Linus Pauling: Difference between revisions
rv good faith addition, unencyclopedic (Wikipedia is not a genealogy site) and uncited. A short mention with a ref might be OK.Undid revision 607114430 by 70.71.19.78 (talk) |
added Category:Delta Upsilon members using HotCat |
||
| Line 1: | Line 1: | ||
{{Short description|American scientist and activist (1901–1994)}} |
|||
{{Use mdy dates|date=May 2012}} |
|||
{{Use mdy dates|date=July 2023|cs1-dates=y}} |
|||
{{Use American English|date=April 2022}} |
|||
{{Infobox scientist |
{{Infobox scientist |
||
| image = |
| image = File:Linus Pauling in the 1940s.jpg |
||
| |
| caption = Pauling in the 1940s |
||
| honorific_suffix = {{post-nominals|country=GBR|size=100%|ForMemRS}} |
|||
| birth_date = {{birth date|1901|2|28}} |
|||
| birth_name = Linus Carl Pauling |
|||
| birth_place = [[Portland, Oregon]], USA |
|||
| birth_date = {{birth date|1901|2|28}} |
|||
| residence = United States |
|||
| birth_place = [[Portland, Oregon]], U.S. |
|||
| nationality = [[American people|American]] |
|||
| nationality = <!-- use only when necessary per [[WP:INFONAT]] --> |
|||
| death_date = {{nowrap|{{death date and age|1994|8|19|1901|2|28}}}} |
|||
| death_date = {{nowrap|{{death date and age|1994|8|19|1901|2|28}}}} |
|||
| death_place = [[Big Sur]], [[California]], USA |
|||
| death_place = [[Big Sur]], California, U.S. |
|||
| field = {{unbulleted list |
|||
| spouse = {{marriage|[[Ava Helen Pauling|Ava Helen Miller]]|1923-06-17|1981-12-07|end=d.}} |
|||
|[[Quantum chemistry]] |
|||
| children = 4 |
|||
|[[Biochemistry]] |
|||
| field = {{ubl|[[Quantum chemistry]]|[[Biochemistry]]}} |
|||
| education = {{ubl|[[Oregon State University]] ([[B. S.|BS]])|[[California Institute of Technology]] ([[PhD]])}} |
|||
| work_institution = {{collapsible list|title={{nobold|''As faculty member''}} |
|||
|[[California Institute of Technology|Caltech]] (1927–1963) |
|||
|[[University of California, San Diego|UC San Diego]] (1967–1969) |
|||
|[[Stanford University|Stanford]] (1969–1975) |
|||
}} |
}} |
||
{{collapsible list|title={{nobold|''As fellow''}} |
|||
| alma_mater = {{unbulleted list |
|||
|[[ |
|[[Cornell University]] (1937–1938) |
||
|[[University of Oxford]] (1948) |
|||
|[[California Institute of Technology|California Institute of Technology (Caltech)]] |
|||
|[[Center for the Study of Democratic Institutions]] (1963–1967) |
|||
}} |
}} |
||
| doctoral_advisor = {{ubl|[[Roscoe G. Dickinson|Roscoe Dickinson]]|[[Richard C. Tolman|Richard Tolman]]<ref name="mathgene">{{MathGenealogy|id=113591}}</ref>}} |
|||
| work_institution = |
|||
| academic_advisors = {{ubl|[[Arnold Sommerfeld]]|[[Niels Bohr]]<ref name="Guggenheim">{{Cite web |title=A Guggenheim Fellow in Europe during the Golden Years of Physics (1926–1927) |url=http://scarc.library.oregonstate.edu/coll/pauling/chronology/page9.html |url-status=live |archive-url=https://web.archive.org/web/20211028235401/http://scarc.library.oregonstate.edu/coll/pauling/chronology/page9.html |archive-date=October 28, 2021 |access-date=May 27, 2015 |website=[[Oregon State University]] |language=en-US}}</ref>}} |
|||
''As faculty member'' |
|||
| doctoral_students = {{plainlist| |
|||
:[[California Institute of Technology|Caltech]] {{small|(1927–1963)}} |
|||
*[[Jerry Donohue]] |
|||
:{{nowrap|[[University of California, San Diego|UC San Diego]] {{small|(1967–1969)}}}} |
|||
*[[Harvey Itano]] |
|||
:[[Stanford University|Stanford]] {{small|(1969–1975)}} |
|||
*[[Barclay Kamb]] |
|||
''As fellow'' |
|||
*[[Martin Karplus]] |
|||
:[[Center for the Study of Democratic Institutions]] {{small|(1963–1967)}} |
|||
*[[Leonard Lerman]] |
|||
| doctoral_advisor = [[Roscoe G. Dickinson|Roscoe Dickinson]]<br>[[Richard C. Tolman|Richard Tolman]]<ref name="mathgene"/> |
|||
*[[William Lipscomb]]<ref name="mathgene" /> |
|||
| academic_advisors =[[Arnold Sommerfeld]]<ref name=Guggenheim/><br>[[Niels Bohr]]<ref name=Guggenheim>{{cite web|title=A Guggenheim Fellow in Europe during the Golden Years of Physics (1926-1927) |work= Special collections |publisher=Oregon State University Libraries |url=http://osulibrary.orst.edu/specialcollections/coll/pauling/chronology/page9.html |accessdate=Dec 18, 2013}}</ref> |
|||
*[[Matthew Meselson]] |
|||
| doctoral_students = [[Martin Karplus]]<br>[[Jerry Donohue]]<br>[[Matthew Meselson]]<br>[[Edgar Bright Wilson]]<br>[[William Lipscomb]]<ref name="mathgene">{{MathGenealogy|id=113591}}</ref> |
|||
*[[Kurt Mislow]] |
|||
| thesis_title = The Determination with X-Rays of the Structures of Crystals |
|||
*[[Arthur Pardee]] |
|||
| thesis_year = 1925<ref name="paulingphd"/> |
|||
*[[Robert E. Rundle]] |
|||
| thesis_url = http://thesis.library.caltech.edu/1791/ |
|||
*[[Edgar Bright Wilson]] |
|||
| known_for = {{collapsible list|title={{nbsp}}|{{unbulleted list |
|||
}} |
|||
| notable_students = ''Undergrads:'' |
|||
{{plainlist | |
|||
* [[Edwin McMillan]] |
|||
}} |
|||
''Post-docs:'' |
|||
{{plainlist | |
|||
*[[Charles D. Coryell]] |
|||
*[[Jack D. Dunitz]] |
|||
*[[Sidney W. Fox]] |
|||
*[[Walter Gordy]] |
|||
*[[Edgar Heilbronner]] |
|||
*[[Jan Ketelaar]] |
|||
*[[Hans Kuhn (chemist)|Hans Kuhn]] |
|||
*[[Leslie Orgel]] |
|||
*[[Alexander Rich]] |
|||
*[[Seymour Jonathan Singer]] |
|||
}} |
|||
| thesis_title = The Determination with X-Rays of the Structures of Crystals |
|||
| thesis_year = 1925<ref name="paulingphd">{{Cite thesis |last=Pauling |first=Linus |title=The determination with x-rays of the structures of crystals |date=1925 |degree=PhD |publisher=[[California Institute of Technology]] |url=https://thesis.library.caltech.edu/1791/ |doi=10.7907/F7V6-4P98 |language=en |author-mask=4 |access-date=April 13, 2022}}</ref> |
|||
| thesis_url = https://thesis.library.caltech.edu/1791 |
|||
| known_for = {{collapsible list|title={{nobold|''See list''}}|{{ubl|item_style={{longitem}} |
|||
|[[Alpha sheet]] |
|[[Alpha sheet]] |
||
|[[Ancestral sequence reconstruction]] |
|||
|[[Pi backbonding|Backbonding]] |
|||
|[[Beta sheet]] |
|[[Beta sheet]] |
||
|[[Bond order]] |
|[[Bond order]] |
||
|[[Breath gas analysis]] |
|||
|[[Coiled coil]] |
|[[Coiled coil]] |
||
|[[Corey-Pauling rules]] |
|||
|[[CPK coloring]] |
|[[CPK coloring]] |
||
|[[Crystal structure prediction]] |
|[[Crystal structure prediction]] |
||
|[[Electronegativity]] |
|[[Electronegativity]] |
||
|Elucidating [[chemical bond]]s and [[molecular structure]]s |
|Elucidating [[chemical bond]]s and [[molecular structure]]s |
||
|[[Geometrical frustration]] |
|||
|[[Orbital hybridisation|Hybridisation theory]] |
|||
|[[Hydrogen bond]]ing |
|||
|[[Ice-type model]] |
|[[Ice-type model]] |
||
|[[Linear combination of atomic orbitals]] |
|[[Linear combination of atomic orbitals]] |
||
|[[Molecular clock]] |
|[[Molecular clock]] |
||
|[[Molecular medicine]] |
|[[Molecular medicine]] |
||
|[[Non-carbon nanotube]] |
|||
|[[Orbital overlap]] |
|[[Orbital overlap]] |
||
|[[Cooperative binding#Pauling equation|Pauling equation]] |
|||
|[[Pauling's rules]] |
|[[Pauling's rules]] |
||
|[[Pauling–Corey–Branson alpha helix]] |
|[[Pauling–Corey–Branson alpha helix]] |
||
|[[Pauling's principle of electroneutrality]] |
|||
|[[Quantum chemistry]] |
|[[Quantum chemistry]] |
||
|[[Quantum graph]] |
|[[Quantum graph]] |
||
|[[Residual entropy]] |
|||
|[[Resonance (chemistry)]] |
|[[Resonance (chemistry)]] |
||
|[[Slater–Pauling rule]] |
|||
|[[Space-filling model]] |
|[[Space-filling model]] |
||
|[[Valence bond theory]] |
|[[Valence bond theory]] |
||
| Line 55: | Line 98: | ||
|Advocating [[nuclear disarmament]] |
|Advocating [[nuclear disarmament]] |
||
}}}} |
}}}} |
||
| prizes = {{ubl|item_style={{longitem}} |
|||
| prizes = |
|||
| [[ACS Award in Pure Chemistry]] (1931) |
|||
{{unbulleted list |
|||
| [[Irving Langmuir Award]] (1931) |
|||
| [[Davy Medal]] (1947) |
|||
| [[Nobel Prize in Chemistry]] (1954) |
| [[Nobel Prize in Chemistry]] (1954) |
||
| [[Nobel Peace Prize]] (1962) |
| [[Nobel Peace Prize]] (1962) |
||
| [[ |
| [[Roebling Medal]] (1967) |
||
| [[Lenin Peace Prize]] (1968–1969) |
|||
| [[National Medal of Science]] (1974) |
|||
| [[Lomonosov Gold Medal]] (1977) |
| [[Lomonosov Gold Medal]] (1977) |
||
| [[NAS Award in Chemical Sciences]] (1979) |
|||
| [[Priestley Medal]] (1984) |
| [[Priestley Medal]] (1984) |
||
| [[Vannevar Bush Award]] (1989) |
|||
}} |
}} |
||
| signature = Linus Pauling signature.svg |
|||
| religion = Raised [[Lutheranism|Lutheran]]{{\}}[[Unitarian Universalism|Unitarian Universalist]]; [[Atheism|Atheist]] as an adult |
|||
| footnotes = The only person to win two unshared Nobel Prizes. |
|||
| signature = Linus Pauling signature.svg |
|||
| footnotes = The only person to win two unshared Nobel Prizes. |
|||
}} |
}} |
||
'''Linus Carl Pauling''' (February 28, 1901 – August 19, 1994)<ref name="frs">{{cite doi|10.1098/rsbm.1996.0020}}</ref> was an American [[chemist]], [[biochemist]], [[peace activist]], author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century.<ref>{{cite web |url=http://www.adherents.com/people/100_scientists.html |title=The Scientific 100: A Ranking of the Most Influential Scientists, Past and Present |accessdate=December 19, 2010 }}</ref><ref>{{cite journal | last = Horgan |first=J | year = 1993 | title = Profile: Linus C. Pauling – Stubbornly Ahead of His Time | url = | journal = [[Scientific American]] | volume = 266 | issue = 3| pages = 36–40 | doi=10.1038/scientificamerican0393-36}}</ref> Pauling was one of the founders of the fields of [[quantum chemistry]] and [[molecular biology]].<ref name="natureobit">{{cite pmid|8090196}}</ref> |
|||
'''Linus Carl Pauling''' {{Post-nominals|country=GBR|FRS}} ({{IPAc-en|ˈ|p|ɔː|l|ɪ|ŋ}} {{respell|PAW|ling}}; February 28, 1901{{snd}}August 19, 1994)<ref name="ChemNobel">{{Cite web |title=Linus Pauling: Facts |url=https://www.nobelprize.org/prizes/chemistry/1954/pauling/facts/ |url-status=live |archive-url=https://web.archive.org/web/20220404140945/https://www.nobelprize.org/prizes/chemistry/1954/pauling/facts/ |archive-date=April 4, 2022 |access-date=April 13, 2022 |website=Nobel Prize |language=en}}</ref> was an American [[chemist]], [[biochemist]], [[chemical engineer]], [[peace activist]], author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific topics.<ref name="VolumeI">{{Cite book |last=Pauling |first=Linus |title=Selected papers of Linus Pauling |date=1997 |publisher=[[World Scientific]] |isbn=978-981-02-2939-9 |editor-last=Pauling |editor-first=Linus Jr. |edition=Volume I |location=[[River Edge, New Jersey]] |page=xvii |author-mask=4}}</ref> ''[[New Scientist]]'' called him one of the 20 greatest scientists of all time.<ref name="Horgan">{{Cite magazine |last=Horgan |first=J |year=1993 |title=Profile: Linus C. Pauling – Stubbornly Ahead of His Time |magazine=[[Scientific American]] |volume=266 |issue=3 |pages=36–40 |bibcode=1993SciAm.266c..36H |doi=10.1038/scientificamerican0393-36}}</ref> For his scientific work, Pauling was awarded the [[Nobel Prize in Chemistry]] in 1954. For his peace activism, he was awarded the [[Nobel Peace Prize]] in 1962. He is one of five people to have [[Nobel Prize#Multiple laureates|won more than one Nobel Prize]] (the others being [[Marie Curie]], [[John Bardeen]], [[Frederick Sanger]], and [[Karl Barry Sharpless]]).<ref name="Nobel">{{Nobelprize|access-date=April 30, 2020}}</ref> Of these, he is the only person to have been awarded two unshared Nobel Prizes,<ref>{{Cite web |date=April 12, 2022 |orig-date=2009-10-05 |title=Nobel Prize Facts |url=https://www.nobelprize.org/nobel_prizes/facts/ |url-status=live |archive-url=https://web.archive.org/web/20170111161907/http://www.nobelprize.org/nobel_prizes/facts/ |archive-date=January 11, 2017|access-date=April 13, 2022 |website=Nobel Prize |language=en }}</ref> and one of two people to be awarded Nobel Prizes in different fields, the other being [[Marie Curie]].<ref name="Nobel" /> |
|||
{{anchor|Nobels}}For his scientific work, Pauling was awarded the [[Nobel Prize in Chemistry]] in 1954. In 1962, for his peace activism, he was awarded the [[Nobel Peace Prize]]. This makes him the only person to be awarded two unshared Nobel Prizes. He is one of only four individuals to have won more than one Nobel Prize (the others being [[Marie Curie]], [[John Bardeen]], and [[Frederick Sanger]]). Pauling is also one of only two people to be awarded Nobel Prizes in different fields, the other being Marie Curie.<ref>As Watson attests, Pauling also came close to being the discoverer of DNA's structure, for which [[Francis Crick]], [[James D. Watson|James Watson]] and [[Maurice Wilkins]] received the Nobel Prize.</ref> |
|||
Pauling was one of the founders of the fields of [[quantum chemistry]] and [[molecular biology]].<ref name="natureobit">{{Cite journal |last=Rich |first=Alexander |author-link=Alexander Rich |year=1994 |title=Linus Pauling (1901–1994) |journal=[[Nature (journal)|Nature]] |volume=371 |issue=6495 |pages=285 |bibcode=1994Natur.371..285R |doi=10.1038/371285a0 |pmid=8090196 |doi-access=free |s2cid=8923975}}</ref> His contributions to the theory of the chemical bond include the concept of [[orbital hybridisation]] and the first accurate scale of [[electronegativity|electronegativities]] of the elements. Pauling also worked on the structures of biological molecules, and showed the importance of the [[alpha helix]] and [[beta sheet]] in [[protein secondary structure]]. Pauling's approach combined methods and results from [[X-ray crystallography]], [[molecular model]] building, and quantum chemistry. His discoveries inspired the work of [[Rosalind Franklin]], [[James Watson]], [[Francis Crick]], and [[Maurice Wilkins]] on the structure of [[DNA]], which in turn made it possible for geneticists to crack the DNA code of all organisms.<ref name="Contributions to DNA double-helix discovery">{{Cite book |last=Gribbin |first=John |title=The Scientists: A History of Science Told Through the Lives of Its Greatest Inventors |date=2004 |publisher=[[Random House]] |isbn=978-0-8129-6788-3 |location=New York City |pages=558–569 |language=en |ol=8020832M |author-link=John Gribbin}}</ref> |
|||
However, his promotion of [[orthomolecular medicine]], [[megavitamin therapy]], [[dietary supplements]], and [[vitamin C]]<!-- References are in body of article. --> has been criticized. |
|||
In his later years, he promoted [[nuclear disarmament]], as well as [[orthomolecular medicine]], [[megavitamin therapy]],<ref name="isbn0-399-50764-7">{{Cite book |last=Stone |first=Irwin |author-link=Irwin Stone |url=https://archive.org/details/healingfactorvit0000ston |title=The healing factor: "vitamin C" against disease |date=1982 |publisher=Perigee Books |isbn=978-0-399-50764-9 |location=New York|language=en |oclc=10169988 |ol=9567597M |via=[[Internet Archive]]}}</ref> and [[dietary supplement]]s, especially [[ascorbic acid]] (commonly known as Vitamin C). None of his ideas concerning the medical usefulness of large doses of vitamins have gained much acceptance in the mainstream scientific community.<ref name="Horgan" /><ref name="Offit">{{Cite magazine |last=Offit |first=Paul |author-link=Paul Offit |date=July 19, 2013 |title=The Vitamin Myth: Why We Think We Need Supplements |url=https://www.theatlantic.com/health/archive/2013/07/the-vitamin-myth-why-we-think-we-need-supplements/277947/ |magazine=[[The Atlantic]] |language=en |issn=2151-9463 |oclc=936540106 |access-date=July 19, 2013 |url-access=subscription}}</ref> He was married to the American human rights activist [[Ava Helen Pauling]]. |
|||
== Early life and education == |
== Early life and education == |
||
[[File:Herman Henry William Pauling.jpg|thumb|left|Herman Henry William Pauling, Linus Pauling's father, {{circa|1900}}]] |
|||
{{Cleanup-rewrite|name=Rewrite section|1=Excessive extraneous genealogical data not directly relevant to Linus' life|2=section|reason=Folksy, conversational tone|date=March 2014}} |
|||
[[File:Pfeifenraucher.jpg|thumb|right|Photo of Herman Henry William Pauling, Linus Pauling's father, taken [[Circa|c.]] 1900.]] |
|||
Pauling was born in [[Portland, Oregon]],<ref name=childhood>{{cite web|title=Linus Pauling's Childhood (1901-1910) |work= Special collections |publisher=Oregon State University Libraries |url=http://osulibrary.orst.edu/specialcollections/coll/pauling/chronology/page3.html |accessdate=April 25, 2013}}</ref><ref>{{cite web|title= |
|||
Linus Pauling |work=NNDB: Tracking the entire world |url=http://www.nndb.com/people/824/000031731/ |publisher=Soylent Communications |accessdate=April 25, 2013}}</ref> as the first-born child of Herman Henry William Pauling (1876–1910) and Lucy Isabelle "Belle" Darling (1881–1926).<ref>Hager, p. 22.</ref> He was named "Linus Carl," in honor of Lucy's father, Linus, and Herman's father, Carl.<ref>Mead and Hager, p. 8.</ref> |
|||
Linus Carl Pauling was born on February 28, 1901, in [[Portland, Oregon]],<ref name="frs">{{Cite journal |last=Dunitz |first=Jack D. |author-link=Jack D. Dunitz |date=1996 |title=Linus Carl Pauling. 28 February 1901–19 August 1994 |journal=[[Biographical Memoirs of Fellows of the Royal Society]] |language=en-gb |volume=42 |issue=9 |pages=316–326 |doi=10.1098/rsbm.1996.0020 |pmid=11619334 |doi-access=free}}</ref><ref name="childhood">{{Cite web |title=Linus Pauling's Childhood (1901–1910) |url=http://scarc.library.oregonstate.edu/coll/pauling/chronology/page3.html |url-status=live |archive-url=https://web.archive.org/web/20220407144827/http://scarc.library.oregonstate.edu/coll/pauling/chronology/page3.html |archive-date=April 7, 2022 |access-date=April 25, 2013 |website=[[Oregon State University]] |language=en-us}}</ref> the firstborn child of Herman Henry William Pauling (1876–1910) and Lucy Isabelle "Belle" Darling (1881–1926).<ref name="Nature">{{Cite book |last=Hager |first=Thomas |author-link=Thomas Hager |url=https://archive.org/details/forceofnaturelif00hage |title=Force of Nature: The Life of Linus Pauling |date=1995 |publisher=[[Simon & Schuster]] |isbn=978-0-684-80909-0 |via=[[Internet Archive]]}}</ref>{{rp|22}} He was named "Linus Carl", in honor of Lucy's father, Linus, and Herman's father, Carl.<ref name="MeadHager">{{Cite book |title=Linus Pauling: Scientist and Peacemaker |date=2001 |publisher=[[Oregon State University Press]] |isbn=978-0-87071-489-4 |editor-last=Mead |editor-first=Clifford |editor-last2=Hager |editor-first2=Thomas |editor-link2=Thomas Hager}}</ref>{{rp|8}} His ancestry included German and English.<ref>{{Cite web |title=Linus Pauling: Biographical |url=https://www.nobelprize.org/prizes/chemistry/1954/pauling/biographical/ |url-status=live |archive-url=https://web.archive.org/web/20220318142845/https://www.nobelprize.org/prizes/chemistry/1954/pauling/biographical/ |archive-date=March 18, 2022 |access-date=September 27, 2021 |website=Nobel Prize |language=en-US}}</ref><ref>{{Cite book |last=Dunitz |first=Jack D. |author-link=Jack D. Dunitz |url=https://www.nap.edu/read/5737/chapter/13 |title=Biographical Memoirs |date=1997 |publisher=[[National Academies Press]] |isbn=978-0-309-05738-7 |volume=71 |pages=221–261 |language=en |chapter=Linus Carl Pauling |doi=10.17226/5737 |archive-url=https://web.archive.org/web/20211028051456/https://www.nap.edu/read/5737/chapter/13 |archive-date=October 28, 2021 |url-status=live |access-date=September 27, 2021 }}</ref> |
|||
Linus Pauling spent his first year living in a one-room apartment with his parents in Portland. In 1902, after his sister Pauline was born, Pauling's parents decided to move out of the city.<ref name="Goertzelp4">Goertzel and Goertzel, p. 4.</ref> They were crowded in their apartment, but could not afford more spacious living quarters in Portland. Lucy stayed with her husband's parents in Lake Oswego, while Herman searched for new housing. Herman brought the family to [[Salem, Oregon|Salem]], where he took up a job as a traveling salesman for the Skidmore Drug Company. Within a year of Lucile's birth in 1904, Herman Pauling moved his family to Oswego, where he opened his own drugstore.<ref name="Goertzelp4"/> The business climate in Oswego was poor, so he moved his family to Condon in 1905.<ref name="Goertzel and Goertzel, p. 5">Goertzel and Goertzel, p. 5.</ref> By 1909, Herman Pauling was suffering from poor health and had regular sharp pains in his [[abdomen]]. His health worsened in the coming months and he finally died of a perforated [[Peptic ulcer|ulcer]] on June 11, 1910, leaving Lucy to care for Linus, Lucile and Pauline.<ref>Mead and Hager, p. 9.</ref> |
|||
In 1902, after his sister Pauline was born, Pauling's parents decided to move out of Portland to find more affordable and spacious living quarters than their one-room apartment.<ref name="GoGo">{{Cite book |last1=Goertzel |first1=Ted |author1-link=Ted Goertzel |url=https://archive.org/details/linuspaulinglife0000goer_i4n1 |title=Linus Pauling: A Life in Science and Politics |last2=Goertzel |first2=Ben |date=1995 |publisher=Basic Books |isbn=978-0-465-00672-4 |language=en |via=[[Internet Archive]]}}</ref>{{rp|4}} Lucy stayed with her husband's parents in Oswego until Herman brought the family to [[Salem, Oregon|Salem]], where he worked briefly as a traveling salesman for the Skidmore Drug Company. Within a year of Lucile's birth in 1904, Herman Pauling moved his family to [[Oswego, Oregon]] where he opened his own drugstore.{{r|GoGo|p=4}} He moved his family to [[Condon, Oregon]], in 1905.{{r|GoGo|p=5}} By 1906, Herman Pauling was suffering from recurrent [[abdominal pain]]. He died of a perforated [[Peptic ulcer|ulcer]] on June 11, 1910, leaving Lucy to care for Linus, Lucile and Pauline.{{r|MeadHager|p=9}} |
|||
At age nine, Linus was a voracious reader. On May 12, 1910 his father wrote a letter to ''[[The Oregonian]]'' inviting suggestions of additional books to occupy his time.<ref name="frs"/> Pauling first planned to become a chemist after being amazed by experiments conducted with a small chemistry lab kit by his friend, Lloyd A. Jeffress.<ref>Goertzel and Goertzel, p. 17.</ref> At high school, Pauling continued to conduct chemistry experiments, scavenging much of the equipment and material from an abandoned steel plant. With an older friend, Lloyd Simon, Pauling set up Palmon Laboratories. Operating from Simon's basement, the two approached local dairies to offer their services in performing butterfat samplings at cheap prices. Dairymen were wary of trusting two boys with the task, and as such, the business ended in failure.<ref>Goertzel and Goertzel, p. 21.</ref> |
|||
Pauling attributes his interest in becoming a chemist to being amazed by experiments conducted by a friend, [[Lloyd A. Jeffress]], who had a small chemistry lab kit.{{r|GoGo|p=17}} He later wrote: "I was simply entranced by chemical phenomena, by the reactions in which substances, often with strikingly different properties, appear; and I hoped to learn more and more about this aspect of the world."<ref name="Abrams">{{Cite book |last=Abrams |first=Irwin |url={{GBurl|id=ny77bPwKxaUC|page=198}} |title=The Nobel Peace Prize and the laureates : an illustrated biographical history, 1901–1987 |date=1988 |publisher=G.K. Hall |isbn=978-0-8161-8609-9 |edition=2. print. |location=[[Boston]]}}</ref> |
|||
By the fall of 1916, Pauling was a 15-year-old high school senior with enough credits to enter [[Oregon State University]] (OSU), known then as Oregon Agricultural College.<ref>Goertzel and Goertzel, p. 22.</ref> He did not have enough credits for two required American history courses that would satisfy the requirements for earning a [[high school diploma]]. He asked the school principal if he could take these courses concurrently during the spring semester, but the principal denied his request, and Pauling decided to leave the school in June without a diploma.<ref>Hager, p. 48.</ref> His high school, [[Washington High School (Portland, Oregon)|Washington High School]] in Portland, awarded him the diploma 45 years later, after he had won two Nobel Prizes.<ref>{{cite web| url=http://nobelprize.org/nobel_prizes/peace/laureates/1962/pauling-bio.html | title=Linus Pauling – Biography | accessdate=August 5, 2007 | publisher=[[Nobel Foundation]]}}</ref><ref>{{cite book |last=Bourgoin |first=Suzanne M. |coauthors=Paula K. Byers| title=Encyclopedia of World Biography | year=1998 | url= | publisher=[[Thomson Gale]] | page=Vol. 12, p. 150 | isbn=0-7876-2221-4 |nopp=true }}</ref> During the summer, Pauling worked part-time at a grocery store, earning eight US dollars a week. His mother set him up with an interview with a Mr. Schwietzerhoff, the owner of a number of manufacturing plants in Portland. Pauling was hired as an apprentice machinist with a salary of 40 dollars per month. Pauling excelled at his job, and saw his salary soon raised to 50 dollars per month.<ref name="Goertzelp23">Goertzel and Goertzel, p. 23.</ref> In his spare time, he set up a photography laboratory with two friends and found business from a local photography company. He hoped that the business would earn him enough money to pay for his future college expenses.<ref>Goertzel and Goertzel, p. 24.</ref> Pauling received a letter of admission from Oregon State University in September 1917 and immediately [[resignation|gave notice]] to his boss and told his mother of his plans.<ref>Goertzel and Goertzel, p. 25.</ref> |
|||
In high school, Pauling conducted chemistry experiments by scavenging equipment and material from an abandoned steel plant. With an older friend, Lloyd Simon, Pauling set up Palmon Laboratories in Simon's basement. They approached local dairies offering to perform butterfat samplings at cheap prices but dairymen were wary of trusting two boys with the task, and the business ended in failure.{{r|GoGo|p=21}} |
|||
===Higher education=== |
|||
[[File:LinusPaulingGraduation1922.jpg|thumb|Pauling's graduation photo from [[Oregon State University]], 1922.]] |
|||
At age 15, the high school senior had enough credits to enter [[Oregon State University]] (OSU), known then as Oregon Agricultural College.{{r|GoGo|p=22}} Lacking two American history courses required for his [[high school diploma]], Pauling asked the school principal if he could take the courses concurrently during the spring semester. Denied, he left [[Washington High School (Portland, Oregon)|Washington High School]] in June without a diploma.{{r|Nature|p=48}} The school awarded him an honorary diploma 45 years later, after he was awarded two Nobel Prizes.<ref name="Nobel" /><ref>{{Cite encyclopedia |year=1998 |title=Pauling, Linus |encyclopedia=Encyclopedia of World Biography |publisher=[[Thomson Gale]] |editor-last=Bourgoin |editor-first=Suzanne M. |volume=12 |page=150 |isbn=978-0-7876-2221-3 |oclc=498136139 |ol=24962233M |editor-first2=Paula K. |editor-last2=Byers}}</ref><ref>{{cite news |title=Pauling Finally Gets High School Diploma |url=https://chroniclingamerica.loc.gov/lccn/sn83045462/1962-06-19/ed-1/seq-1/ |access-date=August 3, 2022 |work=[[The Washington Star|Evening Star]] |date=June 19, 1962 |at=p. 1 col 6}}</ref> |
|||
In October 1917, Pauling lived in a [[boarding house]] on the [[Corvallis, Oregon|Corvallis]] campus with his cousin Mervyn and another man, using the $200 he had saved from odd jobs to finance his education. In his first semester, Pauling registered for two courses in chemistry, two in mathematics, mechanical drawing, introduction to mining and use of explosives, modern English prose, gymnastics and military drill.<ref name="Goertzelp26">Goertzel and Goertzel, p. 26.</ref> Pauling fell in love with a freshman girl named Irene early in the school year, and, by the end of October, he had used up $150 of his savings on her, taking her to shows and games. He soon got a job at the girls' dormitory, working 100 hours a month chopping wood for stoves, cutting up beef and mopping up the kitchen. Despite the salary of 25 cents per hour, Pauling was still having trouble managing his finances. He began eating one hot meal a day at a restaurant off campus to keep his expenses down.<ref name="Goertzelp26"/> Pauling was active in campus life and founded the school's chapter of the [[Delta Upsilon]] fraternity.<ref>{{cite news | last=Swanson |first=Stephen| url=http://oregonstate.edu/ua/ncs/archives/2000/oct/osu-fraternity-donate-pauling-treasures-campus-library | title= OSU fraternity to donate Pauling treasures to campus library | accessdate=April 29, 2013 | date=October 3, 2000 | publisher=[[Oregon State University]]}}</ref> After his second year, he planned to take a job in Portland to help support his mother, but the college offered him a position teaching [[Quantitative analysis (chemistry)|quantitative analysis]], a course he had just finished taking himself. He worked forty hours a week in the laboratory and classroom and earned $100 a month.<ref name="Goertzelp29">Goertzel and Goertzel, p. 29.</ref> This allowed him to continue his studies at the college. |
|||
Pauling held a number of jobs to earn money for his future college expenses, including working part-time at a grocery store for {{currency|8|USD}} per week (equivalent to {{currency|{{inflation|US|8|1916|r=-1}}|USD}} in {{inflation/year|US}}). His mother arranged an interview with the owner of a number of manufacturing plants in Portland, Mr. Schwietzerhoff, who hired him as an apprentice machinist at a salary of {{currency|40|USD}} per month (equivalent to {{currency|{{inflation|US|40|1916|r=-1}}|USD}} in {{inflation/year|US}}). This was soon raised to {{currency|50|USD}} per month.{{r|GoGo|p=23}} Pauling also set up a photography laboratory with two friends.{{r|GoGo|p=24}} In September 1917, Pauling was finally admitted by Oregon State University. He immediately resigned from the machinist's job and informed his mother, who saw no point in a university education, of his plans.{{r|GoGo|p=25}} |
|||
In his last two years at school, Pauling became aware of the work of [[Gilbert N. Lewis]] and [[Irving Langmuir]] on the [[electronic structure]] of atoms and their [[chemical bond|bonding]] to form [[molecule]]s.<ref name="Goertzelp29"/> He decided to focus his research on how the [[physical property|physical]] and [[chemical property|chemical properties]] of substances are related to the structure of the atoms of which they are composed, becoming one of the founders of the new science of quantum chemistry. Pauling began to neglect his studies in humanities and social sciences. He had also exhausted the course offerings in the physics and mathematics departments. Professor Samuel Graf selected Pauling to be his teaching assistant in a high-level mathematics course.<ref>Goertzel and Goertzel, p. 30.</ref> During the winter of his senior year, Pauling was approached by the college to teach a chemistry course for [[family and consumer science|home economics]] majors. It was in one of these classes that Pauling met his future wife, [[Ava Helen Pauling|Ava Helen Miller]].<ref>Goertzel and Goertzel, p. 31.</ref> |
|||
===Higher education=== |
|||
In 1922, Pauling graduated from [[Oregon State University]]<ref name="frs"/> (known then as Oregon Agricultural College) with a degree in [[chemical engineering]] and went on to [[graduate school]] at the [[California Institute of Technology]] (Caltech) in [[Pasadena, California]], under the guidance of [[Roscoe G. Dickinson|Roscoe Dickinson]] and [[Richard C. Tolman|Richard Tolman]].<ref name="mathgene"/> His graduate research involved the use of [[X-ray diffraction]] to determine the structure of [[crystal]]s. He published seven papers on the [[crystal structure]] of minerals while he was at Caltech. He received his PhD in [[physical chemistry]] and [[mathematical physics]],<ref name="paulingphd">{{cite thesis |degree=PhD |first=Linus|last=Pauling |title=The determination with x-rays of the structures of crystals |publisher=California Institute of Technology |date=1925 |url=http://thesis.library.caltech.edu/1791/|authorlink=Linus Pauling}}</ref> ''[[summa cum laude]],'' in 1925.<ref>{{cite web|url=http://caltechcampuspubs.library.caltech.edu/2537/1/1925.pdf |title=Caltech Commencement Program |date=1925-06-12 |publisher=Caltech Campus Publications |accessdate=2013-03-29}}</ref> |
|||
[[File:LinusPaulingGraduation1922.jpg|thumb|left|Pauling's graduation photo from [[Oregon State University]], 1922]] |
|||
In his first semester, Pauling registered for two courses in chemistry, two in mathematics, mechanical drawing, introduction to mining and use of explosives, modern English prose, gymnastics and military drill.{{r|GoGo|p=26}} His roommate was childhood pal and lifelong best friend Lloyd Jeffress.<ref name=Linus>{{cite web|last1=Pauling|first1=Linus|title=Life with Lloyd Jeffress, June 5, 1986|url=https://paulingblog.wordpress.com/2009/07/02/paulings-best-friend-lloyd-jeffress/|website=The Pauling Blog|publisher=Oregon State University Libraries Special Collections and Archives Research Center|access-date=1 June 2016|date=2009-07-02}}</ref> He was active in campus life and founded the school's chapter of the [[Delta Upsilon]] fraternity.<ref>{{Cite web |last=Swanson |first=Stephen |date=October 3, 2000 |title=OSU fraternity to donate Pauling treasures to campus library |url=http://oregonstate.edu/ua/ncs/archives/2000/oct/osu-fraternity-donate-pauling-treasures-campus-library |url-status=dead |archive-url=https://web.archive.org/web/20160303202915/http://oregonstate.edu/ua/ncs/archives/2000/oct/osu-fraternity-donate-pauling-treasures-campus-library |archive-date=March 3, 2016 |access-date=April 29, 2013 |website=[[Oregon State University]] |language=en}}</ref> After his second year, he planned to take a job in Portland to help support his mother. The college offered him a position teaching [[Quantitative analysis (chemistry)|quantitative analysis]], a course he had just finished taking himself. He worked forty hours a week in the laboratory and classroom and earned {{currency|100|USD}} a month (equivalent to {{currency|{{inflation|US|100|1920|r=-2}}|USD}} in {{inflation/year|US}}), enabling him to continue his studies.{{r|GoGo|p=29}} |
|||
== Family Life == |
|||
[[File:Linus Pauling family 1954.jpg|thumb|300px|The Pauling children at a gathering in celebration of the 1954 Nobel Prizes in Stockholm, Sweden. Seated from left: Linus Pauling, Jr., Peter Pauling and Linda Pauling. Standing from left: An unidentified individual and Crellin Pauling.]] |
|||
In his last two years at school, Pauling became aware of the work of [[Gilbert N. Lewis]] and [[Irving Langmuir]] on the [[electronic structure]] of atoms and their [[chemical bond|bonding]] to form [[molecule]]s.{{r|GoGo|p=29}} He decided to focus his research on how the [[physical property|physical]] and [[chemical property|chemical properties]] of substances are related to the structure of the atoms of which they are composed, becoming one of the founders of the new science of quantum chemistry.{{citation needed|date=August 2023}} |
|||
While teaching a class called "Chemistry for Home Economic Majors" at college,<ref>{{cite web | url=http://lpi.oregonstate.edu/lpbio/timeline.html | title=Linus Pauling: A Biographical Timeline | publisher =Linus Pauling Institute | accessdate=November 10, 2011}}</ref> Pauling met his future wife, Ava Helen Miller and they married June 17, 1923. The marriage lasted until Ava Pauling's death in 1981, and had three sons (Linus Jr., Peter and Edward Crellin) and a daughter (Linda).<ref>{{cite web | url = http://profiles.nlm.nih.gov/ps/retrieve/Narrative/MM/p-nid/5e | title = The Linus Pauling Papers: Biographical Information | publisher = United States National Library of Medicine | date = n.d. | accessdate =November 10, 2011 }}</ref> Pauling's sons went on to become scientists and researchers (Linus, a [[psychiatry|psychiatrist]]; Peter, who died in 2003, a [[crystallography|crystallographer]]; and Edward Crellin, who died in 1997, a [[Biology|biologist]]; daughter Linda married the noted Caltech geologist and glaciologist [[Barclay Kamb]]).<ref>{{cite web | url = http://lpi.oregonstate.edu/lpbio/lpbio2.html | title = Linus Pauling Biography | publisher = Linus Pauling Institute | accessdate =November 10, 2011 }}</ref> |
|||
Engineering professor Samuel Graf (1887–1966)<ref>{{cite web|url=https://engineering.oregonstate.edu/alumni-partners/oregon-stater-awards/searchable-awards-database/samuel-graf-engineering-hall-fame |title=Samuel Graf : Engineering Hall of Fame - 1998 | College of Engineering | Oregon State University |date=3 October 2022 }}</ref><ref>{{cite web|url=https://www.newspapers.com/article/corvallis-gazette-times/31584297/ |title=''Long-Time OSU Faculty Man, Sam Graf, Dies'' {Article clipped from Corvallis Gazette-Times) |website=newspapers.com |date=25 July 1966 }}</ref> selected Pauling to be his teaching assistant in a mechanics and materials course.{{r|GoGo|p=29}}<ref name="OSUArchives">{{Cite web |title=Pauling's Years as an Undergraduate at Oregon Agricultural College, Part 2 (1919–1922) |url=http://scarc.library.oregonstate.edu/coll/pauling/chronology/page6.html |url-status=live |archive-url=https://web.archive.org/web/20211031065844/http://scarc.library.oregonstate.edu/coll/pauling/chronology/page6.html |archive-date=October 31, 2021 |access-date=May 27, 2015 |website=[[Oregon State University]]}}</ref><ref name="Marinacci">{{Cite book |last=Pauling |first=Linus |url=https://archive.org/details/linuspaulinginhi0000paul |title=Linus Pauling: in his own words : selected writings, speeches, and interviews |date=1995 |publisher=[[Simon & Schuster]] |isbn=978-0-684-81387-5 |editor-last=Marinacci |editor-first=Barbara |location=New York City |page=39 |language=en-us |author-mask=4 |access-date=May 27, 2015 |url-access=registration |via=[[Internet Archive]]}}</ref> During the winter of his senior year, Pauling taught a chemistry course for [[family and consumer science|home economics]] majors. It was in one of these classes that Pauling met his future wife, [[Ava Helen Pauling|Ava Helen Miller]].{{r|GoGo|p=31}}{{r|Marinacci|p=41}}<ref>{{Cite web |title=Linus Pauling Biographical Timeline |url=http://lpi.oregonstate.edu/lpbio/timeline.html |url-status=live |archive-url=https://web.archive.org/web/20220302113523/https://lpi.oregonstate.edu/about/linus-pauling-biographical-timeline |archive-date=March 2, 2022 |access-date=November 10, 2011 |website=[[Linus Pauling Institute]] |publisher=[[Oregon State University]] |language=en-us}}</ref><ref name="AvaBook">{{Cite news |last=Richard |first=Terry |date=May 3, 2013 |title=Ava Helen Pauling, wife of Linus Pauling, subject of biography by Corvallis author Mina Carson |language=en |work=[[The Oregonian]] |url=http://www.oregonlive.com/books/index.ssf/2013/05/ava_helen_pauling_wife_of_linu.html |url-status=live |url-access=subscription |access-date=June 2, 2015 |archive-url=https://web.archive.org/web/20210513195013/https://www.oregonlive.com/books/2013/05/ava_helen_pauling_wife_of_linu.html |archive-date=May 13, 2021 |issn=8750-1317}}</ref> |
|||
Pauling was raised as a member of the [[Lutheran]] Church, but later joined the [[Unitarian Universalist]] Church and publicly declared his [[atheism]] two years before his death.<ref>{{cite book | author=Linus Pauling & Daisaku Ikeda | title=A Lifelong Quest for Peace: A Dialogue | year=1992 | publisher=Jones & Bartlett | isbn=0-86720-277-7 | page=22 | quote = ...I [Pauling] am not, however, militant in my atheism. The great English theoretical physicist Paul Dirac is a militant atheist. I suppose he is interested in arguing about the existence of God. I am not. It was once quipped that there is no God and Dirac is his prophet.}}</ref> |
|||
In 1922, Pauling graduated with a degree in [[chemical engineering]]. He went on to graduate school at the [[California Institute of Technology]] (Caltech) in [[Pasadena, California]], under the guidance of [[Roscoe G. Dickinson|Roscoe Dickinson]] and [[Richard C. Tolman|Richard Tolman]].<ref name="mathgene" /> His graduate research involved the use of [[X-ray diffraction]] to determine the structure of [[crystal]]s. He published seven papers on the [[crystal structure]] of minerals while he was at Caltech. He received his PhD in [[physical chemistry]] and [[mathematical physics]],<ref name="paulingphd" /> [[summa cum laude]], in 1925.<ref>{{Cite web |date=June 12, 1925 |title=Commencement 1925 California Institute of Technology Pasadena |url=http://caltechcampuspubs.library.caltech.edu/2537/1/1925.pdf |url-status=live |archive-url=https://web.archive.org/web/20201101163715/http://caltechcampuspubs.library.caltech.edu/2537/1/1925.pdf |archive-date=November 1, 2020 |access-date=March 29, 2013 |publisher=[[California Institute of Technology]]}}</ref> |
|||
== Career == |
== Career == |
||
