist|30em}} |
||
== |
== Cited sources == |
||
{{Source-attribution| Brown, R. D., Jr. "Annual Average Bismuth Price", USGS (1998) |
{{Source-attribution| Brown, R. D., Jr. "Annual Average Bismuth Price", USGS (1998)}}. |
||
* Greenwood, N. N. |
* {{cite book|ref=Greenwood|author=Greenwood, N. N.|author2=Earnshaw, A.|name-list-style=amp |date=1997|title=Chemistry of the Elements|edition=2nd|place= Oxford|publisher= Butterworth-Heinemann|isbn=978-0-7506-3365-9}} |
||
* Krüger, Joachim |
* {{cite book|ref=Kruger|author=Krüger, Joachim|author2=Winkler, Peter|author3=Lüderitz, Eberhard|author4=Lück, Manfred|author5=Wolf, Hans Uwe|chapter=Bismuth, Bismuth Alloys, and Bismuth Compounds|title=Ullmann's Encyclopedia of Industrial Chemistry|date= 2003|publisher= Wiley-VCH, Weinheim|pages=171–189 |doi=10.1002/14356007.a04_171|isbn=978-3527306732}} |
||
* {{cite book|author=Suzuki, Hitomi |title=Organobismuth Chemistry|url= |
* {{cite book|ref=Suzuki|author=Suzuki, Hitomi |title=Organobismuth Chemistry|url=https://books.google.com/books?id=qODswAbaBmsC&pg=PA8|date=2001|publisher=Elsevier|isbn=978-0-444-20528-5|pages=1–20}} |
||
* {{cite book |
* {{cite book|ref=Wiberg |
||
| title = Inorganic chemistry |
| title = Inorganic chemistry |
||
| last1= Wiberg | first1=Egon| last2= Holleman | first2=A. F. |last3= Wiberg | first3=Nils |
| last1= Wiberg | first1=Egon| last2= Holleman | first2=A. F. |last3= Wiberg | first3=Nils |
||
| publisher = Academic Press |
| publisher = Academic Press |
||
| |
| date = 2001 |
||
| isbn = 0-12-352651- |
| isbn = 978-0-12-352651-9 |
||
}} |
}} |
||
==External links== |
== External links == |
||
{{Commons |
{{Commons}} |
||
{{ |
{{Wiktionary|bismuth}} |
||
* [[:File:Bismuth-501g.jpg|Laboratory growth of large crystals of Bismuth]] by Jan Kihle Crystal Pulling Laboratories, Norway |
|||
* [http://www.periodicvideos.com/videos/083.htm Bismuth] at ''[[The Periodic Table of Videos]]'' (University of Nottingham) |
* [http://www.periodicvideos.com/videos/083.htm Bismuth] at ''[[The Periodic Table of Videos]]'' (University of Nottingham) |
||
* [http://physicsworld.com/cws/article/news/2003/apr/23/bismuth-breaks-half-life-record-for-alpha-decay Bismuth breaks half-life record for alpha decay] |
|||
* [http://www.amazingrust.com/Experiments/how_to/Bismuth_Crystals.html Bismuth Crystals – Instructions & Pictures] |
* [http://www.amazingrust.com/Experiments/how_to/Bismuth_Crystals.html Bismuth Crystals – Instructions & Pictures] |
||
{{clear}} |
|||
{{ |
{{Periodic table (navbox)}} |
||
{{Bismuth compounds}} |
{{Bismuth compounds}} |
||
{{Bismuthides}} |
|||
{{Authority control}} |
|||
{{Use dmy dates|date=March 2012}} |
|||
{{Good article}} |
|||
[[Category:Bismuth| ]] |
[[Category:Bismuth| ]] |
||
[[Category:Chemical elements]] |
[[Category:Chemical elements]] |
||
[[Category:Minerals in space group 166]] |
|||
[[Category:Native element minerals]] |
|||
[[Category:Pnictogens]] |
|||
[[Category:Post-transition metals]] |
|||
[[Category:Alchemical substances]] |
[[Category:Alchemical substances]] |
||
[[Category:Post-transition metals]] |
|||
[[Category:Poor metals]] |
|||
[[Category:Pnictogens]] |
|||
[[Category:Trigonal minerals]] |
[[Category:Trigonal minerals]] |
||
[[Category:Materials that expand upon freezing]] |
|||
[[Category:Chemical elements with rhombohedral structure]] |
|||
Latest revision as of 16:06, 5 December 2024
 | ||||||||||||||||||||||||||||||||||||||
| Bismuth | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈbɪzməθ/ | |||||||||||||||||||||||||||||||||||||
| Appearance | lustrous brownish silver | |||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Bi) | ||||||||||||||||||||||||||||||||||||||
| Bismuth in the periodic table | ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 83 | |||||||||||||||||||||||||||||||||||||
| Group | group 15 (pnictogens) | |||||||||||||||||||||||||||||||||||||
| Period | period 6 | |||||||||||||||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s2 6p3 | |||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 5 | |||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||||||
| Melting point | 544.7 K (271.5 °C, 520.7 °F) | |||||||||||||||||||||||||||||||||||||
| Boiling point | 1837 K (1564 °C, 2847 °F) | |||||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 9.807 g/cm3 [3] | |||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 10.05 g/cm3 | |||||||||||||||||||||||||||||||||||||
| Heat of fusion | 11.30 kJ/mol | |||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 179 kJ/mol | |||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 25.52 J/(mol·K) | |||||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||
| Oxidation states | common: +3 −3,[4] −2,? −1,? 0,[5] +1,? +2,? +4,? +5[4] | |||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.02 | |||||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 156 pm | |||||||||||||||||||||||||||||||||||||
| Covalent radius | 148±4 pm | |||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 207 pm | |||||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||||
| Crystal structure | rhombohedral (hR2) | |||||||||||||||||||||||||||||||||||||
| Lattice constants | a = 0.47458 nm α = 57.236° ah = 0.45462 nm ch = 1.18617 nm (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||||
| Thermal expansion | 13.09×10−6/K (at 20 °C)[a] | |||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 7.97 W/(m⋅K) | |||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 1.29 µΩ⋅m (at 20 °C) | |||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | |||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −280.1×10−6 cm3/mol[6] | |||||||||||||||||||||||||||||||||||||
| Young's modulus | 32 GPa | |||||||||||||||||||||||||||||||||||||
| Shear modulus | 12 GPa | |||||||||||||||||||||||||||||||||||||
| Bulk modulus | 31 GPa | |||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 1790 m/s (at 20 °C) | |||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.33 | |||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.25 | |||||||||||||||||||||||||||||||||||||
| Brinell hardness | 70–95 MPa | |||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-69-9 | |||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||
| Discovery | Arabic alchemists (before AD 1000) | |||||||||||||||||||||||||||||||||||||
| Isotopes of bismuth | ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
Bismuth is a chemical element with the symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs naturally, and its sulfide and oxide forms are important commercial ores. The free element is 86% as dense as lead. It is a brittle metal with a silvery-white color when freshly produced. Surface oxidation generally gives samples of the metal a somewhat rosy cast. Further oxidation under heat can give bismuth a vividly iridescent appearance due to thin-film interference. Bismuth is both the most diamagnetic element and one of the least thermally conductive metals known.