{{external media |width=190px | float = right | headerimage= [[File:Sickle Cell Blood Smear.JPG|170px]] | video1 = [http://watch.opb.org/video/1954582651/ ''Linus Pauling''], Oregon Experience, [[Oregon Historical Society]]}}In 1926, Pauling was awarded a [[Guggenheim Fellowship]] to travel to Europe, to study under German physicist [[Arnold Sommerfeld]] in Munich, Danish physicist [[Niels Bohr]] in Copenhagen and Austrian physicist [[Erwin Schrödinger]] in [[Zürich]]. All three were experts in the new field of [[quantum mechanics]] and other branches of physics.<ref name="Guggenheim" /> Pauling became interested in how quantum mechanics might be applied in his chosen field of interest, the [[Electron configuration|electronic structure]] of atoms and molecules. In Zürich, Pauling was also exposed to one of the first quantum mechanical analyses of bonding in the [[hydrogen]] molecule, done by [[Walter Heitler]] and [[Fritz London]].<ref name="Realism">{{Cite conference |date=December 9, 2010 |orig-date=1996-10-31 |title=Realism and anti-realism in the philosophy of science |url={{GBurl|id=mvYIkgAACAAJ|page=161}} |conference=Beijing International Conference 1992 |location=[[Dordrecht]] |publisher=[[Springer Nature|Springer]] |page=161 |isbn=978-90-481-4493-8 |ol=28281917M |access-date=May 27, 2015 |editor1-last=Cohen |editor1-first=Robert S. |editor2-last=Hilpinen |editor2-first=Risto |editor3-last=Qiu |editor3-first=Ren-Zong}}</ref> Pauling devoted the two years of his European trip to this work and decided to make it the focus of his future research. He became one of the first scientists in the field of quantum chemistry and a pioneer in the application of quantum theory to the structure of molecules.<ref name="PNNL">{{Cite web |title=About Linus Pauling |url=https://www.pnnl.gov/projects/linus-pauling-distinguished-postdoctoral-fellowship/about |url-status=live |archive-url=https://web.archive.org/web/20220302175453/https://www.pnnl.gov/projects/linus-pauling-distinguished-postdoctoral-fellowship/about |archive-date=March 2, 2022 |access-date=April 13, 2022 |website=[[Pacific Northwest National Laboratory]] |language=en}}</ref> |
|||
In 1927, Pauling took a new position as an assistant professor at [[Caltech]] in [[theoretical chemistry]].<ref name="OH">{{Cite book |last=Pauling |first=Linus |url=https://digital.sciencehistory.org/works/5h73px42w |title=Oral history interview with Linus C. Pauling |date=April 6, 1987 |publisher=[[Science History Institute]] |location=[[Denver]] |publication-place=[[Philadelphia]] |language=en |author-mask=4 |access-date=April 13, 2022 |archive-url=https://web.archive.org/web/20210816110527/https://digital.sciencehistory.org/works/5h73px42w |archive-date=August 16, 2021 |url-status=live |interviewer-last=Sturchio |interviewer-first=Jeffrey L.}}</ref> He launched his faculty career with a very productive five years, continuing with his [[X-ray]] crystal studies and also performing quantum mechanical calculations on atoms and molecules. He published approximately fifty papers in those five years, and created the five rules now known as [[Pauling's rules]].<ref>{{Cite journal |last=Pauling |first=Linus |author-mask=4 |date=April 1, 1929 |title=The principles determining the structure of complex ionic crystals |journal=[[Journal of the American Chemical Society]] |volume=51 |issue=4 |pages=1010–1026 |doi=10.1021/ja01379a006|bibcode=1929JAChS..51.1010P }}</ref><ref name="Pauling1960">{{Cite book |last=Pauling |first=Linus |url={{GBurl|id=L-1K9HmKmUUC}} |title=The nature of the chemical bond and the structure of molecules and crystals; an introduction to modern structural chemistry |date=January 31, 1960 |publisher=[[Cornell University Press]] |isbn=978-0-8014-0333-0 |edition=3rd |location=[[Ithaca, New York]] |pages=543–562 |ol=26811428M |author-mask=4 |orig-date=1939}}</ref> By 1929, he was promoted to associate professor, and by 1930, to full professor.<ref name="OH" /> In 1931, the [[American Chemical Society]] awarded Pauling the Langmuir Prize for the most significant work in pure science by a person 30 years of age or younger.<ref>{{Cite web |last=Hager |first=Thomas |author-link=Thomas Hager |date=December 2004 |title=The Langmuir Prize |url=http://scarc.library.oregonstate.edu/coll/pauling/bond/narrative/page28.html |url-status=live |archive-url=https://web.archive.org/web/20201212140745/http://scarc.library.oregonstate.edu/coll/pauling/bond/narrative/page28.html |archive-date=December 12, 2020 |access-date=February 29, 2008 |website=[[Oregon State University]] |language=en}}</ref> The following year, Pauling published what he regarded as his most important paper, in which he first laid out the concept of [[Orbital hybridization|hybridization of atomic orbitals]] and analyzed the [[tetravalence|tetravalency]] of the [[carbon]] atom.<ref>{{Cite journal |last=Pauling |first=Linus |author-mask=4 |date=March 1, 1932 |title=The nature of the chemical bond. III. The transition from one extreme bond type to another |journal=[[Journal of the American Chemical Society]] |volume=54 |issue=3 |pages=988–1003 |doi=10.1021/ja01342a022|bibcode=1932JAChS..54..988P }}</ref> |
|||
At Caltech, Pauling struck up a close friendship with [[Theoretical physics|theoretical physicist]] [[Robert Oppenheimer]] at the [[University of California, Berkeley]], who spent part of his research and teaching schedule as a visitor at Caltech each year.<ref name="Nature" /><ref name="Oppenheimer">{{Cite book |last=Monk |first=Ray |url={{GBurl|id=EkJ9aWTjWjUC|page=203}} |title=Robert Oppenheimer : a life inside the center |date=March 11, 2014 |publisher=[[Anchor Books]] |isbn=978-0-385-72204-9 |edition=First Anchor Books |page=203 |ol=32935915M |author-link=Ray Monk |orig-date=2012}}</ref> Pauling was also affiliated with Berkeley, serving as a visiting lecturer in physics and chemistry from 1929 to 1934.<ref>{{Cite web |title=Early Career at the California Institute of Technology (1927–1930) |url=http://scarc.library.oregonstate.edu/coll/pauling/chronology/page10.html |url-status=live |archive-url=https://web.archive.org/web/20211112063521/http://scarc.library.oregonstate.edu/coll/pauling/chronology/page10.html |archive-date=November 12, 2021 |access-date=May 18, 2017 |website=[[Oregon State University]] |language=en}}</ref> Oppenheimer even gave Pauling a stunning personal collection of minerals.<ref name="LostAlly">{{Cite web |title=A Lost Ally |url=http://scarc.library.oregonstate.edu/coll/pauling/bond/narrative/page20.html |url-status=live |archive-url=https://web.archive.org/web/20210820230846/http://scarc.library.oregonstate.edu/coll/pauling/bond/narrative/page20.html |archive-date=August 20, 2021 |access-date=May 27, 2015 |website=[[Oregon State University]] |language=en}}</ref> The two men planned to mount a joint attack on the nature of the chemical bond: apparently Oppenheimer would supply the mathematics and Pauling would interpret the results. Their relationship soured when Oppenheimer tried to pursue Pauling's wife, Ava Helen. When Pauling was at work, Oppenheimer came to their home and blurted out an invitation to Ava Helen to join him on a tryst in Mexico. She flatly refused, and reported the incident to Pauling. He immediately cut off his relationship with Oppenheimer.{{r|Nature|p=152}}<ref name="Oppenheimer" /> |
|||
In 1927, Pauling took a new position as an assistant professor at [[Caltech]] in [[theoretical chemistry]]. He launched his faculty career with a very productive five years, continuing with his [[X-ray]] crystal studies and also performing quantum mechanical calculations on atoms and molecules. He published approximately fifty papers in those five years, and created the five rules now known as [[Pauling's rules]]. By 1929, he was promoted to associate professor, and by 1930, to full professor. In 1931, the [[American Chemical Society]] awarded Pauling the Langmuir Prize for the most significant work in pure science by a person 30 years of age or younger.<ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/bond/narrative/page28.html | title=The Langmuir Prize | accessdate=February 29, 2008 | author=Tom Hager |date=December 2004 | publisher=Oregon State University Libraries Special Collections }}</ref> The following year, Pauling published what he regarded as his most important paper, in which he first laid out the concept of [[Orbital hybridization|hybridization of atomic orbitals]] and analyzed the [[tetravalence|tetravalency]] of the [[carbon]] atom.<ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/bond/papers/1932p.2.html | title=The nature of the chemical bond. III. The transition from one extreme bond type to another. | accessdate=February 29, 2008 | author=Linus Pauling |date=March 1932 | publisher=Journal of the American Chemical Society }}</ref> |
|||
In the summer of 1930, Pauling made another European trip, during which he learned about gas-phase electron [[diffraction]] from [[Herman Francis Mark]]. After returning, he built an [[electron diffraction]] instrument at Caltech with a student of his, [[Lawrence O. Brockway|Lawrence Olin Brockway]], and used it to study the [[molecular geometry|molecular structure]] of a large number of chemical substances.<ref name="Hargittai">{{Cite book |last1=Hargittai |first1=István |url={{GBurl|id=KPqac4Y551AC|page=134}} |title=In our own image: personal symmetry in discovery |last2=Hargittai |first2=Magdolna |date=February 29, 2000 |publisher=[[Springer Nature]] |isbn=978-0-306-46091-3 |location=New York City |language=en |lccn=99033173 |ol=9669915M |access-date=May 27, 2015}}</ref> |
|||
At Caltech, Pauling struck up a close friendship with [[Theoretical physics|theoretical physicist]] [[Robert Oppenheimer]], who was spending part of his research and teaching schedule away from [[University of California, Berkeley|U.C. Berkeley]] at Caltech every year. The two men planned to mount a joint attack on the nature of the chemical bond: apparently Oppenheimer would supply the mathematics and Pauling would interpret the results. Their relationship soured when Pauling began to suspect that Oppenheimer was becoming too close to his wife, Ava Helen. Once, when Pauling was at work, Oppenheimer had come to their place and blurted out an invitation to Ava Helen to join him on a tryst in Mexico.<ref>{{cite book | author=Thomas Hager | title=Force of Nature: The Life of Linus Pauling | year=1995 | publisher=Simon & Schuster | isbn=0-684-80909-5}}, pp. 152</ref> She flatly refused, and reported the incident to Pauling. Disquieted by this strange chemistry, and her apparent nonchalance about the incident, he immediately cut off his relationship with Oppenheimer. |
|||
Pauling introduced the concept of [[electronegativity]] in 1932.<ref name="paulingJACS">{{Cite journal |last=Pauling |first=L. |author-mask=4 |date=September 1, 1932 |title=The Nature of the Chemical Bond. IV. The Energy of Single Bonds and the Relative Electronegativity of Atoms |journal=[[Journal of the American Chemical Society]] |volume=54 |issue=9 |pages=3570–3582 |doi=10.1021/ja01348a011 |bibcode=1932JAChS..54.3570P |issn=0002-7863 |lccn=16003159 |oclc=01226990}}</ref> Using the various properties of molecules, such as the energy required to break bonds and the [[dipole]] [[Moment (physics)|moments]] of molecules, he established a scale and an associated numerical value for most of the elements{{thinsp|—}}the [[Pauling scale of electronegativity|Pauling Electronegativity Scale]]{{thinsp|—}}which is useful in predicting the nature of bonds between atoms in molecules.<ref name="Scale2">{{Cite web |date=March 17, 2009 |title=The Pauling Electronegativity Scale: Part 2, Inspired by Biology |url=https://paulingblog.wordpress.com/2009/03/17/the-pauling-electronegativity-scale-part-2-inspired-by-biology/ |url-status=live |archive-url=https://web.archive.org/web/20211117185840/https://paulingblog.wordpress.com/2009/03/17/the-pauling-electronegativity-scale-part-2-inspired-by-biology/ |archive-date=November 17, 2021 |access-date=March 17, 2009 |website=[[Oregon State University]] |language=en}}</ref> |
|||
In the summer of 1930, Pauling made another European trip, during which he learned about the use of [[electron]]s in [[diffraction]] studies similar to the ones he had performed with X-rays. After returning, he built an [[electron diffraction]] instrument at Caltech with a student of his, L. O. Brockway, and used it to study the [[molecular geometry|molecular structure]] of a large number of chemical substances. |
|||
In 1936, Pauling was promoted to chairman of the division of chemistry and chemical engineering at Caltech, and to the position of director of the Gates and Crellin Laboratories of Chemistry. He would hold both positions until 1958.<ref name="OH" /> Pauling also spent a year in 1948 at the [[University of Oxford]] as George Eastman Visiting Professor and Fellow of Balliol.<ref>{{Cite web |date=August 21, 1994 |title=Obituary: Professor Linus Pauling |url=https://www.independent.co.uk/news/people/obituary-professor-linus-pauling-1377923.html |url-status=live |archive-url=https://web.archive.org/web/20210616040502/https://www.independent.co.uk/news/people/obituary-professor-linus-pauling-1377923.html |archive-date=June 16, 2021 |access-date=January 25, 2018 |website=[[The Independent]] |language=en}}</ref> |
|||
Pauling introduced the concept of [[electronegativity]] in 1932. Using the various properties of molecules, such as the energy required to break bonds and the [[dipole]] [[Moment (physics)|moments]] of molecules, he established a scale and an associated numerical value for most of the elements – the [[Pauling scale of electronegativity|Pauling Electronegativity Scale]] – which is useful in predicting the nature of bonds between atoms in molecules. |
|||
===Nature of the chemical bond=== |
===Nature of the chemical bond=== |
||
[[File:Linus Pauling 1955a.jpg|thumb|Linus Pauling with an inset of his Nobel Prize in 1955]] |
|||
In the late 1920s Pauling began publishing papers on the nature of the chemical bond, leading to his famous textbook on the subject published in 1939. It is based primarily on his work in this area that he received the [[Nobel Prize in Chemistry]] in 1954 "for his research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances". Pauling summarized his work on the chemical bond in ''The Nature of the Chemical Bond'', one of the most influential chemistry books ever published.<ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/bond/narrative/page46.html | title=The Nature of the Chemical Bond | accessdate=February 29, 2008 | author=Thomas Hager |date=December 2004 | publisher=Oregon State University Libraries Special Collections }}</ref> In the 30 years after its first edition was published in 1939, the book was cited more than 16,000 times. Even today, many modern scientific papers and articles in important journals cite this work, more than seventy years after the first publication. |
|||
In the late 1920s, Pauling began publishing papers on the nature of the [[chemical bond]]. Between 1937 and 1938, he took a position as George Fischer Baker Non-Resident Lecturer in Chemistry at [[Cornell University]]. While at Cornell, he delivered a series of nineteen lectures<ref>{{Cite web |title=Outline of the George Fischer Baker Lectureship, Cornell University |url=http://scarc.library.oregonstate.edu/coll/pauling/bond/notes/1937s.3-outline-01-large.html |url-status=live |archive-url=https://web.archive.org/web/20211112063624/http://scarc.library.oregonstate.edu/coll/pauling/bond/notes/1937s.3-outline-01-large.html |archive-date=November 12, 2021 |access-date=April 13, 2022 |website=[[Oregon State University]] |language=en}}</ref> and completed the bulk of his famous textbook ''The Nature of the Chemical Bond''.<ref name="The George Fischer Baker Lectureship and the Beginnings of the Manuscript">{{Cite web |date=July 30, 2014 |title=The George Fischer Baker Lectureship and the Beginnings of the Manuscript |url=https://paulingblog.wordpress.com/2014/07/30/the-george-fischer-baker-lectureship-and-the-beginnings-of-the-manuscript/ |url-status=live |archive-url=https://web.archive.org/web/20220307085556/https://paulingblog.wordpress.com/2014/07/30/the-george-fischer-baker-lectureship-and-the-beginnings-of-the-manuscript/ |archive-date=March 7, 2022 |access-date=June 3, 2015 |website=The Pauling Blog |publisher=[[Oregon State University]] |language=en}}</ref><ref name="Pauling1960" />{{rp|Preface}} It is based primarily on his work in this area that he received the [[Nobel Prize in Chemistry]] in 1954 "for his research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances".<ref name="Nobel" /> Pauling's book has been considered "chemistry's most influential book of this century and its effective bible".<ref name="Genes2001">{{Cite book |last=Watson |first=James D. |url=https://archive.org/details/passionfordnagen0000wats |title=A passion for DNA: genes, genomes, and society |date=2001 |publisher=[[Oxford University Press]] |isbn=978-0-19-860428-0 |edition=2003 |location=[[Oxford]] |ol=7401431M |author-link=James Watson |via=[[Internet Archive]]}}</ref> In the 30 years after its first edition was published in 1939, the book was cited more than 16,000 times. Even today, many modern scientific papers and articles in important journals cite this work, more than seventy years after the first publication.<ref name="Google">{{Cite web |title=The nature of the chemical bond (citations and estimated counts) |url=https://scholar.google.com/citations?view_op=view_citation&hl=en&user=b0B12YAAAAAJ&citation_for_view=b0B12YAAAAAJ:j5aT6aphRxQC |access-date=May 27, 2015 |website=Google Scholar}}</ref> |
|||
Part of Pauling's work on the nature of the chemical bond led to his introduction of the concept of orbital hybridization.<ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/bond/notes/sci3.001.21.html | title=London's paper. General ideas on bonds. | accessdate=February 29, 2008 | author=Linus Pauling | year=1928 | publisher=Oregon State University Libraries Special Collections }}</ref> While it is normal to think of the electrons in an atom as being described by [[atomic orbital|orbital]]s of types such as ''s'' and ''p'', it turns out that in describing the bonding in molecules, it is better to construct functions that partake of some of the properties of each. Thus the one 2s and three 2p orbitals in a carbon atom can be combined to make four equivalent orbitals (called sp<sup>3</sup> hybrid orbitals), which would be the appropriate orbitals to describe carbon compounds such as [[methane]], or the 2s orbital may be combined with two of the 2p orbitals to make three equivalent orbitals (called sp<sup>2</sup> hybrid orbitals), with the remaining 2p orbital unhybridized, which would be the appropriate orbitals to describe certain [[alkene|unsaturated]] carbon compounds such as [[ethylene]]. Other hybridization schemes are also found in other types of molecules. |
|||
Part of Pauling's work on the nature of the chemical bond led to his introduction of the concept of [[orbital hybridization]].<ref>{{Cite web |last=Pauling |first=Linus |year=1928 |title=London's paper. General ideas on bonds |url=http://scarc.library.oregonstate.edu/coll/pauling/bond/notes/sci3.001.21.html |access-date=June 2, 2015 |publisher=Oregon State University Libraries Special Collections}}</ref> While it is normal to think of the electrons in an atom as being described by [[atomic orbital|orbital]]s of types such as ''s'' and ''p'', it turns out that in describing the bonding in molecules, it is better to construct functions that partake of some of the properties of each. Thus the one 2s and three 2p orbitals in a carbon atom can be (mathematically) 'mixed' or combined to make four equivalent orbitals (called sp<sup>3</sup> hybrid orbitals), which would be the appropriate orbitals to describe carbon compounds such as [[methane]], or the 2s orbital may be combined with two of the 2p orbitals to make three equivalent orbitals (called sp{{sup|2}} hybrid orbitals), with the remaining 2p orbital unhybridized, which would be the appropriate orbitals to describe certain [[alkene|unsaturated]] carbon compounds such as [[ethylene]].{{r|Pauling1960|pages=111–120}} Other hybridization schemes are also found in other types of molecules. |
|||
Another area which he explored was the relationship between [[ionic bond]]ing, where electrons are transferred between atoms, and [[covalent bond]]ing, where electrons are shared between atoms on an equal basis. Pauling showed that these were merely extremes, between which most actual cases of bonding fall. It was here especially that Pauling's ''[[electronegativity]]'' concept was particularly useful; the electronegativity difference between a pair of atoms will be the surest predictor of the degree of ionicity of the bond.<ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/bond/notes/sci5.001.14.html | title=Notes and Calculations re: Electronegativity and the Electronegativity Scale | accessdate=February 29, 2008 | author=Linus Pauling | date=1930s | publisher=Oregon State University Libraries Special Collections }}</ref> |
|||
Another area which he explored was the relationship between [[ionic bond]]ing, where electrons are transferred between atoms, and [[covalent bond]]ing, where electrons are shared between atoms on an equal basis. Pauling showed that these were merely extremes, and that for most actual cases of bonding, the [[Introduction to quantum mechanics|quantum-mechanical]] [[wave function]] for a polar molecule AB is a [[linear combination|combination]] of wave functions for covalent and ionic molecules.{{r|Pauling1960|page=66}} Here Pauling's ''[[electronegativity]]'' concept is particularly useful; the electronegativity difference between a pair of atoms will be the surest predictor of the degree of ionicity of the bond.<ref>{{Cite web |last=Pauling |first=Linus |date=1930s |title=Notes and Calculations re: Electronegativity and the Electronegativity Scale |url=http://scarc.library.oregonstate.edu/coll/pauling/bond/notes/sci5.001.14.html |access-date=February 29, 2008 |publisher=Oregon State University Libraries Special Collections}}</ref> |
|||
The third of the topics that Pauling attacked under the overall heading of "the nature of the chemical bond" was the accounting of the structure of [[aromatic hydrocarbon]]s, particularly the prototype, [[benzene]].<ref>{{ |
The third of the topics that Pauling attacked under the overall heading of "the nature of the chemical bond" was the accounting of the structure of [[aromatic hydrocarbon]]s, particularly the prototype, [[benzene]].<ref>{{Cite web |last=Pauling |first=Linus |date=January 6, 1934 |title=Benzene |url=http://scarc.library.oregonstate.edu/coll/pauling/bond/notes/sci2.004.6.html |access-date=February 29, 2008 |publisher=Oregon State University Libraries Special Collections}}</ref> The best description of benzene had been made by the German chemist [[Friedrich August Kekulé von Stradonitz|Friedrich Kekulé]]. He had treated it as a rapid interconversion between two structures, each with alternating single and [[double bond]]s, but with the double bonds of one structure in the locations where the single bonds were in the other. Pauling showed that a proper description based on quantum mechanics was an intermediate structure which was a blend of each. The structure was a superposition of structures rather than a rapid interconversion between them. The name "[[resonance (chemistry)|resonance]]" was later applied to this phenomenon.<ref>{{Cite web |last=Pauling |first=Linus |date=July 29, 1946 |title=Resonance |url=http://scarc.library.oregonstate.edu/coll/pauling/bond/notes/1946a.3.html |access-date=February 29, 2008 |publisher=Oregon State University Libraries Special Collections}}</ref> In a sense, this phenomenon resembles those of hybridization and also polar bonding, both described above, because all three phenomena involve combining more than one electronic structure to achieve an intermediate result.{{citation needed|date=August 2023}} |
||
===Ionic crystal structures=== |
|||
In 1929, Pauling published [[Pauling's rules|five rules]] which help to [[crystal structure prediction|predict]] and explain [[crystal structure]]s of [[ionic compound]]s.<ref>{{Cite journal |last=Pauling, Linus |author-link=Pauling L |year=1929 |title=The principles determining the structure of complex ionic crystals |journal=J. Am. Chem. Soc. |volume=51 |issue=4 |pages=1010–1026 |doi=10.1021/ja01379a006|bibcode=1929JAChS..51.1010P }}</ref><ref name=Pauling1960/> |
|||
These rules concern (1) the ratio of cation radius to anion radius, (2) the electrostatic bond strength, (3) the sharing of polyhedron corners, edges and faces, (4) crystals containing different cations, and (5) the rule of parsimony.{{citation needed|date=August 2023}} |
|||
===Biological molecules=== |
===Biological molecules=== |
||
[[File:Linus Pauling 1941.png|thumb|Pauling in 1941]] |
|||
[[Image:Helix electron density myoglobin 2nrl 17-32.jpg|thumb|upright|An alpha helix in ultra-high-resolution electron density contours, with O atoms in red, N atoms in blue, and hydrogen bonds as green dotted lines (PDB file 2NRL, 17-32).]]In the mid-1930s, Pauling, strongly influenced by the biologically oriented funding priorities of the Rockefeller Foundation's [[Warren Weaver]], decided to strike out into new areas of interest. Although Pauling's early interest had focused almost exclusively on inorganic molecular structures, he had occasionally thought about molecules of biological importance, in part because of Caltech's growing strength in biology. Pauling interacted with such great biologists as [[Thomas Hunt Morgan]], [[Theodosius Dobzhanski]], [[Calvin Bridges]] and [[Alfred Sturtevant]]. His early work in this area included studies of the structure of [[hemoglobin]]. He demonstrated that the hemoglobin molecule changes structure when it gains or loses an [[oxygen]] atom. As a result of this observation, he decided to conduct a more thorough study of [[protein]] structure in general. He returned to his earlier use of X-ray diffraction analysis. But protein structures were far less amenable to this technique than the crystalline minerals of his former work. The best X-ray pictures of proteins in the 1930s had been made by the British crystallographer [[William Astbury]], but when Pauling tried, in 1937, to account for Astbury's observations quantum mechanically, he could not. |
|||
[[Image:Helix electron density myoglobin 2nrl 17-32.jpg|thumb|upright|An alpha helix in ultra-high-resolution electron density contours, with O atoms in red, N atoms in blue, and hydrogen bonds as green dotted lines (PDB file 2NRL, 17–32)]] |
|||
In the mid-1930s, Pauling, strongly influenced by the biologically oriented funding priorities of the Rockefeller Foundation's [[Warren Weaver]], decided to strike out into new areas of interest.<ref name="Kay">{{Cite book |last=Kay |first=Lily E. |url=https://books.google.com/books?id=vEHeNI2a8OEC&pg=PA148 |title=The molecular vision of life: Caltech, the Rockefeller Foundation, and the rise of the new biology |date=1996 |publisher=Oxford University Press |isbn=978-0-19-511143-9 |location=New York [u.a.] |pages=148–151 |access-date=May 27, 2015}}</ref> Although Pauling's early interest had focused almost exclusively on inorganic molecular structures, he had occasionally thought about molecules of biological importance, in part because of Caltech's growing strength in biology. Pauling interacted with such great biologists as [[Thomas Hunt Morgan]], [[Theodosius Dobzhanski]], [[Calvin Bridges]] and [[Alfred Sturtevant]].<ref name="Califano">{{Cite book |last=Califano |first=Salvatore |url=https://books.google.com/books?id=s-sCt4RT0bMC&pg=PA198 |title=Pathways to modern chemical physics |date=2012 |publisher=Springer |isbn=978-3-642-28179-2 |location=Heidelberg [Germany] |page=198 |access-date=May 27, 2015}}</ref> His early work in this area included studies of the structure of [[hemoglobin]] with his student [[Charles D. Coryell]]. He demonstrated that the hemoglobin molecule changes structure when it gains or loses an [[oxygen]] molecule.<ref name=Califano/> As a result of this observation, he decided to conduct a more thorough study of [[protein structure]] in general. He returned to his earlier use of X-ray diffraction analysis. But protein structures were far less amenable to this technique than the crystalline minerals of his former work. The best X-ray pictures of proteins in the 1930s had been made by the British crystallographer [[William Astbury]], but when Pauling tried, in 1937, to account for Astbury's observations quantum mechanically, he could not.<ref name="Livio">{{Cite book |last=Livio |first=Mario |url=https://books.google.com/books?id=0XmmAwAAQBAJ&pg=PA285 |title=Brilliant blunders: from Darwin to Einstein: colossal mistakes by great scientists that changed our understanding of life and the universe |date=2014 |publisher=Simon & Schuster |isbn=978-1-4391-9237-5 |location=[S.l.]}}</ref> |
|||
It took eleven years for Pauling to explain the problem: his [[mathematics|mathematical]] analysis was correct, but Astbury's pictures were taken in such a way that the protein molecules were tilted from their expected positions. Pauling had formulated a model for the structure of hemoglobin in which atoms were arranged in a [[helix|helical]] pattern, and applied this idea to proteins in general. |
|||
It took eleven years for Pauling to explain the problem: his [[mathematics|mathematical]] analysis was correct, but Astbury's pictures were taken in such a way that the protein molecules were tilted from their expected positions. Pauling had formulated a model for the structure of hemoglobin in which atoms were arranged in a [[helix|helical]] pattern, and applied this idea to proteins in general.{{citation needed|date=August 2023}} |
|||
In 1951, based on the structures of [[amino acid]]s and [[peptide]]s and the planar nature of the peptide bond, Pauling, [[Robert Corey]] and [[Herman Branson]] correctly proposed the [[alpha helix]] and [[beta sheet]] as the primary structural motifs in protein [[secondary structure]].<ref>{{cite journal | pmc=1063460 | title=Configurations of Polypeptide Chains With Favored Orientations Around Single Bonds: Two New Pleated Sheets | year=1951 | volume=37 | issue=11 | pmid=16578412 | last1=Pauling | first1=L | last2=Corey | first2=RB | pages=729–40 | journal=Proceedings of the National Academy of Sciences of the United States of America|bibcode = 1951PNAS...37..729P |doi = 10.1073/pnas.37.11.729 }}</ref> This work exemplified Pauling's ability to think unconventionally; central to the structure was the unorthodox assumption that one turn of the helix may well contain a non-[[integer]] number of amino acid residues; for the alpha helix it is 3.7 amino acid residues per turn. |
|||
In 1951, based on the structures of [[amino acid]]s and [[peptide]]s and the planar nature of the [[peptide bond]], Pauling, [[Robert Corey]] and [[Herman Branson]] correctly proposed the [[alpha helix]] and [[beta sheet]] as the primary [[structural motif]]s in protein [[secondary structure]].<ref>{{Cite journal |last1=Pauling |first1=L |last2=Corey |first2=RB |year=1951 |title=Configurations of Polypeptide Chains With Favored Orientations Around Single Bonds: Two New Pleated Sheets |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=37 |issue=11 |pages=729–40 |bibcode=1951PNAS...37..729P |doi=10.1073/pnas.37.11.729 |pmc=1063460 |pmid=16578412 |doi-access=free}}</ref><ref name="Goertzel and Goertzel, p. 95-100">Goertzel and Goertzel, p. 95-100.</ref> This work exemplified Pauling's ability to think unconventionally; central to the structure was the unorthodox assumption that one turn of the helix may well contain a non-[[integer]] number of amino acid residues; for the alpha helix it is 3.7 amino acid residues per turn.{{citation needed|date=August 2023}} |
|||
Pauling then proposed that [[deoxyribonucleic acid]] (DNA) was a [[triple helix]];<ref>{{cite web | url=http://www.farooqhussain.org/projects/paulingdnamodel/document_view | title=Linus Pauling's DNA Model | accessdate=August 6, 2007 }}</ref><ref>{{cite journal | journal=Proc Natl Acad Sci U S A. |date=February 1953|volume=39 |issue=2 |pages=84–97 | title= A Proposed Structure For The Nucleic Acids| author=Pauling L, Corey RB| PMID=16578429 | doi=10.1073/pnas.39.2.84 | pmc=1063734 |bibcode = 1953PNAS...39...84P }}</ref> his model contained several basic mistakes, including a proposal of neutral phosphate groups, an idea that conflicted with the acidity of DNA. [[William Lawrence Bragg|Sir Lawrence Bragg]] had been disappointed that Pauling had won the race to find the alpha helix structure of proteins. Bragg's team had made a fundamental error in making their models of protein by not recognizing the planar nature of the peptide bond. When it was learned at the [[Cavendish Laboratory]] that Pauling was working on molecular models of the structure of DNA, [[James Watson]] and [[Francis Crick]] were allowed to make a molecular model of DNA. They later benefited from unpublished data from [[Maurice Wilkins]] and [[Rosalind Franklin]] at [[King's College London|King's College]] which showed evidence for a helix and planar base stacking along the helix axis. Early in 1953 Watson and Crick proposed a correct structure for the DNA double helix. Pauling later cited several reasons to explain how he had been misled about the structure of DNA, among them misleading density data and the lack of high quality X-ray diffraction photographs. During the time Pauling was researching the problem, Rosalind Franklin in England was creating the world's best images. They were key to Watson's and Crick's success. Pauling did not see them before devising his mistaken DNA structure, although his assistant Robert Corey did see at least some of them, while taking Pauling's place at a summer 1952 protein conference in England. Pauling had been prevented from attending because his passport was withheld by the State Department on suspicion that he had Communist sympathies. This led to the legend that Pauling missed the structure of DNA because of the politics of the day (this was at the start of the [[McCarthyism|McCarthy]] period in the United States).<ref>{{cite web | url=http://www.woodrow.org/teachers/ci/1992/Pauling.html | title=Pauling biography citing State Department's revocation of Pauling's passport in 1952 | accessdate=December 11, 2007 }}</ref> Politics did not play a critical role. Not only did Corey see the images at the time, but Pauling himself regained his passport within a few weeks and toured English laboratories well before writing his DNA paper. He had ample opportunity to visit Franklin's lab and see her work, but chose not to.<ref>{{cite book | last=Hager | first=Thomas | year=1995 | title=Force of Nature: The Life of Linus Pauling | publisher=Simon & Schuster | isbn=0-684-80909-5}}, pp. 414–415</ref> |
|||
Pauling then proposed that [[deoxyribonucleic acid]] (DNA) was a [[triple helix]];<ref>{{Cite journal |last1=Pauling |first1=L |last2=Corey |first2=RB |date=February 1953 |title=A Proposed Structure For The Nucleic Acids |journal=Proc Natl Acad Sci U S A |volume=39 |issue=2 |pages=84–97 |bibcode=1953PNAS...39...84P |doi=10.1073/pnas.39.2.84 |pmc=1063734 |pmid=16578429 |doi-access=free}}</ref><ref>{{Cite web |title=Linus Pauling's DNA Model |url=http://www.farooqhussain.org/projects/paulingdnamodel |url-status=dead |archive-url=https://web.archive.org/web/20120204210701/http://www.farooqhussain.org/projects/paulingdnamodel |archive-date=February 4, 2012 |access-date=June 2, 2015}}</ref> his model contained several basic mistakes, including a proposal of neutral phosphate groups, an idea that conflicted with the acidity of DNA. [[William Lawrence Bragg|Sir Lawrence Bragg]] had been disappointed that Pauling had won the race to find the alpha [[Alpha helix|helix structure]] of proteins. Bragg's team had made a fundamental error in making their models of protein by not recognizing the planar nature of the peptide bond. When it was learned at the [[Cavendish Laboratory]] that Pauling was working on molecular models of the structure of DNA, [[James Watson]] and [[Francis Crick]] were allowed to make a molecular model of DNA. They later benefited from unpublished data from [[Maurice Wilkins]] and [[Rosalind Franklin]] at [[King's College London|King's College]] which showed evidence for a helix and planar base stacking along the helix axis. Early in 1953 Watson and Crick proposed a correct structure for the DNA double helix. Pauling later cited several reasons to explain how he had been misled about the structure of DNA, among them misleading density data and the lack of high quality X-ray diffraction photographs. Pauling described this situation as "the biggest disappointment in his life".<ref name="Dye-LATimes">{{Cite web |last=Dye |first=Lee |date=June 2, 1985 |title=The Deeply Personal War of Linus Pauling: Nobel Prize-Winning Chemist Still Battles for His Controversial Vitamin Theory |url=https://www.latimes.com/archives/la-xpm-1985-06-02-vw-15174-story.html |access-date=April 9, 2023 |website=[[Los Angeles Times]] |language=en-US}}</ref> |
|||
Pauling also studied [[enzyme]] reactions and was among the first to point out that enzymes bring about reactions by stabilizing the [[transition state]] of the reaction, a view which is central to understanding their mechanism of action. He was also among the first scientists to postulate that the binding of [[antibodies]] to antigens would be due to a complementarity between their structures. Along the same lines, with the physicist turned biologist [[Max Delbrück]], he wrote an early paper arguing that [[DNA replication]] was likely to be due to [[Complementarity (molecular biology)|complementarity]], rather than similarity, as suggested by a few researchers. This was made clear in the model of the structure of DNA that Watson and Crick discovered. |
|||
During the time Pauling was researching the problem, Rosalind Franklin in England was creating the world's best images. They were key to Watson's and Crick's success. Pauling did not see them before devising his mistaken DNA structure, although his assistant Robert Corey did see at least some of them, while taking Pauling's place at a summer 1952 protein conference in England. Pauling had been prevented from attending because his passport was withheld by the State Department on suspicion that he had Communist sympathies. This led to the legend that Pauling missed the structure of DNA because of the politics of the day (this was at the start of the [[McCarthyism|McCarthy]] period in the United States). Politics did not play a critical role. Not only did Corey see the images at the time, but Pauling himself regained his passport within a few weeks and toured English laboratories well before writing his DNA paper. He had ample opportunity to visit Franklin's lab and see her work, but chose not to.<ref name="Nature" />{{rp|414–415}} Despite these times, Pauling chose to move on from them and be thankful for the discoveries that he had already found.<ref name="Dye-LATimes"/> |
|||
Pauling also studied [[enzyme]] reactions and was among the first to point out that enzymes bring about reactions by stabilizing the [[transition state]] of the reaction, a view which is central to understanding their mechanism of action.<ref>{{Cite book |last=Metzler |first=David E. |url=https://books.google.com/books?id=X194AYXInC8C&pg=PA330 |title=Biochemistry |date=2003 |publisher=Harcourt, Academic Pr. |isbn=978-0-12-492541-0 |edition=2nd |location=San Diego |ref=Metzler}}</ref> He was also among the first scientists to postulate that the binding of [[antibodies]] to antigens would be due to a complementarity between their structures.<ref name="Lewis">{{Cite book |first1=Julius M. |last1=Cruse |first2=Robert E. |last2=Lewis |url=https://books.google.com/books?id=kNI5Lk2z37sC&pg=PA21 |title=Atlas of immunology |date=2010 |publisher=CRC Press/Taylor & Francis |isbn=978-1-4398-0268-7 |edition=3rd |location=Boca Raton, FL |page=21 |access-date=May 27, 2015}}</ref> Along the same lines, with the physicist turned biologist [[Max Delbrück]], he wrote an early paper arguing that [[DNA replication]] was likely to be due to [[Complementarity (molecular biology)|complementarity]], rather than similarity, as suggested by a few researchers. This was made clear in the model of the structure of DNA that Watson and Crick discovered.<ref name="Tudge">{{Cite book |last=Tudge |author-link=Colin Tudge |first=Colin |url=https://books.google.com/books?id=Wprqex2OGY4C&pg=PT74 |title=The engineer in the garden: Genes and genetics: from the idea of heredity to the creation of life |date=1995 |publisher=Hill and Wang |isbn=978-0-8090-4259-3 |edition=1st American |location=New York |access-date=May 27, 2015}}</ref> |
|||
===Molecular genetics=== |
===Molecular genetics=== |
||
[[File:Linus Pauling 1948.png|thumb|Pauling in 1948]] |
|||
In November 1949, Linus Pauling, [[Harvey Itano]], [[S. J. Singer]] and Ibert Wells published "[[Sickle Cell Anemia, a Molecular Disease]]"<ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/blood/papers/1949p.15.html | title=Sickle Cell Anemia, a Molecular Disease | accessdate=August 5, 2007 | last=Pauling | first=Linus | coauthors=Harvey Itano, S. J. Singer, Ibert Wells |date=November 1949 | publisher=Science}}</ref> in the journal ''Science''. It was the first proof of a human disease caused by an abnormal protein, and sickle cell anemia became the first disease understood at the molecular level. Using [[electrophoresis]], they demonstrated that individuals with [[sickle cell disease]] had a modified form of hemoglobin in their [[red blood cell]]s, and that individuals with [[sickle cell trait]] had both the normal and abnormal forms of hemoglobin. This was also the first demonstration that [[Mendelian inheritance]] determined the specific physical properties of proteins, not simply their presence or absence – the dawn of [[molecular genetics]]. |
|||
In November 1949, Pauling, [[Harvey Itano]], [[Seymour Jonathan Singer|S. J. Singer]] and Ibert Wells published "[[Sickle Cell Anemia, a Molecular Disease]]"<ref name="Itano">{{Cite journal |last1=Pauling |first1=L. |last2=Itano |first2=H. A. |last3=Singer |first3=S. J. |author3-link=Seymour Jonathan Singer |last4=Wells |first4=I. C. |date=November 25, 1949 |title=Sickle Cell Anemia, a Molecular Disease |url=http://scarc.library.oregonstate.edu/coll/pauling/blood/papers/1949p.15.htmlfddsf |journal=Science |volume=110 |issue=2865 |pages=543–548 |bibcode=1949Sci...110..543P |doi=10.1126/science.110.2865.543 |pmid=15395398 |s2cid=31674765 |access-date=June 2, 2015}}</ref> in the journal ''Science''. It was the first proof of a human disease being caused by an abnormal protein, and [[sickle cell anemia]] became the first disease understood at the molecular level. (It was not, however, the first demonstration that variant forms of hemoglobin could be distinguished by electrophoresis, which had been shown several years earlier by [[Maud Menten]] and collaborators).<ref>{{Cite journal |last1=Andersch |first1=MA |last2=Wilson |first2=DA |last3=Menten |first3=ML. |year=1944 |title=Sedimentation constants and electrophoretic mobilities of adult and fetal carbonylhemoglobin |journal=Journal of Biological Chemistry |volume=153 |pages=301–305 |doi=10.1016/S0021-9258(18)51237-0 |doi-access=free}}</ref> Using [[electrophoresis]], they demonstrated that individuals with [[sickle cell disease]] have a modified form of hemoglobin in their [[red blood cell]]s, and that individuals with [[sickle cell trait]] have both the normal and abnormal forms of hemoglobin. This was the first demonstration causally linking an abnormal protein to a disease, and also the first demonstration that [[Mendelian inheritance]] determines the specific physical properties of proteins, not simply their presence or absence – the dawn of [[molecular genetics]].<ref name="Strasser">{{Cite journal |last=Strasser |first=Bruno J. |date=August 30, 2002 |title=Linus Pauling's "molecular diseases": Between history and memory |url=http://biologie.unige.ch/assets/brunostrasser/Strasser_AJMG_2002.pdf |archive-url=https://ghostarchive.org/archive/20221009/http://biologie.unige.ch/assets/brunostrasser/Strasser_AJMG_2002.pdf |archive-date=October 9, 2022 |url-status=live |journal=American Journal of Medical Genetics |volume=115 |issue=2 |pages=83–93 |citeseerx=10.1.1.613.5672 |doi=10.1002/ajmg.10542 |pmid=12400054 |access-date=May 27, 2015}}</ref> |
|||
===Activism=== |
|||
Pauling had been practically apolitical until [[World War II]], but the aftermath of the war and his wife's pacifism changed his life profoundly, and he became a peace activist. During the beginning of the [[Manhattan Project]], Robert Oppenheimer invited him to be in charge of the Chemistry division of the project, but he declined, not wanting to uproot his family. He did work on other projects that had military applications, such as explosives, rocket propellants, an oxygen meter for submarines and the patent of an armor-piercing shell; he was awarded a Presidential Medal of Merit.<ref name=NLM>{{cite web | title=The Linus Pauling Papers: Biographical Information | url=http://profiles.nlm.nih.gov/MM/Views/Exhibit/narrative/biographical.html | publisher=United States National Library of Medicine | accessdate=February 11, 2008 }}</ref><ref name=Paulus>{{cite news | title=Pauling's Prizes | last=Paulus | first=John Allen | date =November 5, 1995 | url=http://query.nytimes.com/gst/fullpage.html?res=9D05E3DE1739F936A35752C1A963958260& |work=New York Times | accessdate=December 9, 2007 }}</ref> In 1946, he joined the [[Emergency Committee of Atomic Scientists]], chaired by [[Albert Einstein]].<ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/peace/narrative/page9.html | title=Einstein | accessdate=December 13, 2007 | author=Thomas Hager | date=November 29, 2007 | publisher=Oregon State University Libraries Special Collections }}</ref> Its mission was to warn the public of the dangers associated with the development of nuclear weapons. His political activism prompted the [[United States Department of State|U.S. State Department]] to deny him a passport in 1952, when he was invited to speak at a scientific conference in London.<ref>{{cite web|url=http://www.woodrow.org/teachers/ci/1992/Pauling.html| title=Linus Pauling |quote=[In] January of 1952, Pauling requested a passport to attend a meeting in England ... The passport was denied because granting it "would not be in the best interest of the United States." He applied again and wrote President Eisenhower, asking him to arrange the issuance of the passport since, "I am a loyal citizen of the United States. I have never been guilty of any unpatriotic or criminal act." |accessdate=December 11, 2007 }}</ref><ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/peace/notes/1952a.18.html | title=The Department of State and the Structure of Proteins | accessdate=December 13, 2007 | author=Linus Pauling |date=May 1952 | publisher=Oregon State University Libraries Special Collections}}</ref> In a speech before the [[US senate]] on June 6 of the same year, Senator [[Wayne Morse]] publicly denounced the action of the State Department, and urged the Passport Division to reverse its decision. Pauling and his wife Ava were issued a “limited passport” to attend the aforementioned conference in England.<ref>Robert Paradowski (2011), Oregon State University, Special Collections p.18, [http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/chronology/page18.html Proteins, Passports, and the Prize (1950-1954)], retrieved February 1, 2013</ref><ref>[http://books.google.ch/books?id=sg0AAAAAMBAJ&pg=PA255&lpg=PA255&dq=Pauling+Morse+Senate&source=bl&ots=wq8u_oImE2&sig=jaAUo374rJAymCAi9XUiw-yLho4&hl=de&sa=X&ei=UdILUc2CI8nh4QT9k4CQBA&sqi=2&ved=0CDwQ6AEwBA#v=onepage&q=Morse%20&f=false Bulletin of the Atomic Scientists Vol. VIII, Nr. 7] (Okt. 1952) p. 254, Educational Foundation for Nuclear Science, Inc.</ref> His passport was restored in 1954, shortly before the ceremony in [[Stockholm]] where he received his first Nobel Prize. Joining Einstein, [[Bertrand Russell]] and eight other leading scientists and intellectuals, he signed the [[Russell-Einstein Manifesto]] in 1955.<ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/peace/narrative/page25.html | title=Russell/Einstein | accessdate=December 13, 2007 | author=Thomas Hager | date=November 29, 2007 | publisher=Oregon State University Libraries Special Collections }}</ref> |
|||
His success with sickle cell anemia led Pauling to speculate that a number of other diseases, including mental illnesses such as [[schizophrenia]], might result from flawed genetics. As chairman of the Division of Chemistry and Chemical Engineering and director of the Gates and Crellin Chemical Laboratories, he encouraged the hiring of researchers with a chemical-biomedical approach to mental illness, a direction not always popular with established [[California Institute of Technology|Caltech]] chemists.<ref name="LATimes1994">{{Cite news |date=August 21, 1994 |title=A Flamboyant Scientist's Legacy : Scholar: Linus C. Pauling's supporters and detractors join in calling the two-time Nobel winner one of the most significant figures of this century |work=Los Angeles Times |url=https://www.latimes.com/archives/la-xpm-1994-08-21-mn-29601-story.html |access-date=June 1, 2015}}</ref>{{rp|2}} |
|||
In 1958, Pauling joined a petition drive in cooperation with the founders of the St. Louis Citizen's Committee for Nuclear Information (CNI). This group, headed by [[Washington University in St. Louis]] professors [[Barry Commoner]], Eric Reiss, M. W. Friedlander and John Fowler, set up a study of radioactive [[strontium]]-90 in the [[baby teeth]] of children across North America. The "[[Baby Tooth Survey]]," headed by Dr [[Louise Reiss]], demonstrated conclusively in 1961 that above-ground nuclear testing posed significant public health risks in the form of [[nuclear fallout|radioactive fallout]] spread primarily via milk from cows that had ingested contaminated grass.<ref>{{cite web | url=http://www.sciencemag.org/cgi/reprint/134/3491/1669.pdf | title=Strontium-90 Absorption by Deciduous Teeth: Analysis of teeth provides a practicable method of monitoring strontium-90 uptake by human populations | accessdate=October 13, 2009 | author=Louise Zibold Reiss | date=November 24, 1961 | publisher=Science }}</ref><ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/peace/narrative/page26.html | title=Strontium-90 | accessdate=December 13, 2007 | author=Thomas Hager | date=November 29, 2007 | publisher=Oregon State University Libraries Special Collections }}</ref><ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/peace/narrative/page27.html | title=The Right to Petition | accessdate=December 13, 2007 | author=Thomas Hager | date=November 29, 2007 | publisher=Oregon State University Libraries Special Collections }}</ref> Pauling also participated in a public debate with the atomic physicist [[Edward Teller]] about the actual probability of fallout causing mutations.<ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/peace/video/1958v.3.html | title=Teller vs. Pauling | accessdate=December 13, 2007 | author=Linus Pauling | coauthors=Edward Teller | year=1958 | publisher=Oregon State University Libraries Special Collections }}</ref> In 1958, Pauling and his wife presented the United Nations with the petition signed by more than 11,000 scientists calling for an end to [[nuclear testing|the testing of nuclear weapons]]. Public pressure and the frightening results of the CNI research subsequently led to a moratorium on above-ground nuclear weapons testing, followed by the [[Partial Test Ban Treaty]], signed in 1963 by [[John F. Kennedy]] and [[Nikita Khrushchev]]. On the day that the treaty went into force, the Nobel Prize Committee awarded Pauling the [[Nobel Peace Prize]], describing him as "Linus Carl Pauling, who ever since 1946 has campaigned ceaselessly, not only against nuclear weapons tests, not only against the spread of these armaments, not only against their very use, but against all warfare as a means of solving international conflicts."<ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/peace/notes/rnb23-100.html |title=Notes by Linus Pauling. October 10, 1963. | accessdate=December 13, 2007 | author=Linus Pauling | date=October 10, 1963 | publisher=Oregon State University Libraries Special Collections }}</ref> The Committee for Nuclear Information was never credited for its significant contribution to the test ban, nor was the ground-breaking research conducted by Dr Reiss and the "Baby Tooth Survey". The Caltech Chemistry Department, wary of his political views, did not even formally congratulate him. They did throw him a small party, showing they were more appreciative and sympathetic toward his work on radiation mutation. At Caltech he founded [[Sigma Xi]]'s (The Scientific Research Society) chapter at the school, as he had previously been a member of that organization. He continued his peace activism in the following years co-founding the [[International League of Humanists]] in 1974. He was president of the scientific advisory board of the [[World Union for Protection of Life]] and also one of the signatories of the Dubrovnik-Philadelphia Statement. |
|||
In 1951, Pauling gave a lecture entitled "Molecular Medicine".<ref>{{Cite web |last=Pauling |first=Linus |date=October 1951 |title=Molecular Medicine |url=http://scarc.library.oregonstate.edu/coll/pauling/blood/pictures/1951s.17.html |access-date=August 5, 2007 |publisher=Ava Helen and Linus Pauling Papers}}</ref> In the late 1950s, he studied the role of enzymes in brain function, believing that mental illness may be partly caused by enzyme dysfunction. In the 1960s, as part of his interest in the effects of nuclear weapons, he investigated the role of mutations in evolution, proposing with his student Emile Zuckerkandl, the molecular evolutionary clock, the idea that mutations in proteins and DNA accumulate at a constant rate over time .<ref name="Morgan">{{Cite journal |last=Morgan |first=Gregory J. |date=1998 |title=Emile Zuckerkandl, Linus Pauling, and the molecular evolutionary clock, 1959–1965 |url=https://www.jstor.org/stable/4331476|journal=Journal of the History of Biology |volume=31 |issue=2 |pages=155–78| doi=10.1023/A:1004394418084|jstor=4331476 |pmid=11620303 |s2cid=5660841 |access-date=June 11, 2023}}</ref> |
|||
During the 1960s, President Lyndon Johnson’s policy of increasing America’s involvement in the Vietnam War caused an antiwar movement that the Paulings joined with enthusiasm. Pauling denounced the war as unnecessary and unconstitutional. He made speeches, signed protest letters and communicated personally with the North Vietnamese leader, Ho Chi Minh, and gave the lengthy written response to President Johnson. His efforts were ignored by the government.<ref>{{cite web | url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/peace/narrative/page49.html | title=Linus Pauling and the International Peace Movement: Vietnam. | accessdate=March 12, 2013 | year=2010 | publisher=Oregon State University Libraries }}</ref> By the time Pauling turned 65 in 1966, he was without a research group or a big scientific issue to focus on. A new generation of more radical, younger activists would march, petition, and lead the movement against the Vietnam War. |
|||
===Structure of the atomic nucleus=== |
|||
Many of Pauling's critics, including scientists who appreciated the contributions that he had made in chemistry, disagreed with his political positions and saw him as a naive spokesman for [[Communist Party of the Soviet Union|Soviet communism]]. He was ordered to appear before the [[United States Senate Subcommittee on Internal Security|Senate Internal Security Subcommittee]], which termed him "the number one scientific name in virtually every major activity of the Communist peace offensive in this country." A headline in ''[[Life magazine|Life]]'' magazine characterized his 1962 Nobel Prize as "A Weird Insult from [[Norway]]". Pauling was awarded the [[Lenin Peace Prize|International Lenin Peace Prize]] by the USSR in 1970.<ref>{{cite web | url=http://cabierta.uchile.cl/revista/6/linus.htm | title=The Science and Humanism of Linus Pauling (1901–1994). | accessdate=August 4, 2009 | author=Stephen F Mason | date=July 1999 | publisher=Ciencia Abierta }}</ref> |
|||
[[File:Linus Pauling 1962.jpg|thumb|Pauling in 1962]] |
|||
On September 16, 1952, Pauling opened a new research notebook with the words "I have decided to attack the problem of the structure of nuclei." On October 15, 1965, Pauling published his Close-Packed Spheron Model of the atomic nucleus in two well respected journals, ''Science'' and the ''[[Proceedings of the National Academy of Sciences]]''.<ref name="SpheronPNAS">{{Cite journal |last=Pauling |first=Linus |date=1965 |title=The Close-Packed Spheron Model of atomic nuclei and its relation to the shell model |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=54 |issue=4 |pages=989–994 |bibcode=1965PNAS...54..989P |doi=10.1073/pnas.54.4.989 |pmc=219778 |pmid=16578621 |doi-access=free}}</ref><ref name="SpheronScience">{{Cite journal |last=Pauling |first=L |date=October 15, 1965 |title=The close-packed-spheron theory and nuclear fission |url=http://scarc.library.oregonstate.edu/coll/pauling/rnb/26/26-026.html |journal=Science |volume=150 |issue=3694 |pages=297–305 |bibcode=1965Sci...150..297P |doi=10.1126/science.150.3694.297 |pmid=17742357}}</ref> For nearly three decades, until his death in 1994, Pauling published numerous papers on his spheron cluster model.<ref name=SpheronPNAS/><ref>{{Cite web |last=Pauling |first=Linus |date=July 1966 |title=The close-packed-spheron theory of nuclear structure and the neutron excess for stable nuclei (Dedicated to the seventieth anniversary of Professor Horia Hulubei) |url=http://scarc.library.oregonstate.edu/coll/pauling/rnb/26/26-048.html |access-date=August 5, 2007 |publisher=Revue Roumain de Physique}}</ref><ref>{{Cite journal |last=Pauling |first=Linus |date=December 1967 |title=Magnetic-moment evidence for the polyspheron structure of the lighter atomic nuclei |url=http://scarc.library.oregonstate.edu/coll/pauling/rnb/26/26-068.html |journal=Proceedings of the National Academy of Sciences |volume=58 |issue=6 |pages=2175–2178 |bibcode=1967PNAS...58.2175P |doi=10.1073/pnas.58.6.2175 |pmc=223816 |pmid=16591577 |access-date=August 5, 2007 |doi-access=free}}</ref><ref>{{Cite journal |last=Pauling |first=Linus |date=November 1969 |title=Orbiting clusters in atomic nuclei |url=http://scarc.library.oregonstate.edu/coll/pauling/rnb/26/26-075.html |journal=Proceedings of the National Academy of Sciences of the United States of America |publisher=Proceedings of the National Academy of Sciences |volume=64 |issue=3 |pages=807–9 |bibcode=1969PNAS...64..807P |doi=10.1073/pnas.64.3.807 |pmc=223305 |pmid=16591799 |access-date=August 5, 2007 |doi-access=free}}</ref><ref>{{Cite web |last1=Pauling |first1=Linus |last2=Arthur B. Robinson |year=1975 |title=Rotating clusters in nuclei |url=http://scarc.library.oregonstate.edu/coll/pauling/rnb/26/26-084.html |access-date=August 5, 2007 |publisher=Canadian Journal of Physics}}</ref><ref>{{Cite journal |last=Pauling |first=Linus |date=February 1991 |title=Transition from one revolving cluster to two revolving clusters in the ground-state rotational bands of nuclei in the lanthanon region |url=http://scarc.library.oregonstate.edu/coll/pauling/rnb/26/26-125.html |journal=[[Proceedings of the National Academy of Sciences|Proc. Natl. Acad. Sci.]] |volume=88 |issue=3 |pages=820–823 |bibcode=1991PNAS...88..820P |doi=10.1073/pnas.88.3.820 |pmc=50905 |pmid=11607150 |access-date=August 5, 2007 |doi-access=free}}</ref> |
|||
==Contretemps with William F. Buckley, National Review.== |
|||
The basic idea behind Pauling's spheron model is that a nucleus can be viewed as a set of "clusters of nucleons". The basic nucleon clusters include the [[deuteron]] [np], [[helion (chemistry)|helion]] [pnp], and [[tritium|triton]] [npn]. [[Even-even nucleus|Even–even nuclei]] are described as being composed of clusters of [[alpha particle]]s, as has often been done for light nuclei.<ref name="Clusters">{{Cite journal |last=Pauling |first=Linus |date=November 15, 1969 |title=Orbiting clusters in atomic nuclei |journal=Proceedings of the National Academy of Sciences |volume=64 |issue=3 |pages=807–809 |bibcode=1969PNAS...64..807P |doi=10.1073/pnas.64.3.807 |pmc=223305 |pmid=16591799 |doi-access=free}}</ref> Pauling attempted to derive the shell structure of nuclei from pure geometrical considerations related to [[Platonic solids]] rather than starting from an independent particle model as in the usual [[Nuclear shell model|shell model]]. In an interview given in 1990 Pauling commented on his model:<ref name="AOA">{{Cite web |title=Linus C. Pauling, Ph.D. Biography and Interview |url=https://www.achievement.org/achiever/linus-pauling/#interview/ |website=www.achievement.org |publisher=[[American Academy of Achievement]]}}</ref> |
|||
After Pauling had won the Nobel Peace Prize and the Lenin Peace Prize, he became a frequent target of [[The National Review]] magazine; particularly, in an article entitled "The Collaborators" in the magazine's July 17, 1962 issue. Pauling was not only referred to as a collaborator, but a "fellow traveler" with proponents of Soviet style communism. These National Review articles set off a three year legal battle in the form of federal libel case. In 1965, Pauling sued the magazine, its publisher [[William Rusher]], and its editor [[William F. Buckley, Jr]] for $1 million. Subsequently, he lost both his suit and the 1968 appeal. The loss of both suits and continued attacks by the National Review did nothing to enhance Pauling's reputation. |
|||
<ref>{{cite web | url=http://paulingblog.wordpress.com/2013/01/30/the-national-review-lawsuit/ | title=The National Review Lawsuit | publisher=Paulingblog | accessdate=20 December 2013}}</ref> |
|||
<ref>{{cite web | url=http://paulingblog.wordpress.com/tag/william-f-buckley/ | title=A Tough Conclusion to the National Review Lawsuit | publisher=Paulingblog | accessdate=20 December 2013}}</ref> |
|||
<ref>{{cite web | url=http://law.justia.com/cases/new-york/court-of-appeals/1968/22-n-y-2d-818-0.html | title=Pauling v. NAT'L REVIEW, INC. | work=Justia.com | accessdate=20 December 2013}}</ref> |
|||
<ref>{{cite web | url=http://www.nytimes.com/1998/08/30/nyregion/c-dickerman-williams-97-free-speech-lawyer-is-dead.html | title=http://www.nytimes.com/1998/08/30/nyregion/c-dickerman-williams-97-free-speech-lawyer-is-dead.html | publisher=The New York Times | accessdate=20 December 2013}}</ref> |
|||
{{blockquote|Now recently, I have been trying to determine detailed structures of atomic nuclei by analyzing the ground state and excited state vibrational bends, as observed experimentally. From reading the physics literature, Physical Review Letters and other journals, I know that many physicists are interested in atomic nuclei, but none of them, so far as I have been able to discover, has been attacking the problem in the same way that I attack it. So I just move along at my own speed, making calculations ...}} |
|||
==Molecular medicine, medical research, and vitamin C advocacy== |
|||
[[File:Pauling Vit C Book Cover.jpg|right|150px|thumb|Linus Pauling's book, ''How to Live Longer and Feel Better'', advocated the very high intake of [[Vitamin C]].]] |
|||
==Activism== |
|||
In 1941, at age 40, Pauling was diagnosed with [[Bright's disease]], a renal disease. Following the recommendations of [[Thomas Addis]], Pauling was able to control the disease with Addis' then unusual low-protein salt-free diet and vitamin supplements.<ref>{{cite book |author=Peitzman, Steven J. |title=Dropsy, dialysis, transplant: a short history of failing kidneys |publisher=Johns Hopkins University Press |location=Baltimore |year=2007 |pages= [http://books.google.ca/books?id=8FUDO5K1pAoC&pg=PA72#v=onepage&q&f=false 72–8]; 190 |isbn=0-8018-8734-8 |oclc= }}</ref> |
|||
===Wartime work=== |
|||
[[File:Beckman D2 Oxygen Analyzer 2012 002 5132 cn69m4659.tiff|thumb|right|Beckman D2 Oxygen Analyzer, ca. 1950]] |
|||
Pauling had been practically apolitical until [[World War II]]. At the beginning of the [[Manhattan Project]], Robert Oppenheimer invited him to be in charge of the Chemistry division of the project. He declined, not wanting to uproot his family.<ref name="Hiroshima">{{Cite web |title=Hiroshima |url=http://scarc.library.oregonstate.edu/coll/pauling/peace/narrative/page4.html |access-date=May 27, 2015 |website=Linus Pauling and the International Peace Movement |publisher=Special Collections & Archives Research Center, Oregon State University}}</ref> |
|||
[[File:Beckman Model 735 Dissolved O2 Analyzer 2012 002 3598 jm214p459 crop.tiff|thumb|right| |
|||
In 1951, Pauling gave a lecture entitled "Molecular Medicine".<ref>{{cite web |url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/blood/pictures/1951s.17.html |title=Molecular Medicine |accessdate=August 5, 2007|last=Pauling |first=Linus|date=October 1951|publisher=Ava Helen and Linus Pauling Papers}}</ref> In the late 1950s, Pauling worked on the role of enzymes in brain function, believing that mental illness may be partly caused by enzyme dysfunction. In 1965 Pauling read ''[[Niacin]] Therapy in Psychiatry'' by Abram Hoffer and theorized vitamins might have important biochemical effects unrelated to their prevention of associated deficiency diseases.<ref>{{cite book|editor-last=Nicolle|editor-first=Lorraine|title=Biochemical imbalances in disease a practitioner's handbook|year=2010|publisher=Singing Dragon|location=London|isbn=9780857010285|page=27|editor2-first=Ann Woodriff |editor2-last=Beirne}}</ref> In 1968 Pauling published a brief paper in [[Science (journal)|''Science'']] entitled "Orthomolecular psychiatry"<ref>{{cite journal |author=Pauling L |title=Orthomolecular psychiatry. Varying the concentrations of substances normally present in the human body may control mental disease |journal=Science |volume=160 |issue=3825 |pages=265–71 |date=April 1968 |pmid=5641253 |doi= 10.1126/science.160.3825.265|url=|bibcode = 1968Sci...160..265P }}</ref> that gave name and principle to the popular but controversial [[megavitamin therapy]] movement of the 1970s. Pauling coined the term "orthomolecular" to refer to the practice of varying the concentration of substances normally present in the body to prevent and treat disease. His ideas formed the basis of [[orthomolecular medicine]], which is not generally practiced by conventional medical professionals and has been strongly criticized.<ref>{{cite book |last=Cassileth |first=BR |title=Alternative Medicine Handbook: the Complete Reference Guide to Alternative and Complementary Therapies |year=1998:67 |publisher=W.W. Norton & Co. |location=New York |isbn= }}</ref><ref name="bccancer">{{cite web |url=http://www.bccancer.bc.ca/PPI/UnconventionalTherapies/VitaminTherapyMegadoseOrthomolecularTherapy.htm |title=Vitamin Therapy, Megadose / Orthomolecular Therapy |accessdate=August 5, 2007|date=February 2000|publisher=BC Cancer Agency |archiveurl = https://web.archive.org/web/20070202102734/http://www.bccancer.bc.ca/PPI/UnconventionalTherapies/VitaminTherapyMegadoseOrthomolecularTherapy.htm |archivedate = February 2, 2007}}</ref> His promotion of [[dietary supplements]] has also been criticized. In a 2013 article in ''[[The Atlantic]]'', a pediatrician [[Paul Offit]] wrote that although Pauling was "so spectacularly right" that he won two Nobel Prizes, Pauling's late-career assertions about the benefits of dietary supplements were "so spectacularly wrong that he was arguably the world's greatest quack."<ref name=Offit>{{cite web|title=The Vitamin Myth: Why We Think We Need Supplements |author=[[Paul Offit]] |publisher=''[[The Atlantic]]'' |url=http://www.theatlantic.com/health/archive/2013/07/the-vitamin-myth-why-we-think-we-need-supplements/277947/ | date=19 July 2013 |accessdate=19 July 2013}}</ref> |
|||
Beckman Model 735 Dissolved {{O2|nolink=no}} Analyzer, later model based on Pauling's design, 1968]] |
|||
[[File:Beckman Model D Oxygen Meter with infant incubator 73666474j.tiff|thumb|right|Beckman Model D Oxygen Meter, based on Pauling's design, with infant incubator, 1959]] |
|||
Pauling did, however, work on research for the military. He was a principal investigator on 14 [[Office of Scientific Research and Development|OSRD contracts]].<ref name="Kay2">{{Cite book |last=Kay |first=Lily E. |url=https://books.google.com/books?id=QVtMCAAAQBAJ&pg=PT161 |title=The molecular vision of life : Caltech, the Rockefeller Foundation, and the rise of the new biology |date=1996 |publisher=Oxford University Press |isbn=978-0-19-511143-9 |location=New York |page=179 |access-date=December 27, 2015}}</ref> The [[National Defense Research Committee]] called a meeting on October 3, 1940, wanting an instrument that could reliably measure oxygen content in a mixture of gases, so that they could measure oxygen conditions in submarines and airplanes. In response Pauling designed the Pauling oxygen meter, which was developed and manufactured by [[Arnold O. Beckman|Arnold O. Beckman, Inc.]] After the war, Beckman adapted the oxygen analyzers for use in incubators for premature babies.<ref name="hundred">{{Cite book |last1=Thackray |first1=Arnold |author1-link=Arnold Thackray |title=Arnold O. Beckman : one hundred years of excellence |last2=Minor Myers, Jr. |publisher=Chemical Heritage Foundation |others=foreword by James D. Watson |year=2000 |isbn=978-0-941901-23-9 |location=Philadelphia, Pa. |name-list-style=amp}}</ref>{{rp|180–186}}<ref name="D2OxygenAnalyzer">{{Cite web |title=Beckman D2 Oxygen Analyzer |url=http://www.woodlibrarymuseum.org/museum/item/525/beckman-d2-oxygen-analyzer |access-date=May 28, 2015 |website=Wood Library-Museum of Anesthesiology}}</ref> |
|||
Pauling's work on [[vitamin C]] in his later years generated much controversy. He was first introduced to the concept of high-dose vitamin C by biochemist [[Irwin Stone]] in 1966. After becoming convinced of its worth, Pauling took 3 grams of vitamin C every day to prevent [[colds]].<ref name="frs"/> Excited by his own perceived results, he researched the clinical literature and published ''[[Vitamin C and the Common Cold (book)|Vitamin C and the Common Cold]]'' in 1970. He began a long clinical collaboration with the British cancer surgeon [[Ewan Cameron (physician)|Ewan Cameron]] in 1971 on the use of intravenous and oral vitamin C as cancer therapy for terminal patients.<ref>{{cite web |url=http://www.doctoryourself.com/biblio_cameron.html |title=Cancer bibliography |accessdate=August 5, 2007| author= Ewan Cameron M.D. |publisher=Doctoryourself.com}}</ref> Cameron and Pauling wrote many technical papers and a popular book, ''Cancer and Vitamin C'', that discussed their observations. Pauling made vitamin C popular with the public and eventually published two studies of a group of 100 allegedly [[terminal illness|terminal]] patients that claimed vitamin C increased survival by as much as four times compared to untreated patients.<ref>{{cite journal |author=Cameron E, Pauling L |title=Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer |journal=Proceedings of the National Academy of Sciences |volume=73 |issue=10 |pages=3685–9 |date=October 1976 |pmid=1068480 |pmc=431183 |doi= 10.1073/pnas.73.10.3685|url=|bibcode = 1976PNAS...73.3685C }}</ref><ref>{{cite journal |author=Cameron E, Pauling L |title=Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer |journal=Proceedings of the National Academy of Sciences |volume=75 |issue=9 |pages=4538–42 |date=September 1978 |pmid=279931 |pmc=336151 |doi= 10.1073/pnas.75.9.4538|url=|bibcode = 1978PNAS...75.4538C }}</ref> A re-evaluation of the claims in 1982 found that the patient groups were not actually comparable, with the vitamin C group being less sick on entry to the study, and judged to be "terminal" much earlier than the comparison group.<ref>{{cite journal | last = DeWys | first = WD | title = How to evaluate a new treatment for cancer | journal = Your Patient and Cancer | volume = 2 | issue = 5 | pages = 31–36 | year = 1982 }}</ref> Later clinical trials conducted by the [[Mayo Clinic]] also concluded that high-dose (10,000 mg) vitamin C was no better than [[placebo]] at treating cancer and that there was no benefit to high-dose vitamin C.<ref>{{cite journal |author=Creagan ET, Moertel CG, O'Fallon JR, ''et al.'' |title=Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial |journal=The New England Journal of Medicine |volume=301 |issue=13 |pages=687–90 |date=September 1979 |pmid=384241 |doi= 10.1056/NEJM197909273011303|url=}}</ref><ref>{{cite journal |author=Moertel CG, Fleming TR, Creagan ET, Rubin J, O'Connell MJ, Ames MM |title=High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison |journal=The New England Journal of Medicine |volume=312 |issue=3 |pages=137–41 |date=January 1985 |pmid= 3880867|doi= 10.1056/NEJM198501173120301|url=}}</ref><ref>{{cite journal | last = Tschetter | first = L | coauthors = et al. | title = A community-based study of vitamin C (ascorbic acid) in patients with advanced cancer | journal = Proceedings of the American Society of Clinical Oncology | volume = 2 | page = 92 | year = 1983 }}</ref> The failure of the clinical trials to demonstrate any benefit resulted in the conclusion that vitamin C was not effective in treating cancer; the medical establishment concluding that his claims that vitamin C could prevent colds or treat cancer were [[quackery]].<ref name="frs"/><ref name=PNASChen2007>{{cite journal | last = Chen | first = Q | coauthors = et al | year = 2007 | url = http://www.pnas.org/cgi/content/full/104/21/8749 | title = Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo | journal = Proceedings of the National Academy of Sciences| pages = 8749–54 | volume = 104 | issue = 21 | pmid = 17502596 | doi = 10.1073/pnas.0702854104 | pmc = 1885574 |bibcode = 2007PNAS..104.8749C }}</ref> Pauling denounced the conclusions of these studies and handling of the final study as "fraud and deliberate misrepresentation",<ref>{{cite web | url = http://oregonstate.edu/dept/Special_Collections/subpages/ahp/1995symposium/goertzel.html | title = Analyzing Pauling's Personality: A Three Generational, Three Decade Project |accessdate=August 5, 2007| author= Ted Goertzel |year=1996|publisher=Special Collections, Oregon State University Libraries}}</ref><ref name = Golem>{{cite book |author=Trevor Pinch; Collins, Harry M. |title=Dr. Golem: how to think about medicine |publisher= [[University of Chicago Press]] |location=Chicago |year=2005 |pages= 89–111 | isbn = 0-226-11366-3 |oclc= |accessdate=May 6, 2010 | chapterurl = http://www.press.uchicago.edu/Misc/Chicago/113663.html | chapter = Alternative Medicine: The Cases of Vitamin C and Cancer}}</ref> and criticized the studies for using oral, rather than [[intravenous therapy|intravenous]] vitamin C<ref>{{cite journal | url = http://www.cmaj.ca/cgi/content/full/174/7/937#T217 | title = Intravenously administered vitamin C as cancer therapy: three cases | accessdate=August 5, 2007| author= Levine M et al. | year = 2006 | journal = [[Canadian Medical Association Journal|CMAJ]] | volume = 174 | issue = 7 | doi = 10.1503/cmaj.050346 | pages = 937–942 | pmid = 16567755 | pmc = 1405876 }}</ref> (which was the dosing method used for the first ten days of Pauling's original study<ref name=PNASChen2007/>). Pauling also criticised the Mayo clinic studies because the controls were taking vitamin C during the trial, and because the duration of the treatment with vitamin C was short; Pauling advocated continued high dose vitamin C for the rest of the cancer patient's life whereas the Mayo clinic patients in the second trial were treated with vitamin C for a median of 2.5 months.<ref>{{cite book | author=Linus Pauling | year=1986 | title=How to Live Longer and Feel Better | publisher=[[W.H. Freeman and Company]] | location=New York | pages=173–175 | isbn=0-7167-1781-6}}</ref> The results were publicly debated at length with considerable acrimony between Pauling and Cameron, and Moertel (the lead author of the Mayo Clinic studies), with accusations of misconduct and scientific incompetence on both sides. Ultimately the negative findings of the Mayo Clinic studies ended general interest in vitamin C as a treatment for cancer.<ref name = Golem/> Despite this, Pauling continued to promote vitamin C for treating cancer and the common cold, working with [[The Institutes for the Achievement of Human Potential]] to use vitamin C in the treatment of brain-injured children.<ref name="Pauling1978">{{cite journal | first = L | last = Pauling | editor= Ralph Pelligra, ed. |date=November 1978 | title = Orthomolecular enhancement of human development | journal = Human Neurological Development | pages = 47–51 | url = http://profiles.nlm.nih.gov/MM/B/B/K/G/_/mmbbkg.pdf |format=PDF}}</ref> He later collaborated with the Canadian physician [[Abram Hoffer]] on a micronutrient regimen, including high-dose vitamin C, as adjunctive cancer therapy.<ref>{{cite web |url=http://www.doctoryourself.com/biblio_hoffer.html |title=Abram Hoffer, M.D., PhD 50 Years of Megavitamin Research, Practice and Publication |accessdate=August 5, 2007| author= Andrew W. Saul |coauthors= Dr. Abram Hoffer |publisher=Doctoryourself.com}}</ref> A 2009 review in the journal ''Anticancer Research'' was somewhat partial to Pauling, noting that there were significant methodological differences between the Mayo Clinic's and Pauling's studies (the Mayo clinic did not use intravenous Vitamin C), that other researchers administering intravenous vitamin C to patients reported that the patients gained benefits such as increased survival, improved well-being and reduced pain, that intravenous administration is necessary to achieve high enough plasma levels for a pharmacological effect, and that convergent evidence shows that Vitamin C is effective as an anticancer agent.<ref name ="Cancerreevaluated">{{Cite PMID|19414313}}</ref> |
|||
In 1942, Pauling successfully submitted a proposal on "The Chemical Treatment of Protein Solutions in the Attempt to Find a Substitute for Human Serum for Transfusions". His project group, which included Joseph B. Koepfli and Dan H. Campbell, developed a possible replacement for [[Blood plasma|human blood plasma]] in [[Blood transfusion|transfusions]]: [[polyoxy gelatin]] (Oxypolygelatin).<ref name="Oxypolygelatin">{{Cite web |date=January 27, 2009 |title=Blood and War: The Development of Oxypolygelatin, Part 1 |url=https://paulingblog.wordpress.com/2009/01/27/blood-and-war-the-development-of-oxypolygelatin-part-1/ |access-date=May 28, 2015 |website=The Pauling Blog}}</ref><ref name="Chadarevian">{{Cite book |last=Chadarevian |first=Soraya de |url=https://books.google.com/books?id=A2B4AgAAQBAJ&pg=PA109 |title=Molecularizing biology and medicine new practices and alliances, 1910s–1970s |date=1998 |publisher=Harwood Academic |isbn=978-90-5702-293-7 |location=Amsterdam |page=109 |access-date=May 28, 2015}}</ref> |
|||
With [[Arthur B. Robinson]] and another colleague, Pauling founded the Institute of Orthomolecular Medicine in Menlo Park, California, in 1973, which was soon renamed the [[Linus Pauling Institute]] of Science and Medicine. Pauling directed research on vitamin C, but also continued his theoretical work in chemistry and physics until his death. In his last years, he became especially interested in the possible role of vitamin C in preventing [[atherosclerosis]] and published three case reports on the use of [[lysine]] and vitamin C to relieve [[angina pectoris]]. In 1996, the Linus Pauling Institute moved from Palo Alto, California, to Corvallis, Oregon, to become part of Oregon State University, where it continues to conduct research on [[micronutrient]]s, [[phytochemical]]s (chemicals from plants), and other constituents of the diet in preventing and treating disease. Several researchers that had previously worked at the Linus Pauling Institute in Palo Alto, including the assistant director of research, moved on to form the [[Genetic Information Research Institute]]. |
|||
Other wartime projects with more direct military applications included work on explosives, rocket propellants and the patent for an armor-piercing shell. In October 1948, Pauling, along with [[Lee Alvin DuBridge|Lee A. DuBridge]], [[William Alfred Fowler|William A. Fowler]], [[Max Mason]], and [[Bruce Hornbrook Sage|Bruce H. Sage]], was awarded a [[Presidential Medal for Merit]] by President [[Harry S. Truman]]. The citation credits him for his "imaginative mind", "brilliant success", and "exceptionally meritorious conduct in the performance of outstanding services".<ref name="PMFM">{{Cite web |title=Presidential Medal for Merit |url=http://scarc.library.oregonstate.edu/coll/pauling/awards/1948h.1.html |access-date=May 28, 2015 |website=Linus Pauling Awards Honors and Medals}}</ref><ref name="NLM">{{Cite web |title=The Linus Pauling Papers: Biographical Information |url=https://profiles.nlm.nih.gov/MM/Views/Exhibit/narrative/biographical.html |access-date=February 11, 2008 |publisher=United States National Library of Medicine}}</ref><ref name="Paulus">{{Cite news |last=Paulus |first=John Allen |date=November 5, 1995 |title=Pauling's Prizes |work=[[The New York Times]] |url=https://query.nytimes.com/gst/fullpage.html?res=9D05E3DE1739F936A35752C1A963958260& |access-date=December 9, 2007}}</ref> In 1949, he served as president of the [[American Chemical Society]].<ref name="ACS1949">{{Cite web |title=ACS President: Linus Pauling (1901–1994) |url=http://www.acs.org/content/acs/en/about/president/acspresidents/linus-pauling.html |access-date=June 1, 2015 |website=ACS Chemistry for Life}}</ref> |
|||
===Structure of the atomic nucleus=== |
|||
On September 16, 1952, Pauling opened a new research notebook with the words "I have decided to attack the problem of the structure of nuclei."<ref>{{cite web|url=http://osulibrary.oregonstate.edu/specialcollections/rnb/27/27-124.html |title=Oregon State Special Collections |publisher=Osulibrary.oregonstate.edu |date=2002-02-28 |accessdate=2013-06-25}}</ref> On October 15, 1965, Pauling published his Close-Packed Spheron Model of the atomic nucleus in two well respected journals, ''Science'' and the ''[[Proceedings of the National Academy of Sciences]]''.<ref>{{cite web | url=http://osulibrary.orst.edu/specialcollections/rnb/26/26-026.html | title=The close-packed-spheron theory and nuclear fission | accessdate=August 5, 2007 | last=Pauling | first=Linus |date=October 1965 | publisher=Science}}</ref> For nearly three decades, until his death in 1994, Pauling published numerous papers on his spheron cluster model.<ref>{{cite web | url=http://osulibrary.orst.edu/specialcollections/rnb/26/26-012.html | title=The close-packed spheron model of atomic nuclei and its relation to the shell model | accessdate=August 5, 2007|last=Pauling | first=Linus |date=October 1965 | publisher=Science}}</ref><ref>{{cite web | url=http://osulibrary.orst.edu/specialcollections/rnb/26/26-048.html | title=The close-packed-spheron theory of nuclear structure and the neutron excess for stable nuclei (Dedicated to the seventieth anniversary of Professor Horia Hulubei) | accessdate=August 5, 2007 | last=Pauling | first=Linus |date=July 1966 | publisher=Science}}</ref><ref>{{cite web | url=http://osulibrary.orst.edu/specialcollections/rnb/26/26-068.html | title=Magnetic-moment evidence for the polyspheron structure of the lighter atomic nuclei | accessdate=August 5, 2007 | last=Pauling | first=Linus |date=December 1967 | publisher=Science}}</ref><ref>{{cite web | url=http://osulibrary.orst.edu/specialcollections/rnb/26/26-075.html | title=Orbiting clusters in atomic nuclei | accessdate=August 5, 2007 | last=Pauling | first=Linus |date=November 1969 | publisher=Science}}</ref><ref>{{cite web | url=http://osulibrary.orst.edu/specialcollections/rnb/26/26-084.html | title=Rotating clusters in nuclei | accessdate=August 5, 2007 | last=Pauling | first=Linus | coauthors=Arthur B. Robinson | year=1975 | publisher=Canadian Journal of Physics}}</ref><ref>{{cite journal | url=http://osulibrary.orst.edu/specialcollections/rnb/26/26-125.html | title=Transition from one revolving cluster to two revolving clusters in the ground-state rotational bands of nuclei in the lanthanon region | accessdate=August 5, 2007 | last=Pauling | first=Linus | volume=88 |date=February 1991 |journal=[[Proceedings of the National Academy of Science|Proc. Natl. Acad. Sci.]]}}</ref> |