Bismuth used to be considered the element with the highest atomic mass whose nuclei do not spontaneously decay. However, in 2003 it was discovered to be extremely weakly radioactive. The metal's only primordial isotope, bismuth-209, undergoes alpha decay with a half-life about a billion times the estimated age of the universe.[8][9]
Bismuth metal has been known since ancient times. Before modern analytical methods bismuth's metallurgical similarities to lead and tin often led it to be confused with those metals. The etymology of "bismuth" is uncertain. The name may come from mid-sixteenth century Neo-Latin translations of the German words weiße Masse or Wismuth, meaning 'white mass', which were rendered as bisemutum or bisemutium.
Bismuth compounds account for about half the global production of bismuth. They are used in cosmetics; pigments; and a few pharmaceuticals, notably bismuth subsalicylate, used to treat diarrhea.[9] Bismuth's unusual propensity to expand as it solidifies is responsible for some of its uses, as in the casting of printing type.[9] Bismuth, when in its elemental form, has unusually low toxicity for a heavy metal.[9] As the toxicity of lead and the cost of its environmental remediation became more apparent during the 20th century, suitable bismuth alloys have gained popularity as replacements for lead. Presently, around a third of global bismuth production is dedicated to needs formerly met by lead.
History and etymology
[edit]Bismuth metal has been known since ancient times and it was one of the first 10 metals to have been discovered. The name bismuth dates to around 1665 and is of uncertain etymology. The name possibly comes from obsolete German Bismuth, Wismut, Wissmuth (early 16th century), perhaps related to Old High German hwiz ("white").[10] The Neo-Latin bisemutium (coined by Georgius Agricola, who Latinized many German mining and technical words) is from the German Wismuth, itself perhaps from weiße Masse, meaning "white mass".[11][12]
The element was confused in early times with tin and lead because of its resemblance to those elements. Because bismuth has been known since ancient times, no one person is credited with its discovery. Agricola (1546) states that bismuth is a distinct metal in a family of metals including tin and lead. This was based on observation of the metals and their physical properties.[13]
Miners in the age of alchemy also gave bismuth the name tectum argenti, or "silver being made" in the sense of silver still in the process of being formed within the Earth.[14][15][16]
Bismuth was also known to the Incas and used (along with the usual copper and tin) in a special bronze alloy for knives.[17]

Beginning with Johann Heinrich Pott in 1738,[18] Carl Wilhelm Scheele, and Torbern Olof Bergman, the distinctness of lead and bismuth became clear, and Claude François Geoffroy demonstrated in 1753 that this metal is distinct from lead and tin.[15][19][20]
Characteristics
[edit]
Physical characteristics
[edit]
Bismuth is a brittle metal with a dark, silver-pink hue, often with an iridescent oxide tarnish showing many colors from yellow to blue. The spiral, stair-stepped structure of bismuth crystals is the result of a higher growth rate around the outside edges than on the inside edges. The variations in the thickness of the oxide layer that forms on the surface of the crystal cause different wavelengths of light to interfere upon reflection, thus displaying a rainbow of colors. When burned in oxygen, bismuth burns with a blue flame and its oxide forms yellow fumes.[19] Its toxicity is much lower than that of its neighbors in the periodic table, such as lead and antimony.[21]
No other metal is verified to be more naturally diamagnetic than bismuth.[19][22] (Superdiamagnetism is a different physical phenomenon.) Of any metal, it has one of the lowest values of thermal conductivity (after manganese, neptunium and plutonium) and the highest Hall coefficient.[23] It has a high electrical resistivity.[19] When deposited in sufficiently thin layers on a substrate, bismuth is a semiconductor, despite being a post-transition metal.[24] Elemental bismuth is denser in the liquid phase than the solid, a characteristic it shares with germanium, silicon, gallium, and water.[25] Bismuth expands 3.32% on solidification; therefore, it was long a component of low-melting typesetting alloys, where it compensated for the contraction of the other alloying components[19][26][27][28] to form almost isostatic bismuth-lead eutectic alloys.
Though virtually unseen in nature, high-purity bismuth can form distinctive, colorful hopper crystals. It is relatively nontoxic and has a low melting point just above 271 °C (520 °F), so crystals may be grown using a household stove, although the resulting crystals will tend to be of lower quality than lab-grown crystals.[29]
At ambient conditions, bismuth shares the same layered structure as the metallic forms of arsenic and antimony,[30] crystallizing in the rhombohedral lattice.[31] When compressed at room temperature, this Bi–I structure changes first to the monoclinic Bi-II at 2.55 GPa, then to the tetragonal Bi-III at 2.7 GPa, and finally to the body-centered cubic Bi-V at 7.7 GPa. The corresponding transitions can be monitored via changes in electrical conductivity; they are rather reproducible and abrupt and are therefore used for calibration of high-pressure equipment.[32][33]
Chemical characteristics
[edit]Bismuth is stable to both dry and moist air at ordinary temperatures. When red-hot, it reacts with water to make bismuth(III) oxide.[34]
- 2 Bi + 3 H2O → Bi2O3 + 3 H2
It reacts with fluorine to form bismuth(V) fluoride at 500 °C (932 °F) or bismuth(III) fluoride at lower temperatures (typically from Bi melts); with other halogens it yields only bismuth(III) halides.[35][36][37] The trihalides are corrosive and easily react with moisture, forming oxyhalides with the formula BiOX.[38]
- 4 Bi + 6 X2 → 4 BiX3 (X = F, Cl, Br, I)
- 4 BiX3 + 2 O2 → 4 BiOX + 4 X2
Bismuth dissolves in concentrated sulfuric acid to make bismuth(III) sulfate and sulfur dioxide.[34]
- 6 H2SO4 + 2 Bi → 6 H2O + Bi2(SO4)3 + 3 SO2
It reacts with nitric acid to make bismuth(III) nitrate (which decomposes into nitrogen dioxide when heated[39]).[40]
- Bi + 6 HNO3 → 3 H2O + 3 NO2 + Bi(NO3)3
It also dissolves in hydrochloric acid, but only with oxygen present.[34]
- 4 Bi + 3 O2 + 12 HCl → 4 BiCl3 + 6 H2O
Isotopes
[edit]The only primordial isotope of bismuth, bismuth-209, was regarded as the heaviest stable nuclide, but it had long been suspected[41] to be unstable on theoretical grounds. This was finally demonstrated in 2003, when researchers at the Institut d'astrophysique spatiale in Orsay, France, measured the alpha (α) decay half-life of 209Bi to be 2.01×1019 years (3 Bq/Mg),[42][43] over 109 times longer than the estimated age of the universe.[9] Due to its hugely long half-life, for all known medical and industrial applications, bismuth can be treated as stable. The radioactivity is of academic interest because bismuth is one of a few elements whose radioactivity was suspected and theoretically predicted before being detected in the laboratory.[9] Bismuth has the longest known α-decay half-life, though tellurium-128 has a double beta decay half-life of over 2.2×1024 years.[43]
Six isotopes of bismuth with short half-lives (210–215 inclusive) occur in the natural radioactive decay chains of actinium, radium, thorium, and neptunium; and more have been synthesized. (Though all primordial 237Np has long since decayed, it is continually regenerated by (n,2n) knockout reactions on natural 238U.)[44][45]
Commercially, bismuth-213 can be produced by bombarding radium with bremsstrahlung photons from a linear particle accelerator. In 1997, an antibody conjugate with bismuth-213, which has a 45-minute half-life and α-decays, was used to treat leukemia patients. This isotope has also been tried in cancer treatment, for example, in the targeted alpha therapy (TAT) program.[46][47]
Chemical compounds
[edit]
Chemically, bismuth resembles arsenic and antimony, but is much less toxic.[21] In almost all known compounds, bismuth has oxidation state +3; a few have states +5 or −3.
The trioxide[25][48] and trisulfide can both be made from the elements,[49][36] although the trioxide is extremely corrosive at high temperatures.[37] The pentoxide is not stable at room temperature, and will evolve O
2 gas if heated.[50] Both oxides form complex anions,[51][52] and NaBiO3 is a strong oxidising agent.[52] The trisulfide is common in bismuth ore.[49]
Similarly, bismuth forms all possible trihalides, but the only pentahalide is BiF5. All are Lewis acids.[34] Bismuth forms several formally-BiI halides; these are complex salts with unusually-structured polyatomic cations and anions.[51][53]

In strongly acidic aqueous solution, the Bi3+
ion solvates to form Bi(H
2O)3+
8.[54] As pH increases, the cations polymerize until the octahedral bismuthyl complex [Bi
6O
4(OH)
4]6+
,[55] often abbreviated BiO+. Although bismuth oxychloride and bismuth oxynitrate have stoichiometries suggesting the ion, they are double salts instead.[56] Bismuth nitrate (not oxynitrate) is one of the few aqueous-insoluble nitrate salts.[citation needed]
Bismuth forms very few stable bismuthides, intermetallic compounds in which it attains oxidation state −3.[57] The hydride spontaneously decomposes at room temperature and stabilizes only below −60 °C (−76 °F).[51] Sodium bismuthide has interest as a topological Dirac insulator.[58][59]
Occurrence and production
[edit]

The reported abundance of bismuth in the Earth's crust varies significantly by source from 180ppb (similar to that of silver) to 8ppb (twice as common as gold). The most important ores of bismuth are bismuthinite and bismite.[19] Native bismuth is known from Australia, Bolivia, and China.[60][61]
| Country | Refining[62] |
|---|---|
| China | 16,000 |
| Laos | 2,000 |
| South Korea | 950 |
| Japan | 480 |
| Kazakhstan | 220 |
| Other | 350 |
| Total | 20,000 |
According to the United States Geological Survey (USGS), 10,200 tonnes of bismuth were produced worldwide by mining and 17,100 tonnes by refining in 2016. Since then, USGS does not provide mining data for bismuth, considering them unreliable. Globally, bismuth is mostly produced by refining, as a byproduct of extraction of other metals such as lead, copper, tin, molybdenum and tungsten, though the refining-to-mining ratio depends on the country.[63][64][65][66]
Bismuth travels in crude lead bullion (which can contain up to 10% bismuth) through several stages of refining, until it is removed by the Kroll-Betterton process which separates the impurities as slag, or the electrolytic Betts process. Bismuth will behave similarly with another of its major metals, copper.[64] The raw bismuth metal from both processes contains still considerable amounts of other metals, foremost lead. By reacting the molten mixture with chlorine gas the metals are converted to their chlorides while bismuth remains unchanged. Impurities can also be removed by various other methods for example with fluxes and treatments yielding high-purity bismuth metal (over 99% Bi).[67]
Price
[edit]
The price for pure bismuth metal was relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.[68]
Before World War II, demand for bismuth was small and mainly pharmaceutical—bismuth compounds were used to treat such conditions as digestive disorders, sexually transmitted diseases and burns. Minor amounts of bismuth metal were consumed in fusible alloys for fire sprinkler systems and fuse wire. During World War II bismuth was considered a strategic material, used for solders, fusible alloys, medications and atomic research. To stabilize the market, the producers set the price at $1.25 per pound ($2.75 /kg) during the war and at $2.25 per pound ($4.96 /kg) from 1950 until 1964.[68]
In the early 1970s, the price rose rapidly due to increasing demand for bismuth as a metallurgical additive to aluminium, iron and steel. This was followed by a decline owing to increased world production, stabilized consumption, and the recessions of 1980 and 1981–1982. In 1984, the price began to climb as consumption increased worldwide, especially in the United States and Japan. In the early 1990s, research began on the evaluation of bismuth as a nontoxic replacement for lead in ceramic glazes, fishing sinkers, food-processing equipment, free-machining brasses for plumbing applications, lubricating greases, and shot for waterfowl hunting.[69] Growth in these areas remained slow during the middle 1990s, in spite of the backing of lead replacement by the United States federal government, but intensified around 2005. This resulted in a rapid and continuing increase in price.[68]
Recycling
[edit]Most bismuth is produced as a byproduct of other metal-extraction processes including the smelting of lead, and also of tungsten and copper. Its sustainability is dependent on increased recycling, which is problematic.[70]
It was once believed that bismuth could be practically recycled from the soldered joints in electronic equipment. Recent efficiencies in solder application in electronics mean there is substantially less solder deposited, and thus less to recycle. While recovering the silver from silver-bearing solder may remain economic, recovering bismuth is substantially less so.[71]
Dispersed bismuth is used in certain stomach medicines (bismuth subsalicylate), paints (bismuth vanadate), pearlescent cosmetics (bismuth oxychloride), and bismuth-containing bullets. Recycling bismuth from these uses is impractical.[67]
Applications
[edit]
Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.[67]
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.[67]
Medicines
[edit]Bismuth is an ingredient in some pharmaceuticals,[9] although the use of some of these substances is declining.[56]
- Bismuth subsalicylate is used to treat diarrhea;[9] it is the active ingredient in such "pink bismuth" preparations as Pepto-Bismol, as well as the 2004 reformulation of Kaopectate. It is also used to treat some other gastro-intestinal diseases like shigellosis[72] and cadmium poisoning.[9] The mechanism of action of this substance is still not well documented, although an oligodynamic effect (toxic effect of small doses of heavy metal ions on microbes) may be involved in at least some cases. Salicylic acid from hydrolysis of the compound is antimicrobial for toxogenic E. coli, an important pathogen in traveler's diarrhea.[73]
- A combination of bismuth subsalicylate and bismuth subcitrate is used to treat the bacteria causing peptic ulcers.[74][75]