|||
===Nuclear activism=== |
|||
The aftermath of the [[Manhattan Project]] and his wife Ava's pacifism changed Pauling's life profoundly, and he became a peace activist.{{citation needed|date=August 2023}} |
|||
In June 1945, a "May-Johnson Bill" began<ref>{{Citation |title=Part VI: The Manhattan District in Peacetime: The May-Johnson Bill |date=1998 |url=http://www.atomicarchive.com/History/mp/p6s6.shtml |publisher=Atomic Archive |access-date=October 19, 2019}}</ref><ref>{{Citation |title=Atomic Energy Commission |date=November 18, 2016 |url=https://www.atomicheritage.org/history/atomic-energy-commission |publisher=Atomic Heritage Foundation |access-date=October 19, 2019}}</ref><ref>{{Citation |title=Roy Glauber & Priscilla McMillan on Oppenheimer – Atomic Energy Commission |date=June 6, 2013 |url=https://www.manhattanprojectvoices.org/oral-histories/roy-glauber-priscilla-mcmillan-oppenheimer |publisher=Voices of the Manhattan Project |access-date=October 19, 2019}}</ref> that would become the [[Atomic Energy Act of 1946]] (signed August 1, 1946). In November 1945, Pauling spoke to the [[Independent Citizens Committee of the Arts, Sciences and Professions]] (ICCASP) on [[atomic weapons]]; shortly after, wife Ava and he accepted membership.<ref name="PaulingICCASP">{{Citation |title=The Independent Citizens Committee of the Arts, Sciences and Professions |date=2009 |url=https://paulingblog.wordpress.com/2010/07/15/the-independent-citizens-committee-for-the-arts-sciences-and-professions/amp/ |publisher=Linus Pauling and the International Peace Movement |access-date=October 19, 2019}}</ref> On January 21, 1946, the group met to discuss [[academic freedom]], during which Pauling said, "There is, of course, always a threat to academic freedom – as there is to the other aspects of the freedom and rights of the individual, in the continued attacks which are made on this freedom, these rights, by the selfish, the overly ambitious, the misguided, the unscrupulous, who seek to oppress the great body of mankind in order that they themselves may profit – and we must always be on the alert against this threat, and must fight it with vigor when it becomes dangerous."<ref name=PaulingICCASP/> |
|||
In 1946, he joined the [[Emergency Committee of Atomic Scientists]], chaired by [[Albert Einstein]].<ref>{{Cite web |last=Hager |first=Thomas |author-link=Thomas Hager |date=November 29, 2007 |title=Einstein |url=http://scarc.library.oregonstate.edu/coll/pauling/peace/narrative/page9.html |access-date=December 13, 2007 |publisher=Oregon State University Libraries Special Collections}}</ref> Its mission was to warn the public of the dangers associated with the development of nuclear weapons. |
|||
[[File:Letter from Ruth B. Shipley, Chief Passport Division, Department of State (United States) to Linus Pauling on February 14, 1952.jpg|thumb|Denial letter from [[Ruth B. Shipley]], Chief Passport Division, Department of State to Linus Pauling on February 14, 1952]] |
|||
His political activism prompted the [[United States Department of State|US State Department]] to deny him a passport in 1952, when he was invited to speak at a scientific conference in London.<ref>{{Cite web |title=Linus Pauling |url=http://usstampgallery.com/view.php?id=3228fbec24ea876e8035f61b5fcd643af29a611c |access-date=June 2, 2015 |website=U.S. Stamp Gallery}}</ref><ref>{{Cite web |last=Pauling |first=Linus |date=May 1952 |title=The Department of State and the Structure of Proteins |url=http://scarc.library.oregonstate.edu/coll/pauling/peace/notes/1952a.18.html |access-date=December 13, 2007 |publisher=Oregon State University Libraries Special Collections}}</ref> In a speech before the [[US Senate]] on June 6 of the same year, Senator [[Wayne Morse]] publicly denounced the action of the State Department, and urged the Passport Division to reverse its decision. Pauling and his wife Ava were then issued a "limited passport" to attend the conference.<ref>Robert Paradowski (2011), Oregon State University, Special Collections p.18, [http://scarc.library.oregonstate.edu/coll/pauling/chronology/page18.html Proteins, Passports, and the Prize (1950–1954)], retrieved February 1, 2013</ref><ref>[https://books.google.com/books?id=sg0AAAAAMBAJ&pg=PA255 Bulletin of the Atomic Scientists Vol. VIII, Nr. 7] (Okt. 1952) p. 254, Educational Foundation for Nuclear Science, Inc.</ref> His full passport was restored in 1954, shortly before the ceremony in [[Stockholm]] where he received his first Nobel Prize.{{citation needed|date=August 2023}} |
|||
Joining Einstein, [[Bertrand Russell]] and eight other leading scientists and intellectuals, he signed the [[Russell-Einstein Manifesto]] issued July 9, 1955.<ref>{{Cite web |last=Hager |first=Thomas |date=November 29, 2007 |title=Russell/Einstein |author-link=Thomas Hager |url=http://scarc.library.oregonstate.edu/coll/pauling/peace/narrative/page25.html |access-date=December 13, 2007 |publisher=Oregon State University Libraries Special Collections}}</ref> He also supported the [[Mainau Declaration]] of July 15, 1955, signed by 52 Nobel Prize laureates.<ref name="Snow">{{Cite book |last=Hermann |first=Armin |title=The new physics: the route into the atomic age: in memory of Albert Einstein, Max von Laue, Otto Hahn, Lise Meitner |date=1979 |publisher=Inter Nationes |location=Bonn-Bad Godesberg |page=130}}</ref> |
|||
In May 1957, working with [[Washington University in St. Louis]] professor [[Barry Commoner]], Pauling began to circulate a petition among scientists to stop nuclear testing.<ref name="Tooth">{{Cite web |title=The Baby Tooth Survey |url=https://paulingblog.wordpress.com/tag/committee-for-nuclear-information/ |access-date=June 1, 2011 |website=The Pauling Blog}}</ref> On January 15, 1958, Pauling and his wife presented a petition to United Nations Secretary General [[Dag Hammarskjöld]] calling for an end to [[nuclear testing|the testing of nuclear weapons]]. It was signed by 11,021 scientists representing fifty countries.<ref name="NobelLecture">{{Cite web |title=The Nobel Peace Prize 1962 Linus Pauling: Nobel Lecture |url=https://www.nobelprize.org/nobel_prizes/peace/laureates/1962/pauling-lecture.html |access-date=May 28, 2015 |website=Nobel Prize.org}}</ref><ref name="NobelBlog">{{Cite web |title=Linus Pauling Receives the Nobel Peace Prize |url=https://paulingblog.wordpress.com/tag/united-nations-bomb-test-petition/ |access-date=December 10, 2013 |website=The Pauling Blog}}</ref> |
|||
In February 1958, Pauling participated in a publicly televised debate with the atomic physicist [[Edward Teller]] about the actual probability of fallout causing mutations.<ref name="Moore">{{Cite book |last=Moore |first=Kelly |url=https://books.google.com/books?id=qlBCTlVq_0EC&pg=PA113 |title=Disrupting science : social movements, American scientists, and the politics of the military, 1945–1975 |date=2008 |publisher=Princeton University Press |isbn=978-0-691-11352-4 |location=Princeton |page=113 |access-date=May 28, 2015}}</ref> Later in 1958, Pauling published ''No more war!'', in which he not only called for an end to the testing of nuclear weapons but also an end to war itself. He proposed that a World Peace Research Organization be set up as part of the United Nations to "attack the problem of preserving the peace".<ref name="Nobel" /> |
|||
Pauling also supported the work of the St. Louis Citizen's Committee for Nuclear Information (CNI).<ref name=Tooth/> This group, headed by [[Barry Commoner]], Eric Reiss, M. W. Friedlander and John Fowler, organized a longitudinal study to measure radioactive [[strontium]]-90 in the [[baby teeth]] of children across North America. The "[[Baby Tooth Survey]]", published by [[Louise Reiss]], demonstrated conclusively in 1961 that above-ground nuclear testing posed significant public health risks in the form of [[nuclear fallout|radioactive fallout]] spread primarily via milk from cows that had ingested contaminated grass.<ref>{{Cite journal |last=Reiss |first=Louise Zibold |author-link=Louise Reiss |date=November 24, 1961 |title=Strontium-90 Absorption by Deciduous Teeth: Analysis of teeth provides a practicable method of monitoring strontium-90 uptake by human populations |journal=Science |volume=134 |issue=3491 |pages=1669–1673 |doi=10.1126/science.134.3491.1669 |pmid=14491339}}</ref><ref>{{Cite web |last=Hager |first=Thomas |author-link=Thomas Hager |date=November 29, 2007 |title=Strontium-90 |url=http://scarc.library.oregonstate.edu/coll/pauling/peace/narrative/page26.html |access-date=December 13, 2007 |publisher=Oregon State University Libraries Special Collections}}</ref><ref>{{Cite web |last=Hager |first=Thomas |author-link=Thomas Hager |date=November 29, 2007 |title=The Right to Petition |url=http://scarc.library.oregonstate.edu/coll/pauling/peace/narrative/page27.html |access-date=December 13, 2007 |publisher=Oregon State University Libraries Special Collections}}</ref> The Committee for Nuclear Information is frequently credited for its significant contribution to supporting the test ban,<ref name="McCormick">{{Cite book |last=McCormick |first=John |url=https://books.google.com/books?id=xC16yGp9-HUC&pg=PA70 |title=Reclaiming paradise : the global environmental movement |date=1991 |publisher=Indiana University Press |isbn=978-0-253-20660-2 |edition=1st Midland |location=Bloomington |access-date=May 27, 2015}}</ref> as is the ground-breaking research conducted by Reiss and the "Baby Tooth Survey".<ref name="activism">{{Cite book |last1=Allen |first1=Garland E. |author1-link=Garland E. Allen |url=https://books.google.com/books?id=7axYHFEomgMC&pg=PA302 |title=Science, history and social activism : a tribute to Everett Mendelsohn |last2=MacLeod |first2=Roy M. |date=2001 |publisher=Kluwer Academic |isbn=978-1-4020-0495-7 |location=Dordrecht |page=302 |access-date=May 27, 2015}}</ref> |
|||
Public pressure and the frightening results of the CNI research led to a moratorium on above-ground nuclear weapons testing, followed by the [[Partial Test Ban Treaty]], signed in 1963 by [[John F. Kennedy]] and [[Nikita Khrushchev]]. On the day that the treaty went into force, October 10, 1963, the Nobel Prize Committee awarded Pauling the [[Nobel Peace Prize]] for 1962. (No prize had previously been awarded for that year.)<ref name="PaloAlto">{{Cite news |date=October 10, 1963 |title=Nobel Peace Prize Awarded to Pauling |work=Palo Alto Times |url=http://scarc.library.oregonstate.edu/coll/pauling/peace/newsclips/1963n.30.html |access-date=May 27, 2015}}</ref> They described him as "Linus Carl Pauling, who ever since 1946 has campaigned ceaselessly, not only against nuclear weapons tests, not only against the spread of these armaments, not only against their very use, but against all warfare as a means of solving international conflicts."<ref>{{Cite web |last=Pauling |first=Linus |date=October 10, 1963 |title=Notes by Linus Pauling. October 10, 1963 |url=http://scarc.library.oregonstate.edu/coll/pauling/peace/notes/rnb23-100.html |access-date=December 13, 2007 |publisher=Oregon State University Libraries Special Collections}}</ref> Pauling himself acknowledged his wife Ava's deep involvement in peace work, and regretted that she was not awarded the Nobel Peace Prize with him.<ref name="LPIBio">{{Cite web |date=May 9, 2014 |title=Linus Pauling Biography |url=http://lpi.oregonstate.edu/linus-pauling-biography |access-date=June 2, 2015 |website=Linus Pauling Institute}}</ref> |
|||
===Political criticism=== |
|||
[[File:Linus Pauling's beret at the Nobel Museum (51969).jpg|thumb|Pauling's beret on display at the [[Nobel Prize Museum]]]] |
|||
Many of Pauling's critics, including scientists who appreciated the contributions that he had made in chemistry, disagreed with his political positions and saw him as a naïve spokesman for [[Communist Party of the Soviet Union|Soviet communism]]. In 1960, he was ordered to appear before the [[United States Senate Subcommittee on Internal Security|Senate Internal Security Subcommittee]],<ref name="Senate">{{Cite web |title=issued to Linus Pauling by the Internal Security Subcommittee of the United States Senate. June 20, 1960 |url=http://scarc.library.oregonstate.edu/coll/pauling/peace/papers/bio2.021.3.html |access-date=May 28, 2015 |website=Linus Pauling and the International Peace Movement}}</ref> which termed him "the number one scientific name in virtually every major activity of the Communist peace offensive in this country".<ref name=Humanism/> A headline in ''[[Life magazine|Life]]'' magazine characterized his 1962 Nobel Prize as "A Weird Insult from Norway".<ref name="Kovac">{{Cite journal |last=Kovac |first=Jeffrey |date=1999 |title=A weird insult from Norway: Linus Pauling as public intellectual |journal=Soundings: An Interdisciplinary Journal |volume=82 |issue=1/2 |pages=91–106 |jstor=41178914}}</ref><ref name="Life">{{Cite magazine |date=October 25, 1963 |title=A Weird Insult From Norway |url=https://books.google.com/books?id=UlIEAAAAMBAJ&pg=PA6 |magazine=Life |volume=5 |page=4 |number=17}}</ref> |
|||
Pauling was a frequent target of the ''[[National Review]]'' magazine. In an article entitled "The Collaborators" in the magazine's July 17, 1962, issue, Pauling was referred to not only as a collaborator, but as a "fellow traveler" of proponents of Soviet-style communism. In 1963, Pauling sued the magazine, its publisher [[William Rusher]], and its editor [[William F. Buckley, Jr]] for $1 million. He lost both his libel suits and the 1968 appeal (unlike his earlier 1963 libel case against the [[Hearst Corporation]]), because in the meantime the landmark case ''[[New York Times Co. v. Sullivan]]'' had established the [[actual malice]] standard for libel lawsuits by public figures, requiring that not only falsehood but deliberate lying should be proved by the plaintiff in such cases.<ref>{{Cite web |date=January 30, 2013 |title=The National Review Lawsuit |url=http://paulingblog.wordpress.com/2013/01/30/the-national-review-lawsuit/ |access-date=December 20, 2013 |publisher=Paulingblog}}</ref><ref>{{Cite web |title=A Tough Conclusion to the National Review Lawsuit |url=http://paulingblog.wordpress.com/tag/william-f-buckley/ |access-date=December 20, 2013 |publisher=Paulingblog}}</ref><ref>{{Cite web |title=Pauling v. Nat'l Review, Inc |url=http://law.justia.com/cases/new-york/court-of-appeals/1968/22-n-y-2d-818-0.html |access-date=December 20, 2013 |website=Justia.com}}</ref><ref>{{Cite news |last=Saxon |first=Wolfgang |date=August 30, 1998 |title=C. Dickerman Williams, 97, Free-Speech Lawyer, Is Dead |work=The New York Times |url=https://www.nytimes.com/1998/08/30/nyregion/c-dickerman-williams-97-free-speech-lawyer-is-dead.html |access-date=December 20, 2013}}</ref> |
|||
His peace activism, his frequent travels, and his enthusiastic expansion into chemical-biomedical research all aroused opposition at Caltech. In 1958, the Caltech Board of Trustees demanded that Pauling step down as chairman of the Chemistry and Chemical Engineering Division.<ref name=LATimes1994/>{{rp|2}} Although he had retained tenure as a full professor, Pauling chose to resign from Caltech after he received the Nobel peace prize money. He spent the next three years at the [[Center for the Study of Democratic Institutions]] (1963–1967).<ref name="Abrams" /> In 1967, he moved to the University of California at San Diego, but remained there only briefly, leaving in 1969 in part because of political tensions with the Reagan-era board of regents.<ref name=LATimes1994/>{{rp|3}} From 1969 to 1974, he accepted a position as professor of chemistry at Stanford University.<ref name="OH" /> |
|||
===Vietnam war activism=== |
|||
During the 1960s, President Lyndon Johnson's policy of increasing America's involvement in the Vietnam War caused an [[anti-war movement]] that the Paulings joined with enthusiasm. Pauling denounced the war as unnecessary and unconstitutional. He made speeches, signed protest letters and communicated personally with the North Vietnamese leader, Ho Chi Minh, and gave the lengthy written response to President Johnson. His efforts were ignored by the American government.<ref>{{Cite web |year=2010 |title=Linus Pauling and the International Peace Movement: Vietnam |url=http://scarc.library.oregonstate.edu/coll/pauling/peace/narrative/page49.html |publisher=Oregon State University Libraries}}</ref> |
|||
Pauling was awarded the [[Lenin Peace Prize|International Lenin Peace Prize]] by the USSR in 1970.<ref name="Humanism">{{Cite journal |last=Mason |first=Stephen F. |date=1997 |title=The Science and Humanism of Linus Pauling (1901–1994) |url=http://oregonstate.edu/dept/spc/subpages/ahp/overview/entirearticle.htm |url-status=dead |journal=Chemical Society Reviews |volume=26 |pages=29–39 |doi=10.1039/cs9972600029 |archive-url=https://web.archive.org/web/20090515124732/http://oregonstate.edu/dept/spc/subpages/ahp/overview/entirearticle.htm |archive-date=May 15, 2009 |access-date=May 20, 2015}}</ref><ref name="LeninPrize">{{Cite web |date=May 16, 2011 |title=Lenin Peace Prize Recipients |url=http://www.researchhistory.org/2011/05/16/lenin-peace-prize-recipients/ |website=Research History}}</ref> He continued his peace activism in the following years. He and his wife Ava helped to found the [[International League of Humanists]] in 1974.<ref name="HumanistFounders">{{Cite web |title=Founders |url=http://www.intlh.com/index_enu.html |url-status=dead |archive-url=https://web.archive.org/web/20150611040404/http://www.intlh.com/index_enu.html |archive-date=June 11, 2015 |access-date=May 28, 2015 |website=International League of Humanists for peace and tolerance }}</ref> He was president of the scientific advisory board of the [[World Union for Protection of Life]] and also one of the signatories of the [[Dubrovnik–Philadelphia statement]] of 1974/1976.<ref name="Dubrovnik">{{Cite web |title=The Dubrovnik-Philadelphia Statement /1974–1976/ (short version) |url=http://www.intlh.com/statement.html |url-status=dead |archive-url=https://web.archive.org/web/20150924035630/http://www.intlh.com/statement.html |archive-date=September 24, 2015 |access-date=May 28, 2015 |website=International League of Humanists }}</ref> Linus Carl Pauling was an honorary president and member of the International Academy of Science, Munich, until the end of his life.<ref>{{Cite web |title=History |url=http://www.ias-icsd.org/history.html |access-date=March 16, 2015 |website=International Academy of Science, Munich |archive-date=2015-04-02 |archive-url=https://web.archive.org/web/20150402142953/http://www.ias-icsd.org/history.html |url-status=dead }}</ref> |
|||
Pauling was also a supporter of the [[Fair Play for Cuba Committee]].<ref>{{cite book |editor1-last=Johnson |editor1-first=Loch K. |title=Handbook of Intelligence Studies |date=2007 |publisher=Taylor & Francis |page=275}}</ref> |
|||
=== Global activism === |
|||
He was one of the signatories of the agreement to convene a convention for drafting a [[world constitution]].<ref>{{Cite web |title=Letters from Thane Read asking Helen Keller to sign the World Constitution for world peace. 1961 |url=https://www.afb.org/HelenKellerArchive?a=d&d=A-HK01-07-B149-F04-022.1.8 |access-date=July 1, 2023 |website=Helen Keller Archive |publisher=American Foundation for the Blind}}</ref><ref>{{Cite web |title=Letter from World Constitution Coordinating Committee to Helen, enclosing current materials |url=https://www.afb.org/HelenKellerArchive?a=d&d=A-HK01-07-B154-F05-028.1.6 |access-date=July 3, 2023 |website=Helen Keller Archive |publisher=American Foundation for the Blind}}</ref> As a result, for the first time in human history, a [[World Constituent Assembly]] convened to draft and adopt a [[Constitution for the Federation of Earth]].<ref>{{Cite web |title=Preparing earth constitution {{!}} Global Strategies & Solutions {{!}} The Encyclopedia of World Problems |url=http://encyclopedia.uia.org/en/strategy/193465 |access-date=July 15, 2023 |website=The Encyclopedia of World Problems {{!}} Union of International Associations (UIA) |archive-date=2023-07-19 |archive-url=https://web.archive.org/web/20230719215501/http://encyclopedia.uia.org/en/strategy/193465 |url-status=dead }}</ref> |
|||
===Eugenics=== |
|||
Pauling supported a limited form of [[eugenics]] by suggesting that human carriers of defective genes be given a compulsory visible mark – such as a forehead tattoo – to discourage potential mates with the same defect, in order to reduce the number of babies with diseases such as [[sickle cell anemia]].<ref>{{Cite web |last=Mendelsohn |first=Everett |author-link=Everett Mendelsohn |date=March–April 2000 |title=The Eugenic Temptation |url=http://harvardmagazine.com/2000/03/the-eugenic-temptation.html |website=Harvard Magazine}}</ref><ref>{{Cite web |last=Special Collections & Archives Research Center, Oregon State University Libraries |date=2015 |title=Eugenics for Alleviating Human Suffering |url=http://scarc.library.oregonstate.edu/coll/pauling/blood/narrative/page35.html |access-date=May 30, 2020 |website=It's in the Blood! A Documentary History of Linus Pauling, Hemoglobin and Sickle Cell Anemia}}</ref> |
|||
===Medical research and vitamin C advocacy=== |
|||
{{main|Vitamin C megadosage}} |
|||
[[File:Pauling Vit C Book Cover.jpg|right|150px|thumb|Pauling's book, ''How to Live Longer and Feel Better'', advocated very high intake of [[Vitamin C]].<ref>{{Cite book |last=Pauling |first=Linus |title=How to Live Longer and Feel Better |publisher=Avon Books |year=1987 |edition=1 |location=New York |ol=18076125M}}</ref>]] |
|||
In 1941, at age 40, Pauling was diagnosed with [[Bright's disease]], a renal disease. Following the recommendations of [[Thomas Addis]], who actively recruited Ava Helen Pauling as "nutritionist, cook, and eventually as deputy 'doctor'", Pauling believed he was able to control the disease with Addis's then-unusual low-protein salt-free diet and vitamin supplements.<ref>{{Cite book |last=Peitzman |first=Steven J. |url=https://books.google.com/books?id=8FUDO5K1pAoC&pg=PA72 |title=Dropsy, dialysis, transplant: a short history of failing kidneys |publisher=Johns Hopkins University Press |year=2007 |isbn=978-0-8018-8734-5 |location=Baltimore |pages=72–8; 190}}</ref> Thus Pauling's initial – and intensely personal – exposure to the idea of treating disease with vitamin supplements was positive.{{citation needed|date=August 2023}} |
|||
In 1965, Pauling read ''Niacin Therapy in Psychiatry'' by [[Abram Hoffer]] and theorized vitamins might have important biochemical effects unrelated to their prevention of associated deficiency diseases.<ref>{{Cite book |title=Biochemical imbalances in disease a practitioner's handbook |publisher=Singing Dragon |year=2010 |isbn=978-0-85701-028-5 |editor-last=Nicolle |editor-first=Lorraine |location=London |page=27 |editor-last2=Beirne |editor-first2=Ann Woodriff}}</ref> In 1968, Pauling published a brief paper in [[Science (journal)|''Science'']] entitled "Orthomolecular psychiatry",<ref>{{Cite journal |last=Pauling |first=Linus |date=April 1968 |title=Orthomolecular psychiatry. Varying the concentrations of substances normally present in the human body may control mental disease |journal=Science |volume=160 |issue=3825 |pages=265–71 |bibcode=1968Sci...160..265P |doi=10.1126/science.160.3825.265 |pmid=5641253 |s2cid=20153555}}</ref> giving a name to the popular but controversial [[megavitamin therapy]] movement of the 1970s, and advocating that "orthomolecular therapy, the provision for the individual person of the optimum concentrations of important normal constituents of the brain, may be the preferred treatment for many mentally ill patients." Pauling coined the term "orthomolecular" to refer to the practice of varying the concentration of substances normally present in the body to prevent and treat disease. His ideas formed the basis of [[orthomolecular medicine]], which is not generally practiced by conventional medical professionals and has been strongly criticized.<ref name="Cassileth">{{Cite book |last=Cassileth |first=Barrie R. |author-link=Barrie R. Cassileth |title=The alternative medicine handbook: the complete reference guide to alternative and complementary therapies |publisher=W.W. Norton |year=1998 |isbn=978-0-393-04566-6 |location=New York |pages=67}}</ref><ref name="bccancer">{{Cite web |date=February 2000 |title=Vitamin Therapy, Megadose / Orthomolecular Therapy |url=http://www.bccancer.bc.ca/PPI/UnconventionalTherapies/VitaminTherapyMegadoseOrthomolecularTherapy.htm |archive-url=https://web.archive.org/web/20070202102734/http://www.bccancer.bc.ca/PPI/UnconventionalTherapies/VitaminTherapyMegadoseOrthomolecularTherapy.htm |archive-date=February 2, 2007 |access-date=August 5, 2007 |publisher=BC Cancer Agency}}</ref> |
|||
In 1973, with [[Arthur B. Robinson]] and another colleague, Pauling founded the Institute of Orthomolecular Medicine in Menlo Park, California, which was soon renamed the [[Linus Pauling Institute]] of Science and Medicine. Pauling directed research on vitamin C, but also continued his theoretical work in chemistry and physics until his death. In his last years, he became especially interested in the possible role of vitamin C in preventing [[atherosclerosis]] and published three case reports on the use of [[lysine]] and vitamin C to relieve [[angina pectoris]]. During the 1990s, Pauling put forward a comprehensive plan for the treatment of heart disease using lysine and vitamin C. In 1996, a website was created expounding Pauling's treatment which it referred to as Pauling Therapy. Proponents of Pauling Therapy believe that heart disease can be treated and even cured using only lysine and Vitamin C and without drugs or heart operations.<ref>{{Cite web |title=PaulingTherapy.com – Reversing Heart Disease w/o Drugs is Possible |url=http://www.paulingtherapy.com/ |website=www.paulingtherapy.com}}</ref> |
|||
Pauling's work on [[vitamin C]] in his later years generated much controversy. He was first introduced to the concept of high-dose vitamin C by biochemist [[Irwin Stone]] in 1966. After becoming convinced of its worth, Pauling took 3 grams of vitamin C every day to prevent [[colds]].<ref name="frs" /> Excited by his own perceived results, he researched the clinical literature and published ''[[Vitamin C and the Common Cold (book)|Vitamin C and the Common Cold]]'' in 1970. He began a long clinical collaboration with the British cancer surgeon [[Ewan Cameron (physician)|Ewan Cameron]] in 1971 on the use of intravenous and oral vitamin C as cancer therapy for terminal patients.<ref>{{Cite web |last=Cameron |first=Ewan |author-link=Ewan Cameron |title=Cancer Bibliography: Ewan Cameron, M.D. and Vitamin C Therapy |url=http://www.doctoryourself.com/biblio_cameron.html |access-date=August 5, 2007 |publisher=Doctoryourself.com}}</ref> Cameron and Pauling wrote many technical papers and a popular book, ''Cancer and Vitamin C'', that discussed their observations. Pauling made vitamin C popular with the public<ref name="OnThisDay">{{Cite news |last=Severo |first=Richard |date=August 21, 1994 |title=Linus C. Pauling Dies at 93; Chemist and Voice for Peace |work=The New York Times |url=https://www.nytimes.com/learning/general/onthisday/bday/0228.html |access-date=June 1, 2015}}</ref> and eventually published two studies of a group of 100 allegedly [[terminal illness|terminal]] patients that claimed vitamin C increased survival by as much as four times compared to untreated patients.<ref>{{Cite journal |last1=Cameron |first1=E |author1-link=Ewan Cameron |last2=Pauling |first2=L |date=October 1976 |title=Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer |journal=Proceedings of the National Academy of Sciences |volume=73 |issue=10 |pages=3685–9 |bibcode=1976PNAS...73.3685C |doi=10.1073/pnas.73.10.3685 |pmc=431183 |pmid=1068480 |doi-access=free}}</ref><ref>{{Cite journal |last1=Cameron |first1=E |author1-link=Ewan Cameron |last2=Pauling |first2=L |date=September 1978 |title=Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer |journal=Proceedings of the National Academy of Sciences |volume=75 |issue=9 |pages=4538–42 |bibcode=1978PNAS...75.4538C |doi=10.1073/pnas.75.9.4538 |pmc=336151 |pmid=279931 |doi-access=free}}</ref> |
|||
A re-evaluation of the claims in 1982 found that the patient groups were not actually comparable, with the vitamin C group being less sick on entry to the study, and judged to be "terminal" much earlier than the comparison group.<ref>{{Cite journal |last=DeWys |first=WD |year=1982 |title=How to evaluate a new treatment for cancer |journal=Your Patient and Cancer |volume=2 |issue=5 |pages=31–36}}</ref> Later clinical trials conducted by the [[Mayo Clinic]] led by oncologist [[Edward T. Creagan|Dr. Edward T. Creagan]] also concluded that high-dose (10,000 mg) vitamin C was no better than [[placebo]] at treating cancer and that there was no benefit to high-dose vitamin C.<ref>{{Cite journal |last1=Creagan |first1=ET |last2=Moertel |first2=CG |last3=O'Fallon |first3=JR |date=September 1979 |title=Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial |journal=The New England Journal of Medicine |volume=301 |issue=13 |pages=687–90 |doi=10.1056/NEJM197909273011303 |pmid=384241}}</ref><ref>{{Cite journal |last1=Moertel |first1=CG |last2=Fleming |first2=TR |last3=Creagan |first3=ET |last4=Rubin |first4=J |last5=O'Connell |first5=MJ |last6=Ames |first6=MM |date=January 1985 |title=High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison |journal=The New England Journal of Medicine |volume=312 |issue=3 |pages=137–41 |doi=10.1056/NEJM198501173120301 |pmid=3880867}}</ref><ref>{{Cite journal |last=Tschetter |first=L |display-authors=etal |year=1983 |title=A community-based study of vitamin C (ascorbic acid) in patients with advanced cancer |journal=Proceedings of the American Society of Clinical Oncology |volume=2 |page=92}}</ref> The failure of the clinical trials to demonstrate any benefit resulted in the conclusion that vitamin C was not effective in treating cancer; the medical establishment concluded that his claims that vitamin C could prevent colds or treat cancer were [[quackery]].<ref name="frs" /><ref name="PNASChen2007">{{Cite journal |last1=Chen |first1=Q |last2=Espey |first2=M. G. |last3=Sun |first3=A. Y. |last4=Lee |first4=J.-H. |last5=Krishna |first5=M. C. |last6=Shacter |first6=E. |last7=Choyke |first7=P. L. |last8=Pooput |first8=C. |last9=Kirk |first9=K. L. |last10=Buettner |first10=G. R. |last11=Levine |first11=M. |display-authors=etal |year=2007 |title=Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo |journal=Proceedings of the National Academy of Sciences |volume=104 |issue=21 |pages=8749–54 |bibcode=2007PNAS..104.8749C |doi=10.1073/pnas.0702854104 |pmc=1885574 |pmid=17502596 |doi-access=free}}</ref> Pauling denounced the conclusions of these studies and handling of the final study as "fraud and deliberate misrepresentation",<ref>{{Cite web |last=Goertzel |first=Ted |author-link=Ted Goertzel |year=1996 |title=Analyzing Pauling's Personality: A Three Generational, Three Decade Project |url=http://oregonstate.edu/dept/Special_Collections/subpages/ahp/1995symposium/goertzel.html |url-status=dead |archive-url=https://web.archive.org/web/20071014174038/http://oregonstate.edu/dept/Special_Collections/subpages/ahp/1995symposium/goertzel.html |archive-date=October 14, 2007 |access-date=August 5, 2007 |publisher=Special Collections, Oregon State University Libraries}}</ref><ref name="Golem">{{Cite book |last1=Pinch |first1=Trevor |author1-link=Trevor Pinch |title=Dr. Golem: how to think about medicine |last2=Collins |first2=Harry M. |publisher=[[University of Chicago Press]] |year=2005 |isbn=978-0-226-11366-1 |location=Chicago |pages=89–111 |chapter=Alternative Medicine: The Cases of Vitamin C and Cancer |chapter-url=http://www.press.uchicago.edu/Misc/Chicago/113663.html}}</ref> and criticized the studies for using oral, rather than [[intravenous therapy|intravenous]] vitamin C<ref>{{Cite journal |last=Levine |first=M |display-authors=etal |year=2006 |title=Intravenously administered vitamin C as cancer therapy: three cases |journal=[[Canadian Medical Association Journal|CMAJ]] |volume=174 |issue=7 |pages=937–942 |doi=10.1503/cmaj.050346 |pmc=1405876 |pmid=16567755}}</ref> (which was the dosing method used for the first ten days of Pauling's original study<ref name=PNASChen2007/>). Pauling also criticised the Mayo Clinic studies because the controls were taking vitamin C during the trial, and because the duration of the treatment with vitamin C was short; Pauling advocated continued high-dose vitamin C for the rest of the cancer patient's life whereas the Mayo Clinic patients in the second trial were treated with vitamin C for a median of 2.5 months.<ref>{{Cite book |last=Pauling |first=Linus |url=https://archive.org/details/howtolivelongerf00paul/page/173 |title=How to Live Longer and Feel Better |publisher=[[W. H. Freeman and Company|Freeman]] |year=1986 |isbn=978-0-7167-1781-2 |location=New York |pages=[https://archive.org/details/howtolivelongerf00paul/page/173 173–175]}}</ref> |
|||
Ultimately the negative findings of the Mayo Clinic studies ended general interest in vitamin C as a treatment for cancer.<ref name = Golem/> Despite this, Pauling continued to promote vitamin C for treating cancer and the common cold, working with [[The Institutes for the Achievement of Human Potential]] to use vitamin C in the treatment of brain-injured children.<ref name="Pauling1978">{{Cite journal |last=Pauling |first=L |date=November 1978 |title=Orthomolecular enhancement of human development |url=https://profiles.nlm.nih.gov/MM/B/B/K/G/_/mmbbkg.pdf |archive-url=https://ghostarchive.org/archive/20221009/https://profiles.nlm.nih.gov/MM/B/B/K/G/_/mmbbkg.pdf |archive-date=October 9, 2022 |url-status=live |journal=Human Neurological Development |pages=47–51 |editor=Ralph Pelligra}}</ref> He later collaborated with the Canadian physician [[Abram Hoffer]] on a micronutrient regime, including high-dose vitamin C, as adjunctive cancer therapy.<ref>{{Cite web |last1=Saul |first1=Andrew W. |last2=Dr. Abram Hoffer |title=Abram Hoffer, M.D., PhD 50 Years of Megavitamin Research, Practice and Publication |url=http://www.doctoryourself.com/biblio_hoffer.html |access-date=August 5, 2007 |publisher=Doctoryourself.com}}</ref> A 2009 review also noted differences between the studies, such as the Mayo Clinic not using intravenous Vitamin C, and suggested further studies into the role of vitamin C when given intravenously.<ref name="Cancerreevaluated">{{Cite journal |last1=Ohno |first1=S |last2=Ohno |first2=Y |last3=Suzuki |first3=N |last4=Soma |first4=G |last5=Inoue |first5=M |year=2009 |title=High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer |journal=Anticancer Research |volume=29 |issue=3 |pages=809–15 |pmid=19414313}}</ref> Results from most clinical trials suggest that modest vitamin C supplementation alone or with other nutrients offers no benefit in the prevention of cancer.<ref name="Oncologist">{{Cite journal |last1=Jacobs |first1=Carmel |last2=Hutton |first2=Brian |last3=Ng |first3=Terry |last4=Shorr |first4=Risa |last5=Clemons |first5=Mark |date=2015 |title=Is There a Role for Oral or Intravenous Ascorbate (Vitamin C) in Treating Patients With Cancer? A Systematic Review |journal=The Oncologist |volume=20 |issue=2 |pages=210–223 |doi=10.1634/theoncologist.2014-0381 |pmc=4319640 |pmid=25601965}}</ref><ref name="NIHFactsheet">{{Cite web |title=Vitamin C Fact Sheet for Health Professionals |url=http://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/ |access-date=June 2, 2015 |website=National Institutes of Health}}</ref> |
|||
== Personal life == |
|||
[[File:Linus Pauling family 1954.jpg|thumb|300px|The Pauling children at a gathering in celebration of the 1954 Nobel Prizes in Stockholm, Sweden. Seated from left: Linus Pauling, Jr., Peter Pauling and Linda Pauling. Standing from left: an unidentified person, and Crellin Pauling]] |
|||
Pauling married [[Ava Helen Pauling|Ava Helen Miller]] on June 17, 1923. The marriage lasted until her death in 1981. They had four children.<ref name="family">{{Cite web |date=n.d. |title=The Linus Pauling Papers: Biographical Information |url=https://profiles.nlm.nih.gov/ps/retrieve/Narrative/MM/p-nid/53 |access-date=November 10, 2011 |publisher=United States National Library of Medicine}}</ref> Linus Carl Jr. (1925–2023) became a [[psychiatry|psychiatrist]];<ref>{{cite news|title=Dr. Linus Carl Pauling Jr. Obituary|newspaper=Honolulu Star-Advertiser|date=November 5, 2023|url=https://hawaiiobituaries.com/us/obituaries/hawaiiobituaries/name/linus-pauling-obituary?id=53515681}}</ref> Peter (1931–2003) a [[crystallography|crystallographer]] at [[University College London]];<ref>{{cite book|chapter=Chapter 17. ''Peter Pauling'' by Matt McConnell|pages=203–240|chapter-url=https://books.google.com/books?id=twuhEAAAQBAJ&pg=PA203 | title=Visions of Linus Pauling | isbn=978-981-12-6077-3 | editor=Petersen, Christoffer Eric | date=11 October 2022 | publisher=World Scientific }}</ref> Edward Crellin (1937–1997) a [[biologist]];<ref>{{cite web|url=https://www.reed.edu/reed-magazine/in-memoriam/obituaries/november1997/e-crellin-pauling-1959.html |title=Obituary. E. Crellin Pauling '59 |date=November 1997|website=Reed Magazine, Reed College}}</ref> and Linda Helen (born 1932) married noted Caltech geologist and glaciologist [[Barclay Kamb]].<ref>{{Cite web |title=Linus Pauling Biography |url=http://lpi.oregonstate.edu/lpbio/lpbio2.html |access-date=November 10, 2011 |publisher=Linus Pauling Institute}}</ref> |
|||
Pauling was raised as a member of the [[Lutheran]] Church,<ref name="AIPOralHistory">{{Cite web |title=Oral history interview with Linus Carl Pauling, 1964 March 27 |url=http://www.aip.org/history/ohilist/3448.html |access-date=May 27, 2015 |website=American Institute of Physics |archive-date=2014-08-06 |archive-url=https://web.archive.org/web/20140806110327/http://www.aip.org/history/ohilist/3448.html |url-status=dead }}</ref> but later joined the [[Unitarian Universalist]] Church.<ref name="UUBio">{{Cite web |title=Linus Pauling |url=http://uudb.org/articles/linuspauling.html |access-date=May 27, 2015 |website=Dictionary of Unitarian & Universalist Biography |archive-date=2018-10-16 |archive-url=https://web.archive.org/web/20181016165013/http://uudb.org/articles/linuspauling.html |url-status=dead }}</ref> Two years before his death, in a published dialogue with Buddhist philosopher [[Daisaku Ikeda]], Pauling publicly declared his [[atheism]].<ref>{{Cite book |last1=Pauling |first1=Linus |url=https://archive.org/details/isbn_9780867202786/page/22 |title=A Lifelong Quest for Peace: A Dialogue |last2=Ikeda |first2=Daisaku |publisher=Jones & Bartlett |year=1992 |isbn=978-0-86720-277-9 |page=[https://archive.org/details/isbn_9780867202786/page/22 22] |quote=...I [Pauling] am not, however, militant in my atheism. The great English theoretical physicist Paul Dirac is a militant atheist. I suppose he is interested in arguing about the existence of God. I am not. It was once quipped that there is no God and Dirac is his prophet.}}</ref> |
|||
On January 30, 1960, Pauling and his wife were using a cabin about {{convert|80|mi|km}} south of [[Monterey, California|Monterey]], California, and he decided to go for a walk on a coastal trail. He got lost and tried to climb the rocky cliff, but reached a large overhanging rock about {{convert|300|ft|m|sigfig=1}} above the ocean. He decided it was safest to stay there, and meanwhile he was reported missing. He spent a sleepless night on the cliff before being found after almost 24 hours.<ref>{{Cite web |date=February 1, 1960 |title=Dr. Pauling Rescued, On a Sea Cliff 24 Hrs |url=http://scarc.library.oregonstate.edu/coll/pauling/peace/newsclips/1960n.5.html |access-date=April 22, 2018 |website=scarc.library.oregonstate.edu |publisher=New York Herald Tribune |location=Special Collections & Archives Research Center, Oregon State University Libraries |format=clipping}}</ref> |
|||
===Death and legacy=== |
|||
Pauling died of [[prostate cancer]] on August 19, 1994, at 19:20 at home in [[Big Sur]], California.<ref name="Offit" /> He was 93 years old.<ref>Goertzel and Goertzel, p. 247.</ref> A grave marker for Pauling was placed in Oswego Pioneer Cemetery in [[Lake Oswego, Oregon|Lake Oswego]], Oregon by his sister Pauline, but Pauling's ashes, along with those of his wife, were not buried there until 2005.<ref name="grave">{{Cite web |title=The Centennial: Who's Buried in Linus Pauling's Grave? |url=http://www.ci.oswego.or.us/sites/default/files/fileattachments/publicaffairs/webpage/13678/centennial_july2010.pdf |archive-url=https://ghostarchive.org/archive/20221009/http://www.ci.oswego.or.us/sites/default/files/fileattachments/publicaffairs/webpage/13678/centennial_july2010.pdf |archive-date=October 9, 2022 |url-status=live |access-date=December 26, 2012}}</ref> |
|||
Pauling's discoveries led to decisive contributions in a diverse array of areas including around 350 publications in the fields of quantum mechanics, inorganic chemistry, organic chemistry, protein structure, molecular biology, and medicine.<ref name="California">{{Cite web |title=Linus Pauling |url=http://www.californiamuseum.org/inductee/linus-pauling |access-date=June 1, 2015 |website=California Museum|date=February 17, 2012 }}</ref><ref>{{Cite web |title=Linus Pauling – Biographical |url=https://www.nobelprize.org/nobel_prizes/chemistry/laureates/1954/pauling-bio.html |access-date=October 6, 2016 |website=Nobelprize.org |publisher=Nobel Media AB 2014}}</ref> |
|||
His work on chemical bonding marks him as one of the founders of modern [[quantum chemistry]].<ref name="natureobit" /> ''The Nature of the Chemical Bond'' was the standard work for many years,<ref name="Hamilton">{{Cite book |last=Hamilton |first=Neil A. |url=https://books.google.com/books?id=tKxOpAh78IsC&pg=PA303 |title=American social leaders and activists |date=2002 |publisher=Facts On File |isbn=978-0-8160-4535-8 |location=New York |access-date=June 1, 2015}}</ref> and concepts like [[Orbital hybridisation|hybridization]] and [[electronegativity]] remain part of standard chemistry textbooks. While his [[Valence bond]] approach fell short of accounting quantitatively for some of the characteristics of molecules, such as the color of [[organometallic]] complexes, and would later be eclipsed by the [[molecular orbital theory]] of [[Robert Mulliken]], Valence Bond Theory still competes, in its modern form, with Molecular Orbital Theory and [[density functional theory]] (DFT) as a way of describing chemical phenomena.<ref>{{Cite journal |last1=Hoffmann |first1=Roald |last2=Shaik |first2=Sason |last3=Hiberty |first3=Philippe C. |year=2003 |title=A Conversation on VB vs MO Theory: A Never-Ending Rivalry? |journal=[[Accounts of Chemical Research|Acc Chem Res]] |volume=36 |issue=10 |pages=750–6 |doi=10.1021/ar030162a |pmid=14567708}}</ref> Pauling's work on crystal structure contributed significantly to the prediction and elucidation of the structures of complex minerals and compounds.<ref name=Marinacci/>{{rp|80–81}} His discovery of the alpha helix and beta sheet is a fundamental foundation for the study of protein structure.<ref name="Goertzel and Goertzel, p. 95-100" /> |
|||
[[Francis Crick]] acknowledged Pauling as the "father of [[molecular biology]]".<ref name="natureobit" /><ref>{{Cite news |date=March 1, 1986 |title=Pauling Honored by Scientists at Caltech Event |work=Los Angeles Times |agency=United Press International |url=https://www.latimes.com/archives/la-xpm-1986-03-01-me-13101-story.html |access-date=July 22, 2012}}</ref> His discovery of [[sickle cell anemia]] as a "molecular disease" opened the way toward examining genetically acquired mutations at a molecular level.<ref name=Strasser/> |
|||
The basic idea behind Pauling's spheron model is that a nucleus can be viewed as a set of "clusters of nucleons". The basic nucleon clusters include the [[deuteron]] [np], [[helion (chemistry)|helion]] [pnp], and [[tritium|triton]] [npn]. Even–even nuclei are described as being composed of clusters of [[alpha particle]]s, as has often been done for light nuclei.{{citation needed|date=April 2011}} Pauling attempted to derive the shell structure of nuclei from pure geometrical considerations related to [[Platonic solids]] rather than starting from an independent particle model as in the usual [[Nuclear shell model|shell model]]. In an interview given in 1990 Pauling commented on his model:<ref>{{cite web|url=http://www.achievement.org/autodoc/page/pau0int-9 |title=Linus Pauling Interview – page 9 / 9 – Academy of Achievement |publisher=Achievement.org |date=2008-02-29 |accessdate=2013-06-25}}</ref> |
|||
{{quote|Now recently, I have been trying to determine detailed structures of atomic nuclei by analyzing the ground state and excited state vibrational bends, as observed experimentally. From reading the physics literature, Physical Review Letters and other journals, I know that many physicists are interested in atomic nuclei, but none of them, so far as I have been able to discover, has been attacking the problem in the same way that I attack it. So I just move along at my own speed, making calculations...}} |
|||
Pauling's 1951 publication with Robert B. Corey and H. R. Branson, "The Structure of Proteins: Two Hydrogen-Bonded Helical Configurations of the Polypeptide Chain," was a key early finding in the then newly emerging field of molecular biology. This publication was honored by a Citation for Chemical Breakthrough Award from the Division of History of Chemistry of the American Chemical Society presented to the department of chemistry, Caltech, in 2017.<ref name="breakthrough">{{Cite web |title=Citations for Chemical Breakthrough Awards 2017 Awardees |url=http://www.scs.illinois.edu/~mainzv/HIST/awards/CCB-2017_Awardees.php |access-date=March 12, 2018 |website=Division of the History of Chemistry}}</ref><ref name="configurations">{{Cite journal |last1=Pauling |first1=L. |last2=Corey |first2=R. B. |last3=Branson |first3=H. R. |author3-link=Herman Branson |year=1951 |title=The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain |journal=Proceedings of the National Academy of Sciences |volume=37 |issue=4 |pages=205–11 |bibcode=1951PNAS...37..205P |doi=10.1073/pnas.37.4.205 |pmc=1063337 |pmid=14816373 |doi-access=free}}</ref> |
|||
== Legacy == |
|||
Pauling died of [[prostate cancer]] on August 19, 1994, at 19:20 at home in [[Big Sur, California]]. He was 93 years old.<ref>Goertzel and Goertzel, p. 247.</ref><ref name=obit>Linus Pauling dies at 93. ''The Oregonian'', August 20, 1994.</ref> A grave marker for him is in Oswego Pioneer Cemetery in [[Lake Oswego, Oregon]].<ref name=obit/><ref>{{Find a Grave|22440|Linus Carl Pauling}}</ref> |
|||
Pauling’s ashes, along with those of his wife, were moved from Big Sur to the Oswego Pioneer Cemetery in 2005.<ref>{{cite web |url=http://www.ci.oswego.or.us/sites/default/files/fileattachments/publicaffairs/webpage/13678/centennial_july2010.pdf |title=The Centennial: Who’s Buried in Linus Pauling’s Grave? |accessdate=26 December 2012}}</ref> |
|||
===Commemorations === |
|||
Pauling was included in a list of the 20 greatest scientists of all time by the magazine ''[[New Scientist]]'', with Albert Einstein being the only other scientist from the 20th century on the list. Pauling is notable for the diversity of his interests: quantum mechanics, inorganic chemistry, organic chemistry, protein structure, molecular biology, and medicine. In all these fields, and especially on the boundaries between them, he made decisive contributions. His work on chemical bonding marks the beginning of modern quantum chemistry, and many of his contributions like [[Orbital hybridisation|hybridization]] and [[electronegativity]] have become part of standard chemistry textbooks. While his [[valence bond]] approach fell short of accounting quantitatively for some of the characteristics of molecules, such as the [[photoelectron spectroscopy|photoelectron spectra]] of many molecules, and would later be eclipsed by the [[molecular orbital theory]] of [[Robert Mulliken]], Valence Bond Theory still competes, in its modern form, with both Molecular Orbital Theory and [[density functional theory]] (DFT) for describing the chemical phenomena.<ref>{{cite web |doi=10.1021/ar030162a |title=A Conversation on VB vs MO Theory: A Never-Ending Rivalry? |accessdate=August 5, 2007|first1=Roald |last1=Hoffmann |first2=Sason |last2=Shaik |first3=Philippe C. |last3=Hiberty |year=2003|publisher=ACS Publications|pages=750–756}}</ref> Pauling's work on crystal structure contributed significantly to the prediction and elucidation of the structures of complex minerals and compounds.{{Citation needed|date=January 2009}} His discovery of the alpha helix and beta sheet is a fundamental foundation for the study of protein structure.{{Citation needed|date=January 2009}} |
|||
Oregon State University completed construction of the $77 million, {{convert|100,000|sqft|m2|adj=on}} Linus Pauling Science Center in the late 2000s, now housing the bulk of Oregon State's chemistry classrooms, labs, and instruments.<ref>{{Cite web |title=Linus Pauling Science Center {{!}} Department of Chemistry {{!}} Oregon State University |url=http://chemistry.oregonstate.edu/lpsc |access-date=November 10, 2016 |website=chemistry.oregonstate.edu}}</ref> |
|||
On March 6, 2008, the [[United States Postal Service]] released a 41 cent stamp honoring Pauling designed by artist [[Victor Stabin]].<ref>{{Cite news |date=March 6, 2008 |title=Four Legends of American Science Now on U.S. Postage Stamps |work=United States Postal Service Postal News, Release No. 08-23 |url=https://about.usps.com/news/national-releases/2008/sr08_023.pdf |archive-url=https://ghostarchive.org/archive/20221009/https://about.usps.com/news/national-releases/2008/sr08_023.pdf |archive-date=October 9, 2022 |url-status=live}}</ref><ref name="stamp">{{Cite web |title=OSU Celebrates Linus Pauling and Release of New U.S. Postal Service Stamp |url=http://oregonstate.edu/events/stamp/ |url-status=dead |archive-url=https://web.archive.org/web/20131102050729/http://oregonstate.edu/events/stamp/ |archive-date=November 2, 2013 |access-date=February 25, 2015 |website=Oregon State University – University Events}}</ref> His description reads: "A remarkably versatile scientist, structural chemist Linus Pauling (1901–1994) won the 1954 Nobel Prize in Chemistry for determining the nature of the chemical bond linking atoms into molecules. His work in establishing the field of molecular biology; his studies of hemoglobin led to the classification of sickle cell anemia as a molecular disease."<ref name=Strasser/> The other scientists on this sheet of stamps included [[Gerty Cori]], biochemist, [[Edwin Hubble]], astronomer, and [[John Bardeen]], physicist.<ref name="stamp" /> |
|||
[[Francis Crick]] acknowledged Pauling as the "father of molecular biology"<ref>{{cite news |title=Pauling Honored by Scientists at Caltech Event |url=http://articles.latimes.com/1986-03-01/local/me-13101_1_crick |accessdate=22 July 2012 |newspaper=Los Angeles Times |date=1 March 1986 |agency=United Press International}}</ref> His discovery of [[sickle cell anemia]] as a "molecular disease" opened the way toward examining genetically acquired mutations at a molecular level.{{Citation needed|date=January 2009}} |
|||
California Governor [[Arnold Schwarzenegger]] and First Lady [[Maria Shriver]] announced on May 28, 2008, that Pauling would be inducted into the [[California Hall of Fame]], located at [[The California Museum for History, Women and the Arts]]. The induction ceremony took place December 15, 2008. Pauling's son was asked to accept the honor in his place.<ref name="CA.gov">{{Cite web |title=Governor & First Lady Participate in 2008 CA Hall of Fame Induction Ceremony |url=http://gov.ca.gov/news.php?id=11255 |url-status=dead |archive-url=https://web.archive.org/web/20150602035158/http://gov.ca.gov/news.php?id=11255 |archive-date=June 2, 2015 |access-date=June 1, 2015 |website=CA.gov}}</ref> |
|||
Pauling's work on the molecular basis of disease and its treatment is being carried on by a number of researchers, notably those at the Linus Pauling Institute, which lists a dozen principal investigators and faculty who study the role of micronutrients and phytochemicals in health and disease. |
|||
By proclamation of Gov. [[John Kitzhaber]] in the state of Oregon, February 28 has been named "Linus Pauling Day".<ref name=Notebooks/> The Linus Pauling Institute still exists, but moved in 1996 from Palo Alto, California, to Corvallis, Oregon, where it is part of the Linus Pauling Science Center at [[Oregon State University]].<ref>{{Cite web |title=Linus Pauling Institute |url=http://lpi.oregonstate.edu/ |access-date=June 25, 2013 |publisher=Lpi.oregonstate.edu}}</ref><ref name="CGT2011">{{Cite news |last=Cole |first=Gail |date=October 14, 2011 |title=Linus Pauling Science Center opens at OSU |work=Corvallis Gazette-Times |url=http://www.gazettetimes.com/news/local/linus-pauling-science-center-opens-at-osu/article_4f5a422e-f63a-11e0-af55-001cc4c03286.html |access-date=June 2, 2015}}</ref><ref name="OSUFoundation">{{Cite web |title=Linus Pauling Science Center – A Moment to Celebrate |url=http://osufoundation.org/fundraisingpriorities/facilities/lpsc/landing.htm |access-date=June 2, 2015 |website=Oregon State University Foundation |archive-date=2015-03-29 |archive-url=https://web.archive.org/web/20150329075533/http://osufoundation.org/fundraisingpriorities/facilities/lpsc/landing.htm |url-status=dead }}</ref> [[The Valley Library]] Special Collections at Oregon State University contain the Ava Helen and Linus Pauling Papers, including digitized versions of Pauling's forty-six research notebooks.<ref name="Notebooks">{{Cite web |title=Linus Pauling Research Notebooks Online |url=http://naturalscience.com/ns/news/news40.html |url-status=dead |archive-url=https://web.archive.org/web/20150905114549/http://naturalscience.com/ns/news/news40.html |archive-date=September 5, 2015 |access-date=June 1, 2015 |website=Natural Science }}</ref> |
|||
Items named after Pauling include Pauling Street in Foothill Ranch, California,<ref>The street in Foothill Ranch was once home to vitamin C/Emergen-C maker Alacer Corp. Founder Jay Patrick was a friend of Linus Pauling.</ref> Linus Pauling Drive in Hercules, California, Linus and Ava Helen Pauling Hall at [[Soka University of America]] in Aliso Viejo, California, Linus Pauling Middle School in Corvallis, Oregon, and [[Condon State Airport|Pauling Field]], a small airfield located in Condon, Oregon, where Pauling spent his youth. Additionally, the Linus Pauling Institute<ref>{{cite web|url=http://lpi.oregonstate.edu/ |title=Linus Pauling Institute |publisher=Lpi.oregonstate.edu |accessdate=2013-06-25}}</ref> and also a wing of [[The Valley Library]] at Oregon State University bear his name. There is a psychedelic rock band in Houston, Texas, named [[The Linus Pauling Quartet]]. |
|||
In 1986, Caltech commemorated Linus Pauling with a symposium and lectureship.<ref name="Zewail">{{Cite book |last=Zewail |first=Ahmed |author-link=Ahmed Zewail |url=https://books.google.com/books?id=Y3Y_4BjUj7gC&pg=PR13 |title=The Chemical Bond Structure and Dynamics |date=1992 |publisher=Elsevier Science |isbn=978-0-08-092669-8 |location=Burlington |access-date=June 1, 2015}}</ref> The Pauling Lecture series at Caltech began in 1989 with a lecture by Pauling himself. The Caltech Chemistry Department renamed room 22 of Gates Hall the Linus Pauling Lecture Hall, since Pauling spent so much time there.<ref name="Baum">{{Cite journal |last=Baum |first=Rudy |date=December 11, 1989 |title=Caltech launches Linus Pauling lecture series |journal=Chemical & Engineering News |volume=67 |issue=50 |pages=18–19 |doi=10.1021/cen-v067n050.p018a}}</ref> |
|||
The Caltech Chemistry Department renamed room 22 of Gates Hall the Linus Pauling Lecture Hall, since Linus spent so much time there. |
|||
Other places named after Pauling include Pauling Street in Foothill Ranch, California;<ref name="Factory">{{Cite news |last=Johnson |first=Greg |date=March 20, 1996 |title=Pauling Road Address Fits New Vitamin Factory to a 'C' |work=Los Angeles Times |url=https://www.latimes.com/archives/la-xpm-1996-03-20-fi-49317-story.html |access-date=June 2, 2015}}</ref> Linus Pauling Drive in Hercules, California; Linus and Ava Helen Pauling Hall at [[Soka University of America]] in Aliso Viejo, California;<ref name="Gottlieb">{{Cite news |last=Gottlieb |first=Jeff |date=August 19, 2001 |title=A New-View University |work=Los Angeles Times |url=https://www.latimes.com/archives/la-xpm-2001-aug-19-me-35925-story.html |access-date=June 1, 2015}}</ref> Linus Pauling Middle School in Corvallis, Oregon;<ref name="Woodward">{{Cite news |last=Woodward |first=Raju |date=February 29, 2012 |title=A son's tribute by Linus Pauling Jr. |work=Corvallis Gazette-Times |url=http://www.gazettetimes.com/news/local/a-son-s-tribute-by-linus-pauling-jr/article_9d0f7d7a-627f-11e1-84bb-001871e3ce6c.html |access-date=June 1, 2015}}</ref> and [[Condon State Airport|Pauling Field]], a small airfield located in Condon, Oregon, where Pauling spent his youth.<ref name="Airport">{{Cite news |date=October 19, 1988 |title=Scientist cites Condon years as influential |work=Register-Guard, Eugene, Oregon |url=https://news.google.com/newspapers?nid=1310&dat=19881019&id=8_JVAAAAIBAJ&pg=5043,4737376&hl=en |access-date=June 1, 2015}}</ref> There is a psychedelic rock band in Houston, Texas, named [[The Linus Pauling Quartet]].<ref name="Heberlein">{{Cite book |last=Heberlein |first=L. A. |url=https://books.google.com/books?id=5zV2XWD8q5sC&pg=PA81 |title=The Rough guide to internet radio |date=2002 |publisher=Rough Guides |isbn=978-1-85828-961-8 |location=London |access-date=June 1, 2015}}</ref> |
|||
[[Linus Torvalds]], developer of the [[Linux]] kernel, is named after Pauling.<ref name="moody">{{cite book | last=Moody | first=Glyn | title=Rebel Code: Linux and the Open Source Revolution | publisher=Perseus Books Group | year=2002 | page=336 | url=http://www.perseusbooksgroup.com/perseus/book_detail_redirect.do?imprintCid=BA&isbn=0-7382-0670-9 | isbn=0-7382-0670-9}}</ref> |
|||
The asteroid [[4674 Pauling]] in the inner asteroid belt, discovered by [[Eleanor F. Helin]], was named after Linus Pauling in 1991, on his 90th birthday.<ref name="Schmadel">{{Cite book |last=Schmadel |first=Lutz D. |author-link=Lutz D. Schmadel |url=https://books.google.com/books?id=aeAg1X7afOoC&pg=PA380 |title=Dictionary of minor planet names |date=2012 |publisher=Springer |isbn=978-3-642-29718-2 |edition=6th |location=Berlin |access-date=June 1, 2015}}</ref> |
|||
On March 6, 2008, the [[United States Postal Service]] released a 41 cent stamp honoring Pauling designed by artist [[Victor Stabin]].<ref>{{cite news | title=Linus Pauling stamp debuts at university | first=Kyle | last=Odegard | work=Gazette-Times | url=http://www.gtconnect.com/articles/2008/03/07/news/community/3aaa03_pauling.txt | date=March 7, 2008}}</ref> His description reads: "A remarkably versatile scientist, structural chemist Linus Pauling (1901–1994) won the 1954 Nobel Prize in Chemistry for determining the nature of the chemical bond linking atoms into molecules. His work in establishing the field of molecular biology; his studies of hemoglobin led to the classification of sickle cell anemia as a molecular disease." The other scientists on this sheet include [[Gerty Cori]], biochemist, [[Edwin Hubble]], astronomer, and John Bardeen, physicist. |
|||
[[Linus Torvalds]], developer of the [[Linux]] kernel, is named after Pauling.<ref name="moody">{{Cite book |last=Moody |first=Glyn |author-link=Glyn Moody |url=https://archive.org/details/rebelcodeinside000mood/page/336 |title=Rebel Code: Linux and the Open Source Revolution |publisher=Perseus Books Group |year=2002 |isbn=978-0-7382-0670-7 |page=[https://archive.org/details/rebelcodeinside000mood/page/336 336]}}</ref> |
|||
California Governor [[Arnold Schwarzenegger]] and First Lady [[Maria Shriver]] announced on May 28, 2008 that Pauling would be inducted into the [[California Hall of Fame]], located at [[The California Museum for History, Women and the Arts]]. The induction ceremony took place December 15, 2008. Pauling's son was asked to accept the honor in his place. |
|||
Nobel laureate [[Peter Agre]] has said that Linus Pauling inspired him.<ref>{{ |
Nobel laureate [[Peter Agre]] has said that Linus Pauling inspired him.<ref>{{Cite journal |last=Agre |first=Peter |author-link=Peter Agre |date=December 10, 2013 |title=Fifty Years Ago: Linus Pauling and the Belated Nobel Peace Prize |url=https://www.sciencediplomacy.org/sites/default/files/fifty_years_ago-linus_pauling_and_the_belated_nobel_prize_science__diplomacy.pdf |url-status=live |archive-url=https://web.archive.org/web/20140213004929/https://www.sciencediplomacy.org/letter-field/2013/fifty-years-ago-linus-pauling-and-belated-nobel-peace-prize |archive-date=February 13, 2014 |journal=[[Science & Diplomacy]] |volume=2 |issue=4}}{{cbignore}}</ref> |
||
In 2010, [[Pacific Northwest National Laboratory]] named its distinguished postdoctoral program in his honor, as the Linus Pauling Distinguished Postdoctoral Fellowship Program.<ref>{{Cite web|url=https://www.pnnl.gov/projects/linus-pauling-distinguished-postdoctoral-fellowship|title=Linus Pauling Distinguished Postdoctoral Fellowship | PNNL|website=www.pnnl.gov}}</ref> |
|||
== Quasicrystals == |
|||
Pauling was a stubborn opponent of the idea of [[quasicrystal]]s, relentlessly attacking [[Dan Shechtman|Shechtman]]; Pauling is quoted as saying "There is no such thing as quasicrystals, only quasi-scientists."<ref name=reuters20111005> |
|||
{{cite news |last=Lannin|first=Patrick |title=Ridiculed crystal work wins Nobel for Israeli |url=http://www.reuters.com/article/2011/10/05/nobel-chemistry-idUSL5E7L51U620111005 |accessdate=2011-10-22 |newspaper=Reuters |date=2011-10-05 }}</ref> For his work on quasicrystals, Shechtman was awarded the [[Nobel Prize in Chemistry]] in 2011. |
|||
== Honors and awards == |
== Honors and awards == |
||
Pauling received numerous awards and honors during his career, including the following:<ref name=" |
Pauling received numerous awards and honors during his career, including the following:<ref name="summary">{{Cite web |last=Center for Oral History |title=Linus C. Pauling |url=https://oh.sciencehistory.org/oral-histories/pauling-linus-c |website=[[Science History Institute]]}}</ref><ref name="OH" /><ref name="honors">{{Cite web |title=Linus Pauling: Awards, Honors and Medals |url=http://scarc.library.oregonstate.edu/coll/pauling/awards/ |access-date=April 25, 2013 |website=Special Collections |publisher=Oregon State University Libraries}}</ref> |
||
{{div col}} |
{{div col}} |
||
* 1931 [[ACS Award in Pure Chemistry]]<ref>{{Cite web |title=ACS Award in Pure Chemistry |url=http://www.acs.org/content/acs/en/funding-and-awards/awards/national/bytopic/acs-award-in-pure-chemistry.html |access-date=January 18, 2014 |publisher=American Chemical Society}}</ref> |
|||
*1931 [[Irving Langmuir Award]], American Chemical Society.<ref name="chemher"/><ref name=honors/> |
|||
* |
* 1931 [[Irving Langmuir Award]], American Chemical Society.<ref name="OH" /><ref name=honors/> |
||
* 1933 Elected Member of the United States [[National Academy of Sciences]]<ref>{{cite web|title=Linus Pauling |department=Member Directory |publisher=[[National Academy of Sciences]]|url=https://www.nasonline.org/member-directory/deceased-members/51785.html}}</ref> |
|||
*1946 [[Willard Gibbs Award]], Chicago section of the American Chemical Society.<ref name=honors/> |
|||
* 1936 Elected Member of the United States [[American Philosophical Society]]<ref>{{cite web|title=American Philosophical Society Member History |publisher=[[American Philosophical Society]]|url=https://search.amphilsoc.org/memhist/search?creator=pauling&title=&subject=&subdiv=&mem=&year=&year-max=&dead=&keyword=&smode=advanced}}</ref> |
|||
*1947 [[Davy Medal]], [[Royal Society]].<ref name="chemher"/><ref name=honors/> |
|||
* 1940 [[Alpha Chi Sigma]], professional chemistry fraternity.<ref name="Chi1940">{{Cite web |title=Alpha Chi Sigma Fraternity, Certificate of Membership |url=http://scarc.library.oregonstate.edu/coll/pauling/awards/1940h.1.html |access-date=May 27, 2015 |website=Special Collections & Archives Research Center. |publisher=Oregon State University Libraries}}</ref> |
|||
*1947 T. W. Richards Medal, Northeastern Section of the American Chemical Society.<ref name=honors/> |
|||
* 1941 [[William H. Nichols#Nichols' legacy|Nichols Medal]], New York Section, American Chemical Society.<ref name="OH" /> |
|||
*1948 [[Presidential Medal for Merit]] by President [[Harry S. Truman]] of the United States.<ref name="chemher"/><ref name=honors/> |
|||
* 1944 Elected Member of the [[American Academy of Arts and Sciences]]<ref>{{cite web|title=Linus Carl Pauling |date=February 9, 2023 |department=Member Directory |publisher=[[American Academy of Arts and Sciences]]|url=https://www.amacad.org/person/linus-carl-pauling}}</ref> |
|||
*1948 Elected a Foreign Member of the [[Royal Society]] of London (ForMemRS)<ref name="frs"/> |
|||
* |
* 1946 [[Willard Gibbs Award]], Chicago section of the American Chemical Society.<ref name=honors/> |
||
* |
* 1947 [[Davy Medal]], [[Royal Society]].<ref name="OH" /><ref name=honors/> |
||
* 1947 T. W. Richards Medal, Northeastern Section of the American Chemical Society.<ref name=honors/> |
|||
*1954 [[Nobel Prize in Chemistry]].<ref name="chemher"/><ref name=honors/> |
|||
* |
* 1948 [[Presidential Medal for Merit]] by President [[Harry S. Truman]] of the United States.<ref name="OH" /><ref name=honors/> |
||
* |
* 1948 Elected a Foreign Member of the [[Royal Society]] of London (ForMemRS)<ref name="frs" /> |
||
* |
* 1951 [[Gilbert N. Lewis]] medal, California section of the American Chemical Society.<ref name=honors/> |
||
* 1952 Pasteur Medal, Biochemical Society of France.<ref name="OH" /><ref>{{Cite web |title=Societe de Chimie Biologique, Louis Pasteur Medal. 1952 – Linus Pauling: Awards, Honors and Medals |url=http://scarc.library.oregonstate.edu/coll/pauling/awards/1952h.3.html |website=scarc.library.oregonstate.edu}}</ref> |
|||
*1957 [[Paul Sabatier (chemist)|Paul Sabatier]] Medal. |
|||
* 1954 [[Nobel Prize in Chemistry]].<ref name="OH" /><ref name=honors/> |
|||
*1957 Pierre Fermat Medal in Mathematics (awarded for only the sixth time in three centuries).<ref name="chemher"/><ref name=honors/><ref>[http://oregondigital.org/cdm4/item_viewer.php?CISOROOT=/pawards&CISOPTR=33&CISOBOX=1&REC=2 Pauling's awards and medals] (includes image of Fermat medal).</ref> |
|||
* 1955 Addis Medal, National Nephrosis Foundation.<ref name="OH" /><ref name=honors/> |
|||
*1957 International [[Hugo Grotius|Grotius]] Medal.<ref name="chemher"/> |
|||
* 1955 John Phillips Memorial Award, [[American College of Physicians]].<ref name="OH" /><ref name=honors/> |
|||
*1961 Humanist of the Year, [[American Humanist Association]]. |
|||
* 1956 Avogadro Medal, Italian Academy of Science.<ref name="OH" /><ref name=honors/> |
|||
*1961 [[Gandhi Peace Award]] by [[Promoting Enduring Peace]].<ref>{{cite web |title=Gandhi Peace Award |publisher=Promoting Enduring Peace |url=http://www.pepeace.org/gandhi-peace-award |accessdate=April 26, 2013}}</ref> |
|||
* 1957 [[Paul Sabatier (chemist)|Paul Sabatier]] Medal. |
|||
*1962 [[Nobel Peace Prize]].<ref name="chemher"/><ref name=honors/> |
|||
* 1957 Pierre Fermat Medal in Mathematics (awarded for only the sixth time in three centuries).<ref name="OH" /><ref name=honors/><ref>[http://oregondigital.org/cdm4/item_viewer.php?CISOROOT=/pawards&CISOPTR=33&CISOBOX=1&REC=2 Pauling's awards and medals]{{Dead link|date=January 2025 |bot=InternetArchiveBot |fix-attempted=yes }} (includes image of Fermat medal).</ref> |
|||
*1965 Medal, Academy of the Rumanian People's Republic.<ref name="chemher"/> |
|||
* |
* 1957 International [[Hugo Grotius|Grotius]] Medal.<ref name="OH" /> |
||
* 1959 [[Messenger Lectures]]hip |
|||
*1966 Silver Medal, [[Institute of France]].<ref name="chemher"/> |
|||
* 1960 Fellow, [[Royal Society of Arts]] |
|||
*1966 Supreme Peace Sponsor, World Fellowship of Religion.<ref name="chemher"/> |
|||
* 1961 Humanist of the Year, [[American Humanist Association]]. |
|||
*1967 [[Washington A. Roebling]] Medal, [[Mineralogical Society of America]].<ref name=honors/> |
|||
* 1961 [[Gandhi Peace Award]] by [[Promoting Enduring Peace]].<ref>{{Cite web |title=Gandhi Peace Award |url=http://www.pepeace.org/gandhi-peace-award |access-date=April 26, 2013 |publisher=Promoting Enduring Peace}}</ref> |
|||
*1972 [[Lenin Peace Prize]].<ref name="chemher"/> |
|||
* |
* 1962 [[Nobel Peace Prize]].<ref name="OH" /><ref name=honors/> |
||
* |
* 1965 Medal, Academy of the Rumanian People's Republic.<ref name="OH" /> |
||
* 1966 [[Linus Pauling Award]].<ref name="OH" /> |
|||
*1979 [[NAS Award in Chemical Sciences]], [[United States National Academy of Sciences|National Academy of Sciences]].<ref name="chemher"/><ref name=Chemical>{{cite web |title=NAS Award in Chemical Sciences |url=http://www.nasonline.org/site/PageServer?pagename=AWARDS_chemical_sciences |publisher=National Academy of Sciences |accessdate=February 15, 2011}}</ref> |
|||
* |
* 1966 Silver Medal, [[Institute of France]].<ref name="OH" /> |
||
* |
* 1966 Supreme Peace Sponsor, World Fellowship of Religion.<ref name="OH" /> |
||
* |
* 1967 [[Washington A. Roebling]] Medal, [[Mineralogical Society of America]].<ref name=honors/> |
||
* |
* 1972 [[Lenin Peace Prize]].<ref name="OH" /> |
||
* 1974 [[National Medal of Science]] by President [[Gerald R. Ford]] of the United States.<ref name=honors/> |
|||
*1987 Award in Chemical Education, American Chemical Society.<ref name="chemher"/> |
|||
* |
* 1978 [[Lomonosov Gold Medal]], Presidium of the Academy of the USSR.<ref name="OH" /><ref name=honors/> |
||
* 1979 Gold Medal Honoree, [[National Institute of Social Sciences]].<ref>{{Cite web |title=Gold Medal Honorees |url=http://www.socialsciencesinstitute.org/gold-medal-honorees |url-status=dead |archive-url=https://web.archive.org/web/20190702153336/http://www.socialsciencesinstitute.org/gold-medal-honorees |archive-date=July 2, 2019 |access-date=July 2, 2019 |website=[[National Institute of Social Sciences]]}}</ref> |
|||
*1990 [[Richard C. Tolman]] Medal, American Chemical Society Southern California Section.<ref name="chemher"/> |
|||
* 1979 [[NAS Award in Chemical Sciences]], [[United States National Academy of Sciences|National Academy of Sciences]].<ref name="OH" /><ref name="Chemical">{{Cite web |title=NAS Award in Chemical Sciences |url=http://www.nasonline.org/programs/awards/chemical-sciences.html |access-date=June 2, 2015 |publisher=National Academy of Sciences}}</ref> |
|||
*2008 "American Scientists" [[List of people on stamps of the United States#P|U.S. postage stamp]] series, $0.41, for his sickle cell disease work.<ref>{{cite web|title=OSU Celebrates Linus Pauling and Release of New U.S. Postal Service Stamp |work=Events |publisher=Oregon State University |url=http://oregonstate.edu/events/stamp/ |accessdate=April 25, 2013}}</ref> |
|||
* 1979 Golden Plate Award, [[American Academy of Achievement]]<ref>{{Cite web |title=Golden Plate Awardees of the American Academy of Achievement |url=https://www.achievement.org/our-history/golden-plate-awards/#science-exploration |website=www.achievement.org |publisher=[[American Academy of Achievement]]}}</ref> |
|||
* 1981 [[John K. Lattimer]] Award, [[American Urological Association]].<ref name=honors/> |
|||
* 1984 [[Priestley Medal]], American Chemical Society.<ref name="OH" /><ref name=honors/> |
|||
* 1984 Award for Chemistry, [[Arthur M. Sackler]] Foundation.<ref name="OH" /> |
|||
* 1986 [[Lavoisier Medal]] by Fondation de la Maison de la Chimie.<ref name=honors/> |
|||
* 1987 Award in Chemical Education, American Chemical Society.<ref name="OH" /> |
|||
* 1989 [[Vannevar Bush Award]], [[National Science Board]].<ref name="OH" /><ref name=honors/> |
|||
* 1990 [[Richard C. Tolman]] Medal, American Chemical Society Southern California Section.<ref name="OH" /> |
|||
* 1992 [[Daisaku Ikeda]] Medal, [[Soka Gakkai International]]<ref name=honors/> |
|||
* 2008 "American Scientists" [[List of people on stamps of the United States#P|U.S. postage stamp]] series, $0.41, for his sickle cell disease work.<ref>{{Cite web |title=OSU Celebrates Linus Pauling and Release of New U.S. Postal Service Stamp |url=http://oregonstate.edu/events/stamp/ |url-status=dead |archive-url=https://web.archive.org/web/20131102050729/http://oregonstate.edu/events/stamp/ |archive-date=November 2, 2013 |access-date=April 25, 2013 |website=Events |publisher=Oregon State University}}</ref> |
|||
{{div col end}} |
{{div col end}} |
||
== Publications == |
== Publications == |
||
===Books=== |
===Books=== |
||
{{Library resources box|by=yes|onlinebooksby=yes|viaf= 91892412}} |
|||
*{{cite book |last= Pauling |first= Linus |authormask=— |year= 1939 |title= The Nature of the Chemical Bond and the Structure of Molecules and Crystals |publisher= Cornell University Press}} |
|||
* {{Cite book |last1=Pauling |first1=Linus |title=Introduction to Quantum Mechanics with Applications to Chemistry |last2=Wilson |first2=E. B. |publisher=Reprinted by [[Dover Publications]] |year=1985 |isbn=978-0-486-64871-2 |author-mask=2 |orig-year=Originally published in 1935}} |
|||
*{{cite book |last= Pauling |first= Linus |authormask=— |year= 1947 |title= General Chemistry: An Introduction to Descriptive Chemistry and Modern Chemical Theory |publisher= W. H. Freeman}} |
|||
*{{ |
* {{Cite book |last=Pauling |first=Linus |title=The Nature of the Chemical Bond and the Structure of Molecules and Crystals |publisher=[[Cornell University Press]] |year=1939 |author-mask=——}} |
||
* {{Cite book |last=Pauling |first=Linus |title=General Chemistry: An Introduction to Descriptive Chemistry and Modern Chemical Theory |publisher=[[W. H. Freeman and Company|Freeman]] |year=1947 |author-mask=——}} |
|||
**[http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/bond/index.html Manuscript notes and typescripts (clear images)] |
|||
** Greatly revised and expanded in 1947, 1953, and 1970. Reprinted by [[Dover Publications]] in 1988. |
|||
*{{cite book|last=Pauling|first=Linus|author-mask=1|title=No more war!|year=1958|publisher=Dodd, Mead & Co|isbn=978-1124119663}} |
|||
* {{Cite journal |last1=Pauling |first1=Linus |title=The Architecture of Molecules |last2=Hayward |first2=Roger |journal=[[Proceedings of the National Academy of Sciences of the United States of America|Proceedings of the National Academy of Sciences]] |publisher=[[W. H. Freeman and Company|Freeman]] |year=1964 |isbn=978-0-7167-0158-3 |volume=51 |location=San Francisco |pages=977–84 |doi=10.1073/pnas.51.5.977 |pmc=300194 |pmid=16591181 |author-mask=2 |doi-access=free |issue=5|bibcode=1964PNAS...51..977P }} |
|||
*{{cite book |last= Pauling |first= Linus |authormask=— |year= 1977 |title= Vitamin C, the Common Cold and the Flu |publisher= W.H. Freeman |isbn= 0-7167-0360-2}} |
|||
** [http://scarc.library.oregonstate.edu/coll/pauling/bond/index.html Manuscript notes and typescripts (clear images)] |
|||
*{{cite book |last= Pauling |first= Linus |authormask=— |year= 1987 |title= How to Live Longer and Feel Better |publisher= Avon |isbn= 0-380-70289-4}} |
|||
*{{ |
* {{Cite book |last=Pauling |first=Linus |title=No more war! |publisher=Dodd, Mead & Co |year=1958 |isbn=978-1-124-11966-3 |author-mask=2}} |
||
*{{ |
* {{Cite book |last=Pauling |first=Linus |title=Vitamin C, the Common Cold and the Flu |publisher=[[W. H. Freeman and Company|Freeman]] |year=1977 |isbn=978-0-7167-0360-0 |author-mask=2}} |
||
*{{ |
* {{Cite book |last=Pauling |first=Linus |title=How to Live Longer and Feel Better |publisher=Avon |year=1987 |isbn=978-0-380-70289-3 |author-mask=2}} |
||
* {{Cite book |last1=Cameron |first1=E. |author1-link=Ewan Cameron |title=Cancer and Vitamin C: A Discussion of the Nature, Causes, Prevention, and Treatment of Cancer With Special Reference to the Value of Vitamin C |last2=Pauling |first2=Linus |publisher=Camino |year=1993 |isbn=978-0-940159-21-1 |author-mask2=2}} |
|||
*{{cite book |last= Hoffer |first= Abram |last2= Pauling |first2= Linus |authormask2=— |title=Healing Cancer: Complementary Vitamin & Drug Treatments |year= 2004 |publisher= CCNM Press |location= Toronto |isbn= 978-1897025116}} |
|||
*{{ |
* {{Cite book |last=Pauling |first=Linus |title=Linus Pauling On Peace: A Scientist Speaks Out on Humanism and World Survival |publisher=Rising Star Press |year=1998 |isbn=978-0-933670-03-7 |author-mask=2}} |
||
* {{Cite book |last1=Hoffer |first1=Abram |title=Healing Cancer: Complementary Vitamin & Drug Treatments |last2=Pauling |first2=Linus |publisher=CCNM Press |year=2004 |isbn=978-1-897025-11-6 |location=Toronto |author-mask2=2}} |
|||
* {{Cite book |last1=Ikeda |first1=Daisaku |title=A Lifelong Quest for Peace: A Dialogue |last2=Pauling |first2=Linus |publisher=I. B. Tauris |others=Richard L. Gage (ed., trans.) |year=2008 |isbn=978-1-84511-889-1 |location=London |author-mask2=2}} |
|||
===Journal articles=== |
===Journal articles=== |
||
* {{Cite journal |last=Pauling |first=L. |author-mask=2 |year=1927 |title=The Theoretical Prediction of the Physical Properties of Many-Electron Atoms and Ions. Mole Refraction, Diamagnetic Susceptibility, and Extension in Space |journal=[[Proceedings of the Royal Society|Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences]] |volume=114 |issue=767 |pages=181–211 |bibcode=1927RSPSA.114..181P |doi=10.1098/rspa.1927.0035 |doi-access=free}} |
|||
*{{cite doi|10.1098/rspa.1927.0035}} |
|||
* {{Cite journal |last=Pauling |first=L. |author-mask=2 |year=1929 |title=The Principles Determining the Structure of Complex Ionic Crystals |journal=Journal of the American Chemical Society |volume=51 |issue=4 |pages=1010–1026 |doi=10.1021/ja01379a006|bibcode=1929JAChS..51.1010P }} |
|||
*{{cite doi|10.1021/ja01379a006}} |
|||
* {{Cite journal |last=Pauling |first=L. |author-mask=2 |year=1931 |title=The Nature of the Chemical Bond. I. Application of Results Obtained from the Quantum Mechanics and from a Theory of Paramagnetic Susceptibility to the Structure of Molecules |journal=Journal of the American Chemical Society |volume=53 |issue=4 |pages=1367–1400 |doi=10.1021/ja01355a027|bibcode=1931JAChS..53.1367P }} |
|||
*{{cite doi|10.1021/ja01355a027}} |
|||
* {{Cite journal |last=Pauling |first=L. |author-mask=2 |year=1931 |title=The Nature of the Chemical Bond. II. The One-Electron Bond and the Three-Electron Bond |journal=Journal of the American Chemical Society |volume=53 |issue=9 |pages=3225–3237 |doi=10.1021/ja01360a004|bibcode=1931JAChS..53.3225P }} |
|||
*{{cite doi|10.1021/ja01360a004}} |
|||
* {{Cite journal |last=Pauling |first=L. |author-mask=2 |year=1932 |title=The Nature of the Chemical Bond. III. The Transition from One Extreme Bond Type to Another |journal=Journal of the American Chemical Society |volume=54 |issue=3 |pages=988–1003 |doi=10.1021/ja01342a022|bibcode=1932JAChS..54..988P }} |
|||
*{{cite doi|10.1021/ja01342a022}} |
|||
* {{Cite journal |last=Pauling |first=L. |author-mask=2 |year=1932 |title=The Nature of the Chemical Bond. IV. The Energy of Single Bonds and the Relative Electronegativity of Atoms |journal=Journal of the American Chemical Society |volume=54 |issue=9 |pages=3570–3582 |doi=10.1021/ja01348a011|bibcode=1932JAChS..54.3570P }} |
|||
*{{cite doi|10.1021/ja01348a011}} |
|||
* {{Cite journal |last1=Pauling |first1=L. |last2=Wheland |first2=G. W. |author-mask=2 |year=1933 |title=The Nature of the Chemical Bond. V. The Quantum-Mechanical Calculation of the Resonance Energy of Benzene and Naphthalene and the Hydrocarbon Free Radicals |url=https://authors.library.caltech.edu/11409/1/PAUjcp33c.pdf |archive-url=https://ghostarchive.org/archive/20221009/https://authors.library.caltech.edu/11409/1/PAUjcp33c.pdf |archive-date=October 9, 2022 |url-status=live |journal=The Journal of Chemical Physics |volume=1 |issue=6 |pages=362 |bibcode=1933JChPh...1..362P |doi=10.1063/1.1749304}} |
|||
*{{cite doi|10.1063/1.1749304 }} |
|||
* {{Cite journal |last=Pauling |first=L. |author-mask=2 |year=1935 |title=The Structure and Entropy of Ice and of Other Crystals with Some Randomness of Atomic Arrangement |journal=Journal of the American Chemical Society |volume=57 |issue=12 |pages=2680–2684 |doi=10.1021/ja01315a102|bibcode=1935JAChS..57.2680P }} |
|||
*{{cite doi|10.1021/ja01315a102}} |
|||
* {{Cite journal |last=Pauling |first=L. |author-mask=2 |year=1940 |title=A Theory of the Structure and Process of Formation of Antibodies* |journal=Journal of the American Chemical Society |volume=62 |issue=10 |pages=2643–2657 |doi=10.1021/ja01867a018|bibcode=1940JAChS..62.2643P }} |
|||
*{{cite doi|10.1021/ja01867a018}} |
|||
* {{Cite journal |last=Pauling |first=L. |author-mask=2 |year=1947 |title=Atomic Radii and Interatomic Distances in Metals |journal=Journal of the American Chemical Society |volume=69 |issue=3 |pages=542–553 |doi=10.1021/ja01195a024|bibcode=1947JAChS..69..542P }} |
|||
*{{cite doi|10.1021/ja01195a024}} |
|||
* {{Cite journal |last1=Pauling |first1=L. |last2=Itano |first2=H. A. |last3=Singer |first3=S. J. |author3-link=Seymour Jonathan Singer |last4=Wells |first4=I. C. |author-mask=2 |year=1949 |title=Sickle Cell Anemia, a Molecular Disease |journal=Science |volume=110 |issue=2865 |pages=543–548 |bibcode=1949Sci...110..543P |doi=10.1126/science.110.2865.543 |pmid=15395398|s2cid=31674765 }} |
|||