- Bibrocathol is an organic bismuth-containing compound used to treat eye infections.[76]
- Bismuth subgallate, the active ingredient in Devrom, is used as an internal deodorant to treat malodor from flatulence and feces.[77][78]
- Bismuth compounds (including sodium bismuth tartrate) were formerly used to treat syphilis.[79][80] Arsenic combined with either bismuth or mercury was a mainstay of syphilis treatment from the 1920s until the advent of penicillin in 1943.[81]
- "Milk of bismuth" (an aqueous suspension of bismuth hydroxide and bismuth subcarbonate) was marketed as an alimentary cure-all in the early 20th century, and has been used to treat gastrointestinal disorders.[82]
- Bismuth subnitrate (Bi5O(OH)9(NO3)4) and bismuth subcarbonate (Bi2O2(CO3)) are also used in medicine.[19]
Cosmetics and pigments
[edit]Bismuth oxychloride (BiOCl) is sometimes used in cosmetics, as a pigment in paint for eye shadows, hair sprays and nail polishes.[9][56][83][84] This compound is found as the mineral bismoclite and in crystal form contains layers of atoms (see figure above) that refract light chromatically, resulting in an iridescent appearance similar to nacre of pearl. It was used as a cosmetic in ancient Egypt and in many places since. Bismuth white (also "Spanish white") can refer to either bismuth oxychloride or bismuth oxynitrate (BiONO3), when used as a white pigment.[85] Bismuth vanadate is used as a light-stable non-reactive paint pigment (particularly for artists' paints), often as a replacement for the more toxic cadmium sulfide yellow and orange-yellow pigments. The most common variety in artists' paints is a lemon yellow, visually indistinguishable from its cadmium-containing alternative.[86]
Metal and alloys
[edit]Bismuth is used in alloys with other metals such as tin and lead. Wood's metal, an alloy of bismuth, lead, tin, and cadmium is used in automatic sprinkler systems for fires. It forms the largest part (50%) of Rose's metal, a fusible alloy, which also contains 25–28% lead and 22–25% tin. It was also used to make bismuth bronze, which was used during the Bronze Age, having been found in Inca knives at Machu Picchu.[87]
Lead replacement
[edit]The density difference between lead (11.32 g/cm3) and bismuth (9.78 g/cm3) is small enough that for many ballistics and weighting applications, bismuth can substitute for lead. For example, it can replace lead as a dense material in fishing sinkers. It has been used as a replacement for lead in shot, bullets and less-lethal riot gun ammunition. The Netherlands, Denmark, England, Wales, the United States, and many other countries now prohibit the use of lead shot for the hunting of wetland birds, as many birds are prone to lead poisoning owing to mistaken ingestion of lead (instead of small stones and grit) to aid digestion, or even prohibit the use of lead for all hunting, such as in the Netherlands. Bismuth-tin alloy shot is one alternative that provides similar ballistic performance to lead.[67]
Bismuth, as a dense element of high atomic weight, is used in bismuth-impregnated latex shields to shield from X-ray in medical examinations, such as CTs, mostly as it is considered non-toxic.[88]
The European Union's Restriction of Hazardous Substances Directive (RoHS) for reduction of lead has broadened bismuth's use in electronics as a component of low-melting point solders, as a replacement for traditional tin-lead solders.[67] Its low toxicity will be especially important for solders to be used in food processing equipment and copper water pipes, although it can also be used in other applications including those in the automobile industry, in the European Union, for example.[89]
Bismuth has been evaluated as a replacement for lead in free-machining brasses for plumbing applications,[90] although it does not equal the performance of leaded steels.[89]
Other metal uses and specialty alloys
[edit]Many bismuth alloys have low melting points and are found in specialty applications such as solders. Many automatic sprinklers, electric fuses, and safety devices in fire detection and suppression systems contain the eutectic In19.1-Cd5.3-Pb22.6-Sn8.3-Bi44.7 alloy that melts at 47 °C (117 °F)[19] This is a convenient temperature since it is unlikely to be exceeded in normal living conditions. Low-melting alloys, such as Bi-Cd-Pb-Sn alloy which melts at 70 °C (158 °F), are also used in automotive and aviation industries. Before deforming a thin-walled metal part, it is filled with a melt or covered with a thin layer of the alloy to reduce the chance of breaking. Then the alloy is removed by submerging the part in boiling water.[91]
Bismuth is used to make free-machining steels and free-machining aluminium alloys for precision machining properties. It has similar effect to lead and improves the chip breaking during machining. The shrinking on solidification in lead and the expansion of bismuth compensate each other and therefore lead and bismuth are often used in similar quantities.[92][93] Similarly, alloys containing comparable parts of bismuth and lead exhibit a very small change (on the order 0.01%) upon melting, solidification or aging. Such alloys are used in high-precision casting, e.g. in dentistry, to create models and molds.[91] Bismuth is also used as an alloying agent in production of malleable irons[67] and as a thermocouple material.[19]
Bismuth is also used in aluminium-silicon cast alloys to refine silicon morphology. However, it indicated a poisoning effect on modification of strontium.[94][95] Some bismuth alloys, such as Bi35-Pb37-Sn25, are combined with non-sticking materials such as mica, glass and enamels because they easily wet them allowing to make joints to other parts. Addition of bismuth to caesium enhances the quantum yield of caesium cathodes.[56] Sintering of bismuth and manganese powders at 300 °C (572 °F) produces a permanent magnet and magnetostrictive material, which is used in ultrasonic generators and receivers working in the 10–100 kHz range and in magnetic and holographic memory devices.[96]
Other uses as compounds
[edit]
- Bismuth is included in BSCCO (bismuth strontium calcium copper oxide), which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.[97]
- Bismuth telluride is a semiconductor and an excellent thermoelectric material.[56][98] Bi2Te3 diodes are used in mobile refrigerators, CPU coolers, and as detectors in infrared spectrophotometers.[56]
- Bismuth oxide, in its delta form, is a solid electrolyte for oxygen. This form normally breaks down below a high-temperature threshold, but can be electrodeposited well below this temperature in a highly alkaline solution.[99]
- Bismuth germanate is a scintillator, widely used in X-ray and gamma ray detectors.[100]
- Bismuth vanadate is an opaque yellow pigment used by some artists' oil, acrylic, and watercolor paint companies, primarily as a replacement for the more toxic cadmium sulfide yellows in the greenish-yellow (lemon) to orange-toned yellow range. It performs practically identically to the cadmium pigments, such as in terms of resistance to degradation from UV exposure, opacity, tinting strength, and lack of reactivity when mixed with other pigments. The most commonly-used variety by artists' paint makers is lemon in color. In addition to being a replacement for several cadmium yellows, it also serves as a non-toxic visual replacement for the older chromate pigments made with zinc, lead, and strontium. If a green pigment and barium sulfate (for increased transparency) are added it can also serve as a replacement for barium chromate, which possesses a more greenish cast than the others. In comparison with lead chromate, it does not blacken due to hydrogen sulfide in the air (a process accelerated by UV exposure) and possesses a particularly brighter color than them, especially the lemon, which is the most translucent, dull, and fastest to blacken due to the higher percentage of lead sulfate required to produce that shade. It is also used, on a limited basis due to its cost, as a vehicle paint pigment.[101][102]
- A catalyst for making acrylic fibers.[19]
- As an electrocatalyst in the conversion of CO2 to CO.[103]
- Ingredient in lubricating greases.[104]
- In crackling microstars (dragon's eggs) in pyrotechnics, as the oxide, subcarbonate or subnitrate.[105][106]
- As catalyst for the fluorination of arylboronic pinacol esters through a Bi(III)/Bi(V) catalytic cycle, mimicking transition metals in electrophilic fluorination.[107]
Toxicology and ecotoxicology
[edit]- See also bismuthia, a rare dermatological condition that results from the prolonged use of bismuth.
Scientific literature indicates that some of the compounds of bismuth are less toxic to humans via ingestion than other heavy metals (lead, arsenic, antimony, etc.)[9] presumably due to the comparatively low solubility of bismuth salts.[108] Its biological half-life for whole-body retention is reported to be 5 days but it can remain in the kidney for years in people treated with bismuth compounds.[109]
Bismuth poisoning can occur and has according to some reports been common in relatively recent times.[108][110] As with lead, bismuth poisoning can result in the formation of a black deposit on the gingiva, known as a bismuth line.[111][112][113] Poisoning may be treated with dimercaprol; however, evidence for benefit is unclear.[114][115]
Bismuth's environmental impacts are not well known; it may be less likely to bioaccumulate than some other heavy metals, and this is an area of active research.[116][117]
See also
[edit]Notes
[edit]- ^ The thermal expansion is anisotropic: the coefficients for each crystal axis (at 20 °C) are αah = 11.26×10−6/K, αch = 16.74×10−6/K, and αaverage = αvolume/3 = 13.09×10−6/K.
References
[edit]- ^ "Standard Atomic Weights: Bismuth". CIAAW. 2005.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (4 May 2022). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- ^ Bi(0) state exists in a N-heterocyclic carbene complex of dibismuthene; see Deka, Rajesh; Orthaber, Andreas (9 May 2022). "Carbene chemistry of arsenic, antimony, and bismuth: origin, evolution and future prospects". Royal Society of Chemistry. 51 (22): 8540–8556. doi:10.1039/d2dt00755j. PMID 35578901. S2CID 248675805.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ Dumé, Belle (23 April 2003). "Bismuth breaks half-life record for alpha decay". Physicsworld.
- ^ a b c d e f g h i j k Kean, Sam (2011). The Disappearing Spoon (and other true tales of madness, love, and the history of the world from the Periodic Table of Elements). New York/Boston: Back Bay Books. pp. 158–160. ISBN 978-0-316-051637.
- ^ Harper, Douglas. "bismuth". Online Etymology Dictionary.
- ^ Bismuth Archived 28 August 2019 at the Wayback Machine, The Concise Oxford Dictionary of English Etymology
- ^ Norman, Nicholas C. (1998). Chemistry of Arsenic, Antimony, and Bismuth. Springer. p. 41. ISBN 978-0-7514-0389-3.
- ^ Agricola, Georgious (1955) [1546]. De Natura Fossilium. New York: Mineralogical Society of America. p. 178. Archived from the original on 14 May 2021. Retrieved 8 April 2019.
- ^ Nicholson, William (1819). "Bismuth". American edition of the British encyclopedia: Or, Dictionary of Arts and sciences; comprising an accurate and popular view of the present improved state of human knowledge. p. 181.
- ^ a b Weeks, Mary Elvira (1932). "The discovery of the elements. II. Elements known to the alchemists". Journal of Chemical Education. 9 (1): 11. Bibcode:1932JChEd...9...11W. doi:10.1021/ed009p11.
- ^ Giunta, Carmen J. "Glossary of Archaic Chemical Terms". Le Moyne College. See also for other terms for bismuth, including stannum glaciale (glacial tin or ice-tin).
- ^ Gordon, Robert B.; Rutledge, John W. (1984). "Bismuth Bronze from Machu Picchu, Peru". Science. 223 (4636): 585–586. Bibcode:1984Sci...223..585G. doi:10.1126/science.223.4636.585. JSTOR 1692247. PMID 17749940. S2CID 206572055.
- ^ Pott, Johann Heinrich (1738). "De Wismutho". Exercitationes Chymicae. Berolini: Apud Johannem Andream Rüdigerum. p. 134.
- ^ a b c d e f g h i j Hammond, C. R. (2004). The Elements, in Handbook of Chemistry and Physics (81st ed.). Boca Raton (FL, US): CRC press. p. 4.1. ISBN 978-0-8493-0485-9.
- ^ Geoffroy, C.F. (1753). "Sur Bismuth". Histoire de l'Académie Royale des Sciences ... Avec les Mémoires de Mathématique & de Physique ... Tirez des Registres de Cette Académie: 190.
- ^ a b Levason, W.; Reid, G. (2003). "Coordination Chemistry of the s, p, and f Metals". Comprehensive Coordination Chemistry II. Amsterdam: Elsevier Pergamon. doi:10.1016/B0-08-043748-6/02023-5. ISBN 0-08-043748-6.
- ^ Krüger, p. 171.
- ^ Jones, H. (1936). "The Theory of the Galvomagnetic Effects in Bismuth". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 155 (886): 653–663. Bibcode:1936RSPSA.155..653J. doi:10.1098/rspa.1936.0126. JSTOR 96773.