*{{cite doi|10.1126/science.110.2865.543}} |
|||
* {{Cite journal |last1=Pauling |first1=L. |last2=Corey |first2=R. B. |last3=Branson |first3=H. R. |author3-link=Herman Branson |author-mask=2 |year=1951 |title=The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain |journal=Proceedings of the National Academy of Sciences |volume=37 |issue=4 |pages=205–11 |bibcode=1951PNAS...37..205P |doi=10.1073/pnas.37.4.205 |pmc=1063337 |pmid=14816373 |doi-access=free}} |
|||
*{{cite doi|10.1073/pnas.37.4.205}} |
|||
== |
==See also== |
||
*[[List of peace activists]] |
* [[List of peace activists]] |
||
* [[Niacin (substance)|Niacin]] |
|||
*[[:File:1920 census Pauling.gif|1920 US Census]] with Pauling in Portland, Oregon. |
|||
* [[Nobel disease]] |
|||
==References== |
==References== |
||
===Citations=== |
|||
{{reflist|2}} |
|||
{{reflist}} |
|||
===Bibliography=== |
|||
{{Refbegin}} |
|||
* {{Cite book |last=Hager |first=Thomas |author-link=Thomas Hager |url=https://archive.org/details/linuspaulingchem00hage |title=Linus Pauling and the Chemistry of Life |publisher=Oxford University Press |year=1998 |isbn=978-0-19-513972-3 |via=[[Internet Archive]]}} |
|||
* {{Cite book |last1=Marinacci |first1=Barbara |title=Linus Pauling on Peace |last2=Krishnamurthy |first2=Ramesh |publisher=Rising Star Press |year=1998 |isbn=978-0-933670-03-7}} |
|||
* {{Cite book |last=Serafini |first=Anthony |url=https://archive.org/details/linuspaulingmana00sera |title=Linus Pauling: A Man and His Science |date=1989 |publisher=Paragon House |isbn=978-1-55778-440-7 |via=[[Internet Archive]]}} |
|||
{{Refend}} |
|||
== General and cited references == |
|||
==Notes== |
|||
{{Refbegin}} |
{{Refbegin}} |
||
*{{ |
* {{Cite book |last=Hargittai |first=István |title=Candid Science: Conversations with Famous Chemists |publisher=Imperial College Press |year=2000 |isbn=978-1-86094-151-1 |editor-last=Hargittai |editor-first=Magdolna |edition=Reprinted |location=London}} |
||
* {{Cite book |title=Linus Pauling: In His Own Words: Selected Writings, Speeches, and Interviews |publisher=Simon & Schuster |others=Introduction by Linus Pauling |year=1995 |isbn=978-0-684-81387-5 |editor-last=Marinacci |editor-first=Barbara |location=New York}} [https://archive.org/details/linuspaulinginhi0000paul online] |
|||
*{{cite book | last=Hager | first=Thomas | year=1995 | title=Force of Nature: The Life of Linus Pauling | publisher=Simon & Schuster | isbn=0-684-80909-5}} |
|||
* Pauling, Linus. '' Selected Scientific Papers Vol. II'' [https://archive.org/details/linus-pauling-selected-scientific-papers-vol-ii online] |
|||
*{{cite book | last=Hager | first=Thomas |authormask= — | year=1998 | title=Linus Pauling and the Chemistry of Life | publisher=Oxford University Press | isbn=0-19-513972-0}} |
|||
* {{Cite book |last=Sturchio |first=Jeffrey L. |url=https://oh.sciencehistory.org/sites/default/files/pauling_l_0067_suppl.pdf |title=Linus C. Pauling, Transcript of an Interview Conducted by Jeffrey L. Sturchio in Denver, Colorado on 6 April 1987 |date=April 6, 1987 |publisher=[[Chemical Heritage Foundation]] |location=Philadelphia, PA}} |
|||
*{{cite book | last=Marinacci | first=Barbara | first2=Ramesh |last2= Krishnamurthy | year=1998 | title=Linus Pauling on Peace | publisher=Rising Star Press | isbn=0-933670-03-6}} |
|||
*{{cite book | editor-last=Mead | editor-first=Clifford |editor-first2=Thomas |editor-last2= Hager | year=2001 | title=Linus Pauling: Scientist and Peacemaker | publisher=Oregon State University Press | isbn=0-87071-489-9}} |
|||
*{{cite book | last=Serafini | first=Anthony | year=1989 | title=Linus Pauling: A Man and His Science | publisher=Paragon House | isbn=1-55778-440-X}} |
|||
{{Refend}} |
{{Refend}} |
||
== Further reading == |
== Further reading == |
||
{{Refbegin}} |
{{Refbegin}} |
||
*{{ |
* {{Cite book |last=Coffey |first=Patrick |title=Cathedrals of Science: The Personalities and Rivalries That Made Modern Chemistry |publisher=Oxford University Press |year=2008 |isbn=978-0-19-532134-0}} |
||
*{{ |
* {{Cite journal |last=Davenport |first=Derek A. |year=1996 |title=The Many Lives of Linus Pauling: A Review of Reviews |journal=Journal of Chemical Education |volume=73 |issue=9 |pages=A210 |bibcode=1996JChEd..73A.210D |doi=10.1021/ed073pA210 |doi-access=free}} |
||
* Gormley, Melinda. "The first ‘molecular disease’: a story of Linus Pauling, the intellectual patron." ''Endeavour'' 31.2 (2007): 71–77 [http://gerald.monard.free.fr/MC/m2bio/sickle-cell-anemia-1.pdf online] {{Webarchive|url=https://web.archive.org/web/20201031085636/http://gerald.monard.free.fr/MC/m2bio/sickle-cell-anemia-1.pdf |date=2020-10-31 }}. |
|||
*{{cite book|last=Hargittai|first=István|title=Candid science: conversations with famous chemists|year=2000|publisher=Imperial College Press|location=London|isbn=978-1860941511|edition=Reprinted |editor-first= Magdolna |editor-last= Hargittai}} |
|||
* Mead, Clifford. ''Linus Pauling: Scientist and Peacemaker'' (2008) |
|||
*{{cite book|editor-last=Marinacci|editor-first=Barbara|title=Linus Pauling: In His Own Words; Selected Writings, Speeches, and Interviews |year=1995|publisher=Simon & Schuster|location=New York|isbn=978-0684813875 |others= Introduction by Linus Pauling}} |
|||
* Morgan, G. J. "Emile Zuckerkandl, Linus Pauling, and the molecular evolutionary clock, 1959–1965." ''Journal of the History of Biology'' (1998) 155–178. |
|||
*{{cite interview |last= Pauling |first= Linus |subjectlink= |url= http://www.chemheritage.org/discover/collections/oral-histories/details/pauling-linus-c.aspx |interviewer= Jeffrey L. Sturchio |title= Linus Pauling |callsign = |location= Denver | publication-place= Philadelphia |publisher= Chemical Heritage Foundation |date= April 6, 1987 <!--|publication-date= 1993--> |id= Oral History Transcript # 0067 |accessdate=}} |
|||
* Nakamura, Jeanne, and Mihaly Csikszentmihalyi. "Catalytic creativity: The case of Linus Pauling." ''American Psychologist'' 56.4 (2001): 337+. |
|||
* Strasser, Bruno J. "A world in one dimension: Linus Pauling, Francis Crick and the central dogma of molecular biology." ''History and philosophy of the life sciences'' (2006): 491–512 [https://www.researchgate.net/profile/Bruno_Strasser/publication/5501485_A_world_in_one_dimension_Linus_Pauling_Francis_Crick_and_the_central_dogma_of_molecular_biology/links/00b4951e59db7402f4000000.pdf online]. |
|||
* Strasser, Bruno J. "Linus Pauling's “molecular diseases”: Between history and memory." ''American journal of medical genetics'' 115.2 (2002): 83–93 [http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.613.5672&rep=rep1&type=pdf online]. |
|||
* White, Florence Meiman. ''Linus Pauling Scientist and Crusader'' (1980) [https://archive.org/details/linuspaulingscie00whit online] |
|||
* Zannos, Susan. ''Linus Pauling and the chemical bond'' (2004), 48pp [https://archive.org/details/linuspaulingchem0000zann online], for secondary schools |
|||
{{Refend}} |
{{Refend}} |
||
== External links == |
== External links == |
||
{{wikiquote}} |
{{wikiquote}} |
||
{{ |
{{Commons|Linus C. Pauling}} |
||
{{Scholia|author}} |
|||
*[http://pauling.library.oregonstate.edu Linus Pauling Online] a Pauling portal created by Oregon State University Libraries |
|||
*[http:// |
* [http://scarc.library.oregonstate.edu/digitalresources/pauling/ Linus Pauling Online] a Pauling portal created by Oregon State University Libraries |
||
*[ |
* [https://web.archive.org/web/20071014174033/http://oregonstate.edu/dept/Special_Collections/subpages/ahp/1995symposium/crick.html Crick, Francis, "The Impact of Linus Pauling on Molecular Biology" (transcribed from video at the 1995 Oregon State University symposium)] |
||
* [http://scarc.library.oregonstate.edu/coll/pauling/index.html The Ava Helen and Linus Pauling Papers at the Oregon State University Libraries] |
|||
*[http://paulingcatalogue.org The Pauling Catalogue] |
|||
*[http:// |
* [https://web.archive.org/web/20190719010423/http://www.paulingcatalogue.org/ The Pauling Catalogue] |
||
* {{Cite web |last=Center for Oral History |title=Linus C. Pauling |url=https://oh.sciencehistory.org/oral-histories/pauling-linus-c |website=[[Science History Institute]]}} |
|||
*[http://www.users.muohio.edu/shermalw/honors_2001_fall/honors_papers_2001/Lowrey_LPauling.htm Linus Pauling (1901–1994)] |
|||
* {{Cite book |last=Sturchio |first=Jeffrey L. |url=https://oh.sciencehistory.org/sites/default/files/pauling_l_0067_suppl.pdf |title=Linus C. Pauling, Transcript of an Interview Conducted by Jeffrey L. Sturchio in Denver, Colorado on 6 April 1987 |date=April 6, 1987 |publisher=[[Chemical Heritage Foundation]] |location=Philadelphia, PA}} |
|||
*[http://oralhistories.library.caltech.edu/archive/00000018/00/OH_Pauling_L.pdf Caltech oral history interview] |
|||
* [http://paulingblog.wordpress.com The Pauling Blog] |
|||
*[http://globetrotter.berkeley.edu/conversations/Pauling Berkeley Conversations With History interview] |
|||
*[http:// |
* [http://www.users.muohio.edu/shermalw/honors_2001_fall/honors_papers_2001/Lowrey_LPauling.htm Linus Pauling (1901–1994)] |
||
* [http://globetrotter.berkeley.edu/conversations/Pauling Berkeley Conversations With History interview] |
|||
*[http://www25.uua.org/uuhs/duub/articles/linuspauling.html Linus Pauling from The Dictionary of Unitarian and Universalist Biography] |
|||
*[http:// |
* [http://scarc.library.oregonstate.edu/digitalresources/pauling/ Linus Pauling Centenary Exhibit] |
||
* [http://uudb.org/articles/linuspauling.html Linus Pauling from The Dictionary of Unitarian and Universalist Biography] {{Webarchive|url=https://web.archive.org/web/20181016165013/http://uudb.org/articles/linuspauling.html |date=2018-10-16 }} |
|||
*[http://lpi.oregonstate.edu/ The Linus Pauling Institute] at Oregon State University |
|||
* {{Cite web |title=It's in the Blood! A Documentary History of Linus Pauling, Hemoglobin and Sickle Cell Anemia – Special Collections & Archives Research Center – Oregon State University |url=http://scarc.library.oregonstate.edu/coll/pauling/blood/ |access-date=February 25, 2015 |website=Oregon State University Library}} |
|||
*[http://www.chemheritage.org/exhibits/ex-oral-detail.asp?ID=67&Numb=1 Pauling's CV] |
|||
* [http://lpi.oregonstate.edu/ The Linus Pauling Institute] at Oregon State University |
|||
*[http://charon.girinst.org/~zeke/test.bit.pdf Publications of Pauling] |
|||
* [https://web.archive.org/web/20070621142646/http://charon.girinst.org/~zeke/test.bit.pdf Publications of Pauling] |
|||
*[http://www.soka.edu/page.cfm?p=206 Linus and Ava Helen Pauling Hall] at Soka University of America, devoted to [[pacifism]] in global citizenship. |
|||
*[ |
* [https://profiles.nlm.nih.gov/MM/ The Linus Pauling Papers] – Profiles in Science, National Library of Medicine |
||
*[http:// |
* [http://www.opb.org/television/programs/oregonexperience/segment/linus-pauling/ Linus Pauling] {{Webarchive|url=https://web.archive.org/web/20190719010408/http://www.opb.org/television/programs/oregonexperience/segment/linus-pauling/ |date=July 19, 2019 }} Documentary produced by ''[[Oregon Public Broadcasting]]'' |
||
* [https://digital.sciencehistory.org/works/5h73px42w Oral history interview with Linus C. Pauling] from [https://digital.sciencehistory.org/ Science History Institute Digital Collections] |
|||
*{{worldcat id|id=lccn-n79-144786}} |
|||
{{s-start}} |
|||
{{s-ach}} |
|||
{{succession box |
|||
| title = [[List of Nobel laureates in Chemistry|Laureate]] of the [[Nobel Prize in Chemistry]] |
|||
| years = 1954 |
|||
| before = [[Hermann Staudinger]] |
|||
| after = [[Vincent du Vigneaud]] |
|||
}} |
|||
{{succession box |
|||
| title = [[List of Nobel Peace Prize laureates|Laureate]] of the [[Nobel Peace Prize]] |
|||
| years = 1962 |
|||
| before = [[Dag Hammarskjöld]] |
|||
| after = [[International Committee of the Red Cross|International Committee <br /> of the Red Cross]], <br /> [[International Federation of Red Cross and Red Crescent Societies|League of Red Cross Societies]] |
|||
}} |
|||
{{s-end}} |
|||
{{Linus Pauling}} |
|||
{{Nobel Peace Prize Laureates 1951–1975}} |
{{Nobel Peace Prize Laureates 1951–1975}} |
||
{{Nobel Prize in Chemistry Laureates 1951–1975}} |
{{Nobel Prize in Chemistry Laureates 1951–1975}} |
||
{{1962 Nobel Prize winners}} |
|||
{{Time Persons of the Year 1951–1975}} |
{{Time Persons of the Year 1951–1975}} |
||
{{Presidents of the American Chemical Society}} |
{{Presidents of the American Chemical Society}} |
||
{{U.S. anti-nuclear}} |
{{U.S. anti-nuclear}} |
||
{{Gandhi Peace Award laureates}} |
{{Gandhi Peace Award laureates}} |
||
{{1954 Nobel Prize winners}} |
|||
{{World Constitutional Convention call signatories}} |
|||
{{Authority control}} |
|||
{{Authority control|GND=118789953|LCCN=n/79/144786|VIAF=91892412}} |
|||
{{Persondata |
|||
| NAME =Pauling, Linus Carl |
|||
| ALTERNATIVE NAMES = |
|||
| SHORT DESCRIPTION =American biochemist and theoretical chemist, anti-nuclear testing campaigner, Nobel laureate |
|||
| DATE OF BIRTH =February 28, 1901 |
|||
| PLACE OF BIRTH =Portland, Oregon |
|||
| DATE OF DEATH =August 19, 1994 |
|||
| PLACE OF DEATH =Big Sur, California |
|||
}} |
|||
{{DEFAULTSORT:Pauling, Linus}} |
{{DEFAULTSORT:Pauling, Linus}} |
||
[[Category:1901 births]] |
[[Category:1901 births]] |
||
[[Category:1994 deaths]] |
[[Category:1994 deaths]] |
||
[[Category:20th-century American biochemists]] |
|||
[[Category:20th-century atheists]] |
|||
[[Category:American anti-war activists]] |
[[Category:American anti-war activists]] |
||
[[Category:American anti–nuclear weapons activists]] |
[[Category:American anti–nuclear weapons activists]] |
||
[[Category:American atheists]] |
[[Category:American atheists]] |
||
[[Category:American biochemists]] |
|||
[[Category:American biophysicists]] |
[[Category:American biophysicists]] |
||
[[Category:American chemists]] |
|||
[[Category:American humanists]] |
[[Category:American humanists]] |
||
[[Category:American Nobel laureates]] |
[[Category:American Nobel laureates]] |
||
[[Category:American pacifists]] |
[[Category:American pacifists]] |
||
[[Category:American people of Canadian descent]] |
|||
[[Category:American people of English descent]] |
|||
[[Category:American people of German descent]] |
[[Category:American people of German descent]] |
||
[[Category:American people of Scottish descent]] |
|||
[[Category:American physical chemists]] |
[[Category:American physical chemists]] |
||
[[Category:California Institute of Technology alumni]] |
[[Category:California Institute of Technology alumni]] |
||
[[Category:California Institute of Technology faculty]] |
[[Category:California Institute of Technology faculty]] |
||
[[Category: |
[[Category:Deaths from prostate cancer in California]] |
||
[[Category: |
[[Category:Fellows of the American Academy of Arts and Sciences]] |
||
[[Category:Fellows of the American Physical Society]] |
|||
[[Category:Fellows of the Royal Society of Edinburgh]] |
|||
[[Category:Foreign members of the Royal Society]] |
|||
[[Category:Foreign members of the Russian Academy of Sciences]] |
|||
[[Category:Foreign members of the USSR Academy of Sciences]] |
|||
[[Category:History of genetics]] |
|||
[[Category:American inorganic chemists]] |
|||
[[Category:Medal for Merit recipients]] |
[[Category:Medal for Merit recipients]] |
||
[[Category:Members of the American Chemical Society]] |
|||
[[Category:Members of the American Philosophical Society]] |
[[Category:Members of the American Philosophical Society]] |
||
[[Category: |
[[Category:Members of the Bavarian Academy of Sciences]] |
||
[[Category:Members of the French Academy of Sciences]] |
|||
[[Category:Members of the International Academy of Quantum Molecular Science]] |
|||
[[Category:Members of the Norwegian Academy of Science and Letters]] |
|||
[[Category:Members of the United States National Academy of Sciences]] |
|||
[[Category:National Medal of Science laureates]] |
[[Category:National Medal of Science laureates]] |
||
[[Category:Nobel laureates in Chemistry]] |
[[Category:Nobel laureates in Chemistry]] |
||
[[Category:Nobel laureates with multiple Nobel awards]] |
[[Category:Nobel laureates with multiple Nobel awards]] |
||
[[Category:Nobel Peace Prize laureates]] |
[[Category:Nobel Peace Prize laureates]] |
||
[[Category: |
[[Category:Oregon State University faculty]] |
||
[[Category:Orthomolecular medicine advocates]] |
|||
[[Category:People from Condon, Oregon]] |
|||
[[Category:Proceedings of the National Academy of Sciences of the United States of America editors]] |
|||
[[Category:Recipients of the Lenin Peace Prize]] |
|||
[[Category:Recipients of the Lomonosov Gold Medal]] |
[[Category:Recipients of the Lomonosov Gold Medal]] |
||
[[Category:Theoretical chemists]] |
[[Category:Theoretical chemists]] |
||
[[Category:Time Person of the Year]] |
|||
[[Category:Vannevar Bush Award recipients]] |
[[Category:Vannevar Bush Award recipients]] |
||
[[Category:Guggenheim Fellows]] |
|||
[[Category:People associated with the Human Potential Movement]] |
|||
[[Category:Washington High School (Portland, Oregon) alumni]] |
[[Category:Washington High School (Portland, Oregon) alumni]] |
||
[[Category: |
[[Category:World Constitutional Convention call signatories]] |
||
[[Category:Writers from Portland, Oregon]] |
|||
[[Category:Members of Phi Kappa Phi]] |
|||
{{Link FA|es}} |
|||
[[Category:Fellows of the Royal Society of Arts]] |
|||
{{Link FA|pt}} |
|||
[[Category:Delta Upsilon members]] |
|||
{{Link GA|ja}} |
|||
Latest revision as of 07:32, 5 January 2025
Linus Carl Pauling FRS (/ˈpɔːlɪŋ/ PAW-ling; February 28, 1901 – August 19, 1994)[4] was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific topics.[5] New Scientist called him one of the 20 greatest scientists of all time.[6] For his scientific work, Pauling was awarded the Nobel Prize in Chemistry in 1954. For his peace activism, he was awarded the Nobel Peace Prize in 1962. He is one of five people to have won more than one Nobel Prize (the others being Marie Curie, John Bardeen, Frederick Sanger, and Karl Barry Sharpless).[7] Of these, he is the only person to have been awarded two unshared Nobel Prizes,[8] and one of two people to be awarded Nobel Prizes in different fields, the other being Marie Curie.[7]
Pauling was one of the founders of the fields of quantum chemistry and molecular biology.[9] His contributions to the theory of the chemical bond include the concept of orbital hybridisation and the first accurate scale of electronegativities of the elements. Pauling also worked on the structures of biological molecules, and showed the importance of the alpha helix and beta sheet in protein secondary structure. Pauling's approach combined methods and results from X-ray crystallography, molecular model building, and quantum chemistry. His discoveries inspired the work of Rosalind Franklin, James Watson, Francis Crick, and Maurice Wilkins on the structure of DNA, which in turn made it possible for geneticists to crack the DNA code of all organisms.[10]
In his later years, he promoted nuclear disarmament, as well as orthomolecular medicine, megavitamin therapy,[11] and dietary supplements, especially ascorbic acid (commonly known as Vitamin C). None of his ideas concerning the medical usefulness of large doses of vitamins have gained much acceptance in the mainstream scientific community.[6][12] He was married to the American human rights activist Ava Helen Pauling.
Early life and education
[edit]
Linus Carl Pauling was born on February 28, 1901, in Portland, Oregon,[13][14] the firstborn child of Herman Henry William Pauling (1876–1910) and Lucy Isabelle "Belle" Darling (1881–1926).[15]: 22 He was named "Linus Carl", in honor of Lucy's father, Linus, and Herman's father, Carl.[16]: 8 His ancestry included German and English.[17][18]
In 1902, after his sister Pauline was born, Pauling's parents decided to move out of Portland to find more affordable and spacious living quarters than their one-room apartment.[19]: 4 Lucy stayed with her husband's parents in Oswego until Herman brought the family to Salem, where he worked briefly as a traveling salesman for the Skidmore Drug Company. Within a year of Lucile's birth in 1904, Herman Pauling moved his family to Oswego, Oregon where he opened his own drugstore.[19]: 4 He moved his family to Condon, Oregon, in 1905.[19]: 5 By 1906, Herman Pauling was suffering from recurrent abdominal pain. He died of a perforated ulcer on June 11, 1910, leaving Lucy to care for Linus, Lucile and Pauline.[16]: 9
Pauling attributes his interest in becoming a chemist to being amazed by experiments conducted by a friend, Lloyd A. Jeffress, who had a small chemistry lab kit.[19]: 17 He later wrote: "I was simply entranced by chemical phenomena, by the reactions in which substances, often with strikingly different properties, appear; and I hoped to learn more and more about this aspect of the world."[20]
In high school, Pauling conducted chemistry experiments by scavenging equipment and material from an abandoned steel plant. With an older friend, Lloyd Simon, Pauling set up Palmon Laboratories in Simon's basement. They approached local dairies offering to perform butterfat samplings at cheap prices but dairymen were wary of trusting two boys with the task, and the business ended in failure.[19]: 21
At age 15, the high school senior had enough credits to enter Oregon State University (OSU), known then as Oregon Agricultural College.[19]: 22 Lacking two American history courses required for his high school diploma, Pauling asked the school principal if he could take the courses concurrently during the spring semester. Denied, he left Washington High School in June without a diploma.[15]: 48 The school awarded him an honorary diploma 45 years later, after he was awarded two Nobel Prizes.[7][21][22]
Pauling held a number of jobs to earn money for his future college expenses, including working part-time at a grocery store for US$8 per week (equivalent to US$220 in 2023). His mother arranged an interview with the owner of a number of manufacturing plants in Portland, Mr. Schwietzerhoff, who hired him as an apprentice machinist at a salary of US$40 per month (equivalent to US$1,120 in 2023). This was soon raised to US$50 per month.[19]: 23 Pauling also set up a photography laboratory with two friends.[19]: 24 In September 1917, Pauling was finally admitted by Oregon State University. He immediately resigned from the machinist's job and informed his mother, who saw no point in a university education, of his plans.[19]: 25
Higher education
[edit]
In his first semester, Pauling registered for two courses in chemistry, two in mathematics, mechanical drawing, introduction to mining and use of explosives, modern English prose, gymnastics and military drill.[19]: 26 His roommate was childhood pal and lifelong best friend Lloyd Jeffress.[23] He was active in campus life and founded the school's chapter of the Delta Upsilon fraternity.[24] After his second year, he planned to take a job in Portland to help support his mother. The college offered him a position teaching quantitative analysis, a course he had just finished taking himself. He worked forty hours a week in the laboratory and classroom and earned US$100 a month (equivalent to US$1,500 in 2023), enabling him to continue his studies.[19]: 29
In his last two years at school, Pauling became aware of the work of Gilbert N. Lewis and Irving Langmuir on the electronic structure of atoms and their bonding to form molecules.[19]: 29 He decided to focus his research on how the physical and chemical properties of substances are related to the structure of the atoms of which they are composed, becoming one of the founders of the new science of quantum chemistry.[citation needed]
Engineering professor Samuel Graf (1887–1966)[25][26] selected Pauling to be his teaching assistant in a mechanics and materials course.[19]: 29 [27][28] During the winter of his senior year, Pauling taught a chemistry course for home economics majors. It was in one of these classes that Pauling met his future wife, Ava Helen Miller.[19]: 31 [28]: 41 [29][30]
In 1922, Pauling graduated with a degree in chemical engineering. He went on to graduate school at the California Institute of Technology (Caltech) in Pasadena, California, under the guidance of Roscoe Dickinson and Richard Tolman.[1] His graduate research involved the use of X-ray diffraction to determine the structure of crystals. He published seven papers on the crystal structure of minerals while he was at Caltech. He received his PhD in physical chemistry and mathematical physics,[3] summa cum laude, in 1925.[31]
Career
[edit]| External videos | |
|---|---|
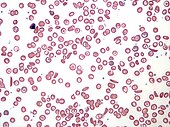 | |
In 1926, Pauling was awarded a Guggenheim Fellowship to travel to Europe, to study under German physicist Arnold Sommerfeld in Munich, Danish physicist Niels Bohr in Copenhagen and Austrian physicist Erwin Schrödinger in Zürich. All three were experts in the new field of quantum mechanics and other branches of physics.[2] Pauling became interested in how quantum mechanics might be applied in his chosen field of interest, the electronic structure of atoms and molecules. In Zürich, Pauling was also exposed to one of the first quantum mechanical analyses of bonding in the hydrogen molecule, done by Walter Heitler and Fritz London.[32] Pauling devoted the two years of his European trip to this work and decided to make it the focus of his future research. He became one of the first scientists in the field of quantum chemistry and a pioneer in the application of quantum theory to the structure of molecules.[33]
In 1927, Pauling took a new position as an assistant professor at Caltech in theoretical chemistry.[34] He launched his faculty career with a very productive five years, continuing with his X-ray crystal studies and also performing quantum mechanical calculations on atoms and molecules. He published approximately fifty papers in those five years, and created the five rules now known as Pauling's rules.[35][36] By 1929, he was promoted to associate professor, and by 1930, to full professor.[34] In 1931, the American Chemical Society awarded Pauling the Langmuir Prize for the most significant work in pure science by a person 30 years of age or younger.[37] The following year, Pauling published what he regarded as his most important paper, in which he first laid out the concept of hybridization of atomic orbitals and analyzed the tetravalency of the carbon atom.[38]
At Caltech, Pauling struck up a close friendship with theoretical physicist Robert Oppenheimer at the University of California, Berkeley, who spent part of his research and teaching schedule as a visitor at Caltech each year.[15][39] Pauling was also affiliated with Berkeley, serving as a visiting lecturer in physics and chemistry from 1929 to 1934.[40] Oppenheimer even gave Pauling a stunning personal collection of minerals.[41] The two men planned to mount a joint attack on the nature of the chemical bond: apparently Oppenheimer would supply the mathematics and Pauling would interpret the results. Their relationship soured when Oppenheimer tried to pursue Pauling's wife, Ava Helen. When Pauling was at work, Oppenheimer came to their home and blurted out an invitation to Ava Helen to join him on a tryst in Mexico. She flatly refused, and reported the incident to Pauling. He immediately cut off his relationship with Oppenheimer.[15]: 152 [39]
In the summer of 1930, Pauling made another European trip, during which he learned about gas-phase electron diffraction from Herman Francis Mark. After returning, he built an electron diffraction instrument at Caltech with a student of his, Lawrence Olin Brockway, and used it to study the molecular structure of a large number of chemical substances.[42]
Pauling introduced the concept of electronegativity in 1932.[43] Using the various properties of molecules, such as the energy required to break bonds and the dipole moments of molecules, he established a scale and an associated numerical value for most of the elements — the Pauling Electronegativity Scale — which is useful in predicting the nature of bonds between atoms in molecules.[44]
In 1936, Pauling was promoted to chairman of the division of chemistry and chemical engineering at Caltech, and to the position of director of the Gates and Crellin Laboratories of Chemistry. He would hold both positions until 1958.[34] Pauling also spent a year in 1948 at the University of Oxford as George Eastman Visiting Professor and Fellow of Balliol.[45]
Nature of the chemical bond
[edit]
In the late 1920s, Pauling began publishing papers on the nature of the chemical bond. Between 1937 and 1938, he took a position as George Fischer Baker Non-Resident Lecturer in Chemistry at Cornell University. While at Cornell, he delivered a series of nineteen lectures[46] and completed the bulk of his famous textbook The Nature of the Chemical Bond.[47][36]: Preface It is based primarily on his work in this area that he received the Nobel Prize in Chemistry in 1954 "for his research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances".[7] Pauling's book has been considered "chemistry's most influential book of this century and its effective bible".[48] In the 30 years after its first edition was published in 1939, the book was cited more than 16,000 times. Even today, many modern scientific papers and articles in important journals cite this work, more than seventy years after the first publication.[49]
Part of Pauling's work on the nature of the chemical bond led to his introduction of the concept of orbital hybridization.[50] While it is normal to think of the electrons in an atom as being described by orbitals of types such as s and p, it turns out that in describing the bonding in molecules, it is better to construct functions that partake of some of the properties of each. Thus the one 2s and three 2p orbitals in a carbon atom can be (mathematically) 'mixed' or combined to make four equivalent orbitals (called sp3 hybrid orbitals), which would be the appropriate orbitals to describe carbon compounds such as methane, or the 2s orbital may be combined with two of the 2p orbitals to make three equivalent orbitals (called sp2 hybrid orbitals), with the remaining 2p orbital unhybridized, which would be the appropriate orbitals to describe certain unsaturated carbon compounds such as ethylene.[36]: 111–120 Other hybridization schemes are also found in other types of molecules. Another area which he explored was the relationship between ionic bonding, where electrons are transferred between atoms, and covalent bonding, where electrons are shared between atoms on an equal basis. Pauling showed that these were merely extremes, and that for most actual cases of bonding, the quantum-mechanical wave function for a polar molecule AB is a combination of wave functions for covalent and ionic molecules.[36]: 66 Here Pauling's electronegativity concept is particularly useful; the electronegativity difference between a pair of atoms will be the surest predictor of the degree of ionicity of the bond.[51]
The third of the topics that Pauling attacked under the overall heading of "the nature of the chemical bond" was the accounting of the structure of aromatic hydrocarbons, particularly the prototype, benzene.[52] The best description of benzene had been made by the German chemist Friedrich Kekulé. He had treated it as a rapid interconversion between two structures, each with alternating single and double bonds, but with the double bonds of one structure in the locations where the single bonds were in the other. Pauling showed that a proper description based on quantum mechanics was an intermediate structure which was a blend of each. The structure was a superposition of structures rather than a rapid interconversion between them. The name "resonance" was later applied to this phenomenon.[53] In a sense, this phenomenon resembles those of hybridization and also polar bonding, both described above, because all three phenomena involve combining more than one electronic structure to achieve an intermediate result.[citation needed]
Ionic crystal structures
[edit]In 1929, Pauling published five rules which help to predict and explain crystal structures of ionic compounds.[54][36] These rules concern (1) the ratio of cation radius to anion radius, (2) the electrostatic bond strength, (3) the sharing of polyhedron corners, edges and faces, (4) crystals containing different cations, and (5) the rule of parsimony.[citation needed]
Biological molecules
[edit]

In the mid-1930s, Pauling, strongly influenced by the biologically oriented funding priorities of the Rockefeller Foundation's Warren Weaver, decided to strike out into new areas of interest.[55] Although Pauling's early interest had focused almost exclusively on inorganic molecular structures, he had occasionally thought about molecules of biological importance, in part because of Caltech's growing strength in biology. Pauling interacted with such great biologists as Thomas Hunt Morgan, Theodosius Dobzhanski, Calvin Bridges and Alfred Sturtevant.[56] His early work in this area included studies of the structure of hemoglobin with his student Charles D. Coryell. He demonstrated that the hemoglobin molecule changes structure when it gains or loses an oxygen molecule.[56] As a result of this observation, he decided to conduct a more thorough study of protein structure in general. He returned to his earlier use of X-ray diffraction analysis. But protein structures were far less amenable to this technique than the crystalline minerals of his former work. The best X-ray pictures of proteins in the 1930s had been made by the British crystallographer William Astbury, but when Pauling tried, in 1937, to account for Astbury's observations quantum mechanically, he could not.[57]
It took eleven years for Pauling to explain the problem: his mathematical analysis was correct, but Astbury's pictures were taken in such a way that the protein molecules were tilted from their expected positions. Pauling had formulated a model for the structure of hemoglobin in which atoms were arranged in a helical pattern, and applied this idea to proteins in general.[citation needed]
In 1951, based on the structures of amino acids and peptides and the planar nature of the peptide bond, Pauling, Robert Corey and Herman Branson correctly proposed the alpha helix and beta sheet as the primary structural motifs in protein secondary structure.[58][59] This work exemplified Pauling's ability to think unconventionally; central to the structure was the unorthodox assumption that one turn of the helix may well contain a non-integer number of amino acid residues; for the alpha helix it is 3.7 amino acid residues per turn.[citation needed]
Pauling then proposed that deoxyribonucleic acid (DNA) was a triple helix;[60][61] his model contained several basic mistakes, including a proposal of neutral phosphate groups, an idea that conflicted with the acidity of DNA. Sir Lawrence Bragg had been disappointed that Pauling had won the race to find the alpha helix structure of proteins. Bragg's team had made a fundamental error in making their models of protein by not recognizing the planar nature of the peptide bond. When it was learned at the Cavendish Laboratory that Pauling was working on molecular models of the structure of DNA, James Watson and Francis Crick were allowed to make a molecular model of DNA. They later benefited from unpublished data from Maurice Wilkins and Rosalind Franklin at King's College which showed evidence for a helix and planar base stacking along the helix axis. Early in 1953 Watson and Crick proposed a correct structure for the DNA double helix. Pauling later cited several reasons to explain how he had been misled about the structure of DNA, among them misleading density data and the lack of high quality X-ray diffraction photographs. Pauling described this situation as "the biggest disappointment in his life".[62]
During the time Pauling was researching the problem, Rosalind Franklin in England was creating the world's best images. They were key to Watson's and Crick's success. Pauling did not see them before devising his mistaken DNA structure, although his assistant Robert Corey did see at least some of them, while taking Pauling's place at a summer 1952 protein conference in England. Pauling had been prevented from attending because his passport was withheld by the State Department on suspicion that he had Communist sympathies. This led to the legend that Pauling missed the structure of DNA because of the politics of the day (this was at the start of the McCarthy period in the United States). Politics did not play a critical role. Not only did Corey see the images at the time, but Pauling himself regained his passport within a few weeks and toured English laboratories well before writing his DNA paper. He had ample opportunity to visit Franklin's lab and see her work, but chose not to.[15]: 414–415 Despite these times, Pauling chose to move on from them and be thankful for the discoveries that he had already found.[62]
Pauling also studied enzyme reactions and was among the first to point out that enzymes bring about reactions by stabilizing the transition state of the reaction, a view which is central to understanding their mechanism of action.[63] He was also among the first scientists to postulate that the binding of antibodies to antigens would be due to a complementarity between their structures.[64] Along the same lines, with the physicist turned biologist Max Delbrück, he wrote an early paper arguing that DNA replication was likely to be due to complementarity, rather than similarity, as suggested by a few researchers. This was made clear in the model of the structure of DNA that Watson and Crick discovered.[65]
Molecular genetics
[edit]
In November 1949, Pauling, Harvey Itano, S. J. Singer and Ibert Wells published "Sickle Cell Anemia, a Molecular Disease"[66] in the journal Science. It was the first proof of a human disease being caused by an abnormal protein, and sickle cell anemia became the first disease understood at the molecular level. (It was not, however, the first demonstration that variant forms of hemoglobin could be distinguished by electrophoresis, which had been shown several years earlier by Maud Menten and collaborators).[67] Using electrophoresis, they demonstrated that individuals with sickle cell disease have a modified form of hemoglobin in their red blood cells, and that individuals with sickle cell trait have both the normal and abnormal forms of hemoglobin. This was the first demonstration causally linking an abnormal protein to a disease, and also the first demonstration that Mendelian inheritance determines the specific physical properties of proteins, not simply their presence or absence – the dawn of molecular genetics.[68]
His success with sickle cell anemia led Pauling to speculate that a number of other diseases, including mental illnesses such as schizophrenia, might result from flawed genetics. As chairman of the Division of Chemistry and Chemical Engineering and director of the Gates and Crellin Chemical Laboratories, he encouraged the hiring of researchers with a chemical-biomedical approach to mental illness, a direction not always popular with established Caltech chemists.[69]: 2
In 1951, Pauling gave a lecture entitled "Molecular Medicine".[70] In the late 1950s, he studied the role of enzymes in brain function, believing that mental illness may be partly caused by enzyme dysfunction. In the 1960s, as part of his interest in the effects of nuclear weapons, he investigated the role of mutations in evolution, proposing with his student Emile Zuckerkandl, the molecular evolutionary clock, the idea that mutations in proteins and DNA accumulate at a constant rate over time .[71]
Structure of the atomic nucleus
[edit]
On September 16, 1952, Pauling opened a new research notebook with the words "I have decided to attack the problem of the structure of nuclei." On October 15, 1965, Pauling published his Close-Packed Spheron Model of the atomic nucleus in two well respected journals, Science and the Proceedings of the National Academy of Sciences.[72][73] For nearly three decades, until his death in 1994, Pauling published numerous papers on his spheron cluster model.[72][74][75][76][77][78]
The basic idea behind Pauling's spheron model is that a nucleus can be viewed as a set of "clusters of nucleons". The basic nucleon clusters include the deuteron [np], helion [pnp], and triton [npn]. Even–even nuclei are described as being composed of clusters of alpha particles, as has often been done for light nuclei.[79] Pauling attempted to derive the shell structure of nuclei from pure geometrical considerations related to Platonic solids rather than starting from an independent particle model as in the usual shell model. In an interview given in 1990 Pauling commented on his model:[80]
Now recently, I have been trying to determine detailed structures of atomic nuclei by analyzing the ground state and excited state vibrational bends, as observed experimentally. From reading the physics literature, Physical Review Letters and other journals, I know that many physicists are interested in atomic nuclei, but none of them, so far as I have been able to discover, has been attacking the problem in the same way that I attack it. So I just move along at my own speed, making calculations ...