- ^ Hoffman, C.; Meyer, J.; Bartoli, F.; Di Venere, A.; Yi, X.; Hou, C.; Wang, H.; Ketterson, J.; Wong, G. (1993). "Semimetal-to-semiconductor transition in bismuth thin films". Phys. Rev. B. 48 (15): 11431–11434. Bibcode:1993PhRvB..4811431H. doi:10.1103/PhysRevB.48.11431. PMID 10007465.
- ^ a b Wiberg, p. 768.
- ^ Tracy, George R.; Tropp, Harry E.; Friedl, Alfred E. (1974). Modern physical science. Holt, Rinehart and Winston. p. 268. ISBN 978-0-03-007381-6.
- ^ Tribe, Alfred (1868). "IX.—Freezing of water and bismuth". Journal of the Chemical Society. 21: 71. doi:10.1039/JS8682100071.
- ^ Papon, Pierre; Leblond, Jacques; Meijer, Paul Herman Ernst (2006). The Physics of Phase Transitions. Springer. p. 82. ISBN 978-3-540-33390-6.
- ^ Tiller, William A. (1991). The science of crystallization: microscopic interfacial phenomena. Cambridge University Press. p. 2. ISBN 978-0-521-38827-6.
- ^ Wiberg, p. 767.
- ^ Krüger, p. 172.
- ^ Boldyreva, Elena (2010). High-Pressure Crystallography: From Fundamental Phenomena to Technological Applications. Springer. pp. 264–265. ISBN 978-90-481-9257-1.
- ^ Manghnani, Murli H. (25–30 July 1999). Science and Technology of High Pressure: Proceedings of the International Conference on High Pressure Science and Technology (AIRAPT-17). Vol. 2. Honolulu, Hawaii: Universities Press (India) (published 2000). p. 1086. ISBN 978-81-7371-339-2.
- ^ a b c d Suzuki, p. 8.
- ^ Wiberg, pp. 769–770.
- ^ a b Greenwood, pp. 559–561.
- ^ a b Krüger, p. 185
- ^ Suzuki, p. 9.
- ^ Krabbe, S.W.; Mohan, R.S. (2012). "Environmentally friendly organic synthesis using Bi(III) compounds". In Ollevier, Thierry (ed.). Topics in Current chemistry 311, Bismuth-Mediated Organic Reactions. Springer. pp. 100–110. ISBN 978-3-642-27239-4.
- ^ Rich, Ronald (2007). Inorganic Reactions in Water (e-book). Springer. ISBN 978-3-540-73962-3.
- ^ Carvalho, H. G.; Penna, M. (1972). "Alpha-activity of 209Bi". Lettere al Nuovo Cimento. 3 (18): 720. doi:10.1007/BF02824346. S2CID 120952231.
- ^ Marcillac, Pierre de; Noël Coron; Gérard Dambier; Jacques Leblanc & Jean-Pierre Moalic (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201. S2CID 4415582.
- ^ a b Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
- ^ Loveland, Walter D.; Morrissey, David J.; Seaborg, Glenn T. (2006). Modern Nuclear Chemistry. John Wiley & Sons. p. 78. Bibcode:2005mnc..book.....L. ISBN 978-0-471-11532-8.
- ^ Peppard, D. F.; Mason, G. W.; Gray, P. R.; Mech, J. F. (1952). "Occurrence of the (4n + 1) series in nature" (PDF). Journal of the American Chemical Society. 74 (23): 6081–6084. Bibcode:1952JAChS..74.6081P. doi:10.1021/ja01143a074.
- ^ Imam, S. (2001). "Advancements in cancer therapy with alpha-emitters: a review". International Journal of Radiation Oncology, Biology, Physics. 51 (1): 271–8. doi:10.1016/S0360-3016(01)01585-1. PMID 11516878.
- ^ Acton, Ashton (2011). Issues in Cancer Epidemiology and Research. ScholarlyEditions. p. 520. ISBN 978-1-4649-6352-0.
- ^ Greenwood, p. 553.
- ^ a b An Introduction to the Study of Chemistry. Forgotten Books. p. 363. ISBN 978-1-4400-5235-4.
- ^ Scott, Thomas; Eagleson, Mary (1994). Concise encyclopedia chemistry. Walter de Gruyter. p. 136. ISBN 978-3-11-011451-5.
- ^ a b c Godfrey, S. M.; McAuliffe, C. A.; Mackie, A. G.; Pritchard, R. G. (1998). Norman, Nicholas C. (ed.). Chemistry of arsenic, antimony, and bismuth. Springer. pp. 67–84. ISBN 978-0-7514-0389-3.
- ^ a b Greenwood, p. 578.
- ^ Gillespie, R. J.; Passmore, J. (1975). Emeléus, H. J.; Sharp A. G. (eds.). Advances in Inorganic Chemistry and Radiochemistry. Academic Press. pp. 77–78. ISBN 978-0-12-023617-6.
- ^ Persson, Ingmar (2010). "Hydrated metal ions in aqueous solution: How regular are their structures?". Pure and Applied Chemistry. 82 (10): 1901–1917. doi:10.1351/PAC-CON-09-10-22.
- ^ Näslund, Jan; Persson, Ingmar; Sandström, Magnus (2000). "Solvation of the Bismuth(III) Ion by Water, Dimethyl Sulfoxide, N,N'-Dimethylpropyleneurea, and N,N-Dimethylthioformamide. An EXAFS, Large-Angle X-ray Scattering, and Crystallographic Structural Study". Inorganic Chemistry. 39 (18): 4012–4021. doi:10.1021/ic000022m. PMID 11198855.
- ^ a b c d e f Krüger, p. 184.
- ^ Okamoto, H. (1 March 2002). "Bi-Nd (Bismuth-Neodymium)". Journal of Phase Equilibria. 23 (2): 191. doi:10.1361/1054971023604224.
- ^ "3D counterpart to graphene discovered [UPDATE]". KurzweilAI. 20 January 2014. Retrieved 28 January 2014.
- ^ Liu, Z. K.; Zhou, B.; Zhang, Y.; Wang, Z. J.; Weng, H. M.; Prabhakaran, D.; Mo, S. K.; Shen, Z. X.; Fang, Z.; Dai, X.; Hussain, Z.; Chen, Y. L. (2014). "Discovery of a Three-Dimensional Topological Dirac Semimetal, Na3Bi". Science. 343 (6173): 864–7. arXiv:1310.0391. Bibcode:2014Sci...343..864L. doi:10.1126/science.1245085. PMID 24436183. S2CID 206552029.
- ^ Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C., eds. (15 April 1990). "Bismuth" (PDF). Handbook of Mineralogy: Elements, Sulfides, Sulfosalts. Chantilly, VA, US: Mineralogical Society of America. ISBN 978-0-9622097-0-3. Retrieved 5 December 2011.
- ^ Krüger, pp. 172–173.
- ^ Merrill, Adam M. "2023 USGS Minerals Yearbook: Bismuth" (PDF). United States Geological Survey.
- ^ Krüger, p. 173.
- ^ a b Ojebuoboh, Funsho K. (1992). "Bismuth—Production, properties, and applications". JOM. 44 (4): 46–49. Bibcode:1992JOM....44d..46O. doi:10.1007/BF03222821. S2CID 52993615.
- ^ Horsley, G. W. (1957). "The preparation of bismuth for use in a liquid-metal fuelled reactor". Journal of Nuclear Energy. 6 (1–2): 41. doi:10.1016/0891-3919(57)90180-8.
- ^ Shevtsov, Yu. V.; Beizel’, N. F. (2011). "Pb distribution in multistep bismuth refining products". Inorganic Materials. 47 (2): 139. doi:10.1134/S0020168511020166. S2CID 96931735.
- ^ a b c d e f g Singerling, Sheryl A.; Callaghan, Robert M. "2018 USGS Minerals Yearbook: Bismuth" (PDF). United States Geological Survey.
- ^ a b c d Bismuth Statistics and Information. see "Metal Prices in the United States through 1998" for a price summary and "Historical Statistics for Mineral and Material Commodities in the United States" for production. USGS.
- ^ Suzuki, p. 14.
- ^ European Commission. Directorate General for Internal Market, Industry, Entrepreneurship and SMEs. (2018). Report on critical raw materials and the circular economy. European Commission. Directorate General for Internal Market, Industry, Entrepreneurship and SMEs. doi:10.2873/167813. ISBN 9789279946264.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Warburg, N. "IKP, Department of Life-Cycle Engineering" (PDF). University of Stuttgart. Archived from the original (PDF) on 25 February 2009. Retrieved 5 May 2009.
- ^ CDC, shigellosis.
- ^ Sox TE; Olson CA (1989). "Binding and killing of bacteria by bismuth subsalicylate". Antimicrob Agents Chemother. 33 (12): 2075–82. doi:10.1128/AAC.33.12.2075. PMC 172824. PMID 2694949.
- ^ "P/74/2009: European Medicines Agency decision of 20 April 2009 on the granting of a product specific waiver for Bismuth subcitrate potassium / Metronidazole / Tetracycline hydrochloride (EMEA-000382-PIP01-08) in accordance with Regulation (EC) No 1901/2006 of the European Parliament and of the Council as amended" (PDF). European Medicines Agency. 10 June 2009. Archived from the original (PDF) on 24 October 2017. Retrieved 13 August 2022.
- ^ Urgesi R, Cianci R, Riccioni ME (2012). "Update on triple therapy for eradication of Helicobacter pylori: current status of the art". Clinical and Experimental Gastroenterology. 5: 151–7. doi:10.2147/CEG.S25416. PMC 3449761. PMID 23028235.
- ^ Gurtler L (January 2002). "Chapter 2: The Eye and Conjunctiva as Target of Entry for Infectious Agents: Prevention by Protection and by Antiseptic Prophylaxis". In Kramer A, Behrens-Baumann W (eds.). Antiseptic prophylaxis and therapy in ocular infections: principles, clinical practice, and infection control. Developments in Ophthalmology. Vol. 33. Basel: Karger. pp. 9–13. doi:10.1159/000065934. ISBN 978-3-8055-7316-0. PMID 12236131.
- ^ Gorbach SL (September 1990). "Bismuth therapy in gastrointestinal diseases". Gastroenterology. 99 (3): 863–75. doi:10.1016/0016-5085(90)90983-8. PMID 2199292.
- ^ Sparberg M (March 1974). "Correspondence: Bismuth subgallate as an effective means for the control of ileostomy odor: a double blind study". Gastroenterology. 66 (3): 476. doi:10.1016/S0016-5085(74)80150-2. PMID 4813513.
- ^ Parnell, R. J. G. (1924). "Bismuth in the Treatment of Syphilis". Journal of the Royal Society of Medicine. 17 (War section): 19–26. doi:10.1177/003591572401702604. PMC 2201253. PMID 19984212.
- ^ Giemsa, Gustav (1924) U.S. patent 1,540,117 "Manufacture of bismuth tartrates"
- ^ Frith, John (November 2012). "Syphilis – Its Early History and Treatment Until Penicillin, and the Debate on its Origins". Journal of Military and Veterans' Health. 20 (4): 54. Retrieved 30 January 2022.
- ^ "Milk of Bismuth". Archived from the original on 4 June 2013. Retrieved 13 August 2022.
- ^ Maile, Frank J.; Pfaff, Gerhard; Reynders, Peter (2005). "Effect pigments—past, present and future". Progress in Organic Coatings. 54 (3): 150. doi:10.1016/j.porgcoat.2005.07.003.
- ^ Pfaff, Gerhard (2008). Special effect pigments: Technical basics and applications. Vincentz Network GmbH. p. 36. ISBN 978-3-86630-905-0.
- ^ Sadler, Peter J (1991). "Chapter 1". In Sykes, A.G. (ed.). ADVANCES IN INORGANIC CHEMISTRY, Volume 36. Academic Press. ISBN 0-12-023636-2.
- ^ Weldon, Dwight G. (2009). Failure analysis of paints and coatings. Chichester, U.K.: Wiley. p. 40. ISBN 978-1-61583-267-5. OCLC 608477934.
- ^ Gordon, Robert B.; Rutledge, John W. (1984). "Bismuth Bronze from Machu Picchu, Peru". Science. 223 (4636). American Association for the Advancement of Science: 585–586. Bibcode:1984Sci...223..585G. doi:10.1126/science.223.4636.585. JSTOR 1692247. PMID 17749940. S2CID 206572055.
- ^ Hopper KD; King SH; Lobell ME; TenHave TR; Weaver JS (1997). "The breast: inplane x-ray protection during diagnostic thoracic CT—shielding with bismuth radioprotective garments". Radiology. 205 (3): 853–8. doi:10.1148/radiology.205.3.9393547. PMID 9393547.
- ^ a b Lohse, Joachim; Zangl, Stéphanie; Groß, Rita; Gensch, Carl-Otto; Deubzer, Otmar (September 2007). "Adaptation to Scientific and Technical Progress of Annex II Directive 2000/53/EC" (PDF). European Commission. Retrieved 11 September 2009.
- ^ La Fontaine, A.; Keast, V. J. (2006). "Compositional distributions in classical and lead-free brasses". Materials Characterization. 57 (4–5): 424. doi:10.1016/j.matchar.2006.02.005.