Activism
[edit]Wartime work
[edit]
Pauling had been practically apolitical until World War II. At the beginning of the Manhattan Project, Robert Oppenheimer invited him to be in charge of the Chemistry division of the project. He declined, not wanting to uproot his family.[81]


Pauling did, however, work on research for the military. He was a principal investigator on 14 OSRD contracts.[82] The National Defense Research Committee called a meeting on October 3, 1940, wanting an instrument that could reliably measure oxygen content in a mixture of gases, so that they could measure oxygen conditions in submarines and airplanes. In response Pauling designed the Pauling oxygen meter, which was developed and manufactured by Arnold O. Beckman, Inc. After the war, Beckman adapted the oxygen analyzers for use in incubators for premature babies.[83]: 180–186 [84]
In 1942, Pauling successfully submitted a proposal on "The Chemical Treatment of Protein Solutions in the Attempt to Find a Substitute for Human Serum for Transfusions". His project group, which included Joseph B. Koepfli and Dan H. Campbell, developed a possible replacement for human blood plasma in transfusions: polyoxy gelatin (Oxypolygelatin).[85][86]
Other wartime projects with more direct military applications included work on explosives, rocket propellants and the patent for an armor-piercing shell. In October 1948, Pauling, along with Lee A. DuBridge, William A. Fowler, Max Mason, and Bruce H. Sage, was awarded a Presidential Medal for Merit by President Harry S. Truman. The citation credits him for his "imaginative mind", "brilliant success", and "exceptionally meritorious conduct in the performance of outstanding services".[87][88][89] In 1949, he served as president of the American Chemical Society.[90]
Nuclear activism
[edit]The aftermath of the Manhattan Project and his wife Ava's pacifism changed Pauling's life profoundly, and he became a peace activist.[citation needed]
In June 1945, a "May-Johnson Bill" began[91][92][93] that would become the Atomic Energy Act of 1946 (signed August 1, 1946). In November 1945, Pauling spoke to the Independent Citizens Committee of the Arts, Sciences and Professions (ICCASP) on atomic weapons; shortly after, wife Ava and he accepted membership.[94] On January 21, 1946, the group met to discuss academic freedom, during which Pauling said, "There is, of course, always a threat to academic freedom – as there is to the other aspects of the freedom and rights of the individual, in the continued attacks which are made on this freedom, these rights, by the selfish, the overly ambitious, the misguided, the unscrupulous, who seek to oppress the great body of mankind in order that they themselves may profit – and we must always be on the alert against this threat, and must fight it with vigor when it becomes dangerous."[94]
In 1946, he joined the Emergency Committee of Atomic Scientists, chaired by Albert Einstein.[95] Its mission was to warn the public of the dangers associated with the development of nuclear weapons.

His political activism prompted the US State Department to deny him a passport in 1952, when he was invited to speak at a scientific conference in London.[96][97] In a speech before the US Senate on June 6 of the same year, Senator Wayne Morse publicly denounced the action of the State Department, and urged the Passport Division to reverse its decision. Pauling and his wife Ava were then issued a "limited passport" to attend the conference.[98][99] His full passport was restored in 1954, shortly before the ceremony in Stockholm where he received his first Nobel Prize.[citation needed]
Joining Einstein, Bertrand Russell and eight other leading scientists and intellectuals, he signed the Russell-Einstein Manifesto issued July 9, 1955.[100] He also supported the Mainau Declaration of July 15, 1955, signed by 52 Nobel Prize laureates.[101]
In May 1957, working with Washington University in St. Louis professor Barry Commoner, Pauling began to circulate a petition among scientists to stop nuclear testing.[102] On January 15, 1958, Pauling and his wife presented a petition to United Nations Secretary General Dag Hammarskjöld calling for an end to the testing of nuclear weapons. It was signed by 11,021 scientists representing fifty countries.[103][104]
In February 1958, Pauling participated in a publicly televised debate with the atomic physicist Edward Teller about the actual probability of fallout causing mutations.[105] Later in 1958, Pauling published No more war!, in which he not only called for an end to the testing of nuclear weapons but also an end to war itself. He proposed that a World Peace Research Organization be set up as part of the United Nations to "attack the problem of preserving the peace".[7]
Pauling also supported the work of the St. Louis Citizen's Committee for Nuclear Information (CNI).[102] This group, headed by Barry Commoner, Eric Reiss, M. W. Friedlander and John Fowler, organized a longitudinal study to measure radioactive strontium-90 in the baby teeth of children across North America. The "Baby Tooth Survey", published by Louise Reiss, demonstrated conclusively in 1961 that above-ground nuclear testing posed significant public health risks in the form of radioactive fallout spread primarily via milk from cows that had ingested contaminated grass.[106][107][108] The Committee for Nuclear Information is frequently credited for its significant contribution to supporting the test ban,[109] as is the ground-breaking research conducted by Reiss and the "Baby Tooth Survey".[110]
Public pressure and the frightening results of the CNI research led to a moratorium on above-ground nuclear weapons testing, followed by the Partial Test Ban Treaty, signed in 1963 by John F. Kennedy and Nikita Khrushchev. On the day that the treaty went into force, October 10, 1963, the Nobel Prize Committee awarded Pauling the Nobel Peace Prize for 1962. (No prize had previously been awarded for that year.)[111] They described him as "Linus Carl Pauling, who ever since 1946 has campaigned ceaselessly, not only against nuclear weapons tests, not only against the spread of these armaments, not only against their very use, but against all warfare as a means of solving international conflicts."[112] Pauling himself acknowledged his wife Ava's deep involvement in peace work, and regretted that she was not awarded the Nobel Peace Prize with him.[113]
Political criticism
[edit]
Many of Pauling's critics, including scientists who appreciated the contributions that he had made in chemistry, disagreed with his political positions and saw him as a naïve spokesman for Soviet communism. In 1960, he was ordered to appear before the Senate Internal Security Subcommittee,[114] which termed him "the number one scientific name in virtually every major activity of the Communist peace offensive in this country".[115] A headline in Life magazine characterized his 1962 Nobel Prize as "A Weird Insult from Norway".[116][117]
Pauling was a frequent target of the National Review magazine. In an article entitled "The Collaborators" in the magazine's July 17, 1962, issue, Pauling was referred to not only as a collaborator, but as a "fellow traveler" of proponents of Soviet-style communism. In 1963, Pauling sued the magazine, its publisher William Rusher, and its editor William F. Buckley, Jr for $1 million. He lost both his libel suits and the 1968 appeal (unlike his earlier 1963 libel case against the Hearst Corporation), because in the meantime the landmark case New York Times Co. v. Sullivan had established the actual malice standard for libel lawsuits by public figures, requiring that not only falsehood but deliberate lying should be proved by the plaintiff in such cases.[118][119][120][121]
His peace activism, his frequent travels, and his enthusiastic expansion into chemical-biomedical research all aroused opposition at Caltech. In 1958, the Caltech Board of Trustees demanded that Pauling step down as chairman of the Chemistry and Chemical Engineering Division.[69]: 2 Although he had retained tenure as a full professor, Pauling chose to resign from Caltech after he received the Nobel peace prize money. He spent the next three years at the Center for the Study of Democratic Institutions (1963–1967).[20] In 1967, he moved to the University of California at San Diego, but remained there only briefly, leaving in 1969 in part because of political tensions with the Reagan-era board of regents.[69]: 3 From 1969 to 1974, he accepted a position as professor of chemistry at Stanford University.[34]
Vietnam war activism
[edit]During the 1960s, President Lyndon Johnson's policy of increasing America's involvement in the Vietnam War caused an anti-war movement that the Paulings joined with enthusiasm. Pauling denounced the war as unnecessary and unconstitutional. He made speeches, signed protest letters and communicated personally with the North Vietnamese leader, Ho Chi Minh, and gave the lengthy written response to President Johnson. His efforts were ignored by the American government.[122]
Pauling was awarded the International Lenin Peace Prize by the USSR in 1970.[115][123] He continued his peace activism in the following years. He and his wife Ava helped to found the International League of Humanists in 1974.[124] He was president of the scientific advisory board of the World Union for Protection of Life and also one of the signatories of the Dubrovnik–Philadelphia statement of 1974/1976.[125] Linus Carl Pauling was an honorary president and member of the International Academy of Science, Munich, until the end of his life.[126]
Pauling was also a supporter of the Fair Play for Cuba Committee.[127]
Global activism
[edit]He was one of the signatories of the agreement to convene a convention for drafting a world constitution.[128][129] As a result, for the first time in human history, a World Constituent Assembly convened to draft and adopt a Constitution for the Federation of Earth.[130]
Eugenics
[edit]Pauling supported a limited form of eugenics by suggesting that human carriers of defective genes be given a compulsory visible mark – such as a forehead tattoo – to discourage potential mates with the same defect, in order to reduce the number of babies with diseases such as sickle cell anemia.[131][132]
Medical research and vitamin C advocacy
[edit]
In 1941, at age 40, Pauling was diagnosed with Bright's disease, a renal disease. Following the recommendations of Thomas Addis, who actively recruited Ava Helen Pauling as "nutritionist, cook, and eventually as deputy 'doctor'", Pauling believed he was able to control the disease with Addis's then-unusual low-protein salt-free diet and vitamin supplements.[134] Thus Pauling's initial – and intensely personal – exposure to the idea of treating disease with vitamin supplements was positive.[citation needed]
In 1965, Pauling read Niacin Therapy in Psychiatry by Abram Hoffer and theorized vitamins might have important biochemical effects unrelated to their prevention of associated deficiency diseases.[135] In 1968, Pauling published a brief paper in Science entitled "Orthomolecular psychiatry",[136] giving a name to the popular but controversial megavitamin therapy movement of the 1970s, and advocating that "orthomolecular therapy, the provision for the individual person of the optimum concentrations of important normal constituents of the brain, may be the preferred treatment for many mentally ill patients." Pauling coined the term "orthomolecular" to refer to the practice of varying the concentration of substances normally present in the body to prevent and treat disease. His ideas formed the basis of orthomolecular medicine, which is not generally practiced by conventional medical professionals and has been strongly criticized.[137][138]
In 1973, with Arthur B. Robinson and another colleague, Pauling founded the Institute of Orthomolecular Medicine in Menlo Park, California, which was soon renamed the Linus Pauling Institute of Science and Medicine. Pauling directed research on vitamin C, but also continued his theoretical work in chemistry and physics until his death. In his last years, he became especially interested in the possible role of vitamin C in preventing atherosclerosis and published three case reports on the use of lysine and vitamin C to relieve angina pectoris. During the 1990s, Pauling put forward a comprehensive plan for the treatment of heart disease using lysine and vitamin C. In 1996, a website was created expounding Pauling's treatment which it referred to as Pauling Therapy. Proponents of Pauling Therapy believe that heart disease can be treated and even cured using only lysine and Vitamin C and without drugs or heart operations.[139]
Pauling's work on vitamin C in his later years generated much controversy. He was first introduced to the concept of high-dose vitamin C by biochemist Irwin Stone in 1966. After becoming convinced of its worth, Pauling took 3 grams of vitamin C every day to prevent colds.[13] Excited by his own perceived results, he researched the clinical literature and published Vitamin C and the Common Cold in 1970. He began a long clinical collaboration with the British cancer surgeon Ewan Cameron in 1971 on the use of intravenous and oral vitamin C as cancer therapy for terminal patients.[140] Cameron and Pauling wrote many technical papers and a popular book, Cancer and Vitamin C, that discussed their observations. Pauling made vitamin C popular with the public[141] and eventually published two studies of a group of 100 allegedly terminal patients that claimed vitamin C increased survival by as much as four times compared to untreated patients.[142][143]
A re-evaluation of the claims in 1982 found that the patient groups were not actually comparable, with the vitamin C group being less sick on entry to the study, and judged to be "terminal" much earlier than the comparison group.[144] Later clinical trials conducted by the Mayo Clinic led by oncologist Dr. Edward T. Creagan also concluded that high-dose (10,000 mg) vitamin C was no better than placebo at treating cancer and that there was no benefit to high-dose vitamin C.[145][146][147] The failure of the clinical trials to demonstrate any benefit resulted in the conclusion that vitamin C was not effective in treating cancer; the medical establishment concluded that his claims that vitamin C could prevent colds or treat cancer were quackery.[13][148] Pauling denounced the conclusions of these studies and handling of the final study as "fraud and deliberate misrepresentation",[149][150] and criticized the studies for using oral, rather than intravenous vitamin C[151] (which was the dosing method used for the first ten days of Pauling's original study[148]). Pauling also criticised the Mayo Clinic studies because the controls were taking vitamin C during the trial, and because the duration of the treatment with vitamin C was short; Pauling advocated continued high-dose vitamin C for the rest of the cancer patient's life whereas the Mayo Clinic patients in the second trial were treated with vitamin C for a median of 2.5 months.[152]
Ultimately the negative findings of the Mayo Clinic studies ended general interest in vitamin C as a treatment for cancer.[150] Despite this, Pauling continued to promote vitamin C for treating cancer and the common cold, working with The Institutes for the Achievement of Human Potential to use vitamin C in the treatment of brain-injured children.[153] He later collaborated with the Canadian physician Abram Hoffer on a micronutrient regime, including high-dose vitamin C, as adjunctive cancer therapy.[154] A 2009 review also noted differences between the studies, such as the Mayo Clinic not using intravenous Vitamin C, and suggested further studies into the role of vitamin C when given intravenously.[155] Results from most clinical trials suggest that modest vitamin C supplementation alone or with other nutrients offers no benefit in the prevention of cancer.[156][157]
Personal life
[edit]
Pauling married Ava Helen Miller on June 17, 1923. The marriage lasted until her death in 1981. They had four children.[158] Linus Carl Jr. (1925–2023) became a psychiatrist;[159] Peter (1931–2003) a crystallographer at University College London;[160] Edward Crellin (1937–1997) a biologist;[161] and Linda Helen (born 1932) married noted Caltech geologist and glaciologist Barclay Kamb.[162]
Pauling was raised as a member of the Lutheran Church,[163] but later joined the Unitarian Universalist Church.[164] Two years before his death, in a published dialogue with Buddhist philosopher Daisaku Ikeda, Pauling publicly declared his atheism.[165]
On January 30, 1960, Pauling and his wife were using a cabin about 80 miles (130 km) south of Monterey, California, and he decided to go for a walk on a coastal trail. He got lost and tried to climb the rocky cliff, but reached a large overhanging rock about 300 feet (90 m) above the ocean. He decided it was safest to stay there, and meanwhile he was reported missing. He spent a sleepless night on the cliff before being found after almost 24 hours.[166]
Death and legacy
[edit]Pauling died of prostate cancer on August 19, 1994, at 19:20 at home in Big Sur, California.[12] He was 93 years old.[167] A grave marker for Pauling was placed in Oswego Pioneer Cemetery in Lake Oswego, Oregon by his sister Pauline, but Pauling's ashes, along with those of his wife, were not buried there until 2005.[168]
Pauling's discoveries led to decisive contributions in a diverse array of areas including around 350 publications in the fields of quantum mechanics, inorganic chemistry, organic chemistry, protein structure, molecular biology, and medicine.[169][170]
His work on chemical bonding marks him as one of the founders of modern quantum chemistry.[9] The Nature of the Chemical Bond was the standard work for many years,[171] and concepts like hybridization and electronegativity remain part of standard chemistry textbooks. While his Valence bond approach fell short of accounting quantitatively for some of the characteristics of molecules, such as the color of organometallic complexes, and would later be eclipsed by the molecular orbital theory of Robert Mulliken, Valence Bond Theory still competes, in its modern form, with Molecular Orbital Theory and density functional theory (DFT) as a way of describing chemical phenomena.[172] Pauling's work on crystal structure contributed significantly to the prediction and elucidation of the structures of complex minerals and compounds.[28]: 80–81 His discovery of the alpha helix and beta sheet is a fundamental foundation for the study of protein structure.[59]
Francis Crick acknowledged Pauling as the "father of molecular biology".[9][173] His discovery of sickle cell anemia as a "molecular disease" opened the way toward examining genetically acquired mutations at a molecular level.[68]
Pauling's 1951 publication with Robert B. Corey and H. R. Branson, "The Structure of Proteins: Two Hydrogen-Bonded Helical Configurations of the Polypeptide Chain," was a key early finding in the then newly emerging field of molecular biology. This publication was honored by a Citation for Chemical Breakthrough Award from the Division of History of Chemistry of the American Chemical Society presented to the department of chemistry, Caltech, in 2017.[174][175]
Commemorations
[edit]Oregon State University completed construction of the $77 million, 100,000-square-foot (9,300 m2) Linus Pauling Science Center in the late 2000s, now housing the bulk of Oregon State's chemistry classrooms, labs, and instruments.[176]
On March 6, 2008, the United States Postal Service released a 41 cent stamp honoring Pauling designed by artist Victor Stabin.[177][178] His description reads: "A remarkably versatile scientist, structural chemist Linus Pauling (1901–1994) won the 1954 Nobel Prize in Chemistry for determining the nature of the chemical bond linking atoms into molecules. His work in establishing the field of molecular biology; his studies of hemoglobin led to the classification of sickle cell anemia as a molecular disease."[68] The other scientists on this sheet of stamps included Gerty Cori, biochemist, Edwin Hubble, astronomer, and John Bardeen, physicist.[178]
California Governor Arnold Schwarzenegger and First Lady Maria Shriver announced on May 28, 2008, that Pauling would be inducted into the California Hall of Fame, located at The California Museum for History, Women and the Arts. The induction ceremony took place December 15, 2008. Pauling's son was asked to accept the honor in his place.[179]
By proclamation of Gov. John Kitzhaber in the state of Oregon, February 28 has been named "Linus Pauling Day".[180] The Linus Pauling Institute still exists, but moved in 1996 from Palo Alto, California, to Corvallis, Oregon, where it is part of the Linus Pauling Science Center at Oregon State University.[181][182][183] The Valley Library Special Collections at Oregon State University contain the Ava Helen and Linus Pauling Papers, including digitized versions of Pauling's forty-six research notebooks.[180]
In 1986, Caltech commemorated Linus Pauling with a symposium and lectureship.[184] The Pauling Lecture series at Caltech began in 1989 with a lecture by Pauling himself. The Caltech Chemistry Department renamed room 22 of Gates Hall the Linus Pauling Lecture Hall, since Pauling spent so much time there.[185]
Other places named after Pauling include Pauling Street in Foothill Ranch, California;[186] Linus Pauling Drive in Hercules, California; Linus and Ava Helen Pauling Hall at Soka University of America in Aliso Viejo, California;[187] Linus Pauling Middle School in Corvallis, Oregon;[188] and Pauling Field, a small airfield located in Condon, Oregon, where Pauling spent his youth.[189] There is a psychedelic rock band in Houston, Texas, named The Linus Pauling Quartet.[190]
The asteroid 4674 Pauling in the inner asteroid belt, discovered by Eleanor F. Helin, was named after Linus Pauling in 1991, on his 90th birthday.[191]
Linus Torvalds, developer of the Linux kernel, is named after Pauling.[192]
Nobel laureate Peter Agre has said that Linus Pauling inspired him.[193]
In 2010, Pacific Northwest National Laboratory named its distinguished postdoctoral program in his honor, as the Linus Pauling Distinguished Postdoctoral Fellowship Program.[194]
Honors and awards
[edit]Pauling received numerous awards and honors during his career, including the following:[195][34][196]
- 1931 ACS Award in Pure Chemistry[197]
- 1931 Irving Langmuir Award, American Chemical Society.[34][196]
- 1933 Elected Member of the United States National Academy of Sciences[198]
- 1936 Elected Member of the United States American Philosophical Society[199]
- 1940 Alpha Chi Sigma, professional chemistry fraternity.[200]
- 1941 Nichols Medal, New York Section, American Chemical Society.[34]
- 1944 Elected Member of the American Academy of Arts and Sciences[201]
- 1946 Willard Gibbs Award, Chicago section of the American Chemical Society.[196]
- 1947 Davy Medal, Royal Society.[34][196]
- 1947 T. W. Richards Medal, Northeastern Section of the American Chemical Society.[196]
- 1948 Presidential Medal for Merit by President Harry S. Truman of the United States.[34][196]
- 1948 Elected a Foreign Member of the Royal Society of London (ForMemRS)[13]
- 1951 Gilbert N. Lewis medal, California section of the American Chemical Society.[196]
- 1952 Pasteur Medal, Biochemical Society of France.[34][202]
- 1954 Nobel Prize in Chemistry.[34][196]
- 1955 Addis Medal, National Nephrosis Foundation.[34][196]
- 1955 John Phillips Memorial Award, American College of Physicians.[34][196]
- 1956 Avogadro Medal, Italian Academy of Science.[34][196]
- 1957 Paul Sabatier Medal.
- 1957 Pierre Fermat Medal in Mathematics (awarded for only the sixth time in three centuries).[34][196][203]
- 1957 International Grotius Medal.[34]
- 1959 Messenger Lectureship
- 1960 Fellow, Royal Society of Arts
- 1961 Humanist of the Year, American Humanist Association.
- 1961 Gandhi Peace Award by Promoting Enduring Peace.[204]
- 1962 Nobel Peace Prize.[34][196]
- 1965 Medal, Academy of the Rumanian People's Republic.[34]
- 1966 Linus Pauling Award.[34]
- 1966 Silver Medal, Institute of France.[34]
- 1966 Supreme Peace Sponsor, World Fellowship of Religion.[34]
- 1967 Washington A. Roebling Medal, Mineralogical Society of America.[196]
- 1972 Lenin Peace Prize.[34]
- 1974 National Medal of Science by President Gerald R. Ford of the United States.[196]
- 1978 Lomonosov Gold Medal, Presidium of the Academy of the USSR.[34][196]
- 1979 Gold Medal Honoree, National Institute of Social Sciences.[205]
- 1979 NAS Award in Chemical Sciences, National Academy of Sciences.[34][206]
- 1979 Golden Plate Award, American Academy of Achievement[207]
- 1981 John K. Lattimer Award, American Urological Association.[196]
- 1984 Priestley Medal, American Chemical Society.[34][196]
- 1984 Award for Chemistry, Arthur M. Sackler Foundation.[34]
- 1986 Lavoisier Medal by Fondation de la Maison de la Chimie.[196]
- 1987 Award in Chemical Education, American Chemical Society.[34]
- 1989 Vannevar Bush Award, National Science Board.[34][196]
- 1990 Richard C. Tolman Medal, American Chemical Society Southern California Section.[34]
- 1992 Daisaku Ikeda Medal, Soka Gakkai International[196]
- 2008 "American Scientists" U.S. postage stamp series, $0.41, for his sickle cell disease work.[208]
Publications
[edit]Books
[edit]- ——; Wilson, E. B. (1985) [Originally published in 1935]. Introduction to Quantum Mechanics with Applications to Chemistry. Reprinted by Dover Publications. ISBN 978-0-486-64871-2.
- —— (1939). The Nature of the Chemical Bond and the Structure of Molecules and Crystals. Cornell University Press.
- —— (1947). General Chemistry: An Introduction to Descriptive Chemistry and Modern Chemical Theory. Freeman.
- Greatly revised and expanded in 1947, 1953, and 1970. Reprinted by Dover Publications in 1988.
- ——; Hayward, Roger (1964). "The Architecture of Molecules". Proceedings of the National Academy of Sciences. 51 (5). San Francisco: Freeman: 977–84. Bibcode:1964PNAS...51..977P. doi:10.1073/pnas.51.5.977. ISBN 978-0-7167-0158-3. PMC 300194. PMID 16591181.
- —— (1958). No more war!. Dodd, Mead & Co. ISBN 978-1-124-11966-3.
- —— (1977). Vitamin C, the Common Cold and the Flu. Freeman. ISBN 978-0-7167-0360-0.
- —— (1987). How to Live Longer and Feel Better. Avon. ISBN 978-0-380-70289-3.
- Cameron, E.; —— (1993). Cancer and Vitamin C: A Discussion of the Nature, Causes, Prevention, and Treatment of Cancer With Special Reference to the Value of Vitamin C. Camino. ISBN 978-0-940159-21-1.
- —— (1998). Linus Pauling On Peace: A Scientist Speaks Out on Humanism and World Survival. Rising Star Press. ISBN 978-0-933670-03-7.
- Hoffer, Abram; —— (2004). Healing Cancer: Complementary Vitamin & Drug Treatments. Toronto: CCNM Press. ISBN 978-1-897025-11-6.
- Ikeda, Daisaku; —— (2008). A Lifelong Quest for Peace: A Dialogue. Richard L. Gage (ed., trans.). London: I. B. Tauris. ISBN 978-1-84511-889-1.
Journal articles
[edit]- —— (1927). "The Theoretical Prediction of the Physical Properties of Many-Electron Atoms and Ions. Mole Refraction, Diamagnetic Susceptibility, and Extension in Space". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 114 (767): 181–211. Bibcode:1927RSPSA.114..181P. doi:10.1098/rspa.1927.0035.
- —— (1929). "The Principles Determining the Structure of Complex Ionic Crystals". Journal of the American Chemical Society. 51 (4): 1010–1026. Bibcode:1929JAChS..51.1010P. doi:10.1021/ja01379a006.
- —— (1931). "The Nature of the Chemical Bond. I. Application of Results Obtained from the Quantum Mechanics and from a Theory of Paramagnetic Susceptibility to the Structure of Molecules". Journal of the American Chemical Society. 53 (4): 1367–1400. Bibcode:1931JAChS..53.1367P. doi:10.1021/ja01355a027.
- —— (1931). "The Nature of the Chemical Bond. II. The One-Electron Bond and the Three-Electron Bond". Journal of the American Chemical Society. 53 (9): 3225–3237. Bibcode:1931JAChS..53.3225P. doi:10.1021/ja01360a004.
- —— (1932). "The Nature of the Chemical Bond. III. The Transition from One Extreme Bond Type to Another". Journal of the American Chemical Society. 54 (3): 988–1003. Bibcode:1932JAChS..54..988P. doi:10.1021/ja01342a022.
- —— (1932). "The Nature of the Chemical Bond. IV. The Energy of Single Bonds and the Relative Electronegativity of Atoms". Journal of the American Chemical Society. 54 (9): 3570–3582. Bibcode:1932JAChS..54.3570P. doi:10.1021/ja01348a011.
- ——; Wheland, G. W. (1933). "The Nature of the Chemical Bond. V. The Quantum-Mechanical Calculation of the Resonance Energy of Benzene and Naphthalene and the Hydrocarbon Free Radicals" (PDF). The Journal of Chemical Physics. 1 (6): 362. Bibcode:1933JChPh...1..362P. doi:10.1063/1.1749304. Archived (PDF) from the original on 2022-10-09.
- —— (1935). "The Structure and Entropy of Ice and of Other Crystals with Some Randomness of Atomic Arrangement". Journal of the American Chemical Society. 57 (12): 2680–2684. Bibcode:1935JAChS..57.2680P. doi:10.1021/ja01315a102.
- —— (1940). "A Theory of the Structure and Process of Formation of Antibodies*". Journal of the American Chemical Society. 62 (10): 2643–2657. Bibcode:1940JAChS..62.2643P. doi:10.1021/ja01867a018.
- —— (1947). "Atomic Radii and Interatomic Distances in Metals". Journal of the American Chemical Society. 69 (3): 542–553. Bibcode:1947JAChS..69..542P. doi:10.1021/ja01195a024.
- ——; Itano, H. A.; Singer, S. J.; Wells, I. C. (1949). "Sickle Cell Anemia, a Molecular Disease". Science. 110 (2865): 543–548. Bibcode:1949Sci...110..543P. doi:10.1126/science.110.2865.543. PMID 15395398. S2CID 31674765.
- ——; Corey, R. B.; Branson, H. R. (1951). "The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain". Proceedings of the National Academy of Sciences. 37 (4): 205–11. Bibcode:1951PNAS...37..205P. doi:10.1073/pnas.37.4.205. PMC 1063337. PMID 14816373.
See also
[edit]References
[edit]Citations
[edit]- ^ a b c Linus Pauling at the Mathematics Genealogy Project
- ^ a b "A Guggenheim Fellow in Europe during the Golden Years of Physics (1926–1927)". Oregon State University. Archived from the original on 2021-10-28. Retrieved 2015-05-27.
- ^ a b ———— (1925). The determination with x-rays of the structures of crystals (PhD thesis). California Institute of Technology. doi:10.7907/F7V6-4P98. Retrieved 2022-04-13.
- ^ "Linus Pauling: Facts". Nobel Prize. Archived from the original on 2022-04-04. Retrieved 2022-04-13.
- ^ ———— (1997). Pauling, Linus Jr. (ed.). Selected papers of Linus Pauling (Volume I ed.). River Edge, New Jersey: World Scientific. p. xvii. ISBN 978-981-02-2939-9.
- ^ a b Horgan, J (1993). "Profile: Linus C. Pauling – Stubbornly Ahead of His Time". Scientific American. Vol. 266, no. 3. pp. 36–40. Bibcode:1993SciAm.266c..36H. doi:10.1038/scientificamerican0393-36.
- ^ a b c d e Linus Pauling on Nobelprize.org , accessed 30 April 2020
- ^ "Nobel Prize Facts". Nobel Prize. 2022-04-12 [2009-10-05]. Archived from the original on 2017-01-11. Retrieved 2022-04-13.
- ^ a b c Rich, Alexander (1994). "Linus Pauling (1901–1994)". Nature. 371 (6495): 285. Bibcode:1994Natur.371..285R. doi:10.1038/371285a0. PMID 8090196. S2CID 8923975.
- ^ Gribbin, John (2004). The Scientists: A History of Science Told Through the Lives of Its Greatest Inventors. New York City: Random House. pp. 558–569. ISBN 978-0-8129-6788-3. OL 8020832M.
- ^ Stone, Irwin (1982). The healing factor: "vitamin C" against disease. New York: Perigee Books. ISBN 978-0-399-50764-9. OCLC 10169988. OL 9567597M – via Internet Archive.
- ^ a b Offit, Paul (2013-07-19). "The Vitamin Myth: Why We Think We Need Supplements". The Atlantic. ISSN 2151-9463. OCLC 936540106. Retrieved 2013-07-19.
- ^ a b c d Dunitz, Jack D. (1996). "Linus Carl Pauling. 28 February 1901–19 August 1994". Biographical Memoirs of Fellows of the Royal Society. 42 (9): 316–326. doi:10.1098/rsbm.1996.0020. PMID 11619334.
- ^ "Linus Pauling's Childhood (1901–1910)". Oregon State University. Archived from the original on 2022-04-07. Retrieved 2013-04-25.
- ^ a b c d e Hager, Thomas (1995). Force of Nature: The Life of Linus Pauling. Simon & Schuster. ISBN 978-0-684-80909-0 – via Internet Archive.
- ^ a b Mead, Clifford; Hager, Thomas, eds. (2001). Linus Pauling: Scientist and Peacemaker. Oregon State University Press. ISBN 978-0-87071-489-4.
- ^ "Linus Pauling: Biographical". Nobel Prize. Archived from the original on 2022-03-18. Retrieved 2021-09-27.
- ^ Dunitz, Jack D. (1997). "Linus Carl Pauling". Biographical Memoirs. Vol. 71. National Academies Press. pp. 221–261. doi:10.17226/5737. ISBN 978-0-309-05738-7. Archived from the original on 2021-10-28. Retrieved 2021-09-27.
- ^ a b c d e f g h i j k l m n Goertzel, Ted; Goertzel, Ben (1995). Linus Pauling: A Life in Science and Politics. Basic Books. ISBN 978-0-465-00672-4 – via Internet Archive.
- ^ a b Abrams, Irwin (1988). The Nobel Peace Prize and the laureates : an illustrated biographical history, 1901–1987 (2. print. ed.). Boston: G.K. Hall. ISBN 978-0-8161-8609-9.
- ^ Bourgoin, Suzanne M.; Byers, Paula K., eds. (1998). "Pauling, Linus". Encyclopedia of World Biography. Vol. 12. Thomson Gale. p. 150. ISBN 978-0-7876-2221-3. OCLC 498136139. OL 24962233M.
- ^ "Pauling Finally Gets High School Diploma". Evening Star. 1962-06-19. p. 1 col 6. Retrieved 2022-08-03.