- ^ a b Krüger, p. 183.
- ^ Llewellyn, D. T.; Hudd, Roger C. (1998). Steels: Metallurgy and applications. Butterworth-Heinemann. p. 239. ISBN 978-0-7506-3757-2.
- ^ Davis, J. R. (1993). Aluminum and Aluminum Alloys. ASM International. p. 41. ISBN 978-0-87170-496-2.
- ^ Farahany, Saeed; A. Ourdjini; M.H. Idris; L.T. Thai (2011). "Poisoning effect of bismuth on modification behavior of strontium in LM25 alloy". Journal of Bulletin of Materials Science. 34 (6): 1223–1231. doi:10.1007/s12034-011-0239-5.
- ^ Farahany, Saeed; A. Ourdjini; M. H. Idris; L.T. Thai (2011). "Effect of bismuth on the microstructure of unmodified and Sr-modified Al-7%Si-0.4Mg alloy". Journal of Transactions of Nonferrous Metals Society of China. 21 (7): 1455–1464. doi:10.1016/S1003-6326(11)60881-9. S2CID 73719425.
- ^ Suzuki, p. 15.
- ^ "BSCCO". National High Magnetic Field Laboratory. Archived from the original on 12 April 2013. Retrieved 18 January 2010.
- ^ Tritt, Terry M. (2000). Recent trends in thermoelectric materials research. Academic Press. p. 12. ISBN 978-0-12-752178-7.
- ^ Maric, Radenka; Mirshekari, Gholamreza (2020). Solid oxide fuel cells : from fundamental principles to complete systems. Boca Raton. p. 70. ISBN 978-0-429-52784-5. OCLC 1228350036.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Saha, Gopal B. (2006). Physics and radiobiology of nuclear medicine. New York: Springer. p. 82. ISBN 978-0-387-36281-6. OCLC 655784658.
- ^ Tücks, Andreas; Beck, Horst P. (2007). "The photochromic effect of bismuth vanadate pigments: Investigations on the photochromic mechanism". Dyes and Pigments. 72 (2): 163. doi:10.1016/j.dyepig.2005.08.027.
- ^ Müller, Albrecht (2003). "Yellow pigments". Coloring of plastics: Fundamentals, colorants, preparations. Hanser Verlag. pp. 91–93. ISBN 978-1-56990-352-0.
- ^ DiMeglio, John L.; Rosenthal, Joel (2013). "Selective conversion of CO2 to CO with high efficiency using an bismuth-based electrocatalyst". Journal of the American Chemical Society. 135 (24): 8798–8801. doi:10.1021/ja4033549. PMC 3725765. PMID 23735115.
- ^ Mortier, Roy M.; Fox, Malcolm F.; Orszulik, Stefan T. (2010). Chemistry and Technology of Lubricants. Springer. p. 430. Bibcode:2010ctl..book.....M. ISBN 978-1-4020-8661-8.
- ^ Croteau, Gerry; Dills, Russell; Beaudreau, Marc; Davis, Mac (2010). "Emission factors and exposures from ground-level pyrotechnics". Atmospheric Environment. 44 (27): 3295. Bibcode:2010AtmEn..44.3295C. doi:10.1016/j.atmosenv.2010.05.048.
- ^ Ledgard, Jared (2006). The Preparatory Manual of Black Powder and Pyrotechnics. Lulu. pp. 207, 319, 370, 518, search. ISBN 978-1-4116-8574-1.
- ^ Planas, Oriol; Wang, Feng; Leutzsch, Markus; Cornella, Josep (2020). "Fluorination of arylboronic esters enabled by bismuth redox catalysis". Science. 367 (6475): 313–317. Bibcode:2020Sci...367..313P. doi:10.1126/science.aaz2258. hdl:21.11116/0000-0005-DB57-3. PMID 31949081. S2CID 210698047.
- ^ a b DiPalma, Joseph R. (2001). "Bismuth Toxicity, Often Mild, Can Result in Severe Poisonings". Emergency Medicine News. 23 (3): 16. doi:10.1097/00132981-200104000-00012.
- ^ Fowler, B.A. & Sexton M.J. (2007). "Bismuth". In Nordberg, Gunnar (ed.). Handbook on the toxicology of metals. Academic Press. pp. 433 ff. ISBN 978-0-12-369413-3.
- ^ Data on Bismuth's health and environmental effects. Lenntech.com. Retrieved on 17 December 2011.
- ^ "Bismuth line" in TheFreeDictionary's Medical dictionary. Farlex, Inc.
- ^ Levantine, Ashley; Almeyda, John (1973). "Drug induced changes in pigmentation". British Journal of Dermatology. 89 (1): 105–12. doi:10.1111/j.1365-2133.1973.tb01932.x. PMID 4132858. S2CID 7175799.
- ^ Krüger, pp. 187–188.
- ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 62. hdl:10665/44053. ISBN 9789241547659.
- ^ "Dimercaprol". The American Society of Health-System Pharmacists. Retrieved 8 December 2016.
- ^ Boriova; et al. (2015). "Bismuth(III) Volatilization and Immobilization by Filamentous Fungus Aspergillus clavatus During Aerobic Incubation". Archives of Environmental Contamination and Toxicology. 68 (2): 405–411. Bibcode:2015ArECT..68..405B. doi:10.1007/s00244-014-0096-5. PMID 25367214. S2CID 30197424.
- ^ Boriova; et al. (2013). "Bioaccumulation and biosorption of bismuth Bi (III) by filamentous fungus Aspergillus clavatus" (PDF). Student Scientific Conference PriF UK 2013. Proceedings of Reviewed Contributions.
Cited sources
[edit]![]() This article incorporates text from this source, which is in the public domain: Brown, R. D., Jr. "Annual Average Bismuth Price", USGS (1998).
This article incorporates text from this source, which is in the public domain: Brown, R. D., Jr. "Annual Average Bismuth Price", USGS (1998).
- Greenwood, N. N. & Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 978-0-7506-3365-9.
- Krüger, Joachim; Winkler, Peter; Lüderitz, Eberhard; Lück, Manfred; Wolf, Hans Uwe (2003). "Bismuth, Bismuth Alloys, and Bismuth Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim. pp. 171–189. doi:10.1002/14356007.a04_171. ISBN 978-3527306732.
- Suzuki, Hitomi (2001). Organobismuth Chemistry. Elsevier. pp. 1–20. ISBN 978-0-444-20528-5.
- Wiberg, Egon; Holleman, A. F.; Wiberg, Nils (2001). Inorganic chemistry. Academic Press. ISBN 978-0-12-352651-9.
External links
[edit]- Bismuth at The Periodic Table of Videos (University of Nottingham)
- Bismuth Crystals – Instructions & Pictures