- ^ Pauling, Linus (2009-07-02). "Life with Lloyd Jeffress, June 5, 1986". The Pauling Blog. Oregon State University Libraries Special Collections and Archives Research Center. Retrieved 2016-06-01.
- ^ Swanson, Stephen (2000-10-03). "OSU fraternity to donate Pauling treasures to campus library". Oregon State University. Archived from the original on 2016-03-03. Retrieved 2013-04-29.
- ^ "Samuel Graf : Engineering Hall of Fame - 1998 | College of Engineering | Oregon State University". 2022-10-03.
- ^ "Long-Time OSU Faculty Man, Sam Graf, Dies {Article clipped from Corvallis Gazette-Times)". newspapers.com. 1966-07-25.
- ^ "Pauling's Years as an Undergraduate at Oregon Agricultural College, Part 2 (1919–1922)". Oregon State University. Archived from the original on 2021-10-31. Retrieved 2015-05-27.
- ^ a b c ———— (1995). Marinacci, Barbara (ed.). Linus Pauling: in his own words : selected writings, speeches, and interviews. New York City: Simon & Schuster. p. 39. ISBN 978-0-684-81387-5. Retrieved 2015-05-27 – via Internet Archive.
- ^ "Linus Pauling Biographical Timeline". Linus Pauling Institute. Oregon State University. Archived from the original on 2022-03-02. Retrieved 2011-11-10.
- ^ Richard, Terry (2013-05-03). "Ava Helen Pauling, wife of Linus Pauling, subject of biography by Corvallis author Mina Carson". The Oregonian. ISSN 8750-1317. Archived from the original on 2021-05-13. Retrieved 2015-06-02.
- ^ "Commencement 1925 California Institute of Technology Pasadena" (PDF). California Institute of Technology. 1925-06-12. Archived (PDF) from the original on 2020-11-01. Retrieved 2013-03-29.
- ^ Cohen, Robert S.; Hilpinen, Risto; Qiu, Ren-Zong, eds. (2010-12-09) [1996-10-31]. Realism and anti-realism in the philosophy of science. Beijing International Conference 1992. Dordrecht: Springer. p. 161. ISBN 978-90-481-4493-8. OL 28281917M. Retrieved 2015-05-27.
- ^ "About Linus Pauling". Pacific Northwest National Laboratory. Archived from the original on 2022-03-02. Retrieved 2022-04-13.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ———— (1987-04-06). Written at Denver. Oral history interview with Linus C. Pauling. Interviewed by Sturchio, Jeffrey L. Philadelphia: Science History Institute. Archived from the original on 2021-08-16. Retrieved 2022-04-13.
- ^ ———— (1929-04-01). "The principles determining the structure of complex ionic crystals". Journal of the American Chemical Society. 51 (4): 1010–1026. Bibcode:1929JAChS..51.1010P. doi:10.1021/ja01379a006.
- ^ a b c d e ———— (1960-01-31) [1939]. The nature of the chemical bond and the structure of molecules and crystals; an introduction to modern structural chemistry (3rd ed.). Ithaca, New York: Cornell University Press. pp. 543–562. ISBN 978-0-8014-0333-0. OL 26811428M.
- ^ Hager, Thomas (December 2004). "The Langmuir Prize". Oregon State University. Archived from the original on 2020-12-12. Retrieved 2008-02-29.
- ^ ———— (1932-03-01). "The nature of the chemical bond. III. The transition from one extreme bond type to another". Journal of the American Chemical Society. 54 (3): 988–1003. Bibcode:1932JAChS..54..988P. doi:10.1021/ja01342a022.
- ^ a b Monk, Ray (2014-03-11) [2012]. Robert Oppenheimer : a life inside the center (First Anchor Books ed.). Anchor Books. p. 203. ISBN 978-0-385-72204-9. OL 32935915M.
- ^ "Early Career at the California Institute of Technology (1927–1930)". Oregon State University. Archived from the original on 2021-11-12. Retrieved 2017-05-18.
- ^ "A Lost Ally". Oregon State University. Archived from the original on 2021-08-20. Retrieved 2015-05-27.
- ^ Hargittai, István; Hargittai, Magdolna (2000-02-29). In our own image: personal symmetry in discovery. New York City: Springer Nature. ISBN 978-0-306-46091-3. LCCN 99033173. OL 9669915M. Retrieved 2015-05-27.
- ^ ———— (1932-09-01). "The Nature of the Chemical Bond. IV. The Energy of Single Bonds and the Relative Electronegativity of Atoms". Journal of the American Chemical Society. 54 (9): 3570–3582. Bibcode:1932JAChS..54.3570P. doi:10.1021/ja01348a011. ISSN 0002-7863. LCCN 16003159. OCLC 01226990.
- ^ "The Pauling Electronegativity Scale: Part 2, Inspired by Biology". Oregon State University. 2009-03-17. Archived from the original on 2021-11-17. Retrieved 2009-03-17.
- ^ "Obituary: Professor Linus Pauling". The Independent. 1994-08-21. Archived from the original on 2021-06-16. Retrieved 2018-01-25.
- ^ "Outline of the George Fischer Baker Lectureship, Cornell University". Oregon State University. Archived from the original on 2021-11-12. Retrieved 2022-04-13.
- ^ "The George Fischer Baker Lectureship and the Beginnings of the Manuscript". The Pauling Blog. Oregon State University. 2014-07-30. Archived from the original on 2022-03-07. Retrieved 2015-06-03.
- ^ Watson, James D. (2001). A passion for DNA: genes, genomes, and society (2003 ed.). Oxford: Oxford University Press. ISBN 978-0-19-860428-0. OL 7401431M – via Internet Archive.
- ^ "The nature of the chemical bond (citations and estimated counts)". Google Scholar. Retrieved 2015-05-27.
- ^ Pauling, Linus (1928). "London's paper. General ideas on bonds". Oregon State University Libraries Special Collections. Retrieved 2015-06-02.
- ^ Pauling, Linus (1930s). "Notes and Calculations re: Electronegativity and the Electronegativity Scale". Oregon State University Libraries Special Collections. Retrieved 2008-02-29.
- ^ Pauling, Linus (1934-01-06). "Benzene". Oregon State University Libraries Special Collections. Retrieved 2008-02-29.
- ^ Pauling, Linus (1946-07-29). "Resonance". Oregon State University Libraries Special Collections. Retrieved 2008-02-29.
- ^ Pauling, Linus (1929). "The principles determining the structure of complex ionic crystals". J. Am. Chem. Soc. 51 (4): 1010–1026. Bibcode:1929JAChS..51.1010P. doi:10.1021/ja01379a006.
- ^ Kay, Lily E. (1996). The molecular vision of life: Caltech, the Rockefeller Foundation, and the rise of the new biology. New York [u.a.]: Oxford University Press. pp. 148–151. ISBN 978-0-19-511143-9. Retrieved 2015-05-27.
- ^ a b Califano, Salvatore (2012). Pathways to modern chemical physics. Heidelberg [Germany]: Springer. p. 198. ISBN 978-3-642-28179-2. Retrieved 2015-05-27.
- ^ Livio, Mario (2014). Brilliant blunders: from Darwin to Einstein: colossal mistakes by great scientists that changed our understanding of life and the universe. [S.l.]: Simon & Schuster. ISBN 978-1-4391-9237-5.
- ^ Pauling, L; Corey, RB (1951). "Configurations of Polypeptide Chains With Favored Orientations Around Single Bonds: Two New Pleated Sheets". Proceedings of the National Academy of Sciences of the United States of America. 37 (11): 729–40. Bibcode:1951PNAS...37..729P. doi:10.1073/pnas.37.11.729. PMC 1063460. PMID 16578412.
- ^ a b Goertzel and Goertzel, p. 95-100.
- ^ Pauling, L; Corey, RB (February 1953). "A Proposed Structure For The Nucleic Acids". Proc Natl Acad Sci U S A. 39 (2): 84–97. Bibcode:1953PNAS...39...84P. doi:10.1073/pnas.39.2.84. PMC 1063734. PMID 16578429.
- ^ "Linus Pauling's DNA Model". Archived from the original on 2012-02-04. Retrieved 2015-06-02.
- ^ a b Dye, Lee (1985-06-02). "The Deeply Personal War of Linus Pauling: Nobel Prize-Winning Chemist Still Battles for His Controversial Vitamin Theory". Los Angeles Times. Retrieved 2023-04-09.
- ^ Metzler, David E. (2003). Biochemistry (2nd ed.). San Diego: Harcourt, Academic Pr. ISBN 978-0-12-492541-0.
- ^ Cruse, Julius M.; Lewis, Robert E. (2010). Atlas of immunology (3rd ed.). Boca Raton, FL: CRC Press/Taylor & Francis. p. 21. ISBN 978-1-4398-0268-7. Retrieved 2015-05-27.
- ^ Tudge, Colin (1995). The engineer in the garden: Genes and genetics: from the idea of heredity to the creation of life (1st American ed.). New York: Hill and Wang. ISBN 978-0-8090-4259-3. Retrieved 2015-05-27.
- ^ Pauling, L.; Itano, H. A.; Singer, S. J.; Wells, I. C. (1949-11-25). "Sickle Cell Anemia, a Molecular Disease". Science. 110 (2865): 543–548. Bibcode:1949Sci...110..543P. doi:10.1126/science.110.2865.543. PMID 15395398. S2CID 31674765. Retrieved 2015-06-02.
- ^ Andersch, MA; Wilson, DA; Menten, ML. (1944). "Sedimentation constants and electrophoretic mobilities of adult and fetal carbonylhemoglobin". Journal of Biological Chemistry. 153: 301–305. doi:10.1016/S0021-9258(18)51237-0.
- ^ a b c Strasser, Bruno J. (2002-08-30). "Linus Pauling's "molecular diseases": Between history and memory" (PDF). American Journal of Medical Genetics. 115 (2): 83–93. CiteSeerX 10.1.1.613.5672. doi:10.1002/ajmg.10542. PMID 12400054. Archived (PDF) from the original on 2022-10-09. Retrieved 2015-05-27.
- ^ a b c "A Flamboyant Scientist's Legacy : Scholar: Linus C. Pauling's supporters and detractors join in calling the two-time Nobel winner one of the most significant figures of this century". Los Angeles Times. 1994-08-21. Retrieved 2015-06-01.
- ^ Pauling, Linus (October 1951). "Molecular Medicine". Ava Helen and Linus Pauling Papers. Retrieved 2007-08-05.
- ^ Morgan, Gregory J. (1998). "Emile Zuckerkandl, Linus Pauling, and the molecular evolutionary clock, 1959–1965". Journal of the History of Biology. 31 (2): 155–78. doi:10.1023/A:1004394418084. JSTOR 4331476. PMID 11620303. S2CID 5660841. Retrieved 2023-06-11.
- ^ a b Pauling, Linus (1965). "The Close-Packed Spheron Model of atomic nuclei and its relation to the shell model". Proceedings of the National Academy of Sciences of the United States of America. 54 (4): 989–994. Bibcode:1965PNAS...54..989P. doi:10.1073/pnas.54.4.989. PMC 219778. PMID 16578621.
- ^ Pauling, L (1965-10-15). "The close-packed-spheron theory and nuclear fission". Science. 150 (3694): 297–305. Bibcode:1965Sci...150..297P. doi:10.1126/science.150.3694.297. PMID 17742357.
- ^ Pauling, Linus (July 1966). "The close-packed-spheron theory of nuclear structure and the neutron excess for stable nuclei (Dedicated to the seventieth anniversary of Professor Horia Hulubei)". Revue Roumain de Physique. Retrieved 2007-08-05.
- ^ Pauling, Linus (December 1967). "Magnetic-moment evidence for the polyspheron structure of the lighter atomic nuclei". Proceedings of the National Academy of Sciences. 58 (6): 2175–2178. Bibcode:1967PNAS...58.2175P. doi:10.1073/pnas.58.6.2175. PMC 223816. PMID 16591577. Retrieved 2007-08-05.
- ^ Pauling, Linus (November 1969). "Orbiting clusters in atomic nuclei". Proceedings of the National Academy of Sciences of the United States of America. 64 (3). Proceedings of the National Academy of Sciences: 807–9. Bibcode:1969PNAS...64..807P. doi:10.1073/pnas.64.3.807. PMC 223305. PMID 16591799. Retrieved 2007-08-05.
- ^ Pauling, Linus; Arthur B. Robinson (1975). "Rotating clusters in nuclei". Canadian Journal of Physics. Retrieved 2007-08-05.
- ^ Pauling, Linus (February 1991). "Transition from one revolving cluster to two revolving clusters in the ground-state rotational bands of nuclei in the lanthanon region". Proc. Natl. Acad. Sci. 88 (3): 820–823. Bibcode:1991PNAS...88..820P. doi:10.1073/pnas.88.3.820. PMC 50905. PMID 11607150. Retrieved 2007-08-05.
- ^ Pauling, Linus (1969-11-15). "Orbiting clusters in atomic nuclei". Proceedings of the National Academy of Sciences. 64 (3): 807–809. Bibcode:1969PNAS...64..807P. doi:10.1073/pnas.64.3.807. PMC 223305. PMID 16591799.
- ^ "Linus C. Pauling, Ph.D. Biography and Interview". www.achievement.org. American Academy of Achievement.
- ^ "Hiroshima". Linus Pauling and the International Peace Movement. Special Collections & Archives Research Center, Oregon State University. Retrieved 2015-05-27.
- ^ Kay, Lily E. (1996). The molecular vision of life : Caltech, the Rockefeller Foundation, and the rise of the new biology. New York: Oxford University Press. p. 179. ISBN 978-0-19-511143-9. Retrieved 2015-12-27.
- ^ Thackray, Arnold & Minor Myers, Jr. (2000). Arnold O. Beckman : one hundred years of excellence. foreword by James D. Watson. Philadelphia, Pa.: Chemical Heritage Foundation. ISBN 978-0-941901-23-9.
- ^ "Beckman D2 Oxygen Analyzer". Wood Library-Museum of Anesthesiology. Retrieved 2015-05-28.
- ^ "Blood and War: The Development of Oxypolygelatin, Part 1". The Pauling Blog. 2009-01-27. Retrieved 2015-05-28.
- ^ Chadarevian, Soraya de (1998). Molecularizing biology and medicine new practices and alliances, 1910s–1970s. Amsterdam: Harwood Academic. p. 109. ISBN 978-90-5702-293-7. Retrieved 2015-05-28.
- ^ "Presidential Medal for Merit". Linus Pauling Awards Honors and Medals. Retrieved 2015-05-28.
- ^ "The Linus Pauling Papers: Biographical Information". United States National Library of Medicine. Retrieved 2008-02-11.
- ^ Paulus, John Allen (1995-11-05). "Pauling's Prizes". The New York Times. Retrieved 2007-12-09.
- ^ "ACS President: Linus Pauling (1901–1994)". ACS Chemistry for Life. Retrieved 2015-06-01.
- ^ Part VI: The Manhattan District in Peacetime: The May-Johnson Bill, Atomic Archive, 1998, retrieved 2019-10-19
- ^ Atomic Energy Commission, Atomic Heritage Foundation, 2016-11-18, retrieved 2019-10-19
- ^ Roy Glauber & Priscilla McMillan on Oppenheimer – Atomic Energy Commission, Voices of the Manhattan Project, 2013-06-06, retrieved 2019-10-19
- ^ a b The Independent Citizens Committee of the Arts, Sciences and Professions, Linus Pauling and the International Peace Movement, 2009, retrieved 2019-10-19
- ^ Hager, Thomas (2007-11-29). "Einstein". Oregon State University Libraries Special Collections. Retrieved 2007-12-13.
- ^ "Linus Pauling". U.S. Stamp Gallery. Retrieved 2015-06-02.
- ^ Pauling, Linus (May 1952). "The Department of State and the Structure of Proteins". Oregon State University Libraries Special Collections. Retrieved 2007-12-13.
- ^ Robert Paradowski (2011), Oregon State University, Special Collections p.18, Proteins, Passports, and the Prize (1950–1954), retrieved February 1, 2013
- ^ Bulletin of the Atomic Scientists Vol. VIII, Nr. 7 (Okt. 1952) p. 254, Educational Foundation for Nuclear Science, Inc.
- ^ Hager, Thomas (2007-11-29). "Russell/Einstein". Oregon State University Libraries Special Collections. Retrieved 2007-12-13.
- ^ Hermann, Armin (1979). The new physics: the route into the atomic age: in memory of Albert Einstein, Max von Laue, Otto Hahn, Lise Meitner. Bonn-Bad Godesberg: Inter Nationes. p. 130.
- ^ a b "The Baby Tooth Survey". The Pauling Blog. Retrieved 2011-06-01.
- ^ "The Nobel Peace Prize 1962 Linus Pauling: Nobel Lecture". Nobel Prize.org. Retrieved 2015-05-28.
- ^ "Linus Pauling Receives the Nobel Peace Prize". The Pauling Blog. Retrieved 2013-12-10.
- ^ Moore, Kelly (2008). Disrupting science : social movements, American scientists, and the politics of the military, 1945–1975. Princeton: Princeton University Press. p. 113. ISBN 978-0-691-11352-4. Retrieved 2015-05-28.
- ^ Reiss, Louise Zibold (1961-11-24). "Strontium-90 Absorption by Deciduous Teeth: Analysis of teeth provides a practicable method of monitoring strontium-90 uptake by human populations". Science. 134 (3491): 1669–1673. doi:10.1126/science.134.3491.1669. PMID 14491339.
- ^ Hager, Thomas (2007-11-29). "Strontium-90". Oregon State University Libraries Special Collections. Retrieved 2007-12-13.
- ^ Hager, Thomas (2007-11-29). "The Right to Petition". Oregon State University Libraries Special Collections. Retrieved 2007-12-13.
- ^ McCormick, John (1991). Reclaiming paradise : the global environmental movement (1st Midland ed.). Bloomington: Indiana University Press. ISBN 978-0-253-20660-2. Retrieved 2015-05-27.
- ^ Allen, Garland E.; MacLeod, Roy M. (2001). Science, history and social activism : a tribute to Everett Mendelsohn. Dordrecht: Kluwer Academic. p. 302. ISBN 978-1-4020-0495-7. Retrieved 2015-05-27.
- ^ "Nobel Peace Prize Awarded to Pauling". Palo Alto Times. 1963-10-10. Retrieved 2015-05-27.
- ^ Pauling, Linus (1963-10-10). "Notes by Linus Pauling. October 10, 1963". Oregon State University Libraries Special Collections. Retrieved 2007-12-13.
- ^ "Linus Pauling Biography". Linus Pauling Institute. 2014-05-09. Retrieved 2015-06-02.
- ^ "issued to Linus Pauling by the Internal Security Subcommittee of the United States Senate. June 20, 1960". Linus Pauling and the International Peace Movement. Retrieved 2015-05-28.
- ^ a b Mason, Stephen F. (1997). "The Science and Humanism of Linus Pauling (1901–1994)". Chemical Society Reviews. 26: 29–39. doi:10.1039/cs9972600029. Archived from the original on 2009-05-15. Retrieved 2015-05-20.
- ^ Kovac, Jeffrey (1999). "A weird insult from Norway: Linus Pauling as public intellectual". Soundings: An Interdisciplinary Journal. 82 (1/2): 91–106. JSTOR 41178914.
- ^ "A Weird Insult From Norway". Life. Vol. 5, no. 17. 1963-10-25. p. 4.
- ^ "The National Review Lawsuit". Paulingblog. 2013-01-30. Retrieved 2013-12-20.
- ^ "A Tough Conclusion to the National Review Lawsuit". Paulingblog. Retrieved 2013-12-20.
- ^ "Pauling v. Nat'l Review, Inc". Justia.com. Retrieved 2013-12-20.
- ^ Saxon, Wolfgang (1998-08-30). "C. Dickerman Williams, 97, Free-Speech Lawyer, Is Dead". The New York Times. Retrieved 2013-12-20.
- ^ "Linus Pauling and the International Peace Movement: Vietnam". Oregon State University Libraries. 2010.
- ^ "Lenin Peace Prize Recipients". Research History. 2011-05-16.
- ^ "Founders". International League of Humanists for peace and tolerance. Archived from the original on 2015-06-11. Retrieved 2015-05-28.
- ^ "The Dubrovnik-Philadelphia Statement /1974–1976/ (short version)". International League of Humanists. Archived from the original on 2015-09-24. Retrieved 2015-05-28.
- ^ "History". International Academy of Science, Munich. Archived from the original on 2015-04-02. Retrieved 2015-03-16.
- ^ Johnson, Loch K., ed. (2007). Handbook of Intelligence Studies. Taylor & Francis. p. 275.
- ^ "Letters from Thane Read asking Helen Keller to sign the World Constitution for world peace. 1961". Helen Keller Archive. American Foundation for the Blind. Retrieved 2023-07-01.
- ^ "Letter from World Constitution Coordinating Committee to Helen, enclosing current materials". Helen Keller Archive. American Foundation for the Blind. Retrieved 2023-07-03.
- ^ "Preparing earth constitution | Global Strategies & Solutions | The Encyclopedia of World Problems". The Encyclopedia of World Problems | Union of International Associations (UIA). Archived from the original on 2023-07-19. Retrieved 2023-07-15.
- ^ Mendelsohn, Everett (March–April 2000). "The Eugenic Temptation". Harvard Magazine.
- ^ Special Collections & Archives Research Center, Oregon State University Libraries (2015). "Eugenics for Alleviating Human Suffering". It's in the Blood! A Documentary History of Linus Pauling, Hemoglobin and Sickle Cell Anemia. Retrieved 2020-05-30.
- ^ Pauling, Linus (1987). How to Live Longer and Feel Better (1 ed.). New York: Avon Books. OL 18076125M.
- ^ Peitzman, Steven J. (2007). Dropsy, dialysis, transplant: a short history of failing kidneys. Baltimore: Johns Hopkins University Press. pp. 72–8, 190. ISBN 978-0-8018-8734-5.
- ^ Nicolle, Lorraine; Beirne, Ann Woodriff, eds. (2010). Biochemical imbalances in disease a practitioner's handbook. London: Singing Dragon. p. 27. ISBN 978-0-85701-028-5.
- ^ Pauling, Linus (April 1968). "Orthomolecular psychiatry. Varying the concentrations of substances normally present in the human body may control mental disease". Science. 160 (3825): 265–71. Bibcode:1968Sci...160..265P. doi:10.1126/science.160.3825.265. PMID 5641253. S2CID 20153555.
- ^ Cassileth, Barrie R. (1998). The alternative medicine handbook: the complete reference guide to alternative and complementary therapies. New York: W.W. Norton. p. 67. ISBN 978-0-393-04566-6.
- ^ "Vitamin Therapy, Megadose / Orthomolecular Therapy". BC Cancer Agency. February 2000. Archived from the original on 2007-02-02. Retrieved 2007-08-05.
- ^ "PaulingTherapy.com – Reversing Heart Disease w/o Drugs is Possible". www.paulingtherapy.com.
- ^ Cameron, Ewan. "Cancer Bibliography: Ewan Cameron, M.D. and Vitamin C Therapy". Doctoryourself.com. Retrieved 2007-08-05.
- ^ Severo, Richard (1994-08-21). "Linus C. Pauling Dies at 93; Chemist and Voice for Peace". The New York Times. Retrieved 2015-06-01.
- ^ Cameron, E; Pauling, L (October 1976). "Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer". Proceedings of the National Academy of Sciences. 73 (10): 3685–9. Bibcode:1976PNAS...73.3685C. doi:10.1073/pnas.73.10.3685. PMC 431183. PMID 1068480.
- ^ Cameron, E; Pauling, L (September 1978). "Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer". Proceedings of the National Academy of Sciences. 75 (9): 4538–42. Bibcode:1978PNAS...75.4538C. doi:10.1073/pnas.75.9.4538. PMC 336151. PMID 279931.
- ^ DeWys, WD (1982). "How to evaluate a new treatment for cancer". Your Patient and Cancer. 2 (5): 31–36.
- ^ Creagan, ET; Moertel, CG; O'Fallon, JR (September 1979). "Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial". The New England Journal of Medicine. 301 (13): 687–90. doi:10.1056/NEJM197909273011303. PMID 384241.
- ^ Moertel, CG; Fleming, TR; Creagan, ET; Rubin, J; O'Connell, MJ; Ames, MM (January 1985). "High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison". The New England Journal of Medicine. 312 (3): 137–41. doi:10.1056/NEJM198501173120301. PMID 3880867.
- ^ Tschetter, L; et al. (1983). "A community-based study of vitamin C (ascorbic acid) in patients with advanced cancer". Proceedings of the American Society of Clinical Oncology. 2: 92.
- ^ a b Chen, Q; Espey, M. G.; Sun, A. Y.; Lee, J.-H.; Krishna, M. C.; Shacter, E.; Choyke, P. L.; Pooput, C.; Kirk, K. L.; Buettner, G. R.; Levine, M.; et al. (2007). "Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo". Proceedings of the National Academy of Sciences. 104 (21): 8749–54. Bibcode:2007PNAS..104.8749C. doi:10.1073/pnas.0702854104. PMC 1885574. PMID 17502596.
- ^ Goertzel, Ted (1996). "Analyzing Pauling's Personality: A Three Generational, Three Decade Project". Special Collections, Oregon State University Libraries. Archived from the original on 2007-10-14. Retrieved 2007-08-05.
- ^ a b Pinch, Trevor; Collins, Harry M. (2005). "Alternative Medicine: The Cases of Vitamin C and Cancer". Dr. Golem: how to think about medicine. Chicago: University of Chicago Press. pp. 89–111. ISBN 978-0-226-11366-1.
- ^ Levine, M; et al. (2006). "Intravenously administered vitamin C as cancer therapy: three cases". CMAJ. 174 (7): 937–942. doi:10.1503/cmaj.050346. PMC 1405876. PMID 16567755.
- ^ Pauling, Linus (1986). How to Live Longer and Feel Better. New York: Freeman. pp. 173–175. ISBN 978-0-7167-1781-2.
- ^ Pauling, L (November 1978). Ralph Pelligra (ed.). "Orthomolecular enhancement of human development" (PDF). Human Neurological Development: 47–51. Archived (PDF) from the original on 2022-10-09.
- ^ Saul, Andrew W.; Dr. Abram Hoffer. "Abram Hoffer, M.D., PhD 50 Years of Megavitamin Research, Practice and Publication". Doctoryourself.com. Retrieved 2007-08-05.
- ^ Ohno, S; Ohno, Y; Suzuki, N; Soma, G; Inoue, M (2009). "High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer". Anticancer Research. 29 (3): 809–15. PMID 19414313.
- ^ Jacobs, Carmel; Hutton, Brian; Ng, Terry; Shorr, Risa; Clemons, Mark (2015). "Is There a Role for Oral or Intravenous Ascorbate (Vitamin C) in Treating Patients With Cancer? A Systematic Review". The Oncologist. 20 (2): 210–223. doi:10.1634/theoncologist.2014-0381. PMC 4319640. PMID 25601965.
- ^ "Vitamin C Fact Sheet for Health Professionals". National Institutes of Health. Retrieved 2015-06-02.
- ^ "The Linus Pauling Papers: Biographical Information". United States National Library of Medicine. n.d. Retrieved 2011-11-10.
- ^ "Dr. Linus Carl Pauling Jr. Obituary". Honolulu Star-Advertiser. 2023-11-05.
- ^ Petersen, Christoffer Eric, ed. (2022-10-11). "Chapter 17. Peter Pauling by Matt McConnell". Visions of Linus Pauling. World Scientific. pp. 203–240. ISBN 978-981-12-6077-3.
- ^ "Obituary. E. Crellin Pauling '59". Reed Magazine, Reed College. November 1997.
- ^ "Linus Pauling Biography". Linus Pauling Institute. Retrieved 2011-11-10.
- ^ "Oral history interview with Linus Carl Pauling, 1964 March 27". American Institute of Physics. Archived from the original on 2014-08-06. Retrieved 2015-05-27.
- ^ "Linus Pauling". Dictionary of Unitarian & Universalist Biography. Archived from the original on 2018-10-16. Retrieved 2015-05-27.
- ^ Pauling, Linus; Ikeda, Daisaku (1992). A Lifelong Quest for Peace: A Dialogue. Jones & Bartlett. p. 22. ISBN 978-0-86720-277-9.
...I [Pauling] am not, however, militant in my atheism. The great English theoretical physicist Paul Dirac is a militant atheist. I suppose he is interested in arguing about the existence of God. I am not. It was once quipped that there is no God and Dirac is his prophet.
- ^ "Dr. Pauling Rescued, On a Sea Cliff 24 Hrs" (clipping). scarc.library.oregonstate.edu. Special Collections & Archives Research Center, Oregon State University Libraries: New York Herald Tribune. 1960-02-01. Retrieved 2018-04-22.
- ^ Goertzel and Goertzel, p. 247.
- ^ "The Centennial: Who's Buried in Linus Pauling's Grave?" (PDF). Archived (PDF) from the original on 2022-10-09. Retrieved 2012-12-26.
- ^ "Linus Pauling". California Museum. 2012-02-17. Retrieved 2015-06-01.
- ^ "Linus Pauling – Biographical". Nobelprize.org. Nobel Media AB 2014. Retrieved 2016-10-06.
- ^ Hamilton, Neil A. (2002). American social leaders and activists. New York: Facts On File. ISBN 978-0-8160-4535-8. Retrieved 2015-06-01.
- ^ Hoffmann, Roald; Shaik, Sason; Hiberty, Philippe C. (2003). "A Conversation on VB vs MO Theory: A Never-Ending Rivalry?". Acc Chem Res. 36 (10): 750–6. doi:10.1021/ar030162a. PMID 14567708.
- ^ "Pauling Honored by Scientists at Caltech Event". Los Angeles Times. United Press International. 1986-03-01. Retrieved 2012-07-22.
- ^ "Citations for Chemical Breakthrough Awards 2017 Awardees". Division of the History of Chemistry. Retrieved 2018-03-12.
- ^ Pauling, L.; Corey, R. B.; Branson, H. R. (1951). "The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain". Proceedings of the National Academy of Sciences. 37 (4): 205–11. Bibcode:1951PNAS...37..205P. doi:10.1073/pnas.37.4.205. PMC 1063337. PMID 14816373.
- ^ "Linus Pauling Science Center | Department of Chemistry | Oregon State University". chemistry.oregonstate.edu. Retrieved 2016-11-10.
- ^ "Four Legends of American Science Now on U.S. Postage Stamps" (PDF). United States Postal Service Postal News, Release No. 08-23. 2008-03-06. Archived (PDF) from the original on 2022-10-09.
- ^ a b "OSU Celebrates Linus Pauling and Release of New U.S. Postal Service Stamp". Oregon State University – University Events. Archived from the original on 2013-11-02. Retrieved 2015-02-25.
- ^ "Governor & First Lady Participate in 2008 CA Hall of Fame Induction Ceremony". CA.gov. Archived from the original on 2015-06-02. Retrieved 2015-06-01.
- ^ a b "Linus Pauling Research Notebooks Online". Natural Science. Archived from the original on 2015-09-05. Retrieved 2015-06-01.
- ^ "Linus Pauling Institute". Lpi.oregonstate.edu. Retrieved 2013-06-25.
- ^ Cole, Gail (2011-10-14). "Linus Pauling Science Center opens at OSU". Corvallis Gazette-Times. Retrieved 2015-06-02.
- ^ "Linus Pauling Science Center – A Moment to Celebrate". Oregon State University Foundation. Archived from the original on 2015-03-29. Retrieved 2015-06-02.
- ^ Zewail, Ahmed (1992). The Chemical Bond Structure and Dynamics. Burlington: Elsevier Science. ISBN 978-0-08-092669-8. Retrieved 2015-06-01.
- ^ Baum, Rudy (1989-12-11). "Caltech launches Linus Pauling lecture series". Chemical & Engineering News. 67 (50): 18–19. doi:10.1021/cen-v067n050.p018a.
- ^ Johnson, Greg (1996-03-20). "Pauling Road Address Fits New Vitamin Factory to a 'C'". Los Angeles Times. Retrieved 2015-06-02.
- ^ Gottlieb, Jeff (2001-08-19). "A New-View University". Los Angeles Times. Retrieved 2015-06-01.
- ^ Woodward, Raju (2012-02-29). "A son's tribute by Linus Pauling Jr". Corvallis Gazette-Times. Retrieved 2015-06-01.
- ^ "Scientist cites Condon years as influential". Register-Guard, Eugene, Oregon. 1988-10-19. Retrieved 2015-06-01.
- ^ Heberlein, L. A. (2002). The Rough guide to internet radio. London: Rough Guides. ISBN 978-1-85828-961-8. Retrieved 2015-06-01.
- ^ Schmadel, Lutz D. (2012). Dictionary of minor planet names (6th ed.). Berlin: Springer. ISBN 978-3-642-29718-2. Retrieved 2015-06-01.
- ^ Moody, Glyn (2002). Rebel Code: Linux and the Open Source Revolution. Perseus Books Group. p. 336. ISBN 978-0-7382-0670-7.
- ^ Agre, Peter (2013-12-10). "Fifty Years Ago: Linus Pauling and the Belated Nobel Peace Prize" (PDF). Science & Diplomacy. 2 (4). Archived from the original on 2014-02-13.
- ^ "Linus Pauling Distinguished Postdoctoral Fellowship | PNNL". www.pnnl.gov.
- ^ Center for Oral History. "Linus C. Pauling". Science History Institute.
- ^ a b c d e f g h i j k l m n o p q r s t u "Linus Pauling: Awards, Honors and Medals". Special Collections. Oregon State University Libraries. Retrieved 2013-04-25.
- ^ "ACS Award in Pure Chemistry". American Chemical Society. Retrieved 2014-01-18.
- ^ "Linus Pauling". Member Directory. National Academy of Sciences.
- ^ "American Philosophical Society Member History". American Philosophical Society.
- ^ "Alpha Chi Sigma Fraternity, Certificate of Membership". Special Collections & Archives Research Center. Oregon State University Libraries. Retrieved 2015-05-27.
- ^ "Linus Carl Pauling". Member Directory. American Academy of Arts and Sciences. 2023-02-09.
- ^ "Societe de Chimie Biologique, Louis Pasteur Medal. 1952 – Linus Pauling: Awards, Honors and Medals". scarc.library.oregonstate.edu.
- ^ Pauling's awards and medals[permanent dead link] (includes image of Fermat medal).
- ^ "Gandhi Peace Award". Promoting Enduring Peace. Retrieved 2013-04-26.
- ^ "Gold Medal Honorees". National Institute of Social Sciences. Archived from the original on 2019-07-02. Retrieved 2019-07-02.
- ^ "NAS Award in Chemical Sciences". National Academy of Sciences. Retrieved 2015-06-02.
- ^ "Golden Plate Awardees of the American Academy of Achievement". www.achievement.org. American Academy of Achievement.
- ^ "OSU Celebrates Linus Pauling and Release of New U.S. Postal Service Stamp". Events. Oregon State University. Archived from the original on 2013-11-02. Retrieved 2013-04-25.
Bibliography
[edit]- Hager, Thomas (1998). Linus Pauling and the Chemistry of Life. Oxford University Press. ISBN 978-0-19-513972-3 – via Internet Archive.
- Marinacci, Barbara; Krishnamurthy, Ramesh (1998). Linus Pauling on Peace. Rising Star Press. ISBN 978-0-933670-03-7.
- Serafini, Anthony (1989). Linus Pauling: A Man and His Science. Paragon House. ISBN 978-1-55778-440-7 – via Internet Archive.
General and cited references
[edit]- Hargittai, István (2000). Hargittai, Magdolna (ed.). Candid Science: Conversations with Famous Chemists (Reprinted ed.). London: Imperial College Press. ISBN 978-1-86094-151-1.
- Marinacci, Barbara, ed. (1995). Linus Pauling: In His Own Words: Selected Writings, Speeches, and Interviews. Introduction by Linus Pauling. New York: Simon & Schuster. ISBN 978-0-684-81387-5. online
- Pauling, Linus. Selected Scientific Papers Vol. II online
- Sturchio, Jeffrey L. (1987-04-06). Linus C. Pauling, Transcript of an Interview Conducted by Jeffrey L. Sturchio in Denver, Colorado on 6 April 1987 (PDF). Philadelphia, PA: Chemical Heritage Foundation.
Further reading
[edit]- Coffey, Patrick (2008). Cathedrals of Science: The Personalities and Rivalries That Made Modern Chemistry. Oxford University Press. ISBN 978-0-19-532134-0.
- Davenport, Derek A. (1996). "The Many Lives of Linus Pauling: A Review of Reviews". Journal of Chemical Education. 73 (9): A210. Bibcode:1996JChEd..73A.210D. doi:10.1021/ed073pA210.
- Gormley, Melinda. "The first ‘molecular disease’: a story of Linus Pauling, the intellectual patron." Endeavour 31.2 (2007): 71–77 online Archived 2020-10-31 at the Wayback Machine.
- Mead, Clifford. Linus Pauling: Scientist and Peacemaker (2008)
- Morgan, G. J. "Emile Zuckerkandl, Linus Pauling, and the molecular evolutionary clock, 1959–1965." Journal of the History of Biology (1998) 155–178.
- Nakamura, Jeanne, and Mihaly Csikszentmihalyi. "Catalytic creativity: The case of Linus Pauling." American Psychologist 56.4 (2001): 337+.
- Strasser, Bruno J. "A world in one dimension: Linus Pauling, Francis Crick and the central dogma of molecular biology." History and philosophy of the life sciences (2006): 491–512 online.
- Strasser, Bruno J. "Linus Pauling's “molecular diseases”: Between history and memory." American journal of medical genetics 115.2 (2002): 83–93 online.
- White, Florence Meiman. Linus Pauling Scientist and Crusader (1980) online
- Zannos, Susan. Linus Pauling and the chemical bond (2004), 48pp online, for secondary schools
External links
[edit]- Linus Pauling Online a Pauling portal created by Oregon State University Libraries
- Crick, Francis, "The Impact of Linus Pauling on Molecular Biology" (transcribed from video at the 1995 Oregon State University symposium)
- The Ava Helen and Linus Pauling Papers at the Oregon State University Libraries
- The Pauling Catalogue
- Center for Oral History. "Linus C. Pauling". Science History Institute.
- Sturchio, Jeffrey L. (1987-04-06). Linus C. Pauling, Transcript of an Interview Conducted by Jeffrey L. Sturchio in Denver, Colorado on 6 April 1987 (PDF). Philadelphia, PA: Chemical Heritage Foundation.
- The Pauling Blog
- Linus Pauling (1901–1994)
- Berkeley Conversations With History interview
- Linus Pauling Centenary Exhibit
- Linus Pauling from The Dictionary of Unitarian and Universalist Biography Archived 2018-10-16 at the Wayback Machine
- "It's in the Blood! A Documentary History of Linus Pauling, Hemoglobin and Sickle Cell Anemia – Special Collections & Archives Research Center – Oregon State University". Oregon State University Library. Retrieved 2015-02-25.
- The Linus Pauling Institute at Oregon State University
- Publications of Pauling
- The Linus Pauling Papers – Profiles in Science, National Library of Medicine
- Linus Pauling Archived July 19, 2019, at the Wayback Machine Documentary produced by Oregon Public Broadcasting
- Oral history interview with Linus C. Pauling from Science History Institute Digital Collections
- 1901 births
- 1994 deaths
- 20th-century American biochemists
- 20th-century atheists
- American anti-war activists
- American anti–nuclear weapons activists
- American atheists
- American biophysicists
- American humanists
- American Nobel laureates
- American pacifists
- American people of German descent
- American physical chemists
- California Institute of Technology alumni
- California Institute of Technology faculty
- Deaths from prostate cancer in California
- Fellows of the American Academy of Arts and Sciences
- Fellows of the American Physical Society
- Fellows of the Royal Society of Edinburgh
- Foreign members of the Royal Society
- Foreign members of the Russian Academy of Sciences
- Foreign members of the USSR Academy of Sciences
- History of genetics
- American inorganic chemists
- Medal for Merit recipients
- Members of the American Chemical Society
- Members of the American Philosophical Society
- Members of the Bavarian Academy of Sciences
- Members of the French Academy of Sciences
- Members of the International Academy of Quantum Molecular Science
- Members of the Norwegian Academy of Science and Letters
- Members of the United States National Academy of Sciences
- National Medal of Science laureates
- Nobel laureates in Chemistry
- Nobel laureates with multiple Nobel awards
- Nobel Peace Prize laureates
- Oregon State University faculty
- Orthomolecular medicine advocates
- People from Condon, Oregon
- Proceedings of the National Academy of Sciences of the United States of America editors
- Recipients of the Lenin Peace Prize
- Recipients of the Lomonosov Gold Medal
- Theoretical chemists
- Time Person of the Year
- Vannevar Bush Award recipients
- Washington High School (Portland, Oregon) alumni
- World Constitutional Convention call signatories
- Writers from Portland, Oregon
- Members of Phi Kappa Phi
- Fellows of the Royal Society of Arts
- Delta Upsilon members